Tracing Earth’s O2 evolution using Zn/Fe ratios in marine carbonates
Affiliations | Corresponding Author | Cite asLiu, X.-M., Kah, L.C., Knoll, A.H., Cui, H., Kaufman, A.J., Shahar, A., Hazen, R.M. (2016) Tracing Earth’s O2 evolution using Zn/Fe ratios in marine carbonates. Geochem. Persp. Let. 2, 24-34.
- Share this article




-
Article views:13,553Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
Abstract
Figures and Tables
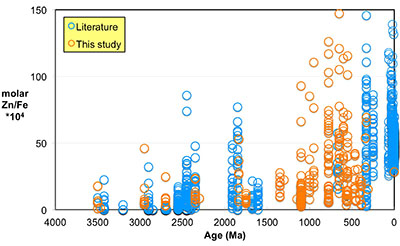 Figure 1 Zn/Fe molar ratios versus time for individual carbonate analyses. The figure contains ~1700 measurements of Zn/Fe data, including literature data (blue), and our 300 new analyses (orange). | 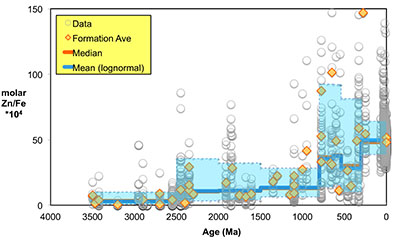 Figure 2 Zn/Fe molar ratio versus time for carbonates, averaged by formation. Formation averages (orange diamonds) were calculated based on simple arithmetic mean of samples within the same formation. Median (orange) and mean from lognormal distribution (blue) lines were calculated based on all samples from the designated time intervals. Estimated Zn/Fe ratio curve through Earth’s history. Uncertainties (light blue fields) are estimated based on one standard deviation from the lognormal distribution. | 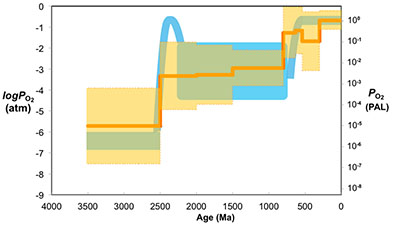 Figure 3 Estimated atmospheric pO2 through Earth’s history. The orange line indicates the best estimate (mean values from lognormal distribution) from carbonate Zn/Fe ratios from this study (yellow fields show the upper and lower range of estimated O2, which is calculated based on one sigma of lognormal distributions). The blue field indicates semi-quantitative interpretation from current understanding of the atmospheric O2 curve (modified from Lyons et al., 2014). |
| Figure 1 | Figure 2 | Figure 3 |
Supplementary Figures and Tables
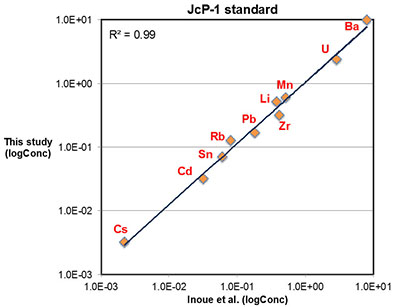 Figure S-1 Comparison of measured trace elements with those reported for Inoue et al. (2004) for the JcP-1 standard. The full analytical method is discussed in the methods section following the main text. | 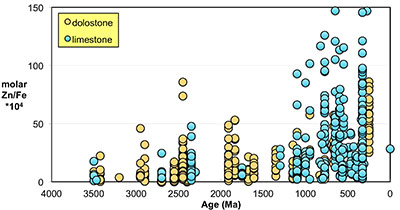 Figure S-2 Zn/Fe ratios in marine carbonate, plotted with information on sample lithology. | 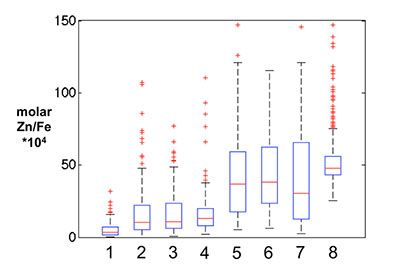 Figure S-3 Box-whisker distribution of all samples. The sample population is divided into eight bins (Bin 1: 3.5-2.5 Ga, Bin 2: 2.5-2.0 Ga, Bin 3: 2.0-1.5 Ga, Bin 4: 1.5-0.8 Ga, Bin 5: 800-635 Ma, Bin 6: 635-541 Ma, Bin 7: 541-300 Ma, Bin 8: 300-0 Ma) of different duration to make sure each bin has statistically meaningful sample numbers (where n > 50, except for one bin with n = 38). Each bin contains samples from at least two different geological formations. We show a Box-whisker plot for each group. Median values are indicated by the red lines and each individual box includes 50 % samples and whiskers mark the 3 sigma boundaries of the group population. Red crosses fall out of whiskers and are considered outliers. | 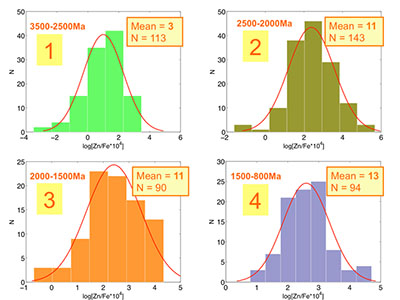 Figure S-4 Histograms of Zn/Fe ratios with lognormal fitting in red. We group all data into eight different bins (age distribution of the bins is provided in Fig. S-2) and plot the lognormal distribution for each group. |
| Figure S-1 | Figure S-2 | Figure S-3 | Figure S-4 |
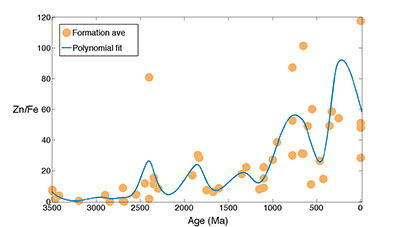 Figure S-5 Zn/Fe molar ratio versus time for carbonates averaged by formation. A polynomial fit through the formation average data. | 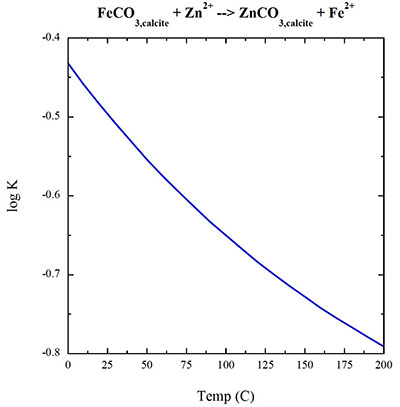 Figure S-6 log K (equilibrium constant) versus temperature plot for chemical reaction: FeCO3 + Zn2+ --> ZnCO3 + Fe2+. |  Table S-1 Zn/Fe ratios with sample name and age information from this study. |
| Figure S-5 | Figure S-6 | Table S-1 |
top
Introduction
Earth’s O2–rich atmosphere, unique among known planets, has played an essential role in evolving feedbacks between life and environment. Atmospheric O2 was extremely low in the Archean Eon (>2.5 Ga), and while multiple lines of evidence suggest that Earth’s oxygenation was protracted (Kah et al., 2004
Kah, L.C., Lyons, T.W., Frank, T.D. (2004) Low marine sulphate and protracted oxygenation of the proterozoic biosphere. Nature 431, 834-838.
; Kah and Bartley, 2011Kah, L.C., Bartley, J.K. (2011) Protracted oxygenation of the Proterozoic biosphere. International Geology Review 53, 1424-1442.
; Lyons et al., 2014Lyons, T.W., Reinhard, C.T., Planavsky, N.J. (2014) The rise of oxygen in Earth's early ocean and atmosphere. Nature 506, 307-315.
; Planavsky et al., 2014Planavsky, N.J., Reinhard, C.T., Wang, X., Thomson, D., McGoldrick, P., Rainbird, R.H., Johnson, T., Fischer, W.W., Lyons, T.W. (2014) Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 346, 635-638.
), pO2 may have risen abruptly at two different points in time: first during the “Great Oxygenation Event” (GOE) at ~2.4 Ga (Canfield, 2005Canfield, D.E. (2005) The early history of atmospheric oxygen: Homage to Robert A. Garrels. Annual Review of Earth and Planetary Sciences 33, 1-36.
; Holland, 2006Holland, H.D. (2006) The oxygenation of the atmosphere and oceans. Philosophical Transactions of the Royal Society B: Biological Sciences 361, 903-915.
; Guo et al., 2009Guo, Q.J., Strauss, H., Kaufman, A.J., Schroder, S., Gutzmer, J., Wing, B., Baker, M.A., Bekker, A., Jin, Q.S., Kim, S.T., Farquhar, J. (2009) Reconstructing Earth's surface oxidation across the Archean-Proterozoic transition. Geology 37, 399-402.
; Farquhar et al., 2011Farquhar, J., Zerkle, A., Bekker, A. (2011) Geological constraints on the origin of oxygenic photosynthesis. Photosynthesis Research 107, 11-36.
), when atmospheric O2 rose from <0.001 % to an intermediate value commonly estimated as 1 to 10 % of the current level (Farquhar et al., 2000Farquhar, J., Bao, H.M., Thiemens, M. (2000) Atmospheric influence of Earth's earliest sulfur cycle. Science 289, 756-758.
; Pavlov and Kasting, 2002Pavlov, A.A., Kasting, J.F. (2002) Mass-independent fractionation of sulfur isotopes in Archean sediments: Strong evidence for an anoxic Archean atmosphere. Astrobiology 2, 27-41.
), and again during a “Neoproterozoic Oxygenation Event” (NOE) at ~800 to 542 million years ago (Canfield and Teske, 1996Canfield, D.E., Teske, A. (1996) Late Proterozoic rise in atmospheric oxygen concentration inferred from phylogenetic and sulphur-isotope studies. Nature 382, 127-132.
; Fike et al., 2006Fike, D.A., Grotzinger, J.P., Pratt, L.M., Summons, R.E. (2006) Oxidation of the Ediacaran Ocean. Nature 444, 744-747.
; Frei et al., 2009Frei, R., Gaucher, C., Poulton, S.W., Canfield, D.E. (2009) Fluctuations in Precambrian atmospheric oxygenation recorded by chromium isotopes. Nature 461, 250-U125.
; Och and Shields-Zhou, 2012Och, L.M., Shields-Zhou, G.A. (2012) The Neoproterozoic oxygenation event: Environmental perturbations and biogeochemical cycling. Earth-Science Reviews 110, 26-57.
). The latter transition may well have continued into the Phanerozoic Eon, eventually resulting in near-present O2 (Berner, 2006Berner, R.A. (2006) GEOCARBSULF: A combined model for Phanerozoic atmospheric O2 and CO2. Geochimica et Cosmochimica Acta 70, 5653-5664.
; Dahl et al., 2010Dahl, T.W., Hammarlund, E.U., Anbar, A.D., Bond, D.P.G., Gill, B.C., Gordon, G.W., Knoll, A.H., Nielsen, A.T., Schovsbo, N.H., Canfield, D.E. (2010) Devonian rise in atmospheric oxygen correlated to the radiations of terrestrial plants and large predatory fish. Proceedings of the National Academy of Sciences of the United States of America 107, 17911-17915.
; Sperling et al., 2015Sperling, E.A., Wolock, C.J., Morgan, A.S., Gill, B.C., Kunzmann, M., Halverson, G.P., Macdonald, F.A., Knoll, A.H., Johnston, D.T. (2015) Statistical analysis of iron geochemical data suggests limited late Proterozoic oxygenation. Nature 523, 451-454.
).Redox-sensitive major and trace elements in iron formations and black shales deposited beneath euxinic waters have been developed as proxies to reconstruct palaeoenvironmental history in deep time (Scott et al., 2008
Scott, C., Lyons, T.W., Bekker, A., Shen, Y., Poulton, S.W., Chu, X., Anbar, A.D. (2008) Tracing the stepwise oxygenation of the Proterozoic ocean. Nature 452, 456-U5.
; Konhauser et al., 2009Konhauser, K.O., Pecoits, E., Lalonde, S.V., Papineau, D., Nisbet, E.G., Barley, M.E., Arndt, N.T., Zahnle, K., Kamber, B.S. (2009) Oceanic nickel depletion and a methanogen famine before the Great Oxidation Event. Nature 458, 750-753.
; Sahoo et al., 2012Sahoo, S.K., Planavsky, N.J., Kendall, B., Wang, X., Shi, X., Scott, C., Anbar, A.D., Lyons, T.W., Jiang, G. (2012) Ocean oxygenation in the wake of the Marinoan glaciation. Nature 489, 546-549.
). The paucity of these facies in many Proterozoic successions, however, limits the continuity of current reconstructions of Earth’s oxygenation. Here we provide evidence for the hypothesis that carbonate-based redox proxies can provide an independent estimate of past pO2, expanding the palaeoredox record in time and space (Hardisty et al., 2014Hardisty, D.S., Lu, Z., Planavsky, N.J., Bekker, A., Philippot, P., Zhou, X., Lyons, T.W. (2014) An iodine record of Paleoproterozoic surface ocean oxygenation. Geology doi: 10.1130/G35439.1.
). Limestone and penecontemporaneous dolomites that retain depositional signatures well (Wilson et al., 2010Wilson, J.P., Fischer, W.W., Johnston, D.T., Knoll, A.H., Grotzinger, J.P., Walter, M.R., McNaughton, N.J., Simon, M., Abelson, J., Schrag, D.P., Summons, R., Allwood, A., Andres, M., Gammon, C., Garvin, J., Rashby, S., Schweizer, M., Watters, W.A. (2010) Geobiology of the late Paleoproterozoic Duck Creek Formation, Western Australia. Precambrian Research 179, 135-149.
) are abundant in the geologic record, typically recording shallow marine environments that would have been in open communication with the overlying atmosphere. Palaeoenvironmental research on carbonate rocks commonly focuses on individual stratigraphic successions; here we adopt a complementary strategy, analysing a large suite of Phanerozoic, Proterozoic, and Archean samples that enables us to make statistical statements (Sperling et al., 2015Sperling, E.A., Wolock, C.J., Morgan, A.S., Gill, B.C., Kunzmann, M., Halverson, G.P., Macdonald, F.A., Knoll, A.H., Johnston, D.T. (2015) Statistical analysis of iron geochemical data suggests limited late Proterozoic oxygenation. Nature 523, 451-454.
) about Zn/Fe in the global surface ocean through geologic time. More importantly, we develop a new tool to provide quantitative constraints on atmospheric pO2 through Earth history.In the modern ocean, zinc input from hydrothermal ridge systems (~4.4 x 109 mol yr-1) is an order of magnitude greater than riverine fluxes (~3.4 x 108 mol yr-1; Robbins et al., 2013
Robbins, L.J., Lalonde, S.V., Saito, M.A., Planavsky, N.J., Mloszewska, A.M., Pecoits, E., Scott, C., Dupont, C.L., Kappler, A., Konhauser, K.O. (2013) Authigenic iron oxide proxies for marine zinc over geological time and implications for eukaryotic metallome evolution. Geobiology 11, 295-306.
). As an essential nutrient in many phytoplankton enzymes, especially those of eukaryotes (Williams and da Silva, 1996Williams, R.J.P., da Silva, J.J.R.F. (1996) The natural selection of the chemical elements. Great Britian, Bath Press Ltd.
), zinc plays an important role in marine primary production, and for this reason, Zn is depleted in surface waters relative to the deep sea (Morel and Price, 2003Morel, F.M.M., Price, N.M. (2003) The Biogeochemical Cycles of Trace Metals in the Oceans. Science 300, 944-947.
). Zn concentrations in euxinic black shale and iron formations (Robbins et al., 2013Robbins, L.J., Lalonde, S.V., Saito, M.A., Planavsky, N.J., Mloszewska, A.M., Pecoits, E., Scott, C., Dupont, C.L., Kappler, A., Konhauser, K.O. (2013) Authigenic iron oxide proxies for marine zinc over geological time and implications for eukaryotic metallome evolution. Geobiology 11, 295-306.
; Scott et al., 2013Scott, C., Planavsky, N.J., Dupont, C.L., Kendall, B., Gill, B.C., Robbins, L.J., Husband, K.F., Arnold, G.L., Wing, B.A., Poulton, S.W., Bekker, A., Anbar, A.D., Konhauser, K.O., Lyons, T.W. (2013) Bioavailability of zinc in marine systems through time. Nature Geoscience 6, 125-128.
), however, suggest that the bioavailability of Zn has not changed dramatically through Earth history. The Fe budget is similar to that of Zn, wherein hydrothermal input dominates over riverine fluxes by a factor of ~9 (Wheat et al., 2002Wheat, C.G., Mottl, M.J., Rudnicki, M. (2002) Trace element and REE composition of a low-temperature ridge-flank hydrothermal spring. Geochimica et Cosmochimica Acta 66, 3693-3705.
). Under sulphidic conditions, dissolved Zn2+ and Fe2+ behave similarly and are rapidly precipitated as sulphides (Morse and Luther III, 1999Morse, J.W., Luther III, G.W. (1999) Chemical influences on trace metal-sulfide interactions in anoxic sediments. Geochimica et Cosmochimica Acta 63, 3373-3378.
). In addition, because both Fe and Zn behave as incompatible elements during mantle partial melting, Zn/Fe has been developed as a tracer of mantle redox, revealing that the oxygen fugacity of the upper mantle has remained relatively constant through Earth history (Lee et al., 2010Lee, C.T.A., Luffi, P., Le Roux, V., Dasgupta, R., Albarede, F., Leeman, W.P. (2010) The redox state of arc mantle using Zn/Fe systematics. Nature 468, 681-685.
