Silicon and oxygen isotopes unravel quartz formation processes in the Icelandic crust
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:5,412Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
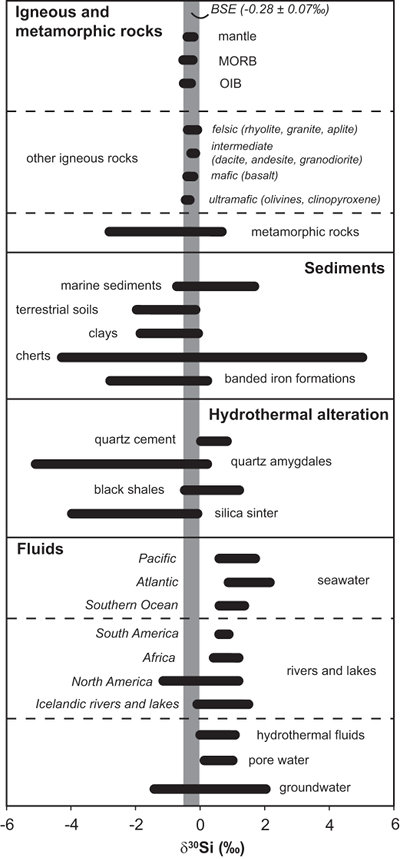 Figure 1 Compilation of δ30Si values for different rock types and fluids. Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016). BSE = bulk silica earth (see Supplementary Information for more details). | 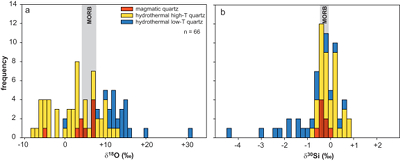 Figure 2 Variation in (a) δ18O and (b) δ30Si values of magmatic quartz (>550 °C), hydrothermal high-temperature (~200-400 °C) quartz and low-temperature (<150 °C) quartz and other silica polymorphs. The isotopic values are compared to δ18O and δ30Si values for MORB (Eiler et al., 2000; Savage et al., 2010). | 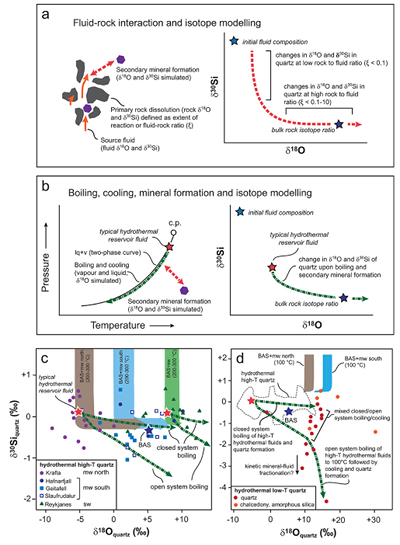 Figure 3 The conceptual chemical and isotope model involves (a) fluid-rock interaction with various source fluids and (b) boiling followed by cooling and mineral formation; (c) isotope systematics in hydrothermal high-temperature quartz largely match the predicted reaction path for progressive fluid-rock interaction (shaded areas) and boiling (arrows). δ30Si decreases with increasing rock-fluid ratio (ξ) whereas δ18O values in quartz both depend on the source water and rock-fluid ratio; (d) most of the isotope variations in hydrothermal low-temperature quartz and silica polymorphs can be explained by boiling and cooling of a hydrothermal fluid (arrows). Silica deposits with low δ30Si and constant δ18O values may result from open system boiling of a high-temperature fluid followed by cooling and quartz or opal formation. BAS = basalt, mw = meteoric water, sw = seawater. Analytical uncertainties of δ30Si and δ18O are listed in Tables S-7 and S-8. | 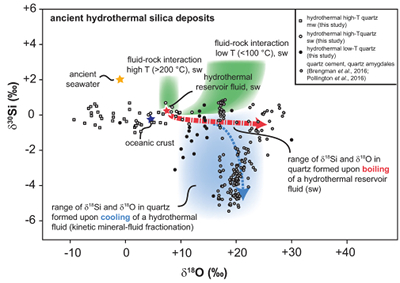 Figure 4 δ30Si versus δ18O values of ancient, hydrothermally altered quartz cement and amygdales (Brengman et al., 2016; Pollington et al., 2016). A highly variable but largely negative range in δ30Si values is likely to result from quartz precipitating out of a boiling and cooling fluid involving kinetic fluid-quartz/opal fractionation during rapid temperature decrease. For modelling, the isotope composition of ancient seawater was used (Marin-Carbonne et al., 2014). mw = meteoric water, sw = seawater. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Quartz is among the most abundant minerals in the continental crust and a major constituent of many plutonic, sedimentary and metamorphic rocks (e.g., Götze, 2009
Götze, J. (2009) Chemistry, textures and physical properties of quartz – geological interpretation and technical application. Mineralogical Magazine 73, 645-671.
). Under magmatic conditions, quartz may crystallise at ~700 °C and numerous metamorphic reactions involve the consumption and production of quartz. Because of its common association with hydrothermal and ore deposits, the origin of quartz and its paragenesis continues to be a subject of considerable discussion.Over the past decades, oxygen isotopes have been applied as a tracer for the source(s) of crustal fluids (e.g., Bowman et al., 1994
Bowman, J.R., Willett, S.D., Cook, S.J. (1994) Oxygen isotopic transport and exchange during fluid flow: one-dimensional models and applications. American Journal of Science 294, 1-55.
). However, Si isotopes have only gained interest recently due to improved analytical techniques. Silicon isotopes of quartz and other silica polymorphs range from -5 to +5 ‰ and have been used for reconstruction of past geological environments (Fig. 1). δ30Si systematics in Precambrian chert deposits have been used to constrain environmental conditions during their formation (e.g., Robert and Chaussidon, 2006Robert, F., Chaussidon, M. (2006) A palaeotemperature curve for the Precambrian oceans based on silicon isotopes in cherts. Nature 443, 969-972.
; Marin-Carbonne et al., 2014Marin-Carbonne, J., Robert, F., Chaussidon, M. (2014) The silicon and oxygen isotope compositions of Precambrian cherts: A record of oceanic paleo-temperatures? Precambrian Research 247, 223-234.
). However, silicon isotope data from more recent quartz deposits formed under variable temperatures and fluid compositions, are still limited (Geilert et al., 2015Geilert, S., Vroon, P.Z., Keller, N.S., Gudbrandsson, S., Stefánsson, A., van Bergen, M.J. (2015) Silicon isotope fractionation during silica precipitation from hot-spring waters: evidence from the Geysir hydrothermal field, Iceland. Geochimica et Cosmochimica Acta 164, 403-427.
). Such data are critical to constrain the processes (e.g., fluid-rock interaction) that lead to the formation of secondary quartz in the Earth’s crust, and can contribute to better understand the origin and formation conditions of ancient silica deposits.
Figure 1 Compilation of δ30Si values for different rock types and fluids. Data taken from Douthitt (1982)
Douthitt, C.B. (1982) The geochemistry of the stable isotopes of silicon. Geochimica et Cosmochimica Acta 46, 1449-1458.
, De La Rocha et al. (2000)De La Rocha, C.L., Brzezinski, M.A., DeNiro, M.J. (2000) A first look at the distribution of the stable isotopes of silicon in natural waters. Geochimica et Cosmochimica Acta 64, 2467-2477.
, André et al. (2006)André, L., Cardinal, D., Alleman, L.Y., Moorbath, S. (2006) Silicon isotopes in ∼3.8 Ga West Greenland rocks as clues to the Eoarchaean supracrustal Si cycle. Earth and Planetary Science Letters 245, 162-173.
, Robert and Chaussidon (2006)Robert, F., Chaussidon, M. (2006) A palaeotemperature curve for the Precambrian oceans based on silicon isotopes in cherts. Nature 443, 969-972.
, Georg et al. (2007aGeorg, R.B., Reynolds, B.C., West, A.J., Burton, K.W., Halliday, A.N. (2007a) Silicon isotope variations accompanying basalt weathering in Iceland. Earth and Planetary Science Letters 261, 476-490.
,bGeorg, R.B., Halliday, A.N., Schauble, E.A., Reynolds, B.C. (2007b) Silicon in the Earth’s core. Nature 447, 1102-1106.
, 2009Georg, R.B., Zhu, C., Reynolds, B.C., Halliday, A.N. (2009) Stable silicon isotopes of groundwater, feldspars, and clay coatings in the Navajo Sandstone aquifer, Black Mesa, Arizona, USA. Geochimica et Cosmochimica Acta 73, 2229-2241.
), Fitoussi et al. (2009)Fitoussi, C., Bourdon, B., Kleine, T., Oberli, F., Reynolds, B.C. (2009) Si isotope systematics of meteorites and terrestrial peridotites: implications for Mg/Si fractionation in the solar nebula and for Si in the Earth's core. Earth and Planetary Science Letters 287, 77-85.
, Steinhoefel, et al. (2009Steinhoefel, G., Horn, I., von Blanckenburg, F. (2009) Micro-scale tracing of Fe and Si isotope signatures in banded iron formation using femtosecond laser ablation. Geochimica et Cosmochimica Acta 73, 5343-5360.
, 2010Steinhoefel, G., von Blanckenburg, F., Horn, I., Konhauser, K.O., Beukes, N.J., Gutzmer, J. (2010) Deciphering formation processes of banded iron formations from the Transvaal and the Hamersley successions by combined Si and Fe isotope analysis using UV femtosecond laser ablation. Geochimica et Cosmochimica Acta 74, 2677-2696.
), Savage et al. (2010Savage, P.S., Georg, R.B., Armytage, R.M.G., Williams, H.M., Halliday, A.N. (2010) Silicon isotope homogeneity in the mantle. Earth and Planetary Science Letters 295, 139-146.
, 2011Savage, P.S., Georg, R.B., Williams, H.M., Burton, K.W., Halliday, A.N. (2011) Silicon isotope fractionation during magmatic differentiation. Geochimica et Cosmochimica Acta 75, 6124-6139.
, 2012Savage, P.S., Georg, R.B., Williams, H.M., Turner, S., Halliday, A.N., Chappell, B.W. (2012) The silicon isotope composition of granites. Geochimica et Cosmochimica Acta 92, 184-202.
