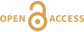Determining subduction-zone fluid composition using a tourmaline mineral probe
Affiliations | Corresponding Author | Cite as | Funding informationKeywords: subduction zone, slab fluid release, element cycling, tourmaline, Tauern Eclogite Zone, arc-signature
- Share this article





Article views:11,291Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
Abstract

Figures and Tables
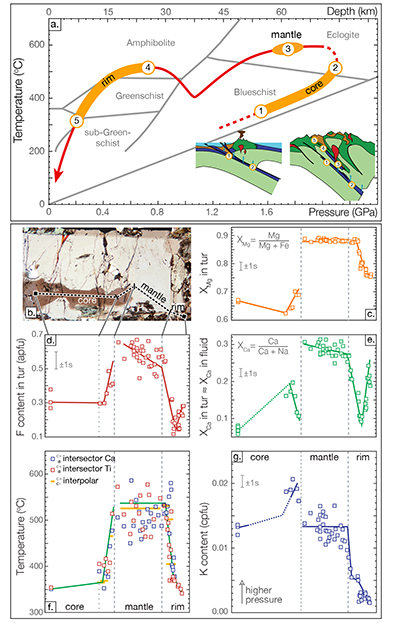 Figure 1 Reading the Tauern tourmaline record. (a) Tauern Eclogite Zone P–T path (Zimmermann et al., 1994) with conditions recorded by tourmaline growth zones shown. Inset cartoons locate these in a schematic subduction-zone cross-section. (b) PPL image of the tourmaline transect. (c) XMg sharply defines the tourmaline growth zones. (d) F-content in tourmaline, >0.5 apfu F in the outer core and mantle. (e) XCa of tourmaline, which tracks the XCa in the coexisting fluid (von Goerne et al., 2011). (f) Temperature transect across the tourmaline grain calculated from inter-sector and inter-polar thermometry. (g) Tourmaline K-content, which acts as a qualitative barometer. Uncertainties shown are the typical 1s analytical precision. | 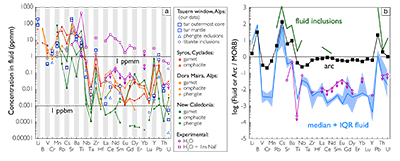 Figure 2 (a) Element concentrations in fluids reconstructed from the Tauern tourmaline outer core and mantle and its mineral inclusions (blue symbols), and eclogite minerals from other subduction-zone terrains show a consistent pattern. (b) MORB-normalised concentrations for average arc magma parallel those of subduction-zone fluids supporting a genetic link. See the Supplementary Information for data sources and calculation method. Uncertainties are given as the Interquartile Range (IQR) and maximum estimates marked with an arrow. | 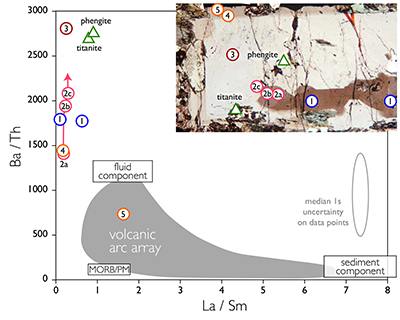 Figure 3 Fluid compositions reconstructed from tourmaline (circles) and its mineral inclusions (triangles), show a consistent, high Ba/Th at low La/Sm signature. Addition of this fluid to the arc-magmatism source region can explain the trend to elevated Ba/Th compared to mid-ocean ridge basalt (MORB) and primitive mantle (PM). |
| Figure 1 | Figure 2 | Figure 3 |
Supplementary Figures and Tables
 Table S-1 WDS electron-microprobe data for tourmaline growth zones, mineral inclusions in tourmaline, and matrix minerals. The values shown are the average for multiple analyses and their associated 1 standard deviation spread, except for omphacite where only one analysis was available. Normalisation factors used are the sum of cations for omphacite, titanite, plagioclase, amphibole and calcite, or the oxygen sum for phengite and biotite. Tourmaline was normalised to the sum of the elements residing at the Y, Z and T sites = 15, or Si = 6 cpfu, if the former resulted in Si > 6 cpfu. |  Table S-2 Laser-ablation ICP-MS data for Ba, Th, La and Sm in tourmaline, and phengite and titanite inclusions. Most data represent the mean of multiple measurements. Uncertainties are the 1 standard deviation on the mean, or the count statistical error if only one measurement is available. Partition coefficients are those of Green and Adam (2003), combined with Dphg/tur from Klemme et al. (2011) and are used to calculate the Ba/Th and La/Sm ratios for the fluid. Analysis locations are shown in Figure 1 |  Table S-3 Laser-ablation ICP-MS trace element data for tourmaline, and phengite and titanite inclusions. The tourmaline data represent the mean of multiple measurements and the uncertainties reported are 1 standard deviation on this mean. For phengite and titanite, we report the count statistical error. Measurements were corrected for differences in ablation behaviour between standards and samples by normalising to EMP Si-content. | 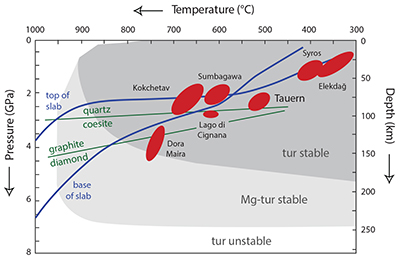 Figure S-1 Tourmaline is stable for most of the P–T range typically found in subduction zones, and is an accessory phase in exposed subduction-zone lithologies for all the terrains shown. Typical subduction path (blue solid lines) from Syracuse et al. (2010) and P–T estimates for subduction-zone terrains from Zimmermann et al. (1994) and Marschall et al. (2009). Tourmaline stability fields from van Hinsberg et al. (2011b). | 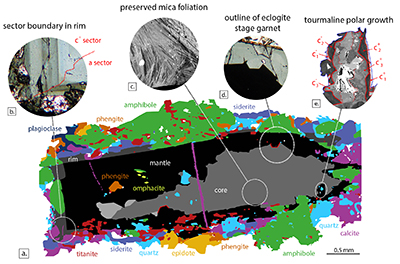 Figure S-2 Petrography of the Tauern tourmaline grain used in this study. (a) Mineral-map showing its inclusion and matrix mineralogy, and the core, mantle and rim zones of the tourmaline grain. This map is based on back-scattered electron imaging and WDS-EMP element mapping for Ca, Mg, Ti, Fe, Si and Na. (b) Example of hourglass sector zoning in the outermost rim in PPL microscopy. (c) Back-scattered electron image of compositional heterogeneity in the tourmaline core, outlining the foliation of protolith mica grains overgrown by tourmaline. (d) Outline of an idiomorphic eclogite-stage garnet grain replaced during growth of the mantle zone in PPL microscopy. (e) Back-scattered electron image of c+-c- polar tourmaline growth. |  Table S-4 Partition coefficients used in this study. Tourmaline–fluid and titanite–fluid coefficients were calculated by combining the phengite–tourmaline and phengite–titanite inter-mineral partition coefficients for Syros sample SY309B (Klemme et al., 2011; Marschall, 2005) with phengite–fluid experimental partition coefficients from Green and Adam (2003). | 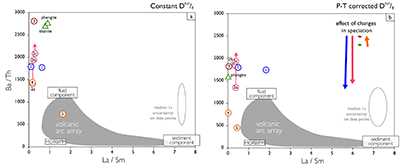 Figure S-3 Reconstructed Ba/Th vs. La/Sm ratios for subduction-zone fluids in the Tauern eclogite zone, as determined from tourmaline compositions (see Fig. 2 for locations of these points). (a) Calculated assuming constant Dtur/fl, obtained by combining experimentally determined Dphg/fl of Green and Adam (2003) with Dphg/tur of Klemme et al. (2011). (b) Calculated from Dphg/fl of Green and Adam (2003) extrapolated to relevant P–T conditions using phengite lattice systematics at constant Dphg/tur (from Klemme et al. 2011). The impact of changing speciation on Dtur/fl is shown by the thick arrows. Reconstructing fluid compositions with extrapolated D values results in lower Ba/Th in the fluid, with only minimal impact on La/Sm. The differences in Ba/Th between core and mantle become smaller, but the predicted effect of changes in speciation would re-introduce this difference. The gray uncertainty ellipses show the 1 standard deviation uncertainty in the ratios resulting from the spread in the trace element analyses of the minerals. |
| Table S-1 | Table S-2 | Table S-3 | Figure S-1 | Figure S-2 | Table S-4 | Figure S-3 |
top
Introduction
The principal material fluxes from the surface into the deep Earth take place at subduction zones. Models of terrestrial element cycling hinge on accurate estimates of these fluxes and of the recycling of material by fluids and melts from the dehydrating slab in the “subduction factory” (Tatsumi, 2005
Tatsumi, Y. (2005) The subduction factory: How it operates in the evolving Earth. GSA today 15, 4–10.
). Estimates of element release from the slab, and hence constraints on the net flux through subduction zones, are derived from three main sources; (i) the compositional signatures of arc magmas (e.g., Elliott et al., 1997Elliott, T., Plank, T., Zindler, A., White, W. Bourdon, B. (1997) Element transport from slab to volcanic front at the Mariana arc. Journal of Geophysical Research-Solid Earth 102, 14991–15019.
), which are Nb-Ta depleted and enriched in Li, B, Sr, Ba and Pb compared to mid-ocean ridge basalts (MORB); (ii) experimentally-determined element partitioning between subduction-zone solids and liquids (e.g., Keppler, 1996Keppler, H. (1996) Constraints from partitioning experiments on the composition of subduction-zone fluids. Nature 380, 237–240.
; Kessel et al., 2005Kessel, R., Schmidt, M.W., Ulmer, P., Pettke, T. (2005) Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120-180 km depth. Nature 437, 724–727.
; Hermann et al., 2006Hermann, J., Spandler, C., Hack, A., Korsakov, A. (2006) Aqueous fluids and hydrous melts in high-pressure and ultra-high pressure rocks: Implications for element transfer in subduction zones. Lithos 92, 399–417.
; Spandler et al., 2007Spandler, C., Mavrogenes, J., Hermann, J. (2007) Experimental constraints on element mobility from subducted sediments using high-P synthetic fluid/melt inclusions. Chemical Geology 239, 228–249.
; Tsay et al., 2017Tsay, A., Zajacz, Z., Ulmer, P., Sanchez-Valle, C. (2017) Mobility of major and trace elements in the eclogite-fluid system and element fluxes upon slab dehydration. Geochimica et Cosmochimica Acta 198, 70–91.
); and (iii) element mass-balance on subducted rock lithologies (e.g., Bebout et al., 1999Bebout, G., Ryan, J., Leeman, W. Bebout, A. (1999) Fractionation of trace elements by subduction-zone metamorphism—effect of convergent-margin thermal evolution. Earth and Planetary Science Letters 171, 63–81.
; Spandler et al., 2003Spandler, C., Hermann, J., Arculus, R., Mavrogenes, J. (2003) Redistribution of trace elements during prograde metamorphism from lawsonite blueschist to eclogite facies; implications for deep subduction-zone processes. Contributions to Mineralogy and Petrology 146, 205–222.
, 2004Spandler, C., Hermann, J., Arculus, R., Mavrogenes, J. (2004) Geochemical heterogeneity and element mobility in deeply subducted oceanic crust; insights from high-pressure mafic rocks from New Caledonia. Chemical Geology 206, 21–42.
). Methods (i) and (iii) provide information on mass transfer from subducting lithologies into the region of melt generation, but no detail on element concentrations in fluids and melt, or total fluxes. Moreover, (iii) depends on the presence of accessory phases (see Klimm et al., 2008Klimm, K., Blundy, J.D., Green, T.H. (2008) Trace element partitioning and accessory phase saturation during H2O-saturated melting of basalt with implications for subduction zone chemical fluxes. Journal of Petrology 49, 523–553.
; Hermann and Rubatto, 2009Hermann, J. Rubatto, D. (2009) Accessory phase control on the trace element signature of sediment melts in subduction zones. Chemical Geology 265, 512–526.
), as these phases can be the dominant repository of certain trace elements in the bulk rock. This fact makes the method critically dependent on preservation of these phases, as well as their representative sampling in bulk-rock studies. Method (ii) provides concentration information, and flux estimates when combined with models of release of volatiles (e.g., Spandler et al., 2003Spandler, C., Hermann, J., Arculus, R., Mavrogenes, J. (2003) Redistribution of trace elements during prograde metamorphism from lawsonite blueschist to eclogite facies; implications for deep subduction-zone processes. Contributions to Mineralogy and Petrology 146, 205–222.
; Connolly, 2005Connolly, J.A.D. (2005) Computation of phase equilibria by linear programming: A tool for geodynamic modeling and its application to subduction zone decarbonation. Earth and Planetary Science Letters 236, 524–541.
), but only under model-dependent conditions of temperature, pressure, mineralogy and bulk composition. What is needed is a more direct record of subduction-zone fluid composition.Here, well-preserved subduction-zone minerals (tourmaline, titanite and phengite) are used to quantitatively reconstruct the compositions of the fluids from which they grew by combining mineral compositions with mineral–fluid element partition coefficients (cf. Keppler, 1996
Keppler, H. (1996) Constraints from partitioning experiments on the composition of subduction-zone fluids. Nature 380, 237–240.
). If D-values are known for the physico-chemical conditions of growth, absolute element concentrations in the fluid result. This approach avoids any assumption of the presence of accessory phases, but rather allows for their presence to be tested. Furthermore, minerals can simultaneously yield P–T conditions and age of growth that can be combined directly with reconstructed fluid compositions into a comprehensive and internally consistent database of the P–T–X–t evolution of a subduction zone.top
Tourmaline as a Mineral Probe
A tourmaline grain from the Tauern Window Eclogite Zone (Austrian Alps) is the principal probe of fluid compositions in this study. The mineral tourmaline has exceptional P–T stability that covers subduction-zone conditions (Fig. S-1); its crystal-chemistry allows it to incorporate a diversity of (trace) elements; and it displays negligible volume-diffusional re-equilibration (e.g., Henry and Dutrow, 1996
Henry D.J., Dutrow, B.L. (1996) Metamorphic tourmaline and its petrologic applications. In: Grew E.S., Anovitz L.M. (Eds.) Boron: Mineralogy, Petrology and Geochemistry. Reviews in Mineralogy 33, 503–557.
; van Hinsberg et al., 2011avan Hinsberg, V.J., Henry, D.J., Dutrow, B.L. (2011a) Tourmaline as a Petrologic Forensic Mineral: A Unique Recorder of Its Geologic Past. Elements 7, 327–332.
,bvan Hinsberg, V.J., Henry, D.J., Marschall, H.R. (2011b) Tourmaline: an Ideal Indicator of Its Host Environment. Canadian Mineralogist 49, 1–16.
). These features allow tourmaline to record its host environment composition throughout subduction and to preserve it for later interrogation. Tourmaline’s presence is dictated by the availability of boron, rather than by P–T conditions. At low grades, B dominantly resides in sheet silicates (Leeman and Sisson, 1996Leeman, W.P., Sisson, V.B. (1996) Geochemistry of boron and its implications for crustal and mantle processes. Reviews in Mineralogy and Geochemistry 33, 645–707.
) and is released during prograde metamorphism in discontinuous reactions resulting in punctuated nucleation and growth, and in continuous reactions producing gradual growth. Owing to negligible diffusive re-equilibration, this growth is expressed as growth zones.The Tauern tourmaline grain occurs in a meta-sedimentary retrogressed eclogite that preserves relics of a garnet–omphacite–phengite–titanite eclogite paragenesis, overprinted by an amphibolite-facies amphibole–plagioclase–epidote–carbonate paragenesis (Fig. S-2a). Peak conditions for this unit have been estimated at 630 ˚C and 2.5 GPa (e.g., Selverstone et al., 1992
Selverstone, J., Franz, G., Thomas, S., Getty, S. (1992) Fluid variability in 2 GPa eclogites as an indicator of fluid behavior during subduction. Contributions to Mineralogy and Petrology 112, 341–357.
; Hoschek, 2007Hoschek, G. (2007) Metamorphic peak conditions of eclogites in the Tauern Window, Eastern Alps, Austria: Thermobarometry of the assemblage garnet + omphacite + phengite + kyanite + quartz. Lithos 93, 1–16.
). There is no evidence for partial melting and tourmaline is interpreted to have formed from aqueous fluid throughout its growth history. Tourmaline is a minor accessory phase in these rocks, and is therefore unable to exert control on element concentrations in the fluid. Rather, it acts as a passive recorder of its environment.Detailed petrographic examination reveals a zoned brown core, a blue-green mantle, and a strongly zoned outer rim (see the Supplementary Information for more details). Hourglass sector-zoning is present (Fig. S-2b), indicating that growth compositions have been preserved (van Hinsberg et al., 2006
van Hinsberg, V.J., Schumacher, J.C., Kearns, S., Mason, P.R.D., Franz, G. (2006) Hourglass sector zoning in metamorphic tourmaline and resultant major and trace-element fractionation. American Mineralogist 91, 717–728.
