FeTiMM – A new oxybarometer for mafic to felsic magmas
Affiliations | Corresponding Author | Cite as | Funding informationKeywords: oxybarometry, FeTiMM, magnetite, element partitioning
- Share this article





Article views:9,202Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
Abstract
Figures and Tables
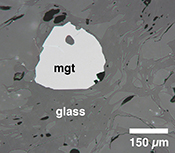
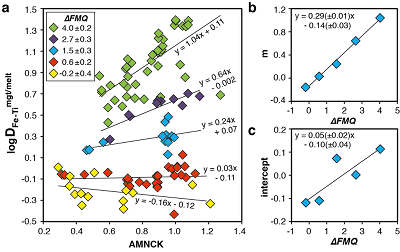 Figure 1 Development of the FeTiMM model. (a) 109 experimental data points (59 ilmenite-saturated; 50 ilmenite-undersaturated) were split into five groups of similar oxygen fugacity, through which linear regressions were fit. DFe–Timgt/melt = (DFeOtotmgt/melt)/(DTiO2mgt/melt); AMCNK = molar Al2O3/(MgO + CaO + Na2O + K2O). (b,c) Variation of the slopes and intercepts of the linear fits in (a) as a function of oxygen fugacity expressed in log unit deviations from the fayalite-magnetite-quartz (FMQ) buffer. | 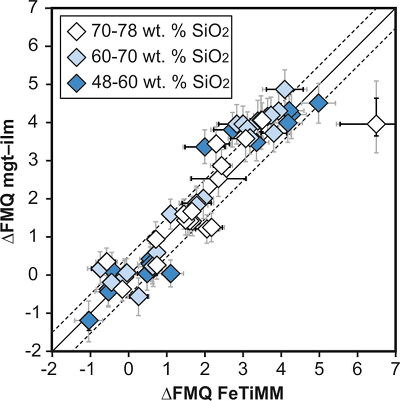 Figure 2 Performance of FeTiMM in ilmenite-saturated magmas. Oxygen fugacities (expressed in log units relative to the FMQ buffer) obtained via FeTiMM are compared with ones obtained via magnetite–ilmenite oxybarometry using the model of Ghiorso and Evans (2008). The data are divided into three groups of contrasting melt SiO2. Black error bars (in most cases smaller than symbol size) denote the analytical error, whereas the grey error bars denote the overall error that includes both the analytical scatter and the error inherent to the model. Details about the calculation of errors are provided in the Supplementary Information. | 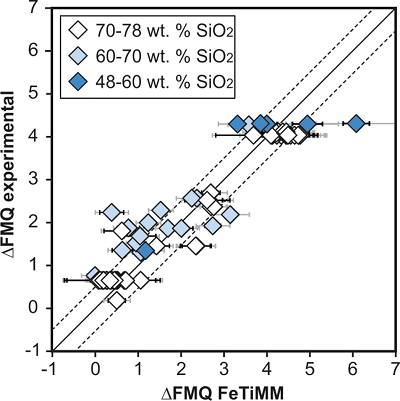 Figure 3 Performance of FeTiMM in ilmenite-undersaturated magmas. Due to the lack of ilmenite, one has to rely on the reported experimentally imposed fO2 values, which are associated with considerable (but unknown) uncertainty. Correspondingly, error bars in y-axis direction cannot be displayed. See Figure 2 for abbreviations and the meaning of the black and grey error bars in x-axis direction. | 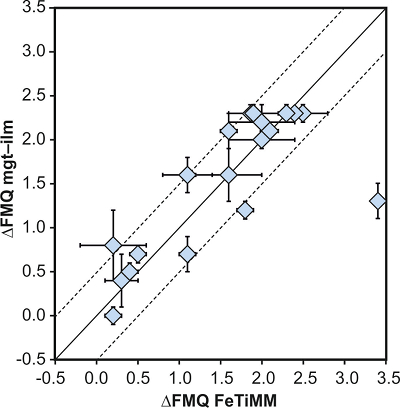 Figure 4 Application of FeTiMM to a set of 19 natural, ilmenite-saturated samples of rhyolitic to dacitic composition (Arató and Audétat, 2017a). Oxygen fugacities (reported in log units relative to the fayalite–magnetite–quartz buffer) obtained via FeTiMM agree within 0.5 log units with those obtained via magnetite–ilmenite oxybarometry (Ghiorso and Evans, 2008) in all but one case. Error bars denote 1σ standard deviation of the fO2 values obtained from several magnetite–melt pairs. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Supplementary Figures and Tables
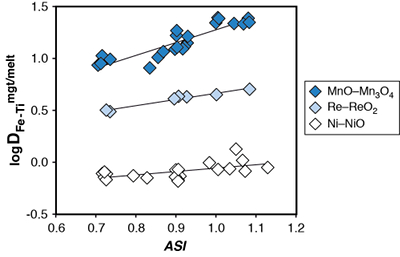 Figure S-1 Dependence of the Fe–Ti exchange coefficient between magnetite and rhyolitic melt on fO2 and melt alumina saturation index. DFe–Timgt/melt refers to (DFeOtotmgt/melt)/(DTiO2mgt/melt), whereas ASI refers to molar Al2O3 /(CaO + Na2O + K2O). The dataset comprises magnetite–melt pairs from 50 different experiments performed at three different oxygen fugacity buffers, temperatures of 800-1000 °C, pressures of 100-500 MPa, with melt ASI values of 0.71-1.12, and magnetite compositions of 0.2-14 wt. % TiO2 (Arató and Audétat, 2017). | 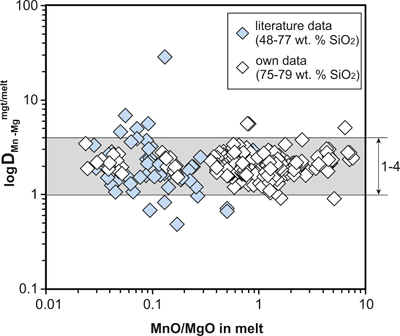 Figure S-2 Variance of the Mn–Mg exchange coefficient between magnetite and silicate melt as a function of the MnO/MgO weight ratio in the silicate melt. DMn–Mgmgt/melt refers to (DMnOmgt/melt)/(DMgOmgt/melt), with MnO and MgO given in weight percent. The data set comprises the 296 magnetite–rhyolite pairs from own experiments, plus the data points shown in Figure 2. The shaded envelope, which encompasses DMn–Mgmgt/melt values of 1-4, comprises 95 % of all data points. | 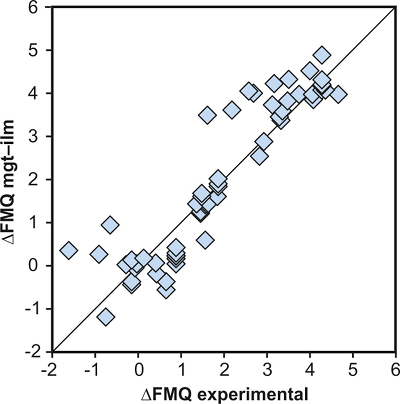 Figure S-3 Comparison of reported experimental fO2 values with fO2 values obtained via magnetite–ilmenite (Ghiorso and Evans, 2008) for 59 ilmenite-bearing experiments taken from the literature. Oxygen fugacities are reported in log units relative to the fayalite-magnetite-quartz (FMQ) buffer. | 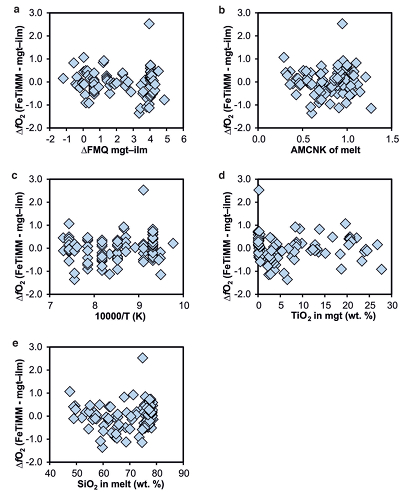 Figure S-4 Difference between the fO2 values calculated via magnetite–ilmenite and ones calculated via FeTiMM [∆fO2 (FeTiMM - mgt–ilm)], as a function of (a) fO2, (b) AMCNK, (c) temperature, (d) magnetite composition, and (e) melt SiO2 content, for the 109 data points that were used to calibrate FeTiMM. | 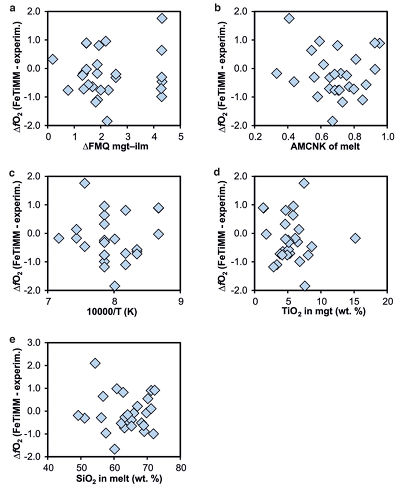 Figure S-5 Difference between reported experimental fO2 and fO2 calculated via FeTiMM [∆fO2 (FeTiMM - experimental)], as a function of (a) fO2, (b) AMCNK, (c) temperature, (d) magnetite composition, and (e) melt SiO2 content, for 27 ilmenite-undersaturated experiments taken from the literature. |  Table S-1 Full data set used to calibrate and test the FeTiMM model. The references listed in this table are detailed in Table S-2. |  Table S-2 List of experimental studies that were screened for the five criteria discussed in the text. |
| Figure S-1 | Figure S-2 | Figure S-3 | Figure S-4 | Figure S-5 | Table S-1 | Table S-2 |
top
Introduction
Oxygen fugacity is an important thermodynamic parameter in magmatic systems because it exerts a first order control on phase equilibria as well as on mineral–melt and fluid–melt partition coefficients. The most commonly used and most reliable way to reconstruct magmatic fO2 is via magnetite–ilmenite oxybarometry (e.g., Buddington and Lindsley, 1964
Buddington, A., Lindsley, D. (1964) Iron-titanium oxide minerals and synthetic equivalents. Journal of Petrology 5, 310-357.
; Carmichael, 1967Carmichael, I.S.E. (1967) The iron-titanium oxides of salic volcanic rocks and their associated ferromagnesian silicates. Contributions to Mineralogy and Petrology 14, 36–64.
; Stormer, 1983Stormer, J.C. (1983) The effects of recalculation on estimates of temperature and oxygen fugacity from analyses of multicomponent iron-titanium oxides. American Mineralogist 68, 586–594.
