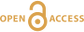Large oxygen excess in the primitive mantle could be the source of the Great Oxygenation Event
Affiliations | Corresponding Author | Cite as | Funding informationAndrault, D., Muñoz, M., Pesce, G., Cerantola, V., Chumakov, A., Kantor, I., Pascarelli, S., Rüffer, R., Hennet, L. (2018) Large oxygen excess in the primitive mantle could be the source of the Great Oxygenation Event. Geochem. Persp. Let. 6, 5–10.
ANR contract "OxyDeep", Laboratory of Excellence ClerVolc, Université Blaise Pascal, Université de Montpellier, CNRS, ESRF.
- Share this article





Article views:10,128Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
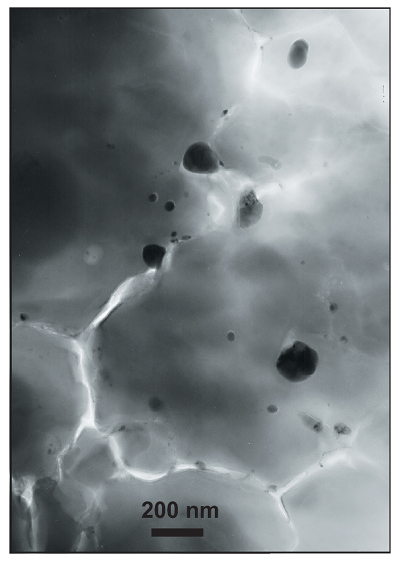 Figure 1 Micrograph of an Al-bearing (Mg,Fe)SiO3 bridgmanite. The starting material consisting of a mixture of (Mg,Fe)SiO3 pyroxene and Al2O3 corundum was compressed to 40 GPa and heated to 2500 K, using the LH-DAC. The reaction between the two phases induced the Fe2+ disproportionation into a mixture of Fe3+-rich, Al-bearing bridgmanite (large grey areas) and metallic Fe0 (dark droplets). Early in the Earth’s history, core segregation drained the metallic Fe0 away from the Fe3+-rich Bg, thus leaving the deep mantle with a large oxygen excess. |  Figure 2 Redox state of Fe probed by X-ray absorption spectroscopy. The pre-edge centroid energy (PCE, Fig. S-4) of our samples (orange and purple dots; see Table S-1) are plotted together with our ferropericlase (red dots, dominantly Fe2+) and bridgmanite (blue dots, 27 % Fe3+) reference compounds. Horizontal grey stripes correspond to PCE values generally found for Fe2+ or Fe3+-bearing minerals at ambient P-T conditions (Wilke et al., 2001). PCEs of our samples plot slightly higher than the reference compounds. Also, they increase regularly with pressure, which suggests significant Fe3+ contents and excludes a decrease of their bulk #Fe3+ up to more than 120 GPa. | 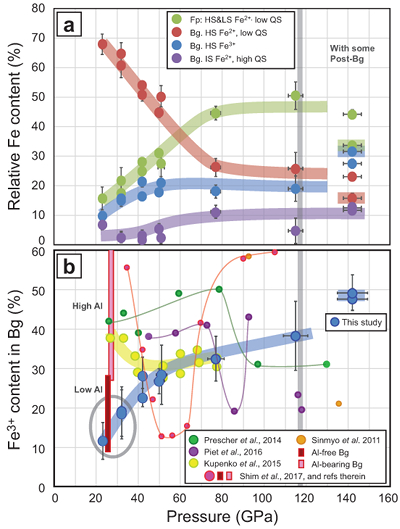 Figure 3 The mantle #Fe3+ refined from SMS measurements. (a) Major Fe contributions found in our sample are high spin and low spin Fe2+ in Fp (Kantor et al., 2009) (both added to each other, green dots), high spin Fe2+ with low QS (red), intermediate spin Fe2+ (purple) and high spin Fe3+ (blue) in Bg (Kupenko et al., 2015). Above ~40 GPa, corresponding to mantle depths greater than ~1000 km, the #Fe3+ in the bulk sample is almost constant at ~20 %. In the pressure field of post-Bg (~142 GPa), we performed two syntheses at ~2500 K (dot with a coloured trend superimposed) and ~3500 K. (b) When focusing on Bg properties, we observe a continuous increase of its #Fe3+ with pressure. At low pressure, our two samples plot in the field of previous Al-free (low Al) Bg compounds (see Shim et al., 2017 and references therein). It could be associated with the remaining presence of Al-bearing garnet in our samples. On the other hand, Kupenko et al. (2015) found higher #Fe3+ in Bg in this pressure range (yellow dots). Above ~40 GPa, our data set produces a smooth trend which lies between the previous findings (Sinmyo et al., 2011; Prescher et al., 2014; Kupenko et al., 2015; Piet et al., 2016; Shim et al., 2017). | 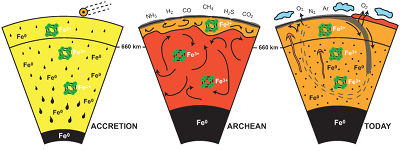 Figure 4 Change in the redox state of the deep mantle. During the Earth's accretion (left), the descending iron droplets equilibrated chemically in a partially molten mantle, which contained a major amount of Bg below the 660 km discontinuity. After loss of the Fe0, the lower mantle was left with a #Fe3+ of ~20 % (Fig. 3a). During the Archean (centre), the oxidised deep mantle remained relatively isolated from the Earth’s surface with a tectonic regime characterised by almost no subduction. At that time, the atmosphere was anoxic. The progressive transition to modern plate tectonics around the Archean to Proterozoic Transition greatly enhanced mantle mixing (right). Subduction became the dominant mechanism, which associated to global mantle convection brought deeper material and then fresh lavas to the Earth’s surface. Large amounts of oxygen were potentially degassed, due to the increase in volcanic fluxes and other sub-surface exchange processes. In this scheme, “Fe2+” and “Fe3+” indicate mantle fractions where the Fe3+/ΣFe ratios are 2–3 % and ~20 %, respectively. Yellow, orange and red colours indicate the Earth’s envelopes of Oxygen/ΣCations ratios that are lower (due to a Fe0 excess), similar and higher (due to a Fe3+ excess), respectively, than the current mantle value. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
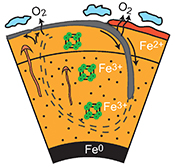
The main lower mantle phase, bridgmanite (Bg: (Mg,Fe)(Si,Al)O3) can easily incorporate Fe3+. This effect is related to a strong coupling of Fe3+ and Al3+ cations on the A and B sites of the ABO3 perovskite-type lattice. The Al-Fe3+ interaction in this phase is so favourable that it can induce the disproportionation of Fe2+ into Fe3+ (which is incorporated into Bg) and metallic iron (Fe0) (Fig. 1) (Frost et al., 2004
Frost, D.J., Liebske, C., Langenhorst, F., McCammon, C.A., Tronnes, R.G., Rubie, D.C. (2004) Experimental evidence for the existence of iron-rich metal in the Earth's lower mantle. Nature 428, 409–412.
). Such electron transfer occurs when a predominantly Fe2+-bearing upper mantle material is buried below the 660 km discontinuity by mantle convection. In the solid lower mantle, the Fe droplets are expected to remain relatively small and separate (Yoshino et al., 2003Yoshino, T., Walter, M.J., Katsura, T. (2003) Core formation in planetesimals triggered by permeable flow. Nature 422, 154–157.
