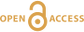Growth of upper plate lithosphere controls tempo of arc magmatism: Constraints from Al-diffusion kinetics and coupled Lu-Hf and Sm-Nd chronology
Affiliations | Corresponding Author | Cite as | Funding informationKeywords: continental arc, Sierra Nevada, Lu-Hf and Sm-Nd chronology, diffusion, garnet peridotite
- Share this article





-
Article views:14,367Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
Abstract

DeCelles, P.G., Ducea, M.N., Kapp, P., Zandt, G. (2009) Cyclicity in Cordilleran orogenic systems. Nature Geoscience 2, 251-257.
). In some cases, such as the Andes, this pattern has recurred several times (Haschke et al., 2002Haschke, M., Siebel, W., Günther, A., Scheuber, E. (2002) Repeated crustal thickening and recycling during the Andean orogeny in north Chile (21-26 S). Journal of Geophysical Research: Solid Earth (1978-2012) 107, ECV 6-1-ECV 6-18.
). Abrupt changes in plate convergence rates and direction (Pilger, 1984Pilger, R.H. (1984) Cenozoic plate kinematics, subduction and magmatism: South American Andes. Journal of the Geological Society 141, 793-802.
) or repeated steepening and shallowing of subducting slabs (Kay and Coira, 2009Kay, S.M., Coira, B.L. (2009) Shallowing and steepening subduction zones, continental lithospheric loss, magmatism, and crustal flow under the Central Andean Altiplano-Puna Plateau. Backbone of the Americas: shallow subduction, plateau uplift, and ridge and terrane collision 204, 229-259.
) have been suggested as triggering flare-ups or terminating magmatism, but such scenarios may not be sufficiently general. Here, we examine the thermal history of deep crustal and lithospheric xenoliths from the Cretaceous Sierra Nevada batholith, California (USA). The deepest samples (~90 km), garnet-bearing spinel peridotites, show cooling-related exsolution of garnet from high-Al pyroxenes originally formed at >1275 °C. Modelling of pyroxene Al diffusion profiles requires rapid cooling from 1275 to 750 °C within ~10 Myr. Also suggesting deep-seated, rapid cooling is a garnet websterite from ~90 km depth with nearly identical Lu-Hf (92.6 ± 1.6 Ma) and Sm-Nd (88.8 ± 3.1 Ma) isochron ages to within error. Thermal modelling shows that this cooling history can be explained by impingement of the base of the Sierran lithosphere against a cold subducting slab at ~90 km depth, precluding cooling by shallowing subduction. Rather, the coincidence of the radiometric ages with the magmatic flare-up (120-80 Ma) suggests that the hot mantle wedge above the subducting slab may have been pinched out by magmatic (± tectonic) thickening of the upper plate, eventually terminating mantle melting. Magmatic flare-ups in continental arcs are thus self-limiting, which explains why continental arc magmatism occurs in narrow time intervals. Convective removal of the deep arc lithosphere can initiate another magmatic cycle.Figures and Tables
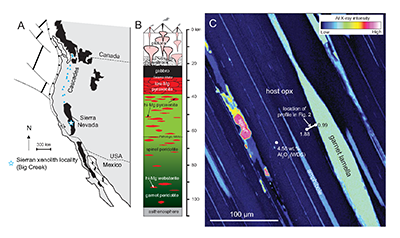 Figure 1 (A) Sample locality map. (B) Vertical architecture of the lithosphere beneath the central Sierra Nevada as sampled by xenoliths (see text for references). (C) Wavelength-dispersive (WDS) elemental map of Al intensity in an orthopyroxene containing garnet and amphibole lamellae in garnet peridotite 1026V from 3 GPa (~90 km). Spots labelled as wt.% Al2O3 correspond to individual, quantitative WDS spot measurements. | 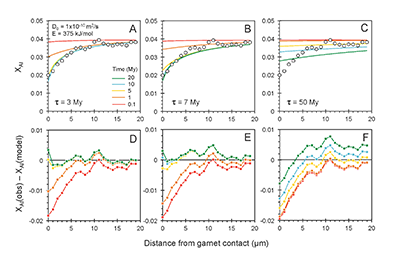 Figure 2 Orthopyroxene Al diffusion modelling of the Al halo transect shown in Figure 1C. Quantitative WDS spot analyses along the transect are plotted as white circles. (A-C) show diffusion profiles at selected times (0.1, 1, 5, 10, 20 Ma) using an activation energy E of 375 kJ/mol and D0 = 10-10 m2/s. Three different cooling scenarios are shown, with e-fold timescales of cooling τ of 3, 7, and 50 Myr. (D) through (F) shows the residual (XAl(observed) – XAl(modelled)) as a function of distance for each of the models, where XAl = atomic Al/2, and Al is the number of cations in the formula for orthopyroxene on a 6 oxygen basis. |  Figure 3 (A) Lu-Hf and (B) Sm-Nd isochrons of Sierran garnet pyroxenite xenoliths. Isochrons were calculated using a MatLab least-squares software by F. Albarède (version 6.0, 2013). | 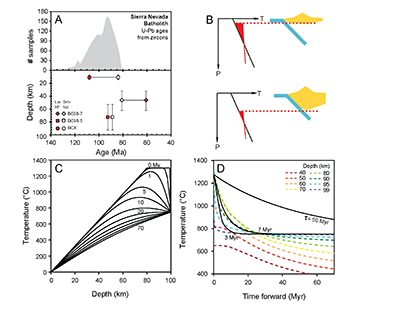 Figure 4 (A) Top panel: U-Pb zircon ages from the Sierra Nevada Batholith (Paterson et al., 2014) showing a major peak at ~95 Ma. Bottom panel: Thermobarometrically constrained depth versus radiometric age of Sierran pyroxenite xenoliths. The vertical bars represent the range of final P, T recorded by several mineral rim pairs in each xenolith (except for BCX, where average REE concentrations were used); the symbols represent the average P, T. (B) Cartoon illustrating the formation of shallow low-MgO pyroxenite (top) and deep high-MgO websterite (bottom). (C) Temperature versus depth diagram showing the initial condition of conductive cooling modelling of a 100-km thick lithosphere at 0 Myr (initial condition) and re-equilibration to a new ambient geotherm after 70 Myr. Thermal diffusivity is 10-6 m2/s. (D) Temperature versus time diagram of depth slices ranging from 40 to 99 km. Superimposed on the diagram are the three cooling scenarios evaluated in the Al-diffusion modelling. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Supplementary Figures and Tables
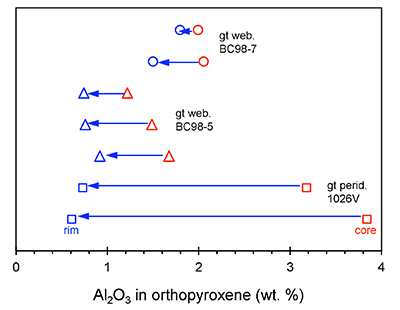 Figure S-1 Al2O3 (wt. %) in orthopyroxene cores and rims in garnet websterites and garnet peridotite. Cores have higher Al contents. |  Figure S-2 Plane-polarised light photograph of thick section (~200 μm) of high-MgO websterite BC98-5. |  Figure S-3 Plane-polarised light photograph of thick section (~200 μm) of high-MgO websterite BC98-7. (IND51), Nagrasu (IND54), Rudraprayag (IND57-59) and Srinagar (IND62, 64, 65, 72 and 73). Flow is from top to bottom of the image. Vertical exaggeration is 3x. |  Figure S-4 Plane-polarised light photograph of thick section (~200 μm) of low-MgO garnet clinopyroxenite BCX. |  Figure S-5 Representative “clean” clinopyroxene and garnet separates from BC98-5. |
| Figure S-1 | Figure S-2 | Figure S-3 | Figure S-4 | Figure S-5 |
 Figure S-6 The thermobarometer of Harley and Green (1982) parameterised as a function of XAl on M1 site of opx versus temperature at constant pressure (top) and XAl on M1 site of opx versus pressure at constant temperature (bottom). | 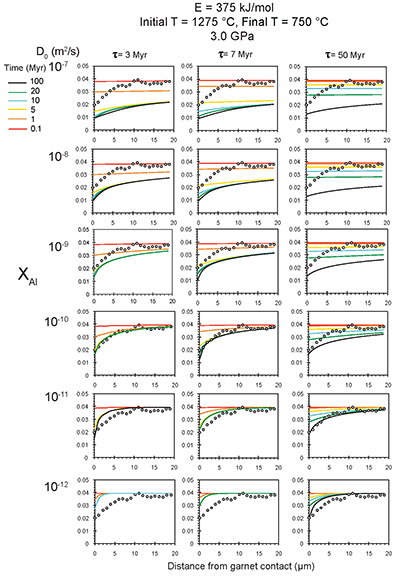 Figure S-7 Modelled Al-diffusion profiles at various times at different values of D0 and constant E (375 kJ/mol), using starting T = 1275 °C, final T = 750 °C. Each column shows models performed at a different cooling scenario. | 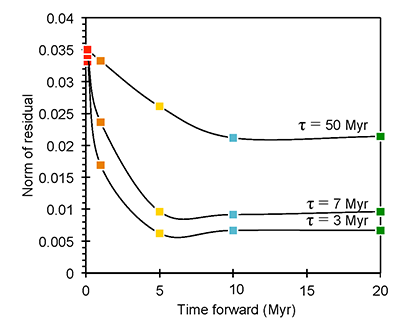 Figure S-8 Norm of residual of Al-diffusion models (E = 375 kJ/mol, D0 = 10-10 m2/s) at 0.1, 1, 5, 10, and 20 Ma and at different cooling scenarios. | 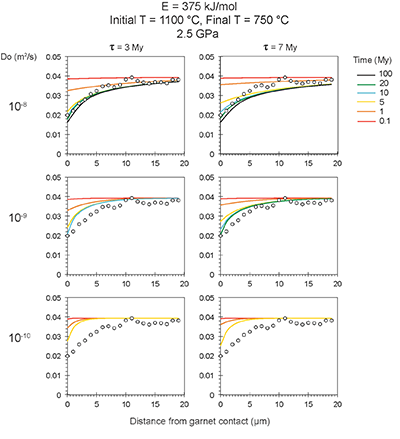 Figure S-9 Modelled Al-diffusion profiles at various times at different values of D0 and constant E (375 kJ/mol), using starting T = 1100 °C, final T = 750 °C. |
| Figure S-6 | Figure S-7 | Figure S-8 | Figure S-9 |
 Table S-1 Major element compositions (wt. %) of orthopyroxene profile in sample 1026V in Figure 1C. |  Table S-2 Orthopyroxene major element compositions (wt. %) in Sierran garnet pyroxenites. |  Table S-3 Clinopyroxene major element compositions (wt. %) in garnet pyroxenites. |  Table S-4 Garnet major element compositions (wt. %) in Sierran garnet pyroxenites. |  Table S-5 Sm-Nd and Lu-Hf concentrations and isotope compositions of Sierran pyroxenites. |
| Table S-1 | Table S-2 | Table S-3 | Table S-4 | Table S-5 |
top
Introduction
Constraining the thermal and temporal evolution of the deep arc lithosphere is critical to understanding the magmatic evolution of a volcanic arc. The Sierra Nevada batholith is one of only a few places world-wide to study deep crustal and lithospheric evolution of an arc because of the availability of lower crustal and mantle xenoliths (Dodge et al., 1988
Dodge, F.C.W., Lockwood, J.P., Calk, L.C. (1988) Fragments of the mantle and crust from beneath the Sierra Nevada batholith: Xenoliths in a volcanic pipe near Big Creek, California. Geological Society of America Bulletin 100, 938-947.
; Mukhopadhyay and Manton, 1994Mukhopadhyay, B., Manton, W.I. (1994) Upper-Mantle Fragments from Beneath the Sierra Nevada Batholith: Partial Fusion, Fractional Crystallization, and Metasomatism in a Subduction Related Ancient Lithosphere. Journal of Petrology 35, 1417-1450.
; Ducea and Saleeby, 1996Ducea, M.N., Saleeby, J.B. (1996) Buoyancy sources for a large, unrooted mountain range, the Sierra Nevada, California: Evidence from xenolith thermobarometry. Journal of Geophysical Research 101, 8229-8244.
; Lee et al., 2006Lee, C.-T.A., Cheng, X., Horodyskyj, U. (2006) The development and refinement of continental arcs by primary basaltic magmatism, garnet pyroxenite accumulation, basaltic recharge and delamination: insights from the Sierra Nevada. Contributions to Mineralogy and Petrology 151, 222-242.
, 2007Lee, C.-T.A., Morton, D.M., Kistler, R.W., Baird, A.K. (2007) Petrology and tectonics of Phanerozoic continent formation: From island arcs to accretion and continental arc magmatism. Earth and Planetary Science Letters 263, 370-387.
; Chin et al., 2012Chin, E.J., Lee, C.-T.A., Luffi, P., Tice, M. (2012) Deep Lithospheric Thickening and Refertilization beneath Continental Arcs: Case Study of the P, T and Compositional Evolution of Peridotite Xenoliths from the Sierra Nevada, California. Journal of Petrology 53, 477-511.
), which are rare elsewhere. Magmatism in the Sierra Nevada occurred primarily between ~120 and ~80 Ma, peaking at ~95 Ma and terminating abruptly at ~80 Ma (Barton, 1996Barton, M.D. (1996) Granitic magmatism and metallogeny of southwestern North America. In: Brown, M., Candela, P.A., Peck, D.L., Stephens, R.J.W., Zen, E.-A. (Eds.) The Third Hutton Symposium on the Origin of Granites and Related Rocks. GSA Special Papers, 261-280.
; Coleman and Glazner, 1997Coleman, D.S., Glazner, A.F. (1997) The Sierra Crest Magmatic Event: Rapid Formation of Juvenile Crust during the Late Cretaceous in California. International Geology Review 39, 768-787.
; Ducea, 2001Ducea, M.N. (2001) The California Arc: Thick Granitic Batholiths, Eclogitic Residues, Lithospheric-Scale Thrusting, and Magmatic Flare-Ups. GSA Today 11, 4-10.
; Paterson et al., 2014Paterson, S.R., Memeti, V., Anderson, L., Cao, W., Lackey, J.S., Putirka, K.D., Miller, R.B., Miller, J.S., Mundil, R. (2014) Day 6: Overview of arc processes and tempos. Field Guides 34, 87-116.
). We examined xenoliths from a late Miocene basaltic dike (Big Creek; N 37.208946° W119.264784°) (Fig. 1A). These xenoliths comprise garnet pyroxenites and peridotites and sample a lithospheric section ranging from the mid to lower crust (~33 to ~45 km) into the mantle (~90 km) (Fig. 1B). Sm-Nd ages between 120 and 84 Ma for several pyroxenite xenoliths (Ducea and Saleeby, 1998Ducea, M.N., Saleeby, J.B. (1998) The age and origin of a thick mafic–ultramafic keel from beneath the Sierra Nevada batholith. Contributions to Mineralogy and Petrology 133, 169-185.
), along with geochemical data (Lee et al., 2006Lee, C.-T.A., Cheng, X., Horodyskyj, U. (2006) The development and refinement of continental arcs by primary basaltic magmatism, garnet pyroxenite accumulation, basaltic recharge and delamination: insights from the Sierra Nevada. Contributions to Mineralogy and Petrology 151, 222-242.
, 2007Lee, C.-T.A., Morton, D.M., Kistler, R.W., Baird, A.K. (2007) Petrology and tectonics of Phanerozoic continent formation: From island arcs to accretion and continental arc magmatism. Earth and Planetary Science Letters 263, 370-387.
), indicate that they represent cumulates left behind by the Cretaceous Sierran granitoids.Garnet pyroxenite cumulates are volumetrically dominant and can be divided into a low-MgO group composed of garnet-rich clinopyroxenites and a high-MgO group composed of garnet websterites (Lee et al., 2006
Lee, C.-T.A., Cheng, X., Horodyskyj, U. (2006) The development and refinement of continental arcs by primary basaltic magmatism, garnet pyroxenite accumulation, basaltic recharge and delamination: insights from the Sierra Nevada. Contributions to Mineralogy and Petrology 151, 222-242.
). The former are interpreted as mid to lower crustal cumulates of an evolved hydrous basalt, whereas the latter are lower crustal and upper mantle cumulates of primitive hydrous basalts, overlapping in final equilibration pressures and therefore interpreted as being interleaved with the spinel and garnet peridotites (Saleeby et al., 2003Saleeby, J., Ducea, M., Clemens-Knott, D. (2003) Production and loss of high-density batholithic root, southern Sierra Nevada, California. Tectonics 22, 1064.
) (Fig. 1B). Garnet-clinopyroxene rare-earth element (REE) thermobarometry of the low-MgO pyroxenite yields equilibration conditions of ~0.33 GPa and 801 °C (Sun and Liang, 2015Sun, C., Liang, Y. (2015) A REE-in-garnet-clinopyroxene thermobarometer for eclogites, granulites and garnet peridotites. Chemical Geology 393-394, 79-92.
) consistent with a mid-crustal origin. In contrast, major element thermobarometry of the high-MgO websterites record equilibration pressures ranging from 1.5 to 3 GPa and temperatures between 700 and 830 °C (Lee et al., 2006Lee, C.-T.A., Cheng, X., Horodyskyj, U. (2006) The development and refinement of continental arcs by primary basaltic magmatism, garnet pyroxenite accumulation, basaltic recharge and delamination: insights from the Sierra Nevada. Contributions to Mineralogy and Petrology 151, 222-242.
). These final P-T conditions are similar to those obtained for the garnet peridotites (2.3 – 3.6 GPa, 651 – 845 °C; Chin et al., 2012Chin, E.J., Lee, C.-T.A., Luffi, P., Tice, M. (2012) Deep Lithospheric Thickening and Refertilization beneath Continental Arcs: Case Study of the P, T and Compositional Evolution of Peridotite Xenoliths from the Sierra Nevada, California. Journal of Petrology 53, 477-511.
).Although both garnet peridotites and high-MgO websterites achieved similar high final pressures and low final temperatures, high-Al orthopyroxene cores in both rock types point to initially high temperatures. In high-MgO websterites, orthopyroxene cores record temperatures of ~1100 °C, supporting an origin as magmatic cumulates precipitated from primary basaltic liquids (Lee et al., 2006
Lee, C.-T.A., Cheng, X., Horodyskyj, U. (2006) The development and refinement of continental arcs by primary basaltic magmatism, garnet pyroxenite accumulation, basaltic recharge and delamination: insights from the Sierra Nevada. Contributions to Mineralogy and Petrology 151, 222-242.
