Calcium stable isotopes place Devonian conodonts as first level consumers
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:6,937Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract

Figures and Tables
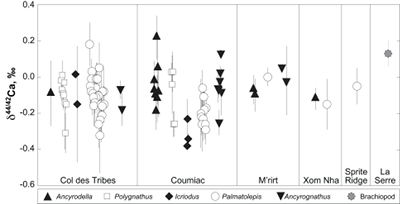 Figure 1 Ca isotope compositions of conodonts (δ44/42Ca) relative to SRM915a (‰) measured in the study. | 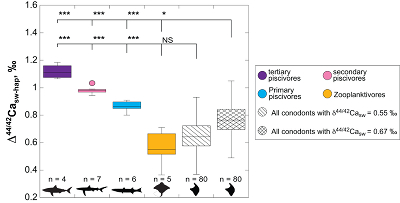 Figure 2 Ca isotopic offset between seawater and hap of modern elasmobranchs compared to that of conodonts (Δ44/42Casw-hap) measured in the study. Modern elasmobranch data are from Martin et al. (2015). The Δ44/42Casw-hap offset is calculated with two δ44/42Casw values, 0.55 ‰ (diagonal lines) and 0.67 ‰ (diagonal cross hatch) corresponding to the range given by Farkaš et al. (2007; Fig. S-3). Boxplots delimit 5, 25, 50, 75 and 95 % percentiles. Depending on the δ44/42Casw value, statistics show that conodonts and zooplanktivores have similar δ44/42Ca values. Under the null hypothesis that there is no difference in the distribution of two groups of δ44/42Ca values, the p value of Student's t-tests provides the smallest level of significance at which null hypothesis would be rejected (NS, non-significant p value; *p = 0.01–0.05; **p = 0.001–0.01; and ***p < 0.001). |
| Figure 1 | Figure 2 |
top
Introduction
Until the publication of the discovery of the first specimen of the conodont animal in 1983 with conodont elements in situ forming a feeding apparatus, the nature and function of the conodont elements was one of palaeontology's great mysteries (Briggs et al., 1983
Briggs, D.E.G., Clarkson, E.N.K., Aldridge, R.J. (1983) The conodont animal. Lethaia 16, 1-14.
). Since then, several other specimens have shown similar schemes for this feeding apparatus, in which the anterior elements form a structure allowing them to trap food that is further processed by the posterior elements (Purnell and Donoghue, 1997Purnell, M.A., Donoghue, P.C.J. (1997) Architecture and functional morphology of the skeletal apparatus of ozarkodinid conodonts. Philosophical Transactions of the Royal Society 352, 1545-1564.
). Today, despite the advancement of synchrotron microtomography that allows reconstructing virtual movements of the elements forming the feeding apparatus (Goudemand et al., 2011Goudemand, N., Orchard, M.J., Urdy, S., Bucher, H., Tafforeau, P. (2011) Synchrotron-aided reconstruction of the conodont feeding apparatus and implications for the mouth of the first vertebrates. Proceedings of the National Academy of Sciences USA 108, 8720-8724.
), the dietary behaviour of conodonts remains an open debate but recently Shirley et al. (2018)Shirley, B., Grohganz, M., Bestmann, M., Jarochowska, E. (2018) Wear, tear and systematic repair: testing models of growth dynamics in conodonts with high-resolution imaging. Proceedings of the Royal Society B: Biological Sciences 285, doi: 10.1098/rspb.2018.1614.
suggested a predatory or scavenger mode of life. Conodonts possessed sclerotic eye capsule and extrinsic eye musculature (Gabbott et al., 1995Gabbott, S.E., Aldridge, R.J., Theron, J.N. (1995) A giant conodont with preserved muscle tissue from the Upper Ordovician of south Africa. Nature 374, 800-803.
; Purnell, 1995aPurnell, M.A. (1995a) Large eyes and vision in conodonts. Lethaia 28, 187-188.
), consistent with conodonts having pattern vision and an active predatory lifestyle. Lastly, microwear patterns were found on conodont elements, which constituted the first direct evidence that they functioned as teeth (Purnell, 1995bPurnell, M.A. (1995b) Microwear on conodont elements and macrophagy in the first vertebrates. Nature 374, 798-800.
).In the present work, which is a pilot study, we use for the first time calcium (Ca) stable isotopes to infer the feeding habit of conodont animals. The first studies showing that trophic levels of animals, including fish, could be inferred from the Ca isotope compositions of their shell or inner skeleton lay back in the 2000's (Skulan et al., 1997
Skulan, J., DePaolo, D.J., Owens, T.L. (1997) Biological control of calcium isotopic abundances in the global calcium cycle. Geochimica et Cosmochimica Acta 61, 2505-2510.
; Skulan and DePaolo, 1999Skulan, J., DePaolo, D.J. (1999) Calcium isotope fractionation between soft and mineralised tissues as a monitor of calcium use in vertebrates. Proceedings of the National Academy of Sciences USA 96, 13709 –13713.
; Clementz et al., 2003Clementz, M. T., Holden, P., Koch, P.L. (2003) Are Calcium isotopes a reliable monitor of trophic level in marine settings? International Journal of Osteoarchaeology 13, 29-36.
