Atmospheric helium isotopic ratio from 1910 to 2016 recorded in stainless steel containers
Affiliations | Corresponding Author | Cite as | Funding informationBoucher, C., Marty, B., Zimmermann, L., Langenfelds, R. (2018) Atmospheric helium isotopic ratio from 1910 to 2016 recorded in stainless steel containers. Geochem. Persp. Let. 6, 23–27.
This work was supported by the European Research Council (grant No. 267255) and by the Deep Carbon Observatory (DCO).
- Share this article





Article views:3,813Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract

Figures and Tables
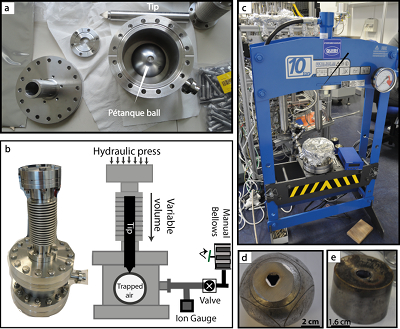 Figure 1 (a) Stainless steel chamber. (b) Sectional view of the chamber. (c) Hydraulic press KSC-10. (d) Pétanque ball pierced. (e) Carburettor float. | 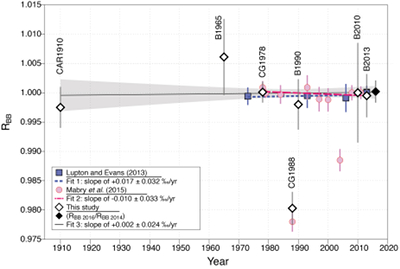 Figure 2 Error-weighted mean (x̅) of the He isotope ratios, expressed as RBB (diamond points; CG = Cape Grim; B = pétanque ball, CAR = carburettor), over time. Mabry et al. (2015) data (pink dots) are also normalised to BB air. Lupton and Evans (2013) data (blue squares) are normalised to 2013 La Jolla air (California, USA). The weighted linear regression fits 1, 2 and 3 are obtained using data of Lupton and Evans (2013), Mabry et al. (2015) and the combination of our data with those of Mabry et al. (2015), respectively. The uncertainty envelope of fit 3 is shown in grey shaded area. Results are reported at 95 % CI (equivalent to 2σ). |
| Figure 1 | Figure 2 |
top
Introduction
Atmospheric helium is a trace gas used as an international standard, whose abundance and isotope composition may be impacted by anthropogenic activities (e.g., Sano et al., 2010
Sano, Y., Furukawa, Y., Takahata, N. (2010) Atmospheric helium isotope ratio: Possible temporal and spatial variations. Geochimica et Cosmochimica Acta 74, 4893–4901.
). It is therefore of the utmost importance to check if the atmospheric 3He/4He ratio (RAIR) has, or has not, been constant over time. According to Oliver et al. (1984)Oliver, B.M., Bradley, J.G., Farrar IV, H. (1984) Helium concentration in the Earth’s lower atmosphere, Geochimica and Cosmochimica Acta 48, 1759–1767.
, the exploitation of natural gases (NG) could have increased the global atmospheric helium content by 1 to 6 ‰ between 1939 and 1981. Because NG are rich in radiogenic 4He generated in the continental crust, the RAIR could have decreased by ≤3 ‰/yr (Brennwald et al., 2013Brennwald, M.S., Vogel, N., Figura, S., Vollmer, M.K., Langenfelds, R., Steele, L.P., Maden, C., Kipfer, R. (2013) Concentrations and isotope ratios of helium and other noble gases in the earth’s atmosphere during 1978–2011. Earth and Planetary Science Letters 366, 27–37.
and references therein; see also Fig. S-1). In line with this possibility, 3He excesses (~3–4 % relative to the present RAIR value) have been reported for old air trapped in vesicles of blast-furnace metallurgical slags (Pierson-Wickman et al., 2001Pierson-Wickman, A.C., Marty, B., Ploquin, A. (2001) Helium trapped in historical slags: a search for temporal variation of the He isotopic composition of air. Earth and Planetary Science Letters 194, 165–175.
; Sano et al., 2010Sano, Y., Furukawa, Y., Takahata, N. (2010) Atmospheric helium isotope ratio: Possible temporal and spatial variations. Geochimica et Cosmochimica Acta 74, 4893–4901.
) and in ancient porcelains (Matsuda et al., 2010Matsuda, J.I., Matsumoto, T., Suzuki, A. (2010) Helium in old porcelain: The historical variation of the He isotopic composition in air. Geochemical Journal 44, e5–e9.