; Supplementary Information). Mineral and bulk rock compositions of the garnet peridotites indicate that their protoliths were spinel peridotites that were melt-depleted at shallow (1 GPa) depths. These spinel peridotites were subsequently refertilised and transported to cold and deep final conditions in the garnet stability field (Chin et al., 2012Chin, E.J., Lee, C.-T.A., Luffi, P., Tice, M. (2012) Deep Lithospheric Thickening and Refertilization beneath Continental Arcs: Case Study of the P, T and Compositional Evolution of Peridotite Xenoliths from the Sierra Nevada, California. Journal of Petrology 53, 477-511.
, 2014Chin, E.J., Lee, C.-T.A., Barnes, J.D. (2014) Thickening, refertilization, and the deep lithosphere filter in continental arcs: Constraints from major and trace elements and oxygen isotopes. Earth and Planetary Science Letters 397, 184-200.
). Temperatures corresponding to melting, and thus initial temperatures, can be obtained using Fe-Mg partitioning between peridotite and melt (Lee and Chin, 2014Lee, C.-T.A., Chin, E.J. (2014) Calculating melting temperatures and pressures of peridotite protoliths: Implications for the origin of cratonic mantle. Earth and Planetary Science Letters 403, 273-286.
). Melting temperatures for the garnet peridotites fall between 1300 and 1400 °C (Chin et al., 2012Chin, E.J., Lee, C.-T.A., Luffi, P., Tice, M. (2012) Deep Lithospheric Thickening and Refertilization beneath Continental Arcs: Case Study of the P, T and Compositional Evolution of Peridotite Xenoliths from the Sierra Nevada, California. Journal of Petrology 53, 477-511.
). These initial temperatures are consistent with the high Al in orthopyroxene away from contact with exsolved garnet lamellae (Fig. 1C). For example, in the profile shown in Figure 1C, the distal Al2O3 of 1.88 wt. % would correspond to 1275 °C at 3 GPa using the garnet-orthopyroxene thermobarometer of Harley and Green (1982)Harley, S.L., Green, D.H. (1982) Garnet-orthopyroxene barometry for granulites and peridotites. Nature 300, 697-701.
.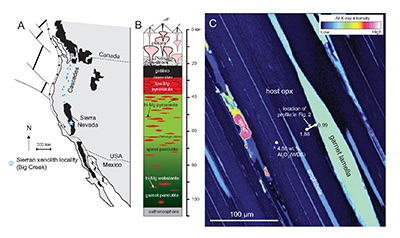
Figure 1 (A) Sample locality map. (B) Vertical architecture of the lithosphere beneath the central Sierra Nevada as sampled by xenoliths (see text for references). (C) Wavelength-dispersive (WDS) elemental map of Al intensity in an orthopyroxene containing garnet and amphibole lamellae in garnet peridotite 1026V from 3 GPa (~90 km). Spots labelled as wt.% Al2O3 correspond to individual, quantitative WDS spot measurements.
Sun, C., Liang, Y. (2015) A REE-in-garnet-clinopyroxene thermobarometer for eclogites, granulites and garnet peridotites. Chemical Geology 393-394, 79-92.
, a high-MgO websterite (BC98-5, average final T of 702 °C and average final P of 2.4 GPa), and a high-MgO amphibole-bearing websterite (BC98-7, average final T of 710 °C and average final P of 1.6 GPa). Sample BC98-7 has an amphibole vein replacing pyroxene and hence was selected deliberately to provide insight into the timing of hydrous metasomatism.top
Methods
High spatial resolution elemental data on sample 1026V were determined by field emission electron microprobe analyser (EPMA) using wavelength-dispersive spectroscopy (WDS) with operating conditions at 15 kV accelerating voltage and 5 nA probe current using the JEOL JXA-8530 F ‘Hyperprobe’ at Yale University. Core and rim mineral compositions in garnet pyroxenites were determined by WDS (15 kV, 20 nA) on the CAMECA SX-50 at Texas A&M University. Typical spot sizes on both electron probes were 1 to 1.5 μm.
Thermobarometry was based on mineral major and trace element compositions published in Lee et al. (2006)
Lee, C.-T.A., Cheng, X., Horodyskyj, U. (2006) The development and refinement of continental arcs by primary basaltic magmatism, garnet pyroxenite accumulation, basaltic recharge and delamination: insights from the Sierra Nevada. Contributions to Mineralogy and Petrology 151, 222-242.
and Chin et al. (2012Chin, E.J., Lee, C.-T.A., Luffi, P., Tice, M. (2012) Deep Lithospheric Thickening and Refertilization beneath Continental Arcs: Case Study of the P, T and Compositional Evolution of Peridotite Xenoliths from the Sierra Nevada, California. Journal of Petrology 53, 477-511.
) and the data reported in this study.For Sm-Nd and Lu-Hf isotopic analysis, clean garnet and clinopyroxene were separated by hand. Between 60 and 680 mg of garnet, clinopyroxene, and whole-rock powders were acid-digested in Parr bombs. Sm, Nd, Lu and Hf were separated by ion-exchange column chromatography and their isotopic compositions measured by multiple-collector inductively coupled plasma mass spectrometry following the procedures outlined in Blichert-Toft et al. (1997
Blichert-Toft, J., Chauvel, C., Albarède, F. (1997) Separation of Hf and Lu for high-precision isotope analysis of rock samples by magnetic sector-multiple collector ICP-MS. Contributions to Mineralogy and Petrology 127, 248-260.
, 2002Blichert-Toft, J., Boyet, M., Télouk, P., Albarède, F. (2002) 147Sm/143Nd and 176Lu/176 Hf in eucrites and the differentiation of the HED parent body. Earth and Planetary Science Letters 204, 167-181.
), Blichert-Toft (2001)Blichert-Toft, J. (2001) On the Lu-Hf Isotope Geochemistry of Silicate Rocks. Geostandards Newsletter 25, 41-56.
, and Blichert-Toft and Puchtel (2010)Blichert-Toft, J., Puchtel, I.S. (2010) Depleted mantle sources through time: Evidence from Lu-Hf and Sm-Nd isotope systematics of Archean komatiites. Earth and Planetary Science Letters 297, 598-606.
. Sm, Nd, Lu and Hf concentrations were determined by isotope dilution using >98 % pure mixed 149Sm-150Nd and 176Lu-180Hf spikes added to the samples at the outset of dissolution. Data and further details of the analytical procedures are in the Supplementary Information.Al diffusion modelling was done by finite-difference, allowing for temperature-dependent diffusivity and boundary conditions. Magnitude of time-steps were adapted with temperature and diffusivity to maintain numerical closure. Thermal modelling was based on conventional finite-difference models of conduction. Additional details on modelling set up and parameters are provided in the Supplementary Information.
top
Results
Al-depletion haloes in orthopyroxene. Field emission electron microprobe measurements show that BC98-7, BC98-5, and 1026V have orthopyroxenes zoned from high-Al cores to low-Al rims (Supplementary Information). In particular, Al in orthopyroxene porphyroclasts becomes depleted towards the contacts with garnet exsolution lamellae. Al-depletion halo thicknesses in orthopyroxene adjacent to the garnet lamellae range from 5-20 μm and are positively correlated with garnet lamellae thickness, confirming that garnet formed by exsolution from an original high-Al, high-temperature pyroxene (Chin et al., 2012
Chin, E.J., Lee, C.-T.A., Luffi, P., Tice, M. (2012) Deep Lithospheric Thickening and Refertilization beneath Continental Arcs: Case Study of the P, T and Compositional Evolution of Peridotite Xenoliths from the Sierra Nevada, California. Journal of Petrology 53, 477-511.
). The Al concentration profiles exhibit strong curvature due to 50 % depletion within the first ~5 μm from the contact followed by only moderate (15 %) depletion 5-20 μm from the contact (Fig. 2).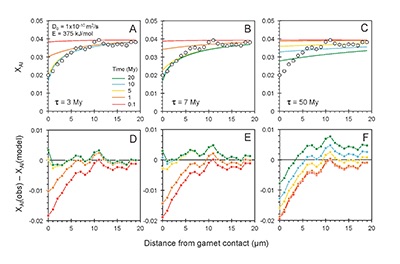
Figure 2 Orthopyroxene Al diffusion modelling of the Al halo transect shown in Figure 1C. Quantitative WDS spot analyses along the transect are plotted as white circles. (A-C) show diffusion profiles at selected times (0.1, 1, 5, 10, 20 Ma) using an activation energy E of 375 kJ/mol and D0 = 10-10 m2/s. Three different cooling scenarios are shown, with e-fold timescales of cooling τ of 3, 7, and 50 Myr. (D) through (F) shows the residual (XAl(observed) – XAl(modelled)) as a function of distance for each of the models, where XAl = atomic Al/2, and Al is the number of cations in the formula for orthopyroxene on a 6 oxygen basis.
Harley, S.L., Green, D.H. (1982) Garnet-orthopyroxene barometry for granulites and peridotites. Nature 300, 697-701.
at 3 GPa.No experimental data are available for Al diffusion in orthopyroxene, but experiments on REE3+ diffusion in orthopyroxene may provide a suitable analogue for estimating the parameters of diffusivity D, that is, D = D0exp(-E/RT), where D0 is the pre-exponent (m2/s), E is the activation energy (kJ/mol), R is the gas constant, and T is temperature (K). Cherniak and Liang (2007)
Cherniak, D.J., Liang, Y. (2007) Rare earth element diffusion in natural enstatite. Geochimica et Cosmochimica Acta 71, 1324-1340.
obtained a D0 of 1.2 × 10-7 m2/s and E of 369 kJ/mol after averaging all experimental data for REE and Y diffusion in orthopyroxene. They showed that E does not vary with cation radius, so E for Al diffusivity is assumed to be similar. We therefore adopted a nominal value of 375 kJ/mol for E though other values are explored in the Supplementary Information. D0, however, is expected to vary with ionic radius based on experiments on clinopyroxene (Van Orman et al., 2001Van Orman, J., Grove, T., Shimizu, N. (2001) Rare earth element diffusion in diopside: influence of temperature, pressure, and ionic radius, and an elastic model for diffusion in silicates. Contributions to Mineralogy and Petrology 141, 687-703.
), but the relationship is not clear for experiments on orthopyroxene. We thus varied D0 from 10-7 to 10-12 m2/s over the three cooling scenarios and evaluated which combination of parameters best match the magnitude and curvature of the Al-depletion halo. For a given cooling scenario, as D0 increases, the modelled profiles progressively flatten because the higher the D0, the faster the Al diffusion front can propagate into the pyroxene. For a given D0, decreasing the cooling rate decreases curvature because longer cooling times allow more time for diffusion.In Figure 2A-C, we show model results of Al profiles, including residuals (Fig. 2D-F), versus elapsed time. The parameters used for the model results in Figure 2 were D0 of 10-10 m2/s, E of 375 kJ/mol, τ of 3, 7, and 50 Myr, starting temperature of 1275 °C, and final temperature of 750 °C (results of models using starting temperature of 1100 °C are shown in the Supplementary Information). The best fit models require closure of the system for Al within 5 Myr for τ of 3 Myr and within 10 Myr for τ of 7 Myr. Model results for other D0S give poor fits or require unreasonable cooling rates to achieve a good fit. For example, the only model using D0 of 10-11 m2/s consistent with the observed Al profile requires τ of 50 Myr and closure at 100 Myr, while it is clear that a cooling duration of 100 Myr for the Sierran deep lithosphere is unacceptably long given the well-constrained magmatic peak at ~95 Ma (Paterson et al., 2014
Paterson, S.R., Memeti, V., Anderson, L., Cao, W., Lackey, J.S., Putirka, K.D., Miller, R.B., Miller, J.S., Mundil, R. (2014) Day 6: Overview of arc processes and tempos. Field Guides 34, 87-116.
) and closure of garnet websterite Sm-Nd ages between 81 and 89 Ma (discussed below). The most robust models are for D0 of 10-10 m2/s and τ of 3-7 Myr and indicate that the base of the Sierran lithosphere (90 km) cooled from 1275 to 750 °C within 10 Myr. We note that if we had assumed a lower initial temperature (1100 °C) corresponding to the maximum Al in orthopyroxene cores in the garnet websterites, we obtain a similar result consistent with cooling within 10 Myr at fast cooling rates (Supplementary Information).Coupled Lu-Hf and Sm-Nd chronology. Additional constraints on the cooling history of the Sierran lithosphere come from coupled Lu-Hf and Sm-Nd chronology. Although the closure temperatures of the Lu-Hf and Sm-Nd systems depend on many factors, such as cooling rate, grain size, and diffusion parameters, which are not always well constrained in texturally complex systems, it is known that the Lu-Hf system closes at higher temperatures than the Sm-Nd system (Kylander-Clark et al., 2007
Kylander-Clark, A.R., Hacker, B.R., Johnson, C.M., Beard, B.L., Mahlen, N.J., Lapen, T.J. (2007) Coupled Lu-Hf and Sm-Nd geochronology constrains prograde and exhumation histories of high-and ultrahigh-pressure eclogites from western Norway. Chemical Geology 242, 137-154.
; Cheng et al., 2008Cheng, H., King, R.L., Nakamura, E., Vervoort, J.D., Zhou, Z. (2008) Coupled Lu–Hf and Sm–Nd geochronology constrains garnet growth in ultra-high-pressure eclogites from the Dabie orogen. Journal of Metamorphic Geology 26, 741-758.
). The slopes of lines regressed through garnet, clinopyroxene, and whole-rock data on 176Hf/177Hf versus 176Lu/177Hf and 143Nd/144Nd versus 147Sm/143Nd diagrams yield ages broadly coeval with magmatism (120-85 Ma) in the Sierra Nevada (Fig. 3). For the deep high-MgO websterite (BC98-5), Lu-Hf and Sm-Nd ages are identical to within analytical uncertainties (92.6 ± 1.6 Ma and 88.8 ± 3.1 Ma, respectively). For the shallow low-MgO pyroxenite (BCX), the Lu-Hf age is considerably older (107.9 ± 1.4 Ma) than the Sm-Nd age (84.0 ± 4.1 Ma), the former coinciding with the main peak of arc magmatism as inferred from the ages of plutons and the latter coinciding with the tail end of the magmatic flare-up. For the amphibole-bearing sample BC98-7, the Lu-Hf age is younger (60.8 ± 3.2 Ma) than the Sm-Nd age (81.0 ± 2.0 Ma), the latter of which is consistent within the quoted uncertainties with the Sm-Nd ages of the other samples.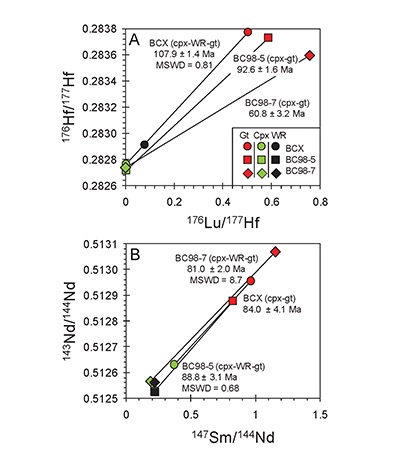
Figure 3 (A) Lu-Hf and (B) Sm-Nd isochrons of Sierran garnet pyroxenite xenoliths. Isochrons were calculated using a MatLab least-squares software by F. Albarède (version 6.0, 2013).
Discussion
The rapid cooling rates of the deep Sierran lithosphere inferred from Al-diffusion modelling are consistent with the near-identical Lu-Hf and Sm-Nd isochron ages of the deep garnet websterite BC98-5. The Lu-Hf and Sm-Nd ages of BC98-5 constrain cooling between the two closure temperatures to be within 5 Myr. Thus, diffusion modelling and chronology suggest that the base of the Sierran lithosphere remained hot until ~92 Ma (the Lu-Hf age), after which it cooled rapidly. In contrast, the coincidence of shallow pyroxenite BCX’s Lu-Hf age with pluton ages suggests that the 108 Ma Lu-Hf age simply may represent a crystallisation age during passage of magmas in the cold shallow parts of the lithosphere (<45 km) (Fig. 4A, B). BCX’s young Sm-Nd age, however, is identical to ages from the base of the lithosphere as represented by BC98-5, indicating that, although BCX crystallised at 108 Ma, it cooled slowly after crystallisation, passing through the Sm-Nd closure temperature at roughly the same time as the deep lithosphere as recorded by BC98-5. This suggests that the entire lithosphere, as represented by the shallow low-MgO pyroxenite and the deep high-MgO websterite and peridotites, experienced cooling at around 92 Ma, just after the peak of arc magmatism. The Lu-Hf and Sm-Nd systematics of amphibole-bearing BC98-7 from the base of the Sierran lithosphere is more perplexing because, although its Sm-Nd age of 81 Ma is within error of Sierran magmatism, its Lu-Hf age is much younger (60.8 Ma). However, we note that the chemical dissimilarity between rare earth element Lu and high field strength element Hf allows for more extreme parent/daughter fractionation in fluid-mediated processes, whereas Sm and Nd, two neighbouring light rare earth elements, are more difficult to fractionate owing to their similar chemical behaviour under all conditions. Thus, the young Lu-Hf age of BC98-7 may reflect resetting by fluid metasomatism of the deep lithosphere continuing well after arc magmatism ended.
One scenario that may explain the rapid cooling inferred at the base of the Sierran lithosphere is if the lithosphere impinged against a cold subducting plate. To satisfy the age constraints, such impingement would have had to occur at ~92 Ma and the depth at which the impingement occurred must have been greater than the deepest xenolith equilibration pressures of ~3 GPa (~100 km). To simulate this scenario, we modelled thermal diffusion in a 100-km thick lithosphere that is instantaneously juxtaposed against a cold slab at 100 km depth. We assume the deepest part of the Sierran lithosphere, represented by interleaved high-MgO garnet websterite and garnet peridotite, to be initially 1300 °C, decreasing with decreasing depth to a surface temperature of 25 °C. We take the temperature of the top of a slab to be 750 °C, based on Syracuse et al. (2010)
Syracuse, E.M., van Keken, P.E., Abers, G.A. (2010) The global range of subduction zone thermal models. Physics of the Earth and Planetary Interiors 183, 73-90.
