Stable vanadium isotopes as a redox proxy in magmatic systems?
Affiliations | Corresponding Author | Cite as | Funding informationKeywords: vanadium, stable isotopes, magmatic fractionation, oxygen fugacity, magnetite
- Share this article





Article views:12,523Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
Abstract
Figures and Tables
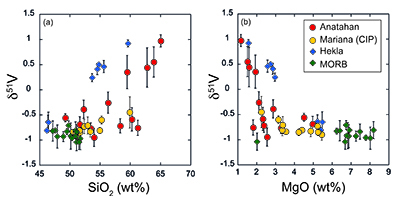 Figure 1 δ51V variations with SiO2 (a) and MgO (b). CIP = Central Island Province. MORB data from Prytulak et al. (2013). Uncertainties on isotope measurements are external 2 sd. | 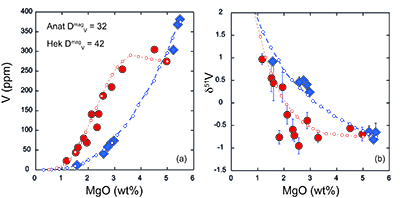 Figure 2 Cotectic fractional crystallisation models. Each small symbol represents a 5 % crystal fractionation increment. See Supplementary Information for input parameters. Symbols and uncertainties as in Figure 1. | 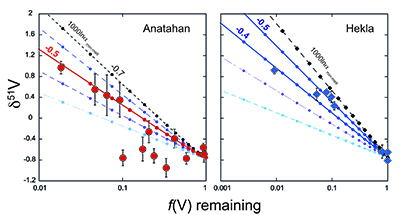 Figure 3 Rayleigh fractionation calculations with same parameters as Figure 2. Symbols and uncertainties as in Figure 1. | 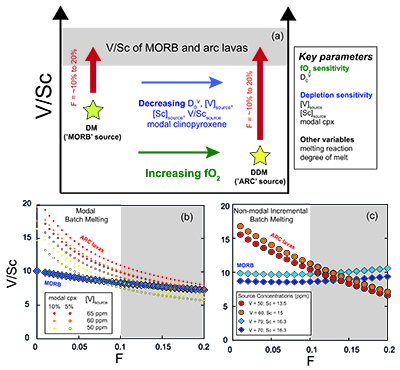 Figure 4 (a) Conceptual effect of depletion and oxidation on V/Sc ratios from two hypothetical spinel peridotite sources. (b,c) Modal and non-modal melting models for the sources in (a). Grey fields highlight reasonable melting degrees. Partition coefficients, modal compositions and melting reactions are detailed in the Supplementary Information. DM = depleted mantle, DDM = depleted MORB mantle, F = melt fraction. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Supplementary Figures and Tables
 Table S-1 Background chemical data. |  Table S-2 Vanadium isotopic data. |  Table S-3 Fractional crystallisation models. |  Table S-4 Example results for non-modal incremental batch melting. |
| Table S-1 | Table S-2 | Table S-3 | Table S-4 |
top
Introduction
Oxygen fugacity (fO2), the chemical potential of oxygen, varies over ten orders of magnitude in igneous systems (Carmichael, 1991). Knowledge of mantle fO2 is of critical importance for understanding the speciation of fluids in subduction zones and thus the efficiency of elemental transfer from slab to surface. Oxygen fugacity is often reported in log units relative to a buffer reaction, commonly fayalite-magnetite-quartz (FMQ). Early study of mantle fO2 employed oxygen thermobarometry, proposing that subduction zone magmas and their sources are more oxidised (>FMQ +1) than those found at mid-ocean ridges (~FMQ) (e.g., see review of Frost and McCammon, 2008
Frost, D.J., McCammon, C.A. (2008) The redox state of Earth’s mantle. Annual Reviews in Earth and Planetary Science 36, 389-420.
).However, the association of mantle fO2 with tectonic setting has become contested following the introduction of novel approaches to its determination. The development of new proxies based on ratios of redox to non-redox sensitive elements, such as V/Sc and Fe/Zn, coupled with synthesis of global chemical databases such as GEOROC (http://georoc.mpch-mainz.gwdg.de/georoc/) and PETDB (http://www.earthchem.org/petdb), has led to the counterintuitive proposal that there is no difference in fO2 between the mantle sources of arc and MORB lavas (Lee et al., 2005
Lee, C.-T.A., Leeman, W.P., Canil, D., Li, Z.-X.A. (2005) Similar V/Sc systematics in MORB and arc basalts: implications for the oxygen fugacities of their mantle source regions. Journal of Petrology 46, 2313-2336.
, 2010Lee, C.-T.A., Luffi, P., Le Roux, V., Dasgupta, R., Albarede, F., Leeman, W.P. (2010) The redox state of arc mantle using Zn/Fe systematics. Nature 468, 681-685.
). Despite this hypothesis, there is a consensus that basaltic melts found in arcs are more oxidised than their MORB counterparts, given, for example, their elevated Fe3+/∑Fe ratios measured in melt inclusions (e.g., Kelley and Cottrell, 2009Kelley, K.A., Cottrell, E. (2009) Water and the oxidation state of subduction zone magmas. Science 325, 605-607.
). In order to explain how oxidised arc basalts and more reduced MORBs can be derived from sources with similar fO2, processes subsequent to magma generation such as degassing and crystal fractionation have been invoked (e.g., Mallmann and O’Neill, 2009Mallmann, G., O’Neill, H.St.C. (2009) The crystal/melt partitioning of V during mantle melting as a function of oxygen fugacity compared with some other elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr, Nb). Journal of Petrology 50, 1765-1794.
; Lee et al., 2010Lee, C.-T.A., Luffi, P., Le Roux, V., Dasgupta, R., Albarede, F., Leeman, W.P. (2010) The redox state of arc mantle using Zn/Fe systematics. Nature 468, 681-685.
). Evidence gleaned from both experimental and natural suites is conflicting, with cases of Fe3+/∑Fe increasing, decreasing and remaining invariant during magmatic degassing and crystallisation (e.g., Brounce et al., 2014Brounce, M.N., Kelley, K.A., Cottrell, E. (2014) Variations in Fe3+/∑Fe of Mariana arc basalts and mantle wedge fO2. Journal of Petrology 55, 2513-2536.
; Waters and Lange, 2016Waters, L.E., Lange, R.A. (2016) No effect of H2O degassing on the oxidation state of magmatic liquids. Earth and Planetary Science Letters 447, 48-59.
). Thus lingering uncertainty as to whether the elevated Fe3+/∑Fe in arc lavas is derived from their sources or during differentiation fuels continued debate.The stable isotope composition of multivalent elements is theoretically linked to fO2. High temperature fractionation of stable isotopes is dictated by the vibrational frequencies of chemical bonds, with higher frequencies having larger bond force constants (K) and preferring heavier isotopes. An instructive estimate of the force constant is given by the mean bond strength: the quotient of average valence and co-ordination number (Pauling, 1929
Pauling, L. (1929) The principles determining the structure of complex ionic crystals. Journal of American Chemical Society 51, 1010-1026.
). Thus, atoms associated with higher oxidation state and/or lower co-ordination should, on average, incorporate a greater proportion of heavier isotopes. Therefore the possibility of resolving transition metal stable isotope variations in high temperature settings has the potential to define mantle fO2 more clearly. Although it is tempting to employ simple comparative geochemistry (i.e. heavier isotope compositions equate to more oxidising conditions), the interplay between co-ordination environment and valence state is vital to interpreting the effect of changing fO2 on isotope fractionation.top
Vanadium as a Redox Proxy
Vanadium is a moderately incompatible, refractory multivalent (2+, 3+, 4+, 5+) element and the strong dependence of its partitioning behaviour on fO2 is well-established (e.g., Canil, 1997
Canil, D. (1997) Vanadium partitioning and the oxidation state of Archaean komatiite magmas. Nature 389, 842-845.
; Toplis and Corgne, 2002Toplis, M.J., Corgne, A. (2002) An experimental study of element partitioning between magnetite, clinopyroxene and iron-bearing silicate liquids with particular emphasis on vanadium. Contributions to Mineralogy and Petrology 144, 22-37.
; Mallmann and O’Neill, 2009Mallmann, G., O’Neill, H.St.C. (2009) The crystal/melt partitioning of V during mantle melting as a function of oxygen fugacity compared with some other elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr, Nb). Journal of Petrology 50, 1765-1794.
). In all major mantle phases, vanadium becomes more incompatible with increasing fO2, leading to the application of V as a redox proxy (e.g., Shervais, 1982Shervais, J.W. (1982) Ti-V plots and the petrogenesis of modern and ophiolitic lavas. Earth and Planetary Science Letters 59, 101-118.
; Canil, 1997Canil, D. (1997) Vanadium partitioning and the oxidation state of Archaean komatiite magmas. Nature 389, 842-845.
; Lee et al., 2005Lee, C.-T.A., Leeman, W.P., Canil, D., Li, Z.-X.A. (2005) Similar V/Sc systematics in MORB and arc basalts: implications for the oxygen fugacities of their mantle source regions. Journal of Petrology 46, 2313-2336.
; Mallmann and O’Neill, 2009Mallmann, G., O’Neill, H.St.C. (2009) The crystal/melt partitioning of V during mantle melting as a function of oxygen fugacity compared with some other elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr, Nb). Journal of Petrology 50, 1765-1794.
). However, using concentrations alone results in fO2 uncertainties on the order of 1-2 log units due to variations in degree of melting, original source concentration, and fractionating mineral assemblages (Lee et al., 2003Lee, C.-T.A., Brandon, A.D., Norman, M.D. (2003) Vanadium in peridotites as a proxy for paleo-fO2 during partial melting: prospects, limitations, and implications. Geochimica et Cosmochimica Acta 67, 3045-3064.
).Vanadium has two stable isotopes, 51V and 50V. Their ratio is reported as per mille deviations, δ51V, relative to the AA (Alfa Aesar) V solution standard, defined as 0 ‰ (see Nielsen et al., 2011
Nielsen, S.G., Prytulak, J., Halliday, A.N. (2011) Determination of precise and accurate 51V/50V isotope ratios by MC-ICP-MS, part 1: chemical separation of vanadium and mass spectrometric protocols. Geostandards and Geoanalytical Research 35, 293-306.
). The extreme natural 51V/50V ratio of ~420 coupled with isobaric interferences from 50Cr and 50Ti on the minor 50V isotope have historically prevented analyses to a precision useful for high temperature applications. These obstacles were recently overcome, and the first analytical protocol to determine δ51V to a precision better than ±0.15 ‰ 2sd (Nielsen et al., 2011Nielsen, S.G., Prytulak, J., Halliday, A.N. (2011) Determination of precise and accurate 51V/50V isotope ratios by MC-ICP-MS, part 1: chemical separation of vanadium and mass spectrometric protocols. Geostandards and Geoanalytical Research 35, 293-306.
; Prytulak et al., 2011Prytulak, J., Nielsen, S.G., Halliday, A.N. (2011) Determination of precise and accurate 51V/50V isotope ratios by multi-collector ICP-MS, part 2: isotopic composition of six reference materials plus the Allende chondrite and verification tests. Geostandards and Geoanalytical Research 35, 307-318.
) demonstrated resolvable variations in igneous rocks of different silica content (δ51Vbasalts < δ51Vandesites). We present the first δ51V measurements directly exploring the potential of δ51V as a redox proxy in magmatic systems. Specifically, we investigate the effect of magmatic evolution and determine if a resolvable δ51V difference exists between arc and non-arc lavas.top
Methods
The oxygen fugacity of the two tectonic settings investigated herein (Mariana arc and Hekla, Iceland) have been previously determined by two independent means: by way of Fe3+/Fe2+ ratios that are converted to fO2, and by Fe-Ti oxybarometry. Both methods indicate that Mariana arc basalts are generally ~1-2 log units more oxidised compared to Icelandic basalts from Hekla (e.g., de Moor et al., 2005
de Moor, J.M., Fischer, T.P., Hilton, D.R., Hauri, E., Jaffe, L.A., Camacho, J.T. (2005) Degassing at Anatahan volcano during the May 2003 eruption: implications from petrology, ash leachates, and SO2 emissions. Journal of Volcanology and Geothermal Research 146, 117-138.
; Moune et al., 2007Moune, S., Sigmarsson, O., Thordarson, T., Gauthier, P.-J. 2007. Recent volatile evolution in the magmatic system of Hekla volcano, Iceland. Earth and Planetary Science Letters 255, 373-389.
; Brounce et al., 2014Brounce, M.N., Kelley, K.A., Cottrell, E. (2014) Variations in Fe3+/∑Fe of Mariana arc basalts and mantle wedge fO2. Journal of Petrology 55, 2513-2536.
; Shorttle et al., 2015Shorttle, O., Moussallam, Y., Hartley, M.E., Maclennan, J., Edmonds, M., Murton, B.J. (2015) Fe-XANES analyses of Reykjanes Ridge basalts: Implications for oceanic crust’s role in the solid Earth oxygen cycle. Earth and Planetary Science Letters 427, 272-285.
). We chose forty whole rock samples from three well-studied lava suites: 1) primitive arc lavas of the Mariana Central Island Province (CIP; Elliott et al., 1997Elliott, T., Plank, T., Zindler, A., White, W.M., Bourdon, B. (1997) Element transport from slab to volcanic front at the Mariana arc. Journal of Geophysical Research 102, 14991-15019.
), 2) co-genetic lavas from Anatahan volcano in the Mariana arc (Wade et al., 2005Wade, J.A., Plank, T., Stern, R.J., Tollstruo, D.L., Gill, J.B., O’Leary, J.C., Eiler, J.M., Moore, R.B., Woodhead, J.D., Trusdell, F., Fischer, T.P., Hilton, D.R. (2005) The May 2003 eruption of Anatahan volcano, Mariana Islands: geochemical evolution of a silicic island-arc volcano. Journal of Volcanology and Geothermal Research 146, 139-170.
) and 3) co-genetic lavas from Hekla volcano, Iceland (Savage et al., 2011Savage, P.S., Georg, R.B., Williams, H.M., Burton, K.W., Halliday, A.N. (2011) Silicon isotope fractionation during magmatic differentiation. Geochimica et Cosmochimica Acta 75, 6124-6139.
). Published major, trace and isotopic data are found in Table S-1.Chemical separation and V isotope measurements were made at Oxford University and Imperial College London, following Nielsen et al. (2011)
Nielsen, S.G., Prytulak, J., Halliday, A.N. (2011) Determination of precise and accurate 51V/50V isotope ratios by MC-ICP-MS, part 1: chemical separation of vanadium and mass spectrometric protocols. Geostandards and Geoanalytical Research 35, 293-306.
. Description of methods and isotopic data are found in the Supplementary Information and Table S-2.top
Results and Discussion
Two key observations arise from Figure 1. First, there is a striking range of ~2 ‰ towards heavy δ51V with progressive differentiation in both suites of lavas, which is an order of magnitude larger than Fe isotope variations in fractionating magmas (e.g., Schuessler et al., 2009
Schuessler, J.A., Schoenberg, R., Sigmarsson, O. (2009) Iron and lithium isotope systematics of the Hekla volcano, Iceland – evidence for Fe isotope fractionation during magma differentiation. Chemical Geology 258, 78-91.
; Sossi et al., 2012Sossi, P.A., Foden, J.D., Halverson, G.P. (2012) Redox-controlled iron isotope fractionation during magmatic differentiation: an example from the Red Hill intrudion, S. Tasmania. Contributions to Mineralogy and Petrology 164, 757-772.
). Second, basaltic lavas from the Marianas, Iceland and MORB have overlapping δ51V.
Figure 1 δ51V variations with SiO2 (a) and MgO (b). CIP = Central Island Province. MORB data from Prytulak et al. (2013)
Prytulak, J., Nielsen, S.G., Ionov, D.A., Halliday, A.N., Harvey, J., Kelley, K.A., Niu, Y.L., Peate, D.W., Shimizu, K., Sims, K.W.W. (2013) The stable vanadium isotope composition of the mantle and mafic lavas. Earth and Planetary Science Letters 365, 177-189.
. Uncertainties on isotope measurements are external 2 sd.The remarkable magnitude of high temperature δ51V fractionation towards heavy values with magmatic evolution is unlikely to be due to the difference in calculated fO2 between Hekla and the Marianas, because the δ51V variation is similar for both suites. Instead, the primary control on isotope fractionation appears to be differences in mineral-melt bonding environment. Oxide minerals such as (titano)magnetite host the majority of V in magmatic systems. Trivalent vanadium is preferentially incorporated in the VI-fold sites of (titano)magnetite, the thermodynamically stable configuration (O’Neill and Navrotsky, 1984
O’Neill, H.St.C., Navrotsky, A. (1984) Cation distributions and thermodynamic properties of binary spinel solid solutions. American Mineralogist 69, 733-753.
) resulting from its high octahedral site preference energy. Vanadium’s oxidation and co-ordination in silicate melts is not constrained by stoichiometry, but is always higher and lower, respectively, than in co-existing (titano)magnetite (Righter et al., 2006Righter, K., Sutton, S.R., Newville, M., Le, L., Schwandt, C.S., Uchida, H., Lavina, B., Downs, R.T. (2006) An experimental study of the oxidation state of vanadium in spinel and basaltic melt with implications for the origin of planetary basalt. American Mineralogist 91, 1643-1656.
). Thus (titano)magnetite (and other ferromagnesian minerals) should be isotopically light, and crystallisation will lead to a progressively heavier residual melt. A significant departure to heavy δ51V is observed at ~60 wt. % SiO2 at Anatahan, and ~55 wt. % at Hekla, although petrographic and chemical evidence demonstrates oxide fractionation occurring earlier in both suites. Indeed, significant (titano)magnetite crystallisation and removal is necessary to impact δ51V signatures whilst melt V concentration is relatively high. We speculate that magma chamber processes at Anatahan, where lavas are generally more evolved, are responsible for the lighter and more variable δ51V in lavas with between 2 and 4 wt. % MgO compared to Hekla (Fig. 1b).Self-consistent models of fractional crystallisation of cotectic phases have been constructed to account for systematic variations in major and trace element concentrations, as well as isotopic compositions in Hekla and Anatahan lavas (Fig. 2). Input parameters and results are provided in the Supplementary Information (Table S-3). Given the strong dependence of V partitioning on fO2, it is of particular interest to assess how the partition coefficient of V in (titano)magnetite differs between the two suites. A DmagV of 32 ± 4 is calculated from analyses of Anatahan lavas (de Moor et al., 2005
de Moor, J.M., Fischer, T.P., Hilton, D.R., Hauri, E., Jaffe, L.A., Camacho, J.T. (2005) Degassing at Anatahan volcano during the May 2003 eruption: implications from petrology, ash leachates, and SO2 emissions. Journal of Volcanology and Geothermal Research 146, 117-138.
), a value that reproduces major, trace and isotopic trends very well. The required DmagV for Hekla lavas, however, must be significantly higher (~42) to reproduce the data. The relative difference in DmagV between the two suites is consistent with lower fO2 at Hekla than Anatahan (e.g., Toplis and Corgne, 2002Toplis, M.J., Corgne, A. (2002) An experimental study of element partitioning between magnetite, clinopyroxene and iron-bearing silicate liquids with particular emphasis on vanadium. Contributions to Mineralogy and Petrology 144, 22-37.
). The same input parameters are used to perform Rayleigh calculations to estimate the bulk V isotope fractionation factor (Fig. 3). Both suites require large ∆51Vmin-melt fractionation factors on the order of 1000lnαmin-melt of -0.4 to -0.5 ‰. Arguably, a larger fractionation factor is required for Anatahan versus Hekla, however, the effect is subtle and difficult to resolve confidently within the current analytical precision.
Figure 2 Cotectic fractional crystallisation models. Each small symbol represents a 5 % crystal fractionation increment. See Supplementary Information for input parameters. Symbols and uncertainties as in Figure 1.

Figure 3 Rayleigh fractionation calculations with same parameters as Figure 2. Symbols and uncertainties as in Figure 1.
Clearly, differences in mineral-melt bonding environment are key to producing large δ51V fractionations. However, prior to significant (titano)magnetite crystallisation, δ51V is identical within uncertainties in arc lavas, Icelandic lavas and MORB at similar MgO contents (Fig. 1). If interpreted as a direct fO2 proxy, this conflicts with oxybarometry in peridotites, but is notionally consistent with their similar V/Sc ratios. This conclusion hinges, however, upon the assumption of a homogeneous source, both with respect to V/Sc and δ51V, an assumption that may be violated. For instance, δ51V becomes isotopically lighter in progressively depleted (clinopyroxene-poor) peridotites (Prytulak et al., 2013
Prytulak, J., Nielsen, S.G., Ionov, D.A., Halliday, A.N., Harvey, J., Kelley, K.A., Niu, Y.L., Peate, D.W., Shimizu, K., Sims, K.W.W. (2013) The stable vanadium isotope composition of the mantle and mafic lavas. Earth and Planetary Science Letters 365, 177-189.
). This is an important observation because the arc mantle wedge has been inferred to be markedly depleted (e.g., Woodhead, 1993Woodhead, J., Eggins, S., Gamble, J. (1993) High field strength and transition element systematics in island arc and back-arc basin basalts: evidence for multi-phase melt extraction and a depleted mantle wedge. Earth and Planetary Science Letters 114, 491-504.
; Nebel et al., 2015Nebel, O., Sossi, P.A., Benard, A., Wille, M., Vroon, P.Z., Arculus, R.J. (2015) Redox-variability and controls in subduction zones from an iron-isotope perspective. Earth and Planetary Science Letters 423, 142-151.
) with respect to the source of MORB. In the absence of a common source, observations of similar V/Sc and δ51V must be explained.Primary arc magmas are notoriously rare, thus most information is garnered from basaltic andesites with Mg# too low (~0.5 assuming 20 % Fe3+) to be in equilibrium with normal mantle (~0.9). Isotope fractionation during partial melting is possible, although high temperatures should minimise the effect. It is, however, perplexing that the well-constrained δ51VMORB is isotopically lighter than most peridotites and the bulk silicate Earth (δ51VMORB = -0.95 ± 0.13 ‰; δ51VBSE = -0.7 ± 0.2 ‰; Prytulak et al., 2013
Prytulak, J., Nielsen, S.G., Ionov, D.A., Halliday, A.N., Harvey, J., Kelley, K.A., Niu, Y.L., Peate, D.W., Shimizu, K., Sims, K.W.W. (2013) The stable vanadium isotope composition of the mantle and mafic lavas. Earth and Planetary Science Letters 365, 177-189.
). The peridotite trend towards lighter δ51V with progressive depletion thus negates a simple explanation of isotope fractionation during melt extraction (Prytulak et al., 2013Prytulak, J., Nielsen, S.G., Ionov, D.A., Halliday, A.N., Harvey, J., Kelley, K.A., Niu, Y.L., Peate, D.W., Shimizu, K., Sims, K.W.W. (2013) The stable vanadium isotope composition of the mantle and mafic lavas. Earth and Planetary Science Letters 365, 177-189.
