Scandium speciation in a world-class lateritic deposit
Affiliations | Corresponding Author | Cite as | Funding informationKeywords: scandium, rare earth elements, laterite, tropical climate, alteration, chemical weathering, Fe oxides, quantitative phase analysis, X-ray absorption spectroscopy, speciation, sorption, incorporation, crystal-chemistry, mineralogy, geochemistry
- Share this article





Article views:10,905Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
Abstract
Figures and Tables
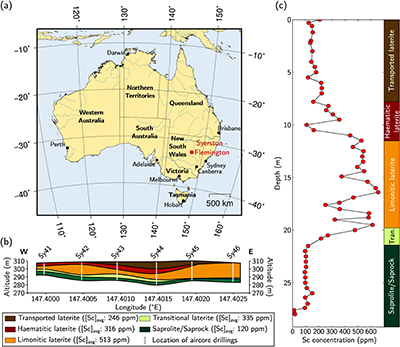 Figure 1 (a) Geographical location of the Syerston–Flemington deposit. (b) Schematic cross section of the lateritic cover forming the Syerston–Flemington deposit, constructed from petrographic analysis of drill-cores. Average Sc concentrations ([Sc]avg) are calculated for each layer from ICP-MS analyses of samples collected every metre on each core. (c) Distribution of Sc with depth along the different levels of a typical profile (Tran.: transitional laterite). | 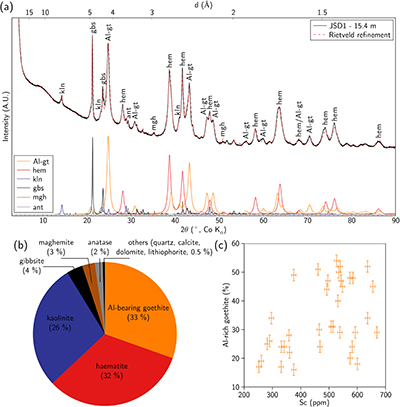 Figure 2 (a) X-ray diffraction pattern of ore sample JSD-1 – 15.4 m and associated multiphase Rietveld refinement (Al-gt: Al-bearing goethite, hem: haematite, kln: kaolinite, gbs: gibbsite, mgh: maghemite, ant: anatase). (b) Average phase proportions in the samples calculated from Rietveld refinement. (c) Bulk Sc concentration versus proportion of Al-bearing goethite estimated from Rietveld refinement. | 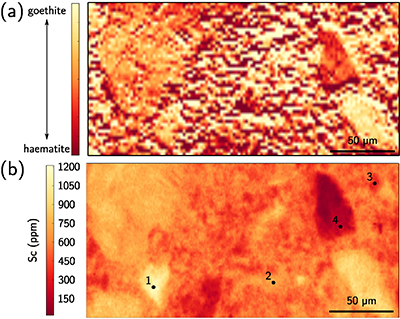 Figure 3 (a) Map of Fe speciation obtained by subtracting normalised synchrotron µ-XRF maps at 7133.9 eV, the energy characteristic of goethite and 7136.7 eV, the energy characteristic of haematite. (b) Synchrotron µ-XRF mapping of Sc. | 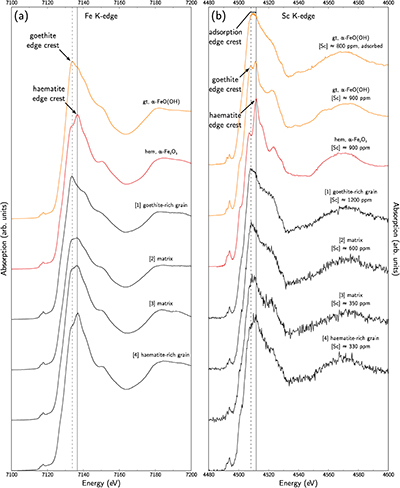 Figure 4 XANES spectra at the (a) Fe K-edge. (b) Sc K-edge (gt: goethite and hem: haematite). The numbers in brackets refer to the position given in Figure 3. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Supplementary Figures and Tables
 Table S-1 Latitude and longitude coordinates, altitude and dip angle of the two diamond core drillings. |  Figure S-1 Geological map of the Tout complex showing the locations of the drilling sites (cpx: clinopyroxene, hbl: hornblende, qtz: quartz). |  Table S-2 Detailed log of the JSD-001 drill core. |  Table S-3 Sc concentration (in ppm) at each metre of the 26 vertical aircore drillings performed in the Syerston–Flemington area. |  Table S-4 Nature and proportion of the phases identified for each sample (b.d.l: below detection limit using Rietveld refinement but present in the sample, abs.: absent from the sample). |
| Table S-1 | Figure S-1 | Table S-2 | Table S-3 | Table S-4 |
 Table S-5 Bulk geochemical analyses for major and minor elements along with Sc and the rare earth elements. | 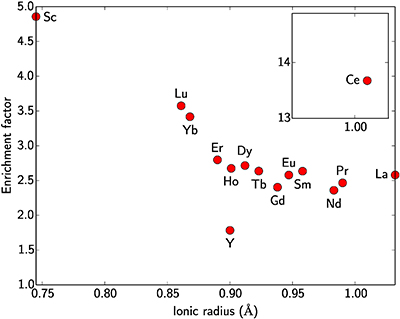 Figure S-2 Enrichment factor of rare earth elements in the lateritic deposit versus ionic radius in octahedral coordination. Values are after Shannon (1976). |  Table S-6 Median, mean and standard deviation of Si, Ti, Fe, Al, analytical total and Sc concentrations in the different grain types distinguished by SEM imaging, EMP and cluster analysis. | 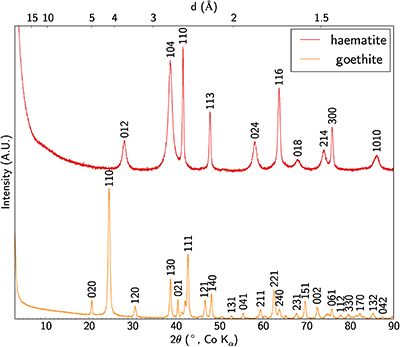 Figure S-3 XRD patterns of synthetic Sc-bearing goethite and haematite. |
| Table S-5 | Figure S-2 | Table S-6 | Figure S-3 |
top
Introduction
Scandium is embedded in the very fabric of modern society, making it one of the most valuable elements. End-uses range from biomedical research to electronics, lasers and ceramics but the demand is driven by energy issues (Emsley, 2014
Emsley, J. (2014) Unsporting Scandium. Nature Chemistry 6, 1025.
). Scandium finds applications in Al–Sc superalloys, increasing tensile strength and improving weldability while maintaining light weight, an energy-saving factor in aerospace and automotive industries. In solid oxide fuel cells, the addition of Sc to the electrolyte improves conductivity and lowers the operating temperature, extending fuel cell life.Despite a crustal abundance of ca. 22 ppm (Rudnick and Gao, 2014
Rudnick, R.L., Gao, S. (2014) Composition of the Continental Crust. In: Holland, H., Turekian, K.K. (Eds.) Treatise on Geochemistry, 2nd Edition. Elsevier Ltd, Amsterdam, Netherlands, 1–51.
), comparable to common elements such as copper or lead, current Sc production is limited to 10 t to 15 t per year (U.S. Geological Survey, 2016U.S. Geological Survey (2016) Scandium. In: Mineral Commodity Summaries. U.S. Geological Survey, Reston, USA, 146–147.
). The large ratio of ionic radius to charge of Sc3+ hinders the concentration of scandium during most geochemical processes (Samson and Chassé, 2016Samson, I.M., Chassé, M. (2016) Scandium. In: White, W.M. (Ed.) Encyclopedia of Geochemistry. Springer International Publishing, Cham, Switzerland, 1–5.
). A notable exception concerns lateritic deposits developed over ultramafic–mafic rocks where Sc concentrations up to 100 ppm make it a potential by-product (Aiglsperger et al., 2016Aiglsperger, T., Proenza, J.A., Lewis, J.F., Labrador, M., Svojtka, M., Rojas-Purón, A., Longo, F., Ďurišová, J. (2016) Critical Metals (REE, Sc, PGE) in Ni Laterites from Cuba and the Dominican Republic. Ore Geology Reviews 73, 127–147.
; Maulana et al., 2016Maulana, A., Sanematsu, K., Sakakibara, M. (2016) An Overview on the Possibility of Scandium and REE Occurrence in Sulawesi, Indonesia. Indonesian Journal on Geoscience 3, 139–147.
). Recently, lateritic deposits with Sc concentrations high enough to mine as a primary product have been reported in Eastern Australia (Jaireth et al., 2014Jaireth, S., Hoatson, D.M., Miezitis, Y. (2014) Geological Setting and Resources of the Major Rare-Earth-Element Deposits in Australia. Ore Geology Reviews 62, 72–128.
). Among these, the Syerston–Flemington deposit contains about 1350 t of Sc at an average concentration of 434 ppm Sc (Pursell, 2016Pursell, D.C. (2016) Quarterly Report. Jervois Mining Ltd, Cheltenham, Australia.
), providing a century-long resource at the present levels of world consumption.We present the first data on Sc speciation in lateritic deposits by combining quantitative mineralogy, geochemical analysis and X-ray absorption near-edge structure (XANES) spectroscopy on drill-core samples from the lateritic profile of the Syerston–Flemington deposit. The results explain the geochemical conditions required to form such exceptional Sc concentrations and improve our understanding of the geochemical behaviour of this under-explored element.
top
Geological Context
In Eastern Australia, lateritic profiles developed under seasonally dry humid tropical climatic conditions that resulted in intensive weathering during the Tertiary; the present occurrences are often erosional remnants of fossil laterite (Milnes et al., 1987
Milnes, A.R., Bourman, R.P., Fitzpatrick, R.W. (1987) Petrology and Mineralogy of ‘Laterites’ in Southern and Eastern Australia and Southern Africa. Chemical Geology 60, 237–250.
). The Syerston–Flemington deposit (Fig. 1a and Table S-1) is part of the lateritic cover developed over the Tout complex, an ultramafic–mafic ‘Alaskan-type’ intrusive complex in the Lachlan Fold Belt (Johan et al., 1989Johan, Z., Ohnenstetter, M., Slansky, E., Barron, L.M., Suppel, D.W. (1989) Platinum Mineralization in the Alaskan-type Intrusive Complexes near Fifield, New South Wales, Australia Part 1. Platinum-Group Minerals in Clinopyroxenites of the Kelvin Grove Prospect, Owendale Intrusion. Mineralogy and Petrology 40, 289–309.
). Scandium anomalies have been found over a body of nearly pure clinopyroxenite (Fig. S-1), with Sc concentrations (ca. 80 ppm; Table S-3) twice as high as those in typical mantle clinopyroxenites (Samson and Chassé, 2016Samson, I.M., Chassé, M. (2016) Scandium. In: White, W.M. (Ed.) Encyclopedia of Geochemistry. Springer International Publishing, Cham, Switzerland, 1–5.
). Similar concentrations (60 ppm to 80 ppm) occur in other ‘Alaskan-type’ clinopyroxenites (Burg et al., 2009Burg, J.-P., Bodinier, J.-L., Gerya, T., Bedini, R.-M., Boudier, F., Dautria, J.-M., Prikhodko, V., Efimov, A., Pupier, E., Balanec, J.-L. (2009) Translithospheric Mantle Diapirism: Geological Evidence and Numerical Modelling of the Kondyor Zoned Ultramafic Complex (Russian Far-East). Journal of Petrology 50, 289–321.
) and in clinopyroxenes from ocean-island basalts (Dorais, 2015Dorais, M.J. (2015) Exploring the Mineralogical Heterogeneities of the Louisville Seamount Trail. Geochemistry, Geophysics, Geosystems 16, 2884–2899.
). This suggests the accumulation of clinopyroxene in subvolcanic feeder conduits during fractional crystallisation of a mantle-derived melt. Anomalies of Sc concentration in the parent rock result from specific conditions of formation and are not shared by all ultramafic–mafic bedrocks.
Figure 1 (a) Geographical location of the Syerston–Flemington deposit. (b) Schematic cross section of the lateritic cover forming the Syerston–Flemington deposit, constructed from petrographic analysis of drill-cores. Average Sc concentrations ([Sc]avg) are calculated for each layer from ICP-MS analyses of samples collected every metre on each core. (c) Distribution of Sc with depth along the different levels of a typical profile (Tran.: transitional laterite).
top
Core Sampling and Analytical Methods
Each metre of 26 vertical aircore holes has been analysed for Sc and two diamond drill-cores of about 30 m length have been sampled to collect rock chips from the limonitic part of the lateritic cover. Quantitative mineralogical compositions were determined by X-ray diffraction (XRD) and Rietveld refinement. Major and trace elements were determined by X-ray fluorescence (XRF) analysis and inductively coupled plasma mass spectrometry (ICP-MS), respectively. Mapping of major elements was done using scanning electron microscopy–energy dispersive X-ray spectroscopy (SEM–EDXS) on polished sections of samples embedded in epoxy resin. The electron microprobe (EMP) gave point analyses for major and minor elements along with Sc. The distribution of Sc was mapped by synchrotron micro-X-ray fluorescence (µ-XRF). Scandium speciation was determined by synchrotron-based micro-X-ray absorption near-edge structure (µ-XANES) spectroscopy at Sc and Fe K-edges. Detailed descriptions are given in the Supplementary Information.
top
Characteristics of the Lateritic Deposit
The lateritic profile shows five levels (Fig. 1b; see also Supplementary Information, Section 2 and Table S-2); from bottom to top: saprolite, with smectite from the weathering of clinopyroxene, which is still visible in the deepest parts; transitional laterite where smectite is progressively replaced by Fe oxides and kaolinite; limonitic laterite dominated by goethite with haematite, kaolinite and gibbsite; haematitic laterite dominated by haematite with goethite, kaolinite and gibbsite; and transported laterite of similar composition but containing clasts and evidence of former alluvial channels. Similar lateritic profiles are developed over other ultramafic–mafic rocks in Australia (Anand and Paine, 2002
Anand, R.R., Paine, M. (2002) Regolith Geology of the Yilgarn Craton, Western Australia: Implications for Exploration. Australian Journal of Earth Sciences 49, 3–162.
), with most of the weathering profile directly inherited from the parent rock except for the transported level. The depth of the profile indicates that the weathering occurred over long time scales, possibly starting as early as 430 million years ago (Fergusson, 2010Fergusson, C.L. (2010) Plate-Driven Extension and Convergence along the East Gondwana Active Margin: Late Silurian–Middle Devonian Tectonics of the Lachlan Fold Belt, Southeastern Australia. Australian Journal of Earth Sciences 57, 627–649.
), in a stable tectonic context. Bulk assays show that Sc concentration increases upward, from ca. 100 ppm in the transitional laterite to ca. 500 ppm in the limonitic laterite (Fig. 1b,c and Table S-3). The concentration then decreases in the haematitic laterite and drops to ca. 250 ppm in the transported laterite.top
Identification of Iron Oxides as Main Scandium Hosts
The mineralogy is typical of lateritic profiles developed over ultramafic rocks (Freyssinet et al., 2005
Freyssinet, P., Butt, C.R.M., Morris, R.C., Piantone, P. (2005) Ore-Forming Processes Related to Lateritic Weathering. Economic Geology 100th Anniversary Volume, 681–722.
; Fig. 2a,b and Table S-4). The ore is dominated by haematite and Al-bearing goethite, associated with kaolinite and gibbsite. Minor phases include anatase, quartz and an Fe oxide with an inverse spinel structure, either maghemite or magnetite. The broad X-ray diffraction lines (Fig. 2a) reflect the poor crystallinity or small particle size (<100 nm) of the Fe oxides. The bulk Sc content is correlated with the proportion of Al-bearing goethite, indicating its potential role as a Sc host in the ore (Fig. 2c).
Figure 2 (a) X-ray diffraction pattern of ore sample JSD-1 – 15.4 m and associated multiphase Rietveld refinement (Al-gt: Al-bearing goethite, hem: haematite, kln: kaolinite, gbs: gibbsite, mgh: maghemite, ant: anatase). (b) Average phase proportions in the samples calculated from Rietveld refinement. (c) Bulk Sc concentration versus proportion of Al-bearing goethite estimated from Rietveld refinement.
Systematic SEM–EDXS mapping confirms the absence of discrete Sc phases, as expected from the scarcity of such phases in nature (Samson and Chassé, 2016
Samson, I.M., Chassé, M. (2016) Scandium. In: White, W.M. (Ed.) Encyclopedia of Geochemistry. Springer International Publishing, Cham, Switzerland, 1–5.
). The EMP analyses of Si, Ti, Al, Fe and Sc have been averaged on each grain type, as identified by combining SEM images, major elements and Sc contents and applying statistical clustering (Supplementary Information, Section 6 and Table S-6). Scandium contents are low to extremely low in Al-rich grains (ca. 300 ppm), Ti-rich oxides (ca. 100 ppm) and quartz (below detection limit, ca. 30 ppm). The highest Sc contents (ca. 1300 ppm) are encountered in Fe oxide grains that contain Al (ca. 7 wt. %) and have low EMP analytical totals (ca. 85 wt. %). These grains appear to correspond to the Al-bearing goethite identified by XRD. Another type of Fe oxide grains, with a lower concentration of Sc (ca. 200 ppm) and high Fe could correspond to the haematite phase identified using XRD.Much of each sample consists of a fine-grained matrix with three distinct compositions. Two correspond to mixtures of Fe oxides with different Sc contents, ca. 650 ppm for one and ca. 400 ppm for the other. The former contains slightly less Fe but more Al and has a lower analytical sum than the latter, reflecting a higher goethite component. A third type of matrix is slightly enriched in both Al and Si, which is consistent with the presence of minor kaolinite mixed with Fe oxides. The Sc content of this matrix is ca. 400 ppm, which suggests that kaolinite is only a minor Sc host.
To distinguish between goethite and haematite, we also used Fe K-edge XANES spectra. Using the relative normalised intensity of the absorption maxima of goethite and haematite, at 7133.8 eV and 7136.9 eV, respectively (Combes et al., 1989
Combes, J.-M., Manceau, A., Calas, G., Bottero, J.-Y. (1989) Formation of Ferric Oxides from Aqueous Solutions: A Polyhedral Approach by X-ray Absorption Spectroscopy: I. Hydrolysis and Formation of Ferric Gels. Geochimica et Cosmochimica Acta 53, 583–594.
), we obtained Fe-speciation maps (Fig. 3a). Comparison with µ-XRF maps of Sc (Fig. 3b) indicate that Sc-poor and Sc-rich grains mostly contain haematite and goethite, respectively. Iron K-edge XANES spectra in the complete range of energy confirm these observation (Fig. 4a), showing a good match between spectra of goethite and Sc-rich zones and between spectra of haematite and Sc-poor zones.
Figure 3 (a) Map of Fe speciation obtained by subtracting normalised synchrotron µ-XRF maps at 7133.9 eV, the energy characteristic of goethite and 7136.7 eV, the energy characteristic of haematite. (b) Synchrotron µ-XRF mapping of Sc.

Figure 4 XANES spectra at the (a) Fe K-edge. (b) Sc K-edge (gt: goethite and hem: haematite). The numbers in brackets refer to the position given in Figure 3.
Using the EMP-derived Sc concentration in goethite, around 1300 ppm, and the proportion of goethite obtained from Rietveld refinement, about 30 %, we calculate that goethite hosts about 400 ppm Sc out of the average ca. 500 ppm of Sc in the samples, i.e. ca. 80 % of total Sc. Average EMP-derived concentrations in haematite are about 200 ppm Sc. As haematite accounts for about 30 % of the mineral phases, Sc in haematite may represent ca. 50 ppm, corresponding to most of the remaining 20 % of total Sc.
top
Scandium Speciation in the Ore
Scandium K-edge XANES spectra of synthetic reference materials show that Sc incorporation in goethite or in haematite can be distinguished using the position of the edge crest at 4508.2 eV and 4511.8 eV, respectively (Fig. 4b), while Sc adsorbed on goethite is identified by a broad, featureless edge crest extending from 4508 eV to 4511.5 eV. On the low energy side, a pre-edge feature bears additional information (e.g., Galoisy et al., 2001
Galoisy, L., Calas, G., Arrio, M.-A. (2001) High-Resolution XANES Spectra of Iron in Minerals and Glasses: Structural Information from the Pre-Edge Region. Chemical Geology 174, 307–319.
). There is a noteworthy enhancement of the intensity of this feature in the substituted samples due to the distortion of the Sc site. The Sc K-edge XANES spectra on lateritic goethite grains show a broad edge crest and a low-intensity pre-edge feature, consistent with Sc adsorbed on goethite. In contrast, lateritic haematite grains show an edge crest at 4512 eV and a more intense pre-edge feature, corresponding to Sc incorporated in haematite. The same distinction holds when investigating the matrix: the high Sc concentrations are associated with Sc-adsorbed goethite. However, lateritic samples give less resolved spectra than the crystalline references. Comparison is limited because Sc K-edge XANES spectra are scarce in the literature, but this may reflect a poor crystallinity in fine-grained natural phases, or some minor contribution of other Sc species.top
A Mechanism for Scandium Enrichment
Due to the high concentration of Sc in clinopyroxenes, weathering leads to Sc-rich waters circulating in the regolith below the water table. Seasonal precipitation of goethite allows the adsorption of Sc3+. During dry periods, haematite develops from goethite, and may incorporate part of the adsorbed Sc in its crystal structure. However, this process is limited by the size difference between six-fold coordinated Sc3+ and Fe3+ but such size differences will not influence the adsorption capacity of goethite under near-neutral pH conditions.
Scandium is often associated with the rare earth elements (REE). The ionic radius of Sc3+ is slightly smaller than those of heavy REE for the same coordination number. During lateritic weathering of ultramafic–mafic rocks, Sc behaves like the heavy REE. The enrichment factor of Sc between the ore and fresh saprolite is ca. 5 (Table S-5, Fig. S-2). For the REE, it increases continuously with decreasing ionic radii, from ca. 2 for the light REE to ca. 3.5 for the heavy REE. The low prospectivity of the lateritic deposits for REE reflects the low REE content in the parent rock compared to average crustal concentrations (two to ten times lower, Rudnick and Gao, 2014
Rudnick, R.L., Gao, S. (2014) Composition of the Continental Crust. In: Holland, H., Turekian, K.K. (Eds.) Treatise on Geochemistry, 2nd Edition. Elsevier Ltd, Amsterdam, Netherlands, 1–51.
