Tin isotopic fractionation during igneous differentiation and Earth’s mantle composition
Affiliations | Corresponding Author | Cite as | Funding informationKeywords: tin isotopes, isotopic fractionation, basalts, Kilauea Iki lava lake, tin
- Share this article





Article views:9,224Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
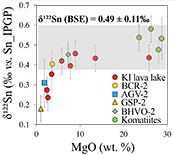
 Table 1 Tin isotopic composition of the samples analysed in this study. | 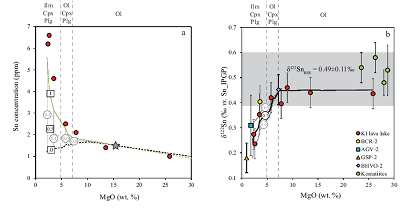 Figure 1 (a) Model showing Sn concentration for KI samples in the melt against MgO content. Red circles = KI samples. Thick lines represent modelled evolution with differing Sn4+/∑Sn, listed in black boxes from 1 (green line), 0.5 (dashed grey line) and 0 (dashed black line). Thin grey line represents the fraction of melt remaining (F), listed in grey circles, with the grey star representing the parental melt composition. (b) δ122Sn and Sn against MgO (wt. %) for the KI lava lake samples. The solid lines represent the modelled δ122Sn for ∆122Snmineral-melt = 0.5 (grey line), 0.7 (black line) and 0.9 (grey line). Geostandard values are taken from Creech et al. (2017). In both cases, the crystallising assemblage is listed at the top of the figure (Ol = olivine, Cpx = clinopyroxene, Plg = plagioclase, Ilm = ilmenite). | 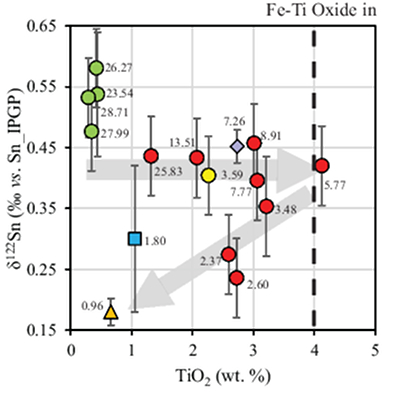 Figure 2 Variation of δ122Sn with TiO2 content, MgO (wt. %) shown next to the data point. Sn isotope fractionation occurs only after Fe-Ti oxide (ilmenite) saturation at Kilauea Iki. Symbols as per Figure 1. |  Table 2 Calculated partition coefficients for Sn2+ and Sn4+ using lattice strain theory. | 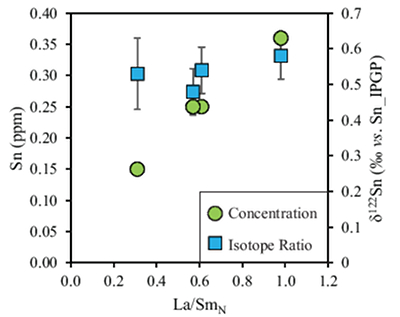 Figure 3 Depletion of komatiite source regions, quantified by La/SmN, as a function of Sn concentration (green circles) and δ122Sn (blue squares). Tin contents are positively correlated with La/SmN, whereas δ122Sn remains constant within uncertainty. |
| Table 1 | Figure 1 | Figure 2 | Table 2 | Figure 3 |
Supplementary Figures and Tables
 Table S-1 Terms used for Sn partition coefficient calculations. |  Table S-2 Average trace element ratio values for Kilauea Iki lava lake. | 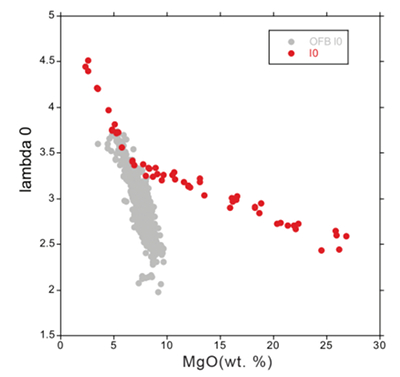 Figure S-1 Calculated λ0, quantifying the mean abundances of the Rare Earth Elements (see O’Neill, 2016) against MgO. Red points correspond to the Kilauea Iki lava lake, and show a rapid drop in MgO and a small increase in λ0 for 7.4 < MgO (wt. %) < 27 during olivine crystallisation/accumulation, before a rapid increase in slope at 7.4 wt. % MgO signalling a change in the crystallising assemblage to cpx + plg + ol. | 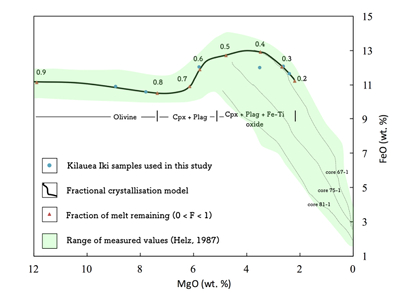 Figure S-2 Modelled abundances of FeO and MgO (wt. %) in the Kilauea Iki lava lake, overlain with the field (green) of literature data (Helz, 1987) for different eruption dates (1967, 1979 and 1981). | 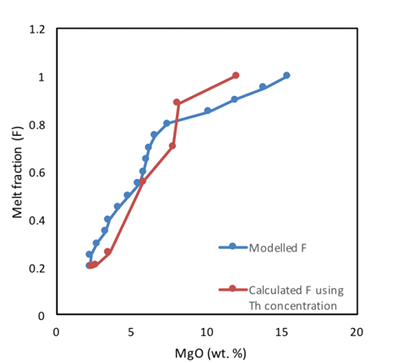 Figure S-3 Plot of melt fraction against MgO (wt. %). The maroon line represents the calculated F assuming that Th behaves as a perfectly incompatible element. The blue line represents the modelled F from the major element modelling of Kilauea Iki lava lake. | 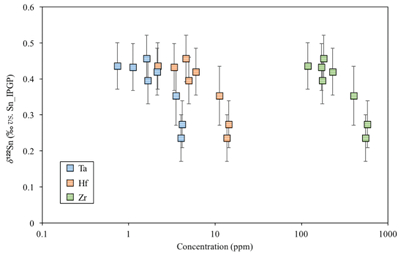 Figure S-4 Plot of δ122Sn against Ta, Hf and Zr (HFSEs). |
| Table S-1 | Table S-2 | Figure S-1 | Figure S-2 | Figure S-3 | Figure S-4 |
top
Letter
Moderately volatile elements (MVEs) and their isotopes are important tracers for planetary and nebular processes such as evaporation and condensation (e.g., O’Neill and Palme, 2008
O’Neill, H.S.C., Palme, H. (2008) Collisional erosion and the non-chondritic composition of the terrestrial planets. Philosophical transactions of the Royal Society A 366, 4205–4238.
; Paniello et al., 2012Paniello, R., Day, J.M.D., Moynier, F. (2012) Zinc isotopic evidence for the origin of the Moon. Nature 490, 376–379.
). A condition that must be met before MVEs can be used to answer these questions is knowledge of the isotopic composition of the bulk silicate Earth (BSE) and hence how their isotopes fractionate during igneous processes.Tin has three oxidation states (Sn0, Sn2+, Sn4+) that confer differing properties as a function of oxygen fugacity (ƒO2). As such, Sn may behave as a siderophile, chalcophile and lithophile element at high temperatures (e.g., Heinrich, 1990
Heinrich, C.A. (1990). The chemistry of hydrothermal tin (-tungsten) ore deposition. Economic Geology 85, 457–481.
; Witt-Eickschen et al., 2009Witt-Eickschen, G., Palme, H., O’Neill, H., Allen, C. (2009) The geochemistry of the volatile trace elements As, Cd, Ga, In and Sn in the Earth’s mantle: new evidence from in situ analyses of mantle xenoliths. Geochimica et Cosmochimica Acta 73, 1755–1778.
). Although the dependence of Sn2+/Sn4+ on melt composition, and the ƒO2 at which this transition occurs in basaltic melts are poorly known (Farges et al., 2006Farges, F., Linnen, R.L., Brown, G.E. (2006) Redox and speciation of tin in hydrous silicate glasses: A comparison with Nb, Ta, Mo and W. Canadian Mineralogist 44, 795–810.
), the behaviour of Sn in igneous systems may be gleaned by comparison with elements of similar incompatibility. Observations of oceanic basalts show that Sn/Sm, Sn/Zr and Sn/Hf ratios are nearly uniform across MORB and OIB (Jochum et al., 1993Jochum, K.P., Hofmann, A.W., Seufert, H.M. (1993) Tin in mantle-derived rocks: Constraints on Earth evolution. Geochimica et Cosmochimica Acta 57, 3585–3595.
; Jenner and O’Neill, 2012Jenner, F., O’Neill, H. (2012) Analysis of 60 elements in 616 ocean floor basaltic glasses. Geochemistry, Geophysics, Geosystems 12, doi: 10.1029/2011GC004009.
). Correlations with tetravalent, incompatible elements suggest Sn may be lithophile and occurs as Sn4+ during igneous processes near FMQ.At equilibrium, all else being equal, isotopic fractionation is controlled by differences in bond stiffness between two phases (e.g., Schauble, 2004). The diversity in Sn bonding environments (Sn-O, Sn-S and Sn0) combined with differing oxidation states offer scope for significant isotope fractionation. Indeed, Creech et al. (2017)
Creech, J.B., Moynier, F., Badullovich, N. (2017) Tin stable isotope analysis of geological materials by double-spike MC-ICPMS. Chemical Geology 457, 61–67.
analysed four genetically unrelated igneous rocks and identified that the 122Sn/118Sn ratio increases with increasing MgO content, suggesting that Sn isotopic fractionation occurs during igneous differentiation. However, the mechanisms driving this fractionation remain unknown.In order to determine the behaviour of Sn isotopes in igneous systems and establish the composition of the BSE, it is necessary to investigate natural terrestrial samples from a common source. Kilauea Iki (KI) lava lake is well-suited to test the effects of fractional crystallisation (e.g., Tomascak et al., 1999
Tomascak, P.B., Tera, F., Helz, R.T., Walker, R.J. (1999) The absence of lithium isotope fractionation during basalt differentiation: new measurements by multicollector sector ICP-MS. Geochimica et Cosmochimica Acta 63, 907–910.
; Teng et al., 2008Teng, F.-Z., Dauphas, N., Helz, R.T. (2008) Iron Isotope Fractionation During Magmatic Differentiation in Kilauea Iki Lava Lake. Science 320, 1620–1622.
; Chen et al. 2013Chen, H., Savage, P., Teng, F.Z., Helz, R., Moynier, F. (2013) Zinc isotope fractionation during magmatic differentiation and the isotopic composition of the bulk silicate Earth. Earth and Planetary Science Letters 369, 34–42.
; Kato et al., 2017Kato, C., Moynier, F., Foriel, J., Teng, F.-Z., Puchtel, I.S. (2017) The gallium isotopic composition of the bulk silicate Earth. Chemical Geology 448, 164–172.
). The cooling lava differentiated in a closed system to form olivine-rich cumulates to andesitic lavas with some rare silicic veins. Extrapolation to the composition of the BSE using a single magmatic suite is tenuous, given the diversity of mantle sources in the present day mantle and the possibility of secular changes in mantle composition (e.g., Maier et al., 2008Maier, W.D., Barnes, S., Campbell, I., Fiorentini, M., Peltonen, P., Barnes, S., Smithies, H. (2008) Progressive mixing of meteoritic veneer into the early Earth's deep mantle. Nature 460, 620–623.
). In order to assess mantle heterogeneity over space and time, komatiites, ranging in age from 3.5 to 2.7 Ga and from 3 cratons (Yilgarn, Kaapvaal, Superior), were analysed for their Sn isotope composition. As komatiites are produced by 25–40 % partial melting (e.g., Herzberg, 1992Herzberg, C. (1992) Depth and degree of melting of komatiites. Journal of Geophysical Research: Solid Earth 97, 4521–4540.