). Given the strong effect of mineral-melt fractionation on δ51V, the potential for early phases to change the δ51V of arc basalts should be considered. Water-rich magmas crystallise Cr-rich spinel before olivine (Feig et al., 2010Feig, S.T., Koepke, J., Snow, J.E. (2010) Effect of oxygen fugacity and water on phase equilibria of a hydrous tholeiitic basalt. Contributions to Mineralogy and Petrology 160, 551-568.
), potentially scavenging V. Oxide fractionation will drive remaining liquid to heavier δ51V, making early spinel crystallisation a viable mechanism to increase δ51V in arc magmas. If arc magmas are derived from a depleted source with a lighter initial V isotope composition than the MORB source, it is therefore possible that the competing effects of source depletion and Cr-spinel fractionation result in similar δ51V in mafic arc and non-arc magmas. Mineral separate data and the analysis of more primitive magmas are required to investigate this premise since it is currently not possible to evaluate the magnitude of isotopic increase due to early Cr-rich spinel, and/or partial melting, as there are no combined V concentration and d51V on spinel and scant peridotite whole rock data.If arc magmas are derived from mantle more depleted than MORB, their sources should have lower δ51V and V/Sc. The decrease of V/Sc in the source will be exacerbated if prior melt-depletion occurs at high fO2. Therefore, the observation of similar δ51V and V/Sc ratios in high MgO lavas from both settings can be interpreted as evidence of melting of more depleted, oxidised sources in arcs. This concept is illustrated with two hypothetical sources in Figure 4a, with examples of simple forward trace element modal (Fig. 4b) and non-modal (Fig. 4c) melting models comparing V/Sc in lavas derived from these two sources. Overall, the effect of source depletion (i.e. less V) coupled with higher fO2 (and thus lower DV), can offset a more fertile (i.e. more V) less oxidised (higher DV) source to yield similar V/Sc in resulting melts. Thus the confluence of δ51V and V/Sc in arc and MORB lavas may paradoxically require differences in their source fO2. Clearly, there are many possible solutions to such models, and the absolute values of V/Sc are very sensitive to input parameters (see Supplementary Information), however, given the assumption of a more oxidised, depleted arc source, the similarity of V/Sc in arc lavas and MORB at 10-15 % melt is relatively straightforward to reproduce.

Figure 4 (a) Conceptual effect of depletion and oxidation on V/Sc ratios from two hypothetical spinel peridotite sources. (b,c) Modal and non-modal melting models for the sources in (a). Grey fields highlight reasonable melting degrees. Partition coefficients, modal compositions and melting reactions are detailed in the Supplementary Information. DM = depleted mantle, DDM = depleted MORB mantle, F = melt fraction.
Irrespective of the trade-offs involved in interpretation of relative oxidation states of arc and non-arc lavas, Rayleigh fractionation of oxide phases is dominantly responsible for the magnitude of observed V isotope fractionation in differentiating magmatic suites. Subtle fO2-related variations are perhaps overprinted onto first order bonding-environment induced fractionations, but these require a much richer understanding of δ51V variations during magmatic processes to be applicable. Therefore, elemental partitioning of V yields a more direct relationship with fO2 than the current understanding of V isotopes at high temperatures permits.
top
Acknowledgements
JP was funded by NERC postdoctoral fellowship NE/H01313X/2, with support of the Oxford laboratories from an Advanced ERC grant (NEWISOTOPEGEOSCIENCE) to ANH. PAS by an APA PhD scholarship and ANU Vice-Chancellor’s Scholarship. We appreciate thoughtful reviews by F. Poitrasson and G. Mallmann. Tim Elliott is thanked for providing CIP samples. Although he may not necessarily agree with all the ideas presented, we thank Hugh O’Neill for many formative discussions.
Editor: Bruce Watson
top
References
Brounce, M.N., Kelley, K.A., Cottrell, E. (2014) Variations in Fe3+/∑Fe of Mariana arc basalts and mantle wedge fO2. Journal of Petrology 55, 2513-2536.
 Show in context
Show in context Evidence gleaned from both experimental and natural suites is conflicting, with cases of Fe3+/∑Fe increasing, decreasing and remaining invariant during magmatic degassing and crystallisation (e.g., Brounce et al., 2014; Waters and Lange, 2016).
View in article
Both methods indicate that Marian arc basalts are generally ~1-2 log units more oxidised compared to Icelandic basalts from Hekla (e.g., de Moor et al., 2005; Moune et al., 2007; Brounce et al., 2014; Shorttle et al., 2015).
View in article
Canil, D. (1997) Vanadium partitioning and the oxidation state of Archaean komatiite magmas. Nature 389, 842-845.
 Show in context
Show in context Vanadium is a moderately incompatible, refractory multivalent (2+, 3+, 4+, 5+) element and the strong dependence of its partitioning behaviour on ƒO2 is well-established (e.g., Canil, 1997; Toplis and Corgne, 2002; Mallmann and O’Neill, 2009).
View in article
In all major mantle phases, vanadium becomes more incompatible with increasing ƒO2, leading to the application of V as a redox proxy (e.g., Shervais, 1982; Canil, 1997; Lee et al., 2005; Mallmann and O’Neill, 2009).
View in article
de Moor, J.M., Fischer, T.P., Hilton, D.R., Hauri, E., Jaffe, L.A., Camacho, J.T. (2005) Degassing at Anatahan volcano during the May 2003 eruption: implications from petrology, ash leachates, and SO2 emissions. Journal of Volcanology and Geothermal Research 146, 117-138.
 Show in context
Show in context Both methods indicate that Marian arc basalts are generally ~1-2 log units more oxidised compared to Icelandic basalts from Hekla (e.g., de Moor et al., 2005; Moune et al., 2007; Brounce et al., 2014; Shorttle et al., 2015).
View in article
A DmagV of 32 ± 4 is calculated from analyses of Anatahan lavas (de Moor et al., 2005), a value that reproduces major, trace and isotopic trends very well.
View in article
Elliott, T., Plank, T., Zindler, A., White, W.M., Bourdon, B. (1997) Element transport from slab to volcanic front at the Mariana arc. Journal of Geophysical Research 102, 14991-15019.
 Show in context
Show in context We choose forty whole rock samples from three well-studied lava suites: 1) primitive arc lavas of the Mariana Central Island Province (CIP; Elliott et al., 1997), 2) co-genetic lavas from Anatahan volcano in the Mariana arc (Wade et al., 2005) and 3) co-genetic lavas from Hekla volcano, Iceland (Savage et al., 2011).
View in article
Feig, S.T., Koepke, J., Snow, J.E. (2010) Effect of oxygen fugacity and water on phase equilibria of a hydrous tholeiitic basalt. Contributions to Mineralogy and Petrology 160, 551-568.
 Show in context
Show in context Water-rich magmas crystallise Cr-rich spinel before olivine (Feig et al., 2010), potentially scavenging V.
View in article
Frost, D.J., McCammon, C.A. (2008) The redox state of Earth’s mantle. Annual Reviews in Earth and Planetary Science 36, 389-420.
 Show in context
Show in context Early study of mantle ƒO2 employed oxygen thermobarometry, proposing that subduction zone magmas and their sources are more oxidised (>FMQ +1) than those found at mid-ocean ridges (~FMQ) (e.g., see review of Frost and McCammon, 2008).
View in article
Kelley, K.A., Cottrell, E. (2009) Water and the oxidation state of subduction zone magmas. Science 325, 605-607.
 Show in context
Show in context Despite this hypothesis, there is a consensus that basaltic melts found in arcs are more oxidised than their MORB counterparts, given, for example, their elevated Fe3+/∑Fe ratios measured in melt inclusions (e.g., Kelley and Cottrell, 2009).
View in article
Lee, C.-T.A., Brandon, A.D., Norman, M.D. (2003) Vanadium in peridotites as a proxy for paleo-fO2 during partial melting: prospects, limitations, and implications. Geochimica et Cosmochimica Acta 67, 3045-3064.
 Show in context
Show in context However, using concentrations alone results in ƒO2 uncertainties on the order of 1-2 log units due to variations in degree of melting, original source concentration, and fractionating mineral assemblages (Lee et al., 2003).
View in article
Lee, C.-T.A., Leeman, W.P., Canil, D., Li, Z.-X.A. (2005) Similar V/Sc systematics in MORB and arc basalts: implications for the oxygen fugacities of their mantle source regions. Journal of Petrology 46, 2313-2336.
 Show in context
Show in context The development of new proxies based on ratios of redox to non-redox sensitive elements, such as V/Sc and Fe/Zn, coupled with synthesis of global chemical databases such as GEOROC (http://georoc.mpch-mainz.gwdg.de/georoc/) and PETDB (http://www.earthchem.org/petdb), has led to the counterintuitive proposal that there is no difference in fO2 between the mantle sources of arc and MORB lavas (Lee et al., 2005, 2010).
View in article
In all major mantle phases, vanadium becomes more incompatible with increasing ƒO2, leading to the application of V as a redox proxy (e.g., Shervais, 1982; Canil, 1997; Lee et al., 2005; Mallmann and O’Neill, 2009).
View in article
Lee, C.-T.A., Luffi, P., Le Roux, V., Dasgupta, R., Albarede, F., Leeman, W.P. (2010) The redox state of arc mantle using Zn/Fe systematics. Nature 468, 681-685.
 Show in context
Show in context The development of new proxies based on ratios of redox to non-redox sensitive elements, such as V/Sc and Fe/Zn, coupled with synthesis of global chemical databases such as GEOROC (http://georoc.mpch-mainz.gwdg.de/georoc/) and PETDB (http://www.earthchem.org/petdb), has led to the counterintuitive proposal that there is no difference in fO2 between the mantle sources of arc and MORB lavas (Lee et al., 2005, 2010).
View in article
In order to explain how oxidised arc basalts and more reduced MORBs can be derived from sources with similar ƒO2, processes subsequent to magma generation such as degassing and crystal fractionation have been invoked (e.g., Mallmann and O’Neill, 2009; Lee et al., 2010).
View in article
Mallmann, G., O’Neill, H.St.C. (2009) The crystal/melt partitioning of V during mantle melting as a function of oxygen fugacity compared with some other elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr, Nb). Journal of Petrology 50, 1765-1794.
 Show in context
Show in context In order to explain how oxidised arc basalts and more reduced MORBs can be derived from sources with similar ƒO2, processes subsequent to magma generation such as degassing and crystal fractionation have been invoked (e.g., Mallmann and O’Neill, 2009; Lee et al., 2010).
View in article
Vanadium is a moderately incompatible, refractory multivalent (2+, 3+, 4+, 5+) element and the strong dependence of its partitioning behaviour on ƒO2 is well-established (e.g., Canil, 1997; Toplis and Corgne, 2002; Mallmann and O’Neill, 2009).
View in article
In all major mantle phases, vanadium becomes more incompatible with increasing ƒO2, leading to the application of V as a redox proxy (e.g., Shervais, 1982; Canil, 1997; Lee et al., 2005; Mallmann and O’Neill, 2009).
View in article
Moune, S., Sigmarsson, O., Thordarson, T., Gauthier, P.-J. 2007. Recent volatile evolution in the magmatic system of Hekla volcano, Iceland. Earth and Planetary Science Letters 255, 373-389.
 Show in context
Show in context Both methods indicate that Marian arc basalts are generally ~1-2 log units more oxidised compared to Icelandic basalts from Hekla (e.g., de Moor et al., 2005; Moune et al., 2007; Brounce et al., 2014; Shorttle et al., 2015).
View in article
Nebel, O., Sossi, P.A., Benard, A., Wille, M., Vroon, P.Z., Arculus, R.J. (2015) Redox-variability and controls in subduction zones from an iron-isotope perspective. Earth and Planetary Science Letters 423, 142-151.
 Show in context
Show in context This is an important observation because the arc mantle wedge has been inferred to be markedly depleted (e.g., Woodhead, 1993; Nebel et al., 2015) with respect to the source of MORB.
View in article
Nielsen, S.G., Prytulak, J., Halliday, A.N. (2011) Determination of precise and accurate 51V/50V isotope ratios by MC-ICP-MS, part 1: chemical separation of vanadium and mass spectrometric protocols. Geostandards and Geoanalytical Research 35, 293-306.
 Show in context
Show in context Their ratio is reported as per mille deviations, δ51V, relative to the AA (Alfa Aesar) V solution standard, defined as 0 ‰ (see Nielsen et al., 2011).
View in article
These obstacles were recently overcome, and the first analytical protocol to determine δ51V to a precision better than ±0.15 ‰ 2sd (Nielsen et al., 2011; Prytulak et al., 2011) demonstrated resolvable variations in igneous rocks of different silica content (δ51Vbasalts < δ51Vandesites).
View in article
Chemical separation and V isotope measurements were made at Oxford University and Imperial College London, following Nielsen et al. (2011).
View in article
O’Neill, H.St.C., Navrotsky, A. (1984) Cation distributions and thermodynamic properties of binary spinel solid solutions. American Mineralogist 69, 733-753.
 Show in context
Show in context Trivalent vanadium is preferentially incorporated in the VI-fold sites of (titano)magnetite, the thermodynamically stable configuration (O’Neill and Navrotsky, 1984) resulting from its high octahedral site preference energy
View in article
Pauling, L. (1929) The principles determining the structure of complex ionic crystals. Journal of American Chemical Society 51, 1010-1026.
 Show in context
Show in context An instructive estimate of the force constant is given by the mean bond strength: the quotient of average valence and co-ordination number (Pauling, 1929).
View in article
Prytulak, J., Nielsen, S.G., Halliday, A.N. (2011) Determination of precise and accurate 51V/50V isotope ratios by multi-collector ICP-MS, part 2: isotopic composition of six reference materials plus the Allende chondrite and verification tests. Geostandards and Geoanalytical Research 35, 307-318.
 Show in context
Show in context These obstacles were recently overcome, and the first analytical protocol to determine δ51V to a precision better than ±0.15 ‰ 2sd (Nielsen et al., 2011; Prytulak et al., 2011) demonstrated resolvable variations in igneous rocks of different silica content (δ51Vbasalts < δ51Vandesites).
View in article
Prytulak, J., Nielsen, S.G., Ionov, D.A., Halliday, A.N., Harvey, J., Kelley, K.A., Niu, Y.L., Peate, D.W., Shimizu, K., Sims, K.W.W. (2013) The stable vanadium isotope composition of the mantle and mafic lavas. Earth and Planetary Science Letters 365, 177-189.
 Show in context
Show in context Figure 1 [...] MORB data from Prytulak et al. (2013).
View in article
For instance, δ51V becomes isotopically lighter in progressively depleted (clinopyroxene-poor) peridotites (Prytulak et al., 2013).
View in article
It is, however, perplexing that the well-constrained δ51VMORB is isotopically lighter than most peridotites and the bulk silicate Earth (δ51VMORB = -0.95 ± 0.13 ‰; δ51VBSE = -0.7 ± 0.2 ‰; Prytulak et al., 2013).
View in article
The peridotite trend towards lighter δ51V with progressive depletion thus negates a simple explanation of isotope fractionation during melt extraction (Prytulak et al., 2013).
View in article
Righter, K., Sutton, S.R., Newville, M., Le, L., Schwandt, C.S., Uchida, H., Lavina, B., Downs, R.T. (2006) An experimental study of the oxidation state of vanadium in spinel and basaltic melt with implications for the origin of planetary basalt. American Mineralogist 91, 1643-1656.
 Show in context
Show in context Vanadium’s oxidation and co-ordination in silicate melts is not constrained by stoichiometry, but is always higher and lower, respectively, than in co-existing (titano)magnetite (Righter et al., 2006).
View in article
Savage, P.S., Georg, R.B., Williams, H.M., Burton, K.W., Halliday, A.N. (2011) Silicon isotope fractionation during magmatic differentiation. Geochimica et Cosmochimica Acta 75, 6124-6139.
 Show in context
Show in context We choose forty whole rock samples from three well-studied lava suites: 1) primitive arc lavas of the Mariana Central Island Province (CIP; Elliott et al., 1997), 2) co-genetic lavas from Anatahan volcano in the Mariana arc (Wade et al., 2005) and 3) co-genetic lavas from Hekla volcano, Iceland (Savage et al., 2011).
View in article
Schuessler, J.A., Schoenberg, R., Sigmarsson, O. (2009) Iron and lithium isotope systematics of the Hekla volcano, Iceland – evidence for Fe isotope fractionation during magma differentiation. Chemical Geology 258, 78-91.
 Show in context
Show in context First, there is a striking range of ~2 ‰ towards heavy δ51V with progressive differentiation in both suites of lavas, which is an order of magnitude larger than Fe isotope variations in fractionating magmas (e.g., Schuessler et al., 2009; Sossi et al., 2012)
View in article
Shervais, J.W. (1982) Ti-V plots and the petrogenesis of modern and ophiolitic lavas. Earth and Planetary Science Letters 59, 101-118.
 Show in context
Show in context In all major mantle phases, vanadium becomes more incompatible with increasing ƒO2, leading to the application of V as a redox proxy (e.g., Shervais, 1982; Canil, 1997; Lee et al., 2005; Mallmann and O’Neill, 2009).
View in article
Shorttle, O., Moussallam, Y., Hartley, M.E., Maclennan, J., Edmonds, M., Murton, B.J. (2015) Fe-XANES analyses of Reykjanes Ridge basalts: Implications for oceanic crust’s role in the solid Earth oxygen cycle. Earth and Planetary Science Letters 427, 272-285.
 Show in context
Show in context Both methods indicate that Marian arc basalts are generally ~1-2 log units more oxidised compared to Icelandic basalts from Hekla (e.g., de Moor et al., 2005; Moune et al., 2007; Brounce et al., 2014; Shorttle et al., 2015).
View in article
Sossi, P.A., Foden, J.D., Halverson, G.P. (2012) Redox-controlled iron isotope fractionation during magmatic differentiation: an example from the Red Hill intrudion, S. Tasmania. Contributions to Mineralogy and Petrology 164, 757-772.
 Show in context
Show in context First, there is a striking range of ~2 ‰ towards heavy δ51V with progressive differentiation in both suites of lavas, which is an order of magnitude larger than Fe isotope variations in fractionating magmas (e.g., Schuessler et al., 2009; Sossi et al., 2012)
View in article
Toplis, M.J., Corgne, A. (2002) An experimental study of element partitioning between magnetite, clinopyroxene and iron-bearing silicate liquids with particular emphasis on vanadium. Contributions to Mineralogy and Petrology 144, 22-37.
 Show in context
Show in context Vanadium is a moderately incompatible, refractory multivalent (2+, 3+, 4+, 5+) element and the strong dependence of its partitioning behaviour on ƒO2 is well-established (e.g., Canil, 1997; Toplis and Corgne, 2002; Mallmann and O’Neill, 2009).
View in article
The relative difference in DmagV between the two suites is consistent with lower ƒO2 at Hekla than Anatahan (e.g., Toplis and Corgne, 2002).
View in article
Wade, J.A., Plank, T., Stern, R.J., Tollstruo, D.L., Gill, J.B., O’Leary, J.C., Eiler, J.M., Moore, R.B., Woodhead, J.D., Trusdell, F., Fischer, T.P., Hilton, D.R. (2005) The May 2003 eruption of Anatahan volcano, Mariana Islands: geochemical evolution of a silicic island-arc volcano. Journal of Volcanology and Geothermal Research 146, 139-170.
 Show in context
Show in context We choose forty whole rock samples from three well-studied lava suites: 1) primitive arc lavas of the Mariana Central Island Province (CIP; Elliott et al., 1997), 2) co-genetic lavas from Anatahan volcano in the Mariana arc (Wade et al., 2005) and 3) co-genetic lavas from Hekla volcano, Iceland (Savage et al., 2011).
View in article
Waters, L.E., Lange, R.A. (2016) No effect of H2O degassing on the oxidation state of magmatic liquids. Earth and Planetary Science Letters 447, 48-59.
 Show in context
Show in context Evidence gleaned from both experimental and natural suites is conflicting, with cases of Fe3+/∑Fe increasing, decreasing and remaining invariant during magmatic degassing and crystallisation (e.g., Brounce et al., 2014; Waters and Lange, 2016).
View in article
Woodhead, J., Eggins, S., Gamble, J. (1993) High field strength and transition element systematics in island arc and back-arc basin basalts: evidence for multi-phase melt extraction and a depleted mantle wedge. Earth and Planetary Science Letters 114, 491-504.
 Show in context
Show in context This is an important observation because the arc mantle wedge has been inferred to be markedly depleted (e.g., Woodhead, 1993; Nebel et al., 2015) with respect to the source of MORB.
View in article
top
Supplementary Information
Methods and Model Descriptions
Table S-1 presents a compilation of published major and trace element and radiogenic isotope data on the samples used in this study. We have also compiled available information on other stable isotope systems such as Mo (Freymuth et al., 2015; Yang et al., 2015), Zn (Chen et al., 2013), Cu (Savage et al., 2015), and Tl (Prytulak et al., 2013).