). Thus, they are gravitationally metastable in the lower mantle. When this mineral mixture migrates upwards to the upper mantle, the coexisting Fe3+ and Fe0 can eventually recombine with each other (i.e. 2Fe3+ + Fe0 → 3Fe2+), leading to a dominantly Fe2+-bearing upper mantle with a true Fe3+ excess of only 2–3 % (Frost et al., 2008Frost, D.J., Mann, U., Asahara, Y., Rubie, D.C. (2008) The redox state of the mantle during and just after core formation. Philosophical Transactions of the Royal Society A-Mathematical Physical and Engineering Sciences 366, 4315–4337.
). This ongoing cycle of Fe2+ disproportionation followed by Fe3+ plus Fe0 recombination results in a neutral budget of the cation/oxygen ratio of the mantle. It remains difficult, however, to provide quantitative estimates of the #Fe3+ in the deep mantle, because different studies present a controversial range of #Fe3+ values from 10 % to 60 % or more, for pressures up to the 135 GPa found at the core-mantle boundary (see Shim et al., 2017Shim, S.-H., Grocholski, B., Ye, Y., Alp, E.E., Xu, S., Morgan, D., Meng, Y., Prakapenka, V.B. (2017) Stability of ferrous-iron-rich bridgmanite under reducing midmantle conditions. Proceedings of the National Academy of Sciences of the United States of America 114, 6468–6473.
).The redox equilibrium between Bg with a high #Fe3+ and metallic Fe0 was established early in the Earth’s history. However, the core-mantle segregation induced drainage of Fe0 droplets from the molten mantle down into the core, which resulted in a large Fe3+ excess in the primordial lower mantle (Frost et al., 2008
Frost, D.J., Mann, U., Asahara, Y., Rubie, D.C. (2008) The redox state of the mantle during and just after core formation. Philosophical Transactions of the Royal Society A-Mathematical Physical and Engineering Sciences 366, 4315–4337.
). This excess became a potential source of oxygen for the shallow mantle and the Earth’s surface. This is highly important with respect to the Great Oxygenation Event (GOE) which occurred 2.2–2.5 Ga ago (Bekker and Holland, 2012Bekker, A., Holland, H.D. (2012) Oxygen overshoot and recovery during the early Paleoproterozoic. Earth and Planetary Science Letters 317, 295–304.
). Different scenarios were proposed to explain the major change in redox state at the Earth’s surface at this time, including the emergence of oxygenic cyanobacteria (Buick, 2008Buick, R. (2008) When did oxygenic photosynthesis evolve? Philosophical Transactions of the Royal Society B-Biological Sciences 363, 2731–2743.
), and, in relation to the deep Earth, to a change in the oxidation state of sulphur in volcanic gases (Gaillard et al., 2011Gaillard, F., Scaillet, B., Arndt, N.T. (2011) Atmospheric oxygenation caused by a change in volcanic degassing pressure. Nature 478, 229–232.
). This subject is still hotly debated.
Figure 1 Micrograph of an Al-bearing (Mg,Fe)SiO3 bridgmanite. The starting material consisting of a mixture of (Mg,Fe)SiO3 pyroxene and Al2O3 corundum was compressed to 40 GPa and heated to 2500 K, using the LH-DAC. The reaction between the two phases induced the Fe2+ disproportionation into a mixture of Fe3+-rich, Al-bearing bridgmanite (large grey areas) and metallic Fe0 (dark droplets). Early in the Earth’s history, core segregation drained the metallic Fe0 away from the Fe3+-rich Bg, thus leaving the deep mantle with a large oxygen excess.
top
Experimental Determination of the #Fe3+ in the Deep Lower Mantle
Our experiments were aimed at refining the #Fe3+ in the Earth's lower mantle. Starting materials consisted of homogeneous glasses with a primitive mantle composition, but different Fe and Al contents (Tables S-1 and S-2). Their #Fe3+ was typically 7–8 % (Fig. S-5). Samples were synthesised at P-T conditions ranging from 25 to 140 GPa and 2000 to 3500 K, using a laser-heated diamond-anvil cell (LH-DAC). The DAC provides a closed system in which the cation/oxygen ratio remains constant. This represents a good proxy for natural conditions. During laser heating, chemical segregation associated with temperature gradients was minimised thanks to scanning of the lasers over the sample surface on both sides of the DAC. The valence state of Fe was determined in situ using synchrotron-based X-ray absorption (Fe K-edge XANES) and Mossbauer (SMS) spectroscopy (see Supplementary Information).
XANES measurements. We modelled the pre-edge features (Figs. S-1 to S-4, Wilke et al., 2001
Wilke, M., Farges, F., Petit, P.E., Brown, J.G.E., Martin, F. (2001) Oxidation state and coordination of Fe in minerals: An Fe K-XANES spectroscopic study. American Mineralogist 86, 714–730.
) to retrieve information on the sample #Fe3+ as a function of pressure. To apply this technique, we first determined the centroid energy position (PCE) of the pre-edge features in two Fp and Bg reference compounds, before comparing them to samples which contained a mixture of both phases. PCEs for both Fp and Bg increase with pressure by 0.3–0.4 eV up to ~30 GPa, before decreasing progressively at higher pressures. Because the iron valence of the reference compounds does not change under compression, such an evolution is likely to be due to pressure effects which are as yet undocumented in the literature.XANES spectra of our samples (Fig. 2) show PCE values slightly higher than our Bg reference, which suggests an #Fe3+ higher than 27 %, as found in our Bg reference using SMS (Fig. S-6). PCEs of both samples increase regularly with pressure up to more than 120 GPa, in strong contrast with decreasing trends observed for Fp and Bg references. This observation clearly supports a progressive increase in #Fe3+ in CI-NoAl and CI-2Fe samples with increasing pressure.

Figure 2 Redox state of Fe probed by X-ray absorption spectroscopy. The pre-edge centroid energy (PCE, Fig. S-4) of our samples (orange and purple dots; see Table S-1) are plotted together with our ferropericlase (red dots, dominantly Fe2+) and bridgmanite (blue dots, 27 % Fe3+) reference compounds. Horizontal grey stripes correspond to PCE values generally found for Fe2+ or Fe3+-bearing minerals at ambient P-T conditions (Wilke et al., 2001
Wilke, M., Farges, F., Petit, P.E., Brown, J.G.E., Martin, F. (2001) Oxidation state and coordination of Fe in minerals: An Fe K-XANES spectroscopic study. American Mineralogist 86, 714–730.
). PCEs of our samples plot slightly higher than the reference compounds. Also, they increase regularly with pressure, which suggests significant Fe3+ contents and excludes a decrease of their bulk #Fe3+ up to more than 120 GPa.SMS measurements. Previous studies present detailed analyses of the main SMS parameters of Fe species located in Bg and Fp (Kantor et al., 2009
Kantor, I., Dubrovinsky, L., McCammon, C., Steinle-Neumann, G., Kantor, A., Skorodumova, N., Pascarelli, S., Aquilanti, G. (2009) Short-range order and Fe clustering in Mg1-xFexO under high pressure. Physical Review B 80, 014204.
; Kupenko et al., 2015Kupenko, I., McCammon, C., Sinmyo, R., Cerantola, V., Potapkin, V., Chumakov, A.I., Kantor, A., Ruffer, R., Dubrovinsky, L. (2015) Oxidation state of the lower mantle: In situ observations of the iron electronic configuration in bridgmanite at extreme conditions. Earth and Planetary Science Letters 423, 78–86.
; Figs. S-7 and S-8). In our sample, which contains a mixture of both phases, we observe an increase in the FeO component in Fp up to ~80 GPa (Fig. 3a), which could be related to the Fe2+ spin transition in Fp (Badro et al., 2004Badro, J., Rueff, J.P., Vanko, G., Monaco, G., Fiquet, G., Guyot, F. (2004) Electronic transitions in perovskite: possible nonconvecting layers in the lower mantle. Science 305, 383–386.