), and Sn is incompatible during mantle melting (being predominantly hosted in clinopyroxene; Witt-Eickschen et al., 2009Witt-Eickschen, G., Palme, H., O’Neill, H., Allen, C. (2009) The geochemistry of the volatile trace elements As, Cd, Ga, In and Sn in the Earth’s mantle: new evidence from in situ analyses of mantle xenoliths. Geochimica et Cosmochimica Acta 73, 1755–1778.
), melting quantitatively extracts Sn from their mantle sources. Komatiite sources, especially for older 3.5 Ga examples, have primitive mantle-like compositions (Sossi et al., 2016Sossi, P.A., Eggins, S.M., Nesbitt, R.W., Nebel, O., Hergt, J.M., Campbell, I.H., O'Neill, H., VanKranendonk, M., Davies, D. (2016) Petrogenesis and geochemistry of Archean komatiites. Journal of Petrology 57, 147–184.
), and thus permit assessment of the temporal and chemical heterogeneity in Earth’s mantle.Here, we have analysed the isotopic composition of a series of samples from KI lava lake and komatiites utilising the 117Sn-122Sn double spike MC-ICPMS method Creech et al. (2017)
Creech, J.B., Moynier, F., Badullovich, N. (2017) Tin stable isotope analysis of geological materials by double-spike MC-ICPMS. Chemical Geology 457, 61–67.
(see Supplementary Information). The differentiation sequence samples form a sub-alkali series with MgO content ranging from 25.83–2.37 wt. % (Helz et al., 1994Helz, R.T., Kirschenbaum, H., Marinenko, J.W., Qian, R. (1994) Whole-rock analyses of core samples from the 1967, 1979 and 1981 drillings of Kilauea Iki lava lake, Hawaii. U.S. Geological Survey Report 94-684.
) (see Supplementary Information). The four spinifex-textured komatiites have 23.54 < MgO (wt. %) < 28.71, and thus chemical compositions close to their respective parental magma (Sossi et al., 2016Sossi, P.A., Eggins, S.M., Nesbitt, R.W., Nebel, O., Hergt, J.M., Campbell, I.H., O'Neill, H., VanKranendonk, M., Davies, D. (2016) Petrogenesis and geochemistry of Archean komatiites. Journal of Petrology 57, 147–184.
).The data are reported in Table 1, with δ122Sn being the ‰ deviation of the 122Sn/118Sn ratio relative to the Sn_IPGP standard. In the KI samples, δ122Sn ranges from 0.24 ± 0.07 to 0.46 ± 0.07 ‰, which represents a variation (~0.05 ‰/amu) similar to Zn (~0.05 ‰/amu; Chen et al. 2013
Chen, H., Savage, P., Teng, F.Z., Helz, R., Moynier, F. (2013) Zinc isotope fractionation during magmatic differentiation and the isotopic composition of the bulk silicate Earth. Earth and Planetary Science Letters 369, 34–42.
). The samples with less than 5 wt. % MgO show a progressive depletion in the heavier isotopes of Sn as MgO decreases (Fig. 1b). In addition, Sn concentration inversely correlates with the MgO content of the samples, defining a parabolic curve typical of highly incompatible elements (Fig. 1a). The Sn/E ratios (E = Sm, Hf or Zr; data from Helz, 2012Helz, R.T. (2012) Trace-element analyses of core samples from the 1967-1988 drillings of Kilauea Iki Lava Lake, Hawaii. U.S. Geological Survey Report 2010-1093.
) in samples across the differentiation sequence are similar to OIBs and MORBs reported in Jochum et al. (1993)Jochum, K.P., Hofmann, A.W., Seufert, H.M. (1993) Tin in mantle-derived rocks: Constraints on Earth evolution. Geochimica et Cosmochimica Acta 57, 3585–3595.
and Jenner and O’Neill (2012)Jenner, F., O’Neill, H. (2012) Analysis of 60 elements in 616 ocean floor basaltic glasses. Geochemistry, Geophysics, Geosystems 12, doi: 10.1029/2011GC004009.
(see Supplementary Information). The four komatiites have different Sn contents between 0.15 and 0.36 ppm (BSE = 0.14 ppm), however, their δ122Sn fall between 0.48 ± 0.065 ‰ and 0.58 ± 0.065 ‰, and show no systematic differences.Table 1 Tin isotopic composition of the samples analysed in this study.
| Sample ID | Rock type | Weight (g) | MgO (wt. %) | Sn conc. (μg/g) | δ122Sn (‰) | 2 s.d.b | n |
| KI67-3-6.8 | picro-Basalt | 0.3546 | 25.83a | 1 | 0.44 | 0.07 | 1 |
| KI79-3-150.4 | Basalt | 0.2506 | 13.51a | 1.4 | 0.43 | 0.07 | 2 |
| KI67-3-58.0 | Basalt | 0.2664 | 8.91a | 1.4 | 0.46 | 0.07 | 2 |
| KI75-1-121.5 | Basalt | 0.2483 | 7.77a | 2.1 | 0.4 | 0.07 | 2 |
| KI75-1-75.2 | Basalt | 0.246 | 5.77a | 2.5 | 0.42 | 0.07 | 2 |
| KI79-1R1-170.9 | Basaltic-andesite | 0.241 | 3.48a | 4.6 | 0.35 | 0.08 | 2 |
| KI67-2-85.7 | Basaltic-andesite | 0.2475 | 2.60a | 6.6 | 0.24 | 0.07 | 2 |
| KI81-2-88.6 | Andesite | 0.2512 | 2.37a | 6.2 | 0.27 | 0.07 | 2 |
| 331/783 | Komatiite (Barberton. 3.48 Ga) | 1.5415 | 26.27c | 0.36 | 0.58 | 0.07 | 2 |
| 331/948 | Komatiite (Yilgarn. 2.7 Ga) | 1.181 | 23.54c | 0.25 | 0.54 | 0.07 | 2 |
| SD6/400 | Komatiite (Yilgarn. 2.7 Ga) | 1.2648 | 27.99c | 0.25 | 0.48 | 0.07 | 2 |
| 422/96 | Komatiite (Munro. 2.7 Ga) | 1.1968 | 28.71c | 0.15 | 0.53 | 0.10 | 2 |
a SiO2/MgO values for Kilauea Iki lava lake taken from Helz et al. (1994) Helz, R.T., Kirschenbaum, H., Marinenko, J.W., Qian, R. (1994) Whole-rock analyses of core samples from the 1967, 1979 and 1981 drillings of Kilauea Iki lava lake, Hawaii. U.S. Geological Survey Report 94-684. b Maximum of either 2 times the standard deviation on the sample measurement or BCR-2 external reproducibility. c MgO value for komatiites taken from Sossi et al. (2016) Sossi, P.A., Eggins, S.M., Nesbitt, R.W., Nebel, O., Hergt, J.M., Campbell, I.H., O'Neill, H., VanKranendonk, M., Davies, D. (2016) Petrogenesis and geochemistry of Archean komatiites. Journal of Petrology 57, 147–184.
Download in Excel
Figure 1 (a) Model showing Sn concentration for KI samples in the melt against MgO content. Red circles = KI samples. Thick lines represent modelled evolution with differing Sn4+/∑Sn, listed in black boxes from 1 (green line), 0.5 (dashed grey line) and 0 (dashed black line). Thin grey line represents the fraction of melt remaining (F), listed in grey circles, with the grey star representing the parental melt composition. (b) δ122Sn and Sn against MgO (wt. %) for the KI lava lake samples. The solid lines represent the modelled δ122Sn for ∆122Snmineral-melt = 0.5 (grey line), 0.7 (black line) and 0.9 (grey line). Geostandard values are taken from Creech et al. (2017)
Creech, J.B., Moynier, F., Badullovich, N. (2017) Tin stable isotope analysis of geological materials by double-spike MC-ICPMS. Chemical Geology 457, 61–67.
. In both cases, the crystallising assemblage is listed at the top of the figure (Ol = olivine, Cpx = clinopyroxene, Plg = plagioclase, Ilm = ilmenite).To understand the Sn isotopic variations, it is necessary first to estimate the partition coefficients of Sn2+ and Sn4+ between major minerals and melt. Given the limited experimental data currently available (Adam and Green, 2006
Adam, J., Green, T. (2006) Trace element partitioning between mica-and amphibole-bearing garnet lherzolite and hydrous basanitic melt:1. Experimental results and the investigation of controls on partitioning behaviour. Contributions to Mineralogy and Petrology 152, 1–17.
; Klemme et al., 2006Klemme, S., Günther, D., Hametner, K., Prowatke, S., Zack, T. (2006) The partitioning of trace elements between ilmenite, ulvospinel, armalcolite and silicate melts with implications for the early differentiation of the moon. Chemical Geology 234, 251–263.
; Michely et al., 2017Michely, L.T., Leitzke, F.P., Speelmanns, I.M., Fonseca, R.O.C. (2017) Competing effects of crystal chemistry and silicate melt composition on trace element behavior in magmatic systems: insights from crystal/silicate melt partitioning of the REE, HFSE, Sn, In, Ga, Ba, Pt and Rh. Contributions to Mineralogy and Petrology 172, 39.
), we address this question using lattice strain theory and then model the Sn abundance and speciation during the differentiation of the KI lava lake.Calculating elemental partitioning of Sn. Partition coefficients for Sn2+ and Sn4+ between crystals and melt were calculated for olivine, clinopyroxene, orthopyroxene, plagioclase and ilmenite using the lattice strain model (Blundy and Wood, 1994
Blundy, J., Wood, B. (1994) Prediction of crystal-melt partition coefficients from elastic moduli. Nature 372, 452–454.
; Supplementary Information). The calculated values were then compared to experimentally derived partition coefficients (Michely et al., 2017Michely, L.T., Leitzke, F.P., Speelmanns, I.M., Fonseca, R.O.C. (2017) Competing effects of crystal chemistry and silicate melt composition on trace element behavior in magmatic systems: insights from crystal/silicate melt partitioning of the REE, HFSE, Sn, In, Ga, Ba, Pt and Rh. Contributions to Mineralogy and Petrology 172, 39.
; Adam and Green, 2006Adam, J., Green, T. (2006) Trace element partitioning between mica-and amphibole-bearing garnet lherzolite and hydrous basanitic melt:1. Experimental results and the investigation of controls on partitioning behaviour. Contributions to Mineralogy and Petrology 152, 1–17.
; Klemme et al., 2006Klemme, S., Günther, D., Hametner, K., Prowatke, S., Zack, T. (2006) The partitioning of trace elements between ilmenite, ulvospinel, armalcolite and silicate melts with implications for the early differentiation of the moon. Chemical Geology 234, 251–263.
; Table 2). Stannous Sn2+ (rVI = 0.96 Å) substitutes poorly into the M1 sites of Fe-Mg silicates (Dmin/melt ≈ 0.1), but has Dmin/melt of unity in the larger, Ca-bearing VIII-fold sites of clinopyroxene and plagioclase (rVIII ≈ 1.15 Å). Divalent tin is more compatible in all minerals except ilmenite compared to Sn4+ (r = 0.69 Å) due to its substitution for VITi4+ (r = 0.605 Å). The experiments of Michely et al. (2017)Michely, L.T., Leitzke, F.P., Speelmanns, I.M., Fonseca, R.O.C. (2017) Competing effects of crystal chemistry and silicate melt composition on trace element behavior in magmatic systems: insights from crystal/silicate melt partitioning of the REE, HFSE, Sn, In, Ga, Ba, Pt and Rh. Contributions to Mineralogy and Petrology 172, 39.