. Cooling histories are shown in Figures 4C and 4D, where it can be seen that the deepest part of the lithosphere cools the fastest. In Figure 4D, the cooling histories determined from Al-diffusion kinetics, using 1275 °C as the initial temperature and 750 °C as the final temperature (Fig. 2), are also compared with the cooling histories from the conductive cooling model. Thus, Al-diffusion kinetics, coupled Sm-Nd and Lu-Hf chronology, and thermal modelling all independently lead to the same conclusion that the base of the Sierran lithosphere cooled from >1250 °C to 750 °C in <10 Myr, just after the peak of arc magmatism.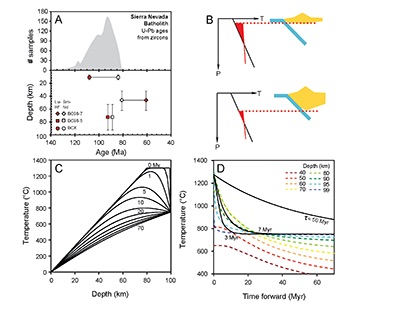
Figure 4 (A) Top panel: U-Pb zircon ages from the Sierra Nevada Batholith (Paterson et al., 2014
Paterson, S.R., Memeti, V., Anderson, L., Cao, W., Lackey, J.S., Putirka, K.D., Miller, R.B., Miller, J.S., Mundil, R. (2014) Day 6: Overview of arc processes and tempos. Field Guides 34, 87-116.
) showing a major peak at ~95 Ma. Bottom panel: Thermobarometrically constrained depth versus radiometric age of Sierran pyroxenite xenoliths. The vertical bars represent the range of final P, T recorded by several mineral rim pairs in each xenolith (except for BCX, where average REE concentrations were used); the symbols represent the average P, T. (B) Cartoon illustrating the formation of shallow low-MgO pyroxenite (top) and deep high-MgO websterite (bottom). (C) Temperature versus depth diagram showing the initial condition of conductive cooling modelling of a 100-km thick lithosphere at 0 Myr (initial condition) and re-equilibration to a new ambient geotherm after 70 Myr. Thermal diffusivity is 10-6 m2/s. (D) Temperature versus time diagram of depth slices ranging from 40 to 99 km. Superimposed on the diagram are the three cooling scenarios evaluated in the Al-diffusion modelling.Dickinson, W.R., Snyder, W.S. (1978) Plate tectonics of the Laramide orogeny. In: Matthews, V. (Ed.) Laramide folding associated with basement block faulting in the Western United States. Geological Society of America Memoir 151, 355-366.
; Dumitru et al., 1991Dumitru, T.A., Gans, P.B., Foster, D.A., Miller, E.L. (1991) Refrigeration of the western Cordilleran lithosphere during Laramide shallow-angle subduction. Geology 19, 1145-1148.
). However, the presence of cold xenoliths at ~100 km depth requires, instead, that the Sierran arc lithosphere thickened downward and impinged upon a normally dipping slab, ~100 km beneath the arc front. The coincidence of thickening with the peak of arc magmatism suggests that crustal thickening must have, in part, been driven by magmatic additions to the crust (Flowers et al., 2005Flowers, R.M., Bowring, S.A., Tulloch, A.J., Klepeis, K.A. (2005) Tempo of burial and exhumation within the deep roots of a magmatic arc, Fiordland, New Zealand. Geology 33, 17-20.
; Jagoutz, 2010Jagoutz, O. (2010) Construction of the granitoid crust of an island arc. Part II: a quantitative petrogenetic model. Contributions to Mineralogy and Petrology 160, 359-381.
). We therefore propose that magmatic thickening may be a key mechanism that modulates the tempo of arc magmas, specifically the abrupt cessation of magmatism observed in many continental arcs. With progressive magmatic additions to the crust, the upper plate thickens, eventually impinging against the cold subducting lower plate and terminating magmatism. In other words, magmatic flare-ups, whatever their origin, cannot be sustained for long periods of time if magmatism leads to thickening. The ability for magmatism to thicken the upper plate depends on the stress state of the upper plate (Karlstrom et al., 2014Karlstrom, L., Lee, C.-T.A., Manga, M. (2014) The role of magmatically driven lithospheric thickening on arc front migration. Geochemistry, Geophysics, Geosystems 15, 2655-2675.
). In many island arcs, the upper plate is in extension, which could compensate for magmatic thickening. In contrast, the upper plate in continental arcs tends to be in compression, adding to the effects of magmatic thickening (DeCelles, 2004DeCelles, P.G. (2004) Late Jurassic to Eocene evolution of the Cordilleran thrust belt and foreland basin system, western U.S.A. American Journal of Science 304, 105-168.
; Paterson et al., 2011Paterson, S.R., Okaya, D., Memeti, V., Economos, R., Miller, R.B. (2011) Magma addition and flux calculations of incrementally constructed magma chambers in continental margin arcs: Combined field, geochronologic, and thermal modeling studies. Geosphere 7, 1439-1468.
). Because of these intimate relationships between magmatism and crustal thickness, we predict that continental arc magmatism should rise and fall over tens of millions of years, while magmatism in island arcs should be long-lived.top
Acknowledgements
We thank Simon Harley and Chenguang Sun for thorough and critical reviews. We also thank Wenrong Cao and Scott Paterson for insightful discussions while writing the manuscript. This work was supported by the US-NSF grant EAR-1119315 to C.-T.A. Lee, a Geological Society of America Grant-in-Aid and the Martha Lou Broussard Fellowship to E.J. Chin, and the French Agence Nationale de la Recherche grant ANR-10-BLAN-0603 (M&Ms — Mantle Melting — Measurements, Models, Mechanisms) to J. Blichert-Toft.
Editor: Bruce Watson
top
Author Contributions
E.J. Chin collected the electron microprobe data; J. Blichert-Toft and E.J. Chin did the Lu-Hf and Sm-Nd isotope measurements; E.J. Chin and C.-T.A. Lee modelled the Al and thermal diffusion; and E.J. Chin wrote the manuscript with help from C.-T.A. Lee and J. Blichert-Toft. All authors discussed the results and commented on the manuscript.
top
References
Barton, M.D. (1996) Granitic magmatism and metallogeny of southwestern North America. In: Brown, M., Candela, P.A., Peck, D.L., Stephens, R.J.W., Zen, E.-A. (Eds.) The Third Hutton Symposium on the Origin of Granites and Related Rocks. GSA Special Papers, 261-280.
 Show in context
Show in context Magmatism in the Sierra Nevada occurred primarily between ~120 and ~80 Ma, peaking at ~95 Ma and terminating abruptly at ~80 Ma (Barton, 1996; Coleman and Glazner, 1997; Ducea, 2001; Paterson et al., 2014).
View in article
Blichert-Toft, J. (2001) On the Lu-Hf Isotope Geochemistry of Silicate Rocks. Geostandards Newsletter 25, 41-56.
 Show in context
Show in context For Sm-Nd and Lu-Hf isotopic analysis, clean garnet and clinopyroxene were separated by hand. Between 60 and 680 mg of garnet, clinopyroxene, and whole-rock powders were acid-digested in Parr bombs. Sm, Nd, Lu and Hf were separated by ion-exchange column chromatography and their isotopic compositions measured by multiple-collector inductively coupled plasma mass spectrometry following the procedures outlined in Blichert-Toft et al. (1997, 2002), Blichert-Toft (2001), and Blichert-Toft and Puchtel (2010).
View in article
Blichert-Toft, J., Puchtel, I.S. (2010) Depleted mantle sources through time: Evidence from Lu-Hf and Sm-Nd isotope systematics of Archean komatiites. Earth and Planetary Science Letters 297, 598-606.
 Show in context
Show in context For Sm-Nd and Lu-Hf isotopic analysis, clean garnet and clinopyroxene were separated by hand. Between 60 and 680 mg of garnet, clinopyroxene, and whole-rock powders were acid-digested in Parr bombs. Sm, Nd, Lu and Hf were separated by ion-exchange column chromatography and their isotopic compositions measured by multiple-collector inductively coupled plasma mass spectrometry following the procedures outlined in Blichert-Toft et al. (1997, 2002), Blichert-Toft (2001), and Blichert-Toft and Puchtel (2010).
View in article
Blichert-Toft, J., Chauvel, C., Albarède, F. (1997) Separation of Hf and Lu for high-precision isotope analysis of rock samples by magnetic sector-multiple collector ICP-MS. Contributions to Mineralogy and Petrology 127, 248-260.
 Show in context
Show in context For Sm-Nd and Lu-Hf isotopic analysis, clean garnet and clinopyroxene were separated by hand. Between 60 and 680 mg of garnet, clinopyroxene, and whole-rock powders were acid-digested in Parr bombs. Sm, Nd, Lu and Hf were separated by ion-exchange column chromatography and their isotopic compositions measured by multiple-collector inductively coupled plasma mass spectrometry following the procedures outlined in Blichert-Toft et al. (1997, 2002), Blichert-Toft (2001), and Blichert-Toft and Puchtel (2010).
View in article
Blichert-Toft, J., Boyet, M., Télouk, P., Albarède, F. (2002) 147Sm/143Nd and 176Lu/176 Hf in eucrites and the differentiation of the HED parent body. Earth and Planetary Science Letters 204, 167-181.
 Show in context
Show in context For Sm-Nd and Lu-Hf isotopic analysis, clean garnet and clinopyroxene were separated by hand. Between 60 and 680 mg of garnet, clinopyroxene, and whole-rock powders were acid-digested in Parr bombs. Sm, Nd, Lu and Hf were separated by ion-exchange column chromatography and their isotopic compositions measured by multiple-collector inductively coupled plasma mass spectrometry following the procedures outlined in Blichert-Toft et al. (1997, 2002), Blichert-Toft (2001), and Blichert-Toft and Puchtel (2010).
View in article
Cheng, H., King, R.L., Nakamura, E., Vervoort, J.D., Zhou, Z. (2008) Coupled Lu–Hf and Sm–Nd geochronology constrains garnet growth in ultra-high-pressure eclogites from the Dabie orogen. Journal of Metamorphic Geology 26, 741-758.
 Show in context
Show in context Although the closure temperatures of the Lu-Hf and Sm-Nd systems depend on many factors, such as cooling rate, grain size, and diffusion parameters, which are not always well constrained in texturally complex systems, it is known that the Lu-Hf system closes at higher temperatures than the Sm-Nd system (Kylander-Clark et al., 2007; Cheng et al., 2008).
View in article
Cherniak, D.J., Liang, Y. (2007) Rare earth element diffusion in natural enstatite. Geochimica et Cosmochimica Acta 71, 1324-1340.
 Show in context
Show in context Cherniak and Liang (2007) obtained a D0 of 1.2 × 10-7 m2/s and E of 369 kJ/mol after averaging all experimental data for REE and Y diffusion in orthopyroxene.
View in article
Chin, E.J., Lee, C.-T.A., Luffi, P., Tice, M. (2012) Deep Lithospheric Thickening and Refertilization beneath Continental Arcs: Case Study of the P, T and Compositional Evolution of Peridotite Xenoliths from the Sierra Nevada, California. Journal of Petrology 53, 477-511.
 Show in context
Show in context The Sierra Nevada batholith is one of only a few places world-wide to study deep crustal and lithospheric evolution of an arc because of the availability of lower crustal and mantle xenoliths (Dodge et al., 1988; Mukhopadhyay and Manton, 1994; Ducea and Saleeby, 1996; Lee et al., 2006, 2007; Chin et al. 2012), which are rare elsewhere.
View in article
These final P-T conditions are similar to those obtained for the garnet peridotites (2.3 – 3.6 GPa, 651 – 845 °C; Chin et al., 2012).
View in article
These spinel peridotites were subsequently refertilised and transported to cold and deep final conditions in the garnet stability field (Chin et al., 2012, 2014).
View in article
Temperatures corresponding to melting, and thus initial temperatures, can be obtained using Fe-Mg partitioning between peridotite and melt (Lee and Chin, 2014). Melting temperatures for the garnet peridotites fall between 1300 and 1400 °C (Chin et al. 2012).
View in article
Thermobarometry was based on mineral major and trace element compositions published in Lee et al. (2006) and Chin et al. (2012) and the data reported in this study.
View in article
Al-depletion halo thicknesses in orthopyroxene adjacent to the garnet lamellae range from 5-20 μm and are positively correlated with garnet lamellae thickness, confirming that garnet formed by exsolution from an original high-Al, high-temperature pyroxene (Chin et al. 2012).
View in article
Chin, E.J., Lee, C.-T.A., Barnes, J.D. (2014) Thickening, refertilization, and the deep lithosphere filter in continental arcs: Constraints from major and trace elements and oxygen isotopes. Earth and Planetary Science Letters 397, 184-200.
 Show in context
Show in context These spinel peridotites were subsequently refertilised and transported to cold and deep final conditions in the garnet stability field (Chin et al., 2012, 2014).
View in article
Coleman, D.S., Glazner, A.F. (1997) The Sierra Crest Magmatic Event: Rapid Formation of Juvenile Crust during the Late Cretaceous in California. International Geology Review 39, 768-787.
 Show in context
Show in context Magmatism in the Sierra Nevada occurred primarily between ~120 and ~80 Ma, peaking at ~95 Ma and terminating abruptly at ~80 Ma (Barton, 1996; Coleman and Glazner, 1997; Ducea, 2001; Paterson et al., 2014).
View in article
DeCelles, P.G. (2004) Late Jurassic to Eocene evolution of the Cordilleran thrust belt and foreland basin system, western U.S.A. American Journal of Science 304, 105-168.
 Show in context
Show in contextIn many island arcs, the upper plate is in extension, which could compensate for magmatic thickening. In contrast, the upper plate in continental arcs tends to be in compression, adding to the effects of magmatic thickening (DeCelles, 2004; Paterson et al., 2011).
View in article
DeCelles, P.G., Ducea, M.N., Kapp, P., Zandt, G. (2009) Cyclicity in Cordilleran orogenic systems. Nature Geoscience 2, 251-257.
 Show in context
Show in context While plate spreading and subduction are continuous, arc magmatism, particularly in continental arcs, is characterised by >10-50 Myr intervals of enhanced magmatic activity followed by rapid decline (DeCelles et al., 2009).
View in article
Dickinson, W.R., Snyder, W.S. (1978) Plate tectonics of the Laramide orogeny. In: Matthews, V. (Ed.) Laramide folding associated with basement block faulting in the Western United States. Geological Society of America Memoir 151, 355-366.
 Show in context
Show in context Although cooling of the deep Sierran lithosphere by slab “refrigeration” has been suggested previously, shallowing of the slab dip typically has been invoked (Dickinson and Snyder, 1978; Dumitru et al., 1991).
View in article
Dodge, F.C.W., Lockwood, J.P., Calk, L.C. (1988) Fragments of the mantle and crust from beneath the Sierra Nevada batholith: Xenoliths in a volcanic pipe near Big Creek, California. Geological Society of America Bulletin 100, 938-947.
 Show in context
Show in context The Sierra Nevada batholith is one of only a few places world-wide to study deep crustal and lithospheric evolution of an arc because of the availability of lower crustal and mantle xenoliths (Dodge et al., 1988; Mukhopadhyay and Manton, 1994; Ducea and Saleeby, 1996; Lee et al., 2006, 2007; Chin et al., 2012), which are rare elsewhere.
View in article
Ducea, M.N. (2001) The California Arc: Thick Granitic Batholiths, Eclogitic Residues, Lithospheric-Scale Thrusting, and Magmatic Flare-Ups. GSA Today 11, 4-10.
 Show in context
Show in context Magmatism in the Sierra Nevada occurred primarily between ~120 and ~80 Ma, peaking at ~95 Ma and terminating abruptly at ~80 Ma (Barton, 1996; Coleman and Glazner, 1997; Ducea, 2001; Paterson et al., 2014).
View in article
Ducea, M.N., Saleeby, J.B. (1996) Buoyancy sources for a large, unrooted mountain range, the Sierra Nevada, California: Evidence from xenolith thermobarometry. Journal of Geophysical Research 101, 8229-8244.
 Show in context
Show in context The Sierra Nevada batholith is one of only a few places world-wide to study deep crustal and lithospheric evolution of an arc because of the availability of lower crustal and mantle xenoliths (Dodge et al., 1988; Mukhopadhyay and Manton, 1994; Ducea and Saleeby, 1996; Lee et al., 2006, 2007; Chin et al., 2012), which are rare elsewhere.
View in article
Ducea, M.N., Saleeby, J.B. (1998) The age and origin of a thick mafic–ultramafic keel from beneath the Sierra Nevada batholith. Contributions to Mineralogy and Petrology 133, 169-185.
 Show in context
Show in context Sm-Nd ages between 120 and 84 Ma for several pyroxenite xenoliths (Ducea and Saleeby, 1998), along with geochemical data (Lee et al., 2006, 2007), indicate that they represent cumulates left behind by the Cretaceous Sierran granitoids.
View in article
Dumitru, T.A., Gans, P.B., Foster, D.A., Miller, E.L. (1991) Refrigeration of the western Cordilleran lithosphere during Laramide shallow-angle subduction. Geology 19, 1145-1148.
 Show in context
Show in context Although cooling of the deep Sierran lithosphere by slab “refrigeration” has been suggested previously, shallowing of the slab dip typically has been invoked (Dickinson and Snyder, 1978; Dumitru et al., 1991).
View in article
Flowers, R.M., Bowring, S.A., Tulloch, A.J., Klepeis, K.A. (2005) Tempo of burial and exhumation within the deep roots of a magmatic arc, Fiordland, New Zealand. Geology 33, 17-20.
 Show in context
Show in context The coincidence of thickening with the peak of arc magmatism suggests that crustal thickening must have, in part, been driven by magmatic additions to the crust (Flowers et al., 2005; Jagoutz, 2010).
View in article
Harley, S.L., Green, D.H. (1982) Garnet-orthopyroxene barometry for granulites and peridotites. Nature 300, 697-701.
 Show in context
Show in context For example, in the profile shown in Figure 1C, the distal Al2O3 of 1.88 wt. % would correspond to 1275 °C using the garnet-orthopyroxene thermobarometer of Harley and Green (1982).
View in article
The temperature-dependent boundary condition was determined by parameterising the garnet-orthopyroxene thermobarometer of Harley and Green (1982) at 3 GPa.
View in article
Haschke, M., Siebel, W., Günther, A., Scheuber, E. (2002) Repeated crustal thickening and recycling during the Andean orogeny in north Chile (21-26 S). Journal of Geophysical Research: Solid Earth (1978-2012) 107, ECV 6-1-ECV 6-18.