). Inter-sector thermometry (van Hinsberg and Schumacher, 2007van Hinsberg, V.J., Schumacher, J.C. (2007) Intersector element partitioning in tourmaline: a potentially powerful single crystal thermometer. Contributions to Mineralogy and Petrology 153, 289–301.
), combined with qualitative K-barometry and inclusion mineralogy suggests growth of the tourmaline core during prograde subduction and its associated progressive internal B-release, formation of the mantle zone following detachment from the subducting slab from external fluids that were released in deeper slab devolatilisation, and rim growth during retrogression that records the orogenic uplift of the Eclogite Zone (Fig. 1a). This single tourmaline grain thus chronicles the subduction to uplift history, and can provide information on the associated fluids at multiple stages along this path.
Figure 1 Reading the Tauern tourmaline record. (a) Tauern Eclogite Zone P–T path (Zimmermann et al., 1994
Zimmermann, R., Hammerschmidt, K., Franz, G. (1994) Eocene high pressure metamorphism in the Penninic units of the Tauern Window (Eastern Alps): evidence from 40Ar-39Ar dating and petrological investigations. Contributions to Mineralogy and Petrology 117, 175–186.
) with conditions recorded by tourmaline growth zones shown. Inset cartoons locate these in a schematic subduction-zone cross-section. (b) PPL image of the tourmaline transect. (c) XMg sharply defines the tourmaline growth zones. (d) F-content in tourmaline, >0.5 apfu F in the outer core and mantle. (e) XCa of tourmaline, which tracks the XCa in the coexisting fluid (von Goerne et al., 2011von Goerne, G., Franz, G. van Hinsberg, V.J. (2011) Experimental determination of Na–Ca distribution between tourmaline and fluid in the system CaO–Na2O–MgO–Al2O3–SiO2–B2O3–H2O. Canadian Mineralogist 49, 137–152.
). (f) Temperature transect across the tourmaline grain calculated from inter-sector and inter-polar thermometry. (g) Tourmaline K-content, which acts as a qualitative barometer. Uncertainties shown are the typical 1s analytical precision.top
Tourmaline Composition and Fluid Reconstruction
Compositions of individual tourmaline growth zones and mineral inclusions were determined by EMP (major elements) and LA-ICP-MS (trace elements). Tourmaline compositions are dominated by the dravite end-member with lesser schorl, uvite and foitite, have variable XCa and XMg, and high F contents in core and mantle (Fig. 1, Table S-1). Tourmaline and mineral inclusion compositions constrain growth to be from an acidic aqueous solution with Na concentrations from 0.45 to 0.75 mol L-1, variable XCa and an F-content between 2 and 1400 ppmm (Supplementary Information). Trace element concentrations in tourmaline are low, from tens of ppmm for the LILE to ppbm for the HFSE (Table S-3).
Tourmaline–fluid trace element partition coefficients, required to convert tourmaline compositions to those of their formation fluids, have not been determined experimentally and were therefore estimated (see Supplementary Information for details and discussion). As a result, absolute concentrations in the fluid calculated from tourmaline are interpreted to have an associated uncertainty of an order of magnitude. However, element ratios, patterns and the overall elemental signature are robust. A further confirmation of reconstructed fluid compositions is the good agreement between the fluid composition reconstructed from the tourmaline mantle, and the same fluid reconstructed from phengite and titanite inclusions within this growth zone (Tables S-2, S-3).
top
Controls on Fluid Composition
The high-P Tauern subduction-zone fluids, reconstructed from tourmaline, phengite and titanite, are dilute aqueous solutions with <0.75 mol L-1 Na and total trace element concentrations <500 ppmm (Fig. 2a). Titanium concentrations suggest saturation in rutile when experimental solubilities (Manning et al., 2008
Manning, C.E., Wilke, M., Schmidt, C., Cauzid, J. (2008) Rutile solubility in albite-H2O and Na2Si3O7-H2O at high temperatures and pressures by in-situ synchrotron radiation micro-XRF. Earth and Planetary Science Letters 272, 730–737.
; Rapp et al., 2010Rapp, J.F., Klemme, S., Butler, I.B., Harley, S.L. (2010) Extremely high solubility of rutile in chloride and fluoride-bearing metamorphic fluids: An experimental investigation. Geology 38, 323–326.
; Tsay et al., 2017Tsay, A., Zajacz, Z., Ulmer, P., Sanchez-Valle, C. (2017) Mobility of major and trace elements in the eclogite-fluid system and element fluxes upon slab dehydration. Geochimica et Cosmochimica Acta 198, 70–91.
) are extrapolated to Tauern conditions, consistent with the presence of rutile inclusions in tourmaline’s core and mantle. In contrast, fluid REE concentrations are at a median level of only tens of ppbm (Fig. 2a), which is at least 2 orders of magnitude below that required for saturation in the common REE-minerals (Tropper et al., 2011Tropper, P., Manning, C.E., Harlov, D.E. (2011) Solubility of CePO4 monazite and YPO4 xenotime in H2O and H2O-NaCl at 800oC and 1 GPa: Implications for REE and Y transport during high-grade metamorphism. Chemical Geology 282, 58–66.
). This level is consistent with REE concentrations in eclogite–fluid experiments with allanitic zoisite (Tsay et al., 2017Tsay, A., Zajacz, Z., Ulmer, P., Sanchez-Valle, C. (2017) Mobility of major and trace elements in the eclogite-fluid system and element fluxes upon slab dehydration. Geochimica et Cosmochimica Acta 198, 70–91.
), where mineral–fluid partitioning, rather than mineral solubility would control REE concentrations.Fluid compositions reconstructed from well-preserved high-P minerals from the Dora Maira, Syros and New Caledonia palaeo-subduction zones using a similar methodology overlap with those reconstructed for the Tauern Eclogite Zone (Fig. 2a). Absolute concentrations vary by 2–3 orders of magnitude, but elemental patterns are consistent, and similarly suggest rutile-saturated, but REE-phase-undersaturated conditions. Hence, Ti concentrations will be controlled by solubility and are therefore independent of bulk-rock composition and mineral paragenesis until the saturating phase is exhausted (see Klimm et al., 2008
Klimm, K., Blundy, J.D., Green, T.H. (2008) Trace element partitioning and accessory phase saturation during H2O-saturated melting of basalt with implications for subduction zone chemical fluxes. Journal of Petrology 49, 523–553.
). However, for elements controlled by partitioning, such as the REE, differences in slab bulk-rock composition will impart differences in absolute element content of slab-derived fluids. Elemental patterns will persist, assuming no changes in major mineral paragenesis, as is indeed observed (Fig. 2a). Hence, arc magmas will share a characteristic element signature, but absolute concentrations can vary, even on a local scale, depending on the composition and mineralogy of the material that enters the trench.
Figure 2 (a) Element concentrations in fluids reconstructed from the Tauern tourmaline outer core and mantle and its mineral inclusions (blue symbols), and eclogite minerals from other subduction-zone terrains show a consistent pattern. (b) MORB-normalised concentrations for average arc magma parallel those of subduction-zone fluids supporting a genetic link. See the Supplementary Information for data sources and calculation method. Uncertainties are given as the Interquartile Range (IQR) and maximum estimates marked with an arrow.
top
Arc Volcanic Compositional Signature
To evaluate the resulting fluid compositions, these are compared in a typical arc-magma plot of Ba/Th vs. La/Sm (Fig. 3). La/Sm is controlled by residual garnet in the slab and high values signify a meta-sedimentary input (e.g., Elliott et al., 1997
Elliott, T., Plank, T., Zindler, A., White, W. Bourdon, B. (1997) Element transport from slab to volcanic front at the Mariana arc. Journal of Geophysical Research-Solid Earth 102, 14991–15019.
), whereas Ba/Th indicates fluid involvement, given the contrasting fluid mobility of Ba and Th (e.g., Keppler, 1996Keppler, H. (1996) Constraints from partitioning experiments on the composition of subduction-zone fluids. Nature 380, 237–240.
; Elliott et al., 1997Elliott, T., Plank, T., Zindler, A., White, W. Bourdon, B. (1997) Element transport from slab to volcanic front at the Mariana arc. Journal of Geophysical Research-Solid Earth 102, 14991–15019.
). Tourmaline-reconstructed fluids show the high Ba/Th at low La/Sm signature expected for subduction-zone fluids. This is moreover most distinct for the outermost core and mantle zones, which are interpreted to record the fluid released in slab devolatilisation and that which flushes the subduction channel, respectively. The latter is modified en-route to enrich fluid-compatible Ba over incompatible Th, consistent with models for subduction-zone fluids (Manning, 2004Manning, C.E. (2004) The chemistry of subduction-zone fluids. Earth and Planetary Science Letters 223, 1–16.
). Fluids reconstructed from phengite and titanite inclusions in the mantle zone are within error of those derived from tourmaline. Ba/Th values are lowest for the orogenic uplift part of the history, and suggest that a high Ba/Th ratio is characteristic for slab-derived fluids.Normalised to MORB, the element patterns of the reconstructed fluids, as well as those of fluid inclusions in subduction-zone rocks (e.g., Scambelluri et al., 2001
Scambelluri, M., Bottazzi, P., Trommsdorff, V., Vannucci, R., Hermann, J., Gomez-Pugnaire, M.T., Sanchez-Vizcaino, V.L. (2001) Incompatible element-rich fluids released by antigorite breakdown in deeply subducted mantle. Earth and Planetary Science Letters 192, 457–470.
) parallel that of average primitive arc magma; elevated in large-ion lithophile elements while depleted in V, Cr, and Ti (Fig. 2b). This shows that addition of these slab-derived fluids could confer the arc-characteristic element signature, as was concluded for Ba/Th.
Figure 3 Fluid compositions reconstructed from tourmaline (circles) and its mineral inclusions (triangles), show a consistent, high Ba/Th at low La/Sm signature. Addition of this fluid to the arc-magmatism source region can explain the trend to elevated Ba/Th compared to mid-ocean ridge basalt (MORB) and primitive mantle (PM).
top
Material Transfer from the Slab to Mantle
A fluid to primitive mantle ratio of 10:3 is required to produce the observed range of Ba/Th in arc magmas. Such high fluid-to-rock ratios are incompatible with the common conceptual model of arc magmatism where mantle is metasomatised by fluids, and subsequently partially molten, to initiate magmatism. Even when fully hydrated, the mantle cannot accommodate more than ~10 wt. % H2O. Moreover, arc-magmas are incompatible with the compositional dilution that high fluid-rock ratios impart. MORB-normalised fluid compositions (Fig. 2b) are also incompatible with a model of simple fluid addition to the mantle, with reconstructed concentrations of Sr and Ba, for example, unable to lead to the observed enrichment in arc magmas. This suggests a decoupling of water and its solutes during arc magma genesis.
Rather than batch fluid transfer to the mantle, we envision channelling of fluids along the slab interface mélange at high fluid-to-rock ratios. The arc-signature is indeed observed in palaeo-subduction mélanges (Marschall and Schumacher, 2012
Marschall, H.R., Schumacher, J.C. (2012) Arc magmas sourced from mélange diapirs in subduction zones. Nature Geoscience 5, 862–867.
), and the compositional range therein is in agreement with a progressive, and locally variable imprint of this signature by fluids flushing the mélange. Diapirism of this mélange into the mantle can subsequently transfer the compositional signature to the source region of arc magmas (cf. Marschall and Schumacher, 2012Marschall, H.R., Schumacher, J.C. (2012) Arc magmas sourced from mélange diapirs in subduction zones. Nature Geoscience 5, 862–867.
and references therein).top
Conclusions
Reconstruction of subduction-zone fluid compositions from eclogite minerals is a powerful method that allows absolute element concentrations in these fluids to be constrained when partition coefficients for the relevant P–T–X conditions are known. Tourmaline-reconstructed subduction-zone fluid compositions show that fluid-induced selective element release from the subducting slab can imprint the compositional signature characteristic of arc magmas. The compositions of these fluids appear controlled by mineral–fluid partitioning, except for Ti, and subduction-zone fluids are therefore expected to have variable compositions, but share a common elemental signature. Conversion of these fluid compositions into a net element flux in subduction zones is still imprecise, not least because our data indicate that a simple batch model of element transfer from slab to mantle and crust is untenable. Fluids instead likely migrate along the slab interface and progressively redistribute elements as recorded in refractory minerals.
top
Acknowledgements
VvH acknowledges financial support from the European Union Seventh Framework Program (FP7/2007-2013) under grant agreement no. 254015. We thank Barbara Dutrow and an anonymous reviewer for their suggestions.
Editor: Helen Williams
top
References
Bebout, G., Ryan, J., Leeman, W. Bebout, A. (1999) Fractionation of trace elements by subduction-zone metamorphism—effect of convergent-margin thermal evolution. Earth and Planetary Science Letters 171, 63–81.
 Show in context
Show in context Estimates of element release from the slab, and hence constraints on the net flux through subduction zones, are derived from three main sources; (i) the compositional signatures of arc magmas (e.g., Elliott et al., 1997), which are Nb-Ta depleted and enriched in Li, B, Sr, Ba and Pb compared to mid-ocean ridge basalts (MORB); (ii) experimentally-determined element partitioning between subduction-zone solids and liquids (e.g., Keppler, 1996; Kessel et al., 2005; Hermann et al., 2006; Spandler et al., 2007; Tsay et al., 2017); and (iii) element mass-balance on subducted rock lithologies (e.g., Bebout et al., 1999; Spandler et al., 2003, 2004).
View in article
Connolly, J.A.D. (2005) Computation of phase equilibria by linear programming: A tool for geodynamic modeling and its application to subduction zone decarbonation. Earth and Planetary Science Letters 236, 524–541.
 Show in context
Show in context Method (ii) provides concentration information, and flux estimates when combined with models of release of volatiles (e.g., Spandler et al., 2003; Connolly, 2005), but only under model-dependent conditions of temperature, pressure, mineralogy and bulk composition
View in article
Elliott, T., Plank, T., Zindler, A., White, W. Bourdon, B. (1997) Element transport from slab to volcanic front at the Mariana arc. Journal of Geophysical Research-Solid Earth 102, 14991–15019.
 Show in context
Show in context Estimates of element release from the slab, and hence constraints on the net flux through subduction zones, are derived from three main sources; (i) the compositional signatures of arc magmas (e.g., Elliott et al., 1997), which are Nb-Ta depleted and enriched in Li, B, Sr, Ba and Pb compared to mid-ocean ridge basalts (MORB); (ii) experimentally-determined element partitioning between subduction-zone solids and liquids (e.g., Keppler, 1996; Kessel et al., 2005; Hermann et al., 2006; Spandler et al., 2007; Tsay et al., 2017); and (iii) element mass-balance on subducted rock lithologies (e.g., Bebout et al., 1999; Spandler et al., 2003, 2004).
View in article
La/Sm is controlled by residual garnet in the slab and high values signify a meta-sedimentary input (e.g., Elliott et al., 1997), whereas Ba/Th indicates fluid involvement, given the contrasting fluid mobility of Ba and Th (e.g., Keppler, 1996; Elliott et al., 1997).
View in article
Henry D.J., Dutrow, B.L. (1996) Metamorphic tourmaline and its petrologic applications. In: Grew E.S., Anovitz L.M. (Eds.) Boron: Mineralogy, Petrology and Geochemistry. Reviews in Mineralogy 33, 503–557.
 Show in context
Show in context The mineral tourmaline has exceptional P–T stability that covers subduction-zone conditions (Fig. S-1); its crystal-chemistry allows it to incorporate a diversity of (trace) elements; and it displays negligible volume-diffusional re-equilibration (e.g., Henry and Dutrow, 1996; van Hinsberg et al., 2011a,b).
View in article
Hermann, J. Rubatto, D. (2009) Accessory phase control on the trace element signature of sediment melts in subduction zones. Chemical Geology 265, 512–526.
 Show in context
Show in context Moreover, (iii) depends on the presence of accessory phases (see Klimm et al., 2008; Hermann and Rubatto, 2009), as these phases can be the dominant repository of certain trace elements in the bulk rock.
View in article
Hermann, J., Spandler, C., Hack, A., Korsakov, A. (2006) Aqueous fluids and hydrous melts in high-pressure and ultra-high pressure rocks: Implications for element transfer in subduction zones. Lithos 92, 399–417.
 Show in context
Show in context Estimates of element release from the slab, and hence constraints on the net flux through subduction zones, are derived from three main sources; (i) the compositional signatures of arc magmas (e.g., Elliott et al., 1997), which are Nb-Ta depleted and enriched in Li, B, Sr, Ba and Pb compared to mid-ocean ridge basalts (MORB); (ii) experimentally-determined element partitioning between subduction-zone solids and liquids (e.g., Keppler, 1996; Kessel et al., 2005; Hermann et al., 2006; Spandler et al., 2007; Tsay et al., 2017); and (iii) element mass-balance on subducted rock lithologies (e.g., Bebout et al., 1999; Spandler et al., 2003, 2004).