Table S-1 Background chemical data.
| A. MAJOR ELEMENTS | ||||||||||||||||||||||||||||||||||||||
| eruption age | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | LOI | |||||||||||||||||||||||||||
| Hekla | ||||||||||||||||||||||||||||||||||||||
| hek 6 | 1913 AD | 46.20 | 4.508 | 13.73 | 17.28 | 0.254 | 5.40 | 9.45 | 2.74 | 0.64 | 0.538 | -0.40 | ||||||||||||||||||||||||||
| hek 12 | 1913AD | 46.42 | 4.469 | 13.59 | 17.18 | 0.259 | 5.23 | 9.22 | 2.81 | 0.67 | 0.566 | -0.29 | ||||||||||||||||||||||||||
| hek 5 | 1913AD | 46.47 | 4.493 | 13.85 | 17.28 | 0.253 | 5.48 | 9.53 | 2.76 | 0.63 | 0.529 | -0.38 | ||||||||||||||||||||||||||
| hek 14 | 1991 AD | 53.71 | 2.120 | 14.80 | 13.26 | 0.274 | 2.97 | 6.98 | 3.95 | 1.23 | 1.066 | -0.15 | ||||||||||||||||||||||||||
| hek 17 | 1980 AD | 54.57 | 2.060 | 14.75 | 13.05 | 0.276 | 2.86 | 6.80 | 3.94 | 1.26 | 1.007 | -0.13 | ||||||||||||||||||||||||||
| hek 16 | 1980 AD | 54.81 | 1.961 | 14.90 | 12.71 | 0.275 | 2.74 | 6.67 | 4.02 | 1.28 | 0.929 | -0.11 | ||||||||||||||||||||||||||
| hek 21 | 1390 AD | 55.64 | 1.773 | 15.14 | 12.56 | 0.273 | 2.58 | 6.34 | 4.12 | 1.35 | 0.865 | -0.09 | ||||||||||||||||||||||||||
| hek 15 | 1980 AD | 59.64 | 1.164 | 15.22 | 10.27 | 0.248 | 1.57 | 5.07 | 4.55 | 1.66 | 0.425 | 0.03 | ||||||||||||||||||||||||||
| Anatahan | ||||||||||||||||||||||||||||||||||||||
| AN-10 | 63.91 | 0.75 | 15.47 | 7.026 | 0.20 | 1.52 | 4.56 | 4.38 | 1.88 | 0.30 | ||||||||||||||||||||||||||||
| Anat9 | 65.06 | 0.83 | 15.00 | 7.515 | 0.19 | 1.17 | 4.22 | 4.27 | 2.05 | 0.30 | ||||||||||||||||||||||||||||
| 04-Anat-01 | 59.51 | 0.87 | 17.14 | 8.163 | 0.21 | 1.94 | 5.56 | 4.02 | 1.49 | 0.31 | ||||||||||||||||||||||||||||
| 04-Anat-03 | 56.35 | 0.75 | 18.89 | 7.899 | 0.17 | 2.12 | 8.17 | 3.60 | 1.26 | 0.22 | ||||||||||||||||||||||||||||
| 04-Anat-04 | 54.03 | 0.78 | 19.41 | 8.688 | 0.18 | 2.56 | 9.08 | 3.44 | 1.00 | 0.19 | ||||||||||||||||||||||||||||
| AN-2 | 52.31 | 0.71 | 21.18 | 8.772 | 0.16 | 2.88 | 10.59 | 2.76 | 0.51 | 0.12 | ||||||||||||||||||||||||||||
| AN-1 | 51.66 | 0.73 | 18.54 | 10.340 | 0.19 | 4.97 | 10.34 | 2.53 | 0.57 | 0.13 | ||||||||||||||||||||||||||||
| AN-8 | 49.28 | 0.68 | 20.09 | 10.649 | 0.19 | 4.51 | 11.81 | 2.22 | 0.46 | 0.11 | ||||||||||||||||||||||||||||
| Anat4-s | 61.26 | 0.80 | 15.67 | 8.360 | 0.21 | 1.82 | 5.44 | 3.99 | 1.52 | 0.32 | ||||||||||||||||||||||||||||
| AN-12D | 62.82 | 0.83 | 15.12 | 8.270 | 0.21 | 1.60 | 4.82 | 4.33 | 1.67 | 0.32 | ||||||||||||||||||||||||||||
| AN-7 | 53.49 | 0.79 | 18.63 | 10.370 | 0.20 | 3.29 | 9.33 | 3.06 | 0.68 | 0.16 | ||||||||||||||||||||||||||||
| Anat-26-01 | 60.29 | 0.84 | 16.10 | 8.568 | 0.20 | 2.33 | 6.04 | 3.73 | 1.37 | 0.32 | ||||||||||||||||||||||||||||
| Anat-26-02 | 58.36 | 0.88 | 16.13 | 9.040 | 0.22 | 2.39 | 6.22 | 4.41 | 1.36 | 0.25 | ||||||||||||||||||||||||||||
| Mariana Central Island Province | ||||||||||||||||||||||||||||||||||||||
| ALAM 2 | 54.95 | 0.783 | 17.4 | 9.58 | 0.184 | 4.4 | 8.98 | 2.98 | 0.944 | 0.145 | ||||||||||||||||||||||||||||
| AGR 8B | 50.5 | 0.726 | 20.2 | 9.83 | 0.186 | 3.55 | 11.15 | 2.58 | 0.889 | 0.175 | ||||||||||||||||||||||||||||
| PAG 1 | 51.34 | 0.908 | 15.87 | 12.19 | 0.218 | 5.45 | 10.6 | 2.8 | 0.749 | 0.142 | ||||||||||||||||||||||||||||
| PAG 3 | 51.64 | 0.925 | 15.73 | 12.23 | 0.218 | 5.25 | 10.25 | 2.72 | 0.78 | 0.149 | ||||||||||||||||||||||||||||
| GUG 4 | 52.37 | 0.824 | 17.54 | 10.26 | 0.197 | 5.22 | 10.41 | 2.64 | 0.549 | 0.105 | ||||||||||||||||||||||||||||
| GUG 9 | 50.985 | 0.82 | 19.6 | 10.07 | 0.18 | 4.23 | 11.05 | 2.56 | 0.4325 | 0.0865 | ||||||||||||||||||||||||||||
| MM-92-6 | 55.24 | 0.872 | 16.49 | 10.67 | 0.236 | 3.15 | 7.68 | 3.56 | 1.41 | 0.269 | ||||||||||||||||||||||||||||
| GUG3 | 51.61 | 0.858 | 19.75 | 9.93 | 0.191 | 3.36 | 10.5 | 2.69 | 0.908 | 0.174 | ||||||||||||||||||||||||||||
| GUG11 | 52.96 | 0.807 | 19.88 | 9.32 | 0.177 | 3.38 | 10.22 | 2.95 | 0.507 | 0.098 | ||||||||||||||||||||||||||||
| ALAM5 | 53.39 | 0.815 | 17.17 | 10.05 | 0.19 | 4.86 | 9.97 | 2.7 | 0.835 | 0.144 | ||||||||||||||||||||||||||||
| URA6 | 59.92 | 0.826 | 15.39 | 9.99 | 0.234 | 2.25 | 6.2 | 3.85 | 1.099 | 0.186 | ||||||||||||||||||||||||||||
| SAG1 | 53.37 | 0.777 | 16.23 | 9.83 | 0.186 | 5.57 | 10.45 | 2.71 | 0.716 | 0.126 | ||||||||||||||||||||||||||||
| GUG13 | 51.78 | 0.807 | 19.94 | 9.67 | 0.179 | 3.47 | 10.66 | 2.55 | 0.442 | 0.087 | ||||||||||||||||||||||||||||
| GUG12 | 52.16 | 0.814 | 20.04 | 9.73 | 0.183 | 3.49 | 10.73 | 2.67 | 0.448 | 0.089 | ||||||||||||||||||||||||||||
| URA5 | 53.57 | 0.628 | 16.63 | 9.61 | 0.184 | 6.13 | 10.52 | 2.29 | 0.53 | 0.094 | ||||||||||||||||||||||||||||
| URA7 | 54.17 | 0.775 | 19.2 | 9.73 | 0.193 | 2.712 | 9.64 | 2.88 | 0.756 | 0.134 | ||||||||||||||||||||||||||||
| URA12 | 53.77 | 0.75 | 19.62 | 9.55 | 0.188 | 2.68 | 9.93 | 2.81 | 0.705 | 0.128 | ||||||||||||||||||||||||||||
| AGR1 | 51.58 | 0.752 | 20.19 | 9.77 | 0.188 | 3.04 | 10.71 | 2.72 | 1.03 | 0.189 | ||||||||||||||||||||||||||||
| AGR2 | 50.94 | 0.794 | 16.84 | 12.45 | 0.235 | 4.98 | 10.46 | 2.85 | 0.744 | 0.147 | ||||||||||||||||||||||||||||
| | ||||||||||||||||||||||||||||||||||||||
| B. TRACE ELEMENTS | ||||||||||||||||||||||||||||||||||||||
| Zn (Savage) | Zn (ICP) | Li | Be | Sc | V | Cr | Co | Ni | Cu | Cu | Rb | Sr | Y | Zr | Nb | Cs | Tl | Ba | La | Ce | Pr | Nd | Sm | Eu | Tb | Ga | Gd | Dy | Ho | Er | Yb | Lu | Hf | Ta | Pb | Th | U | |
| Hekla | XRF | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | (savage XRF) | ppm | ppm | ppm | ppm | ppm | ppm | ppb | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm |
| hek 6 | 134.00 | 136.56 | 8.04 | 1.96 | 34.53 | 368.43 | 12.09 | 47.43 | 13.64 | 42.01 | 38.00 | 11.83 | 363.83 | 46.23 | 271.21 | 43.78 | 0.15 | 20.29 | 178.08 | 27.14 | 63.19 | 8.72 | 38.77 | 9.57 | 3.14 | 1.62 | 23.93 | 10.03 | 9.11 | 1.79 | 4.67 | 4.06 | 0.59 | 6.82 | 2.99 | 1.72 | 2.20 | 0.67 |
| hek 12 | 2.00 | 31.00 | 26.00 | 14.00 | 371.00 | 49.20 | 275.00 | 41.00 | ||||||||||||||||||||||||||||||
| hek 5 | 136.00 | 139.95 | 8.18 | 1.89 | 34.80 | 381.44 | 15.24 | 48.81 | 17.14 | 50.80 | 47.00 | 12.04 | 370.35 | 46.50 | 272.22 | 43.66 | 0.15 | 21.96 | 177.77 | 27.07 | 62.86 | 8.69 | 38.25 | 9.50 | 3.10 | 1.60 | 24.42 | 9.90 | 8.97 | 1.76 | 4.60 | 3.99 | 0.58 | 6.69 | 2.95 | 1.70 | 2.14 | 0.66 |
| hek 14 | 163.00 | 188.23 | 15.63 | 3.32 | 22.40 | 74.02 | 16.90 | 18.08 | 4.24 | 15.56 | 17.00 | 25.20 | 400.40 | 77.67 | 476.38 | 66.16 | 0.32 | 48.68 | 332.54 | 51.76 | 117.54 | 15.93 | 67.18 | 16.04 | 4.81 | 2.59 | 27.59 | 16.25 | 14.43 | 2.83 | 7.41 | 6.49 | 0.96 | 11.14 | 4.14 | 3.39 | 4.54 | 1.37 |
| hek 17 | 135.00 | 186.71 | 15.81 | 3.32 | 22.45 | 67.80 | 5.26 | 16.71 | 4.02 | 15.25 | 15.00 | 25.67 | 388.72 | 76.94 | 484.92 | 66.83 | 0.32 | 50.64 | 335.54 | 51.76 | 116.91 | 15.78 | 65.90 | 15.75 | 4.69 | 2.54 | 27.40 | 15.92 | 14.22 | 2.79 | 7.33 | 6.44 | 0.95 | 11.23 | 4.15 | 3.40 | 4.57 | 1.39 |
| hek 16 | 163.00 | 189.30 | 16.22 | 3.47 | 22.23 | 58.24 | 9.29 | 15.69 | 3.62 | 14.32 | 16.00 | 26.33 | 393.96 | 77.60 | 494.48 | 67.87 | 0.33 | 50.59 | 344.19 | 52.40 | 118.18 | 15.90 | 66.34 | 15.86 | 4.75 | 2.56 | 27.68 | 15.97 | 14.35 | 2.82 | 7.48 | 6.61 | 0.97 | 11.55 | 4.22 | 3.49 | 4.70 | 1.42 |
| hek 21 | 162.00 | 188.91 | 15.98 | 3.60 | 20.75 | 41.11 | 5.42 | 14.77 | 3.18 | 14.85 | 18.00 | 27.31 | 393.07 | 77.98 | 509.15 | 68.05 | 0.35 | 53.79 | 353.76 | 53.27 | 119.98 | 16.02 | 66.43 | 15.82 | 4.70 | 2.55 | 27.75 | 15.86 | 14.34 | 2.83 | 7.46 | 6.66 | 0.98 | 11.81 | 4.20 | 3.67 | 4.88 | 1.46 |
| hek 15 | 163.00 | 195.84 | 20.58 | 4.28 | 19.44 | 11.71 | 2.79 | 8.47 | 4.01 | 10.17 | 13.00 | 34.39 | 368.61 | 82.67 | 627.62 | 78.59 | 0.44 | 59.17 | 433.10 | 59.43 | 131.16 | 17.12 | 68.91 | 16.01 | 4.74 | 2.63 | 30.27 | 15.96 | 15.00 | 3.00 | 8.13 | 7.55 | 1.12 | 14.64 | 4.84 | 4.24 | 6.10 | 1.83 |
| Anatahan | ||||||||||||||||||||||||||||||||||||||
| AN-10 | 13.71 | 1.07 | 20.48 | 44 | 0.20 | 9.15 | 1.46 | 14.74 | 36.77 | 323.09 | 43.12 | 140.94 | 3.37 | 0.81 | 114 | 478.7675645548 | 15.07 | 32.58 | 4.79 | 21.27 | 5.64 | 1.60 | 1.13 | 6.62 | 6.87 | 1.49 | 4.21 | 4.26 | 0.67 | 3.72 | 0.27 | 5.55 | 2.22 | 0.92 | ||||
| Anat9 | 13.75 | 1.27 | 20.53 | 23 | 5.30 | 11.65 | 2.33 | 24.67 | 42.58 | 302.48 | 46.95 | 170.80 | 3.64 | 0.94 | 106 | 538.2747115042 | 15.95 | 33.66 | 4.92 | 22.58 | 6.08 | 1.76 | 1.24 | 7.32 | 7.78 | 1.65 | 4.76 | 4.77 | 0.76 | 4.51 | 0.27 | 7.13 | 2.67 | 1.11 | ||||
| 04-Anat-01 | 11.64 | 0.99 | 23.10 | 69 | 0.52 | 13.72 | 0.56 | 32.86 | 28.68 | 342.00 | 39.07 | 117.70 | 2.94 | 0.72 | 97 | 405.3645918868 | 12.61 | 27.74 | 4.27 | 18.80 | 5.10 | 1.58 | 1.01 | 6.02 | 6.33 | 1.37 | 3.87 | 3.86 | 0.61 | 3.32 | 0.20 | 4.95 | 1.84 | 0.73 | ||||
| 04-Anat-03 | 8.56 | 0.77 | 22.07 | 141 | 0.45 | 17.19 | 1.45 | 62.83 | 23.52 | 417.00 | 29.69 | 90.93 | 2.23 | 0.60 | 75 | 331.2030084306 | 9.82 | 21.36 | 3.13 | 14.45 | 3.90 | 1.27 | 0.77 | 4.57 | 4.85 | 1.04 | 2.95 | 2.93 | 0.46 | 2.57 | 0.15 | 4.09 | 1.44 | 0.59 | ||||
| 04-Anat-04 | 7.77 | 0.66 | 25.67 | 188 | 1.39 | 21.23 | 2.74 | 63.30 | 20.32 | 428.00 | 27.34 | 79.49 | 2.59 | 0.53 | 66 | 279.9777078064 | 8.19 | 17.88 | 2.72 | 12.35 | 3.41 | 1.15 | 0.69 | 4.05 | 4.38 | 0.95 | 2.70 | 2.69 | 0.42 | 2.24 | 0.18 | 3.50 | 1.23 | 0.51 | ||||
| AN-2 | 5.28 | 0.45 | 25.47 | 210 | 4.32 | 30.64 | 9.90 | 85.40 | 9.50 | 445.44 | 20.60 | 50.06 | 1.14 | 0.24 | 38 | 191.4824170528 | 5.25 | 11.52 | 1.83 | 8.60 | 2.49 | 0.90 | 0.54 | 3.10 | 3.38 | 0.72 | 2.03 | 2.07 | 0.32 | 1.41 | 2.34 | 0.66 | 0.26 | |||||
| AN-1 | 5.25 | 0.47 | 31.74 | 275 | 17.93 | 31.56 | 17.84 | 116.05 | 11.58 | 381.51 | 20.65 | 51.78 | 1.23 | 0.28 | 26 | 198.5843640904 | 5.69 | 12.30 | 1.88 | 8.78 | 2.52 | 0.87 | 0.54 | 3.11 | 3.42 | 0.74 | 2.08 | 2.10 | 0.33 | 1.49 | 0.09 | 2.42 | 0.86 | 0.31 | ||||
| AN-8 | 4.40 | 0.37 | 32.30 | 305 | 4.42 | 33.04 | 14.72 | 103.63 | 10.55 | 447.37 | 16.13 | 35.35 | 0.84 | 0.21 | 24 | 147.8840365564 | 4.89 | 10.38 | 1.65 | 7.30 | 2.07 | 0.77 | 0.43 | 2.51 | 2.64 | 0.57 | 1.60 | 1.57 | 0.25 | 1.02 | 0.06 | 2.37 | 0.62 | 0.23 | ||||
| Anat4-s | 12.04 | 1.06 | 23.44 | 80 | 0.77 | 15.77 | 5.25 | 38.81 | 30.54 | 339.63 | 41.56 | 126.31 | 2.88 | 0.76 | 434.7408859364 | 13.70 | 29.13 | 4.28 | 19.90 | 5.46 | 1.68 | 1.11 | 6.57 | 6.97 | 1.49 | 4.26 | 4.16 | 0.68 | 3.41 | 0.22 | 5.75 | 2.02 | 0.79 | |||||
| AN-12D | 8.18 | 1.04 | 23.14 | 57 | 0.07 | 12.22 | 0.38 | 31.58 | 30.59 | 331.28 | 44.18 | 132.55 | 3.17 | 0.63 | 444.3276469033 | 13.99 | 30.25 | 4.57 | 20.74 | 5.61 | 1.69 | 1.16 | 6.86 | 7.02 | 1.51 | 4.35 | 4.32 | 0.69 | 3.71 | 0.23 | 5.56 | 2.08 | 0.84 | |||||
| AN-7 | 6.36 | 0.51 | 30.27 | 255 | 2.32 | 25.68 | 6.08 | 105.06 | 11.73 | 414.14 | 24.72 | 61.72 | 1.20 | 0.18 | 240.8067628495 | 5.79 | 13.39 | 2.04 | 9.67 | 2.89 | 0.99 | 0.63 | 3.61 | 3.97 | 0.86 | 2.42 | 2.43 | 0.39 | 1.67 | 0.11 | 2.34 | 0.80 | 0.35 | |||||
| Anat-26-01 | 12.44 | 0.89 | 24.69 | 108 | 2.72 | 14.92 | 0.86 | 39.79 | 28.31 | 345.00 | 35.04 | 105.54 | 2.54 | 0.68 | 374.2473001959 | 11.34 | 24.78 | 3.65 | 16.79 | 4.56 | 1.43 | 0.91 | 5.37 | 5.66 | 1.23 | 3.46 | 3.48 | 0.55 | 2.99 | 0.18 | 4.81 | 1.69 | 0.68 | |||||
| Anat-26-02 | 8.10 | 0.82 | 26.30 | 142 | 0.96 | 22.37 | 2.98 | 66.39 | 23.62 | 366.00 | 33.28 | 97.55 | 2.48 | 0.58 | 356.7788087856 | 10.77 | 24.05 | 3.70 | 16.82 | 4.59 | 1.44 | 0.89 | 5.24 | 5.50 | 1.18 | 3.38 | 3.44 | 0.54 | 2.80 | 0.17 | 4.32 | 1.53 | 0.63 | |||||
| Mariana Central Island Province | ||||||||||||||||||||||||||||||||||||||
| ALAM 2 | 78.7 | 32 | 218 | 19.7 | 20 | 16.34 | 303 | 26.7 | 82.9 | 1.305 | 0.605 | 59 | 251 | 5.90 | 14.04 | 2.17 | 10.33 | 3.05 | 0.967 | 0.727 | 17.24 | 3.86 | 4.29 | 0.933 | 2.69 | 2.73 | 0.443 | 2.33 | 0.0931 | 3.14 | 0.816 | 0.409 | ||||||
| AGR 8B | 76.1 | 30.45 | 257 | 6.5 | 12.3 | 19.89 | 395 | 18.1 | 59.2 | 1.126 | 0.463 | 54 | 165 | 7.45 | 15.76 | 2.34 | 10.73 | 2.79 | 0.992 | 0.563 | 18.71 | 3.17 | 3.16 | 0.667 | 1.87 | 1.86 | 0.288 | 1.60 | 0.0881 | 1.79 | 0.894 | 0.377 | ||||||
| PAG 1 | 98.7 | 41.0 | 386.1 | 33.706 | 22.6 | 11.92 | 334 | 1.016 | 0.524 | 25 | 221 | 5.242 | 12.051 | 1.913 | 9.225 | 2.827 | 0.960 | 0.584 | 17.45 | 3.380 | 3.749 | 0.789 | 2.218 | 2.219 | 0.334 | 1.579 | 0.073 | 2.801 | 0.542 | 0.263 | ||||||||
| PAG 3 | 100.0 | 41.3 | 373 | 44.9 | 26.4 | 12.46 | 317 | 23.2 | 60.8 | 0.965 | 0.608 | 27 | 230 | 5.18 | 12.45 | 1.95 | 9.57 | 2.95 | 1.015 | 0.670 | 17.15 | 3.69 | 3.93 | 0.853 | 2.40 | 2.36 | 0.369 | 1.80 | 0.0706 | 2.71 | 0.537 | 0.280 | ||||||
| GUG 4 | 86.4 | 34.8 | 258.9 | 37.4 | 24 | 8.07 | 291 | 22.8 | 57.9 | 0.808 | 0.357 | 44 | 167 | 3.55 | 9.11 | 1.50 | 7.58 | 2.39 | 0.861 | 0.600 | 17.5 | 3.23 | 3.57 | 0.791 | 2.26 | 2.30 | 0.365 | 1.63 | 0.0705 | 2.42 | 0.350 | 0.201 | ||||||
| GUG 9 | 81.0 | 30.1 | 272.4 | 12.3 | 14.9 | 6.53 | 299 | 20.1 | 50.4 | 0.731 | 0.297 | 36 | 139 | 2.85 | 7.63 | 1.30 | 6.57 | 2.14 | 0.809 | 0.547 | 18.5 | 2.86 | 3.29 | 0.727 | 2.11 | 2.07 | 0.331 | 1.42 | 0.0613 | 2.03 | 0.271 | 0.147 | ||||||
| MM-92-6 | 116.1 | 31.8 | 204.5 | 11.1 | 31.45 | 324 | 29.8 | 96.3 | 1.856 | 0.733 | 115 | 277.7 | 11.41 | 23.98 | 3.53 | 15.88 | 4.11 | 1.373 | 0.874 | 18.22 | 4.84 | 4.93 | 1.058 | 2.98 | 2.94 | 0.472 | 2.59 | 0.1168 | 3.01 | 1.422 | 0.563 | |||||||
| GUG3 | 82.4 | 28.6 | 278.6 | 4.7 | 12.6 | 16.9 | 401 | 23.0 | 66.3 | 1.241 | 0.467 | 67 | 217 | 7.40 | 15.86 | 2.40 | 11.11 | 3.08 | 1.084 | 0.657 | 17.7 | 3.65 | 3.81 | 0.816 | 2.30 | 2.29 | 0.356 | 1.78 | 0.0916 | 2.82 | 0.760 | 0.352 | ||||||
| GUG11 | 77.8 | 28.3 | 241 | 4.3 | 15.2 | 7.2 | 306 | 23.5 | 60.0 | 0.699 | 0.271 | 31 | 190 | 3.18 | 8.53 | 1.46 | 7.47 | 2.43 | 0.920 | 0.626 | 18.44 | 3.19 | 3.72 | 0.830 | 2.38 | 2.46 | 0.391 | 1.70 | 0.0689 | 2.44 | 0.297 | 0.175 | ||||||
| ALAM5 | 82.0 | 36.5 | 264 | 22.1 | 18.4 | 15.41 | 307 | 24.1 | 74.4 | 1.244 | 0.589 | 56 | 238 | 5.85 | 13.51 | 2.12 | 10.12 | 2.99 | 1.011 | 0.693 | 15.55 | 3.73 | 4.10 | 0.890 | 2.54 | 2.53 | 0.394 | 2.18 | 0.0945 | 3.24 | 0.824 | 0.395 | ||||||
| URA6 | 111.4 | 31.1 | 120 | 0.01 | 5.4 | 18.32 | 319 | 36.4 | 96.1 | 2.107 | 0.675 | 80 | 424 | 10.05 | 22.20 | 3.19 | 14.92 | 4.30 | 1.448 | 1.014 | 17.29 | 5.33 | 6.04 | 1.345 | 3.85 | 3.92 | 0.617 | 2.78 | 0.1426 | 4.23 | 1.501 | 0.511 | ||||||