). We find a low #Fe3+ in our samples synthesised below 30–35 GPa, with #Fe3+ similar to those previously reported for Al-free or low-Al Bg phases (Fig. 3b). In this pressure range, Al-rich garnet remains stable (e.g., Irifune et al., 1996Irifune, T., Koizumi, T., Ando, J. (1996) An experimental study of the garnet-perovskite transformation in the system MgSiO3-Mg3Al2Si3O12. Physics of the Earth and Planetary Interiors 96, 147–157.
), thus inducing a low Al content in Bg. On the other hand, previous SMS measurements performed on a Bg sample containing 6 % Al per formula unit suggest #Fe3+ of 30–35 % in this pressure range (Kupenko et al., 2015Kupenko, I., McCammon, C., Sinmyo, R., Cerantola, V., Potapkin, V., Chumakov, A.I., Kantor, A., Ruffer, R., Dubrovinsky, L. (2015) Oxidation state of the lower mantle: In situ observations of the iron electronic configuration in bridgmanite at extreme conditions. Earth and Planetary Science Letters 423, 78–86.
).Increasing pressure up to ~120 GPa produces a weak and continuous increase in #Fe3+ in Bg up to ~40 %. Our measurements are overall compatible with previous work (see Shim et al., 2017
Shim, S.-H., Grocholski, B., Ye, Y., Alp, E.E., Xu, S., Morgan, D., Meng, Y., Prakapenka, V.B. (2017) Stability of ferrous-iron-rich bridgmanite under reducing midmantle conditions. Proceedings of the National Academy of Sciences of the United States of America 114, 6468–6473.
and references therein). At the highest lower mantle pressures, when post-Bg becomes a major component, the bulk mantle #Fe3+ becomes 25–30 %, which corresponds to an #Fe3+ in the mixture of Bg plus post-Bg of 45–50 %.Our XANES and SMS analyses are consistent with each other. The more quantitative SMS results yield an average mantle #Fe3+ of ~20 % (Fig. 3a). It corresponds to ~2.1023 mol of Fe3+ in the lower mantle and an excess of ~1023 mol of oxygen compared to a predominantly Fe2+-bearing silicate mantle. Today, this oxygen excess is compensated by the presence of ~1023 mol of Fe0 (as in Fig. 1).

Figure 3 The mantle #Fe3+ refined from SMS measurements. (a) Major Fe contributions found in our sample are high spin and low spin Fe2+ in Fp (Kantor et al., 2009
Kantor, I., Dubrovinsky, L., McCammon, C., Steinle-Neumann, G., Kantor, A., Skorodumova, N., Pascarelli, S., Aquilanti, G. (2009) Short-range order and Fe clustering in Mg1-xFexO under high pressure. Physical Review B 80, 014204.
) (both added to each other, green dots), high spin Fe2+ with low QS (red), intermediate spin Fe2+ (purple) and high spin Fe3+ (blue) in Bg (Kupenko et al., 2015Kupenko, I., McCammon, C., Sinmyo, R., Cerantola, V., Potapkin, V., Chumakov, A.I., Kantor, A., Ruffer, R., Dubrovinsky, L. (2015) Oxidation state of the lower mantle: In situ observations of the iron electronic configuration in bridgmanite at extreme conditions. Earth and Planetary Science Letters 423, 78–86.
). Above ~40 GPa, corresponding to mantle depths greater than ~1000 km, the #Fe3+ in the bulk sample is almost constant at ~20 %. In the pressure field of post-Bg (~142 GPa), we performed two syntheses at ~2500 K (dot with a coloured trend superimposed) and ~3500 K. (b) When focusing on Bg properties, we observe a continuous increase of its #Fe3+ with pressure. At low pressure, our two samples plot in the field of previous Al-free (low Al) Bg compounds (see Shim et al., 2017Shim, S.-H., Grocholski, B., Ye, Y., Alp, E.E., Xu, S., Morgan, D., Meng, Y., Prakapenka, V.B. (2017) Stability of ferrous-iron-rich bridgmanite under reducing midmantle conditions. Proceedings of the National Academy of Sciences of the United States of America 114, 6468–6473.
and references therein). It could be associated with the remaining presence of Al-bearing garnet in our samples. On the other hand, Kupenko et al. (2015)Kupenko, I., McCammon, C., Sinmyo, R., Cerantola, V., Potapkin, V., Chumakov, A.I., Kantor, A., Ruffer, R., Dubrovinsky, L. (2015) Oxidation state of the lower mantle: In situ observations of the iron electronic configuration in bridgmanite at extreme conditions. Earth and Planetary Science Letters 423, 78–86.
found higher #Fe3+ in Bg in this pressure range (yellow dots). Above ~40 GPa, our data set produces a smooth trend which lies between the previous findings (Sinmyo et al., 2011Sinmyo, R., Hirose, K., Muto, S., Ohishi, Y., Akira, Y. (2011) The valence state and partitioning of iron in the Earth's lowermost mantle. Journal of Geophysical Research 116, B07205.
; Prescher et al., 2014Prescher, C., Langenhorst, F., Dubrovinsky, L.S., Prakapenka, V.B., Miyajima, N. (2014) The effect of Fe spin crossovers on its partitioning behavior and oxidation state in a pyrolitic Earth's lower mantle system. Earth and Planetary Science Letters 399, 86–91.
; Kupenko et al., 2015Kupenko, I., McCammon, C., Sinmyo, R., Cerantola, V., Potapkin, V., Chumakov, A.I., Kantor, A., Ruffer, R., Dubrovinsky, L. (2015) Oxidation state of the lower mantle: In situ observations of the iron electronic configuration in bridgmanite at extreme conditions. Earth and Planetary Science Letters 423, 78–86.
; Piet et al., 2016Piet, H., Badro, J., Nabiei, F., Dennenwaldt, T., Shim, S.H., Cantoni, M., Hebert, C., Gillet, P. (2016) Spin and valence dependence of iron partitioning in Earth's deep mantle. Proceedings of the National Academy of Sciences of the United States of America 113, 11127–11130.
; Shim et al., 2017Shim, S.-H., Grocholski, B., Ye, Y., Alp, E.E., Xu, S., Morgan, D., Meng, Y., Prakapenka, V.B. (2017) Stability of ferrous-iron-rich bridgmanite under reducing midmantle conditions. Proceedings of the National Academy of Sciences of the United States of America 114, 6468–6473.
).top
An Oxygen Excess Stored in the Primordial Lower Mantle
As mentioned in the introduction, the redox state of the primitive mantle was largely different from that prevailing today, because Fe0 droplets drained down from the magma ocean into the core. The removal of Fe0 left the primitive lower mantle with a relative Fe3+ excess (Frost et al., 2008
Frost, D.J., Mann, U., Asahara, Y., Rubie, D.C. (2008) The redox state of the mantle during and just after core formation. Philosophical Transactions of the Royal Society A-Mathematical Physical and Engineering Sciences 366, 4315–4337.
), and thus a relative excess of oxygen, compared to the present day mantle. The true oxygen excess in the primitive lower mantle was controlled by two main parameters: (i) the amount of Bg present in the lower mantle when the Fe0 segregation stopped. Since the magma ocean would have undergone a steep increase in viscosity at 60–70 % fractional crystallisation (Abe, 1997Abe, Y. (1997) Thermal and chemical evolution of the terrestrial magma ocean. Physics of the Earth and Planetary Interiors 100, 27–39.