).Lateritic profiles over ultramafic–mafic rocks usually show Sc concentrations lower than 100 ppm, corresponding to enrichment by a factor of ten during lateritic weathering (Aiglsperger et al., 2016
Aiglsperger, T., Proenza, J.A., Lewis, J.F., Labrador, M., Svojtka, M., Rojas-Purón, A., Longo, F., Ďurišová, J. (2016) Critical Metals (REE, Sc, PGE) in Ni Laterites from Cuba and the Dominican Republic. Ore Geology Reviews 73, 127–147.
; Maulana et al., 2016Maulana, A., Sanematsu, K., Sakakibara, M. (2016) An Overview on the Possibility of Scandium and REE Occurrence in Sulawesi, Indonesia. Indonesian Journal on Geoscience 3, 139–147.
). This is similar to the maximum factor of enrichment found in the Syerston–Flemington deposit where the limonitic laterite reaches 800 ppm Sc compared to ca. 80 ppm in the parent rock. This observation rules out any need for peculiar lateritic conditions in Eastern Australia, and the proposed genetic model for Sc concentration during lateritic weathering may thus be extrapolated to other lateritic deposits developed over ultramafic–mafic rocks. Nonetheless, the duration of the weathering process and tectonic stability are key factors leading to the development of such volumes of ore.Exceptional concentration of Sc in lateritic deposits thus results from a combination of three circumstances: (1) anomalously high Sc concentration in the parent rock, (2) long time scales of alteration in stable tectonic environment and (3) lateritic conditions during weathering, allowing the trapping of Sc by Fe oxides.
top
Acknowledgements
We thank Benoît Baptiste, Omar Bouddouma, Ludovic Delbes, Michel Fialin, Thierry Pilorge, Jean-Louis Robert and Peter Wieland for help in sample preparation and analyses. ESRF and SOLEIL facilities are acknowledged for beamtime allocation. We are grateful to Marine Cotte and Delphine Vantelon for providing help in these experiments. We also thank Christian Polak, Guillaume Morin and Martine Gérard for fruitful discussions and Hal Aral, Rod Ewing, Adam Simon and two anonymous reviewers for constructive comments on the manuscript. This work was funded by scholarships from the École Normale Supérieure (ENS) and Macquarie University, by the Institut Universitaire de France (IUF), a Foundation Grant from the ARC Centre of Excellence for Core to Crust Fluid Systems (CCFS) and Jervois Mining Ltd. This is contribution 867 from the ARC Centre of Excellence for Core to Crust Fluid Systems (http://www.ccfs.mq.edu.au) and 1123 from the GEMOC Key Centre (http://www.gemoc.mq.edu.au).
Editor: Rodney C. Ewing
top
References
Aiglsperger, T., Proenza, J.A., Lewis, J.F., Labrador, M., Svojtka, M., Rojas-Purón, A., Longo, F., Ďurišová, J. (2016) Critical Metals (REE, Sc, PGE) in Ni Laterites from Cuba and the Dominican Republic. Ore Geology Reviews 73, 127–147.
 Show in context
Show in context A notable exception concerns lateritic deposits developed over ultramafic–mafic rocks where Sc concentrations up to 100 ppm make it a potential by-product (Aiglsperger et al., 2016; Maulana et al., 2016).
View in article
Lateritic profiles over ultramafic–mafic rocks usually show Sc concentrations lower than 100 ppm, corresponding to enrichment by a factor of ten during lateritic weathering (Aiglsperger et al., 2016; Maulana et al., 2016).
View in article
Anand, R.R., Paine, M. (2002) Regolith Geology of the Yilgarn Craton, Western Australia: Implications for Exploration. Australian Journal of Earth Sciences 49, 3–162.
 Show in context
Show in context Similar lateritic profiles are developed over other ultramafic–mafic rocks in Australia (Anand and Paine, 2002), with most of the weathering profile directly inherited from the parent rock except for the transported level.
View in article
Burg, J.-P., Bodinier, J.-L., Gerya, T., Bedini, R.-M., Boudier, F., Dautria, J.-M., Prikhodko, V., Efimov, A., Pupier, E., Balanec, J.-L. (2009) Translithospheric Mantle Diapirism: Geological Evidence and Numerical Modelling of the Kondyor Zoned Ultramafic Complex (Russian Far-East). Journal of Petrology 50, 289–321.
 Show in context
Show in context Similar concentrations (60 ppm to 80 ppm) occur in other ‘Alaskan-type’ clinopyroxenites (Burg et al., 2009) and in clinopyroxenes from ocean-island basalts (Dorais, 2015).
View in article
Combes, J.-M., Manceau, A., Calas, G., Bottero, J.-Y. (1989) Formation of Ferric Oxides from Aqueous Solutions: A Polyhedral Approach by X-ray Absorption Spectroscopy: I. Hydrolysis and Formation of Ferric Gels. Geochimica et Cosmochimica Acta 53, 583–594.
 Show in context
Show in context Using the relative normalised intensity of the absorption maxima of goethite and haematite, at 7133.8 eV and 7136.9 eV, respectively (Combes et al., 1989), we obtained Fe-speciation maps (Fig. 3a).
View in article
Dorais, M.J. (2015) Exploring the Mineralogical Heterogeneities of the Louisville Seamount Trail. Geochemistry, Geophysics, Geosystems 16, 2884–2899.
 Show in context
Show in context Similar concentrations (60 ppm to 80 ppm) occur in other ‘Alaskan-type’ clinopyroxenites (Burg et al., 2009) and in clinopyroxenes from ocean-island basalts (Dorais, 2015).
View in article
Emsley, J. (2014) Unsporting Scandium. Nature Chemistry 6, 1025.
 Show in context
Show in context End-uses range from biomedical research to electronics, lasers and ceramics but the demand is driven by energy issues (Emsley, 2014).
View in article
Fergusson, C.L. (2010) Plate-Driven Extension and Convergence along the East Gondwana Active Margin: Late Silurian–Middle Devonian Tectonics of the Lachlan Fold Belt, Southeastern Australia. Australian Journal of Earth Sciences 57, 627–649.
 Show in context
Show in contextThe depth of the profile indicates that the weathering occurred over long time scales, possibly starting as early as 430 million years ago (Fergusson, 2010), in a stable tectonic context.
View in article
Freyssinet, P., Butt, C.R.M., Morris, R.C., Piantone, P. (2005) Ore-Forming Processes Related to Lateritic Weathering. Economic Geology 100th Anniversary Volume, 681–722.
 Show in context
Show in context The mineralogy is typical of lateritic profiles developed over ultramafic rocks (Freyssinet et al., 2005; Fig. 2a,b and Table S-4).
View in article
Galoisy, L., Calas, G., Arrio, M.-A. (2001) High-Resolution XANES Spectra of Iron in Minerals and Glasses: Structural Information from the Pre-Edge Region. Chemical Geology 174, 307–319.
 Show in context
Show in context On the low energy side, a pre-edge feature bears additional information (e.g., Galoisy et al., 2001).
View in article
Jaireth, S., Hoatson, D.M., Miezitis, Y. (2014) Geological Setting and Resources of the Major Rare-Earth-Element Deposits in Australia. Ore Geology Reviews 62, 72–128.
 Show in context
Show in context Recently, lateritic deposits with Sc concentrations high enough to mine as a primary product have been reported in Eastern Australia (Jaireth et al., 2014).
View in article
Johan, Z., Ohnenstetter, M., Slansky, E., Barron, L.M., Suppel, D.W. (1989) Platinum Mineralization in the Alaskan-type Intrusive Complexes near Fifield, New South Wales, Australia Part 1. Platinum-Group Minerals in Clinopyroxenites of the Kelvin Grove Prospect, Owendale Intrusion. Mineralogy and Petrology 40, 289–309.
 Show in context
Show in context The Syerston–Flemington deposit (Fig. 1a and Table S-1) is part of the lateritic cover developed over the Tout complex, an ultramafic–mafic ‘Alaskan-type’ intrusive complex in the Lachlan Fold Belt (Johan et al., 1989).
View in article
Maulana, A., Sanematsu, K., Sakakibara, M. (2016) An Overview on the Possibility of Scandium and REE Occurrence in Sulawesi, Indonesia. Indonesian Journal on Geoscience 3, 139–147.
 Show in context
Show in context A notable exception concerns lateritic deposits developed over ultramafic–mafic rocks where Sc concentrations up to 100 ppm make it a potential by-product (Aiglsperger et al., 2016; Maulana et al., 2016).
View in article
Lateritic profiles over ultramafic–mafic rocks usually show Sc concentrations lower than 100 ppm, corresponding to enrichment by a factor of ten during lateritic weathering (Aiglsperger et al., 2016; Maulana et al., 2016).
View in article
Milnes, A.R., Bourman, R.P., Fitzpatrick, R.W. (1987) Petrology and Mineralogy of ‘Laterites’ in Southern and Eastern Australia and Southern Africa. Chemical Geology 60, 237–250.
 Show in context
Show in context In Eastern Australia, lateritic profiles developed under seasonally dry humid tropical climatic conditions that resulted in intensive weathering during the Tertiary; the present occurrences are often erosional remnants of fossil laterite (Milnes et al., 1987).
View in article
Pursell, D.C. (2016) Quarterly Report. Jervois Mining Ltd, Cheltenham, Australia.
 Show in context
Show in context Among these, the Syerston–Flemington deposit contains about 1350 t of Sc at an average concentration of 434 ppm Sc (Pursell, 2016), providing a century-long resource at the present levels of world consumption.
View in article
Rudnick, R.L., Gao, S. (2014) Composition of the Continental Crust. In: Holland, H., Turekian, K.K. (Eds.) Treatise on Geochemistry, 2nd Edition. Elsevier Ltd, Amsterdam, Netherlands, 1–51.
 Show in context
Show in context Despite a crustal abundance of ca. 22 ppm (Rudnick and Gao, 2014), comparable to common elements such as copper or lead, current Sc production is limited to 10 t to 15 t per year (U.S. Geological Survey, 2016).
View in article
The low prospectivity of the lateritic deposits for REE reflects the low REE content in the parent rock compared to average crustal concentrations (two to ten times lower, Rudnick and Gao, 2014).
View in article
Samson, I.M., Chassé, M. (2016) Scandium. In: White, W.M. (Ed.) Encyclopedia of Geochemistry. Springer International Publishing, Cham, Switzerland, 1–5.
 Show in context
Show in context The large ratio of ionic radius to charge of Sc3+ hinders the concentration of scandium during most geochemical processes (Samson and Chassé, 2016).
View in article
Scandium anomalies have been found over a body of nearly pure clinopyroxenite (Fig. S-1), with Sc concentrations (ca. 80 ppm; Table S-3) twice as high as those in typical mantle clinopyroxenites (Samson and Chassé, 2016).
View in article
Systematic SEM–EDXS mapping confirms the absence of discrete Sc phases, as expected from the scarcity of such phases in nature (Samson and Chassé, 2016).
View in article
U.S. Geological Survey (2016) Scandium. In: Mineral Commodity Summaries. U.S. Geological Survey, Reston, USA, 146–147.
 Show in context
Show in context Despite a crustal abundance of ca. 22 ppm (Rudnick and Gao, 2014), comparable to common elements such as copper or lead, current Sc production is limited to 10 t to 15 t per year (U.S. Geological Survey, 2016).
View in article
top
Supplementary Information
1. Location of the Diamond Core Drillings and Geology of the Area
Table S-1 Latitude and longitude coordinates, altitude and dip angle of the two diamond core drillings.
| Drill hole | Latitude | Longitude | Altitude | Dip |
| JSD-001 | −32°44′42.91901′′ | 147°24′7.42757′′ | 309.211 m | −90° |
| JSD-002 | −32°44′43.326 11′′ | 147°24′3.72856′′ | 309.067 m | −90° |

Figure S-1 Geological map of the Tout complex showing the locations of the drilling sites (cpx: clinopyroxene, hbl: hornblende, qtz: quartz).
2. Typical Core Logging of a Core Drilling
Table S-2 Detailed log of the JSD-001 drill core.
| Horizon | Depth | Colour | Fabric and/or texture |
| Transported laterite | 0 m – 0.3 m | Red-brown | Brecciated; coarsely grained (>5 mm); mixture of soil (≃25 %) and residual lateritic material (≃25 %), supporting Fe pisoliths (≃50 %); cavernous and interstitial porosity. |
| Transported laterite | 0.3 m – 2.5 m | Red mottled with orange spots | Massive with a breccia-like texture; fine to coarse grains (<0.002 mm – 5 mm); matrix of lateritic material, probably a mixture of Fe oxides and oxyhydroxides with kaolinites (>80 %), associated with few Fe pisoliths (≃10 %), lateritic clasts (≃10 %) and fine sandy grains, possibly quartz, accessory orange areas (<5 %) without pisoliths or clasts and probably richer in Fe oxyhydroxides; interstitial porosity. |
| Transported laterite | 2.5 m – 5.2 m | Dark red | Massive; fine grains when visible (<1 mm); dominated by a matrix of fine grains with clayey content (>95 %), probably a mixture of Fe oxides and oxyhydroxides with kaolinite, rare sandy grains (<2 mm), possibly quartz; interstitial porosity. |
| Transported laterite | 5.2 m – 8 m | Dark red to dark orange-red | Massive with breccia-like texture; fine grains when visible (<1 mm) with coarse clasts (>3 mm); dominated by a fine grained matrix with minor clay content (≃75 %), probably a mixture of Fe oxides and oxyhydroxides with kaolinite, common angular clasts of lateritic material (≃20 %); interstitial porosity. |
| Haematitic laterite | 8 m – 10.2 m | Dark red to dark orange-red | Massive to cemented granular material; fine to coarse grains (<0.002 mm – 5 mm); dominated by a clayey matrix with fine grains (>70 %), probably a mixture of Fe oxides and oxyhydroxides with kaolinite, common to minor Fe pisoliths (5 % – 25 %); interstitial porosity. |
| Haematitic laterite | 10.2 m – 11.2 m | Dark brown to red | Massive; fine grains when visible (<1 mm); dominant matrix of fine grains and clays (>90 %), probably a mixture of Fe oxides and oxyhydroxides with kaolinite, a homogeneous dark-brown vein at the bottom of the level, possibly rich in Al oxyhydroxides; interstitial and cavernous porosity. |
| Limonitic laterite | 11.2 m – 14.2 m | Dark to medium orange-red | Massive to cemented granular material; fine to coarse grains (<0.002 mm – >5 mm); dominant to minor Fe pisoliths (5 % – 70 %) in a common to dominant matrix of fine grains and clays (30 % – 90 %), probably a mixture of Fe oxides and oxyhydroxides with kaolinite, rare to common orange areas, richer in Fe oxyhydroxides; interstitial and cavernous porosity. |
| Limonitic laterite | 14.2 m – 16.7 m | Medium to light orange-red mottled with rare orange spots | Massive to cemented granular material; similar to the above level, with rarer orange areas (<10 %). |
| Limonitic laterite | 16.7 m – 18.2 m | Red-pink mottled with orange spots | Massive to cemented granular material; fine grains when visible (<1 mm); dominated by a matrix of fine pinkish grains associated with clayey material (>90 %), probably Fe oxides and oxyhydroxides associated with kaolinites, with minor orange areas (≃10 %), probably rich in Fe oxyhydroxides; interstitial to cavernous porosity. |
| Limonitic laterite | 18.2 m – 18.7 m | Tan-brown to khaki-brown | Massive; fine grains when visible (<1 mm); dominated by a matrix of fine grains and clayey material (>80 %), probably Fe oxides and oxyhydroxides associated with kaolinite, accessory white crystals (≃1 %); interstitial porosity. |
| Transitional laterite | 18.7 m – 20.5 m | Variable, mottled with green, orange, red, purple, brown and black spots | Granular; medium to fine grains (<0.002 mm – 3 mm); dominated by a red-brown finely grained matrix (50 % – 80 %), probably a mixture of Fe oxides and oxyhydroxides, common green clayey areas becoming accessory in the upper parts (≃20 % – <1 %), two types of veins, filled-in by a black fine-grained matrix, possibly Mn oxides or by a white homogeneous matrix, possibly calcite; important interstitial porosity with fractures. |
| Transitional laterite | 20.5 m – 21.5 m | Green-grey mottled with orange and black spots | Clay texture; dominant clay matrix (≃70 %), possibly smectite, minor fine grained orange areas (<10 %) possibly clays coated with Fe oxides and oxyhydroxides, accessory crystalline black areas (<5 %), remnants of primary pyroxenes; probably important porosity but pores are not visible. |
| Saprolite | 21.5 m – 27.6 m | Green to light green-grey mottled with orange and black spots | Crystalline to granular; medium to fine grains (<0.002 mm – 3 mm); dominated by green clay minerals (>70 %), possibly smectite, with accessory remnants of black crystals (<5 %) identified as pyroxenes and minor rust-coloured areas (<10 %) formed by weathered crystals covered by Fe oxides; limited interstitial porosity increasing in the upper levels. |
| Saprolite | 27.6 m – 29.2 m | Dark brown-grey with light green to green brown fractures | Mesh texture with filled-in fractures; rare sandy grains (<5 %) in a dominant serpentinized groundmass (≃90 %), fractures filled with clay fibres, probably serpentines; low porosity except in fractures. |
3. Procedures and Results of the Chemical Analyses on Aircore Drillings
Twenty-six vertical aircore drillings have been carried out in the EL 7805 mining tenement, in the Syerston–Flemington area. Every metre, a sample has been analysed for Sc by ALS in Brisbane using a fusion inductively coupled plasma-atomic emission spectroscopy (ICP-AES) method (Table S-3).
Table S-3 Sc concentration (in ppm) at each metre of the 26 vertical aircore drillings performed in the Syerston–Flemington area.
| Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc |
| (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) |
| SY34-0-1 | 344 | SY35-0-1 | 362 | SY36-0-1 | 114 | SY37-0-1 | 205 | SY38-0-1 | 432 | SY39-0-1 | 140 | SY40-0-1 | 189 |
| SY34-1-2 | 464 | SY35-1-2 | 330 | SY36-1-2 | 136 | SY37-1-2 | 186 | SY38-1-2 | 394 | SY39-1-2 | 90 | SY40-1-2 | 82 |
| SY34-2-3 | 484 | SY35-2-3 | 368 | SY36-2-3 | 137 | SY37-2-3 | 161 | SY38-2-3 | 443 | SY39-2-3 | 95 | SY40-2-3 | 78 |
| SY34-3-4 | 460 | SY35-3-4 | 418 | SY36-3-4 | 123 | SY37-3-4 | 158 | SY38-3-4 | 451 | SY40-3-4 | 81 | ||
| SY34-4-5 | 562 | SY35-4-5 | 482 | SY36-4-5 | 120 | SY37-4-5 | 161 | SY38-4-5 | 455 | SY40-4-5 | 79 | ||
| SY34-5-6 | 532 | SY35-5-6 | 519 | SY36-5-6 | 176 | SY37-5-6 | 188 | SY38-5-6 | 461 | SY40-5-6 | 92 | ||
| SY34-6-7 | 706 | SY35-6-7 | 544 | SY36-6-7 | 211 | SY37-6-7 | 203 | SY38-6-7 | 457 | ||||
| SY34-7-8 | 800 | SY35-7-8 | 550 | SY36-7-8 | 243 | SY37-7-8 | 224 | SY38-7-8 | 467 | ||||
| SY34-8-9 | 724 | SY35-8-9 | 606 | SY36-8-9 | 320 | SY37-8-9 | 265 | SY38-8-9 | 479 | ||||
| SY34-9-10 | 874 | SY35-9-10 | 646 | SY36-9-10 | 361 | SY37-9-10 | 318 | SY38-9-10 | 507 | ||||
| SY34-10-11 | 796 | SY35-10-11 | 642 | SY36-10-11 | 324 | SY37-10-11 | 331 | SY38-10-11 | 519 | ||||
| SY34-11-12 | 565 | SY35-11-12 | 583 | SY36-11-12 | 300 | SY37-11-12 | 409 | SY38-11-12 | 578 | ||||
| SY34-12-13 | 502 | SY35-12-13 | 610 | SY36-12-13 | 349 | SY37-12-13 | 506 | SY38-12-13 | 547 | ||||
| SY34-13-14 | 505 | SY35-13-14 | 576 | SY36-13-14 | 353 | SY37-13-14 | 438 | SY38-13-14 | 438 | ||||
| SY34-14-15 | 410 | SY35-14-15 | 665 | SY36-14-15 | 362 | SY37-14-15 | 446 | SY38-14-15 | 392 | ||||
| SY34-15-16 | 322 | SY35-15-16 | 764 | SY36-15-16 | 255 | SY37-15-16 | 507 | SY38-15-16 | 439 | ||||
| SY34-16-17 | 226 | SY35-16-17 | 945 | SY36-16-17 | 160 | SY37-16-17 | 606 | SY38-16-17 | 445 | ||||
| SY34-17-18 | 200 | SY35-17-18 | 770 | SY36-17-18 | 181 | SY37-17-18 | 514 | SY38-17-18 | 455 | ||||
| SY34-18-19 | 266 | SY35-18-19 | 690 | SY36-18-19 | 283 | SY37-18-19 | 808 | SY38-18-19 | 385 | ||||
| SY34-19-20 | 174 | SY35-19-20 | 535 | SY36-19-20 | 354 | SY37-19-20 | 661 | SY38-19-20 | 267 | ||||
| SY34-20-21 | 128 | SY35-20-21 | 446 | SY36-20-21 | 298 | SY37-20-21 | 449 | SY38-20-21 | 268 | ||||
| SY35-21-22 | 374 | SY36-21-22 | 274 | SY37-21-22 | 281 | SY38-21-22 | 222 | ||||||
| SY35-22-23 | 343 | SY36-22-23 | 268 | SY37-22-23 | 440 | SY38-22-23 | 140 | ||||||
| SY35-23-24 | 329 | SY36-23-24 | 128 | SY37-23-24 | 155 | SY38-23-24 | 117 | ||||||
| SY35-24-25 | 331 | SY36-24-25 | 106 | SY37-24-25 | 133 | ||||||||
| SY35-25-26 | 338 | SY36-25-26 | 97 | SY37-25-26 | 114 | ||||||||
| SY35-26-27 | 304 | SY36-0-26 | 232 | SY37-26-27 | 109 | ||||||||
| SY35-27-28 | 159 | ||||||||||||
| SY35-28-29 | 126 | ||||||||||||
| SY35-29-30 | 126 | ||||||||||||
| Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc |
| (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) |
| SY41-0-1 | 200 | SY42-0-1 | 373 | SY43-0-1 | 128 | SY44-0-1 | 107 | SY45-0-1 | 147 | SY46-0-1 | 358 | SY47-0-1 | 158 |
| SY41-1-2 | 306 | SY42-1-2 | 460 | SY43-1-2 | 178 | SY44-1-2 | 129 | SY45-1-2 | 157 | SY46-1-2 | 514 | SY47-1-2 | 381 |
| SY41-2-3 | 496 | SY42-2-3 | 477 | SY43-2-3 | 179 | SY44-2-3 | 134 | SY45-2-3 | 165 | SY46-2-3 | 512 | SY47-2-3 | 424 |
| SY41-3-4 | 590 | SY42-3-4 | 569 | SY43-3-4 | 183 | SY44-3-4 | 147 | SY45-3-4 | 182 | SY46-3-4 | 612 | SY47-3-4 | 475 |
| SY41-4-5 | 747 | SY42-4-5 | 795 | SY43-4-5 | 225 | SY44-4-5 | 117 | SY45-4-5 | 191 | SY46-4-5 | 546 | SY47-4-5 | 451 |
| SY41-5-6 | 598 | SY42-5-6 | 655 | SY43-5-6 | 213 | SY44-5-6 | 107 | SY45-5-6 | 235 | SY46-5-6 | 603 | SY47-5-6 | 505 |
| SY41-6-7 | 591 | SY42-6-7 | 588 | SY43-6-7 | 195 | SY44-6-7 | 110 | SY45-6-7 | 500 | SY46-6-7 | 594 | SY47-6-7 | 567 |
| SY41-7-8 | 566 | SY42-7-8 | 595 | SY43-7-8 | 229 | SY44-7-8 | 120 | SY45-7-8 | 511 | SY46-7-8 | 540 | SY47-7-8 | 588 |
| SY41-8-9 | 525 | SY42-8-9 | 540 | SY43-8-9 | 323 | SY44-8-9 | 152 | SY45-8-9 | 564 | SY46-8-9 | 577 | SY47-8-9 | 492 |
| SY41-9-10 | 476 | SY42-9-10 | 618 | SY43-9-10 | 396 | SY44-9-10 | 201 | SY45-9-10 | 545 | SY46-9-10 | 600 | SY47-9-10 | 641 |
| SY41-10-11 | 469 | SY42-10-11 | 574 | SY43-10-11 | 506 | SY44-10-11 | 239 | SY45-10-11 | 475 | SY46-10-11 | 590 | SY47-10-11 | 865 |
| SY41-11-12 | 361 | SY42-11-12 | 505 | SY43-11-12 | 618 | SY44-11-12 | 282 | SY45-11-12 | 525 | SY46-11-12 | 607 | SY47-11-12 | 689 |
| SY41-12-13 | 300 | SY42-12-13 | 617 | SY43-12-13 | 647 | SY44-12-13 | 253 | SY45-12-13 | 401 | SY46-12-13 | 504 | SY47-12-13 | 810 |
| SY41-13-14 | 275 | SY42-13-14 | 756 | SY43-13-14 | 667 | SY44-13-14 | 226 | SY45-13-14 | 453 | SY46-13-14 | 397 | SY47-13-14 | 847 |
| SY41-14-15 | 208 | SY42-14-15 | 606 | SY43-14-15 | 695 | SY44-14-15 | 249 | SY45-14-15 | 514 | SY46-14-15 | 485 | SY47-14-15 | 874 |
| SY41-15-16 | 132 | SY42-15-16 | 577 | SY43-15-16 | 553 | SY44-15-16 | 312 | SY45-15-16 | 482 | SY46-15-16 | 433 | SY47-15-16 | 622 |
| SY42-16-17 | 556 | SY43-16-17 | 484 | SY44-16-17 | 359 | SY45-16-17 | 516 | SY46-16-17 | 469 | SY47-16-17 | 268 | ||
| SY42-17-18 | 549 | SY43-17-18 | 463 | SY44-17-18 | 407 | SY45-17-18 | 596 | SY46-17-18 | 304 | SY47-17-18 | 184 | ||
| SY42-18-19 | 343 | SY43-18-19 | 349 | SY44-18-19 | 380 | SY45-18-19 | 625 | SY46-18-19 | 152 | ||||
| SY42-19-20 | 230 | SY43-19-20 | 248 | SY44-19-20 | 335 | SY45-19-20 | 595 | SY46-19-20 | 204 | ||||
| SY42-20-21 | 133 | SY43-20-21 | 268 | SY44-20-21 | 532 | SY45-20-21 | 835 | SY46-20-21 | 212 | ||||
| SY42-21-22 | 119 | SY43-21-22 | 160 | SY44-21-22 | 630 | SY45-21-22 | 980 | SY46-21-22 | 163 | ||||
| SY42-22-23 | 121 | SY43-22-23 | 240 | SY44-22-23 | 492 | SY45-22-23 | 687 | SY46-22-23 | 121 | ||||
| SY42-23-24 | 105 | SY43-23-24 | 212 | SY44-23-24 | 322 | SY45-23-24 | 404 | SY46-23-24 | 101 | ||||
| SY44-24-25 | 285 | SY45-24-25 | 243 | SY46-24-25 | 83 | ||||||||
| SY44-25-26 | 257 | SY45-25-26 | 280 | SY46-25-26 | 81 | ||||||||
| SY44-26-27 | 238 | ||||||||||||
| SY44-27-28 | 225 | ||||||||||||
| SY44-28-29 | 261 | ||||||||||||
| SY44-29-30 | 242 | ||||||||||||
| Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc |
| (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) |
| SY48-0-1 | 203 | SY49-0-1 | 150 | SY50-0-1 | 111 | SY51-0-1 | 358 | SY52-0-1 | 156 | SY53-0-1 | 206 | SY54-0-1 | 206 |
| SY48-1-2 | 216 | SY49-1-2 | 120 | SY50-1-2 | 108 | SY51-1-2 | 388 | SY52-1-2 | 118 | SY53-1-2 | 299 | SY54-1-2 | 136 |
| SY48-2-3 | 229 | SY49-2-3 | 102 | SY50-2-3 | 106 | SY51-2-3 | 401 | SY52-2-3 | 96 | SY53-2-3 | 341 | SY54-2-3 | 136 |
| SY48-3-4 | 211 | SY49-3-4 | 106 | SY50-3-4 | 114 | SY51-3-4 | NSS | SY52-3-4 | 98 | SY53-3-4 | 393 | SY54-3-4 | 140 |
| SY48-4-5 | 217 | SY49-4-5 | 122 | SY50-4-5 | 153 | SY51-4-5 | 513 | SY52-4-5 | 116 | SY53-4-5 | 457 | SY54-4-5 | 200 |
| SY48-5-6 | 222 | SY49-5-6 | 113 | SY50-5-6 | 199 | SY51-5-6 | 500 | SY52-5-6 | 103 | SY53-5-6 | 457 | SY54-5-6 | 192 |
| SY48-6-7 | 264 | SY49-6-7 | 116 | SY50-6-7 | 245 | SY51-6-7 | 425 | SY52-6-7 | 108 | SY53-6-7 | 479 | SY54-6-7 | 212 |
| SY48-7-8 | 335 | SY49-7-8 | 118 | SY50-7-8 | 274 | SY51-7-8 | 327 | SY52-7-8 | 121 | SY53-7-8 | 400 | SY54-7-8 | 247 |
| SY48-8-9 | 416 | SY49-8-9 | 133 | SY50-8-9 | 277 | SY51-8-9 | 347 | SY52-8-9 | 115 | SY53-8-9 | 510 | SY54-8-9 | 251 |
| SY48-9-10 | 427 | SY49-9-10 | 156 | SY50-9-10 | 212 | SY51-9-10 | 386 | SY52-9-10 | 142 | SY53-9-10 | 574 | SY54-9-10 | 308 |
| SY48-10-11 | 486 | SY49-10-11 | 155 | SY50-10-11 | 278 | SY51-10-11 | 522 | SY52-10-11 | 166 | SY53-10-11 | 466 | SY54-10-11 | 416 |
| SY48-11-12 | 480 | SY49-11-12 | 144 | SY50-11-12 | 282 | SY51-11-12 | 371 | SY52-11-12 | 213 | SY53-11-12 | 597 | SY54-11-12 | 433 |
| SY48-12-13 | 427 | SY49-12-13 | 163 | SY50-12-13 | 349 | SY51-12-13 | 456 | SY52-12-13 | 265 | SY53-12-13 | 656 | SY54-12-13 | 275 |
| SY48-13-14 | 500 | SY49-13-14 | 187 | SY50-13-14 | 341 | SY51-13-14 | 365 | SY52-13-14 | 393 | SY53-13-14 | 671 | SY54-13-14 | 264 |
| SY48-14-15 | 552 | SY49-14-15 | 236 | SY50-14-15 | 472 | SY51-14-15 | 384 | SY52-14-15 | 431 | SY53-14-15 | 968 | SY54-14-15 | 225 |
| SY48-15-16 | 612 | SY49-15-16 | 351 | SY50-15-16 | 502 | SY51-15-16 | 470 | SY52-15-16 | 499 | SY53-15-16 | 531 | SY54-15-16 | 369 |
| SY48-16-17 | 672 | SY49-16-17 | 400 | SY50-16-17 | 600 | SY51-16-17 | 475 | SY52-16-17 | 561 | SY53-16-17 | 348 | SY54-16-17 | 400 |
| SY48-17-18 | 659 | SY49-17-18 | 451 | SY50-17-18 | 513 | SY51-17-18 | 614 | SY52-17-18 | 650 | SY53-17-18 | 321 | SY54-17-18 | 360 |
| SY48-18-19 | 582 | SY49-18-19 | 353 | SY50-18-19 | 404 | SY51-18-19 | 533 | SY52-18-19 | 611 | SY53-18-19 | 232 | SY54-18-19 | 419 |
| SY48-19-20 | 513 | SY49-19-20 | 338 | SY50-19-20 | 228 | SY51-19-20 | 540 | SY52-19-20 | 455 | SY53-19-20 | 256 | SY54-19-20 | 354 |
| SY48-20-21 | 376 | SY49-20-21 | 217 | SY50-20-21 | 322 | SY51-20-21 | 600 | SY52-20-21 | 449 | SY53-20-21 | 315 | SY54-20-21 | 400 |
| SY48-21-22 | 316 | SY49-21-22 | 143 | SY50-21-22 | 332 | SY51-21-22 | 480 | SY52-21-22 | 340 | SY53-21-22 | 274 | SY54-21-22 | 494 |
| SY48-22-23 | 318 | SY49-22-23 | 114 | SY50-22-23 | 309 | SY51-22-23 | 396 | SY52-22-23 | 316 | SY53-22-23 | 258 | SY54-22-23 | 313 |
| SY49-23-24 | 89 | SY50-23-24 | 277 | SY51-23-24 | 290 | SY52-23-24 | 314 | SY53-23-24 | 191 | SY54-23-24 | 263 | ||
| SY50-24-25 | 217 | SY51-24-25 | 213 | SY52-24-25 | 307 | SY53-24-25 | 105 | SY54-24-25 | 234 | ||||
| SY50-25-26 | 213 | SY51-25-26 | 295 | SY52-25-26 | 140 | SY54-25-26 | 281 | ||||||
| SY50-26-27 | 159 | SY51-26-27 | 192 | SY52-26-27 | 107 | SY54-26-27 | 268 | ||||||
| SY50-27-28 | 178 | SY51-27-28 | 133 | SY54-27-28 | 266 | ||||||||
| SY50-28-29 | 137 | SY54-28-29 | 289 | ||||||||||
| SY54-29-30 | 140 | ||||||||||||
| SY54-30-31 | 84 | ||||||||||||
| Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | ||||
| (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | ||||
| SY55-1-2 | 576 | SY56-1-2 | 441 | SY57-1-2 | 390 | SY58-1-2 | 137 | SY59-1-2 | 137 | ||||
| SY55-2-3 | 574 | SY56-2-3 | 479 | SY57-2-3 | 582 | SY58-2-3 | 186 | SY59-2-3 | 142 | ||||
| SY55-3-4 | 664 | SY56-3-4 | 492 | SY57-3-4 | 598 | SY58-3-4 | 191 | SY59-3-4 | 133 | ||||
| SY55-4-5 | 569 | SY56-4-5 | 490 | SY57-4-5 | 500 | SY58-4-5 | 212 | SY59-4-5 | 117 | ||||
| SY55-5-6 | 481 | SY56-5-6 | 493 | SY57-5-6 | 594 | SY58-5-6 | 223 | SY59-5-6 | 103 | ||||
| SY55-6-7 | 429 | SY56-6-7 | 475 | SY57-6-7 | 594 | SY58-6-7 | 243 | SY59-6-7 | 110 | ||||
| SY55-7-8 | 358 | SY56-7-8 | 432 | SY57-7-8 | 673 | SY58-7-8 | 257 | SY59-7-8 | 108 | ||||
| SY55-8-9 | 379 | SY56-8-9 | 488 | SY57-8-9 | 783 | SY58-8-9 | 298 | SY59-8-9 | 104 | ||||
| SY55-9-10 | 244 | SY56-9-10 | 500 | SY57-9-10 | 899 | SY58-9-10 | 315 | SY59-9-10 | 101 | ||||
| SY55-10-11 | 161 | SY56-10-11 | 543 | SY57-10-11 | 617 | SY58-10-11 | 327 | SY59-10-11 | 109 | ||||
| SY55-11-12 | 120 | SY56-11-12 | 508 | SY57-11-12 | 214 | SY58-11-12 | 329 | SY59-11-12 | 120 | ||||
| SY56-12-13 | 375 | SY57-12-13 | 153 | SY58-12-13 | 384 | SY59-12-13 | 133 | ||||||
| SY56-13-14 | 411 | SY57-13-14 | 118 | SY58-13-14 | 500 | SY59-13-14 | 161 | ||||||
| SY56-14-15 | 426 | SY57-14-15 | 110 | SY58-14-15 | 558 | SY59-14-15 | 150 | ||||||
| SY56-15-16 | 471 | SY58-15-16 | 688 | SY59-15-16 | 148 | ||||||||
| SY56-16-17 | 314 | SY58-16-17 | 444 | SY59-16-17 | 133 | ||||||||
| SY56-17-18 | 214 | SY58-17-18 | 195 | SY59-17-18 | 130 | ||||||||
| SY56-18-19 | 230 | SY58-18-19 | 133 | SY59-18-19 | 130 | ||||||||
| SY56-19-20 | 192 | SY58-19-20 | 99 | SY59-19-20 | 137 | ||||||||
| SY56-20-21 | 119 | SY58-20-21 | 104 | SY59-20-21 | 187 | ||||||||
| SY59-21-22 | 252 | ||||||||||||
| SY59-22-23 | 228 | ||||||||||||
| SY59-23-24 | 149 |
4. Mineralogical Analyses Procedures
The mineralogical composition of bulk powder samples was determined by X-ray diffraction (XRD). The data were collected in Bragg-Brentano geometry with a PANalytical X’Pert PRO MPD diffractometer equipped with an X’Celerator detector. Co Kα radiation (λKα1 = 1.78897 Å, λKα2 = 1.79285 Å) was used in order to minimise the effect of Fe fluorescence on background. Data were recorded over the 3° 2θ to 90° 2θ range, with 0.017° 2θ steps and a total counting time of 6 hours. Aluminium-bearing goethite, anatase, calcite, dolomite, gibbsite, haematite, kaolinite, lithiophorite, magnetite/maghemite and quartz were identified using International Centre for Diffraction Data (ICDD) references (PDF-2 database).
Least squares refinement of the experimental XRD patterns were performed using the Rietveld method, fitting the experimental patterns with theoretical ones calculated from the crystal structure of each phase identified in the sample. Those refinements were carried out with the FullProf suite of programmes (Rodríguez-Carvajal, 1993). Unit cell parameters, atomic positions, and isotropic Debye–Waller factors were refined from the following structure refinements: Hazemann et al. (1991) for goethite [α-FeOOH]; Parker (1923) for anatase [TiO2]; Chessin et al. (1965) for calcite [CaCO3]; Wasastjerna (1924) for dolomite [CaMg(CO3)2]; Saalfeld and Wedde (1974) for gibbsite [α-Al(OH)3]; Blake and Hessevic (1966) for haematite [α-Fe2O3]; Bish and Von Dreele (1989) for kaolinite [Al2Si2O5(OH)4]; Post and Appleman (1994) for lithiophorite; Pecharromán et al. (1995) for maghemite [γ-Fe2O3] and Wei (1935) for α-quartz [α-SiO2]. Mineral proportions were estimated from those refinements (Table S-4).
Table S-4 Nature and proportion of the phases identified for each sample (b.d.l: below detection limit using Rietveld refinement but present in the sample, abs.: absent from the sample).
| Core | Sample Name | Depth (m) | Kaolinite | Al-rich goethite | Haematite | Maghemite | Gibbsite | Quartz | Anatase | Calcite | Dolomite | Lithiophorite |
| JSD-001 | jsd1_115 | 11.5 | abs. | 54 | 30 | 1 | 13 | abs. | 1 | abs. | 1 | abs. |
| JSD-001 | jsd1_121 | 12.1 | abs. | 51 | 33 | 1 | 13 | abs. | 1 | abs. | 2 | abs. |
| JSD-001 | jsd1_125 | 12.5 | abs. | 47 | 34 | 1 | 17 | abs. | 1 | abs. | abs. | abs. |
| JSD-001 | jsd1_130 | 13.0 | abs. | 50 | 28 | 1 | 20 | abs. | 1 | abs. | abs. | abs. |
| JSD-001 | jsd1_135 | 13.5 | abs. | 52 | 26 | 1 | 21 | abs. | b.d.l. | abs. | abs. | abs. |
| JSD-001 | jsd1_140 | 14.0 | abs. | 47 | 27 | 1 | 25 | abs. | b.d.l. | abs. | abs. | abs. |
| JSD-001 | jsd1_144 | 14.4 | 1 | 45 | 23 | 1 | 29 | abs. | 1 | abs. | abs. | abs. |
| JSD-001 | jsd1_149 | 14.9 | 22 | 49 | 27 | 1 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-001 | jsd1_154 | 15.4 | 6 | 48 | 29 | 1 | 15 | abs. | 1 | abs. | abs. | abs. |
| JSD-001 | jsd1_160 | 16.0 | 23 | 52 | 23 | b.d.l. | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-001 | jsd1_164 | 16.4 | 33 | 45 | 29 | 1 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-001 | jsd1_169 | 16.9 | 42 | 30 | 23 | 1 | abs. | abs. | 3 | abs. | abs. | abs. |
| JSD-001 | jsd1_174 | 17.4 | 43 | 28 | 22 | 4 | abs. | abs. | 3 | abs. | abs. | abs. |
| JSD-001 | jsd1_176 | 17.6 | 6 | 17 | 60 | 16 | abs. | b.d.l. | 1 | abs. | abs. | abs. |
| JSD-001 | jsd1_180 | 18.0 | 38 | 22 | 33 | 5 | abs. | b.d.l. | 2 | abs. | abs. | abs. |
| JSD-001 | jsd1_184 | 18.4 | 9 | 48 | 37 | 6 | abs. | b.d.l. | 1 | abs. | abs. | abs. |
| JSD-002 | jsd2_083 | 8.3 | 28 | 31 | 34 | 2 | abs. | abs. | 1 | abs. | 4 | abs. |
| JSD-002 | jsd2_089 | 8.9 | 36 | 34 | 27 | 1 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-002 | jsd2_096 | 9.6 | 59 | 25 | 12 | 1 | abs. | abs. | 2 | abs. | abs. | 1 |
| JSD-002 | jsd2_100 | 10.0 | 28 | 40 | 28 | 1 | abs. | abs. | 2 | abs. | abs. | 1 |
| JSD-002 | jsd2_107 | 10.7 | 35 | 31 | 31 | 1 | abs. | abs. | 2 | abs. | 0 | abs. |
| JSD-002 | jsd2_110 | 11.0 | 44 | 17 | 34 | 2 | abs. | abs. | 2 | abs. | 1 | abs. |
| JSD-002 | jsd2_117 | 11.7 | 42 | 24 | 29 | 2 | abs. | abs. | 2 | abs. | 1 | abs. |
| JSD-002 | jsd2_120 | 12.0 | 26 | 24 | 36 | 10 | abs. | abs. | 1 | abs. | 3 | abs. |
| JSD-002 | jsd2_125 | 12.5 | 33 | 27 | 36 | 2 | abs. | abs. | 2 | abs. | 0 | abs. |
| JSD-002 | jsd2_130 | 13.0 | 32 | 26 | 37 | 3 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-002 | jsd2_140 | 14.0 | 39 | 20 | 36 | 2 | abs. | abs. | 2 | abs. | 1 | abs. |
| JSD-002 | jsd2_145 | 14.5 | 42 | 24 | 31 | 1 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-002 | jsd2_150 | 15.0 | 38 | 18 | 38 | 3 | abs. | abs. | 2 | abs. | 1 | abs. |
| JSD-002 | jsd2_160 | 16.0 | 41 | 16 | 38 | 3 | abs. | abs. | 2 | abs. | 0 | abs. |
| JSD-002 | jsd2_165 | 16.5 | 36 | 29 | 31 | 3 | abs. | abs. | 1 | abs. | abs. | abs. |
| JSD-002 | jsd2_170 | 17.0 | 31 | 29 | 36 | 2 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-002 | jsd2_175 | 17.5 | 22 | 24 | 50 | 2 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-002 | jsd2_180 | 18.0 | 29 | 29 | 38 | 2 | abs. | abs. | 2 | 0 | abs. | abs. |
| JSD-002 | jsd2_185 | 18.5 | 20 | 44 | 31 | 3 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-002 | jsd2_190 | 19.0 | 28 | 34 | 34 | 3 | abs. | abs. | 1 | abs. | abs. | abs. |
| JSD-002 | jsd2_195 | 19.5 | 34 | 19 | 42 | 3 | abs. | abs. | 2 | abs. | abs. | abs. |
| Average | 25.6 | 33.5 | 32.2 | 2.5 | 4.1 | 0 | 1.6 | 0 | 0.4 | 0.1 |
5. Chemical Analyses Procedures and Data
Chemical analyses have been carried out in the Geochemical Analytical Unit (GAU) of the ARC Centre of Excellence for Core to Crust Fluid Systems (CCFS) and the Department of Earth and Planetary Sciences at Macquarie University. Selected chip samples were powdered using a tungsten carbide mill to obtain a fine powder (below 50 µm in size). Thorough care was taken when cleaning the mill with ethanol and Milli-Q® water between samples to avoid cross-contamination.