), suggesting that pre-industrial air contained less 4He than the present-day air. However, these excesses might also be related to: (i) the release of cosmogenic/nucleogenic 3He from the sample matrix; (ii) isotope fractionation during helium extraction and (iii) the capture of fractionated RAIR during manufacturing (Pierson-Wickman et al., 2001Pierson-Wickman, A.C., Marty, B., Ploquin, A. (2001) Helium trapped in historical slags: a search for temporal variation of the He isotopic composition of air. Earth and Planetary Science Letters 194, 165–175.
).The occurrence of temporal variations of the RAIR value has been questioned in several studies. Lupton and Evans (2013)
Lupton, J., Evans, L. (2013) Changes in the atmospheric helium isotope ratio over the past 40 years. Geophysical Research Letters 40, 6271–6275.
did not detect significant variations in Pacific marine air from La Jolla, California, USA (trend of -0.014 ± 0.045 ‰/yr obtained by direct comparison of La Jolla air collected in 1973 and 2013). From the analysis of air sampled in stainless steel bottles in Tasmania since 1978 (Cape Grim Air Archive, CGAA), Mabry et al. (2015)Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
concluded that the RAIR value has been stable (trend of -0.0095 ± 0.0330 ‰/yr) over the last three decades. These authors argued that the mean helium content of globally produced NG has been overestimated by ~3 times in early studies, so that 4He released by NG may not have impacted the RAIR value within the precision of measurements.Here, we aim to constrain temporal variation of RAIR that could be related to the beginning of the commercial helium production in 1921 (Mohr and Ward, 2014
Mohr, S., Ward, J. (2014) Helium Production and Possible Projection. Minerals 4, 130–144.
). To do so, we used large amounts of air trapped in stainless steel materials insuring good preservation of helium over time since 1910. The air volume trapped in our selected samples (≥50 cm3), larger than that trapped in vesicles of slags/porcelains, allows repeated measurements and therefore more precision.top
Methods
Air sample collection. At the same location in the Brabois Park (Villers-lès-Nancy, France), two air samples, called BB, were collected in 2014 and 2016, in stainless steel tanks previously put under vacuum. The BB 2014 air, used as an external standard, has been trapped in a 2.3 L tank. The BB 2016 air was collected in a 500 cm3 tank (Swagelok® 304L-HDF4-500) welded with two stainless steel valves (Swagelok® SS-8BG-TW-VD) placed in series. These valves ensure a safe isolation of the trapped air.
In 500 cm3 tanks, we also subsampled two air samples from CGAA (1978, 1988). Mabry et al. (2015)
Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
have previously analysed the He composition of these two samples, through copper tube subsamples (~10–15 cm3). We selected these samples because: (i) the 1978 sample was collected during the first storage year of CGAA samples, and (ii) the 1988 sample has previously shown a He isotope composition slightly different from those of other measured CGAA samples.We obtained a carburettor float of a vintage Renault car (1910) from the Musée de l’Histoire du Fer (Jarville, France) and pétanque balls (manufactured in 1965, 1990, 2010, 2013) from the OBUT® company (France). These objects have trapped the air at their manufacturing time. The wall and welding of the carburettor float were inspected for possible microfractures, but none was found. The pétanque balls are made of two half-spheres that trapped ~150 cm3 of air when welded together. The thickness of the ball walls (~6 mm), observed on X-ray photography, minimises potential fractionation effects linked to microcrack formation. No evidence of leaking was found for the two oldest samples (See Supplementary Information).
RAIR measurements. Two stainless steel chambers have been designed to pierce the welded containers (i.e. pétanque balls, float reservoir, respectively). They are both composed of a cylindrical volume in which each container is placed, a variable bellows in which a sharp metal tip is positioned, a Pirani gauge to monitor the pressure, and a manual valve to isolate the chamber (Fig. 1). Their cylindrical volumes and tips were designed to fit the respective sizes of the welded containers. The balls were first abraded to obtain a thin flat surface, easier to pierce with the tip. Each container was placed in its respective chamber, which was closed and pre-evacuated down to ~10-7 mbar using a turbomolecular pump. After isolation from the pumping system, the tip under the bellows was pressed to the container’s wall in order to pierce it using an external hydraulic press (KSC-10 model, Quiri Hydromécanique). The trapped air released in the chamber was left equilibrating to obtain a stable pressure. The connection between the closed chamber and the purification line were baked and degassed for a few hours before each analysis.

Figure 1 (a) Stainless steel chamber. (b) Sectional view of the chamber. (c) Hydraulic press KSC-10. (d) Pétanque ball pierced. (e) Carburettor float.