, performed in air, contain exclusively Sn4+, whereas those of Adam and Green (2006)Adam, J., Green, T. (2006) Trace element partitioning between mica-and amphibole-bearing garnet lherzolite and hydrous basanitic melt:1. Experimental results and the investigation of controls on partitioning behaviour. Contributions to Mineralogy and Petrology 152, 1–17.
may contain both Sn2+ and Sn4+ because Sn falls between calculated lattice strain curves for a given redox state and site property. The graphite capsules used imposed ƒO2 conditions near FMQ-2 (Médard et al., 2008Médard, E., McCammon, C.A., Barr, J.A., Grove, T.L. (2008) Oxygen fugacity, temperature reproducibility, and H2O contents of nominally anhydrous piston-cylinder experiments using graphite capsules. American Mineralogist 93, 1838–1844.
), at least 2 orders of magnitude lower than in the KI lava lake, thereby increasing the stability of divalent Sn, according to:Eq. 1
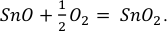
Based on the relative compatibility of Sn2+, Sn4+ would accumulate in the melt as fractional crystallisation progresses, such that
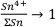 as the fraction of melt remaining, F → O.
as the fraction of melt remaining, F → O.Sn speciation during fractional crystallisation. The relative proportions of MgO and FeO(T) in the KI samples were modelled assuming fractional crystallisation (Fig. 2). The mineral/melt partition coefficients calculated for Sn (Table 2) were used to model the evolution of Sn in the melt.

Figure 2 Variation of δ122Sn with TiO2 content, MgO (wt. %) shown next to the data point. Sn isotope fractionation occurs only after Fe-Ti oxide (ilmenite) saturation at Kilauea Iki. Symbols as per Figure 1.
Table 2 Calculated partition coefficients for Sn2+ and Sn4+ using lattice strain theory.
| Coordination | Olivine | Orthopyroxene | Clinopyroxene | Plagioclase | Ilmenite | |
| 2+ | Lattice Strain | |||||
| VI | 0.12 – 0.20 | 0.09 – 0.14 | 0.04 – 0.07 | - | - | |
| VIII | - | - | 1.0 – 1.2 | 0.94 – 1.27 | - | |
| Bulk Sn2+ | 0.12 – 0.20 | 0.09 – 0.14 | 1.04 – 1.27 | 0.94 – 1.27 | - | |
| 4+ | IV | 1x10-5 | 1x10-5 | 1x10-5 | 1x10-5 | - |
| VI | 0.005 – 0.01 | 0.01 – 0.07 | 0.05 – 0.39 | - | 4.5 – 6.8 | |
| Bulk Sn4+ | 0.005 – 0.01 | 0.01 – 0.07 | 0.05 – 0.39 | - | - | |
| Experimental | ||||||
| Adam and Green (2006) Adam, J., Green, T. (2006) Trace element partitioning between mica-and amphibole-bearing garnet lherzolite and hydrous basanitic melt:1. Experimental results and the investigation of controls on partitioning behaviour. Contributions to Mineralogy and Petrology 152, 1–17. | 0.002 – 0.014 | 0.015 | 0.024 – 1 | - | - | |
| Klemme et al. (2006) Klemme, S., Günther, D., Hametner, K., Prowatke, S., Zack, T. (2006) The partitioning of trace elements between ilmenite, ulvospinel, armalcolite and silicate melts with implications for the early differentiation of the moon. Chemical Geology 234, 251–263. | - | - | - | - | 5.1 | |
| Michely et al. (2017) Michely, L.T., Leitzke, F.P., Speelmanns, I.M., Fonseca, R.O.C. (2017) Competing effects of crystal chemistry and silicate melt composition on trace element behavior in magmatic systems: insights from crystal/silicate melt partitioning of the REE, HFSE, Sn, In, Ga, Ba, Pt and Rh. Contributions to Mineralogy and Petrology 172, 39. | | | 0.19 – 0.46* | | | |
*Only partition coefficients for the natural, komatiitic ‘W’ composition are reported here.
Download in ExcelThe crystallising phase proportions of olivine, augite, plagioclase and Fe-Ti oxides were adjusted such that the modelled evolution of MgO and FeO contents closely follow the natural data, assuming KDol,aug-meltFe-Mg= 0.3 (Fig. 2). Olivine is the dominant crystallising phase until the MgO content falls to ~7.4 wt. % in the melt, as noted in Helz et al. (1994)
Helz, R.T., Kirschenbaum, H., Marinenko, J.W., Qian, R. (1994) Whole-rock analyses of core samples from the 1967, 1979 and 1981 drillings of Kilauea Iki lava lake, Hawaii. U.S. Geological Survey Report 94-684.
, at which point the liquid reaches the cpx + plg + ol cotectic, as shown by a change in slope of REE abundances against MgO (O’Neill, 2016O’Neill, H.S.C. (2016) The smoothness and shapes of chondrite-normalized rare Earth element patterns in basalts. Journal of Petrology 57, 1463–1508.
; see Supplementary Information). Ilmenite then crystallises at the expense of olivine below ≈5 wt. % MgO (Fig. 2).To evaluate the proportion of Sn2+ and Sn4+ in the melt, three distinct scenarios were considered: a) 100 % Sn2+, b) Sn2+/Sn4+ = 1 and c) 100 % Sn4+ (see Fig. 1a). The first two cases produce a) no increase in Sn concentration with MgO or b) a rise that is insufficient to describe the observed increase with MgO. Only scenario c), in which Sn is entirely stannic and thus strongly incompatible (0.01 < ∑Dmin/melt < 0.21) reproduces the Sn evolution of the melt. Therefore, the Sn2+ species is likely very minor and the majority of Sn occurs as Sn4+.
No experimental constraints exist for the equilibrium constant of Eq. 1 in basaltic liquids. However, a first order estimate of
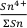 expected in magmatic systems comes from the experimentally derived relative reduction potential for the redox couple Sn4+ and Sn2+; -4.4 V in Na2Si2O5 melt (Schreiber, 1987
expected in magmatic systems comes from the experimentally derived relative reduction potential for the redox couple Sn4+ and Sn2+; -4.4 V in Na2Si2O5 melt (Schreiber, 1987Schreiber, H.D. (1987) An Electrochemical Series of Redox Couples in Silicate Melts: a review and applications to geochemistry. Journal of Geophysical Research 92, 9225–9232.
). Using this value enables calculation of Sn4+/∑Sn at the ƒO2 (FMQ) and temperature (≈1200 oC) of the parental liquid, yielding a value of ≈0.9 (10 % Sn2+). A caveat to this approach is that this result may not be applicable to basaltic melts. However, it also independently suggests that Sn4+ should predominate in terrestrial magmatic environments.Isotopic behaviour of Sn during magmatic processes. Since Sn is likely tetravalent and coordinated by oxygen in the KI lava lake, the ~0.2 ‰ isotopic fractionation must reflect the difference in coordination number of Sn between the melt and the mineral assemblage. Sample KI79-3-150.4, with ~14 wt. % MgO (δ122Sn = 0.46 ± 0.07 ‰), is close to the parental magma composition (Helz et al. (1994)
Helz, R.T., Kirschenbaum, H., Marinenko, J.W., Qian, R. (1994) Whole-rock analyses of core samples from the 1967, 1979 and 1981 drillings of Kilauea Iki lava lake, Hawaii. U.S. Geological Survey Report 94-684.
. As olivine crystallises and accumulates at the bottom of the sequence, δ122Sn is unchanged, because DSnol/melt≈ 0 (Fig. 1b). This behaviour contrasts with both Fe and Zn, whose isotopic fractionation is indeed controlled by olivine due to their relative compatibility in this phase, where DFe,Znol/melt= 1, driving both δ57Fe and δ66Zn to heavier compositions to lower MgO (Teng et al., 2008Teng, F.-Z., Dauphas, N., Helz, R.T. (2008) Iron Isotope Fractionation During Magmatic Differentiation in Kilauea Iki Lava Lake. Science 320, 1620–1622.
; Chen et al. 2013Chen, H., Savage, P., Teng, F.Z., Helz, R., Moynier, F. (2013) Zinc isotope fractionation during magmatic differentiation and the isotopic composition of the bulk silicate Earth. Earth and Planetary Science Letters 369, 34–42.
). Only in samples with <5 wt. % MgO does δ122Sn begin to decrease. Tin is weakly chalcophile, and its DSnsulf/melt= 5.3 ± 3.6 in KI samples (Greaney et al., 2017Greaney, A.T., Rudnick, R.L., Helz, R.T., Gaschnig, R.M., Piccoli, P.M., Ash, R.D. (2017) The behavior of chalcophile elements during magmatic differentiation as observed in Kilauea Iki lava lake, Hawaii. Geochimica et Cosmochimica Acta 210, 71–96.
). Sulphides are present in evolved basalts as isocubanite and bornite (having exsolved from high temperature intermediate solid solution), however their low modal abundance (<0.01 %; Stone and Fleet, 1991Stone, W.E., Fleet, M.E. (1991) Nickel-copper sulfides from the 1959 eruption of Kilauea Volcano, Hawaii; contrasting compositions and phase relations in eruption pumice and Kilauea Iki lava lake. American Mineralogist 76, 1363–1372.
) means they have a negligible effect on the Sn budget of the magma. Furthermore, by analogy with Fe isotopes (Wawryk and Foden, 2017Wawryk, C.M., Foden, J.D. (2017). Iron-isotope systematics from the Batu Hijau Cu-Au deposit, Sumbawa, Indonesia. Chemical Geology 466, 159–172.
), Cu-bearing sulphides should harbour light Sn isotopes (contrary to observations), as also suggested by the long Sn-S bond length of 2.56 Å in SnS2 (Hazen and Finger, 1979Hazen, R.M., Finger, L.W. (1979) Bulk modulus —volume relationship for cation-anion polyhedra. Journal of Geophysical Research: Solid Earth 84, 6723–6728.
). Rather, comparison with TiO2 shows that the decrease in δ122Sn coincides with crystallisation of Fe-Ti oxides, predominantly ilmenite, in which Sn4+ is highly compatible (Klemme et al., 2006Klemme, S., Günther, D., Hametner, K., Prowatke, S., Zack, T. (2006) The partitioning of trace elements between ilmenite, ulvospinel, armalcolite and silicate melts with implications for the early differentiation of the moon. Chemical Geology 234, 251–263.
; Table 2), further attesting to the predominance of Sn4+ in the melt. The TiO2 content reaches a maximum of 4.12 wt. % at 5.77 wt. % MgO before dropping to 2.59 wt. % at 2.37 wt. % MgO (Fig. 2). Although the measured DSnilm/melt= 0.63 (Greaney et al., 2017Greaney, A.T., Rudnick, R.L., Helz, R.T., Gaschnig, R.M., Piccoli, P.M., Ash, R.D. (2017) The behavior of chalcophile elements during magmatic differentiation as observed in Kilauea Iki lava lake, Hawaii. Geochimica et Cosmochimica Acta 210, 71–96.
) is less than that determined experimentally (5.1), ilmenite nevertheless hosts 50 % of the Sn in solid phases. Coupled with its low modal abundance (≈5 %), ∑DSnmin/melt remains below unity (0.2), and Sn continues to increase.In order to replicate the change in Sn content and isotopic fractionation in the KI samples, a fractionation factor (Δmineral-melt122Sn) of 0.7 ± 0.2 ‰ is required (Supplementary Information), equivalent to 0.175 ± 0.050 ‰/amu. Sossi and O’Neill (2017)
Sossi, P.A., O’Neill, H.S.C. (2017) The effect of bonding environment on iron isotope fractionation between minerals at high temperature. Geochimica et Cosmochimica Acta 196, 121–143.
explored the effect of changing coordination environment on iron isotope fractionation, and found that the difference between IV-fold Fe2+ in chromite and VI-fold Fe2+ in ilmenite is only 0.05 ‰/amu at 1073 K, significantly smaller than that calculated for Sn. Nonetheless, the degree of enrichment of the light Sn isotopes in the more evolved samples is consistent with the observation of Creech et al. (2017)Creech, J.B., Moynier, F., Badullovich, N. (2017) Tin stable isotope analysis of geological materials by double-spike MC-ICPMS. Chemical Geology 457, 61–67.