). In the following discussion, we assume that Zn/Fe in hydrothermal inputs into the ocean have not changed significantly through time. We recognise, however, that a number of factors could limit this assumption, and consider these below.Zn/Fe in the sedimentary record thus has the potential to document Earth surface redox evolution if we consider the following assumptions: 1) Zn and Fe budgets in the oceans are dominated by hydrothermal inputs and are therefore not significantly influenced by secular evolution of continental inputs; 2) Fe2+ and Zn2+ have similar solubility in the oceans; 3) the partition coefficient of Zn/Fe ratios into carbonates has remained the same through time; and 4) when Fe2+ is oxidised to Fe3+, it precipitates from seawater and thus is not incorporated into carbonate; zinc, however, remains divalent as Zn2+.
top
Methods
Major, trace, and rare earth elements (REE) concentrations were determined with a Thermo Scientific® iCAP-Q ICP-MS (Inductively Coupled Plasma – Mass Spectrometry) at the Carnegie Institution of Washington. Approximately 5 to 10 mg of micro-drilled sample powders were weighed and dissolved in 2 ml distilled 0.4 M HNO3 and reacted for 12 hours. The resulting solutions were centrifuged for 5 minutes at ~6000 rps and 1 ml of the supernatant was pipetted and diluted with distilled 4 ml 0.4 M HNO3 for elemental analysis. Calibration curves were created using multi-elemental standards with different dilutions made from pure element solutions (Alfa Aesar®). Both standard and sample solutions were doped with 4 ppb In to correct for instrumental drift. Precision of the analyses was determined by repeated analyses of an in-house carbonate standard, and was typically better than 5 % (2σ) for major elements, and better than 10 % (2σ) for most trace elements including REE. Accuracy of the analyses was determined by replicates of an international coral standard (JCp-1), as shown in Figure S-1.
top
Results and Discussion
Here, we report Zn/Fe molar ratios in marine carbonate rock through Earth history (Fig. 1) and provide a quantification of atmospheric O2 evolution since the Mesoarchean Era. Samples (n = 1700) come from our analyses (n = 300), as well as a literature compilation (see SI-1 Table S-1). In all carbonate samples, the potential for diagenetic alteration is of concern. To evaluate the degree of sample alteration, we selected specimens with known sedimentological and stratigraphic context and investigated their petrography and elemental and isotope geochemistry. Samples used in this study were primarily composed of fine-grained limestone and penecontemporaenous, fabric-retentive dolostone, including both micrites and stromatolites. We micro-sampled carbonate specimens from polished billets to avoid weathering alteration, secondary veins/precipitation, and areas with visible non-carbonate phases. In addition to geological and petrographic criteria, we further selected samples based on primary isotopic and trace element patterns (see SI-2).

Figure 1 Zn/Fe molar ratios versus time for individual carbonate analyses. The figure contains ~1700 measurements of Zn/Fe data, including literature data (blue), and our 300 new analyses (orange).
Even if we carefully select the most primary samples, we cannot ignore diagenetic influences on the elemental composition of sampled carbonates, as this can contribute to local variation of Zn/Fe (Fig. 1). Both Zn and Fe partition coefficients (Kd) from fluid to carbonates increase with increasing diagenesis as shown by earlier work of Brand and Veizer (1980)
Brand, U., Veizer, J. (1980) Chemical diagenesis of a multicomponent carbonate system; 1, Trace elements. Journal of Sedimentary Research 50, 1219-1236.
. Also, Kd (Fe) increases faster compared to Kd (Zn), from 1 to 20 and from 5.2 to 5.5 for Fe and Zn, respectively. According to this work, diagenesis will cause a decrease in Zn/Fe ratios by incorporating more Fe than Zn in carbonates. We acknowledge that all of the carbonates examined here have undergone some degree of burial diagenesis, and this will be reflected in the variance of Zn/Fe within individual time intervals. Also, local primary production differences may contribute to Zn/Fe variability of different formations from the same interval. In the modern oxidised shallow ocean, particulate Fe sourced from eroding continents remains biogeochemically labile and may be cycled back to a dissolved phase during diagenesis in reducing continental margin sediments (Raiswell et al., 2006Raiswell, R., Tranter, M., Benning, L.G., Siegert, M., De’ath, R., Huybrechts, P., Payne, T. (2006) Contributions from glacially derived sediment to the global iron (oxyhydr)oxide cycle: Implications for iron delivery to the oceans. Geochimica et Cosmochimica Acta 70, 2765-2780.
). Therefore, there is also a potentially large and variable source of reactive Fe to shallow marine settings that is decoupled from the Zn flux, which likely causes Zn/Fe ratios to be lower and therefore could contribute to the variations observed in Zn/Fe data. In addition, theoretical calculations suggest that kinetic effects on trace element partitioning in carbonate may contribute to Zn/Fe variability in samples from the same locality (Watson, 2004Watson, E.B. (2004) A conceptual model for near-surface kinetic controls on the trace-element and stable isotope composition of abiogenic calcite crystals. Geochimica et Cosmochimica Acta 68, 1473-1488.
; DePaolo, 2011DePaolo, D.J. (2011) Surface kinetic model for isotopic and trace element fractionation during precipitation of calcite from aqueous solutions. Geochimica et Cosmochimica Acta 75, 1039-1056.
). Importantly, however, these influences should not result in systematic variations that would contribute to observed first-order secular changes. We plot all carbonate samples based on their lithology in Figure S-2; this shows that there is no systematic difference between limestone and dolomite samples through time - not unexpected, as many Proterozoic dolomites formed penecontemporaneously and preserve geochemical signatures as well as coeval limestones that underwent neomorphism during burial.We observe a distinct trend of increasing Zn/Fe through time (Fig. 1), especially around the GOE and NOE. Our Palaeoproterozoic data are also consistent with earlier suggestions that pO2 may have risen substantially during the GOE and then declined again to persistent Proterozoic values (Lyons et al., 2014
Lyons, T.W., Reinhard, C.T., Planavsky, N.J. (2014) The rise of oxygen in Earth's early ocean and atmosphere. Nature 506, 307-315.
). Employing three statistically complementary approaches (see details in SI-3), carbonate Zn/Fe could follow “step” or “smooth” fits through Earth’s history (Figs. S-3, S-4 and S-5), where we prefer the “step” approach with lognormal distributions (see Figs. 2 and S-3). Using lognormal distributions to estimate Zn/Fe through time, we can provide quantitative constraints on Earth’s atmospheric O2 evolution, as follows.From the chemical reaction of Fe oxidisation from Fe2+ to Fe3+:
4Fe2+ (aq) + O2 (aq) + 10H2O (aq) = 4Fe(OH)3 (s) + 8H+ (aq),
Eq. 1
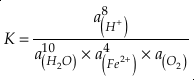
where K is the equilibrium constant and
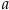 is activity. In this equation, we assume that when Fe2+ oxidises to Fe3+ and is precipitated from the aqueous system as iron hydroxide, and only Fe2+ gets incorporated into carbonates. We are aware that secular variations in seawater sulphate might modulate hydrothermal iron fluxes through time via the formation of iron sulphides (Kump and Seyfried, 2005), we do not know the extent to which Zn abundances might similarly be buffered and so do not consider this in our first-order model. Assuming O2 equilibrium between atmosphere and surface ocean on hundred million year time scales, we can write the equation using atmospheric oxygen fugacity,
is activity. In this equation, we assume that when Fe2+ oxidises to Fe3+ and is precipitated from the aqueous system as iron hydroxide, and only Fe2+ gets incorporated into carbonates. We are aware that secular variations in seawater sulphate might modulate hydrothermal iron fluxes through time via the formation of iron sulphides (Kump and Seyfried, 2005), we do not know the extent to which Zn abundances might similarly be buffered and so do not consider this in our first-order model. Assuming O2 equilibrium between atmosphere and surface ocean on hundred million year time scales, we can write the equation using atmospheric oxygen fugacity, 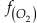 , as
, asEq. 2
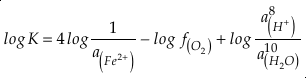
if we assume the Zn concentrations in seawater and partitioning of Zn/Fe from seawater to carbonate minerals are constant over Earth history. Therefore, we can write the equation normalised to Zn2+ as
Eq. 3
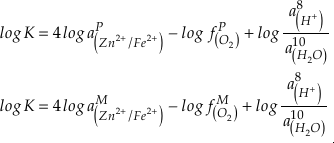
in which superscripts P and M indicate the past and modern parameters. Assuming pH and K are constant (see SI-4), we can simplify the relationship between Fe/Zn ratios and
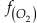 , as
, asEq. 4
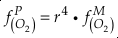
where
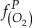 is the oxygen fugacity in the past (any time in Earth’s history),
is the oxygen fugacity in the past (any time in Earth’s history), 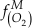 is the oxygen fugacity in modern time, and
is the oxygen fugacity in modern time, andEq. 5
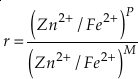
provides Zn/Fe ratios in past carbonate normalised to modern values. If we assume that atmospheric O2 is in equilibrium with the shallow marine environment, and that we know the current atmospheric pO2 (0.21) and the modern seawater Zn/Fe ratios as reflected in Zn/Fe ratios of marine carbonates, we can use Zn/Fe to calculate fO2 (also expressed as pO2) at any given time of Earth history (Fig. 3). This pO2 curve provides a more continuous coverage of atmospheric O2 levels compared to compilations derived from multiple geochemical tracers, such as mass-independent S isotopes and palaeosol records (Rye and Holland, 1998
Rye, R., Holland, H.D. (1998) Paleosols and the evolution of atmospheric oxygen: A critical review. American Journal of Science 298, 621-672.
; Catling and Claire, 2005Catling, D.C., Claire, M.W. (2005) How Earth's atmosphere evolved to an oxic state: A status report. Earth and Planetary Science Letters 237, 1-20.
).
Figure 2 Zn/Fe molar ratio versus time for carbonates, averaged by formation. Formation averages (orange diamonds) were calculated based on simple arithmetic mean of samples within the same formation. Median (orange) and mean from lognormal distribution (blue) lines were calculated based on all samples from the designated time intervals. Estimated Zn/Fe ratio curve through Earth’s history. Uncertainties (light blue fields) are estimated based on one standard deviation from the lognormal distribution.

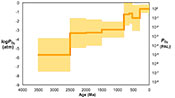
Figure 3 Estimated atmospheric pO2 through Earth’s history. The orange line indicates the best estimate (mean values from lognormal distribution) from carbonate Zn/Fe ratios from this study (yellow fields show the upper and lower range of estimated O2, which is calculated based on one sigma of lognormal distributions). The blue field indicates semi-quantitative interpretation from current understanding of the atmospheric O2 curve (modified from Lyons et al., 2014
Lyons, T.W., Reinhard, C.T., Planavsky, N.J. (2014) The rise of oxygen in Earth's early ocean and atmosphere. Nature 506, 307-315.
).The log pO2 curve in Figure 3 reproduces what we think we know about oxygen history: estimated pO2 is extremely low in the Archean and reaches modern levels only in the mid-Palaeozoic Era. Moreover, the estimates match our current understanding (Lyons et al., 2014
Lyons, T.W., Reinhard, C.T., Planavsky, N.J. (2014) The rise of oxygen in Earth's early ocean and atmosphere. Nature 506, 307-315.
) of a general two-step increase of atmospheric O2 around the GOE and the NOE. Importantly, our study provides an estimate of the upper and lower bounds on pO2 in the mid-Proterozoic atmosphere, with a preferred value between 0.1 and 1 % PAL. This value is substantially lower than traditional estimates based on palaeosol work (Canfield, 1998Canfield, D.E. (1998) A new model for Proterozoic ocean chemistry. Nature 396, 450-453.
; Rye and Holland, 1998Rye, R., Holland, H.D. (1998) Paleosols and the evolution of atmospheric oxygen: A critical review. American Journal of Science 298, 621-672.
), but consistent with recent estimates based on an independent tracer, a kinetic model for Cr-Mn oxidation and Cr isotopes in ironstones (Planavsky et al., 2014Planavsky, N.J., Reinhard, C.T., Wang, X., Thomson, D., McGoldrick, P., Rainbird, R.H., Johnson, T., Fischer, W.W., Lyons, T.W. (2014) Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 346, 635-638.
). We conducted sensitivity tests of temperature and pH variations on our pO2 estimates and found that the influence of temperature is negligible. pH, however, could potentially lower pO2 estimates, especially for earlier samples when pCO2 was high (see SI-4 for details); thus our estimates of Proterozoic pO2 should be considered conservative and may overestimate past oxygen levels. There are hints of biologically interesting structure in the Neoproterozoic and Cambrian records, but at present our sample numbers and bin sizes are too small to address this in detail. As more carbonate data become available for key transitional time periods such as those around GOE and NOE, potentially complex secular patterns of redox change may become clearer. Further investigations on well-constrained modern and Phanerozoic marine carbonates are currently underway to evaluate with more quantitative rigour the potential effects of diagenesis, mineralogy, and ocean depth gradient distributions on the proxy proposed here.top
Conclusion
In summary, we have demonstrated the potential for using divalent cations in carbonates as sensitive proxies for the evolution of Earth’s near surface environment. Because many marine carbonate rocks were deposited in shallow marine environments, in direct contact with the atmosphere, elemental ratios are likely to reflect equilibrium atmospheric conditions extending back to the Archean Eon and including time intervals poorly represented by other lithologies. Although further work will be needed to fully validate this promising palaeoredox proxy, carbonate-based redox proxies show great potential to expand the palaeoredox record and to provide self-consistent and quantitative constraints on atmospheric O2 through Earth’s history.
top
Acknowledgements
We are grateful to T. Mock for assistance with the Q-ICP-MS analyses, and M. Horan for help in the clean lab. We are grateful to Mahrnaz Siahy and Axel Hofmann for providing the Pongola samples. We are grateful to M. Van Kranendonk for sampling help in Western Australia and M. Evans, J. Hao, T. Lyons, D. Sverjensky and J. Veizer for discussions. The Alfred P. Sloan Foundation, the Deep Carbon Observatory, the National Science Foundation, the NASA Astrobiology Institute, and the Carnegie Institution of Washington provided financial support to RMH and X-ML. AHK thanks the NASA Astrobiology Institute.
Editor: Eric H. Oelkers
top
Author Contributions
X-ML and RMH designed the project with inputs from all authors. X-ML performed the chemical analyses. X-ML wrote the manuscript with inputs from all authors. LK, AHK, HC, AJK, and RMH provided samples.
top
References
Berner, R.A. (2006) GEOCARBSULF: A combined model for Phanerozoic atmospheric O2 and CO2. Geochimica et Cosmochimica Acta 70, 5653-5664.
 Show in context
Show in context The latter transition may well have continued into the Phanerozoic Eon, eventually resulting in near-present O2 (Berner, 2006; Dahl et al., 2010; Sperling et al., 2015).
View in article
Brand, U., Veizer, J. (1980) Chemical diagenesis of a multicomponent carbonate system; 1, Trace elements. Journal of Sedimentary Research 50, 1219-1236.
 Show in context
Show in context Both Zn and Fe partition coefficients (Kd) from fluid to carbonates increase with increasing diagenesis as shown by earlier work of Brand and Veizer (1980).
View in article
Canfield, D.E. (1998) A new model for Proterozoic ocean chemistry. Nature 396, 450-453.
 Show in context
Show in context This value is substantially lower than traditional estimates based on palaeosol work (Canfield, 1998; Rye and Holland, 1998), but consistent with recent estimates based on an independent tracer, a kinetic model for Cr-Mn oxidation and Cr isotopes in ironstones (Planavsky et al., 2014).
View in article
Canfield, D.E. (2005) The early history of atmospheric oxygen: Homage to Robert A. Garrels. Annual Review of Earth and Planetary Sciences 33, 1-36.
 Show in context
Show in context Atmospheric O2 was extremely low in the Archean Eon (>2.5 Ga), and while multiple lines of evidence suggest that Earth’s oxygenation was protracted (Kah et al., 2004; Kah and Bartley, 2011; Lyons et al., 2014; Planavsky et al., 2014), pO2 may have risen abruptly at two different points in time: first during the “Great Oxygenation Event” (GOE) at ~2.4 Ga (Canfield, 2005; Holland, 2006; Guo et al., 2009; Farquhar et al., 2011), when atmospheric O2 rose from <0.001 % to an intermediate value commonly estimated as 1 to 10 % of the current level (Farquhar et al., 2000; Pavlov and Kasting, 2002), and again during a “Neoproterozoic Oxygenation Event” (NOE) at ~800 to 542 million years ago (Canfield and Teske, 1996; Fike et al., 2006; Frei et al., 2009; Och and Shields-Zhou, 2012).
View in article
Canfield, D.E., Teske, A. (1996) Late Proterozoic rise in atmospheric oxygen concentration inferred from phylogenetic and sulphur-isotope studies. Nature 382, 127-132.
 Show in context
Show in contextAtmospheric O2 was extremely low in the Archean Eon (>2.5 Ga), and while multiple lines of evidence suggest that Earth’s oxygenation was protracted (Kah et al., 2004; Kah and Bartley, 2011; Lyons et al., 2014; Planavsky et al., 2014), pO2 may have risen abruptly at two different points in time: first during the “Great Oxygenation Event” (GOE) at ~2.4 Ga (Canfield, 2005; Holland, 2006; Guo et al., 2009; Farquhar et al., 2011), when atmospheric O2 rose from <0.001 % to an intermediate value commonly estimated as 1 to 10 % of the current level (Farquhar et al., 2000; Pavlov and Kasting, 2002), and again during a “Neoproterozoic Oxygenation Event” (NOE) at ~800 to 542 million years ago (Canfield and Teske, 1996; Fike et al., 2006; Frei et al., 2009; Och and Shields-Zhou, 2012).
View in article
Catling, D.C., Claire, M.W. (2005) How Earth's atmosphere evolved to an oxic state: A status report. Earth and Planetary Science Letters 237, 1-20.
 Show in context
Show in context This pO2 curve provides a more continuous coverage of atmospheric O2 levels compared to compilations derived from multiple geochemical tracers, such as mass-independent S isotopes and palaeosol records (Rye and Holland, 1998; Catling and Claire, 2005).