For each complete analysis (lasting 9 hours), about 15–20 cm3 of an air sample (i.e. pétanque ball, float reservoir, BB, CGAA) was subsampled and purified with a combination of active charcoal traps held at liquid nitrogen temperature and getters. For the welded containers, the air was transferred into the purification line using a tube and an adjustable bellows connecting the chamber to the line (Fig. 1c). Following the purification, we used a sample-standard bracketing method, which consisted of measuring multiple aliquots of an air sample alternately with an internal He standard. Simultaneous measurements of 3He and 4He have been achieved with a split flight tube mass spectrometer (Helix SFT, Thermo Fisher ScientificTM). More information about the purification procedure and the analysis are available in Mabry et al. (2013)
Mabry, J., Lan, T., Burnard, P., Marty, B. (2013) High-precision helium isotope measurements in air. Journal of Analytical Atomic Spectrometry 28, 1903–1910.
and in the Supplementary Information.For each analysis, we normalised the He isotopic composition of the air sample (R) to the average composition of our external standard BB (RBB2014) given its long term reproducibility (RBB2014 =1.0300 ± 0.0014 at 95 % CI; number of complete analysis = N = 26). The internal standard is fractionated relative to BB air since the beginning of its preparation (Mabry et al., 2013
Mabry, J., Lan, T., Burnard, P., Marty, B. (2013) High-precision helium isotope measurements in air. Journal of Analytical Atomic Spectrometry 28, 1903–1910.
). In the following discussion, all data and related estimations are given at the 95 % confidence interval (CI) level.top
Result and Discussion
RAIR from France (BB tanks, pétanque balls, carburettor float reservoir) and Cape Grim (CGAA). The normalised isotope ratios (RBB = R/RBB2014) of each complete analysis done on air trapped in tanks (CGAA, BB), pétanque balls (B) and in the carburettor float reservoir (CAR) are reported in Table S-1. Each x̅ value represents the error-weighted mean of the RBB ratios obtained for one air sample, with its related uncertainty (Table S-1). Figure 2 presents the results of Mabry et al. (2015)
Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
on CGAA as x̅ values when more than one RBB has been measured, our x̅ values and the results of Lupton and Evans (2013)Lupton, J., Evans, L. (2013) Changes in the atmospheric helium isotope ratio over the past 40 years. Geophysical Research Letters 40, 6271–6275.
on Pacific marine air.
Figure 2 Error-weighted mean (x̅) of the He isotope ratios, expressed as RBB (diamond points; CG = Cape Grim; B = pétanque ball, CAR = carburettor), over time. Mabry et al. (2015)
Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
data (pink dots) are also normalised to BB air. Lupton and Evans (2013)Lupton, J., Evans, L. (2013) Changes in the atmospheric helium isotope ratio over the past 40 years. Geophysical Research Letters 40, 6271–6275.
data (blue squares) are normalised to 2013 La Jolla air (California, USA). The weighted linear regression fits 1, 2 and 3 are obtained using data of Lupton and Evans (2013)Lupton, J., Evans, L. (2013) Changes in the atmospheric helium isotope ratio over the past 40 years. Geophysical Research Letters 40, 6271–6275.
, Mabry et al. (2015)Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
and the combination of our data with those of Mabry et al. (2015)Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
, respectively. The uncertainty envelope of fit 3 is shown in grey shaded area. Results are reported at 95 % CI (equivalent to 2σ).1. The CGAA (1978, 1988). The x̅ value of the CGAA samples (CG1978, CG1988) are consistent with those previously reported (x̅1978: a = 1.0000 ± 0.0018, b = 1.0009 ± 0.0011; x̅1988: a = 0.9802 ± 0.0028, b = 0.9780 ± 0.0017; [a] this study, [b] Mabry et al., 2015
Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
). This confirms that (i) the CG1988 sample presents a substantially lower He isotope ratio, and that (ii) both the copper tubes and stainless steel reservoirs well preserved the initial He signature. For the anomalous CG1988 air, trace species (CO2, CH4, N2O) measured in its parent tank through time do not show variation since its collection in 1988. However, its composition is anomalous with respect to other records from Cape Grim, including another CGAA sample collected on the same day (Langenfelds et al., 1996Langenfelds, R.L., Francey, R.J., Steele, L.P., Keeling, R.F., Bender, M.L., Battle, M., Budd, W.F. (1996) Measurements of O2/N2 ratio from the Cape Grim Air Archive and three independent flask sampling programs. In: Gras, J.L., Derek, N., Tindale, N.W., A.L. Dick (Eds.) Baseline Atmospheric Program Australia, Bureau of Meteorology and CSIRO, Melbourne, 57–70.