| SAG1 | 77.8 | 34.8 | 233 | 63.1 | 29.4 | 9.45 | 352 | 23.5 | 68.5 | 1.641 | 0.353 | 44 | 253 | 6.17 | 13.98 | 2.14 | 10.01 | 2.86 | 1.014 | 0.644 | 16.27 | 3.51 | 3.75 | 0.787 | 2.29 | 2.28 | 0.350 | 1.90 | 0.1203 | 3.63 | 0.886 | 0.366 | ||||||
| GUG13 | 77.5 | 29.5 | 265 | 1.7 | 12 | 6.06 | 303 | 21.6 | 53.4 | 0.674 | 0.223 | 15 | 162 | 2.75 | 7.53 | 1.28 | 6.59 | 2.21 | 0.829 | 0.573 | 18.44 | 2.96 | 3.51 | 0.781 | 2.17 | 2.22 | 0.358 | 1.55 | 0.0590 | 1.92 | 0.244 | 0.146 | ||||||
| GUG12 | 78.6 | 285.7 | 53.2 | 28.9 | 7.35 | 281 | 21.7 | 53.5 | 0.720 | 0.198 | 49 | 172 | 3.26 | 8.43 | 1.40 | 7.08 | 2.27 | 0.841 | 0.559 | 16.9 | 2.94 | 3.38 | 0.743 | 2.15 | 2.18 | 0.345 | 1.50 | 0.0663 | 1.81 | 0.316 | 0.206 | |||||||
| URA5 | 72.3 | 39.65 | 235 | 48.9 | 26.5 | 8.15 | 307 | 20.2 | 49.0 | 1.044 | 0.365 | 38 | 198 | 4.45 | 10.17 | 1.54 | 7.25 | 2.15 | 0.814 | 0.528 | 14.17 | 2.80 | 3.19 | 0.696 | 1.99 | 2.05 | 0.324 | 1.37 | 0.0722 | 2.06 | 0.657 | 0.240 | ||||||
| URA7 | 85.8 | 29.9 | 232 | 2.4 | 11 | 12.01 | 384 | 24.0 | 62.6 | 1.663 | 0.401 | 47 | 290 | 7.97 | 16.80 | 2.41 | 10.75 | 2.97 | 1.066 | 0.699 | 18.2 | 3.68 | 4.19 | 0.882 | 2.56 | 2.55 | 0.406 | 1.76 | 0.1015 | 2.76 | 1.214 | 0.399 | ||||||
| URA12 | 82.6 | 30.1 | 208 | 2.3 | 9.3 | 11.9 | 398 | 23.0 | 59.4 | 1.596 | 0.390 | 51 | 245 | 7.29 | 15.57 | 2.21 | 9.98 | 2.79 | 1.004 | 0.638 | 18.09 | 3.41 | 3.80 | 0.827 | 2.34 | 2.35 | 0.385 | 1.66 | 0.0917 | 2.59 | 1.157 | 0.373 | ||||||
| AGR1 | 75.7 | 246 | 5.2 | 9.7 | 22.5 | 394 | 0.526 | 181 | 20.16 | 0.68 | ||||||||||||||||||||||||||||
| AGR2 | 89.6 | 341 | 5.7 | 12.4 | 12.96 | 344 | 20.1 | 51.9 | 1.007 | 0.160 | 23 | 158 | 6.84 | 14.71 | 2.24 | 10.48 | 2.90 | 1.028 | 0.603 | 17.11 | 3.33 | 3.47 | 0.741 | 2.09 | 2.01 | 0.316 | 1.52 | 0.0648 | 1.83 | 0.822 | 0.313 | |||||||
| C. ISOTOPIC DATA | ||||||||||||||||||||||||||||||||||||||
| 87Sr/86Sr | 143Nd/144Nd | 206Pb/204Pb | 2sd | 207Pb/204Pb | 2sd | 208Pb/204Pb | 2sd | d30Si | 2sd | d29Si | 2sd | Mo | d98Mo | 2se | d66Zn | d67Zn | d68Zn | d65Cu | 2sd | e205Tl | 2sd | |||||||||||||||||
| Hekla | µg/g | |||||||||||||||||||||||||||||||||||||
| hek 6 | -0.31 | 0.09 | -0.16 | 0.05 | 1.3 | -0.16 | 0.06 | |||||||||||||||||||||||||||||||
| hek 12 | -0.29 | 0.1 | -0.17 | 0.05 | 1.4 | -0.12 | 0.06 | 0.29 | 0.4 | 0.53 | 0.13 | 0.08 | ||||||||||||||||||||||||||
| hek 5 | -0.32 | 0.12 | -0.19 | 0.11 | 1.3 | -0.18 | 0.06 | 0.26 | 0.37 | 0.52 | 0 | 0.08 | ||||||||||||||||||||||||||
| hek 14 | -0.26 | 0.06 | -0.16 | 0.05 | 2.4 | -0.15 | 0.06 | 0.23 | 0.37 | 0.47 | ||||||||||||||||||||||||||||
| hek 17 | -0.25 | 0.18 | -0.16 | 0.04 | 2.6 | -0.11 | 0.06 | 0.26 | 0.39 | 0.5 | ||||||||||||||||||||||||||||
| hek 16 | -0.27 | 0.07 | -0.12 | 0.05 | 2.6 | -0.15 | 0.06 | 0.28 | 0.4 | 0.56 | ||||||||||||||||||||||||||||
| hek 21 | -0.29 | 0.15 | -0.12 | 0.07 | 2.7 | -0.17 | 0.06 | 0.28 | 0.4 | 0.56 | ||||||||||||||||||||||||||||
| hek 15 | -0.22 | 0.03 | -0.13 | 0.03 | 3.2 | -0.12 | 0.06 | 0.32 | 0.45 | 0.62 | ||||||||||||||||||||||||||||
| Anatahan | ||||||||||||||||||||||||||||||||||||||
| AN-10 | 18.7970 | 15.5658 | 38.4281 | |||||||||||||||||||||||||||||||||||
| Anat9 | 0.70340 | 18.7900 | 15.5640 | 38.4178 | ||||||||||||||||||||||||||||||||||
| 04-Anat-01 | 18.7790 | 15.5640 | 38.4190 | |||||||||||||||||||||||||||||||||||
| 04-Anat-03 | 0.70339 | 0.513001 | 18.7690 | 15.5630 | 38.4160 | |||||||||||||||||||||||||||||||||
| 04-Anat-04 | 18.7889 | 15.5589 | 38.4068 | |||||||||||||||||||||||||||||||||||
| AN-2 | 18.8000 | 15.5680 | 38.4270 | |||||||||||||||||||||||||||||||||||
| AN-1 | 18.7971 | 0.0035 | 15.5694 | 0.0031 | 38.4398 | 0.0099 | ||||||||||||||||||||||||||||||||
| AN-8 | 18.8236 | 0.0011 | 15.5657 | 0.0010 | 38.4124 | 0.0030 | ||||||||||||||||||||||||||||||||
| Anat4-s | 18.8228 | 0.0009 | 15.5663 | 0.0008 | 38.4021 | 0.0026 | ||||||||||||||||||||||||||||||||
| AN-12D | 18.7553 | 0.0009 | 15.5509 | 0.0008 | 38.3351 | 0.0026 | ||||||||||||||||||||||||||||||||
| AN-7 | 18.8228 | 0.0009 | 15.5663 | 0.0008 | 38.4021 | 0.0026 | ||||||||||||||||||||||||||||||||
| Anat-26-01 | 18.7671 | 0.0012 | 15.5533 | 0.0010 | 38.3413 | 0.0033 | ||||||||||||||||||||||||||||||||
| Anat-26-02 | 18.8604 | 0.0022 | 15.5774 | 0.0020 | 38.5062 | 0.0064 | ||||||||||||||||||||||||||||||||
| Mariana Central Island Province | ||||||||||||||||||||||||||||||||||||||
| ALAM 2 | 1.277 | 0.082 | 0.021 | |||||||||||||||||||||||||||||||||||
| AGR 8B | 0.595 | -0.113 | 0.014 | -1.1 | 0.5 | |||||||||||||||||||||||||||||||||
| PAG 1 | -1.5 | 0.5 | ||||||||||||||||||||||||||||||||||||
| PAG 3 | -0.4 | 0.5 | ||||||||||||||||||||||||||||||||||||
| GUG 4 | -1.2 | 0.5 | ||||||||||||||||||||||||||||||||||||
| GUG 9 | 0.776 | 0.067 | 0.020 | -1.1 | 0.5 | |||||||||||||||||||||||||||||||||
| MM-92-6 | 0.998 | -0.083 | 0.022 | -1.2 | 0.5 | |||||||||||||||||||||||||||||||||
| GUG3 | -0.5 | 0.5 | ||||||||||||||||||||||||||||||||||||
| GUG11 | 1.003 | 0.049 | 0.016 | -1.2 | 0.5 | |||||||||||||||||||||||||||||||||
| ALAM5 | 1.072 | 0.047 | 0.014 | -0.6 | 0.5 | |||||||||||||||||||||||||||||||||
| URA6 | -1.7 | 0.5 | ||||||||||||||||||||||||||||||||||||
| SAG1 | -0.6 | 0.5 | ||||||||||||||||||||||||||||||||||||
| GUG13 | 0.856 | 0.038 | 0.031 | 1.2 | 0.5 | |||||||||||||||||||||||||||||||||
| GUG12 | 0.894 | 0.021 | 0.025 | -0.8 | 0.5 | |||||||||||||||||||||||||||||||||
| URA5 | 0.710 | 0.059 | 0.015 | -1.8 | 0.5 | |||||||||||||||||||||||||||||||||
| URA7 | 0.907 | 0.054 | 0.017 | -1.1 | 0.5 | |||||||||||||||||||||||||||||||||
| URA12 | 0.909 | 0.043 | 0.022 | -1.7 | 0.5 | |||||||||||||||||||||||||||||||||
| AGR1 | ||||||||||||||||||||||||||||||||||||||
| AGR2 | | | | 0.492 | -0.092 | 0.018 | -1.2 | 0.5 | ||||||||||||||||||||||||||||||
References
Hekla
Major elements: Savage et al., 2011
Trace elements: Prytulak et al., 2016
Si isotopes: Savage et al., 2011
Mo isotopes: Yang et al., 2015
Zn isotopes: Chen et al., 2013
Cu isotopes: Savage et al., 2015
Anatahan
Major, trace elements: Wade et al., 2005
Radiogenic isotopes: Woodhead, 1989; Woodhead et Fraser, 1985
Mariana Central Island Province
Major elements: Elliott et al., 1997
Trace elements: Elliott et al., 1997 & Prytulak et al., 2013
Mo isotopes: Freymuth et al., 2015
Pb isotopes: Freymuth et al., 2015
Tl isotopes: Prytulak et al., 2013
Measurement of vanadium isotopes
Table S-2 presents new whole rock stable vanadium isotope data. Measurement took place at University of Oxford and Imperial College London following the protocols outlined in Nielsen et al. (2011).
Table S-2 Vanadium isotopic data.
| Sample | d51V | 2sd | number dissolutions | number of measurements | number of sessions | Oxford or Imperial College |
| Marianas Central Island Province | ||||||
| SAG1 | -0.80 | 0.14 | 1 | 5 | 2 | OX |
| GUG13 | -0.98 | 0.17 | 2 | 8 | 2 | OX |
| GUG12 | -0.84 | 0.13 | 1 | 5 | 1 | OX |
| URA5 | -0.75 | 0.15 | 3 | 8 | 2 | OX |
| URA7 | -0.63 | 0.21 | 2 | 7 | 2 | OX |
| URA12 | -0.77 | 0.13 | 2 | 11 | 2 | OX |
| AGR1 | -0.83 | 0.09 | 1 | 9 | 3 | OX |
| AGR2 | -0.91 | 0.15 | 2 | 21 | 4 | OX |
| AGR8b | -0.84 | 0.05 | 2 | 8 | 2 | OX |
| M-92-6 | -0.60 | 0.08 | 2 | 7 | 2 | OX |
| GUG3 | -0.81 | 0.11 | 1 | 3 | 1 | OX |
| GUG4 | -0.73 | 0.14 | 1 | 4 | 1 | OX |
| GUG11 | -0.71 | 0.17 | 3 | 11 | 2 | OX |
| PAG1 | -0.90 | 0.11 | 1 | 4 | 2 | OX |
| PAG3 | -0.85 | 0.07 | 2 | 12 | 2 | OX |
| ALAM2 | -0.81 | 0.06 | 2 | 6 | 3 | OX and IC |
| ALAM5 | -0.83 | 0.11 | 1 | 4 | 1 | OX |
| URA6 | -0.45 | 0.31 | 4 | 11 | 5 | OX |
| GUG9 | -0.85 | 0.07 | 1 | 6 | 2 | OX |
| Anatahan. Marianas | ||||||
| 04 ANAT 4 | -0.95 | 0.18 | 1 | 3 | 1 | IC |
| 04 ANAT 03 | -0.26 | 0.21 | 2 | 8 | 3 | IC |
| ANAT 26-01 | -0.59 | 0.23 | 1 | 2 | 1 | IC |
| 04 ANAT 1 | 0.35 | 0.33 | 1 | 5 | 2 | IC |
| ANAT 26-02 | -0.72 | 0.14 | 1 | 4 | 2 | IC |
| ANAT 4 as | -0.76 | 0.15 | 1 | 9 | 2 | IC |
| AN2 | -0.39 | 0.18 | 2 | 6 | 2 | IC |
| AN7 | -0.77 | 0.11 | 1 | 4 | 1 | IC |
| AN12D | 0.44 | 0.39 | 2 | 2 | 1 | IC |
| Anat9 | 0.97 | 0.12 | 1 | 2 | 2 | IC |
| AN10 | 0.55 | 0.30 | 2 | 4 | 2 | IC |
| AN1 | -0.69 | 0.15 | 2 | 9 | 2 | IC |
| AN8 | -0.56 | 0.08 | 1 | 5 | 1 | IC |
| Hekla. Iceland | ||||||
| HEK05 | -0.65 | 0.20 | 2 | 16 | 2 | OX |
| HEK06 | -0.81 | 0.01 | 1 | 2 | 1 | OX |
| HEK12 | -0.64 | 0.13 | 1 | 11 | 3 | OX |
| HEK14 | 0.24 | 0.08 | 1 | 5 | 2 | OX |
| HEK15 | 0.92 | 0.07 | 1 | 2 | 1 | OX |
| HEK16 | 0.50 | 0.04 | 1 | 5 | 1 | OX |
| HEK17 | 0.41 | 0.11 | 1 | 4 | 1 | OX |
| HEK21 | 0.46 | 0.12 | 1 | 5 | 2 | OX |
| USGS Standards (from Prytulak et al.. 2011 as lavas data are contemporaneous) | ||||||
| BIR1a | -0.92 | 0.16 | 12 | 50 | 8 | OX |
| BHVO1 | -0.92 | 0.04 | 1 | 4 | 1 | OX |
| BHVO2 | -0.88 | 0.10 | 7 | 14 | 4 | OX |
| BCR2 | -0.92 | 0.16 | 13 | 27 | 7 | OX |
| AGV2 | -0.58 | 0.10 | 4 | 6 | 4 | OX and IC |
| GSP2 | -0.63 | 0.10 | 3 | 6 | 1 | OX |
| PCC1 | -1.01 | 0.09 | 2 | 8 | 3 | OX |
| DTS | -0.95 | na | 1 | 1 | 1 | OX |
Briefly, 50-150 mg of sample were dissolved with standard HF-HNO3 techniques and put through a seven-column procedure to separate V quantitatively from Cr, Ti and other matrix elements. Isotope ratio measurement was conducted on Nu Plasma HR machines at both Oxford and Imperial, using a 109 Ω resistor to collect 51V and standard 1011 Ω resistors for all other masses. Sample solutions were 5 ppm and total procedural blanks were <1.5 ng, which is negligible compared to the amount of V processed (5-20 µg).
Sample measurements were contemporaneous with those of USGS reference materials and verification tests presented in Prytulak et al. (2011). Many of the samples were measured multiple times and across both Oxford and Imperial as the first inter-laboratory vanadium isotope effort (Table S-2). Measurements of the secondary V solution standard, BDH, were δ51V = -1.17 ± 0.17 2sd (n = 1329) over the Oxford-Imperial cross calibration period. The isotopic offset of AA-BDH has been confirmed in subsequent studies using vastly different measurement protocols and consuming significantly less vanadium (Nielsen et al., 2016; Wu et al., 2016).
Modelling δ51V-MgO-V during magmatic differentiation
Both lava suites are modelled assuming that they are related by fractional crystallisation using the equations of Shaw (1970). Such a relationship has been demonstrated for Anatahan lavas by Wade et al. (2005). This assumption does not hold in detail for Hekla, where there is evidence for mixing between basaltic andesites and dacites (e.g., Sigmarsson et al., 1992), but is an adequate approximation. Note that (titano)magnetite is always crystallising in the investigated lavas at Hekla due to their Fe-rich nature (e.g., Sigmarsson et al., 1992). The delayed onset of major magnetite fractionation in Anatahan lavas may be due to suppression by water (e.g., Feig et al., 2010), although this remains controversial.
The objective of the calculations is not to model all aspects of magmatic differentiation, but rather to produce an internally consistent model whereby the chemical trends of Mg, Fe, V (and its isotopes) and inferred melt fractions are reproduced. As a first step, the degree of melt remaining (the operative variable in the modal calculations) is calibrated against natural samples by their MgO-Rb systematics. For fractional crystallisation, the concentration of a perfectly incompatible element (i.e. DMin-Melt = 0) in the melt is given by C l = C0/F, where C0 is the bulk concentration and F the melt fraction. The melt fraction calculated from Rb abundances (FRb) shows a linear relationship with MgO for both suites, where FRb = 0.19 x MgO wt. %. This is used to anchor the model in terms of Mg-Fe evolution, where both elements are treated as trace elements for simplicity. Modelling of the two evolving suites was performed at 5 % intervals of melt remaining (F). At Anatahan, mineral assemblages from de Moor et al. (2005) of 60 % plagioclase, 15 % opx and cpx with 5-10 % Fe-Ti oxides is used as a guide. The onset of significant (titano)magnetite crystallisation is estimated at 4 wt. % MgO from the precipitous decrease in V concentration. The fractionating assemblage at Hekla consists of olivine (and later orthopyroxene), plagioclase, clinopyroxene and titanomagnetite (Sigmarsson et al., 1992), though the proportions are not reported, they are adjusted so as to fit the MgO-FeO evolution defined by the whole rocks.
The V partition coefficient for magnetite at Anatahan is calculated from the measurements of de Moor et al. (2005), DMagV = 32. The DV for magnetite at Hekla is determined by the best fit to the data (DMagV = 42). For pyroxenes, values for the NNO experiments of Mallmann and O’Neill (2009) were used.
We use the most primitive (highest V contents) lavas from each suite as a starting point. Clearly Hekla (375 ppm V) has less evolved magmas than Anatahan (275 ppm V), but since their most primitive lavas available have similar δ51V, we use δ51V = -0.75 as a starting composition for both. We prescribe initial V, MgO and FeO contents according to the most magnesian lava in the suite.
Temperatures are important when evaluating bulk isotope fractionation factors, particularly since contrasting temperatures are expected between the two suites of lavas. de Moor et al. (2005) used 2-pyroxene equilibrium thermometry to estimate magmatic temperatures of 1050-1100 oC. This matches well with the empirical relationship developed for mafic magmas (T (1atm) = 1000 + 20*MgO) from Nisbet (1982). This relationship is used to estimate similar magmatic temperatures at Hekla. Table S-3 tabulates the results of the fractional crystallisation model and gives all input parameters.
The extent of vanadium isotope fractionation is calculated by the Rayleigh distillation equation, δ51VMelt = δ51VBulk + ∆51VMin-Meltln(FV), where FV is the fraction of V remaining in the liquid. As each phase partitions V differently, a weighting factor, W, is given by Wn = (PnDn)/ ∑n(P,D), where P is the modal proportion of phase, n, and D is the partition coefficient. When (titano)magnetite crystallises, it hosts >75 % of the V budget, thereby controlling its isotope fractionation in magmatic suites.