(Fig. 1b). This vector of fractionation to lighter Sn isotopes in the melt precludes the presence of Sn2+, which, as it is concentrated in all crystallising phases relative to Sn4+, and as a more reduced species, should result in heavy isotope enrichment in the melt. Rather, this argues for the lower coordination of Sn4+ in minerals relative to the melt. While the lattice strain model predicts that Sn4+ coordination (N) in minerals is 6-fold (Table 2), its coordination in the melt is poorly known. In melts with higher NBO/T (number of non-bridging oxygens per tetrahedral cation) than the hydrous granitic melts studied by Farges et al. (2006)Farges, F., Linnen, R.L., Brown, G.E. (2006) Redox and speciation of tin in hydrous silicate glasses: A comparison with Nb, Ta, Mo and W. Canadian Mineralogist 44, 795–810.
, Sn4+ may exist in 6 to 8-fold coordination by analogy with Sn2+, which is in 8-fold coordination. While further XANES studies will help elucidate this issue, empirically, the shift to lighter Sn isotope compositions requires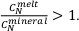 .
.The komatiites plot within uncertainty of the δ122Sn plateau defined by KI lava lake samples with MgO > 5 wt. % (Fig. 1b). The komatiites analysed display spinifex textures, indicative of their formation from quenched liquids, representative of the parental magma. This consideration is not crucial for Sn, however, as olivine is the only mineral on the komatiite liquidus and its removal does not cause resolvable Sn isotope fractionation (Fig. 1b), that is, δ122Sn should remain constant irrespective of its position on an olivine control line.
Tin contents of the four komatiites positively correlate with La/SmN ratios (Fig. 3). The high degree of melting (25–40 %) undergone during komatiite formation results in quantitative extraction of incompatible elements, including Sn. Therefore, Sn depletion reflects that of its mantle source, where younger (2.7 Ga) komatiites experienced greater (up to 5 %) source depletion than the primitive mantle-derived 3.48 Ga komatiites (see Sossi et al., 2016
Sossi, P.A., Eggins, S.M., Nesbitt, R.W., Nebel, O., Hergt, J.M., Campbell, I.H., O'Neill, H., VanKranendonk, M., Davies, D. (2016) Petrogenesis and geochemistry of Archean komatiites. Journal of Petrology 57, 147–184.
). Despite this variation in Sn contents (Fig. 3), the absence of isotopic fractionation between komatiite groups indicates that prior melt extraction (≤5 %) leaves the Sn isotopic composition of their mantle sources unchanged. Hawaiian basalts (0.44 ± 0.03, 2 s.d.) are isotopically indistinguishable (0.53 ± 0.08, 2 s.d.), and these asthenosphere-derived igneous rocks yield an average d122Sn = 0.49 ± 0.11 (2 s.d., n = 7). Owing to the constancy of Sn isotope composition in high MgO igneous rocks, and across several spatial and temporal domains, this value is taken to estimate the Sn isotope composition of the bulk silicate Earth.
Figure 3 Depletion of komatiite source regions, quantified by La/SmN, as a function of Sn concentration (green circles) and δ122Sn (blue squares). Tin contents are positively correlated with La/SmN, whereas δ122Sn remains constant within uncertainty.
top
Acknowledgements
FM acknowledges funding from the European Research Council under the H2020 framework program/ERC grant agreement #637503 (Pristine) and support from the ANR through the Cradle project and the UnivEarthS Labex program at Sorbonne Paris Cité (ANR-10-LABX-0023 and ANR-11-IDEX-0005-02). Parts of this work were supported by IPGP multidisciplinary program PARI, and by Region Île-de-France SESAME Grant no. 12015908. We thank the two reviewers for constructive comments that have greatly improved the quality of the manuscript and Helen Williams for her careful and efficient edition of the paper.
Editor: Helen Williams
top
References
Adam, J., Green, T. (2006) Trace element partitioning between mica-and amphibole-bearing garnet lherzolite and hydrous basanitic melt:1. Experimental results and the investigation of controls on partitioning behaviour. Contributions to Mineralogy and Petrology 152, 1–17.
 Show in context
Show in context Given the limited experimental data currently available (Adam and Green, 2006; Klemme et al., 2006; Michely et al., 2017), we address this question using lattice strain theory and then model the Sn abundance and speciation during the differentiation of the KI lava lake.
View in article
The calculated values were then compared to experimentally derived partition coefficients (Michely et al., 2017; Adam and Green, 2006; Klemme et al. 2006; Table 2).
View in article
The experiments of Michely et al. (2017), performed in air, contain exclusively Sn4+, whereas those of Adam and Green (2006) may contain both Sn2+ and Sn4+ because Sn falls between calculated lattice strain curves for a given redox state and site property.
View in article
Table 2
View in article
Blundy, J., Wood, B. (1994) Prediction of crystal-melt partition coefficients from elastic moduli. Nature 372, 452–454.
 Show in context
Show in context Partition coefficients for Sn2+ and Sn4+ between crystals and melt were calculated for olivine, clinopyroxene, orthopyroxene, plagioclase and ilmenite using the lattice strain model (Blundy and Wood, 1994; Supplementary Information).
View in article
Chen, H., Savage, P., Teng, F.Z., Helz, R., Moynier, F. (2013) Zinc isotope fractionation during magmatic differentiation and the isotopic composition of the bulk silicate Earth. Earth and Planetary Science Letters 369, 34–42.
 Show in context
Show in context In order to determine the behaviour of Sn isotopes in igneous systems and establish the composition of the BSE, it is necessary to investigate natural terrestrial samples from a common source. Kilauea Iki (KI) lava lake is well-suited to test the effects of fractional crystallisation (e.g., Tomascak et al., 1999; Teng et al., 2008; Chen et al. 2013; Kato et al., 2017).
View in article
In the KI samples, δ122Sn ranges from 0.24 ± 0.07 to 0.46 ± 0.07 ‰, which represents a variation (~0.05 ‰/amu) similar to Zn (~0.05 ‰/amu; Chen et al., 2013).
View in article
This behaviour contrasts with both Fe and Zn, whose isotopic fractionation is indeed controlled by olivine due to their relative compatibility in this phase, where DFe,Znol/melt= 1, driving both δ57Fe and δ66Zn to heavier compositions to lower MgO (Teng et al., 2008; Chen et al., 2013).
View in article
Creech, J.B., Moynier, F., Badullovich, N. (2017) Tin stable isotope analysis of geological materials by double-spike MC-ICPMS. Chemical Geology 457, 61–67.
 Show in context
Show in context Indeed, Creech et al. (2017) analysed four genetically unrelated igneous rocks and identified that the 122Sn/118Sn ratio increases with increasing MgO content, suggesting that Sn isotopic fractionation occurs during igneous differentiation.
View in article
Here, we have analysed the isotopic composition of a series of samples from KI lava lake and komatiites utilising the 117Sn-122Sn double spike MC-ICPMS method Creech et al. (2017) (see Supplementary Information).
View in article
Figure 1 [...] Geostandard values are taken from Creech et al. (2017).
View in article
Nonetheless, the degree of enrichment of the light Sn isotopes in the more evolved samples is consistent with the observation of Creech et al. (2017) (Fig. 1b).
View in article
Farges, F., Linnen, R.L., Brown, G.E. (2006) Redox and speciation of tin in hydrous silicate glasses: A comparison with Nb, Ta, Mo and W. Canadian Mineralogist 44, 795–810.
 Show in context
Show in context Although the dependence of Sn2+/Sn4+ on melt composition, and the fO2 at which this transition occurs in basaltic melts are poorly known (Farges et al., 2006), the behaviour of Sn in igneous systems may be gleaned by comparison with elements of similar incompatibility.
View in article
In melts with higher NBO/T (number of non-bridging oxygens per tetrahedral cation) than the hydrous granitic melts studied by Farges et al. (2006), Sn4+ may exist in 6 to 8-fold coordination by analogy with Sn2+, which is in 8-fold coordination.
View in article
Greaney, A.T., Rudnick, R.L., Helz, R.T., Gaschnig, R.M., Piccoli, P.M., Ash, R.D. (2017) The behavior of chalcophile elements during magmatic differentiation as observed in Kilauea Iki lava lake, Hawaii. Geochimica et Cosmochimica Acta 210, 71–96.
 Show in context
Show in context Tin is weakly chalcophile, and its DSnsulf/melt = 5.3 ± 3.6 in KI samples (Greaney et al., 2017).
View in article
Although the measured DSnilm/melt= 0.63 (Greaney et al., 2017) is less than that determined experimentally (5.1), ilmenite nevertheless hosts 50 % of the Sn in solid phases.
View in article
Hazen, R.M., Finger, L.W. (1979) Bulk modulus —volume relationship for cation-anion polyhedra. Journal of Geophysical Research: Solid Earth 84, 6723–6728.
 Show in context
Show in context Furthermore, by analogy with Fe isotopes (Wawryk and Foden, 2017), Cu-bearing sulphides should harbour light Sn isotopes (contrary to observations), as also suggested by the long Sn-S bond length of 2.56 Å in SnS2 (Hazen and Finger, 1979).
View in article
Heinrich, C.A. (1990). The chemistry of hydrothermal tin (-tungsten) ore deposition. Economic Geology 85, 457–481.
 Show in context
Show in context As such, Sn may behave as a siderophile, chalcophile and lithophile element at high temperatures (e.g., Heinrich, 1990; Witt-Eickschen et al., 2009).
View in article
Helz, R.T. (2012) Trace-element analyses of core samples from the 1967-1988 drillings of Kilauea Iki Lava Lake, Hawaii. U.S. Geological Survey Report 2010-1093.
 Show in context
Show in context The Sn/E ratios (E = Sm, Hf or Zr; data from Helz, 2012) in samples across the differentiation sequence are similar to OIBs and MORBs reported in Jochum et al. (1993) and Jenner and O’Neill (2012) (see Supplementary Information).
View in article
Helz, R.T., Kirschenbaum, H., Marinenko, J.W., Qian, R. (1994) Whole-rock analyses of core samples from the 1967, 1979 and 1981 drillings of Kilauea Iki lava lake, Hawaii. U.S. Geological Survey Report 94-684.
 Show in context
Show in context The differentiation sequence samples form a sub-alkali series with MgO content ranging from 25.83–2.37 wt. % (Helz et al., 1994) (see Supplementary Information).
View in article
Table 1 a SiO2/MgO values for Kilauea Iki lava lake taken from Helz et al. (1994).
View in article
Olivine is the dominant crystallising phase until the MgO content falls to ~7.4 wt. % in the melt, as noted in Helz (1994), at which point the liquid reaches the cpx + plg + ol cotectic, as shown by a change in slope of REE abundances against MgO (O’Neill, 2016; see Supplementary Information).
View in article
Sample KI79-3-150.4, with ~14 wt. % MgO (δ122Sn = 0.46 ± 0.07 ‰), is close to the parental magma composition (Helz et al., 1994).
View in article
Herzberg, C. (1992) Depth and degree of melting of komatiites. Journal of Geophysical Research: Solid Earth 97, 4521–4540.
 Show in context
Show in context As komatiites are produced by 25–40 % partial melting (e.g., Herzberg, 1992), and Sn is incompatible during mantle melting (being predominantly hosted in clinopyroxene; Witt-Eickschen et al., 2009), melting quantitatively extracts Sn from their mantle sources.
View in article
Jenner, F., O’Neill, H. (2012) Analysis of 60 elements in 616 ocean floor basaltic glasses. Geochemistry, Geophysics, Geosystems 12, doi: 10.1029/2011GC004009.