View in article
Hoschek, G. (2007) Metamorphic peak conditions of eclogites in the Tauern Window, Eastern Alps, Austria: Thermobarometry of the assemblage garnet + omphacite + phengite + kyanite + quartz. Lithos 93, 1–16.
 Show in context
Show in context Peak conditions for this unit have been estimated at 630 ˚C and 2.5 GPa (e.g., Selverstone et al., 1992; Hoschek, 2007).
View in article
Keppler, H. (1996) Constraints from partitioning experiments on the composition of subduction-zone fluids. Nature 380, 237–240.
 Show in context
Show in context Estimates of element release from the slab, and hence constraints on the net flux through subduction zones, are derived from three main sources; (i) the compositional signatures of arc magmas (e.g., Elliott et al., 1997), which are Nb-Ta depleted and enriched in Li, B, Sr, Ba and Pb compared to mid-ocean ridge basalts (MORB); (ii) experimentally-determined element partitioning between subduction-zone solids and liquids (e.g., Keppler, 1996; Kessel et al., 2005; Hermann et al., 2006; Spandler et al., 2007; Tsay et al., 2017); and (iii) element mass-balance on subducted rock lithologies (e.g., Bebout et al., 1999; Spandler et al., 2003, 2004).
View in article
Here, well-preserved subduction-zone minerals (tourmaline, titanite and phengite) are used quantitatively to reconstruct the compositions of the fluids from which they grew by combining mineral compositions with mineral–fluid element partition coefficients (cf. Keppler, 1996).
View in article
La/Sm is controlled by residual garnet in the slab and high values signify a meta-sedimentary input (e.g., Elliott et al., 1997), whereas Ba/Th indicates fluid involvement, given the contrasting fluid mobility of Ba and Th (e.g., Keppler, 1996; Elliott et al., 1997).
View in article
Kessel, R., Schmidt, M.W., Ulmer, P., Pettke, T. (2005) Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120-180 km depth. Nature 437, 724–727.
 Show in context
Show in context Estimates of element release from the slab, and hence constraints on the net flux through subduction zones, are derived from three main sources; (i) the compositional signatures of arc magmas (e.g., Elliott et al., 1997), which are Nb-Ta depleted and enriched in Li, B, Sr, Ba and Pb compared to mid-ocean ridge basalts (MORB); (ii) experimentally-determined element partitioning between subduction-zone solids and liquids (e.g., Keppler, 1996; Kessel et al., 2005; Hermann et al., 2006; Spandler et al., 2007; Tsay et al., 2017); and (iii) element mass-balance on subducted rock lithologies (e.g., Bebout et al., 1999; Spandler et al., 2003, 2004).
View in article
Klimm, K., Blundy, J.D., Green, T.H. (2008) Trace element partitioning and accessory phase saturation during H2O-saturated melting of basalt with implications for subduction zone chemical fluxes. Journal of Petrology 49, 523–553.
 Show in context
Show in context Moreover, (iii) depends on the presence of accessory phases (see Klimm et al., 2008; Hermann and Rubatto, 2009), as these phases can be the dominant repository of certain trace elements in the bulk rock.
View in article
Hence, Ti concentrations will be controlled by solubility and are therefore independent of bulk-rock composition and mineral paragenesis until the saturating phase is exhausted (see Klimm et al., 2008).
View in article
Leeman, W.P., Sisson, V.B. (1996) Geochemistry of boron and its implications for crustal and mantle processes. Reviews in Mineralogy and Geochemistry 33, 645–707.
 Show in context
Show in context At low grades, B dominantly resides in sheet silicates (Leeman and Sisson, 1996) and is released during prograde metamorphism in discontinuous reactions resulting in punctuated nucleation and growth, and in continuous reactions producing gradual growth.
View in article
Manning, C.E. (2004) The chemistry of subduction-zone fluids. Earth and Planetary Science Letters 223, 1–16.
 Show in context
Show in context The latter is modified en-route to enrich fluid-compatible Ba over incompatible Th, consistent with models for subduction-zone fluids (Manning, 2004).
View in article
Manning, C.E., Wilke, M., Schmidt, C., Cauzid, J. (2008) Rutile solubility in albite-H2O and Na2Si3O7-H2O at high temperatures and pressures by in-situ synchrotron radiation micro-XRF. Earth and Planetary Science Letters 272, 730–737.
 Show in context
Show in context Titanium concentrations suggest saturation in rutile when experimental solubilities (Manning et al., 2008; Rapp et al., 2010; Tsay et al., 2017) are extrapolated to Tauern conditions, consistent with the presence of rutile inclusions in tourmaline’s core and mantle.
View in article
Marschall, H.R., Schumacher, J.C. (2012) Arc magmas sourced from mélange diapirs in subduction zones. Nature Geoscience 5, 862–867.
 Show in context
Show in context The arc-signature is indeed observed in palaeo-subduction mélanges (Marschall and Schumacher, 2012), and the compositional range therein is in agreement with a progressive, and locally variable imprint of this signature by fluids flushing the mélange.
View in article
Diapirism of this mélange into the mantle can subsequently transfer the compositional signature to the source region of arc magmas (cf. Marschall and Schumacher, 2012 and references therein).
View in article
Rapp, J.F., Klemme, S., Butler, I.B., Harley, S.L. (2010) Extremely high solubility of rutile in chloride and fluoride-bearing metamorphic fluids: An experimental investigation. Geology 38, 323–326.
 Show in context
Show in context Titanium concentrations suggest saturation in rutile when experimental solubilities (Manning et al., 2008; Rapp et al., 2010; Tsay et al., 2017) are extrapolated to Tauern conditions, consistent with the presence of rutile inclusions in tourmaline’s core and mantle.
View in article
Scambelluri, M., Bottazzi, P., Trommsdorff, V., Vannucci, R., Hermann, J., Gomez-Pugnaire, M.T., Sanchez-Vizcaino, V.L. (2001) Incompatible element-rich fluids released by antigorite breakdown in deeply subducted mantle. Earth and Planetary Science Letters 192, 457–470.
 Show in context
Show in context Normalised to MORB, the element patterns of the reconstructed fluids, as well as those of fluid inclusions in subduction-zone rocks (e.g., Scambelluri et al., 2001) parallel that of average primitive arc magma; elevated in large-ion lithophile elements while depleted in V, Cr, and Ti (Fig. 2b).
View in article
Selverstone, J., Franz, G., Thomas, S., Getty, S. (1992) Fluid variability in 2 GPa eclogites as an indicator of fluid behavior during subduction. Contributions to Mineralogy and Petrology 112, 341–357.
 Show in context
Show in context Peak conditions for this unit have been estimated at 630 ˚C and 2.5 GPa (e.g., Selverstone et al., 1992; Hoschek, 2007).
View in article
Spandler, C., Hermann, J., Arculus, R., Mavrogenes, J. (2003) Redistribution of trace elements during prograde metamorphism from lawsonite blueschist to eclogite facies; implications for deep subduction-zone processes. Contributions to Mineralogy and Petrology 146, 205–222.
 Show in context
Show in context Estimates of element release from the slab, and hence constraints on the net flux through subduction zones, are derived from three main sources; (i) the compositional signatures of arc magmas (e.g., Elliott et al., 1997), which are Nb-Ta depleted and enriched in Li, B, Sr, Ba and Pb compared to mid-ocean ridge basalts (MORB); (ii) experimentally-determined element partitioning between subduction-zone solids and liquids (e.g., Keppler, 1996; Kessel et al., 2005; Hermann et al., 2006; Spandler et al., 2007; Tsay et al., 2017); and (iii) element mass-balance on subducted rock lithologies (e.g., Bebout et al., 1999; Spandler et al., 2003, 2004).
View in article
Method (ii) provides concentration information, and flux estimates when combined with models of release of volatiles (e.g., Spandler et al., 2003; Connolly, 2005), but only under model-dependent conditions of temperature, pressure, mineralogy and bulk composition
View in article
Spandler, C., Hermann, J., Arculus, R., Mavrogenes, J. (2004) Geochemical heterogeneity and element mobility in deeply subducted oceanic crust; insights from high-pressure mafic rocks from New Caledonia. Chemical Geology 206, 21–42.
 Show in context
Show in context Estimates of element release from the slab, and hence constraints on the net flux through subduction zones, are derived from three main sources; (i) the compositional signatures of arc magmas (e.g., Elliott et al., 1997), which are Nb-Ta depleted and enriched in Li, B, Sr, Ba and Pb compared to mid-ocean ridge basalts (MORB); (ii) experimentally-determined element partitioning between subduction-zone solids and liquids (e.g., Keppler, 1996; Kessel et al., 2005; Hermann et al., 2006; Spandler et al., 2007; Tsay et al., 2017); and (iii) element mass-balance on subducted rock lithologies (e.g., Bebout et al., 1999; Spandler et al., 2003, 2004).
View in article
Spandler, C., Mavrogenes, J., Hermann, J. (2007) Experimental constraints on element mobility from subducted sediments using high-P synthetic fluid/melt inclusions. Chemical Geology 239, 228–249.
 Show in context
Show in context Estimates of element release from the slab, and hence constraints on the net flux through subduction zones, are derived from three main sources; (i) the compositional signatures of arc magmas (e.g., Elliott et al., 1997), which are Nb-Ta depleted and enriched in Li, B, Sr, Ba and Pb compared to mid-ocean ridge basalts (MORB); (ii) experimentally-determined element partitioning between subduction-zone solids and liquids (e.g., Keppler, 1996; Kessel et al., 2005; Hermann et al., 2006; Spandler et al., 2007; Tsay et al., 2017); and (iii) element mass-balance on subducted rock lithologies (e.g., Bebout et al., 1999; Spandler et al., 2003, 2004).
View in article
Tatsumi, Y. (2005) The subduction factory: How it operates in the evolving Earth. GSA today 15, 4–10.
 Show in context
Show in context Models of terrestrial element cycling hinge on accurate estimates of these fluxes and of the recycling of material by fluids and melts from the dehydrating slab in the “subduction factory” (Tatsumi, 2005).
View in article
Tropper, P., Manning, C.E., Harlov, D.E. (2011) Solubility of CePO4 monazite and YPO4 xenotime in H2O and H2O-NaCl at 800oC and 1 GPa: Implications for REE and Y transport during high-grade metamorphism. Chemical Geology 282, 58–66.
 Show in context
Show in context In contrast, fluid REE concentrations are at a median level of only tens of ppbm (Fig. 2a), which is at least 2 orders of magnitude below that required for saturation in the common REE-minerals (Tropper et al., 2011).
View in article
Tsay, A., Zajacz, Z., Ulmer, P., Sanchez-Valle, C. (2017) Mobility of major and trace elements in the eclogite-fluid system and element fluxes upon slab dehydration. Geochimica et Cosmochimica Acta 198, 70–91.
 Show in context
Show in context Estimates of element release from the slab, and hence constraints on the net flux through subduction zones, are derived from three main sources; (i) the compositional signatures of arc magmas (e.g., Elliott et al., 1997), which are Nb-Ta depleted and enriched in Li, B, Sr, Ba and Pb compared to mid-ocean ridge basalts (MORB); (ii) experimentally-determined element partitioning between subduction-zone solids and liquids (e.g., Keppler, 1996; Kessel et al., 2005; Hermann et al., 2006; Spandler et al., 2007; Tsay et al., 2017); and (iii) element mass-balance on subducted rock lithologies (e.g., Bebout et al., 1999; Spandler et al., 2003, 2004).
View in article
Titanium concentrations suggest saturation in rutile when experimental solubilities (Manning et al., 2008; Rapp et al., 2010; Tsay et al., 2017) are extrapolated to Tauern conditions, consistent with the presence of rutile inclusions in tourmaline’s core and mantle.
View in article
This level is consistent with REE concentrations in eclogite-fluid experiments with allanitic zoisite (Tsay et al., 2017), where mineral–fluid partitioning, rather than mineral solubility would control REE concentrations.
View in article
van Hinsberg, V.J., Schumacher, J.C. (2007) Intersector element partitioning in tourmaline: a potentially powerful single crystal thermometer. Contributions to Mineralogy and Petrology 153, 289–301.
 Show in context
Show in context Inter-sector thermometry (van Hinsberg and Schumacher, 2007), combined with qualitative K-barometry and inclusion mineralogy suggests growth of the tourmaline core during prograde subduction and its associated progressive internal B-release, formation of the mantle zone following detachment from the subducting slab from external fluids that were released in deeper slab devolatilisation, and rim growth during retrogression that records the orogenic uplift of the Eclogite Zone (Fig. 1a).
View in article
van Hinsberg, V.J., Schumacher, J.C., Kearns, S., Mason, P.R.D., Franz, G. (2006) Hourglass sector zoning in metamorphic tourmaline and resultant major and trace-element fractionation. American Mineralogist 91, 717–728.
 Show in context
Show in context Hourglass sector-zoning is present (Fig. S-2b), indicating that growth compositions have been preserved (van Hinsberg et al., 2006).
View in article
van Hinsberg, V.J., Henry, D.J., Dutrow, B.L. (2011a) Tourmaline as a Petrologic Forensic Mineral: A Unique Recorder of Its Geologic Past. Elements 7, 327–332.
 Show in context
Show in context The mineral tourmaline has exceptional P–T stability that covers subduction-zone conditions (Fig. S-1); its crystal-chemistry allows it to incorporate a diversity of (trace) elements; and it displays negligible volume-diffusional re-equilibration (e.g., Henry and Dutrow, 1996; van Hinsberg et al., 2011a,b).
View in article
van Hinsberg, V.J., Henry, D.J., Marschall, H.R. (2011b) Tourmaline: an Ideal Indicator of Its Host Environment. Canadian Mineralogist 49, 1–16.
 Show in context
Show in context The mineral tourmaline has exceptional P–T stability that covers subduction-zone conditions (Fig. S-1); its crystal-chemistry allows it to incorporate a diversity of (trace) elements; and it displays negligible volume-diffusional re-equilibration (e.g., Henry and Dutrow, 1996; van Hinsberg et al., 2011a,b).
View in article
von Goerne, G., Franz, G. van Hinsberg, V.J. (2011) Experimental determination of Na–Ca distribution between tourmaline and fluid in the system CaO–Na2O–MgO–Al2O3–SiO2–B2O3–H2O. Canadian Mineralogist 49, 137–152.
 Show in context
Show in context Figure 1 [...] (e) XCa of tourmaline, which tracks the XCa in the coexisting fluid (von Goerne et al., 2011).
View in article
Zimmermann, R., Hammerschmidt, K., Franz, G. (1994) Eocene high pressure metamorphism in the Penninic units of the Tauern Window (Eastern Alps): evidence from 40Ar-39Ar dating and petrological investigations. Contributions to Mineralogy and Petrology 117, 175–186.
 Show in context
Show in context Figure 1 [...] (a) Tauern Eclogite Zone P–T path (Zimmermann et al., 1994) with conditions recorded by tourmaline growth zones shown.
View in article
top
Supplementary Information
Analytical Methods
Mineral major element compositions were determined by WDS-EMP, using the McGill University JEOL JXA-8900 microprobe (20kV, 15nA, focused beam). Natural minerals were used as primary standards with the tourmaline standards of Dyar et al. (2001) and an in-house tourmaline standard (UoB) as secondary references. Tourmaline analyses were converted to cations per formula unit using a Y + Z + T = 15 normalisation, or Si = 6 for analyses where the former resulted in Si in excess of 6. The precision, expressed as % RSD, is SiO2 (1.1), TiO2 (2.1) Al2O3 (1.1), MgO (2.9), MnO (11), FeO (2.0), CaO (4.8), Na2O (2.0), K2O (8), and F (15). Precision values for calculated cpfu are Si (0.7), Ti (1.6), Al (0.5), Mg (2.6), Mn (11), Fe (1.4), Ca (4.4), Na (1.8), K (8), and F (15). Accuracy, expressed as the average relative deviation in % for the secondary reference materials is SiO2 (3), TiO2 (-8) Al2O3 (4), MgO (-8), MnO (20), FeO (3), CaO (4), Na2O (-1), K2O (-22), and F (37), and for the calculated cpfu Si (0.3), Ti (-11), Al (0.5), Mg (-11), Mn (18), Fe (0.01), Ca (0.5), Na (-4.6), K (-26), and F (35). Trace element concentrations were determined by laser-ablation ICP-MS using a NewWave 213nm Nd:YAG laser at 10 Hz coupled to a Finnigan Element 2 magnetic sector mass spectrometer at the University of Bristol (La, Sm, Ba and Th; 15 and 30 µm spot size), and a PerkinElmer 6100 Elan DRC quadrupole ICP-MS at McGill University (60 µm spot size). Concentrations were standardised to NIST SRM 610 with Si from EMP as the internal reference element. Accuracy was estimated from measurements of the tourmaline standards of Dyar et al. (2001) and an in-house tourmaline standard (UoB), and is approximately 30 % relative, with significant exceptions V (6 %), Sr (-1 %), La (3 %), Nd (10 %), Hf (57 %) and U (95 %). EMP major element data are presented in Table S-1 and LA-ICP-MS trace element data in Tables S-2 and S-3. The major and trace element compositions of the tourmaline a-sectors have been used for all interpretations, because this sector is unaffected by sector zoning induced preferential elemental uptake or exclusion (see van Hinsberg et al., 2006).