Standard X-ray fluorescence (XRF) technique was used for bulk sample major and minor elements analysis (e.g., Potts et al., 1984). Approximately 1 g of powder of each sample was mixed with 10 g of lithium tetraborate–meta-borate flux (12:22 mixture), fused in a Pt crucible and cooled to give glass discs. The discs were analysed using a PANalytical Axios 1 kW energy-dispersive XRF spectrometer. Loss on ignition was determined for each sample at 1100 °C overnight. The deviations from reference values from GeoReM (Jochum and Nohl, 2008) for standards BIR-1 and BHVO-2 have been used, along with replicates, to estimate the error. Analyses of the samples are listed in Table S-5.
Scandium concentrations in the bulk samples, along with the other rare earth elements, have been determined by standard inductively coupled plasma mass spectrometry (ICP-MS) methods (e.g., Eggins et al., 1998). Approximately 100 mg of powder samples were weighed into clean Savillex® Teflon beakers. Samples were then digested following the next steps:
(1) Digestion in a 1:1 mixture of HF (Merck, Suprapur grade) and HNO3 (Merck, AR grade, Teflon distilled) for 24 h at 160 °C followed by evaporation to dryness at 150 °C.
(2) Digestion in a 1:1 mixture of HF and perchloric acid (HClO4, Merck, Suprapur grade) for 3 days at 170 °C followed by evaporation to dryness at 120 °C, 150 °C and then 170 °C.
(3) Digestion in a 1:1:1 mixture of HF, HCl (Merck, AR grade, Teflon distilled) and HClO4 for 3 days at 170 °C followed by evaporation to dryness at 170 °C to 190 °C.
(4) Digestion in aqua regia (HNO3 + 3 HCl) for 3 days at 150 °C followed by evaporation to dryness at 150 °C.
(5) Digestion in 6 N HCl solution for 12 h at 150 °C followed by evaporation to dryness at 150 °C.
(6) Digestion in 6 N HNO3 for 12 h at 150 °C followed by evaporation at 120 °C.
The samples were then diluted to 100 mL in a 2 % HNO3 and 0.25 % HF solution. These 1:1000 dilutions of each sample were individually spiked with a 15 µL aliquot of a solution of Li6, As, Rh, In, Tm and Bi in 2 % HNO3. The samples and standards were analysed by quadrupole ICP-MS on an Agilent 7500c/s ICP-MS system. BCR-2 standard was used as a calibration standard to correct for instrument sensitivity. Run drift was corrected using the aliquot of solution added to each sample. The background was measured on a 2 % HNO3 rinse solution. The deviations from reference values from GeoReM (Jochum and Nohl, 2008) for standards BIR-1 and BHVO-2 have been used, along with replicates, to estimate the error. Analyses of the samples are given in Table S-5.
From these analyses, the enrichment factor of each REE, including Sc, has been calculated and shows a correlation with the ionic radii of these elements in octahedral coordination (Fig. S-2). Cerium does not follow this trend as under atmospheric conditions, Ce3+ can be oxidised to Ce4+ which is easily trapped by Mn oxides, commonly formed in lateritic profiles.
Table S-5 Bulk geochemical analyses for major and minor elements along with Sc and the rare earth elements.
| Core | Sample Name | Depth (m) | SiO2 (wt. %) | TiO2 (wt. %) | Al2O3 (wt. %) | Fe2O3 (wt. %) | Cr2O3 (wt. %) | MnO (wt. %) | NiO (wt. %) | MgO (wt. %) | CaO (wt. %) | BaO (wt. %) | Na2O (wt. %) | K2O (wt. %) | P2O5 (wt. %) | SO3 (wt. %) | L.O.I. (wt. %) | Total (wt. %) |
| JSD-001 | jsd1_115 | 11.5 | 3.41 | 2.25 | 17.69 | 59.69 | 0.40 | 0.15 | 0.04 | 0.38 | 0.19 | -0.01 | 0.09 | -0.01 | 0.06 | 0.12 | 14.45 | 98.90 |
| JSD-001 | jsd1_121 | 12.1 | 3.10 | 2.19 | 17.36 | 59.09 | 0.39 | 0.17 | 0.05 | 0.68 | 0.59 | 0.00 | 0.12 | -0.01 | 0.06 | 0.12 | 15.37 | 99.27 |
| JSD-001 | jsd1_125 | 12.5 | 4.60 | 1.83 | 17.03 | 57.42 | 0.33 | 0.21 | 0.06 | 0.40 | 0.12 | -0.01 | 0.10 | -0.01 | 0.03 | 0.09 | 15.42 | 97.63 |
| JSD-001 | jsd1_130 | 13.0 | 3.30 | 2.04 | 17.25 | 60.28 | 0.34 | 0.21 | 0.05 | 0.32 | 0.08 | -0.01 | 0.02 | -0.01 | 0.05 | 0.10 | 14.77 | 98.77 |
| JSD-001 | jsd1_135 | 13.5 | 3.51 | 1.85 | 17.21 | 59.38 | 0.34 | 0.23 | 0.07 | 0.41 | 0.07 | -0.02 | 0.06 | -0.01 | 0.03 | 0.10 | 15.57 | 98.81 |
| JSD-001 | jsd1_140 | 14.0 | 4.95 | 1.79 | 18.30 | 57.00 | 0.33 | 0.21 | 0.07 | 0.44 | 0.08 | 0.00 | 0.10 | -0.01 | 0.03 | 0.09 | 15.76 | 99.13 |
| JSD-001 | jsd1_144 | 14.4 | 6.20 | 1.46 | 19.97 | 45.10 | 0.27 | 0.16 | 0.05 | 0.52 | 0.20 | -0.02 | 0.04 | -0.01 | 0.03 | 0.07 | 17.21 | 91.26 |
| JSD-001 | jsd1_149 | 14.9 | 14.53 | 1.62 | 15.82 | 52.61 | 0.41 | 0.16 | 0.08 | 0.21 | 0.08 | 0.00 | 0.10 | -0.01 | 0.03 | 0.05 | 14.00 | 99.69 |
| JSD-001 | jsd1_154 | 15.4 | 8.35 | 1.84 | 17.00 | 55.01 | 0.35 | 0.22 | 0.07 | 0.46 | 0.09 | -0.01 | 0.09 | -0.01 | 0.04 | 0.07 | 15.60 | 99.16 |
| JSD-001 | jsd1_160 | 16.0 | 16.07 | 1.65 | 16.88 | 48.76 | 0.31 | 0.19 | 0.08 | 0.23 | 0.08 | 0.00 | 0.11 | -0.01 | 0.04 | 0.05 | 14.46 | 98.90 |
| JSD-001 | jsd1_164 | 16.4 | 17.88 | 1.70 | 17.45 | 47.51 | 0.32 | 0.17 | 0.07 | 0.28 | 0.10 | -0.01 | 0.18 | 0.00 | 0.02 | 0.05 | 13.24 | 98.97 |
| JSD-001 | jsd1_169 | 16.9 | 24.57 | 1.32 | 20.34 | 37.88 | 0.24 | 0.13 | 0.05 | 0.26 | 0.11 | 0.01 | 0.12 | 0.00 | 0.02 | 0.03 | 14.69 | 99.76 |
| JSD-001 | jsd1_174 | 17.4 | 21.89 | 1.19 | 17.71 | 44.87 | 0.23 | 0.20 | 0.04 | 0.27 | 0.12 | 0.00 | 0.14 | 0.00 | 0.02 | 0.04 | 11.94 | 98.65 |
| JSD-001 | jsd1_176 | 17.6 | 4.59 | 0.39 | 3.78 | 83.90 | 0.10 | 0.62 | 0.06 | 0.21 | 0.08 | 0.01 | 0.09 | -0.01 | 0.01 | 0.02 | 4.81 | 98.66 |
| JSD-001 | jsd1_180 | 18.0 | 17.98 | 1.17 | 14.95 | 52.25 | 0.15 | 0.47 | 0.05 | 0.25 | 0.15 | 0.00 | 0.11 | 0.00 | 0.04 | 0.02 | 11.17 | 98.76 |
| JSD-001 | jsd1_184 | 18.4 | 4.77 | 0.55 | 5.53 | 76.62 | 0.21 | 0.42 | 0.20 | 0.34 | 0.09 | 0.01 | 0.16 | -0.01 | 0.03 | 0.07 | 8.54 | 97.52 |
| JSD-002 | jsd2_083 | 8.3 | 17.37 | 1.53 | 15.42 | 48.03 | 0.20 | 0.84 | 0.06 | 0.96 | 1.35 | 0.01 | 0.06 | 0.00 | 0.02 | 0.00 | 13.41 | 99.26 |
| JSD-002 | jsd2_089 | 8.9 | 18.37 | 1.55 | 17.27 | 47.20 | 0.19 | 1.16 | 0.06 | 0.23 | 0.17 | 0.01 | 0.09 | 0.00 | 0.02 | 0.01 | 13.21 | 99.52 |
| JSD-002 | jsd2_096 | 9.6 | 24.98 | 1.08 | 22.60 | 33.13 | 0.15 | 1.62 | 0.10 | 0.21 | 0.12 | 0.04 | 0.07 | 0.00 | 0.02 | 0.00 | 14.92 | 99.04 |
| JSD-002 | jsd2_100 | 10.0 | 15.23 | 1.64 | 14.28 | 52.20 | 0.19 | 1.55 | 0.13 | 0.24 | 0.14 | 0.03 | 0.05 | 0.00 | 0.03 | 0.00 | 13.03 | 98.75 |
| JSD-002 | jsd2_107 | 10.7 | 17.79 | 1.59 | 15.35 | 50.32 | 0.16 | 0.75 | 0.06 | 0.28 | 0.24 | 0.01 | 0.06 | 0.00 | 0.03 | 0.00 | 12.70 | 99.32 |
| JSD-002 | jsd2_110 | 11.0 | 20.41 | 1.40 | 16.06 | 47.78 | 0.11 | 0.61 | 0.04 | 0.33 | 0.51 | 0.00 | 0.06 | 0.00 | 0.03 | -0.01 | 12.02 | 99.34 |
| JSD-002 | jsd2_117 | 11.7 | 20.08 | 1.27 | 16.50 | 47.49 | 0.20 | 0.54 | 0.06 | 0.29 | 0.32 | 0.01 | 0.06 | 0.00 | 0.03 | 0.00 | 12.83 | 99.68 |
| JSD-002 | jsd2_120 | 12.0 | 12.68 | 0.85 | 10.25 | 61.98 | 0.16 | 0.57 | 0.06 | 0.83 | 1.15 | 0.04 | 0.05 | 0.00 | 0.03 | 0.00 | 10.70 | 99.34 |
| JSD-002 | jsd2_125 | 12.5 | 16.87 | 1.51 | 13.47 | 54.18 | 0.44 | 0.43 | 0.06 | 0.23 | 0.22 | 0.02 | 0.05 | 0.00 | 0.03 | -0.01 | 11.93 | 99.43 |
| JSD-002 | jsd2_130 | 13.0 | 17.20 | 1.37 | 13.46 | 52.70 | 0.50 | 1.05 | 0.07 | 0.20 | 0.20 | 0.01 | 0.06 | 0.00 | 0.02 | -0.01 | 12.30 | 99.12 |
| JSD-002 | jsd2_140 | 14.0 | 17.92 | 1.45 | 14.17 | 52.17 | 0.19 | 0.35 | 0.04 | 0.35 | 0.56 | 0.01 | 0.10 | 0.00 | 0.06 | -0.01 | 12.43 | 99.79 |
| JSD-002 | jsd2_145 | 14.5 | 17.49 | 1.47 | 12.99 | 48.27 | 0.29 | 0.42 | 0.05 | 0.16 | 0.19 | 0.01 | 0.02 | 0.00 | 0.02 | -0.01 | 11.61 | 92.98 |
| JSD-002 | jsd2_150 | 15.0 | 17.12 | 1.44 | 13.31 | 51.26 | 0.35 | 0.98 | 0.11 | 0.40 | 0.41 | 0.05 | 0.04 | 0.00 | 0.01 | -0.01 | 12.58 | 98.05 |
| JSD-002 | jsd2_160 | 16.0 | 18.84 | 1.48 | 14.41 | 50.82 | 0.16 | 0.41 | 0.05 | 0.33 | 0.48 | 0.02 | 0.03 | -0.01 | 0.04 | -0.01 | 11.59 | 98.65 |
| JSD-002 | jsd2_165 | 16.5 | 17.84 | 1.48 | 13.61 | 52.30 | 0.20 | 0.42 | 0.10 | 0.28 | 0.19 | 0.00 | 0.07 | -0.01 | 0.05 | -0.01 | 12.65 | 99.16 |
| JSD-002 | jsd2_170 | 17.0 | 17.28 | 1.26 | 12.75 | 45.81 | 0.12 | 0.41 | 0.08 | 0.24 | 0.20 | 0.01 | 0.04 | 0.00 | 0.03 | -0.01 | 12.89 | 91.11 |
| JSD-002 | jsd2_175 | 17.5 | 16.47 | 1.58 | 11.62 | 53.52 | 0.16 | 2.24 | 0.10 | 0.22 | 0.28 | 0.02 | 0.02 | 0.00 | 0.05 | -0.01 | 12.23 | 98.50 |
| JSD-002 | jsd2_180 | 18.0 | 18.28 | 1.56 | 13.07 | 50.49 | 0.61 | 0.06 | 0.07 | 0.27 | 0.87 | 0.00 | 0.02 | 0.00 | 0.05 | -0.01 | 13.04 | 98.35 |
| JSD-002 | jsd2_185 | 18.5 | 16.61 | 1.60 | 13.22 | 52.40 | 0.43 | 0.14 | 0.09 | 0.29 | 0.21 | 0.00 | 0.05 | 0.00 | 0.04 | -0.01 | 13.28 | 98.34 |
| JSD-002 | jsd2_190 | 19.0 | 15.53 | 1.49 | 12.35 | 54.40 | 0.57 | 0.12 | 0.12 | 0.39 | 0.17 | 0.02 | 0.08 | -0.01 | 0.03 | -0.01 | 13.13 | 98.36 |
| JSD-002 | jsd2_195 | 19.5 | 20.91 | 1.48 | 13.35 | 48.85 | 0.10 | 0.64 | 0.08 | 0.34 | 0.26 | -0.01 | 0.09 | 0.00 | 0.02 | -0.01 | 12.59 | 98.69 |
| Error (wt. %) | 0.10 | 0.01 | 0.02 | 0.11 | 0.05 | 0.00 | 0.05 | 0.01 | 0.02 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.36 | |||
| Saprolite (average) | 49.99 | 0.27 | 2.32 | 8.31 | 0.26 | 0.17 | 0.05 | 14.66 | 19.45 | -0.01 | 0.21 | -0.01 | 0.00 | -0.01 | 4.64 | 100.31 | ||
| Core | Sample Name | Depth (m) | La (ppm) | Ce (ppm) | Pr (ppm) | Nd (ppm) | Sm (ppm) | Eu (ppm) | Gd (ppm) | Tb (ppm) | Dy (ppm) | Ho (ppm) | Er (ppm) | Yb (ppm) | Lu (ppm) | Y (ppm) | Sc (ppm) | |
| JSD-001 | jsd1_115 | 11.5 | 11.3 | 50.9 | 4.2 | 19.3 | 6.2 | 1.7 | 5.6 | 0.9 | 5.3 | 1.0 | 2.9 | 3.2 | 0.5 | 23.9 | 526.7 | |
| JSD-001 | jsd1_121 | 12.1 | 6.0 | 47.7 | 2.2 | 10.5 | 3.5 | 1.0 | 3.2 | 0.5 | 3.1 | 0.6 | 1.7 | 1.9 | 0.3 | 11.6 | 461.2 | |
| JSD-001 | jsd1_125 | 12.5 | 12.0 | 152.7 | 5.4 | 24.5 | 8.7 | 2.4 | 7.2 | 1.2 | 7.2 | 1.4 | 3.9 | 4.7 | 0.7 | 20.1 | 542.6 | |
| JSD-001 | jsd1_130 | 13.0 | 11.3 | 234.9 | 4.7 | 21.3 | 7.1 | 2.0 | 6.3 | 1.1 | 6.1 | 1.2 | 3.4 | 3.8 | 0.6 | 21.0 | 533.8 | |
| JSD-001 | jsd1_135 | 13.5 | 8.1 | 183.4 | 4.1 | 18.6 | 6.9 | 1.9 | 5.7 | 1.0 | 5.8 | 1.1 | 3.1 | 3.9 | 0.6 | 12.9 | 539.8 | |
| JSD-001 | jsd1_140 | 14.0 | 6.8 | 66.2 | 3.1 | 14.0 | 5.2 | 1.4 | 4.1 | 0.7 | 4.2 | 0.8 | 2.2 | 2.7 | 0.4 | 11.4 | 495.8 | |
| JSD-001 | jsd1_144 | 14.4 | 9.5 | 77.5 | 4.5 | 20.4 | 7.6 | 2.0 | 6.1 | 1.1 | 6.3 | 1.2 | 3.4 | 4.3 | 0.6 | 17.4 | 547.9 | |
| JSD-001 | jsd1_149 | 14.9 | 25.3 | 56.2 | 10.8 | 48.9 | 15.1 | 3.9 | 11.6 | 1.8 | 9.7 | 1.8 | 5.0 | 4.8 | 0.7 | 24.8 | 375.2 | |
| JSD-001 | jsd1_154 | 15.4 | 17.0 | 62.6 | 5.9 | 28.5 | 9.5 | 2.6 | 8.4 | 1.3 | 7.2 | 1.4 | 3.9 | 3.7 | 0.5 | 31.0 | 573.8 | |
| JSD-001 | jsd1_160 | 16.0 | 26.1 | 124.8 | 11.3 | 53.8 | 16.8 | 4.6 | 13.7 | 2.2 | 11.9 | 2.2 | 6.0 | 6.2 | 0.9 | 32.9 | 635.9 | |
| JSD-001 | jsd1_164 | 16.4 | 27.9 | 138.9 | 12.0 | 54.9 | 15.7 | 4.4 | 13.9 | 2.2 | 12.1 | 2.2 | 6.0 | 6.0 | 0.9 | 32.7 | 654.8 | |
| JSD-001 | jsd1_169 | 16.9 | 29.5 | 234.2 | 11.7 | 52.7 | 12.1 | 3.3 | 10.6 | 1.6 | 8.1 | 1.5 | 3.9 | 3.4 | 0.5 | 24.8 | 465.5 | |
| JSD-001 | jsd1_174 | 17.4 | 22.9 | 116.9 | 8.7 | 38.5 | 8.9 | 2.6 | 9.2 | 1.4 | 7.5 | 1.4 | 3.8 | 3.3 | 0.5 | 25.8 | 358.3 | |
| JSD-001 | jsd1_176 | 17.6 | 33.9 | 138.8 | 10.4 | 45.3 | 10.5 | 3.1 | 11.1 | 1.6 | 8.6 | 1.7 | 4.5 | 3.8 | 0.6 | 30.5 | 251.6 | |
| JSD-001 | jsd1_180 | 18.0 | 25.1 | 71.5 | 8.6 | 36.6 | 8.6 | 2.5 | 8.9 | 1.3 | 7.1 | 1.3 | 3.5 | 3.0 | 0.4 | 23.0 | 360.5 | |
| JSD-001 | jsd1_184 | 18.4 | 28.5 | 38.3 | 7.7 | 34.9 | 8.7 | 2.7 | 12.2 | 1.7 | 9.8 | 2.1 | 5.7 | 4.2 | 0.7 | 47.0 | 584.7 | |
| JSD-002 | jsd2_083 | 8.3 | 24.6 | 77.1 | 7.1 | 31.6 | 6.7 | 1.9 | 7.5 | 1.0 | 5.2 | 1.1 | 2.8 | 1.9 | 0.3 | 32.0 | 507.7 | |
| JSD-002 | jsd2_089 | 8.9 | 23.4 | 155.4 | 6.1 | 27.1 | 5.5 | 1.6 | 6.1 | 0.8 | 3.9 | 0.8 | 1.9 | 1.2 | 0.2 | 17.9 | 296.3 | |
| JSD-002 | jsd2_096 | 9.6 | 18.2 | 320.2 | 4.8 | 21.5 | 4.8 | 1.4 | 5.6 | 0.8 | 3.9 | 0.8 | 2.0 | 1.3 | 0.2 | 16.8 | 281.8 | |
| JSD-002 | jsd2_100 | 10.0 | 13.0 | 61.0 | 4.1 | 19.4 | 4.9 | 1.5 | 5.8 | 0.8 | 4.6 | 0.9 | 2.5 | 1.9 | 0.3 | 28.4 | 531.3 | |
| JSD-002 | jsd2_107 | 10.7 | 7.5 | 39.6 | 2.4 | 11.0 | 2.8 | 0.8 | 3.4 | 0.5 | 2.7 | 0.6 | 1.4 | 1.1 | 0.2 | 19.6 | 513.7 | |
| JSD-002 | jsd2_110 | 11.0 | 9.8 | 59.2 | 3.2 | 14.3 | 3.5 | 1.0 | 3.6 | 0.5 | 2.8 | 0.5 | 1.4 | 1.2 | 0.2 | 15.7 | 331.1 | |
| JSD-002 | jsd2_117 | 11.7 | 12.4 | 90.1 | 4.0 | 18.4 | 4.6 | 1.3 | 4.8 | 0.7 | 3.9 | 0.7 | 2.0 | 1.7 | 0.2 | 15.9 | 468.0 | |
| JSD-002 | jsd2_120 | 12.0 | 10.6 | 66.5 | 3.9 | 18.6 | 5.6 | 1.7 | 6.1 | 0.9 | 5.3 | 1.1 | 2.9 | 2.8 | 0.4 | 27.7 | 582.4 | |
| JSD-002 | jsd2_125 | 12.5 | 5.2 | 21.0 | 2.0 | 9.5 | 2.7 | 0.8 | 2.8 | 0.4 | 2.4 | 0.5 | 1.3 | 1.0 | 0.2 | 15.6 | 749.6 | |
| JSD-002 | jsd2_130 | 13.0 | 8.8 | 71.2 | 2.8 | 12.5 | 3.1 | 0.9 | 3.0 | 0.4 | 2.1 | 0.4 | 1.0 | 0.8 | 0.1 | 9.6 | 290.0 | |
| JSD-002 | jsd2_140 | 14.0 | 5.9 | 29.0 | 2.4 | 11.3 | 3.3 | 1.0 | 3.5 | 0.5 | 3.1 | 0.6 | 1.7 | 1.5 | 0.2 | 17.4 | 574.9 | |
| JSD-002 | jsd2_145 | 14.5 | 4.2 | 18.8 | 1.6 | 7.9 | 2.2 | 0.6 | 2.2 | 0.3 | 1.7 | 0.3 | 0.9 | 0.7 | 0.1 | 8.6 | 331.5 | |
| JSD-002 | jsd2_150 | 15.0 | 8.9 | 55.9 | 3.0 | 13.5 | 3.5 | 1.1 | 3.3 | 0.5 | 2.6 | 0.5 | 1.3 | 1.0 | 0.1 | 12.3 | 600.3 | |
| JSD-002 | jsd2_160 | 16.0 | 5.1 | 11.9 | 2.3 | 10.6 | 3.2 | 0.9 | 2.7 | 0.4 | 2.4 | 0.4 | 1.2 | 1.3 | 0.2 | 7.9 | 375.2 | |
| JSD-002 | jsd2_165 | 16.5 | 8.4 | 15.6 | 3.6 | 18.3 | 6.0 | 1.8 | 6.1 | 1.0 | 6.0 | 1.2 | 3.3 | 3.7 | 0.6 | 16.2 | 538.1 | |
| JSD-002 | jsd2_170 | 17.0 | 11.6 | 18.6 | 4.3 | 20.6 | 6.5 | 2.0 | 6.7 | 1.1 | 6.5 | 1.3 | 3.7 | 4.3 | 0.7 | 22.0 | 593.0 | |
| JSD-002 | jsd2_175 | 17.5 | 20.9 | 61.5 | 6.4 | 29.8 | 8.1 | 2.3 | 7.5 | 1.1 | 6.2 | 1.2 | 3.2 | 3.0 | 0.4 | 21.9 | 342.0 | |
| JSD-002 | jsd2_180 | 18.0 | 12.3 | 3.8 | 5.1 | 24.8 | 7.5 | 2.2 | 7.6 | 1.2 | 6.9 | 1.3 | 3.7 | 3.7 | 0.6 | 24.8 | 669.3 | |
| JSD-002 | jsd2_185 | 18.5 | 6.0 | 5.6 | 3.3 | 16.9 | 5.4 | 1.6 | 5.3 | 0.9 | 5.0 | 0.9 | 2.5 | 2.4 | 0.4 | 14.6 | 492.4 | |
| JSD-002 | jsd2_190 | 19.0 | 8.3 | 5.4 | 4.4 | 22.9 | 7.3 | 2.3 | 7.7 | 1.2 | 7.2 | 1.4 | 3.7 | 3.3 | 0.5 | 23.2 | 636.3 | |
| JSD-002 | jsd2_195 | 19.5 | 21.8 | 20.2 | 9.0 | 44.9 | 11.6 | 3.3 | 10.7 | 1.5 | 8.2 | 1.6 | 4.0 | 3.2 | 0.4 | 28.4 | 260.9 | |
| Error (ppm) | 0.3 | 1.7 | 0.2 | 0.5 | 0.2 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.5 | 8.0 | |||
| Saprolite (average) | 5.7 | 5.9 | 2.2 | 10.6 | 2.7 | 0.8 | 2.8 | 0.4 | 2.1 | 0.4 | 1.1 | 0.8 | 0.1 | 11.9 | 99.4 | |||
| Enrichment factor (JSD-001) | 2.6 | 13.7 | 2.5 | 2.4 | 2.6 | 2.6 | 2.4 | 2.6 | 2.7 | 2.7 | 2.8 | 3.4 | 3.6 | 1.8 | 4.9 |

Figure S-2 Enrichment factor of rare earth elements in the lateritic deposit versus ionic radius in octahedral coordination. Values are after Shannon (1976).