), a transition that would stop the descent of Fe0 droplets by gravity, we use a Bg fraction of 50 % of the total lower mantle mass to estimate the Fe3+ content of the primitive lower mantle at the chemical closure; (ii) the efficiency of Fe0 droplet segregation. This depends on complex parameters, such as the level of turbulence in the magma ocean. We consider that 50 % of the Fe0 droplets produced by Bg crystallisation (which induces partial Fe2+ disproportionation into Fe3+ and Fe0) were efficiently segregated into the core.Based on these assumptions, we calculate that 1/4 of the total amount of Fe3+ stored in the lower mantle Bg phase (with average #Fe3+ of 20 %) was not counterbalanced by the presence of Fe0 after core segregation was completed. It corresponds to an O2 excess of more than ~2.5 x 1022 mol O2, compared to the current predominantly Fe2+-bearing bulk mantle. This oxygen excess represents 500 to 1000 times the O2 content in the Earth’s atmosphere today.
top
Geodynamical Changes at the APT Possibly Produced the GOE
The fate of such a large oxygen excess (coupled to Fe3+ in Bg) trapped in the primordial lower mantle is a major question. It might have reached the Earth’s surface (crust plus atmosphere) through volcanism. Analyses of Archean magma and mantle residues suggest that mantle sources have retained a constant, relatively oxidised, redox state for the last 3.5 Gyr or more (Delano, 2001
Delano, J.W. (2001) Redox history of the Earth's interior since similar to 3900 Ma: Implications for prebiotic molecules. Origins of Life and Evolution of the Biosphere 31, 311–341.
; Canil, 2002Canil, D. (2002) Vanadium in peridotites, mantle redox and tectonic environments: Archean to present. Earth and Planetary Science Letters 195, 75–90.
). It is paradoxical that while early volcanic eruptions should have favoured an Earth’s atmosphere dominated by oxidised volatile species (H2O, CO2, O2), the Archean atmosphere remained anoxic (Shaw, 2008Shaw, G.H. (2008) Earth's atmosphere - Hadean to early Proterozoic. Chemie Der Erde-Geochemistry 68, 235–264.
). This suggests a limited and/or inefficient mantle degassing during the Archean.The tectonic regime prevailing during the Archean was dominated by microplates floating at the Earth’s surface with a limited amount of subducted material (Condie and Kröner, 2013
Condie, K.C., Kröner, A. (2013) The building blocks of continental crust: Evidence for a major change in the tectonic setting of continental growth at the end of the Archean. Gondwana Research 23, 394–402.
; Kamber, 2015Kamber, B.S. (2015) The evolving nature of terrestrial crust from the Hadean, through the Archaean, into the Proterozoic. Precambrian Research 258, 48–82.
). In such a regime, the lower mantle material could have remained relatively isolated from shallow mantle processes, with only sporadic volcanism associated to deep mantle sources. Another argument in favour of a deep mantle which remained isolated for a long period of time is the relatively constant Lu/Hf ratio reported in rising plumes, suggesting that continents grew from nearly primordial unfractionated material (Guitreau et al., 2012Guitreau, M., Blichert-Toft, J., Martin, H., Mojzsis, S.J., Albarede, F. (2012) Hafnium isotope evidence from Archean granitic rocks for deep-mantle origin of continental crust. Earth and Planetary Science Letters 337, 211–223.
).Around the APT, the early no subduction regime was progressively replaced by a modern style subduction regime (Fig. 4). This evolution could have been fairly chaotic, because episodic subductions have been identified during the late Archean (van Hunen and Moyen, 2012
van Hunen, J., Moyen, J.-F. (2012) Archean Subduction: Fact or Fiction? Annual Review of Earth and Planetary Sciences 40, 195–219.
). Nevertheless, the transition resulted in major ultramafic volcanic events, greatly increasing the amount of fresh lava rising to the Earth’s surface (Arndt and Davaille, 2013Arndt, N., Davaille, A. (2013) Episodic Earth evolution. Tectonophysics 609, 661–674.
). We can estimate the flux of lower mantle material carried up by convection by assuming that it was equivalent to the slab volume subducting into the deep mantle today (Wen and Anderson, 1995Wen, L.X., Anderson, D.L. (1995) The fate of slabs inferred from seismic tomography and 130 million years of subduction. Earth and Planetary Science Letters 133, 185–198.
). An upwelling flux of ~2.5 x 1011 m3/year of primitive, oxidised, lower mantle material would correspond to the rise in excess of oxygen of ~1.5 x 1013 mol/year of O2. With such a flux, it would take ~2.5 x 106 years for the ascent of an amount of oxygen “equivalent” to the current oxygen content of the Earth’s atmosphere (Shaw, 2008Shaw, G.H. (2008) Earth's atmosphere - Hadean to early Proterozoic. Chemie Der Erde-Geochemistry 68, 235–264.
).
Figure 4 Change in the redox state of the deep mantle. During the Earth’s accretion (left), the descending iron droplets equilibrated chemically in a partially molten mantle, which contained a major amount of Bg below the 660 km discontinuity. After loss of the Fe0, the lower mantle was left with a #Fe3+ of ~20 % (Fig. 3a). During the Archean (centre), the oxidised deep mantle remained relatively isolated from the Earth’s surface with a tectonic regime characterised by almost no subduction. At that time, the atmosphere was anoxic. The progressive transition to modern plate tectonics around the Archean to Proterozoic Transition greatly enhanced mantle mixing (right). Subduction became the dominant mechanism, which associated to global mantle convection brought deeper material and then fresh lavas to the Earth’s surface. Large amounts of oxygen were potentially degassed, due to the increase in volcanic fluxes and other sub-surface exchange processes. In this scheme, “Fe2+” and “Fe3+” indicate mantle fractions where the Fe3+/ΣFe ratios are 2–3 % and ~20 %, respectively. Yellow, orange and red colours indicate the Earth’s envelopes of Oxygen/ΣCations ratios that are lower (due to a Fe0 excess), similar and higher (due to a Fe3+ excess), respectively, than the current mantle value.
Oscillations and overshoots in atmospheric oxygen pressure have been reported for a few 100 Myr around the APT (Bekker and Holland, 2012
Bekker, A., Holland, H.D. (2012) Oxygen overshoot and recovery during the early Paleoproterozoic. Earth and Planetary Science Letters 317, 295–304.
). They could be related to the chaotic transition between the two tectonic regimes, which might have resulted in an irregular rate of mantle mixing. In addition, the geological processes that could have accommodated the transfer of oxygen from a deep mantle source to the Earth’s surface are multiple and complex. Partial melting is obviously a major component, because Fe3+ is an incompatible element. Fluids might also have played a role, because the nature of molecular components changes with the redox state (Gaillard et al., 2011Gaillard, F., Scaillet, B., Arndt, N.T. (2011) Atmospheric oxygenation caused by a change in volcanic degassing pressure. Nature 478, 229–232.
). At the time of the GOE, the increased ƒO2 at the Earth’s surface led to the precipitation of a large variety of minerals containing more oxidised ionic species, including carbonates, phosphates, sulphates, etc. (Sverjensky and Lee, 2010Sverjensky, D.A., Lee, N. (2010) The Great Oxidation Event and Mineral Diversification. Elements 6, 31–36.
). Consequently, the amount of oxygen needed to induce the GOE must have far exceeded the current oxygen content of the Earth’s atmosphere. The massive amount of oxygen that can be stored in Fe3+-bearing Bg appears to be a unique redox source which can meet these requirements.top
Acknowledgements
We warmly thank N. Bolfan-Casanova, P. Bonnand, J.-L. Devidal, M. Guitreau, I. Kupenko, H. Martin, G. Manthilake, M. Murakami, C. Prescher, A. Rosa, F. van Wyk de Vries, two anonymous reviewers and the editor W.L. Mao, for help and fruitful discussions. This work has been supported by the ANR contract “OxyDeep”. This research was financed by ANR OxyDeep. This is Laboratory of Excellence ClerVolc contribution number 280.