; DePaolo, 2004DePaolo, D.J. (2004) Calcium Isotopic Variations Produced by Biological, Kinetic, Radiogenic and Nucleosynthetic Processes. Reviews in Mineralogy and Geochemistry 55, 255-288.
). These results were recently confirmed on modern and fossil elasmobranchs (Martin et al., 2015Martin, J.E., Tacail, T., Adnet, S., Girard, C., Balter, V. (2015) Calcium isotopes reveal the trophic position of extant and fossil elasmobranchs. Chemical Geology 415, 118-125.
), a subclass of cartilaginous fish, including the sharks, rays and skates, and sawfish. Some authors have already measured the Ca isotope composition of conodonts but with the aim to reconstruct variations of the seawater composition (Hinojosa et al. 2012Hinojosa, J.L., Brown, S.T., Chen, J., DePaolo, D.J., Paytan, A., Shen, S.-Z., Payne, J.L. (2012) Evidence for end-Permian ocean acidification from calcium isotopes in biogenic apatite. Geology 40, 743-746.
; Jost et al. 2014Jost, A., Mundil, R., He, B., Brown, S.T., Altiner, D., Sun, Y., DePaolo, D.J., Payne, J.L (2014) Constraining the cause of the end-Guadalupian extinction with coupled records of carbon and calcium isotopes. Earth and Planeraty Science Letters 396, 201-212.
; Le Houedec et al. 2017Le Houedec, S., McCulloch, M., Trotter, J., Rankenburg, K. (2017) Conodont apatite δ88/86Sr and δ44/40Ca compositions and implications for the evolution of Palaeozoic to early Mesozoic seawater. Chemical Geology 453, 55-65.
). Here, the Late Devonian period, particularly the Frasnian-Famennian boundary (F/F), was chosen because it is accompanied by important variations in the shape of conodont elements, suggestive of changes in the feeding behaviour of several genera (Balter et al., 2008Balter V., Renaud S., Girard C., Joachimski, M. (2008) The record of climate-driven morphological changes in 376 Ma old Devonian fossils. Geology 36, 907-910.
; Girard and Renaud, 2008Girard, C., Renaud, S. (2008) Disentangling allometry and response to Kellwasser anoxic events in the Late Devonian conodont genus Ancyrodella. Lethaia 41, 383-394.
). The Material and Method sections are described in the Supplementary Information.top
Results
All values presented in this work are expressed as δ44/42Ca and defined as δ44/42Ca = ((44Ca/42Casample) / (44Ca/42CaSRM915a) – 1) * 1000. All measured samples were plotted as δ43/42Ca against δ44/42Ca and fall on a line with a slope of 0.557 close to the theoretical 0.507 slope predicted by the exponential approximation of mass dependent fractionation (Tacail et al., 2014
Tacail T., Albalat E., Télouk P., Balter V. (2014) A simplified protocol for the measurement of Ca isotopes in biological samples. Journal of Analytical Atomic Spectrometry 29, 529-535.
; Fig. S-2). Quality control assessment is given in Table S-2, and Ca isotope values of conodonts measured in this study (Table S-3; Fig. 1) range from -0.38 ‰ to 0.22 ‰, with an average value of -0.10 ± 0.22 ‰ (±2 s.d., n = 80). The average δ44/42Ca value is -0.10 ± 0.20 ‰ (±2 s.d., n = 39) at Col des Tribes, -0.12 ± 0.28 ‰ (±2 s.d., n = 39) at Coumiac, -0.03 ± 0.12 ‰ (±2 s.d., n = 5) at M'rirt, -0.14 ± 0.04 ‰ (±2 s.d., n = 2) at Xom Nha and -0.05 ‰ at Sprite Ridge (Table S-3). Comparison between conodonts at Coumiac and Col des Tribes, the two most abundant sites, reveals no significant difference of the δ44/42Ca value (Student's t-test, p = 0.373). Comparisons between conodonts grouped by genus reveal no taxonomic difference (Table S-4). The brachiopod yielded a δ44/42Ca value of 0.13 ‰ (Table S-1), which represents one of the highest values of the dataset.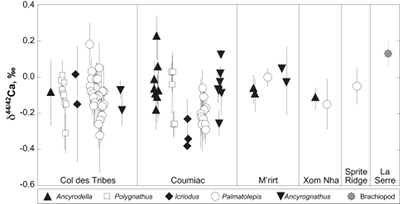
Figure 1 Ca isotope compositions of conodonts (δ44/42Ca) relative to SRM915a (‰) measured in the study.
top
Discussion
The present paper focuses on the trophic position of conodont animals based on their Ca isotope compositions, but these could have been affected by diagenetic processes. Discussion on the effects of diagenesis is developed in the Supplementary Information. We conclude, in the absence of any evidence of Ca isotope compositions being diagenetically reworked, that the measured δ44/42Ca values of conodonts are biogenic.