 Show in context
Show in context Observations of oceanic basalts show that Sn/Sm, Sn/Zr and Sn/Hf ratios are nearly uniform across MORB and OIB (Jochum et al., 1993; Jenner and O’Neill, 2012).
View in article
The Sn/E ratios (E = Sm, Hf or Zr; data from Helz, 2012) in samples across the differentiation sequence are similar to OIBs and MORBs reported in Jochum et al. (1993) and Jenner and O’Neill (2012) (see Supplementary Information).
View in article
Jochum, K.P., Hofmann, A.W., Seufert, H.M. (1993) Tin in mantle-derived rocks: Constraints on Earth evolution. Geochimica et Cosmochimica Acta 57, 3585–3595.
 Show in context
Show in context Observations of oceanic basalts show that Sn/Sm, Sn/Zr and Sn/Hf ratios are nearly uniform across MORB and OIB (Jochum et al., 1993; Jenner and O’Neill, 2012).
View in article
The Sn/E ratios (E = Sm, Hf or Zr; data from Helz, 2012) in samples across the differentiation sequence are similar to OIBs and MORBs reported in Jochum et al. (1993) and Jenner and O’Neill (2012) (see Supplementary Information).
View in article
Kato, C., Moynier, F., Foriel, J., Teng, F.-Z., Puchtel, I.S. (2017) The gallium isotopic composition of the bulk silicate Earth. Chemical Geology 448, 164–172.
 Show in context
Show in context In order to determine the behaviour of Sn isotopes in igneous systems and establish the composition of the BSE, it is necessary to investigate natural terrestrial samples from a common source. Kilauea Iki (KI) lava lake is well-suited to test the effects of fractional crystallisation (e.g., Tomascak et al., 1999; Teng et al., 2008; Chen et al. 2013; Kato et al., 2017).
View in article
Klemme, S., Günther, D., Hametner, K., Prowatke, S., Zack, T. (2006) The partitioning of trace elements between ilmenite, ulvospinel, armalcolite and silicate melts with implications for the early differentiation of the moon. Chemical Geology 234, 251–263.
 Show in context
Show in context Given the limited experimental data currently available (Adam and Green, 2006; Klemme et al., 2006; Michely et al., 2017), we address this question using lattice strain theory and then model the Sn abundance and speciation during the differentiation of the KI lava lake.
View in article
The calculated values were then compared to experimentally derived partition coefficients (Michely et al., 2017; Adam and Green, 2006; Klemme et al. 2006; Table 2).
View in article
Table 2
View in article
Rather, comparison with TiO2 shows that the decrease in d122Sn coincides with crystallisation of Fe-Ti oxides, predominantly ilmenite, in which Sn4+ is highly compatible (Klemme et al., 2006; Table 2), further attesting to the predominance of Sn4+ in the melt.
View in article
Maier, W.D., Barnes, S., Campbell, I., Fiorentini, M., Peltonen, P., Barnes, S., Smithies, H. (2008) Progressive mixing of meteoritic veneer into the early Earth's deep mantle. Nature 460, 620–623.
 Show in context
Show in context Extrapolation to the composition of the BSE using a single magmatic suite is tenuous, given the diversity of mantle sources in the present day mantle and the possibility of secular changes in mantle composition (e.g., Maier et al., 2008).
View in article
Médard, E., McCammon, C.A., Barr, J.A., Grove, T.L. (2008) Oxygen fugacity, temperature reproducibility, and H2O contents of nominally anhydrous piston-cylinder experiments using graphite capsules. American Mineralogist 93, 1838–1844.
 Show in context
Show in context The graphite capsules used imposed ƒO2 conditions near FMQ-2 (Médard et al., 2008), at least 2 orders of magnitude lower than in the KI lava lake, thereby increasing the stability of divalent Sn, according to: 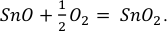 Eq. 1
Eq. 1
View in article
Michely, L.T., Leitzke, F.P., Speelmanns, I.M., Fonseca, R.O.C. (2017) Competing effects of crystal chemistry and silicate melt composition on trace element behavior in magmatic systems: insights from crystal/silicate melt partitioning of the REE, HFSE, Sn, In, Ga, Ba, Pt and Rh. Contributions to Mineralogy and Petrology 172, 39.
 Show in context
Show in context Given the limited experimental data currently available (Adam and Green, 2006; Klemme et al., 2006; Michely et al., 2017), we address this question using lattice strain theory and then model the Sn abundance and speciation during the differentiation of the KI lava lake.
View in article
The calculated values were then compared to experimentally derived partition coefficients (Michely et al., 2017; Adam and Green, 2006; Klemme et al. 2006; Table 2).
View in article
The experiments of Michely et al. (2017), performed in air, contain exclusively Sn4+, whereas those of Adam and Green (2006) may contain both Sn2+ and Sn4+ because Sn falls between calculated lattice strain curves for a given redox state and site property.
View in article
Table 2
View in article
O’Neill, H.S.C. (2016) The smoothness and shapes of chondrite-normalized rare Earth element patterns in basalts. Journal of Petrology 57, 1463–1508.
 Show in context
Show in context Olivine is the dominant crystallising phase until the MgO content falls to ~7.4 wt. % in the melt, as noted in Helz (1994), at which point the liquid reaches the cpx + plg + ol cotectic, as shown by a change in slope of REE abundances against MgO (O’Neill, 2016; see Supplementary Information).
View in article
O’Neill, H.S.C., Palme, H. (2008) Collisional erosion and the non-chondritic composition of the terrestrial planets. Philosophical transactions of the Royal Society A 366, 4205–4238.
 Show in context
Show in context Moderately volatile elements (MVEs) and their isotopes are important tracers for planetary and nebular processes such as evaporation and condensation (e.g., O’Neill and Palme, 2008; Paniello et al., 2012).
View in article
Paniello, R., Day, J.M.D., Moynier, F. (2012) Zinc isotopic evidence for the origin of the Moon. Nature 490, 376–379.
 Show in context
Show in context Moderately volatile elements (MVEs) and their isotopes are important tracers for planetary and nebular processes such as evaporation and condensation (e.g., O’Neill and Palme, 2008; Paniello et al., 2012).
View in article
Schreiber, H.D. (1987) An Electrochemical Series of Redox Couples in Silicate Melts: a review and applications to geochemistry. Journal of Geophysical Research 92, 9225–9232.
 Show in context
Show in context However, a first order estimate of 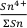 expected in magmatic systems comes from the experimentally derived relative reduction potential for the redox couple Sn4+ and Sn2+; -4.4 V in Na2Si2O5 melt (Schreiber, 1987).
expected in magmatic systems comes from the experimentally derived relative reduction potential for the redox couple Sn4+ and Sn2+; -4.4 V in Na2Si2O5 melt (Schreiber, 1987).
View in article
Sossi, P.A., O’Neill, H.S.C. (2017) The effect of bonding environment on iron isotope fractionation between minerals at high temperature. Geochimica et Cosmochimica Acta 196, 121–143.
 Show in context
Show in context Sossi and O’Neill (2017) explored the effect of changing coordination environment on iron isotope fractionation, and found that the difference between IV-fold Fe2+ in chromite and VI-fold Fe2+ in ilmenite is only 0.05 ‰/amu at 1073 K, significantly smaller than that calculated for Sn.
View in article
Sossi, P.A., Eggins, S.M., Nesbitt, R.W., Nebel, O., Hergt, J.M., Campbell, I.H., O'Neill, H., VanKranendonk, M., Davies, D. (2016) Petrogenesis and geochemistry of Archean komatiites. Journal of Petrology 57, 147–184.
 Show in context
Show in context Komatiite sources, especially for older 3.5 Ga examples, have primitive mantle-like compositions (Sossi et al., 2016), and thus permit assessment of the temporal and chemical heterogeneity in Earth’s mantle.
View in article
The four spinifex-textured komatiites have 23.54 < MgO (wt. %) < 28.71, and thus chemical compositions close to their respective parental magma (Sossi et al., 2016).
View in article
Table 1 [...] c MgO value for komatiites taken from Sossi et al. (2016).
View in article
Therefore, Sn depletion reflects that of its mantle source, where younger (2.7 Ga) komatiites experienced greater (up to 5 %) source depletion than the primitive mantle-derived 3.48 Ga komatiites (see Sossi et al., 2016).
View in article
Stone, W.E., Fleet, M.E. (1991) Nickel-copper sulfides from the 1959 eruption of Kilauea Volcano, Hawaii; contrasting compositions and phase relations in eruption pumice and Kilauea Iki lava lake. American Mineralogist 76, 1363–1372.
 Show in context
Show in context Sulphides are present in evolved basalts as isocubanite and bornite (having exsolved from high temperature intermediate solid solution), however their low modal abundance (<0.01 %; Stone and Fleet, 1991) means they have a negligible effect on the Sn budget of the magma.
View in article
Teng, F.-Z., Dauphas, N., Helz, R.T. (2008) Iron Isotope Fractionation During Magmatic Differentiation in Kilauea Iki Lava Lake. Science 320, 1620–1622.
 Show in context
Show in context In order to determine the behaviour of Sn isotopes in igneous systems and establish the composition of the BSE, it is necessary to investigate natural terrestrial samples from a common source. Kilauea Iki (KI) lava lake is well-suited to test the effects of fractional crystallisation (e.g., Tomascak et al., 1999; Teng et al., 2008; Chen et al. 2013; Kato et al., 2017).
View in article
This behaviour contrasts with both Fe and Zn, whose isotopic fractionation is indeed controlled by olivine due to their relative compatibility in this phase, where DFe,Znol/melt= 1, driving both δ57Fe and δ66Zn to heavier compositions to lower MgO (Teng et al., 2008; Chen et al., 2013).
View in article
Tomascak, P.B., Tera, F., Helz, R.T., Walker, R.J. (1999) The absence of lithium isotope fractionation during basalt differentiation: new measurements by multicollector sector ICP-MS. Geochimica et Cosmochimica Acta 63, 907–910.
 Show in context
Show in context In order to determine the behaviour of Sn isotopes in igneous systems and establish the composition of the BSE, it is necessary to investigate natural terrestrial samples from a common source. Kilauea Iki (KI) lava lake is well-suited to test the effects of fractional crystallisation (e.g., Tomascak et al., 1999; Teng et al., 2008; Chen et al. 2013; Kato et al., 2017).
View in article
Wawryk, C.M., Foden, J.D. (2017). Iron-isotope systematics from the Batu Hijau Cu-Au deposit, Sumbawa, Indonesia. Chemical Geology 466, 159–172.
 Show in context
Show in context Furthermore, by analogy with Fe isotopes (Wawryk and Foden, 2017), Cu-bearing sulphides should harbour light Sn isotopes (contrary to observations), as also suggested by the long Sn-S bond length of 2.56 Å in SnS2 (Hazen and Finger, 1979).
View in article
Witt-Eickschen, G., Palme, H., O’Neill, H., Allen, C. (2009) The geochemistry of the volatile trace elements As, Cd, Ga, In and Sn in the Earth’s mantle: new evidence from in situ analyses of mantle xenoliths. Geochimica et Cosmochimica Acta 73, 1755–1778.
 Show in context
Show in context As such, Sn may behave as a siderophile, chalcophile and lithophile element at high temperatures (e.g., Heinrich, 1990; Witt-Eickschen et al., 2009).
View in article
As komatiites are produced by 25–40 % partial melting (e.g., Herzberg, 1992), and Sn is incompatible during mantle melting (being predominantly hosted in clinopyroxene; Witt-Eickschen et al., 2009), melting quantitatively extracts Sn from their mantle sources.