Editor: Wendy Mao
top
Author Contributions
GP and DA prepared the DACs. MM, IK, DA, GP, SP performed XANES measurements, which were subsequently treated by MM. VC, DA, GP, AC, RR performed SMS measurements, which were subsequently treated by DA. DA and MM wrote the manuscript. All authors participated to discussions.
top
References
Abe, Y. (1997) Thermal and chemical evolution of the terrestrial magma ocean. Physics of the Earth and Planetary Interiors 100, 27–39.
 Show in context
Show in contextSince the magma ocean would have undergone a steep increase in viscosity at 60–70 % fractional crystallisation (Abe, 1997), a transition that would stop the descent of Fe0 droplets by gravity, we use a Bg fraction of 50 % of the total lower mantle mass to estimate the Fe3+ content of the primitive lower mantle at the chemical closure; (ii) the efficiency of Fe0 droplet segregation.
View in article
Arndt, N., Davaille, A. (2013) Episodic Earth evolution. Tectonophysics 609, 661–674.
 Show in context
Show in context Nevertheless, the transition resulted in major ultramafic volcanic events, greatly increasing the amount of fresh lava rising to the Earth’s surface (Arndt and Davaille, 2013).
View in article
Badro, J., Rueff, J.P., Vanko, G., Monaco, G., Fiquet, G., Guyot, F. (2004) Electronic transitions in perovskite: possible nonconvecting layers in the lower mantle. Science 305, 383–386.
 Show in context
Show in context In our sample, which contains a mixture of both phases, we observe an increase in the FeO component in Fp up to ~80 GPa (Fig. 3a), which could be related to the Fe2+ spin transition in Fp (Badro et al., 2004).
View in article
Bekker, A., Holland, H.D. (2012) Oxygen overshoot and recovery during the early Paleoproterozoic. Earth and Planetary Science Letters 317, 295–304.
 Show in context
Show in context This is highly important with respect to the Great Oxygenation Event (GOE) which occurred 2.2–2.5 Ga ago (Bekker and Holland, 2012).
View in article
Oscillations and overshoots in atmospheric oxygen pressure have been reported for a few 100 Myr around the APT (Bekker and Holland, 2012).
View in article
Boujibar, A., Bolfan-Casanova, N., Andrault, D., Bouhifd, M.A., Trcera, N. (2016) Incorporation of Fe2+ and Fe3+ in bridgmanite during magma ocean crystallization. American Mineralogist 101, 1560–1570.
 Show in context
Show in context Figure 2
View in article
Buick, R. (2008) When did oxygenic photosynthesis evolve? Philosophical Transactions of the Royal Society B-Biological Sciences 363, 2731–2743.
 Show in context
Show in context Different scenarios were proposed to explain the major change in redox state at the Earth’s surface at this time, including the emergence of oxygenic cyanobacteria (Buick, 2008), and, in relation to the deep Earth, to a change in the oxidation state of sulphur in volcanic gases (Gaillard et al., 2011).
View in article
Canil, D. (2002) Vanadium in peridotites, mantle redox and tectonic environments: Archean to present. Earth and Planetary Science Letters 195, 75–90.
 Show in context
Show in contextAnalyses of Archean magma and mantle residues suggest that mantle sources have retained a constant, relatively oxidised, redox state for the last 3.5 Gyr or more (Delano, 2001; Canil, 2002)
View in article
Condie, K.C., Kröner, A. (2013) The building blocks of continental crust: Evidence for a major change in the tectonic setting of continental growth at the end of the Archean. Gondwana Research 23, 394–402.
 Show in context
Show in context The tectonic regime prevailing during the Archean was dominated by microplates floating at the Earth’s surface with a limited amount of subducted material (Condie and Kröner, 2013; Kamber, 2015).
View in article
Delano, J.W. (2001) Redox history of the Earth's interior since similar to 3900 Ma: Implications for prebiotic molecules. Origins of Life and Evolution of the Biosphere 31, 311–341.
 Show in context
Show in context Analyses of Archean magma and mantle residues suggest that mantle sources have retained a constant, relatively oxidised, redox state for the last 3.5 Gyr or more (Delano, 2001; Canil, 2002)
View in article
Frost, D.J., Liebske, C., Langenhorst, F., McCammon, C.A., Tronnes, R.G., Rubie, D.C. (2004) Experimental evidence for the existence of iron-rich metal in the Earth's lower mantle. Nature 428, 409–412.
 Show in context
Show in context The Al-Fe3+ interaction in this phase is so favourable that it can induce the disproportionation of Fe2+ into Fe3+ (which is incorporated into Bg) and metallic iron (Fe0) (Fig. 1) (Frost et al., 2004).
View in article
Frost, D.J., Mann, U., Asahara, Y., Rubie, D.C. (2008) The redox state of the mantle during and just after core formation. Philosophical Transactions of the Royal Society A-Mathematical Physical and Engineering Sciences 366, 4315–4337.
 Show in context
Show in context When this mineral mixture migrates upwards to the upper mantle, the coexisting Fe3+ and Fe0 can eventually recombine with each other (i.e. 2Fe3+ + Fe0 → 3Fe2+), leading to a dominantly Fe2+-bearing upper mantle with a true Fe3+ excess of only 2–3 % (Frost et al., 2008).
View in article
However, the core-mantle segregation induced drainage of Fe0 droplets from the molten mantle down into the core, which resulted in a large Fe3+ excess in the primordial lower mantle (Frost et al., 2008).
View in article
The removal of Fe0 left the primitive lower mantle with a relative Fe3+ excess (Frost et al., 2008), and thus a relative excess of oxygen, compared to the present day mantle.
View in article
Gaillard, F., Scaillet, B., Arndt, N.T. (2011) Atmospheric oxygenation caused by a change in volcanic degassing pressure. Nature 478, 229–232.
 Show in context
Show in context Different scenarios were proposed to explain the major change in redox state at the Earth’s surface at this time, including the emergence of oxygenic cyanobacteria (Buick, 2008), and, in relation to the deep Earth, to a change in the oxidation state of sulphur in volcanic gases (Gaillard et al., 2011).
View in article
Fluids might also have played a role, because the nature of molecular components changes with the redox state (Gaillard et al., 2011).
View in article
Guitreau, M., Blichert-Toft, J., Martin, H., Mojzsis, S.J., Albarede, F. (2012) Hafnium isotope evidence from Archean granitic rocks for deep-mantle origin of continental crust. Earth and Planetary Science Letters 337, 211–223.
 Show in context
Show in context Another argument in favour of a deep mantle which remained isolated for a long period of time is the relatively constant Lu/Hf ratio reported in rising plumes, suggesting that continents grew from nearly primordial unfractionated material (Guitreau et al., 2012).
View in article
Irifune, T., Koizumi, T., Ando, J. (1996) An experimental study of the garnet-perovskite transformation in the system MgSiO3-Mg3Al2Si3O12. Physics of the Earth and Planetary Interiors 96, 147–157.
 Show in context
Show in context In this pressure range, Al-rich garnet remains stable (e.g., Irifune et al., 1996), thus inducing a low Al content in Bg.
View in article
Kamber, B.S. (2015) The evolving nature of terrestrial crust from the Hadean, through the Archaean, into the Proterozoic. Precambrian Research 258, 48–82.
 Show in context
Show in contextThe tectonic regime prevailing during the Archean was dominated by microplates floating at the Earth’s surface with a limited amount of subducted material (Condie and Kröner, 2013; Kamber, 2015).
View in article
Kantor, I., Dubrovinsky, L., McCammon, C., Steinle-Neumann, G., Kantor, A., Skorodumova, N., Pascarelli, S., Aquilanti, G. (2009) Short-range order and Fe clustering in Mg1-xFexO under high pressure. Physical Review B 80, 014204.