Table S-3 Fractional crystallisation models.
| Anatahan | ||||||||||||||||
| Global Input Variables | ||||||||||||||||
| DV | DMgO | DFeO | ∆51Vmin-melt | |||||||||||||
| Plagioclase | 0 | 0 | 0 | 0 | ||||||||||||
| Magnetite | 32 | 0 | 6.5 | -0.85*106/T2 | ||||||||||||
| OPX | 1.5 | 6.75 | 3 | -0.85*106/T2 | ||||||||||||
| CPX | 2.5 | 3.75 | 1.8 | -0.85*106/T2 | ||||||||||||
| Modes | Bulk D | Model Output | ||||||||||||||
| F | Plagioclase | Magnetite | OPX | CPX | DV | DMgO | DFeO | ∆51Vmin-melt | V | δ51V | MgO | FeO | T (°C) | |||
| 1 | 275 | -0.75 | 5 | 9 | 1100 | |||||||||||
| 0.95 | 0.6 | 0.2 | 0.2 | 0.81 | 2.13 | 0.99 | -0.31 | 277.8 | -0.74 | 4.7 | 9 | 1094.4 | ||||
| 0.9 | 0.6 | 0.2 | 0.2 | 0.81 | 2.13 | 0.99 | -0.31 | 280.7 | -0.72 | 4.4 | 9 | 1088.8 | ||||
| 0.85 | 0.6 | 0.2 | 0.2 | 0.81 | 2.13 | 0.99 | -0.31 | 283.8 | -0.71 | 4.2 | 9 | 1083.2 | ||||
| 0.8 | 0.6 | 0.2 | 0.2 | 0.81 | 2.13 | 0.99 | -0.31 | 287.2 | -0.69 | 3.9 | 9 | 1077.7 | ||||
| 0.75 | 0.6 | 0.2 | 0.2 | 0.81 | 2.13 | 0.99 | -0.31 | 290.8 | -0.68 | 3.6 | 9 | 1072.2 | ||||
| 0.7 | 0.6 | 0.01 | 0.2 | 0.2 | 1 | 2.13 | 1.03 | -0.35 | 275.2 | -0.63 | 3.3 | 8.9 | 1066.8 | |||
| 0.65 | 0.6 | 0.01 | 0.2 | 0.2 | 1.13 | 2.13 | 1.06 | -0.36 | 260.5 | -0.57 | 3.1 | 8.8 | 1061.4 | |||
| 0.6 | 0.6 | 0.02 | 0.19 | 0.19 | 1.26 | 2.05 | 1.05 | -0.38 | 241.3 | -0.5 | 2.9 | 8.8 | 1058.4 | |||
| 0.55 | 0.6 | 0.02 | 0.19 | 0.19 | 1.41 | 2.03 | 1.07 | -0.4 | 215.7 | -0.42 | 2.7 | 8.6 | 1054.2 | |||
| 0.5 | 0.6 | 0.03 | 0.19 | 0.19 | 1.56 | 2 | 1.09 | -0.41 | 187.1 | -0.31 | 2.5 | 8.4 | 1050 | |||
| 0.45 | 0.6 | 0.03 | 0.19 | 0.19 | 1.71 | 1.97 | 1.11 | -0.42 | 156.5 | -0.18 | 2.3 | 8.2 | 1045.9 | |||
| 0.4 | 0.6 | 0.04 | 0.18 | 0.18 | 1.86 | 1.95 | 1.13 | -0.42 | 125.5 | -0.03 | 2.1 | 8 | 1042 | |||
| 0.35 | 0.6 | 0.04 | 0.18 | 0.18 | 2.01 | 1.92 | 1.15 | -0.43 | 95.6 | 0.16 | 1.9 | 7.7 | 1038 | |||
| 0.3 | 0.6 | 0.05 | 0.18 | 0.18 | 2.16 | 1.9 | 1.17 | -0.44 | 68.4 | 0.38 | 1.7 | 7.3 | 1034 | |||
| 0.25 | 0.6 | 0.05 | 0.18 | 0.18 | 2.31 | 1.87 | 1.2 | -0.44 | 45 | 0.66 | 1.5 | 6.9 | 1029.9 | |||
| 0.2 | 0.6 | 0.06 | 0.17 | 0.17 | 2.46 | 1.84 | 1.22 | -0.45 | 26.4 | 1.01 | 1.3 | 6.4 | 1025.7 | |||
| 0.15 | 0.6 | 0.06 | 0.17 | 0.17 | 2.61 | 1.82 | 1.24 | -0.45 | 13.1 | 1.47 | 1.1 | 5.8 | 1021.2 | |||
| 0.1 | 0.6 | 0.07 | 0.17 | 0.17 | 2.76 | 1.79 | 1.26 | -0.45 | 4.8 | 2.13 | 0.8 | 5 | 1016.1 | |||
| 0.05 | 0.6 | 0.07 | 0.17 | 0.17 | 2.91 | 1.77 | 1.28 | -0.46 | 0.9 | 3.22 | 0.5 | 3.9 | 1010.1 | |||
| Hekla | ||||||||||||||||
| Global Input Variables | ||||||||||||||||
| DV | DMgO | DFeO | ∆51Vmin-melt | |||||||||||||
| Plagioclase | 0 | 0 | 0 | 0 | ||||||||||||
| Magnetite | 42 | 0 | 8 | -0.75*106/T2 | ||||||||||||
| OPX | 1.5 | 7.25 | 3 | -0.75*106/T2 | ||||||||||||
| CPX | 2.5 | 4 | 1.8 | -0.75*106/T2 | ||||||||||||
| Modes | Bulk D | Model Output | ||||||||||||||
| F | Plagioclase | Magnetite | OPX | CPX | DV | DMgO | DFeO | ∆51Vmin-melt | V | δ51V | MgO | FeO | T (°C) | |||
| 1 | 375 | -0.75 | 5.5 | 15.75 | 1110 | |||||||||||
| 0.95 | 0.6 | 0.06 | 0.17 | 0.17 | 3.24 | 1.95 | 1.33 | -0.39 | 334.4 | -0.69 | 5.2 | 15.5 | 1104.8 | |||
| 0.9 | 0.6 | 0.06 | 0.17 | 0.17 | 3.24 | 1.95 | 1.33 | -0.39 | 296.1 | -0.62 | 5 | 15.2 | 1099.6 | |||
| 0.85 | 0.6 | 0.06 | 0.17 | 0.17 | 3.25 | 1.95 | 1.33 | -0.39 | 259.9 | -0.55 | 4.7 | 14.9 | 1094.3 | |||
| 0.8 | 0.6 | 0.06 | 0.17 | 0.17 | 3.27 | 1.95 | 1.33 | -0.39 | 225.9 | -0.47 | 4.5 | 14.6 | 1089.1 | |||
| 0.75 | 0.6 | 0.06 | 0.17 | 0.17 | 3.29 | 1.95 | 1.34 | -0.39 | 193.8 | -0.38 | 4.2 | 14.3 | 1083.8 | |||
| 0.7 | 0.6 | 0.06 | 0.17 | 0.17 | 3.32 | 1.95 | 1.34 | -0.39 | 163.9 | -0.29 | 3.9 | 13.9 | 1078.5 | |||
| 0.65 | 0.6 | 0.06 | 0.17 | 0.17 | 3.35 | 1.95 | 1.35 | -0.39 | 136.3 | -0.19 | 3.7 | 13.6 | 1073.2 | |||
| 0.6 | 0.6 | 0.06 | 0.17 | 0.17 | 3.38 | 1.95 | 1.35 | -0.39 | 111.1 | -0.08 | 3.4 | 13.1 | 1067.9 | |||
| 0.55 | 0.6 | 0.06 | 0.17 | 0.17 | 3.42 | 1.95 | 1.36 | -0.39 | 88.4 | 0.04 | 3.1 | 12.7 | 1062.5 | |||
| 0.5 | 0.6 | 0.07 | 0.17 | 0.17 | 3.45 | 1.95 | 1.37 | -0.39 | 68.4 | 0.18 | 2.9 | 12.2 | 1057.1 | |||
| 0.45 | 0.6 | 0.07 | 0.17 | 0.17 | 3.49 | 1.95 | 1.37 | -0.39 | 51.2 | 0.33 | 2.6 | 11.7 | 1051.7 | |||
| 0.4 | 0.6 | 0.07 | 0.17 | 0.17 | 3.53 | 1.95 | 1.38 | -0.39 | 36.8 | 0.51 | 2.3 | 11.1 | 1046.2 | |||
| 0.35 | 0.6 | 0.07 | 0.17 | 0.17 | 3.57 | 1.95 | 1.39 | -0.39 | 25.2 | 0.71 | 2 | 10.5 | 1040.8 | |||
| 0.3 | 0.6 | 0.07 | 0.17 | 0.17 | 3.62 | 1.95 | 1.4 | -0.39 | 16.1 | 0.95 | 1.8 | 9.8 | 1035.2 | |||
| 0.25 | 0.6 | 0.07 | 0.17 | 0.17 | 3.66 | 1.95 | 1.41 | -0.39 | 9.4 | 1.23 | 1.5 | 9 | 1029.6 | |||
| 0.2 | 0.6 | 0.07 | 0.17 | 0.17 | 3.7 | 1.95 | 1.41 | -0.39 | 4.9 | 1.57 | 1.2 | 8.1 | 1024 | |||
| 0.15 | 0.6 | 0.07 | 0.17 | 0.17 | 3.74 | 1.95 | 1.42 | -0.39 | 2.1 | 2.02 | 0.9 | 7.1 | 1018.3 | |||
| 0.1 | 0.6 | 0.07 | 0.17 | 0.17 | 3.78 | 1.95 | 1.43 | -0.39 | 0.6 | 2.66 | 0.6 | 5.9 | 1012.5 | |||
| 0.05 | | 0.6 | 0.07 | 0.17 | 0.17 | 3.83 | 1.95 | 1.44 | -0.39 | 0.1 | 3.74 | 0.3 | 4.3 | 1006.5 | ||
Modelling V/Sc ratios with variable fO2 and source depletion
1. Model Rationale. Here we detail the example forward models shown in Figure 4b,c. The majority of melt in both subduction zone and MORB are generated at pressures in the spinel stability field, thus we focus on the melting of spinel peridotite. Furthermore, the REE pattern of both suites of lavas are flat and lack obvious evidence for involvement of garnet in their sources.
The models explore aspects of the parameter space to give a sense of the dominant sensitivities and are intended to be illustrative rather than quantitatively comparable to natural samples. We focus on comparing the expected V/Sc ratios of lavas from a ‘MORB’ source and an ‘ARC’ source. The most critical assumption is that the arc source is one log unit more oxidised than the MORB source. Internally consistent partition coefficients for V and Sc from Mallmann and O’Neill (2009) are used, which have data for the same starting composition with variable fO2 and a focus on vanadium partitioning. We use their experiments for FMQ + 0.3 (experiments V1, V7, V8) and FMQ +1.3 (experiments V1, V7, V5). Because the degrees of melting in arc and MORB (and Iceland) are large (>10 %), batch and fractional melting models should yield similar results for liquid compositions. Given that neither V nor Sc are highly incompatible, the residues from a batch and fractional approach will not be markedly different either. Simple modal batch melting is used to explore the effects of changing modal compositions and source concentrations (Fig. 4b) and results are compared with a more detailed incremental non-modal melting approach that specifically models residual concentrations and recalculated partition coefficients and modal abundances at every step (Fig. 4c).
2. Modal batch melting. The depleted mantle MORB source is modelled with the following parameters from Salters and Stracke (2004): source [V] = 79 ppm, [Sc] = 16.3 ppm. Modal composition is 60 % olivine, 20 % orthopyroxene, 15 % clinopyroxene and 5 % spinel. Even with a set choice for partition coefficients, the bulk DV in a spinel lherozolite assemblage varies a great deal depending on modal abundances. Two scenarios of 5 % and 10 % modal clinopyroxene in the source of arc lavas are explored. Curves calculated for how V/Sc ratio changes with degree of melting in MORB and arc lavas are depicted in Figure 4b.
3. Non-modal incremental melting. A more realistic scenario with the non-modal melting equations of Shaw (1970) are explored in Figure 4c. Self-consistent V and Sc partition coefficients (Table S-4) from Mallmann and O’Neill (2009) and the melting reaction coefficients of Kinzler (1997) experiment L134 to L138 (0.13opx + 0.89cpx +0.12sp = liquid + 0.13ol) are used. This experiment was chosen because it is relevant for spinel peridotite melting up to 24 % at 1.5 GPa, conditions reasonable for the exploratory calculations here. Each 1 % increment of melt is formed and removed, and subsequent modal abundances, residual concentration of V and Sc, and bulk DV and DSc are recalculated at each step. Table S-4 gives an example of how modes and bulk partition coefficients change with progressive melting.
Table S-4 Example results for non-modal incremental batch melting.
Using melting reaction of 0.89 cpx + 0.13 opx + 0.12 sp = 0.13 ol + liquid.
Partition coefficients from Mallmann and O'Neill (2009).
| "MORB" | "ARC" | ||||||||||
| FMQ +0.3 | V | Sc | FMQ +1.3 | V | Sc | ||||||
| D olvine (V8) | 0.0646876241 | 0.2837600644 | D olivine | 0.021411957 | 0.2284364764 | ||||||
| D cpx (V1) | 0.4179636039 | 1.4403029494 | D cpx (V1) | 0.1717294109 | 1.4932144217 | ||||||
| D opx | 0.1960168624 | 0.3386847915 | D opx (V5) | 0.0933871639 | 0.3394884245 | ||||||
| D spinel | 1.5516253802 | 0.0434309112 | D spinel | 0.4937800944 | 0.0401772021 | ||||||
| "MORB" | |||||||||||
| Modes 'MORB' source | Bulk Partition Coefficients | Cl (V) | Cs (V) | Cl (Sc) | Cs (Sc) | ||||||
| Total Melt F | olivine | opx | cpx | spinel | V | Sc | |||||
| 0 | 79.00 | 16.30 | |||||||||
| 0.01 | 0.600 | 0.200 | 0.150 | 0.050 | 0.218 | 0.456 | 354.99 | 77.77 | 35.96 | 15.98 | |
| 0.02 | 0.608 | 0.197 | 0.137 | 0.049 | 0.212 | 0.438 | 359.99 | 76.49 | 36.70 | 15.64 | |
| 0.03 | 0.616 | 0.195 | 0.124 | 0.049 | 0.206 | 0.422 | 364.18 | 75.17 | 37.31 | 15.30 | |
| 0.04 | 0.624 | 0.192 | 0.113 | 0.048 | 0.200 | 0.408 | 367.54 | 73.81 | 37.80 | 14.94 | |
| 0.05 | 0.632 | 0.190 | 0.103 | 0.048 | 0.195 | 0.394 | 370.09 | 72.42 | 38.16 | 14.58 | |
| 0.06 | 0.640 | 0.187 | 0.094 | 0.047 | 0.190 | 0.383 | 371.85 | 71.00 | 38.40 | 14.21 | |
| 0.07 | 0.648 | 0.185 | 0.086 | 0.047 | 0.186 | 0.372 | 372.82 | 69.55 | 38.50 | 13.84 | |
| 0.08 | 0.657 | 0.182 | 0.078 | 0.046 | 0.182 | 0.363 | 373.04 | 68.09 | 38.48 | 13.47 | |
| 0.09 | 0.665 | 0.180 | 0.071 | 0.045 | 0.179 | 0.354 | 372.53 | 66.61 | 38.34 | 13.09 | |
| 0.1 | 0.674 | 0.178 | 0.065 | 0.045 | 0.175 | 0.347 | 371.34 | 65.12 | 38.08 | 12.72 | |
| 0.11 | 0.683 | 0.175 | 0.059 | 0.044 | 0.172 | 0.340 | 369.49 | 63.63 | 37.72 | 12.35 | |
| 0.12 | 0.692 | 0.173 | 0.054 | 0.044 | 0.169 | 0.334 | 367.03 | 62.13 | 37.26 | 11.98 | |
| 0.13 | 0.701 | 0.171 | 0.049 | 0.043 | 0.166 | 0.329 | 364.00 | 60.63 | 36.71 | 11.61 | |
| 0.14 | 0.710 | 0.169 | 0.045 | 0.043 | 0.164 | 0.325 | 360.45 | 59.13 | 36.09 | 11.25 | |
| 0.15 | 0.719 | 0.167 | 0.041 | 0.042 | 0.162 | 0.321 | 356.41 | 57.64 | 35.39 | 10.90 | |
| 0.16 | 0.728 | 0.164 | 0.037 | 0.042 | 0.160 | 0.318 | 351.94 | 56.17 | 34.64 | 10.55 | |
| 0.17 | 0.738 | 0.162 | 0.034 | 0.041 | 0.158 | 0.315 | 347.06 | 54.70 | 33.83 | 10.21 | |
| 0.18 | 0.747 | 0.160 | 0.031 | 0.041 | 0.156 | 0.312 | 341.83 | 53.25 | 32.98 | 9.87 | |
| 0.19 | 0.757 | 0.158 | 0.028 | 0.040 | 0.154 | 0.310 | 336.29 | 51.81 | 32.11 | 9.55 | |
| 0.2 | 0.767 | 0.156 | 0.026 | 0.040 | 0.153 | 0.309 | 330.47 | 50.39 | 31.20 | 9.23 | |
| "ARC" | |||||||||||
| Modes 'ARC' source | Bulk Partition Coefficients | Cl (V) | Cs (V) | Cl (Sc) | Cs (Sc) | ||||||
| Total Melt F | olivine | opx | cpx | spinel | V | Sc | |||||
| 0 | 50.00 | 13.50 | |||||||||
| 0.01 | 0.650 | 0.250 | 0.050 | 0.050 | 0.071 | 0.310 | 668.56 | 46.40 | 42.96 | 13.06 | |
| 0.02 | 0.651 | 0.247 | 0.046 | 0.049 | 0.069 | 0.302 | 631.80 | 42.99 | 42.57 | 12.61 | |
| 0.03 | 0.659 | 0.244 | 0.041 | 0.049 | 0.068 | 0.297 | 594.26 | 39.76 | 41.83 | 12.17 | |
| 0.04 | 0.668 | 0.240 | 0.038 | 0.048 | 0.067 | 0.293 | 557.69 | 36.73 | 41.02 | 11.74 | |
| 0.05 | 0.677 | 0.237 | 0.034 | 0.048 | 0.066 | 0.288 | 522.24 | 33.88 | 40.12 | 11.32 | |
| 0.06 | 0.685 | 0.234 | 0.031 | 0.047 | 0.065 | 0.285 | 488.03 | 31.21 | 39.16 | 10.90 | |
| 0.07 | 0.694 | 0.231 | 0.029 | 0.047 | 0.064 | 0.282 | 455.16 | 28.71 | 38.15 | 10.50 | |
| 0.08 | 0.703 | 0.228 | 0.026 | 0.046 | 0.064 | 0.279 | 423.69 | 26.38 | 37.09 | 10.11 | |
| 0.09 | 0.712 | 0.225 | 0.024 | 0.045 | 0.063 | 0.276 | 393.69 | 24.21 | 36.00 | 9.72 | |
| 0.1 | 0.722 | 0.222 | 0.022 | 0.045 | 0.062 | 0.274 | 365.17 | 22.20 | 34.89 | 9.35 | |
| 0.11 | 0.731 | 0.219 | 0.020 | 0.044 | 0.061 | 0.273 | 338.15 | 20.33 | 33.76 | 8.99 | |
| 0.12 | 0.741 | 0.216 | 0.018 | 0.044 | 0.061 | 0.271 | 312.62 | 18.59 | 32.63 | 8.64 | |
| 0.13 | 0.750 | 0.214 | 0.016 | 0.043 | 0.060 | 0.270 | 288.58 | 16.99 | 31.49 | 8.30 | |
| 0.14 | 0.760 | 0.211 | 0.015 | 0.043 | 0.060 | 0.269 | 266.00 | 15.51 | 30.36 | 7.98 | |
| 0.15 | 0.770 | 0.208 | 0.014 | 0.042 | 0.059 | 0.268 | 244.84 | 14.15 | 29.24 | 7.66 | |
| 0.16 | 0.780 | 0.205 | 0.012 | 0.042 | 0.059 | 0.268 | 225.06 | 12.89 | 28.14 | 7.36 | |
| 0.17 | 0.790 | 0.203 | 0.011 | 0.041 | 0.058 | 0.268 | 206.61 | 11.73 | 27.06 | 7.07 | |
| 0.18 | 0.800 | 0.200 | 0.010 | 0.041 | 0.058 | 0.268 | 189.43 | 10.67 | 25.99 | 6.79 | |
| 0.19 | 0.811 | 0.198 | 0.009 | 0.040 | 0.057 | 0.268 | 173.48 | 9.70 | 24.96 | 6.52 | |
| 0.2 | 0.821 | 0.195 | 0.009 | 0.040 | 0.057 | 0.268 | 158.69 | 8.81 | 23.95 | 6.26 | |
| Cl | concentration in liquid | ||||||||||
| | Cs | concentration in residue | |||||||||
Absolute values of V/Sc change dependent on the input parameters such as source concentrations and partition coefficients, when comparing lavas of similar, reasonable melting degrees (i.e. 10-20 %). Critically, a common feature of all forward models, assuming that arc lavas generally derive from a more depleted, oxidised source compared to MORB, is the overlap in V/Sc ratio due to decreasing V bulk partition coefficient and source V concentration in arc sources. This can counterbalance the higher V source concentration and V bulk partition coefficient of more fertile sources, leading to the generation of similar V/Sc in both settings. The variation in V/Sc with degree of melt in MORB is muted compared to arc lavas due to the more compatible behaviour of V in our models which means, although DV < DSc, the two absolute values are similar. It is interesting to note that the V/Sc ratio of arc lavas from this calculation are in good agreement with V/ScMORB = 6.74 ± 1.11 determined by Li and Lee (2004) from a global database. The V/Sc ratios of lavas from our ‘MORB’ source do not reproduce this value very well, but can be shifted to higher and lower values simply by choice of starting composition (Fig. 4c). Models incorporating a greater pressure range extending into the garnet stability field may also be able to better reconcile this difference, however, this is beyond the scope of the current contribution.
Supplementary Information References
Chen, H., Savage, P.S., Teng, F.-Z., Helz, R.T., Moynier, F. (2013) Zinc isotope fractionation during magmatic differentiation and the isotopic composition of the bulk Earth. Earth and Planetary Science Letters 369-370, 34-42.
Elliott, T., Plank, T., Zindler, A., White, W.M., Bourdon, B. (1997) Element transport from slab to volcanic front at the Mariana arc. Journal of Geophysical Research 102, 14991-15019.
Feig, S.T., Koepke, J., Snow, J.E. (2010) Effect of oxygen fugacity and water on phase equilibria of a hydrous tholeiitic basalt. Contributions to Mineralogy and Petrology 160, 551-568.
Freymuth, H., Vils, F., Willbold, M., Taylor, R.N., Elliott, T. (2015) Molybdenum mobility and isotopic fractionation during subduction at the Mariana arc. Earth and Planetary Science Letters 432, 176-186.
Kinzler, R.J. (1997) Melting of mantle peridotite at pressures approaching spinel to garnet transition: application to mid-ocean ridge basalt petrogenesis. Journal of Geophysical Research 102, 853-874.
Li, Z-W.A., Lee, C.-T.A. (2004) The constancy of upper mantle fO2 through time inferred from V/Sc ratios in basalts. Earth and Planetary Science Letters 228, 483-493.
Mallmann, G., O’Neill, H.St.C. (2009) The crystal/melt partitioning of V during mantle melting as a function of oxygen fugacity compared with some other elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr, Nb). Journal of Petrology 50, 1765-1794.
Nielsen, S.G., Prytulak, J., Halliday, A.N. (2011) Determination of precise and accurate 51V/50V isotope ratios by MC-ICP-MS, part 1: chemical separation of vanadium and mass spectrometric protocols. Geostandards and Geoanalytical Research 35, 293-306.
Nielsen, S.G., Owens, J.D., Horner, T.J. (2016) Analysis of high-precision vanadium isotope ratios by medium resolution MC-IC-MS. Journal of Analytical Atomic Spectrometry 31, 531-536.
Nisbet, e.g. (1982) The tectonic setting and petrogenesis of komatiites. In: Arndt, N.T., Nisbet, e.g. (Eds.) Komatiite. George Allen and Unwin, London, pp. 501-520.
Prytulak, J., Nielsen, S.G., Halliday, A.N. (2011) Determination of precise and accurate 51V/50V isotope ratios by multi-collector ICP-MS, part 2: isotopic composition of six reference materials plus the Allende chondrite and verification tests. Geostandards and Geoanalytical Research 35, 307-318.