Table S-1 WDS electron-microprobe data for tourmaline growth zones, mineral inclusions in tourmaline, and matrix minerals. The values shown are the average for multiple analyses and their associated 1 standard deviation spread, except for omphacite where only one analysis was available. Normalisation factors used are the sum of cations for omphacite, titanite, plagioclase, amphibole and calcite, or the oxygen sum for phengite and biotite. Tourmaline was normalised to the sum of the elements residing at the Y, Z and T sites = 15, or Si = 6 cpfu, if the former resulted in Si > 6 cpfu.
| Tourmaline growth zones | ||||||||||||||||
| inner core | 1s | mid core | 1s | outer core 1 | 1s | outer core 2 | 1s | mantle | 1s | inner rim | 1s | mid rim | 1s | outer rim | 1s | |
| SiO2 | 37.9 | 0.4 | 38.1 | 0.4 | 38.3 | 0.2 | 38.2 | 0.7 | 38.7 | 0.5 | 36.0 | 0.2 | 35.9 | 0.3 | 36.2 | 0.3 |
| TiO2 | 0.44 | 0.05 | 0.39 | 0.03 | 0.42 | 0.10 | 0.25 | 0.03 | 0.19 | 0.02 | 0.18 | 0.05 | 0.36 | 0.09 | 0.35 | 0.08 |
| Al2O3 | 29.1 | 0.3 | 29.3 | 0.2 | 29.7 | 0.7 | 30.8 | 0.1 | 31.8 | 0.3 | 28.2 | 0.3 | 28.7 | 0.2 | 28.4 | 0.4 |
| MgO | 10.3 | 0.4 | 10.0 | 0.2 | 10.2 | 0.2 | 10.1 | 0.1 | 12.8 | 0.1 | 10.7 | 0.4 | 9.5 | 0.3 | 9.5 | 0.3 |
| MnO | 0.004 | 0.008 | 0.01 | 0.01 | 0.004 | 0.006 | 0.01 | 0.02 | 0.007 | 0.009 | 0.008 | 0.007 | 0.002 | 0.004 | 0.007 | 0.008 |
| FeO | 9.67 | 0.84 | 10.30 | 0.43 | 9.06 | 1.01 | 8.08 | 0.05 | 3.04 | 0.10 | 2.73 | 0.31 | 3.79 | 0.48 | 4.43 | 0.82 |
| CaO | 1.2 | 0.1 | 0.93 | 0.06 | 1.1 | 0.1 | 0.64 | 0.05 | 1.6 | 0.2 | 1.3 | 0.3 | 0.56 | 0.08 | 0.9 | 0.2 |
| Na2O | 2.48 | 0.05 | 2.59 | 0.01 | 2.54 | 0.10 | 2.83 | 0.02 | 2.32 | 0.10 | 2.1 | 0.2 | 2.49 | 0.09 | 2.1 | 0.2 |
| K2O | 0.09 | 0.02 | 0.095 | 0.002 | 0.09 | 0.01 | 0.092 | 0.006 | 0.06 | 0.01 | 0.010 | 0.003 | 0.013 | 0.002 | 0.012 | 0.002 |
| F | 0.6 | 0.3 | 0.60 | 0.01 | 0.9 | 0.1 | 0.82 | 0.02 | 1.2 | 0.1 | 0.54 | 0.06 | 0.35 | 0.08 | 0.51 | 0.10 |
| norm. factor | Y + Z + T = 15 | Y + Z + T = 15 | Y + Z + T = 15 | Y + Z + T = 15 | Y + Z + T = 15 | Si = 6 | Si = 6 | Si = 6 | ||||||||
| Si | 5.92 | 0.02 | 5.93 | 0.02 | 5.95 | 0.03 | 5.94 | 0.08 | 5.93 | 0.04 | 6 | - | 6 | - | 6 | - |
| Ti | 0.052 | 0.006 | 0.046 | 0.003 | 0.049 | 0.012 | 0.029 | 0.004 | 0.022 | 0.002 | 0.022 | 0.006 | 0.05 | 0.01 | 0.04 | 0.01 |
| Al | 5.36 | 0.02 | 5.37 | 0.01 | 5.4 | 0.1 | 5.65 | 0.04 | 5.74 | 0.05 | 5.54 | 0.08 | 5.66 | 0.06 | 5.56 | 0.04 |
| Mg | 2.40 | 0.08 | 2.31 | 0.02 | 2.37 | 0.05 | 2.33 | 0.02 | 2.91 | 0.04 | 2.66 | 0.10 | 2.37 | 0.06 | 2.34 | 0.07 |
| Mn | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 | 0.002 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 |
| Fe | 1.3 | 0.1 | 1.34 | 0.06 | 1.2 | 0.1 | 1.05 | 0.01 | 0.39 | 0.01 | 0.38 | 0.04 | 0.53 | 0.07 | 0.6 | 0.1 |
| Ca | 0.19 | 0.02 | 0.156 | 0.009 | 0.18 | 0.02 | 0.11 | 0.01 | 0.26 | 0.03 | 0.23 | 0.05 | 0.10 | 0.01 | 0.16 | 0.03 |
| Na | 0.75 | 0.02 | 0.781 | 0.007 | 0.76 | 0.03 | 0.85 | 0.01 | 0.69 | 0.03 | 0.68 | 0.05 | 0.81 | 0.04 | 0.68 | 0.06 |
| K | 0.018 | 0.003 | 0.0189 | 0.0002 | 0.018 | 0.003 | 0.018 | 0.001 | 0.012 | 0.003 | 0.002 | 0.001 | 0.0027 | 0.0005 | 0.0026 | 0.0004 |
| F | 0.3 | 0.1 | 0.295 | 0.002 | 0.42 | 0.07 | 0.41 | 0.01 | 0.57 | 0.06 | 0.28 | 0.03 | 0.18 | 0.04 | 0.27 | 0.06 |
| XMg | 0.66 | 0.03 | 0.63 | 0.01 | 0.67 | 0.03 | 0.69 | 0.00 | 0.88 | 0.00 | 0.87 | 0.02 | 0.82 | 0.02 | 0.79 | 0.04 |
| dravite | 0.50 | 0.01 | 0.51 | 0.01 | 0.52 | 0.04 | 0.60 | 0.01 | 0.62 | 0.03 | 0.60 | 0.04 | 0.5 | 0.3 | 0.5 | 0.2 |
| schorl | 0.27 | 0.03 | 0.29 | 0.01 | 0.26 | 0.01 | 0.27 | 0.00 | 0.08 | 0.00 | 0.09 | 0.02 | 0.12 | 0.07 | 0.13 | 0.05 |
| uvite | 0.19 | 0.02 | 0.16 | 0.01 | 0.18 | 0.02 | 0.11 | 0.01 | 0.26 | 0.03 | 0.23 | 0.05 | 0.08 | 0.04 | 0.14 | 0.06 |
| foitite | 0.037 | 0.004 | 0.044 | 0.002 | 0.042 | 0.015 | 0.023 | 0.018 | 0.040 | 0.014 | 0.09 | 0.01 | 0.3 | 0.4 | 0.3 | 0.3 |
| Mineral inclusions in tourmaline mantle | Matrix minerals | |||||||||||||||
| omphacite | 1s | phengite | 1s | titanite | 1s | biotite | 1s | plagioclase | amphibole | calcite | ||||||
| SiO2 | 53.9 | - | 48 | 1 | 29.6 | 0.4 | 37.1 | 1.7 | 59.1 | 0.2 | 51.5 | 0.8 | 0.03 | 0.02 | ||
| TiO2 | 0.04 | - | 0.20 | 0.07 | 34 | 3 | 0.5 | 0.4 | < d.l. | - | 0.09 | 0.03 | 0.01 | 0.02 | ||
| Al2O3 | 5.9 | - | 24 | 1 | 4 | 2 | 14.6 | 0.9 | 21.5 | 0.2 | 6 | 1 | 0.001 | 0.001 | ||
| MgO | 11.3 | - | 4.6 | 0.5 | 0.04 | 0.02 | 19.7 | 0.7 | 0.01 | 0.01 | 17.7 | 0.8 | 0.47 | 0.09 | ||
| MnO | 0.03 | - | 0.004 | 0.006 | 0.009 | 0.008 | 0.02 | 0.01 | < d.l. | - | 0.02 | 0.01 | 0.09 | 0.10 | ||
| FeO | 5.3 | - | 2.0 | 0.3 | 0.7 | 0.3 | 9.7 | 0.8 | 0.14 | 0.06 | 8.3 | 0.5 | 0.33 | 0.09 | ||
| CaO | 17.9 | - | < d.l. | - | 28.3 | 0.3 | < d.l. | - | 3.47 | 0.09 | 10 | 2 | 55 | 1 | ||
| Na2O | 4.2 | - | 0.2 | 0.2 | 0.02 | 0.01 | 0.16 | 0.07 | 9.5 | 0.1 | 2.0 | 0.8 | < d.l. | - | ||
| K2O | 0.002 | - | 10.8 | 0.5 | 0.001 | 0.002 | 9 | 2 | 0.06 | 0.01 | 0.17 | 0.04 | < d.l. | - | ||
| F | 0.01 | - | 0.6 | 0.2 | 1.1 | 0.6 | 1.6 | 0.4 | 0.05 | 0.08 | 0.54 | 0.09 | 0.02 | 0.03 | ||
| norm. factor | ∑cat = 4 | O = 11 | ∑cat = 3 | O = 11 | ∑cat = 5 | ∑cat = 15 | ∑cat = 1 | |||||||||
| Si | 1.97 | - | 3.44 | 0.06 | 0.978 | 0.008 | 2.83 | 0.08 | 2.783 | 0.001 | 7.3 | 0.1 | 0.0005 | 0.0003 | ||
| Ti | 0.001 | - | 0.011 | 0.004 | 0.84 | 0.07 | 0.03 | 0.02 | - | - | 0.010 | 0.003 | 0.0002 | 0.0002 | ||
| Al | 0.25 | - | 1.99 | 0.09 | 0.15 | 0.06 | 1.3 | 0.1 | 1.191 | 0.007 | 0.9 | 0.2 | 0.00002 | 0.00003 | ||
| Mg | 0.62 | - | 0.49 | 0.06 | 0.0018 | 0.0009 | 2.24 | 0.09 | 0.001 | 0.001 | 3.7 | 0.2 | 0.012 | 0.002 | ||
| Mn | 0.001 | - | 0.0002 | 0.0004 | 0.0003 | 0.0002 | 0.001 | 0.001 | - | - | 0.003 | 0.001 | 0.001 | 0.001 | ||
| Fe | 0.16 | - | 0.12 | 0.02 | 0.019 | 0.008 | 0.62 | 0.05 | 0.005 | 0.002 | 0.99 | 0.05 | 0.005 | 0.001 | ||
| Ca | 0.70 | - | - | - | 1.004 | 0.003 | - | - | 0.175 | 0.004 | 1.5 | 0.2 | 0.982 | 0.002 | ||
| Na | 0.29 | - | 0.03 | 0.02 | 0.001 | 0.001 | 0.02 | 0.01 | 0.86 | 0.01 | 0.6 | 0.2 | - | - | ||
| K | 0.0001 | - | 0.98 | 0.04 | 0.00005 | 0.00008 | 0.9 | 0.2 | 0.004 | 0.001 | 0.03 | 0.01 | - | - | ||
| F | 0.001 | - | 0.14 | 0.05 | 0.12 | 0.06 | 0.4 | 0.1 | 0.01 | 0.01 | 0.24 | 0.04 | 0.001 | 0.002 | ||
Table S-2 Laser-ablation ICP-MS data for Ba, Th, La and Sm in tourmaline, and phengite and titanite inclusions. Most data represent the mean of multiple measurements. Uncertainties are the 1 standard deviation on the mean, or the count statistical error if only one measurement is available. Partition coefficients are those of Green and Adam (2003), combined with Dphg/tur from Klemme et al. (2011) and are used to calculate the Ba/Th and La/Sm ratios for the fluid. Analysis locations are shown in Figure 1.
| Concentrations in ppm | Partition coefficients (min/fl) | Ratios | ||||||||||||||
| Mineral | Ba | 1 std | Th | 1 std | La | 1 std | Sm | 1 std | Ba | Th | La | Sm | Ba/Th fluid | La/Sm fluid | ||
| Tur core - 1 | 1.1 | 0.3 | 0.009 | 0.006 | 0.008 | 0.007 | 0.09 | 0.07 | 0.06 | 0.88 | 1.08 | 1.50 | 1795 | 0.12 | ||
| Tur core - 1 | 3.0 | 0.7 | 0.03 | 0.02 | 0.04 | 0.02 | 0.08 | 0.10 | 0.06 | 0.88 | 1.08 | 1.50 | 1770 | 0.64 | ||
| Tur core - 2a | 1.7 | 0.4 | 0.02 | 0.01 | 0.03 | 0.02 | 0.20 | 0.16 | 0.06 | 0.88 | 1.08 | 1.50 | 1397 | 0.18 | ||
| Tur core - 2b | 1.9 | 1.0 | 0.02 | 0.02 | 0.012 | 0.006 | 0.08 | 0.09 | 0.06 | 0.88 | 1.08 | 1.50 | 1950 | 0.22 | ||
| Tur core - 2c | 0.9 | 0.2 | 0.007 | 0.008 | 0.03 | 0.01 | 0.13 | 0.06 | 0.06 | 0.88 | 1.08 | 1.50 | 2088 | 0.30 | ||
| Tur mantle - 3 | 2 | 2 | 0.011 | 0.005 | 0.005 | 0.006 | 0.028 | 0.008 | 0.06 | 0.88 | 1.08 | 1.50 | 2813 | 0.27 | ||
| Tur rim - 4 | 0.81 | 0.07 | 0.009 | 0.001 | 0.004 | 0.003 | 0.04 | 0.01 | 0.06 | 0.88 | 1.08 | 1.50 | 1443 | 0.17 | ||
| Tur rim - 5 | 0.8 | 0.9 | 0.02 | 0.02 | 0.036 | 0.003 | 0.031 | 0.005 | 0.06 | 0.88 | 1.08 | 1.50 | 729 | 1.64 | ||
| Phengite | 1908 | 183 | 0.012 | 0.005 | 0.02 | 0.01 | 0.04 | 0.04 | 91.2 | 1.53 | 3.84 | 6.07 | 2782 | 0.92 | ||
| Titanite | 55 | 73 | 0.6 | 0.7 | 0.45 | 0.04 | 5 | 3 | 0.15 | 4.4 | 18.503 | 173.83 | 2710 | 0.79 | ||
Table S-3 Laser-ablation ICP-MS trace element data for tourmaline, and phengite and titanite inclusions. The tourmaline data represent the mean of multiple measurements and the uncertainties reported are 1 standard deviation on this mean. For phengite and titanite, we report the count statistical error. Measurements were corrected for differences in ablation behaviour between standards and samples by normalising to EMP Si-content.
| Conc in ppm | Tourmaline | Inclusions | ||||||
| Element | Rim of core | 1 std | Mantle | 1 std | Phengite | 1 std cse | Titanite | 1 std cse |
| Li | 31 | 3 | 74 | 10 | 250 | 6 | 19 | 2 |
| V | 65 | 4 | 64.1 | 0.8 | 71.6 | 0.9 | 94 | 3 |
| Cr | 209 | 20 | 139 | 44 | 122 | 2 | 299 | 9 |
| Mn | 26.6 | 0.6 | 17 | 1 | 11.6 | 0.4 | 17.1 | 0.8 |
| Cs | 0.07 | 0.04 | 0.07 | 0.02 | 26.1 | 0.3 | 0.07 | 0.03 |
| Sr | 1107 | 28 | 1376 | 132 | 42.4 | 0.8 | 387 | 13 |
| Ba | 0.9 | 0.6 | 0.6 | 0.2 | 1778 | 17 | 3.8 | 0.6 |
| Ti | 805 | 116 | 1312 | 461 | 941 | 90 | 188700 | 15341 |
| Nb | < d.l. | 0.02 | 1.04 | 0.07 | 77 | 2 | ||
| Ta | 0.007 | 0.006 | 0.02 | 0.12 | 0.02 | 3.5 | 0.2 | |
| Zr | < d.l. | 3 | 6 | 0.07 | 0.03 | 16 | 4 | |
| Hf | < d.l. | 0.1 | 0.1 | 0.003 | 0.005 | 0.5 | 0.1 | |
| La | < d.l. | 0.001 | 0.001 | 0.01 | 0.01 | 0.48 | 0.05 | |
| Ce | < d.l. | 0.014 | 0.002 | 0.010 | 0.006 | 3.4 | 0.2 | |
| Sm | < d.l. | 0.024 | 0.006 | 0.04 | 0.04 | 3.4 | 0.4 | |
| Lu | < d.l. | 0.007 | 0.002 | < d.l. | 0.56 | 0.07 | ||
| Y | 0.03 | 0.02 | 0.02 | 0.03 | 0.06 | 0.02 | 36 | 2 |
| Pb | 13 | 4 | 6.7 | 0.3 | 2.4 | 0.7 | 14 | 4 |
| Th | 0.01 | 0.01 | 0.01 | 0.01 | 0.015 | 0.009 | 0.09 | 0.03 |
| U | 0.002 | 0.001 | 0.008 | 0.006 | 0.000 | 0.004 | 1.3 | 0.1 |
< d.l. Value below detection limit
Download in ExcelTauern Tourmaline

Figure S-1 Tourmaline is stable for most of the P–T range typically found in subduction zones, and is an accessory phase in exposed subduction-zone lithologies for all the terrains shown. Typical subduction path (blue solid lines) from Syracuse et al. (2010) and P–T estimates for subduction-zone terrains from Zimmermann et al. (1994) and Marschall et al. (2009). Tourmaline stability fields from van Hinsberg et al. (2011b).