 Show in context
Show in contextPrevious studies present detailed analyses of the main SMS parameters of Fe species located in Bg and Fp (Kantor et al., 2009; Kupenko et al., 2015; Figs. S-7 and S-8).
View in article
Figure 3 [...](a) Major Fe contributions found in our sample are high spin and low spin Fe2+ in Fp (Kantor et al., 2009) (both added to each other, green dots), high spin Fe2+ with low QS (red), intermediate spin Fe2+ (purple) and high spin Fe3+ (blue) in Bg (Kupenko et al., 2015).
View in article
Kupenko, I., McCammon, C., Sinmyo, R., Cerantola, V., Potapkin, V., Chumakov, A.I., Kantor, A., Ruffer, R., Dubrovinsky, L. (2015) Oxidation state of the lower mantle: In situ observations of the iron electronic configuration in bridgmanite at extreme conditions. Earth and Planetary Science Letters 423, 78–86.
 Show in context
Show in context Previous studies present detailed analyses of the main SMS parameters of Fe species located in Bg and Fp (Kantor et al., 2009; Kupenko et al., 2015; Figs. S-7 and S-8).
View in article
On the other hand, previous SMS measurements performed on a Bg sample containing 6 % Al per formula unit suggest #Fe3+ of 30–35 % in this pressure range (Kupenko et al., 2015).
View in article
Figure 3 [...](a) Major Fe contributions found in our sample are high spin and low spin Fe2+ in Fp (Kantor et al., 2009) (both added to each other, green dots), high spin Fe2+ with low QS (red), intermediate spin Fe2+ (purple) and high spin Fe3+ (blue) in Bg (Kupenko et al., 2015).
View in article
Figure 3 [...] On the other hand, Kupenko et al. (2015) found higher #Fe3+ in Bg in this pressure range (yellow dots). Above ~40 GPa, our data set produces a smooth trend which lies between the previous findings (Sinmyo et al., 2011; Prescher et al., 2014; Kupenko et al., 2015; Piet et al., 2016; Shim et al., 2017).
View in article
Piet, H., Badro, J., Nabiei, F., Dennenwaldt, T., Shim, S.H., Cantoni, M., Hebert, C., Gillet, P. (2016) Spin and valence dependence of iron partitioning in Earth's deep mantle. Proceedings of the National Academy of Sciences of the United States of America 113, 11127–11130.
 Show in context
Show in context Figure 3 [...] On the other hand, Kupenko et al. (2015) found higher #Fe3+ in Bg in this pressure range (yellow dots). Above ~40 GPa, our data set produces a smooth trend which lies between the previous findings (Sinmyo et al., 2011; Prescher et al., 2014; Kupenko et al., 2015; Piet et al., 2016; Shim et al., 2017).
View in article
Prescher, C., Langenhorst, F., Dubrovinsky, L.S., Prakapenka, V.B., Miyajima, N. (2014) The effect of Fe spin crossovers on its partitioning behavior and oxidation state in a pyrolitic Earth's lower mantle system. Earth and Planetary Science Letters 399, 86–91.
 Show in context
Show in contextFigure 3 [...] On the other hand, Kupenko et al. (2015) found higher #Fe3+ in Bg in this pressure range (yellow dots). Above ~40 GPa, our data set produces a smooth trend which lies between the previous findings (Sinmyo et al., 2011; Prescher et al., 2014; Kupenko et al., 2015; Piet et al., 2016; Shim et al., 2017).
View in article
Shaw, G.H. (2008) Earth's atmosphere - Hadean to early Proterozoic. Chemie Der Erde-Geochemistry 68, 235–264.
 Show in context
Show in context It is paradoxical that while early volcanic eruptions should have favoured an Earth’s atmosphere dominated by oxidised volatile species (H2O, CO2, O2), the Archean atmosphere remained anoxic (Shaw, 2008).
View in article
With such a flux, it would take ~2.5 x 106 years for the ascent of an amount of oxygen “equivalent” to the current oxygen content of the Earth’s atmosphere (Shaw, 2008).
View in article
Shim, S.-H., Grocholski, B., Ye, Y., Alp, E.E., Xu, S., Morgan, D., Meng, Y., Prakapenka, V.B. (2017) Stability of ferrous-iron-rich bridgmanite under reducing midmantle conditions. Proceedings of the National Academy of Sciences of the United States of America 114, 6468–6473.
 Show in context
Show in context It remains difficult, however, to provide quantitative estimates of the #Fe3+ in the deep mantle, because different studies present a controversial range of #Fe3+ values from 10 % to 60 % or more, for pressures up to the 135 GPa found at the core-mantle boundary (see Shim et al., 2017).
View in article
Our measurements are overall compatible with previous work (see Shim et al., 2017 and references therein).
View in article
Figure 3 [...] At low pressure, our two samples plot in the field of previous Al-free (low Al) Bg compounds (see Shim et al., 2017 and references therein).
View in article
Figure 3 [...] On the other hand, Kupenko et al. (2015) found higher #Fe3+ in Bg in this pressure range (yellow dots). Above ~40 GPa, our data set produces a smooth trend which lies between the previous findings (Sinmyo et al., 2011; Prescher et al., 2014; Kupenko et al., 2015; Piet et al., 2016; Shim et al., 2017).
View in article
Sinmyo, R., Hirose, K., Muto, S., Ohishi, Y., Akira, Y. (2011) The valence state and partitioning of iron in the Earth's lowermost mantle. Journal of Geophysical Research 116, B07205.
 Show in context
Show in contextFigure 3 [...] On the other hand, Kupenko et al. (2015) found higher #Fe3+ in Bg in this pressure range (yellow dots). Above ~40 GPa, our data set produces a smooth trend which lies between the previous findings (Sinmyo et al., 2011; Prescher et al., 2014; Kupenko et al., 2015; Piet et al., 2016; Shim et al., 2017).
View in article
Sverjensky, D.A., Lee, N. (2010) The Great Oxidation Event and Mineral Diversification. Elements 6, 31–36.
 Show in context
Show in context At the time of the GOE, the increased ƒO2 at the Earth’s surface led to the precipitation of a large variety of minerals containing more oxidised ionic species, including carbonates, phosphates, sulphates, etc. (Sverjensky and Lee, 2010).
View in article
van Hunen, J., Moyen, J.-F. (2012) Archean Subduction: Fact or Fiction? Annual Review of Earth and Planetary Sciences 40, 195–219.
 Show in context
Show in contextThis evolution could have been fairly chaotic, because episodic subductions have been identified during the late Archean (van Hunen and Moyen, 2012).
View in article
Wen, L.X., Anderson, D.L. (1995) The fate of slabs inferred from seismic tomography and 130 million years of subduction. Earth and Planetary Science Letters 133, 185–198.
 Show in context
Show in context We can estimate the flux of lower mantle material carried up by convection by assuming that it was equivalent to the slab volume subducting into the deep mantle today (Wen and Anderson, 1995).
View in article
Wilke, M., Farges, F., Petit, P.E., Brown, J.G.E., Martin, F. (2001) Oxidation state and coordination of Fe in minerals: An Fe K-XANES spectroscopic study. American Mineralogist 86, 714–730.
 Show in context
Show in context We modelled the pre-edge features (Figs. S-1 to S-4, Wilke et al., 2001) to retrieve information on the sample #Fe3+ as a function of pressure.
View in article
Figure 2 [...] Horizontal grey stripes correspond to PCE values generally found for Fe2+ or Fe3+-bearing minerals at ambient P-T conditions (Wilke et al., 2001).