The present paper focuses on the trophic position of conodont animals based on their Ca isotope composition, but accurate comparisons with modern analogues first necessitate calibrating the Ca isotopic values of the conodont elements relative to that of the contemporaneous seawater. Based on the existing Ca isotopic fractionation factor between modern brachiopods (br) Terebratalia and seawater (sw), αbr-sw = 0.99915 (Gussone et al., 2005
Gussone, N., Böhm, F., Eisenhauer, A., Dietzel, M., Heuser, A., Teichert, B.M.A., Reitner, J., Wörheide, G., Dullo, W.C. (2005) Calcium isotope fractionation in calcite and aragonite. Geochimica et Cosmochimica Acta 69, 4485-4494.
), the δ44/42Ca value of end-Devonian seawater (δ44/42Casw) was estimated at 0.55 ‰, i.e. ~0.4 ‰ lower than that of modern oceans (Blättler et al. 2012Blättler, C.L., Henderson, G.M., Jenkyns, H.C. (2012) Explaining the Phanerozoic Ca isotope history of seawater. Geology 40, 843-846.
). With an age estimated slightly younger than the Devonian-Carboniferous boundary of 360 Ma, this value falls in the range, but in the lower limit, of the reconstructed Phanerozoic seawater Ca isotope composition of Farkaš et al. (2007Farkaš, J., Böhm, F., Wallmann, K., Blenkinsop, J., Eisenhauer, A., van Geldern, R., Munnecke, A., Voigt, S., Veizer, J. (2007) Calcium isotope record of Phanerozoic oceans: implications for chemical evolution of seawater and its causative mechanisms: Geochimica et Cosmochimica Acta 71, 5117-5134.
; Fig. S-3). We can now calculate the Ca isotopic offset between seawater and conodonts, which is equal to 0.65 ± 0.25 ‰, (± s.d., n = 80). Conodonts are made up of hydroxylapatite (hap), which is more or less fluorinated, but it is the same mineral phase as that of elasmobranch teeth. This allows comparing the average Ca isotopic offset between Devonian seawater and conodonts with that of modern seawater and extant elasmobranch tooth enameloid (Martin et al., 2015Martin, J.E., Tacail, T., Adnet, S., Girard, C., Balter, V. (2015) Calcium isotopes reveal the trophic position of extant and fossil elasmobranchs. Chemical Geology 415, 118-125.
), which is annotated Δ44/42Casw-hap (Fig. 2). Using a modern seawater δ44/42Casw value of 0.92 ‰, an offset of ~0.65 ‰ is observed nowadays between seawater and the zooplanktivore and primary piscivores group, which are characterised by average Δ44/42Casw-hap values of 0.56 ± 0.27 ‰ (±2 s.d., n = 5) and 0.86 ± 0.08 ‰ (±2 s.d., n = 6), respectively (Fig. 2). To fully encompass the δ44/42Casw variability at that time, which is well described by the study of Farkaš et al. (2007)Farkaš, J., Böhm, F., Wallmann, K., Blenkinsop, J., Eisenhauer, A., van Geldern, R., Munnecke, A., Voigt, S., Veizer, J. (2007) Calcium isotope record of Phanerozoic oceans: implications for chemical evolution of seawater and its causative mechanisms: Geochimica et Cosmochimica Acta 71, 5117-5134.
, we can also calculate the Δ44/42Casw-hap with the upper limit of the contemporaneous δ44/42Casw value, i.e. ~0.67 ‰. Even with this higher value, the calculated Δ44/42Casw-hap offset shows that conodonts are still in the the zooplanktivore - primary piscivores group (Fig. 2). The observation that conodonts fall as first level consumers is in accordance with the macrophagous hypothesis (i.e. feeding on relatively large particles of food), but is at odds with the view that conodont animals had a purely predatory lifestyle, which would have implied a δ44/42Ca value of conodont elements around 1 ‰. Scavenging of fish cannot be ruled out, but must have involved small fish above all, otherwise the δ44/42Ca values would have been those of predators.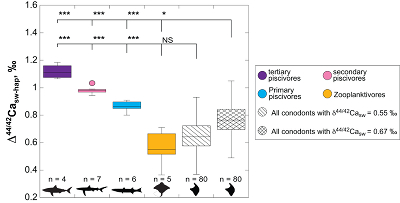
Figure 2 Ca isotopic offset between seawater and hap of modern elasmobranchs compared to that of conodonts (Δ44/42Casw-hap) measured in the study. Modern elasmobranch data are from Martin et al. (2015)
Martin, J.E., Tacail, T., Adnet, S., Girard, C., Balter, V. (2015) Calcium isotopes reveal the trophic position of extant and fossil elasmobranchs. Chemical Geology 415, 118-125.
. The Δ44/42Casw-hap offset is calculated with two δ44/42Casw values, 0.55 ‰ (diagonal lines) and 0.67 ‰ (diagonal cross hatch) corresponding to the range given by Farkaš et al. (2007Farkaš, J., Böhm, F., Wallmann, K., Blenkinsop, J., Eisenhauer, A., van Geldern, R., Munnecke, A., Voigt, S., Veizer, J. (2007) Calcium isotope record of Phanerozoic oceans: implications for chemical evolution of seawater and its causative mechanisms: Geochimica et Cosmochimica Acta 71, 5117-5134.
