Influence of metasomatism on vanadium-based redox proxies for mantle peridotite
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:3,936Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
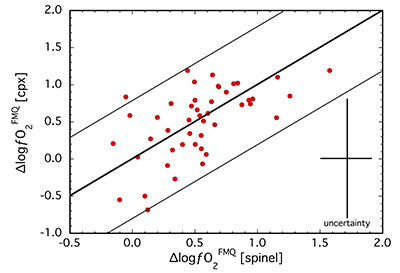 Figure 1 Comparison of ∆logƒO2 determined from spinel-based and clinopyroxene-based Fe3+-Fe2+ equilibria (see Supplementary Information). The 1:1 correlation line is shown for reference, along with the estimated uncertainty of the clinopyroxene-based oxybarometer of ±0.8 log units (Luth and Canil, 1993). | 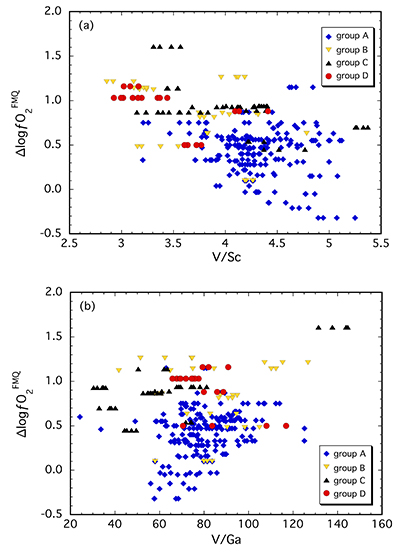 Figure 2 (a) V/Sc and (b) V/Ga of clinopyroxene plotted as a function of ∆logƒO2 determined by spinel-based Fe3+-Fe2+ oxybarometry (see Supplementary Information). | 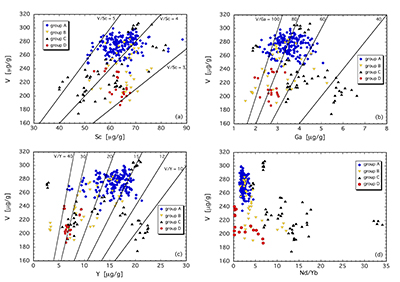 Figure 3 V concentration in different types of clinopyroxene plotted against their corresponding (a) Sc, (b) Ga and (c) Y contents, as well as (d) their Nd/Yb ratio. | 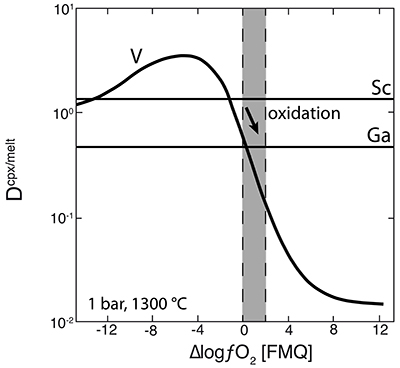 Figure 4 Variation in the clinopyroxene/silicate melt partition coefficient with ƒO2 at 1 bar and 1300 °C, along with Sc and Ga for reference as reported by Mallmann and O’Neill (2009). The arrow emphasises the effect of oxidation on DV in the ƒO2 range observed in our xenolith suite from the Massif Central (shaded area). See text. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
The oxidation state of the Earth’s upper mantle is an important parameter that influences many geochemical processes, including partial melting and whether carbon can migrate as carbonate in a metasomatising fluid/melt or is sequestered as graphite/diamond. Therefore, constraining this parameter is an essential goal in petrology. At the conditions of the Earth’s interior, free oxygen is an insignificant component, even though oxidation state is usually expressed in terms of oxygen fugacity (ƒO2). Instead, the reigning ƒO2 is related to the bulk Fe3+/∑Fe via ferric-ferrous equilibria involving mantle silicates and oxides. The Fe3+-partitioning between these phases is determinant, since this controls the activities of the Fe3+-bearing components involved in these equilibria. Thus, the Fe3+ content of key phases (spinel, garnet, or clinopyroxene) reflects the oxidation state. However, Fe3+ contents will be continually modified through interactions with migrating melts/fluids or juxtaposition of rock domains with different ƒO2’s. In practice, the ƒO2 determined for a given rock represents the most recent state of equilibration.
The multi-valence nature of vanadium (V2+, V3+, V4+, V5+) means that its geochemical behaviour is influenced by the prevailing ƒO2 and that its concentration could serve as a proxy for oxidation state in mantle peridotites (e.g., Canil and Fedortchouk, 2000
Canil, D., Fedortchouk, Y. (2000) Clinopyroxene-liquid partitioning for vanadium and the oxygen fugacity during formation of cratonic and oceanic mantle lithosphere. Journal of Geophysical Research 105, 26003-26016.
; Canil, 2002Canil, D. (2002) Vanadium in peridotites, mantle redox and tectonic environments: Archean to present. Earth and Planetary Science Letters 195, 75-90.
; Lee et al., 2003Lee, C.-T.A., Brandon, A.D., Norman, M.D. (2003). Vanadium in peridotites as a proxy for paleo-fO2 during partial melting: prospects, limitations, and implications. Geochimica et Cosmochimica Acta 67, 3045–3064
). It has been suggested that V concentrations or V/Sc of peridotites are less sensitive to metasomatic processes than Fe3+/Fe2+ and thus can “see through” such interactions and provide a picture of oxidation state during the last episode of partial melting (Lee et al., 2003Lee, C.-T.A., Brandon, A.D., Norman, M.D. (2003). Vanadium in peridotites as a proxy for paleo-fO2 during partial melting: prospects, limitations, and implications. Geochimica et Cosmochimica Acta 67, 3045–3064
, 2005Lee, C.-T.A., Leeman, W.P., Canil, D., Li, Z.-X. (2005) Similar V/Sc Systematics in MORB and Arc Basalts: Implications for the Oxygen Fugacities of their Mantle Source Regions. Journal of Petrology 46, 2313-2336.
). The advantage of using V/Sc, or similarly V/Ga, is that while only V is redox-sensitive, all three are mildly incompatible (e.g., Canil, 2004Canil, D. (2004) Mildly incompatible elements in peridotites and origins of the mantle lithosphere. Lithos 77, 375-393.