View in article
top
Supplementary Information
Samples and Methods
Kilauea Iki lava lake
Located in the southeast of the Island of Hawai’i is the crystallised pit crater of Kilauea Iki. An eruption in 1959 saw lava pond into a pre-existing crater and fill to a depth of ~135 m (Richter et al., 1970). As a result, lava cooled and crystallised resulting in a mostly closed system of fractional crystallisation. The interior was initially sampled in 1960–62 and the resulting cores are described in Richter and Moore (1966). Subsequent studies (Helz and Thornber, 1987; Helz et al., 1994) have further documented the rocks in terms of geothermometry and trace element analysis. The parent magma is a picritic tholeiite with an average MgO content of 15.43 wt. % (Wright, 1973) and large-scale differentiation ceased around 1980 making the cooling period less than 30 years (Helz and Thornber, 1987). The surrounding country rock has a similar composition to the lava lake meaning country rock assimilation has a negligible effect on chemical composition. A study by Tomascak et al. (1999) identified that the time for the differentiation sequence to form, and subsequently be cored and collected, was significantly short on geological timescales. This means there has been insufficient time for fluid-rock interaction to occur. The samples utilised in this study from Kilauea Iki consist of eight rocks from the differentiation sequence with a marked systematic decrease in MgO content. The differentiation sequence samples form a sub-alkali series with MgO content ranging from 25.83 wt. % to 2.37 wt. % (Helz et al., 1994). Rare Earth Elements are particularly diagnostic tracers of igneous processes because, as a group, their behaviour varies as a smooth function of ionic radius where their charge (3+) is constant across the lanthanide period. The properties of smooth, REE patterns may be quantified by fitting a polynomial to them. In order to identify which aspect of the pattern affects the polynomial fit, the polynomial can be written in an orthogonal form. That is, its coefficients are independent of each another, taking the form: ln([REE])/([REE]N) = λ0 + λ1x1 + λ2x2 + λ3x3…λnxn, where [REE] refers to the concentration of the REE pattern, normalised by CI chondrites, N. The x terms are defined such that the coefficients, λ, are independent of each other and the number of terms, n, in the equation. The λ coefficients describe the shape of the pattern, with each of them defining a specific property. The simplest, λ0, is the average abundance of REE, λ1 the average linear slope, and λ2 the quadratic curvature (see O’Neill, 2016). A fundamental change in the fractionating mineral assemblage is observed at 7.4 wt. % MgO, where the mean Rare Earth Element abundance increase (quantified by λ0) with MgO changes slope. This corresponds to the transition from olivine-dominated to crystallisation cpx + plg + ol cotectic, occurring at 1170 °C (Helz, 1987).
Analytical method
All samples were already in powdered form and around 0.250 g were measured out, depending on the Sn concentration in order to have ~0.5 µg total of Sn for each sample. The powders were transferred to Teflon beakers where concentrated HNO3-HF (3:1) was added to facilitate the digestion process. All samples spent two days on the hotplate at 140 °C with 15 minutes in an ultrasonic bath after each day to break down any conglomerated sample. After total digestion was achieved the samples were dried down on the hotplate and then taken up in aqua regia (3:1 of concentrated HCl-HNO3) to ensure all samples were in solution and to break down fluoride complexes. This stage was accompanied by the addition of the 117Sn-122 Sn double spike to all samples. The double spike amounts varied depending on sample weight and Sn concentration (which was estimated to be 2 µg/g in all Kilauea Iki samples in order to spike). The samples were returned to the hotplate for another day to allow requilibration of the double spike with the sample. The samples were then dried down once again and taken up in concentrated HCl for conversion and then dried down again and taken up in 0.5 N HCl for column chemistry.
To achieve Sn purifcation, the method outlined in Creech et al. (2017) was applied to all samples. Columns were loaded with 2 mL of TRU spec anion exchange resin and after cleaning were equilibrated with 10 mL of 0.5 N HCl. After matrix elements were eluted using 0.5 N and 0.25 N HCl, the Sn remaining was liberated from the column and collected using 0.5 N HNO3. Measurement of the procedural blanks yielded Sn concentrations of <1 ng which is insignificant considering the amount of Sn in the samples (~0.5 µg). The final Sn cuts were dried down and taken up in 0.5 N HCl and diluted to achieve Sn concentration of 100 ppb for analysis on the Thermo Scientific Neptune Plus high resolution MC-ICP-MS at the Institut de Physique du Globe de Paris (procedures following Creech et al., 2017). To visualise the Sn isotopic data from this study, delta notation is used relative to an in house standard produced at the Institut de Physique du Globe de Paris, Sn_IPGP (Creech et al., 2017):
Eq. S-1
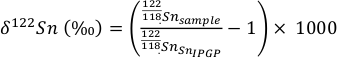
Measurements of samples were bracketed with measurements of the Sn_IPGP standard during the course of the different analytical sessions. All the samples were run during the same session as the results reported in Creech et al. (2017) with the exception of the komatiite samples that were run in a different session. Uncertainty was taken from the external reproducibility of the USGS standard BCR-2 (±0.065 ‰; see Creech et al. 2017), or as the 2 s.d. of the two measurements performed on each sample, whichever was larger.
Lattice strain theory
The lattice strain theory outlined in Blundy and Wood (1994) reports that the partitioning behaviour of any series of isovalent cations can be understood with a model where the main controls are the size and elasticity of the crystal lattice site. The calculated partition coefficient of an element (Di) can be related to the partition coefficient of an ideal element (D0) (for a particular site), the ideal radius (r0) and Young’s modulus (E) (Eq. S-2):
Eq. S-2
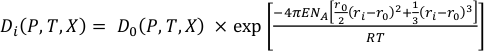
The calculated partition coefficient will be a function of pressure, temperature and composition and the Young’s modulus can be calculated for a different valence cations using the Hazen and Finger (1979) relationship. The values of D0, E and r0 used for the Sn partitioning calculations are outlined in Table S-1. The Sn2+ species can be hosted in the M1 sites of olivine, orthopyroxene and clinopyroxene in VI-fold coordination (based on charge and ionic radius constraints). Similarly, it can also be present in the M2 site of clinopyroxene and plagioclase in VIII-fold coordination. Its substitution is a simple one for other M2+ cations, and is thus energetically favourable.
The Sn4+ species in olivine, orthopyroxene and clinopyroxene partitions into the M1 site in VI-fold coordination and the T site in IV-fold coordination. Finally, ilmenite can only host Sn4+ in its M site in VI-fold coordination. In all cases, Sn4+ (r = 0.69 Å) behaves in a similar manner to Ti4+ (r = 0.605 Å), as shown for example, by the remarkable correlation between DTi and DSn in natural olivines (Witt-Eickschen et al., 2009). In olivine, Ti is dominantly incorporated as the Ti-clinohumite end member, MgTiO2(OH)2, as point defects (Berry et al., 2007), suggesting that Sn4+ contents in olivine may also be controlled by this mechanism. As Sn4+ is highly incompatible in olivine the mechanism is difficult to assess. In quadrilateral pyroxenes, however, Ti4+ is strongly ordered into the M1 site (e.g., Adam and Green, 1994). Tetravalent titanium may be incorporated via two prevailing reactions (Morimoto, 1988), or a combination thereof:
Eq. S-3
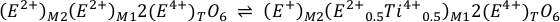
and
Eq. S-4
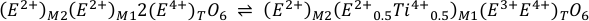
which are in both cases, the coupled substitutions 3E2+(2E+Ti4+)-1, abbreviated NAT, and 2E4+E2+(2E3+Ti4+)-1, abbreviated TAL, respectively. In natural pyroxenes, E+ = Na+, E2+ = Fe2+ or Mg2+, and E3+ = Al3+, where an assessment of terrestrial pyroxenes (Cameron and Papike, 1981) reveals that the TAL substitution is the most important mechanism for Ti substitution in all tectonic settings, particularly in Hawaiian basalts. It is therefore likely that Sn4+ is partitioned into pyroxenes via an analogous reaction to Eq. S-4. Indeed, the data of Michely et al. (2017) show that DSncpx/melt increases systematically with the IVAl3+ in clinopyroxene, indicative of the TAL substitution. In their experiments, D0 for 4+ cations on the M1 site increases with melt Na2O content (0.5 < D0 < 2.3) and E is higher (≈3,100 GPa) than derived from the Hazen-Finger relationship (575 GPa). Nonetheless, the measured DSncpx/melt in natural komatiitic melt compositions (0.19 to 0.67) from Michely et al. (2017) overlaps with our calculated values (0.05 to 0.39) and is thus deemed fit for purpose considering differences in melt composition. In ilmenite, Sn4+ partitions by the simple direct substitution for octahedrally coordinated Ti4+, yielding high DSnilm/melt ≈ 5 (Klemme et al., 2006). Table S-1 summarises the values used for the ideal parameters and bulk mineral properties when calculating Sn partition coefficients utilising the lattice strain theory outlined in Blundy and Wood (1994).
Table S-1 Terms used for Sn partition coefficient calculations.
| Phase | Olivine | Orthopyroxene | Clinopyroxene | Plagioclase | Ilmenite | ||
| Coordination | M1 (VI-fold) | M1 (VI-fold) | M1 (VI-fold) | M2 (VIII-fold) | M2 (VIII-fold) | M (VI-fold | |
| 2+ | ri2+ | 0.96 Å | 0.96 Å | 0.96 Å | 1.15 Å | 1.15 Å | - |
| ro2+ | 0.72 Å (Mg2+) | 0.72 Å (Mg2+) | 0.72 Å (Mg2+) | 1.06 Å (Ca2+) | 1.06 Å (Ca2+) | - | |
| Do2+ | 4.2 - 7.0 | 3.1 | 1.5 - 2.5 | 1.0 - 1.35 | 1.0 - 1.35 | - | |
| Ē2+ | 243 (GPa) | 243 (GPa) | 243 (GPa) | 116 (GPa) | 116 (GPa) | - | |
| 4+ | ri4+ | 0.69 Å | 0.69 Å | 0.69 Å | - | - | 0.69 Å |
| ro4+ | 0.605 Å (Ti4+) | 0.605 Å (Ti4+) | 0.605 Å (Ti4+) | - | - | 0.605 Å (Ti4+) | |
| Do4+ | 0.01 - 0.1 | 0.1 | 0.1 - 0.87 | - | - | 10 - 15 | |
| Ē4+ | 575 (GPa) | 575 (GPa) | 575 (GPa) | - | - | 575 (GPa) | |
Temperature (T) was taken as 1573 K, in the range of a typical basaltic melt.
Download in ExcelDeriving the VIII-fold radius of Sn2+
There is no data available for this parameter and so we estimated the Sn2+ radius for VIII-fold coordination using the radius of Cd (1.11 Å). Tin is larger than Cd and so the estimate for its ionic radius is slightly higher at 1.15 Å. The difference between the VI-fold and VIII-fold coordination between other 2+ cations is between about 0.15 and 0.2 Å. We adopted this reasoning and assumed the difference between the VI-fold and VIII-fold coordinated Sn2+ to be 0.19 Å.
Major element modelling using fractional crystallisation equation
The Mg and Fe contents in the Kilauea Iki lava lake were modelled using the fractional crystallisation equation outlined in Shaw (1970). The purpose of the model was to replicate the trends of MgO and FeO described in Helz (1987) so that the phase proportions (olivine, clinopyroxene, plagioclase and Fe-Ti oxide) could be deduced and ultimately also the Sn concentration and Sn isotopic values (see below). Assuming perfect Rayleigh distillation and considering a perfectly incompatible element (e.g., Th) then the fractional crystallisation equation simplifies to:
Eq. S-5
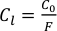
Sn isotopic modelling of Kilauea Iki lava lake
The Sn isotopic fractionation in the lava lake is the result of fractional crystallisation and so the modelling is governed by the same Rayleigh equation:
Eq. S-6
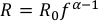
Eq. S-7
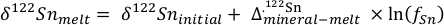
The two MgO-poorest samples record the lowest δ122Sn values and this is the point of crystallising of Fe-Ti oxides (including ilmenite), at melt fraction of ~0.3. Table S-1 shows that Sn4+ is highly compatible in ilmenite (DSn ≈ 5, substituting for Ti4+) which agrees with experimental studies from Klemme et al. (2006). The Ti-O (octahedrally coordinated) bond length in ilmenite is 1.98 Å (Wechsler and Prewitt, 1984) whereas the Sn-O (octahedrally coordinated) bond length in a hydrous granitic melt was found by Farges et al. (2006) to be 2.03 Å. An Sn-O bond length of 1.98 Å in ilmenite would qualitatively describe its isotopic fractionation, as the δ122Sn values continue decreasing for the two MgO-poorest samples, caused by partitioning of heavy Sn isotopes into ilmenite, resulting in an isotopically lighter melt (as observed in our data).