), Van den Boorn et al. (2010)Van den Boorn, S.H.J.M., Van Bergen, M.J., Vroon, P.Z., De Vries, S.T., Nijman, W. (2010) Silicon isotope and trace element constraints on the origin of ∼3.5 Ga cherts: implications for Early Archaean marine environments. Geochimica et Cosmochimica Acta 74, 1077-1103.
, Abraham et al. (2011)Abraham, K., Hofmann, A., Foley, S.F., Cardinal, D., Harris, C., Barth, M.G., André, L. (2011) Coupled silicon-oxygen isotope fractionation traces Archaean silicification. Earth and Planetary Science Letters 301, 222-230.
, Armytage et al. (2011)Armytage, R.M.G., Georg, R.B., Savage, P.S., Williams, H.M., Halliday, A.N. (2011) Silicon isotopes in meteorites and planetary core formation. Geochimica et Cosmochimica Acta 75, 3662-3676.
, Heck et al. (2011)Heck, P.R., Huberty, J.M., Kita, N.T., Ushikubo, T., Kozdon, R., Valley, J.W. (2011) SIMS analyses of silicon and oxygen isotope ratios for quartz from Archean and Paleoproterozoic banded iron formations. Geochimica et Cosmochimica Acta 75, 5879-5891.
, Chakrabarti et al. (2012)Chakrabarti, R., Knoll, A.H., Jacobsen, S.B., Fischer, W.W. (2012) Si isotope variability in Proterozoic cherts. Geochimica et Cosmochimica Acta 91, 187-201.
, Delvigne et al. (2012)Delvigne, C., Cardinal, D., Hofmann, A., André, L. (2012) Stratigraphic changes of Ge/Si, REE+ Y and silicon isotopes as insights into the deposition of a Mesoarchaean banded iron formation. Earth and Planetary Science Letters 355, 109-118.
, Marin-Carbonne et al. (2012Marin-Carbonne, J., Chaussidon, M., Robert, F. (2012) Micrometer-scale chemical and isotopic criteria (O and Si) on the origin and history of Precambrian cherts: implications for paleo-temperature reconstructions. Geochimica et Cosmochimica Acta 92, 129-147.
, 2014Marin-Carbonne, J., Robert, F., Chaussidon, M. (2014) The silicon and oxygen isotope compositions of Precambrian cherts: A record of oceanic paleo-temperatures? Precambrian Research 247, 223-234.
), Opfergelt et al. (2012)Opfergelt, S., Georg, R.B., Delvaux, B., Cabidoche, Y.-M., Burton, K.W., Halliday, A.N. (2012) Silicon isotopes and the tracing of desilication in volcanic soil weathering sequences, Guadeloupe. Chemical Geology 326, 113-122.
, von Strandmann et al. (2012)Von Strandmann, P.A.E.P., Opfergelt, S., Lai, Y.-J., Sigfússon, B., Gislason, S.R., Burton, K.W. (2012) Lithium, magnesium and silicon isotope behaviour accompanying weathering in a basaltic soil and pore water profile in Iceland. Earth and Planetary Science Letters 339, 11-23.
, Fan et al. (2013)Fan, H., Wen, H., Zhu, X., Hu, R., Tian, S. (2013) Hydrothermal activity during Ediacaran–Cambrian transition: Silicon isotopic evidence. Precambrian Research 224, 23-35.
, Zambardi et al. (2013)Zambardi, T., Poitrasson, F., Corgne, A., Méheut, M., Quitté, G., Anand, M. (2013) Silicon isotope variations in the inner solar system: Implications for planetary formation, differentiation and composition. Geochimica et Cosmochimica Acta 121, 67-83.
, Geilert et al. (2015)Geilert, S., Vroon, P.Z., Keller, N.S., Gudbrandsson, S., Stefánsson, A., van Bergen, M.J. (2015) Silicon isotope fractionation during silica precipitation from hot-spring waters: evidence from the Geysir hydrothermal field, Iceland. Geochimica et Cosmochimica Acta 164, 403-427.
, Stefurak et al. (2015)Stefurak, E.J.T., Fischer, W.W., Lowe, D.R. (2015) Texture-specific Si isotope variations in Barberton Greenstone Belt cherts record low temperature fractionations in early Archean seawater. Geochimica et Cosmochimica Acta 150, 26-52.
, Brengman et al. (2016)Brengman, L.A., Fedo, C.M., Whitehouse, M.J. (2016) Micro‐scale silicon isotope heterogeneity observed in hydrothermal quartz precipitates from the >3.7 Ga Isua Greenstone Belt, SW Greenland. Terra Nova 28, 70-75.
, Chen et al. (2016)Chen, X., Chafetz, H.S., Andreasen, R., Lapen, T.J. (2016) Silicon isotope compositions of euhedral authigenic quartz crystals: Implications for abiotic fractionation at surface temperatures. Chemical Geology 423, 61-73.
, Pollington et al. (2016)Pollington, A.D., Kozdon, R., Anovitz, L.M., Georg, R.B., Spicuzza, M.J., Valley, J.W. (2016) Experimental calibration of silicon and oxygen isotope fractionations between quartz and water at 250° C by in situ microanalysis of experimental products and application to zoned low δ30Si quartz overgrowths. Chemical Geology 421, 127-142.
. BSE = bulk silica earth (see Supplementary Information for more details).Often adopted as a potential analogue for Earth’s earliest continental crust (Reimink et al., 2014
Reimink, J.R., Chacko, T., Stern, R.A., Heaman, L.M. (2014) Earth's earliest evolved crust generated in an Iceland-like setting. Nature Geoscience 7, 529-533.
), the Icelandic crust represents a continental-type crust with characteristics typical of the oceanic crust. Iceland’s crustal lithology is dominated by basalts with silicic volcanics and volcaniclastic sediments also being common (Sæmundsson, 1979Sæmundsson, K. (1979) Outline of the geology of Iceland. Jökull 29, 7-28.
). Hydrothermal activity occurs over a wide temperature range (<10 to >400 °C; Arnórsson et al., 2008Arnórsson, S., Axelsson, G., Sæmundsson, K. (2008) Hydrothermal systems in Iceland. Jökull 58, 269-302.
), resulting in low-grade metamorphism within the crust (Kristmannsdóttir, 1979Kristmannsdóttir, H. (1979) Alteration of Basaltic Rocks by Hydrothermal-Activity at 100-300° C. Developments in sedimentology 27, 359-367.
). Quartz is a common secondary mineral in hydrothermal systems whereas primary quartz is not common within the dominantly basaltic crust (Browne, 1978Browne, P.R.L. (1978) Hydrothermal alteration in active geothermal fields. Annual Review of Earth and Planetary Sciences 6, 229-250.
). This makes Iceland an ideal locality for studying secondary quartz formation processes taking place in the crust.To assess quartz formation processes over a wide temperature range (<150 to >550 °C), δ18O and δ30Si isotopic and chemical composition in quartz and silica polymorphs from magmatic and hydrothermal settings in Iceland were studied using secondary ion mass spectrometry (SIMS) and electron microprobe analysis (EMPA). Applying isotope modelling approaches (Stefánsson et al., 2017
Stefánsson, A., Hilton, D.R., Sveinbjörnsdóttir, Á.E., Torssander, P., Heinemeier, J., Barnes, J.D., Ono, S., Halldórsson, S.A., Fiebig, J., Arnórsson, S. (2017) Isotope systematics of Icelandic thermal fluids. Journal of Volcanology and Geothermal Research 337, 146-164.
), fluid sources, chemical reactions and associated isotope fractionations were quantified for various processes occurring in the curst, including fluid-rock interaction, fluid phase separation (boiling) and cooling. Implications of this dataset and the isotope modelling were further extended to explain the origin of ancient hydrothermal silica deposits.top
Methods and Results
Quartz-bearing samples were collected from multiple locations throughout Iceland (Fig. S-1) including (1) magmatic (primary) quartz (n = 9) associated with crustal xenoliths and micro-granites (>550 °C), (2) hydrothermal (secondary) high-temperature quartz (~200 to 400 °C; n = 48) and (3) hydrothermal low-temperature quartz and silica polymorphs (<150 °C; n = 20). Oxygen (δ18OV-SMOW) and silicon (δ30SiNBS28) isotope composition of quartz and silica polymorphs were analysed in situ using SIMS and trace elements of the same grains using EMPA. Details on sample selection, processing, analysis and results are given in the Supplementary Information (SI).
Oxygen isotopes in magmatic quartz display δ18O values of -5.6 to +6.6 ‰, while silicon isotopes show a very limited range of δ30Si values of -0.7 to -0.2 ‰ (Fig. 2). In contrast, both δ18O (-9.3 to +30.1 ‰) and δ30Si (-4.6 to +0.5 ‰) of hydrothermal quartz display a much greater range. Trace element concentrations of quartz agree with existing quartz data from other magmatic and hydrothermal settings (Götze, 2009
Götze, J. (2009) Chemistry, textures and physical properties of quartz – geological interpretation and technical application. Mineralogical Magazine 73, 645-671.
; Table S-2). Al and Ti in magmatic quartz range from 57 to 77 ppm and 56 to 140 ppm, respectively, while Al and Ti in hydrothermal quartz and silica polymorphs span a wide range (Al = 28-2140 ppm; Ti = 20-80 ppm). Additionally, trace element concentrations in hydrothermal quartz do not show any distinct correlation with δ18O and δ30Si that would allow us to further distinguish different formation conditions (Fig. S-3).
Figure 2 Variation in (a) δ18O and (b) δ30Si values of magmatic quartz (>550 °C), hydrothermal high-temperature (~200-400 °C) quartz and low-temperature (<150 °C) quartz and other silica polymorphs. The isotopic values are compared to δ18O and δ30Si values for MORB (Eiler et al., 2000
Eiler, J.M., Schiano, P., Kitchen, N., Stolper, E.M. (2000) Oxygen isotope evidence for recycled crust in the sources of mid ocean ridge basalts. Nature 403, 530-534.
; Savage et al., 2010Savage, P.S., Georg, R.B., Armytage, R.M.G., Williams, H.M., Halliday, A.N. (2010) Silicon isotope homogeneity in the mantle. Earth and Planetary Science Letters 295, 139-146.