; Mallmann and O’Neill, 2009Mallmann, G., O’Neill, H.St.C. (2009) The Crystal/Melt Partitioning of V during Mantle Melting as a Function of Oxygen Fugacity Compared with some other Elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr and Nb). Journal of Petrology 50, 1765-1794.
). Thus, variations in V/Sc and V/Ga derive from partial melting under different ƒO2 conditions rather than other processes causing relative fractionation. The relative partitioning of V and Sc at least between clinopyroxene and silicate melt also does not seem to be influenced by pressure (Li, 2018Li, Y. (2018) Temperature and pressure effects on the partitioning of V and Sc between clinopyroxene and silicate melt: Implications for mantle oxygen fugacity. American Mineralogist 103, 819-823.
). Modelling by Lee et al. (2005)Lee, C.-T.A., Leeman, W.P., Canil, D., Li, Z.-X. (2005) Similar V/Sc Systematics in MORB and Arc Basalts: Implications for the Oxygen Fugacities of their Mantle Source Regions. Journal of Petrology 46, 2313-2336.
indicates that the V/Sc of residual peridotite will decrease with partial melting when ƒO2 is ≥1 log unit below the fayalite-magnetite-quartz (i.e. ≥FMQ-1) reference oxygen buffer. Under more reducing conditions V/Sc will increase as V becomes more compatible than Sc in clinopyroxene and spinel.We have undertaken a study of V, Sc and Ga contents in clinopyroxene from a suite of well-characterised spinel peridotite xenoliths from the Massif Central, France (Uenver-Thiele et al., 2014
Uenver-Thiele, L., Woodland, A.B., Downes, H., Altherr, R. (2014) Oxidation state of the lithospheric mantle below the Massif Central, France. Journal of Petrology 55, 2457-2480.
, 2017Uenver-Thiele, L., Woodland, A.B., Seitz, H.-M., Downes, H., Altherr, R. (2017) Metasomatic processes revealed by trace element and redox signatures of the lithospheric mantle beneath the Massif Central, France. Journal of Petrology 58, 395-422.
). Some samples exhibit little or no evidence of metasomatism, while others have been strongly metasomatised by a variety of different agents. For all samples, oxidation states have already been determined by spinel oxybarometry using Fe3+ contents measured by Mössbauer spectroscopy (Uenver-Thiele et al., 2014Uenver-Thiele, L., Woodland, A.B., Downes, H., Altherr, R. (2014) Oxidation state of the lithospheric mantle below the Massif Central, France. Journal of Petrology 55, 2457-2480.
). Although we do not have bulk concentrations to estimate ƒO2 independently, elemental concentrations in clinopyroxene can provide insight into the geochemical behaviour of these trace elements in the subcontinental lithospheric mantle (SCLM). On the other hand, the Fe3+ contents of clinopyroxene were also measured for a subset of samples, allowing application of the clinopyroxene-based oxybarometer of Luth and Canil (1993)Luth. R.W., Canil, D. (1993) Ferric iron in mantle-derived pyroxenes and a new oxybarometer for the mantle. Contributions to Mineralogy and Petrology 113, 236-248.
to test whether clinopyroxene and spinel are in Fe3+-Fe2+ redox equilibrium. V and other trace element concentrations are then discussed in this context, including their behaviour during metasomatism.top
Samples
The samples are spinel lherzolites and harzburgites from 36 localities across the Massif Central, France. Uenver-Thiele et al. (2017)
Uenver-Thiele, L., Woodland, A.B., Seitz, H.-M., Downes, H., Altherr, R. (2017) Metasomatic processes revealed by trace element and redox signatures of the lithospheric mantle beneath the Massif Central, France. Journal of Petrology 58, 395-422.
divided the samples into four groups (A, B, C, D) based upon the REE signatures of clinopyroxene, which reflect different styles of metasomatic interaction. Locality, rock type, and REE signature of clinopyroxene are summarised in the Supplementary Information (Table S-1). Group A has mildly depleted to flat patterns, indicating minor metasomatic effects. Group B exhibits LREE enrichment with La/Ce between 0.4 and 1.4 and Nd/Yb ranging from 2 to 11 (Uenver-Thiele et al., 2017Uenver-Thiele, L., Woodland, A.B., Seitz, H.-M., Downes, H., Altherr, R. (2017) Metasomatic processes revealed by trace element and redox signatures of the lithospheric mantle beneath the Massif Central, France. Journal of Petrology 58, 395-422.
). Group C clinopyroxene is enriched in both LREE and MREE, with Nd/Yb = 5-35, but limited variation in La/Ce (0.2-0.7). Group D has a characteristic “tick-shape” REE pattern, with MREE depletion (Nd/Yb < 1.5), but strong LREE enrichment (La/Ce = 0.6-2). Each group is further characterised by other elemental enrichments or depletions and the reader is referred to Uenver-Thiele et al. (2017)Uenver-Thiele, L., Woodland, A.B., Seitz, H.-M., Downes, H., Altherr, R. (2017) Metasomatic processes revealed by trace element and redox signatures of the lithospheric mantle beneath the Massif Central, France. Journal of Petrology 58, 395-422.
for a thorough description. Some samples from groups B and C have Lu/Hf systematics interpreted as indicating a secondary origin for the clinopyroxene rather than just being overprinted.While group D clinopyroxenes are considered to have interacted with oxidised subduction zone fluids, the signatures of groups B and C were interpreted by Uenver-Thiele et al. (2017)
Uenver-Thiele, L., Woodland, A.B., Seitz, H.-M., Downes, H., Altherr, R. (2017) Metasomatic processes revealed by trace element and redox signatures of the lithospheric mantle beneath the Massif Central, France. Journal of Petrology 58, 395-422.
to have been affected by carbonatitic and carbonated mafic silicate melts (group C), as well as by mafic silicate melts (group B and some group A). In some samples, multiple metasomatic events by different agents are apparent.top
Oxybarometry Using Fe3+-Fe2+ Equilibria
The oxidation state of spinel peridotite is most often determined using the olivine-orthopyroxene-spinel equilibrium (e.g., Wood et al., 1990
Wood, B.J., Bryndzia, L.T., Johnson, K.E. (1990) Mantle oxidation state and its relation to tectonic environment. Science 248, 337-345.