; Fig. S-3). Boxplots delimit 5, 25, 50, 75 and 95 % percentiles. Depending on the δ44/42Casw value, statistics show that conodonts and zooplanktivores have similar δ44/42Ca values. Under the null hypothesis that there is no difference in the distribution of two groups of δ44/42Ca values, the p value of Student's t-tests provides the smallest level of significance at which null hypothesis would be rejected (NS, non-significant p value; *p = 0.01–0.05; **p = 0.001–0.01; and ***p < 0.001).Another argument in favour of a basal trophic position for conodonts, is that modern piscivore elasmobranchs exhibit a much tighter grouping of the δ44/42Ca values than modern zooplanktivore elasmobranchs and conodonts (Figs. 1 and 2). The range of δ44/42Ca values for a given trophic level of piscivore elasmobranchs never exceeds 0.1 ‰ while it is higher than 0.2 ‰ for modern zooplanktivore elasmobranchs and conodonts. No definitive explanation can be put forward from the state of the results, but a reasonable hypothesis could be that animals at the bottom of the trophic chain are more likely to sample local isotopic heterogeneities. This variability is subsequently contracted at higher trophic level probably thanks to a biopurification process such as in the case of the Sr/Ca and Ba/Ca ratios (Peek and Clementz, 2012
Peek, S., Clementz, M.T. (2012) Sr/Ca and Ba/Ca variations in environmental and biological sources: A survey of marine and terrestrial systems. Geochimica et Cosmochimica Acta 95, 36-52.
).The present results suggest that no significant difference in trophic level may have existed among conodonts, because genera exhibit indistinguishable δ44/42Ca values (Table S-2). This overall similarity suggests that competition must have existed between some genera occupying similar trophic levels at the same time, i.e. between Palmatolepis and Polygnathus for instance. It is noteworthy that Ancyrodella is the only genus analysed in the study that disappeared at the F/F boundary, questioning the possibility that a distinct ecological trait would have triggered the extinction of this conodont genus.
Using a similar Δ44/42Casw-hap for conodont elements and modern elasmobranchs to retrieve the trophic position of conodont animals implies similar vital effects (i.e. isotope fractionation due to biological processes) in both groups. This approach is however probably simplistic. In fish, Ca is taken up along three pathways, (1) directly from the water via the gills, which contain a lot of ion-transporting cells or chloride cells (also known as ionocytes), but also through the intestine from (2) drinking water and (3) food (Flik and Verbost, 1993
Flik, G., Verbost, P.M. (1993) Calcium transport in fish gills and intestine. Journal of Experimental Biology 184, 17-29.
). No evidence for gills has ever been reported in preserved specimens of conodont animals (Aldridge and Purnell, 1996Aldridge, R.J., Purnell, M.A. (1996) The conodont controversies. Trends in Ecology and Evolution 11, 463-468.
), which would suggest distinct Δ44/42Casw-hap values between conodont animals and elasmobranchs. Total intestinal absorption of calcium in marine fish represents around 30 % of the total calcium intake (Björnsson and Nilsson, 1985Björnsson, B.T., Nilsson, S. (1985) Renal and extrarenal excretion of calcium in the marine teleost, Gadus morhua. American Journal of Physiology 248, 18–22.
; Sundell and Björnsson, 1988Sundell, K., Björnsson, B.T. (1988) Kinetics of calcium fluxes across the intestinal mucosa of the marine teleost, Gadus morhua, measured using an in vitro perfusion method. Journal of Experimental Biology 140, 170–186.
). To our knowledge, relative proportions of drinking water and food in fish have never been determined, but the isotopic results of Martin et al. (2015)Martin, J.E., Tacail, T., Adnet, S., Girard, C., Balter, V. (2015) Calcium isotopes reveal the trophic position of extant and fossil elasmobranchs. Chemical Geology 415, 118-125.
in elasmobranchs demonstrate that food must make a sizable proportion, otherwise no trophic effect would have been observed. Indeed, the most likely explanation to account for the depletion of Ca heavy isotopes up trophic chains, being marine or terrestrial, is that preys are wholly ingested along with their skeleton which is depleted in heavy Ca isotopes. If the three types of Ca uptake described above are characterised by different isotope fractionation intensity, and their relative proportions vary between fish groups, this should in principle result in a different Δ44/42Casw-hap fractionation. Analysis of dietary relevant trace elements for marine organisms, such as Sr/Ca and Ba/Ca ratios (Balter and Lécuyer, 2004Balter V., Lécuyer C. (2004) Determination of Sr and Ba partitioning coefficients between apatite and water from 5°C to 60°C: a new thermometer for aquatic environments. Geochimica et Cosmochimica Acta 68, 423-432.
, 2010Balter V., Lécuyer C. (2010) Determination of Sr and Ba partition coefficients between apatite from fish (Sparus aurata) and seawater: the influence of temperature. Geochimica et Cosmochimica Acta 74, 3449-3458.
; Le Houedec et al., 2013Le Houedec, S., Girard, C., Balter, V. (2013) Conodont Sr/Ca and δ18O record seawater changes at the Frasnian-Famennian boundary. Palaeogeography, Palaeoclimatology, Palaeoecology 376, 114-121.