; Andersen and Lindsley, 1988Andersen, D.J., Lindsley, D.H. (1988) Internally consistent solution models for Fe-Mg-Mn-Ti oxides: Fe-Ti oxides. American Mineralogist 73, 714–726.
; Ghiorso and Sack, 1991Ghiorso, M.S., Sack, O. (1991) Fe-Ti oxide geothermometry: thermodynamic formulation and the estimation of intensive variables in silicic magmas. Contributions to Mineralogy and Petrology 108, 485–510.
; Lattard et al., 2005Lattard, D., Sauerzapf, U., Käsemann, M. (2005) New calibration data for the Fe-Ti oxide thermo-oxybarometers from experiments in the Fe-Ti-O system at 1 bar, 1,000-1,300°C and a large range of oxygen fugacities. Contributions to Mineralogy and Petrology 149, 735–754.
; Ghiorso and Evans, 2008Ghiorso, M.S., Evans, B.W. (2008) Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. American Journal of Science 308, 957–1039.
). Alternative approaches are based on (i) the Fe2+/Fe3+ ratio of whole rocks (Kress and Carmichael, 1989Kress, V.C., Carmichael, I.S. (1989) The lime-iron-silicate melt system: Redox and volume systematics. Geochimica et Cosmochimica Acta 53, 2883–2892.
; Putirka, 2016Putirka, K. (2016) Rates and styles of planetary cooling on Earth, Moon, Mars, and Vesta, using new models for oxygen fugacity, ferric-ferrous ratios, olivine-liquid Fe-Mg exchange, and mantle potential temperature. American Mineralogist 101, 819–840.
), (ii) mineral reactions involving olivine, pyroxene, and/or sphene (Frost and Lindsley, 1992Frost, B.R., Lindsley, D. (1992) Equilibria among Fe-Ti oxides, pyroxenes, olivine, and quartz: Part II. Application. American Mineralogist 77, 1004–1004.
; Lindsley and Frost, 1992Lindsley, D.H., Frost, B.R. (1992) Equilibria among Fe-Ti oxides, pyroxenes, olivine, and quartz; Part I, Theory. American Mineralogist 77, 987–1003.
; Andersen et al., 1993Andersen, D.J., Lindsley, D.H., Davidson, P.M. (1993) QUILF: A pascal program to assess equilibria among Fe–Mg–Mn–Ti oxides, pyroxenes, olivine, and quartz. Computers & Geosciences 19, 1333–1350.
; Xirouchakis et al., 2001Xirouchakis, D., Lindsley, D.H., Frost, B.R. (2001) Assemblages with titanite (CaTiOSiO4), Ca-Mg-Fe olivine and pyroxenes, Fe-Mg-Ti oxides, and quartz: Part II. Application. American Mineralogist 86, 254–264.
), (iii) biotite, K-feldspar and magnetite (Wones and Eugster, 1965Wones, D., Eugster, H. (1965) Stability of biotite–experiment theory and application. American Mineralogist 50, 1228–1272.
; Wones, 1981Wones, D. (1981) Mafic silicates as indicators of intensive variables in granitic magmas. Mining Geology 31, 191–212.
) , (iv) zircon Ce (and Eu) anomalies (Ballard et al., 2002Ballard, J.R., Palin, M.J., Campbell, I.H. (2002) Relative oxidation states of magmas inferred from Ce(IV)/Ce(III) in zircon: application to porphyry copper deposits of northern Chile. Contributions to Mineralogy and Petrology 144, 347–364.
; Trail et al., 2012Trail, D., Watson, E.B., Tailby, N.D. (2012) Ce and Eu anomalies in zircon as proxies for the oxidation state of magmas. Geochimica et Cosmochimica Acta 97, 70–87.
; Smythe and Brenan, 2016Smythe, D.J., Brenan, J.M. (2016) Magmatic oxygen fugacity estimated using zircon-melt partitioning of cerium. Earth and Planetary Science Letters 453, 260–266.
), and (v) single amphibole oxybarometry (Ridolfi et al., 2010), and (v) single amphibole oxybarometry (Ridolfi et al., 2010
Ridolfi, F., Renzulli, A., Puerini, M. (2010) Stability and chemical equilibrium of amphibole in calc-alkaline magmas: an overview, new thermobarometric formulations and application to subduction-related volcanoes. Contributions to Mineralogy and Petrology 160, 45–66.
). However, despite these various approaches, reconstruction of magmatic fO2 in igneous rocks remains difficult, particularly in the case of intrusives, because during slow cooling Fe-Ti-oxide minerals usually get either reset or altered. Furthermore, many magmas do not contain ilmenite but only magnetite (Frost and Lindsley, 1991Frost, B., Lindsley, D.H. (1991) Occurrence of iron-titanium oxides in igneous rocks. Reviews in Mineralogy and Geochemistry 25, 433–468.
), precluding the application of magnetite–ilmenite oxybarometry.top
Calibration of the FeTiMM Oxybarometer
The aim of this study was to develop an oxybarometer that is based on element partitioning between a mineral and silicate melt, such that it can be applied to samples in which these phases occur as inclusions within phenocrysts and thus were protected from re-equilibration and alteration during slow cooling. Iron partitioning between magnetite and melt is a promising candidate because magnetite is a common mineral and because magnetite solubility in silicic melts has been shown to depend on fO2 (Gaillard et al., 2001
Gaillard, F., Scaillet, B., Pichavant, M., Bény, J.-M. (2001) The effect of water and fO2 on the ferric–ferrous ratio of silicic melts. Chemical Geology 174, 255–273.
). However, magnetite solubility (and thus Fe partitioning between magnetite and silicate melt) also depends strongly on temperature (T) and melt composition. At constant fO2 and T, magnetite solubility increases by a factor of up to 6 as the alumina saturation index (ASI; defined as the molar Al2O3/(Na2O + K2O + CaO) ratio) decreases from 1.0 to 0.6. Magnetite solubility is thus not suitable as an oxybarometer unless T and ASI can be extremely well constrained, which is commonly difficult in natural samples (Arató and Audétat, 2017aArató, R., Audétat, A. (2017a) Vanadium magnetite–melt oxybarometry of natural, silicic magmas: a comparison of various oxybarometers and thermometers. Contributions to Mineralogy and Petrology 172, doi: 10.1007/s00410-017-1369-6.
). However, we noticed that the effect of ASI on magnetite solubility is similar to that on TiO2 solubility (Kularatne and Audétat, 2014Kularatne, K., Audétat, A. (2014) Rutile solubility in hydrous rhyolite melts at 750–900°C and 2kbar, with application to titanium-in-quartz (TitaniQ) thermobarometry. Geochimica et Cosmochimica Acta 125, 196–209.
), with the latter being independent on fO2. Hence, the effect of melt composition may be diminished by dividing the mgt–melt partition coefficient of Fe (DFemgt/melt) by that of Ti (DTimgt/melt).We tested this idea first on a set of 50 of our own experiments conducted in the system magnetite–H2O–rhyolite melt at various oxygen fugacities, temperatures, pressures, melt ASIs, and magnetite compositions (Arató and Audétat, 2017b
Arató, R., Audétat, A. (2017b) Experimental calibration of a new oxybarometer for silicic magmas based on vanadium partitioning between magnetite and silicate melt. Geochimica et Cosmochimica Acta 209, 284–295.
; Table S-1). The results (Fig. S-1) revealed that the melt composition effect indeed gets greatly reduced in this way, and that the Fe–Ti exchange coefficient between magnetite and silicate melt, DFe–Timgt/melt = (DFeOtotmgt/melt)/(DTiO2mgt/melt), depends most strongly on fO2, with the effect of temperature becoming negligible. In a second step we extended the dataset by 59 experiments from 14 different studies performed at 750-1100 °C, 0.1-700 MPa, oxygen fugacities of -1.3 to +5.5 log units relative to the fayalite-magnetite-quartz buffer (∆FMQ-1.3 to ∆FMQ+5.5), with melt compositions of 48-79 wt. % SiO2 and ASI = 0.3-1.3, and magnetite compositions of 0.01-28 wt. % TiO2. These 59 experiments were left after screening 33 experimental studies with a total of >1600 experiments (Table S-2) for the following criteria: (i) fO2 was controlled experimentally, (ii) magnetite coexists with ilmenite in at least some of the experiments, (iii) compositional data for magnetite, silicate melt, ±ilmenite are available, (iv) the reported average compositions of magnetite and ilmenite represent equilibrium pairs (Bacon and Hirschmann, 1988Bacon, C.R., Hirschmann, M.M. (1988) Mg/Mn partitioning as a test for equilibrium between coexisting Fe-Ti oxides. American Mineralogist 73, 57–61.
), and (v) the average compositions of magnetite and silicate melt pass a similar test that we developed to check for magnetite–melt equilibrium (Fig. S-2). In this second step, we focused on ilmenite-saturated experiments to be able to constrain fO2 values independently via magnetite–ilmenite oxybarometry. In nine cases, the reported experimental fO2 values deviated by more than 1.0 log unit fO2 from the values obtained via magnetite–ilmenite oxybarometry (Ghiorso and Evans, 2008Ghiorso, M.S., Evans, B.W. (2008) Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. American Journal of Science 308, 957–1039.
; Fig. S-3), suggesting problems with the control of experimental fO2 (Matjuschkin et al., 2015Matjuschkin, V., Brooker, R.A., Tattitch, B., Blundy, J.D., Stamper, C.C. (2015) Control and monitoring of oxygen fugacity in piston cylinder experiments. Contributions to Mineralogy and Petrology 169, 9.
). We thus relied on the fO2 values calculated via magnetite–ilmenite oxybarometry for all 59 ilmenite-saturated experiments. To account for the large range of melt compositions, it was necessary to include MgO in the melt compositional parameter. Based on the extended dataset of 109 experiments we developed a model (which we hereinafter call FeTiMM) that allows fO2 to be calculated as a function of DFe–Timgt/melt (with FeOtot and TiO2 measured in weight percent, and the melt composition reported dry) and the melt compositional parameter AMCNK = molar Al2O3/(CaO + Na2O + K2O + MgO):Eq. 1
[ΔFMQ = (log(DFeOtotmgt/melt/DTiO2mgt/melt) + 0.137*AMCNK + 0.102)/(0.288*AMCNK + 0.054) ]
The rationale behind this equation is depicted in Figure 1. The overall uncertainty of the FeTiMM model, calculated from the errors of the fits in Figure 1b,c propagated into Equation 1 (see Supplementary Information) increases from ±0.2-0.3 log units fO2 at ≤∆FMQ+1.5, to ±0.3-0.5 log units fO2 at ∆FMQ+4.5 (Supplementary Table S-1). The performance of FeTiMM on the 59 ilmenite-saturated experiments is shown in Figure 2. As explained above, we relied on fO2 values calculated via magnetite–ilmenite oxybarometry (Ghiorso and Evans, 2008
Ghiorso, M.S., Evans, B.W. (2008) Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. American Journal of Science 308, 957–1039.
