Aqueous concentration of CO2 in carbon-saturated fluids as a highly sensitive oxybarometer
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:1,914Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract

Tables and Figures
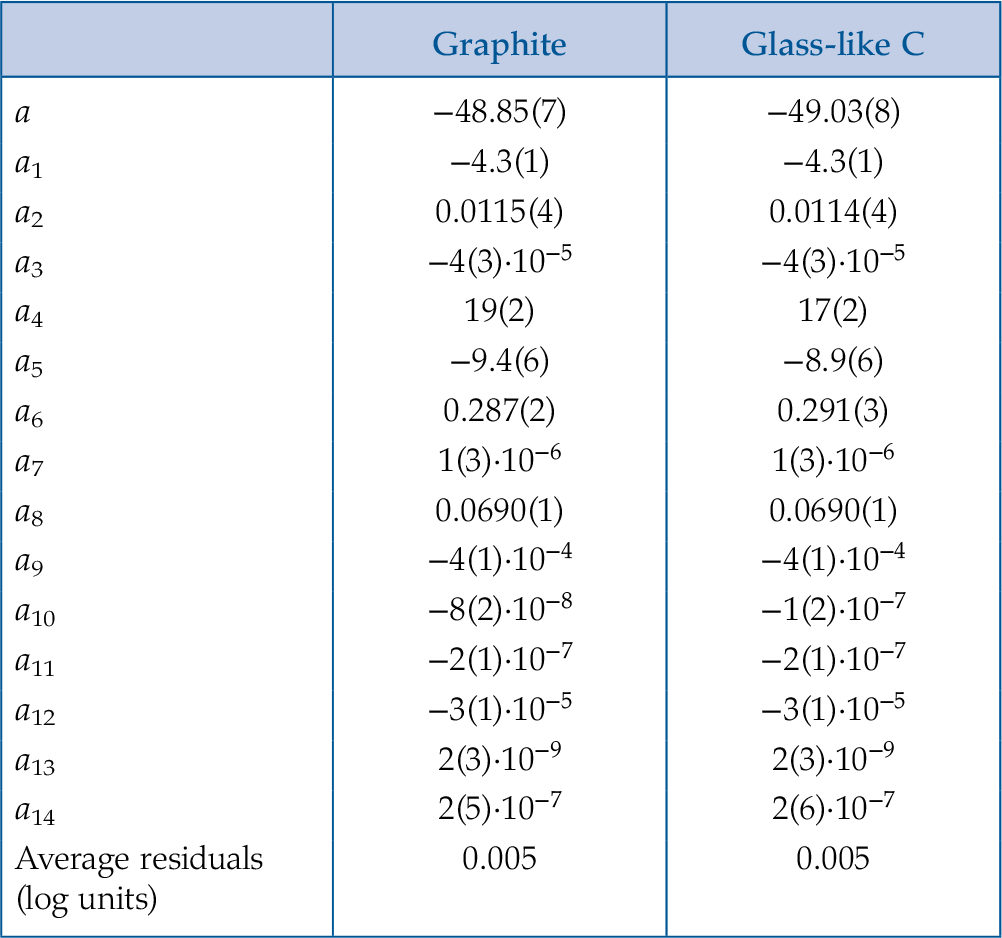 Table 1 Parameters of Eq. 1 provided for graphite and glass-like C. Numbers in parentheses indicate the standard error. | 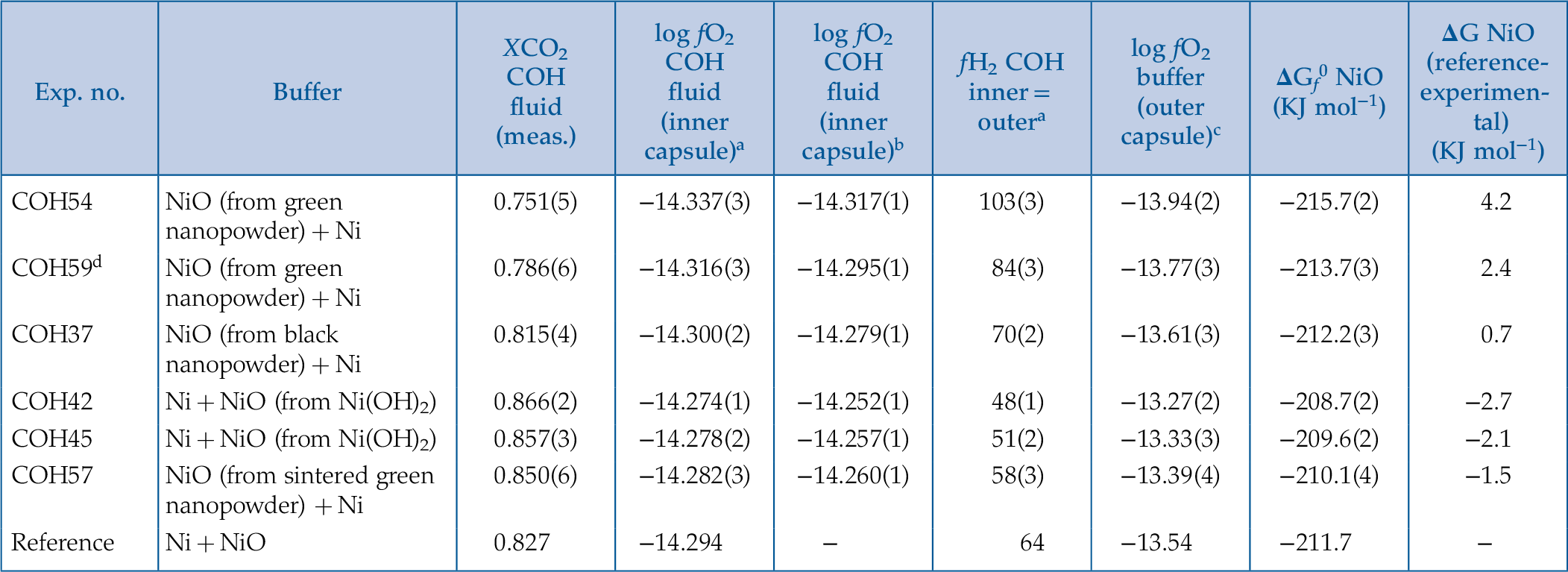 Table 2 Run table including CO2 measured in the experimental aqueous fluids. fO2 resulting from the thermodynamic modelling of glass-like C-saturated fluids buffered at 10 kbar and 800 °C and derived free energies of formation of different nickel oxide precursors are provided. Numbers in parentheses indicate the standard error. | 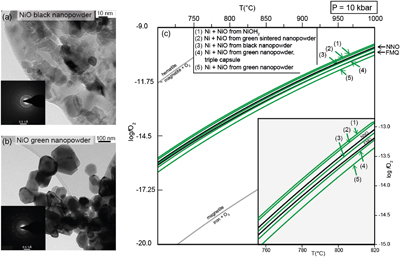 Figure 1 (a) and (b) Transmission electron microscopy images and diffraction rings (insets) of the two commercial NiO nanopowders used as reagents in the buffering assemblages. (c) Isobaric evolution of the NNO buffer oxygen fugacity for buffering assemblages with different NiO reagents (green lines). Black lines represent the evolution predicted by thermodynamic models of the NNO and FMQ buffers. The line representing FMQ (fayalite + O2 = magnetite + quartz), iron + O2 = magnetite and magnetite + O2 = hematite buffers are provided for reference. | 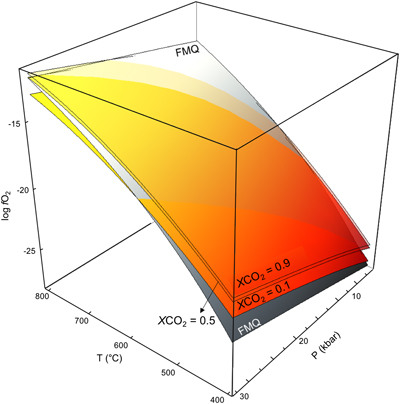 Figure 2 Graphite saturated COH fluid in the P-T-log fO2 space. Coloured surfaces represent isopleths of low (0.1), intermediate (0.5) and high (0.9) CO2 content in the COH fluid expressed as XCO2 (XCO2 = CO2/(CO2 + H2O)). The grey shaded FMQ surface represents the univariant equilibrium of the reaction: 2 Fe3O4 + 3 SiO2 = 3 Fe2SiO4 + O2. |
| Table 1 | Table 2 | Figure 1 | Figure 2 |
top
Introduction
The definition and calibration of new and accurate oxybarometers aid in the investigation and interpretation of geological processes (e.g., Arató and Audetat, 2017
Arató, R., Audétat, A. (2017) FeTiMM–A new oxybarometer for mafic to felsic magmas. Geochemical Perspectives Letters 5, 19–23.