; Peek and Clementz, 2012Peek, S., Clementz, M.T. (2012) Sr/Ca and Ba/Ca variations in environmental and biological sources: A survey of marine and terrestrial systems. Geochimica et Cosmochimica Acta 95, 36-52.
) would corroborate the present results, but we question whether this would be feasible in light of the difference of vital effects discussed above, and of potential diagenetic effects. Further analysis of Ca isotopes in conodont assemblages will document the diversity of their ecological niches within Palaeozoic oceanic trophic chains.top
Acknowledgements
We thank two anonymous reviewers for their insightful comments on an earlier version of the manuscript and R. Feist (Montpellier) who provided us with the brachiopod shell analysed in this work. This study was supported by the ANR project Ecodev (ANR-13-BSV7-0005). ISEM contribution 2019-071.
Editor: Ariel Anbar
top
References
Aldridge, R.J., Purnell, M.A. (1996) The conodont controversies. Trends in Ecology and Evolution 11, 463-468.
 Show in context
Show in context No evidence for gills has ever been reported in preserved specimens of conodont animals (Aldridge and Purnell, 1996), which would suggest distinct Δ44/42Casw-hap values between conodont animals and elasmobranchs.
View in article
Balter V., Lécuyer C. (2004) Determination of Sr and Ba partitioning coefficients between apatite and water from 5°C to 60°C: a new thermometer for aquatic environments. Geochimica et Cosmochimica Acta 68, 423-432.
 Show in context
Show in context Analysis of dietary relevant trace elements for marine organisms, such as Sr/Ca and Ba/Ca ratios (Balter and Lécuyer, 2004, 2010; Le Houedec et al., 2013; Peek and Clementz, 2012) would corroborate the present results, but we question whether this would be feasible in light of the difference of vital effects discussed above, and of potential diagenetic effects.
View in article
Balter V., Lécuyer C. (2010) Determination of Sr and Ba partition coefficients between apatite from fish (Sparus aurata) and seawater: the influence of temperature. Geochimica et Cosmochimica Acta 74, 3449-3458.
 Show in context
Show in context Analysis of dietary relevant trace elements for marine organisms, such as Sr/Ca and Ba/Ca ratios (Balter and Lécuyer, 2004, 2010; Le Houedec et al., 2013; Peek and Clementz, 2012) would corroborate the present results, but we question whether this would be feasible in light of the difference of vital effects discussed above, and of potential diagenetic effects.
View in article
Balter V., Renaud S., Girard C., Joachimski, M. (2008) The record of climate-driven morphological changes in 376 Ma old Devonian fossils. Geology 36, 907-910.
 Show in context
Show in context Here, the Late Devonian period, particularly the Frasnian-Famennian boundary (F/F), was chosen because it is accompanied by important variations in the shape of conodont elements, suggestive of changes in the feeding behaviour of several genera (Balter et al., 2008; Girard and Renaud, 2008).
View in article
Blättler, C.L., Henderson, G.M., Jenkyns, H.C. (2012) Explaining the Phanerozoic Ca isotope history of seawater. Geology 40, 843-846.
 Show in context
Show in context Based on the existing Ca isotopic fractionation factor between modern brachiopods (br) Terebratalia and seawater (sw), αbr-sw = 0.99915 (Gussone et al., 2005), the δ44/42Ca value of end-Devonian seawater (δ44/42Casw) was estimated at 0.55 ‰, i.e. ~0.4 ‰ lower than that of modern oceans (Blättler et al. 2012).
View in article
Björnsson, B.T., Nilsson, S. (1985) Renal and extrarenal excretion of calcium in the marine teleost, Gadus morhua. American Journal of Physiology 248, 18–22.
 Show in context
Show in context Total intestinal absorption of calcium in marine fish represents around 30 % of the total calcium intake (Björnsson and Nilsson, 1985; Sundell and Björnsson, 1988).
View in article
Briggs, D.E.G., Clarkson, E.N.K., Aldridge, R.J. (1983) The conodont animal. Lethaia 16, 1-14.
 Show in context
Show in context Until the publication of the discovery of the first specimen of the conodont animal in 1983 with conodont elements in situ forming a feeding apparatus, the nature and function of the conodont elements was one of palaeontology's great mysteries (Briggs et al., 1983).
View in article
Clementz, M. T., Holden, P., Koch, P.L. (2003) Are Calcium isotopes a reliable monitor of trophic level in marine settings? International Journal of Osteoarchaeology 13, 29-36.
 Show in context
Show in context The first studies showing that trophic levels of animals, including fish, could be inferred from the Ca isotope compositions of their shell or inner skeleton lay back in the 2000's (Skulan et al., 1997; Skulan and DePaolo, 1999; Clementz et al., 2003; DePaolo, 2004).
View in article
DePaolo, D.J. (2004) Calcium Isotopic Variations Produced by Biological, Kinetic, Radiogenic and Nucleosynthetic Processes. Reviews in Mineralogy and Geochemistry 55, 255-288.
 Show in context
Show in context The first studies showing that trophic levels of animals, including fish, could be inferred from the Ca isotope compositions of their shell or inner skeleton lay back in the 2000's (Skulan et al., 1997; Skulan and DePaolo, 1999; Clementz et al., 2003; DePaolo, 2004).