) for this test. The uncertainty of the latter model was not explicitly stated (Ghiorso and Evans, 2008Ghiorso, M.S., Evans, B.W. (2008) Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. American Journal of Science 308, 957–1039.
), but based on their Figure 27 it can be estimated at ca. ±0.3 log units at reducing conditions (∆FMQ-1 to ∆FMQ+1) to ca. ±0.5 log units at strongly oxidising conditions (∆FMQ+4.5; not considering a group of outliers), i.e. similar to the uncertainty associated with our model. Within these errors, 63 out of the 109 experiments show perfect agreement between the two methods and all but five experiments return fO2 values that agree within ≤0.5 log units. No correlations are evident between the degree of correspondence and fO2, temperature, melt SiO2 content, ASI, or magnetite composition (Fig. S-4), suggesting that FeTiMM works equally well over the entire range of the investigated P-T-X conditions. It should be mentioned that any misfit can result from various sources including (i) analytical errors, (ii) experimental problems, as well as (iii) weaknesses in either model.
Figure 1 Development of the FeTiMM model. (a) 109 experimental data points (59 ilmenite-saturated; 50 ilmenite-undersaturated) were split into five groups of similar oxygen fugacity, through which linear regressions were fit. DFe–Timgt/melt = (DFeOtotmgt/melt)/(DTiO2mgt/melt); AMCNK = molar Al2O3/(MgO + CaO + Na2O + K2O). (b,c) Variation of the slopes and intercepts of the linear fits in (a) as a function of oxygen fugacity expressed in log unit deviations from the fayalite-magnetite-quartz (FMQ) buffer.

Figure 2 Performance of FeTiMM in ilmenite-saturated magmas. Oxygen fugacities (expressed in log units relative to the FMQ buffer) obtained via FeTiMM are compared with ones obtained via magnetite–ilmenite oxybarometry using the model of Ghiorso and Evans (2008)
Ghiorso, M.S., Evans, B.W. (2008) Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. American Journal of Science 308, 957–1039.
. The data are divided into three groups of contrasting melt SiO2. Black error bars (in most cases smaller than symbol size) denote the analytical error, whereas the grey error bars denote the overall error that includes both the analytical scatter and the error inherent to the model. Details about the calculation of errors are provided in the Supplementary Information.top
Tests on Additional Ilmenite-Undersaturated Experiments
As discussed above, Equation 1 was derived from a database that comprises 50 ilmenite-undersaturated, rhyolitic experiments, plus 59 ilmenite-saturated, basaltic to rhyolitic experiments. It remains to be tested whether FeTiMM works also for ilmenite-undersaturated intermediate to mafic magmas. However, in ilmenite-undersaturated experiments fO2 cannot be independently constrained via magnetite–ilmenite thermobarometry, which forces us to rely on reported experimental fO2 values even if the test with the ilmenite-saturated experiments (Fig. S-3) revealed that these values are not always reliable. To reduce the chance of including erroneous fO2 reference values, we restricted our choice of ilmenite-undersaturated experiments to studies which comprised both ilmenite-undersaturated and ilmenite-saturated experiments, and which in the latter case showed good agreement between the reported experimental and magnetite–ilmenite-based fO2 values. This approach returned 27 data points from 5 different studies (Table S-1), excluding 22 data points that did not pass the Mn/Mg magnetite–melt equilibrium test mentioned above. The performance of FeTiMM on these 27 literature-based experiments plus 50 of our own ilmenite-undersaturated experiments is shown in Figure 3. The data in Figure 3 show a larger scatter than those in Figure 2, which is likely due to errors in the reported experimental fO2 values (cf. Fig. S-3). Nevertheless, within the error quoted for FeTiMM, 7 of the 27 experiments return fO2 values that agree between the two methods and all but 5 experiments show a correspondence within 0.5 log units. Again, no correlations are evident between the degree of fO2 correspondence and other key variables (Fig. S-5), suggesting that FeTiMM works equally well for mafic as for silicic rocks.

Figure 3 Performance of FeTiMM in ilmenite-undersaturated magmas. Due to the lack of ilmenite, one has to rely on the reported experimentally imposed fO2 values, which are associated with considerable (but unknown) uncertainty. Correspondingly, error bars in y-axis direction cannot be displayed. See Figure 2 for abbreviations and the meaning of the black and grey error bars in x-axis direction.
In summary, the results in Figures 2 and 3 suggest that FeTiMM provides reliable fO2 estimates for both ilmenite-saturated and ilmenite-undersaturated magmas over the entire range of basaltic to rhyolitic compositions. It should be mentioned that none of the investigated melt compositions plot in the alkaline field (Macdonald and Katsura, 1964
MacDonald, G.A., Katsura, T. (1964) Chemical composition of Hawaiian lavas. Journal of Petrology 5, 82–133.
) in the total alkali vs. SiO2 (TAS) diagram (Le Maitre et al., 1989Le Maitre, R.W.B., Dudek, P., Keller, A., Lameyre, J., Le Bas, J., Sabine, M., Schmid, P., Sorensen, R., Streckeisen, H., Woolley, A. (1989) A classification of igneous rocks and glossary of terms: Recommendations of the International Union of Geological Sciences, Subcommission on the Systematics of Igneous Rocks. Blackwell Scientific Publications, Oxford.
), so the performance of FeTiMM in highly alkaline magmas is not known yet. However, 102 out of the 136 data points plotted in Figures 2 and 3 are peralkaline according to molar Al2O3/(Na2O + K2O + CaO) (= ACNK), with 17 melts having ACNK values <0.7.top
Application to Natural Samples
The results of a first application of FeTiMM to 19 natural samples of rhyolitic to dacitic composition are shown in Figure 4. Details on the samples and analytical methods can be found in Arató and Audétat (2017a)
Arató, R., Audétat, A. (2017a) Vanadium magnetite–melt oxybarometry of natural, silicic magmas: a comparison of various oxybarometers and thermometers. Contributions to Mineralogy and Petrology 172, doi: 10.1007/s00410-017-1369-6.
. All samples were ilmenite-saturated, such that fO2 could be independently constrained via magnetite–ilmenite oxybarometry. Coexistence of analysed magnetite, ilmenite and silicate melt was verified by means of the Mg/Mn magnetite–ilmenite partitioning test (Bacon and Hirschmann, 1988Bacon, C.R., Hirschmann, M.M. (1988) Mg/Mn partitioning as a test for equilibrium between coexisting Fe-Ti oxides. American Mineralogist 73, 57–61.
) plus our own Mn/Mg magnetite–melt partitioning test described in the Supplementary Information. In all but one sample, FeTiMM returned fO2 values that agree within 0.5 log units with those obtained via magnetite–ilmenite oxybarometry (Fig. 4).One of the main advantages of FeTiMM is that it can be applied to magmas that do not contain ilmenite, which is true for many igneous rocks of mafic to felsic composition, particularly for those that are alkali-rich (Lindsley and Frost, 1992
Lindsley, D.H., Frost, B.R. (1992) Equilibria among Fe-Ti oxides, pyroxenes, olivine, and quartz; Part I, Theory. American Mineralogist 77, 987–1003.
). Another major advantage of the method is that it can be applied to slowly cooled and/or altered rocks if magnetite and silicate melt are present as inclusions within phenocrysts (preferably quartz) and are analysed as entities by LA-ICP-MS, thereby effectively reversing compositional heterogeneities that developed within the inclusions during slow cooling. The sole disadvantage of FeTiMM is that it requires knowledge of the silicate melt composition. This can be readily accomplished in rapidly quenched, volcanic samples with glassy matrix, but is a bit more difficult in holocrystalline, porphyritic samples (for fresh samples selective analysis of the matrix suffices), and can be challenging in holocrystalline, coarse grained samples. In the latter samples, both magnetite and silicate melt need to be analysed as inclusions within phenocrysts, with the quantification of melt compositions requiring re-homogenisation experiments and/or constraints from whole rock data (Audétat and Lowenstern, 2014Audétat, A., Lowenstern, J.B. (2014) Melt inclusions. In: Scott, S.D. (Ed.) Geochemistry of Mineral Deposits. Treatise on Geochemistry, 2nd Edition, Volume 13. Elsevier, Amsterdam, Oxford, Waltham, 143–173.
; Arató and Audétat, 2017aArató, R., Audétat, A. (2017a) Vanadium magnetite–melt oxybarometry of natural, silicic magmas: a comparison of various oxybarometers and thermometers. Contributions to Mineralogy and Petrology 172, doi: 10.1007/s00410-017-1369-6.
).
Figure 4 Application of FeTiMM to a set of 19 natural, ilmenite-saturated samples of rhyolitic to dacitic composition (Arató and Audétat, 2017a
Arató, R., Audétat, A. (2017a) Vanadium magnetite–melt oxybarometry of natural, silicic magmas: a comparison of various oxybarometers and thermometers. Contributions to Mineralogy and Petrology 172, doi: 10.1007/s00410-017-1369-6.
). Oxygen fugacities (reported in log units relative to the fayalite–magnetite–quartz buffer) obtained via FeTiMM agree within 0.5 log units with those obtained via magnetite–ilmenite oxybarometry (Ghiorso and Evans, 2008Ghiorso, M.S., Evans, B.W. (2008) Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. American Journal of Science 308, 957–1039.
) in all but one case. Error bars denote 1σ standard deviation of the fO2 values obtained from several magnetite–melt pairs.top
Acknowledgements
This study was financed by German Science Foundation project AU 314/5-1 to A.A.
Editor: Helen Williams
top
References
Andersen, D.J., Lindsley, D.H. (1988) Internally consistent solution models for Fe-Mg-Mn-Ti oxides: Fe-Ti oxides. American Mineralogist 73, 714–726.
 Show in context
Show in context The most commonly used and most reliable way to reconstruct magmatic ƒO2 is via magnetite–ilmenite oxybarometry (e.g., Buddington and Lindsley, 1964; Carmichael, 1967; Stormer, 1983; Andersen and Lindsley, 1988; Ghiorso and Sack, 1991; Lattard et al., 2005; Ghiorso and Evans, 2008).