 Show in context
Show in context In some cases, such as the Andes, this pattern has recurred several times (Haschke et al., 2002).
View in article
Jagoutz, O. (2010) Construction of the granitoid crust of an island arc. Part II: a quantitative petrogenetic model. Contributions to Mineralogy and Petrology 160, 359-381.
 Show in context
Show in context The coincidence of thickening with the peak of arc magmatism suggests that crustal thickening must have, in part, been driven by magmatic additions to the crust (Flowers et al., 2005; Jagoutz, 2010).
View in article
Karlstrom, L., Lee, C.-T.A., Manga, M. (2014) The role of magmatically driven lithospheric thickening on arc front migration. Geochemistry, Geophysics, Geosystems 15, 2655-2675.
 Show in context
Show in context The ability for magmatism to thicken the upper plate depends on the stress state of the upper plate (Karlstrom et al., 2014).
View in article
Kay, S.M., Coira, B.L. (2009) Shallowing and steepening subduction zones, continental lithospheric loss, magmatism, and crustal flow under the Central Andean Altiplano-Puna Plateau. Backbone of the Americas: shallow subduction, plateau uplift, and ridge and terrane collision 204, 229-259.
 Show in context
Show in context Abrupt changes in plate convergence rates and direction (Pilger, 1984) or repeated steepening and shallowing of subducting slabs (Kay and Coira, 2009) have been suggested as triggering flare-ups or terminating magmatism, but such scenarios may not be sufficiently general.
View in article
Kylander-Clark, A.R., Hacker, B.R., Johnson, C.M., Beard, B.L., Mahlen, N.J., Lapen, T.J. (2007) Coupled Lu-Hf and Sm-Nd geochronology constrains prograde and exhumation histories of high-and ultrahigh-pressure eclogites from western Norway. Chemical Geology 242, 137-154.
 Show in context
Show in context Although the closure temperatures of the Lu-Hf and Sm-Nd systems depend on many factors, such as cooling rate, grain size, and diffusion parameters, which are not always well constrained in texturally complex systems, it is known that the Lu-Hf system closes at higher temperatures than the Sm-Nd system (Kylander-Clark et al., 2007; Cheng et al., 2008).
View in article
Lee, C.-T.A., Chin, E.J. (2014) Calculating melting temperatures and pressures of peridotite protoliths: Implications for the origin of cratonic mantle. Earth and Planetary Science Letters 403, 273-286.
 Show in context
Show in context Temperatures corresponding to melting, and thus initial temperatures, can be obtained using Fe-Mg partitioning between peridotite and melt (Lee and Chin, 2014).
View in article
Lee, C.-T.A., Cheng, X., Horodyskyj, U. (2006) The development and refinement of continental arcs by primary basaltic magmatism, garnet pyroxenite accumulation, basaltic recharge and delamination: insights from the Sierra Nevada, California. Contributions to Mineralogy and Petrology 151, 222-242.
 Show in context
Show in context The Sierra Nevada batholith is one of only a few places world-wide to study deep crustal and lithospheric evolution of an arc because of the availability of lower crustal and mantle xenoliths (Dodge et al., 1988; Mukhopadhyay and Manton, 1994; Ducea and Saleeby, 1996; Lee et al., 2006, 2007; Chin et al., 2012), which are rare elsewhere.
View in article
Sm-Nd ages between 120 and 84 Ma for several pyroxenite xenoliths (Ducea and Saleeby, 1998), along with geochemical data (Lee et al., 2006, 2007), indicate that they represent cumulates left behind by the Cretaceous Sierran granitoids.
View in article
Garnet pyroxenite cumulates are volumetrically dominant and can be divided into a low-MgO group composed of garnet-rich clinopyroxenites and a high-MgO group composed of garnet websterites (Lee et al., 2006).
View in article
In contrast, major element thermobarometry of the high-MgO websterites record equilibration pressures ranging from 1.5 to 3 GPa and temperatures between 700 and 830 °C (Lee et al., 2006).
View in article
Although both garnet peridotites and high-MgO websterites achieved similar high final pressures and low final temperatures, high-Al orthopyroxene cores in both rock types point to initially high temperatures. In high-MgO websterites, orthopyroxene cores record temperatures of ~1100 °C, supporting an origin as magmatic cumulates precipitated from primary basaltic liquids (Lee et al., 2006; Supplementary Information).
View in article
Thermobarometry was based on mineral major and trace element compositions published in Lee et al. (2006) and Chin et al. (2012) and the data reported in this study.
View in article
Lee, C.-T.A., Morton, D.M., Kistler, R.W., Baird, A.K. (2007) Petrology and tectonics of Phanerozoic continent formation: From island arcs to accretion and continental arc magmatism. Earth and Planetary Science Letters 263, 370-387.
 Show in context
Show in context The Sierra Nevada batholith is one of only a few places world-wide to study deep crustal and lithospheric evolution of an arc because of the availability of lower crustal and mantle xenoliths (Dodge et al., 1988; Mukhopadhyay and Manton, 1994; Ducea and Saleeby, 1996; Lee et al., 2006, 2007; Chin et al., 2012), which are rare elsewhere.
View in article
Sm-Nd ages between 120 and 84 Ma for several pyroxenite xenoliths (Ducea and Saleeby, 1998), along with geochemical data (Lee et al., 2006, 2007), indicate that they represent cumulates left behind by the Cretaceous Sierran granitoids.
View in article
Mukhopadhyay, B., Manton, W.I. (1994) Upper-Mantle Fragments from Beneath the Sierra Nevada Batholith: Partial Fusion, Fractional Crystallization, and Metasomatism in a Subduction Related Ancient Lithosphere. Journal of Petrology 35, 1417-1450.
 Show in context
Show in context The Sierra Nevada batholith is one of only a few places world-wide to study deep crustal and lithospheric evolution of an arc because of the availability of lower crustal and mantle xenoliths (Dodge et al., 1988; Mukhopadhyay and Manton, 1994; Ducea and Saleeby, 1996; Lee et al., 2006, 2007; Chin et al., 2012), which are rare elsewhere.
View in article
Paterson, S.R., Okaya, D., Memeti, V., Economos, R., Miller, R.B. (2011) Magma addition and flux calculations of incrementally constructed magma chambers in continental margin arcs: Combined field, geochronologic, and thermal modeling studies. Geosphere 7, 1439-1468.
 Show in context
Show in context In many island arcs, the upper plate is in extension, which could compensate for magmatic thickening. In contrast, the upper plate in continental arcs tends to be in compression, adding to the effects of magmatic thickening (DeCelles, 2004; Paterson et al., 2011).
View in article
Paterson, S.R., Memeti, V., Anderson, L., Cao, W., Lackey, J.S., Putirka, K.D., Miller, R.B., Miller, J.S., Mundil, R. (2014) Day 6: Overview of arc processes and tempos. Field Guides 34, 87-116.
 Show in context
Show in context Magmatism in the Sierra Nevada occurred primarily between ~120 and ~80 Ma, peaking at ~95 Ma and terminating abruptly at ~80 Ma (Barton, 1996; Coleman and Glazner, 1997; Ducea, 2001; Paterson et al., 2014).
View in article
For example, the only model using D0 of 10-11 m2/s consistent with the observed Al profile requires τ of 50 Myr and closure at 100 Myr, while it is clear that a cooling duration of 100 Myr for the Sierran deep lithosphere is unacceptably long given the well-constrained magmatic peak at ~95 Ma (Paterson et al., 2014) and closure of garnet websterite Sm-Nd ages between 81 and 89 Ma (discussed below).
View in article
U-Pb zircon ages from the Sierra Nevada Batholith (Paterson et al., 2014) showing a major peak at ~95 Ma.
View in article
Pilger, R.H. (1984) Cenozoic plate kinematics, subduction and magmatism: South American Andes. Journal of the Geological Society 141, 793-802.
 Show in context
Show in context Abrupt changes in plate convergence rates and direction (Pilger, 1984) or repeated steepening and shallowing of subducting slabs (Kay and Coira, 2009) have been suggested as triggering flare-ups or terminating magmatism, but such scenarios may not be sufficiently general.
View in article
Saleeby, J., Ducea, M., Clemens-Knott, D. (2003) Production and loss of high-density batholithic root, southern Sierra Nevada, California. Tectonics 22, 1064.
 Show in context
Show in context The former are interpreted as mid to lower crustal cumulates of an evolved hydrous basalt, whereas the latter are lower crustal and upper mantle cumulates of primitive hydrous basalts, overlapping in final equilibration pressures and therefore interpreted as being interleaved with the spinel and garnet peridotites (Saleeby et al., 2003) (Fig. 1B).
View in article
Sun, C., Liang, Y. (2015) A REE-in-garnet-clinopyroxene thermobarometer for eclogites, granulites and garnet peridotites. Chemical Geology 393-394, 79-92.
 Show in context
Show in context Garnet-clinopyroxene rare-earth element (REE) thermobarometry of the low-MgO pyroxenite yields equilibration conditions of ~0.33 GPa and 801 °C (Sun and Liang, 2015) consistent with a mid-crustal origin.
View in article
We chose a low-MgO pyroxenite (BCX; final T of 801 °C and final P of 0.33 GPa using the REE garnet-clinopyroxene thermobarometer of Sun and Liang (2015)), a high-MgO websterite (BC98-5, average final T of 702 °C and average final P of 2.4 GPa), and a high-MgO amphibole-bearing websterite (BC98-7, average final T of 710 °C and average final P of 1.6 GPa).
View in article
Syracuse, E.M., van Keken, P.E., Abers, G.A. (2010) The global range of subduction zone thermal models. Physics of the Earth and Planetary Interiors 183, 73-90.
 Show in context
Show in context We take the temperature of the top of a slab to be 750 °C, based on Syracuse et al. (2010).
View in article
Van Orman, J., Grove, T., Shimizu, N. (2001) Rare earth element diffusion in diopside: influence of temperature, pressure, and ionic radius, and an elastic model for diffusion in silicates. Contributions to Mineralogy and Petrology 141, 687-703.
 Show in context
Show in context D0, however, is expected to vary with ionic radius based on experiments on clinopyroxene (Van Orman et al., 2001), but the relationship is not clear for experiments on orthopyroxene.
View in article
top
Supplementary Information
Supplementary Data
Mineral compositions and petrography of Sierran pyroxenites and garnet peridotite 1026V
Our study investigated four Sierran xenoliths. Of these, one xenolith is a garnet peridotite (1026V). Major element compositions of the transect shown in Figure 1C are reported in Table S-1. The remaining three xenoliths are pyroxenites: one low-MgO (<15 wt.% MgO) garnet clinopyroxenite (BCX) and two high-MgO (>15 wt.% MgO) garnet websterites (BC98-5, BC98-7). Whole-rock major element and trace element geochemistry, major and trace element mineral compositions, and petrography for 1026V were previously published in Lee et al. (2001)
Lee, C.-T.A., Rudnick, R.L., Brimhall Jr, G.H. (2001) Deep lithospheric dynamics beneath the Sierra Nevada during the Mesozoic and Cenozoic as inferred from xenolith petrology. Geochemistry Geophysics Geosystems 2.
, Lee (2005)Lee, C.-T.A. (2005) Trace Element Evidence for Hydrous Metasomatism at the Base of the North American Lithosphere and Possible Association with Laramide Low-Angle Subduction. The Journal of Geology 113, 673-685.
, and Chin et al. (2012)Chin, E.J., Lee, C.-T.A., Luffi, P., Tice, M. (2012) Deep Lithospheric Thickening and Refertilization beneath Continental Arcs: Case Study of the P, T and Compositional Evolution of Peridotite Xenoliths from the Sierra Nevada, California. Journal of Petrology 53, 477-511.
. For microphotographs and additional details on petrography and textures in 1026V, the reader is referred to Chin et al. (2012)Chin, E.J., Lee, C.-T.A., Luffi, P., Tice, M. (2012) Deep Lithospheric Thickening and Refertilization beneath Continental Arcs: Case Study of the P, T and Compositional Evolution of Peridotite Xenoliths from the Sierra Nevada, California. Journal of Petrology 53, 477-511.
. Here, we focus on the garnet exsolution within orthopyroxene texture in 1026V and its utility in Al diffusion modelling. We also report new electron microprobe measurements of minerals in BCX, BC98-5, and BC98-7. In Figure S-1 we show that the two high-MgO websterites BC98-5 and BC98-7 have orthopyroxenes zoned from high-Al cores to low-Al rims, similar to previously observed Al zonation in 1026V. All probe data are reported in Tables S2 – S4.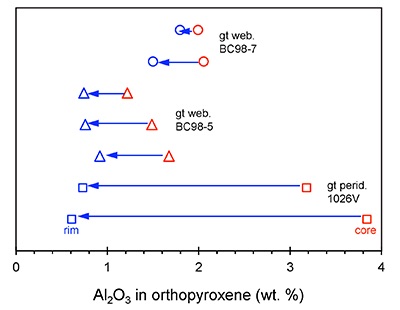
Figure S-1 Al2O3 (wt. %) in orthopyroxene cores and rims in garnet websterites and garnet peridotite. Cores have higher Al contents.
Supplementary Methods
a. Lu-Hf and Sm-Nd Isotope Geochemistry
For combined Sm-Nd and Lu-Hf isotope analyses, garnet and clinopyroxene were separated from the pyroxenites by first gently disaggregating ~5 cm whole-rock chunks using a rock hammer. Samples were wrapped in paper towels to minimise contact with the metal hammer, and only the freshest, interior gravel-size pieces were selected for further hand crushing in an agate mortar. Samples were then sieved for grain sizes between 250 to 500 μm. From this fraction, minerals were handpicked under a binocular microscope. Owing to ubiquitous kelyphite rims (Figs. S2-S4), garnets were repeatedly re-crushed by hand in agate after several rounds of picking to separate out a “clean”, kelyphite-free fraction and a “dirty”, kelyphite-bearing fraction. Representative “clean” mineral separates are shown in Figure S-5.While every effort was made to separate as much “clean” garnet as possible, the ubiquitous nature of kelyphite made it difficult for BC98-5. For this sample, kelyphite-bearing garnet therefore was analysed. The presence of kelyphite rims apparently did not disturb the Sm-Nd and Lu-Hf ages – garnet, clinopyroxene, and the whole-rock defined a 3-point Sm-Nd isochron of 88.8 ± 3.1 Ma (MSWD = 0.68) and a 2-point Lu-Hf “isochron” of 92.6 ± 1.6 Ma.

Figure S-2 Plane-polarised light photograph of thick section (~200 μm) of high-MgO websterite BC98-5.

Figure S-3 Plane-polarised light photograph of thick section (~200 μm) of high-MgO websterite BC98-7.

Figure S-4 Plane-polarised light photograph of thick section (~200 μm) of low-MgO garnet clinopyroxenite BCX.
Figure S-5 Representative “clean” clinopyroxene and garnet separates from BC98-5.
Prior to sample dissolution, mineral separates were cleaned in dilute HCl, ultrasonicated for several hours, and rinsed with ultrapure de-ionised water. All sample dissolution procedures and Sm, Nd, Lu, and Hf chemical separation and isotopic analysis were carried out at the Ecole Normale Supérieure in Lyon. After dissolution in Parr bombs, Sm, Nd, Lu, and Hf were separated from ca. 60-680 mg sized aliquots (depending on degree of sample depletion and sample availability) of garnet and clinopyroxene separates and whole-rock powders by ion-exchange column chromatography, and measured for their isotopic compositions by MC-ICP-MS (Nu Plasma 500 HR) coupled with a desolvating nebuliser DSN-100 according to the procedures of Blichert-Toft et al. (1997
Blichert-Toft, J., Chauvel, C., Albarède, F. (1997) Separation of Hf and Lu for high-precision isotope analysis of rock samples by magnetic sector-multiple collector ICP-MS. Contributions to Mineralogy and Petrology 127, 248-260.
, 2002Blichert-Toft, J., Boyet, M., Télouk, P., Albarède, F. (2002) 147Sm/143Nd and 176Lu/176 Hf in eucrites and the differentiation of the HED parent body. Earth and Planetary Science Letters 204, 167-181.
), Blichert-Toft (2001)Blichert-Toft, J. (2001) On the Lu-Hf Isotope Geochemistry of Silicate Rocks. Geostandards Newsletter 25, 41-56.
, and Blichert-Toft and Puchtel (2010)Blichert-Toft, J., Puchtel, I.S. (2010) Depleted mantle sources through time: Evidence from Lu-Hf and Sm-Nd isotope systematics of Archean komatiites. Earth and Planetary Science Letters 297, 598-606.
.Samarium, Nd, Lu, and Hf concentrations were determined by isotope dilution using >98 % pure mixed 149Sm-150Nd and 176Lu-180Hf spikes added to the samples at the outset of the dissolution procedure. The “Rennes” in-house Nd and JMC-475 Hf standards were analysed systematically every one or two samples to monitor machine performance and allow for sample-standard bracketing of the unknowns. The measured sample values were normalised to the accepted values of 0.511961 ± 0.000013 (corresponding to 0.511856 for La Jolla (Chauvel and Blichert-Toft, 2001
The measured sample values were normalised to the accepted values of 0.511961 ± 0.000013 (corresponding to 0.511856 for La Jolla (Chauvel and Blichert-Toft, 2001)) for 143Nd/144Nd of the “Rennes” in-house Nd standard and 0.282163 ± 0.000009 for 176Hf/177Hf of the JMC-475 Hf standard using sample-standard bracketing.
)) for 143Nd/144Nd of the “Rennes” in-house Nd standard and 0.282163 ± 0.000009 for 176Hf/177Hf of the JMC-475 Hf standard using sample-standard bracketing. Mass bias were corrected relative to 146Nd/144Nd = 0.7219 and 179Hf/177Hf = 0.7325 using an exponential law. Total procedural blanks for Sm, Nd, Lu, and Hf were less than 20, 50, 20, and 20 pg, respectively. Isochron calculations (ages, initial isotopic compositions, and MSWDs) were done using a MatLab least-squares software by F. Albarède (version 6.0, 2013). Errors applied to the measured 147Sm/144Nd and 176Lu/177Hf ratios were ± 0.2%, while errors applied to the measured 143Nd/144Nd and 176Hf/177Hf ratios were ± 0.00001, which was the external reproducibility of the present analytical sessions as determined by the repeated standard measurements. In those cases where the in-run error for a sample was larger than the external reproducibility, the in-run error was used for the least-squares regressions. The decay constants used were 6.54 × 10-12 y-1 for 147Sm and 1.865 × 10-11 y-1 for 176Lu (Scherer et al., 2001Scherer, E., Münker, C., Mezger, K. (2001) Calibration of the Lutetium-Hafnium Clock. Science 293, 683-687.