View in article
Farkaš, J., Böhm, F., Wallmann, K., Blenkinsop, J., Eisenhauer, A., van Geldern, R., Munnecke, A., Voigt, S., Veizer, J. (2007) Calcium isotope record of Phanerozoic oceans: implications for chemical evolution of seawater and its causative mechanisms: Geochimica et Cosmochimica Acta 71, 5117-5134.
 Show in context
Show in context With an age estimated slightly younger than the Devonian-Carboniferous boundary of 360 Ma, this value falls in the range, but in the lower limit, of the reconstructed Phanerozoic seawater Ca isotope composition of Farkaš et al. (2007; Fig. S-3).
View in article
To fully encompass the δ44/42Casw variability at that time, which is well described by the study of Farkaš et al. (2007), we can also calculate the Δ44/42Casw-hap with the upper limit of the contemporaneous δ44/42Casw value, i.e. ~0.67 ‰.
View in article
Figure 2 [...] The Δ44/42Casw-hap offset is calculated with two δ44/42Casw values, 0.55 ‰ (diagonal lines) and 0.67 ‰ (diagonal cross hatch) corresponding to the range given by Farkaš et al. (2007; Fig. S-3).
View in article
Flik, G., Verbost, P.M. (1993) Calcium transport in fish gills and intestine. Journal of Experimental Biology 184, 17-29.
 Show in context
Show in context In fish, Ca is taken up along three pathways, (1) directly from the water via the gills, which contain a lot of ion-transporting cells or chloride cells (also known as ionocytes), but also through the intestine from (2) drinking water and (3) food (Flik and Verbost, 1993).
View in article
Gabbott, S.E., Aldridge, R.J., Theron, J.N. (1995) A giant conodont with preserved muscle tissue from the Upper Ordovician of south Africa. Nature 374, 800-803.
 Show in context
Show in context Conodonts possessed sclerotic eye capsule and extrinsic eye musculature (Gabbott et al., 1995; Purnell, 1995a), consistent with conodonts having pattern vision and an active predatory lifestyle.
View in article
Girard, C., Renaud, S. (2008) Disentangling allometry and response to Kellwasser anoxic events in the Late Devonian conodont genus Ancyrodella. Lethaia 41, 383-394.
 Show in context
Show in context Here, the Late Devonian period, particularly the Frasnian-Famennian boundary (F/F), was chosen because it is accompanied by important variations in the shape of conodont elements, suggestive of changes in the feeding behaviour of several genera (Balter et al., 2008; Girard and Renaud, 2008).
View in article
Goudemand, N., Orchard, M.J., Urdy, S., Bucher, H., Tafforeau, P. (2011) Synchrotron-aided reconstruction of the conodont feeding apparatus and implications for the mouth of the first vertebrates. Proceedings of the National Academy of Sciences USA 108, 8720-8724.
 Show in context
Show in context Today, despite the advancement of synchrotron microtomography that allows reconstructing virtual movements of the elements forming the feeding apparatus (Goudemand et al., 2011), the dietary behaviour of conodonts remains an open debate but recently Shirley et al. (2018) suggest a predatory or scavenger mode of life.
View in article
Gussone, N., Böhm, F., Eisenhauer, A., Dietzel, M., Heuser, A., Teichert, B.M.A., Reitner, J., Wörheide, G., Dullo, W.C. (2005) Calcium isotope fractionation in calcite and aragonite. Geochimica et Cosmochimica Acta 69, 4485-4494.
 Show in context
Show in context Based on the existing Ca isotopic fractionation factor between modern brachiopods (br) Terebratalia and seawater (sw), αbr-sw = 0.99915 (Gussone et al., 2005), the δ44/42Ca value of end-Devonian seawater (δ44/42Casw) was estimated at 0.55 ‰, i.e. ~0.4 ‰ lower than that of modern oceans (Blättler et al. 2012).
View in article
Hinojosa, J.L., Brown, S.T., Chen, J., DePaolo, D.J., Paytan, A., Shen, S.-Z., Payne, J.L. (2012) Evidence for end-Permian ocean acidification from calcium isotopes in biogenic apatite. Geology 40, 743-746.
 Show in context
Show in context Some authors have already measured the Ca isotope composition of conodonts but with the aim to reconstruct variations of the seawater composition (Hinojosa et al. 2012; Jost et al. 2014; Le Houedec et al. 2017).
View in article
Jost, A., Mundil, R., He, B., Brown, S.T., Altiner, D., Sun, Y., DePaolo, D.J., Payne, J.L (2014) Constraining the cause of the end-Guadalupian extinction with coupled records of carbon and calcium isotopes. Earth and Planeraty Science Letters 396, 201-212.
 Show in context
Show in context Some authors have already measured the Ca isotope composition of conodonts but with the aim to reconstruct variations of the seawater composition (Hinojosa et al. 2012; Jost et al. 2014; Le Houedec et al. 2017).
View in article
Le Houedec, S., Girard, C., Balter, V. (2013) Conodont Sr/Ca and δ18O record seawater changes at the Frasnian-Famennian boundary. Palaeogeography, Palaeoclimatology, Palaeoecology 376, 114-121.