View in article
Andersen, D.J., Lindsley, D.H., Davidson, P.M. (1993) QUILF: A pascal program to assess equilibria among Fe–Mg–Mn–Ti oxides, pyroxenes, olivine, and quartz. Computers & Geosciences 19, 1333–1350.
 Show in context
Show in context Alternative approaches are based on (i) the Fe2+/Fe3+ ratio of whole rocks (Kress and Carmichael, 1989; Putirka, 2016), (ii) mineral reactions involving olivine, pyroxene, and/or sphene (Frost and Lindsley, 1992; Lindsley and Frost, 1992; Andersen et al., 1993; Xirouchakis et al., 2001), (iii) biotite, K-feldspar and magnetite (Wones and Eugster, 1965; Wones, 1981) , (iv) zircon Ce (and Eu) anomalies (Ballard et al., 2002; Trail et al., 2012; Smythe and Brenan, 2016), and (v) single amphibole oxybarometry (Ridolfi et al., 2010).
View in article
Arató, R., Audétat, A. (2017a) Vanadium magnetite–melt oxybarometry of natural, silicic magmas: a comparison of various oxybarometers and thermometers. Contributions to Mineralogy and Petrology 172, doi: 10.1007/s00410-017-1369-6.
 Show in context
Show in context Magnetite solubility is thus not suitable as an oxybarometer unless T and ASI can be extremely well constrained, which is commonly difficult in natural samples (Arató and Audétat, 2017a).
View in article
Details on the samples and analytical methods can be found in Arató and Audétat (2017a).
View in article
In the latter samples, both magnetite and silicate melt need to be analysed as inclusions within phenocrysts, with the quantification of melt compositions requiring re-homogenisation experiments and/or constraints from whole rock data (Audétat and Lowenstern, 2014; Arató and Audétat, 2017a).
View in article
Figure 4 Application of FeTiMM to a set of 19 natural, ilmenite-saturated samples of rhyolitic to dacitic composition (Arató and Audétat, 2017a).
View in article
Arató, R., Audétat, A. (2017b) Experimental calibration of a new oxybarometer for silicic magmas based on vanadium partitioning between magnetite and silicate melt. Geochimica et Cosmochimica Acta 209, 284–295.
 Show in context
Show in context We tested this idea first on a set of 50 of our own experiments conducted in the system magnetite–H2O–rhyolite melt at various oxygen fugacities, temperatures, pressures, melt ASIs, and magnetite compositions (Arató and Audétat, 2017b; Table S-1).
View in article
Audétat, A., Lowenstern, J.B. (2014) Melt inclusions. In: Scott, S.D. (Ed.) Geochemistry of Mineral Deposits. Treatise on Geochemistry, 2nd Edition, Volume 13. Elsevier, Amsterdam, Oxford, Waltham, 143–173.
 Show in context
Show in context In the latter samples, both magnetite and silicate melt need to be analysed as inclusions within phenocrysts, with the quantification of melt compositions requiring re-homogenisation experiments and/or constraints from whole rock data (Audétat and Lowenstern, 2014; Arató and Audétat, 2017a).
View in article
Bacon, C.R., Hirschmann, M.M. (1988) Mg/Mn partitioning as a test for equilibrium between coexisting Fe-Ti oxides. American Mineralogist 73, 57–61.
 Show in context
Show in context These 59 experiments were left after screening 33 experimental studies with a total of >1600 experiments (Table S-2) for the following criteria: (i) ƒO2 was controlled experimentally, (ii) magnetite coexists with ilmenite in at least some of the experiments, (iii) compositional data for magnetite, silicate melt, ±ilmenite are available, (iv) the reported average compositions of magnetite and ilmenite represent equilibrium pairs (Bacon and Hirschmann, 1988), and (v) the average compositions of magnetite and silicate melt pass a similar test that we developed to check for magnetite–melt equilibrium (Fig. S-2).
View in article
Coexistence of analysed magnetite, ilmenite and silicate melt was verified by means of the Mg/Mn magnetite–ilmenite partitioning test (Bacon and Hirschmann, 1988) plus our own Mn/Mg magnetite–melt partitioning test described in the Supplementary Information.
View in article
Ballard, J.R., Palin, M.J., Campbell, I.H. (2002) Relative oxidation states of magmas inferred from Ce(IV)/Ce(III) in zircon: application to porphyry copper deposits of northern Chile. Contributions to Mineralogy and Petrology 144, 347–364.
 Show in context
Show in context Alternative approaches are based on (i) the Fe2+/Fe3+ ratio of whole rocks (Kress and Carmichael, 1989; Putirka, 2016), (ii) mineral reactions involving olivine, pyroxene, and/or sphene (Frost and Lindsley, 1992; Lindsley and Frost, 1992; Andersen et al., 1993; Xirouchakis et al., 2001), (iii) biotite, K-feldspar and magnetite (Wones and Eugster, 1965; Wones, 1981) , (iv) zircon Ce (and Eu) anomalies (Ballard et al., 2002; Trail et al., 2012; Smythe and Brenan, 2016), and (v) single amphibole oxybarometry (Ridolfi et al., 2010).
View in article
Buddington, A., Lindsley, D. (1964) Iron-titanium oxide minerals and synthetic equivalents. Journal of Petrology 5, 310-357.
 Show in context
Show in context The most commonly used and most reliable way to reconstruct magmatic ƒO2 is via magnetite–ilmenite oxybarometry (e.g., Buddington and Lindsley, 1964; Carmichael, 1967; Stormer, 1983; Andersen and Lindsley, 1988; Ghiorso and Sack, 1991; Lattard et al., 2005; Ghiorso and Evans, 2008).
View in article
Carmichael, I.S.E. (1967) The iron-titanium oxides of salic volcanic rocks and their associated ferromagnesian silicates. Contributions to Mineralogy and Petrology 14, 36–64.
 Show in context
Show in context The most commonly used and most reliable way to reconstruct magmatic ƒO2 is via magnetite–ilmenite oxybarometry (e.g., Buddington and Lindsley, 1964; Carmichael, 1967; Stormer, 1983; Andersen and Lindsley, 1988; Ghiorso and Sack, 1991; Lattard et al., 2005; Ghiorso and Evans, 2008).
View in article
Frost, B., Lindsley, D.H. (1991) Occurrence of iron-titanium oxides in igneous rocks. Reviews in Mineralogy and Geochemistry 25, 433–468.
 Show in context
Show in context Furthermore, many magmas do not contain ilmenite but only magnetite (Frost and Lindsley, 1991), precluding the application of magnetite–ilmenite oxybarometry.
View in article
Frost, B.R., Lindsley, D. (1992) Equilibria among Fe-Ti oxides, pyroxenes, olivine, and quartz: Part II. Application. American Mineralogist 77, 1004–1004.
 Show in context
Show in context Alternative approaches are based on (i) the Fe2+/Fe3+ ratio of whole rocks (Kress and Carmichael, 1989; Putirka, 2016), (ii) mineral reactions involving olivine, pyroxene, and/or sphene (Frost and Lindsley, 1992; Lindsley and Frost, 1992; Andersen et al., 1993; Xirouchakis et al., 2001), (iii) biotite, K-feldspar and magnetite (Wones and Eugster, 1965; Wones, 1981) , (iv) zircon Ce (and Eu) anomalies (Ballard et al., 2002; Trail et al., 2012; Smythe and Brenan, 2016), and (v) single amphibole oxybarometry (Ridolfi et al., 2010).
View in article
Gaillard, F., Scaillet, B., Pichavant, M., Bény, J.-M. (2001) The effect of water and fO2 on the ferric–ferrous ratio of silicic melts. Chemical Geology 174, 255–273.
 Show in context
Show in context Iron partitioning between magnetite and melt is a promising candidate because magnetite is a common mineral and because magnetite solubility in silicic melts has been shown to depend on fO2 (Gaillard et al., 2001).
View in article
Ghiorso, M.S., Evans, B.W. (2008) Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. American Journal of Science 308, 957–1039.
 Show in context
Show in context The most commonly used and most reliable way to reconstruct magmatic ƒO2 is via magnetite–ilmenite oxybarometry (e.g., Buddington and Lindsley, 1964; Carmichael, 1967; Stormer, 1983; Andersen and Lindsley, 1988; Ghiorso and Sack, 1991; Lattard et al., 2005; Ghiorso and Evans, 2008).
View in article
In nine cases, the reported experimental fO2 values deviated by more than 1.0 log unit fO2 from the values obtained via magnetite–ilmenite oxybarometry (Ghiorso and Evans, 2008; Fig. S-3), suggesting problems with the control of experimental fO2 (Matjuschkin et al., 2015).
View in article
As explained above, we relied on fO2 values calculated via magnetite–ilmenite oxybarometry (Ghiorso and Evans, 2008) for this test.
View in article
The uncertainty of the latter model was not explicitly stated (Ghiorso and Evans, 2008), but based on their Figure 27 it can be estimated at ca. ±0.3 log units at reducing conditions (∆FMQ-1 to ∆FMQ+1) to ca. ±0.5 log units at strongly oxidising conditions (∆FMQ+4.5; not considering a group of outliers), i.e. similar to the uncertainty associated with our model.
View in article
Figure 2 [...] Oxygen fugacities (expressed in log units relative to the FMQ buffer) obtained via FeTiMM are compared with ones obtained via magnetite–ilmenite oxybarometry using the model of Ghiorso and Evans (2008).
View in article
Figure 4 [...] Oxygen fugacities (reported in log units relative to the fayalite–magnetite–quartz buffer) obtained via FeTiMM agree within 0.5 log units with those obtained via magnetite–ilmenite oxybarometry (Ghiorso and Evans, 2008) in all but one case.
View in article
Ghiorso, M.S., Sack, O. (1991) Fe-Ti oxide geothermometry: thermodynamic formulation and the estimation of intensive variables in silicic magmas. Contributions to Mineralogy and Petrology 108, 485–510.
 Show in context
Show in context The most commonly used and most reliable way to reconstruct magmatic ƒO2 is via magnetite–ilmenite oxybarometry (e.g., Buddington and Lindsley, 1964; Carmichael, 1967; Stormer, 1983; Andersen and Lindsley, 1988; Ghiorso and Sack, 1991; Lattard et al., 2005; Ghiorso and Evans, 2008).