The grain investigated here (inventory number 2013/13, Mineralogical Museum, Technical University Berlin) is a ~2 mm long black tourmaline. It has an irregular brown core that likely grew as an interstitial phase, mantled by an idiomorphic, compositionally homogenous rim, and a strongly zoned outer rim that fingers into the enclosing minerals (Fig. S-2a). The inner core and mantle are homogenous in composition, whereas the outer core and rim are strongly compositionally zoned (Fig. 1), suggesting that the former represent punctuated growth events, whereas the latter record growth by gradual boron release. Only quartz and rutile inclusions are found in the core, whereas the mantle contains inclusions of the peak metamorphic minerals (see Zimmermann et al., 1994; Hoschek, 2007), omphacite (partially replaced by amphibole), titanite and phengite, as well as negative crystal forms of garnet (Fig. S-2d). The outermost rim is in equilibrium with the matrix minerals. These inclusion parageneses suggest formation of the core prior to peak metamorphism, i.e. prograde; formation of the mantle at, or after peak metamorphism; and late-stage growth of the rim following re-appearance of amphibole. Back-scattered electron images reveal fine compositional heterogeneity in the core (Fig. S-2c), interpreted as outlining an original mica foliation and suggests that tourmaline overgrew mica during growth.
The tourmaline grain has a dravite-dominated composition (Henry et al., 2011), with variable proportions of the uvite (0.05 to 0.3), schorl (0.05 to 0.3) and foitite (0.01 to 0.3) end-members (Table S-1). Al-contents are consistently below 6, indicating significant Mg and/or Fe at the Z-site. The X-site vacancy content is low, but shows a marked increase in the rim, in agreement with the negative correlation between metamorphic grade and X-site vacancy content (Henry and Dutrow, 1996).

Figure S-2 Petrography of the Tauern tourmaline grain used in this study. (a) Mineral-map showing its inclusion and matrix mineralogy, and the core, mantle and rim zones of the tourmaline grain. This map is based on back-scattered electron imaging and WDS-EMP element mapping for Ca, Mg, Ti, Fe, Si and Na. (b) Example of hourglass sector zoning in the outermost rim in PPL microscopy. (c) Back-scattered electron image of compositional heterogeneity in the tourmaline core, outlining the foliation of protolith mica grains overgrown by tourmaline. (d) Outline of an idiomorphic eclogite-stage garnet grain replaced during growth of the mantle zone in PPL microscopy. (e) Back-scattered electron image of c+-c- polar tourmaline growth.
P–T Constraints for Tourmaline Growth
The tourmaline grain displays compositional hourglass-style sector zoning in core, mantle and rim, as well as polar growth (Fig. S-2b,e), and this allows temperatures of formation to be determined from inter-sector thermometry (Henry and Dutrow, 1996; van Hinsberg and Schumacher, 2007b). Temperatures calculated using the Ca and Ti thermometric formulations of van Hinsberg and Schumacher (2007b) are consistent, and results for inter-polar and inter-sector pairs agree within uncertainty (Fig. 1f). Mantle zone temperatures show significant scatter, which is attributed to rapid growth of this zone leading to diffusion unable to keep up with growth. Equilibrium separation of Ca and Ti was, therefore, not achieved. As such, inter-sector partition coefficients, and hence temperatures will be underestimates for the mantle growth zone.
Tourmaline barometry has yet to be established, but its K-content has been suggested as a pressure-indicator based on characteristically high K-contents in UHP tourmalines (e.g., Shimizu and Ogasawara, 2013), thermodynamic considerations (van Hinsberg and Schumacher, 2007a) and experimental studies (Berryman et al., 2014, 2015). However, this correlation between K-content and P is not always consistent (e.g., Marschall et al., 2009), as it also depends on the exchanging mineral phases and bulk K-content (Berryman et al., 2015). For the Tauern tourmaline, the dominant K-exchanging phase is phengite, and its presence as inclusions (Fig. S-2a) and as a matrix phase, as well as its ubiquitous occurrence in the Tauern Eclogite Zone at various metamorphic grades (Zimmermann et al., 1994; Hoschek, 2007) suggests that phengite was present throughout tourmaline’s growth history. The K-content in the a-sector of the tourmaline grain is therefore interpreted as predominantly controlled by P, and used as a qualitative barometer (Fig. 1g).
Constraints on the Nature and Composition of the Fluids
Sector zoning in tourmaline develops in equilibrium on the growth surfaces, but is subsequently in disequilibrium in the crystal bulk (van Hinsberg et al., 2006; see also Hollister, 1970). Re-equilibration is governed by volume diffusion rates, and the preservation of sector zoning in this Tauern tourmaline grain proves that primary compositions are preserved. The compositions of the subsequent growth zones can, therefore, be used to track the evolving composition of its host environment throughout subduction and uplift. Based on tourmaline compositions, textures, and P–T history, we interpret the core to have formed from internal release of B, thus recording the compositions of fluids released from the progressively subducting slab (Fig. 1a); the mantle zone to represent growth by metasomatic input of B after detachment of the Tauern Eclogite Zone from the slab and its transfer to the overriding plate, and hence recording fluids flushing the subduction channel that were released in deeper slab devolatilisation (i.e. those recorded by the outermost core); and the rim to track the uplift after merging with the hanging and footwall tectonic units. In this interpretation, the core represents growth from internal release of B in prograde metamorphic reactions, and hence records locally derived fluids in equilibrium with the evolving blueschist to eclogite mineral paragenesis. Fluid heterogeneity on a local scale is possible at this stage (cf. Selverstone et al., 1992), but this will decrease as the temperature, and hence element diffusivities, increase. The inner core growth zones are large and subsequent core zones progressively narrower, which is in agreement with most B being released early in prograde metamorphism (e.g., Bebout et al., 1999; van Hinsberg et al., 2011a). The eclogite-facies minerals can be expected to contain little B, except for that sequestered in tourmaline (e.g., Bebout et al., 1993, 1999; Marschall et al., 2006), and the mantle growth zone is, therefore, interpreted to have formed from externally derived, metasomatising fluids. Addition of B can also explain the replacement of garnet by tourmaline (Fig. S-2d) despite it being a stable phase at these P–T conditions (see Zimmermann et al., 1994; Hoschek, 2007), because tourmaline is predicted to replace the aluminous phases preferentially as B is progressively added (van Hinsberg and Schumacher, 2007a). The lack of compositional zoning in the mantle growth zone suggests growth in a single event, followed by continuous rim growth. The lack of a clear compositional break between mantle and inner rim suggests a common fluid source, although this fluid changes in composition as the rocks are exhumed. The outer rim has a distinctly different composition, especially in XCa, and T and XMg display a kink (Fig. 1).
For these specific tourmaline compositions there is no crystal-chemical limitation to F incorporation (Henry and Dutrow, 2011), and F-contents likely track that of the fluid. High F-contents in the mantle zone match elevated F in associated titanite, which has been attributed to a high fluid F-activity (Franz and Spear, 1985). Whereas the outermost core has a similar F-content to that of the mantle zone and is, therefore, consistent with a fluid link between these growth zones as proposed above, this is not true for XCa (Fig. 1e). This indicates modification of slab-released fluids in the subduction channel prior to being recorded by tourmaline in the overriding plate, and is in agreement with predicted Ca and Na behaviour (e.g, Manning, 2004). Na concentrations in the fluid can be estimated from experimental tourmaline–fluid partitioning relationships (von Goerne et al., 2001; Berryman et al., 2015) combined with tourmaline a-sector Na concentrations. Calculated Na contents vary from 0.45 to 0.75 mol L-1 with P–T, and are highest for the outermost core. Sodium is likely a dominant solute in subduction-zone fluids (e.g., Manning, 2004), and these reconstructed Na contents indicate that the fluids released from the subducting slab are relatively dilute, in good agreement with theoretical considerations and experimental results (Selverstone et al., 1992; Manning, 2004; Kessel et al., 2005; Hermann et al., 2006; Spandler et al., 2007; Tsay et al., 2017).
Tourmaline is stable in acidic aqueous solutions and dissolves at high pH (e.g., Henry and Dutrow, 1996). A constraint on pH for the tourmaline mantle zone can be obtained from the reaction:
| 3 NaAlSi2O6 | + | 2 H+(aq) | = | NaAl3Si3O10(OH)2 | + | 2 Na+(aq) | + | 3 SiO2 |
| 3 jadeite | + | 2 H+(aq) | = | paragonite | + | 2 Na+(aq) | + | 3 quartz |
the Na concentration reconstructed above, and the jadeite and paragonite activities in omphacite and phengite mineral inclusions, respectively. Thermodynamic properties for minerals and aqueous species were calculated using the Holland and Powell (2011) database and its associated activity models (this database uses a modified version of the density model of Anderson et al. (1991) to calculate properties for aqueous species at elevated P and T). This results in a pH estimate of 0.8, which is 1.7 pH units below the neutral point of water at these conditions.
The F content of this solution can similarly be estimated from the reaction:
| CaTiSiO5 | + | NaAlSi2O6 | + | HF(aq) | = | CaAlSiO4F | + | TiO2 | + | 2 SiO2 | + | Na+(aq) | + | OH-(aq) |
| titanite | + | jadeite | + | HF(aq) | = | “F-titanite” | + | rutile | + | 2 quartz | + | Na+(aq) | + | OH-(aq) |
Thermodynamic data were sourced as above, except for thermodynamic properties and activity models for titanite, which were taken from Tropper et al. (2002), and the ∆Gf (T,P) for HF(aq), which was estimated from the fluorite solubility data of Tropper and Manning (2007). Estimated F-concentrations are 1.8 ppmm for the average composition of titanite inclusions, and 3.1 ppmm for the most F-rich titanite zones in these inclusions. This concentration is surprisingly low, which could indicate that species other than HF are the dominant F-species at these conditions (see also Tropper and Manning, 2007). An upper constraint on F-content is given by the saturation concentration of fluorite, which is approximately 1400 ppmm at these conditions (Tropper and Manning, 2007), when assuming m Cl(aq) = m Na(aq). Fluorite has been found in marbles of the Tauern Eclogite Zone (Franz and Spear, 1985), but not in this sample.
Tourmaline–Fluid Partition Coefficients
Mineral–fluid trace element partition coefficients allow fluid composition to be calculated from the compositions of the minerals that grew from this fluid, assuming equilibrium between minerals and fluid. At present, these D-values are not known for tourmaline, nor for titanite. These were estimated here by combining partitioning data for natural tourmaline–phengite and titanite–phengite mineral pairs in subduction-zone rocks from Syros, Greece (Marschall, 2005; Klemme et al., 2011) with experimentally determined high-P phengite–fluid D-values (Green and Adam, 2003) in an approach similar to that used by Keppler (1996). The D-values used are given in Table S-4.
Table S-4 Partition coefficients used in this study. Tourmaline–fluid and titanite–fluid coefficients were calculated by combining the phengite–tourmaline and phengite–titanite inter-mineral partition coefficients for Syros sample SY309B (Klemme et al., 2011; Marschall, 2005) with phengite–fluid experimental partition coefficients from Green and Adam (2003).
| Element | Phg/Tur | Phg/fluid | Tur/fluid | Ttn/fluid |
| Li | 5.3 | 2.0 | 0.4 | 0.004 |
| B | 0.004 | 0.94 | 233 | 0.001 |
| V | 1.25 | 223 | 179 | 327 |
| Cr | 3.0 | 287 | 95 | |
| Mn | 2.8 | 11 | 4.0 | 29 |
| Rb | 250 | 108 | 0.4 | 0.9 |
| Cs | 25 | 7.8 | 0.3 | 0.8 |
| Sr | 0.20 | 1.4 | 6.9 | 13 |
| Ba | 1635 | 91 | 0.06 | 0.15 |
| Ti | 0.40 | 75 | 187 | |
| Nb | 24 | 7.1 | 0.3 | 1929 |
| Ta | 34 | 51 | 1.5 | 471 |
| Zr | 2.2 | 2.9 | 1.4 | 21 |
| Hf | 4.0 | |||
| La | 3.6 | 3.8 | 1.1 | 19 |
| Ce | 3.1 | 3.3 | 1.1 | 155 |
| Nd | 3.3 | |||
| Sm | 4.0 | 6.1 | 1.5 | 174 |
| Eu | 5.4 | |||
| Gd | 1.6 | |||
| Dy | 1.7 | |||
| Er | 2.8 | |||
| Yb | 9.3 | |||
| Lu | 4.0 | 11 | 2.8 | 1618 |
| Y | 4.0 | 24 | 6.0 | |
| Pb | 4.2 | 43 | 10 | 116 |
| Th | 1.7 | 1.5 | 0.9 | 0.9 |
| U | 0.93 | 1.6 | 1.7 | 4.5 |
There are two main uncertainties in this approach. First, fluid concentrations reported by Green and Adam (2003) are based on an assumed total dissolved solid (TDS) content of 50 wt. %. This is higher than most experimental values (e.g., Kessel et al., 2005; Tsay et al., 2017), although high TDS values have been reported for fluid inclusions in eclogite minerals (e.g., Scambelluri and Phillipot, 2001). The 50 wt. % TDS assumption of Green and Adam (2003) only impacts the absolute concentrations calculated from the estimated Dtur/fl. Element ratios are unaffected. For example, if a TDS of 25 wt. % would be assumed instead, all fluid concentrations in Figure 2 would half, but the ratios plotted in Figure 3 would be identical.
Secondly, the Syros Dphg/tur values used to derive Dtur/fl are for ~1 GPa at 450 ˚C, whereas tourmaline growth took place from peak conditions of 2.1 GPa at 500 ˚C to rim retrograde conditions of 0.3 GPa and 350 ˚C (Fig. 1a). Moreover, the Dphg/fl data of Green and Adam (2003) are for experimental conditions of 3 GPa and 600–700 ˚C. This mismatch in P–T conditions can impact calculated fluid compositions, because D-values have a P–T dependence, both in their absolute values and in the relative D-values among the elements (e.g., Blundy and Wood, 2003). This therefore introduces uncertainty in both the absolute concentrations of the fluid calculated from tourmaline compositions and elemental patterns.
To evaluate the impact on calculated fluid compositions from changes in D-values with P–T, the D-values were modelled using Lattice-Strain Theory (LST; see Onuma et al., 1968; Brice, 1975; Blundy and Wood, 1994, 2003; Wood and Blundy, 2003). This allows for extrapolating a LST fit of Dphg/fl to the P–T conditions of interest using the P–T dependence of phengite lattice dimensions and elasticity and phengite solubility to account for changes in the partitioning behaviour of the mineral, and changes in the aqueous element speciation to model the fluid. Lattice dimensions, and changes therein with P–T, control a mineral’s trace element size preferences, lattice elasticity determines the contrast in partition coefficients among the elements, and mineral solubility their absolute values, whereas aqueous speciation impacts the availability of elements for incorporation into the mineral (see Blundy and Wood, 1994; Wood and Blundy, 2003; van Hinsberg et al., 2010, and references therein).
The experimental Dphg/fl values of Green and Adam (2003) were fit to LST equations to obtain r0, D0 and E parameters (the ideal site radius, D at this ideal radius, and site elasticity, respectively; Blundy and Wood, 1994). The r0 and E parameters were subsequently extrapolated to relevant P–T conditions using independently-determined P–T dependence of white mica lattice constants (r0: Guggenheim et al., 1987; Gatta et al., 2010, E: McNeil and Grimsditch, 1993; Curetti et al., 2006). The D0 parameter tracks changes in mineral solubility, but, unfortunately, literature data on white mica solubility and its dependence on P–T are inconsistent (cf. Melzer and Wunder, 2000; Green and Adam, 2003). Qualitatively, mineral solubility is expected to increase with temperature, but decrease with pressure, and the net effect on D0 may thus be small for the 200 ˚C and 2.4 GPa extrapolation required from experimental to Syros conditions. D0 was therefore assumed to be constant. Phengite–fluid partition coefficients can then be extrapolated to the P–T conditions of interest.