6. Procedures for Spatially Resolved Chemical Analyses
Polished sections were obtained by impregnating dry chip samples in polyester resin diluted with acetone and polishing on cloth with diamond pastes. Microscopic observations and chemical mapping of major elements of these polished sections have been carried out on a ZEISS SUPRATM 55VP scanning electron microscope (SEM) at University Pierre et Marie Curie (UPMC), Paris. The SEM is equipped with a field emission gun (FEG) operating at 15 kV. The working distance was set to 15 mm. Backscattered electron (BSE) images were collected using an AsB detector. Chemical mapping was performed using the energy dispersive X-ray (EDX) microanalysis system (Brucker XFlash® QUAD silicon drift detector) coupled to the SEM.
Electron microprobe (EMP) analyses were performed for major and minor elements on selected points of the polished sections using a Cameca SXFive EMP equipped with five wavelength-dispersive spectrometers (WDS) at the Centre d’Analyse des Minéraux de PARIS (CAMPARIS, Université Pierre et Marie Curie, Paris, France). Each point has been probed with a focused 20 kV, 40 nA beam for major elements and with a focused 20 kV, 200 nA beam for minor and trace elements. Major elements: Na, Mg, Al, Si, P, Cl, K, Ca, Ti, Cr, Mn, Fe and Ba were analysed with a 20 s (peak + background) counting time. Minor and trace elements were Sc, V, Ni; all of these were analysed with 60 s (peak + background) counting time. The WDS were used with the following monochromators: a large area (1320 mm2) thallium acid pthalate (TAP); a regular (660 mm2) TAP; a regular (660 mm2) pentaerythritol (PET) and two large (1320 mm2) lithium fluoride (LiF) to collect, respectively, Na, Mg and P Kα X-rays; Si and Al Kα X-rays; K, Ca and Cl Kα X-rays and Ba Lα, Cr, Mn, Fe, Ti, V, Ni and Sc Kα X-rays. The two large LiF monochromators were used simultaneously to analyse each of the minor and trace elements. This allowed us to achieve detection limits as low as 60 ppm to 30 ppm. WDS analyses were performed using the following standards: albite for Na; diopside for Mg, Si and Ca; apatite Durango for P; orthoclase for Al and K; scapolite for Cl, Cr2O3 for Cr; MnTiO3 for Mn and Ti; haematite for Fe; BaSO4 for Ba; vanadite for V; NiO for Ni and Sc2O3 for Sc.
The different EMP analyses have been classified in groups in order to distinguish between different types of grains or phases. In order to do that, analyses for Si, Ti, Fe, Al, Sc and the analytical total have been selected as they exhibited repeated patterns with similar concentrations. Using the R software (version 3.1.3) and the k-means function of the “stats’’ package (version 3.4.0), a k-means clustering method has been applied to our EMP analyses distinguishing 5 clusters. Based on those results, each cluster has been carefully studied by analysing the position of the analysis on the SEM images as well as the concentrations in the selected element. From these analyses, 8 groups of grains could be distinguished (Table S-6): Fe-rich oxyhydroxides, Fe-rich oxides, Ti-rich oxides, Al-rich oxyhydroxides, quartz, Sc-rich groundmass, kaolinite-rich groundmass and Sc-poor groundmass.
Table S-6 Median, mean and standard deviation of Si, Ti, Fe, Al, analytical total and Sc concentrations in the different grain types distinguished by SEM imaging, EMP and cluster analysis.
| Grain type | Num. Obs. | SiO2 (wt. %) | TiO2 (wt. %) | Al2O3 (wt. %) | Fe2O3 (wt. %) | Sc (ppm) | Total (wt. %) | ||||||||||||
| Median | Mean | Std. Dev. | Median | Mean | Std. Dev. | Median | Mean | Std. Dev. | Median | Mean | Std. Dev. | Median | Mean | Std. Dev. | Median | Mean | Std. Dev. | ||
| Fe-rich oxyhydroxide | 43 | 4.30 | 4.50 | 1.06 | 1.72 | 1.76 | 0.75 | 6.27 | 6.58 | 2.16 | 70.56 | 68.10 | 8.84 | 1190 | 1339 | 351 | 85.49 | 83.01 | 9.01 |
| Fe-rich oxide | 16 | 0.07 | 0.25 | 0.67 | 1.24 | 1.28 | 1.02 | 0.31 | 0.48 | 0.51 | 95.61 | 95.93 | 3.25 | 201 | 195 | 85 | 100.34 | 100.45 | 2.41 |
| Ti-rich oxide | 3 | 0.05 | 0.04 | 0.02 | 57.38 | 62.63 | 11.50 | -1.23 | -1.27 | 0.12 | 41.75 | 37.22 | 9.77 | 102 | 92 | 50 | 104.96 | 104.16 | 2.10 |
| Al-rich oxyhydroxide | 7 | 3.67 | 5.50 | 5.16 | 1.05 | 1.02 | 0.70 | 52.30 | 48.28 | 8.26 | 19.65 | 15.84 | 10.72 | 336 | 271 | 161 | 68.49 | 71.64 | 12.38 |
| Quartz | 4 | 99.13 | 98.58 | 2.80 | 0.02 | 0.02 | 0.00 | 0.04 | 0.04 | 0.04 | 0.39 | 0.37 | 0.06 | -2 | 0 | 10 | 99.61 | 99.08 | 2.75 |
| High-Sc matrix | 31 | 4.43 | 5.02 | 3.19 | 1.88 | 2.23 | 1.40 | 7.13 | 9.05 | 7.39 | 61.52 | 65.28 | 12.44 | 640 | 658 | 107 | 85.61 | 83.45 | 8.81 |
| Kaolinite-rich matrix | 9 | 38.91 | 36.30 | 9.97 | 0.08 | 0.32 | 0.42 | 31.14 | 30.26 | 7.67 | 3.77 | 12.33 | 14.64 | 364 | 408 | 139 | 82.41 | 80.01 | 6.45 |
| Low-Sc matrix | 12 | 2.73 | 5.54 | 6.02 | 1.79 | 1.91 | 0.60 | 6.42 | 7.30 | 4.31 | 76.05 | 70.53 | 13.62 | 391 | 382 | 83 | 90.48 | 86.59 | 8.93 |
7. Procedures for the Synthesis of Reference Materials
Sc-Bearing Goethite
Ferrihydrite was precipitated by adding 50 mL of 1.35 M NaOH to 25 mL of 0.5 M Fe(NO3)3 and 25 mL of 1 M ScCl3 solutions. The suspension was then diluted to 1 L with a 33 mM NaOH solution. The solution was heated to 65 °C in a water-bath for 48 h, transforming the ferrihydrite suspension into a precipitate of goethite. The solid product was separated by centrifugation, washed at 45 °C for 2 h in a 3 M H2SO4 solution, in order to remove any adsorbed species and dried at 45 °C for 24 h giving 1 g of Sc-bearing goethite.
Sc-Bearing Hematite
Sc-bearing haematite was obtained by dehydroxylation of the previously synthesised goethite at 300 °C.
Sc-Adsorbed Goethite
10 mL of ScCl3 solution at 1000 ppm Sc was added to 1 g of pure α-FeOOH obtained following the synthesis method previously described without addition of ScCl3. The near neutral pH of the solution prohibits any dissolution and reprecipitation of Sc-bearing goethite, considering the stability diagram of goethite. The solid and the solution were shaken together for 24 h. The solid was then separated by centrifugation and dried at 45 °C for 24 h.
8. Characterisation of the Synthetic Compounds
The crystal structure of the mineral phases obtained has been checked by XRD (Fig. S-3) using the method described in SI-4.

Figure S-3 XRD patterns of synthetic Sc-bearing goethite and haematite.
The incorporation or adsorption of Sc was then verified using ICP-MS. The amount of Sc incorporated in the crystal lattice of the synthetic Sc-bearing goethite, Sc-bearing haematite or adsorbed on goethite has been determined to be 898 ppm ± 0.025 %, 872 ppm ± 0.025 % and 819 ppm ± 0.025 %, respectively. The analyses were performed on a Thermo Scientific XSERIES 2 quadrupole instrument at the Université Pierre et Marie Curie. About 50 mg of powdered sample were digested in 2 mL of concentrated HNO3-HCl solution at 90 °C for 24 h and then evaporated to dryness at 60 °C. The residue was then diluted in a 2 % HNO3 solution (1:1000 dilution) for analysis. A standard solution of ScCl3 at 10 ppb in Sc was prepared by dilution of commercial 99.99 % ScCl3 powder and used to correct for instrumental shifts and to calculate the Sc content of the samples.
9. Synchrotron Measurement Procedures
Micro-X-ray absorption near-edge structure (XANES) and micro-fluorescence X-ray spectroscopy (µ-XRF) data were collected on the LUCIA beamline at the SOLEIL synchrotron in Paris (France) and on the ID21 beamline at the European Synchrotron Radiation Facility (ESRF) in Grenoble (France). The former operated with a storage ring current of 450 mA and an energy of 2.75 GeV while the latter operated with a storage ring current of 200 mA and an energy of 6 GeV. The layouts of the LUCIA and ID21 beamlines are described in Vantelon et al. (2016) and Salomé et al. (2013), respectively. XANES spectra were collected using an Si(311) and an Si(220) double crystal monochromator on LUCIA and ID21, respectively. The monochromators were calibrated against the maximum intensity of the first inflection of the first derivative of a Fe foil (7112.0 eV) or a Sc2O3 standard (4492.8 eV) for XANES measurements at the Fe or Sc K-edge, respectively.
Iron and Sc K-edge XANES measurements were performed in the energy range 7060 eV to 7300 eV and 4400 eV to 4800 eV, respectively. The energy step at the Fe K-edge was (5, 0.1, 0.3, 0.5 and 1) eV for energy ranges of (7060 to 7110) eV, (7110 to 7125) eV, (7125 to 7170) eV, (7170 to 7219) eV and (7219 to 7300) eV, respectively. The energy step at the Sc K-edge was (5, 0.2, 0.5 and 1) eV for energy ranges of (4400 to 4485) eV, (4485 to 4534) eV, (4534 to 4586) eV and (4586 to 4800) eV, respectively. Spectra were collected at room temperature, under vacuum. For Fe reference materials, the spectra were collected on ID21 in transmission mode on powder samples diluted in a cellulose pellet and mounted on a holder between two Ultralene® films. Two spectra were recorded for each reference with a counting time of about 50 s per spectrum. For Sc reference materials, the spectra were collected on LUCIA in XRF mode on pellets obtained from powdered material and mounted on a holder between two Ultralene® films, using a four-element silicon drift detector (SDD).
For natural samples, the data were collected on ID21 in XRF mode on polished sections for both Fe and Sc K-edges using a photodiode and a large area (80 mm2 collimated area) SDD, respectively. The beam was focused with a Kirkpatrick-Baez mirror system, down to 0.7 µm (horizontal) x 0.3 µm (vertical) at the Fe X-edge and 0.9 µm (horizontal) x 0.3 µm (vertical) at the Sc K-edge. The selection of the spots for analysis was made by combining prior SEM and EMP analyses and imaging with chemical mapping of Sc using µ-XRF mapping. The maps were recorded with step sizes of 1 µm x 1 µm with an incident energy of 4800 eV and a dwell time of 100 ms. The XRF spectra obtained for each pixel was then fitted using the PyMCA software to obtain Sc maps. At the Sc K-edge, 10 to 40 XANES spectra were acquired for each spot with a counting time of about 80 s per spectrum. At the Fe X-edge, 2 XANES spectra were acquired for each spot with a counting time of about 50 s. Individual spectra acquired on each spot were merged to obtain average spectra before background subtraction and normalisation using the PyMCA software (Solé et al., 2007). Due to the high Fe concentration in the natural samples, self-absorption occurred during XRF Fe K-edge XANES measurements. This phenomenon has been corrected using the FLUO algorithm from the ATHENA software (Ravel and Newville, 2005). The input parameters have been chosen considering the geometry of the experiment and the chemical composition obtained from EMP measurement on the same spot.
In order to map qualitative Fe speciation, we recorded µ-XRF maps on ID21 in the same area but with three incident energies: 7133.8 eV, corresponding to the maximum intensity of the Fe K-edge XANES spectrum of goethite; 7136.9 eV, corresponding to the maximum intensity of the Fe K-edge XANES spectrum of haematite and 7165.0 eV corresponding to an identical intensity in the normalised Fe K-edge XANES spectra of goethite and haematite. These maps were recorded with step sizes of 2 µm x 2 µm and a dwell time of 50 ms. As for µ-XRF mapping of Sc, the three maps have been obtained by fitting the spectra at each pixel using the PyMCA software. The map of Fe speciation shown in the paper corresponds to the difference between the 7133.8 eV and the 7136.9 eV maps normalised to the 7165.0 eV map.
Supplementary Information References
Blake, R.L., Hessevic, R.E. (1966) Refinement of the Hematite Structure. American Mineralogist 51, 123–129.
Chessin, H., Hamilton, W.C., Post, B. (1965) Position and Thermal Parameters of Oxygen Atoms in Calcite. Acta Crystallographica 18, 689–693.
Eggins, S.M., Rudnick, R.L., McDonough, W.F. (1998) The Composition of Peridotites and their Minerals: a Laser-Ablation ICP–MS Study. Earth and Planetary Science Letters 154, 53–71.
Hazemann, J.-L., Bérar, J.F., Manceau, A. (1991) Rietveld Studies of the Aluminium-Iron Substitution in Synthetic Goethite. Material Sciences Forum 79, 821–826.
Jochum, K.P., Nohl, U. (2008) Reference Materials in Geochemistry and Environmental Research and the GeoReM Database. Chemical Geology 253, 50–53.
Parker, R.L. (1923) Zur Kristallographie von Anatas und Rutil. Zeitschrift für Kristallographie – Crystalline Materials 58, 522–582.
Pecharromán, C., González-Carreño, T., Iglesias, J.E. (1995) The Infrared Dielectric Properties of Maghemite, γ–Fe2O3, from Reflectance Measurement on Pressed Powders. Physics and Chemistry of Minerals 22, 21–29.
Post, J.E., Appleman, D.E. (1994) Crystal Structure Refinement of Lithiophorite. American Mineralogist 79, 370–374.
Potts, P.J., Webb, P.C., Watson, J.S. (1984) Energy-Dispersive X-Ray Fluorescence Analysis of Silicate Rocks for Major and Trace Elements. X-Ray Spectrometry 13, 2–15.
Ravel, B., Newville, M. (2005) ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. Journal of Synchrotron Radiation 12, 537–541.
Rodríguez-Carvajal, J. (1993) Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Physica B: Condensed Matter 192, 55–69.
Saalfeld, H., Wedde, M. (1974) Refinement of the Crystal Structure of Gibbsite, Al(OH)3. Zeitschrift für Kristallographie – Crystalline Materials 139, 129–135.
Salomé, M., Cotte, M., Baker, R., Barrett, R., Benseny-Cases, N., Berruyer, G., Bugnazet, D., Castillo-Michel, H., Cornu, C., Fayard, B., Gagliardini, E., Hino, R., Morse, J., Papillon, E., Pouyet, E., Rivard, C., Solé, V.A., Susini, J., Veronesi, G. (2013) The ID21 Scanning X-ray Microscope at ESRF. Journal of Physics: Conference Series 425, 1–5.
Shannon, R.D. (1976) Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallographica Section A: Foundations and Advances A32, 751–767.
Solé, V.A., Papillon, E., Cotte, M., Walter, P., Susini, J. (2007) A Multiplatform Code for the Analysis of Energy-Dispersive X-ray Fluorescence Spectra. Spectrochimica Acta – Part B: Atomic Spectroscopy 62, 63–68.
Vantelon, D., Trcera, N., Roy, D., Moreno, T., Mailly, D., Guilet, S., Metchalkov, E., Delmotte, F., Lassalle, B., Lagarde, P., Flank, A.M. (2016) The LUCIA Beamline at SOLEIL. Journal of Synchrotron Radiation 23, 635–640.
Wasastjerna, J.A. (1924) Crystal Structure of Dolomite. Societas Scientiarum Fennica. Commentationes Physicomathematicae II 11, 14–14.
Wei, P.H. (1935) Die Bindung im Quarz. Zeitschrift für Kristallographie – Crystalline Materials 92, 355–362.
Figures and Tables
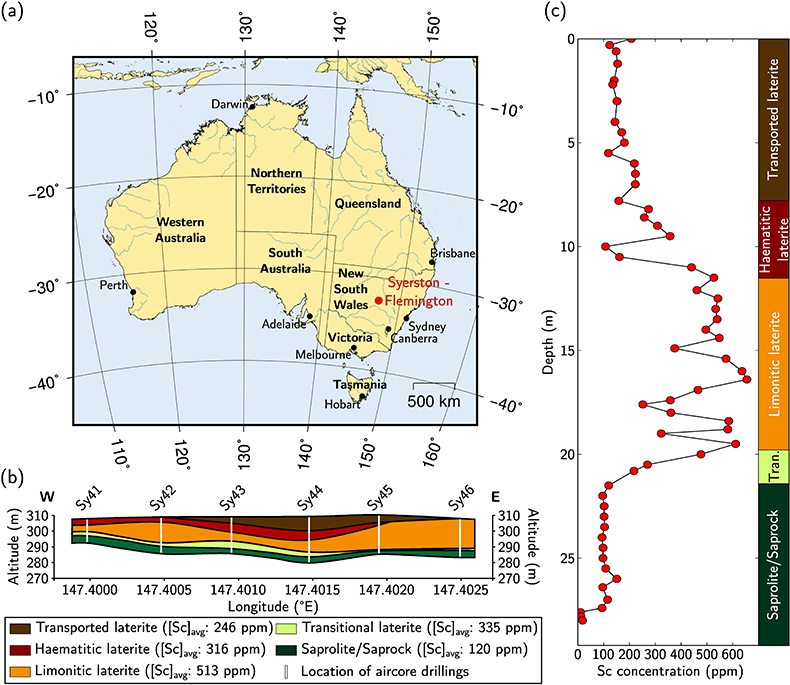
Figure 1 (a) Geographical location of the Syerston–Flemington deposit. (b) Schematic cross section of the lateritic cover forming the Syerston–Flemington deposit, constructed from petrographic analysis of drill-cores. Average Sc concentrations ([Sc]avg) are calculated for each layer from ICP-MS analyses of samples collected every metre on each core. (c) Distribution of Sc with depth along the different levels of a typical profile (Tran.: transitional laterite).