View in article
Kress, V.C., Carmichael, I.S. (1989) The lime-iron-silicate melt system: Redox and volume systematics. Geochimica et Cosmochimica Acta 53, 2883–2892.
 Show in context
Show in context Alternative approaches are based on (i) the Fe2+/Fe3+ ratio of whole rocks (Kress and Carmichael, 1989; Putirka, 2016), (ii) mineral reactions involving olivine, pyroxene, and/or sphene (Frost and Lindsley, 1992; Lindsley and Frost, 1992; Andersen et al., 1993; Xirouchakis et al., 2001), (iii) biotite, K-feldspar and magnetite (Wones and Eugster, 1965; Wones, 1981) , (iv) zircon Ce (and Eu) anomalies (Ballard et al., 2002; Trail et al., 2012; Smythe and Brenan, 2016), and (v) single amphibole oxybarometry (Ridolfi et al., 2010).
View in article
Kularatne, K., Audétat, A. (2014) Rutile solubility in hydrous rhyolite melts at 750–900°C and 2kbar, with application to titanium-in-quartz (TitaniQ) thermobarometry. Geochimica et Cosmochimica Acta 125, 196–209.
 Show in context
Show in context However, we noticed that the effect of ASI on magnetite solubility is similar to that on TiO2 solubility (Kularatne and Audétat, 2014), with the latter being independent on fO2. Hence, the effect of melt composition may be diminished by dividing the mgt–melt partition coefficient of Fe (DFemgt/melt) by that of Ti (DTimgt/melt).
View in article
Lattard, D., Sauerzapf, U., Käsemann, M. (2005) New calibration data for the Fe-Ti oxide thermo-oxybarometers from experiments in the Fe-Ti-O system at 1 bar, 1,000-1,300°C and a large range of oxygen fugacities. Contributions to Mineralogy and Petrology 149, 735–754.
 Show in context
Show in context The most commonly used and most reliable way to reconstruct magmatic ƒO2 is via magnetite–ilmenite oxybarometry (e.g., Buddington and Lindsley, 1964; Carmichael, 1967; Stormer, 1983; Andersen and Lindsley, 1988; Ghiorso and Sack, 1991; Lattard et al., 2005; Ghiorso and Evans, 2008).
View in article
Le Maitre, R.W.B., Dudek, P., Keller, A., Lameyre, J., Le Bas, J., Sabine, M., Schmid, P., Sorensen, R., Streckeisen, H., Woolley, A. (1989) A classification of igneous rocks and glossary of terms: Recommendations of the International Union of Geological Sciences, Subcommission on the Systematics of Igneous Rocks. Blackwell Scientific Publications, Oxford.
 Show in context
Show in context It should be mentioned that none of the investigated melt compositions plot in the alkaline field (Macdonald and Katsura, 1964) in the total alkali vs. SiO2 (TAS) diagram (Le Maitre et al., 1989), so the performance of FeTiMM in highly alkaline magmas is not known yet.
View in article
Lindsley, D.H., Frost, B.R. (1992) Equilibria among Fe-Ti oxides, pyroxenes, olivine, and quartz; Part I, Theory. American Mineralogist 77, 987–1003.
 Show in context
Show in context Alternative approaches are based on (i) the Fe2+/Fe3+ ratio of whole rocks (Kress and Carmichael, 1989; Putirka, 2016), (ii) mineral reactions involving olivine, pyroxene, and/or sphene (Frost and Lindsley, 1992; Lindsley and Frost, 1992; Andersen et al., 1993; Xirouchakis et al., 2001), (iii) biotite, K-feldspar and magnetite (Wones and Eugster, 1965; Wones, 1981) , (iv) zircon Ce (and Eu) anomalies (Ballard et al., 2002; Trail et al., 2012; Smythe and Brenan, 2016), and (v) single amphibole oxybarometry (Ridolfi et al., 2010).
View in article
One of the main advantages of FeTiMM is that it can be applied to magmas that do not contain ilmenite, which is true for many igneous rocks of mafic to felsic composition, particularly for those that are alkali-rich (Lindsley and Frost, 1992).
View in article
MacDonald, G.A., Katsura, T. (1964) Chemical composition of Hawaiian lavas. Journal of Petrology 5, 82–133.
 Show in context
Show in context It should be mentioned that none of the investigated melt compositions plot in the alkaline field (Macdonald and Katsura, 1964) in the total alkali vs. SiO2 (TAS) diagram (Le Maitre et al., 1989), so the performance of FeTiMM in highly alkaline magmas is not known yet.
View in article
Matjuschkin, V., Brooker, R.A., Tattitch, B., Blundy, J.D., Stamper, C.C. (2015) Control and monitoring of oxygen fugacity in piston cylinder experiments. Contributions to Mineralogy and Petrology 169, 9.
 Show in context
Show in context In nine cases, the reported experimental fO2 values deviated by more than 1.0 log unit fO2 from the values obtained via magnetite–ilmenite oxybarometry (Ghiorso and Evans, 2008; Fig. S-3), suggesting problems with the control of experimental fO2 (Matjuschkin et al., 2015).
View in article
Putirka, K. (2016) Rates and styles of planetary cooling on Earth, Moon, Mars, and Vesta, using new models for oxygen fugacity, ferric-ferrous ratios, olivine-liquid Fe-Mg exchange, and mantle potential temperature. American Mineralogist 101, 819–840.
 Show in context
Show in context Alternative approaches are based on (i) the Fe2+/Fe3+ ratio of whole rocks (Kress and Carmichael, 1989; Putirka, 2016), (ii) mineral reactions involving olivine, pyroxene, and/or sphene (Frost and Lindsley, 1992; Lindsley and Frost, 1992; Andersen et al., 1993; Xirouchakis et al., 2001), (iii) biotite, K-feldspar and magnetite (Wones and Eugster, 1965; Wones, 1981) , (iv) zircon Ce (and Eu) anomalies (Ballard et al., 2002; Trail et al., 2012; Smythe and Brenan, 2016), and (v) single amphibole oxybarometry (Ridolfi et al., 2010).
View in article
Ridolfi, F., Renzulli, A., Puerini, M. (2010) Stability and chemical equilibrium of amphibole in calc-alkaline magmas: an overview, new thermobarometric formulations and application to subduction-related volcanoes. Contributions to Mineralogy and Petrology 160, 45–66.
 Show in context
Show in context Alternative approaches are based on (i) the Fe2+/Fe3+ ratio of whole rocks (Kress and Carmichael, 1989; Putirka, 2016), (ii) mineral reactions involving olivine, pyroxene, and/or sphene (Frost and Lindsley, 1992; Lindsley and Frost, 1992; Andersen et al., 1993; Xirouchakis et al., 2001), (iii) biotite, K-feldspar and magnetite (Wones and Eugster, 1965; Wones, 1981) , (iv) zircon Ce (and Eu) anomalies (Ballard et al., 2002; Trail et al., 2012; Smythe and Brenan, 2016), and (v) single amphibole oxybarometry (Ridolfi et al., 2010).
View in article
Smythe, D.J., Brenan, J.M. (2016) Magmatic oxygen fugacity estimated using zircon-melt partitioning of cerium. Earth and Planetary Science Letters 453, 260–266.
 Show in context
Show in context Alternative approaches are based on (i) the Fe2+/Fe3+ ratio of whole rocks (Kress and Carmichael, 1989; Putirka, 2016), (ii) mineral reactions involving olivine, pyroxene, and/or sphene (Frost and Lindsley, 1992; Lindsley and Frost, 1992; Andersen et al., 1993; Xirouchakis et al., 2001), (iii) biotite, K-feldspar and magnetite (Wones and Eugster, 1965; Wones, 1981) , (iv) zircon Ce (and Eu) anomalies (Ballard et al., 2002; Trail et al., 2012; Smythe and Brenan, 2016), and (v) single amphibole oxybarometry (Ridolfi et al., 2010).
View in article
Stormer, J.C. (1983) The effects of recalculation on estimates of temperature and oxygen fugacity from analyses of multicomponent iron-titanium oxides. American Mineralogist 68, 586–594.
 Show in context
Show in context The most commonly used and most reliable way to reconstruct magmatic ƒO2 is via magnetite–ilmenite oxybarometry (e.g., Buddington and Lindsley, 1964; Carmichael, 1967; Stormer, 1983; Andersen and Lindsley, 1988; Ghiorso and Sack, 1991; Lattard et al., 2005; Ghiorso and Evans, 2008).
View in article
Trail, D., Watson, E.B., Tailby, N.D. (2012) Ce and Eu anomalies in zircon as proxies for the oxidation state of magmas. Geochimica et Cosmochimica Acta 97, 70–87.
 Show in context
Show in context Alternative approaches are based on (i) the Fe2+/Fe3+ ratio of whole rocks (Kress and Carmichael, 1989; Putirka, 2016), (ii) mineral reactions involving olivine, pyroxene, and/or sphene (Frost and Lindsley, 1992; Lindsley and Frost, 1992; Andersen et al., 1993; Xirouchakis et al., 2001), (iii) biotite, K-feldspar and magnetite (Wones and Eugster, 1965; Wones, 1981) , (iv) zircon Ce (and Eu) anomalies (Ballard et al., 2002; Trail et al., 2012; Smythe and Brenan, 2016), and (v) single amphibole oxybarometry (Ridolfi et al., 2010).
View in article
Wones, D. (1981) Mafic silicates as indicators of intensive variables in granitic magmas. Mining Geology 31, 191–212.
 Show in context
Show in context Alternative approaches are based on (i) the Fe2+/Fe3+ ratio of whole rocks (Kress and Carmichael, 1989; Putirka, 2016), (ii) mineral reactions involving olivine, pyroxene, and/or sphene (Frost and Lindsley, 1992; Lindsley and Frost, 1992; Andersen et al., 1993; Xirouchakis et al., 2001), (iii) biotite, K-feldspar and magnetite (Wones and Eugster, 1965; Wones, 1981) , (iv) zircon Ce (and Eu) anomalies (Ballard et al., 2002; Trail et al., 2012; Smythe and Brenan, 2016), and (v) single amphibole oxybarometry (Ridolfi et al., 2010).
View in article
Wones, D., Eugster, H. (1965) Stability of biotite–experiment theory and application. American Mineralogist 50, 1228–1272.