; see Supplementary Information). Alternatively, Luth and Canil (1993)Luth. R.W., Canil, D. (1993) Ferric iron in mantle-derived pyroxenes and a new oxybarometer for the mantle. Contributions to Mineralogy and Petrology 113, 236-248.
calibrated several olivine-orthopyroxene-clinopyroxene equilibria for estimating oxidation state based on the Fe3+ content of clinopyroxene (see Supplementary Information). For a subset of samples (N = 46), ∆logƒO2 values were obtained from both spinel-based and clinopyroxene-based oxybarometers and are compared in Figure 1. The two oxybarometers agree within their uncertainties (±0.8 log units; see Supplementary Information), implying redox equilibrium generally exists among the four main mantle phases, most specifically in terms of Fe3+ contents of spinel and clinopyroxene.Ranging from FMQ-0.3 to FMQ+1.6 for spinel-based and FMQ-0.7 to FMQ+1.2 for clinopyroxene-based equilibria (Table S-1), ∆logƒO2 values for our suite are relatively oxidised compared to the global average for non-cratonic SCLM of FMQ-0.68 (Foley, 2011
Foley, S.F. (2011) A reappraisal of redox melting in the Earth’s mantle as a function of tectonic setting and time. Journal of Petrology 52,1363-1391.
). This high ƒO2 is attributable to various types and degrees of metasomatic interactions, with the highest ƒO2 correlating with the strongest LREE enrichments in clinopyroxene (Uenver-Thiele et al., 2014Uenver-Thiele, L., Woodland, A.B., Downes, H., Altherr, R. (2014) Oxidation state of the lithospheric mantle below the Massif Central, France. Journal of Petrology 55, 2457-2480.
, 2017Uenver-Thiele, L., Woodland, A.B., Seitz, H.-M., Downes, H., Altherr, R. (2017) Metasomatic processes revealed by trace element and redox signatures of the lithospheric mantle beneath the Massif Central, France. Journal of Petrology 58, 395-422.
).
Figure 1 Comparison of ∆logƒO2 determined from spinel-based and clinopyroxene-based Fe3+-Fe2+ equilibria (see Supplementary Information). The 1:1 correlation line is shown for reference, along with the estimated uncertainty of the clinopyroxene-based oxybarometer of ±0.8 log units (Luth and Canil, 1993
Luth. R.W., Canil, D. (1993) Ferric iron in mantle-derived pyroxenes and a new oxybarometer for the mantle. Contributions to Mineralogy and Petrology 113, 236-248.
).top
The Redox Proxies V/Sc, V/Ga and V/Y
Clinopyroxene in our suite contains 170-310 µg/g V and 40-90 µg/g and 1.5-6.5 µg/g Sc and Ga, respectively (Table S-1). In Figure 2, V/Sc and V/Ga are plotted against ∆logƒO2. The spinel-based oxybarometer was used since it is a more robust measure of ƒO2 with smaller uncertainties. A broad decrease in V/Sc is observed with increasing ∆logƒO2, with clinopyroxenes belonging to groups B, C and D tending to have lower ratios than those from group A (Fig. 2a). Given the number of data points (n = 247) and the correlation coefficient (r = 0.49), there is <0.05 % probability that the two parameters are not correlated. Thus, the correlation is considered to be statistically “highly significant” (e.g., Taylor, 1982
Taylor, J.R. (1982) An Introduction to Error Analysis. University Science Books, Mill Valley, California, U.S.A., pp. 270.
). Some scatter is due to variable element concentrations between clinopyroxene grains from the same sample (Table S-1). However, a link does exist between the oxidation state recorded by Fe3+-Fe2+ equilibrium and V/Sc. On the other hand, a correlation between V/Ga and ∆logƒO2 is less clear, although clinopyroxene from group A tends to higher V/Ga with increasing ƒO2 (Fig. 2b). This is not the case for clinopyroxene from more metasomatised samples (groups B, C and D). Such varied behaviour of V/Sc and V/Ga cannot be attributed to subsolidus re-equilibration (i.e. Witt-Eickschen and O’Neill, 2005Witt-Eickschen, G., O’Neill, H.St.C. (2005) The effect of temperature on the equilibrium distribution of trace elements between clinopyroxene, orthopyroxene, olivine and spinel in upper mantle peridotite. Chemical Geology 221, 65-101.
) since both relatively oxidised and reduced samples (and those with low and high V/Sc) exhibit a similar range in equilibration temperature (Uenver-Thiele et al., 2014Uenver-Thiele, L., Woodland, A.B., Downes, H., Altherr, R. (2014) Oxidation state of the lithospheric mantle below the Massif Central, France. Journal of Petrology 55, 2457-2480.
). Thus, understanding these differences in geochemical behaviour requires consideration of the potential effects of metasomatic interactions.
Figure 2 (a) V/Sc and (b) V/Ga of clinopyroxene plotted as a function of ∆logƒO2 determined by spinel-based Fe3+-Fe2+ oxybarometry (see Supplementary Information).
top
V Systematics with Sc, Ga and Y and Their Relation to Metasomatism
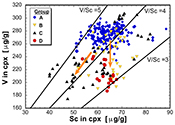
The least metasomatised samples (group A) form a cluster in element-element plots (Fig. 3a-d), providing a baseline for comparing clinopyroxene compositions that are more strongly overprinted. V, Sc and Ga concentrations are 250-300 µg/g, 50-70 µg/g and 2-4.5 µg/g, yielding V/Sc and V/Ga of 3.8-5.0 and 60-100, respectively (Fig. 3a,b). In contrast, clinopyroxene belonging to groups B, C and D exhibits variable V, Sc and Ga systematics, depending on the metasomatising agent involved. Most obvious is a lower V concentration in virtually all these samples. In many cases, a near constant Sc content relative to group A leads to systematically lower V/Sc, particularly for group B and D clinopyroxene. V and Sc concentrations of some group C clinopyroxenes are both relatively depleted, maintaining a near constant V/Sc. Gallium behaves differently, with noted enrichment in some group C clinopyroxenes, driving V/Ga down to <40. Other group C clinopyroxenes, as well as those of groups B and D, have V/Ga similar to the unmetasomatised (or weakly metasomatised) group A clinopyroxenes, but with decreased concentrations of both elements (Fig. 3b).