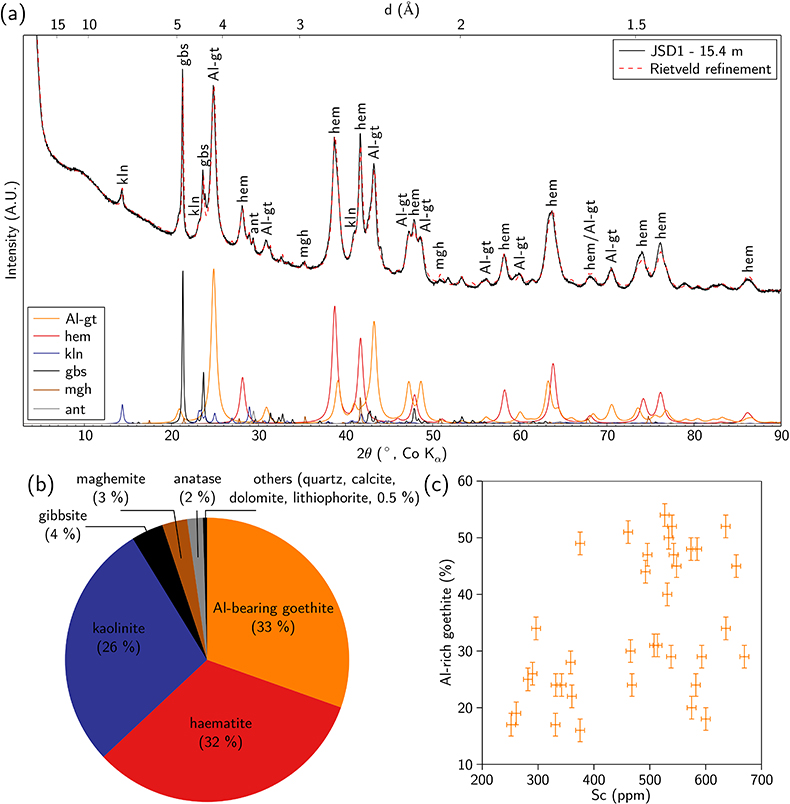
Figure 2 (a) X-ray diffraction pattern of ore sample JSD-1 – 15.4 m and associated multiphase Rietveld refinement (Al-gt: Al-bearing goethite, hem: haematite, kln: kaolinite, gbs: gibbsite, mgh: maghemite, ant: anatase). (b) Average phase proportions in the samples calculated from Rietveld refinement. (c) Bulk Sc concentration versus proportion of Al-bearing goethite estimated from Rietveld refinement.
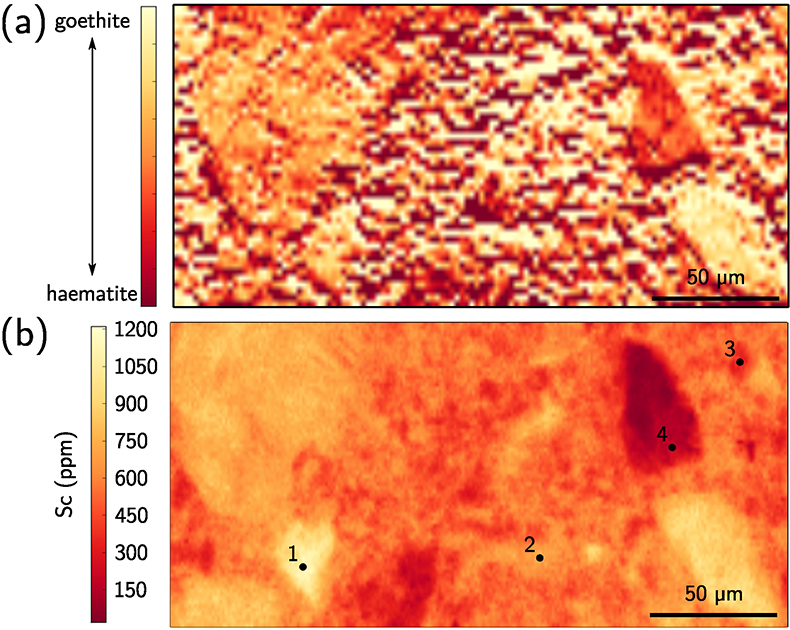
Figure 3 (a) Map of Fe speciation obtained by subtracting normalised synchrotron µ-XRF maps at 7133.9 eV, the energy characteristic of goethite and 7136.7 eV, the energy characteristic of haematite. (b) Synchrotron µ-XRF mapping of Sc.
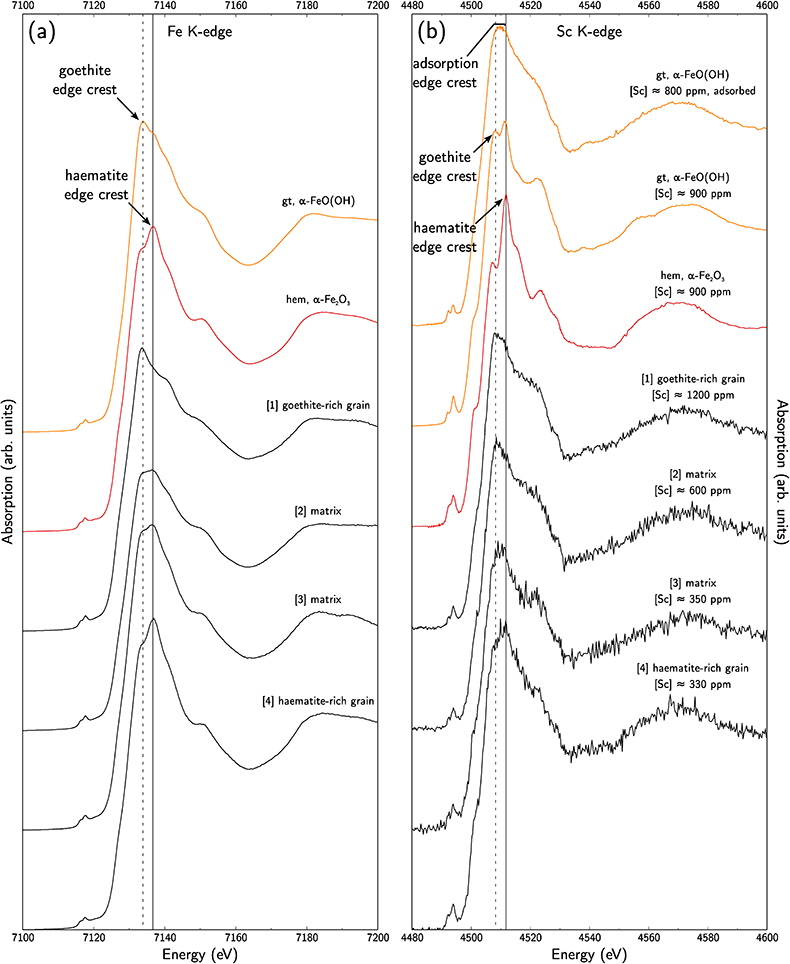
Figure 4 XANES spectra at the (a) Fe K-edge. (b) Sc K-edge (gt: goethite and hem: haematite). The numbers in brackets refer to the position given in Figure 3.
Back to article
Supplementary Figures and Tables
Table S-1 Latitude and longitude coordinates, altitude and dip angle of the two diamond core drillings.
| Drill hole | Latitude | Longitude | Altitude | Dip |
| JSD-001 | −32°44′42.91901′′ | 147°24′7.42757′′ | 309.211 m | −90° |
| JSD-002 | −32°44′43.326 11′′ | 147°24′3.72856′′ | 309.067 m | −90° |
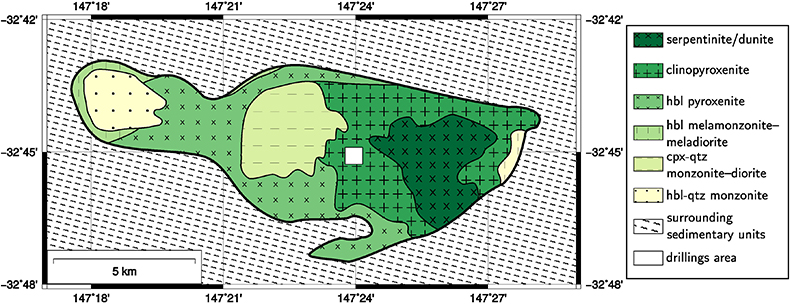
Figure S-1 Geological map of the Tout complex showing the locations of the drilling sites (cpx: clinopyroxene, hbl: hornblende, qtz: quartz).
Table S-2 Detailed log of the JSD-001 drill core.
| Horizon | Depth | Colour | Fabric and/or texture |
| Transported laterite | 0 m – 0.3 m | Red-brown | Brecciated; coarsely grained (>5 mm); mixture of soil (≃25 %) and residual lateritic material (≃25 %), supporting Fe pisoliths (≃50 %); cavernous and interstitial porosity. |
| Transported laterite | 0.3 m – 2.5 m | Red mottled with orange spots | Massive with a breccia-like texture; fine to coarse grains (<0.002 mm – 5 mm); matrix of lateritic material, probably a mixture of Fe oxides and oxyhydroxides with kaolinites (>80 %), associated with few Fe pisoliths (≃10 %), lateritic clasts (≃10 %) and fine sandy grains, possibly quartz, accessory orange areas (<5 %) without pisoliths or clasts and probably richer in Fe oxyhydroxides; interstitial porosity. |
| Transported laterite | 2.5 m – 5.2 m | Dark red | Massive; fine grains when visible (<1 mm); dominated by a matrix of fine grains with clayey content (>95 %), probably a mixture of Fe oxides and oxyhydroxides with kaolinite, rare sandy grains (<2 mm), possibly quartz; interstitial porosity. |
| Transported laterite | 5.2 m – 8 m | Dark red to dark orange-red | Massive with breccia-like texture; fine grains when visible (<1 mm) with coarse clasts (>3 mm); dominated by a fine grained matrix with minor clay content (≃75 %), probably a mixture of Fe oxides and oxyhydroxides with kaolinite, common angular clasts of lateritic material (≃20 %); interstitial porosity. |
| Haematitic laterite | 8 m – 10.2 m | Dark red to dark orange-red | Massive to cemented granular material; fine to coarse grains (<0.002 mm – 5 mm); dominated by a clayey matrix with fine grains (>70 %), probably a mixture of Fe oxides and oxyhydroxides with kaolinite, common to minor Fe pisoliths (5 % – 25 %); interstitial porosity. |
| Haematitic laterite | 10.2 m – 11.2 m | Dark brown to red | Massive; fine grains when visible (<1 mm); dominant matrix of fine grains and clays (>90 %), probably a mixture of Fe oxides and oxyhydroxides with kaolinite, a homogeneous dark-brown vein at the bottom of the level, possibly rich in Al oxyhydroxides; interstitial and cavernous porosity. |
| Limonitic laterite | 11.2 m – 14.2 m | Dark to medium orange-red | Massive to cemented granular material; fine to coarse grains (<0.002 mm – >5 mm); dominant to minor Fe pisoliths (5 % – 70 %) in a common to dominant matrix of fine grains and clays (30 % – 90 %), probably a mixture of Fe oxides and oxyhydroxides with kaolinite, rare to common orange areas, richer in Fe oxyhydroxides; interstitial and cavernous porosity. |
| Limonitic laterite | 14.2 m – 16.7 m | Medium to light orange-red mottled with rare orange spots | Massive to cemented granular material; similar to the above level, with rarer orange areas (<10 %). |
| Limonitic laterite | 16.7 m – 18.2 m | Red-pink mottled with orange spots | Massive to cemented granular material; fine grains when visible (<1 mm); dominated by a matrix of fine pinkish grains associated with clayey material (>90 %), probably Fe oxides and oxyhydroxides associated with kaolinites, with minor orange areas (≃10 %), probably rich in Fe oxyhydroxides; interstitial to cavernous porosity. |
| Limonitic laterite | 18.2 m – 18.7 m | Tan-brown to khaki-brown | Massive; fine grains when visible (<1 mm); dominated by a matrix of fine grains and clayey material (>80 %), probably Fe oxides and oxyhydroxides associated with kaolinite, accessory white crystals (≃1 %); interstitial porosity. |
| Transitional laterite | 18.7 m – 20.5 m | Variable, mottled with green, orange, red, purple, brown and black spots | Granular; medium to fine grains (<0.002 mm – 3 mm); dominated by a red-brown finely grained matrix (50 % – 80 %), probably a mixture of Fe oxides and oxyhydroxides, common green clayey areas becoming accessory in the upper parts (≃20 % – <1 %), two types of veins, filled-in by a black fine-grained matrix, possibly Mn oxides or by a white homogeneous matrix, possibly calcite; important interstitial porosity with fractures. |
| Transitional laterite | 20.5 m – 21.5 m | Green-grey mottled with orange and black spots | Clay texture; dominant clay matrix (≃70 %), possibly smectite, minor fine grained orange areas (<10 %) possibly clays coated with Fe oxides and oxyhydroxides, accessory crystalline black areas (<5 %), remnants of primary pyroxenes; probably important porosity but pores are not visible. |
| Saprolite | 21.5 m – 27.6 m | Green to light green-grey mottled with orange and black spots | Crystalline to granular; medium to fine grains (<0.002 mm – 3 mm); dominated by green clay minerals (>70 %), possibly smectite, with accessory remnants of black crystals (<5 %) identified as pyroxenes and minor rust-coloured areas (<10 %) formed by weathered crystals covered by Fe oxides; limited interstitial porosity increasing in the upper levels. |
| Saprolite | 27.6 m – 29.2 m | Dark brown-grey with light green to green brown fractures | Mesh texture with filled-in fractures; rare sandy grains (<5 %) in a dominant serpentinized groundmass (≃90 %), fractures filled with clay fibres, probably serpentines; low porosity except in fractures. |
Table S-3 Sc concentration (in ppm) at each metre of the 26 vertical aircore drillings performed in the Syerston–Flemington area.
| Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc |
| (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) |
| SY34-0-1 | 344 | SY35-0-1 | 362 | SY36-0-1 | 114 | SY37-0-1 | 205 | SY38-0-1 | 432 | SY39-0-1 | 140 | SY40-0-1 | 189 |
| SY34-1-2 | 464 | SY35-1-2 | 330 | SY36-1-2 | 136 | SY37-1-2 | 186 | SY38-1-2 | 394 | SY39-1-2 | 90 | SY40-1-2 | 82 |
| SY34-2-3 | 484 | SY35-2-3 | 368 | SY36-2-3 | 137 | SY37-2-3 | 161 | SY38-2-3 | 443 | SY39-2-3 | 95 | SY40-2-3 | 78 |
| SY34-3-4 | 460 | SY35-3-4 | 418 | SY36-3-4 | 123 | SY37-3-4 | 158 | SY38-3-4 | 451 | SY40-3-4 | 81 | ||
| SY34-4-5 | 562 | SY35-4-5 | 482 | SY36-4-5 | 120 | SY37-4-5 | 161 | SY38-4-5 | 455 | SY40-4-5 | 79 | ||
| SY34-5-6 | 532 | SY35-5-6 | 519 | SY36-5-6 | 176 | SY37-5-6 | 188 | SY38-5-6 | 461 | SY40-5-6 | 92 | ||
| SY34-6-7 | 706 | SY35-6-7 | 544 | SY36-6-7 | 211 | SY37-6-7 | 203 | SY38-6-7 | 457 | ||||
| SY34-7-8 | 800 | SY35-7-8 | 550 | SY36-7-8 | 243 | SY37-7-8 | 224 | SY38-7-8 | 467 | ||||
| SY34-8-9 | 724 | SY35-8-9 | 606 | SY36-8-9 | 320 | SY37-8-9 | 265 | SY38-8-9 | 479 | ||||
| SY34-9-10 | 874 | SY35-9-10 | 646 | SY36-9-10 | 361 | SY37-9-10 | 318 | SY38-9-10 | 507 | ||||
| SY34-10-11 | 796 | SY35-10-11 | 642 | SY36-10-11 | 324 | SY37-10-11 | 331 | SY38-10-11 | 519 | ||||
| SY34-11-12 | 565 | SY35-11-12 | 583 | SY36-11-12 | 300 | SY37-11-12 | 409 | SY38-11-12 | 578 | ||||
| SY34-12-13 | 502 | SY35-12-13 | 610 | SY36-12-13 | 349 | SY37-12-13 | 506 | SY38-12-13 | 547 | ||||
| SY34-13-14 | 505 | SY35-13-14 | 576 | SY36-13-14 | 353 | SY37-13-14 | 438 | SY38-13-14 | 438 | ||||
| SY34-14-15 | 410 | SY35-14-15 | 665 | SY36-14-15 | 362 | SY37-14-15 | 446 | SY38-14-15 | 392 | ||||
| SY34-15-16 | 322 | SY35-15-16 | 764 | SY36-15-16 | 255 | SY37-15-16 | 507 | SY38-15-16 | 439 | ||||
| SY34-16-17 | 226 | SY35-16-17 | 945 | SY36-16-17 | 160 | SY37-16-17 | 606 | SY38-16-17 | 445 | ||||
| SY34-17-18 | 200 | SY35-17-18 | 770 | SY36-17-18 | 181 | SY37-17-18 | 514 | SY38-17-18 | 455 | ||||
| SY34-18-19 | 266 | SY35-18-19 | 690 | SY36-18-19 | 283 | SY37-18-19 | 808 | SY38-18-19 | 385 | ||||
| SY34-19-20 | 174 | SY35-19-20 | 535 | SY36-19-20 | 354 | SY37-19-20 | 661 | SY38-19-20 | 267 | ||||
| SY34-20-21 | 128 | SY35-20-21 | 446 | SY36-20-21 | 298 | SY37-20-21 | 449 | SY38-20-21 | 268 | ||||
| SY35-21-22 | 374 | SY36-21-22 | 274 | SY37-21-22 | 281 | SY38-21-22 | 222 | ||||||
| SY35-22-23 | 343 | SY36-22-23 | 268 | SY37-22-23 | 440 | SY38-22-23 | 140 | ||||||
| SY35-23-24 | 329 | SY36-23-24 | 128 | SY37-23-24 | 155 | SY38-23-24 | 117 | ||||||
| SY35-24-25 | 331 | SY36-24-25 | 106 | SY37-24-25 | 133 | ||||||||
| SY35-25-26 | 338 | SY36-25-26 | 97 | SY37-25-26 | 114 | ||||||||
| SY35-26-27 | 304 | SY36-0-26 | 232 | SY37-26-27 | 109 | ||||||||
| SY35-27-28 | 159 | ||||||||||||
| SY35-28-29 | 126 | ||||||||||||
| SY35-29-30 | 126 | ||||||||||||
| Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc |
| (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) |
| SY41-0-1 | 200 | SY42-0-1 | 373 | SY43-0-1 | 128 | SY44-0-1 | 107 | SY45-0-1 | 147 | SY46-0-1 | 358 | SY47-0-1 | 158 |
| SY41-1-2 | 306 | SY42-1-2 | 460 | SY43-1-2 | 178 | SY44-1-2 | 129 | SY45-1-2 | 157 | SY46-1-2 | 514 | SY47-1-2 | 381 |
| SY41-2-3 | 496 | SY42-2-3 | 477 | SY43-2-3 | 179 | SY44-2-3 | 134 | SY45-2-3 | 165 | SY46-2-3 | 512 | SY47-2-3 | 424 |
| SY41-3-4 | 590 | SY42-3-4 | 569 | SY43-3-4 | 183 | SY44-3-4 | 147 | SY45-3-4 | 182 | SY46-3-4 | 612 | SY47-3-4 | 475 |
| SY41-4-5 | 747 | SY42-4-5 | 795 | SY43-4-5 | 225 | SY44-4-5 | 117 | SY45-4-5 | 191 | SY46-4-5 | 546 | SY47-4-5 | 451 |
| SY41-5-6 | 598 | SY42-5-6 | 655 | SY43-5-6 | 213 | SY44-5-6 | 107 | SY45-5-6 | 235 | SY46-5-6 | 603 | SY47-5-6 | 505 |
| SY41-6-7 | 591 | SY42-6-7 | 588 | SY43-6-7 | 195 | SY44-6-7 | 110 | SY45-6-7 | 500 | SY46-6-7 | 594 | SY47-6-7 | 567 |
| SY41-7-8 | 566 | SY42-7-8 | 595 | SY43-7-8 | 229 | SY44-7-8 | 120 | SY45-7-8 | 511 | SY46-7-8 | 540 | SY47-7-8 | 588 |
| SY41-8-9 | 525 | SY42-8-9 | 540 | SY43-8-9 | 323 | SY44-8-9 | 152 | SY45-8-9 | 564 | SY46-8-9 | 577 | SY47-8-9 | 492 |
| SY41-9-10 | 476 | SY42-9-10 | 618 | SY43-9-10 | 396 | SY44-9-10 | 201 | SY45-9-10 | 545 | SY46-9-10 | 600 | SY47-9-10 | 641 |
| SY41-10-11 | 469 | SY42-10-11 | 574 | SY43-10-11 | 506 | SY44-10-11 | 239 | SY45-10-11 | 475 | SY46-10-11 | 590 | SY47-10-11 | 865 |