View in article
Dahl, T.W., Hammarlund, E.U., Anbar, A.D., Bond, D.P.G., Gill, B.C., Gordon, G.W., Knoll, A.H., Nielsen, A.T., Schovsbo, N.H., Canfield, D.E. (2010) Devonian rise in atmospheric oxygen correlated to the radiations of terrestrial plants and large predatory fish. Proceedings of the National Academy of Sciences of the United States of America 107, 17911-17915.
 Show in context
Show in context The latter transition may well have continued into the Phanerozoic Eon, eventually resulting in near-present O2 (Berner, 2006; Dahl et al., 2010; Sperling et al., 2015).
View in article
DePaolo, D.J. (2011) Surface kinetic model for isotopic and trace element fractionation during precipitation of calcite from aqueous solutions. Geochimica et Cosmochimica Acta 75, 1039-1056.
 Show in context
Show in context In addition, theoretical calculations suggest that kinetic effects on trace element partitioning in carbonate may contribute to Zn/Fe variability in samples from the same locality (Watson, 2004; DePaolo, 2011).
View in article
Farquhar, J., Bao, H.M., Thiemens, M. (2000) Atmospheric influence of Earth's earliest sulfur cycle. Science 289, 756-758.
 Show in context
Show in context Atmospheric O2 was extremely low in the Archean Eon (>2.5 Ga), and while multiple lines of evidence suggest that Earth’s oxygenation was protracted (Kah et al., 2004; Kah and Bartley, 2011; Lyons et al., 2014; Planavsky et al., 2014), pO2 may have risen abruptly at two different points in time: first during the “Great Oxygenation Event” (GOE) at ~2.4 Ga (Canfield, 2005; Holland, 2006; Guo et al., 2009; Farquhar et al., 2011), when atmospheric O2 rose from <0.001 % to an intermediate value commonly estimated as 1 to 10 % of the current level (Farquhar et al., 2000; Pavlov and Kasting, 2002), and again during a “Neoproterozoic Oxygenation Event” (NOE) at ~800 to 542 million years ago (Canfield and Teske, 1996; Fike et al., 2006; Frei et al., 2009; Och and Shields-Zhou, 2012).
View in article
Farquhar, J., Zerkle, A., Bekker, A. (2011) Geological constraints on the origin of oxygenic photosynthesis. Photosynthesis Research 107, 11-36.
 Show in context
Show in context Atmospheric O2 was extremely low in the Archean Eon (>2.5 Ga), and while multiple lines of evidence suggest that Earth’s oxygenation was protracted (Kah et al., 2004; Kah and Bartley, 2011; Lyons et al., 2014; Planavsky et al., 2014), pO2 may have risen abruptly at two different points in time: first during the “Great Oxygenation Event” (GOE) at ~2.4 Ga (Canfield, 2005; Holland, 2006; Guo et al., 2009; Farquhar et al., 2011), when atmospheric O2 rose from <0.001 % to an intermediate value commonly estimated as 1 to 10 % of the current level (Farquhar et al., 2000; Pavlov and Kasting, 2002), and again during a “Neoproterozoic Oxygenation Event” (NOE) at ~800 to 542 million years ago (Canfield and Teske, 1996; Fike et al., 2006; Frei et al., 2009; Och and Shields-Zhou, 2012).
View in article
Fike, D.A., Grotzinger, J.P., Pratt, L.M., Summons, R.E. (2006) Oxidation of the Ediacaran Ocean. Nature 444, 744-747.
 Show in context
Show in contextAtmospheric O2 was extremely low in the Archean Eon (>2.5 Ga), and while multiple lines of evidence suggest that Earth’s oxygenation was protracted (Kah et al., 2004; Kah and Bartley, 2011; Lyons et al., 2014; Planavsky et al., 2014), pO2 may have risen abruptly at two different points in time: first during the “Great Oxygenation Event” (GOE) at ~2.4 Ga (Canfield, 2005; Holland, 2006; Guo et al., 2009; Farquhar et al., 2011), when atmospheric O2 rose from <0.001 % to an intermediate value commonly estimated as 1 to 10 % of the current level (Farquhar et al., 2000; Pavlov and Kasting, 2002), and again during a “Neoproterozoic Oxygenation Event” (NOE) at ~800 to 542 million years ago (Canfield and Teske, 1996; Fike et al., 2006; Frei et al., 2009; Och and Shields-Zhou, 2012).
View in article
Frei, R., Gaucher, C., Poulton, S.W., Canfield, D.E. (2009) Fluctuations in Precambrian atmospheric oxygenation recorded by chromium isotopes. Nature 461, 250-U125.
 Show in context
Show in context Atmospheric O2 was extremely low in the Archean Eon (>2.5 Ga), and while multiple lines of evidence suggest that Earth’s oxygenation was protracted (Kah et al., 2004; Kah and Bartley, 2011; Lyons et al., 2014; Planavsky et al., 2014), pO2 may have risen abruptly at two different points in time: first during the “Great Oxygenation Event” (GOE) at ~2.4 Ga (Canfield, 2005; Holland, 2006; Guo et al., 2009; Farquhar et al., 2011), when atmospheric O2 rose from <0.001 % to an intermediate value commonly estimated as 1 to 10 % of the current level (Farquhar et al., 2000; Pavlov and Kasting, 2002), and again during a “Neoproterozoic Oxygenation Event” (NOE) at ~800 to 542 million years ago (Canfield and Teske, 1996; Fike et al., 2006; Frei et al., 2009; Och and Shields-Zhou, 2012).
View in article
Guo, Q.J., Strauss, H., Kaufman, A.J., Schroder, S., Gutzmer, J., Wing, B., Baker, M.A., Bekker, A., Jin, Q.S., Kim, S.T., Farquhar, J. (2009) Reconstructing Earth's surface oxidation across the Archean-Proterozoic transition. Geology 37, 399-402.
 Show in context
Show in context Atmospheric O2 was extremely low in the Archean Eon (>2.5 Ga), and while multiple lines of evidence suggest that Earth’s oxygenation was protracted (Kah et al., 2004; Kah and Bartley, 2011; Lyons et al., 2014; Planavsky et al., 2014), pO2 may have risen abruptly at two different points in time: first during the “Great Oxygenation Event” (GOE) at ~2.4 Ga (Canfield, 2005; Holland, 2006; Guo et al., 2009; Farquhar et al., 2011), when atmospheric O2 rose from <0.001 % to an intermediate value commonly estimated as 1 to 10 % of the current level (Farquhar et al., 2000; Pavlov and Kasting, 2002), and again during a “Neoproterozoic Oxygenation Event” (NOE) at ~800 to 542 million years ago (Canfield and Teske, 1996; Fike et al., 2006; Frei et al., 2009; Och and Shields-Zhou, 2012).
View in article
Hardisty, D.S., Lu, Z., Planavsky, N.J., Bekker, A., Philippot, P., Zhou, X., Lyons, T.W. (2014) An iodine record of Paleoproterozoic surface ocean oxygenation. Geology doi: 10.1130/G35439.1.
 Show in context
Show in context Here we provide evidence for the hypothesis that carbonate-based redox proxies can provide an independent estimate of past pO2, expanding the palaeoredox record in time and space (Hardisty et al., 2014).
View in article
Holland, H.D. (2006) The oxygenation of the atmosphere and oceans. Philosophical Transactions of the Royal Society B: Biological Sciences 361, 903-915.
 Show in context
Show in context Atmospheric O2 was extremely low in the Archean Eon (>2.5 Ga), and while multiple lines of evidence suggest that Earth’s oxygenation was protracted (Kah et al., 2004; Kah and Bartley, 2011; Lyons et al., 2014; Planavsky et al., 2014), pO2 may have risen abruptly at two different points in time: first during the “Great Oxygenation Event” (GOE) at ~2.4 Ga (Canfield, 2005; Holland, 2006; Guo et al., 2009; Farquhar et al., 2011), when atmospheric O2 rose from <0.001 % to an intermediate value commonly estimated as 1 to 10 % of the current level (Farquhar et al., 2000; Pavlov and Kasting, 2002), and again during a “Neoproterozoic Oxygenation Event” (NOE) at ~800 to 542 million years ago (Canfield and Teske, 1996; Fike et al., 2006; Frei et al., 2009; Och and Shields-Zhou, 2012).
View in article
Kah, L.C., Bartley, J.K. (2011) Protracted oxygenation of the Proterozoic biosphere. International Geology Review 53, 1424-1442.
 Show in context
Show in context Atmospheric O2 was extremely low in the Archean Eon (>2.5 Ga), and while multiple lines of evidence suggest that Earth’s oxygenation was protracted (Kah et al., 2004; Kah and Bartley, 2011; Lyons et al., 2014; Planavsky et al., 2014), pO2 may have risen abruptly at two different points in time: first during the “Great Oxygenation Event” (GOE) at ~2.4 Ga (Canfield, 2005; Holland, 2006; Guo et al., 2009; Farquhar et al., 2011), when atmospheric O2 rose from <0.001 % to an intermediate value commonly estimated as 1 to 10 % of the current level (Farquhar et al., 2000; Pavlov and Kasting, 2002), and again during a “Neoproterozoic Oxygenation Event” (NOE) at ~800 to 542 million years ago (Canfield and Teske, 1996; Fike et al., 2006; Frei et al., 2009; Och and Shields-Zhou, 2012).
View in article
Kah, L.C., Lyons, T.W., Frank, T.D. (2004) Low marine sulphate and protracted oxygenation of the proterozoic biosphere. Nature 431, 834-838.
 Show in context
Show in context Atmospheric O2 was extremely low in the Archean Eon (>2.5 Ga), and while multiple lines of evidence suggest that Earth’s oxygenation was protracted (Kah et al., 2004; Kah and Bartley, 2011; Lyons et al., 2014; Planavsky et al., 2014), pO2 may have risen abruptly at two different points in time: first during the “Great Oxygenation Event” (GOE) at ~2.4 Ga (Canfield, 2005; Holland, 2006; Guo et al., 2009; Farquhar et al., 2011), when atmospheric O2 rose from <0.001 % to an intermediate value commonly estimated as 1 to 10 % of the current level (Farquhar et al., 2000; Pavlov and Kasting, 2002), and again during a “Neoproterozoic Oxygenation Event” (NOE) at ~800 to 542 million years ago (Canfield and Teske, 1996; Fike et al., 2006; Frei et al., 2009; Och and Shields-Zhou, 2012).
View in article
Konhauser, K.O., Pecoits, E., Lalonde, S.V., Papineau, D., Nisbet, E.G., Barley, M.E., Arndt, N.T., Zahnle, K., Kamber, B.S. (2009) Oceanic nickel depletion and a methanogen famine before the Great Oxidation Event. Nature 458, 750-753.
 Show in context
Show in context Redox-sensitive major and trace elements in iron formations and black shales deposited beneath euxinic waters have been developed as proxies to reconstruct palaeoenvironmental history in deep time (Scott et al., 2008; Konhauser et al., 2009; Sahoo et al., 2012).
View in article
Lee, C.T.A., Luffi, P., Le Roux, V., Dasgupta, R., Albarede, F., Leeman, W.P. (2010) The redox state of arc mantle using Zn/Fe systematics. Nature 468, 681-685.
 Show in context
Show in contextIn addition, because both Fe and Zn behave as incompatible elements during mantle partial melting, Zn/Fe has been developed as a tracer of mantle redox, revealing that the oxygen fugacity of the upper mantle has remained relatively constant through Earth history (Lee et al., 2010).
View in article
Lyons, T.W., Reinhard, C.T., Planavsky, N.J. (2014) The rise of oxygen in Earth's early ocean and atmosphere. Nature 506, 307-315.
 Show in context
Show in context Atmospheric O2 was extremely low in the Archean Eon (>2.5 Ga), and while multiple lines of evidence suggest that Earth’s oxygenation was protracted (Kah et al., 2004; Kah and Bartley, 2011; Lyons et al., 2014; Planavsky et al., 2014), pO2 may have risen abruptly at two different points in time: first during the “Great Oxygenation Event” (GOE) at ~2.4 Ga (Canfield, 2005; Holland, 2006; Guo et al., 2009; Farquhar et al., 2011), when atmospheric O2 rose from <0.001 % to an intermediate value commonly estimated as 1 to 10 % of the current level (Farquhar et al., 2000; Pavlov and Kasting, 2002), and again during a “Neoproterozoic Oxygenation Event” (NOE) at ~800 to 542 million years ago (Canfield and Teske, 1996; Fike et al., 2006; Frei et al., 2009; Och and Shields-Zhou, 2012).
View in article
Our Palaeoproterozoic data are also consistent with earlier suggestions that pO2 may have risen substantially during the GOE and then declined again to persistent Proterozoic values (Lyons et al., 2014).
View in article
Figure 3 [...] The blue field indicates semi-quantitative interpretation from current understanding of the atmospheric O2 curve (modified from Lyons et al., 2014).
View in article
Moreover, the estimates match our current understanding (Lyons et al., 2014) of a general two-step increase of atmospheric O2 around the GOE and the NOE.
View in article
Morel, F.M.M., Price, N.M. (2003) The Biogeochemical Cycles of Trace Metals in the Oceans. Science 300, 944-947.
 Show in context
Show in context As an essential nutrient in many phytoplankton enzymes, especially those of eukaryotes (Williams and da Silva, 1996), zinc plays an important role in marine primary production, and for this reason, Zn is depleted in surface waters relative to the deep sea (Morel and Price, 2003).
View in article
Morse, J.W., Luther III, G.W. (1999) Chemical influences on trace metal-sulfide interactions in anoxic sediments. Geochimica et Cosmochimica Acta 63, 3373-3378.
 Show in context
Show in context Under sulphidic conditions, dissolved Zn2+ and Fe2+ behave similarly and are rapidly precipitated as sulphides (Morse and Luther III, 1999).
View in article
Och, L.M., Shields-Zhou, G.A. (2012) The Neoproterozoic oxygenation event: Environmental perturbations and biogeochemical cycling. Earth-Science Reviews 110, 26-57.
 Show in context
Show in context Atmospheric O2 was extremely low in the Archean Eon (>2.5 Ga), and while multiple lines of evidence suggest that Earth’s oxygenation was protracted (Kah et al., 2004; Kah and Bartley, 2011; Lyons et al., 2014; Planavsky et al., 2014), pO2 may have risen abruptly at two different points in time: first during the “Great Oxygenation Event” (GOE) at ~2.4 Ga (Canfield, 2005; Holland, 2006; Guo et al., 2009; Farquhar et al., 2011), when atmospheric O2 rose from <0.001 % to an intermediate value commonly estimated as 1 to 10 % of the current level (Farquhar et al., 2000; Pavlov and Kasting, 2002), and again during a “Neoproterozoic Oxygenation Event” (NOE) at ~800 to 542 million years ago (Canfield and Teske, 1996; Fike et al., 2006; Frei et al., 2009; Och and Shields-Zhou, 2012).
View in article
Pavlov, A.A., Kasting, J.F. (2002) Mass-independent fractionation of sulfur isotopes in Archean sediments: Strong evidence for an anoxic Archean atmosphere. Astrobiology 2, 27-41.
 Show in context
Show in context Atmospheric O2 was extremely low in the Archean Eon (>2.5 Ga), and while multiple lines of evidence suggest that Earth’s oxygenation was protracted (Kah et al., 2004; Kah and Bartley, 2011; Lyons et al., 2014; Planavsky et al., 2014), pO2 may have risen abruptly at two different points in time: first during the “Great Oxygenation Event” (GOE) at ~2.4 Ga (Canfield, 2005; Holland, 2006; Guo et al., 2009; Farquhar et al., 2011), when atmospheric O2 rose from <0.001 % to an intermediate value commonly estimated as 1 to 10 % of the current level (Farquhar et al., 2000; Pavlov and Kasting, 2002), and again during a “Neoproterozoic Oxygenation Event” (NOE) at ~800 to 542 million years ago (Canfield and Teske, 1996; Fike et al., 2006; Frei et al., 2009; Och and Shields-Zhou, 2012).
View in article
Planavsky, N.J., Reinhard, C.T., Wang, X., Thomson, D., McGoldrick, P., Rainbird, R.H., Johnson, T., Fischer, W.W., Lyons, T.W. (2014) Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 346, 635-638.
 Show in context
Show in context Raiswell, R., Tranter, M., Benning, L.G., Siegert, M., De’ath, R., Huybrechts, P., Payne, T. (2006) Contributions from glacially derived sediment to the global iron (oxyhydr)oxide cycle: Implications for iron delivery to the oceans. Geochimica et Cosmochimica Acta 70, 2765-2780.
 Show in context
Show in contextAlso, local primary production differences may contribute to Zn/Fe variability of different formations from the same interval. In the modern oxidised shallow ocean, particulate Fe sourced from eroding continents remains biogeochemically labile and may be cycled back to a dissolved phase during diagenesis in reducing continental margin sediments (Raiswell et al., 2006).
View in article
Robbins, L.J., Lalonde, S.V., Saito, M.A., Planavsky, N.J., Mloszewska, A.M., Pecoits, E., Scott, C., Dupont, C.L., Kappler, A., Konhauser, K.O. (2013) Authigenic iron oxide proxies for marine zinc over geological time and implications for eukaryotic metallome evolution. Geobiology 11, 295-306.
 Show in context
Show in context In the modern ocean, zinc input from hydrothermal ridge systems (~4.4 x 109 mol yr-1) is an order of magnitude greater than riverine fluxes (~3.4 x 108 mol yr-1; Robbins et al., 2013).
View in article
Zn concentrations in euxinic black shale and iron formations (Robbins et al., 2013; Scott et al., 2013), however, suggest that the bioavailability of Zn has not changed dramatically through Earth history.
View in article
Rye, R., Holland, H.D. (1998) Paleosols and the evolution of atmospheric oxygen: A critical review. American Journal of Science 298, 621-672.