). If the decay constant for 176Lu by Söderlund et al. (2004)Söderlund, U., Patchett, P.J., Vervoort, J.D., Isachsen, C.E. (2004) The 176Lu decay constant determined by Lu-Hf and U-Pb isotope systematics of Precambrian mafic intrusions. Earth and Planetary Science Letters 219, 311-324.
of 1.867 × 10-11 is used, the calculated ages are older by 1 per mil, which is within the error of the measurements. All Sm-Nd and Lu-Hf concentration and isotope data are reported in Table S-5.Table S-1 Major element compositions (wt. %) of orthopyroxene profile in sample 1026V in Figure 1C.
| ID | 1b#7 | 1b#8 | 1b#9 | 1b#10 | 1b#11 | 1b#12 | 1b#13 | 1b#14 | 1b#15 | 1b#16 | 1b#17 | 1b#18 | 1b#19 | 1b#20 | 1b#21 | 1b#22 | 1b#23 | 1b#24 | 1b#25 | 1b#26 | |
| Xmm | 32.2384 | 32.2394 | 32.2404 | 32.2414 | 32.2424 | 32.2434 | 32.2444 | 32.2454 | 32.2464 | 32.2474 | 32.2484 | 32.2494 | 32.2504 | 32.2514 | 32.2524 | 32.2534 | 32.2544 | 32.2554 | 32.2564 | 32.2574 | |
| Ymm | 26.0967 | 26.0972 | 26.0977 | 26.0982 | 26.0987 | 26.0992 | 26.0997 | 26.1002 | 26.1007 | 26.1012 | 26.1017 | 26.1022 | 26.1027 | 26.1032 | 26.1037 | 26.1042 | 26.1047 | 26.1052 | 26.1057 | 26.1062 | |
| at contact with garnet lamella | |||||||||||||||||||||
| SiO2 | 57.53 | 57.37 | 57.13 | 56.86 | 57.44 | 57.00 | 56.97 | 57.10 | 57.22 | 57.03 | 56.59 | 56.41 | 56.65 | 57.25 | 56.90 | 57.20 | 57.16 | 57.08 | 56.77 | 56.57 | |
| TiO2 | 0.013 | 0.022 | 0.028 | 0.002 | 0.014 | 0.005 | 0.035 | 0.035 | 0.017 | 0.023 | 0.01 | 0.019 | 0.023 | 0.005 | 0 | 0.024 | 0.029 | 0.023 | 0.029 | 0.001 | |
| Al2O3 | 0.99 | 1.09 | 1.27 | 1.37 | 1.52 | 1.60 | 1.71 | 1.74 | 1.69 | 1.74 | 1.85 | 1.88 | 1.84 | 1.82 | 1.78 | 1.83 | 1.82 | 1.79 | 1.88 | 1.87 | |
| Cr2O3 | 0.31 | 0.29 | 0.35 | 0.47 | 0.49 | 0.60 | 0.65 | 0.50 | 0.55 | 0.54 | 0.57 | 0.50 | 0.51 | 0.43 | 0.58 | 0.42 | 0.37 | 0.52 | 0.36 | 0.47 | |
| FeOT | 5.84 | 5.58 | 5.45 | 5.39 | 5.34 | 5.45 | 5.64 | 5.75 | 5.52 | 5.48 | 5.68 | 5.74 | 5.64 | 5.68 | 5.36 | 5.68 | 5.53 | 5.74 | 5.83 | 5.91 | |
| MnO | 0.16 | 0.10 | 0.11 | 0.13 | 0.09 | 0.16 | 0.14 | 0.09 | 0.15 | 0.10 | 0.11 | 0.11 | 0.08 | 0.05 | 0.13 | 0.11 | 0.08 | 0.11 | 0.14 | 0.12 | |
| MgO | 35.28 | 34.91 | 34.90 | 34.65 | 34.71 | 34.79 | 34.58 | 34.63 | 34.64 | 34.26 | 32.57 | 31.76 | 34.50 | 34.95 | 34.69 | 34.69 | 34.71 | 34.56 | 34.55 | 34.47 | |
| CaO | 0.10 | 0.10 | 0.11 | 0.13 | 0.14 | 0.13 | 0.13 | 0.11 | 0.12 | 0.12 | 0.11 | 0.14 | 0.12 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.13 | |
| Na2O | 0.022 | 0.032 | 0.007 | 0.007 | 0.003 | 0.007 | 0.001 | 0 | 0.009 | 0.015 | 0.011 | 0.007 | 0.011 | 0.026 | 0 | 0.023 | 0 | 0 | 0 | 0.005 | |
| NiO | 0.06 | 0.10 | 0.07 | 0.06 | 0.02 | 0.06 | 0.05 | 0.08 | 0.06 | 0.07 | 0.02 | 0.08 | 0.17 | 0.10 | 0.01 | 0.14 | 0.14 | 0.05 | 0.14 | 0.08 | |
| Total | 100.29 | 99.59 | 99.42 | 99.06 | 99.76 | 99.79 | 99.91 | 100.04 | 99.98 | 99.38 | 97.51 | 96.65 | 99.54 | 100.42 | 99.56 | 100.21 | 99.94 | 99.98 | 99.80 | 99.61 | |
Table S-2 Orthopyroxene major element compositions (wt. %) in Sierran garnet pyroxenites.
| BC98-7 | BC98-5 | |||||||||
| ID | BC987_01-opxR | BC987_02-opxC | BC987_07-opxR | BC987_08-opxC | BC985_03-opxR | BC985_04-opxC | BC985_07-opxR | BC985_08-opxC | BC985_17-opxR | BC985_18-opxC |
| rim | core | rim | core | rim | core | rim | core | rim | core | |
| x (μm) | 12871 | 12793 | 657 | 273 | 3731 | 3919 | -122 | -96 | 7631 | 7345 |
| y (μm) | 13416 | 13785 | 8599 | 8825 | 23191 | 23429 | 26133 | 25878 | 27889 | 27423 |
| SiO2 | 54.13 | 54.29 | 54.94 | 54.10 | 55.19 | 54.64 | 55.12 | 54.47 | 54.86 | 53.72 |
| TiO2 | 0.043 | 0.02 | 0.034 | 0.02 | 0.02 | 0.013 | 0 | 0.034 | 0.028 | 0.01 |
| Al2O3 | 1.80 | 1.99 | 1.50 | 2.05 | 0.74 | 1.22 | 0.76 | 1.49 | 0.92 | 1.68 |
| Cr2O3 | 0.13 | 0.11 | 0.10 | 0.20 | 0.08 | 0.11 | 0.08 | 0.09 | 0.08 | 0.11 |
| FeOT | 12.80 | 12.85 | 12.92 | 13.07 | 12.32 | 12.38 | 12.71 | 13.14 | 13.19 | 13.55 |
| MnO | 0.14 | 0.14 | 0.17 | 0.15 | 0.11 | 0.11 | 0.11 | 0.15 | 0.16 | 0.16 |
| MgO | 30.80 | 30.57 | 31.14 | 30.76 | 31.45 | 31.07 | 31.32 | 30.45 | 30.53 | 30.18 |
| CaO | 0.25 | 0.21 | 0.20 | 0.16 | 0.12 | 0.17 | 0.10 | 0.19 | 0.20 | 0.20 |
| Na2O | 0.014 | 0.025 | 0.014 | 0.017 | 0.012 | 0.002 | 0.005 | 0.012 | 0.006 | 0 |
| Total | 100.09 | 100.22 | 101.02 | 100.53 | 100.04 | 99.70 | 100.21 | 100.02 | 99.98 | 99.61 |
Table S-3 Clinopyroxene major element compositions (wt. %) in garnet pyroxenites.
| BCX | BC98-7 | BC98-5 | |||||||||||
| ID | BCX_03-cpx | BCX_05-cpx | BCX_08-cpx | BC987_05-cpxR | BC987_06-cpxC | BC987_09-cpxR | BC987_10-cpxC | BC985_05-cpxR | BC985_06-cpxC | BC985_11-cpxR | BC985_12-cpxC | BC985_15-cpxR | BC985_16-cpxC |
| rim next to gt | rim next to gt | rim next to gt | rim | core | rim | core | rim | core | rim | core | rim | core | |
| x (μm) | -6 | -5075 | -4444 | 12686 | 12656 | 769 | 849 | 4675 | 5089 | -470 | -299 | 7454 | 8124 |
| y (μm) | -15851 | -6058 | -13636 | 14152 | 14420 | 8874 | 9171 | 23847 | 24479 | 26791 | 27289 | 28170 | 28080 |
| SiO2 | 54.85 | 54.80 | 54.53 | 53.79 | 53.62 | 54.45 | 53.86 | 54.35 | 53.59 | 54.31 | 53.41 | 54.06 | 52.98 |
| TiO2 | 0.18 | 0.19 | 0.21 | 0.15 | 0.24 | 0.09 | 0.14 | 0.05 | 0.15 | 0.05 | 0.13 | 0.04 | 0.13 |
| Al2O3 | 9.34 | 9.42 | 9.24 | 3.91 | 4.49 | 3.56 | 4.03 | 1.97 | 4.19 | 2.32 | 4.42 | 1.66 | 4.70 |
| Cr2O3 | 0.04 | 0.00 | 0.00 | 0.44 | 0.44 | 0.45 | 0.44 | 0.26 | 0.22 | 0.27 | 0.29 | 0.26 | 0.36 |
| FeOT | 4.14 | 4.17 | 4.19 | 3.73 | 4.04 | 3.50 | 3.65 | 3.60 | 4.18 | 3.59 | 4.22 | 3.48 | 4.37 |
| MnO | 0.002 | 0.029 | 0.037 | 0.084 | 0.056 | 0.033 | 0.037 | 0.076 | 0.098 | 0.037 | 0.051 | 0.041 | 0.057 |
| MgO | 10.57 | 10.57 | 10.60 | 15.00 | 14.50 | 15.16 | 14.96 | 16.17 | 14.77 | 16.03 | 14.73 | 16.37 | 14.41 |
| CaO | 17.04 | 16.76 | 16.97 | 21.81 | 21.38 | 21.83 | 21.61 | 23.22 | 21.97 | 22.95 | 22.12 | 23.25 | 22.08 |
| Na2O | 4.61 | 4.70 | 4.53 | 1.61 | 1.67 | 1.65 | 1.71 | 0.90 | 1.23 | 1.03 | 1.36 | 0.79 | 1.36 |
| Total | 100.78 | 100.64 | 100.30 | 100.51 | 100.44 | 100.72 | 100.44 | 100.59 | 100.40 | 100.58 | 100.72 | 99.93 | 100.46 |
Table S-4 Garnet major element compositions (wt. %) in Sierran garnet pyroxenites.
| BC98-5 | BCX | BC98-7 | ||||||||||||||
| ID | BC985_01-GT1 | BC985_02-GT1 | BC985_09-GTr | BC985_10-GTc | BC985_13-GTc | BC985_14-GTr | BCX_01-GTC | BCX_02-GTr | BCX_04-GTr | BCX_06-GTr | BCX_07-GTC | BCX_09-GT | BC987_03-GTC | BC987_04-GTr | BC987_11-GT1 | BC987_12-GT2 |
| rim | core | core | rim | core | rim | rim | rim | core | core | rim | ||||||
| x (μm) | 3639 | 3671 | -308 | -593 | 7284 | 7366 | 3793 | 8008 | 338 | -4877 | -4680 | -4976 | 11863 | 12152 | 57 | 60 |
| y (μm) | 22892 | 22835 | 26127 | 26358 | 28034 | 28111 | -12444 | -12849 | -16003 | -5904 | -5797 | -13521 | 13995 | 13891 | 9855 | 9811 |
| SiO2 | 40.15 | 40.13 | 40.01 | 40.23 | 39.77 | 40.00 | 39.35 | 39.16 | 39.31 | 39.08 | 39.05 | 39.19 | 40.23 | 40.30 | 40.28 | 39.96 |
| TiO2 | 0.044 | 0.093 | 0.048 | 0.104 | 0.104 | 0.017 | 0.14 | 0.073 | 0.16 | 0.05 | 0.107 | 0.093 | 0.077 | 0.053 | 0.062 | 0.058 |
| Al2O3 | 22.94 | 22.86 | 22.84 | 22.62 | 22.58 | 22.60 | 22.20 | 22.10 | 22.56 | 22.58 | 22.25 | 22.46 | 22.72 | 22.90 | 22.84 | 22.81 |
| Cr2O3 | 0.34 | 0.37 | 0.42 | 0.34 | 0.39 | 0.38 | 0 | 0.04 | 0 | 0.03 | 0.02 | 0.02 | 0.47 | 0.52 | 0.51 | 0.49 |
| FeOT | 18.00 | 18.16 | 18.97 | 18.09 | 18.94 | 19.20 | 22.73 | 21.14 | 20.73 | 20.69 | 20.96 | 20.74 | 17.93 | 18.79 | 18.45 | 18.43 |
| MnO | 0.56 | 0.57 | 0.53 | 0.42 | 0.54 | 0.57 | 0.66 | 0.56 | 0.53 | 0.56 | 0.53 | 0.60 | 0.56 | 0.57 | 0.53 | 0.57 |
| MgO | 13.10 | 12.94 | 12.31 | 12.81 | 12.28 | 12.14 | 9.43 | 7.41 | 7.95 | 7.40 | 7.48 | 7.36 | 13.42 | 12.70 | 13.14 | 13.21 |
| CaO | 5.61 | 5.61 | 5.61 | 5.65 | 5.51 | 5.50 | 6.26 | 9.89 | 9.58 | 10.38 | 10.17 | 10.46 | 5.19 | 5.22 | 5.22 | 5.29 |
| Na2O | 0.02 | 0.025 | 0.014 | 0.02 | 0.005 | 0.019 | 0.056 | 0.024 | 0.067 | 0.003 | 0.039 | 0.02 | 0.014 | 0.012 | 0.02 | 0.002 |
| Total | 100.76 | 100.75 | 100.75 | 100.27 | 100.11 | 100.42 | 100.82 | 100.41 | 100.88 | 100.77 | 100.60 | 100.94 | 100.62 | 101.07 | 101.05 | 100.81 |
Table S-5 Sm-Nd and Lu-Hf concentrations and isotope compositions of Sierran pyroxenites.
| Sample | Sm (ppm) | Nd (ppm) | 147Sm/144Nd | 143Nd/144Nd | 2σ | Lu (ppm) | Hf (ppm) | 176Lu/177Hf | 176Hf/177Hf | 2σ |
| BC98-7 gt | 0.563 | 0.295 | 1.152 | 0.513068 | 0.000008 | 0.455 | 0.0852 | 0.7581 | 0.283596 | 0.000044 |
| BC98-7 cpx | 2.00 | 6.50 | 0.1858 | 0.512566 | 0.000004 | 0.00628 | 0.912 | 0.0009766 | 0.282737 | 0.000005 |
| BC98-7 wr | 0.769 | 2.13 | 0.2178 | 0.512562 | 0.000004 | 0.134 | 0.360 | 0.05285 | 0.283094 | 0.000005 |
| BC98-5 kgt | 0.727 | 0.533 | 0.8257 | 0.512877 | 0.000006 | 0.769 | 0.186 | 0.5871 | 0.283728 | 0.000014 |
| BC98-5 cpx | 2.34 | 6.51 | 0.2173 | 0.512526 | 0.000005 | 0.00946 | 1.07 | 0.001252 | 0.282716 | 0.000004 |
| BC98-5 wr | 1.48 | 4.04 | 0.2215 | 0.512523 | 0.000005 | 0.158 | 0.817 | 0.02749 | 0.282841 | 0.000014 |
| BCX gt | 3.50 | 2.19 | 0.9639 | 0.512956 | 0.000005 | 1.22 | 0.344 | 0.5037 | 0.283774 | 0.000010 |
| BCX gt dupl | 1.25 | |||||||||
| BCX cpx | 1.11 | 1.80 | 0.3718 | 0.512630 | 0.000012 | 0.0197 | 1.10 | 0.002529 | 0.282767 | 0.000003 |
| BCX cpx dupl | 0.0197 | 1.09 | 0.002574 | |||||||
| BCX wr | 2.79 | 4.73 | 0.3563 | 0.512702 | 0.000004 | 0.514 | 0.936 | 0.07795 | 0.282913 | 0.000004 |
Download in Excel
b. Al Diffusion Modelling
To model Al diffusion in orthopyroxene, we solved the general diffusion equation,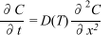
using an explicit finite difference program with adaptive time-steps using a VisualBasic Macro in Excel developed by C.-T. Lee. The program is available upon request.
The temperature-dependent diffusion coefficient D follows an Arrhenius relation

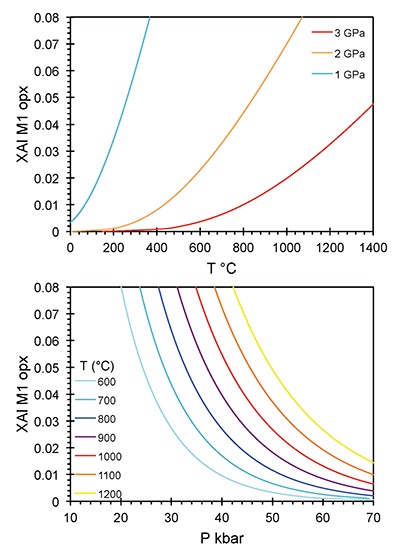
Figure S-6 The thermobarometer of Harley and Green (1982)
Harley, S.L., Green, D.H. (1982) Garnet-orthopyroxene barometry for granulites and peridotites. Nature 300, 697-701.
parameterised as a function of XAl on M1 site of opx versus temperature at constant pressure (top) and XAl on M1 site of opx versus pressure at constant temperature (bottom).Harley, S.L., Green, D.H. (1982) Garnet-orthopyroxene barometry for granulites and peridotites. Nature 300, 697-701.
. We assumed that the Fe/Mg ratio in orthopyroxene is roughly constant given that it is buffered by the bulk rock composition and that the exchange coefficient of Fe/Mg between pyroxenes and olivines are close to 1 and relatively insensitive to temperature and pressure (von Seckendorff and O’Neill, 1993von Seckendorff, V., O'Neill, H.S.C. (1993) An experimental study of Fe-Mg partitioning between olivine and orthopyroxene at 1173, 1273 and 1423 K and 1.6 GPa. Contributions to Mineralogy and Petrology 113, 196-207.