| SY41-11-12 | 361 | SY42-11-12 | 505 | SY43-11-12 | 618 | SY44-11-12 | 282 | SY45-11-12 | 525 | SY46-11-12 | 607 | SY47-11-12 | 689 |
| SY41-12-13 | 300 | SY42-12-13 | 617 | SY43-12-13 | 647 | SY44-12-13 | 253 | SY45-12-13 | 401 | SY46-12-13 | 504 | SY47-12-13 | 810 |
| SY41-13-14 | 275 | SY42-13-14 | 756 | SY43-13-14 | 667 | SY44-13-14 | 226 | SY45-13-14 | 453 | SY46-13-14 | 397 | SY47-13-14 | 847 |
| SY41-14-15 | 208 | SY42-14-15 | 606 | SY43-14-15 | 695 | SY44-14-15 | 249 | SY45-14-15 | 514 | SY46-14-15 | 485 | SY47-14-15 | 874 |
| SY41-15-16 | 132 | SY42-15-16 | 577 | SY43-15-16 | 553 | SY44-15-16 | 312 | SY45-15-16 | 482 | SY46-15-16 | 433 | SY47-15-16 | 622 |
| SY42-16-17 | 556 | SY43-16-17 | 484 | SY44-16-17 | 359 | SY45-16-17 | 516 | SY46-16-17 | 469 | SY47-16-17 | 268 | ||
| SY42-17-18 | 549 | SY43-17-18 | 463 | SY44-17-18 | 407 | SY45-17-18 | 596 | SY46-17-18 | 304 | SY47-17-18 | 184 | ||
| SY42-18-19 | 343 | SY43-18-19 | 349 | SY44-18-19 | 380 | SY45-18-19 | 625 | SY46-18-19 | 152 | ||||
| SY42-19-20 | 230 | SY43-19-20 | 248 | SY44-19-20 | 335 | SY45-19-20 | 595 | SY46-19-20 | 204 | ||||
| SY42-20-21 | 133 | SY43-20-21 | 268 | SY44-20-21 | 532 | SY45-20-21 | 835 | SY46-20-21 | 212 | ||||
| SY42-21-22 | 119 | SY43-21-22 | 160 | SY44-21-22 | 630 | SY45-21-22 | 980 | SY46-21-22 | 163 | ||||
| SY42-22-23 | 121 | SY43-22-23 | 240 | SY44-22-23 | 492 | SY45-22-23 | 687 | SY46-22-23 | 121 | ||||
| SY42-23-24 | 105 | SY43-23-24 | 212 | SY44-23-24 | 322 | SY45-23-24 | 404 | SY46-23-24 | 101 | ||||
| SY44-24-25 | 285 | SY45-24-25 | 243 | SY46-24-25 | 83 | ||||||||
| SY44-25-26 | 257 | SY45-25-26 | 280 | SY46-25-26 | 81 | ||||||||
| SY44-26-27 | 238 | ||||||||||||
| SY44-27-28 | 225 | ||||||||||||
| SY44-28-29 | 261 | ||||||||||||
| SY44-29-30 | 242 | ||||||||||||
| Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc |
| (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) |
| SY48-0-1 | 203 | SY49-0-1 | 150 | SY50-0-1 | 111 | SY51-0-1 | 358 | SY52-0-1 | 156 | SY53-0-1 | 206 | SY54-0-1 | 206 |
| SY48-1-2 | 216 | SY49-1-2 | 120 | SY50-1-2 | 108 | SY51-1-2 | 388 | SY52-1-2 | 118 | SY53-1-2 | 299 | SY54-1-2 | 136 |
| SY48-2-3 | 229 | SY49-2-3 | 102 | SY50-2-3 | 106 | SY51-2-3 | 401 | SY52-2-3 | 96 | SY53-2-3 | 341 | SY54-2-3 | 136 |
| SY48-3-4 | 211 | SY49-3-4 | 106 | SY50-3-4 | 114 | SY51-3-4 | NSS | SY52-3-4 | 98 | SY53-3-4 | 393 | SY54-3-4 | 140 |
| SY48-4-5 | 217 | SY49-4-5 | 122 | SY50-4-5 | 153 | SY51-4-5 | 513 | SY52-4-5 | 116 | SY53-4-5 | 457 | SY54-4-5 | 200 |
| SY48-5-6 | 222 | SY49-5-6 | 113 | SY50-5-6 | 199 | SY51-5-6 | 500 | SY52-5-6 | 103 | SY53-5-6 | 457 | SY54-5-6 | 192 |
| SY48-6-7 | 264 | SY49-6-7 | 116 | SY50-6-7 | 245 | SY51-6-7 | 425 | SY52-6-7 | 108 | SY53-6-7 | 479 | SY54-6-7 | 212 |
| SY48-7-8 | 335 | SY49-7-8 | 118 | SY50-7-8 | 274 | SY51-7-8 | 327 | SY52-7-8 | 121 | SY53-7-8 | 400 | SY54-7-8 | 247 |
| SY48-8-9 | 416 | SY49-8-9 | 133 | SY50-8-9 | 277 | SY51-8-9 | 347 | SY52-8-9 | 115 | SY53-8-9 | 510 | SY54-8-9 | 251 |
| SY48-9-10 | 427 | SY49-9-10 | 156 | SY50-9-10 | 212 | SY51-9-10 | 386 | SY52-9-10 | 142 | SY53-9-10 | 574 | SY54-9-10 | 308 |
| SY48-10-11 | 486 | SY49-10-11 | 155 | SY50-10-11 | 278 | SY51-10-11 | 522 | SY52-10-11 | 166 | SY53-10-11 | 466 | SY54-10-11 | 416 |
| SY48-11-12 | 480 | SY49-11-12 | 144 | SY50-11-12 | 282 | SY51-11-12 | 371 | SY52-11-12 | 213 | SY53-11-12 | 597 | SY54-11-12 | 433 |
| SY48-12-13 | 427 | SY49-12-13 | 163 | SY50-12-13 | 349 | SY51-12-13 | 456 | SY52-12-13 | 265 | SY53-12-13 | 656 | SY54-12-13 | 275 |
| SY48-13-14 | 500 | SY49-13-14 | 187 | SY50-13-14 | 341 | SY51-13-14 | 365 | SY52-13-14 | 393 | SY53-13-14 | 671 | SY54-13-14 | 264 |
| SY48-14-15 | 552 | SY49-14-15 | 236 | SY50-14-15 | 472 | SY51-14-15 | 384 | SY52-14-15 | 431 | SY53-14-15 | 968 | SY54-14-15 | 225 |
| SY48-15-16 | 612 | SY49-15-16 | 351 | SY50-15-16 | 502 | SY51-15-16 | 470 | SY52-15-16 | 499 | SY53-15-16 | 531 | SY54-15-16 | 369 |
| SY48-16-17 | 672 | SY49-16-17 | 400 | SY50-16-17 | 600 | SY51-16-17 | 475 | SY52-16-17 | 561 | SY53-16-17 | 348 | SY54-16-17 | 400 |
| SY48-17-18 | 659 | SY49-17-18 | 451 | SY50-17-18 | 513 | SY51-17-18 | 614 | SY52-17-18 | 650 | SY53-17-18 | 321 | SY54-17-18 | 360 |
| SY48-18-19 | 582 | SY49-18-19 | 353 | SY50-18-19 | 404 | SY51-18-19 | 533 | SY52-18-19 | 611 | SY53-18-19 | 232 | SY54-18-19 | 419 |
| SY48-19-20 | 513 | SY49-19-20 | 338 | SY50-19-20 | 228 | SY51-19-20 | 540 | SY52-19-20 | 455 | SY53-19-20 | 256 | SY54-19-20 | 354 |
| SY48-20-21 | 376 | SY49-20-21 | 217 | SY50-20-21 | 322 | SY51-20-21 | 600 | SY52-20-21 | 449 | SY53-20-21 | 315 | SY54-20-21 | 400 |
| SY48-21-22 | 316 | SY49-21-22 | 143 | SY50-21-22 | 332 | SY51-21-22 | 480 | SY52-21-22 | 340 | SY53-21-22 | 274 | SY54-21-22 | 494 |
| SY48-22-23 | 318 | SY49-22-23 | 114 | SY50-22-23 | 309 | SY51-22-23 | 396 | SY52-22-23 | 316 | SY53-22-23 | 258 | SY54-22-23 | 313 |
| SY49-23-24 | 89 | SY50-23-24 | 277 | SY51-23-24 | 290 | SY52-23-24 | 314 | SY53-23-24 | 191 | SY54-23-24 | 263 | ||
| SY50-24-25 | 217 | SY51-24-25 | 213 | SY52-24-25 | 307 | SY53-24-25 | 105 | SY54-24-25 | 234 | ||||
| SY50-25-26 | 213 | SY51-25-26 | 295 | SY52-25-26 | 140 | SY54-25-26 | 281 | ||||||
| SY50-26-27 | 159 | SY51-26-27 | 192 | SY52-26-27 | 107 | SY54-26-27 | 268 | ||||||
| SY50-27-28 | 178 | SY51-27-28 | 133 | SY54-27-28 | 266 | ||||||||
| SY50-28-29 | 137 | SY54-28-29 | 289 | ||||||||||
| SY54-29-30 | 140 | ||||||||||||
| SY54-30-31 | 84 | ||||||||||||
| Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | Drill hole-depth | Sc | ||||
| (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | (m) | (ppm) | ||||
| SY55-1-2 | 576 | SY56-1-2 | 441 | SY57-1-2 | 390 | SY58-1-2 | 137 | SY59-1-2 | 137 | ||||
| SY55-2-3 | 574 | SY56-2-3 | 479 | SY57-2-3 | 582 | SY58-2-3 | 186 | SY59-2-3 | 142 | ||||
| SY55-3-4 | 664 | SY56-3-4 | 492 | SY57-3-4 | 598 | SY58-3-4 | 191 | SY59-3-4 | 133 | ||||
| SY55-4-5 | 569 | SY56-4-5 | 490 | SY57-4-5 | 500 | SY58-4-5 | 212 | SY59-4-5 | 117 | ||||
| SY55-5-6 | 481 | SY56-5-6 | 493 | SY57-5-6 | 594 | SY58-5-6 | 223 | SY59-5-6 | 103 | ||||
| SY55-6-7 | 429 | SY56-6-7 | 475 | SY57-6-7 | 594 | SY58-6-7 | 243 | SY59-6-7 | 110 | ||||
| SY55-7-8 | 358 | SY56-7-8 | 432 | SY57-7-8 | 673 | SY58-7-8 | 257 | SY59-7-8 | 108 | ||||
| SY55-8-9 | 379 | SY56-8-9 | 488 | SY57-8-9 | 783 | SY58-8-9 | 298 | SY59-8-9 | 104 | ||||
| SY55-9-10 | 244 | SY56-9-10 | 500 | SY57-9-10 | 899 | SY58-9-10 | 315 | SY59-9-10 | 101 | ||||
| SY55-10-11 | 161 | SY56-10-11 | 543 | SY57-10-11 | 617 | SY58-10-11 | 327 | SY59-10-11 | 109 | ||||
| SY55-11-12 | 120 | SY56-11-12 | 508 | SY57-11-12 | 214 | SY58-11-12 | 329 | SY59-11-12 | 120 | ||||
| SY56-12-13 | 375 | SY57-12-13 | 153 | SY58-12-13 | 384 | SY59-12-13 | 133 | ||||||
| SY56-13-14 | 411 | SY57-13-14 | 118 | SY58-13-14 | 500 | SY59-13-14 | 161 | ||||||
| SY56-14-15 | 426 | SY57-14-15 | 110 | SY58-14-15 | 558 | SY59-14-15 | 150 | ||||||
| SY56-15-16 | 471 | SY58-15-16 | 688 | SY59-15-16 | 148 | ||||||||
| SY56-16-17 | 314 | SY58-16-17 | 444 | SY59-16-17 | 133 | ||||||||
| SY56-17-18 | 214 | SY58-17-18 | 195 | SY59-17-18 | 130 | ||||||||
| SY56-18-19 | 230 | SY58-18-19 | 133 | SY59-18-19 | 130 | ||||||||
| SY56-19-20 | 192 | SY58-19-20 | 99 | SY59-19-20 | 137 | ||||||||
| SY56-20-21 | 119 | SY58-20-21 | 104 | SY59-20-21 | 187 | ||||||||
| SY59-21-22 | 252 | ||||||||||||
| SY59-22-23 | 228 | ||||||||||||
| SY59-23-24 | 149 |
Table S-4 Nature and proportion of the phases identified for each sample (b.d.l: below detection limit using Rietveld refinement but present in the sample, abs.: absent from the sample).
| Core | Sample Name | Depth (m) | Kaolinite | Al-rich goethite | Haematite | Maghemite | Gibbsite | Quartz | Anatase | Calcite | Dolomite | Lithiophorite |
| JSD-001 | jsd1_115 | 11.5 | abs. | 54 | 30 | 1 | 13 | abs. | 1 | abs. | 1 | abs. |
| JSD-001 | jsd1_121 | 12.1 | abs. | 51 | 33 | 1 | 13 | abs. | 1 | abs. | 2 | abs. |
| JSD-001 | jsd1_125 | 12.5 | abs. | 47 | 34 | 1 | 17 | abs. | 1 | abs. | abs. | abs. |
| JSD-001 | jsd1_130 | 13.0 | abs. | 50 | 28 | 1 | 20 | abs. | 1 | abs. | abs. | abs. |
| JSD-001 | jsd1_135 | 13.5 | abs. | 52 | 26 | 1 | 21 | abs. | b.d.l. | abs. | abs. | abs. |
| JSD-001 | jsd1_140 | 14.0 | abs. | 47 | 27 | 1 | 25 | abs. | b.d.l. | abs. | abs. | abs. |
| JSD-001 | jsd1_144 | 14.4 | 1 | 45 | 23 | 1 | 29 | abs. | 1 | abs. | abs. | abs. |
| JSD-001 | jsd1_149 | 14.9 | 22 | 49 | 27 | 1 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-001 | jsd1_154 | 15.4 | 6 | 48 | 29 | 1 | 15 | abs. | 1 | abs. | abs. | abs. |
| JSD-001 | jsd1_160 | 16.0 | 23 | 52 | 23 | b.d.l. | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-001 | jsd1_164 | 16.4 | 33 | 45 | 29 | 1 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-001 | jsd1_169 | 16.9 | 42 | 30 | 23 | 1 | abs. | abs. | 3 | abs. | abs. | abs. |
| JSD-001 | jsd1_174 | 17.4 | 43 | 28 | 22 | 4 | abs. | abs. | 3 | abs. | abs. | abs. |
| JSD-001 | jsd1_176 | 17.6 | 6 | 17 | 60 | 16 | abs. | b.d.l. | 1 | abs. | abs. | abs. |
| JSD-001 | jsd1_180 | 18.0 | 38 | 22 | 33 | 5 | abs. | b.d.l. | 2 | abs. | abs. | abs. |
| JSD-001 | jsd1_184 | 18.4 | 9 | 48 | 37 | 6 | abs. | b.d.l. | 1 | abs. | abs. | abs. |
| JSD-002 | jsd2_083 | 8.3 | 28 | 31 | 34 | 2 | abs. | abs. | 1 | abs. | 4 | abs. |
| JSD-002 | jsd2_089 | 8.9 | 36 | 34 | 27 | 1 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-002 | jsd2_096 | 9.6 | 59 | 25 | 12 | 1 | abs. | abs. | 2 | abs. | abs. | 1 |
| JSD-002 | jsd2_100 | 10.0 | 28 | 40 | 28 | 1 | abs. | abs. | 2 | abs. | abs. | 1 |
| JSD-002 | jsd2_107 | 10.7 | 35 | 31 | 31 | 1 | abs. | abs. | 2 | abs. | 0 | abs. |
| JSD-002 | jsd2_110 | 11.0 | 44 | 17 | 34 | 2 | abs. | abs. | 2 | abs. | 1 | abs. |
| JSD-002 | jsd2_117 | 11.7 | 42 | 24 | 29 | 2 | abs. | abs. | 2 | abs. | 1 | abs. |
| JSD-002 | jsd2_120 | 12.0 | 26 | 24 | 36 | 10 | abs. | abs. | 1 | abs. | 3 | abs. |
| JSD-002 | jsd2_125 | 12.5 | 33 | 27 | 36 | 2 | abs. | abs. | 2 | abs. | 0 | abs. |
| JSD-002 | jsd2_130 | 13.0 | 32 | 26 | 37 | 3 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-002 | jsd2_140 | 14.0 | 39 | 20 | 36 | 2 | abs. | abs. | 2 | abs. | 1 | abs. |
| JSD-002 | jsd2_145 | 14.5 | 42 | 24 | 31 | 1 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-002 | jsd2_150 | 15.0 | 38 | 18 | 38 | 3 | abs. | abs. | 2 | abs. | 1 | abs. |
| JSD-002 | jsd2_160 | 16.0 | 41 | 16 | 38 | 3 | abs. | abs. | 2 | abs. | 0 | abs. |
| JSD-002 | jsd2_165 | 16.5 | 36 | 29 | 31 | 3 | abs. | abs. | 1 | abs. | abs. | abs. |
| JSD-002 | jsd2_170 | 17.0 | 31 | 29 | 36 | 2 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-002 | jsd2_175 | 17.5 | 22 | 24 | 50 | 2 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-002 | jsd2_180 | 18.0 | 29 | 29 | 38 | 2 | abs. | abs. | 2 | 0 | abs. | abs. |
| JSD-002 | jsd2_185 | 18.5 | 20 | 44 | 31 | 3 | abs. | abs. | 2 | abs. | abs. | abs. |
| JSD-002 | jsd2_190 | 19.0 | 28 | 34 | 34 | 3 | abs. | abs. | 1 | abs. | abs. | abs. |
| JSD-002 | jsd2_195 | 19.5 | 34 | 19 | 42 | 3 | abs. | abs. | 2 | abs. | abs. | abs. |
| Average | 25.6 | 33.5 | 32.2 | 2.5 | 4.1 | 0 | 1.6 | 0 | 0.4 | 0.1 |
Table S-5 Bulk geochemical analyses for major and minor elements along with Sc and the rare earth elements.