).top
Controls on δ18O and δ30Si in Hydrothermal Quartz
Oxygen and silicon isotope values of both ancient and modern hydrothermal silica deposits vary significantly with δ18O ranging from -10 to +30 ‰ and δ30Si ranging from -5 to +2 ‰ (Geilert et al., 2015
Geilert, S., Vroon, P.Z., Keller, N.S., Gudbrandsson, S., Stefánsson, A., van Bergen, M.J. (2015) Silicon isotope fractionation during silica precipitation from hot-spring waters: evidence from the Geysir hydrothermal field, Iceland. Geochimica et Cosmochimica Acta 164, 403-427.
; Brengman et al., 2016Brengman, L.A., Fedo, C.M., Whitehouse, M.J. (2016) Micro‐scale silicon isotope heterogeneity observed in hydrothermal quartz precipitates from the >3.7 Ga Isua Greenstone Belt, SW Greenland. Terra Nova 28, 70-75.
; Pollington et al., 2016Pollington, A.D., Kozdon, R., Anovitz, L.M., Georg, R.B., Spicuzza, M.J., Valley, J.W. (2016) Experimental calibration of silicon and oxygen isotope fractionations between quartz and water at 250° C by in situ microanalysis of experimental products and application to zoned low δ30Si quartz overgrowths. Chemical Geology 421, 127-142.
; this study). Silica and oxygen are both reactive elements and their isotope ratios in minerals and fluids may change significantly upon crustal processes and associated chemical reactions. The observed wide range in isotopic values of hydrothermal silica deposits highlights the need for quantification of such processes and their effects on the isotope systematics. Processes of importance in hydrothermal systems include fluid-rock interaction, phase separation (boiling) and temperature changes (cooling). Such processes lead to changes in the relative abundance of elements of various sources, e.g., the water to rock ratio, changes in aqueous species and gas concentrations and quantity of secondary minerals formed, which may all influence isotopic characteristics of fluids and minerals (Stefánsson et al., 2017Stefánsson, A., Hilton, D.R., Sveinbjörnsdóttir, Á.E., Torssander, P., Heinemeier, J., Barnes, J.D., Ono, S., Halldórsson, S.A., Fiebig, J., Arnórsson, S. (2017) Isotope systematics of Icelandic thermal fluids. Journal of Volcanology and Geothermal Research 337, 146-164.
).top
Origin of Primary δ18O and δ30Si Characteristics
The δ18O values of igneous rocks in Iceland are generally thought to represent derivation from a mantle source ranging in δ18O from +5 to +6 ‰ (Hemond et al., 1993
Hemond, C., Arndt, N.T., Lichtenstein, U., Hofmann, A.W., Oskarsson, N., Steinthorsson, S. (1993) The heterogeneous Iceland plume: Nd‐Sr‐O isotopes and trace element constraints. Journal of Geophyscal Research: Solid Earth 98, 15833-15850.
). However, as a result of extensive fluid-rock interaction involving a low-18O meteoric water component, strong δ18O-depletions from such a mantle source are commonly observed in Icelandic rocks (Muehlenbachs et al., 1974Muehlenbachs, K., Anderson, A.T., Sigvaldason, G.E. (1974) Low-O18 basalts from Iceland. Geochimica et Cosmochimica Acta 38, 577-588.
). Such fluid-rock interaction likely affected xenoliths from the 1875 Askja eruption from our sample suite that show notable depletion in δ18O (-5.5 ± 0.4 ‰; Fig. 2a).Despite strong δ18O-depletions in the Askja xenoliths, their δ30Si values display only a limited range (Fig. 2b). Indeed, δ30Si values measured in magmatic quartz (-0.75 to -0.19 ‰) in this study agree with values reported for whole rock and glass samples of igneous rocks from Iceland and elsewhere (Figs. 1, 2b; Table S-1). Recent studies showed that the silicon isotopic composition of Earth’s mantle is relatively homogenous (δ30Si = -0.29 ± 0.07 ‰) and not greatly affected by magmatic processes (Savage et al., 2010
Savage, P.S., Georg, R.B., Armytage, R.M.G., Williams, H.M., Halliday, A.N. (2010) Silicon isotope homogeneity in the mantle. Earth and Planetary Science Letters 295, 139-146.
). As a result, the δ30Si values of magmatic quartz (this study) likely represent magmatic values. This implies that the measured δ30Si values have not been affected by interaction of the xenoliths with meteoric water and/or other secondary processes.top
Assessing Secondary Silica Mineralisation Processes in the Icelandic Crust
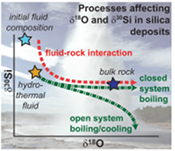
The large range in δ18O and δ30Si values of hydrothermal quartz and silica polymorphs (Fig. 2) are considered to reflect the source(s) of the elements and isotope fractionations associated with hydrothermal processes and reactions. These processes and their effects on the δ18O and δ30Si values in quartz can be quantified using isotope modelling approaches (Fig. 3a,b; detailed description in SI).
Comparison of our dataset with the results of the isotope models reveals that silica in hydrothermal high-temperature quartz predominantly originates from the primary (basaltic) rocks that generally display a limited range in δ30Si values (-0.29 ± 0.07 ‰; Savage et al., 2010
Savage, P.S., Georg, R.B., Armytage, R.M.G., Williams, H.M., Halliday, A.N. (2010) Silicon isotope homogeneity in the mantle. Earth and Planetary Science Letters 295, 139-146.
, Fig. 3c,d). Upon progressive fluid-rock interaction, the silica is dissolved from the rock by the hydrothermal fluid and precipitates as hydrothermal quartz. The model predicts that the observed decrease in δ30Si values (+0.66 to -1.01 ‰) compared to the isotopic values characteristic for basaltic rocks and meteoric water (+0.68 ‰, Georg et al., 2007aGeorg, R.B., Reynolds, B.C., West, A.J., Burton, K.W., Halliday, A.N. (2007a) Silicon isotope variations accompanying basalt weathering in Iceland. Earth and Planetary Science Letters 261, 476-490.
) and/or seawater (+1.16 ‰; De La Rocha et al., 2000De La Rocha, C.L., Brzezinski, M.A., DeNiro, M.J. (2000) A first look at the distribution of the stable isotopes of silicon in natural waters. Geochimica et Cosmochimica Acta 64, 2467-2477.
) results from isotope fractionation upon progressive fluid-rock interaction, boiling and cooling. In contrast, oxygen in quartz has multiple sources with the δ18O values dominated by the source water at low rock-water ratio (ξ < 0.1 mol basalt/kg water) and the primary rock at high rock-water ratio (ξ = 0.1-10 mol basalt/kg water; Figs. 3, S-4). The δ18O value of the source water also varies. Seawater can be approximated to a value of 0 ± 1 ‰ (vSMOW), while meteoric water will depend on the geographical location, with values of -7.5 ± 1 ‰ in the south but -12.5 ± 1 ‰ in the north of Iceland (Árnason, 1976Árnason, B. (1976) Groundwater systems in Iceland traced by deuterium. Vísindafélag Íslendinga 42, 60-66.
).
Figure 3 The conceptual chemical and isotope model involves (a) fluid-rock interaction with various source fluids and (b) boiling followed by cooling and mineral formation; (c) isotope systematics in hydrothermal high-temperature quartz largely match the predicted reaction path for progressive fluid-rock interaction (shaded areas) and boiling (arrows). δ30Si decreases with increasing rock-fluid ratio (ξ) whereas δ18O values in quartz both depend on the source water and rock-fluid ratio; (d) most of the isotope variations in hydrothermal low-temperature quartz and silica polymorphs can be explained by boiling and cooling of a hydrothermal fluid (arrows). Silica deposits with low δ30Si and constant δ18O values may result from open system boiling of a high-temperature fluid followed by cooling and quartz or opal formation. BAS = basalt, mw = meteoric water, sw = seawater. Analytical uncertainties of δ30Si and δ18O are listed in Tables S-7 and S-8.
Hydrothermal low-temperature quartz and silica polymorphs are typically formed by precipitation of dissolved silica upon cooling at or near the surface (e.g., Neuhoff et al., 1999
Neuhoff, P.S., Fridriksson, T., Arnórsson, S., Bird, D.K. (1999) Porosity evolution and mineral paragenesis during low-grade metamorphism of basaltic lavas at Teigarhorn, Eastern Iceland. American Journal of Science 299, 467-501.
; Geilert et al., 2015Geilert, S., Vroon, P.Z., Keller, N.S., Gudbrandsson, S., Stefánsson, A., van Bergen, M.J. (2015) Silicon isotope fractionation during silica precipitation from hot-spring waters: evidence from the Geysir hydrothermal field, Iceland. Geochimica et Cosmochimica Acta 164, 403-427.
). In most cases, the source waters are high-temperature hydrothermal fluids with elevated silica concentrations (Geilert et al., 2015Geilert, S., Vroon, P.Z., Keller, N.S., Gudbrandsson, S., Stefánsson, A., van Bergen, M.J. (2015) Silicon isotope fractionation during silica precipitation from hot-spring waters: evidence from the Geysir hydrothermal field, Iceland. Geochimica et Cosmochimica Acta 164, 403-427.
). The trends observed in Figure 3d are consistent with these silica phases having δ30Si values similar to, or more negative than, the primary host rocks. The depletion of 30Si in the silica minerals cannot be explained by simple fluid-rock interaction and equilibrium fractionation. According to our model, equilibrium fractionation associated with fluid-rock interaction would result in precipitation of quartz with δ30Si values ranging from +2 to +0.5 ‰ and, depending on the fluid source, δ18O values of +10 ± 5 ‰ or +15 ± 5 ‰. Instead, the observed δ18O and δ30Si values follow a trend predicted by boiling of high-temperature hydrothermal fluids to surface (100 °C) followed by cooling (Fig. 3d). The model predicts that the fluid becomes progressively more negative in δ30Si than the corresponding silica minerals. However, recent measurements of silica precipitates and coexisting hydrothermal water suggest the opposite, i.e. that the boiled water is enriched in 30Si relative to the solid precipitate (Geilert et al., 2015Geilert, S., Vroon, P.Z., Keller, N.S., Gudbrandsson, S., Stefánsson, A., van Bergen, M.J. (2015) Silicon isotope fractionation during silica precipitation from hot-spring waters: evidence from the Geysir hydrothermal field, Iceland. Geochimica et Cosmochimica Acta 164, 403-427.