 Show in context
Show in context Alternative approaches are based on (i) the Fe2+/Fe3+ ratio of whole rocks (Kress and Carmichael, 1989; Putirka, 2016), (ii) mineral reactions involving olivine, pyroxene, and/or sphene (Frost and Lindsley, 1992; Lindsley and Frost, 1992; Andersen et al., 1993; Xirouchakis et al., 2001), (iii) biotite, K-feldspar and magnetite (Wones and Eugster, 1965; Wones, 1981) , (iv) zircon Ce (and Eu) anomalies (Ballard et al., 2002; Trail et al., 2012; Smythe and Brenan, 2016), and (v) single amphibole oxybarometry (Ridolfi et al., 2010).
View in article
Xirouchakis, D., Lindsley, D.H., Frost, B.R. (2001) Assemblages with titanite (CaTiOSiO4), Ca-Mg-Fe olivine and pyroxenes, Fe-Mg-Ti oxides, and quartz: Part II. Application. American Mineralogist 86, 254–264.
 Show in context
Show in context Alternative approaches are based on (i) the Fe2+/Fe3+ ratio of whole rocks (Kress and Carmichael, 1989; Putirka, 2016), (ii) mineral reactions involving olivine, pyroxene, and/or sphene (Frost and Lindsley, 1992; Lindsley and Frost, 1992; Andersen et al., 1993; Xirouchakis et al., 2001), (iii) biotite, K-feldspar and magnetite (Wones and Eugster, 1965; Wones, 1981) , (iv) zircon Ce (and Eu) anomalies (Ballard et al., 2002; Trail et al., 2012; Smythe and Brenan, 2016), and (v) single amphibole oxybarometry (Ridolfi et al., 2010).
View in article
top
Supplementary Information
Mn/Mg Test to Check for Equilibrium between Magnetite and Silicate Melt
To be able to check for equilibrium between a given magnetite–silicate melt pair, a new test was developed which is based on the same two elements (Mn, Mg) as the equilibrium test between magnetite and ilmenite (Bacon and Hirschmann, 1988), although the underlying rationale is quite different. We searched for two elements whose magnetite–melt partition coefficients depend in a similar manner on melt composition, but whose absolute concentrations in the silicate melt vary strongly during magma differentiation. With this approach, melts that do not coexist with a given magnetite composition should be easily identifiable. We thus use the Mn–Mg exchange coefficient DMn–Mgmgt/melt = (DMnOmgt/melt)/(DMgOmgt/melt), with MnO and MgO given in weight percent. A plot of DMn–Mgmgt/melt versus MnO/MgO ratio in the silicate melt for 356 magnetite–melt pairs with melts ranging from basaltic composition (48 wt. % SiO2) to high silica rhyolitic composition (79 wt. % SiO2) is shown in Figure S-2. It turns out that 95 % of the data points with reported Mn and Mg concentrations display a DMn–Mgmgt/melt value between 1 and 4, with the remaining 5 % apparently representing outliers because no correlation between magnitude of mismatch and the MnO/MgO ratio in the silicate melt (Fig. S-2) or other parameters (Table S-1) is evident.
Estimation of the Uncertainty of FeTiMM
We assume that f is an n-dimensional differentiable function and X1, X2, ..., Xn are independent variables with σ1, σ2, ..., σn standard deviations. Therefore, the overall uncertainty can be estimated from the first order linear estimate of f (X1, X2, ..., Xn ):
Eq. S-1
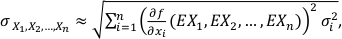
where
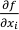 is the i-th partial derivative of the function f. If the expected values (EXi) of the variables (in our case chemical analyses) are unknown, one can replace the expected values by the variables themselves:
is the i-th partial derivative of the function f. If the expected values (EXi) of the variables (in our case chemical analyses) are unknown, one can replace the expected values by the variables themselves:Eq. S-2
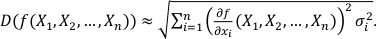
Our function has the following form:
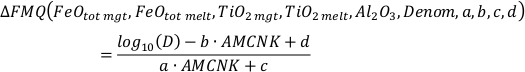
where
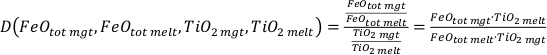
and
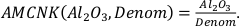
The variables CaO, Na2O, K2O, and MgO appear only as a sum in the equation (i.e. as CaO + Na2O + K2O + MgO) and their variances are independent, therefore they can be treated as a single variable in the calculation of the partial derivatives – named “Denom” – the variance of which is the sum of the individual elements variances:
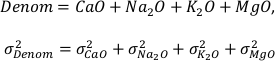
It is important to note that Al2O3, CaO, Na2O, K2O, and MgO (as well as their standard deviations) refer to molar abundances, whereas FeOtot mgt, FeOtot melt, TiO2 mgt and TiO2 melt are given in weight percent.
The partial derivatives used for the propagation of uncertainty are the following:
a) For the analytical uncertainty:
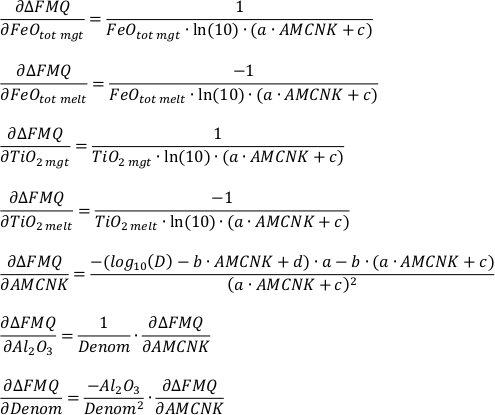
b) For the uncertainty inherent to the model:
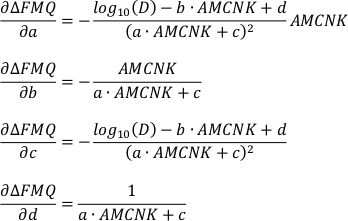
From the individual partial derivatives and variances (σ2i) of FeOtot mgt, FeOtot melt, TiO2 mgt, TiO2 melt, Al2O3, Denom, a, b, c and d the overall uncertainty can be estimated according to Equation S-2. For the estimation of the propagated analytical uncertainty only the partial derivatives and variances of FeOtot mgt, FeOtot melt, TiO2 mgt, TiO2 melt, Al2O3 and Denom have to be considered.
Supplementary Figures

Figure S-1 Dependence of the Fe–Ti exchange coefficient between magnetite and rhyolitic melt on fO2 and melt alumina saturation index. DFe–Timgt/melt refers to (DFeOtotmgt/melt)/(DTiO2mgt/melt), whereas ASI refers to molar Al2O3 /(CaO + Na2O + K2O). The dataset comprises magnetite–melt pairs from 50 different experiments performed at three different oxygen fugacity buffers, temperatures of 800-1000 °C, pressures of 100-500 MPa, with melt ASI values of 0.71-1.12, and magnetite compositions of 0.2-14 wt. % TiO2 (Arató and Audétat, 2017).

Figure S-2 Variance of the Mn–Mg exchange coefficient between magnetite and silicate melt as a function of the MnO/MgO weight ratio in the silicate melt. DMn–Mgmgt/melt refers to (DMnOmgt/melt)/(DMgOmgt/melt), with MnO and MgO given in weight percent. The data set comprises the 296 magnetite–rhyolite pairs from own experiments, plus the data points shown in Figure 2. The shaded envelope, which encompasses DMn–Mgmgt/melt values of 1-4, comprises 95 % of all data points.

Figure S-3 Comparison of reported experimental fO2 values with fO2 values obtained via magnetite–ilmenite (Ghiorso and Evans, 2008) for 59 ilmenite-bearing experiments taken from the literature. Oxygen fugacities are reported in log units relative to the fayalite-magnetite-quartz (FMQ) buffer.

Figure S-4 Difference between the fO2 values calculated via magnetite–ilmenite and ones calculated via FeTiMM [∆fO2 (FeTiMM - mgt–ilm)], as a function of (a) fO2, (b) AMCNK, (c) temperature, (d) magnetite composition, and (e) melt SiO2 content, for the 109 data points that were used to calibrate FeTiMM.

Figure S-5 Difference between reported experimental fO2 and fO2 calculated via FeTiMM [∆fO2 (FeTiMM - experimental)], as a function of (a) fO2, (b) AMCNK, (c) temperature, (d) magnetite composition, and (e) melt SiO2 content, for 27 ilmenite-undersaturated experiments taken from the literature.
Supplementary Tables
Supplementary Tables S-1 and S-2 can be downloaded as Excel files from the links below.
Table S-1 Full data set used to calibrate and test the FeTiMM model. The references listed in this table are detailed in Table S-2.
Table S-2 List of experimental studies that were screened for the five criteria discussed in the text.
Supplementary Information References
Arató, R., Audétat, A. (2017) Experimental calibration of a new oxybarometer for silicic magmas based on vanadium partitioning between magnetite and silicate melt. Geochimica et Cosmochimica Acta 209, 284–295.
Ghiorso, M.S., Evans, B.W. (2008) Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. American Journal of Science 308, 957–1039.
Figures and Tables
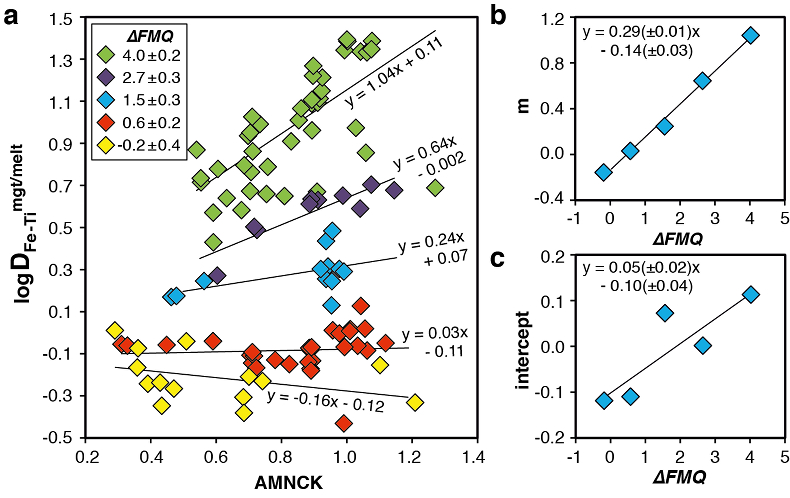
Figure 1 Development of the FeTiMM model. (a) 109 experimental data points (59 ilmenite-saturated; 50 ilmenite-undersaturated) were split into five groups of similar oxygen fugacity, through which linear regressions were fit. DFe–Timgt/melt = (DFeOtotmgt/melt)/(DTiO2mgt/melt); AMCNK = molar Al2O3/(MgO + CaO + Na2O + K2O). (b,c) Variation of the slopes and intercepts of the linear fits in (a) as a function of oxygen fugacity expressed in log unit deviations from the fayalite-magnetite-quartz (FMQ) buffer.