Table S-2 Average trace element ratio values for Kilauea Iki lava lake.
| Trace element ratio* | Value | Jochum et al. (1993) value | Jenner and O’Neill (2012) | |
| Kilauea Iki | Sn/Hf | 0.42 ± 0.08 | 0.41 | 0.45 |
| Sn/Zr | 0.010 ± 0.0018 | 0.011 | 0.012 | |
| Sn/Ta | 1.3 ± 0.25 | - | - | |
| Sn/Sm | 0.26 ± 0.06 | 0.32 | 0.32 |
* Hf, Zr, Ta and Sm values taken from Helz and Taggart (2012).
Download in ExcelSupplementary Figures

Figure S-1 Calculated λ0, quantifying the mean abundances of the Rare Earth Elements (see O’Neill, 2016) against MgO. Red points correspond to the Kilauea Iki lava lake, and show a rapid drop in MgO and a small increase in λ0 for 7.4 < MgO (wt. %) < 27 during olivine crystallisation/accumulation, before a rapid increase in slope at 7.4 wt. % MgO signalling a change in the crystallising assemblage to cpx + plg + ol.

Figure S-2 Modelled abundances of FeO and MgO (wt. %) in the Kilauea Iki lava lake, overlain with the field (green) of literature data (Helz, 1987) for different eruption dates (1967, 1979 and 1981).

Figure S-3 Plot of melt fraction against MgO (wt. %). The maroon line represents the calculated F assuming that Th behaves as a perfectly incompatible element. The blue line represents the modelled F from the major element modelling of Kilauea Iki lava lake.

Figure S-4 Plot of δ122Sn against Ta, Hf and Zr (HFSEs).
Supplementary Information References
Berry, A.J., O'Neill, H.S.C., Hermann, J., Scott, D.R. (2007) The infrared signature of water associated with trivalent cations in olivine. Earth and Planetary Science Letters 261, 134–142.
Blundy, J., Wood, B. (1994) Prediction of crystal-melt partition coefficients from elastic moduli. Nature 372, 452–454.
Cameron, M., Papike, J.J. (1981) Structural and chemical variations in pyroxenes. American Mineralogist 66, 1–50.
Creech, J.B., Moynier, F., Badullovich, N. (2017) Tin stable isotope analysis of geological materials by double-spike MC-ICPMS. Chemical Geology 457, 61–67.
Farges, F., Linnen, R.L., Brown, G.E. (2006) Redox and speciation of tin in hydrous silicate glasses: A comparison with Nb, Ta, Mo and W. Canadian Mineralogist 44, 795–810.
Hazen, R.M., Finger, L.W. (1979) Bulk modulus —volume relationship for cation-anion polyhedra. Journal of Geophysical Research: Solid Earth 84, 6723–6728.
Helz, R.T. (1987) Differentiation behavior of Kilauea Iki lava lake, Kilauea volcano, Hawaii: an overview of past and current work. Physicochemical principles 1, 241–258.
Helz, R.T., Taggart Jr., J.E. (2012) Trace-Element Analyses of Core Samples from the 1967–1988 Drillings of Kilauea Iki Lava Lake, Hawaii. U.S. Geological Survey Open-File Report 2010-1093.
Helz, R.T., Thornber, C.R. (1987) Geothermometry of Kilauea Iki lava lake, Hawaii. Bulletin of Volcanology 49, 651–668.
Helz, R.T., Kirschenbaum, H., Marinenko, J.W., Qian, R. (1994) Whole-rock analyses of core samples from the 1967, 1979 and 1981 drillings of Kilauea Iki lava lake, Hawaii. U.S. Geological Survey Open-File Report 94-684.
Jenner, F., O’Neill, H. (2012) Analysis of 60 elements in 616 ocean floor basaltic glasses. Geochemistry, Geophysics, Geosystems 12, doi: 10.1029/2011GC004009.
Jochum, K.P., Hofmann, A.W., Seufert, H.M. (1993) Tin in mantle-derived rocks: Constraints on Earth evolution. Geochimica et Cosmochimica Acta 57, 3585–3595.
Klemme, S., Günther, D., Hametner, K., Prowatke, S., Zack, T. (2006) The partitioning of trace elements between ilmenite, ulvospinel, armalcolite and silicate melts with implications for the early differentiation of the moon. Chemical Geology 234, 251–263.
Michely, L.T., Leitzke, F.P., Speelmanns, I.M., Fonseca, R.O.C. (2017) Competing effects of crystal chemistry and silicate melt composition on trace element behavior in magmatic systems: insights from crystal/silicate melt partitioning of the REE, HFSE, Sn, In, Ga, Ba, Pt and Rh. Contributions to Mineralogy and Petrology 172, 39.
Morimoto, N. (1988) Nomenclature of pyroxenes. Mineralogy and Petrology 39, 55–76.
O’Neill, H.S.C. (2016) The smoothness and shapes of chondrite-normalized rare Earth element patterns in basalts. Journal of Petrology 57, 1463–1508.
Richter, D.H., Moore, J.G. (1966) Petrology of the Kilauea Iki Lava Lake, Hawaii. U.S. Geological Survey Professional Paper 537-B.
Richter, D.H., Eaton, J.P., Murata, K.J., Ault, W.U., Krivoy, H.L. (1970) Chronological narrative of the 1959-60 eruption of Kilauea Volcano, Hawaii. U.S. Geological Survey Professional Paper 537-E.
Shaw, D.M. (1970) Trace element fractionation during anatexis. Geochimica et Cosmochimica Acta 34, 237–243.
Tomascak, P.B., Tera, F., Helz, R.T., Walker, R.J. (1999) The absence of lithium isotope fractionation during basalt differentiation: new measurements by multicollector sector ICP-MS. Geochimica et Cosmochimica Acta 63, 907–910.
Wechsler, B.A., Prewitt, C.T. (1984) Crystal structure of ilmenite (FeTiO3) at high temperature and at high pressure. American Mineralogist 69, 176–185.
Witt-Eickschen, G., Palme, H., O’Neill, H.S.C., Allen, C.M. (2009) The geochemistry of the volatile trace elements As, Cd, Ga, In and Sn in the Earth’s mantle: new evidence from in situ analyses of mantle xenoliths. Geochimica et Cosmochimica Acta 73, 1755–1778.
Wright, T.L. (1973) Magma Mixing as Illustrated by the 1959 Eruption , Kilauea Volcano, Hawaii Magma Mixing as Illustrated by the 1959 Eruption , Kilauea Volcano, Hawaii. Geological Society of America Bulletin 84, 849–858.
Figures and Tables
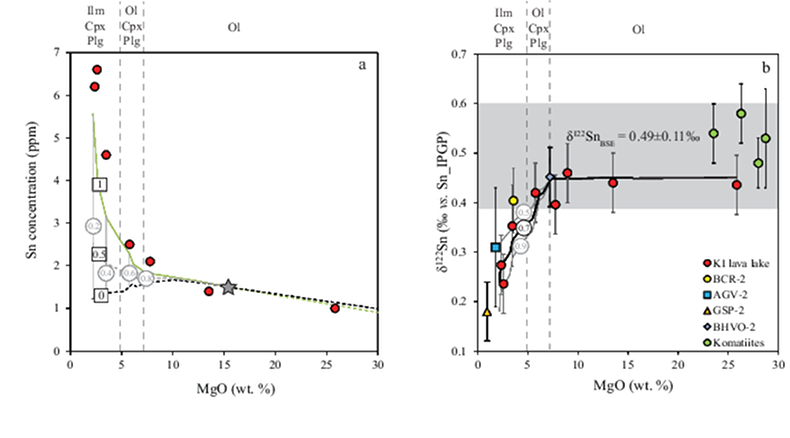
Figure 1 (a) Model showing Sn concentration for KI samples in the melt against MgO content. Red circles = KI samples. Thick lines represent modelled evolution with differing Sn4+/∑Sn, listed in black boxes from 1 (green line), 0.5 (dashed grey line) and 0 (dashed black line). Thin grey line represents the fraction of melt remaining (F), listed in grey circles, with the grey star representing the parental melt composition. (b) δ122Sn and Sn against MgO (wt. %) for the KI lava lake samples. The solid lines represent the modelled δ122Sn for ∆122Snmineral-melt = 0.5 (grey line), 0.7 (black line) and 0.9 (grey line). Geostandard values are taken from Creech et al. (2017)
Creech, J.B., Moynier, F., Badullovich, N. (2017) Tin stable isotope analysis of geological materials by double-spike MC-ICPMS. Chemical Geology 457, 61–67.
. In both cases, the crystallising assemblage is listed at the top of the figure (Ol = olivine, Cpx = clinopyroxene, Plg = plagioclase, Ilm = ilmenite).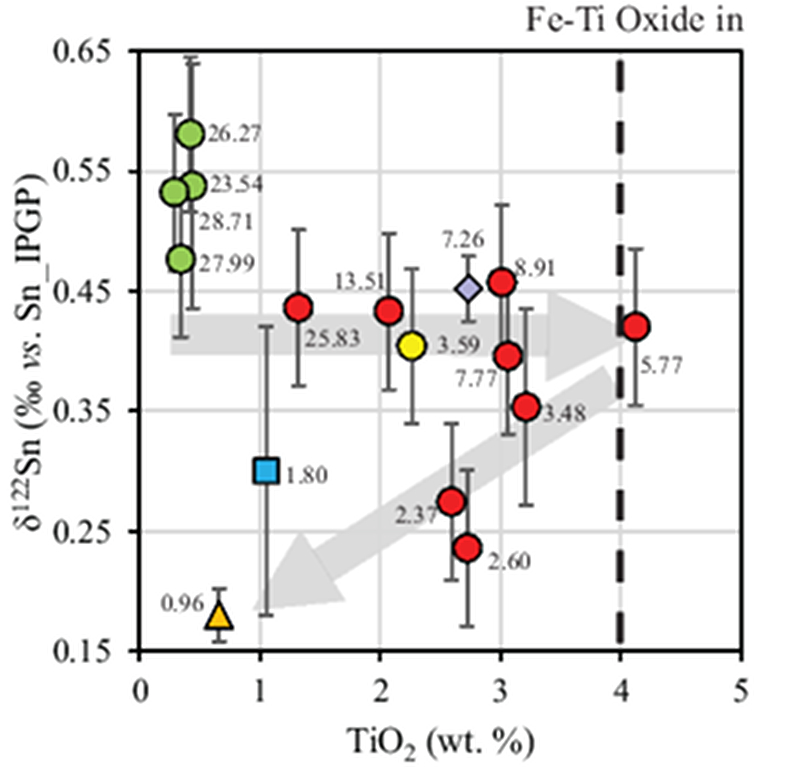
Figure 2 Variation of δ122Sn with TiO2 content, MgO (wt. %) shown next to the data point. Sn isotope fractionation occurs only after Fe-Ti oxide (ilmenite) saturation at Kilauea Iki. Symbols as per Figure 1.