Other trace elements also exhibit variable behaviour. For example, clinopyroxenes affected by subduction fluids (group D) and alkali silicate melts (group B) underwent different extents of depletion in Y (Fig. 3c). In contrast, those influenced by carbonatitic or carbonated silicate melts (group C) can be either relatively enriched or depleted in Y, the latter having a secondary origin.
As illustrated in Figure 3d, where Nd/Yb serves to distinguish between different types of metasomatised clinopyroxenes, a common feature is a systematically lower V concentration compared to unmetasomatised or weakly metasomatised samples. Thus, although metasomatic interactions are often associated with element enrichment, under certain conditions elements can be extracted from the mineral assemblage to the mobile phase and further transported. In the case of V, this occurs via a variety of metasomatic agents. Selective removal of V during metasomatism can be understood in terms of changing solid/liquid partition coefficients. The clinopyroxene/melt partition coefficient decreases with increasing ƒO2 (Canil and Fedortchouk, 2000
Canil, D., Fedortchouk, Y. (2000) Clinopyroxene-liquid partitioning for vanadium and the oxygen fugacity during formation of cratonic and oceanic mantle lithosphere. Journal of Geophysical Research 105, 26003-26016.
; Mallmann and O’Neill, 2009Mallmann, G., O’Neill, H.St.C. (2009) The Crystal/Melt Partitioning of V during Mantle Melting as a Function of Oxygen Fugacity Compared with some other Elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr and Nb). Journal of Petrology 50, 1765-1794.
). For 1300 °C and 1 bar, Mallmann and O’Neill (2009)Mallmann, G., O’Neill, H.St.C. (2009) The Crystal/Melt Partitioning of V during Mantle Melting as a Function of Oxygen Fugacity Compared with some other Elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr and Nb). Journal of Petrology 50, 1765-1794.
give DVcpx/melt of 0.83 and 0.13 at ∆logƒO2 values of FMQ-0.5 and FMQ+2, respectively (Fig. 4). They report DVcpx/melt increases by a factor of ~2 between 1 bar and 3 GPa, but this may be an overestimate (Davis et al., 2013Davis, F.A., Humayun, M., Hirschmann, M.M., Cooper, R.S. (2013) Experimentally determined mineral/melt partitioning of first-row transition elements (FRTE) during partial melting of peridotite at 3 GPa. Geochimica et Cosmochimica Acta 104, 232-260.
). In any case, the relationship between DVcpx/melt and ƒO2 depicted in Figure 4 for 1 bar is not expected to change significantly at elevated pressures as V4+ and V5+ become more important compared to V3+. Given the range in ƒO2 recorded in our suite of samples (shaded area in Fig. 4), the decreasing partition coefficient implies that clinopyroxene should give up some of its V as it re-equilibrated with a migrating V-poor melt or fluid that is relatively oxidised (i.e. ∆logƒO2 ≥ FMQ+1; Fig. 4).One exception is sample 78-LU3b that has clinopyroxene somewhat enriched in V, modestly elevated Nd/Yb, but similar Sc, Ga and Y concentrations to unmetasomatised clinopyroxene (Fig. 3a-d). Uenver-Thiele et al. (2017)
Uenver-Thiele, L., Woodland, A.B., Seitz, H.-M., Downes, H., Altherr, R. (2017) Metasomatic processes revealed by trace element and redox signatures of the lithospheric mantle beneath the Massif Central, France. Journal of Petrology 58, 395-422.
considered this sample to have been refertilised by re-assimilating silicate melt trapped within the peridotite matrix. Thus, in this case the geochemical signature represents the sum of a harzburgitic protolith and an aliquot of trapped melt, leading to an overall increased budget of incompatible trace elements.
Figure 3 V concentration in different types of clinopyroxene plotted against their corresponding (a) Sc, (b) Ga and (c) Y contents, as well as (d) their Nd/Yb ratio.

Figure 4 Variation in the clinopyroxene/silicate melt partition coefficient with ƒO2 at 1 bar and 1300 °C, along with Sc and Ga for reference as reported by Mallmann and O’Neill (2009)
Mallmann, G., O’Neill, H.St.C. (2009) The Crystal/Melt Partitioning of V during Mantle Melting as a Function of Oxygen Fugacity Compared with some other Elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr and Nb). Journal of Petrology 50, 1765-1794.
. The arrow emphasises the effect of oxidation on DV in the ƒO2 range observed in our xenolith suite from the Massif Central (shaded area). See text.top
Implications
This study demonstrates that metasomatic interactions can have a direct influence on V concentrations in clinopyroxene. This occurs when the ƒO2 of the agent is different from the initial ambient peridotite. Considering that metasomatism is usually associated with oxidation (i.e. Woodland et al., 2006
Woodland, A.B., Kornprobst, J., Tabit, A. (2006) Ferric iron in orogenic lherzolite massifs and controls of oxygen fugacity in the upper mantle. Lithos 89, 222-241.
; Foley, 2011Foley, S.F. (2011) A reappraisal of redox melting in the Earth’s mantle as a function of tectonic setting and time. Journal of Petrology 52,1363-1391.
), modification of V content might be expected to be the rule rather than the exception. However, whether or not a change is evident will depend on the extent of interaction and the relative difference in oxidation state. Note that even some group A clinopyroxenes that are less strongly overprinted also exhibit subtly lower V concentrations than many others from this group (Fig. 3a-d). Assuming that relative partitioning of V, Sc and Ga is similar at 1bar and pressures corresponding to spinel peridotite facies (Li, 2018Li, Y. (2018) Temperature and pressure effects on the partitioning of V and Sc between clinopyroxene and silicate melt: Implications for mantle oxygen fugacity. American Mineralogist 103, 819-823.
), V/Sc should decrease with increasing ƒO2 above FMQ as DV and DSc diverge, but V/Ga may not vary significantly since DV and DGa become more similar at higher ƒO2 (Fig. 4, Lee et al., 2005Lee, C.-T.A., Leeman, W.P., Canil, D., Li, Z.-X. (2005) Similar V/Sc Systematics in MORB and Arc Basalts: Implications for the Oxygen Fugacities of their Mantle Source Regions. Journal of Petrology 46, 2313-2336.
; Mallmann and O’Neill, 2009Mallmann, G., O’Neill, H.St.C. (2009) The Crystal/Melt Partitioning of V during Mantle Melting as a Function of Oxygen Fugacity Compared with some other Elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr and Nb). Journal of Petrology 50, 1765-1794.