Two approaches were explored to calculate the Dtur/fl at conditions of Tauern tourmaline growth. In the first, the same approach as that used for Dphg/fl extrapolation was used. Dphg/fl extrapolated to 1 GPa and 450 ˚C was combined with minimum Syros Dphg/tur to estimate Dtur/fl at these conditions. These tourmaline-fluid D-values were subsequently fit to the LST equation, and r0 and E parameters were then extrapolated to Tauern conditions using tourmaline lattice systematics reported by Xu et al. (2016). Scarcity of D-values (e.g., only three data points for the 3+ elements), as well as uncertainties in element site occupancy in the tourmaline structure, complicates the LST fits. Moreover, changes in D0 cannot be modelled for lack of data on tourmaline solubility. As an alternative, Dtur/fl was calculated by extrapolating Dphg/fl to Tauern conditions and assuming constant Dphg/tur. Phengite–tourmaline D-values do appear to increase with pressure (Klemme et al., 2011), and this approach will thus lead to underestimated trace element concentrations in the fluid at high pressure. However, the LST fit of Dphg/fl is better constrained than that of Dtur/fl, as is the P–T dependence of phengite lattice dimensions and elasticity. The second approach is therefore preferred.
Aqueous element speciation and changes therein with P and T also impact partition coefficients by changing the availability of the species dominantly involved in element uptake (van Hinsberg et al., 2010). Whereas free ions become more abundant with increasing P, increasing T favours associated species (e.g., Manning, 2004). Metal-silicate species are likely important at subduction zone conditions (Manning et al., 2008; Wilke et al., 2012; Tsay et al., 2017) and may represent the dominant element uptake vector into silicate minerals, but lack of data on their stability constants precludes modelling of these species. Instead, the relative abundance of metal-(hydr)oxide species at varying P–T was modelled for a Tauern fluid composition at a tourmaline-reconstructed salinity of 0.6 mol L-1 NaCl using the hkf-model and the latest version of the SUPCRT database (Johnson et al., 1992). This makes the implicit assumption that (hydr)oxide species are a more important element uptake vector in phengite and tourmaline than free ions and Cl-species.
Figure S-3 shows the results of this modelling by comparing Ba/Th vs. La/Sm ratios in the reconstructed Tauern fluids as calculated assuming, in Figure S-3a, constant Dtur/fl, and, in Figure S-3b, variable Dtur/fl calculated from extrapolated Dphg/fl, assuming constant Dphg/tur. The overall trend is identical, but Ba/Th and La/Sm both shift to lower values. Fluids reconstructed from phengite inclusions in the tourmaline mantle remain consistent when using the extrapolated Dphg/fl (titanite D-values were not extrapolated and the titanite-reconstructed fluid is therefore not plotted). Changes in speciation are predicted to lower Ba/Th for the prograde tourmaline zones, and increase it for the retrograde rim, with the mantle zone unaffected. These shifts are entirely owing to changes in Ba-speciation, as changes in Th-speciation are expected to be negligible (Bailey and Ragnarsdottir, 1994). The impact on La/Sm is estimated to lower the ratio for all growth zones, but the shift is small given the similarity in speciation among the REE.

Figure S-3 Reconstructed Ba/Th vs. La/Sm ratios for subduction-zone fluids in the Tauern eclogite zone, as determined from tourmaline compositions (see Fig. 2 for locations of these points). (a) Calculated assuming constant D tur/fl, obtained by combining experimentally determined D phg/fl of Green and Adam (2003) with D phg/tur of Klemme et al. (2011). (b) Calculated from D phg/fl of Green and Adam (2003) extrapolated to relevant P–T conditions using phengite lattice systematics at constant D phg/tur (from Klemme et al., 2011). The impact of changing speciation on D tur/fl is shown by the thick arrows. Reconstructing fluid compositions with extrapolated D values results in lower Ba/Th in the fluid, with only minimal impact on La/Sm. The differences in Ba/Th between core and mantle become smaller, but the predicted effect of changes in speciation would re-introduce this difference. The gray uncertainty ellipses show the 1 standard deviation uncertainty in the ratios resulting from the spread in the trace element analyses of the minerals.
In conclusion, the impact of using estimated tourmaline–fluid element partition coefficients on results presented in Figure 3 is small and does not change its interpretation. There is too much uncertainty in the D-value LST extrapolations to make a quantitative assessment of the impact on the absolute concentrations shown in Figure 2. However, there is good agreement among the fluid concentrations reconstructed from tourmaline and from co-genetic phengite and titanite, suggesting that the precision in reconstructed fluid compositions is approximately an order of magnitude. The reconstructed concentrations of Ti are consistent with a rutile-saturated fluid as determined from rutile solubility experiments (Manning et al., 2008; Rapp et al., 2010; Hayden and Manning, 2011; Tsay et al., 2017), and LILE and REE concentrations similarly agree within an order of magnitude with eclogite-fluid experiments buffered by non-end-member allanite (Tsay et al., 2017) for the estimated F-contents in Tauern solutions derived above, as shown in Figure 2. The best estimate of the uncertainty in the absolute concentrations presented in Figure 2 is thus that these are precise and accurate to within an order of magnitude.
Data Sources for Figure 2
Fluid concentrations reconstructed from eclogite-facies minerals for other palaeo-subduction zones shown in Figure 2a were calculated from the average garnet, omphacite and phengite compositions reported for samples DM9 and DM30 from Dora Maira (Hermann, 2002), samples SY309, 412, 413 and 420 from Syros (Marschall, 2005) and samples 803, 1008 and 1101 from New Caledonia (Spandler et al., 2003). These mineral data were combined with the experimental mineral–fluid trace element partition coefficients of Green and Adam (2003) to reconstruct the compositions of the fluids from which these minerals formed. Experimental data shown are from Tsay et al. (2017) and represent the composition of a fluid in equilibrium with eclogite at 590 ˚C and 2.4 GPa in H2O, and at 680 ˚C and 2.5 GPa in a fluid with 1 mol L-1 NaF. Symbols with arrows indicate maximum values as defined by the relevant detection limit. Figure 2b shows MORB-normalised compositions of average volcanic arc rocks (calculated from the Georoc database – http://georoc.mpch-mainz.gwdg.de/), fluid inclusions in eclogite-facies olivine (samples AL9854B and AL9854, Scambelluri et al., 2001), the H2O-experiment of Tsay et al., (2017), and the median and associated interquartile range of the reconstructed fluids shown in Figure 2a for the Tauern, Dora Maira, Syros and New Caledonia palaeo-subduction zones.
Supplementary Information References
Bailey, E.H., Ragnarsdottir, K.V. (1994) Uranium and thorium solubilities in subduction zone fluids. Earth and Planetary Science Letters 124, 119–129.
Bebout, G.E., Ryan, J.G., Leeman, W.P. (1993) B–Be systematics in subduction-related metamorphic rocks: characterization of the subducted component. Geochimica et Cosmochimica Acta 57, 2227–2237.
Berryman, E., Wunder, B., Rhede, D. (2014) Synthesis of K-dominant tourmaline. American Mineralogist 99, 539–542.
Berryman, E., Wunder, B., Wirth, R., Rhede, D., Schettler, G., Franz, G., Heinrich, W. (2015) An experimental study on K and Na incorporation in dravitic tourmaline and insight into the origin of diamondiferous tourmaline from the Kokchetav Massif, Kazakhstan. Contributions to Mineralogy and Petrology 169, 1–16.
Blundy, J., Wood, B. (1994) Prediction of crystal-melt partition-coefficients from elastic-moduli. Nature 372, 452–454.
Blundy, J., Wood, B. (2003) Partitioning of trace elements between crystals and melts. Earth and Planetary Science Letters 210, 383–397.
Brice, J. (1975) Some thermodynamic aspects of the growth of strained crystals. Journal of Crystal Growth 28, 249–253.
Curetti, N., Levy, D., Pavese, A., Ivaldi, G. (2006) Elastic properties and stability of coexisting 3T and 2M(1) phengite polytypes. Physics and Chemistry of Minerals 32, 670–678.
Dyar, M., Wiedenbeck, M., Robertson, D., Cross, L., Delaney, J., Ferguson, K., Francis, C., Grew, E., Guidotti, C., Hervig, R., Hughes, J., Husler, J., Leeman, W., McGuire, A., Rhede, D., Rothe, H., Paul, R., Richards, I., Yates, M. (2001) Reference minerals for the Microanalysis of light elements. Geostandards Newsletter-The Journal of Geostandards and Geoanalysis 25, 441–463.
Franz, G., Spear, F. (1985) Aluminous titanite(sphene) from the eclogite zone, south-central Tauern Window, Austria. Chemical Geology 50, 33–46.
Gatta, G.D., Rotiroti, N., Lotti, P., Pavese, A., Curetti, N. (2010) Structural evolution of a 2M (1) phengite mica up to 11 GPa: an in situ single-crystal X-ray diffraction study. Physics and Chemistry of Minerals 37, 581–591.
Green, T.H., Adam, J. (2003) Experimentally-determined trace element characteristics of aqueous fluid from partially dehydrated mafic oceanic crust at 3.0 GPa, 650-700 oC. European Journal of Mineralogy 15, 815–830.
Guggenheim, S., Chang, Y.-H., Koster van Groos, A.F. (1987) Muscovite Dehydroxylation - High-Temperature Studies. American Mineralogist 72, 537–550.
Hayden, L.A., Manning, C.E. (2011) Rutile solubility in supercritical NaAlSi3O8-H2O fluids. Chemical Geology 284, 74–81. h Henry D.J. Dutrow B.L. (1996) Metamorphic tourmaline and its petrologic applications. In: Grew E.S., Anovitz L.M. (Eds.) Boron: Mineralogy, Petrology and Geochemistry. Reviews in Mineralogy 33, 503-557.
Henry, D.J., Dutrow, B.L. (2011) The Incorporation of Fluorine in Tourmaline: Internal Crystallographic Controls or External Environmental Influences? Canadian Mineralogist 49, 41–56.
Henry, D.J., Novak, M., Hawthorne, F.C., Ertl, A., Dutrow, B.L., Uher, P., Pezzotta, F. (2011) Nomenclature of the tourmaline-supergroup minerals. American Mineralogist 96, 895–913.
Hermann, J. (2002) Allanite: thorium and light rare earth element carrier in subducted crust. Chemical Geology 192, 289–306.
Hermann, J., Spandler, C., Hack, A., Korsakov, A. (2006) Aqueous fluids and hydrous melts in high-pressure and ultra-high pressure rocks: Implications for element transfer in subduction zones. Lithos 92, 399–417.
Holland, T.J.B., Powell, R. (2011) An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. Journal of Metamorphic Geology 29, 333–383.
Hollister, L. (1970) Origin, mechanism, and consequences of compositional sector-zoning in staurolite. American Mineralogist 55, 742–766.
Hoschek, G. (2007) Metamorphic peak conditions of eclogites in the Tauern Window, Eastern Alps, Austria: Thermobarometry of the assemblage garnet + omphacite + phengite + kyanite + quartz. Lithos 93, 1–16.
Johnson, J.W., Oelkers, E.H., Helgeson, H.C. (1992) SUPCRT92 - A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000˚C. Computers and Geosciences 18, 899–947.
Keppler, H. (1996) Constraints from partitioning experiments on the composition of subduction-zone fluids. Nature 380, 237–240.
Klemme, S., Marschall, H.R., Jacob, D.E., Prowatke, S., Ludwig, T. (2011) Trace-Element Partitioning and Boron Isotope Fractionation Between White Mica and Tourmaline. Canadian Mineralogist 49, 165–176.
Klimm, K., Blundy, J.D., Green, T.H. (2008) Trace element partitioning and accessory phase saturation during H2O-saturated melting of basalt with implications for subduction zone chemical fluxes. Journal of Petrology 49, 523–553.
Manning, C.E. (2004) The chemistry of subduction-zone fluids. Earth and Planetary Science Letters 223, 1–16.
Manning, C.E., Wilke, M., Schmidt, C., Cauzid, J. (2008) Rutile solubility in albite-H2O and Na2Si3O7-H2O at high temperatures and pressures by in-situ synchrotron radiation micro-XRF. Earth and Planetary Science Letters 272, 730–737.
Marschall, H.R. (2005) Lithium, Beryllium and Boron in High-Pressure Metamorphic Rocks from Syros (Greece). PhD thesis, Universität Heidelberg. http://www.ub.uni-heidelberg.de/archiv/5634.
Marschall, H.R., Altherr, R., Ludwig, T., Kalt, A., Gméling, K., Kasztovszky, Z.S. (2006) Partitioning and budget of Li, Be and B in high-pressure metamorphic rocks. Geochimica et Cosmochimica Acta 70, 4750–4769.
Marschall, H.R., Korsakov, A.V., Luvizotto, G.L., Nasdala, L, Ludwig, T. (2009) On the occurrence and boron isotopic composition of tourmaline in (ultra)high-pressure metamorphic rocks. Journal of the Geological Society 166, 811–823.
McNeil, L.E., Grimsditch, M. (1993) Elastic-Moduli of Muscovite Mica. Journal of Physics-Condensed Matter 5, 1681–1690.
Melzer, S., Wunder, B. (2000) Island-arc basalt alkali ratios: Constraints from phengite-fluid partitioning experiments. Geology 28, 583–586.
Onuma, N., Higuchi, H., Wakita, H., Nagasawa, H. (1968) Trace element partitioning between two pyroxenes and the host lava. Earth and Planetary Science Letters 5, 47–51.
Rapp, J.F., Klemme, S., Butler, I.B., Harley, S.L. (2010) Extremely high solubility of rutile in chloride and fluoride-bearing metamorphic fluids: An experimental investigation. Geology 38, 323–326.
Scambelluri, M., Phillipot, P. (2001) Deep fluids in subduction zones, Lithos 55, 213–227.
Scambelluri, M., Bottazzi, P., Trommsdorff, V., Vannucci, R., Hermann, J., Gomez-Pugnaire, M.T., Sanchez-Vizcaino, V.L. (2001) Incompatible element-rich fluids released by antigorite breakdown in deeply subducted mantle. Earth and Planetary Science Letters 192, 457–470.
Selverstone, J., Franz, G., Thomas, S., Getty, S. (1992) Fluid variability in 2 GPa eclogites as an indicator of fluid behavior during subduction. Contributions to Mineralogy and Petrology 112, 341–357.
Shimizu, R., Ogasawara, Y. (2013) Diversity of potassium-bearing tourmalines in diamondiferous Kokchetav UHP metamorphic rocks: A geochemical recorder from peak to retrograde metamorphic stages. Journal of Asian Earth Sciences 63, 39–55.
Spandler, C., Hermann, J., Arculus, R., Mavrogenes, J. (2003) Redistribution of trace elements during prograde metamorphism from lawsonite blueschist to eclogite facies; implications for deep subduction-zone processes. Contributions to Mineralogy and Petrology 146, 205–222.
Spandler, C., Mavrogenes, J., Hermann, J. (2007) Experimental constraints on element mobility from subducted sediments using high-P synthetic fluid/melt inclusions. Chemical Geology 239, 228–249.
Syracuse, E.M., van Keken, P.E., Abers, G.A. (2010) The global range of subduction zone thermal models. Physics of Earth and Planetary Interiors 183, 73–90.
Tropper, P., Manning, C.E. (2007). The solubility of fluorite in H2O and H2O-NaCl at high pressure and temperature. Chemical Geology 242, 299–306.
Tropper, P., Manning, C.E., Essene, E.J. (2002). The substitution of Al and F in titanite at high pressure and temperature: Experimental constraints on phase relations and solid solution properties. Journal of Petrology 43, 1787–1814.
Tsay, A., Zajacz, Z., Ulmer, P., Sanchez-Valle, C. (2017) Mobility of major and trace elements in the eclogite-fluid system and element fluxes upon slab dehydration. Geochimica et Cosmochimica Acta 198, 70–91.
van Hinsberg, V.J., Schumacher, J.C. (2007a) Using estimated thermodynamic properties to model accessory phases: the case of tourmaline. Journal of Metamorphic Geology 25, 769–779.
van Hinsberg, V.J., Schumacher, J.C. (2007b) Intersector element partitioning in tourmaline: a potentially powerful single crystal thermometer. Contributions to Mineralogy and Petrology 153, 289–301.
van Hinsberg, V.J., Schumacher, J.C., Kearns, S., Mason, P.R.D., Franz, G. (2006) Hourglass sector zoning in metamorphic tourmaline and resultant major and trace-element fractionation. American Mineralogist 91, 717–728.
van Hinsberg, V.J., Migdisov, A.A., Williams-Jones, A.E. (2010) Reading the mineral record of fluid composition from element partitioning. Geology 38, 847–850.
van Hinsberg, V.J., Henry, D.J., Dutrow, B.L. (2011a) Tourmaline as a petrologic forensic mineral: A unique recorder of its geologic past. Elements 7, 327–332.
van Hinsberg, V.J., Henry, D.J., Marschall, H.R. (2011b) Tourmaline: an Ideal Indicator of Its Host Environment. Canadian Mineralogist 49, 1–16.
von Goerne, G., Franz, G., Heinrich, W. (2001) Synthesis of tourmaline solid solutions in the system Na2O-MgO-Al2O3-SiO2-B2O3-H2O-HCl and the distribution of Na between tourmaline and fluid at 300 to 700 °C and 200 MPa. Contributions to Mineralogy and Petrology 141, 160–173.