). As oxygen fugacity (fO2) influences processes involving solids, aqueous fluids and melts, affecting phase equilibria and the behaviour of multivalent elements (e.g., C, S, Fe), its precise determination is of primary importance.All the chemical reactions dependent on fO2 have the potential to serve as fO2 sensors. Many mineral assemblages (e.g., Ballhaus et al., 1991
Ballhaus, C., Berry, R.F., Green, D.H. (1991) High pressure experimental calibration of the olivine-orthopyroxene-spinel oxygen geobarometer: implications for the oxidation state of the upper mantle. Contributions to Mineralogy and Petrology 107, 27–40.
), oxides and metal-oxide couples (e.g., Tao et al., 2017Tao, R., Zhang, L., Stagno, V., Chu, X., Liu, X. (2017) High-Pressure experimental verification of rutile-ilmenite oxybarometer: Implications for the redox state of the subduction zone. Science China Earth Sciences 60, 1817–1825.
) and binary/ternary alloys (e.g., Balta et al., 2011Balta, J.B., Beckett, J.R., Asimow, P.D. (2011) Thermodynamic properties of alloys of gold-74/palladium-26 with variable amounts of iron and the use of Au-Pd-Fe alloys as containers for experimental petrology. American Mineralogist 96, 1467–1474.
) have been calibrated as solid oxybarometers and redox sensors, allowing the determination of fO2 in both natural and experimental systems with an uncertainty of the order of 0.3 log units. Recently the aptness as oxybarometers of non-solid phases, for instance melts, where their CO2 content depends on fO2 (Stagno and Frost, 2010Stagno, V., Frost, D.J. (2010). Carbon speciation in the asthenosphere: Experimental measurements of the redox conditions at which carbonate-bearing melts coexist with graphite or diamond in peridotite assemblages. Earth and Planetary Science Letters 300, 72–84.
), has been shown. Using non-solid phases has the advantage of avoiding issues related to structure behaviour (phase transitions, melting processes etc.) at different pressure and temperatures.Here we present the first calibration of an oxybarometer based on the CO2 content of aqueous fluids. As carbon saturation constrains the fluid composition to the carbon-saturation surface, which is univariant in the COH system once the P-T conditions are fixed (e.g, Connolly, 1995
Connolly, J.A.D. (1995) Phase diagram methods for graphitic rocks and application to the system C−O−H−FeO−TiO2−SiO2. Contributions to Mineralogy and Petrology 119, 94–116.
; Tumiati and Malaspina, 2019Tumiati, S., Malaspina, N. (2019) Redox processes and the role of carbon-bearing volatiles from the slab–mantle interface to the mantle wedge. Journal of the Geological Society 176, 388–397.
), the independent definition of the fluid composition (e.g., XCO2 = CO2,aq/(H2O + CO2,aq)molar) can be used to retrieve other intensive variables (e.g., fO2), and vice versa. In this study, we parameterised log (fO2/1 bar)fluid as a function of P, T and the fluid XCO2. The ideal conditions for the application of the CO2-in-fluid oxybarometer require an appreciable CO2 content and carbon saturation. This means oxidised fluids, where XO (= O/H + O) > 1/3 (e.g., Connolly and Cesare, 1993Connolly, J.A.D., Cesare, B. (1993) C-O-H-S Fluid Composition and Oxygen Fugacity in Graphitic Metapelites. Journal of Metamorphic Geology 11, 379–388.
; Huizenga, 2001Huizenga, J.M. (2001). Thermodynamic modelling of C–O–H fluids. Lithos 55, 101–114.
; Zhang and Duan, 2009Zhang, C., Duan, Z. (2009) A model for C–O–H fluid in the Earth’s mantle. Geochimica et Cosmochimica Acta 73, 2089–2102.
) where there is saturation of ordered (graphite) or disordered (glass-like C) carbon. Glass-like carbon is considered an analogue of natural “disordered” carbon deriving from organic matter and has different thermodynamic properties in respect to graphite (e.g., Tumiati et al., 2020Tumiati, S., Tiraboschi, C., Miozzi, F., Vitale-Brovarone, A., Manning, C.E., Sverjensky, D.A., Milani, S., Poli, S. (2020) Dissolution susceptibility of glass-like carbon versus crystalline graphite in high-pressure aqueous fluids and implications for the behavior of organic matter in subduction zones. Geochimica et Cosmochimica Acta 273, 383–402.
).top
Parameterisation of the CO2-in-fluid Oxybarometer
The fO2 values of COH fluids in equilibrium with ideal graphite and glass-like C, a disordered but very homogenous X-ray amorphous graphitic carbon form often used in experimental petrology (e.g., Spandler et al., 2008
Spandler, C., Yaxley, G., Green, D.H., Rosenthal, A. (2008) Phase relations and melting of anhydrous K-bearing eclogite from 1200 to 1600 C and 3 to 5 GPa. Journal of Petrology 49, 771–795.
), were fitted using a specific routine written in the Wolfram Mathematica® computation environment. The parametric equation Eq. 1, (P in kbar, T in °C and XCO2 in mol. %) represents the best polynomial, evaluated statistically in more than 300 thousand other possible models (see Supplementary Information S-1). The fitted fO2 values were calculated in the P-T-XCO2 range 5–30 kbar, 600–1000 °C, 0.1–0.9 using the thermodynamic model from Zhang and Duan (2009)Zhang, C., Duan, Z. (2009) A model for C–O–H fluid in the Earth’s mantle. Geochimica et Cosmochimica Acta 73, 2089–2102.
, assessed by previous authors as the more robust fit to experimental data within those available (Tumiati et al., 2020Tumiati, S., Tiraboschi, C., Miozzi, F., Vitale-Brovarone, A., Manning, C.E., Sverjensky, D.A., Milani, S., Poli, S. (2020) Dissolution susceptibility of glass-like carbon versus crystalline graphite in high-pressure aqueous fluids and implications for the behavior of organic matter in subduction zones. Geochimica et Cosmochimica Acta 273, 383–402.
). The model was used in its original version for graphite-saturated fluid and with modified equilibrium constants (Kps) for glass-like C-saturated fluids (cf. Tumiati et al., 2020Tumiati, S., Tiraboschi, C., Miozzi, F., Vitale-Brovarone, A., Manning, C.E., Sverjensky, D.A., Milani, S., Poli, S. (2020) Dissolution susceptibility of glass-like carbon versus crystalline graphite in high-pressure aqueous fluids and implications for the behavior of organic matter in subduction zones. Geochimica et Cosmochimica Acta 273, 383–402.