). We have also assumed that the Ca in the garnet does not change significantly, being more of a function of the bulk rock composition than on temperature and pressure (Pearson et al., 2003Pearson, D., Canil, D., Shirey, S. (2003) Mantle samples included in volcanic rocks: xenoliths and diamonds. Treatise on Geochemistry 2, 171-275.
). The parameterised Al concentration in orthopyroxene as a function of T or P is shown in Figure S-6. Al concentration is shown in units of cation fraction XAl on the orthopyroxene M1 site, where XAl = Al/2, and Al is the number of cations in the formula for orthopyroxene on a 6 oxygen basis.We assumed isobaric cooling at 3 GPa from 1275 °C to 750 °C (we explore different starting temperatures in a later section). The starting temperature of 1275 °C is obtained using a parameterised relationship between Al in orthopyroxene as a function of temperature at a constant pressure of 3 GPa,
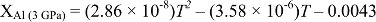
Chin, E.J., Lee, C.-T.A., Luffi, P., Tice, M. (2012) Deep Lithospheric Thickening and Refertilization beneath Continental Arcs: Case Study of the P, T and Compositional Evolution of Peridotite Xenoliths from the Sierra Nevada, California. Journal of Petrology 53, 477-511.
).Temperature-time scenarios, D0 and E
Case 1: Starting temperature = 1275 °C, final temperature = 750 °C
In our Al diffusion modelling of garnet exsolution lamellae in host orthopyroxene, we evaluated cooling scenarios that followed an exponential decay from an initial temperature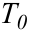 to a final temperature
to a final temperature 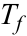
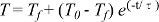
Chin, E.J., Lee, C.-T.A., Luffi, P., Tice, M. (2012) Deep Lithospheric Thickening and Refertilization beneath Continental Arcs: Case Study of the P, T and Compositional Evolution of Peridotite Xenoliths from the Sierra Nevada, California. Journal of Petrology 53, 477-511.
). Thus, the initial temperature effectively represents the temperature at which garnet first nucleated and Al in orthopyroxene began diffusing out to support further garnet growth. Alternative cooling scenarios, such as a linear decay, could have also been explored, but exponential decay to a constant final temperature is physically more reasonable.In Figure S-7 we plot all model results using an activation energy E of 375 kJ/mol, D0 from 10-7 to 10-12 m2/s, and for cooling scenarios usingτof 3, 7, and 50 Myr. We adopt an activation energy of ~375 kJ/mol as recommended by Cherniak and Liang (2007)
Cherniak, D.J., Liang, Y. (2007) Rare earth element diffusion in natural enstatite. Geochimica et Cosmochimica Acta 71, 1324-1340.
for diffusion of rare earth elements in orthopyroxene. Cherniak and Liang showed that activation energy is not sensitive to ionic radius, so we assume that the activation energy for Al is similar. Values for the pre-exponent D0, however, do vary systematically with ionic radius for diffusion of trivalent cations in clinopyroxene, but there is insufficient data to evaluate this dependency on radius in orthopyroxene. For these reasons, we have explored a range of D0 as shown in Figure S-7. D0S of 10-7 to 10-9 m2/s produce diffusion profiles with much less curvature than observed, indicating that input diffusivities are too high. D0S of 10-11 m2/s produce too much curvature and diffusion haloes too thin compared to observation, indicating that input diffusivities are too low. We find that D0 of ~10-10 m2/s best matches the observed curvature. We thus adopt D0 of 10-10 m2/s for our models.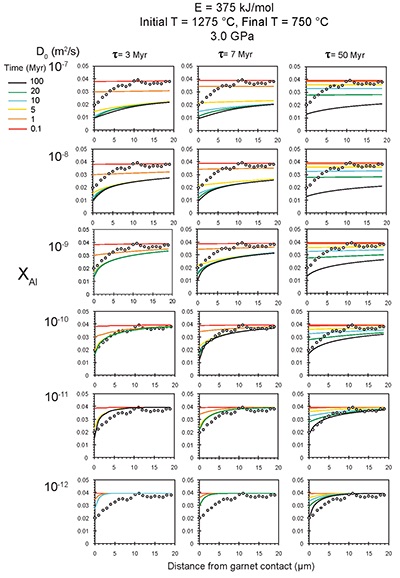
Figure S-7 Modelled Al-diffusion profiles at various times at different values of D0 and constant E (375 kJ/mol), using starting T = 1275 °C, final T = 750 °C. Each column shows models performed at a different cooling scenario.
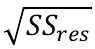 , where
, where 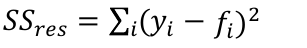 ,
, 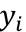 = observed, and
= observed, and 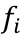 = model, for models using 375 kJ/mol and D0 of 10-10 m2/s for cooling rates with τ = 3, 7, and 50 Ma. The norm of residual for each model at times 0.1, 1, 5, 10, and 20 Ma is plotted. From Figure S-8, the lowest norm of residual occurs at a modelled diffusion profile at 5 Myr after cooling from initially hot (1275 °C) conditions and using the most rapid cooling scenario (τ = 3 Ma). It can be seen from Figure S-8 that cooling scenarios of τ = 3 and 7 Myr both reproduce the observed diffusion profiles, but much slower cooling scenarios (τ = 50 Myr) do not.
= model, for models using 375 kJ/mol and D0 of 10-10 m2/s for cooling rates with τ = 3, 7, and 50 Ma. The norm of residual for each model at times 0.1, 1, 5, 10, and 20 Ma is plotted. From Figure S-8, the lowest norm of residual occurs at a modelled diffusion profile at 5 Myr after cooling from initially hot (1275 °C) conditions and using the most rapid cooling scenario (τ = 3 Ma). It can be seen from Figure S-8 that cooling scenarios of τ = 3 and 7 Myr both reproduce the observed diffusion profiles, but much slower cooling scenarios (τ = 50 Myr) do not. 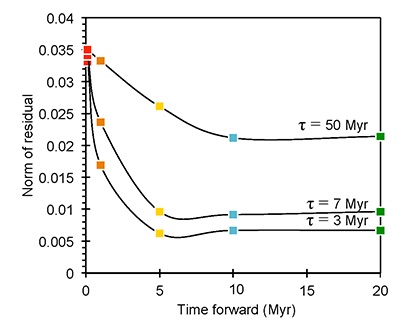
Figure S-8 Norm of residual of Al-diffusion models (E = 375 kJ/mol, D0 = 10-10 m2/s) at 0.1, 1, 5, 10, and 20 Ma and at different cooling scenarios.
Case 2: Starting temperature = 1100 °C, final temperature = 750 °C
Harley, S.L., Green, D.H. (1982) Garnet-orthopyroxene barometry for granulites and peridotites. Nature 300, 697-701.
parameterisation scheme discussed above. Keeping all other model parameters constant (E = 375 kJ/mol, τ = 3, 7, 50 Myr, and varyng D- from 10-7 to 10-12 m2/s) and using a starting temperature of 1100 °C, we find that modelled profiles after 5 – 10 Myr at D0 = 10-8 m2/s and τ = 3 Myr provide consistent fits to the data (Fig. S-9).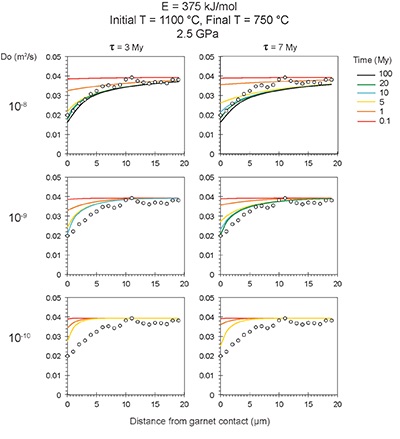
Figure S-9 Modelled Al-diffusion profiles at various times at different values of D0 and constant E (375 kJ/mol), using starting T = 1100 °C, final T = 750 °C.
c. Thermal Evolution of Lithosphere
To model the thermal evolution of the lithosphere after impingement on a cold subducting slab, we assumed the thermal evolution is controlled only by conduction. We thus solved the heat diffusion equation using finite-difference (thermal diffusivity was 10-6 m2/s). The lithosphere was assumed to be 100 km thick. For the initial thermal state, we assumed that the temperature was zero at the surface and 1350 °C at the base of the lithosphere and that the temperature within the lithosphere changed gradually between these two boundary values. Temperature between 90 and 99 km was initially held at 1300 °C to reflect the initially hot magmatic conditions associated with peak Sierran arc magmatism. To model the cooling of the lithosphere by impingement against the cold (750 °C) slab, the boundary condition at the base of the lithosphere was instantaneously changed to 750 °C.Supplementary Information References
Blichert-Toft, J. (2001) On the Lu-Hf Isotope Geochemistry of Silicate Rocks. Geostandards Newsletter 25, 41-56.
 Show in context
Show in context After dissolution in Parr bombs, Sm, Nd, Lu, and Hf were separated from ca. 60-680 mg sized aliquots (depending on degree of sample depletion and sample availability) of garnet and clinopyroxene separates and whole-rock powders by ion-exchange column chromatography, and measured for their isotopic compositions by MC-ICP-MS (Nu Plasma 500 HR) coupled with a desolvating nebuliser DSN-100 according to the procedures of Blichert-Toft et al. (1997, 2002), Blichert-Toft (2001), and Blichert-Toft and Puchtel (2010).
View in article
Blichert-Toft, J., Puchtel, I.S. (2010) Depleted mantle sources through time: Evidence from Lu-Hf and Sm-Nd isotope systematics of Archean komatiites. Earth and Planetary Science Letters 297, 598-606.
 Show in context
Show in context After dissolution in Parr bombs, Sm, Nd, Lu, and Hf were separated from ca. 60-680 mg sized aliquots (depending on degree of sample depletion and sample availability) of garnet and clinopyroxene separates and whole-rock powders by ion-exchange column chromatography, and measured for their isotopic compositions by MC-ICP-MS (Nu Plasma 500 HR) coupled with a desolvating nebuliser DSN-100 according to the procedures of Blichert-Toft et al. (1997, 2002), Blichert-Toft (2001), and Blichert-Toft and Puchtel (2010).
View in article
Blichert-Toft, J., Chauvel, C., Albarède, F. (1997) Separation of Hf and Lu for high-precision isotope analysis of rock samples by magnetic sector-multiple collector ICP-MS. Contributions to Mineralogy and Petrology 127, 248-260.
 Show in context
Show in contextAfter dissolution in Parr bombs, Sm, Nd, Lu, and Hf were separated from ca. 60-680 mg sized aliquots (depending on degree of sample depletion and sample availability) of garnet and clinopyroxene separates and whole-rock powders by ion-exchange column chromatography, and measured for their isotopic compositions by MC-ICP-MS (Nu Plasma 500 HR) coupled with a desolvating nebuliser DSN-100 according to the procedures of Blichert-Toft et al. (1997, 2002), Blichert-Toft (2001), and Blichert-Toft and Puchtel (2010).
View in article
Blichert-Toft, J., Boyet, M., Télouk, P., Albarède, F. (2002) 147Sm/143Nd and 176Lu/176 Hf in eucrites and the differentiation of the HED parent body. Earth and Planetary Science Letters 204, 167-181.
 Show in context
Show in context After dissolution in Parr bombs, Sm, Nd, Lu, and Hf were separated from ca. 60-680 mg sized aliquots (depending on degree of sample depletion and sample availability) of garnet and clinopyroxene separates and whole-rock powders by ion-exchange column chromatography, and measured for their isotopic compositions by MC-ICP-MS (Nu Plasma 500 HR) coupled with a desolvating nebuliser DSN-100 according to the procedures of Blichert-Toft et al. (1997, 2002), Blichert-Toft (2001), and Blichert-Toft and Puchtel (2010).
View in article
Chauvel, C., Blichert-Toft, J. (2001) A hafnium isotope and trace element perspective on melting of the depleted mantle. Earth and Planetary Science Letters 190, 137-151.
 Show in context
Show in context The measured sample values were normalised to the accepted values of 0.511961 ± 0.000013 (corresponding to 0.511856 for La Jolla (Chauvel and Blichert-Toft, 2001)) for 143Nd/144Nd of the “Rennes” in-house Nd standard and 0.282163 ± 0.000009 for 176Hf/177Hf of the JMC-475 Hf standard using sample-standard bracketing.
View in article
Cherniak, D.J., Liang, Y. (2007) Rare earth element diffusion in natural enstatite. Geochimica et Cosmochimica Acta 71, 1324-1340.
 Show in context
Show in context In Figure S-7 we plot all model results using an activation energy E of 375 kJ/mol, D0 from 10-7 to 10-12 m2/s, and for cooling scenarios usingτof 3, 7, and 50 Myr. We adopt an activation energy of ~375 kJ/mol as recommended by Cherniak and Liang (2007) for diffusion of rare earth elements in orthopyroxene. Cherniak and Liang showed that activation energy is not sensitive to ionic radius, so we assume that the activation energy for Al is similar.
View in article
Chin, E.J., Lee, C.-T.A., Luffi, P., Tice, M. (2012) Deep Lithospheric Thickening and Refertilization beneath Continental Arcs: Case Study of the P, T and Compositional Evolution of Peridotite Xenoliths from the Sierra Nevada, California. Journal of Petrology 53, 477-511.
 Show in context
Show in context Whole-rock major element and trace element geochemistry, major and trace element mineral compositions, and petrography for 1026V were previously published in Lee et al. (2001), Lee (2005), and Chin et al. (2012).
View in article
For microphotographs and additional details on petrography and textures in 1026V, the reader is referred to Chin et al. (2012)
View in article
We adopted 750 °C as the final temperature based on an average of temperatures obtained from mineral rim pairs in 1026V (Chin et al., 2012).
View in article
The final temperature of 750 °C corresponds to the final equilibration temperature recorded by garnet-orthopyroxene pairs in 1026V (Chin et al., 2012).
View in article
Harley, S.L., Green, D.H. (1982) Garnet-orthopyroxene barometry for granulites and peridotites. Nature 300, 697-701.
 Show in context
Show in context The thermobarometer of Harley and Green (1982) parameterised as a function of XAl on M1 site of opx versus temperature at constant pressure (top) and XAl on M1 site of opx versus pressure at constant temperature (bottom).
View in article
Al in orthopyroxene in equilibrium with garnet was parameterised as a function of temperature using the garnet-orthopyroxene thermobarometer of Harley and Green (1982).
View in article
Owing to the positive slope in P-T space of Al isopleths for mantle assemblages, at a temperature of 1100 °C, XAl = 0.039 would occur at 2.5 GPa using the Harley and Green (1982) parameterisation scheme discussed above.
View in article
Lee, C.-T.A. (2005) Trace Element Evidence for Hydrous Metasomatism at the Base of the North American Lithosphere and Possible Association with Laramide Low-Angle Subduction. The Journal of Geology 113, 673-685.
 Show in context
Show in context Whole-rock major element and trace element geochemistry, major and trace element mineral compositions, and petrography for 1026V were previously published in Lee et al. (2001), Lee (2005), and Chin et al. (2012).
View in article
Lee, C.-T.A., Rudnick, R.L., Brimhall Jr, G.H. (2001) Deep lithospheric dynamics beneath the Sierra Nevada during the Mesozoic and Cenozoic as inferred from xenolith petrology. Geochemistry Geophysics Geosystems 2.
 Show in context
Show in context Whole-rock major element and trace element geochemistry, major and trace element mineral compositions, and petrography for 1026V were previously published in Lee et al. (2001), Lee (2005), and Chin et al. (2012).
View in article
Pearson, D., Canil, D., Shirey, S. (2003) Mantle samples included in volcanic rocks: xenoliths and diamonds. Treatise on Geochemistry 2, 171-275.
 Show in context
Show in context We have also assumed that the Ca in the garnet does not change significantly, being more of a function of the bulk rock composition than on temperature and pressure (Pearson et al., 2003).
View in article
Scherer, E., Münker, C., Mezger, K. (2001) Calibration of the Lutetium-Hafnium Clock. Science 293, 683-687.
 Show in context
Show in context The decay constants used were 6.54 × 10-12 y-1 for 147Sm and 1.865 × 10-11 y-1 for 176Lu (Scherer et al., 2001).
View in article
Söderlund, U., Patchett, P.J., Vervoort, J.D., Isachsen, C.E. (2004) The 176Lu decay constant determined by Lu-Hf and U-Pb isotope systematics of Precambrian mafic intrusions. Earth and Planetary Science Letters 219, 311-324.
 Show in context
Show in context If the decay constant for 176Lu by Söderlund et al. (2004) of 1.867 × 10-11 is used, the calculated ages are older by 1 per mil, which is within the error of the measurements.
View in article
von Seckendorff, V., O'Neill, H.S.C. (1993) An experimental study of Fe-Mg partitioning between olivine and orthopyroxene at 1173, 1273 and 1423 K and 1.6 GPa. Contributions to Mineralogy and Petrology 113, 196-207.
 Show in context
Show in context We assumed that the Fe/Mg ratio in orthopyroxene is roughly constant given that it is buffered by the bulk rock composition and that the exchange coefficient of Fe/Mg between pyroxenes and olivines are close to 1 and relatively insensitive to temperature and pressure (von Seckendorff and O'Neill, 1993).
View in article
Figures and Tables
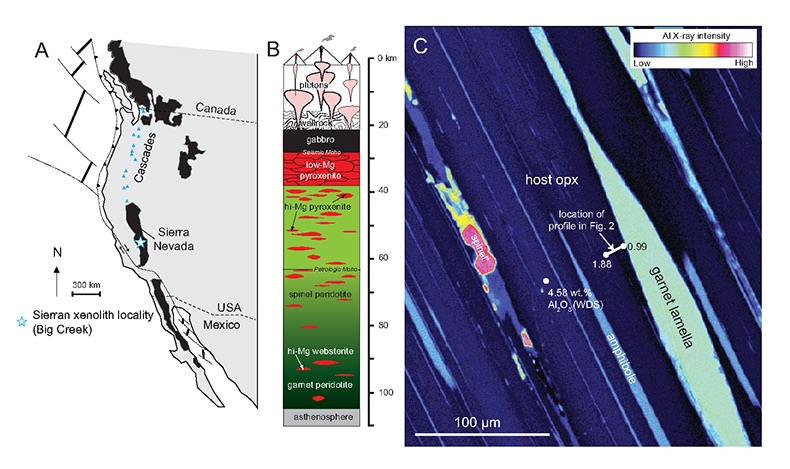
Figure 1 (A) Sample locality map. (B) Vertical architecture of the lithosphere beneath the central Sierra Nevada as sampled by xenoliths (see text for references). (C) Wavelength-dispersive (WDS) elemental map of Al intensity in an orthopyroxene containing garnet and amphibole lamellae in garnet peridotite 1026V from 3 GPa (~90 km). Spots labelled as wt.% Al2O3 correspond to individual, quantitative WDS spot measurements.