). In line with previous findings (Geilert et al., 2015Geilert, S., Vroon, P.Z., Keller, N.S., Gudbrandsson, S., Stefánsson, A., van Bergen, M.J. (2015) Silicon isotope fractionation during silica precipitation from hot-spring waters: evidence from the Geysir hydrothermal field, Iceland. Geochimica et Cosmochimica Acta 164, 403-427.
; Roerdink et al., 2015Roerdink, D.L., van den Boorn, S.H.J.M., Geilert, S., Vroon, P.Z., van Bergen, M.J. (2015) Experimental constraints on kinetic and equilibrium silicon isotope fractionation during the formation of non-biogenic chert deposits. Chemical Geology 402, 40-51.
; Brengman et al., 2016Brengman, L.A., Fedo, C.M., Whitehouse, M.J. (2016) Micro‐scale silicon isotope heterogeneity observed in hydrothermal quartz precipitates from the >3.7 Ga Isua Greenstone Belt, SW Greenland. Terra Nova 28, 70-75.
), this suggests that fractionation between silica deposits and fluids under low-temperature (<150 °C) hydrothermal conditions is likely controlled by kinetics and may strongly depend on precipitation rates.top
Implications for Hydrothermal Silica deposits in the Geological Record
Coupled δ18O and δ30Si studies of ancient hydrothermal silica are still rare (Brengman et al., 2016
Brengman, L.A., Fedo, C.M., Whitehouse, M.J. (2016) Micro‐scale silicon isotope heterogeneity observed in hydrothermal quartz precipitates from the >3.7 Ga Isua Greenstone Belt, SW Greenland. Terra Nova 28, 70-75.
; Pollington et al., 2016Pollington, A.D., Kozdon, R., Anovitz, L.M., Georg, R.B., Spicuzza, M.J., Valley, J.W. (2016) Experimental calibration of silicon and oxygen isotope fractionations between quartz and water at 250° C by in situ microanalysis of experimental products and application to zoned low δ30Si quartz overgrowths. Chemical Geology 421, 127-142.
). Hydrothermal quartz precipitates such as quartz amygdales encased by quartz cement occur in a brecciated pillow basalt from the Isua Greenstone Belt in SW Greenland and display a broad range of δ30Si values with insignificant variations of δ18O (Brengman et al., 2016Brengman, L.A., Fedo, C.M., Whitehouse, M.J. (2016) Micro‐scale silicon isotope heterogeneity observed in hydrothermal quartz precipitates from the >3.7 Ga Isua Greenstone Belt, SW Greenland. Terra Nova 28, 70-75.
). A similar isotopic trend has been observed in quartz cements of hydrothermal origin within the Cambrian Mt. Simon Formation in central North America (Pollington et al., 2016Pollington, A.D., Kozdon, R., Anovitz, L.M., Georg, R.B., Spicuzza, M.J., Valley, J.W. (2016) Experimental calibration of silicon and oxygen isotope fractionations between quartz and water at 250° C by in situ microanalysis of experimental products and application to zoned low δ30Si quartz overgrowths. Chemical Geology 421, 127-142.
). Such trends have been interpreted as the result of (1) rapid quartz formation from thermal fluids and kinetic isotope fractionation (Brengman et al., 2016Brengman, L.A., Fedo, C.M., Whitehouse, M.J. (2016) Micro‐scale silicon isotope heterogeneity observed in hydrothermal quartz precipitates from the >3.7 Ga Isua Greenstone Belt, SW Greenland. Terra Nova 28, 70-75.
) or (2) quartz cement precipitation from hydrothermal fluids that leached 30Si-depleted silicon from the surrounding highly weathered Precambrian rocks (Pollington et al., 2016Pollington, A.D., Kozdon, R., Anovitz, L.M., Georg, R.B., Spicuzza, M.J., Valley, J.W. (2016) Experimental calibration of silicon and oxygen isotope fractionations between quartz and water at 250° C by in situ microanalysis of experimental products and application to zoned low δ30Si quartz overgrowths. Chemical Geology 421, 127-142.
). The results of the present study indicate that such trends are also typical for open system silica precipitation from a hydrothermal fluid conduit and near-surface cooling, where the chemical and isotope composition of the fluid and silicates changes along the fluid flow path (Fig. 4). Differences in δ18O values between silica deposits may not only result from varying temperature conditions but may also derive from variation in the source water. Furthermore, despite the sedimentary origin of cherts and banded iron formations (BIFs), these rocks have often been exposed to hydrothermal alteration (e.g., Van den Boorn et al., 2007Van den Boorn, S.H.J.M., Van Bergen, M.J., Nijman, W., Vroon, P.Z. (2007) Dual role of seawater and hydrothermal fluids in Early Archean chert: Evidence from silicon isotopes. Geology 35, 939-942.
). Thus, the interaction between seawater, hydrothermal fluids and host rocks might have an effect on the Si and O composition even in those silica deposits.Despite the lack of fractionation factors between fluids and precipitating minerals, this study demonstrates that progressive fluid-rock interaction and the reactions involved may have a strong influence on the oxygen and silicon isotopic characteristics of hydrothermal fluids and associated secondary minerals over a wide temperature range. Fractionation between fluids and minerals of reactive elements like silicon and oxygen are dependent on the source(s) and chemical reactions, aqueous and gas speciation changes as well as secondary mineral formation. Such processes could lead to δ30Si and δ18O variations in hydrothermal silica deposits of >2 ‰ and >20 ‰, respectively, for a system dominated by a single source of both primary rock and water.

Figure 4 δ30Si versus δ18O values of ancient, hydrothermally altered quartz cement and amygdales (Brengman et al., 2016
Brengman, L.A., Fedo, C.M., Whitehouse, M.J. (2016) Micro‐scale silicon isotope heterogeneity observed in hydrothermal quartz precipitates from the >3.7 Ga Isua Greenstone Belt, SW Greenland. Terra Nova 28, 70-75.
; Pollington et al., 2016Pollington, A.D., Kozdon, R., Anovitz, L.M., Georg, R.B., Spicuzza, M.J., Valley, J.W. (2016) Experimental calibration of silicon and oxygen isotope fractionations between quartz and water at 250° C by in situ microanalysis of experimental products and application to zoned low δ30Si quartz overgrowths. Chemical Geology 421, 127-142.
). A highly variable but largely negative range in δ30Si values is likely to result from quartz precipitating out of a boiling and cooling fluid involving kinetic fluid-quartz/opal fractionation during rapid temperature decrease. For modelling, the isotope composition of ancient seawater was used (Marin-Carbonne et al., 2014Marin-Carbonne, J., Robert, F., Chaussidon, M. (2014) The silicon and oxygen isotope compositions of Precambrian cherts: A record of oceanic paleo-temperatures? Precambrian Research 247, 223-234.
). mw = meteoric water, sw = seawater.top
Conclusions
The δ18O and δ30Si systematics of quartz were determined in situ using SIMS to assess crustal quartz formation processes in Iceland. Magmatic quartz records δ18O of -5.6 to +6.6 ‰ and δ30Si of -0.4 ± 0.2 ‰ representative of mantle- and crustally-derived melts in Iceland. Hydrothermal quartz and silica polymorphs (<150 to 400 °C) record a much greater range with δ18O of -9.3 to +30.1 ‰ and δ30Si of -4.6 to +0.7 ‰. Isotope modelling reveals that these large variations in the δ18O and δ30Si values are caused by a combination of processes such as fluid-rock interaction, cooling, boiling and associated changes in aqueous and gas speciation of the fluids as well as type and quantity of secondary minerals formed upon these processes. Comparison of the results of this study suggests that the large ranges observed in δ30Si values and insignificant δ18O variations observed in quartz cements and quartz amygdales of hydrothermal origin may result from silica formation in a hydrothermal fluid conduit at or near the surface associated with cooling. Equilibrium fractionation of δ18O and δ30Si between fluids and minerals seems to prevail at hydrothermal high-temperature conditions (~200-400 °C), whereas kinetic fractionation is likely to influence isotope systematics at lower temperatures.
top
Acknowledgements
This project was financially supported by NordVulk and Landsvirkjun. HS Orka and Landsvirkjun kindly provided access to the drill cuttings. H. Franzson, J.S. Gunnarsdóttir, G.H. Guðfinnsson, S. Harðardóttir, M. Hermanska, L. Ilyinsky, K. Lindén, J. Prikryl and H. Sigurjónsson are thanked for assistance during field work, sample preparation and/or data acquisition. We thank J. Marin-Carbonne and an anonymous reviewer for their constructive reviews. A. Anbar is thanked for careful editorial handling. This is NordSIM contribution 551.
Editor: Ariel Anbar
top
References
Abraham, K., Hofmann, A., Foley, S.F., Cardinal, D., Harris, C., Barth, M.G., André, L. (2011) Coupled silicon-oxygen isotope fractionation traces Archaean silicification. Earth and Planetary Science Letters 301, 222-230.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
André, L., Cardinal, D., Alleman, L.Y., Moorbath, S. (2006) Silicon isotopes in ∼3.8 Ga West Greenland rocks as clues to the Eoarchaean supracrustal Si cycle. Earth and Planetary Science Letters 245, 162-173.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Armytage, R.M.G., Georg, R.B., Savage, P.S., Williams, H.M., Halliday, A.N. (2011) Silicon isotopes in meteorites and planetary core formation. Geochimica et Cosmochimica Acta 75, 3662-3676.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Árnason, B. (1976) Groundwater systems in Iceland traced by deuterium. Vísindafélag Íslendinga 42, 60-66.
 Show in context
Show in context Seawater can be approximated to a value of 0 ± 1 ‰ (vSMOW), while meteoric water will depend on the geographical location, with values of -7.5 ± 1 ‰ in the south but -12.5 ± 1 ‰ in the north of Iceland (Árnason, 1976).
View in article
Arnórsson, S., Axelsson, G., Sæmundsson, K. (2008) Hydrothermal systems in Iceland. Jökull 58, 269-302.