) (see Supplementary Information S-2). Therefore, two sets of parameters (Table 1) were determined depending on the type of carbon considered (i.e. graphite or glass-like C).Eq. 1
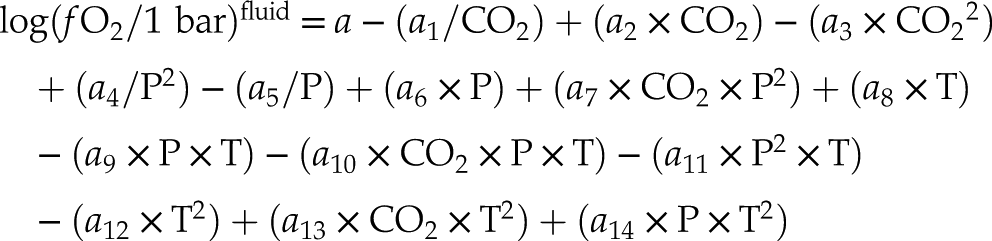
Table 1 Parameters of Eq. 1 provided for graphite and glass-like C. Numbers in parentheses indicate the standard error.
| Graphite | Glass-like C | |
| a | −48.85(7) | −49.03(8) |
| a1 | −4.3(1) | −4.3(1) |
| a2 | 0.0115(4) | 0.0114(4) |
| a3 | −4(3)·10−5 | −4(3)·10−5 |
| a4 | 19(2) | 17(2) |
| a5 | −9.4(6) | −8.9(6) |
| a6 | 0.287(2) | 0.291(3) |
| a7 | 1(3)·10−6 | 1(3)·10−6 |
| a8 | 0.0690(1) | 0.0690(1) |
| a9 | −4(1)·10−4 | −4(1)·10−4 |
| a10 | −8(2)·10−8 | −1(2)·10−7 |
| a11 | −2(1)·10−7 | −2(1)·10−7 |
| a12 | −3(1)·10−5 | −3(1)·10−5 |
| a13 | 2(3)·10−9 | 2(3)·10−9 |
| a14 | 2(5)·10−7 | 2(6)·10−7 |
| Average residuals (log units) | 0.005 | 0.005 |
The average residuals, 0.005 log units (Table 1), represent the uncertainty of the model (see also Fig. S-1). This value does not take into account the uncertainties associated with the measurement of CO2 in aqueous fluids, which is of the order of 1 mol. % for quadrupole mass spectrometry analyses (Tiraboschi et al., 2016
Tiraboschi, C., Tumiati, S., Recchia, S., Miozzi, F., Poli, S. (2016) Quantitative analysis of COH fluids synthesized at HP–HT conditions: an optimized methodology to measure volatiles in experimental capsules. Geofluids 16, 841–855.
and Tumiati et al., 2020Tumiati, S., Tiraboschi, C., Miozzi, F., Vitale-Brovarone, A., Manning, C.E., Sverjensky, D.A., Milani, S., Poli, S. (2020) Dissolution susceptibility of glass-like carbon versus crystalline graphite in high-pressure aqueous fluids and implications for the behavior of organic matter in subduction zones. Geochimica et Cosmochimica Acta 273, 383–402.
). Accordingly, we performed a series of experiments to evaluate realistic uncertainties associated with the CO2-in-fluid oxybarometer and assess its sensitivity to resolve small variations of oxygen fugacity.top
Testing the CO2-in-fluid Oxybarometer: Carbon-saturated Fluids Buffered with Ni-NiO
The nickel-nickel oxide (NNO) oxygen buffer, whose position in the P-T-fO2 field depends on the thermodynamic properties of the Ni and NiO, was used to control externally the oxygen fugacity (and therefore the CO2 content) of a glass-like C saturated aqueous fluid. Different NiO reagents have slightly different thermodynamic properties (e.g., O’Neill and Pownceby, 1993
O’Neill, H.S.C., Pownceby, M.I. (1993) Thermodynamic data from redox reactions at high temperatures. I. An experimental and theoretical assessment of the electrochemical method using stabilized zirconia electrolytes, with revised values for the Fe-“FeO”, Co-CoO, Ni-NiO and Cu-Cu2O oxygen buffers, and new data for the W-WO2 buffer. Contributions to Mineralogy and Petrology 114, 296–314.
). We built up the buffering assemblages employing different NiO precursors to test the sensitivity of the oxybarometer and investigate their thermodynamic properties. Reagent grade Ni metal powder was mixed with: (i) two commercial nickel oxide nanopowders, green and black, with an average crystallite size of 100 and 10 nm respectively; (ii) sintered nickel oxide, produced by heating green nanopowder for three days at 1300 °C and (iii) nickel hydroxide (Ni(OH)2), which decomposes to NiO at T > 230 °C (details in Supplementary Information S-3, Fig. S-2). As a source of carbon, glass-like C, a NIST standard material (Cappelletti et al., 2018Cappelletti, R.L., Udovic, T.J., Li, H., Paul, R.L. (2018) Glassy carbon, NIST Standard Reference Material (SRM 3600): hydrogen content, neutron vibrational density of states and heat capacity. Journal of Applied Crystallography 51, 1323–1328.
) was preferred to graphite in order to avoid impurities often present in natural samples and to have a material with well characterised thermodynamic properties (cf. Tumiati et al., 2020Tumiati, S., Tiraboschi, C., Miozzi, F., Vitale-Brovarone, A., Manning, C.E., Sverjensky, D.A., Milani, S., Poli, S. (2020) Dissolution susceptibility of glass-like carbon versus crystalline graphite in high-pressure aqueous fluids and implications for the behavior of organic matter in subduction zones. Geochimica et Cosmochimica Acta 273, 383–402.
).Experiments were performed using the double capsule (e.g., Eugster and Skippen, 1967
Eugster, H.P., Skippen, G.B. (1967) Igneous and metamorphic reactions involving gas equilibria. Researches in Geochemistry 2, 492–520.
) and the triple capsule (Matjuschkin et al., 2015Matjuschkin, V., Brooker, R.A., Tattitch, B., Blundy, J.D., Stamper, C.C. (2015) Control and monitoring of oxygen fugacity in piston cylinder experiments. Contributions to Mineralogy and Petrology 169, 9.
) techniques (Fig. S-4) to avoid the direct contact of the fluids with the NNO buffer. Fluids were equilibrated at 10 kbar and 800 °C for 24 hours in an end load piston cylinder. After the experiments, quenched capsules were pierced in a gas tight vessel and the gases conveyed to a quadrupole mass spectrometer (QMS) for the analysis of volatiles (Tiraboschi et al., 2016Tiraboschi, C., Tumiati, S., Recchia, S., Miozzi, F., Poli, S. (2016) Quantitative analysis of COH fluids synthesized at HP–HT conditions: an optimized methodology to measure volatiles in experimental capsules. Geofluids 16, 841–855.
).On the basis of the measured CO2 content, we retrieved the log fO2 of the fluids using: (i) the thermodynamic calculations suggested by Tumiati et al., (2017)
Tumiati, S., Tiraboschi, C., Sverjensky, D.A., Pettke, T., Recchia, S., Ulmer, P., Miozzi, F., Poli, S. (2017) Silicate dissolution boosts the CO2 concentrations in subduction fluids. Nature Communications 8, 1–11.
and Tumiati et al., (2020)Tumiati, S., Tiraboschi, C., Miozzi, F., Vitale-Brovarone, A., Manning, C.E., Sverjensky, D.A., Milani, S., Poli, S. (2020) Dissolution susceptibility of glass-like carbon versus crystalline graphite in high-pressure aqueous fluids and implications for the behavior of organic matter in subduction zones. Geochimica et Cosmochimica Acta 273, 383–402.
, and (ii) the CO2-in-fluid oxybarometer (Table 2). The susceptibility of XCO2 to fO2 variations gives the methodology enough sensitivity to see variations of 0.001 log units. The uncertainties on fO2 value, propagated from the analytical error on the XCO2, are of the order of 0.01 log units. The results obtained by thermodynamic calculations are well reproduced by the CO2-in-fluid oxybarometer, with the constant discrepancy of 0.02 being the result of the different equilibrium constants (Kps) used. Those derived from experiments (Tumiati et al., 2020Tumiati, S., Tiraboschi, C., Miozzi, F., Vitale-Brovarone, A., Manning, C.E., Sverjensky, D.A., Milani, S., Poli, S. (2020) Dissolution susceptibility of glass-like carbon versus crystalline graphite in high-pressure aqueous fluids and implications for the behavior of organic matter in subduction zones. Geochimica et Cosmochimica Acta 273, 383–402.