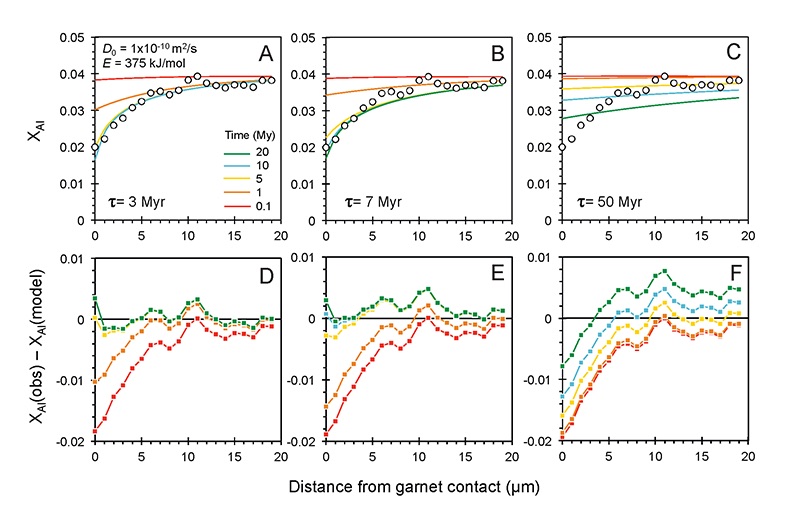
Figure 2 Orthopyroxene Al diffusion modelling of the Al halo transect shown in Figure 1C. Quantitative WDS spot analyses along the transect are plotted as white circles. (A-C) show diffusion profiles at selected times (0.1, 1, 5, 10, 20 Ma) using an activation energy E of 375 kJ/mol and D0 = 10-10 m2/s. Three different cooling scenarios are shown, with e-fold timescales of cooling τ of 3, 7, and 50 Myr. (D) through (F) shows the residual (XAl(observed) – XAl(modelled)) as a function of distance for each of the models, where XAl = atomic Al/2, and Al is the number of cations in the formula for orthopyroxene on a 6 oxygen basis.
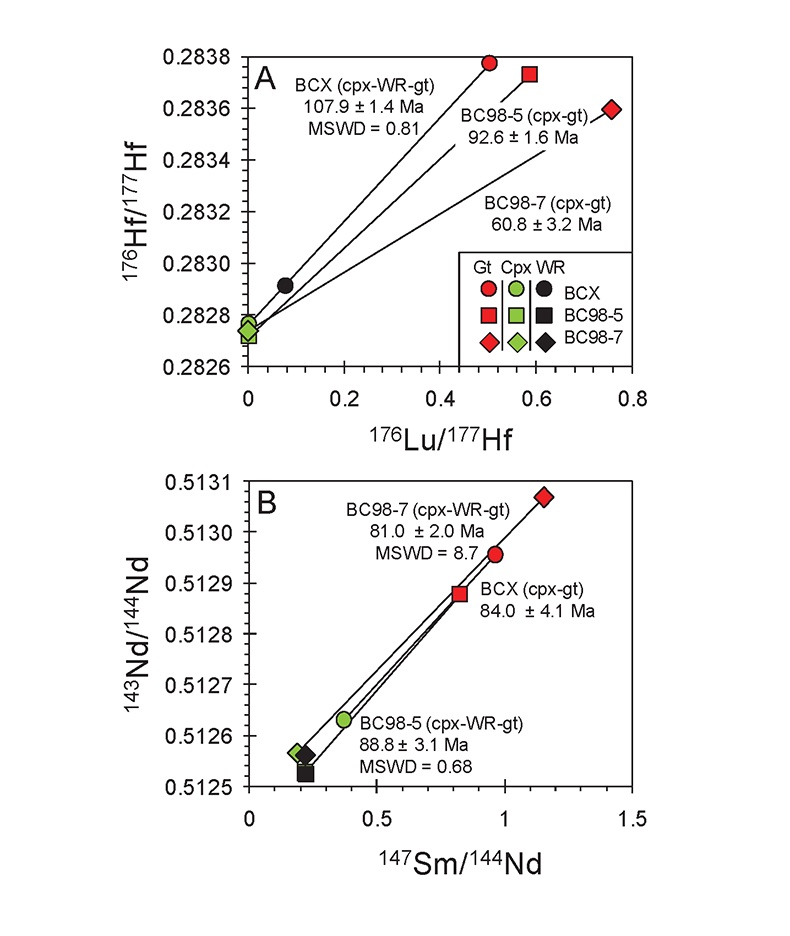
Figure 3 (A) Lu-Hf and (B) Sm-Nd isochrons of Sierran garnet pyroxenite xenoliths. Isochrons were calculated using a MatLab least-squares software by F. Albarède (version 6.0, 2013).
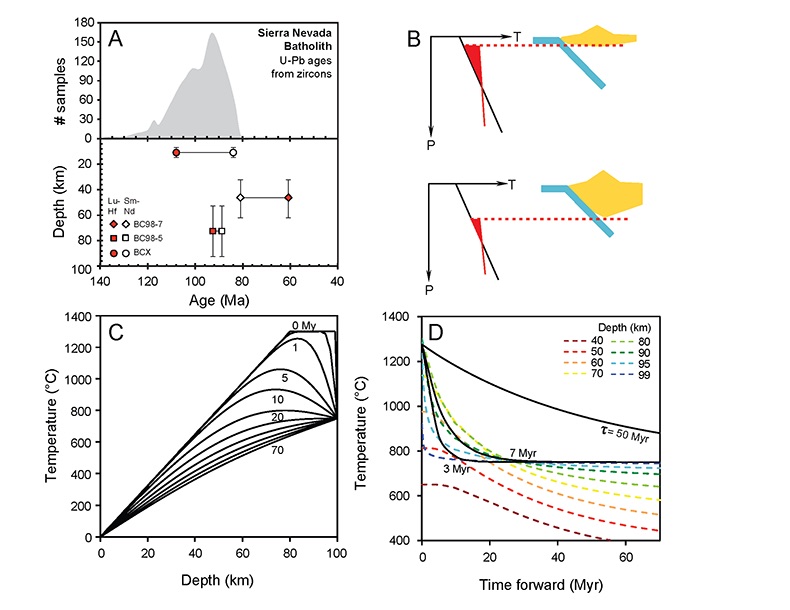
Figure 4 (A) Top panel: U-Pb zircon ages from the Sierra Nevada Batholith (Paterson et al., 2014) showing a major peak at ~95 Ma. Bottom panel: Thermobarometrically constrained depth versus radiometric age of Sierran pyroxenite xenoliths. The vertical bars represent the range of final P, T recorded by several mineral rim pairs in each xenolith (except for BCX, where average REE concentrations were used); the symbols represent the average P, T. (B) Cartoon illustrating the formation of shallow low-MgO pyroxenite (top) and deep high-MgO websterite (bottom). (C) Temperature versus depth diagram showing the initial condition of conductive cooling modelling of a 100-km thick lithosphere at 0 Myr (initial condition) and re-equilibration to a new ambient geotherm after 70 Myr. Thermal diffusivity is 10-6 m2/s. (D) Temperature versus time diagram of depth slices ranging from 40 to 99 km. Superimposed on the diagram are the three cooling scenarios evaluated in the Al-diffusion modelling.
Back to article
Supplementary Figures and Tables
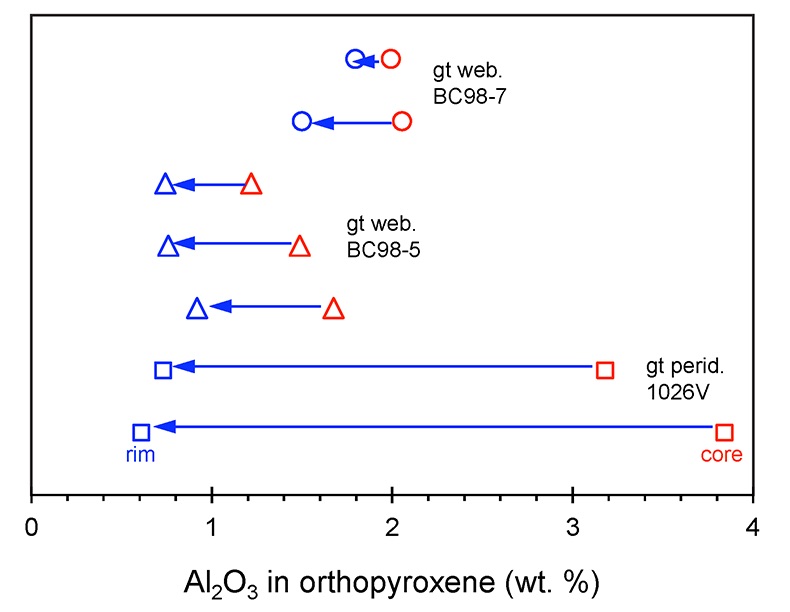
Figure S-1 Al2O3 (wt. %) in orthopyroxene cores and rims in garnet websterites and garnet peridotite. Cores have higher Al contents.

Figure S-2 Plane-polarised light photograph of thick section (~200 μm) of high-MgO websterite BC98-5.
Figure S-3 Plane-polarised light photograph of thick section (~200 μm) of high-MgO websterite BC98-7. (IND51), Nagrasu (IND54), Rudraprayag (IND57-59) and Srinagar (IND62, 64, 65, 72 and 73). Flow is from top to bottom of the image. Vertical exaggeration is 3x.

Figure S-4 Plane-polarised light photograph of thick section (~200 μm) of low-MgO garnet clinopyroxenite BCX.
Figure S-5 Representative “clean” clinopyroxene and garnet separates from BC98-5.
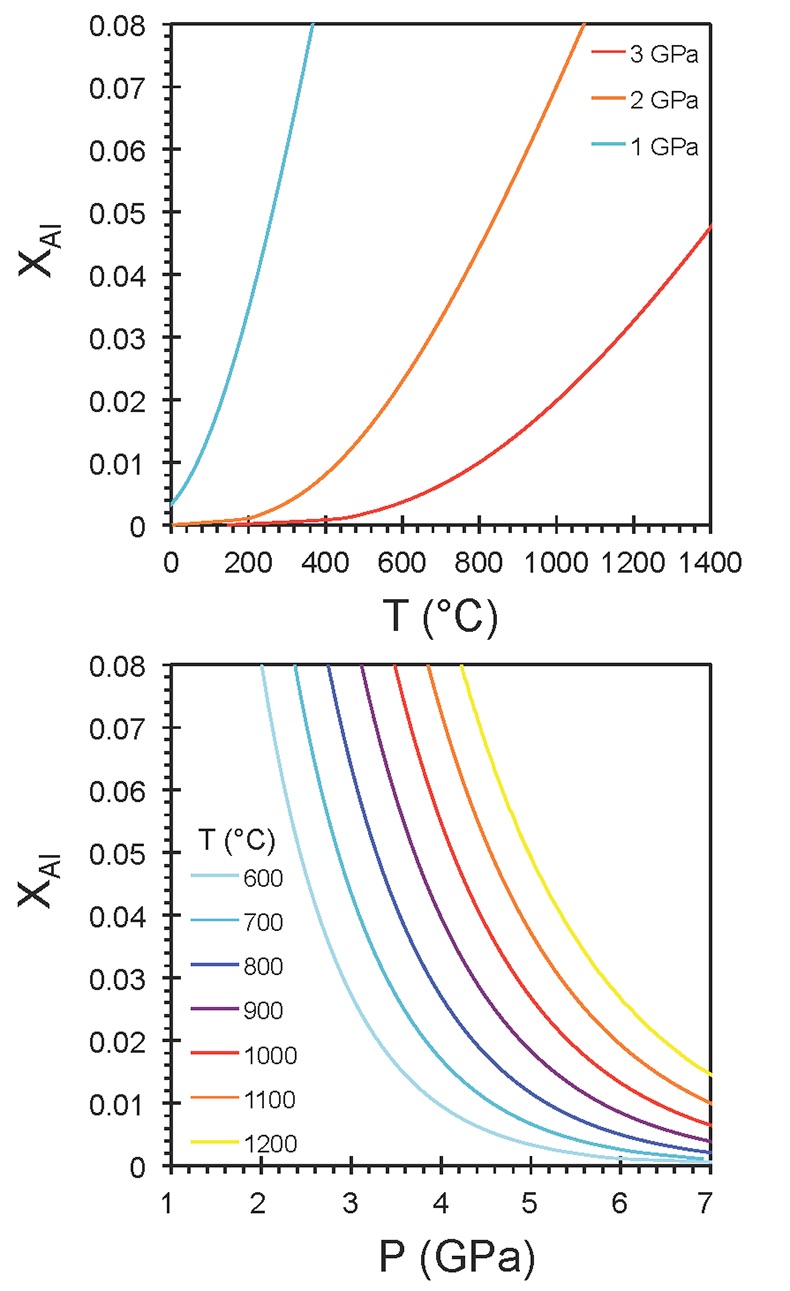
Figure S-6 The thermobarometre of Harley and Green (1982) parameterised as a function of XAl on M1 site of opx versus temperature at constant pressure (top) and XAl on M1 site of opx versus pressure at constant temperature (bottom).
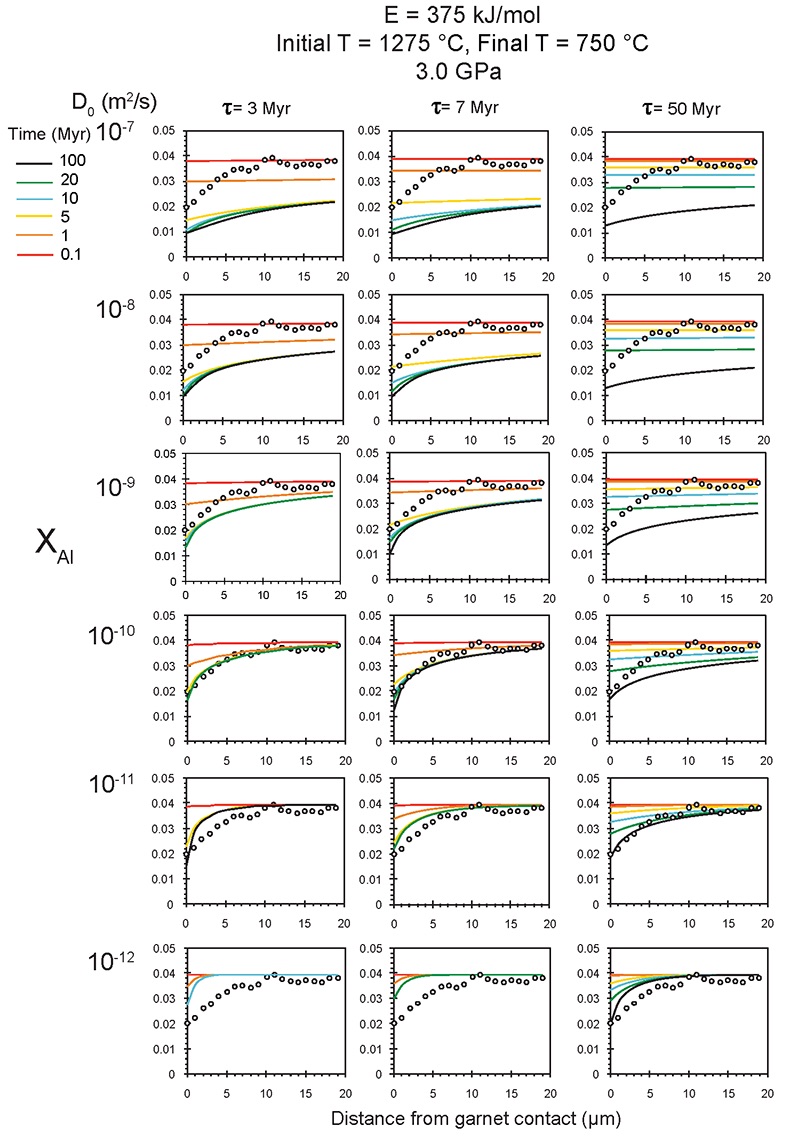
Figure S-7 Modelled Al-diffusion profiles at various times at different values of D0 and constant E (375 kJ/mol), using starting T = 1275 °C, final T = 750 °C. Each column shows models performed at a different cooling scenario.
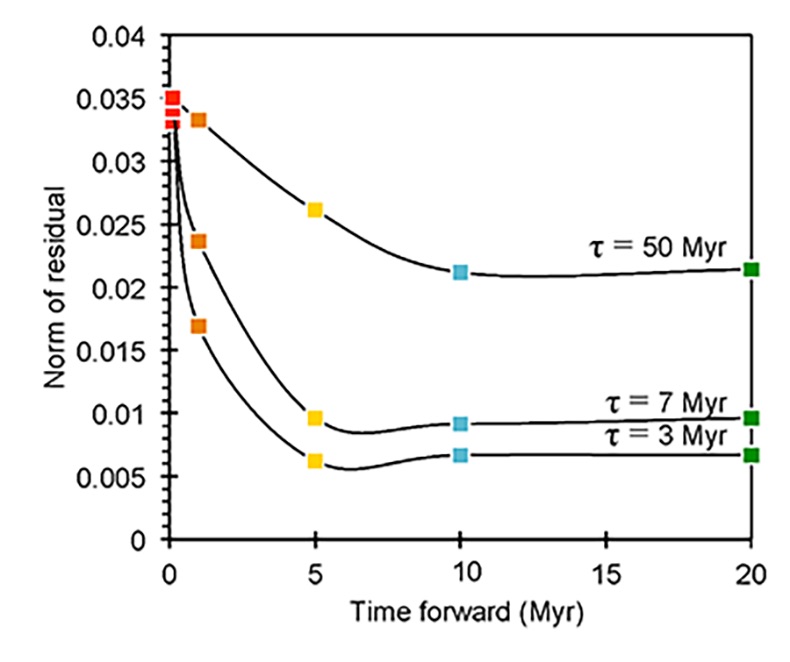
Figure S-8 Norm of residual of Al-diffusion models (E = 375 kJ/mol, D0 = 10-10 m2/s) at 0.1, 1, 5, 10, and 20 Ma and at different cooling scenarios.