View in article
Yoshino, T., Walter, M.J., Katsura, T. (2003) Core formation in planetesimals triggered by permeable flow. Nature 422, 154–157.
 Show in context
Show in contextIn the solid lower mantle, the Fe droplets are expected to remain relatively small and separate (Yoshino et al., 2003).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Methods
- Tables S-1 to S-3
- Figures S-1 to S-8
- Supplementary Information References
Figures and Tables
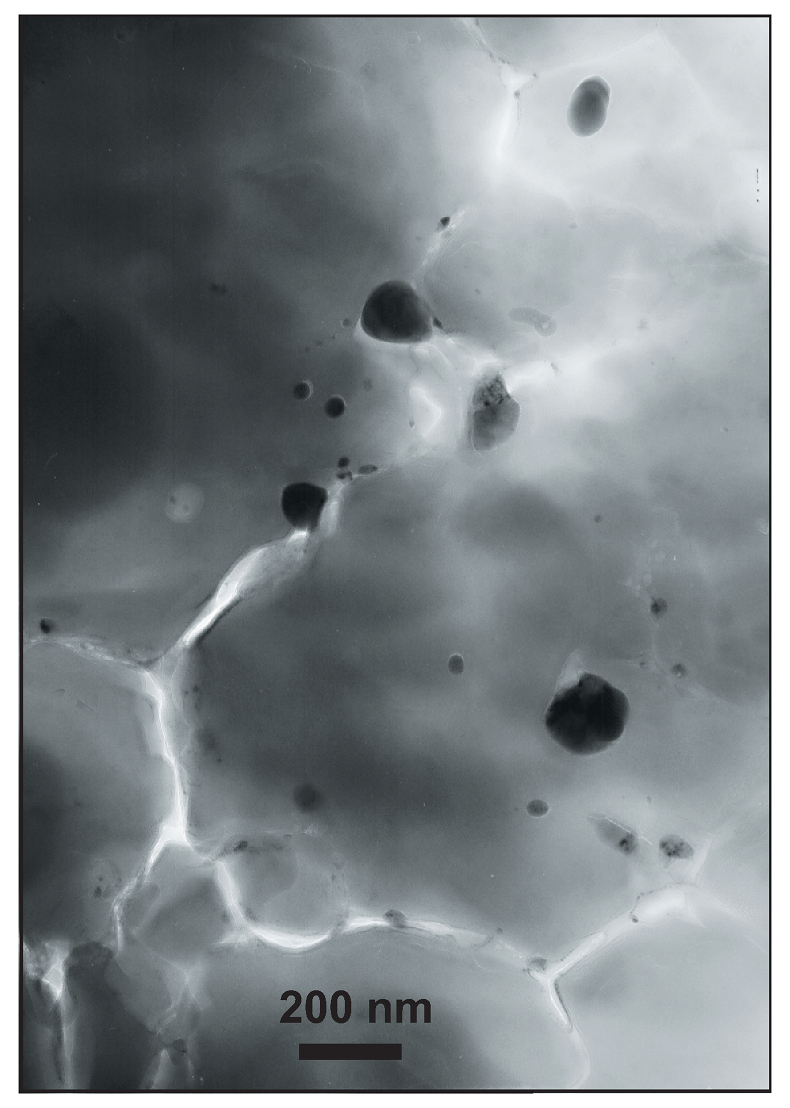
Figure 1 Micrograph of an Al-bearing (Mg,Fe)SiO3 bridgmanite. The starting material consisting of a mixture of (Mg,Fe)SiO3 pyroxene and Al2O3 corundum was compressed to 40 GPa and heated to 2500 K, using the LH-DAC. The reaction between the two phases induced the Fe2+ disproportionation into a mixture of Fe3+-rich, Al-bearing bridgmanite (large grey areas) and metallic Fe0 (dark droplets). Early in the Earth’s history, core segregation drained the metallic Fe0 away from the Fe3+-rich Bg, thus leaving the deep mantle with a large oxygen excess.
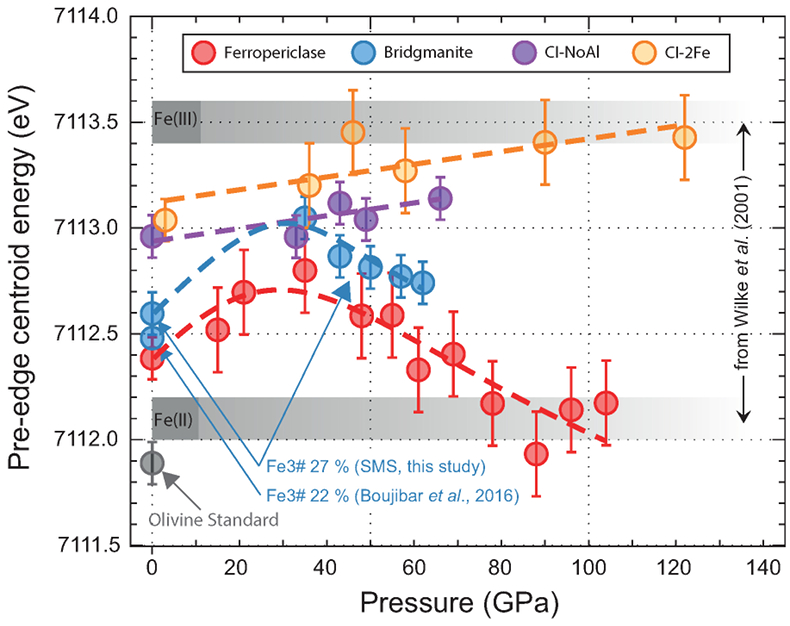
Figure 2 Redox state of Fe probed by X-ray absorption spectroscopy. The pre-edge centroid energy (PCE, Fig. S-4) of our samples (orange and purple dots; see Table S-1) are plotted together with our ferropericlase (red dots, dominantly Fe2+) and bridgmanite (blue dots, 27 % Fe3+) reference compounds. Horizontal grey stripes correspond to PCE values generally found for Fe2+ or Fe3+-bearing minerals at ambient P-T conditions (Wilke et al., 2001
Wilke, M., Farges, F., Petit, P.E., Brown, J.G.E., Martin, F. (2001) Oxidation state and coordination of Fe in minerals: An Fe K-XANES spectroscopic study. American Mineralogist 86, 714–730.