Wilke, M., Schmidt, C., Dubrail, J., Appel, K., Borchert, M., Kvashnina, K., Manning, C.E. (2012) Zircon solubility and zirconium complexation in H2O+Na2O+SiO2 ± Al2O3 fluids at high pressure and temperature. Earth and Planetary Science Letters 349, 15–25.
Wood, B.J., Blundy, J.D. (2003) Trace Element Partitioning under Crustal and Uppermost Mantle Conditions: The Influences of Ionic Radius, Cation Charge, Pressure, and Temperature. Treatise on Geochemistry 2, 395–424.
Xu, J., Kuang, Y., Zhang, B., Liu, Y., Fan, D., Li, X., Xie, H. (2016) Thermal equation of state of natural tourmaline at high pressure and temperature. Physics and Chemistry of Minerals 43, 315–326.
Zimmermann, R., Hammerschmidt, K., Franz, G. (1994) Eocene high pressure metamorphism in the Penninic units of the Tauern Window (Eastern Alps): evidence from 40Ar-39Ar dating and petrological investigations. Contributions to Mineralogy and Petrology 117, 175–186.
Figures and Tables
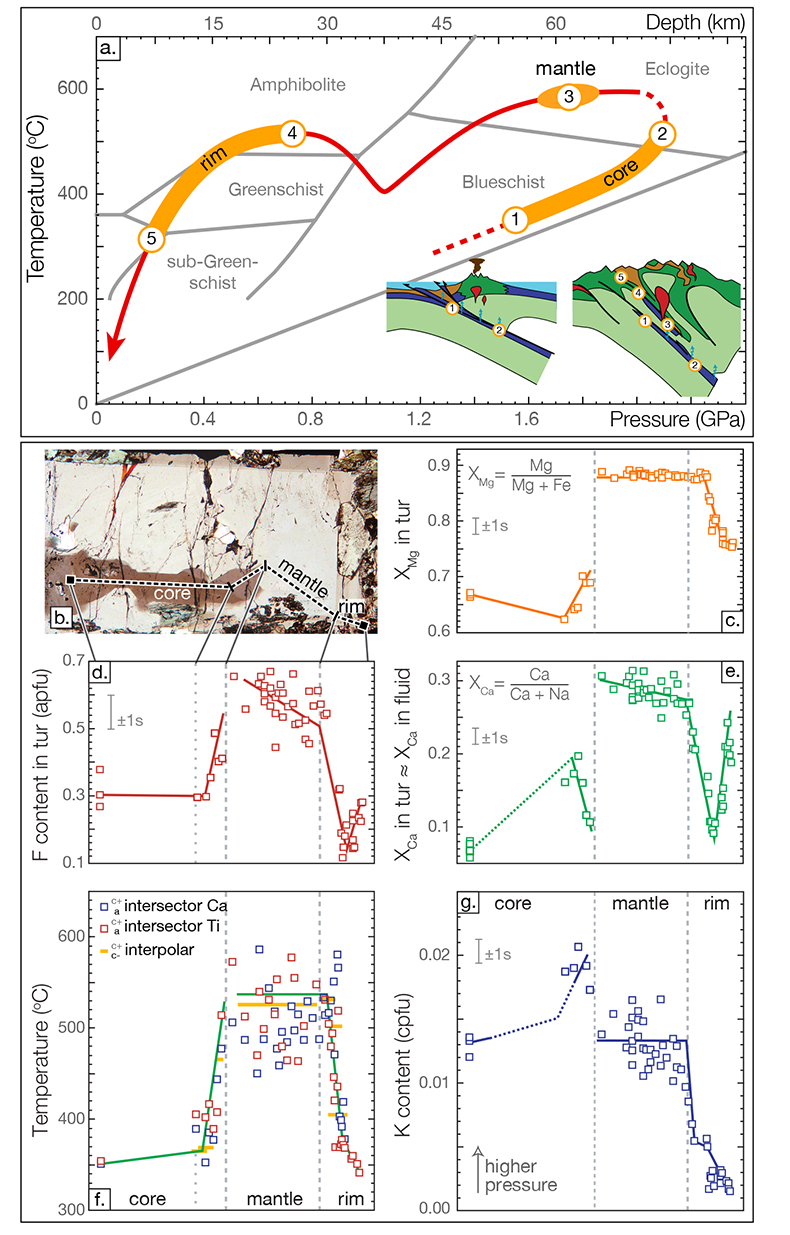
Figure 1 Reading the Tauern tourmaline record. (a) Tauern Eclogite Zone P–T path (Zimmermann et al., 1994
Zimmermann, R., Hammerschmidt, K., Franz, G. (1994) Eocene high pressure metamorphism in the Penninic units of the Tauern Window (Eastern Alps): evidence from 40Ar-39Ar dating and petrological investigations. Contributions to Mineralogy and Petrology 117, 175–186.
) with conditions recorded by tourmaline growth zones shown. Inset cartoons locate these in a schematic subduction-zone cross-section. (b) PPL image of the tourmaline transect. (c) XMg sharply defines the tourmaline growth zones. (d) F-content in tourmaline, >0.5 apfu F in the outer core and mantle. (e) XCa of tourmaline, which tracks the XCa in the coexisting fluid (von Goerne et al., 2011von Goerne, G., Franz, G. van Hinsberg, V.J. (2011) Experimental determination of Na–Ca distribution between tourmaline and fluid in the system CaO–Na2O–MgO–Al2O3–SiO2–B2O3–H2O. Canadian Mineralogist 49, 137–152.
). (f) Temperature transect across the tourmaline grain calculated from inter-sector and inter-polar thermometry. (g) Tourmaline K-content, which acts as a qualitative barometer. Uncertainties shown are the typical 1s analytical precision.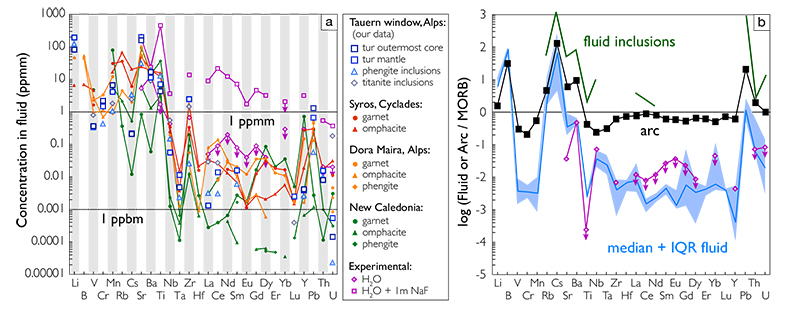
Figure 2 (a) Element concentrations in fluids reconstructed from the Tauern tourmaline outer core and mantle and its mineral inclusions (blue symbols), and eclogite minerals from other subduction-zone terrains show a consistent pattern. (b) MORB-normalised concentrations for average arc magma parallel those of subduction-zone fluids supporting a genetic link. See the Supplementary Information for data sources and calculation method. Uncertainties are given as the Interquartile Range (IQR) and maximum estimates marked with an arrow.
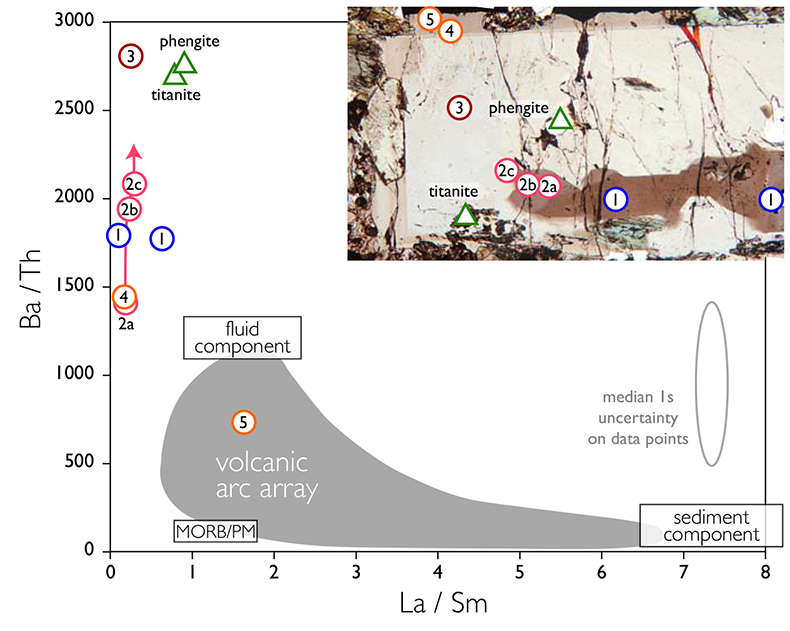
Figure 3 Fluid compositions reconstructed from tourmaline (circles) and its mineral inclusions (triangles), show a consistent, high Ba/Th at low La/Sm signature. Addition of this fluid to the arc-magmatism source region can explain the trend to elevated Ba/Th compared to mid-ocean ridge basalt (MORB) and primitive mantle (PM).
Back to article
Supplementary Figures and Tables
Table S-1 WDS electron-microprobe data for tourmaline growth zones, mineral inclusions in tourmaline, and matrix minerals. The values shown are the average for multiple analyses and their associated 1 standard deviation spread, except for omphacite where only one analysis was available. Normalisation factors used are the sum of cations for omphacite, titanite, plagioclase, amphibole and calcite, or the oxygen sum for phengite and biotite. Tourmaline was normalised to the sum of the elements residing at the Y, Z and T sites = 15, or Si = 6 cpfu, if the former resulted in Si > 6 cpfu.
| Tourmaline growth zones | ||||||||||||||||
| inner core | 1s | mid core | 1s | outer core 1 | 1s | outer core 2 | 1s | mantle | 1s | inner rim | 1s | mid rim | 1s | outer rim | 1s | |
| SiO2 | 37.9 | 0.4 | 38.1 | 0.4 | 38.3 | 0.2 | 38.2 | 0.7 | 38.7 | 0.5 | 36.0 | 0.2 | 35.9 | 0.3 | 36.2 | 0.3 |
| TiO2 | 0.44 | 0.05 | 0.39 | 0.03 | 0.42 | 0.10 | 0.25 | 0.03 | 0.19 | 0.02 | 0.18 | 0.05 | 0.36 | 0.09 | 0.35 | 0.08 |
| Al2O3 | 29.1 | 0.3 | 29.3 | 0.2 | 29.7 | 0.7 | 30.8 | 0.1 | 31.8 | 0.3 | 28.2 | 0.3 | 28.7 | 0.2 | 28.4 | 0.4 |
| MgO | 10.3 | 0.4 | 10.0 | 0.2 | 10.2 | 0.2 | 10.1 | 0.1 | 12.8 | 0.1 | 10.7 | 0.4 | 9.5 | 0.3 | 9.5 | 0.3 |
| MnO | 0.004 | 0.008 | 0.01 | 0.01 | 0.004 | 0.006 | 0.01 | 0.02 | 0.007 | 0.009 | 0.008 | 0.007 | 0.002 | 0.004 | 0.007 | 0.008 |
| FeO | 9.67 | 0.84 | 10.30 | 0.43 | 9.06 | 1.01 | 8.08 | 0.05 | 3.04 | 0.10 | 2.73 | 0.31 | 3.79 | 0.48 | 4.43 | 0.82 |
| CaO | 1.2 | 0.1 | 0.93 | 0.06 | 1.1 | 0.1 | 0.64 | 0.05 | 1.6 | 0.2 | 1.3 | 0.3 | 0.56 | 0.08 | 0.9 | 0.2 |
| Na2O | 2.48 | 0.05 | 2.59 | 0.01 | 2.54 | 0.10 | 2.83 | 0.02 | 2.32 | 0.10 | 2.1 | 0.2 | 2.49 | 0.09 | 2.1 | 0.2 |
| K2O | 0.09 | 0.02 | 0.095 | 0.002 | 0.09 | 0.01 | 0.092 | 0.006 | 0.06 | 0.01 | 0.010 | 0.003 | 0.013 | 0.002 | 0.012 | 0.002 |
| F | 0.6 | 0.3 | 0.60 | 0.01 | 0.9 | 0.1 | 0.82 | 0.02 | 1.2 | 0.1 | 0.54 | 0.06 | 0.35 | 0.08 | 0.51 | 0.10 |
| norm. factor | Y + Z + T = 15 | Y + Z + T = 15 | Y + Z + T = 15 | Y + Z + T = 15 | Y + Z + T = 15 | Si = 6 | Si = 6 | Si = 6 | ||||||||
| Si | 5.92 | 0.02 | 5.93 | 0.02 | 5.95 | 0.03 | 5.94 | 0.08 | 5.93 | 0.04 | 6 | - | 6 | - | 6 | - |
| Ti | 0.052 | 0.006 | 0.046 | 0.003 | 0.049 | 0.012 | 0.029 | 0.004 | 0.022 | 0.002 | 0.022 | 0.006 | 0.05 | 0.01 | 0.04 | 0.01 |
| Al | 5.36 | 0.02 | 5.37 | 0.01 | 5.4 | 0.1 | 5.65 | 0.04 | 5.74 | 0.05 | 5.54 | 0.08 | 5.66 | 0.06 | 5.56 | 0.04 |
| Mg | 2.40 | 0.08 | 2.31 | 0.02 | 2.37 | 0.05 | 2.33 | 0.02 | 2.91 | 0.04 | 2.66 | 0.10 | 2.37 | 0.06 | 2.34 | 0.07 |
| Mn | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 | 0.002 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 |
| Fe | 1.3 | 0.1 | 1.34 | 0.06 | 1.2 | 0.1 | 1.05 | 0.01 | 0.39 | 0.01 | 0.38 | 0.04 | 0.53 | 0.07 | 0.6 | 0.1 |
| Ca | 0.19 | 0.02 | 0.156 | 0.009 | 0.18 | 0.02 | 0.11 | 0.01 | 0.26 | 0.03 | 0.23 | 0.05 | 0.10 | 0.01 | 0.16 | 0.03 |
| Na | 0.75 | 0.02 | 0.781 | 0.007 | 0.76 | 0.03 | 0.85 | 0.01 | 0.69 | 0.03 | 0.68 | 0.05 | 0.81 | 0.04 | 0.68 | 0.06 |
| K | 0.018 | 0.003 | 0.0189 | 0.0002 | 0.018 | 0.003 | 0.018 | 0.001 | 0.012 | 0.003 | 0.002 | 0.001 | 0.0027 | 0.0005 | 0.0026 | 0.0004 |
| F | 0.3 | 0.1 | 0.295 | 0.002 | 0.42 | 0.07 | 0.41 | 0.01 | 0.57 | 0.06 | 0.28 | 0.03 | 0.18 | 0.04 | 0.27 | 0.06 |
| XMg | 0.66 | 0.03 | 0.63 | 0.01 | 0.67 | 0.03 | 0.69 | 0.00 | 0.88 | 0.00 | 0.87 | 0.02 | 0.82 | 0.02 | 0.79 | 0.04 |
| dravite | 0.50 | 0.01 | 0.51 | 0.01 | 0.52 | 0.04 | 0.60 | 0.01 | 0.62 | 0.03 | 0.60 | 0.04 | 0.5 | 0.3 | 0.5 | 0.2 |
| schorl | 0.27 | 0.03 | 0.29 | 0.01 | 0.26 | 0.01 | 0.27 | 0.00 | 0.08 | 0.00 | 0.09 | 0.02 | 0.12 | 0.07 | 0.13 | 0.05 |
| uvite | 0.19 | 0.02 | 0.16 | 0.01 | 0.18 | 0.02 | 0.11 | 0.01 | 0.26 | 0.03 | 0.23 | 0.05 | 0.08 | 0.04 | 0.14 | 0.06 |
| foitite | 0.037 | 0.004 | 0.044 | 0.002 | 0.042 | 0.015 | 0.023 | 0.018 | 0.040 | 0.014 | 0.09 | 0.01 | 0.3 | 0.4 | 0.3 | 0.3 |
| Mineral inclusions in tourmaline mantle | Matrix minerals | |||||||||||||||
| omphacite | 1s | phengite | 1s | titanite | 1s | biotite | 1s | plagioclase | amphibole | calcite | ||||||
| SiO2 | 53.9 | - | 48 | 1 | 29.6 | 0.4 | 37.1 | 1.7 | 59.1 | 0.2 | 51.5 | 0.8 | 0.03 | 0.02 | ||
| TiO2 | 0.04 | - | 0.20 | 0.07 | 34 | 3 | 0.5 | 0.4 | < d.l. | - | 0.09 | 0.03 | 0.01 | 0.02 | ||
| Al2O3 | 5.9 | - | 24 | 1 | 4 | 2 | 14.6 | 0.9 | 21.5 | 0.2 | 6 | 1 | 0.001 | 0.001 | ||
| MgO | 11.3 | - | 4.6 | 0.5 | 0.04 | 0.02 | 19.7 | 0.7 | 0.01 | 0.01 | 17.7 | 0.8 | 0.47 | 0.09 | ||
| MnO | 0.03 | - | 0.004 | 0.006 | 0.009 | 0.008 | 0.02 | 0.01 | < d.l. | - | 0.02 | 0.01 | 0.09 | 0.10 | ||
| FeO | 5.3 | - | 2.0 | 0.3 | 0.7 | 0.3 | 9.7 | 0.8 | 0.14 | 0.06 | 8.3 | 0.5 | 0.33 | 0.09 | ||
| CaO | 17.9 | - | < d.l. | - | 28.3 | 0.3 | < d.l. | - | 3.47 | 0.09 | 10 | 2 | 55 | 1 | ||
| Na2O | 4.2 | - | 0.2 | 0.2 | 0.02 | 0.01 | 0.16 | 0.07 | 9.5 | 0.1 | 2.0 | 0.8 | < d.l. | - | ||
| K2O | 0.002 | - | 10.8 | 0.5 | 0.001 | 0.002 | 9 | 2 | 0.06 | 0.01 | 0.17 | 0.04 | < d.l. | - | ||
| F | 0.01 | - | 0.6 | 0.2 | 1.1 | 0.6 | 1.6 | 0.4 | 0.05 | 0.08 | 0.54 | 0.09 | 0.02 | 0.03 | ||
| norm. factor | ∑cat = 4 | O = 11 | ∑cat = 3 | O = 11 | ∑cat = 5 | ∑cat = 15 | ∑cat = 1 | |||||||||
| Si | 1.97 | - | 3.44 | 0.06 | 0.978 | 0.008 | 2.83 | 0.08 | 2.783 | 0.001 | 7.3 | 0.1 | 0.0005 | 0.0003 | ||
| Ti | 0.001 | - | 0.011 | 0.004 | 0.84 | 0.07 | 0.03 | 0.02 | - | - | 0.010 | 0.003 | 0.0002 | 0.0002 | ||
| Al | 0.25 | - | 1.99 | 0.09 | 0.15 | 0.06 | 1.3 | 0.1 | 1.191 | 0.007 | 0.9 | 0.2 | 0.00002 | 0.00003 | ||
| Mg | 0.62 | - | 0.49 | 0.06 | 0.0018 | 0.0009 | 2.24 | 0.09 | 0.001 | 0.001 | 3.7 | 0.2 | 0.012 | 0.002 | ||
| Mn | 0.001 | - | 0.0002 | 0.0004 | 0.0003 | 0.0002 | 0.001 | 0.001 | - | - | 0.003 | 0.001 | 0.001 | 0.001 | ||
| Fe | 0.16 | - | 0.12 | 0.02 | 0.019 | 0.008 | 0.62 | 0.05 | 0.005 | 0.002 | 0.99 | 0.05 | 0.005 | 0.001 | ||
| Ca | 0.70 | - | - | - | 1.004 | 0.003 | - | - | 0.175 | 0.004 | 1.5 | 0.2 | 0.982 | 0.002 | ||
| Na | 0.29 | - | 0.03 | 0.02 | 0.001 | 0.001 | 0.02 | 0.01 | 0.86 | 0.01 | 0.6 | 0.2 | - | - | ||
| K | 0.0001 | - | 0.98 | 0.04 | 0.00005 | 0.00008 | 0.9 | 0.2 | 0.004 | 0.001 | 0.03 | 0.01 | - | - | ||
| F | 0.001 | - | 0.14 | 0.05 | 0.12 | 0.06 | | 0.4 | 0.1 | 0.01 | 0.01 | 0.24 | 0.04 | 0.001 | 0.002 | |
Table S-2 Laser-ablation ICP-MS data for Ba, Th, La and Sm in tourmaline, and phengite and titanite inclusions. Most data represent the mean of multiple measurements. Uncertainties are the 1 standard deviation on the mean, or the count statistical error if only one measurement is available. Partition coefficients are those of Green and Adam (2003), combined with Dphg/tur from Klemme et al. (2011) and are used to calculate the Ba/Th and La/Sm ratios for the fluid. Analysis locations are shown in Figure 1.