 Show in context
Show in contextThis pO2 curve provides a more continuous coverage of atmospheric O2 levels compared to compilations derived from multiple geochemical tracers, such as mass-independent S isotopes and palaeosol records (Rye and Holland, 1998; Catling and Claire, 2005).
View in article
This value is substantially lower than traditional estimates based on palaeosol work (Canfield, 1998; Rye and Holland, 1998), but consistent with recent estimates based on an independent tracer, a kinetic model for Cr-Mn oxidation and Cr isotopes in ironstones (Planavsky et al., 2014).
View in article
Sahoo, S.K., Planavsky, N.J., Kendall, B., Wang, X., Shi, X., Scott, C., Anbar, A.D., Lyons, T.W., Jiang, G. (2012) Ocean oxygenation in the wake of the Marinoan glaciation. Nature 489, 546-549.
 Show in context
Show in context Redox-sensitive major and trace elements in iron formations and black shales deposited beneath euxinic waters have been developed as proxies to reconstruct palaeoenvironmental history in deep time (Scott et al., 2008; Konhauser et al., 2009; Sahoo et al., 2012).
View in article
Scott, C., Lyons, T.W., Bekker, A., Shen, Y., Poulton, S.W., Chu, X., Anbar, A.D. (2008) Tracing the stepwise oxygenation of the Proterozoic ocean. Nature 452, 456-U5.
 Show in context
Show in context Redox-sensitive major and trace elements in iron formations and black shales deposited beneath euxinic waters have been developed as proxies to reconstruct palaeoenvironmental history in deep time (Scott et al., 2008; Konhauser et al., 2009; Sahoo et al., 2012).
View in article
Scott, C., Planavsky, N.J., Dupont, C.L., Kendall, B., Gill, B.C., Robbins, L.J., Husband, K.F., Arnold, G.L., Wing, B.A., Poulton, S.W., Bekker, A., Anbar, A.D., Konhauser, K.O., Lyons, T.W. (2013) Bioavailability of zinc in marine systems through time. Nature Geoscience 6, 125-128.
 Show in context
Show in context Zn concentrations in euxinic black shale and iron formations (Robbins et al., 2013; Scott et al., 2013), however, suggest that the bioavailability of Zn has not changed dramatically through Earth history.
View in article
Sperling, E.A., Wolock, C.J., Morgan, A.S., Gill, B.C., Kunzmann, M., Halverson, G.P., Macdonald, F.A., Knoll, A.H., Johnston, D.T. (2015) Statistical analysis of iron geochemical data suggests limited late Proterozoic oxygenation. Nature 523, 451-454.
 Show in context
Show in context The latter transition may well have continued into the Phanerozoic Eon, eventually resulting in near-present O2 (Berner, 2006; Dahl et al., 2010; Sperling et al., 2015).
View in article
Palaeoenvironmental research on carbonate rocks commonly focuses on individual stratigraphic successions; here we adopt a complementary strategy, analysing a large suite of Phanerozoic, Proterozoic, and Archean samples that enables us to make statistical statements (Sperling et al., 2015) about Zn/Fe in the global surface ocean through geologic time.
View in article
Watson, E.B. (2004) A conceptual model for near-surface kinetic controls on the trace-element and stable isotope composition of abiogenic calcite crystals. Geochimica et Cosmochimica Acta 68, 1473-1488.
 Show in context
Show in context In addition, theoretical calculations suggest that kinetic effects on trace element partitioning in carbonate may contribute to Zn/Fe variability in samples from the same locality (Watson, 2004; DePaolo, 2011).
View in article
Wheat, C.G., Mottl, M.J., Rudnicki, M. (2002) Trace element and REE composition of a low-temperature ridge-flank hydrothermal spring. Geochimica et Cosmochimica Acta 66, 3693-3705.
 Show in context
Show in contextThe Fe budget is similar to that of Zn, wherein hydrothermal input dominates over riverine fluxes by a factor of ~9 (Wheat et al., 2002).
View in article
Williams, R.J.P., da Silva, J.J.R.F. (1996) The natural selection of the chemical elements. Great Britian, Bath Press Ltd.
 Show in context
Show in context As an essential nutrient in many phytoplankton enzymes, especially those of eukaryotes (Williams and da Silva, 1996), zinc plays an important role in marine primary production, and for this reason, Zn is depleted in surface waters relative to the deep sea (Morel and Price, 2003).
View in article
Wilson, J.P., Fischer, W.W., Johnston, D.T., Knoll, A.H., Grotzinger, J.P., Walter, M.R., McNaughton, N.J., Simon, M., Abelson, J., Schrag, D.P., Summons, R., Allwood, A., Andres, M., Gammon, C., Garvin, J., Rashby, S., Schweizer, M., Watters, W.A. (2010) Geobiology of the late Paleoproterozoic Duck Creek Formation, Western Australia. Precambrian Research 179, 135-149.
 Show in context
Show in context Limestone and penecontemporaneous dolomites that retain depositional signatures well (Wilson et al., 2010) are abundant in the geologic record, typically recording shallow marine environments that would have been in open communication with the overlying atmosphere.
View in article
top
Supplementary Information
SI-1: Table S-1, Figures S-1 and S-2
Table S-1 Zn/Fe ratios with sample name and age information from this study.
| Geologic Unit | Sample # | Age (Ma) | Zn/Fe*104 | Reference |
| Strelley Pool Fm, Warrawoona Group, Pilbara, Western Australia | BH2 | 3400 | 0.9 | Hazen (unpublished) |
| Strelley Pool Fm, Warrawoona Group, Pilbara, Western Australia | BH3 | 3400 | 17.6 | Hazen (unpublished) |
| Strelley Pool Fm, Warrawoona Group, Pilbara, Western Australia | BH7 | 3400 | 5.3 | Hazen (unpublished) |
| Strelley Pool Fm, Warrawoona Group, Pilbara, Western Australia | BH10 | 3400 | 6.5 | Hazen (unpublished) |
| Calcite filling in Mt. Ada basalt | BH18 | 3470 | 1.5 | Hazen (unpublished) |
| Pongola Fm, Sourth Africa | Po1 | 2950 | 16.1 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po2 | 2950 | 45.9 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po3 | 2950 | 4.5 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po4 | 2950 | 6.4 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po5 | 2950 | 5.9 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po6 | 2950 | 7.8 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po7 | 2950 | 5.8 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po8 | 2950 | 4.0 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po9 | 2950 | 3.8 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po10 | 2950 | 4.8 | Beukes and Lowe (1989) |
| Tumbiana Fm, Fortesue Group, Western Australia | BH11 | 2700 | 5.8 | Hazen (unpublished) |
| Tumbiana Fm, Fortesue Group, Western Australia | BH12 | 2700 | 24.6 | Hazen (unpublished) |
| Tumbiana Fm, Fortesue Group, Western Australia | BH13 | 2700 | 8.9 | Hazen (unpublished) |
| Tumbiana Fm, Fortesue Group, Western Australia | BH14 | 2700 | 8.0 | Hazen (unpublished) |
| Tumbiana Fm, Fortesue Group, Western Australia | BH15 | 2700 | 2.8 | Hazen (unpublished) |
| Tumbiana Fm, Fortesue Group, Western Australia | BH16 | 2700 | 3.4 | Hazen (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN94 | 2350 | 7.6 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN95 | 2350 | 8.4 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN96 | 2350 | 9.9 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN97 | 2350 | 7.7 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN98 | 2350 | 8.2 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN99 | 2350 | 5.2 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN100 | 2350 | 7.8 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN101 | 2350 | 10.4 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN102 | 2350 | 11.5 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN103 | 2350 | 13.1 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN104 | 2350 | 23.0 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN105 | 2350 | 23.5 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | BH19 | 2350 | 8.5 | Hazen (unpublished) |
| Duck Creek, Auatralia | AN80 | 1835 | 53.0 | Wilson et al. (2010) |
| Duck Creek, Auatralia | AN82 | 1835 | 8.0 | Wilson et al. (2010) |
| Duck Creek, Auatralia | AN83 | 1835 | 36.6 | Wilson et al. (2010) |
| Duck Creek, Auatralia | AN84 | 1835 | 53.0 | Wilson et al. (2010) |
| Duck Creek, Auatralia | AN85 | 1835 | 26.5 | Wilson et al. (2010) |
| Duck Creek, Auatralia | AN86 | 1835 | 16.2 | Wilson et al. (2010) |
| Duck Creek, Auatralia | AN87 | 1835 | 27.6 | Wilson et al. (2010) |
| Duck Creek, Auatralia | AN88 | 1835 | 6.2 | Wilson et al. (2010) |
| Vempalle Fm, Cuddapah Basin, India | V-SC/1 | 1750 | 8.5 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-SC/2 | 1750 | 4.8 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-SC/3 | 1750 | 5.3 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-SC/5 | 1750 | 10.6 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-SC/6-1 | 1750 | 6.8 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-P2/1 | 1750 | 5.5 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-P8/1 | 1750 | 12.2 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-P10/1 | 1750 | 7.5 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-P11/1 | 1750 | 6.9 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-P12/1 | 1750 | 6.1 | Chakrabarti et al. (2014) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C133-3252.3 | 1350 | 17.8 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C133-3253.3 | 1350 | 12.8 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C133-3038 | 1350 | 9.5 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C133-2767.5 | 1350 | 27.7 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C133-3114.5 | 1350 | 20.1 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C203-3852A | 1350 | 24.4 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C203-2459.8 | 1350 | 10.0 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C203-2459.6-1 | 1350 | 18.1 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C203-2353 | 1350 | 20.9 | Kah et al. (2007) |
| Sulky Fm, Dismal Lakes, Canada | DL1-364-1 | 1300 | 13.9 | Kah et al. (2006) |
| Sulky Fm, Dismal Lakes, Canada | DL1-306-1 | 1300 | 28.0 | Kah et al. (2006) |
| Sulky Fm, Dismal Lakes, Canada | DL1-332 | 1300 | 22.4 | Kah et al. (2006) |
| Sulky Fm, Dismal Lakes, Canada | SL16-1-1 | 1300 | 2.7 | Kah et al. (2006) |
| Sulky Fm, Dismal Lakes, Canada | SL17-10-1 | 1300 | 4.5 | Kah et al. (2006) |
| Avzyan Fm, Southern Urals, Russia | M1(AZ)-39 | 1150 | 3.0 | Bartley et al. (2007) |
| Avzyan Fm, Southern Urals, Russia | M1(AZ)-47 | 1150 | 31.7 | Bartley et al. (2007) |
| Avzyan Fm, Southern Urals, Russia | RV(AZ)-15 | 1150 | 8.8 | Bartley et al. (2007) |
| Avzyan Fm, Southern Urals, Russia | RV(AZ)-33 | 1150 | 13.8 | Bartley et al. (2007) |
| Avzyan Fm, Southern Urals, Russia | KT(AZ)-49.5 | 1150 | 17.6 | Bartley et al. (2007) |
| Avzyan Fm, Southern Urals, Russia | KT(AZ)-131.6 | 1150 | 3.0 | Bartley et al. (2007) |
| Avzyan Fm, Southern Urals, Russia | KT(AZ)-236-1 | 1150 | 7.0 | Bartley et al. (2007) |
| Avzyan Fm, Southern Urals, Russia | KT(AZ)-373.5 | 1150 | 8.0 | Bartley et al. (2007) |
| El Mreiti,Atar Group, West Africa | F4-10-1 | 1100 | 42.6 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-19-1 | 1100 | 17.7 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-50-1 | 1100 | 13.9 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-53-1 | 1100 | 16.4 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-90-1 | 1100 | 17.8 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-95-1 | 1100 | 11.9 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-98-1 | 1100 | 17.7 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-99-1 | 1100 | 39.6 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-102-1 | 1100 | 20.0 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-104-1 | 1100 | 8.6 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-106-1 | 1100 | 11.4 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-107-1 | 1100 | 13.0 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-108-1 | 1100 | 6.8 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-109-1 | 1100 | 6.1 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-113-1 | 1100 | 13.1 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-114-1 | 1100 | 7.2 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-115-1 | 1100 | 7.8 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-116-1 | 1100 | 7.8 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-117-1 | 1100 | 7.9 | Gilleaudeau and Kah (2013) |
| Atar Group, West Africa | ATS-31 | 1100 | 14.8 | Kah et al. (2012) |
| Atar Group, West Africa | ATS-53 | 1100 | 92.9 | Kah et al. (2012) |
| Atar Group, West Africa | ATS-61 | 1100 | 16.5 | Kah et al. (2012) |
| Atar Group, West Africa | ATS-5-1 | 1100 | 13.8 | Kah et al. (2012) |
| Atar Group, West Africa | ATS-119 | 1100 | 11.6 | Kah et al. (2012) |
| Atar Group, West Africa | ATS-154 | 1100 | 12.8 | Kah et al. (2012) |
| Atar Group, West Africa | ATD-17 | 1100 | 18.4 | Kah et al. (2012) |
| Atar Group, West Africa | ATD-61 | 1100 | 18.2 | Kah et al. (2012) |
| Atar Group, West Africa | ATL-51-1 | 1100 | 9.3 | Kah et al. (2012) |
| Atar Group, West Africa | ATL-58 | 1100 | 66.2 | Kah et al. (2012) |
| Atar Group, West Africa | ATL-68 | 1100 | 4.9 | Kah et al. (2012) |
| Atar Group, West Africa | ATL-105 | 1100 | 19.3 | Kah et al. (2012) |
| Atar Group, West Africa | ATL-110-1 | 1100 | 6.3 | Kah et al. (2012) |
| Atar Group, West Africa | ATD-27.5 | 1100 | 12.3 | Kah et al. (2012) |
| Atar Group, West Africa | ATD-45 | 1100 | 18.4 | Kah et al. (2012) |
| Atar Group, West Africa | R1-Δ-1 | 1100 | 16.4 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-7 | 1100 | 25.5 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-13 | 1100 | 14.9 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-25 | 1100 | 12.7 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-29 | 1100 | 12.0 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-30 | 1100 | 10.3 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-31 | 1100 | 5.8 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-38 | 1100 | 12.2 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-39 | 1100 | 6.5 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-41 | 1100 | 9.6 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-42 | 1100 | 6.7 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-48 | 1100 | 8.1 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-54-1 | 1100 | 9.6 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-55 | 1100 | 7.1 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-58 | 1100 | 7.6 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-60 | 1100 | 4.7 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-61 | 1100 | 4.6 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-65 | 1100 | 9.9 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-68-1 | 1100 | 18.3 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-72 | 1100 | 6.6 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-76 | 1100 | 12.4 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-79-1 | 1100 | 8.3 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-81-1 | 1100 | 5.2 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-85 | 1100 | 6.0 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-89 | 1100 | 16.8 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-90 | 1100 | 4.4 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-97 | 1100 | 3.1 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-98 | 1100 | 9.2 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-100 | 1100 | 3.6 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-103 | 1100 | 3.1 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-106 | 1100 | 2.2 | Manning-Berg and Kah (in prep) |
| Chattisgarh, India | SRJ-2I | 1000 | 25.9 | Bickford et al. (2011) |
| Chattisgarh, India | SRJ-3I | 1000 | 22.8 | Bickford et al. (2011) |
| Chattisgarh, India | SRJ-4I | 1000 | 13.0 | Bickford et al. (2011) |
| Chattisgarh, India | SRJ-6I | 1000 | 20.6 | Bickford et al. (2011) |
| Chattisgarh, India | SRJ-8I | 1000 | 37.8 | Bickford et al. (2011) |
| Chattisgarh, India | SRJ-9I | 1000 | 85.2 | Bickford et al. (2011) |
| Chattisgarh, India | TML-3I | 1000 | 7.7 | Bickford et al. (2011) |
| Chattisgarh, India | TML-6I | 1000 | 17.8 | Bickford et al. (2011) |
| Chattisgarh, India | TML-7I | 1000 | 14.9 | Bickford et al. (2011) |
| Sukhaya Tunguska Formation, Russia | AN59 | 950 | 29.5 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN60 | 950 | 46.1 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN61 | 950 | 25.7 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN62 | 950 | 76.4 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN63 | 950 | 110.5 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN64 | 950 | 17.0 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN66 | 950 | 28.1 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN67 | 950 | 20.0 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN68 | 950 | 20.2 | Sergeev et al. (1997) |
| Akademikerbeen Group, Spitsbergen | AN1 | 775 | 29.2 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN2 | 775 | 40.3 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN3 | 775 | 17.3 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN4 | 775 | 6.2 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN5 | 775 | 63.2 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN6 | 775 | 65.0 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN7 | 775 | 27.6 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN8 | 775 | 74.3 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN9 | 775 | 95.4 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN10 | 775 | 59.4 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN12 | 775 | 71.5 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN13 | 775 | 15.8 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN14 | 775 | 49.4 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN16 | 775 | 15.1 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN18 | 775 | 17.6 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN19 | 775 | 9.4 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN20 | 775 | 34.4 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN21 | 775 | 276.2 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN22 | 775 | 34.6 | Knoll and Swett (1990) |
| Limestone-Dolomite Series, East Greenland | AN24 | 775 | 126.1 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN25 | 775 | 59.3 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN26 | 775 | 45.7 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN28 | 775 | 5.3 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN29 | 775 | 61.2 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN30 | 775 | 307.6 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN31 | 775 | 310.7 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN32 | 775 | 38.3 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN33 | 775 | 33.3 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN34 | 775 | 15.5 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN35 | 775 | 8.3 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN36 | 775 | 102.9 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN37 | 775 | 67.0 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN38 | 775 | 41.4 | Knoll et al. (1986) |