) for the thermodynamic calculations (Table 2) and those obtained by modelling the thermodynamic properties (cf. “Parameterisation of the CO2-in-fluid oxybarometer” above) for the CO2-in-fluid oxybarometer (Table 2).Table 2 Run table including CO2 measured in the experimental aqueous fluids. fO2 resulting from the thermodynamic modelling of glass-like C-saturated fluids buffered at 10 kbar and 800 °C and derived free energies of formation of different nickel oxide precursors are provided. Numbers in parentheses indicate the standard error.
| Exp. no. | Buffer | XCO2 COH fluid (meas.) | log fO2 COH fluid (inner capsule)a | log fO2 COH fluid (inner capsule)b | fH2 COH inner = outera | log fO2 buffer (outer capsule)c | ΔGf0 NiO (KJ mol−1) | ΔG NiO (reference-experimental) (KJ mol−1) |
| COH54 | NiO (from green nanopowder) + Ni | 0.751(5) | −14.337(3) | −14.317(1) | 103(3) | −13.94(2) | −215.7(2) | 4.2 |
| COH59d | NiO (from green nanopowder) + Ni | 0.786(6) | −14.316(3) | −14.295(1) | 84(3) | −13.77(3) | −213.7(3) | 2.4 |
| COH37 | NiO (from black nanopowder) + Ni | 0.815(4) | −14.300(2) | −14.279(1) | 70(2) | −13.61(3) | −212.2(3) | 0.7 |
| COH42 | Ni + NiO (from Ni(OH)2) | 0.866(2) | −14.274(1) | −14.252(1) | 48(1) | −13.27(2) | −208.7(2) | −2.7 |
| COH45 | Ni + NiO (from Ni(OH)2) | 0.857(3) | −14.278(2) | −14.257(1) | 51(2) | −13.33(3) | −209.6(2) | −2.1 |
| COH57 | NiO (from sintered green nanopowder) + Ni | 0.850(6) | −14.282(3) | −14.260(1) | 58(3) | −13.39(4) | −210.1(4) | −1.5 |
| Reference | Ni + NiO | 0.827 | −14.294 | − | 64 | −13.54 | −211.7 | − |
aEoS by Zhang and Duan (2009)Zhang, C., Duan, Z. (2009) A model for C–O–H fluid in the Earth’s mantle. Geochimica et Cosmochimica Acta 73, 2089–2102. modified to include the dynamic γH2 taken from Connolly and Cesare (1993)Connolly, J.A.D., Cesare, B. (1993) C-O-H-S Fluid Composition and Oxygen Fugacity in Graphitic Metapelites. Journal of Metamorphic Geology 11, 379–388., changing as a function of P, T and XO (γH 2= A•(XO)3 + B• (XO)2 + C•XO + D; where A = 43.919, B = 114.55, C = −105.75, D = 41.215 at 10 kbar and 800 °C; A = −11208; B = 26723, C = −21949, D = 6979.2 at 3 GPa and 800 °C) (Tumiati et al., 2017Tumiati, S., Tiraboschi, C., Sverjensky, D.A., Pettke, T., Recchia, S., Ulmer, P., Miozzi, F., Poli, S. (2017) Silicate dissolution boosts the CO2 concentrations in subduction fluids. Nature Communications 8, 1–11.), and the equilibrium constants (Kps) for glass-like C from Tumiati et al., (2020)Tumiati, S., Tiraboschi, C., Miozzi, F., Vitale-Brovarone, A., Manning, C.E., Sverjensky, D.A., Milani, S., Poli, S. (2020) Dissolution susceptibility of glass-like carbon versus crystalline graphite in high-pressure aqueous fluids and implications for the behavior of organic matter in subduction zones. Geochimica et Cosmochimica Acta 273, 383–402..
bretrieved with the CO2-in-fluid oxybarometer
cretrieved using the routine ‘‘fluids” of the Perple_X package (H-O HSMRK/MRK hybrid EoS).ΔGf0 Gibbs free energy of formation from the elements in their standard state (298 K; 1 bar). Determined by changing the thermodynamic database hp04ver.dat in the Perple_X computer package (http://www.perplex.ethz.ch; Connolly, 2005Connolly, J.A. (2005) Computation of phase equilibria by linear programming: a tool for geodynamic modeling and its application to subduction zone decarbonation. Earth and Planetary Science Letters 236, 524–541.) to match the oxygen fugacity obtained using different nickel oxide precursors in the buffer.ΔG NiO = ΔGf0 NNO reference – ΔGf0 NNO experimental.
dtriple capsule design. Two smaller capsules, respectively containing the carbon-saturated fluid and the buffering assemblage, are vertically positioned on top of each other and inside a bigger capsule, embedded in Al2O3 (Matjuschkin et al., 2015Matjuschkin, V., Brooker, R.A., Tattitch, B., Blundy, J.D., Stamper, C.C. (2015) Control and monitoring of oxygen fugacity in piston cylinder experiments. Contributions to Mineralogy and Petrology 169, 9.).
With the fluid’s log fO2, fH2 can be retrieved and used to obtain the log fO2 of the buffering assemblage (see Supplementary Information S-4 for the procedure), thus allowing comparison with the NNO reference calculated with Perple_X (Connolly, 2005
Connolly, J.A. (2005) Computation of phase equilibria by linear programming: a tool for geodynamic modeling and its application to subduction zone decarbonation. Earth and Planetary Science Letters 236, 524–541.
) and the hp04ver.dat thermodynamic database updated with the most recent re-assessment for both Ni and NiO of ΔGf0 and S0 (Gamsjäger et al., 2005Gamsjäger, H., Bugajski, J., Preis, W. (2005) Chemical thermodynamics of nickel. Elsevier, Amsterdam.
) and equation of state parameters K0 and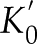 (Campbell et al., 2009
(Campbell et al., 2009Campbell, A.J., Danielson, L., Righter, K., Seagle, C.T., Wang, Y., Prakapenka, V.B. (2009) High pressure effects on the iron–iron oxide and nickel–nickel oxide oxygen fugacity buffers. Earth and Planetary Science Letters 286, 556–564.
) (Table 2).Within the different buffering assemblages, those made with black nickel oxide and sintered nickel oxide reproduce the reference fO2 better. The discrepancy in the oxygen fugacity imposed by the sintered, in comparison to standard green NiO, confirms the need to sinter the material to increase its reactivity, as previously reported in literature (e.g., Mattioli and Wood, 1988
Mattioli, G.S., Wood, B.J. (1988) Magnetite activities across the MgAl2O4-Fe3O4 spinel join, with application to thermobarometric estimates of upper mantle oxygen fugacity. Contributions to Mineralogy and Petrology 98, 148–162.
; O’Neill and Pownceby, 1993O’Neill, H.S.C., Pownceby, M.I. (1993) Thermodynamic data from redox reactions at high temperatures. I. An experimental and theoretical assessment of the electrochemical method using stabilized zirconia electrolytes, with revised values for the Fe-“FeO”, Co-CoO, Ni-NiO and Cu-Cu2O oxygen buffers, and new data for the W-WO2 buffer. Contributions to Mineralogy and Petrology 114, 296–314.
). For black nickel oxide, the high temperature of the experiments coupled with the long duration likely induced the sintering of this disordered material (cf. TEM images, Fig. 1a) during the experimental run, hence the imposed fO2 closely reproduces the NNO reference. As such, its usage for experiments at low T or of short duration is discouraged without previous sintering. Sintering during the experimental run is not easily achieved for the NiO green nanopowder, due to its relatively ordered (and therefore stable) state, with larger sizes and well shaped crystals (cf. TEM images, Fig. 1b).
Figure 1 (a) and (b) Transmission electron microscopy images and diffraction rings (insets) of the two commercial NiO nanopowders used as reagents in the buffering assemblages. (c) Isobaric evolution of the NNO buffer oxygen fugacity for buffering assemblages with different NiO reagents (green lines). Black lines represent the evolution predicted by thermodynamic models of the NNO and FMQ buffers. The line representing FMQ (fayalite + O2 = magnetite + quartz), iron + O2 = magnetite and magnetite + O2 = hematite buffers are provided for reference.
Finally, the retrieved fO2 of the buffering assemblages were used to determine the Gibbs free energy of formation (ΔGf0) of the different NiO reagents and subsequently model the evolution in temperature of their log fO2 (Fig. 1c).
Using the dependency between the two parameters, we iteratively changed the ΔGf0 for NiO in the updated hp04ver.dat thermodynamic database of Perple_X, to match the fO2 derived from the measured fluid compositions. Compared to the NNO reference, the minimum variation in ΔGf0 detected is 0.7 KJ mol−1 for a 0.07 log fO2 variation. The fO2 imposed by the different precursors have a consistent evolution in the investigated T range. While the assemblages with black NiO, sintered NiO and Ni(OH)2 plot closer to the NNO reference, green NiO plots even below the FMQ equilibrium. The different capsule assemblage (i.e. triple instead of double) slightly increases the fO2 but not enough to reach the reference. Accordingly NiO sintering before the experiments is needed in order to impose an fO2 close to the thermodynamic reference.
top
Applications
The CO2-in-fluid oxybarometer has been calibrated for CO2-dominated aqueous fluids saturated in carbon, either ordered (graphite) or disordered (glass-like C) and can be used at P-T-pH conditions where the carbon-bearing dissolved species mostly exist in their molecular state (i.e. CO2,aq) and not as charged anionic or cationic species (Sverjensky et al., 2014
Sverjensky, D.A., Stagno, V., Huang, F. (2014) Important role for organic carbon in subduction-zone fluids in the deep carbon cycle. Nature Geoscience 7, 909–913.