). Its relative depletion in CO2 (1 ppm, ~ 2.8 ‰), CH4 (36 ppb, ~ 21 ‰) and N2O (2 ppb, ~ 6.5 ‰) suggests a sampling artefact, the nature of which has not been established.2. Air sampled in France (1910, 1965, 1990, 2010, 2013, 2016). The He isotopic composition of BB 2014 air is consistent with that of 2016 (RBB2016/RBB2014 = 1.0002 ± 0.0019, N = 26 for each sample). The He composition of BB air is also comparable to that of air trapped in recent balls, which indicates no detectable fractionation effects due to the manufacturing environment. It is also comparable to that of air trapped in the float reservoir CAR1910 (x̅ = 0.9974 ± 0.0036) and to all French air samples taking together (weighted mean of x̅ = 0.9991 ± 0.0034). The weighted least squares regression fit of the French air x̅ data (B1965, B1990, B2010, B2013, CAR1910) yields a slope of +0.019 ± 0.042 ‰/yr over the period 1910–2016 (+2.0 ± 4.5 ‰ over 106 years; Fig. 2). Therefore, we could not detect significant RAIR variations within the range of uncertainties.
No evidence of RAIR variation since 1910. The air samples of Tasmania and France have indistinguishable helium isotope compositions, whatever the sampling periods (Table S-1; Fig. 2). When including the Mabry et al. (2015)
Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
data for CGAA (excluding the anomalous results from 1988 and 2004 samples but including the 1984 air sample corrected for effusion; see Mabry et al., 2015Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
), the temporal trend is +0.002 ± 0.024 ‰/yr between 1910 and 2016 (Fig. 2). This trend is consistent with those of Mabry et al. (2015)Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
and Lupton and Evans (2013)Lupton, J., Evans, L. (2013) Changes in the atmospheric helium isotope ratio over the past 40 years. Geophysical Research Letters 40, 6271–6275.
within uncertainties.NG and nuclear tests impacts on RAIR. The global He production from NG has been estimated to be ~954 kilotons over a 79-year span (1935–2014; U.S. Geological Survey, 2014
U.S. Geological Survey (2014) Helium statistics. In: Kelly, T.D., Matos, G.R. (Comps.) Historical statistics for mineral and material commodities in the United States: U.S. Geological Survey Data Series 140. Accessed 11 July 2017, at http://minerals.usgs.gov/minerals/pubs/historical-statistics/.
) or ~789 kilotons over a 91-year span (1921–2012; Mohr and Ward, 2014Mohr, S., Ward, J. (2014) Helium Production and Possible Projection. Minerals 4, 130–144.
). Assuming that NG helium is purely radiogenic, these amounts would correspond to ~2.0–2.4 × 1011 moles of 4He. If such helium was globally released and homogenised in the atmosphere (4He atmospheric inventory: 9.3 × 1014 mol), the RAIR could have decreased by ~0.22 ‰ (1921–2012; Mohr and Ward, 2014Mohr, S., Ward, J. (2014) Helium Production and Possible Projection. Minerals 4, 130–144.
; Mabry et al. 2015Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
) or ~0.26 ‰ (1935–2014; U.S. Geological Survey, 2014U.S. Geological Survey (2014) Helium statistics. In: Kelly, T.D., Matos, G.R. (Comps.) Historical statistics for mineral and material commodities in the United States: U.S. Geological Survey Data Series 140. Accessed 11 July 2017, at http://minerals.usgs.gov/minerals/pubs/historical-statistics/.
). Such estimates are rough since the global He production of NG (i) includes an amount of stocked helium not released into the atmosphere; (ii) neglects an unknown amount of He lost into the air by venting/extraction during its production (e.g., Mohr and Ward, 2014Mohr, S., Ward, J. (2014) Helium Production and Possible Projection. Minerals 4, 130–144.
) (iii) neglects the poorly defined average He composition (some are rich in 3He; e.g., Pinti and Marty, 2000Pinti, D.L., Marty, B. (2000) Chapter 7. Noble gases in oil and gas fields: origins and processes. In: Kyser, K. (Ed). Fluids and Basin Evolution, Mineralogical Association of Canada Short Course 28, 160–196.
).Another potential anthropogenic source of He isotopes is the production of 3H by nuclear tests and industry (Lupton and Evans, 2004
Lupton, J., Evans, L. (2004) The atmospheric helium isotope ratio: Is it changing? Geophysical Research Letters 31, 1–4.
), as 3H decays to 3He with a half-life of 12.32 ± 0.02 yr (Lucas and Unterweger, 2000Lucas, L.L., Unterweger, M.P. (2000) Comprehensive review and critical evaluation of the half-life of Tritium. Journal of Research of the National Institute of Standards and Technology 105, 541.
). 4He from NG exploitation might have been partly counter-balanced by that of 3He produced by nuclear tests. Over the period 1945–1963, the natural tritium background of ~ 3.6 kg has been upset by the input of ~560 kg of tritium from weapon tests in the atmosphere (Guétat et al., 2008Guétat, P., Douche, C., Hubinois, J.C. (2008) Le tritium et l’environnement: sources, mesures et transferts. Radioprotection 43, 547– 569.