| Shaler Group, Arctic Canada | AN41 | 775 | 5.6 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN42 | 775 | 17.3 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN43 | 775 | 45.7 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN44 | 775 | 7.0 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN45 | 775 | 154.8 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN46 | 775 | 6.2 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN47 | 775 | 40.3 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN48 | 775 | 14.8 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN49 | 775 | 116.9 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN50 | 775 | 8.4 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN51 | 775 | 10.9 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN52 | 775 | 57.5 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN53 | 775 | 6.9 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN54 | 775 | 6.0 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN55 | 775 | 12.8 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN56 | 775 | 17.8 | Jones et al. (2010) |
| Lagoa Do Jacare Formation, Brazil | KM7-14.0.0 | 650 | 38.1 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-01.0 | 650 | 21.9 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-02.0 | 650 | 49.0 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-03.0 | 650 | 17.6 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-04.0 | 650 | 12.5 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-05.0 | 650 | 22.0 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-06.0 | 650 | 38.9 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-07.0 | 650 | 54.1 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-08.0 | 650 | 32.8 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-09.0 | 650 | 23.8 | Misi et al. (2007) |
| Huttenburg Formation, Namibia | S86A-971.2 | 650 | 105.2 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-976.0 | 650 | 77.5 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-977.0 | 650 | 120.9 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-980.8 | 650 | 53.5 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-985.1 | 650 | 147.1 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-987.8 | 650 | 28.4 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-988.2 | 650 | 41.8 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-1033.8 | 650 | 424.1 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-1060.2 | 650 | 156.2 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-1077.1 | 650 | 57.4 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-1144.8 | 650 | 52.8 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-1145.1 | 650 | 47.7 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-1148.4 | 650 | 55.6 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-1213.2 | 650 | 51.1 | Kaufman et al. (2009) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-7 | 600 | 61.0 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-21 | 600 | 62.5 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-26 | 600 | 30.7 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-34 | 600 | 37.5 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-38 | 600 | 19.8 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-38b | 600 | 40.1 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-39 | 600 | 38.7 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-46 | 600 | 24.9 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-49 | 600 | 59.0 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-51 | 600 | 19.8 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-54 | 600 | 24.9 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | M1-with fossil | 600 | 62.2 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | N-2-3 | 600 | 113.4 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | N-2-11 | 600 | 73.1 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | N-2-16 | 600 | 68.9 | Miller et al. (2008) |
| Yangjiaping, Doushantuo Formation, South China | YD-01 | 551 | 12.9 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-02 | 551 | 47.1 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-03 | 551 | 11.7 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-04 | 551 | 25.1 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-05 | 551 | 71.0 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-06 | 551 | 13.9 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-07 | 551 | 28.4 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-08 | 551 | 6.6 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-09 | 551 | 6.2 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-10 | 551 | 26.9 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-11 | 551 | 50.4 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-12 | 551 | 25.1 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-13 | 551 | 23.7 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-14 | 551 | 55.0 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-15 | 551 | 115.4 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-16 | 551 | 221.5 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-17 | 551 | 253.8 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-18 | 551 | 195.9 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-19 | 551 | 101.2 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-20 | 551 | 14.5 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-21 | 551 | 10.9 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-22 | 551 | 29.1 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-23 | 551 | 40.1 | Cui et al. (2015) |
| Orthoceras limestone, Ohio | BH21 | 465 | 27.5 | Hazen (unpublished) |
| Bryozoan limestone, Ohio | BH24 | 465 | 7.2 | Hazen (unpublished) |
| Branch Hill limestone, Ohio | BH25 | 465 | 10.9 | Hazen (unpublished) |
| Jersey Shore Station, Pennsylvania | BH26 | 465 | 7.0 | Hazen (unpublished) |
| Madison County, Kentucky | BH31 | 465 | 14.3 | Hazen (unpublished) |
| Ripley, Ohio | BH36 | 465 | 8.3 | Hazen (unpublished) |
| Washington, Kentucky | BH37 | 465 | 11.4 | Hazen (unpublished) |
| Bryozoan limestone, Ohio | BH33 | 465 | 3.5 | Hazen (unpublished) |
| Coburn Fm, Pennsylvania | BH41 | 465 | 10.9 | Hazen (unpublished) |
| La Silla Fm, Argentina | AF-23 | 464 | 22.9 | Thompson and Kah (2012) |
| La Silla Fm, Argentina | AF-30 | 464 | 12.4 | Thompson and Kah (2012) |
| La Silla Fm, Argentina | SJF08-14 | 464 | 16.1 | Thompson and Kah (2012) |
| La Silla Fm, Argentina | LFG-27 | 464 | 40.5 | Thompson and Kah (2012) |
| La Silla Fm, Argentina | LFG-55 | 464 | 17.6 | Thompson and Kah (2012) |
| La Silla Fm, Argentina | LS-01 | 464 | 29.7 | Thompson and Kah (2012) |
| La Silla Fm, Argentina | SJC-116 | 464 | 42.1 | Thompson and Kah (2012) |
| West Newfoundland | TH-1 | 464 | 15.3 | Thompson and Kah (2012) |
| West Newfoundland | TH-18 | 464 | 41.7 | Thompson and Kah (2012) |
| Clay's Ferry Fm, Kentucky | BH49 | 457 | 7.1 | Hazen (unpublished) |
| Cincinnati, Ohio | BH32 | 451 | 6.5 | Hazen (unpublished) |
| Richmond, Indiana | BH27 | 451 | 7.3 | Hazen (unpublished) |
| Ludlow Fm, Silurian | BH28 | 432 | 30.3 | Hazen (unpublished) |
| Limestone Quarry, Dickensonville, Virginia | BH29 | 406 | 7.7 | Hazen (unpublished) |
| John Boyd Thatchen State Park, New York | BH30 | 406 | 12.8 | Hazen (unpublished) |
| Blue Stone limestone Quarry, New York | BH34 | 406 | 19.0 | Hazen (unpublished) |
| Isle La Motte, Vermont | BH38 | 406 | 21.6 | Hazen (unpublished) |
| Isle La Motte, Vermont | BH39 | 406 | 12.2 | Hazen (unpublished) |
| Isle La Motte, Vermont | BH40 | 406 | 22.9 | Hazen (unpublished) |
| Hamilton Group, New York | BH22 | 388 | 3.6 | Hazen (unpublished) |
| Plainville Quarry, Ohio | BH44 | 388 | 17.4 | Hazen (unpublished) |
| Limestone Quarry, Ohio | BH52 | 388 | 4.2 | Hazen (unpublished) |
| Coral limestone, Michigan | BH53 | 388 | 21.0 | Hazen (unpublished) |
| Madision limestone, Garrett Co., Maryland | BH23 | 341 | 31.7 | Hazen (unpublished) |
| Mammoth Cave National Park, Kentucky | BH47 | 341 | 190.6 | Hazen (unpublished) |
| Sheep Mountain | BH51 | 341 | 53.6 | Hazen (unpublished) |
| Lydstep Haven coral, Wales | BH35 | 329 | 70.4 | Hazen (unpublished) |
| Tenby, Wales | BH43 | 329 | 69.0 | Hazen (unpublished) |
| Cheddar Gorge, England | BH46 | 329 | 65.5 | Hazen (unpublished) |
| Everett Quarry, Missouri | BH45 | 311 | 15.4 | Hazen (unpublished) |
| Shark Bay, Australia | BH20 | 0 | 28.4 | Hazen (unpublished) |
*See references in SI-5.
Download in Excel
Figure S-1 Comparison of measured trace elements with those reported for Inoue et al. (2004)
Inoue, M., Nohara, M., Okai, T., Suzuki, A., Kawahata, H. (2004) Concentrations of trace elements in carbonate reference materials coral JCp-1 and giant clam JCt-1 by Inductively Coupled Plasma-Mass Spectrometry. Geostandards and Geoanalytical Research 28, 411-416.
for the JcP-1 standard. The full analytical method is discussed in the methods section following the main text.
Figure S-2 Zn/Fe ratios in marine carbonate, plotted with information on sample lithology.
SI-2: Data Filtering
Diagenetic alteration can be problematic when interpreting the chemical composition of carbonate rocks; therefore, we carefully screened carbonate specimens for a range of diagenetic effects and hydrothermal alteration by combining geologic, petrographic, and element and isotope geochemical analyses. For the published literature, we compiled data only for samples that are considered to reflect primary depositional environments. For our own analyses, we selected samples from within known sedimentological and stratigraphic context, most of which are from previously investigated rock units. Petrographic analysis was used to select sample areas preserving original sedimentary fabrics, which suggests the least interaction with diagenetic fluids, and samples were microdrilled from these localities. To identify possible affects of diagenesis, we screened the samples based on the classic geochemical tracers of late diagenesis, including major and minor elements (e.g., Fe, Mn, Sr) as well as C and O isotopic signatures. We did not fix the selection criteria; rather, we adopted the selection criteria used by individual researchers for the different localities and published in their original descriptions. Readers can refer to the original papers that describe the samples in the reference lists below (see SI-5). In addition, we studied REE patterns and Eu anomalies to avoid samples with significant hydrothermal alternation (e.g., Frimmel, 2009
Frimmel, H.E. (2009) Trace element distribution in Neoproterozoic carbonates as palaeoenvironmental indicator. Chemical Geology 258, 338-353.
). Both Zn and Fe partition coefficients (Kd) from fluid to carbonates increase with increasing diagenesis as shown by earlier work of Brand and Veizer (1980)Brand, U., Veizer, J. (1980) Chemical diagenesis of a multicomponent carbonate system; 1, Trace elements. Journal of Sedimentary Research 50, 1219-1236.
. Also, (Kd) (Fe) increases faster compared to (Kd) (Zn), from 1 to 20 and from 5.2 to 5.5 for Fe and Zn, respectively. So, diagenesis will cause a decrease in Zn/Fe ratios by incorporating more Fe than Zn in carbonates. Even though, every carbonates we studied today have been through diagenesis. That is why we are adopting a statistic treatment of our global data.SI-3: Statistical Analysis of Data
Temporal data sets are subject to biases associated with sampling: recent geological eras are commonly represented by more samples, and some formations are represented by multiple analyses. Since we do not know exactly how the effect of diagenesis, local primary production, mineralogy and kinetic effect influence the Zn/Fe values, we therefore adopted three discrete approaches to evaluate Zn/Fe data statistically through time aiming to investigate the population behaviour. First, we divided the entire sample population into eight bins of different duration to make sure each bin has statistically meaningful sample numbers (where n > 50, expect for one bin with n = 38). The bins were chosen to reflect current hypotheses for pO2 evolution through time; specifically, we chose a bin boundary at 800 Ma to reflect the recent hypothesis of Planavsky et al. (2014) concerning mid-Neoproterozoic oxygenation, and we broke out Cryogenian, Ediacaran and Lower Palaeozoic bins in an attempt to illuminate existing hypothesis concerning Ediacaran-Cambrian oxygen increase. Each bin contains samples from at least two different geological formations. The choice of the bin breaking points is based on convenience and what is already known about pO2 evolution through Earth history. For example, the first bin is from 3.5–2.5 Ga, which is the pre-GOE time, where we expect low pO2 and we observe low Zn/Fe and little variation of Zn/Fe. We divide the following bin into 2.5-2.0 Ga and 2.0-1.5 Ga, to have statistically meaningful number of samples in each bin. Then we have three different formations from ~800 Ma with high Zn/Fe compared to pre-1.0 Ga samples and therefore we make a bin from 1.5-0.8 Ga. For the following time periods, we divide bins into several geological meaningful groups, as 800-635 Ma (Cryogenian), 635-541 Ma (Ediacaran), 541-300 Ma (earlier Palaeozoic), and 300-0 Ma (later Palaeozoic, Mesozoic and Cenozoic) to make self-consistent and statistically meaningful sample subsets.
We then performed a box-whisker plot for all data (Fig. S-3), where median, 50 %, and outliers (outside of 3 sigma of the population) of Zn/Fe values were calculated for each bin, which are shown with red lines (orange lines in Fig. 2), blue boxes, and red crosses, respectively in Fig. S-3. In a second approach, we divided data into the same ten bins as in the previous approach, but we plotted histograms for each bin (Fig. S-4). Data in nearly all of the bins follows a lognormal distribution, which permits calculation of means and standard deviations for each of the 10 bins (shown with blue lines in Fig. 2). In a third approach, we calculated average composition for each geological formation, which reduces the influence from sampling bias. For example, some localities/formations are represented by 20 samples, whereas others incorporate 10 or fewer. The formation averages are shown in Figure 2 and a polynomial fit is calculated and displayed in Figure S-5.

Figure S-3 Box-whisker distribution of all samples. The sample population is divided into eight bins (Bin 1: 3.5-2.5 Ga, Bin 2: 2.5-2.0 Ga, Bin 3: 2.0-1.5 Ga, Bin 4: 1.5-0.8 Ga, Bin 5: 800-635 Ma, Bin 6: 635-541 Ma, Bin 7: 541-300 Ma, Bin 8: 300-0 Ma) of different duration to make sure each bin has statistically meaningful sample numbers (where n > 50, except for one bin with n = 38). Each bin contains samples from at least two different geological formations. We show a Box-whisker plot for each group. Median values are indicated by the red lines and each individual box includes 50 % samples and whiskers mark the 3 sigma boundaries of the group population. Red crosses fall out of whiskers and are considered outliers.

Figure S-4 Histograms of Zn/Fe ratios with lognormal fitting in red. We group all data into eight different bins (age distribution of the bins is provided in Fig. S-2) and plot the lognormal distribution for each group.

Figure S-5 Zn/Fe molar ratio versus time for carbonates averaged by formation. A polynomial fit through the formation average data.
SI-4: Assumptions, Deviation of Equations and Sensitivity Tests
Fe oxidisation a chemical reaction can be written as:
4Fe2+ (aq) + O2 (aq) + 10H2O (aq) = 4Fe(OH)3 (s) + 8H+ (aq)
Eq. S-1
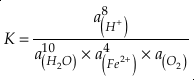
where K is the equilibrium constant and
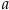 is activity. In this equation, we assume that when Fe2+ oxidises to Fe3+ and is precipitated from the aqueous system as iron hydroxide [Fe(OH)3], only Fe2+ is incorporated into carbonates. Assuming equilibrium between atmosphere and surface ocean on hundred million year time scales (the smallest bin size is around 100 million years, Bin 6: 635-541 Ma), we replace
is activity. In this equation, we assume that when Fe2+ oxidises to Fe3+ and is precipitated from the aqueous system as iron hydroxide [Fe(OH)3], only Fe2+ is incorporated into carbonates. Assuming equilibrium between atmosphere and surface ocean on hundred million year time scales (the smallest bin size is around 100 million years, Bin 6: 635-541 Ma), we replace 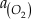 in Equation S-1 with oxygen fugacity in the atmosphere,
in Equation S-1 with oxygen fugacity in the atmosphere, 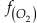 , as
, asEq. S-2
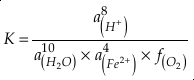
Then we can reorganise Equation S-2 to
Eq. S-3
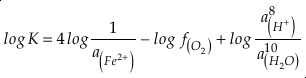
if we assume the Zn concentrations in seawater and partitioning of Zn/Fe from seawater to carbonate minerals are constant over Earth’s history. Therefore, we can write the equation normalised to Zn2+ as
Eq. S-4
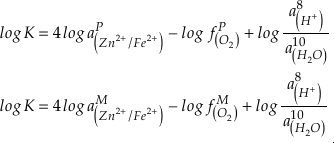
in which superscripts P and M indicate the past and modern parameters. Assuming pH and K are constant, we can simplify the relationship between Fe/Zn ratios and
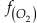 , as
, asEq. S-5
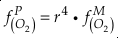
where
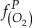 is the oxygen fugacity in the past (any time in Earth’s history),
is the oxygen fugacity in the past (any time in Earth’s history), 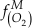 is the oxygen fugacity in modern time, and
is the oxygen fugacity in modern time, andEq. S-6
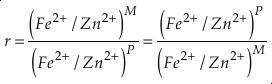
provides Zn/Fe ratios in past carbonate normalised to the modern values. If we assume that atmospheric O2 is in equilibrium with the shallow marine environment, and that we know the current atmospheric pO2 (0.21) and the modern seawater Zn/Fe ratios as reflected in Zn/Fe ratios of marine carbonates, we can therefore calculate fO2 at any given time of Earth’s history if we know the Zn/Fe ratio.
Discussion of Assumptions
This approach requires several critical assumptions, one of which is that we assume the pH of the ocean has not changed significantly through Earth’s history—an assumption supported by some studies (Grotzinger and Kasting, 1993
Grotzinger, J.P., Kasting, J.F. (1993) New constraints on precambrian ocean composition. Journal of Geology 101, 235-243.
; Sumner and Grotzinger, 2004Sumner, D.Y., Grotzinger, J.P. (2004) Implications for Neoarchaean ocean chemistry from primary carbonate mineralogy of the Campbellrand-Malmani Platform, South Africa. Sedimentology 51, 1273-1299.
). However, we do recognise the possibly large influence of pH change on the quantitative constraint of atmospheric O2. We also assume a constant Zn concentration in seawater through time. Although Zn concentrations, theoretically may have responded to changes in enzymatic use during biosphere evolution, there is no evidence for change in Zn bioavailability change through time (Robbins et al., 2013Robbins, L.J., Lalonde, S.V., Saito, M.A., Planavsky, N.J., Mloszewska, A.M., Pecoits, E., Scott, C., Dupont, C.L., Kappler, A., Konhauser, K.O. (2013) Authigenic iron oxide proxies for marine zinc over geological time and implications for eukaryotic metallome evolution. Geobiology 11, 295-306.
; Scott et al., 2013Scott, C., Planavsky, N.J., Dupont, C.L., Kendall, B., Gill, B.C., Robbins, L.J., Husband, K.F., Arnold, G.L., Wing, B.A., Poulton, S.W., Bekker, A., Anbar, A.D., Konhauser, K.O., Lyons, T.W. (2013) Bioavailability of zinc in marine systems through time. Nature Geoscience 6, 125-128.