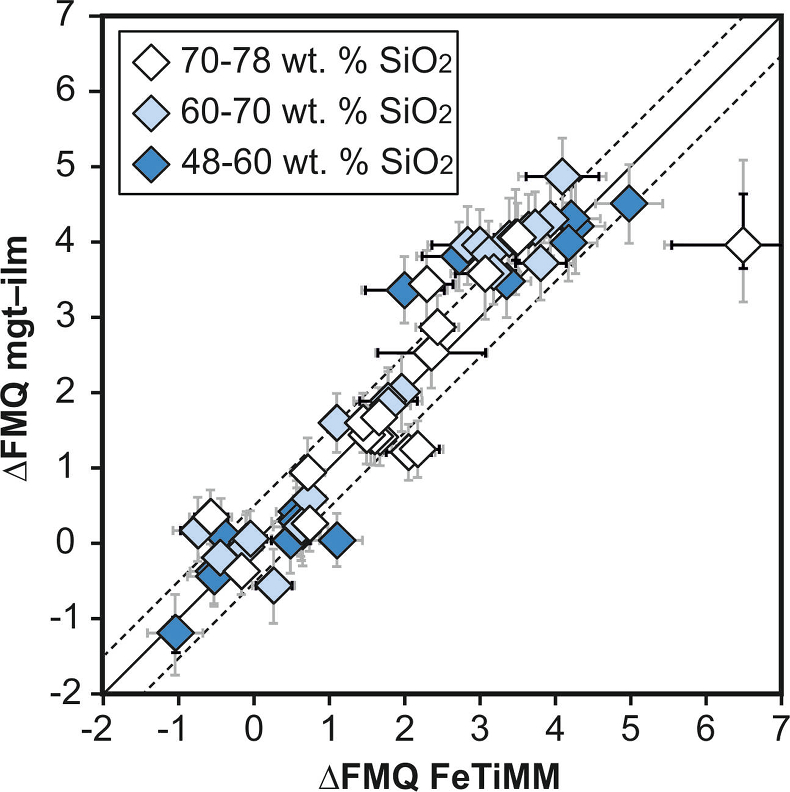
Figure 2 Performance of FeTiMM in ilmenite-saturated magmas. Oxygen fugacities (expressed in log units relative to the FMQ buffer) obtained via FeTiMM are compared with ones obtained via magnetite–ilmenite oxybarometry using the model of Ghiorso and Evans (2008)
Ghiorso, M.S., Evans, B.W. (2008) Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. American Journal of Science 308, 957–1039.
. The data are divided into three groups of contrasting melt SiO2. Black error bars (in most cases smaller than symbol size) denote the analytical error, whereas the grey error bars denote the overall error that includes both the analytical scatter and the error inherent to the model. Details about the calculation of errors are provided in the Supplementary Information.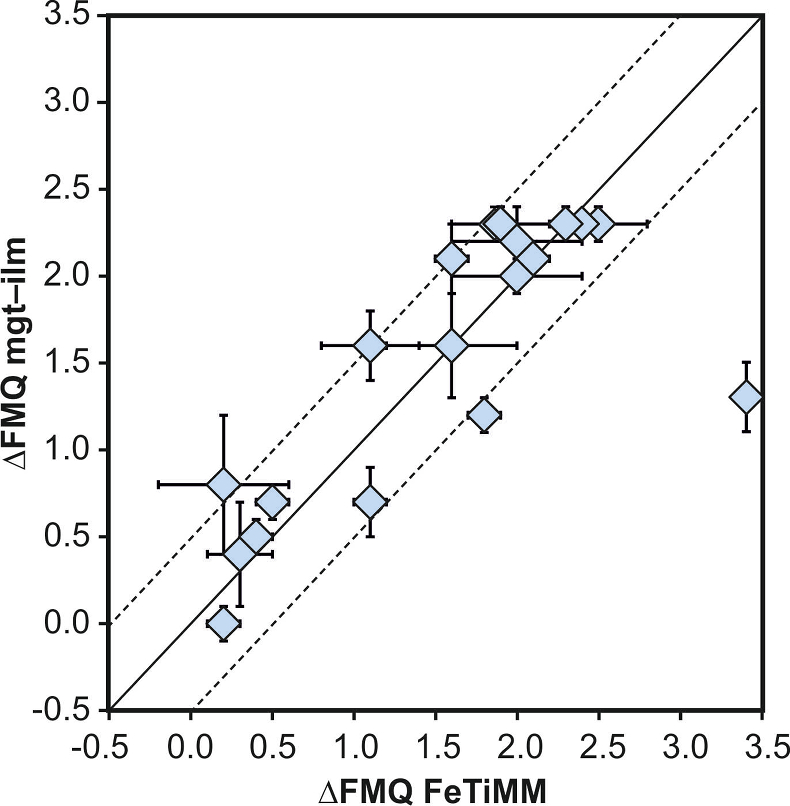
Figure 3 Performance of FeTiMM in ilmenite-undersaturated magmas. Due to the lack of ilmenite, one has to rely on the reported experimentally imposed fO2 values, which are associated with considerable (but unknown) uncertainty. Correspondingly, error bars in y-axis direction cannot be displayed. See Figure 2 for abbreviations and the meaning of the black and grey error bars in x-axis direction.
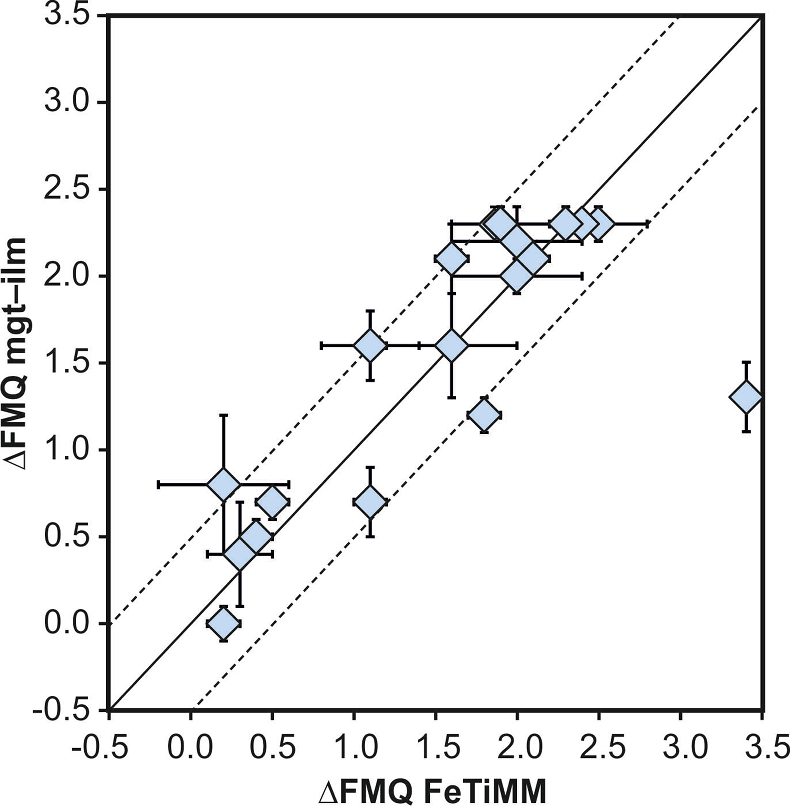
Figure 4 Application of FeTiMM to a set of 19 natural, ilmenite-saturated samples of rhyolitic to dacitic composition (Arató and Audétat, 2017a
Arató, R., Audétat, A. (2017a) Vanadium magnetite–melt oxybarometry of natural, silicic magmas: a comparison of various oxybarometers and thermometers. Contributions to Mineralogy and Petrology 172, doi: 10.1007/s00410-017-1369-6.
). Oxygen fugacities (reported in log units relative to the fayalite–magnetite–quartz buffer) obtained via FeTiMM agree within 0.5 log units with those obtained via magnetite–ilmenite oxybarometry (Ghiorso and Evans, 2008Ghiorso, M.S., Evans, B.W. (2008) Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. American Journal of Science 308, 957–1039.
) in all but one case. Error bars denote 1σ standard deviation of the fO2 values obtained from several magnetite–melt pairs.Back to article
Supplementary Figures and Tables
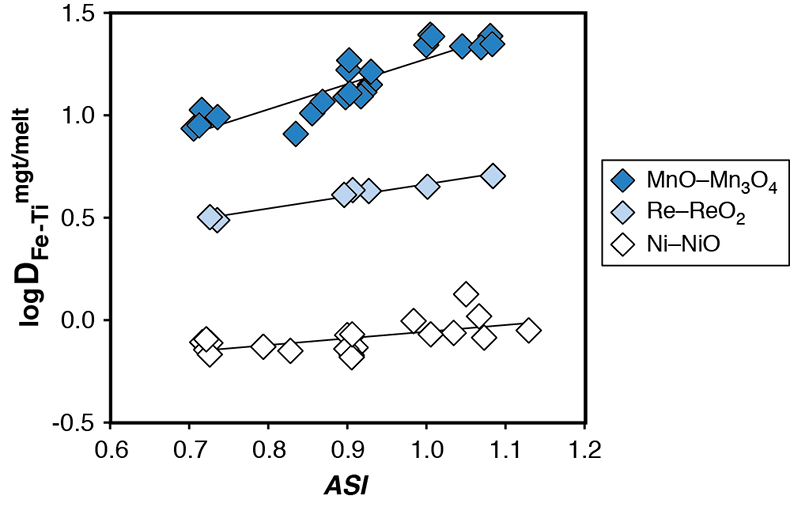
Figure S-1 Dependence of the Fe–Ti exchange coefficient between magnetite and rhyolitic melt on fO2 and melt alumina saturation index. DFe–Timgt/melt refers to (DFeOtotmgt/melt)/(DTiO2mgt/melt), whereas ASI refers to molar Al2O3 /(CaO + Na2O + K2O). The dataset comprises magnetite–melt pairs from 50 different experiments performed at three different oxygen fugacity buffers, temperatures of 800-1000 °C, pressures of 100-500 MPa, with melt ASI values of 0.71-1.12, and magnetite compositions of 0.2-14 wt. % TiO2 (Arató and Audétat, 2017).