Prytulak, J., Nielsen, S.G., Plank, T., Barker, M., Elliott, T. (2013) Assessing the utility of thallium and thallium isotopes for tracing subduction zone input to the Mariana arc. Chemical Geology 345, 139-149.
Prytulak, J., Brett, A., Webb, M., Plank, T., Rehkamper, M., Savage, P.S., Woodhead, J. (2016) Thallium elemental behavior and stable isotope fractionation during magmatic processes. Chemical Geology, doi: 10.1016/j.chemgeo.2016.11.007.
Salters, V.J.M., Stracke, A. (2004) Composition of the depleted mantle. Geochemistry, Geophysics, Geosystems 5, doi:10.1029/2003GC000597.
Savage, P.S., Georg, R.B., Williams, H.M., Burton, K.W., Halliday, A.N. (2011) Silicon isotope fractionation during magmatic differentiation. Geochimica et Cosmochimica Acta 75, 6124-6139.
Savage, P.S., Moynier, F., Chen, H., Shofner, G., Siebert, J., Badro, J., Puchtel, I.S. (2015) Copper isotope evidence for large-scale sulphide fractionation during Earth’s differentiation. Geochemical Perspectives Letters 1, 53-64.
Shaw, D.M. (1970) Trace element fractionation during anatexis. Geochimica et Cosmochimica Acta 34, 237-243.
Sigmarsson, O., Condomines, M., Fourcade, S. (1992) A detailed Th, Sr, and O isotope study of Hekla: differentiation processes in an Icelandic volcano. Contributions to Mineralogy and Petrology 112, 20-34.
Toplis, M.J., Corgne, A. (2002) An experimental study of element partitioning between magnetite, clinopyroxene and iron-bearing silicate liquids with particular emphasis on vanadium. Contributions to Mineralogy and Petrology 144, 22-37.
Wade, J.A., Plank, T., Stern, R.J., Tollstrup, D.L., Gill, J.B., O’Leary, J.C., Eiler, J.M., Moore, R.B., Woodhead, J.D., Trusdell, F., Fischer, T.P., Hilton, D.R. (2005) The May 2003 eruption of Anatahan volcano, Mariana Islands: geochemical evolution of a silicic island-arc volcano. Journal of Volcanology and Geothermal Research 146, 139-170.
Woodhead, J.D. (1989) Geochemistry of the Mariana arc (western Pacific): source composition and processes. Chemical Geology 76, 1-24.
Wu, F., Qi, Y., Yu, H., Tian, S., Hou, Z., Huang, F. (2016) Vanadium isotope measurment by MC-ICP-MS. Chemical Geology, 421, 17-25.
Yang, J., Siebert, C., Barling, J., Savage, P.S., Liang, Y.-H., Halliday, A.N. (2015) Absence of molybdenum isotope fractionation during magmatic differentiation at Hekla volcano, Iceland. Geochimica et Cosmochimica Acta 162, 126-136.
Figures and Tables
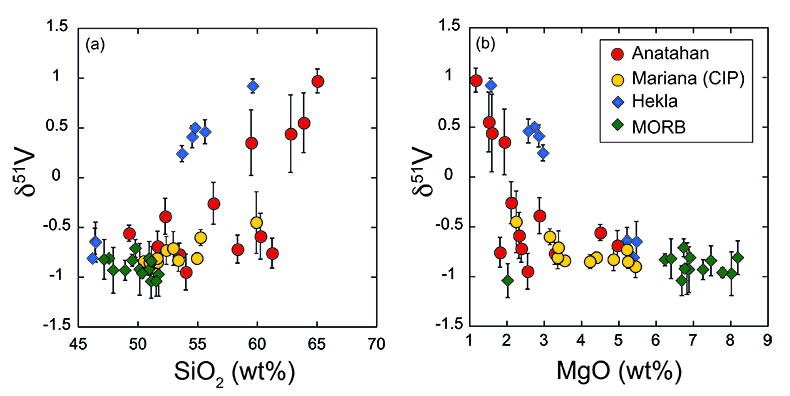
Figure 1 δ51V variations with SiO2 (a) and MgO (b). CIP = Central Island Province. MORB data from Prytulak et al. (2013)
Prytulak, J., Nielsen, S.G., Ionov, D.A., Halliday, A.N., Harvey, J., Kelley, K.A., Niu, Y.L., Peate, D.W., Shimizu, K., Sims, K.W.W. (2013) The stable vanadium isotope composition of the mantle and mafic lavas. Earth and Planetary Science Letters 365, 177-189.
. Uncertainties on isotope measurements are external 2 sd.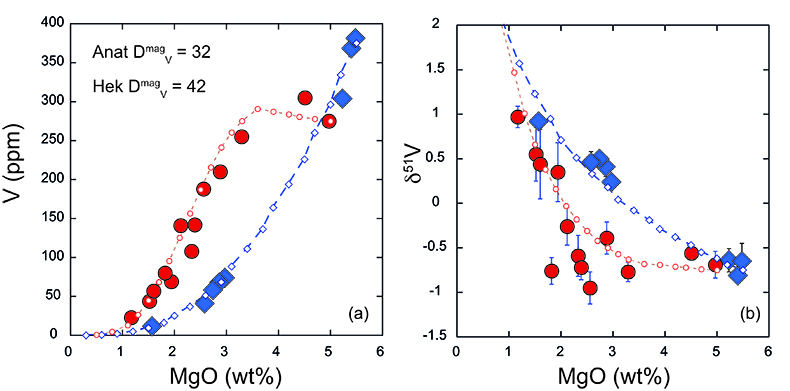
Figure 2 Cotectic fractional crystallisation models. Each small symbol represents a 5 % crystal fractionation increment. See Supplementary Information for input parameters. Symbols and uncertainties as in Figure 1.
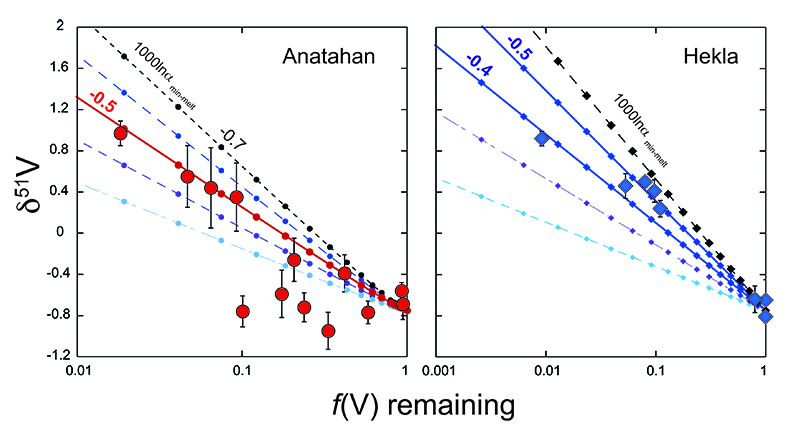
Figure 3 Rayleigh fractionation calculations with same parameters as Figure 2. Symbols and uncertainties as in Figure 1.
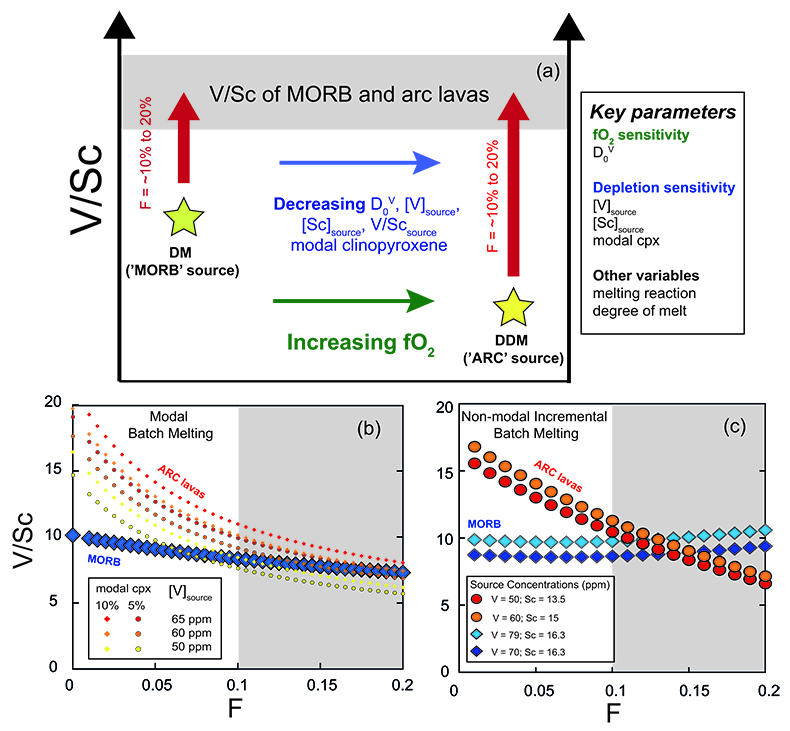
Figure 4 (a) Conceptual effect of depletion and oxidation on V/Sc ratios from two hypothetical spinel peridotite sources. (b,c) Modal and non-modal melting models for the sources in (a). Grey fields highlight reasonable melting degrees. Partition coefficients, modal compositions and melting reactions are detailed in the Supplementary Information. DM = depleted mantle, DDM = depleted MORB mantle, F = melt fraction.
Back to article
Supplementary Figures and Tables
Table S-1 presents a compilation of published major and trace element and radiogenic isotope data on the samples used in this study. We have also compiled available information on other stable isotope systems such as Mo (Freymuth et al., 2015; Yang et al., 2015), Zn (Chen et al., 2013), Cu (Savage et al., 2015), and Tl (Prytulak et al., 2013).Table S-1 Background chemical data.
| A. MAJOR ELEMENTS | ||||||||||||||||||||||||||||||||||||||
| eruption age | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | LOI | |||||||||||||||||||||||||||
| Hekla | ||||||||||||||||||||||||||||||||||||||
| hek 6 | 1913 AD | 46.20 | 4.508 | 13.73 | 17.28 | 0.254 | 5.40 | 9.45 | 2.74 | 0.64 | 0.538 | -0.40 | ||||||||||||||||||||||||||
| hek 12 | 1913AD | 46.42 | 4.469 | 13.59 | 17.18 | 0.259 | 5.23 | 9.22 | 2.81 | 0.67 | 0.566 | -0.29 | ||||||||||||||||||||||||||
| hek 5 | 1913AD | 46.47 | 4.493 | 13.85 | 17.28 | 0.253 | 5.48 | 9.53 | 2.76 | 0.63 | 0.529 | -0.38 | ||||||||||||||||||||||||||
| hek 14 | 1991 AD | 53.71 | 2.120 | 14.80 | 13.26 | 0.274 | 2.97 | 6.98 | 3.95 | 1.23 | 1.066 | -0.15 | ||||||||||||||||||||||||||
| hek 17 | 1980 AD | 54.57 | 2.060 | 14.75 | 13.05 | 0.276 | 2.86 | 6.80 | 3.94 | 1.26 | 1.007 | -0.13 | ||||||||||||||||||||||||||
| hek 16 | 1980 AD | 54.81 | 1.961 | 14.90 | 12.71 | 0.275 | 2.74 | 6.67 | 4.02 | 1.28 | 0.929 | -0.11 | ||||||||||||||||||||||||||
| hek 21 | 1390 AD | 55.64 | 1.773 | 15.14 | 12.56 | 0.273 | 2.58 | 6.34 | 4.12 | 1.35 | 0.865 | -0.09 | ||||||||||||||||||||||||||
| hek 15 | 1980 AD | 59.64 | 1.164 | 15.22 | 10.27 | 0.248 | 1.57 | 5.07 | 4.55 | 1.66 | 0.425 | 0.03 | ||||||||||||||||||||||||||
| Anatahan | ||||||||||||||||||||||||||||||||||||||
| AN-10 | 63.91 | 0.75 | 15.47 | 7.026 | 0.20 | 1.52 | 4.56 | 4.38 | 1.88 | 0.30 | ||||||||||||||||||||||||||||
| Anat9 | 65.06 | 0.83 | 15.00 | 7.515 | 0.19 | 1.17 | 4.22 | 4.27 | 2.05 | 0.30 | ||||||||||||||||||||||||||||
| 04-Anat-01 | 59.51 | 0.87 | 17.14 | 8.163 | 0.21 | 1.94 | 5.56 | 4.02 | 1.49 | 0.31 | ||||||||||||||||||||||||||||
| 04-Anat-03 | 56.35 | 0.75 | 18.89 | 7.899 | 0.17 | 2.12 | 8.17 | 3.60 | 1.26 | 0.22 | ||||||||||||||||||||||||||||
| 04-Anat-04 | 54.03 | 0.78 | 19.41 | 8.688 | 0.18 | 2.56 | 9.08 | 3.44 | 1.00 | 0.19 | ||||||||||||||||||||||||||||
| AN-2 | 52.31 | 0.71 | 21.18 | 8.772 | 0.16 | 2.88 | 10.59 | 2.76 | 0.51 | 0.12 | ||||||||||||||||||||||||||||
| AN-1 | 51.66 | 0.73 | 18.54 | 10.340 | 0.19 | 4.97 | 10.34 | 2.53 | 0.57 | 0.13 | ||||||||||||||||||||||||||||
| AN-8 | 49.28 | 0.68 | 20.09 | 10.649 | 0.19 | 4.51 | 11.81 | 2.22 | 0.46 | 0.11 | ||||||||||||||||||||||||||||
| Anat4-s | 61.26 | 0.80 | 15.67 | 8.360 | 0.21 | 1.82 | 5.44 | 3.99 | 1.52 | 0.32 | ||||||||||||||||||||||||||||
| AN-12D | 62.82 | 0.83 | 15.12 | 8.270 | 0.21 | 1.60 | 4.82 | 4.33 | 1.67 | 0.32 | ||||||||||||||||||||||||||||
| AN-7 | 53.49 | 0.79 | 18.63 | 10.370 | 0.20 | 3.29 | 9.33 | 3.06 | 0.68 | 0.16 | ||||||||||||||||||||||||||||
| Anat-26-01 | 60.29 | 0.84 | 16.10 | 8.568 | 0.20 | 2.33 | 6.04 | 3.73 | 1.37 | 0.32 | ||||||||||||||||||||||||||||
| Anat-26-02 | 58.36 | 0.88 | 16.13 | 9.040 | 0.22 | 2.39 | 6.22 | 4.41 | 1.36 | 0.25 | ||||||||||||||||||||||||||||
| Mariana Central Island Province | ||||||||||||||||||||||||||||||||||||||
| ALAM 2 | 54.95 | 0.783 | 17.4 | 9.58 | 0.184 | 4.4 | 8.98 | 2.98 | 0.944 | 0.145 | ||||||||||||||||||||||||||||
| AGR 8B | 50.5 | 0.726 | 20.2 | 9.83 | 0.186 | 3.55 | 11.15 | 2.58 | 0.889 | 0.175 | ||||||||||||||||||||||||||||
| PAG 1 | 51.34 | 0.908 | 15.87 | 12.19 | 0.218 | 5.45 | 10.6 | 2.8 | 0.749 | 0.142 | ||||||||||||||||||||||||||||
| PAG 3 | 51.64 | 0.925 | 15.73 | 12.23 | 0.218 | 5.25 | 10.25 | 2.72 | 0.78 | 0.149 | ||||||||||||||||||||||||||||
| GUG 4 | 52.37 | 0.824 | 17.54 | 10.26 | 0.197 | 5.22 | 10.41 | 2.64 | 0.549 | 0.105 | ||||||||||||||||||||||||||||
| GUG 9 | 50.985 | 0.82 | 19.6 | 10.07 | 0.18 | 4.23 | 11.05 | 2.56 | 0.4325 | 0.0865 | ||||||||||||||||||||||||||||
| MM-92-6 | 55.24 | 0.872 | 16.49 | 10.67 | 0.236 | 3.15 | 7.68 | 3.56 | 1.41 | 0.269 | ||||||||||||||||||||||||||||
| GUG3 | 51.61 | 0.858 | 19.75 | 9.93 | 0.191 | 3.36 | 10.5 | 2.69 | 0.908 | 0.174 | ||||||||||||||||||||||||||||
| GUG11 | 52.96 | 0.807 | 19.88 | 9.32 | 0.177 | 3.38 | 10.22 | 2.95 | 0.507 | 0.098 | ||||||||||||||||||||||||||||
| ALAM5 | 53.39 | 0.815 | 17.17 | 10.05 | 0.19 | 4.86 | 9.97 | 2.7 | 0.835 | 0.144 | ||||||||||||||||||||||||||||
| URA6 | 59.92 | 0.826 | 15.39 | 9.99 | 0.234 | 2.25 | 6.2 | 3.85 | 1.099 | 0.186 | ||||||||||||||||||||||||||||
| SAG1 | 53.37 | 0.777 | 16.23 | 9.83 | 0.186 | 5.57 | 10.45 | 2.71 | 0.716 | 0.126 | ||||||||||||||||||||||||||||
| GUG13 | 51.78 | 0.807 | 19.94 | 9.67 | 0.179 | 3.47 | 10.66 | 2.55 | 0.442 | 0.087 | ||||||||||||||||||||||||||||
| GUG12 | 52.16 | 0.814 | 20.04 | 9.73 | 0.183 | 3.49 | 10.73 | 2.67 | 0.448 | 0.089 | ||||||||||||||||||||||||||||
| URA5 | 53.57 | 0.628 | 16.63 | 9.61 | 0.184 | 6.13 | 10.52 | 2.29 | 0.53 | 0.094 | ||||||||||||||||||||||||||||
| URA7 | 54.17 | 0.775 | 19.2 | 9.73 | 0.193 | 2.712 | 9.64 | 2.88 | 0.756 | 0.134 | ||||||||||||||||||||||||||||
| URA12 | 53.77 | 0.75 | 19.62 | 9.55 | 0.188 | 2.68 | 9.93 | 2.81 | 0.705 | 0.128 | ||||||||||||||||||||||||||||
| AGR1 | 51.58 | 0.752 | 20.19 | 9.77 | 0.188 | 3.04 | 10.71 | 2.72 | 1.03 | 0.189 | ||||||||||||||||||||||||||||
| AGR2 | 50.94 | 0.794 | 16.84 | 12.45 | 0.235 | 4.98 | 10.46 | 2.85 | 0.744 | 0.147 | ||||||||||||||||||||||||||||
| | ||||||||||||||||||||||||||||||||||||||
| B. TRACE ELEMENTS | ||||||||||||||||||||||||||||||||||||||
| Zn (Savage) | Zn (ICP) | Li | Be | Sc | V | Cr | Co | Ni | Cu | Cu | Rb | Sr | Y | Zr | Nb | Cs | Tl | Ba | La | Ce | Pr | Nd | Sm | Eu | Tb | Ga | Gd | Dy | Ho | Er | Yb | Lu | Hf | Ta | Pb | Th | U | |
| Hekla | XRF | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | (savage XRF) | ppm | ppm | ppm | ppm | ppm | ppm | ppb | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm |
| hek 6 | 134.00 | 136.56 | 8.04 | 1.96 | 34.53 | 368.43 | 12.09 | 47.43 | 13.64 | 42.01 | 38.00 | 11.83 | 363.83 | 46.23 | 271.21 | 43.78 | 0.15 | 20.29 | 178.08 | 27.14 | 63.19 | 8.72 | 38.77 | 9.57 | 3.14 | 1.62 | 23.93 | 10.03 | 9.11 | 1.79 | 4.67 | 4.06 | 0.59 | 6.82 | 2.99 | 1.72 | 2.20 | 0.67 |
| hek 12 | 2.00 | 31.00 | 26.00 | 14.00 | 371.00 | 49.20 | 275.00 | 41.00 | ||||||||||||||||||||||||||||||
| hek 5 | 136.00 | 139.95 | 8.18 | 1.89 | 34.80 | 381.44 | 15.24 | 48.81 | 17.14 | 50.80 | 47.00 | 12.04 | 370.35 | 46.50 | 272.22 | 43.66 | 0.15 | 21.96 | 177.77 | 27.07 | 62.86 | 8.69 | 38.25 | 9.50 | 3.10 | 1.60 | 24.42 | 9.90 | 8.97 | 1.76 | 4.60 | 3.99 | 0.58 | 6.69 | 2.95 | 1.70 | 2.14 | 0.66 |
| hek 14 | 163.00 | 188.23 | 15.63 | 3.32 | 22.40 | 74.02 | 16.90 | 18.08 | 4.24 | 15.56 | 17.00 | 25.20 | 400.40 | 77.67 | 476.38 | 66.16 | 0.32 | 48.68 | 332.54 | 51.76 | 117.54 | 15.93 | 67.18 | 16.04 | 4.81 | 2.59 | 27.59 | 16.25 | 14.43 | 2.83 | 7.41 | 6.49 | 0.96 | 11.14 | 4.14 | 3.39 | 4.54 | 1.37 |
| hek 17 | 135.00 | 186.71 | 15.81 | 3.32 | 22.45 | 67.80 | 5.26 | 16.71 | 4.02 | 15.25 | 15.00 | 25.67 | 388.72 | 76.94 | 484.92 | 66.83 | 0.32 | 50.64 | 335.54 | 51.76 | 116.91 | 15.78 | 65.90 | 15.75 | 4.69 | 2.54 | 27.40 | 15.92 | 14.22 | 2.79 | 7.33 | 6.44 | 0.95 | 11.23 | 4.15 | 3.40 | 4.57 | 1.39 |
| hek 16 | 163.00 | 189.30 | 16.22 | 3.47 | 22.23 | 58.24 | 9.29 | 15.69 | 3.62 | 14.32 | 16.00 | 26.33 | 393.96 | 77.60 | 494.48 | 67.87 | 0.33 | 50.59 | 344.19 | 52.40 | 118.18 | 15.90 | 66.34 | 15.86 | 4.75 | 2.56 | 27.68 | 15.97 | 14.35 | 2.82 | 7.48 | 6.61 | 0.97 | 11.55 | 4.22 | 3.49 | 4.70 | 1.42 |
| hek 21 | 162.00 | 188.91 | 15.98 | 3.60 | 20.75 | 41.11 | 5.42 | 14.77 | 3.18 | 14.85 | 18.00 | 27.31 | 393.07 | 77.98 | 509.15 | 68.05 | 0.35 | 53.79 | 353.76 | 53.27 | 119.98 | 16.02 | 66.43 | 15.82 | 4.70 | 2.55 | 27.75 | 15.86 | 14.34 | 2.83 | 7.46 | 6.66 | 0.98 | 11.81 | 4.20 | 3.67 | 4.88 | 1.46 |
| hek 15 | 163.00 | 195.84 | 20.58 | 4.28 | 19.44 | 11.71 | 2.79 | 8.47 | 4.01 | 10.17 | 13.00 | 34.39 | 368.61 | 82.67 | 627.62 | 78.59 | 0.44 | 59.17 | 433.10 | 59.43 | 131.16 | 17.12 | 68.91 | 16.01 | 4.74 | 2.63 | 30.27 | 15.96 | 15.00 | 3.00 | 8.13 | 7.55 | 1.12 | 14.64 | 4.84 | 4.24 | 6.10 | 1.83 |
| Anatahan | ||||||||||||||||||||||||||||||||||||||
| AN-10 | 13.71 | 1.07 | 20.48 | 44 | 0.20 | 9.15 | 1.46 | 14.74 | 36.77 | 323.09 | 43.12 | 140.94 | 3.37 | 0.81 | 114 | 478.7675645548 | 15.07 | 32.58 | 4.79 | 21.27 | 5.64 | 1.60 | 1.13 | 6.62 | 6.87 | 1.49 | 4.21 | 4.26 | 0.67 | 3.72 | 0.27 | 5.55 | 2.22 | 0.92 | ||||