).The nature of the metasomatising agent will also determine if other trace elements, including Sc and Ga will be added to or removed from clinopyroxene. This depends on the elemental concentrations in the agent relative to that dictated by the partition coefficient for equilibrium with peridotitic clinopyroxene. Thus, V/Sc and V/Ga can potentially shift independently of oxidation state, since the denominator element cannot necessarily be treated as immobile during metasomatism. This is illustrated by various outliers of group A clinopyroxene in Figure 3a-d that have V concentrations below ~275 µg/g and exhibit relative addition or removal of Sc, Ga and Y. However, the correlation observed between V/Sc in clinopyroxene and Fe3+/Fe2+-based ∆logƒO2 (Fig. 2a) indicates not only that V/Sc can be reset to reflect a changed oxidation state, but that both V/Sc and Fe3+-Fe2+ equilibria can equally track such changes related to metasomatic interactions.
We have investigated the trace element behaviour only of clinopyroxene, which has not yet been calibrated to give a direct estimate of ƒO2 from our measured V/Sc. Although bulk-rock V/Sc is generally applied as a redox proxy (Lee et al., 2003
Lee, C.-T.A., Brandon, A.D., Norman, M.D. (2003). Vanadium in peridotites as a proxy for paleo-fO2 during partial melting: prospects, limitations, and implications. Geochimica et Cosmochimica Acta 67, 3045–3064
, 2005Lee, C.-T.A., Leeman, W.P., Canil, D., Li, Z.-X. (2005) Similar V/Sc Systematics in MORB and Arc Basalts: Implications for the Oxygen Fugacities of their Mantle Source Regions. Journal of Petrology 46, 2313-2336.
), the DV-ƒO2 systematics of clinopyroxene, olivine, orthopyroxene and spinel all exhibit the same trend of decreasing DV with increasing logƒO2 at conditions more oxidising than ~FMQ-2 (see Supplementary Information). In addition, the partitioning systematics of Sc among the peridotite minerals indicate that clinopyroxene should dominate the bulk Sc budget (Mallmann and O’Neill, 2009Mallmann, G., O’Neill, H.St.C. (2009) The Crystal/Melt Partitioning of V during Mantle Melting as a Function of Oxygen Fugacity Compared with some other Elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr and Nb). Journal of Petrology 50, 1765-1794.
). Thus, our observations for clinopyroxene should directly mirror changes in bulk V/Sc. This implies that metasomatism can influence the bulk V/Sc of mantle peridotite, being wholly or partially reset to reflect changing oxidation states during melt/fluid-rock interaction.top
Acknowledgements
This manuscript benefited from discussions with A. Zeh and S. Aulbach as well as the reviews of G. Mallmann and an anonymous reviewer.
Editor: Cin-Ty Lee
top
References
Canil, D. (2002) Vanadium in peridotites, mantle redox and tectonic environments: Archean to present. Earth and Planetary Science Letters 195, 75-90.
 Show in context
Show in contextThe multi-valence nature of vanadium (V2+, V3+, V4+, V5+) means that its geochemical behaviour is influenced by the prevailing ƒO2 and that its concentration could serve as a proxy for oxidation state in mantle peridotites (e.g., Canil and Fedortchouk, 2000; Canil, 2002; Lee et al., 2003).
View in article
Canil, D. (2004) Mildly incompatible elements in peridotites and origins of the mantle lithosphere. Lithos 77, 375-393.
 Show in context
Show in context The advantage of using V/Sc, or similarly V/Ga, is that while only V is redox-sensitive, all three are mildly incompatible (e.g., Canil, 2004; Mallmann and O’Neill, 2009).
View in article
Canil, D., Fedortchouk, Y. (2000) Clinopyroxene-liquid partitioning for vanadium and the oxygen fugacity during formation of cratonic and oceanic mantle lithosphere. Journal of Geophysical Research 105, 26003-26016.
 Show in context
Show in context The multi-valence nature of vanadium (V2+, V3+, V4+, V5+) means that its geochemical behaviour is influenced by the prevailing ƒO2 and that its concentration could serve as a proxy for oxidation state in mantle peridotites (e.g., Canil and Fedortchouk, 2000; Canil, 2002; Lee et al., 2003).
View in article
The clinopyroxene/melt partition coefficient decreases with increasing ƒO2 (Canil and Fedortchouk, 2000; Mallmann and O’Neill, 2009).
View in article
Davis, F.A., Humayun, M., Hirschmann, M.M., Cooper, R.S. (2013) Experimentally determined mineral/melt partitioning of first-row transition elements (FRTE) during partial melting of peridotite at 3 GPa. Geochimica et Cosmochimica Acta 104, 232-260.
 Show in context
Show in contextThey report DVcpx/melt increases by a factor of ~2 between 1 bar and 3 GPa, but this may be an overestimate (Davis et al., 2013).
View in article
Foley, S.F. (2011) A reappraisal of redox melting in the Earth’s mantle as a function of tectonic setting and time. Journal of Petrology 52,1363-1391.
 Show in context
Show in contextRanging from FMQ-0.3 to FMQ+1.6 for spinel-based and FMQ-0.7 to FMQ+1.2 for clinopyroxene-based equilibria (Table S-1), ∆logƒO2 values for our suite are relatively oxidised compared to the global average for non-cratonic SCLM of FMQ-0.68 (Foley, 2011).
View in article
Considering that metasomatism is usually associated with oxidation (i.e. Woodland et al., 2006; Foley, 2011), modification of V content might be expected to be the rule rather than the exception.
View in article
Lee, C.-T.A., Brandon, A.D., Norman, M.D. (2003). Vanadium in peridotites as a proxy for paleo-fO2 during partial melting: prospects, limitations, and implications. Geochimica et Cosmochimica Acta 67, 3045–3064
 Show in context
Show in contextThe multi-valence nature of vanadium (V2+, V3+, V4+, V5+) means that its geochemical behaviour is influenced by the prevailing ƒO2 and that its concentration could serve as a proxy for oxidation state in mantle peridotites (e.g., Canil and Fedortchouk, 2000; Canil, 2002; Lee et al., 2003).
View in article
It has been suggested that V concentrations or V/Sc of peridotites are less sensitive to metasomatic processes than Fe3+/Fe2+ and thus can “see through” such interactions and provide a picture of oxidation state during the last episode of partial melting (Lee et al., 2003, 2005).