 Show in context
Show in context Analysis of dietary relevant trace elements for marine organisms, such as Sr/Ca and Ba/Ca ratios (Balter and Lécuyer, 2004, 2010; Le Houedec et al., 2013; Peek and Clementz, 2012) would corroborate the present results, but we question whether this would be feasible in light of the difference of vital effects discussed above, and of potential diagenetic effects.
View in article
Le Houedec, S., McCulloch, M., Trotter, J., Rankenburg, K. (2017) Conodont apatite δ88/86Sr and δ44/40Ca compositions and implications for the evolution of Palaeozoic to early Mesozoic seawater. Chemical Geology 453, 55-65.
 Show in context
Show in context Some authors have already measured the Ca isotope composition of conodonts but with the aim to reconstruct variations of the seawater composition (Hinojosa et al. 2012; Jost et al. 2014; Le Houedec et al. 2017).
View in article
Martin, J.E., Tacail, T., Adnet, S., Girard, C., Balter, V. (2015) Calcium isotopes reveal the trophic position of extant and fossil elasmobranchs. Chemical Geology 415, 118-125.
 Show in context
Show in context These results were recently confirmed on modern and fossil elasmobranchs (Martin et al., 2015), a subclass of cartilaginous fish, including the sharks, rays and skates, and sawfish.
View in article
This allows comparing the average Ca isotopic offset between Devonian seawater and conodonts with that of modern seawater and extant elasmobranch tooth enameloid (Martin et al. 2015), which is annotated Δ44/42Casw-hap (Fig. 2).
View in article
Figure 2 [...] Modern elasmobranch data are from Martin et al. (2015).
View in article
To our knowledge, relative proportions of drinking water and food in fish have never been determined, but the isotopic results of Martin et al. (2015) in elasmobranchs demonstrate that food must make a sizable proportion, otherwise no trophic effect would have been observed.
View in article
Peek, S., Clementz, M.T. (2012) Sr/Ca and Ba/Ca variations in environmental and biological sources: A survey of marine and terrestrial systems. Geochimica et Cosmochimica Acta 95, 36-52.
 Show in context
Show in context This variability is subsequently contracted at higher trophic level probably thanks to a biopurification process such as in the case of the Sr/Ca and Ba/Ca ratios (Peek and Clementz, 2012).
View in article
Analysis of dietary relevant trace elements for marine organisms, such as Sr/Ca and Ba/Ca ratios (Balter and Lécuyer, 2004, 2010; Le Houedec et al., 2013; Peek and Clementz, 2012) would corroborate the present results, but we question whether this would be feasible in light of the difference of vital effects discussed above, and of potential diagenetic effects.
View in article
Purnell, M.A. (1995a) Large eyes and vision in conodonts. Lethaia 28, 187-188.
 Show in context
Show in context Conodonts possessed sclerotic eye capsule and extrinsic eye musculature (Gabbott et al., 1995; Purnell, 1995a), consistent with conodonts having pattern vision and an active predatory lifestyle.
View in article
Purnell, M.A. (1995b) Microwear on conodont elements and macrophagy in the first vertebrates. Nature 374, 798-800.
 Show in context
Show in context Lastly, microwear patterns were found on conodont elements, which constituted the first direct evidence that they functioned as teeth (Purnell, 1995b).
View in article
Purnell, M.A., Donoghue, P.C.J. (1997) Architecture and functional morphology of the skeletal apparatus of ozarkodinid conodonts. Philosophical Transactions of the Royal Society 352, 1545-1564.
 Show in context
Show in context Since then, several other specimens have shown similar schemes for this feeding apparatus, in which the anterior elements form a structure allowing them to trap food that is further processed by the posterior elements (Purnell and Donoghue, 1997).
View in article
Shirley, B., Grohganz, M., Bestmann, M., Jarochowska, E. (2018) Wear, tear and systematic repair: testing models of growth dynamics in conodonts with high-resolution imaging. Proceedings of the Royal Society B: Biological Sciences 285, doi: 10.1098/rspb.2018.1614.
 Show in context
Show in context Today, despite the advancement of synchrotron microtomography that allows reconstructing virtual movements of the elements forming the feeding apparatus (Goudemand et al., 2011), the dietary behaviour of conodonts remains an open debate but recently Shirley et al. (2018) suggested a predatory or scavenger mode of life.
View in article
Skulan, J., DePaolo, D.J. (1999) Calcium isotope fractionation between soft and mineralised tissues as a monitor of calcium use in vertebrates. Proceedings of the National Academy of Sciences USA 96, 13709 –13713.
 Show in context
Show in context The first studies showing that trophic levels of animals, including fish, could be inferred from the Ca isotope compositions of their shell or inner skeleton lay back in the 2000's (Skulan et al., 1997; Skulan and DePaolo, 1999; Clementz et al., 2003; DePaolo, 2004).
View in article
Skulan, J., DePaolo, D.J., Owens, T.L. (1997) Biological control of calcium isotopic abundances in the global calcium cycle. Geochimica et Cosmochimica Acta 61, 2505-2510.