Table 2 Calculated partition coefficients for Sn2+ and Sn4+ using lattice strain theory.
| Coordination | Olivine | Orthopyroxene | Clinopyroxene | Plagioclase | Ilmenite | |
| 2+ | Lattice Strain | |||||
| VI | 0.12 – 0.20 | 0.09 – 0.14 | 0.04 – 0.07 | - | - | |
| VIII | - | - | 1.0 – 1.2 | 0.94 – 1.27 | - | |
| Bulk Sn2+ | 0.12 – 0.20 | 0.09 – 0.14 | 1.04 – 1.27 | 0.94 – 1.27 | - | |
| 4+ | IV | 1x10-5 | 1x10-5 | 1x10-5 | 1x10-5 | - |
| VI | 0.005 – 0.01 | 0.01 – 0.07 | 0.05 – 0.39 | - | 4.5 – 6.8 | |
| Bulk Sn4+ | 0.005 – 0.01 | 0.01 – 0.07 | 0.05 – 0.39 | - | - | |
| Experimental | ||||||
| Adam and Green (2006) Adam, J., Green, T. (2006) Trace element partitioning between mica-and amphibole-bearing garnet lherzolite and hydrous basanitic melt:1. Experimental results and the investigation of controls on partitioning behaviour. Contributions to Mineralogy and Petrology 152, 1–17. | 0.002 – 0.014 | 0.015 | 0.024 – 1 | - | - | |
| Klemme et al. (2006) Klemme, S., Günther, D., Hametner, K., Prowatke, S., Zack, T. (2006) The partitioning of trace elements between ilmenite, ulvospinel, armalcolite and silicate melts with implications for the early differentiation of the moon. Chemical Geology 234, 251–263. | - | - | - | - | 5.1 | |
| Michely et al. (2017) Michely, L.T., Leitzke, F.P., Speelmanns, I.M., Fonseca, R.O.C. (2017) Competing effects of crystal chemistry and silicate melt composition on trace element behavior in magmatic systems: insights from crystal/silicate melt partitioning of the REE, HFSE, Sn, In, Ga, Ba, Pt and Rh. Contributions to Mineralogy and Petrology 172, 39. | | | 0.19 – 0.46* | | | |
*Only partition coefficients for the natural, komatiitic ‘W’ composition are reported here.
Back to article | Download in Excel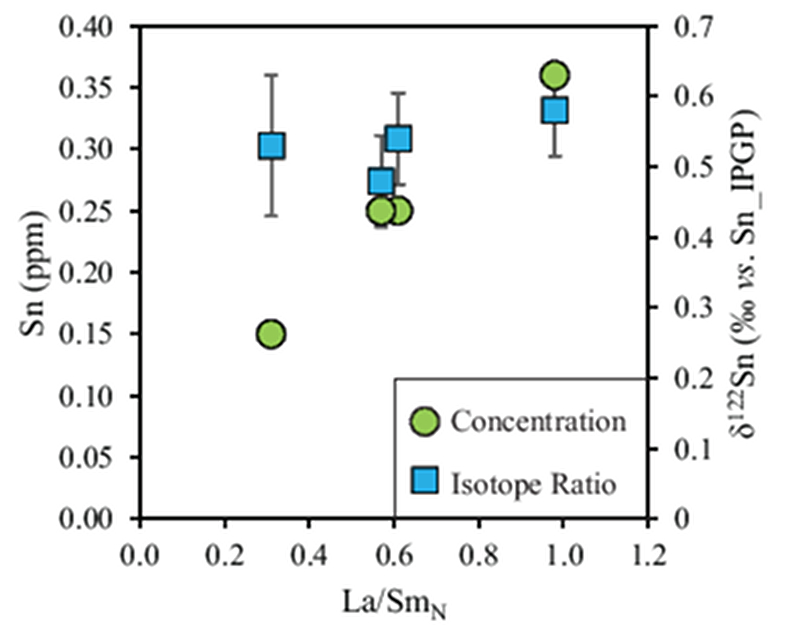
Figure 3 Depletion of komatiite source regions, quantified by La/SmN, as a function of Sn concentration (green circles) and δ122Sn (blue squares). Tin contents are positively correlated with La/SmN, whereas δ122Sn remains constant within uncertainty.
Back to article
Supplementary Figures and Tables
Table S-1 Terms used for Sn partition coefficient calculations.
| Phase | Olivine | Orthopyroxene | Clinopyroxene | Plagioclase | Ilmenite | ||
| Coordination | M1 (VI-fold) | M1 (VI-fold) | M1 (VI-fold) | M2 (VIII-fold) | M2 (VIII-fold) | M (VI-fold | |
| 2+ | ri2+ | 0.96 Å | 0.96 Å | 0.96 Å | 1.15 Å | 1.15 Å | - |
| ro2+ | 0.72 Å (Mg2+) | 0.72 Å (Mg2+) | 0.72 Å (Mg2+) | 1.06 Å (Ca2+) | 1.06 Å (Ca2+) | - | |
| Do2+ | 4.2 - 7.0 | 3.1 | 1.5 - 2.5 | 1.0 - 1.35 | 1.0 - 1.35 | - | |
| Ē2+ | 243 (GPa) | 243 (GPa) | 243 (GPa) | 116 (GPa) | 116 (GPa) | - | |
| 4+ | ri4+ | 0.69 Å | 0.69 Å | 0.69 Å | - | - | 0.69 Å |
| ro4+ | 0.605 Å (Ti4+) | 0.605 Å (Ti4+) | 0.605 Å (Ti4+) | - | - | 0.605 Å (Ti4+) | |
| Do4+ | 0.01 - 0.1 | 0.1 | 0.1 - 0.87 | - | - | 10 - 15 | |
| Ē4+ | 575 (GPa) | 575 (GPa) | 575 (GPa) | - | - | 575 (GPa) | |
Temperature (T) was taken as 1573 K, in the range of a typical basaltic melt.
Back to article | Download in ExcelTable S-2 Average trace element ratio values for Kilauea Iki lava lake.
| Trace element ratio* | Value | Jochum et al. (1993) value | Jenner and O’Neill (2012) | |
| Kilauea Iki | Sn/Hf | 0.42 ± 0.08 | 0.41 | 0.45 |
| Sn/Zr | 0.010 ± 0.0018 | 0.011 | 0.012 | |
| Sn/Ta | 1.3 ± 0.25 | - | - | |
| Sn/Sm | 0.26 ± 0.06 | 0.32 | 0.32 |
* Hf, Zr, Ta and Sm values taken from Helz and Taggart (2012).
Back to article | Download in Excel
Figure S-1 Calculated λ0, quantifying the mean abundances of the Rare Earth Elements (see O’Neill, 2016) against MgO. Red points correspond to the Kilauea Iki lava lake, and show a rapid drop in MgO and a small increase in λ0 for 7.4 < MgO (wt. %) < 27 during olivine crystallisation/accumulation, before a rapid increase in slope at 7.4 wt. % MgO signalling a change in the crystallising assemblage to cpx + plg + ol.
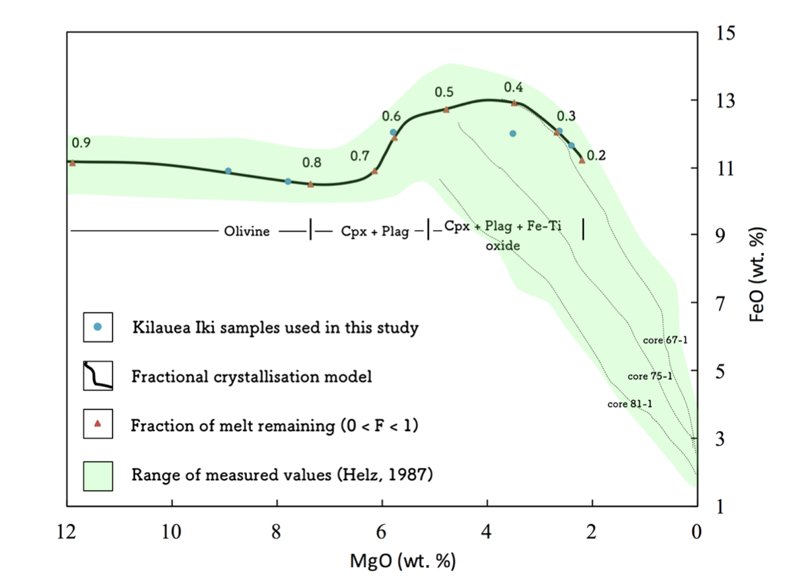
Figure S-2 Modelled abundances of FeO and MgO (wt. %) in the Kilauea Iki lava lake, overlain with the field (green) of literature data (Helz, 1987) for different eruption dates (1967, 1979 and 1981).
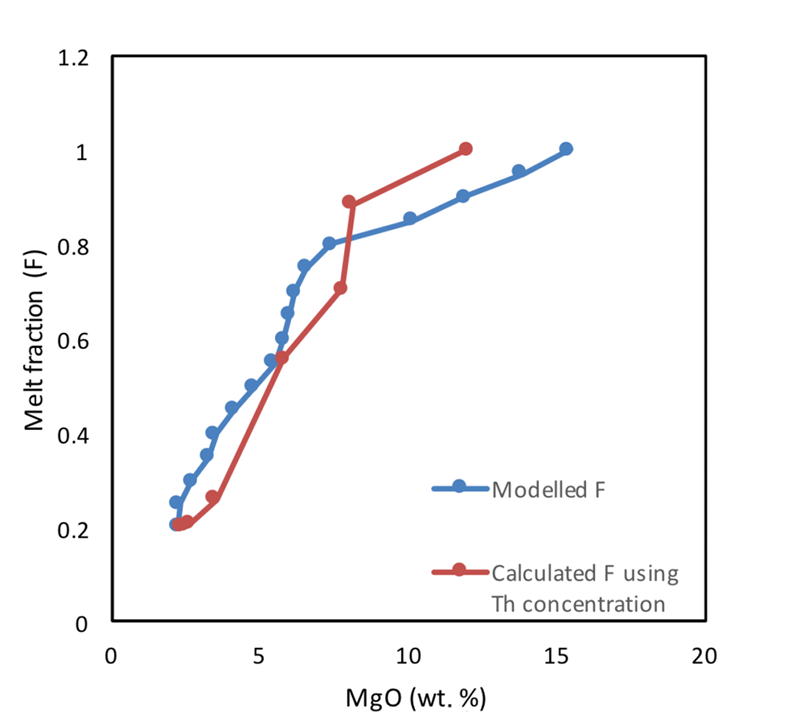
Figure S-3 Plot of melt fraction against MgO (wt. %). The maroon line represents the calculated F assuming that Th behaves as a perfectly incompatible element. The blue line represents the modelled F from the major element modelling of Kilauea Iki lava lake.
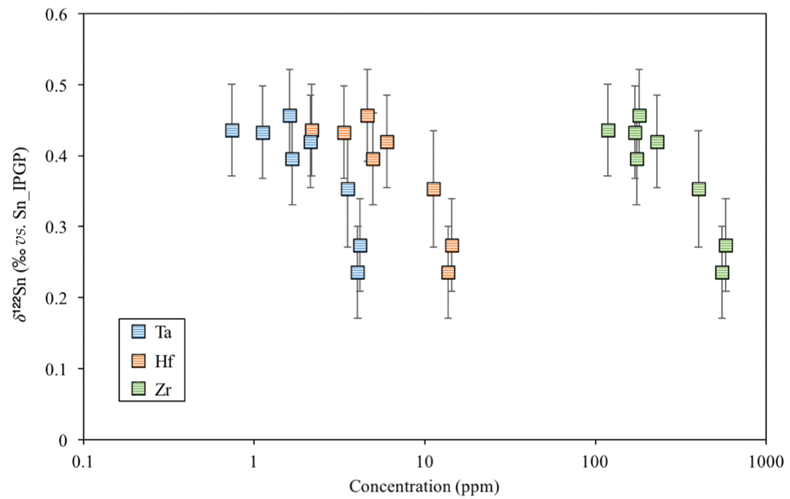
Figure S-4 Plot of δ122Sn against Ta, Hf and Zr (HFSEs).