). By 2008, only 40 kg of anthropogenic 3H remained in the environment, indicating that ~520 kg of 3H has decayed in ~1.7 × 105 mol of 3He. Over the period 1945–2008, the RAIR might have increased by ~0.13 ‰ assuming efficient mixing of 3He in the atmosphere (3He atmospheric inventory: 1.29 × 109 mol). This estimate neglects the amount of 3H produced by the nuclear industry (∼0.06 kg yr-1; Belot et al., 1996Belot, Y., Roy, M., Metivier, H. (1996) Le tritium de l’environnement à l’homme. Éditions de Physique, Paris.
).top
Conclusion
Cape Grim (Tasmania, Australia) and French air samples show a similar He isotope composition. We found no evidence of RAIR variation in France, between 1910 and 2016 (trend of 0.019 ± 0.042 ‰/yr; 2σ). From the combination of previous CGAA results reported by Mabry et al. (2015)
Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
with the results of this study, we determine a trend of 0.002 ± 0.024 ‰/yr over the same period of time (0.21 ± 2.54 ‰ over 106 years). This trend is coherent with the potential RAIR variations from 4He produced by NG and 3He derived from nuclear tests, which could have partly balanced each other out. The global helium production from NG could have lowered RAIR by ~0.22 ‰ over 91 years (Mabry et al., 2015Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
), while the release of tritiogenic 3He by nuclear tests (1940s–1980s) could have increased this ratio by ~0.13 ‰. Exploring the recent cycle of helium isotopes in air will require improved analytical precision by at least one order of magnitude.top
Acknowledgements
This work was supported by the European Research Council (grant No. 267255) and the Deep Carbon Observatory (DCO). Christian France-Lanord and the Musée de l’Histoire du Fer (Jarville, France) are thanked for providing the Renault 1910 float reservoir. The OBUT company and its technical director M. André Dupuy are also gratefully thanked for providing the pétanque balls. We are grateful to the Cape Grim and CSIRO staff, supported by the Australian Bureau of Meteorology, who over many years have been involved in the collection and management of the CGAA. This work is dedicated to our colleague and friend Pete Burnard.
Editor: Bruce Watson
top
References
Belot, Y., Roy, M., Metivier, H. (1996) Le tritium de l’environnement à l’homme. Éditions de Physique, Paris.
 Show in context
Show in context This estimate neglects the amount of 3H produced by the nuclear industry (∼0.06 kg yr-1; Belot et al., 1996).
View in article
Brennwald, M.S., Vogel, N., Figura, S., Vollmer, M.K., Langenfelds, R., Steele, L.P., Maden, C., Kipfer, R. (2013) Concentrations and isotope ratios of helium and other noble gases in the earth’s atmosphere during 1978–2011. Earth and Planetary Science Letters 366, 27–37.
 Show in context
Show in context Because NG are rich in radiogenic 4He generated in the continental crust, the RAIR could have decreased by ≤3 ‰/yr (Brennwald et al., 2013 and references therein; see also Fig. S-1).
View in article
Guétat, P., Douche, C., Hubinois, J.C. (2008) Le tritium et l’environnement: sources, mesures et transferts. Radioprotection 43, 547– 569.
 Show in context
Show in context Over the period 1945–1963, the natural tritium background of ~ 3.6 kg has been upset by the input of ~560 kg of tritium from weapon tests in the atmosphere (Guétat et al., 2008)
View in article
Langenfelds, R.L., Francey, R.J., Steele, L.P., Keeling, R.F., Bender, M.L., Battle, M., Budd, W.F. (1996) Measurements of O2/N2 ratio from the Cape Grim Air Archive and three independent flask sampling programs. In: Gras, J.L., Derek, N., Tindale, N.W., A.L. Dick (Eds.) Baseline Atmospheric Program Australia. Bureau of Meteorology and CSIRO, Melbourne, 57–70.
 Show in context
Show in context However, its composition is anomalous with respect to other records from Cape Grim, including another CGAA sample collected on the same day (Langenfelds et al., 1996)
View in article
Lucas, L.L., Unterweger, M.P. (2000) Comprehensive review and critical evaluation of the half-life of Tritium. Journal of Research of the National Institute of Standards and Technology 105, 541.
 Show in context
Show in context Another potential anthropogenic source of He isotopes is the production of 3H by nuclear tests and industry (Lupton and Evans, 2004), as 3H decays to 3He with a half-life of 12.32 ± 0.02 yr (Lucas and Unterweger, 2000).
View in article
Lupton, J., Evans, L. (2004) The atmospheric helium isotope ratio: Is it changing? Geophysical Research Letters 31, 1–4.