; Tumiati et al., 2020Tumiati, S., Tiraboschi, C., Miozzi, F., Vitale-Brovarone, A., Manning, C.E., Sverjensky, D.A., Milani, S., Poli, S. (2020) Dissolution susceptibility of glass-like carbon versus crystalline graphite in high-pressure aqueous fluids and implications for the behavior of organic matter in subduction zones. Geochimica et Cosmochimica Acta 273, 383–402.
).The model is easily accessible in the CO2-in-fluid Excel spreadsheet available for download in the Supplementary Information. The calculation requires as input data P, T, XCO2 and the type of carbon considered (i.e. graphite or glass-like C). The calculator provides the COH fluid’s fO2 and its uncertainties both expressed as absolute value (log unit) and in relation to the FMQ reference (i.e. ΔFMQ = logfO2FMQ – logfO2) as well as the fO2 for the FMQ reference itself. The latter is calculated using an improved parameterisation, established in the present study in order for the uncertainties to be consistent with those determined for the oxybarometer (see Supplementary Information S-5). In particular, the average residuals of the present FMQ fit are 0.01629 and the maximum 0.09674 while the commonly used equation, established by O’Neill (1987)
O’Neill, H.S. (1987). Quartz-fayalite-iron and quartz-fayalite-magnetite equilibria and the free energy of formation of fayalite (Fe2SiO4) and magnetite (Fe3O4). American Mineralogist, 72(1-2), 67–75.
, has average and maximum residuals of 0.06347 and 0.32023 respectively.The extensive use of fluids in experimental petrology guarantees a wide range of applications in this field. The CO2-in-fluid oxybarometers give the opportunity to retrieve the oxygen fugacity of the studied system in experiments focused on fluids or fluid-rock interactions (e.g. Stagno and Frost, 2010
Stagno, V., Frost, D.J. (2010). Carbon speciation in the asthenosphere: Experimental measurements of the redox conditions at which carbonate-bearing melts coexist with graphite or diamond in peridotite assemblages. Earth and Planetary Science Letters 300, 72–84.
) and those involving a glass-like C melt-trap or graphite linings to isolate the capsule from the experimental charge (e.g., Spandler et al., 2008Spandler, C., Yaxley, G., Green, D.H., Rosenthal, A. (2008) Phase relations and melting of anhydrous K-bearing eclogite from 1200 to 1600 C and 3 to 5 GPa. Journal of Petrology 49, 771–795.
; Tiraboschi et al., 2018Tiraboschi, C., Tumiati, S., Sverjensky, D., Pettke, T., Ulmer, P., Poli, S. (2018) Experimental determination of magnesia and silica solubilities in graphite-saturated and redox-buffered high-pressure COH fluids in equilibrium with forsterite+enstatite and magnesite+enstatite. Contributions to Mineralogy and Petrology 173, 2.
). Its use is also extended to double capsule buffered experiments where it represents a helpful tool to assess the attainment of equilibrium on the basis of the expected fO2 conditions, thus excluding any influence of the experimental assembly. Using the XCO2 to determine the fO2 in experiments involving fluids represents a reliable and more accurate alternative to solid state sensors provided attainment of the needed conditions. It would avoid potential issues related to the possible interaction of the solid sensor with the starting materials, which would modify the initial petrological system, and to the possible disequilibrium composition of solid state sensors, e.g., alloys, in low T experiments.The CO2-in-fluid oxybarometer could be a useful tool also in natural systems, provided that some important requirements are met. The most important is the attainment of equilibrium between carbon and COH fluid. This is not a trivial issue. For instance, previous studies showed that graphite is poorly reactive below 500–600 °C (Ziegenbein and Johannes, 1980
Ziegenbein, D., Johannes, W. (1980) Graphite in CHO fluids: An unsuitable compound to buffer fluid composition at temperatures up to 700°C. Neues Jahrbuch fur Mineralogie-Monatshefte 7, 289–305.
; Luque et al., 1998Luque, F.J., Pasteris, J.D., Wopenka, B., Rodas, M., Fernández Barrenechea, J.M. (1998) Natural fluid-deposited graphite: mineralogical characteristics and mechanisms of formation. American Journal of Science 298, 471–498.
). At these low temperatures, thermodynamic equilibrium is hampered by slow kinetics, thus implying that results coming from thermodynamic modelling of COH fluids in equilibrium with carbon, independently from the chosen model, should be handled with care. Another variable that obviously could affect the approach to equilibrium is time. Short lived processes or an opening of the system could result in fluids that are not representative of the equilibrium state. Finally, the complexity of the systems should also be taken into account as complex systems might have a different CO2 content with respect to the pure COH (e.g., SiO2-bearing, Tumiati etal., 2017Tumiati, S., Tiraboschi, C., Sverjensky, D.A., Pettke, T., Recchia, S., Ulmer, P., Miozzi, F., Poli, S. (2017) Silicate dissolution boosts the CO2 concentrations in subduction fluids. Nature Communications 8, 1–11.
).
Figure 2 Graphite saturated COH fluid in the P-T-log fO2 space. Coloured surfaces represent isopleths of low (0.1), intermediate (0.5) and high (0.9) CO2 content in the COH fluid expressed as XCO2 (XCO2 = CO2/(CO2 + H2O)). The grey shaded FMQ surface represents the univariant equilibrium of the reaction: 2 Fe3O4 + 3 SiO2 = 3 Fe2SiO4 + O2.
top
Acknowledgements
Authors are indebted to A. Risplendente for the assistance at scanning electron microscope and electron microprobe, to N. Rotiroti for the assistance with the transmission electron microscope and to C. Tiraboschi for preparing some of the experiments. FM and ST acknowledge support of the Italian program MIUR PRIN 2017ZE49E7_002.
Editor: Eric H. Oelkers
top
References
Arató, R., Audétat, A. (2017) FeTiMM–A new oxybarometer for mafic to felsic magmas. Geochemical Perspectives Letters 5, 19–23.
 Show in context
Show in context The definition and calibration of new and accurate oxybarometers aid in the investigation and interpretation of geological processes (e.g., Arató and Audetat, 2017).
View in article
Balta, J.B., Beckett, J.R., Asimow, P.D. (2011) Thermodynamic properties of alloys of gold-74/palladium-26 with variable amounts of iron and the use of Au-Pd-Fe alloys as containers for experimental petrology. American Mineralogist 96, 1467–1474.
 Show in context
Show in context Many mineral assemblages (e.g., Ballhaus et al., 1991), oxides and metal-oxide couples (e.g., Tao et al., 2017) and binary/ternary alloys (e.g., Balta et al., 2011) have been calibrated as solid oxybarometers and redox sensors, allowing the determination of fO2 in both natural and experimental systems with an uncertainty of the order of 0.3 log units.
View in article
Ballhaus, C., Berry, R.F., Green, D.H. (1991) High pressure experimental calibration of the olivine-orthopyroxene-spinel oxygen geobarometer: implications for the oxidation state of the upper mantle. Contributions to Mineralogy and Petrology 107, 27–40.
 Show in context
Show in context Many mineral assemblages (e.g., Ballhaus et al., 1991), oxides and metal-oxide couples (e.g., Tao et al., 2017) and binary/ternary alloys (e.g., Balta et al., 2011) have been calibrated as solid oxybarometers and redox sensors, allowing the determination of fO2 in both natural and experimental systems with an uncertainty of the order of 0.3 log units.
View in article
Campbell, A.J., Danielson, L., Righter, K., Seagle, C.T., Wang, Y., Prakapenka, V.B. (2009) High pressure effects on the iron–iron oxide and nickel–nickel oxide oxygen fugacity buffers. Earth and Planetary Science Letters 286, 556–564.