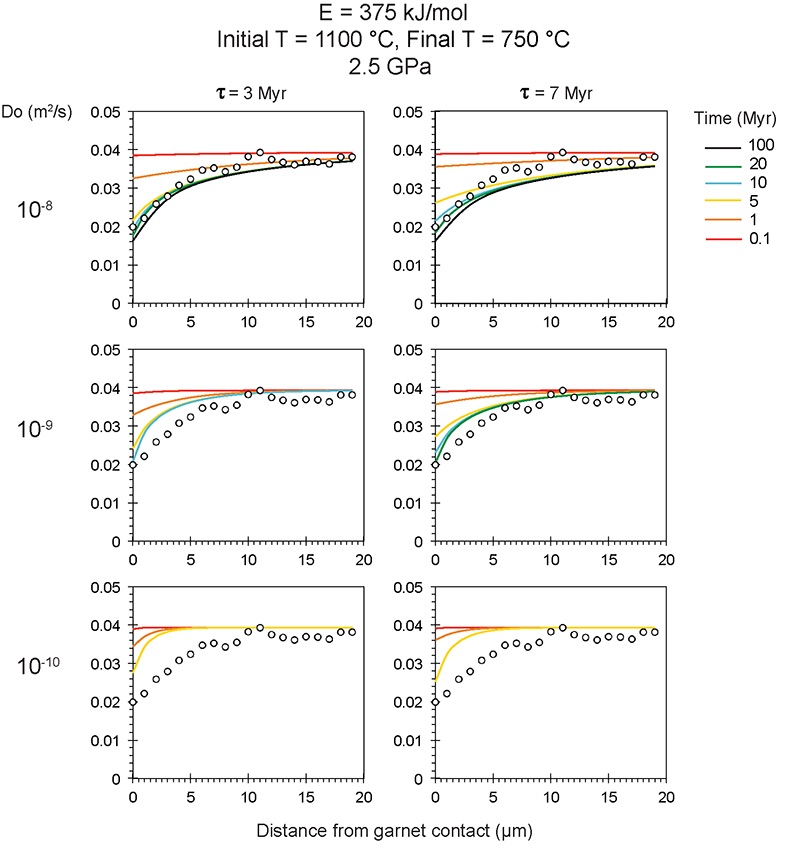
Figure S-9 Modelled Al-diffusion profiles at various times at different values of D0 and constant E (375 kJ/mol), using starting T = 1100 °C, final T = 750 °C.
Table S-1 Major element compositions (wt. %) of orthopyroxene profile in sample 1026V in Figure 1C.
| ID | 1b#7 | 1b#8 | 1b#9 | 1b#10 | 1b#11 | 1b#12 | 1b#13 | 1b#14 | 1b#15 | 1b#16 | 1b#17 | 1b#18 | 1b#19 | 1b#20 | 1b#21 | 1b#22 | 1b#23 | 1b#24 | 1b#25 | 1b#26 | |
| Xmm | 32.2384 | 32.2394 | 32.2404 | 32.2414 | 32.2424 | 32.2434 | 32.2444 | 32.2454 | 32.2464 | 32.2474 | 32.2484 | 32.2494 | 32.2504 | 32.2514 | 32.2524 | 32.2534 | 32.2544 | 32.2554 | 32.2564 | 32.2574 | |
| Ymm | 26.0967 | 26.0972 | 26.0977 | 26.0982 | 26.0987 | 26.0992 | 26.0997 | 26.1002 | 26.1007 | 26.1012 | 26.1017 | 26.1022 | 26.1027 | 26.1032 | 26.1037 | 26.1042 | 26.1047 | 26.1052 | 26.1057 | 26.1062 | |
| at contact with garnet lamella | |||||||||||||||||||||
| SiO2 | 57.53 | 57.37 | 57.13 | 56.86 | 57.44 | 57.00 | 56.97 | 57.10 | 57.22 | 57.03 | 56.59 | 56.41 | 56.65 | 57.25 | 56.90 | 57.20 | 57.16 | 57.08 | 56.77 | 56.57 | |
| TiO2 | 0.013 | 0.022 | 0.028 | 0.002 | 0.014 | 0.005 | 0.035 | 0.035 | 0.017 | 0.023 | 0.01 | 0.019 | 0.023 | 0.005 | 0 | 0.024 | 0.029 | 0.023 | 0.029 | 0.001 | |
| Al2O3 | 0.99 | 1.09 | 1.27 | 1.37 | 1.52 | 1.60 | 1.71 | 1.74 | 1.69 | 1.74 | 1.85 | 1.88 | 1.84 | 1.82 | 1.78 | 1.83 | 1.82 | 1.79 | 1.88 | 1.87 | |
| Cr2O3 | 0.31 | 0.29 | 0.35 | 0.47 | 0.49 | 0.60 | 0.65 | 0.50 | 0.55 | 0.54 | 0.57 | 0.50 | 0.51 | 0.43 | 0.58 | 0.42 | 0.37 | 0.52 | 0.36 | 0.47 | |
| FeOT | 5.84 | 5.58 | 5.45 | 5.39 | 5.34 | 5.45 | 5.64 | 5.75 | 5.52 | 5.48 | 5.68 | 5.74 | 5.64 | 5.68 | 5.36 | 5.68 | 5.53 | 5.74 | 5.83 | 5.91 | |
| MnO | 0.16 | 0.10 | 0.11 | 0.13 | 0.09 | 0.16 | 0.14 | 0.09 | 0.15 | 0.10 | 0.11 | 0.11 | 0.08 | 0.05 | 0.13 | 0.11 | 0.08 | 0.11 | 0.14 | 0.12 | |
| MgO | 35.28 | 34.91 | 34.90 | 34.65 | 34.71 | 34.79 | 34.58 | 34.63 | 34.64 | 34.26 | 32.57 | 31.76 | 34.50 | 34.95 | 34.69 | 34.69 | 34.71 | 34.56 | 34.55 | 34.47 | |
| CaO | 0.10 | 0.10 | 0.11 | 0.13 | 0.14 | 0.13 | 0.13 | 0.11 | 0.12 | 0.12 | 0.11 | 0.14 | 0.12 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.13 | |
| Na2O | 0.022 | 0.032 | 0.007 | 0.007 | 0.003 | 0.007 | 0.001 | 0 | 0.009 | 0.015 | 0.011 | 0.007 | 0.011 | 0.026 | 0 | 0.023 | 0 | 0 | 0 | 0.005 | |
| NiO | 0.06 | 0.10 | 0.07 | 0.06 | 0.02 | 0.06 | 0.05 | 0.08 | 0.06 | 0.07 | 0.02 | 0.08 | 0.17 | 0.10 | 0.01 | 0.14 | 0.14 | 0.05 | 0.14 | 0.08 | |
| Total | 100.29 | 99.59 | 99.42 | 99.06 | 99.76 | 99.79 | 99.91 | 100.04 | 99.98 | 99.38 | 97.51 | 96.65 | 99.54 | 100.42 | 99.56 | 100.21 | 99.94 | 99.98 | 99.80 | 99.61 | |
Table S-2 Orthopyroxene major element compositions (wt. %) in Sierran garnet pyroxenites.
| BC98-7 | BC98-5 | |||||||||
| ID | BC987_01-opxR | BC987_02-opxC | BC987_07-opxR | BC987_08-opxC | BC985_03-opxR | BC985_04-opxC | BC985_07-opxR | BC985_08-opxC | BC985_17-opxR | BC985_18-opxC |
| rim | core | rim | core | rim | core | rim | core | rim | core | |
| x (μm) | 12871 | 12793 | 657 | 273 | 3731 | 3919 | -122 | -96 | 7631 | 7345 |
| y (μm) | 13416 | 13785 | 8599 | 8825 | 23191 | 23429 | 26133 | 25878 | 27889 | 27423 |
| SiO2 | 54.13 | 54.29 | 54.94 | 54.10 | 55.19 | 54.64 | 55.12 | 54.47 | 54.86 | 53.72 |
| TiO2 | 0.043 | 0.02 | 0.034 | 0.02 | 0.02 | 0.013 | 0 | 0.034 | 0.028 | 0.01 |
| Al2O3 | 1.80 | 1.99 | 1.50 | 2.05 | 0.74 | 1.22 | 0.76 | 1.49 | 0.92 | 1.68 |
| Cr2O3 | 0.13 | 0.11 | 0.10 | 0.20 | 0.08 | 0.11 | 0.08 | 0.09 | 0.08 | 0.11 |
| FeOT | 12.80 | 12.85 | 12.92 | 13.07 | 12.32 | 12.38 | 12.71 | 13.14 | 13.19 | 13.55 |
| MnO | 0.14 | 0.14 | 0.17 | 0.15 | 0.11 | 0.11 | 0.11 | 0.15 | 0.16 | 0.16 |
| MgO | 30.80 | 30.57 | 31.14 | 30.76 | 31.45 | 31.07 | 31.32 | 30.45 | 30.53 | 30.18 |
| CaO | 0.25 | 0.21 | 0.20 | 0.16 | 0.12 | 0.17 | 0.10 | 0.19 | 0.20 | 0.20 |
| Na2O | 0.014 | 0.025 | 0.014 | 0.017 | 0.012 | 0.002 | 0.005 | 0.012 | 0.006 | 0 |
| Total | 100.09 | 100.22 | 101.02 | 100.53 | 100.04 | 99.70 | 100.21 | 100.02 | 99.98 | 99.61 |
Table S-3 Clinopyroxene major element compositions (wt. %) in garnet pyroxenites.
| BCX | BC98-7 | BC98-5 | |||||||||||
| ID | BCX_03-cpx | BCX_05-cpx | BCX_08-cpx | BC987_05-cpxR | BC987_06-cpxC | BC987_09-cpxR | BC987_10-cpxC | BC985_05-cpxR | BC985_06-cpxC | BC985_11-cpxR | BC985_12-cpxC | BC985_15-cpxR | BC985_16-cpxC |
| rim next to gt | rim next to gt | rim next to gt | rim | core | rim | core | rim | core | rim | core | rim | core | |
| x (μm) | -6 | -5075 | -4444 | 12686 | 12656 | 769 | 849 | 4675 | 5089 | -470 | -299 | 7454 | 8124 |
| y (μm) | -15851 | -6058 | -13636 | 14152 | 14420 | 8874 | 9171 | 23847 | 24479 | 26791 | 27289 | 28170 | 28080 |
| SiO2 | 54.85 | 54.80 | 54.53 | 53.79 | 53.62 | 54.45 | 53.86 | 54.35 | 53.59 | 54.31 | 53.41 | 54.06 | 52.98 |
| TiO2 | 0.18 | 0.19 | 0.21 | 0.15 | 0.24 | 0.09 | 0.14 | 0.05 | 0.15 | 0.05 | 0.13 | 0.04 | 0.13 |
| Al2O3 | 9.34 | 9.42 | 9.24 | 3.91 | 4.49 | 3.56 | 4.03 | 1.97 | 4.19 | 2.32 | 4.42 | 1.66 | 4.70 |
| Cr2O3 | 0.04 | 0.00 | 0.00 | 0.44 | 0.44 | 0.45 | 0.44 | 0.26 | 0.22 | 0.27 | 0.29 | 0.26 | 0.36 |
| FeOT | 4.14 | 4.17 | 4.19 | 3.73 | 4.04 | 3.50 | 3.65 | 3.60 | 4.18 | 3.59 | 4.22 | 3.48 | 4.37 |
| MnO | 0.002 | 0.029 | 0.037 | 0.084 | 0.056 | 0.033 | 0.037 | 0.076 | 0.098 | 0.037 | 0.051 | 0.041 | 0.057 |
| MgO | 10.57 | 10.57 | 10.60 | 15.00 | 14.50 | 15.16 | 14.96 | 16.17 | 14.77 | 16.03 | 14.73 | 16.37 | 14.41 |
| CaO | 17.04 | 16.76 | 16.97 | 21.81 | 21.38 | 21.83 | 21.61 | 23.22 | 21.97 | 22.95 | 22.12 | 23.25 | 22.08 |
| Na2O | 4.61 | 4.70 | 4.53 | 1.61 | 1.67 | 1.65 | 1.71 | 0.90 | 1.23 | 1.03 | 1.36 | 0.79 | 1.36 |
| Total | 100.78 | 100.64 | 100.30 | 100.51 | 100.44 | 100.72 | 100.44 | 100.59 | 100.40 | 100.58 | 100.72 | 99.93 | 100.46 |
Table S-4 Garnet major element compositions (wt. %) in Sierran garnet pyroxenites.
| BC98-5 | BCX | BC98-7 | ||||||||||||||
| ID | BC985_01-GT1 | BC985_02-GT1 | BC985_09-GTr | BC985_10-GTc | BC985_13-GTc | BC985_14-GTr | BCX_01-GTC | BCX_02-GTr | BCX_04-GTr | BCX_06-GTr | BCX_07-GTC | BCX_09-GT | BC987_03-GTC | BC987_04-GTr | BC987_11-GT1 | BC987_12-GT2 |
| rim | core | core | rim | core | rim | rim | rim | core | core | rim | ||||||
| x (μm) | 3639 | 3671 | -308 | -593 | 7284 | 7366 | 3793 | 8008 | 338 | -4877 | -4680 | -4976 | 11863 | 12152 | 57 | 60 |
| y (μm) | 22892 | 22835 | 26127 | 26358 | 28034 | 28111 | -12444 | -12849 | -16003 | -5904 | -5797 | -13521 | 13995 | 13891 | 9855 | 9811 |
| SiO2 | 40.15 | 40.13 | 40.01 | 40.23 | 39.77 | 40.00 | 39.35 | 39.16 | 39.31 | 39.08 | 39.05 | 39.19 | 40.23 | 40.30 | 40.28 | 39.96 |
| TiO2 | 0.044 | 0.093 | 0.048 | 0.104 | 0.104 | 0.017 | 0.14 | 0.073 | 0.16 | 0.05 | 0.107 | 0.093 | 0.077 | 0.053 | 0.062 | 0.058 |
| Al2O3 | 22.94 | 22.86 | 22.84 | 22.62 | 22.58 | 22.60 | 22.20 | 22.10 | 22.56 | 22.58 | 22.25 | 22.46 | 22.72 | 22.90 | 22.84 | 22.81 |
| Cr2O3 | 0.34 | 0.37 | 0.42 | 0.34 | 0.39 | 0.38 | 0 | 0.04 | 0 | 0.03 | 0.02 | 0.02 | 0.47 | 0.52 | 0.51 | 0.49 |
| FeOT | 18.00 | 18.16 | 18.97 | 18.09 | 18.94 | 19.20 | 22.73 | 21.14 | 20.73 | 20.69 | 20.96 | 20.74 | 17.93 | 18.79 | 18.45 | 18.43 |
| MnO | 0.56 | 0.57 | 0.53 | 0.42 | 0.54 | 0.57 | 0.66 | 0.56 | 0.53 | 0.56 | 0.53 | 0.60 | 0.56 | 0.57 | 0.53 | 0.57 |
| MgO | 13.10 | 12.94 | 12.31 | 12.81 | 12.28 | 12.14 | 9.43 | 7.41 | 7.95 | 7.40 | 7.48 | 7.36 | 13.42 | 12.70 | 13.14 | 13.21 |
| CaO | 5.61 | 5.61 | 5.61 | 5.65 | 5.51 | 5.50 | 6.26 | 9.89 | 9.58 | 10.38 | 10.17 | 10.46 | 5.19 | 5.22 | 5.22 | 5.29 |
| Na2O | 0.02 | 0.025 | 0.014 | 0.02 | 0.005 | 0.019 | 0.056 | 0.024 | 0.067 | 0.003 | 0.039 | 0.02 | 0.014 | 0.012 | 0.02 | 0.002 |
| Total | 100.76 | 100.75 | 100.75 | 100.27 | 100.11 | 100.42 | 100.82 | 100.41 | 100.88 | 100.77 | 100.60 | 100.94 | 100.62 | 101.07 | 101.05 | 100.81 |
Table S-5 Sm-Nd and Lu-Hf concentrations and isotope compositions of Sierran pyroxenites.
gt = garnet
cpx = clinopyroxene
wr = whole-rock
kgt = kelyphite-rimmed garnet
| Sample | Sm (ppm) | Nd (ppm) | 147Sm/144Nd | 143Nd/144Nd | 2σ | Lu (ppm) | Hf (ppm) | 176Lu/177Hf | 176Hf/177Hf | 2σ |
| BC98-7 gt | 0.563 | 0.295 | 1.152 | 0.513068 | 0.000008 | 0.455 | 0.0852 | 0.7581 | 0.283596 | 0.000044 |
| BC98-7 cpx | 2.00 | 6.50 | 0.1858 | 0.512566 | 0.000004 | 0.00628 | 0.912 | 0.0009766 | 0.282737 | 0.000005 |
| BC98-7 wr | 0.769 | 2.13 | 0.2178 | 0.512562 | 0.000004 | 0.134 | 0.360 | 0.05285 | 0.283094 | 0.000005 |
| BC98-5 kgt | 0.727 | 0.533 | 0.8257 | 0.512877 | 0.000006 | 0.769 | 0.186 | 0.5871 | 0.283728 | 0.000014 |
| BC98-5 cpx | 2.34 | 6.51 | 0.2173 | 0.512526 | 0.000005 | 0.00946 | 1.07 | 0.001252 | 0.282716 | 0.000004 |
| BC98-5 wr | 1.48 | 4.04 | 0.2215 | 0.512523 | 0.000005 | 0.158 | 0.817 | 0.02749 | 0.282841 | 0.000014 |
| BCX gt | 3.50 | 2.19 | 0.9639 | 0.512956 | 0.000005 | 1.22 | 0.344 | 0.5037 | 0.283774 | 0.000010 |
| BCX gt dupl | 1.25 | |||||||||
| BCX cpx | 1.11 | 1.80 | 0.3718 | 0.512630 | 0.000012 | 0.0197 | 1.10 | 0.002529 | 0.282767 | 0.000003 |
| BCX cpx dupl | 0.0197 | 1.09 | 0.002574 | |||||||
| BCX wr | 2.79 | 4.73 | 0.3563 | 0.512702 | 0.000004 | 0.514 | 0.936 | 0.07795 | 0.282913 | 0.000004 |