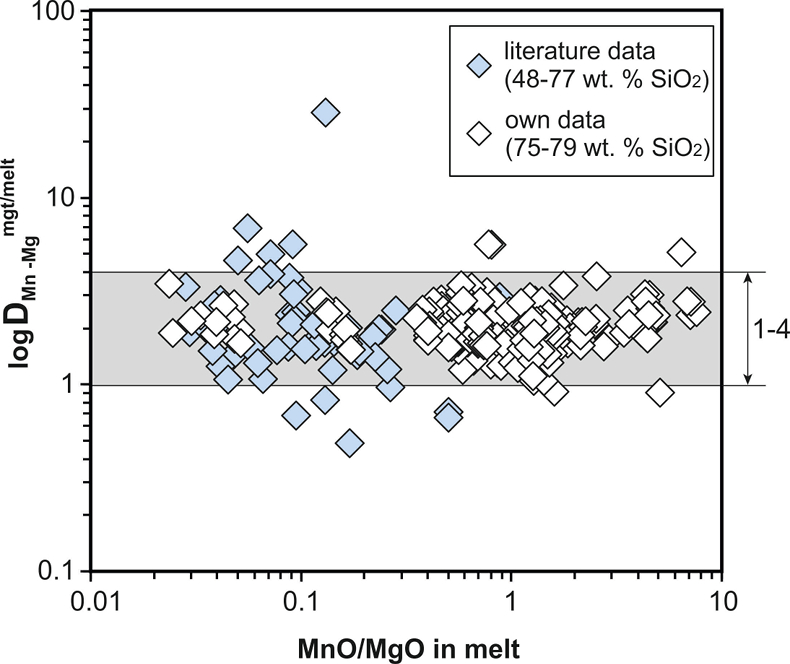
Figure S-2 Variance of the Mn–Mg exchange coefficient between magnetite and silicate melt as a function of the MnO/MgO weight ratio in the silicate melt. DMn–Mgmgt/melt refers to (DMnOmgt/melt)/(DMgOmgt/melt), with MnO and MgO given in weight percent. The data set comprises the 296 magnetite–rhyolite pairs from own experiments, plus the data points shown in Figure 2. The shaded envelope, which encompasses DMn–Mgmgt/melt values of 1-4, comprises 95 % of all data points.
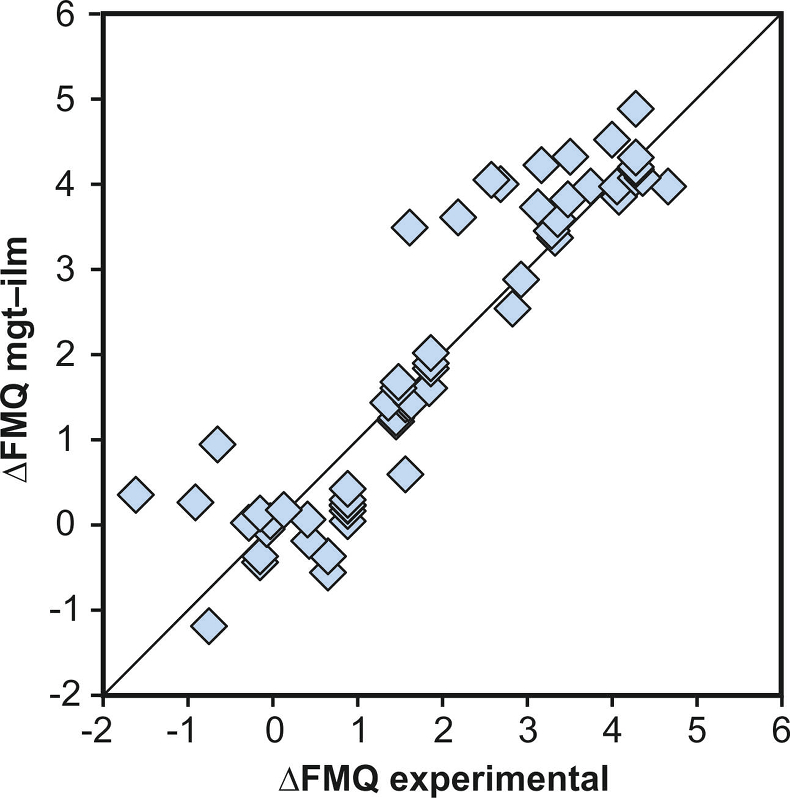
Figure S-3 Comparison of reported experimental fO2 values with fO2 values obtained via magnetite–ilmenite (Ghiorso and Evans, 2008) for 59 ilmenite-bearing experiments taken from the literature. Oxygen fugacities are reported in log units relative to the fayalite-magnetite-quartz (FMQ) buffer.
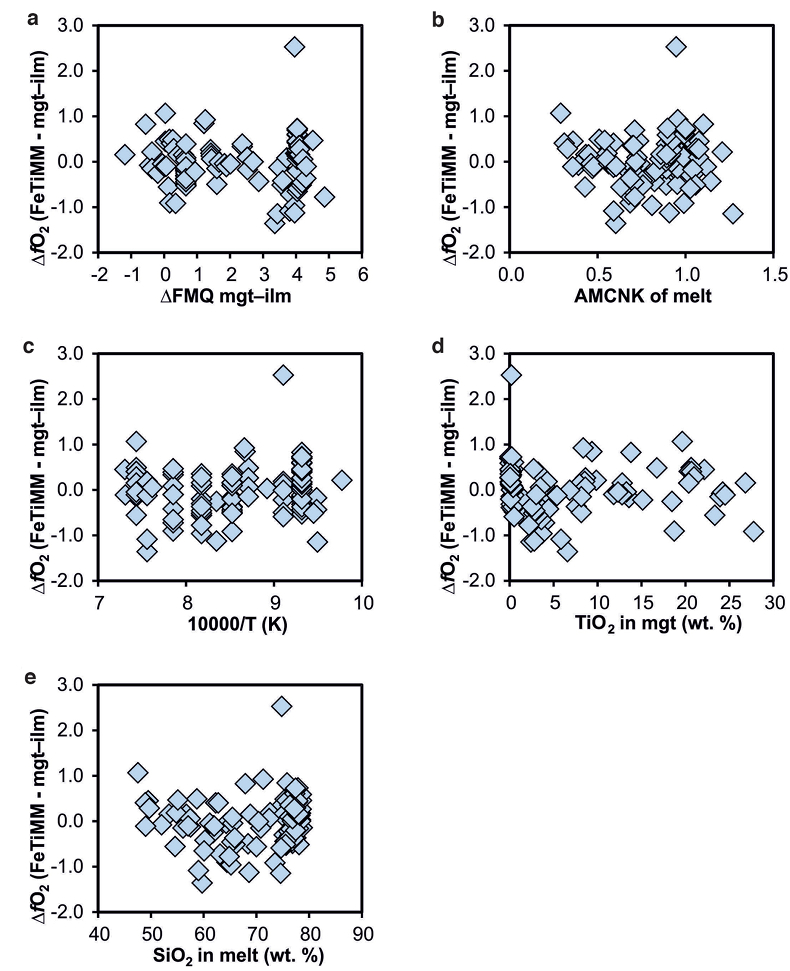
Figure S-4 Difference between the fO2 values calculated via magnetite–ilmenite and ones calculated via FeTiMM [∆fO2 (FeTiMM - mgt–ilm)], as a function of (a) fO2, (b) AMCNK, (c) temperature, (d) magnetite composition, and (e) melt SiO2 content, for the 109 data points that were used to calibrate FeTiMM.
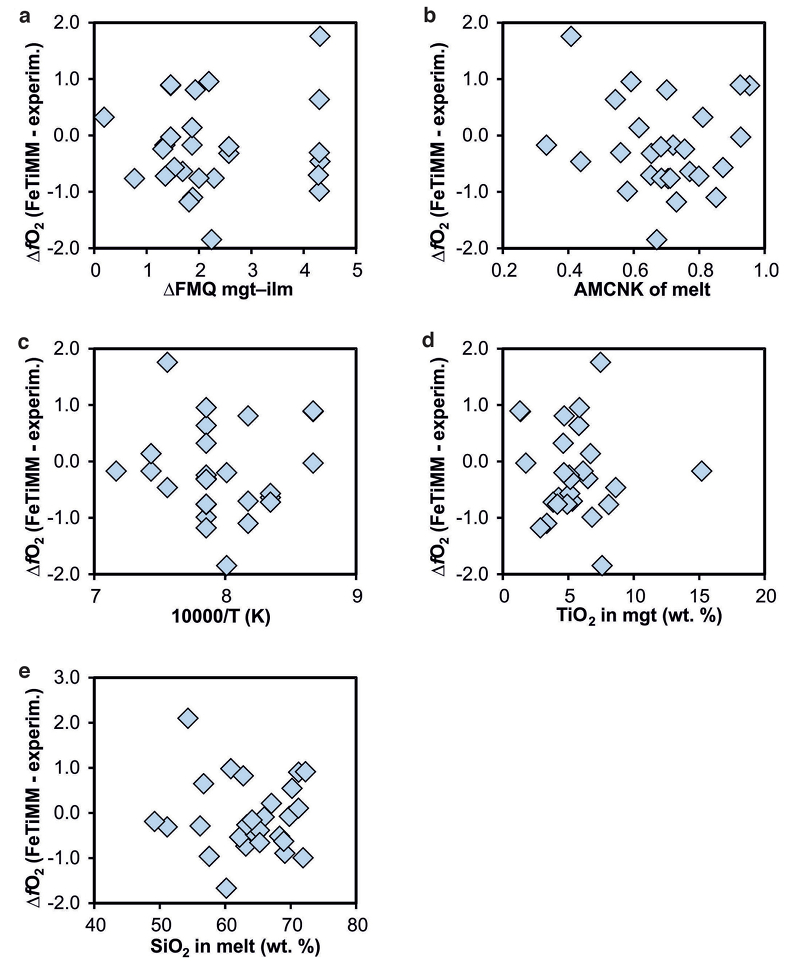
Figure S-5 Difference between reported experimental fO2 and fO2 calculated via FeTiMM [∆fO2 (FeTiMM - experimental)], as a function of (a) fO2, (b) AMCNK, (c) temperature, (d) magnetite composition, and (e) melt SiO2 content, for 27 ilmenite-undersaturated experiments taken from the literature.
Table S-1 Full data set used to calibrate and test the FeTiMM model. The references listed in this table are detailed in Table S-2.
Table S-2 List of experimental studies that were screened for the five criteria discussed in the text.