 Show in context
Show in context With the fluid’s log fO2, fH2 can be retrieved and used to obtain the log fO2 of the buffering assemblage (see Supplementary Information S-4 for the procedure), thus allowing comparison with the NNO reference calculated with Perple_X (Connolly, 2005) and the hp04ver.dat thermodynamic database updated with the most recent re-assessment for both Ni and NiO of ΔGf0 and S0 (Gamsjäger et al., 2005) and equation of state parameters K0 and 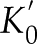 (Campbell et al., 2009) (Table 2).
(Campbell et al., 2009) (Table 2).
View in article
Cappelletti, R.L., Udovic, T.J., Li, H., Paul, R.L. (2018) Glassy carbon, NIST Standard Reference Material (SRM 3600): hydrogen content, neutron vibrational density of states and heat capacity. Journal of Applied Crystallography 51, 1323–1328.
 Show in context
Show in context As a source of carbon, glass-like C, a NIST standard material (Cappelletti et al., 2018) was preferred to graphite in order to avoid impurities often present in natural samples and to have a material with well characterised thermodynamic properties (cf. Tumiati et al., 2020).
View in article
Connolly, J.A.D. (1995) Phase diagram methods for graphitic rocks and application to the system C−O−H−FeO−TiO2−SiO2. Contributions to Mineralogy and Petrology 119, 94–116.
 Show in context
Show in context As carbon saturation constrains the fluid composition to the carbon-saturation surface, which is univariant in the COH system once the P-T conditions are fixed (e.g, Connolly, 1995; Tumiati and Malaspina, 2019), the independent definition of the fluid composition (e.g., XCO2 = CO2,aq/(H2O + CO2,aq)molar) can be used to retrieve other intensive variables (e.g., fO2), and vice versa.
View in article
Connolly, J.A. (2005) Computation of phase equilibria by linear programming: a tool for geodynamic modeling and its application to subduction zone decarbonation. Earth and Planetary Science Letters 236, 524–541.
 Show in context
Show in context Determined by changing the thermodynamic database hp04ver.dat in the Perple_X computer package (http://www.perplex.ethz.ch; Connolly, 2005) to match the oxygen fugacity obtained using different nickel oxide precursors in the buffer.
View in article
With the fluid’s log fO2, fH2 can be retrieved and used to obtain the log fO2 of the buffering assemblage (see Supplementary Information S-4 for the procedure), thus allowing comparison with the NNO reference calculated with Perple_X (Connolly, 2005) and the hp04ver.dat thermodynamic database updated with the most recent re-assessment for both Ni and NiO of ΔGf0 and S0 (Gamsjäger et al., 2005) and equation of state parameters K0 and 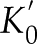 (Campbell et al., 2009) (Table 2).
(Campbell et al., 2009) (Table 2).
View in article
Connolly, J.A.D., Cesare, B. (1993) C-O-H-S Fluid Composition and Oxygen Fugacity in Graphitic Metapelites. Journal of Metamorphic Geology 11, 379–388.
 Show in context
Show in context This means oxidised fluids, where XO (= O/H + O) > 1/3 (e.g., Connolly and Cesare, 1993; Huizenga, 2001; Zhang and Duan, 2009) where there is saturation of ordered (graphite) or disordered (glass-like C) carbon.
View in article
EoS by Zhang and Duan (2009) modified to include the dynamic γH2 taken from Connolly and Cesare (1993), changing as a function of P, T and XO (γH 2= A•(XO)3 + B• (XO)2 + C•XO + D; where A = 43.919, B = 114.55, C = −105.75, D = 41.215 at 10 kbar and 800 °C; A = −11208; B = 26723, C = −21949, D = 6979.2 at 3 GPa and 800 °C) (Tumiati et al., 2017), and the equilibrium constants (Kps) for glass-like C from Tumiati et al., (2020).
View in article
Eugster, H.P., Skippen, G.B. (1967) Igneous and metamorphic reactions involving gas equilibria. Researches in Geochemistry 2, 492–520.
 Show in context
Show in context Experiments were performed using the double capsule (e.g., Eugster and Skippen, 1967) and the triple capsule (Matjuschkin et al., 2015) techniques (Fig. S-4) to avoid the direct contact of the fluids with the NNO buffer.
View in article
Gamsjäger, H., Bugajski, J., Preis, W. (2005) Chemical thermodynamics of nickel. Elsevier, Amsterdam.
 Show in context
Show in context With the fluid’s log fO2, fH2 can be retrieved and used to obtain the log fO2 of the buffering assemblage (see Supplementary Information S-4 for the procedure), thus allowing comparison with the NNO reference calculated with Perple_X (Connolly, 2005) and the hp04ver.dat thermodynamic database updated with the most recent re-assessment for both Ni and NiO of ΔGf0 and S0 (Gamsjäger et al., 2005) and equation of state parameters K0 and 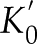 (Campbell et al., 2009) (Table 2).
(Campbell et al., 2009) (Table 2).
View in article
Luque, F.J., Pasteris, J.D., Wopenka, B., Rodas, M., Fernández Barrenechea, J.M. (1998) Natural fluid-deposited graphite: mineralogical characteristics and mechanisms of formation. American Journal of Science 298, 471–498.
 Show in context
Show in context For instance, previous studies showed that graphite is poorly reactive below 500–600 °C (Ziegenbein and Johannes, 1980; Luque et al., 1998).
View in article
Huizenga, J.M. (2001). Thermodynamic modelling of C–O–H fluids. Lithos 55, 101–114.
 Show in context
Show in context This means oxidised fluids, where XO (= O/H + O) > 1/3 (e.g., Connolly and Cesare, 1993; Huizenga, 2001; Zhang and Duan, 2009) where there is saturation of ordered (graphite) or disordered (glass-like C) carbon.
View in article
Matjuschkin, V., Brooker, R.A., Tattitch, B., Blundy, J.D., Stamper, C.C. (2015) Control and monitoring of oxygen fugacity in piston cylinder experiments. Contributions to Mineralogy and Petrology 169, 9.
 Show in context
Show in context Experiments were performed using the double capsule (e.g., Eugster and Skippen, 1967) and the triple capsule (Matjuschkin et al., 2015) techniques (Fig. S-4) to avoid the direct contact of the fluids with the NNO buffer.
View in article
triple capsule design. Two smaller capsules, respectively containing the carbon-saturated fluid and the buffering assemblage, are vertically positioned on top of each other and inside a bigger capsule, embedded in Al2O3 (Matjuschkin et al., 2015).
View in article
Mattioli, G.S., Wood, B.J. (1988) Magnetite activities across the MgAl2O4-Fe3O4 spinel join, with application to thermobarometric estimates of upper mantle oxygen fugacity. Contributions to Mineralogy and Petrology 98, 148–162.
 Show in context
Show in context The discrepancy in the oxygen fugacity imposed by the sintered, in comparison to standard green NiO, confirms the need to sinter the material to increase its reactivity, as previously reported in literature (e.g., Mattioli and Wood, 1988; O’Neill and Pownceby, 1993).
View in article
O’Neill, H.S. (1987). Quartz-fayalite-iron and quartz-fayalite-magnetite equilibria and the free energy of formation of fayalite (Fe2SiO4) and magnetite (Fe3O4). American Mineralogist, 72(1-2), 67–75.
 Show in context
Show in context In particular, the average residuals of the present FMQ fit are 0.01629 and the maximum 0.09674 while the commonly used equation, established by O’Neill (1987), has average and maximum residuals of 0.06347 and 0.32023 respectively.
View in article
O’Neill, H.S.C., Pownceby, M.I. (1993) Thermodynamic data from redox reactions at high temperatures. I. An experimental and theoretical assessment of the electrochemical method using stabilized zirconia electrolytes, with revised values for the Fe-“FeO”, Co-CoO, Ni-NiO and Cu-Cu2O oxygen buffers, and new data for the W-WO2 buffer. Contributions to Mineralogy and Petrology 114, 296–314.
 Show in context
Show in context Different NiO reagents have slightly different thermodynamic properties (e.g., O’Neill and Pownceby, 1993).
View in article
The discrepancy in the oxygen fugacity imposed by the sintered, in comparison to standard green NiO, confirms the need to sinter the material to increase its reactivity, as previously reported in literature (e.g., Mattioli and Wood, 1988; O’Neill and Pownceby, 1993).