). However, Zn concentrations in seawater may drop during the Mid-Proterozoic due to some extent of euxinia (~1~10 % of modern seafloor area; Reinhard et al., 2013Reinhard, C.T., Planavsky, N.J., Robbins, L.J., Partin, C.A., Gill, B.C., Lalonde, S.V., Bekker, A., Konhauser, K.O., Lyons, T.W. (2013) Proterozoic ocean redox and biogeochemical stasis. Proceedings of the National Academy of Sciences of the United States of America 110, 5357-5362.
). These two important factors (pH and Zn content) may contribute in various degrees to the accuracy of atmospheric O2 estimation in our model. However, we do not have a quantitative understanding of either at present. Therefore, we develop a first order quantification of secular evolution of atmospheric O2 with the assumptions that neither pH nor Zn content in seawater change significantly during the time intervals we investigated.Sensitivity Tests of the Modelling
Here, we evaluate how temperature and pH changes influence the modelling results in seawater through Earth’s history.
First, we perform a sensitivity test that assuming three different temperatures:
Eq. S-7
Eq. S-8
By combining Equations S-7 and S-8, we have FeCO3 + Zn2+ --> ZnCO3 + Fe2+, where equilibrium constant K can be written as log K = log (a(ZnCO3)/a(FeCO3)) - log (a(Zn2+)/a(Fe2+)), where a are activities for different components. We then calculate K values at different temperatures using SUPCRT92 (Johnson et al., 1992
Johnson, J.W., Oelkers, E.H., Helgeson, H.C. (1992) SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000°C. Computers & Geosciences 18, 899-947.
) and the results are shown in Figure S-6.
Figure S-6 log K (equilibrium constant) versus temperature plot for chemical reaction: FeCO3 + Zn2+ --> ZnCO3 + Fe2+.
Generally, when T increases, log K (also K) decreases, but still not so much.
When T = 25 oC, log K = -0.496, K = 0.32;
When T = 60 oC, log K = -0.575, K = 0.27;
When T = 100 oC, log K = -0.65, K = 0.22.
If we take the most extreme example: assuming modern T is 25 oC and Archean T is 100 oC. The fO2 estimate in the Archean will increase by approximately 40 %. Therefore, even if we assume the most extreme ocean temperature (100 oC), the uncertainty caused by temperature change on fO2 estimate is much smaller compared to uncertainties generated by Zn/Fe variations in carbonates (Fig. 3), which is at least one order of magnitude.
Second, we carry out a sensitivity test on pH change:
Eq. S-9
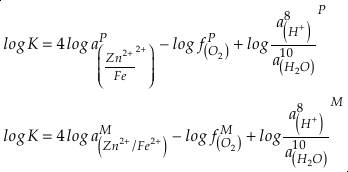
The modern day seawater has a pH of ~8 (M in the equation). If we assume a lower pH in the past (P in the equation), we can rearrange the equations as
Eq. S-10
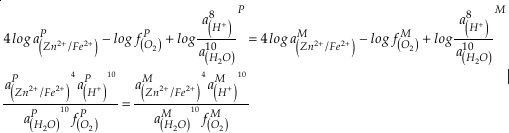
Finally, we get the new pH dependent relationship as
Eq. S-11
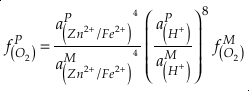
Therefore, if the pH is lower in the past, this will cause the O2 estimate to be lower. This also means that the O2 level we estimate is the maximum values assuming the pH was lower in the past. Also, if we change the pH from 8.1 to 7.6, estimated O2 is four orders of magnitude lower because the O2 estimate is sensitive to pH change. Since we do not know how seawater pH changes through time, and there is no evidence that it changes dramatically on the order of hundreds of million years, we keep the pH constant in our model. However, if we understand better how pH change through time, we can plug in pH in above equations and get a more accurate estimate of O2 evolution through time.
SI-5: Supplementary Information References
For Table S-1
Beukes, N.J., Lowe, D.R. (1989) Environmental control on diverse stromatolite morphologies in the 3000 Myr Pongola Supergroup, South Africa. Sedimentology 36, 383-397.
Bickford, M.E., Basu, A., Patranabis-Deb, S., Dhang, P.C., Schieber, J. (2011) Depositional History of the Chhattisgarh Basin, Central India: Constraints from New SHRIMP Zircon Ages. Journal of Geology 119, 33-50.
Chakrabarti, G., Shome, D., Kumar, S., Stephens, G.M., III, Kah, L.C. (2014) Carbonate platform development in a Paleoproterozoic extensional basin, Vempalle Formation, Cuddapah Basin, India. Journal of Asian Earth Sciences 91, 263-279.
Cui, H., Kaufman, A.J., Xiao, S., Zhu, M., Zhou, C., Liu, X.-M. (2015) Redox architecture of an Ediacaran ocean margin: Integrated chemo-stratigraphic (δ13C–δ34S–87Sr/86Sr–Ce/Ce*) correlation of the Doushantuo Formation. Chemical Geology 405, 48-62.
Fio, K., Spangenberg, J.E., Vlahovic, I., Sremac, J., Velic, I., Mrinjek, E. (2010) Stable isotope and trace element stratigraphy across the Permian-Triassic transition: A redefinition of the boundary in the Velebit Mountain, Croatia. Chemical Geology 278, 38-57.
Gilleaudeau, G.J., Kah, L.C. (2013) Carbon isotope records in a Mesoproterozoic epicratonic sea: Carbon cycling in a low-oxygen world. Precambrian Research 228, 85-101.
Grotzinger, J.P., Kasting, J.F. (1993) New constraints on precambrian ocean composition. Journal of Geology 101, 235-243.
Inoue, M., Nohara, M., Okai, T., Suzuki, A., Kawahata, H. (2004) Concentrations of trace elements in carbonate reference materials coral JCp-1 and giant clam JCt-1 by Inductively Coupled Plasma-Mass Spectrometry. Geostandards and Geoanalytical Research 28, 411-416.
Jones, D.S., Maloof, A.C., Hurtgen, M.T., Rainbird, R.H., Schrag, D.P. (2010) Regional and global chemostratigraphic correlation of the early Neoproterozoic Shaler Supergroup, Victoria Island, Northwestern Canada. Precambrian Research 181, 43-63.
Kah, L.C., Bartley, J.K., Frank, T.D., Lyons, T.W. (2006) Reconstructing sea-level change from the internal architecture of stromatolite reefs: an example from the Mesoproterozoic Sulky Formation, Dismal Lakes Group, arctic Canada. Canadian Journal of Earth Sciences 43, 653-669.
Kah, L.C., Crawford, D.C., Bartley, J.K., Kozlov, V.I., Sergeeva, N.D., Puchkov, V.N. (2007) C- and Sr-isotope chemostratigraphy as a tool for verifying age of Riphean deposits in the Kama-Belaya aulacogen, the east European platform. Stratigraphy and Geological Correlation 15, 12-29.
Kah, L.C., Bartley, J.K., Teal, D.A. (2012) Chemostratigraphy of the Late Mesoproterozoic Atar Group, Taoudeni Basin, Mauritania: Muted isotopic variability, facies correlation, and global isotopic trends. Precambrian Research 200, 82-103.
Kaufman, A.J., Sial, A.N., Frimmel, H.E., Misi, A. (2009) Neoproterozoic to Cambrian Palaeoclimatic Events in Southwestern Gondwana. In: Gaucher, C., Sial, A.N., Frimmel, H.E., Halverson, G., P. (Eds.) Developments in Precambrian Geology. Elsevier, pp. 369-388.
Knoll, A.H., Swett, K. (1990) Carbonate deposition during the late proterozoic era - an example from Spitsbergen. American Journal of Science 290A, 104-132.
Knoll, A.H., Hayes, J.M., Kaufman, A.J., Swett, K., Lambert, I.B. (1986) Secular variation in carbon isotope ratios from upper proterozoic successions of svalbard and east greenland. Nature 321, 832-838.
Meyer, E.E., Quicksall, A.N., Landis, J.D., Link, P.K., Bostick, B.C. (2012) Trace and rare earth elemental investigation of a Sturtian cap carbonate, Pocatello, Idaho: Evidence for ocean redox conditions before and during carbonate deposition. Precambrian Research 192-95, 89-106.
Miller, N., Johnson, P.R., Stern, R.J. (2008) Marine versus non-marine environments for the Jibalah Group, NW Arabian shield: A sedimentologic and geochemical survey and report of possible metazoa in the Dhaiqa formation. Arabian Journal for Science and Engineering 33, 55-77.
Mirota, M.D., Veizer, J. (1994) Geochemistry of Precambrian carbonates. 6. Aphebian Albanel Formations, Quebec, Canada. Geochimica et Cosmochimica Acta 58, 1735-1745.
Misi, A., Kaufman, A.J., Veizer, J., Powis, K., Azmy, K., Boggiani, P.C., Gaucher, C., Teixeira, J.B.G., Sanches, A.L., Iyer, S.S.S. (2007) Chemostratigraphic correlation of neoproterozoic successions in South America. Chemical Geology 237, 143-167.
Morgan, R., Orberger, B., Rosiere, C.A., Wirth, R., Carvalho, C.d.M., Bellver-Baca, M.T. (2013) The origin of coexisting carbonates in banded iron formations: A micro-mineralogical study of the 2.4 Ga Itabira Group, Brazil. Precambrian Research 224, 491-511.
Planavsky, N.J., Reinhard, C.T., Wang, X., Thomson, D., McGoldrick, P., Rainbird, R.H., Johnson, T., Fischer, W.W., Lyons, T.W. (2014) Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 346, 635-638.
Reinhard, C.T., Planavsky, N.J., Robbins, L.J., Partin, C.A., Gill, B.C., Lalonde, S.V., Bekker, A., Konhauser, K.O., Lyons, T.W. (2013) Proterozoic ocean redox and biogeochemical stasis. Proceedings of the National Academy of Sciences of the United States of America 110, 5357-5362.
Robbins, L.J., Lalonde, S.V., Saito, M.A., Planavsky, N.J., Mloszewska, A.M., Pecoits, E., Scott, C., Dupont, C.L., Kappler, A., Konhauser, K.O. (2013) Authigenic iron oxide proxies for marine zinc over geological time and implications for eukaryotic metallome evolution. Geobiology 11, 295-306.
Scott, C., Planavsky, N.J., Dupont, C.L., Kendall, B., Gill, B.C., Robbins, L.J., Husband, K.F., Arnold, G.L., Wing, B.A., Poulton, S.W., Bekker, A., Anbar, A.D., Konhauser, K.O., Lyons, T.W. (2013) Bioavailability of zinc in marine systems through time. Nature Geoscience 6, 125-128.
Sergeev, V.N., Knoll, A.H., Petrov, P.Y. (1997) Paleobiology of the Mesoproterozoic-Neoproterozoic transition: The Sukhaya Tunguska Formation, Turukhansk Uplift, Siberia. Precambrian Research 85, 201-239.
Sumner, D.Y., Grotzinger, J.P. (2004) Implications for Neoarchaean ocean chemistry from primary carbonate mineralogy of the Campbellrand-Malmani Platform, South Africa. Sedimentology 51, 1273-1299.
Thompson, C.K., Kah, L.C. (2012) Sulfur isotope evidence for widespread euxinia and a fluctuating oxycline in Early to Middle Ordovician greenhouse oceans. Palaeogeography Palaeoclimatology Palaeoecology 313, 189-214.
Veizer, J., Hoefs, J., Lowe, D.R., Thurston, P.C. (1989a) Geochemistry of precambrian carbonates. 2. Archean greenstone belts and archean sea-water. Geochimica et Cosmochimica Acta 53, 859-871.
Veizer, J., Hoefs, J., Ridler, R.H., Jensen, L.S., Lowe, D.R. (1989b) Geochemistry of Precambrian carbonates . 1. Archean hydrothermal systems. Geochimica et Cosmochimica Acta 53, 845-857.
Veizer, J., Clayton, R.N., Hinton, R.W., Vonbrunn, V., Mason, T.R., Buck, S.G., Hoefs, J. (1990) Geochemistry of Precambrian carbonates. 3. Shelf seas and nonmarine environments of the Archean. Geochimica et Cosmochimica Acta 54, 2717-2729.
Veizer, J., Clayton, R.N., Hinton, R.W. (1992a) Geochemistry of Precambrian carbonates .4. Early Paleoproterozoic (2.25 +/- 0.25 ga) seawater. Geochimica et Cosmochimica Acta 56, 875-885.
Veizer, J., Plumb, K.A., Clayton, R.N., Hinton, R.W., Grotzinger, J.P. (1992b) Geochemistry of Precambrian carbonates. 5. Late Paleoproterozoic seawater. Geochimica et Cosmochimica Acta 56, 2487-2501.
Wilson, J.P., Fischer, W.W., Johnston, D.T., Knoll, A.H., Grotzinger, J.P., Walter, M.R., McNaughton, N.J., Simon, M., Abelson, J., Schrag, D.P., Summons, R., Allwood, A., Andres, M., Gammon, C., Garvin, J., Rashby, S., Schweizer, M., Watters, W.A. (2010) Geobiology of the late Paleoproterozoic Duck Creek Formation, Western Australia. Precambrian Research 179, 135-149.
Zhao, M.-Y., Zheng, Y.-F. (2014) Marine carbonate records of terrigenous input into Paleotethyan seawater: Geochemical constraints from Carboniferous limestones. Geochimica Et Cosmochimica Acta 141, 508-531.
For the rest of SI
Brand, U., Veizer, J. (1980) Chemical diagenesis of a multicomponent carbonate system; 1, Trace elements. Journal of Sedimentary Research 50, 1219-1236.
 Show in context
Show in context Both Zn and Fe partition coefficients (Kd) from fluid to carbonates increase with increasing diagenesis as shown by earlier work of Brand and Veizer (1980).
View in article
Frimmel, H.E. (2009) Trace element distribution in Neoproterozoic carbonates as palaeoenvironmental indicator. Chemical Geology 258, 338-353.
 Show in context
Show in context In addition, we studied REE patterns and Eu anomalies to avoid samples with significant hydrothermal alternation (e.g., Frimmel, 2009).
View in article
Grotzinger, J.P., Kasting, J.F. (1993) New constraints on precambrian ocean composition. Journal of Geology 101, 235-243.
 Show in context
Show in context This approach requires several critical assumptions, one of which is that we assume the pH of the ocean has not changed significantly through Earth’s history—an assumption supported by some studies (Grotzinger and Kasting, 1993; Sumner and Grotzinger, 2004).
View in article
Inoue, M., Nohara, M., Okai, T., Suzuki, A., Kawahata, H. (2004) Concentrations of trace elements in carbonate reference materials coral JCp-1 and giant clam JCt-1 by Inductively Coupled Plasma-Mass Spectrometry. Geostandards and Geoanalytical Research 28, 411-416.
 Show in context
Show in context Figure S-1 Comparison of measured trace elements with those reported for Inoue et al. (2004) for the JcP-1 standard.
View in article
Johnson, J.W., Oelkers, E.H., Helgeson, H.C. (1992) SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000°C. Computers & Geosciences 18, 899-947.
 Show in context
Show in context We then calculate K values at different temperatures using SUPCRT92 (Johnson et al., 1992) and the results are shown in Figure S-6.
View in article
Reinhard, C.T., Planavsky, N.J., Robbins, L.J., Partin, C.A., Gill, B.C., Lalonde, S.V., Bekker, A., Konhauser, K.O., Lyons, T.W. (2013) Proterozoic ocean redox and biogeochemical stasis. Proceedings of the National Academy of Sciences of the United States of America 110, 5357-5362.
 Show in context
Show in context However, Zn concentrations in seawater may drop during the Mid-Proterozoic due to some extent of euxinia (~1~10 % of modern seafloor area; Reinhard et al., 2013).
View in article
Robbins, L.J., Lalonde, S.V., Saito, M.A., Planavsky, N.J., Mloszewska, A.M., Pecoits, E., Scott, C., Dupont, C.L., Kappler, A., Konhauser, K.O. (2013) Authigenic iron oxide proxies for marine zinc over geological time and implications for eukaryotic metallome evolution. Geobiology 11, 295-306.
 Show in context
Show in context Although Zn concentrations, theoretically may have responded to changes in enzymatic use during biosphere evolution, there is no evidence for change in Zn bioavailability change through time (Robbins et al., 2013; Scott et al., 2013).
View in article
Scott, C., Planavsky, N.J., Dupont, C.L., Kendall, B., Gill, B.C., Robbins, L.J., Husband, K.F., Arnold, G.L., Wing, B.A., Poulton, S.W., Bekker, A., Anbar, A.D., Konhauser, K.O., Lyons, T.W. (2013) Bioavailability of zinc in marine systems through time. Nature Geoscience 6, 125-128.
 Show in context
Show in context Although Zn concentrations, theoretically may have responded to changes in enzymatic use during biosphere evolution, there is no evidence for change in Zn bioavailability change through time (Robbins et al., 2013; Scott et al., 2013).
View in article
Sumner, D.Y., Grotzinger, J.P. (2004) Implications for Neoarchaean ocean chemistry from primary carbonate mineralogy of the Campbellrand-Malmani Platform, South Africa. Sedimentology 51, 1273-1299.
 Show in context
Show in context This approach requires several critical assumptions, one of which is that we assume the pH of the ocean has not changed significantly through Earth’s history—an assumption supported by some studies (Grotzinger and Kasting, 1993; Sumner and Grotzinger, 2004).