 Show in context
Show in context Hydrothermal activity occurs over a wide temperature range (<10 to >400 °C; Arnórsson et al., 2008), resulting in low-grade metamorphism within the crust (Kristmannsdóttir, 1979).
View in article
Bowman, J.R., Willett, S.D., Cook, S.J. (1994) Oxygen isotopic transport and exchange during fluid flow: one-dimensional models and applications. American Journal of Science 294, 1-55.
 Show in context
Show in context Over the past decades, oxygen isotopes have been applied as a tracer for the source(s) of crustal fluids (e.g., Bowman et al., 1994).
View in article
Brengman, L.A., Fedo, C.M., Whitehouse, M.J. (2016) Micro‐scale silicon isotope heterogeneity observed in hydrothermal quartz precipitates from the >3.7 Ga Isua Greenstone Belt, SW Greenland. Terra Nova 28, 70-75.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Oxygen and silicon isotope values of both ancient and modern hydrothermal silica deposits vary significantly with δ18O ranging from -10 to +30 ‰ and δ30Si ranging from -5 to +2 ‰ (Geilert et al., 2015; Brengman et al., 2016; Pollington et al., 2016; this study).
View in article
In line with previous findings (Geilert et al., 2015; Roerdink et al., 2015; Brengman et al., 2016), this suggests that fractionation between silica deposits and fluids under low-temperature (<150 °C) hydrothermal conditions is likely controlled by kinetics and may strongly depend on precipitation rates.
View in article
Coupled δ18O and δ30Si studies of ancient hydrothermal silica are still rare (Brengman et al., 2016; Pollington et al., 2016).
View in article
Hydrothermal quartz precipitates such as quartz amygdales encased by quartz cement occur in a brecciated pillow basalt from the Isua Greenstone Belt in SW Greenland and display a broad range of δ30Si values with insignificant variations of δ18O (Brengman et al., 2016).
View in article
Such trends have been interpreted as the result of (1) rapid quartz formation from thermal fluids and kinetic isotope fractionation (Brengman et al., 2016) or (2) quartz cement precipitation from hydrothermal fluids that leached 30Si-depleted silicon from the surrounding highly weathered Precambrian rocks (Pollington et al., 2016).
View in article
Figure 4 δ30Si versus δ18O values of ancient, hydrothermally altered quartz cement and amygdales (Brengman et al., 2016; Pollington et al., 2016).
View in article
Browne, P.R.L. (1978) Hydrothermal alteration in active geothermal fields. Annual Review of Earth and Planetary Sciences 6, 229-250.
 Show in context
Show in context Quartz is a common secondary mineral in hydrothermal systems whereas primary quartz is not common within the dominantly basaltic crust (Browne, 1978).
View in article
Chakrabarti, R., Knoll, A.H., Jacobsen, S.B., Fischer, W.W. (2012) Si isotope variability in Proterozoic cherts. Geochimica et Cosmochimica Acta 91, 187-201.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Chen, X., Chafetz, H.S., Andreasen, R., Lapen, T.J. (2016) Silicon isotope compositions of euhedral authigenic quartz crystals: Implications for abiotic fractionation at surface temperatures. Chemical Geology 423, 61-73.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
De La Rocha, C.L., Brzezinski, M.A., DeNiro, M.J. (2000) A first look at the distribution of the stable isotopes of silicon in natural waters. Geochimica et Cosmochimica Acta 64, 2467-2477.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
The model predicts that the observed decrease in δ30Si values (+0.66 to -1.01 ‰) compared to the isotopic values characteristic for basaltic rocks and meteoric water (+0.68 ‰, Georg et al., 2007a) and/or seawater (+1.16 ‰; De La Rocha et al., 2000) results from isotope fractionation upon progressive fluid-rock interaction, boiling and cooling.
View in article
Delvigne, C., Cardinal, D., Hofmann, A., André, L. (2012) Stratigraphic changes of Ge/Si, REE+ Y and silicon isotopes as insights into the deposition of a Mesoarchaean banded iron formation. Earth and Planetary Science Letters 355, 109-118.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Douthitt, C.B. (1982) The geochemistry of the stable isotopes of silicon. Geochimica et Cosmochimica Acta 46, 1449-1458.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Eiler, J.M., Schiano, P., Kitchen, N., Stolper, E.M. (2000) Oxygen isotope evidence for recycled crust in the sources of mid ocean ridge basalts. Nature 403, 530-534.
 Show in context
Show in context Figure 2 [...] The isotopic values are compared to δ18O and δ30Si values for MORB (Eiler et al., 2000; Savage et al., 2010).
View in article
Fan, H., Wen, H., Zhu, X., Hu, R., Tian, S. (2013) Hydrothermal activity during Ediacaran–Cambrian transition: Silicon isotopic evidence. Precambrian Research 224, 23-35.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Fitoussi, C., Bourdon, B., Kleine, T., Oberli, F., Reynolds, B.C. (2009) Si isotope systematics of meteorites and terrestrial peridotites: implications for Mg/Si fractionation in the solar nebula and for Si in the Earth's core. Earth and Planetary Science Letters 287, 77-85.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Geilert, S., Vroon, P.Z., Keller, N.S., Gudbrandsson, S., Stefánsson, A., van Bergen, M.J. (2015) Silicon isotope fractionation during silica precipitation from hot-spring waters: evidence from the Geysir hydrothermal field, Iceland. Geochimica et Cosmochimica Acta 164, 403-427.
 Show in context
Show in context However, silicon isotope data from more recent quartz deposits formed under variable temperatures and fluid compositions, are still limited (Geilert et al., 2015).
View in article
Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Oxygen and silicon isotope values of both ancient and modern hydrothermal silica deposits vary significantly with δ18O ranging from -10 to +30 ‰ and δ30Si ranging from -5 to +2 ‰ (Geilert et al., 2015; Brengman et al., 2016; Pollington et al., 2016; this study).
View in article
Hydrothermal low-temperature quartz and silica polymorphs are typically formed by precipitation of dissolved silica upon cooling at or near the surface (e.g., Neuhoff et al., 1999; Geilert et al., 2015).
View in article
In most cases, the source waters are high-temperature hydrothermal fluids with elevated silica concentrations (Geilert et al., 2015).
View in article
However, recent measurements of silica precipitates and coexisting hydrothermal water suggest the opposite, i.e. that the boiled water is enriched in 30Si relative to the solid precipitate (Geilert et al., 2015).
View in article
In line with previous findings (Geilert et al., 2015; Roerdink et al., 2015; Brengman et al., 2016), this suggests that fractionation between silica deposits and fluids under low-temperature (<150 °C) hydrothermal conditions is likely controlled by kinetics and may strongly depend on precipitation rates.
View in article
Georg, R.B., Reynolds, B.C., West, A.J., Burton, K.W., Halliday, A.N. (2007a) Silicon isotope variations accompanying basalt weathering in Iceland. Earth and Planetary Science Letters 261, 476-490.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
The model predicts that the observed decrease in δ30Si values (+0.66 to -1.01 ‰) compared to the isotopic values characteristic for basaltic rocks and meteoric water (+0.68 ‰, Georg et al., 2007a) and/or seawater (+1.16 ‰; De La Rocha et al., 2000) results from isotope fractionation upon progressive fluid-rock interaction, boiling and cooling.
View in article
Georg, R.B., Halliday, A.N., Schauble, E.A., Reynolds, B.C. (2007b) Silicon in the Earth’s core. Nature 447, 1102-1106.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Georg, R.B., Zhu, C., Reynolds, B.C., Halliday, A.N. (2009) Stable silicon isotopes of groundwater, feldspars, and clay coatings in the Navajo Sandstone aquifer, Black Mesa, Arizona, USA. Geochimica et Cosmochimica Acta 73, 2229-2241.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Götze, J. (2009) Chemistry, textures and physical properties of quartz – geological interpretation and technical application. Mineralogical Magazine 73, 645-671.
 Show in context
Show in context Quartz is among the most abundant minerals in the continental crust and a major constituent of many plutonic, sedimentary and metamorphic rocks (e.g., Götze, 2009).
View in article
Trace element concentrations of quartz agree with existing quartz data from other magmatic and hydrothermal settings (Götze, 2009; Table S-2).
View in article
Heck, P.R., Huberty, J.M., Kita, N.T., Ushikubo, T., Kozdon, R., Valley, J.W. (2011) SIMS analyses of silicon and oxygen isotope ratios for quartz from Archean and Paleoproterozoic banded iron formations. Geochimica et Cosmochimica Acta 75, 5879-5891.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Hemond, C., Arndt, N.T., Lichtenstein, U., Hofmann, A.W., Oskarsson, N., Steinthorsson, S. (1993) The heterogeneous Iceland plume: Nd‐Sr‐O isotopes and trace element constraints. Journal of Geophyscal Research: Solid Earth 98, 15833-15850.
 Show in context
Show in context The δ18O values of igneous rocks in Iceland are generally thought to represent derivation from a mantle source ranging in δ18O from +5 to +6 ‰ (Hemond et al., 1993).
View in article
Kristmannsdóttir, H. (1979) Alteration of Basaltic Rocks by Hydrothermal-Activity at 100-300° C. Developments in sedimentology 27, 359-367.
 Show in context
Show in context Hydrothermal activity occurs over a wide temperature range (<10 to >400 °C; Arnórsson et al., 2008), resulting in low-grade metamorphism within the crust (Kristmannsdóttir, 1979).
View in article
Marin-Carbonne, J., Chaussidon, M., Robert, F. (2012) Micrometer-scale chemical and isotopic criteria (O and Si) on the origin and history of Precambrian cherts: implications for paleo-temperature reconstructions. Geochimica et Cosmochimica Acta 92, 129-147.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Marin-Carbonne, J., Robert, F., Chaussidon, M. (2014) The silicon and oxygen isotope compositions of Precambrian cherts: A record of oceanic paleo-temperatures? Precambrian Research 247, 223-234.
 Show in context
Show in context δ30Si systematics in Precambrian chert deposits have been used to constrain environmental conditions during their formation (e.g., Robert and Chaussidon, 2006; Marin-Carbonne et al., 2014).
View in article
Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Figure 4 [...] For modelling, the isotope composition of ancient seawater was used (Marin-Carbonne et al., 2014).