| Concentrations in ppm | Partition coefficients (min/fl) | Ratios | ||||||||||||||
| Mineral | Ba | 1 std | Th | 1 std | La | 1 std | Sm | 1 std | Ba | Th | La | Sm | Ba/Th fluid | La/Sm fluid | ||
| Tur core - 1 | 1.1 | 0.3 | 0.009 | 0.006 | 0.008 | 0.007 | 0.09 | 0.07 | 0.06 | 0.88 | 1.08 | 1.50 | 1795 | 0.12 | ||
| Tur core - 1 | 3.0 | 0.7 | 0.03 | 0.02 | 0.04 | 0.02 | 0.08 | 0.10 | 0.06 | 0.88 | 1.08 | 1.50 | 1770 | 0.64 | ||
| Tur core - 2a | 1.7 | 0.4 | 0.02 | 0.01 | 0.03 | 0.02 | 0.20 | 0.16 | 0.06 | 0.88 | 1.08 | 1.50 | 1397 | 0.18 | ||
| Tur core - 2b | 1.9 | 1.0 | 0.02 | 0.02 | 0.012 | 0.006 | 0.08 | 0.09 | 0.06 | 0.88 | 1.08 | 1.50 | 1950 | 0.22 | ||
| Tur core - 2c | 0.9 | 0.2 | 0.007 | 0.008 | 0.03 | 0.01 | 0.13 | 0.06 | 0.06 | 0.88 | 1.08 | 1.50 | 2088 | 0.30 | ||
| Tur mantle - 3 | 2 | 2 | 0.011 | 0.005 | 0.005 | 0.006 | 0.028 | 0.008 | 0.06 | 0.88 | 1.08 | 1.50 | 2813 | 0.27 | ||
| Tur rim - 4 | 0.81 | 0.07 | 0.009 | 0.001 | 0.004 | 0.003 | 0.04 | 0.01 | 0.06 | 0.88 | 1.08 | 1.50 | 1443 | 0.17 | ||
| Tur rim - 5 | 0.8 | 0.9 | 0.02 | 0.02 | 0.036 | 0.003 | 0.031 | 0.005 | 0.06 | 0.88 | 1.08 | 1.50 | 729 | 1.64 | ||
| Phengite | 1908 | 183 | 0.012 | 0.005 | 0.02 | 0.01 | 0.04 | 0.04 | 91.2 | 1.53 | 3.84 | 6.07 | 2782 | 0.92 | ||
| Titanite | 55 | 73 | 0.6 | 0.7 | 0.45 | 0.04 | 5 | 3 | 0.15 | 4.4 | 18.503 | 173.83 | 2710 | 0.79 | ||
Table S-3 Laser-ablation ICP-MS trace element data for tourmaline, and phengite and titanite inclusions. The tourmaline data represent the mean of multiple measurements and the uncertainties reported are 1 standard deviation on this mean. For phengite and titanite, we report the count statistical error. Measurements were corrected for differences in ablation behaviour between standards and samples by normalising to EMP Si-content.
| Conc in ppm | Tourmaline | Inclusions | ||||||
| Element | Rim of core | 1 std | Mantle | 1 std | Phengite | 1 std cse | Titanite | 1 std cse |
| Li | 31 | 3 | 74 | 10 | 250 | 6 | 19 | 2 |
| V | 65 | 4 | 64.1 | 0.8 | 71.6 | 0.9 | 94 | 3 |
| Cr | 209 | 20 | 139 | 44 | 122 | 2 | 299 | 9 |
| Mn | 26.6 | 0.6 | 17 | 1 | 11.6 | 0.4 | 17.1 | 0.8 |
| Cs | 0.07 | 0.04 | 0.07 | 0.02 | 26.1 | 0.3 | 0.07 | 0.03 |
| Sr | 1107 | 28 | 1376 | 132 | 42.4 | 0.8 | 387 | 13 |
| Ba | 0.9 | 0.6 | 0.6 | 0.2 | 1778 | 17 | 3.8 | 0.6 |
| Ti | 805 | 116 | 1312 | 461 | 941 | 90 | 188700 | 15341 |
| Nb | < d.l. | 0.02 | 1.04 | 0.07 | 77 | 2 | ||
| Ta | 0.007 | 0.006 | 0.02 | 0.12 | 0.02 | 3.5 | 0.2 | |
| Zr | < d.l. | 3 | 6 | 0.07 | 0.03 | 16 | 4 | |
| Hf | < d.l. | 0.1 | 0.1 | 0.003 | 0.005 | 0.5 | 0.1 | |
| La | < d.l. | 0.001 | 0.001 | 0.01 | 0.01 | 0.48 | 0.05 | |
| Ce | < d.l. | 0.014 | 0.002 | 0.010 | 0.006 | 3.4 | 0.2 | |
| Sm | < d.l. | 0.024 | 0.006 | 0.04 | 0.04 | 3.4 | 0.4 | |
| Lu | < d.l. | 0.007 | 0.002 | < d.l. | 0.56 | 0.07 | ||
| Y | 0.03 | 0.02 | 0.02 | 0.03 | 0.06 | 0.02 | 36 | 2 |
| Pb | 13 | 4 | 6.7 | 0.3 | 2.4 | 0.7 | 14 | 4 |
| Th | 0.01 | 0.01 | 0.01 | 0.01 | 0.015 | 0.009 | 0.09 | 0.03 |
| U | 0.002 | 0.001 | 0.008 | 0.006 | 0.000 | 0.004 | 1.3 | 0.1 |
< d.l. Value below detection limit
Back to article | Download in Excel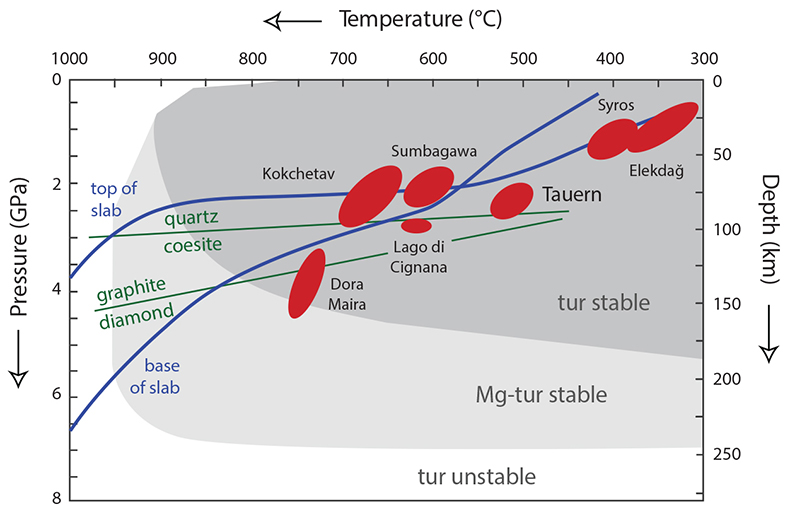
Figure S-1 Tourmaline is stable for most of the P–T range typically found in subduction zones, and is an accessory phase in exposed subduction-zone lithologies for all the terrains shown. Typical subduction path (blue solid lines) from Syracuse et al. (2010) and P–T estimates for subduction-zone terrains from Zimmermann et al. (1994) and Marschall et al. (2009). Tourmaline stability fields from van Hinsberg et al. (2011b).
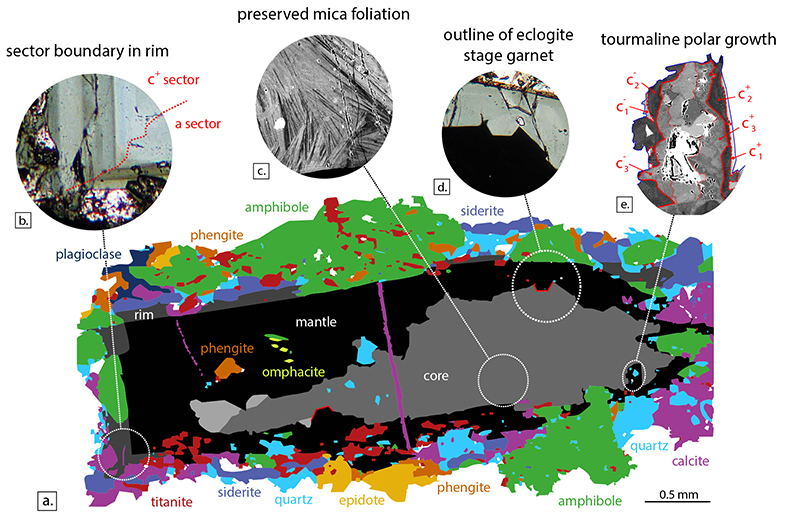
Figure S-2 Petrography of the Tauern tourmaline grain used in this study. (a) Mineral-map showing its inclusion and matrix mineralogy, and the core, mantle and rim zones of the tourmaline grain. This map is based on back-scattered electron imaging and WDS-EMP element mapping for Ca, Mg, Ti, Fe, Si and Na. (b) Example of hourglass sector zoning in the outermost rim in PPL microscopy. (c) Back-scattered electron image of compositional heterogeneity in the tourmaline core, outlining the foliation of protolith mica grains overgrown by tourmaline. (d) Outline of an idiomorphic eclogite-stage garnet grain replaced during growth of the mantle zone in PPL microscopy. (e) Back-scattered electron image of c+-c- polar tourmaline growth.
Table S-4 Partition coefficients used in this study. Tourmaline–fluid and titanite–fluid coefficients were calculated by combining the phengite–tourmaline and phengite–titanite inter-mineral partition coefficients for Syros sample SY309B (Klemme et al., 2011; Marschall, 2005) with phengite-fluid experimental partition coefficients from Green and Adam (2003).
| Element | Phg/Tur | Phg/fluid | Tur/fluid | Ttn/fluid |
| Li | 5.3 | 2.0 | 0.4 | 0.004 |
| B | 0.004 | 0.94 | 233 | 0.001 |
| V | 1.25 | 223 | 179 | 327 |
| Cr | 3.0 | 287 | 95 | |
| Mn | 2.8 | 11 | 4.0 | 29 |
| Rb | 250 | 108 | 0.4 | 0.9 |
| Cs | 25 | 7.8 | 0.3 | 0.8 |
| Sr | 0.20 | 1.4 | 6.9 | 13 |
| Ba | 1635 | 91 | 0.06 | 0.15 |
| Ti | 0.40 | 75 | 187 | |
| Nb | 24 | 7.1 | 0.3 | 1929 |
| Ta | 34 | 51 | 1.5 | 471 |
| Zr | 2.2 | 2.9 | 1.4 | 21 |
| Hf | 4.0 | |||
| La | 3.6 | 3.8 | 1.1 | 19 |
| Ce | 3.1 | 3.3 | 1.1 | 155 |
| Nd | 3.3 | |||
| Sm | 4.0 | 6.1 | 1.5 | 174 |
| Eu | 5.4 | |||
| Gd | 1.6 | |||
| Dy | 1.7 | |||
| Er | 2.8 | |||
| Yb | 9.3 | |||
| Lu | 4.0 | 11 | 2.8 | 1618 |
| Y | 4.0 | 24 | 6.0 | |
| Pb | 4.2 | 43 | 10 | 116 |
| Th | 1.7 | 1.5 | 0.9 | 0.9 |
| U | 0.93 | 1.6 | 1.7 | 4.5 |

Figure S-3 Reconstructed Ba/Th vs. La/Sm ratios for subduction-zone fluids in the Tauern eclogite zone, as determined from tourmaline compositions (see Fig. 2 for locations of these points). (a) Calculated assuming constant D tur/fl, obtained by combining experimentally determined D phg/fl of Green and Adam (2003) with D phg/tur of Klemme et al. (2011). (b) Calculated from D phg/fl of Green and Adam (2003) extrapolated to relevant P–T conditions using phengite lattice systematics at constant D phg/tur (from Klemme et al., 2011). The impact of changing speciation on D tur/fl is shown by the thick arrows. Reconstructing fluid compositions with extrapolated D values results in lower Ba/Th in the fluid, with only minimal impact on La/Sm. The differences in Ba/Th between core and mantle become smaller, but the predicted effect of changes in speciation would re-introduce this difference. The gray uncertainty ellipses show the 1 standard deviation uncertainty in the ratios resulting from the spread in the trace element analyses of the minerals.