View in article
Spandler, C., Yaxley, G., Green, D.H., Rosenthal, A. (2008) Phase relations and melting of anhydrous K-bearing eclogite from 1200 to 1600 C and 3 to 5 GPa. Journal of Petrology 49, 771–795.
 Show in context
Show in context The fO2 values of COH fluids in equilibrium with ideal graphite and glass-like C, a disordered but very homogenous X-ray amorphous graphitic carbon form often used in experimental petrology (e.g., Spandler et al., 2008), were fitted using a specific routine written in the Wolfram Mathematica® computation environment.
View in article
The CO2-in-fluid oxybarometers give the opportunity to retrieve the oxygen fugacity of the studied system in experiments focused on fluids or fluid-rock interactions (e.g. Stagno and Frost, 2010) and those involving a glass-like C melt-trap or graphite linings to isolate the capsule from the experimental charge (e.g., Spandler et al., 2008; Tiraboschi et al., 2018).
View in article
Stagno, V., Frost, D.J. (2010). Carbon speciation in the asthenosphere: Experimental measurements of the redox conditions at which carbonate-bearing melts coexist with graphite or diamond in peridotite assemblages. Earth and Planetary Science Letters 300, 72–84.
 Show in context
Show in context Recently the aptness as oxybarometers of non-solid phases, for instance melts, where their CO2 content depends on fO2 (Stagno and Frost, 2010), has been shown. Using non-solid phases has the advantage of avoiding issues related to structure behaviour (phase transitions, melting processes etc.) at different pressure and temperatures.
View in article
The CO2-in-fluid oxybarometers give the opportunity to retrieve the oxygen fugacity of the studied system in experiments focused on fluids or fluid-rock interactions (e.g. Stagno and Frost, 2010) and those involving a glass-like C melt-trap or graphite linings to isolate the capsule from the experimental charge (e.g., Spandler et al., 2008; Tiraboschi et al., 2018).
View in article
Sverjensky, D.A., Stagno, V., Huang, F. (2014) Important role for organic carbon in subduction-zone fluids in the deep carbon cycle. Nature Geoscience 7, 909–913.
 Show in context
Show in context The CO2-in-fluid oxybarometer has been calibrated for CO2-dominated aqueous fluids saturated in carbon, either ordered (graphite) or disordered (glass-like C) and can be used at P-T-pH conditions where the carbon-bearing dissolved species mostly exist in their molecular state (i.e. CO2,aq) and not as charged anionic or cationic species (Sverjensky et al., 2014; Tumiati et al., 2020).
View in article
Tao, R., Zhang, L., Stagno, V., Chu, X., Liu, X. (2017) High-Pressure experimental verification of rutile-ilmenite oxybarometer: Implications for the redox state of the subduction zone. Science China Earth Sciences 60, 1817–1825.
 Show in context
Show in context Many mineral assemblages (e.g., Ballhaus et al., 1991), oxides and metal-oxide couples (e.g., Tao et al., 2017) and binary/ternary alloys (e.g., Balta et al., 2011) have been calibrated as solid oxybarometers and redox sensors, allowing the determination of fO2 in both natural and experimental systems with an uncertainty of the order of 0.3 log units.
View in article
Tiraboschi, C., Tumiati, S., Recchia, S., Miozzi, F., Poli, S. (2016) Quantitative analysis of COH fluids synthesized at HP–HT conditions: an optimized methodology to measure volatiles in experimental capsules. Geofluids 16, 841–855.
 Show in context
Show in context This value does not take into account the uncertainties associated with the measurement of CO2 in aqueous fluids, which is of the order of 1 mol. % for quadrupole mass spectrometry analyses (Tiraboschi et al., 2016 and Tumiati et al., 2020).
View in article
After the experiments, quenched capsules were pierced in a gas tight vessel and the gases conveyed to a quadrupole mass spectrometer (QMS) for the analysis of volatiles (Tiraboschi et al., 2016).
View in article
Tiraboschi, C., Tumiati, S., Sverjensky, D., Pettke, T., Ulmer, P., Poli, S. (2018) Experimental determination of magnesia and silica solubilities in graphite-saturated and redox-buffered high-pressure COH fluids in equilibrium with forsterite+enstatite and magnesite+enstatite. Contributions to Mineralogy and Petrology 173, 2.
 Show in context
Show in context The CO2-in-fluid oxybarometers give the opportunity to retrieve the oxygen fugacity of the studied system in experiments focused on fluids or fluid-rock interactions (e.g. Stagno and Frost, 2010) and those involving a glass-like C melt-trap or graphite linings to isolate the capsule from the experimental charge (e.g., Spandler et al., 2008; Tiraboschi et al., 2018).
View in article
Tumiati, S., Malaspina, N. (2019) Redox processes and the role of carbon-bearing volatiles from the slab–mantle interface to the mantle wedge. Journal of the Geological Society 176, 388–397.
 Show in context
Show in context As carbon saturation constrains the fluid composition to the carbon-saturation surface, which is univariant in the COH system once the P-T conditions are fixed (e.g, Connolly, 1995; Tumiati and Malaspina, 2019), the independent definition of the fluid composition (e.g., XCO2 = CO2,aq/(H2O + CO2,aq)molar) can be used to retrieve other intensive variables (e.g., fO2), and vice versa.
View in article
Tumiati, S., Tiraboschi, C., Sverjensky, D.A., Pettke, T., Recchia, S., Ulmer, P., Miozzi, F., Poli, S. (2017) Silicate dissolution boosts the CO2 concentrations in subduction fluids. Nature Communications 8, 1–11.
 Show in context
Show in context On the basis of the measured CO2 content, we retrieved the log fO2 of the fluids using: (i) the thermodynamic calculations suggested by Tumiati et al., (2017) and Tumiati et al., (2020), and (ii) the CO2-in-fluid oxybarometer (Table 2).
View in article
EoS by Zhang and Duan (2009) modified to include the dynamic γH2 taken from Connolly and Cesare (1993), changing as a function of P, T and XO (γH 2= A•(XO)3 + B• (XO)2 + C•XO + D; where A = 43.919, B = 114.55, C = −105.75, D = 41.215 at 10 kbar and 800 °C; A = −11208; B = 26723, C = −21949, D = 6979.2 at 3 GPa and 800 °C) (Tumiati et al., 2017), and the equilibrium constants (Kps) for glass-like C from Tumiati et al., (2020).
View in article
Finally, the complexity of the systems should also be taken into account as complex systems might have a different CO2 content with respect to the pure COH (e.g., SiO2-bearing, Tumiati etal., 2017).
View in article
Tumiati, S., Tiraboschi, C., Miozzi, F., Vitale-Brovarone, A., Manning, C.E., Sverjensky, D.A., Milani, S., Poli, S. (2020) Dissolution susceptibility of glass-like carbon versus crystalline graphite in high-pressure aqueous fluids and implications for the behavior of organic matter in subduction zones. Geochimica et Cosmochimica Acta 273, 383–402.
 Show in context
Show in context Glass-like carbon is considered an analogue of natural “disordered” carbon deriving from organic matter and has different thermodynamic properties in respect to graphite (e.g., Tumiati et al., 2020).
View in article
The fitted fO2 values were calculated in the P-T-XCO2 range 5–30 kbar, 600–1000 °C, 0.1–0.9 using the thermodynamic model from Zhang and Duan (2009), assessed by previous authors as the more robust fit to experimental data within those available (Tumiati et al., 2020).
View in article
The model was used in its original version for graphite-saturated fluid and with modified equilibrium constants (Kps) for glass-like C-saturated fluids (cf. Tumiati et al., 2020) (see Supplementary Information S-2).
View in article
This value does not take into account the uncertainties associated with the measurement of CO2 in aqueous fluids, which is of the order of 1 mol. % for quadrupole mass spectrometry analyses (Tiraboschi et al., 2016 and Tumiati et al., 2020).
View in article
As a source of carbon, glass-like C, a NIST standard material (Cappelletti et al., 2018) was preferred to graphite in order to avoid impurities often present in natural samples and to have a material with well characterised thermodynamic properties (cf. Tumiati et al., 2020).