View in article
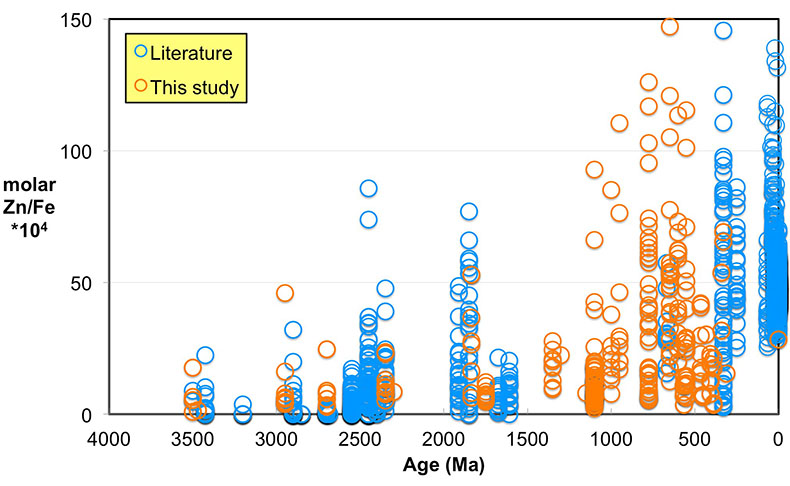
Figure 1 Zn/Fe molar ratios versus time for individual carbonate analyses. The figure contains ~1700 measurements of Zn/Fe data, including literature data (blue), and our 300 new analyses (orange).
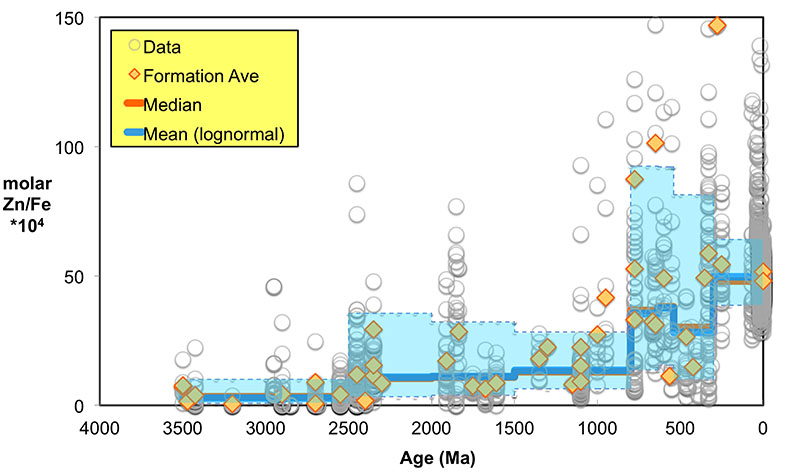
Figure 2 Zn/Fe molar ratio versus time for carbonates, averaged by formation. Formation averages (orange diamonds) were calculated based on simple arithmetic mean of samples within the same formation. Median (orange) and mean from lognormal distribution (blue) lines were calculated based on all samples from the designated time intervals. Estimated Zn/Fe ratio curve through Earth’s history. Uncertainties (light blue fields) are estimated based on one standard deviation from the lognormal distribution.
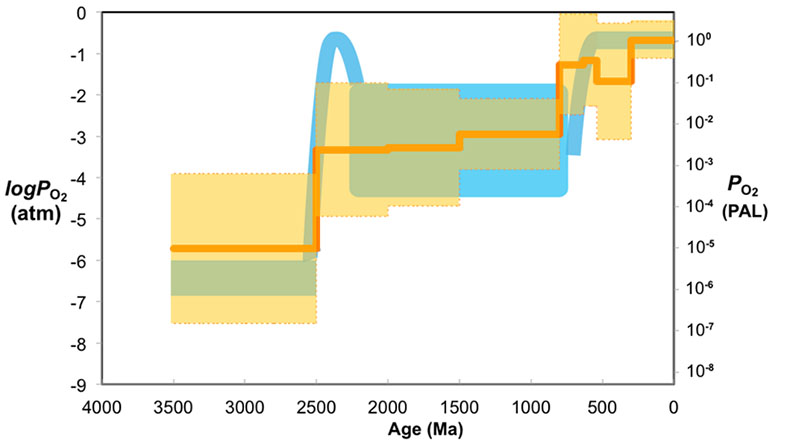
Figure 3 Estimated atmospheric pO2 through Earth’s history. The orange line indicates the best estimate (mean values from lognormal distribution) from carbonate Zn/Fe ratios from this study (yellow fields show the upper and lower range of estimated O2, which is calculated based on one sigma of lognormal distributions). The blue field indicates semi-quantitative interpretation from current understanding of the atmospheric O2 curve (modified from Lyons et al., 2014).
Back to article
Supplementary Figures and Tables
Table S-1 Zn/Fe ratios with sample name and age information from this study.
| Geologic Unit | Sample # | Age (Ma) | Zn/Fe*104 | Reference |
| Strelley Pool Fm, Warrawoona Group, Pilbara, Western Australia | BH2 | 3400 | 0.9 | Hazen (unpublished) |
| Strelley Pool Fm, Warrawoona Group, Pilbara, Western Australia | BH3 | 3400 | 17.6 | Hazen (unpublished) |
| Strelley Pool Fm, Warrawoona Group, Pilbara, Western Australia | BH7 | 3400 | 5.3 | Hazen (unpublished) |
| Strelley Pool Fm, Warrawoona Group, Pilbara, Western Australia | BH10 | 3400 | 6.5 | Hazen (unpublished) |
| Calcite filling in Mt. Ada basalt | BH18 | 3470 | 1.5 | Hazen (unpublished) |
| Pongola Fm, Sourth Africa | Po1 | 2950 | 16.1 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po2 | 2950 | 45.9 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po3 | 2950 | 4.5 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po4 | 2950 | 6.4 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po5 | 2950 | 5.9 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po6 | 2950 | 7.8 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po7 | 2950 | 5.8 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po8 | 2950 | 4.0 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po9 | 2950 | 3.8 | Beukes and Lowe (1989) |
| Pongola Fm, Sourth Africa | Po10 | 2950 | 4.8 | Beukes and Lowe (1989) |
| Tumbiana Fm, Fortesue Group, Western Australia | BH11 | 2700 | 5.8 | Hazen (unpublished) |
| Tumbiana Fm, Fortesue Group, Western Australia | BH12 | 2700 | 24.6 | Hazen (unpublished) |
| Tumbiana Fm, Fortesue Group, Western Australia | BH13 | 2700 | 8.9 | Hazen (unpublished) |
| Tumbiana Fm, Fortesue Group, Western Australia | BH14 | 2700 | 8.0 | Hazen (unpublished) |
| Tumbiana Fm, Fortesue Group, Western Australia | BH15 | 2700 | 2.8 | Hazen (unpublished) |
| Tumbiana Fm, Fortesue Group, Western Australia | BH16 | 2700 | 3.4 | Hazen (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN94 | 2350 | 7.6 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN95 | 2350 | 8.4 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN96 | 2350 | 9.9 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN97 | 2350 | 7.7 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN98 | 2350 | 8.2 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN99 | 2350 | 5.2 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN100 | 2350 | 7.8 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN101 | 2350 | 10.4 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN102 | 2350 | 11.5 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN103 | 2350 | 13.1 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN104 | 2350 | 23.0 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | AN105 | 2350 | 23.5 | Knoll et al. (unpublished) |
| Kazput Fm, Turee Creek, Australia | BH19 | 2350 | 8.5 | Hazen (unpublished) |
| Duck Creek, Auatralia | AN80 | 1835 | 53.0 | Wilson et al. (2010) |
| Duck Creek, Auatralia | AN82 | 1835 | 8.0 | Wilson et al. (2010) |
| Duck Creek, Auatralia | AN83 | 1835 | 36.6 | Wilson et al. (2010) |
| Duck Creek, Auatralia | AN84 | 1835 | 53.0 | Wilson et al. (2010) |
| Duck Creek, Auatralia | AN85 | 1835 | 26.5 | Wilson et al. (2010) |
| Duck Creek, Auatralia | AN86 | 1835 | 16.2 | Wilson et al. (2010) |
| Duck Creek, Auatralia | AN87 | 1835 | 27.6 | Wilson et al. (2010) |
| Duck Creek, Auatralia | AN88 | 1835 | 6.2 | Wilson et al. (2010) |
| Vempalle Fm, Cuddapah Basin, India | V-SC/1 | 1750 | 8.5 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-SC/2 | 1750 | 4.8 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-SC/3 | 1750 | 5.3 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-SC/5 | 1750 | 10.6 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-SC/6-1 | 1750 | 6.8 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-P2/1 | 1750 | 5.5 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-P8/1 | 1750 | 12.2 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-P10/1 | 1750 | 7.5 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-P11/1 | 1750 | 6.9 | Chakrabarti et al. (2014) |
| Vempalle Fm, Cuddapah Basin, India | V-P12/1 | 1750 | 6.1 | Chakrabarti et al. (2014) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C133-3252.3 | 1350 | 17.8 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C133-3253.3 | 1350 | 12.8 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C133-3038 | 1350 | 9.5 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C133-2767.5 | 1350 | 27.7 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C133-3114.5 | 1350 | 20.1 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C203-3852A | 1350 | 24.4 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C203-2459.8 | 1350 | 10.0 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C203-2459.6-1 | 1350 | 18.1 | Kah et al. (2007) |
| Kyrpy Group, East Eurapean Platform, Southern Urals, Russia | C203-2353 | 1350 | 20.9 | Kah et al. (2007) |
| Sulky Fm, Dismal Lakes, Canada | DL1-364-1 | 1300 | 13.9 | Kah et al. (2006) |
| Sulky Fm, Dismal Lakes, Canada | DL1-306-1 | 1300 | 28.0 | Kah et al. (2006) |
| Sulky Fm, Dismal Lakes, Canada | DL1-332 | 1300 | 22.4 | Kah et al. (2006) |
| Sulky Fm, Dismal Lakes, Canada | SL16-1-1 | 1300 | 2.7 | Kah et al. (2006) |
| Sulky Fm, Dismal Lakes, Canada | SL17-10-1 | 1300 | 4.5 | Kah et al. (2006) |
| Avzyan Fm, Southern Urals, Russia | M1(AZ)-39 | 1150 | 3.0 | Bartley et al. (2007) |
| Avzyan Fm, Southern Urals, Russia | M1(AZ)-47 | 1150 | 31.7 | Bartley et al. (2007) |
| Avzyan Fm, Southern Urals, Russia | RV(AZ)-15 | 1150 | 8.8 | Bartley et al. (2007) |
| Avzyan Fm, Southern Urals, Russia | RV(AZ)-33 | 1150 | 13.8 | Bartley et al. (2007) |
| Avzyan Fm, Southern Urals, Russia | KT(AZ)-49.5 | 1150 | 17.6 | Bartley et al. (2007) |
| Avzyan Fm, Southern Urals, Russia | KT(AZ)-131.6 | 1150 | 3.0 | Bartley et al. (2007) |
| Avzyan Fm, Southern Urals, Russia | KT(AZ)-236-1 | 1150 | 7.0 | Bartley et al. (2007) |
| Avzyan Fm, Southern Urals, Russia | KT(AZ)-373.5 | 1150 | 8.0 | Bartley et al. (2007) |
| El Mreiti,Atar Group, West Africa | F4-10-1 | 1100 | 42.6 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-19-1 | 1100 | 17.7 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-50-1 | 1100 | 13.9 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-53-1 | 1100 | 16.4 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-90-1 | 1100 | 17.8 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-95-1 | 1100 | 11.9 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-98-1 | 1100 | 17.7 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-99-1 | 1100 | 39.6 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-102-1 | 1100 | 20.0 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-104-1 | 1100 | 8.6 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-106-1 | 1100 | 11.4 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-107-1 | 1100 | 13.0 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-108-1 | 1100 | 6.8 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-109-1 | 1100 | 6.1 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-113-1 | 1100 | 13.1 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-114-1 | 1100 | 7.2 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-115-1 | 1100 | 7.8 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-116-1 | 1100 | 7.8 | Gilleaudeau and Kah (2013) |
| El Mreiti,Atar Group, West Africa | F4-117-1 | 1100 | 7.9 | Gilleaudeau and Kah (2013) |
| Atar Group, West Africa | ATS-31 | 1100 | 14.8 | Kah et al. (2012) |
| Atar Group, West Africa | ATS-53 | 1100 | 92.9 | Kah et al. (2012) |
| Atar Group, West Africa | ATS-61 | 1100 | 16.5 | Kah et al. (2012) |
| Atar Group, West Africa | ATS-5-1 | 1100 | 13.8 | Kah et al. (2012) |
| Atar Group, West Africa | ATS-119 | 1100 | 11.6 | Kah et al. (2012) |
| Atar Group, West Africa | ATS-154 | 1100 | 12.8 | Kah et al. (2012) |
| Atar Group, West Africa | ATD-17 | 1100 | 18.4 | Kah et al. (2012) |
| Atar Group, West Africa | ATD-61 | 1100 | 18.2 | Kah et al. (2012) |
| Atar Group, West Africa | ATL-51-1 | 1100 | 9.3 | Kah et al. (2012) |
| Atar Group, West Africa | ATL-58 | 1100 | 66.2 | Kah et al. (2012) |
| Atar Group, West Africa | ATL-68 | 1100 | 4.9 | Kah et al. (2012) |
| Atar Group, West Africa | ATL-105 | 1100 | 19.3 | Kah et al. (2012) |
| Atar Group, West Africa | ATL-110-1 | 1100 | 6.3 | Kah et al. (2012) |
| Atar Group, West Africa | ATD-27.5 | 1100 | 12.3 | Kah et al. (2012) |
| Atar Group, West Africa | ATD-45 | 1100 | 18.4 | Kah et al. (2012) |
| Atar Group, West Africa | R1-Δ-1 | 1100 | 16.4 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-7 | 1100 | 25.5 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-13 | 1100 | 14.9 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-25 | 1100 | 12.7 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-29 | 1100 | 12.0 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-30 | 1100 | 10.3 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-31 | 1100 | 5.8 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-38 | 1100 | 12.2 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-39 | 1100 | 6.5 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-41 | 1100 | 9.6 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-42 | 1100 | 6.7 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-48 | 1100 | 8.1 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-54-1 | 1100 | 9.6 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-55 | 1100 | 7.1 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-58 | 1100 | 7.6 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-60 | 1100 | 4.7 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-61 | 1100 | 4.6 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-65 | 1100 | 9.9 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-68-1 | 1100 | 18.3 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-72 | 1100 | 6.6 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-76 | 1100 | 12.4 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-79-1 | 1100 | 8.3 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-81-1 | 1100 | 5.2 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-85 | 1100 | 6.0 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-89 | 1100 | 16.8 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-90 | 1100 | 4.4 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-97 | 1100 | 3.1 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-98 | 1100 | 9.2 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-100 | 1100 | 3.6 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-103 | 1100 | 3.1 | Manning-Berg and Kah (in prep) |
| Atar Group, West Africa | R1-Δ-106 | 1100 | 2.2 | Manning-Berg and Kah (in prep) |
| Chattisgarh, India | SRJ-2I | 1000 | 25.9 | Bickford et al. (2011) |
| Chattisgarh, India | SRJ-3I | 1000 | 22.8 | Bickford et al. (2011) |
| Chattisgarh, India | SRJ-4I | 1000 | 13.0 | Bickford et al. (2011) |
| Chattisgarh, India | SRJ-6I | 1000 | 20.6 | Bickford et al. (2011) |
| Chattisgarh, India | SRJ-8I | 1000 | 37.8 | Bickford et al. (2011) |
| Chattisgarh, India | SRJ-9I | 1000 | 85.2 | Bickford et al. (2011) |
| Chattisgarh, India | TML-3I | 1000 | 7.7 | Bickford et al. (2011) |
| Chattisgarh, India | TML-6I | 1000 | 17.8 | Bickford et al. (2011) |
| Chattisgarh, India | TML-7I | 1000 | 14.9 | Bickford et al. (2011) |
| Sukhaya Tunguska Formation, Russia | AN59 | 950 | 29.5 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN60 | 950 | 46.1 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN61 | 950 | 25.7 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN62 | 950 | 76.4 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN63 | 950 | 110.5 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN64 | 950 | 17.0 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN66 | 950 | 28.1 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN67 | 950 | 20.0 | Sergeev et al. (1997) |