View in article
Muehlenbachs, K., Anderson, A.T., Sigvaldason, G.E. (1974) Low-O18 basalts from Iceland. Geochimica et Cosmochimica Acta 38, 577-588.
 Show in context
Show in context However, as a result of extensive fluid-rock interaction involving a low-18O meteoric water component, strong δ18O-depletions from such a mantle source are commonly observed in Icelandic rocks (Muehlenbachs et al., 1974).
View in article
Neuhoff, P.S., Fridriksson, T., Arnórsson, S., Bird, D.K. (1999) Porosity evolution and mineral paragenesis during low-grade metamorphism of basaltic lavas at Teigarhorn, Eastern Iceland. American Journal of Science 299, 467-501.
 Show in context
Show in context Hydrothermal low-temperature quartz and silica polymorphs are typically formed by precipitation of dissolved silica upon cooling at or near the surface (e.g., Neuhoff et al., 1999; Geilert et al., 2015).
View in article
Opfergelt, S., Georg, R.B., Delvaux, B., Cabidoche, Y.-M., Burton, K.W., Halliday, A.N. (2012) Silicon isotopes and the tracing of desilication in volcanic soil weathering sequences, Guadeloupe. Chemical Geology 326, 113-122.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Pollington, A.D., Kozdon, R., Anovitz, L.M., Georg, R.B., Spicuzza, M.J., Valley, J.W. (2016) Experimental calibration of silicon and oxygen isotope fractionations between quartz and water at 250° C by in situ microanalysis of experimental products and application to zoned low δ30Si quartz overgrowths. Chemical Geology 421, 127-142.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Oxygen and silicon isotope values of both ancient and modern hydrothermal silica deposits vary significantly with δ18O ranging from -10 to +30 ‰ and δ30Si ranging from -5 to +2 ‰ (Geilert et al., 2015; Brengman et al., 2016; Pollington et al., 2016; this study).
View in article
Coupled δ18O and δ30Si studies of ancient hydrothermal silica are still rare (Brengman et al., 2016; Pollington et al., 2016).
View in article
A similar isotopic trend has been observed in quartz cements of hydrothermal origin within the Cambrian Mt. Simon Formation in central North America (Pollington et al., 2016).
View in article
Such trends have been interpreted as the result of (1) rapid quartz formation from thermal fluids and kinetic isotope fractionation (Brengman et al., 2016) or (2) quartz cement precipitation from hydrothermal fluids that leached 30Si-depleted silicon from the surrounding highly weathered Precambrian rocks (Pollington et al., 2016).
View in article
Figure 4 δ30Si versus δ18O values of ancient, hydrothermally altered quartz cement and amygdales (Brengman et al., 2016; Pollington et al., 2016).
View in article
Reimink, J.R., Chacko, T., Stern, R.A., Heaman, L.M. (2014) Earth's earliest evolved crust generated in an Iceland-like setting. Nature Geoscience 7, 529-533.
 Show in context
Show in context Often adopted as a potential analogue for Earth’s earliest continental crust (Reimink et al., 2014), the Icelandic crust represents a continental-type crust with characteristics typical of the oceanic crust.
View in article
Robert, F., Chaussidon, M. (2006) A palaeotemperature curve for the Precambrian oceans based on silicon isotopes in cherts. Nature 443, 969-972.
 Show in context
Show in context δ30Si systematics in Precambrian chert deposits have been used to constrain environmental conditions during their formation (e.g., Robert and Chaussidon, 2006; Marin-Carbonne et al., 2014).
View in article
Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Roerdink, D.L., van den Boorn, S.H.J.M., Geilert, S., Vroon, P.Z., van Bergen, M.J. (2015) Experimental constraints on kinetic and equilibrium silicon isotope fractionation during the formation of non-biogenic chert deposits. Chemical Geology 402, 40-51.
 Show in context
Show in context In line with previous findings (Geilert et al., 2015; Roerdink et al., 2015; Brengman et al., 2016), this suggests that fractionation between silica deposits and fluids under low-temperature (<150 °C) hydrothermal conditions is likely controlled by kinetics and may strongly depend on precipitation rates.
View in article
Sæmundsson, K. (1979) Outline of the geology of Iceland. Jökull 29, 7-28.
 Show in context
Show in context Iceland’s crustal lithology is dominated by basalts with silicic volcanics and volcaniclastic sediments also being common (Sæmundsson, 1979).
View in article
Savage, P.S., Georg, R.B., Armytage, R.M.G., Williams, H.M., Halliday, A.N. (2010) Silicon isotope homogeneity in the mantle. Earth and Planetary Science Letters 295, 139-146.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Figure 2 [...] The isotopic values are compared to δ18O and δ30Si values for MORB (Eiler et al., 2000; Savage et al., 2010).
View in article
Recent studies showed that the silicon isotopic composition of Earth’s mantle is relatively homogenous (δ30Si = -0.29 ± 0.07 ‰) and not greatly affected by magmatic processes (Savage et al., 2010).
View in article
Comparison of our dataset with the results of the isotope models reveals that silica in hydrothermal high-temperature quartz predominantly originates from the primary (basaltic) rocks that generally display a limited range in δ30Si values (-0.29 ± 0.07 ‰; Savage et al., 2010, Fig. 3c,d).
View in article
Savage, P.S., Georg, R.B., Williams, H.M., Burton, K.W., Halliday, A.N. (2011) Silicon isotope fractionation during magmatic differentiation. Geochimica et Cosmochimica Acta 75, 6124-6139.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Savage, P.S., Georg, R.B., Williams, H.M., Turner, S., Halliday, A.N., Chappell, B.W. (2012) The silicon isotope composition of granites. Geochimica et Cosmochimica Acta 92, 184-202.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Stefánsson, A., Hilton, D.R., Sveinbjörnsdóttir, Á.E., Torssander, P., Heinemeier, J., Barnes, J.D., Ono, S., Halldórsson, S.A., Fiebig, J., Arnórsson, S. (2017) Isotope systematics of Icelandic thermal fluids. Journal of Volcanology and Geothermal Research 337, 146-164.
 Show in context
Show in context Applying isotope modelling approaches (Stefánsson et al., 2017), fluid sources, chemical reactions and associated isotope fractionations were quantified for various processes occurring in the curst, including fluid-rock interaction, fluid phase separation (boiling) and cooling.
View in article
Such processes lead to changes in the relative abundance of elements of various sources, e.g., the water to rock ratio, changes in aqueous species and gas concentrations and quantity of secondary minerals formed, which may all influence isotopic characteristics of fluids and minerals (Stefánsson et al., 2017).
View in article
Stefurak, E.J.T., Fischer, W.W., Lowe, D.R. (2015) Texture-specific Si isotope variations in Barberton Greenstone Belt cherts record low temperature fractionations in early Archean seawater. Geochimica et Cosmochimica Acta 150, 26-52.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Steinhoefel, G., Horn, I., von Blanckenburg, F. (2009) Micro-scale tracing of Fe and Si isotope signatures in banded iron formation using femtosecond laser ablation. Geochimica et Cosmochimica Acta 73, 5343-5360.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Steinhoefel, G., von Blanckenburg, F., Horn, I., Konhauser, K.O., Beukes, N.J., Gutzmer, J. (2010) Deciphering formation processes of banded iron formations from the Transvaal and the Hamersley successions by combined Si and Fe isotope analysis using UV femtosecond laser ablation. Geochimica et Cosmochimica Acta 74, 2677-2696.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Van den Boorn, S.H.J.M., Van Bergen, M.J., Nijman, W., Vroon, P.Z. (2007) Dual role of seawater and hydrothermal fluids in Early Archean chert: Evidence from silicon isotopes. Geology 35, 939-942.
 Show in context
Show in context Furthermore, despite the sedimentary origin of cherts and banded iron formations (BIFs), these rocks have often been exposed to hydrothermal alteration (e.g., Van den Boorn et al., 2007).
View in article
Van den Boorn, S.H.J.M., Van Bergen, M.J., Vroon, P.Z., De Vries, S.T., Nijman, W. (2010) Silicon isotope and trace element constraints on the origin of ∼3.5 Ga cherts: implications for Early Archaean marine environments. Geochimica et Cosmochimica Acta 74, 1077-1103.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Von Strandmann, P.A.E.P., Opfergelt, S., Lai, Y.-J., Sigfússon, B., Gislason, S.R., Burton, K.W. (2012) Lithium, magnesium and silicon isotope behaviour accompanying weathering in a basaltic soil and pore water profile in Iceland. Earth and Planetary Science Letters 339, 11-23.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
Zambardi, T., Poitrasson, F., Corgne, A., Méheut, M., Quitté, G., Anand, M. (2013) Silicon isotope variations in the inner solar system: Implications for planetary formation, differentiation and composition. Geochimica et Cosmochimica Acta 121, 67-83.
 Show in context
Show in context Figure 1 [...] Data taken from Douthitt (1982), De La Rocha et al. (2000), André et al. (2006), Robert and Chaussidon (2006), Georg et al. (2007a,b, 2009), Fitoussi et al. (2009), Steinhoefel, et al. (2009, 2010), Savage et al. (2010, 2011, 2012), Van den Boorn et al. (2010), Abraham et al. (2011), Armytage et al. (2011), Heck et al. (2011), Chakrabarti et al. (2012), Delvigne et al. (2012), Marin-Carbonne et al. (2012, 2014), Opfergelt et al. (2012), von Strandmann et al. (2012), Fan et al. (2013), Zambardi et al. (2013), Geilert et al. (2015), Stefurak et al. (2015), Brengman et al. (2016), Chen et al. (2016), Pollington et al. (2016).