View in article
On the basis of the measured CO2 content, we retrieved the log fO2 of the fluids using: (i) the thermodynamic calculations suggested by Tumiati et al., (2017) and Tumiati et al., (2020), and (ii) the CO2-in-fluid oxybarometer (Table 2).
View in article
Those derived from experiments (Tumiati et al., 2020) for the thermodynamic calculations (Table 2) and those obtained by modelling the thermodynamic properties (cf. “Parameterisation of the CO2-in-fluid oxybarometer” above) for the CO2-in-fluid oxybarometer (Table 2).
View in article
EoS by Zhang and Duan (2009) modified to include the dynamic γH2 taken from Connolly and Cesare (1993), changing as a function of P, T and XO (γH 2= A•(XO)3 + B• (XO)2 + C•XO + D; where A = 43.919, B = 114.55, C = −105.75, D = 41.215 at 10 kbar and 800 °C; A = −11208; B = 26723, C = −21949, D = 6979.2 at 3 GPa and 800 °C) (Tumiati et al., 2017), and the equilibrium constants (Kps) for glass-like C from Tumiati et al., (2020).
View in article
The CO2-in-fluid oxybarometer has been calibrated for CO2-dominated aqueous fluids saturated in carbon, either ordered (graphite) or disordered (glass-like C) and can be used at P-T-pH conditions where the carbon-bearing dissolved species mostly exist in their molecular state (i.e. CO2,aq) and not as charged anionic or cationic species (Sverjensky et al., 2014; Tumiati et al., 2020).
View in article
Ziegenbein, D., Johannes, W. (1980) Graphite in CHO fluids: An unsuitable compound to buffer fluid composition at temperatures up to 700°C. Neues Jahrbuch fur Mineralogie-Monatshefte 7, 289–305.
 Show in context
Show in context For instance, previous studies showed that graphite is poorly reactive below 500–600 °C (Ziegenbein and Johannes, 1980; Luque et al., 1998).
View in article
Zhang, C., Duan, Z. (2009) A model for C–O–H fluid in the Earth’s mantle. Geochimica et Cosmochimica Acta 73, 2089–2102.
 Show in context
Show in context This means oxidised fluids, where XO (= O/H + O) > 1/3 (e.g., Connolly and Cesare, 1993; Huizenga, 2001; Zhang and Duan, 2009) where there is saturation of ordered (graphite) or disordered (glass-like C) carbon.
View in article
The fitted fO2 values were calculated in the P-T-XCO2 range 5–30 kbar, 600–1000 °C, 0.1–0.9 using the thermodynamic model from Zhang and Duan (2009), assessed by previous authors as the more robust fit to experimental data within those available (Tumiati et al., 2020).
View in article
EoS by Zhang and Duan (2009) modified to include the dynamic γH2 taken from Connolly and Cesare (1993), changing as a function of P, T and XO (γH 2= A•(XO)3 + B• (XO)2 + C•XO + D; where A = 43.919, B = 114.55, C = −105.75, D = 41.215 at 10 kbar and 800 °C; A = −11208; B = 26723, C = −21949, D = 6979.2 at 3 GPa and 800 °C) (Tumiati et al., 2017), and the equilibrium constants (Kps) for glass-like C from Tumiati et al., (2020).
View in article
top
Supplementary Information
The Supplementary Information includes:
- CO2-in-fluid Oxybarometer (Excel Spreadsheet)
- S-1 Details on the Evaluation of the Best Fit Model
- S-2 Modelling of Glass-like C Saturated COH Fluids for the Calibration of the Oxybarometer
- S-3 NiO Reagents and Starting Materials
- S-4 Thermodynamic Modelling in Double and Triple Capsule Experiments
- S-5 Parameterisation of the fO2 Dependency from P and T for the Fayalite-Quartz-Magnetite (FMQ) Equilibrium.
- Figures S-1 to S-4
- Supplementary Information References
Download the CO2-in-fluid Oxybarometer (Excel).
Download the Supplementary Information (PDF).
Figures
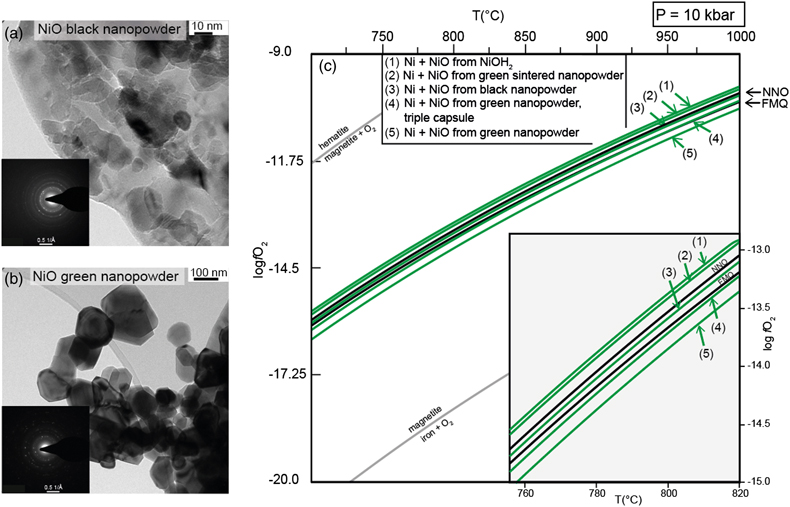
Figure 1 (a) and (b) Transmission electron microscopy images and diffraction rings (insets) of the two commercial NiO nanopowders used as reagents in the buffering assemblages. (c) Isobaric evolution of the NNO buffer oxygen fugacity for buffering assemblages with different NiO reagents (green lines). Black lines represent the evolution predicted by thermodynamic models of the NNO and FMQ buffers. The line representing FMQ (fayalite + O2 = magnetite + quartz), iron + O2 = magnetite and magnetite + O2 = hematite buffers are provided for reference.
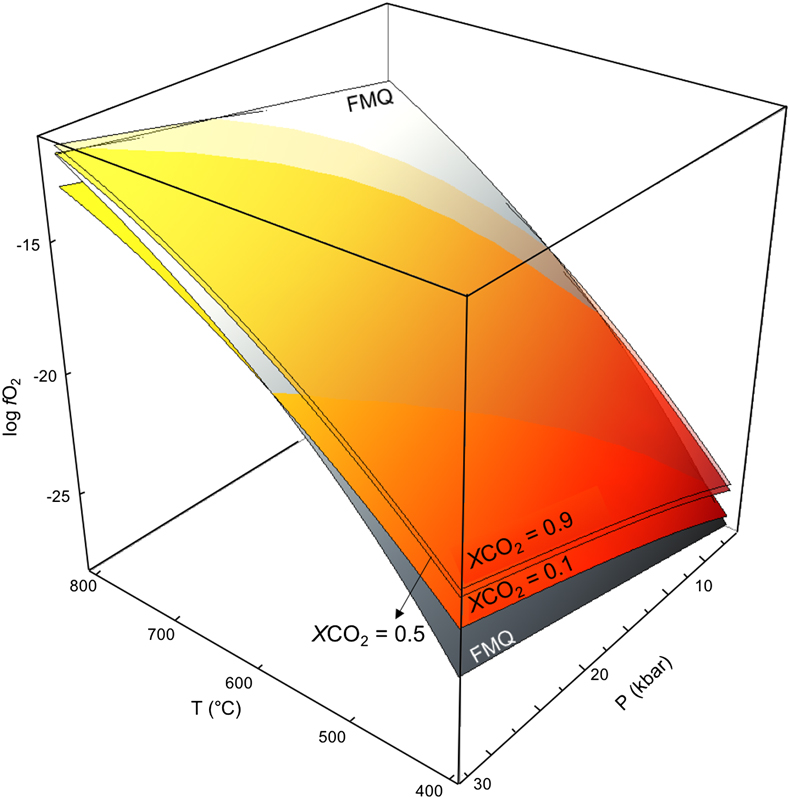
Figure 2 Graphite saturated COH fluid in the P-T-log fO2 space. Coloured surfaces represent isopleths of low (0.1), intermediate (0.5) and high (0.9) CO2 content in the COH fluid expressed as XCO2 (XCO2 = CO2/(CO2 + H2O)). The grey shaded FMQ surface represents the univariant equilibrium of the reaction: 2 Fe3O4 + 3 SiO2 = 3 Fe2SiO4 + O2.