). PCEs of our samples plot slightly higher than the reference compounds. Also, they increase regularly with pressure, which suggests significant Fe3+ contents and excludes a decrease of their bulk #Fe3+ up to more than 120 GPa.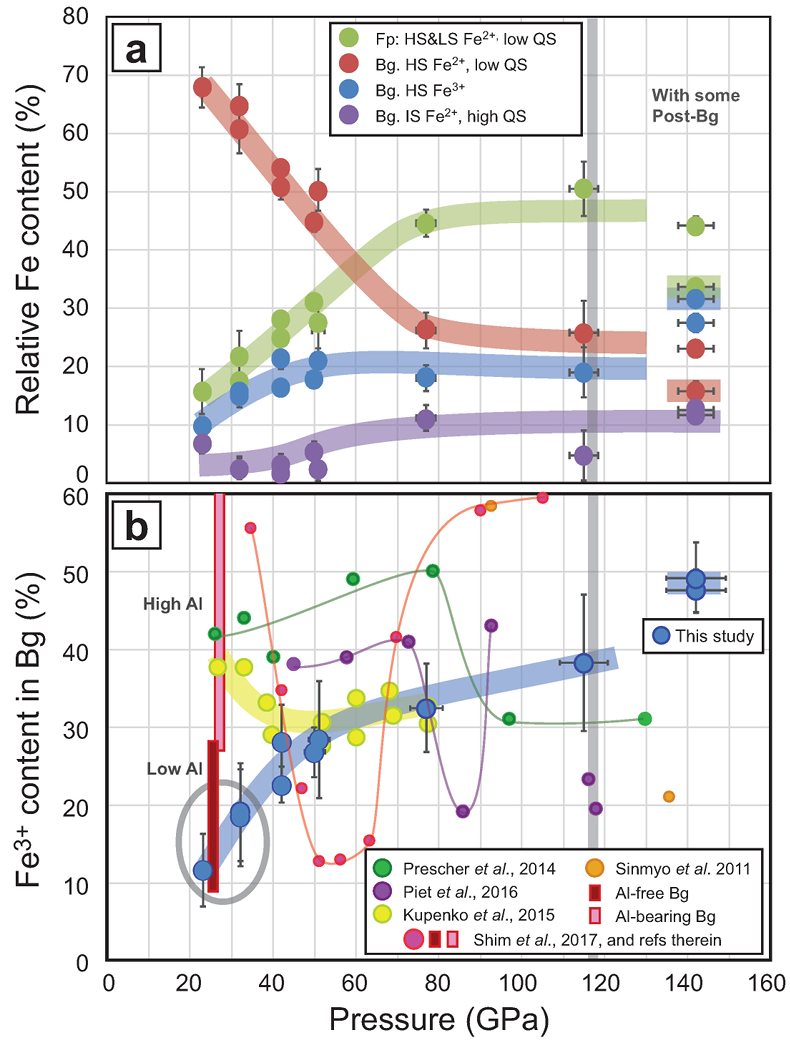
Figure 3 The mantle #Fe3+ refined from SMS measurements. (a) Major Fe contributions found in our sample are high spin and low spin Fe2+ in Fp (Kantor et al., 2009
Kantor, I., Dubrovinsky, L., McCammon, C., Steinle-Neumann, G., Kantor, A., Skorodumova, N., Pascarelli, S., Aquilanti, G. (2009) Short-range order and Fe clustering in Mg1-xFexO under high pressure. Physical Review B 80, 014204.
) (both added to each other, green dots), high spin Fe2+ with low QS (red), intermediate spin Fe2+ (purple) and high spin Fe3+ (blue) in Bg (Kupenko et al., 2015Kupenko, I., McCammon, C., Sinmyo, R., Cerantola, V., Potapkin, V., Chumakov, A.I., Kantor, A., Ruffer, R., Dubrovinsky, L. (2015) Oxidation state of the lower mantle: In situ observations of the iron electronic configuration in bridgmanite at extreme conditions. Earth and Planetary Science Letters 423, 78–86.
). Above ~40 GPa, corresponding to mantle depths greater than ~1000 km, the #Fe3+ in the bulk sample is almost constant at ~20 %. In the pressure field of post-Bg (~142 GPa), we performed two syntheses at ~2500 K (dot with a coloured trend superimposed) and ~3500 K. (b) When focusing on Bg properties, we observe a continuous increase of its #Fe3+ with pressure. At low pressure, our two samples plot in the field of previous Al-free (low Al) Bg compounds (see Shim et al., 2017Shim, S.-H., Grocholski, B., Ye, Y., Alp, E.E., Xu, S., Morgan, D., Meng, Y., Prakapenka, V.B. (2017) Stability of ferrous-iron-rich bridgmanite under reducing midmantle conditions. Proceedings of the National Academy of Sciences of the United States of America 114, 6468–6473.
and references therein). It could be associated with the remaining presence of Al-bearing garnet in our samples. On the other hand, Kupenko et al. (2015)Kupenko, I., McCammon, C., Sinmyo, R., Cerantola, V., Potapkin, V., Chumakov, A.I., Kantor, A., Ruffer, R., Dubrovinsky, L. (2015) Oxidation state of the lower mantle: In situ observations of the iron electronic configuration in bridgmanite at extreme conditions. Earth and Planetary Science Letters 423, 78–86.
found higher #Fe3+ in Bg in this pressure range (yellow dots). Above ~40 GPa, our data set produces a smooth trend which lies between the previous findings (Sinmyo et al., 2011Sinmyo, R., Hirose, K., Muto, S., Ohishi, Y., Akira, Y. (2011) The valence state and partitioning of iron in the Earth's lowermost mantle. Journal of Geophysical Research 116, B07205.
; Prescher et al., 2014Prescher, C., Langenhorst, F., Dubrovinsky, L.S., Prakapenka, V.B., Miyajima, N. (2014) The effect of Fe spin crossovers on its partitioning behavior and oxidation state in a pyrolitic Earth's lower mantle system. Earth and Planetary Science Letters 399, 86–91.
; Kupenko et al., 2015Kupenko, I., McCammon, C., Sinmyo, R., Cerantola, V., Potapkin, V., Chumakov, A.I., Kantor, A., Ruffer, R., Dubrovinsky, L. (2015) Oxidation state of the lower mantle: In situ observations of the iron electronic configuration in bridgmanite at extreme conditions. Earth and Planetary Science Letters 423, 78–86.
; Piet et al., 2016Piet, H., Badro, J., Nabiei, F., Dennenwaldt, T., Shim, S.H., Cantoni, M., Hebert, C., Gillet, P. (2016) Spin and valence dependence of iron partitioning in Earth's deep mantle. Proceedings of the National Academy of Sciences of the United States of America 113, 11127–11130.
; Shim et al., 2017Shim, S.-H., Grocholski, B., Ye, Y., Alp, E.E., Xu, S., Morgan, D., Meng, Y., Prakapenka, V.B. (2017) Stability of ferrous-iron-rich bridgmanite under reducing midmantle conditions. Proceedings of the National Academy of Sciences of the United States of America 114, 6468–6473.
).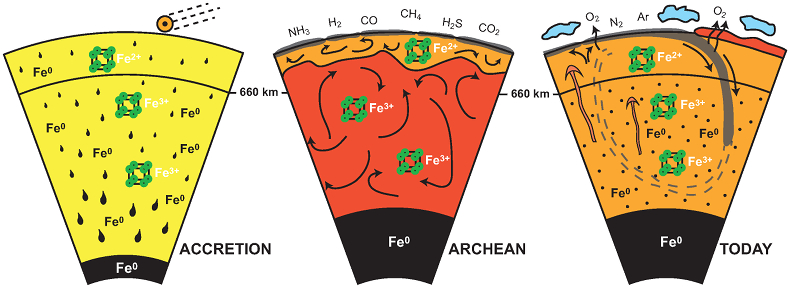
Figure 4 Change in the redox state of the deep mantle. During the Earth's accretion (left), the descending iron droplets equilibrated chemically in a partially molten mantle, which contained a major amount of Bg below the 660 km discontinuity. After loss of the Fe0, the lower mantle was left with a #Fe3+ of ~20 % (Fig. 3a). During the Archean (centre), the oxidised deep mantle remained relatively isolated from the Earth’s surface with a tectonic regime characterised by almost no subduction. At that time, the atmosphere was anoxic. The progressive transition to modern plate tectonics around the Archean to Proterozoic Transition greatly enhanced mantle mixing (right). Subduction became the dominant mechanism, which associated to global mantle convection brought deeper material and then fresh lavas to the Earth’s surface. Large amounts of oxygen were potentially degassed, due to the increase in volcanic fluxes and other sub-surface exchange processes. In this scheme, “Fe2+” and “Fe3+” indicate mantle fractions where the Fe3+/ΣFe ratios are 2–3 % and ~20 %, respectively. Yellow, orange and red colours indicate the Earth’s envelopes of Oxygen/ΣCations ratios that are lower (due to a Fe0 excess), similar and higher (due to a Fe3+ excess), respectively, than the current mantle value.