View in article
Although bulk-rock V/Sc is generally applied as a redox proxy (Lee et al., 2003, 2005), the DV-ƒO2 systematics of clinopyroxene, olivine, orthopyroxene and spinel all exhibit the same trend of decreasing DV with increasing logƒO2 at conditions more oxidising than ~FMQ-2 (see Supplementary Information).
View in article
Lee, C.-T.A., Leeman, W.P., Canil, D., Li, Z.-X. (2005) Similar V/Sc Systematics in MORB and Arc Basalts: Implications for the Oxygen Fugacities of their Mantle Source Regions. Journal of Petrology 46, 2313-2336.
 Show in context
Show in contextIt has been suggested that V concentrations or V/Sc of peridotites are less sensitive to metasomatic processes than Fe3+/Fe2+ and thus can “see through” such interactions and provide a picture of oxidation state during the last episode of partial melting (Lee et al., 2003, 2005).
View in article
Modelling by Lee et al. (2005) indicates that the V/Sc of residual peridotite will decrease with partial melting when ƒO2 is ≥1 log unit below the fayalite-magnetite-quartz (i.e. ≥FMQ-1) reference oxygen buffer.
View in article
Assuming that relative partitioning of V, Sc and Ga is similar at 1bar and pressures corresponding to spinel peridotite facies (Li, 2018), V/Sc should decrease with increasing ƒO2 above FMQ as DV and DSc diverge, but V/Ga may not vary significantly since DV and DGa become more similar at higher ƒO2 (Fig. 4, Lee et al., 2005; Mallmann and O’Neill, 2009).
View in article
Although bulk-rock V/Sc is generally applied as a redox proxy (Lee et al., 2003, 2005), the DV-ƒO2 systematics of clinopyroxene, olivine, orthopyroxene and spinel all exhibit the same trend of decreasing DV with increasing logƒO2 at conditions more oxidising than ~FMQ-2 (see Supplementary Information).
View in article
Li, Y. (2018) Temperature and pressure effects on the partitioning of V and Sc between clinopyroxene and silicate melt: Implications for mantle oxygen fugacity. American Mineralogist 103, 819-823.
 Show in context
Show in context Assuming that relative partitioning of V, Sc and Ga is similar at 1bar and pressures corresponding to spinel peridotite facies (Li, 2018), V/Sc should decrease with increasing ƒO2 above FMQ as DV and DSc diverge, but V/Ga may not vary significantly since DV and DGa become more similar at higher ƒO2 (Fig. 4, Lee et al., 2005; Mallmann and O’Neill, 2009).
View in article
The relative partitioning of V and Sc at least between clinopyroxene and silicate melt also does not seem to be influenced by pressure (Li, 2018).
View in article
Luth. R.W., Canil, D. (1993) Ferric iron in mantle-derived pyroxenes and a new oxybarometer for the mantle. Contributions to Mineralogy and Petrology 113, 236-248.
 Show in context
Show in context On the other hand, the Fe3+ contents of clinopyroxene were also measured for a subset of samples, allowing application of the clinopyroxene-based oxybarometer of Luth and Canil (1993) to test whether clinopyroxene and spinel are in Fe3+-Fe2+ redox equilibrium.
View in article
Alternatively, Luth and Canil (1993) calibrated several olivine-orthopyroxene-clinopyroxene equilibria for estimating oxidation state based on the Fe3+ content of clinopyroxene (see Supplementary Information).
View in article
Mallmann, G., O’Neill, H.St.C. (2009) The Crystal/Melt Partitioning of V during Mantle Melting as a Function of Oxygen Fugacity Compared with some other Elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr and Nb). Journal of Petrology 50, 1765-1794.
 Show in context
Show in context The advantage of using V/Sc, or similarly V/Ga, is that while only V is redox-sensitive, all three are mildly incompatible (e.g., Canil, 2004; Mallmann and O’Neill, 2009).
View in article
The clinopyroxene/melt partition coefficient decreases with increasing ƒO2 (Canil and Fedortchouk, 2000; Mallmann and O’Neill, 2009).
View in article
For 1300 °C and 1 bar, Mallmann and O’Neill (2009) give DVcpx/melt of 0.83 and 0.13 at ∆logƒO2 values of FMQ-0.5 and FMQ+2, respectively (Fig. 4).
View in article
Figure 4 Variation in the clinopyroxene/silicate melt partition coefficient with ƒO2 at 1 bar and 1300 °C, along with Sc and Ga for reference as reported by Mallmann and O’Neill (2009).
View in article
Assuming that relative partitioning of V, Sc and Ga is similar at 1bar and pressures corresponding to spinel peridotite facies (Li, 2018), V/Sc should decrease with increasing ƒO2 above FMQ as DV and DSc diverge, but V/Ga may not vary significantly since DV and DGa become more similar at higher ƒO2 (Fig. 4, Lee et al., 2005; Mallmann and O’Neill, 2009).
View in article
In addition, the partitioning systematics of Sc among the peridotite minerals indicate that clinopyroxene should dominate the bulk Sc budget (Mallmann and O’Neill, 2009).
View in article
Taylor, J.R. (1982) An Introduction to Error Analysis. University Science Books, Mill Valley, California, U.S.A., pp. 270.
 Show in context
Show in context Thus, the correlation is considered to be statistically “highly significant” (e.g., Taylor, 1982).
View in article
Uenver-Thiele, L., Woodland, A.B., Downes, H., Altherr, R. (2014) Oxidation state of the lithospheric mantle below the Massif Central, France. Journal of Petrology 55, 2457-2480.
 Show in context
Show in contextWe have undertaken a study of V, Sc and Ga contents in clinopyroxene from a suite of well-characterised spinel peridotite xenoliths from the Massif Central, France (Uenver-Thiele et al., 2014, 2017).
View in article
For all samples, oxidation states have already been determined by spinel oxybarometry using Fe3+ contents measured by Mössbauer spectroscopy (Uenver-Thiele et al., 2014).
View in article
This high ƒO2 is attributable to various types and degrees of metasomatic interactions, with the highest ƒO2 correlating with the strongest LREE enrichments in clinopyroxene (Uenver-Thiele et al., 2014, 2017).