 Show in context
Show in context Another potential anthropogenic source of He isotopes is the production of 3H by nuclear tests and industry (Lupton and Evans, 2004), as 3H decays to 3He with a half-life of 12.32 ± 0.02 yr (Lucas and Unterweger, 2000).
View in article
Lupton, J., Evans, L. (2013) Changes in the atmospheric helium isotope ratio over the past 40 years. Geophysical Research Letters 40, 6271–6275.
 Show in context
Show in context Lupton and Evans (2013) did not detect significant variations in Pacific marine air from La Jolla, California, USA (trend of -0.014 ± 0.045 ‰/yr obtained by direct comparison of La Jolla air collected in 1973 and 2013).
View in article
Figure 2 presents the results of Mabry et al. (2015) on CGAA as x̅ values when more than one RBB has been measured, our x̅ values and the results of Lupton and Evans (2013) on Pacific marine air.
View in article
Figure 2 [...] Lupton and Evans (2013) data (blue squares) are normalised to 2013 La Jolla air (California, USA).
View in article
Figure 2 [...] The weighted linear regression fits 1, 2 and 3 are obtained using data of Lupton and Evans (2013), Mabry et al. (2015) and the combination of our data with those of Mabry et al. (2015), respectively.
View in article
This trend is consistent with those of Mabry et al. (2015) and Lupton and Evans (2013) within uncertainties.
View in article
Mabry, J., Lan, T., Burnard, P., Marty, B. (2013) High-precision helium isotope measurements in air. Journal of Analytical Atomic Spectrometry 28, 1903–1910.
 Show in context
Show in contextMore information about the purification procedure and the analysis are available in Mabry et al. (2013) and in the Supplementary Information.
View in article
The internal standard is fractionated relative to BB air since the beginning of its preparation (Mabry et al., 2013).
View in article
Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
 Show in context
Show in contextFrom the analysis of air sampled in stainless steel bottles in Tasmania since 1978 (Cape Grim Air Archive, CGAA), Mabry et al. (2015) concluded that the RAIR value has been stable (trend of -0.0095 ± 0.0330 ‰/yr) over the last three decades.
View in article
Mabry et al. (2015) have previously analysed the He composition of these two samples, through copper tube subsamples (~10–15 cm3).
View in article
Figure 2 presents the results of Mabry et al. (2015) on CGAA as x̅ values when more than one RBB has been measured, our x̅ values and the results of Lupton and Evans (2013) on Pacific marine air.
View in article
Figure 2 [...] Mabry et al. (2015) data (pink dots) are also normalised to BB air.
View in article
Figure 2 [...] The weighted linear regression fits 1, 2 and 3 are obtained using data of Lupton and Evans (2013), Mabry et al. (2015) and the combination of our data with those of Mabry et al. (2015), respectively.
View in article
The x̅ value of the CGAA samples (CG1978, CG1988) are consistent with those previously reported (x̅1978: a = 1.0000 ± 0.0018, b = 1.0009 ± 0.0011; x̅1988: a = 0.9802 ± 0.0028, b = 0.9780 ± 0.0017; [a] this study, [b] Mabry et al., 2015).
View in article
When including the Mabry et al. (2015) data for CGAA (excluding the anomalous results from 1988 and 2004 samples but including the 1984 air sample corrected for effusion; see Mabry et al., 2015), the temporal trend is +0.002 ± 0.024 ‰/yr between 1910 and 2016 (Fig. 2).
View in article
This trend is consistent with those of Mabry et al. (2015) and Lupton and Evans (2013) within uncertainties.
View in article
If such helium was globally released and homogenised in the atmosphere (4He atmospheric inventory: 9.3 × 1014 mol), the RAIR could have decreased by ~0.22 ‰ (1921–2012; Mohr and Ward, 2014; Mabry et al. 2015) or ~0.26 ‰ (1935–2014; U.S. Geological Survey, 2014).
View in article
From the combination of previous CGAA results reported by Mabry et al. (2015) with the results of this study, we determine a trend of 0.002 ± 0.024 ‰/yr over the same period of time (0.21 ± 2.54 ‰ over 106 years).
View in article
The global helium production from NG could have lowered RAIR by ~0.22 ‰ over 91 years (Mabry et al., 2015), while the release of tritiogenic 3He by nuclear tests (1940s–1980s) could have increased this ratio by ~0.13 ‰.
View in article
Matsuda, J.I., Matsumoto, T., Suzuki, A. (2010) Helium in old porcelain: The historical variation of the He isotopic composition in air. Geochemical Journal 44, e5–e9.
 Show in context
Show in contextIn line with this possibility, 3He excesses (~3–4 % relative to the present RAIR value) have been reported for old air trapped in vesicles of blast-furnace metallurgical slags (Pierson-Wickman et al., 2001; Sano et al., 2010) and in ancient porcelains (Matsuda et al., 2010), suggesting that pre-industrial air contained less 4He than the present-day air.