View in article
top
Supplementary Information
The Supplementary Information includes:
- 1. δ30Si Variations in Earth's Material
- 2. Sample Selection
- 3. Sample Processing and Analysis
- 4. δ18O, δ30Si and Trace Elements in Quartz and Silica Polymorphs
- 5. Quantitative Geochemical and Isotope Modelling
- 6. Summary
- 7. Analytical Details of Si and O Isotope Analyses
- Tables S-1 to S-8
- Figures S-1 to S-4
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures and Tables
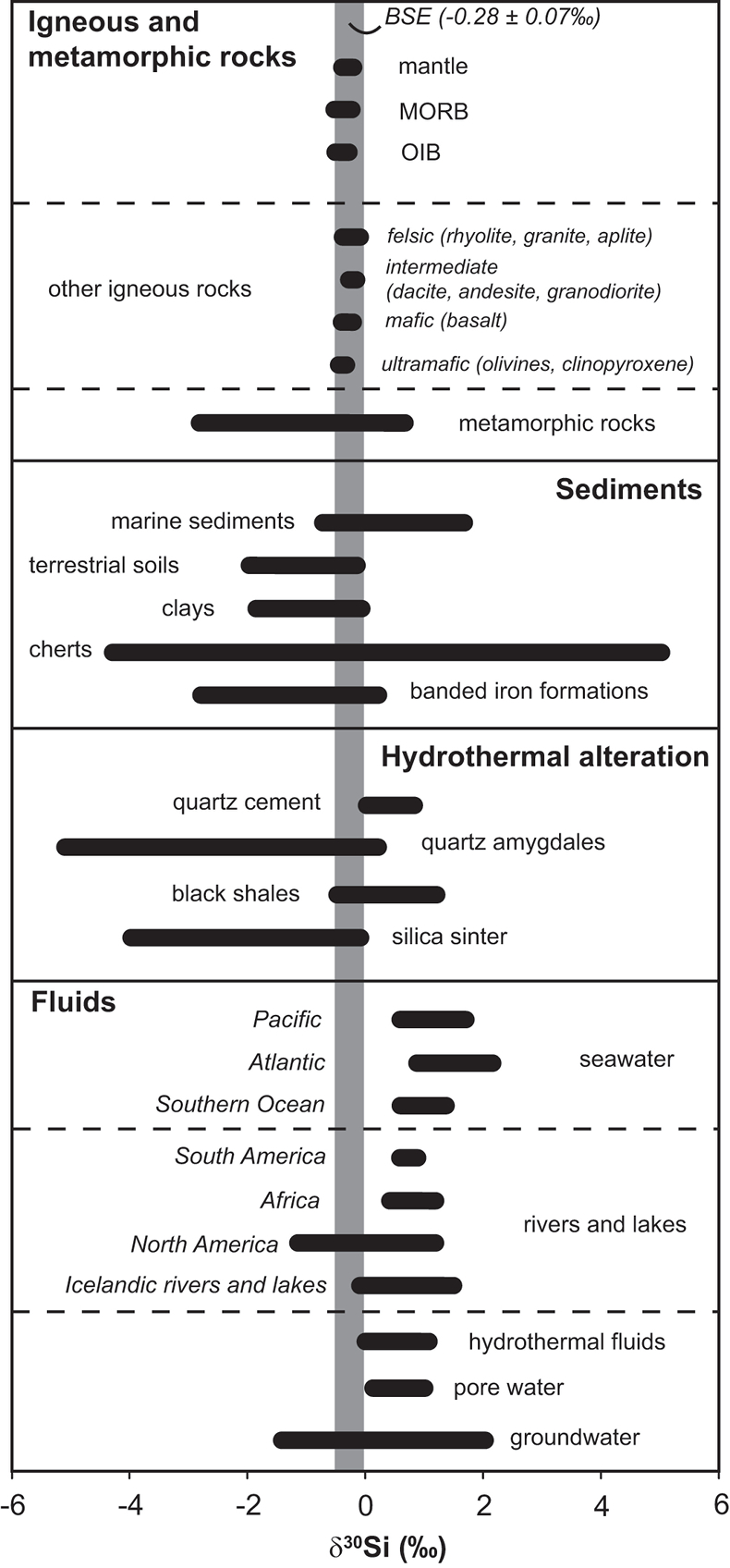
Figure 1 Compilation of δ30Si values for different rock types and fluids. Data taken from Douthitt (1982)
Douthitt, C.B. (1982) The geochemistry of the stable isotopes of silicon. Geochimica et Cosmochimica Acta 46, 1449-1458.
, De La Rocha et al. (2000)De La Rocha, C.L., Brzezinski, M.A., DeNiro, M.J. (2000) A first look at the distribution of the stable isotopes of silicon in natural waters. Geochimica et Cosmochimica Acta 64, 2467-2477.
, André et al. (2006)André, L., Cardinal, D., Alleman, L.Y., Moorbath, S. (2006) Silicon isotopes in ∼3.8 Ga West Greenland rocks as clues to the Eoarchaean supracrustal Si cycle. Earth and Planetary Science Letters 245, 162-173.
, Robert and Chaussidon (2006)Robert, F., Chaussidon, M. (2006) A palaeotemperature curve for the Precambrian oceans based on silicon isotopes in cherts. Nature 443, 969-972.
, Georg et al. (2007aGeorg, R.B., Reynolds, B.C., West, A.J., Burton, K.W., Halliday, A.N. (2007a) Silicon isotope variations accompanying basalt weathering in Iceland. Earth and Planetary Science Letters 261, 476-490.
,bGeorg, R.B., Halliday, A.N., Schauble, E.A., Reynolds, B.C. (2007b) Silicon in the Earth’s core. Nature 447, 1102-1106.
, 2009Georg, R.B., Zhu, C., Reynolds, B.C., Halliday, A.N. (2009) Stable silicon isotopes of groundwater, feldspars, and clay coatings in the Navajo Sandstone aquifer, Black Mesa, Arizona, USA. Geochimica et Cosmochimica Acta 73, 2229-2241.
), Fitoussi et al. (2009)Fitoussi, C., Bourdon, B., Kleine, T., Oberli, F., Reynolds, B.C. (2009) Si isotope systematics of meteorites and terrestrial peridotites: implications for Mg/Si fractionation in the solar nebula and for Si in the Earth's core. Earth and Planetary Science Letters 287, 77-85.
, Steinhoefel, et al. (2009Steinhoefel, G., Horn, I., von Blanckenburg, F. (2009) Micro-scale tracing of Fe and Si isotope signatures in banded iron formation using femtosecond laser ablation. Geochimica et Cosmochimica Acta 73, 5343-5360.
, 2010Steinhoefel, G., von Blanckenburg, F., Horn, I., Konhauser, K.O., Beukes, N.J., Gutzmer, J. (2010) Deciphering formation processes of banded iron formations from the Transvaal and the Hamersley successions by combined Si and Fe isotope analysis using UV femtosecond laser ablation. Geochimica et Cosmochimica Acta 74, 2677-2696.
), Savage et al. (2010Savage, P.S., Georg, R.B., Armytage, R.M.G., Williams, H.M., Halliday, A.N. (2010) Silicon isotope homogeneity in the mantle. Earth and Planetary Science Letters 295, 139-146.
, 2011Savage, P.S., Georg, R.B., Williams, H.M., Burton, K.W., Halliday, A.N. (2011) Silicon isotope fractionation during magmatic differentiation. Geochimica et Cosmochimica Acta 75, 6124-6139.
, 2012Savage, P.S., Georg, R.B., Williams, H.M., Turner, S., Halliday, A.N., Chappell, B.W. (2012) The silicon isotope composition of granites. Geochimica et Cosmochimica Acta 92, 184-202.
), Van den Boorn et al. (2010)Van den Boorn, S.H.J.M., Van Bergen, M.J., Vroon, P.Z., De Vries, S.T., Nijman, W. (2010) Silicon isotope and trace element constraints on the origin of ∼3.5 Ga cherts: implications for Early Archaean marine environments. Geochimica et Cosmochimica Acta 74, 1077-1103.
, Abraham et al. (2011)Abraham, K., Hofmann, A., Foley, S.F., Cardinal, D., Harris, C., Barth, M.G., André, L. (2011) Coupled silicon-oxygen isotope fractionation traces Archaean silicification. Earth and Planetary Science Letters 301, 222-230.
, Armytage et al. (2011)Armytage, R.M.G., Georg, R.B., Savage, P.S., Williams, H.M., Halliday, A.N. (2011) Silicon isotopes in meteorites and planetary core formation. Geochimica et Cosmochimica Acta 75, 3662-3676.
, Heck et al. (2011)Heck, P.R., Huberty, J.M., Kita, N.T., Ushikubo, T., Kozdon, R., Valley, J.W. (2011) SIMS analyses of silicon and oxygen isotope ratios for quartz from Archean and Paleoproterozoic banded iron formations. Geochimica et Cosmochimica Acta 75, 5879-5891.
, Chakrabarti et al. (2012)Chakrabarti, R., Knoll, A.H., Jacobsen, S.B., Fischer, W.W. (2012) Si isotope variability in Proterozoic cherts. Geochimica et Cosmochimica Acta 91, 187-201.
, Delvigne et al. (2012)Delvigne, C., Cardinal, D., Hofmann, A., André, L. (2012) Stratigraphic changes of Ge/Si, REE+ Y and silicon isotopes as insights into the deposition of a Mesoarchaean banded iron formation. Earth and Planetary Science Letters 355, 109-118.
, Marin-Carbonne et al. (2012Marin-Carbonne, J., Chaussidon, M., Robert, F. (2012) Micrometer-scale chemical and isotopic criteria (O and Si) on the origin and history of Precambrian cherts: implications for paleo-temperature reconstructions. Geochimica et Cosmochimica Acta 92, 129-147.
, 2014Marin-Carbonne, J., Robert, F., Chaussidon, M. (2014) The silicon and oxygen isotope compositions of Precambrian cherts: A record of oceanic paleo-temperatures? Precambrian Research 247, 223-234.
), Opfergelt et al. (2012)Opfergelt, S., Georg, R.B., Delvaux, B., Cabidoche, Y.-M., Burton, K.W., Halliday, A.N. (2012) Silicon isotopes and the tracing of desilication in volcanic soil weathering sequences, Guadeloupe. Chemical Geology 326, 113-122.
, von Strandmann et al. (2012)Von Strandmann, P.A.E.P., Opfergelt, S., Lai, Y.-J., Sigfússon, B., Gislason, S.R., Burton, K.W. (2012) Lithium, magnesium and silicon isotope behaviour accompanying weathering in a basaltic soil and pore water profile in Iceland. Earth and Planetary Science Letters 339, 11-23.