View in article
Such varied behaviour of V/Sc and V/Ga cannot be attributed to subsolidus re-equilibration (i.e. Witt-Eickschen and O’Neill, 2005) since both relatively oxidised and reduced samples (and those with low and high V/Sc) exhibit a similar range in equilibration temperature (Uenver-Thiele et al., 2014).
View in article
Uenver-Thiele, L., Woodland, A.B., Seitz, H.-M., Downes, H., Altherr, R. (2017) Metasomatic processes revealed by trace element and redox signatures of the lithospheric mantle beneath the Massif Central, France. Journal of Petrology 58, 395-422.
 Show in context
Show in contextWe have undertaken a study of V, Sc and Ga contents in clinopyroxene from a suite of well-characterised spinel peridotite xenoliths from the Massif Central, France (Uenver-Thiele et al., 2014, 2017).
View in article
Uenver-Thiele et al. (2017) divided the samples into four groups (A, B, C, D) based upon the REE signatures of clinopyroxene, which reflect different styles of metasomatic interaction.
View in article
Group B exhibits LREE enrichment with La/Ce between 0.4 and 1.4 and Nd/Yb ranging from 2 to 11 (Uenver-Thiele et al., 2017).
View in article
While group D clinopyroxenes are considered to have interacted with oxidised subduction zone fluids, the signatures of groups B and C were interpreted by Uenver-Thiele et al. (2017) to have been affected by carbonatitic and carbonated mafic silicate melts (group C), as well as by mafic silicate melts (group B and some group A).
View in article
Uenver-Thiele et al. (2017) considered this sample to have been refertilised by re-assimilating silicate melt trapped within the peridotite matrix.
View in article
Witt-Eickschen, G., O’Neill, H.St.C. (2005) The effect of temperature on the equilibrium distribution of trace elements between clinopyroxene, orthopyroxene, olivine and spinel in upper mantle peridotite. Chemical Geology 221, 65-101.
 Show in context
Show in context Such varied behaviour of V/Sc and V/Ga cannot be attributed to subsolidus re-equilibration (i.e. Witt-Eickschen and O’Neill, 2005) since both relatively oxidised and reduced samples (and those with low and high V/Sc) exhibit a similar range in equilibration temperature (Uenver-Thiele et al., 2014).
View in article
Wood, B.J., Bryndzia, L.T., Johnson, K.E. (1990) Mantle oxidation state and its relation to tectonic environment. Science 248, 337-345.
 Show in context
Show in context The oxidation state of spinel peridotite is most often determined using the olivine-orthopyroxene-spinel equilibrium (e.g., Wood et al., 1990; see Supplementary Information).
View in article
Woodland, A.B., Kornprobst, J., Tabit, A. (2006) Ferric iron in orogenic lherzolite massifs and controls of oxygen fugacity in the upper mantle. Lithos 89, 222-241.
 Show in context
Show in context Considering that metasomatism is usually associated with oxidation (i.e. Woodland et al., 2006; Foley, 2011), modification of V content might be expected to be the rule rather than the exception.
View in article
top
Supplementary Information
The Supplementary Information includes:
- Analytical Methods
- Oxybarometry based on Fe3+-Fe2+ in Spinel and Clinopyroxene
- Bulk DV for Peridotitic Compositions
- Tables S-1 (Excel Download) and S-2
- Figures S-1 and S-2
- Supplementary Information References
Download the Supplementary Information (PDF).
Download Table S-1 (Excel).
Figures and Tables
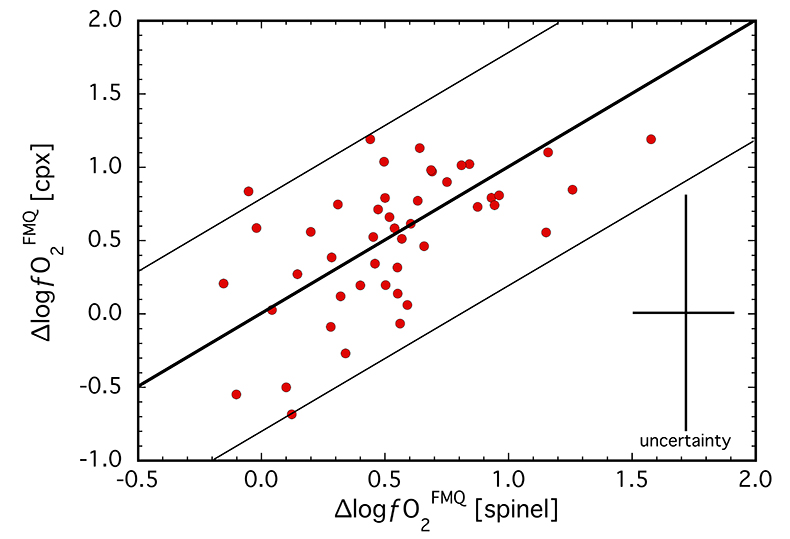
Figure 1 Comparison of ∆logƒO2 determined from spinel-based and clinopyroxene-based Fe3+-Fe2+ equilibria (see Supplementary Information). The 1:1 correlation line is shown for reference, along with the estimated uncertainty of the clinopyroxene-based oxybarometer of ±0.8 log units (Luth and Canil, 1993
Luth. R.W., Canil, D. (1993) Ferric iron in mantle-derived pyroxenes and a new oxybarometer for the mantle. Contributions to Mineralogy and Petrology 113, 236-248.