| Core | Sample Name | Depth (m) | SiO2 (wt. %) | TiO2 (wt. %) | Al2O3 (wt. %) | Fe2O3 (wt. %) | Cr2O3 (wt. %) | MnO (wt. %) | NiO (wt. %) | MgO (wt. %) | CaO (wt. %) | BaO (wt. %) | Na2O (wt. %) | K2O (wt. %) | P2O5 (wt. %) | SO3 (wt. %) | L.O.I. (wt. %) | Total (wt. %) |
| JSD-001 | jsd1_115 | 11.5 | 3.41 | 2.25 | 17.69 | 59.69 | 0.40 | 0.15 | 0.04 | 0.38 | 0.19 | -0.01 | 0.09 | -0.01 | 0.06 | 0.12 | 14.45 | 98.90 |
| JSD-001 | jsd1_121 | 12.1 | 3.10 | 2.19 | 17.36 | 59.09 | 0.39 | 0.17 | 0.05 | 0.68 | 0.59 | 0.00 | 0.12 | -0.01 | 0.06 | 0.12 | 15.37 | 99.27 |
| JSD-001 | jsd1_125 | 12.5 | 4.60 | 1.83 | 17.03 | 57.42 | 0.33 | 0.21 | 0.06 | 0.40 | 0.12 | -0.01 | 0.10 | -0.01 | 0.03 | 0.09 | 15.42 | 97.63 |
| JSD-001 | jsd1_130 | 13.0 | 3.30 | 2.04 | 17.25 | 60.28 | 0.34 | 0.21 | 0.05 | 0.32 | 0.08 | -0.01 | 0.02 | -0.01 | 0.05 | 0.10 | 14.77 | 98.77 |
| JSD-001 | jsd1_135 | 13.5 | 3.51 | 1.85 | 17.21 | 59.38 | 0.34 | 0.23 | 0.07 | 0.41 | 0.07 | -0.02 | 0.06 | -0.01 | 0.03 | 0.10 | 15.57 | 98.81 |
| JSD-001 | jsd1_140 | 14.0 | 4.95 | 1.79 | 18.30 | 57.00 | 0.33 | 0.21 | 0.07 | 0.44 | 0.08 | 0.00 | 0.10 | -0.01 | 0.03 | 0.09 | 15.76 | 99.13 |
| JSD-001 | jsd1_144 | 14.4 | 6.20 | 1.46 | 19.97 | 45.10 | 0.27 | 0.16 | 0.05 | 0.52 | 0.20 | -0.02 | 0.04 | -0.01 | 0.03 | 0.07 | 17.21 | 91.26 |
| JSD-001 | jsd1_149 | 14.9 | 14.53 | 1.62 | 15.82 | 52.61 | 0.41 | 0.16 | 0.08 | 0.21 | 0.08 | 0.00 | 0.10 | -0.01 | 0.03 | 0.05 | 14.00 | 99.69 |
| JSD-001 | jsd1_154 | 15.4 | 8.35 | 1.84 | 17.00 | 55.01 | 0.35 | 0.22 | 0.07 | 0.46 | 0.09 | -0.01 | 0.09 | -0.01 | 0.04 | 0.07 | 15.60 | 99.16 |
| JSD-001 | jsd1_160 | 16.0 | 16.07 | 1.65 | 16.88 | 48.76 | 0.31 | 0.19 | 0.08 | 0.23 | 0.08 | 0.00 | 0.11 | -0.01 | 0.04 | 0.05 | 14.46 | 98.90 |
| JSD-001 | jsd1_164 | 16.4 | 17.88 | 1.70 | 17.45 | 47.51 | 0.32 | 0.17 | 0.07 | 0.28 | 0.10 | -0.01 | 0.18 | 0.00 | 0.02 | 0.05 | 13.24 | 98.97 |
| JSD-001 | jsd1_169 | 16.9 | 24.57 | 1.32 | 20.34 | 37.88 | 0.24 | 0.13 | 0.05 | 0.26 | 0.11 | 0.01 | 0.12 | 0.00 | 0.02 | 0.03 | 14.69 | 99.76 |
| JSD-001 | jsd1_174 | 17.4 | 21.89 | 1.19 | 17.71 | 44.87 | 0.23 | 0.20 | 0.04 | 0.27 | 0.12 | 0.00 | 0.14 | 0.00 | 0.02 | 0.04 | 11.94 | 98.65 |
| JSD-001 | jsd1_176 | 17.6 | 4.59 | 0.39 | 3.78 | 83.90 | 0.10 | 0.62 | 0.06 | 0.21 | 0.08 | 0.01 | 0.09 | -0.01 | 0.01 | 0.02 | 4.81 | 98.66 |
| JSD-001 | jsd1_180 | 18.0 | 17.98 | 1.17 | 14.95 | 52.25 | 0.15 | 0.47 | 0.05 | 0.25 | 0.15 | 0.00 | 0.11 | 0.00 | 0.04 | 0.02 | 11.17 | 98.76 |
| JSD-001 | jsd1_184 | 18.4 | 4.77 | 0.55 | 5.53 | 76.62 | 0.21 | 0.42 | 0.20 | 0.34 | 0.09 | 0.01 | 0.16 | -0.01 | 0.03 | 0.07 | 8.54 | 97.52 |
| JSD-002 | jsd2_083 | 8.3 | 17.37 | 1.53 | 15.42 | 48.03 | 0.20 | 0.84 | 0.06 | 0.96 | 1.35 | 0.01 | 0.06 | 0.00 | 0.02 | 0.00 | 13.41 | 99.26 |
| JSD-002 | jsd2_089 | 8.9 | 18.37 | 1.55 | 17.27 | 47.20 | 0.19 | 1.16 | 0.06 | 0.23 | 0.17 | 0.01 | 0.09 | 0.00 | 0.02 | 0.01 | 13.21 | 99.52 |
| JSD-002 | jsd2_096 | 9.6 | 24.98 | 1.08 | 22.60 | 33.13 | 0.15 | 1.62 | 0.10 | 0.21 | 0.12 | 0.04 | 0.07 | 0.00 | 0.02 | 0.00 | 14.92 | 99.04 |
| JSD-002 | jsd2_100 | 10.0 | 15.23 | 1.64 | 14.28 | 52.20 | 0.19 | 1.55 | 0.13 | 0.24 | 0.14 | 0.03 | 0.05 | 0.00 | 0.03 | 0.00 | 13.03 | 98.75 |
| JSD-002 | jsd2_107 | 10.7 | 17.79 | 1.59 | 15.35 | 50.32 | 0.16 | 0.75 | 0.06 | 0.28 | 0.24 | 0.01 | 0.06 | 0.00 | 0.03 | 0.00 | 12.70 | 99.32 |
| JSD-002 | jsd2_110 | 11.0 | 20.41 | 1.40 | 16.06 | 47.78 | 0.11 | 0.61 | 0.04 | 0.33 | 0.51 | 0.00 | 0.06 | 0.00 | 0.03 | -0.01 | 12.02 | 99.34 |
| JSD-002 | jsd2_117 | 11.7 | 20.08 | 1.27 | 16.50 | 47.49 | 0.20 | 0.54 | 0.06 | 0.29 | 0.32 | 0.01 | 0.06 | 0.00 | 0.03 | 0.00 | 12.83 | 99.68 |
| JSD-002 | jsd2_120 | 12.0 | 12.68 | 0.85 | 10.25 | 61.98 | 0.16 | 0.57 | 0.06 | 0.83 | 1.15 | 0.04 | 0.05 | 0.00 | 0.03 | 0.00 | 10.70 | 99.34 |
| JSD-002 | jsd2_125 | 12.5 | 16.87 | 1.51 | 13.47 | 54.18 | 0.44 | 0.43 | 0.06 | 0.23 | 0.22 | 0.02 | 0.05 | 0.00 | 0.03 | -0.01 | 11.93 | 99.43 |
| JSD-002 | jsd2_130 | 13.0 | 17.20 | 1.37 | 13.46 | 52.70 | 0.50 | 1.05 | 0.07 | 0.20 | 0.20 | 0.01 | 0.06 | 0.00 | 0.02 | -0.01 | 12.30 | 99.12 |
| JSD-002 | jsd2_140 | 14.0 | 17.92 | 1.45 | 14.17 | 52.17 | 0.19 | 0.35 | 0.04 | 0.35 | 0.56 | 0.01 | 0.10 | 0.00 | 0.06 | -0.01 | 12.43 | 99.79 |
| JSD-002 | jsd2_145 | 14.5 | 17.49 | 1.47 | 12.99 | 48.27 | 0.29 | 0.42 | 0.05 | 0.16 | 0.19 | 0.01 | 0.02 | 0.00 | 0.02 | -0.01 | 11.61 | 92.98 |
| JSD-002 | jsd2_150 | 15.0 | 17.12 | 1.44 | 13.31 | 51.26 | 0.35 | 0.98 | 0.11 | 0.40 | 0.41 | 0.05 | 0.04 | 0.00 | 0.01 | -0.01 | 12.58 | 98.05 |
| JSD-002 | jsd2_160 | 16.0 | 18.84 | 1.48 | 14.41 | 50.82 | 0.16 | 0.41 | 0.05 | 0.33 | 0.48 | 0.02 | 0.03 | -0.01 | 0.04 | -0.01 | 11.59 | 98.65 |
| JSD-002 | jsd2_165 | 16.5 | 17.84 | 1.48 | 13.61 | 52.30 | 0.20 | 0.42 | 0.10 | 0.28 | 0.19 | 0.00 | 0.07 | -0.01 | 0.05 | -0.01 | 12.65 | 99.16 |
| JSD-002 | jsd2_170 | 17.0 | 17.28 | 1.26 | 12.75 | 45.81 | 0.12 | 0.41 | 0.08 | 0.24 | 0.20 | 0.01 | 0.04 | 0.00 | 0.03 | -0.01 | 12.89 | 91.11 |
| JSD-002 | jsd2_175 | 17.5 | 16.47 | 1.58 | 11.62 | 53.52 | 0.16 | 2.24 | 0.10 | 0.22 | 0.28 | 0.02 | 0.02 | 0.00 | 0.05 | -0.01 | 12.23 | 98.50 |
| JSD-002 | jsd2_180 | 18.0 | 18.28 | 1.56 | 13.07 | 50.49 | 0.61 | 0.06 | 0.07 | 0.27 | 0.87 | 0.00 | 0.02 | 0.00 | 0.05 | -0.01 | 13.04 | 98.35 |
| JSD-002 | jsd2_185 | 18.5 | 16.61 | 1.60 | 13.22 | 52.40 | 0.43 | 0.14 | 0.09 | 0.29 | 0.21 | 0.00 | 0.05 | 0.00 | 0.04 | -0.01 | 13.28 | 98.34 |
| JSD-002 | jsd2_190 | 19.0 | 15.53 | 1.49 | 12.35 | 54.40 | 0.57 | 0.12 | 0.12 | 0.39 | 0.17 | 0.02 | 0.08 | -0.01 | 0.03 | -0.01 | 13.13 | 98.36 |
| JSD-002 | jsd2_195 | 19.5 | 20.91 | 1.48 | 13.35 | 48.85 | 0.10 | 0.64 | 0.08 | 0.34 | 0.26 | -0.01 | 0.09 | 0.00 | 0.02 | -0.01 | 12.59 | 98.69 |
| Error (wt. %) | 0.10 | 0.01 | 0.02 | 0.11 | 0.05 | 0.00 | 0.05 | 0.01 | 0.02 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.36 | |||
| Saprolite (average) | 49.99 | 0.27 | 2.32 | 8.31 | 0.26 | 0.17 | 0.05 | 14.66 | 19.45 | -0.01 | 0.21 | -0.01 | 0.00 | -0.01 | 4.64 | 100.31 | ||
| Core | Sample Name | Depth (m) | La (ppm) | Ce (ppm) | Pr (ppm) | Nd (ppm) | Sm (ppm) | Eu (ppm) | Gd (ppm) | Tb (ppm) | Dy (ppm) | Ho (ppm) | Er (ppm) | Yb (ppm) | Lu (ppm) | Y (ppm) | Sc (ppm) | |
| JSD-001 | jsd1_115 | 11.5 | 11.3 | 50.9 | 4.2 | 19.3 | 6.2 | 1.7 | 5.6 | 0.9 | 5.3 | 1.0 | 2.9 | 3.2 | 0.5 | 23.9 | 526.7 | |
| JSD-001 | jsd1_121 | 12.1 | 6.0 | 47.7 | 2.2 | 10.5 | 3.5 | 1.0 | 3.2 | 0.5 | 3.1 | 0.6 | 1.7 | 1.9 | 0.3 | 11.6 | 461.2 | |
| JSD-001 | jsd1_125 | 12.5 | 12.0 | 152.7 | 5.4 | 24.5 | 8.7 | 2.4 | 7.2 | 1.2 | 7.2 | 1.4 | 3.9 | 4.7 | 0.7 | 20.1 | 542.6 | |
| JSD-001 | jsd1_130 | 13.0 | 11.3 | 234.9 | 4.7 | 21.3 | 7.1 | 2.0 | 6.3 | 1.1 | 6.1 | 1.2 | 3.4 | 3.8 | 0.6 | 21.0 | 533.8 | |
| JSD-001 | jsd1_135 | 13.5 | 8.1 | 183.4 | 4.1 | 18.6 | 6.9 | 1.9 | 5.7 | 1.0 | 5.8 | 1.1 | 3.1 | 3.9 | 0.6 | 12.9 | 539.8 | |
| JSD-001 | jsd1_140 | 14.0 | 6.8 | 66.2 | 3.1 | 14.0 | 5.2 | 1.4 | 4.1 | 0.7 | 4.2 | 0.8 | 2.2 | 2.7 | 0.4 | 11.4 | 495.8 | |
| JSD-001 | jsd1_144 | 14.4 | 9.5 | 77.5 | 4.5 | 20.4 | 7.6 | 2.0 | 6.1 | 1.1 | 6.3 | 1.2 | 3.4 | 4.3 | 0.6 | 17.4 | 547.9 | |
| JSD-001 | jsd1_149 | 14.9 | 25.3 | 56.2 | 10.8 | 48.9 | 15.1 | 3.9 | 11.6 | 1.8 | 9.7 | 1.8 | 5.0 | 4.8 | 0.7 | 24.8 | 375.2 | |
| JSD-001 | jsd1_154 | 15.4 | 17.0 | 62.6 | 5.9 | 28.5 | 9.5 | 2.6 | 8.4 | 1.3 | 7.2 | 1.4 | 3.9 | 3.7 | 0.5 | 31.0 | 573.8 | |
| JSD-001 | jsd1_160 | 16.0 | 26.1 | 124.8 | 11.3 | 53.8 | 16.8 | 4.6 | 13.7 | 2.2 | 11.9 | 2.2 | 6.0 | 6.2 | 0.9 | 32.9 | 635.9 | |
| JSD-001 | jsd1_164 | 16.4 | 27.9 | 138.9 | 12.0 | 54.9 | 15.7 | 4.4 | 13.9 | 2.2 | 12.1 | 2.2 | 6.0 | 6.0 | 0.9 | 32.7 | 654.8 | |
| JSD-001 | jsd1_169 | 16.9 | 29.5 | 234.2 | 11.7 | 52.7 | 12.1 | 3.3 | 10.6 | 1.6 | 8.1 | 1.5 | 3.9 | 3.4 | 0.5 | 24.8 | 465.5 | |
| JSD-001 | jsd1_174 | 17.4 | 22.9 | 116.9 | 8.7 | 38.5 | 8.9 | 2.6 | 9.2 | 1.4 | 7.5 | 1.4 | 3.8 | 3.3 | 0.5 | 25.8 | 358.3 | |
| JSD-001 | jsd1_176 | 17.6 | 33.9 | 138.8 | 10.4 | 45.3 | 10.5 | 3.1 | 11.1 | 1.6 | 8.6 | 1.7 | 4.5 | 3.8 | 0.6 | 30.5 | 251.6 | |
| JSD-001 | jsd1_180 | 18.0 | 25.1 | 71.5 | 8.6 | 36.6 | 8.6 | 2.5 | 8.9 | 1.3 | 7.1 | 1.3 | 3.5 | 3.0 | 0.4 | 23.0 | 360.5 | |
| JSD-001 | jsd1_184 | 18.4 | 28.5 | 38.3 | 7.7 | 34.9 | 8.7 | 2.7 | 12.2 | 1.7 | 9.8 | 2.1 | 5.7 | 4.2 | 0.7 | 47.0 | 584.7 | |
| JSD-002 | jsd2_083 | 8.3 | 24.6 | 77.1 | 7.1 | 31.6 | 6.7 | 1.9 | 7.5 | 1.0 | 5.2 | 1.1 | 2.8 | 1.9 | 0.3 | 32.0 | 507.7 | |
| JSD-002 | jsd2_089 | 8.9 | 23.4 | 155.4 | 6.1 | 27.1 | 5.5 | 1.6 | 6.1 | 0.8 | 3.9 | 0.8 | 1.9 | 1.2 | 0.2 | 17.9 | 296.3 | |
| JSD-002 | jsd2_096 | 9.6 | 18.2 | 320.2 | 4.8 | 21.5 | 4.8 | 1.4 | 5.6 | 0.8 | 3.9 | 0.8 | 2.0 | 1.3 | 0.2 | 16.8 | 281.8 | |
| JSD-002 | jsd2_100 | 10.0 | 13.0 | 61.0 | 4.1 | 19.4 | 4.9 | 1.5 | 5.8 | 0.8 | 4.6 | 0.9 | 2.5 | 1.9 | 0.3 | 28.4 | 531.3 | |
| JSD-002 | jsd2_107 | 10.7 | 7.5 | 39.6 | 2.4 | 11.0 | 2.8 | 0.8 | 3.4 | 0.5 | 2.7 | 0.6 | 1.4 | 1.1 | 0.2 | 19.6 | 513.7 | |
| JSD-002 | jsd2_110 | 11.0 | 9.8 | 59.2 | 3.2 | 14.3 | 3.5 | 1.0 | 3.6 | 0.5 | 2.8 | 0.5 | 1.4 | 1.2 | 0.2 | 15.7 | 331.1 | |
| JSD-002 | jsd2_117 | 11.7 | 12.4 | 90.1 | 4.0 | 18.4 | 4.6 | 1.3 | 4.8 | 0.7 | 3.9 | 0.7 | 2.0 | 1.7 | 0.2 | 15.9 | 468.0 | |
| JSD-002 | jsd2_120 | 12.0 | 10.6 | 66.5 | 3.9 | 18.6 | 5.6 | 1.7 | 6.1 | 0.9 | 5.3 | 1.1 | 2.9 | 2.8 | 0.4 | 27.7 | 582.4 | |
| JSD-002 | jsd2_125 | 12.5 | 5.2 | 21.0 | 2.0 | 9.5 | 2.7 | 0.8 | 2.8 | 0.4 | 2.4 | 0.5 | 1.3 | 1.0 | 0.2 | 15.6 | 749.6 | |
| JSD-002 | jsd2_130 | 13.0 | 8.8 | 71.2 | 2.8 | 12.5 | 3.1 | 0.9 | 3.0 | 0.4 | 2.1 | 0.4 | 1.0 | 0.8 | 0.1 | 9.6 | 290.0 | |
| JSD-002 | jsd2_140 | 14.0 | 5.9 | 29.0 | 2.4 | 11.3 | 3.3 | 1.0 | 3.5 | 0.5 | 3.1 | 0.6 | 1.7 | 1.5 | 0.2 | 17.4 | 574.9 | |
| JSD-002 | jsd2_145 | 14.5 | 4.2 | 18.8 | 1.6 | 7.9 | 2.2 | 0.6 | 2.2 | 0.3 | 1.7 | 0.3 | 0.9 | 0.7 | 0.1 | 8.6 | 331.5 | |
| JSD-002 | jsd2_150 | 15.0 | 8.9 | 55.9 | 3.0 | 13.5 | 3.5 | 1.1 | 3.3 | 0.5 | 2.6 | 0.5 | 1.3 | 1.0 | 0.1 | 12.3 | 600.3 | |
| JSD-002 | jsd2_160 | 16.0 | 5.1 | 11.9 | 2.3 | 10.6 | 3.2 | 0.9 | 2.7 | 0.4 | 2.4 | 0.4 | 1.2 | 1.3 | 0.2 | 7.9 | 375.2 | |
| JSD-002 | jsd2_165 | 16.5 | 8.4 | 15.6 | 3.6 | 18.3 | 6.0 | 1.8 | 6.1 | 1.0 | 6.0 | 1.2 | 3.3 | 3.7 | 0.6 | 16.2 | 538.1 | |
| JSD-002 | jsd2_170 | 17.0 | 11.6 | 18.6 | 4.3 | 20.6 | 6.5 | 2.0 | 6.7 | 1.1 | 6.5 | 1.3 | 3.7 | 4.3 | 0.7 | 22.0 | 593.0 | |
| JSD-002 | jsd2_175 | 17.5 | 20.9 | 61.5 | 6.4 | 29.8 | 8.1 | 2.3 | 7.5 | 1.1 | 6.2 | 1.2 | 3.2 | 3.0 | 0.4 | 21.9 | 342.0 | |
| JSD-002 | jsd2_180 | 18.0 | 12.3 | 3.8 | 5.1 | 24.8 | 7.5 | 2.2 | 7.6 | 1.2 | 6.9 | 1.3 | 3.7 | 3.7 | 0.6 | 24.8 | 669.3 | |
| JSD-002 | jsd2_185 | 18.5 | 6.0 | 5.6 | 3.3 | 16.9 | 5.4 | 1.6 | 5.3 | 0.9 | 5.0 | 0.9 | 2.5 | 2.4 | 0.4 | 14.6 | 492.4 | |
| JSD-002 | jsd2_190 | 19.0 | 8.3 | 5.4 | 4.4 | 22.9 | 7.3 | 2.3 | 7.7 | 1.2 | 7.2 | 1.4 | 3.7 | 3.3 | 0.5 | 23.2 | 636.3 | |
| JSD-002 | jsd2_195 | 19.5 | 21.8 | 20.2 | 9.0 | 44.9 | 11.6 | 3.3 | 10.7 | 1.5 | 8.2 | 1.6 | 4.0 | 3.2 | 0.4 | 28.4 | 260.9 | |
| Error (ppm) | 0.3 | 1.7 | 0.2 | 0.5 | 0.2 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.5 | 8.0 | |||
| Saprolite (average) | 5.7 | 5.9 | 2.2 | 10.6 | 2.7 | 0.8 | 2.8 | 0.4 | 2.1 | 0.4 | 1.1 | 0.8 | 0.1 | 11.9 | 99.4 | |||
| Enrichment factor (JSD-001) | 2.6 | 13.7 | 2.5 | 2.4 | 2.6 | 2.6 | 2.4 | 2.6 | 2.7 | 2.7 | 2.8 | 3.4 | 3.6 | 1.8 | 4.9 |

Figure S-2 Enrichment factor of rare earth elements in the lateritic deposit versus ionic radius in octahedral coordination. Values are after Shannon (1976).
Table S-6 Median, mean and standard deviation of Si, Ti, Fe, Al, analytical total and Sc concentrations in the different grain types distinguished by SEM imaging, EMP and cluster analysis.
| Grain type | Num. Obs. | SiO2 (wt. %) | TiO2 (wt. %) | Al2O3 (wt. %) | Fe2O3 (wt. %) | Sc (ppm) | Total (wt. %) | ||||||||||||
| Median | Mean | Std. Dev. | Median | Mean | Std. Dev. | Median | Mean | Std. Dev. | Median | Mean | Std. Dev. | Median | Mean | Std. Dev. | Median | Mean | Std. Dev. | ||
| Fe-rich oxyhydroxide | 43 | 4.30 | 4.50 | 1.06 | 1.72 | 1.76 | 0.75 | 6.27 | 6.58 | 2.16 | 70.56 | 68.10 | 8.84 | 1190 | 1339 | 351 | 85.49 | 83.01 | 9.01 |
| Fe-rich oxide | 16 | 0.07 | 0.25 | 0.67 | 1.24 | 1.28 | 1.02 | 0.31 | 0.48 | 0.51 | 95.61 | 95.93 | 3.25 | 201 | 195 | 85 | 100.34 | 100.45 | 2.41 |
| Ti-rich oxide | 3 | 0.05 | 0.04 | 0.02 | 57.38 | 62.63 | 11.50 | -1.23 | -1.27 | 0.12 | 41.75 | 37.22 | 9.77 | 102 | 92 | 50 | 104.96 | 104.16 | 2.10 |
| Al-rich oxyhydroxide | 7 | 3.67 | 5.50 | 5.16 | 1.05 | 1.02 | 0.70 | 52.30 | 48.28 | 8.26 | 19.65 | 15.84 | 10.72 | 336 | 271 | 161 | 68.49 | 71.64 | 12.38 |
| Quartz | 4 | 99.13 | 98.58 | 2.80 | 0.02 | 0.02 | 0.00 | 0.04 | 0.04 | 0.04 | 0.39 | 0.37 | 0.06 | -2 | 0 | 10 | 99.61 | 99.08 | 2.75 |
| High-Sc matrix | 31 | 4.43 | 5.02 | 3.19 | 1.88 | 2.23 | 1.40 | 7.13 | 9.05 | 7.39 | 61.52 | 65.28 | 12.44 | 640 | 658 | 107 | 85.61 | 83.45 | 8.81 |
| Kaolinite-rich matrix | 9 | 38.91 | 36.30 | 9.97 | 0.08 | 0.32 | 0.42 | 31.14 | 30.26 | 7.67 | 3.77 | 12.33 | 14.64 | 364 | 408 | 139 | 82.41 | 80.01 | 6.45 |
| Low-Sc matrix | 12 | 2.73 | 5.54 | 6.02 | 1.79 | 1.91 | 0.60 | 6.42 | 7.30 | 4.31 | 76.05 | 70.53 | 13.62 | 391 | 382 | 83 | 90.48 | 86.59 | 8.93 |
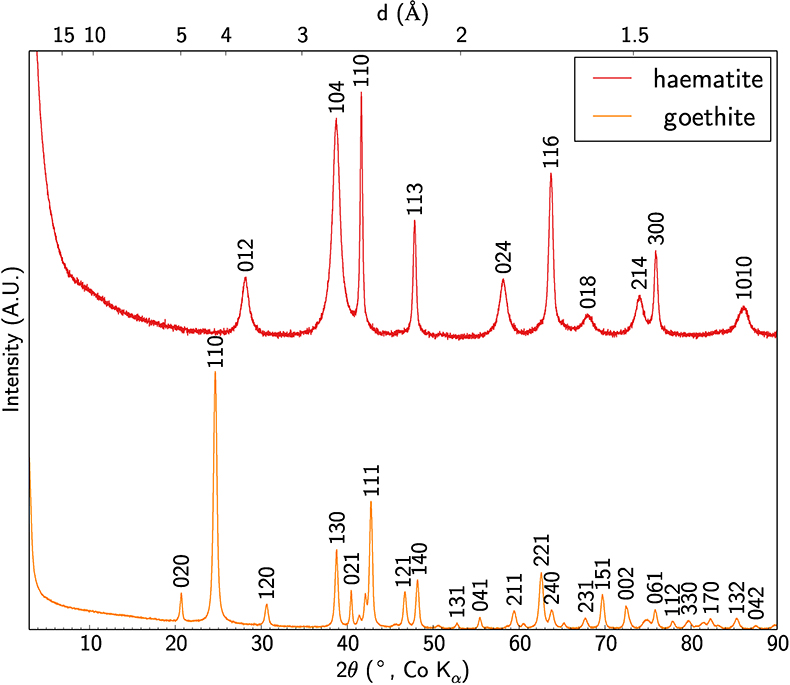
Figure S-3 XRD patterns of synthetic Sc-bearing goethite and haematite.