, Fan et al. (2013)Fan, H., Wen, H., Zhu, X., Hu, R., Tian, S. (2013) Hydrothermal activity during Ediacaran–Cambrian transition: Silicon isotopic evidence. Precambrian Research 224, 23-35.
, Zambardi et al. (2013)Zambardi, T., Poitrasson, F., Corgne, A., Méheut, M., Quitté, G., Anand, M. (2013) Silicon isotope variations in the inner solar system: Implications for planetary formation, differentiation and composition. Geochimica et Cosmochimica Acta 121, 67-83.
, Geilert et al. (2015)Geilert, S., Vroon, P.Z., Keller, N.S., Gudbrandsson, S., Stefánsson, A., van Bergen, M.J. (2015) Silicon isotope fractionation during silica precipitation from hot-spring waters: evidence from the Geysir hydrothermal field, Iceland. Geochimica et Cosmochimica Acta 164, 403-427.
, Stefurak et al. (2015)Stefurak, E.J.T., Fischer, W.W., Lowe, D.R. (2015) Texture-specific Si isotope variations in Barberton Greenstone Belt cherts record low temperature fractionations in early Archean seawater. Geochimica et Cosmochimica Acta 150, 26-52.
, Brengman et al. (2016)Brengman, L.A., Fedo, C.M., Whitehouse, M.J. (2016) Micro‐scale silicon isotope heterogeneity observed in hydrothermal quartz precipitates from the >3.7 Ga Isua Greenstone Belt, SW Greenland. Terra Nova 28, 70-75.
, Chen et al. (2016)Chen, X., Chafetz, H.S., Andreasen, R., Lapen, T.J. (2016) Silicon isotope compositions of euhedral authigenic quartz crystals: Implications for abiotic fractionation at surface temperatures. Chemical Geology 423, 61-73.
, Pollington et al. (2016)Pollington, A.D., Kozdon, R., Anovitz, L.M., Georg, R.B., Spicuzza, M.J., Valley, J.W. (2016) Experimental calibration of silicon and oxygen isotope fractionations between quartz and water at 250° C by in situ microanalysis of experimental products and application to zoned low δ30Si quartz overgrowths. Chemical Geology 421, 127-142.
. BSE = bulk silica earth (see Supplementary Information for more details).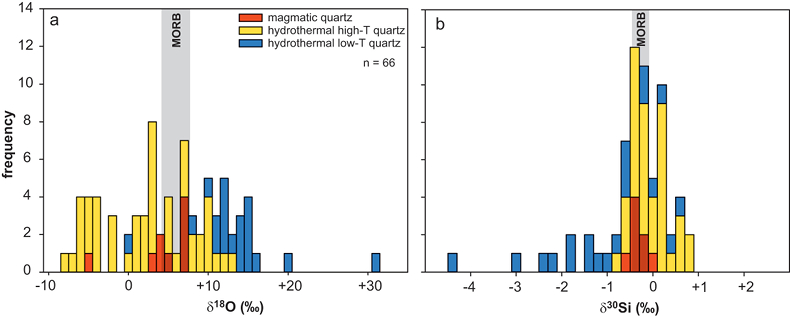
Figure 2 Variation in (a) δ18O and (b) δ30Si values of magmatic quartz (>550 °C), hydrothermal high-temperature (~200-400 °C) quartz and low-temperature (<150 °C) quartz and other silica polymorphs. The isotopic values are compared to δ18O and δ30Si values for MORB (Eiler et al., 2000
Eiler, J.M., Schiano, P., Kitchen, N., Stolper, E.M. (2000) Oxygen isotope evidence for recycled crust in the sources of mid ocean ridge basalts. Nature 403, 530-534.
; Savage et al., 2010Savage, P.S., Georg, R.B., Armytage, R.M.G., Williams, H.M., Halliday, A.N. (2010) Silicon isotope homogeneity in the mantle. Earth and Planetary Science Letters 295, 139-146.
).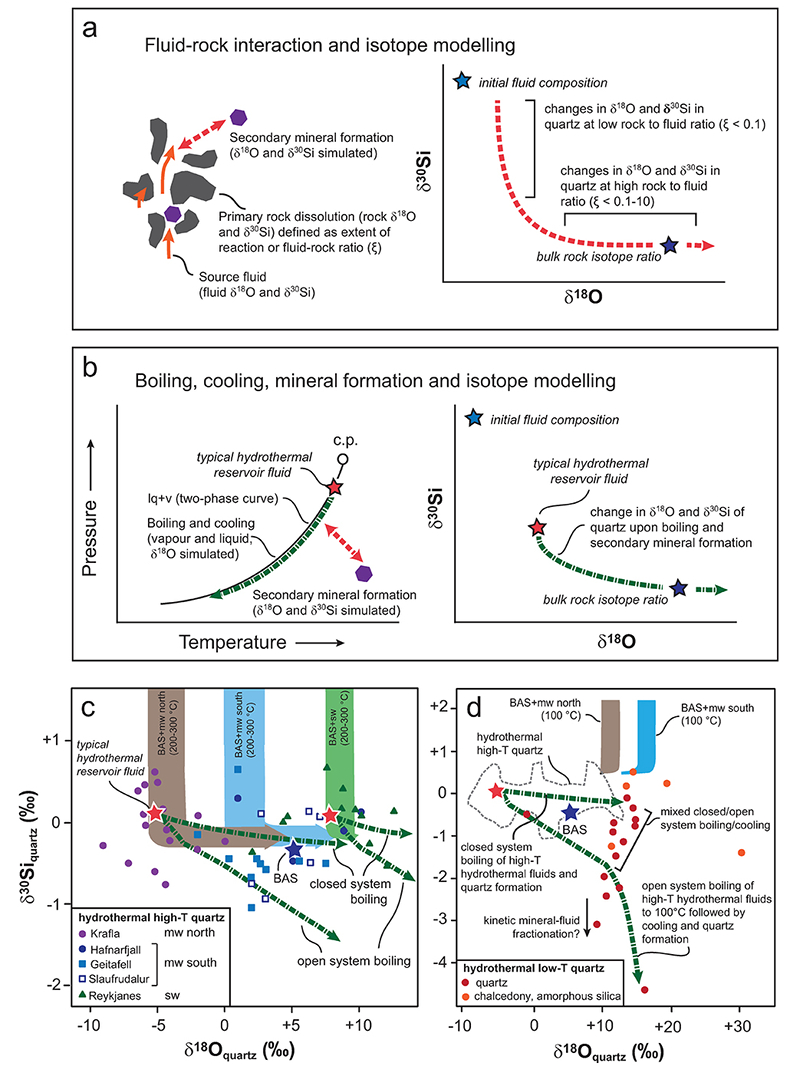
Figure 3 The conceptual chemical and isotope model involves (a) fluid-rock interaction with various source fluids and (b) boiling followed by cooling and mineral formation; (c) isotope systematics in hydrothermal high-temperature quartz largely match the predicted reaction path for progressive fluid-rock interaction (shaded areas) and boiling (arrows). δ30Si decreases with increasing rock-fluid ratio (ξ) whereas δ18O values in quartz both depend on the source water and rock-fluid ratio; (d) most of the isotope variations in hydrothermal low-temperature quartz and silica polymorphs can be explained by boiling and cooling of a hydrothermal fluid (arrows). Silica deposits with low δ30Si and constant δ18O values may result from open system boiling of a high-temperature fluid followed by cooling and quartz or opal formation. BAS = basalt, mw = meteoric water, sw = seawater. Analytical uncertainties of δ30Si and δ18O are listed in Tables S-7 and S-8.
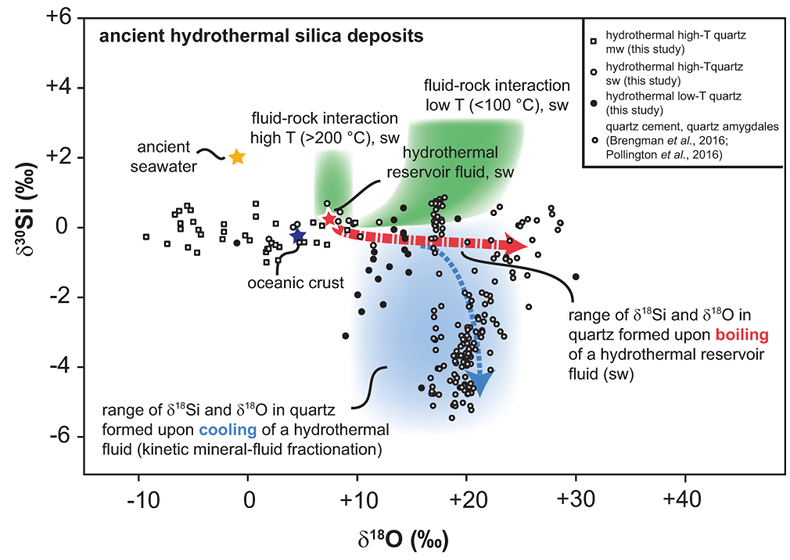
Figure 4 δ30Si versus δ18O values of ancient, hydrothermally altered quartz cement and amygdales (Brengman et al., 2016
Brengman, L.A., Fedo, C.M., Whitehouse, M.J. (2016) Micro‐scale silicon isotope heterogeneity observed in hydrothermal quartz precipitates from the >3.7 Ga Isua Greenstone Belt, SW Greenland. Terra Nova 28, 70-75.
; Pollington et al., 2016Pollington, A.D., Kozdon, R., Anovitz, L.M., Georg, R.B., Spicuzza, M.J., Valley, J.W. (2016) Experimental calibration of silicon and oxygen isotope fractionations between quartz and water at 250° C by in situ microanalysis of experimental products and application to zoned low δ30Si quartz overgrowths. Chemical Geology 421, 127-142.
). A highly variable but largely negative range in δ30Si values is likely to result from quartz precipitating out of a boiling and cooling fluid involving kinetic fluid-quartz/opal fractionation during rapid temperature decrease. For modelling, the isotope composition of ancient seawater was used (Marin-Carbonne et al., 2014Marin-Carbonne, J., Robert, F., Chaussidon, M. (2014) The silicon and oxygen isotope compositions of Precambrian cherts: A record of oceanic paleo-temperatures? Precambrian Research 247, 223-234.
). mw = meteoric water, sw = seawater.