).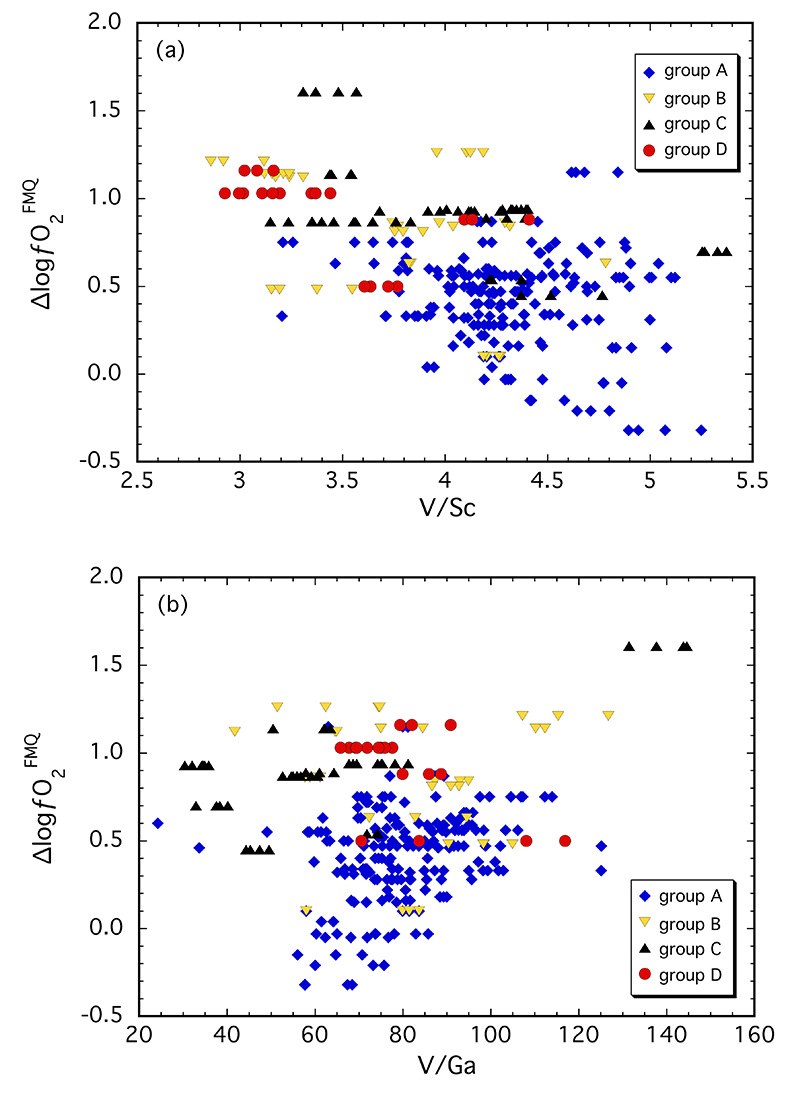
Figure 2 (a) V/Sc and (b) V/Ga of clinopyroxene plotted as a function of ∆logƒO2 determined by spinel-based Fe3+-Fe2+ oxybarometry (see Supplementary Information).
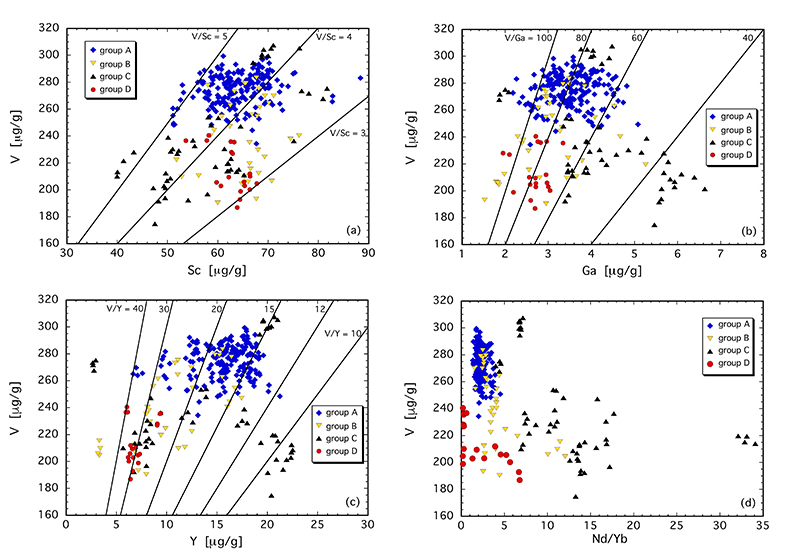
Figure 3 V concentration in different types of clinopyroxene plotted against their corresponding (a) Sc, (b) Ga and (c) Y contents, as well as (d) their Nd/Yb ratio.
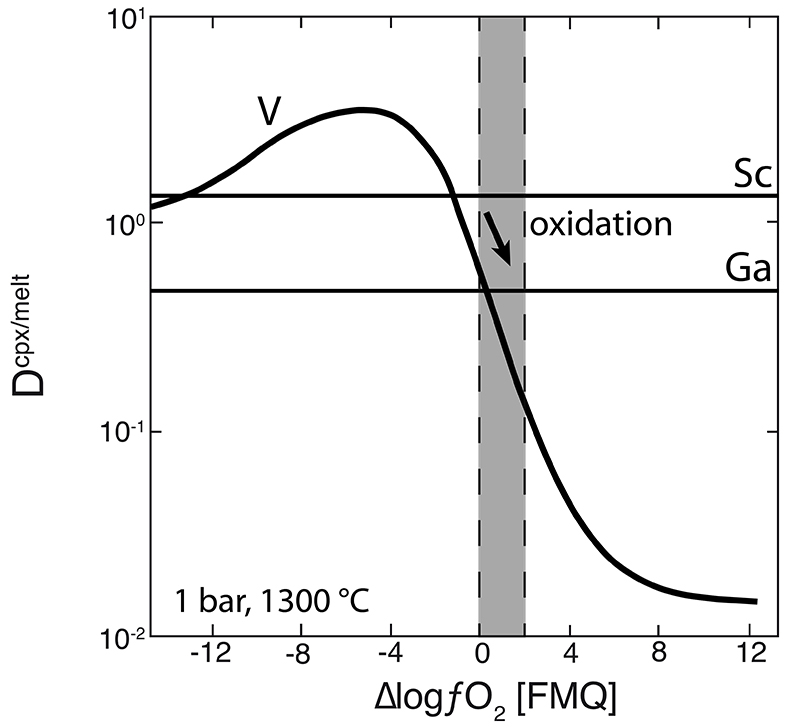
Figure 4 Variation in the clinopyroxene/silicate melt partition coefficient with ƒO2 at 1 bar and 1300 °C, along with Sc and Ga for reference as reported by Mallmann and O’Neill (2009)
Mallmann, G., O’Neill, H.St.C. (2009) The Crystal/Melt Partitioning of V during Mantle Melting as a Function of Oxygen Fugacity Compared with some other Elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr and Nb). Journal of Petrology 50, 1765-1794.
. The arrow emphasises the effect of oxidation on DV in the ƒO2 range observed in our xenolith suite from the Massif Central (shaded area). See text.