View in article
Mohr, S., Ward, J. (2014) Helium Production and Possible Projection. Minerals 4, 130–144.
 Show in context
Show in context Here, we aim to constrain temporal variation of RAIR that could be related to the beginning of the commercial helium production in 1921 (Mohr and Ward, 2014).
View in article
The global He production from NG has been estimated to be ~954 kilotons over a 79-year span (1935–2014; U.S. Geological Survey, 2014) or ~789 kilotons over a 91-year span (1921–2012; Mohr and Ward, 2014).
View in article
If such helium was globally released and homogenised in the atmosphere (4He atmospheric inventory: 9.3 × 1014 mol), the RAIR could have decreased by ~0.22 ‰ (1921–2012; Mohr and Ward, 2014; Mabry et al. 2015) or ~0.26 ‰ (1935–2014; U.S. Geological Survey, 2014).
View in article
Such estimates are rough since the global He production of NG (i) includes an amount of stocked helium not released into the atmosphere; (ii) neglects an unknown amount of He lost into the air by venting/extraction during its production (e.g., Mohr and Ward, 2014) (iii) neglects the poorly defined average He composition (some are rich in 3He; e.g., Pinti and Marty, 2000).
View in article
Oliver, B.M., Bradley, J.G., Farrar IV, H. (1984) Helium concentration in the Earth’s lower atmosphere, Geochimica and Cosmochimica Acta 48, 1759–1767.
 Show in context
Show in context According to Oliver et al. (1984), the exploitation of natural gases (NG) could have increased the global atmospheric helium content by 1 to 6 ‰ between 1939 and 1981.
View in article
Pierson-Wickman, A.C., Marty, B., Ploquin, A. (2001) Helium trapped in historical slags: a search for temporal variation of the He isotopic composition of air. Earth and Planetary Science Letters 194, 165–175.
 Show in context
Show in contextIn line with this possibility, 3He excesses (~3–4 % relative to the present RAIR value) have been reported for old air trapped in vesicles of blast-furnace metallurgical slags (Pierson-Wickman et al., 2001; Sano et al., 2010) and in ancient porcelains (Matsuda et al., 2010), suggesting that pre-industrial air contained less 4He than the present-day air.
View in article
However, these excesses might also be related to: (i) the release of cosmogenic/nucleogenic 3He from the sample matrix; (ii) isotope fractionation during helium extraction and (iii) the capture of fractionated RAIR during manufacturing (Pierson-Wickman et al., 2001).
View in article
Pinti, D.L., Marty, B. (2000) Chapter 7. Noble gases in oil and gas fields: origins and processes. In: Kyser, K. (Ed). Fluids and Basin Evolution, Mineralogical Association of Canada Short Course 28, 160–196.
 Show in context
Show in contextSuch estimates are rough since the global He production of NG (i) includes an amount of stocked helium not released into the atmosphere; (ii) neglects an unknown amount of He lost into the air by venting/extraction during its production (e.g., Mohr and Ward, 2014) (iii) neglects the poorly defined average He composition (some are rich in 3He; e.g., Pinti and Marty, 2000).
View in article
Sano, Y., Furukawa, Y., Takahata, N. (2010) Atmospheric helium isotope ratio: Possible temporal and spatial variations. Geochimica et Cosmochimica Acta 74, 4893–4901.
 Show in context
Show in context Atmospheric helium is a trace gas used as an international standard, whose abundance and isotope composition may be impacted by anthropogenic activities (e.g., Sano et al., 2010).
View in article
In line with this possibility, 3He excesses (~3–4 % relative to the present RAIR value) have been reported for old air trapped in vesicles of blast-furnace metallurgical slags (Pierson-Wickman et al., 2001; Sano et al., 2010) and in ancient porcelains (Matsuda et al., 2010), suggesting that pre-industrial air contained less 4He than the present-day air.
View in article
U.S. Geological Survey (2014) Helium statistics. In: Kelly, T.D., Matos, G.R. (Comps.) Historical statistics for mineral and material commodities in the United States: U.S. Geological Survey Data Series 140. Accessed 11 July 2017, at http://minerals.usgs.gov/minerals/pubs/historical-statistics/.
 Show in context
Show in context The global He production from NG has been estimated to be ~954 kilotons over a 79-year span (1935–2014; U.S. Geological Survey, 2014) or ~789 kilotons over a 91-year span (1921–2012; Mohr and Ward, 2014).