 Show in context
Show in context The first studies showing that trophic levels of animals, including fish, could be inferred from the Ca isotope compositions of their shell or inner skeleton lay back in the 2000's (Skulan et al., 1997; Skulan and DePaolo, 1999; Clementz et al., 2003; DePaolo, 2004).
View in article
Sundell, K., Björnsson, B.T. (1988) Kinetics of calcium fluxes across the intestinal mucosa of the marine teleost, Gadus morhua, measured using an in vitro perfusion method. Journal of Experimental Biology 140, 170–186.
 Show in context
Show in context Total intestinal absorption of calcium in marine fish represents around 30 % of the total calcium intake (Björnsson and Nilsson, 1985; Sundell and Björnsson, 1988).
View in article
Tacail T., Albalat E., Télouk P., Balter V. (2014) A simplified protocol for the measurement of Ca isotopes in biological samples. Journal of Analytical Atomic Spectrometry 29, 529-535.
 Show in context
Show in context All measured samples were plotted as δ43/42Ca against δ44/42Ca and fall on a line with a slope of 0.557 close to the theoretical 0.507 slope predicted by the exponential approximation of mass dependent fractionation (Tacail et al., 2014; Fig. S-2).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Supplementary Material
- Supplementary Method
- Supplementary Discussion
- Tables S-1 to S-5
- Figures S-1 to S-3
- Supplementary Information References
Download the Supplementary Information (PDF)
Figures and Tables
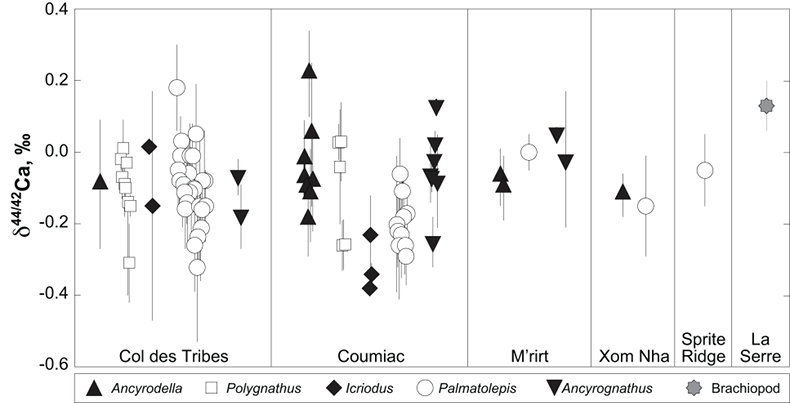
Figure 1 Ca isotope compositions of conodonts (δ44/42Ca) relative to SRM915a (‰) measured in the study.
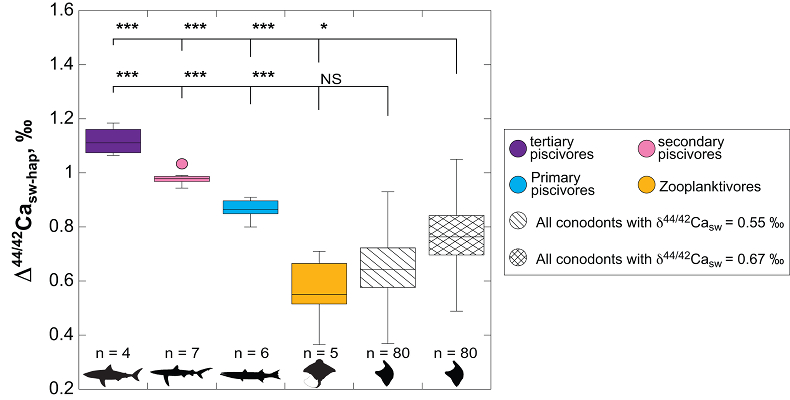
Figure 2 Ca isotopic offset between seawater and hap of modern elasmobranchs compared to that of conodonts (Δ44/42Casw-hap) measured in the study. Modern elasmobranch data are from Martin et al. (2015)
Martin, J.E., Tacail, T., Adnet, S., Girard, C., Balter, V. (2015) Calcium isotopes reveal the trophic position of extant and fossil elasmobranchs. Chemical Geology 415, 118-125.
. The Δ44/42Casw-hap offset is calculated with two δ44/42Casw values, 0.55 ‰ (diagonal lines) and 0.67 ‰ (diagonal cross hatch) corresponding to the range given by Farkaš et al. (2007Farkaš, J., Böhm, F., Wallmann, K., Blenkinsop, J., Eisenhauer, A., van Geldern, R., Munnecke, A., Voigt, S., Veizer, J. (2007) Calcium isotope record of Phanerozoic oceans: implications for chemical evolution of seawater and its causative mechanisms: Geochimica et Cosmochimica Acta 71, 5117-5134.
; Fig. S-3). Boxplots delimit 5, 25, 50, 75 and 95 % percentiles. Depending on the δ44/42Casw value, statistics show that conodonts and zooplanktivores have similar δ44/42Ca values. Under the null hypothesis that there is no difference in the distribution of two groups of δ44/42Ca values, the p value of Student's t-tests provides the smallest level of significance at which null hypothesis would be rejected (NS, non-significant p value; *p = 0.01–0.05; **p = 0.001–0.01; and ***p < 0.001).