| Anat9 | 13.75 | 1.27 | 20.53 | 23 | 5.30 | 11.65 | 2.33 | 24.67 | 42.58 | 302.48 | 46.95 | 170.80 | 3.64 | 0.94 | 106 | 538.2747115042 | 15.95 | 33.66 | 4.92 | 22.58 | 6.08 | 1.76 | 1.24 | 7.32 | 7.78 | 1.65 | 4.76 | 4.77 | 0.76 | 4.51 | 0.27 | 7.13 | 2.67 | 1.11 | ||||
| 04-Anat-01 | 11.64 | 0.99 | 23.10 | 69 | 0.52 | 13.72 | 0.56 | 32.86 | 28.68 | 342.00 | 39.07 | 117.70 | 2.94 | 0.72 | 97 | 405.3645918868 | 12.61 | 27.74 | 4.27 | 18.80 | 5.10 | 1.58 | 1.01 | 6.02 | 6.33 | 1.37 | 3.87 | 3.86 | 0.61 | 3.32 | 0.20 | 4.95 | 1.84 | 0.73 | ||||
| 04-Anat-03 | 8.56 | 0.77 | 22.07 | 141 | 0.45 | 17.19 | 1.45 | 62.83 | 23.52 | 417.00 | 29.69 | 90.93 | 2.23 | 0.60 | 75 | 331.2030084306 | 9.82 | 21.36 | 3.13 | 14.45 | 3.90 | 1.27 | 0.77 | 4.57 | 4.85 | 1.04 | 2.95 | 2.93 | 0.46 | 2.57 | 0.15 | 4.09 | 1.44 | 0.59 | ||||
| 04-Anat-04 | 7.77 | 0.66 | 25.67 | 188 | 1.39 | 21.23 | 2.74 | 63.30 | 20.32 | 428.00 | 27.34 | 79.49 | 2.59 | 0.53 | 66 | 279.9777078064 | 8.19 | 17.88 | 2.72 | 12.35 | 3.41 | 1.15 | 0.69 | 4.05 | 4.38 | 0.95 | 2.70 | 2.69 | 0.42 | 2.24 | 0.18 | 3.50 | 1.23 | 0.51 | ||||
| AN-2 | 5.28 | 0.45 | 25.47 | 210 | 4.32 | 30.64 | 9.90 | 85.40 | 9.50 | 445.44 | 20.60 | 50.06 | 1.14 | 0.24 | 38 | 191.4824170528 | 5.25 | 11.52 | 1.83 | 8.60 | 2.49 | 0.90 | 0.54 | 3.10 | 3.38 | 0.72 | 2.03 | 2.07 | 0.32 | 1.41 | 2.34 | 0.66 | 0.26 | |||||
| AN-1 | 5.25 | 0.47 | 31.74 | 275 | 17.93 | 31.56 | 17.84 | 116.05 | 11.58 | 381.51 | 20.65 | 51.78 | 1.23 | 0.28 | 26 | 198.5843640904 | 5.69 | 12.30 | 1.88 | 8.78 | 2.52 | 0.87 | 0.54 | 3.11 | 3.42 | 0.74 | 2.08 | 2.10 | 0.33 | 1.49 | 0.09 | 2.42 | 0.86 | 0.31 | ||||
| AN-8 | 4.40 | 0.37 | 32.30 | 305 | 4.42 | 33.04 | 14.72 | 103.63 | 10.55 | 447.37 | 16.13 | 35.35 | 0.84 | 0.21 | 24 | 147.8840365564 | 4.89 | 10.38 | 1.65 | 7.30 | 2.07 | 0.77 | 0.43 | 2.51 | 2.64 | 0.57 | 1.60 | 1.57 | 0.25 | 1.02 | 0.06 | 2.37 | 0.62 | 0.23 | ||||
| Anat4-s | 12.04 | 1.06 | 23.44 | 80 | 0.77 | 15.77 | 5.25 | 38.81 | 30.54 | 339.63 | 41.56 | 126.31 | 2.88 | 0.76 | 434.7408859364 | 13.70 | 29.13 | 4.28 | 19.90 | 5.46 | 1.68 | 1.11 | 6.57 | 6.97 | 1.49 | 4.26 | 4.16 | 0.68 | 3.41 | 0.22 | 5.75 | 2.02 | 0.79 | |||||
| AN-12D | 8.18 | 1.04 | 23.14 | 57 | 0.07 | 12.22 | 0.38 | 31.58 | 30.59 | 331.28 | 44.18 | 132.55 | 3.17 | 0.63 | 444.3276469033 | 13.99 | 30.25 | 4.57 | 20.74 | 5.61 | 1.69 | 1.16 | 6.86 | 7.02 | 1.51 | 4.35 | 4.32 | 0.69 | 3.71 | 0.23 | 5.56 | 2.08 | 0.84 | |||||
| AN-7 | 6.36 | 0.51 | 30.27 | 255 | 2.32 | 25.68 | 6.08 | 105.06 | 11.73 | 414.14 | 24.72 | 61.72 | 1.20 | 0.18 | 240.8067628495 | 5.79 | 13.39 | 2.04 | 9.67 | 2.89 | 0.99 | 0.63 | 3.61 | 3.97 | 0.86 | 2.42 | 2.43 | 0.39 | 1.67 | 0.11 | 2.34 | 0.80 | 0.35 | |||||
| Anat-26-01 | 12.44 | 0.89 | 24.69 | 108 | 2.72 | 14.92 | 0.86 | 39.79 | 28.31 | 345.00 | 35.04 | 105.54 | 2.54 | 0.68 | 374.2473001959 | 11.34 | 24.78 | 3.65 | 16.79 | 4.56 | 1.43 | 0.91 | 5.37 | 5.66 | 1.23 | 3.46 | 3.48 | 0.55 | 2.99 | 0.18 | 4.81 | 1.69 | 0.68 | |||||
| Anat-26-02 | 8.10 | 0.82 | 26.30 | 142 | 0.96 | 22.37 | 2.98 | 66.39 | 23.62 | 366.00 | 33.28 | 97.55 | 2.48 | 0.58 | 356.7788087856 | 10.77 | 24.05 | 3.70 | 16.82 | 4.59 | 1.44 | 0.89 | 5.24 | 5.50 | 1.18 | 3.38 | 3.44 | 0.54 | 2.80 | 0.17 | 4.32 | 1.53 | 0.63 | |||||
| Mariana Central Island Province | ||||||||||||||||||||||||||||||||||||||
| ALAM 2 | 78.7 | 32 | 218 | 19.7 | 20 | 16.34 | 303 | 26.7 | 82.9 | 1.305 | 0.605 | 59 | 251 | 5.90 | 14.04 | 2.17 | 10.33 | 3.05 | 0.967 | 0.727 | 17.24 | 3.86 | 4.29 | 0.933 | 2.69 | 2.73 | 0.443 | 2.33 | 0.0931 | 3.14 | 0.816 | 0.409 | ||||||
| AGR 8B | 76.1 | 30.45 | 257 | 6.5 | 12.3 | 19.89 | 395 | 18.1 | 59.2 | 1.126 | 0.463 | 54 | 165 | 7.45 | 15.76 | 2.34 | 10.73 | 2.79 | 0.992 | 0.563 | 18.71 | 3.17 | 3.16 | 0.667 | 1.87 | 1.86 | 0.288 | 1.60 | 0.0881 | 1.79 | 0.894 | 0.377 | ||||||
| PAG 1 | 98.7 | 41.0 | 386.1 | 33.706 | 22.6 | 11.92 | 334 | 1.016 | 0.524 | 25 | 221 | 5.242 | 12.051 | 1.913 | 9.225 | 2.827 | 0.960 | 0.584 | 17.45 | 3.380 | 3.749 | 0.789 | 2.218 | 2.219 | 0.334 | 1.579 | 0.073 | 2.801 | 0.542 | 0.263 | ||||||||
| PAG 3 | 100.0 | 41.3 | 373 | 44.9 | 26.4 | 12.46 | 317 | 23.2 | 60.8 | 0.965 | 0.608 | 27 | 230 | 5.18 | 12.45 | 1.95 | 9.57 | 2.95 | 1.015 | 0.670 | 17.15 | 3.69 | 3.93 | 0.853 | 2.40 | 2.36 | 0.369 | 1.80 | 0.0706 | 2.71 | 0.537 | 0.280 | ||||||
| GUG 4 | 86.4 | 34.8 | 258.9 | 37.4 | 24 | 8.07 | 291 | 22.8 | 57.9 | 0.808 | 0.357 | 44 | 167 | 3.55 | 9.11 | 1.50 | 7.58 | 2.39 | 0.861 | 0.600 | 17.5 | 3.23 | 3.57 | 0.791 | 2.26 | 2.30 | 0.365 | 1.63 | 0.0705 | 2.42 | 0.350 | 0.201 | ||||||
| GUG 9 | 81.0 | 30.1 | 272.4 | 12.3 | 14.9 | 6.53 | 299 | 20.1 | 50.4 | 0.731 | 0.297 | 36 | 139 | 2.85 | 7.63 | 1.30 | 6.57 | 2.14 | 0.809 | 0.547 | 18.5 | 2.86 | 3.29 | 0.727 | 2.11 | 2.07 | 0.331 | 1.42 | 0.0613 | 2.03 | 0.271 | 0.147 | ||||||
| MM-92-6 | 116.1 | 31.8 | 204.5 | 11.1 | 31.45 | 324 | 29.8 | 96.3 | 1.856 | 0.733 | 115 | 277.7 | 11.41 | 23.98 | 3.53 | 15.88 | 4.11 | 1.373 | 0.874 | 18.22 | 4.84 | 4.93 | 1.058 | 2.98 | 2.94 | 0.472 | 2.59 | 0.1168 | 3.01 | 1.422 | 0.563 | |||||||
| GUG3 | 82.4 | 28.6 | 278.6 | 4.7 | 12.6 | 16.9 | 401 | 23.0 | 66.3 | 1.241 | 0.467 | 67 | 217 | 7.40 | 15.86 | 2.40 | 11.11 | 3.08 | 1.084 | 0.657 | 17.7 | 3.65 | 3.81 | 0.816 | 2.30 | 2.29 | 0.356 | 1.78 | 0.0916 | 2.82 | 0.760 | 0.352 | ||||||
| GUG11 | 77.8 | 28.3 | 241 | 4.3 | 15.2 | 7.2 | 306 | 23.5 | 60.0 | 0.699 | 0.271 | 31 | 190 | 3.18 | 8.53 | 1.46 | 7.47 | 2.43 | 0.920 | 0.626 | 18.44 | 3.19 | 3.72 | 0.830 | 2.38 | 2.46 | 0.391 | 1.70 | 0.0689 | 2.44 | 0.297 | 0.175 | ||||||
| ALAM5 | 82.0 | 36.5 | 264 | 22.1 | 18.4 | 15.41 | 307 | 24.1 | 74.4 | 1.244 | 0.589 | 56 | 238 | 5.85 | 13.51 | 2.12 | 10.12 | 2.99 | 1.011 | 0.693 | 15.55 | 3.73 | 4.10 | 0.890 | 2.54 | 2.53 | 0.394 | 2.18 | 0.0945 | 3.24 | 0.824 | 0.395 | ||||||
| URA6 | 111.4 | 31.1 | 120 | 0.01 | 5.4 | 18.32 | 319 | 36.4 | 96.1 | 2.107 | 0.675 | 80 | 424 | 10.05 | 22.20 | 3.19 | 14.92 | 4.30 | 1.448 | 1.014 | 17.29 | 5.33 | 6.04 | 1.345 | 3.85 | 3.92 | 0.617 | 2.78 | 0.1426 | 4.23 | 1.501 | 0.511 | ||||||
| SAG1 | 77.8 | 34.8 | 233 | 63.1 | 29.4 | 9.45 | 352 | 23.5 | 68.5 | 1.641 | 0.353 | 44 | 253 | 6.17 | 13.98 | 2.14 | 10.01 | 2.86 | 1.014 | 0.644 | 16.27 | 3.51 | 3.75 | 0.787 | 2.29 | 2.28 | 0.350 | 1.90 | 0.1203 | 3.63 | 0.886 | 0.366 | ||||||
| GUG13 | 77.5 | 29.5 | 265 | 1.7 | 12 | 6.06 | 303 | 21.6 | 53.4 | 0.674 | 0.223 | 15 | 162 | 2.75 | 7.53 | 1.28 | 6.59 | 2.21 | 0.829 | 0.573 | 18.44 | 2.96 | 3.51 | 0.781 | 2.17 | 2.22 | 0.358 | 1.55 | 0.0590 | 1.92 | 0.244 | 0.146 | ||||||
| GUG12 | 78.6 | 285.7 | 53.2 | 28.9 | 7.35 | 281 | 21.7 | 53.5 | 0.720 | 0.198 | 49 | 172 | 3.26 | 8.43 | 1.40 | 7.08 | 2.27 | 0.841 | 0.559 | 16.9 | 2.94 | 3.38 | 0.743 | 2.15 | 2.18 | 0.345 | 1.50 | 0.0663 | 1.81 | 0.316 | 0.206 | |||||||
| URA5 | 72.3 | 39.65 | 235 | 48.9 | 26.5 | 8.15 | 307 | 20.2 | 49.0 | 1.044 | 0.365 | 38 | 198 | 4.45 | 10.17 | 1.54 | 7.25 | 2.15 | 0.814 | 0.528 | 14.17 | 2.80 | 3.19 | 0.696 | 1.99 | 2.05 | 0.324 | 1.37 | 0.0722 | 2.06 | 0.657 | 0.240 | ||||||
| URA7 | 85.8 | 29.9 | 232 | 2.4 | 11 | 12.01 | 384 | 24.0 | 62.6 | 1.663 | 0.401 | 47 | 290 | 7.97 | 16.80 | 2.41 | 10.75 | 2.97 | 1.066 | 0.699 | 18.2 | 3.68 | 4.19 | 0.882 | 2.56 | 2.55 | 0.406 | 1.76 | 0.1015 | 2.76 | 1.214 | 0.399 | ||||||
| URA12 | 82.6 | 30.1 | 208 | 2.3 | 9.3 | 11.9 | 398 | 23.0 | 59.4 | 1.596 | 0.390 | 51 | 245 | 7.29 | 15.57 | 2.21 | 9.98 | 2.79 | 1.004 | 0.638 | 18.09 | 3.41 | 3.80 | 0.827 | 2.34 | 2.35 | 0.385 | 1.66 | 0.0917 | 2.59 | 1.157 | 0.373 | ||||||
| AGR1 | 75.7 | 246 | 5.2 | 9.7 | 22.5 | 394 | 0.526 | 181 | 20.16 | 0.68 | ||||||||||||||||||||||||||||
| AGR2 | 89.6 | 341 | 5.7 | 12.4 | 12.96 | 344 | 20.1 | 51.9 | 1.007 | 0.160 | 23 | 158 | 6.84 | 14.71 | 2.24 | 10.48 | 2.90 | 1.028 | 0.603 | 17.11 | 3.33 | 3.47 | 0.741 | 2.09 | 2.01 | 0.316 | 1.52 | 0.0648 | 1.83 | 0.822 | 0.313 | |||||||
| C. ISOTOPIC DATA | ||||||||||||||||||||||||||||||||||||||
| 87Sr/86Sr | 143Nd/144Nd | 206Pb/204Pb | 2sd | 207Pb/204Pb | 2sd | 208Pb/204Pb | 2sd | d30Si | 2sd | d29Si | 2sd | Mo | d98Mo | 2se | d66Zn | d67Zn | d68Zn | d65Cu | 2sd | e205Tl | 2sd | |||||||||||||||||
| Hekla | µg/g | |||||||||||||||||||||||||||||||||||||
| hek 6 | -0.31 | 0.09 | -0.16 | 0.05 | 1.3 | -0.16 | 0.06 | |||||||||||||||||||||||||||||||
| hek 12 | -0.29 | 0.1 | -0.17 | 0.05 | 1.4 | -0.12 | 0.06 | 0.29 | 0.4 | 0.53 | 0.13 | 0.08 | ||||||||||||||||||||||||||
| hek 5 | -0.32 | 0.12 | -0.19 | 0.11 | 1.3 | -0.18 | 0.06 | 0.26 | 0.37 | 0.52 | 0 | 0.08 | ||||||||||||||||||||||||||
| hek 14 | -0.26 | 0.06 | -0.16 | 0.05 | 2.4 | -0.15 | 0.06 | 0.23 | 0.37 | 0.47 | ||||||||||||||||||||||||||||
| hek 17 | -0.25 | 0.18 | -0.16 | 0.04 | 2.6 | -0.11 | 0.06 | 0.26 | 0.39 | 0.5 | ||||||||||||||||||||||||||||
| hek 16 | -0.27 | 0.07 | -0.12 | 0.05 | 2.6 | -0.15 | 0.06 | 0.28 | 0.4 | 0.56 | ||||||||||||||||||||||||||||
| hek 21 | -0.29 | 0.15 | -0.12 | 0.07 | 2.7 | -0.17 | 0.06 | 0.28 | 0.4 | 0.56 | ||||||||||||||||||||||||||||
| hek 15 | -0.22 | 0.03 | -0.13 | 0.03 | 3.2 | -0.12 | 0.06 | 0.32 | 0.45 | 0.62 | ||||||||||||||||||||||||||||
| Anatahan | ||||||||||||||||||||||||||||||||||||||
| AN-10 | 18.7970 | 15.5658 | 38.4281 | |||||||||||||||||||||||||||||||||||
| Anat9 | 0.70340 | 18.7900 | 15.5640 | 38.4178 | ||||||||||||||||||||||||||||||||||
| 04-Anat-01 | 18.7790 | 15.5640 | 38.4190 | |||||||||||||||||||||||||||||||||||
| 04-Anat-03 | 0.70339 | 0.513001 | 18.7690 | 15.5630 | 38.4160 | |||||||||||||||||||||||||||||||||
| 04-Anat-04 | 18.7889 | 15.5589 | 38.4068 | |||||||||||||||||||||||||||||||||||
| AN-2 | 18.8000 | 15.5680 | 38.4270 | |||||||||||||||||||||||||||||||||||
| AN-1 | 18.7971 | 0.0035 | 15.5694 | 0.0031 | 38.4398 | 0.0099 | ||||||||||||||||||||||||||||||||
| AN-8 | 18.8236 | 0.0011 | 15.5657 | 0.0010 | 38.4124 | 0.0030 | ||||||||||||||||||||||||||||||||
| Anat4-s | 18.8228 | 0.0009 | 15.5663 | 0.0008 | 38.4021 | 0.0026 | ||||||||||||||||||||||||||||||||
| AN-12D | 18.7553 | 0.0009 | 15.5509 | 0.0008 | 38.3351 | 0.0026 | ||||||||||||||||||||||||||||||||
| AN-7 | 18.8228 | 0.0009 | 15.5663 | 0.0008 | 38.4021 | 0.0026 | ||||||||||||||||||||||||||||||||
| Anat-26-01 | 18.7671 | 0.0012 | 15.5533 | 0.0010 | 38.3413 | 0.0033 | ||||||||||||||||||||||||||||||||
| Anat-26-02 | 18.8604 | 0.0022 | 15.5774 | 0.0020 | 38.5062 | 0.0064 | ||||||||||||||||||||||||||||||||
| Mariana Central Island Province | ||||||||||||||||||||||||||||||||||||||
| ALAM 2 | 1.277 | 0.082 | 0.021 | |||||||||||||||||||||||||||||||||||
| AGR 8B | 0.595 | -0.113 | 0.014 | -1.1 | 0.5 | |||||||||||||||||||||||||||||||||
| PAG 1 | -1.5 | 0.5 | ||||||||||||||||||||||||||||||||||||
| PAG 3 | -0.4 | 0.5 | ||||||||||||||||||||||||||||||||||||
| GUG 4 | -1.2 | 0.5 | ||||||||||||||||||||||||||||||||||||
| GUG 9 | 0.776 | 0.067 | 0.020 | -1.1 | 0.5 | |||||||||||||||||||||||||||||||||
| MM-92-6 | 0.998 | -0.083 | 0.022 | -1.2 | 0.5 | |||||||||||||||||||||||||||||||||
| GUG3 | -0.5 | 0.5 | ||||||||||||||||||||||||||||||||||||
| GUG11 | 1.003 | 0.049 | 0.016 | -1.2 | 0.5 | |||||||||||||||||||||||||||||||||
| ALAM5 | 1.072 | 0.047 | 0.014 | -0.6 | 0.5 | |||||||||||||||||||||||||||||||||
| URA6 | -1.7 | 0.5 | ||||||||||||||||||||||||||||||||||||
| SAG1 | -0.6 | 0.5 | ||||||||||||||||||||||||||||||||||||
| GUG13 | 0.856 | 0.038 | 0.031 | 1.2 | 0.5 | |||||||||||||||||||||||||||||||||
| GUG12 | 0.894 | 0.021 | 0.025 | -0.8 | 0.5 | |||||||||||||||||||||||||||||||||
| URA5 | 0.710 | 0.059 | 0.015 | -1.8 | 0.5 | |||||||||||||||||||||||||||||||||
| URA7 | 0.907 | 0.054 | 0.017 | -1.1 | 0.5 | |||||||||||||||||||||||||||||||||
| URA12 | 0.909 | 0.043 | 0.022 | -1.7 | 0.5 | |||||||||||||||||||||||||||||||||
| AGR1 | ||||||||||||||||||||||||||||||||||||||
| AGR2 | | | | 0.492 | -0.092 | 0.018 | -1.2 | 0.5 | ||||||||||||||||||||||||||||||
References
Hekla
Major elements: Savage et al., 2011
Trace elements: Prytulak et al., 2016
Si isotopes: Savage et al., 2011
Mo isotopes: Yang et al., 2015
Zn isotopes: Chen et al., 2013
Cu isotopes: Savage et al., 2015
Anatahan
Major, trace elements: Wade et al., 2005
Radiogenic isotopes: Woodhead, 1989; Woodhead et Fraser, 1985
Mariana Central Island Province
Major elements: Elliott et al., 1997
Trace elements: Elliott et al., 1997 & Prytulak et al., 2013
Mo isotopes: Freymuth et al., 2015
Pb isotopes: Freymuth et al., 2015
Tl isotopes: Prytulak et al., 2013
Table S-2 Vanadium isotopic data.