View in article
If such helium was globally released and homogenised in the atmosphere (4He atmospheric inventory: 9.3 × 1014 mol), the RAIR could have decreased by ~0.22 ‰ (1921–2012; Mohr and Ward, 2014; Mabry et al. 2015) or ~0.26 ‰ (1935–2014; U.S. Geological Survey, 2014).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Historical Data
- Global Helium Production Database
- He Isotopic Analyses and Results
- Sample Quality
- Table S-1
- Figures S-1 to S-3
- Supplementary Information References
Figures and Tables
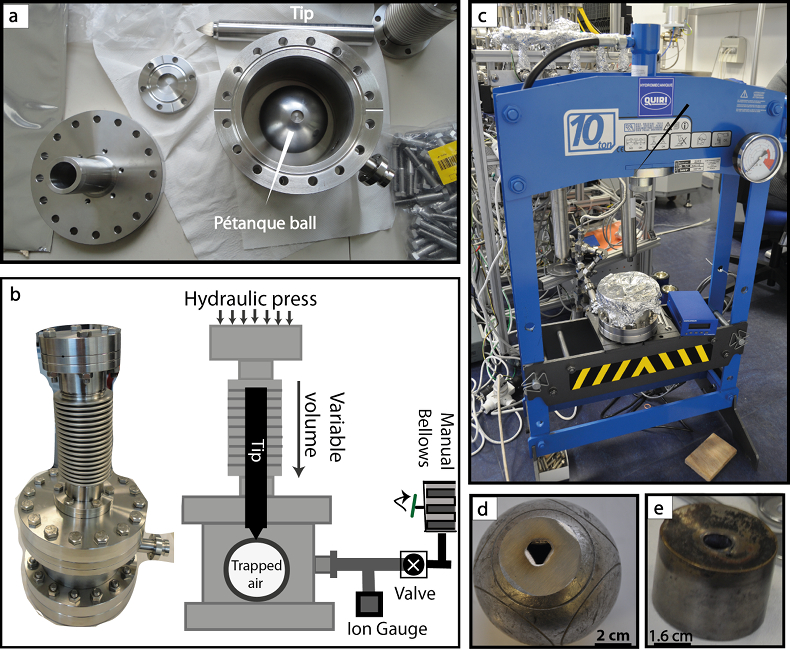
Figure 1 (a) Stainless steel chamber. (b) Sectional view of the chamber. (c) Hydraulic press KSC-10. (d) Pétanque ball pierced. (e) Carburettor float.
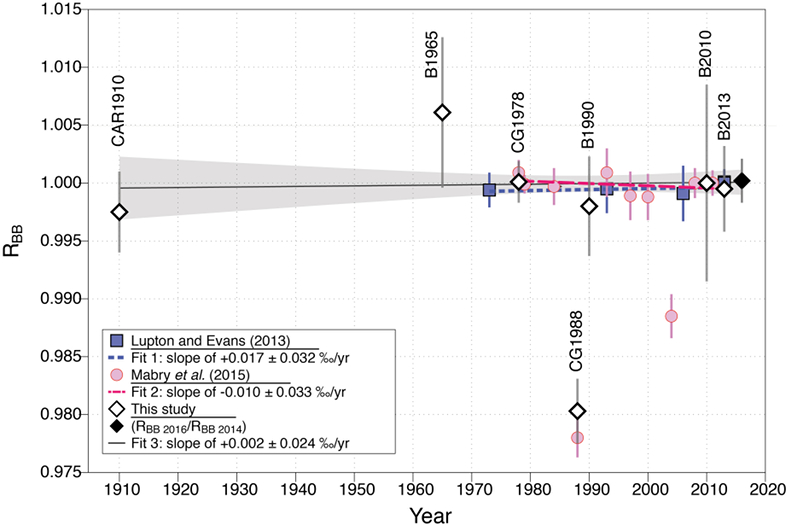
Figure 2 Error-weighted mean (x̅) of the He isotope ratios, expressed as RBB (diamond points; CG = Cape Grim; B = pétanque ball, CAR = carburettor), over time. Mabry et al. (2015)
Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
data (pink dots) are also normalised to BB air. Lupton and Evans (2013)Lupton, J., Evans, L. (2013) Changes in the atmospheric helium isotope ratio over the past 40 years. Geophysical Research Letters 40, 6271–6275.
data (blue squares) are normalised to 2013 La Jolla air (California, USA). The weighted linear regression fits 1, 2 and 3 are obtained using data of Lupton and Evans (2013)Lupton, J., Evans, L. (2013) Changes in the atmospheric helium isotope ratio over the past 40 years. Geophysical Research Letters 40, 6271–6275.
, Mabry et al. (2015)Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
and the combination of our data with those of Mabry et al. (2015)Mabry, J., Lan, T., Boucher, C., Burnard, P., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the cape grim air archive. Earth and Planetary Science Letters 428, 134–138.
, respectively. The uncertainty envelope of fit 3 is shown in grey shaded area. Results are reported at 95 % CI (equivalent to 2σ).