| Sukhaya Tunguska Formation, Russia | AN68 | 950 | 20.2 | Sergeev et al. (1997) |
| Akademikerbeen Group, Spitsbergen | AN1 | 775 | 29.2 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN2 | 775 | 40.3 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN3 | 775 | 17.3 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN4 | 775 | 6.2 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN5 | 775 | 63.2 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN6 | 775 | 65.0 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN7 | 775 | 27.6 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN8 | 775 | 74.3 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN9 | 775 | 95.4 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN10 | 775 | 59.4 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN12 | 775 | 71.5 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN13 | 775 | 15.8 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN14 | 775 | 49.4 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN16 | 775 | 15.1 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN18 | 775 | 17.6 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN19 | 775 | 9.4 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN20 | 775 | 34.4 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN21 | 775 | 276.2 | Knoll and Swett (1990) |
| Akademikerbeen Group, Spitsbergen | AN22 | 775 | 34.6 | Knoll and Swett (1990) |
| Limestone-Dolomite Series, East Greenland | AN24 | 775 | 126.1 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN25 | 775 | 59.3 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN26 | 775 | 45.7 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN28 | 775 | 5.3 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN29 | 775 | 61.2 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN30 | 775 | 307.6 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN31 | 775 | 310.7 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN32 | 775 | 38.3 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN33 | 775 | 33.3 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN34 | 775 | 15.5 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN35 | 775 | 8.3 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN36 | 775 | 102.9 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN37 | 775 | 67.0 | Knoll et al. (1986) |
| Limestone-Dolomite Series, East Greenland | AN38 | 775 | 41.4 | Knoll et al. (1986) |
| Shaler Group, Arctic Canada | AN41 | 775 | 5.6 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN42 | 775 | 17.3 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN43 | 775 | 45.7 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN44 | 775 | 7.0 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN45 | 775 | 154.8 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN46 | 775 | 6.2 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN47 | 775 | 40.3 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN48 | 775 | 14.8 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN49 | 775 | 116.9 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN50 | 775 | 8.4 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN51 | 775 | 10.9 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN52 | 775 | 57.5 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN53 | 775 | 6.9 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN54 | 775 | 6.0 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN55 | 775 | 12.8 | Jones et al. (2010) |
| Shaler Group, Arctic Canada | AN56 | 775 | 17.8 | Jones et al. (2010) |
| Lagoa Do Jacare Formation, Brazil | KM7-14.0.0 | 650 | 38.1 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-01.0 | 650 | 21.9 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-02.0 | 650 | 49.0 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-03.0 | 650 | 17.6 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-04.0 | 650 | 12.5 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-05.0 | 650 | 22.0 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-06.0 | 650 | 38.9 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-07.0 | 650 | 54.1 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-08.0 | 650 | 32.8 | Misi et al. (2007) |
| Lagoa Do Jacare Formation, Brazil | KM7-14-09.0 | 650 | 23.8 | Misi et al. (2007) |
| Huttenburg Formation, Namibia | S86A-971.2 | 650 | 105.2 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-976.0 | 650 | 77.5 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-977.0 | 650 | 120.9 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-980.8 | 650 | 53.5 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-985.1 | 650 | 147.1 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-987.8 | 650 | 28.4 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-988.2 | 650 | 41.8 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-1033.8 | 650 | 424.1 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-1060.2 | 650 | 156.2 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-1077.1 | 650 | 57.4 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-1144.8 | 650 | 52.8 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-1145.1 | 650 | 47.7 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-1148.4 | 650 | 55.6 | Kaufman et al. (2009) |
| Huttenburg Formation, Namibia | S86A-1213.2 | 650 | 51.1 | Kaufman et al. (2009) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-7 | 600 | 61.0 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-21 | 600 | 62.5 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-26 | 600 | 30.7 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-34 | 600 | 37.5 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-38 | 600 | 19.8 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-38b | 600 | 40.1 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-39 | 600 | 38.7 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-46 | 600 | 24.9 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-49 | 600 | 59.0 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-51 | 600 | 19.8 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | Dhaiqa-54 | 600 | 24.9 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | M1-with fossil | 600 | 62.2 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | N-2-3 | 600 | 113.4 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | N-2-11 | 600 | 73.1 | Miller et al. (2008) |
| Dhaiqa Formation, NW Arabian shield, Saudi Arabia | N-2-16 | 600 | 68.9 | Miller et al. (2008) |
| Yangjiaping, Doushantuo Formation, South China | YD-01 | 551 | 12.9 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-02 | 551 | 47.1 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-03 | 551 | 11.7 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-04 | 551 | 25.1 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-05 | 551 | 71.0 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-06 | 551 | 13.9 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-07 | 551 | 28.4 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-08 | 551 | 6.6 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-09 | 551 | 6.2 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-10 | 551 | 26.9 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-11 | 551 | 50.4 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-12 | 551 | 25.1 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-13 | 551 | 23.7 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-14 | 551 | 55.0 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-15 | 551 | 115.4 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-16 | 551 | 221.5 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-17 | 551 | 253.8 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-18 | 551 | 195.9 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-19 | 551 | 101.2 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-20 | 551 | 14.5 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-21 | 551 | 10.9 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-22 | 551 | 29.1 | Cui et al. (2015) |
| Yangjiaping, Doushantuo Formation, South China | YD-23 | 551 | 40.1 | Cui et al. (2015) |
| Orthoceras limestone, Ohio | BH21 | 465 | 27.5 | Hazen (unpublished) |
| Bryozoan limestone, Ohio | BH24 | 465 | 7.2 | Hazen (unpublished) |
| Branch Hill limestone, Ohio | BH25 | 465 | 10.9 | Hazen (unpublished) |
| Jersey Shore Station, Pennsylvania | BH26 | 465 | 7.0 | Hazen (unpublished) |
| Madison County, Kentucky | BH31 | 465 | 14.3 | Hazen (unpublished) |
| Ripley, Ohio | BH36 | 465 | 8.3 | Hazen (unpublished) |
| Washington, Kentucky | BH37 | 465 | 11.4 | Hazen (unpublished) |
| Bryozoan limestone, Ohio | BH33 | 465 | 3.5 | Hazen (unpublished) |
| Coburn Fm, Pennsylvania | BH41 | 465 | 10.9 | Hazen (unpublished) |
| La Silla Fm, Argentina | AF-23 | 464 | 22.9 | Thompson and Kah (2012) |
| La Silla Fm, Argentina | AF-30 | 464 | 12.4 | Thompson and Kah (2012) |
| La Silla Fm, Argentina | SJF08-14 | 464 | 16.1 | Thompson and Kah (2012) |
| La Silla Fm, Argentina | LFG-27 | 464 | 40.5 | Thompson and Kah (2012) |
| La Silla Fm, Argentina | LFG-55 | 464 | 17.6 | Thompson and Kah (2012) |
| La Silla Fm, Argentina | LS-01 | 464 | 29.7 | Thompson and Kah (2012) |
| La Silla Fm, Argentina | SJC-116 | 464 | 42.1 | Thompson and Kah (2012) |
| West Newfoundland | TH-1 | 464 | 15.3 | Thompson and Kah (2012) |
| West Newfoundland | TH-18 | 464 | 41.7 | Thompson and Kah (2012) |
| Clay's Ferry Fm, Kentucky | BH49 | 457 | 7.1 | Hazen (unpublished) |
| Cincinnati, Ohio | BH32 | 451 | 6.5 | Hazen (unpublished) |
| Richmond, Indiana | BH27 | 451 | 7.3 | Hazen (unpublished) |
| Ludlow Fm, Silurian | BH28 | 432 | 30.3 | Hazen (unpublished) |
| Limestone Quarry, Dickensonville, Virginia | BH29 | 406 | 7.7 | Hazen (unpublished) |
| John Boyd Thatchen State Park, New York | BH30 | 406 | 12.8 | Hazen (unpublished) |
| Blue Stone limestone Quarry, New York | BH34 | 406 | 19.0 | Hazen (unpublished) |
| Isle La Motte, Vermont | BH38 | 406 | 21.6 | Hazen (unpublished) |
| Isle La Motte, Vermont | BH39 | 406 | 12.2 | Hazen (unpublished) |
| Isle La Motte, Vermont | BH40 | 406 | 22.9 | Hazen (unpublished) |
| Hamilton Group, New York | BH22 | 388 | 3.6 | Hazen (unpublished) |
| Plainville Quarry, Ohio | BH44 | 388 | 17.4 | Hazen (unpublished) |
| Limestone Quarry, Ohio | BH52 | 388 | 4.2 | Hazen (unpublished) |
| Coral limestone, Michigan | BH53 | 388 | 21.0 | Hazen (unpublished) |
| Madision limestone, Garrett Co., Maryland | BH23 | 341 | 31.7 | Hazen (unpublished) |
| Mammoth Cave National Park, Kentucky | BH47 | 341 | 190.6 | Hazen (unpublished) |
| Sheep Mountain | BH51 | 341 | 53.6 | Hazen (unpublished) |
| Lydstep Haven coral, Wales | BH35 | 329 | 70.4 | Hazen (unpublished) |
| Tenby, Wales | BH43 | 329 | 69.0 | Hazen (unpublished) |
| Cheddar Gorge, England | BH46 | 329 | 65.5 | Hazen (unpublished) |
| Everett Quarry, Missouri | BH45 | 311 | 15.4 | Hazen (unpublished) |
| Shark Bay, Australia | BH20 | 0 | 28.4 | Hazen (unpublished) |
*See references in SI-5.
Back to article | Download in Excel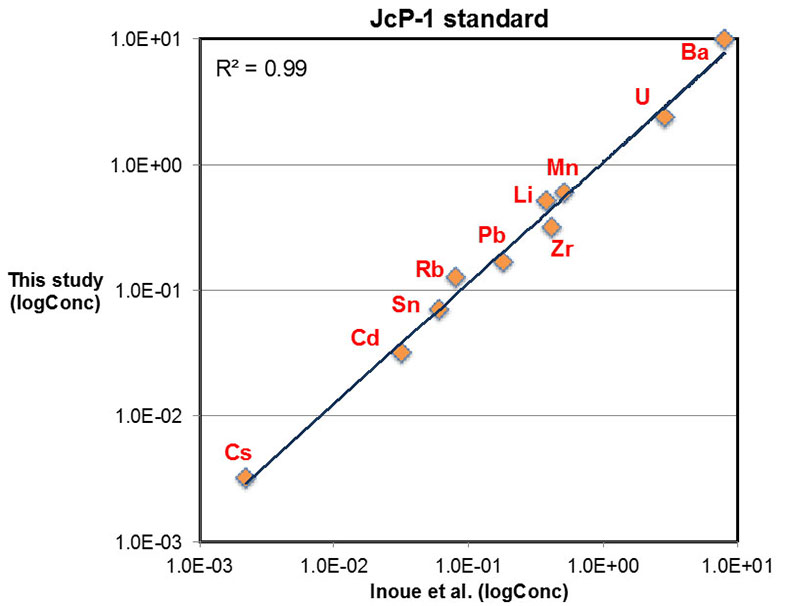
Figure S-1 Comparison of measured trace elements with those reported for Inoue et al. (2004) for the JcP-1 standard. The full analytical method is discussed in the methods section following the main text.
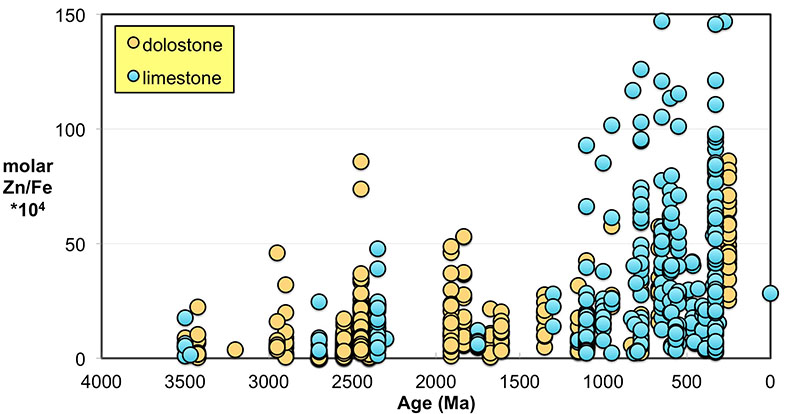
Figure S-2 Zn/Fe ratios in marine carbonate, plotted with information on sample lithology.
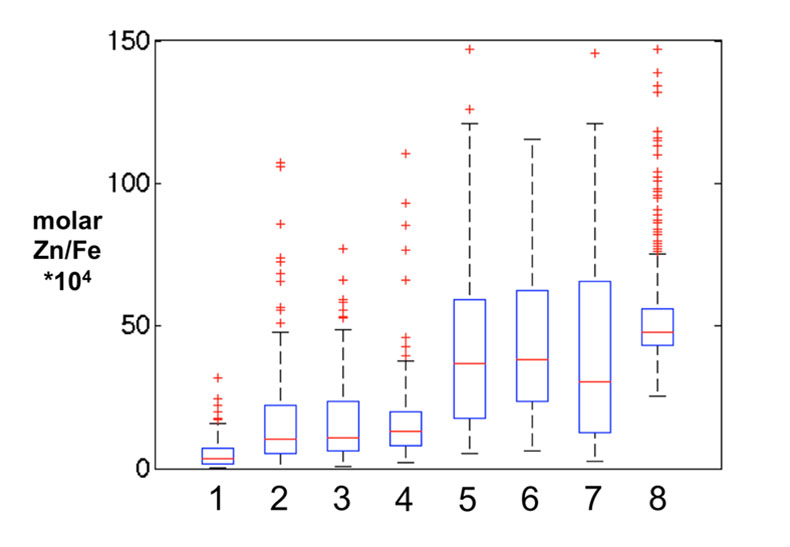
Figure S-3 Box-whisker distribution of all samples. The sample population is divided into eight bins (Bin 1: 3.5-2.5 Ga, Bin 2: 2.5-2.0 Ga, Bin 3: 2.0-1.5 Ga, Bin 4: 1.5-0.8 Ga, Bin 5: 800-635 Ma, Bin 6: 635-541 Ma, Bin 7: 541-300 Ma, Bin 8: 300-0 Ma) of different duration to make sure each bin has statistically meaningful sample numbers (where n > 50, except for one bin with n = 38). Each bin contains samples from at least two different geological formations. We show a Box-whisker plot for each group. Median values are indicated by the red lines and each individual box includes 50 % samples and whiskers mark the 3 sigma boundaries of the group population. Red crosses fall out of whiskers and are considered outliers.
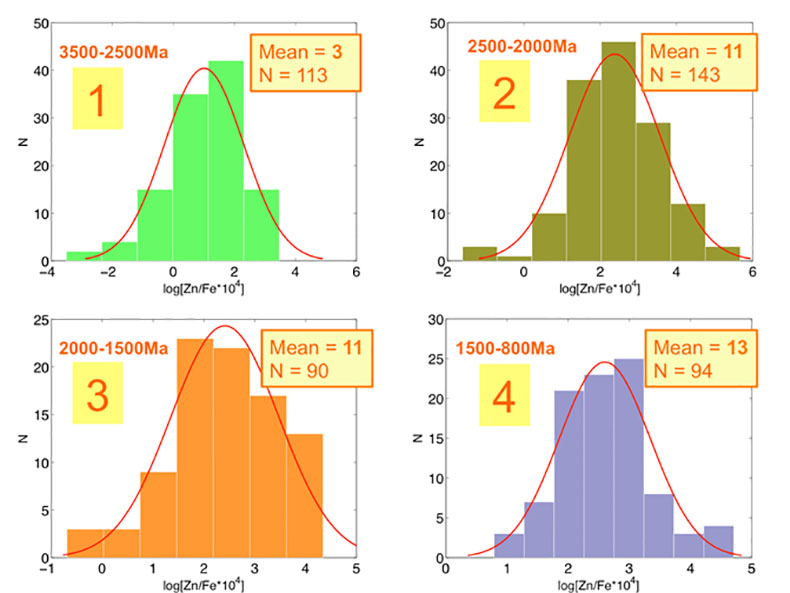
Figure S-4 Histograms of Zn/Fe ratios with lognormal fitting in red. We group all data into eight different bins (age distribution of the bins is provided in Fig. S-2) and plot the lognormal distribution for each group.
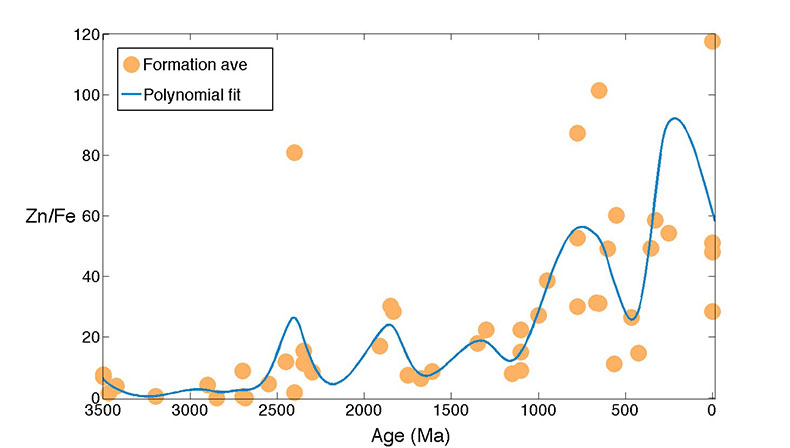
Figure S-5 Zn/Fe molar ratio versus time for carbonates averaged by formation. A polynomial fit through the formation average data.
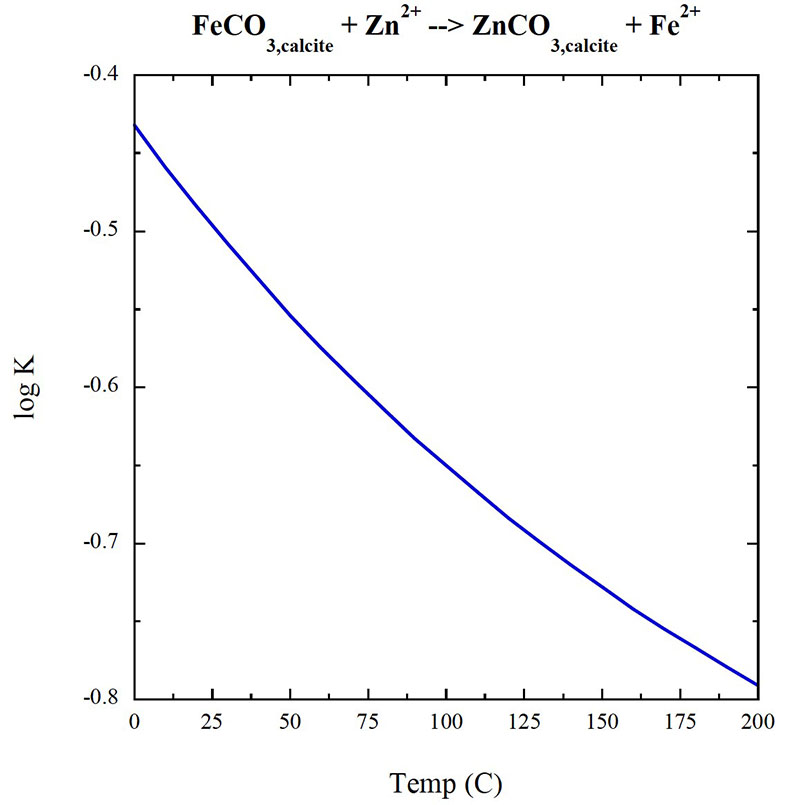
Figure S-6 log K (equilibrium constant) versus temperature plot for chemical reaction: FeCO3 + Zn2+ --> ZnCO3 + Fe2+.