| Sample | d51V | 2sd | number dissolutions | number of measurements | number of sessions | Oxford or Imperial College |
| Marianas Central Island Province | ||||||
| SAG1 | -0.80 | 0.14 | 1 | 5 | 2 | OX |
| GUG13 | -0.98 | 0.17 | 2 | 8 | 2 | OX |
| GUG12 | -0.84 | 0.13 | 1 | 5 | 1 | OX |
| URA5 | -0.75 | 0.15 | 3 | 8 | 2 | OX |
| URA7 | -0.63 | 0.21 | 2 | 7 | 2 | OX |
| URA12 | -0.77 | 0.13 | 2 | 11 | 2 | OX |
| AGR1 | -0.83 | 0.09 | 1 | 9 | 3 | OX |
| AGR2 | -0.91 | 0.15 | 2 | 21 | 4 | OX |
| AGR8b | -0.84 | 0.05 | 2 | 8 | 2 | OX |
| M-92-6 | -0.60 | 0.08 | 2 | 7 | 2 | OX |
| GUG3 | -0.81 | 0.11 | 1 | 3 | 1 | OX |
| GUG4 | -0.73 | 0.14 | 1 | 4 | 1 | OX |
| GUG11 | -0.71 | 0.17 | 3 | 11 | 2 | OX |
| PAG1 | -0.90 | 0.11 | 1 | 4 | 2 | OX |
| PAG3 | -0.85 | 0.07 | 2 | 12 | 2 | OX |
| ALAM2 | -0.81 | 0.06 | 2 | 6 | 3 | OX and IC |
| ALAM5 | -0.83 | 0.11 | 1 | 4 | 1 | OX |
| URA6 | -0.45 | 0.31 | 4 | 11 | 5 | OX |
| GUG9 | -0.85 | 0.07 | 1 | 6 | 2 | OX |
| Anatahan. Marianas | ||||||
| 04 ANAT 4 | -0.95 | 0.18 | 1 | 3 | 1 | IC |
| 04 ANAT 03 | -0.26 | 0.21 | 2 | 8 | 3 | IC |
| ANAT 26-01 | -0.59 | 0.23 | 1 | 2 | 1 | IC |
| 04 ANAT 1 | 0.35 | 0.33 | 1 | 5 | 2 | IC |
| ANAT 26-02 | -0.72 | 0.14 | 1 | 4 | 2 | IC |
| ANAT 4 as | -0.76 | 0.15 | 1 | 9 | 2 | IC |
| AN2 | -0.39 | 0.18 | 2 | 6 | 2 | IC |
| AN7 | -0.77 | 0.11 | 1 | 4 | 1 | IC |
| AN12D | 0.44 | 0.39 | 2 | 2 | 1 | IC |
| Anat9 | 0.97 | 0.12 | 1 | 2 | 2 | IC |
| AN10 | 0.55 | 0.30 | 2 | 4 | 2 | IC |
| AN1 | -0.69 | 0.15 | 2 | 9 | 2 | IC |
| AN8 | -0.56 | 0.08 | 1 | 5 | 1 | IC |
| Hekla. Iceland | ||||||
| HEK05 | -0.65 | 0.20 | 2 | 16 | 2 | OX |
| HEK06 | -0.81 | 0.01 | 1 | 2 | 1 | OX |
| HEK12 | -0.64 | 0.13 | 1 | 11 | 3 | OX |
| HEK14 | 0.24 | 0.08 | 1 | 5 | 2 | OX |
| HEK15 | 0.92 | 0.07 | 1 | 2 | 1 | OX |
| HEK16 | 0.50 | 0.04 | 1 | 5 | 1 | OX |
| HEK17 | 0.41 | 0.11 | 1 | 4 | 1 | OX |
| HEK21 | 0.46 | 0.12 | 1 | 5 | 2 | OX |
| USGS Standards (from Prytulak et al.. 2011 as lavas data are contemporaneous) | ||||||
| BIR1a | -0.92 | 0.16 | 12 | 50 | 8 | OX |
| BHVO1 | -0.92 | 0.04 | 1 | 4 | 1 | OX |
| BHVO2 | -0.88 | 0.10 | 7 | 14 | 4 | OX |
| BCR2 | -0.92 | 0.16 | 13 | 27 | 7 | OX |
| AGV2 | -0.58 | 0.10 | 4 | 6 | 4 | OX and IC |
| GSP2 | -0.63 | 0.10 | 3 | 6 | 1 | OX |
| PCC1 | -1.01 | 0.09 | 2 | 8 | 3 | OX |
| DTS | -0.95 | na | 1 | 1 | 1 | OX |
Table S-3 Fractional crystallisation models.
| Anatahan | ||||||||||||||||
| Global Input Variables | ||||||||||||||||
| DV | DMgO | DFeO | ∆51Vmin-melt | |||||||||||||
| Plagioclase | 0 | 0 | 0 | 0 | ||||||||||||
| Magnetite | 32 | 0 | 6.5 | -0.85*106/T2 | ||||||||||||
| OPX | 1.5 | 6.75 | 3 | -0.85*106/T2 | ||||||||||||
| CPX | 2.5 | 3.75 | 1.8 | -0.85*106/T2 | ||||||||||||
| Modes | Bulk D | Model Output | ||||||||||||||
| F | Plagioclase | Magnetite | OPX | CPX | DV | DMgO | DFeO | ∆51Vmin-melt | V | δ51V | MgO | FeO | T (°C) | |||
| 1 | 275 | -0.75 | 5 | 9 | 1100 | |||||||||||
| 0.95 | 0.6 | 0.2 | 0.2 | 0.81 | 2.13 | 0.99 | -0.31 | 277.8 | -0.74 | 4.7 | 9 | 1094.4 | ||||
| 0.9 | 0.6 | 0.2 | 0.2 | 0.81 | 2.13 | 0.99 | -0.31 | 280.7 | -0.72 | 4.4 | 9 | 1088.8 | ||||
| 0.85 | 0.6 | 0.2 | 0.2 | 0.81 | 2.13 | 0.99 | -0.31 | 283.8 | -0.71 | 4.2 | 9 | 1083.2 | ||||
| 0.8 | 0.6 | 0.2 | 0.2 | 0.81 | 2.13 | 0.99 | -0.31 | 287.2 | -0.69 | 3.9 | 9 | 1077.7 | ||||
| 0.75 | 0.6 | 0.2 | 0.2 | 0.81 | 2.13 | 0.99 | -0.31 | 290.8 | -0.68 | 3.6 | 9 | 1072.2 | ||||
| 0.7 | 0.6 | 0.01 | 0.2 | 0.2 | 1 | 2.13 | 1.03 | -0.35 | 275.2 | -0.63 | 3.3 | 8.9 | 1066.8 | |||
| 0.65 | 0.6 | 0.01 | 0.2 | 0.2 | 1.13 | 2.13 | 1.06 | -0.36 | 260.5 | -0.57 | 3.1 | 8.8 | 1061.4 | |||
| 0.6 | 0.6 | 0.02 | 0.19 | 0.19 | 1.26 | 2.05 | 1.05 | -0.38 | 241.3 | -0.5 | 2.9 | 8.8 | 1058.4 | |||
| 0.55 | 0.6 | 0.02 | 0.19 | 0.19 | 1.41 | 2.03 | 1.07 | -0.4 | 215.7 | -0.42 | 2.7 | 8.6 | 1054.2 | |||
| 0.5 | 0.6 | 0.03 | 0.19 | 0.19 | 1.56 | 2 | 1.09 | -0.41 | 187.1 | -0.31 | 2.5 | 8.4 | 1050 | |||
| 0.45 | 0.6 | 0.03 | 0.19 | 0.19 | 1.71 | 1.97 | 1.11 | -0.42 | 156.5 | -0.18 | 2.3 | 8.2 | 1045.9 | |||
| 0.4 | 0.6 | 0.04 | 0.18 | 0.18 | 1.86 | 1.95 | 1.13 | -0.42 | 125.5 | -0.03 | 2.1 | 8 | 1042 | |||
| 0.35 | 0.6 | 0.04 | 0.18 | 0.18 | 2.01 | 1.92 | 1.15 | -0.43 | 95.6 | 0.16 | 1.9 | 7.7 | 1038 | |||
| 0.3 | 0.6 | 0.05 | 0.18 | 0.18 | 2.16 | 1.9 | 1.17 | -0.44 | 68.4 | 0.38 | 1.7 | 7.3 | 1034 | |||
| 0.25 | 0.6 | 0.05 | 0.18 | 0.18 | 2.31 | 1.87 | 1.2 | -0.44 | 45 | 0.66 | 1.5 | 6.9 | 1029.9 | |||
| 0.2 | 0.6 | 0.06 | 0.17 | 0.17 | 2.46 | 1.84 | 1.22 | -0.45 | 26.4 | 1.01 | 1.3 | 6.4 | 1025.7 | |||
| 0.15 | 0.6 | 0.06 | 0.17 | 0.17 | 2.61 | 1.82 | 1.24 | -0.45 | 13.1 | 1.47 | 1.1 | 5.8 | 1021.2 | |||
| 0.1 | 0.6 | 0.07 | 0.17 | 0.17 | 2.76 | 1.79 | 1.26 | -0.45 | 4.8 | 2.13 | 0.8 | 5 | 1016.1 | |||
| 0.05 | 0.6 | 0.07 | 0.17 | 0.17 | 2.91 | 1.77 | 1.28 | -0.46 | 0.9 | 3.22 | 0.5 | 3.9 | 1010.1 | |||
| Hekla | ||||||||||||||||
| Global Input Variables | ||||||||||||||||
| DV | DMgO | DFeO | ∆51Vmin-melt | |||||||||||||
| Plagioclase | 0 | 0 | 0 | 0 | ||||||||||||
| Magnetite | 42 | 0 | 8 | -0.75*106/T2 | ||||||||||||
| OPX | 1.5 | 7.25 | 3 | -0.75*106/T2 | ||||||||||||
| CPX | 2.5 | 4 | 1.8 | -0.75*106/T2 | ||||||||||||
| Modes | Bulk D | Model Output | ||||||||||||||
| F | Plagioclase | Magnetite | OPX | CPX | DV | DMgO | DFeO | ∆51Vmin-melt | V | δ51V | MgO | FeO | T (°C) | |||
| 1 | 375 | -0.75 | 5.5 | 15.75 | 1110 | |||||||||||
| 0.95 | 0.6 | 0.06 | 0.17 | 0.17 | 3.24 | 1.95 | 1.33 | -0.39 | 334.4 | -0.69 | 5.2 | 15.5 | 1104.8 | |||
| 0.9 | 0.6 | 0.06 | 0.17 | 0.17 | 3.24 | 1.95 | 1.33 | -0.39 | 296.1 | -0.62 | 5 | 15.2 | 1099.6 | |||
| 0.85 | 0.6 | 0.06 | 0.17 | 0.17 | 3.25 | 1.95 | 1.33 | -0.39 | 259.9 | -0.55 | 4.7 | 14.9 | 1094.3 | |||
| 0.8 | 0.6 | 0.06 | 0.17 | 0.17 | 3.27 | 1.95 | 1.33 | -0.39 | 225.9 | -0.47 | 4.5 | 14.6 | 1089.1 | |||
| 0.75 | 0.6 | 0.06 | 0.17 | 0.17 | 3.29 | 1.95 | 1.34 | -0.39 | 193.8 | -0.38 | 4.2 | 14.3 | 1083.8 | |||
| 0.7 | 0.6 | 0.06 | 0.17 | 0.17 | 3.32 | 1.95 | 1.34 | -0.39 | 163.9 | -0.29 | 3.9 | 13.9 | 1078.5 | |||
| 0.65 | 0.6 | 0.06 | 0.17 | 0.17 | 3.35 | 1.95 | 1.35 | -0.39 | 136.3 | -0.19 | 3.7 | 13.6 | 1073.2 | |||
| 0.6 | 0.6 | 0.06 | 0.17 | 0.17 | 3.38 | 1.95 | 1.35 | -0.39 | 111.1 | -0.08 | 3.4 | 13.1 | 1067.9 | |||
| 0.55 | 0.6 | 0.06 | 0.17 | 0.17 | 3.42 | 1.95 | 1.36 | -0.39 | 88.4 | 0.04 | 3.1 | 12.7 | 1062.5 | |||
| 0.5 | 0.6 | 0.07 | 0.17 | 0.17 | 3.45 | 1.95 | 1.37 | -0.39 | 68.4 | 0.18 | 2.9 | 12.2 | 1057.1 | |||
| 0.45 | 0.6 | 0.07 | 0.17 | 0.17 | 3.49 | 1.95 | 1.37 | -0.39 | 51.2 | 0.33 | 2.6 | 11.7 | 1051.7 | |||
| 0.4 | 0.6 | 0.07 | 0.17 | 0.17 | 3.53 | 1.95 | 1.38 | -0.39 | 36.8 | 0.51 | 2.3 | 11.1 | 1046.2 | |||
| 0.35 | 0.6 | 0.07 | 0.17 | 0.17 | 3.57 | 1.95 | 1.39 | -0.39 | 25.2 | 0.71 | 2 | 10.5 | 1040.8 | |||
| 0.3 | 0.6 | 0.07 | 0.17 | 0.17 | 3.62 | 1.95 | 1.4 | -0.39 | 16.1 | 0.95 | 1.8 | 9.8 | 1035.2 | |||
| 0.25 | 0.6 | 0.07 | 0.17 | 0.17 | 3.66 | 1.95 | 1.41 | -0.39 | 9.4 | 1.23 | 1.5 | 9 | 1029.6 | |||
| 0.2 | 0.6 | 0.07 | 0.17 | 0.17 | 3.7 | 1.95 | 1.41 | -0.39 | 4.9 | 1.57 | 1.2 | 8.1 | 1024 | |||
| 0.15 | 0.6 | 0.07 | 0.17 | 0.17 | 3.74 | 1.95 | 1.42 | -0.39 | 2.1 | 2.02 | 0.9 | 7.1 | 1018.3 | |||
| 0.1 | 0.6 | 0.07 | 0.17 | 0.17 | 3.78 | 1.95 | 1.43 | -0.39 | 0.6 | 2.66 | 0.6 | 5.9 | 1012.5 | |||
| 0.05 | | 0.6 | 0.07 | 0.17 | 0.17 | 3.83 | 1.95 | 1.44 | -0.39 | 0.1 | 3.74 | 0.3 | 4.3 | 1006.5 | ||
Table S-4 Example results for non-modal incremental batch melting.
Using melting reaction of 0.89 cpx + 0.13 opx + 0.12 sp = 0.13 ol + liquid.
Partition coefficients from Mallmann and O'Neill (2009).
| "MORB" | "ARC" | ||||||||||
| FMQ +0.3 | V | Sc | FMQ +1.3 | V | Sc | ||||||
| D olvine (V8) | 0.0646876241 | 0.2837600644 | D olivine | 0.021411957 | 0.2284364764 | ||||||
| D cpx (V1) | 0.4179636039 | 1.4403029494 | D cpx (V1) | 0.1717294109 | 1.4932144217 | ||||||
| D opx | 0.1960168624 | 0.3386847915 | D opx (V5) | 0.0933871639 | 0.3394884245 | ||||||
| D spinel | 1.5516253802 | 0.0434309112 | D spinel | 0.4937800944 | 0.0401772021 | ||||||
| "MORB" | |||||||||||
| Modes 'MORB' source | Bulk Partition Coefficients | Cl (V) | Cs (V) | Cl (Sc) | Cs (Sc) | ||||||
| Total Melt F | olivine | opx | cpx | spinel | V | Sc | |||||
| 0 | 79.00 | 16.30 | |||||||||
| 0.01 | 0.600 | 0.200 | 0.150 | 0.050 | 0.218 | 0.456 | 354.99 | 77.77 | 35.96 | 15.98 | |
| 0.02 | 0.608 | 0.197 | 0.137 | 0.049 | 0.212 | 0.438 | 359.99 | 76.49 | 36.70 | 15.64 | |
| 0.03 | 0.616 | 0.195 | 0.124 | 0.049 | 0.206 | 0.422 | 364.18 | 75.17 | 37.31 | 15.30 | |
| 0.04 | 0.624 | 0.192 | 0.113 | 0.048 | 0.200 | 0.408 | 367.54 | 73.81 | 37.80 | 14.94 | |
| 0.05 | 0.632 | 0.190 | 0.103 | 0.048 | 0.195 | 0.394 | 370.09 | 72.42 | 38.16 | 14.58 | |
| 0.06 | 0.640 | 0.187 | 0.094 | 0.047 | 0.190 | 0.383 | 371.85 | 71.00 | 38.40 | 14.21 | |
| 0.07 | 0.648 | 0.185 | 0.086 | 0.047 | 0.186 | 0.372 | 372.82 | 69.55 | 38.50 | 13.84 | |
| 0.08 | 0.657 | 0.182 | 0.078 | 0.046 | 0.182 | 0.363 | 373.04 | 68.09 | 38.48 | 13.47 | |
| 0.09 | 0.665 | 0.180 | 0.071 | 0.045 | 0.179 | 0.354 | 372.53 | 66.61 | 38.34 | 13.09 | |
| 0.1 | 0.674 | 0.178 | 0.065 | 0.045 | 0.175 | 0.347 | 371.34 | 65.12 | 38.08 | 12.72 | |
| 0.11 | 0.683 | 0.175 | 0.059 | 0.044 | 0.172 | 0.340 | 369.49 | 63.63 | 37.72 | 12.35 | |
| 0.12 | 0.692 | 0.173 | 0.054 | 0.044 | 0.169 | 0.334 | 367.03 | 62.13 | 37.26 | 11.98 | |
| 0.13 | 0.701 | 0.171 | 0.049 | 0.043 | 0.166 | 0.329 | 364.00 | 60.63 | 36.71 | 11.61 | |
| 0.14 | 0.710 | 0.169 | 0.045 | 0.043 | 0.164 | 0.325 | 360.45 | 59.13 | 36.09 | 11.25 | |
| 0.15 | 0.719 | 0.167 | 0.041 | 0.042 | 0.162 | 0.321 | 356.41 | 57.64 | 35.39 | 10.90 | |
| 0.16 | 0.728 | 0.164 | 0.037 | 0.042 | 0.160 | 0.318 | 351.94 | 56.17 | 34.64 | 10.55 | |
| 0.17 | 0.738 | 0.162 | 0.034 | 0.041 | 0.158 | 0.315 | 347.06 | 54.70 | 33.83 | 10.21 | |
| 0.18 | 0.747 | 0.160 | 0.031 | 0.041 | 0.156 | 0.312 | 341.83 | 53.25 | 32.98 | 9.87 | |
| 0.19 | 0.757 | 0.158 | 0.028 | 0.040 | 0.154 | 0.310 | 336.29 | 51.81 | 32.11 | 9.55 | |
| 0.2 | 0.767 | 0.156 | 0.026 | 0.040 | 0.153 | 0.309 | 330.47 | 50.39 | 31.20 | 9.23 | |
| "ARC" | |||||||||||
| Modes 'ARC' source | Bulk Partition Coefficients | Cl (V) | Cs (V) | Cl (Sc) | Cs (Sc) | ||||||
| Total Melt F | olivine | opx | cpx | spinel | V | Sc | |||||
| 0 | 50.00 | 13.50 | |||||||||
| 0.01 | 0.650 | 0.250 | 0.050 | 0.050 | 0.071 | 0.310 | 668.56 | 46.40 | 42.96 | 13.06 | |
| 0.02 | 0.651 | 0.247 | 0.046 | 0.049 | 0.069 | 0.302 | 631.80 | 42.99 | 42.57 | 12.61 | |
| 0.03 | 0.659 | 0.244 | 0.041 | 0.049 | 0.068 | 0.297 | 594.26 | 39.76 | 41.83 | 12.17 | |
| 0.04 | 0.668 | 0.240 | 0.038 | 0.048 | 0.067 | 0.293 | 557.69 | 36.73 | 41.02 | 11.74 | |
| 0.05 | 0.677 | 0.237 | 0.034 | 0.048 | 0.066 | 0.288 | 522.24 | 33.88 | 40.12 | 11.32 | |
| 0.06 | 0.685 | 0.234 | 0.031 | 0.047 | 0.065 | 0.285 | 488.03 | 31.21 | 39.16 | 10.90 | |
| 0.07 | 0.694 | 0.231 | 0.029 | 0.047 | 0.064 | 0.282 | 455.16 | 28.71 | 38.15 | 10.50 | |
| 0.08 | 0.703 | 0.228 | 0.026 | 0.046 | 0.064 | 0.279 | 423.69 | 26.38 | 37.09 | 10.11 | |
| 0.09 | 0.712 | 0.225 | 0.024 | 0.045 | 0.063 | 0.276 | 393.69 | 24.21 | 36.00 | 9.72 | |
| 0.1 | 0.722 | 0.222 | 0.022 | 0.045 | 0.062 | 0.274 | 365.17 | 22.20 | 34.89 | 9.35 | |
| 0.11 | 0.731 | 0.219 | 0.020 | 0.044 | 0.061 | 0.273 | 338.15 | 20.33 | 33.76 | 8.99 | |
| 0.12 | 0.741 | 0.216 | 0.018 | 0.044 | 0.061 | 0.271 | 312.62 | 18.59 | 32.63 | 8.64 | |
| 0.13 | 0.750 | 0.214 | 0.016 | 0.043 | 0.060 | 0.270 | 288.58 | 16.99 | 31.49 | 8.30 | |
| 0.14 | 0.760 | 0.211 | 0.015 | 0.043 | 0.060 | 0.269 | 266.00 | 15.51 | 30.36 | 7.98 | |
| 0.15 | 0.770 | 0.208 | 0.014 | 0.042 | 0.059 | 0.268 | 244.84 | 14.15 | 29.24 | 7.66 | |
| 0.16 | 0.780 | 0.205 | 0.012 | 0.042 | 0.059 | 0.268 | 225.06 | 12.89 | 28.14 | 7.36 | |
| 0.17 | 0.790 | 0.203 | 0.011 | 0.041 | 0.058 | 0.268 | 206.61 | 11.73 | 27.06 | 7.07 | |
| 0.18 | 0.800 | 0.200 | 0.010 | 0.041 | 0.058 | 0.268 | 189.43 | 10.67 | 25.99 | 6.79 | |
| 0.19 | 0.811 | 0.198 | 0.009 | 0.040 | 0.057 | 0.268 | 173.48 | 9.70 | 24.96 | 6.52 | |
| 0.2 | 0.821 | 0.195 | 0.009 | 0.040 | 0.057 | 0.268 | 158.69 | 8.81 | 23.95 | 6.26 | |
| Cl | concentration in liquid | ||||||||||
| | Cs | concentration in residue | |||||||||