Concentration of meteoritic free organic matter by fluid transport and adsorption
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:2,153Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
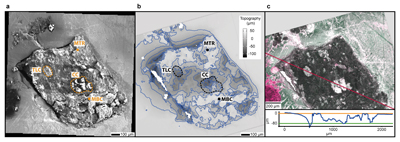 Figure 1 (a) An SEM image indicating the positions of chondrules (orange dashed lines) and matrix areas where EDX analyses were performed. (b) A topographic image of the sample surface obtained using a laser profiler. (c) An optical microscope image and topographic profile across the sample (red line). | 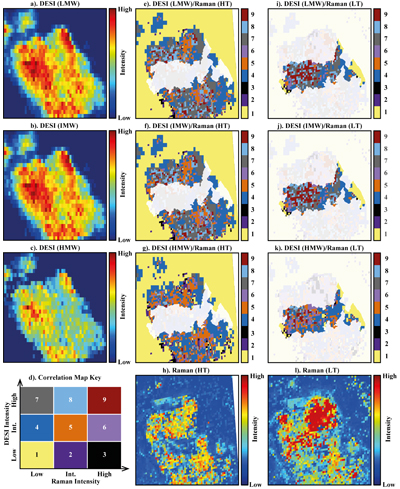 Figure 2 A compilation of DESI, Raman and correlation maps for selected alkylimidazole homologues. (a-c) DESI intensity maps for mass 139.12293, 181.16978 and 237.23225, respectively. Highest normalised intensity is 8.79 x 10-3, 1.22 x 10-2 and 3.84 x 10-3, respectively. (d) A schematic outlining the criteria for the generation of DESI vs. Raman correlation maps. (e-g) Correlation maps showing the similarity between a given DESI and high topography (HT) Raman map. (h) A Raman intensity map focused on high topography, for the interlayer/adsorbed water band (3000-3800 cm-1). (i-k) Correlation maps showing the similarity between a given DESI and low topography (LT) Raman map. (l) As for h except focused on low topography. Note that for the correlation maps a partially transparent white mask has been used to highlight the high topography (top 40 µm) or low topography (below top 40 µm). | 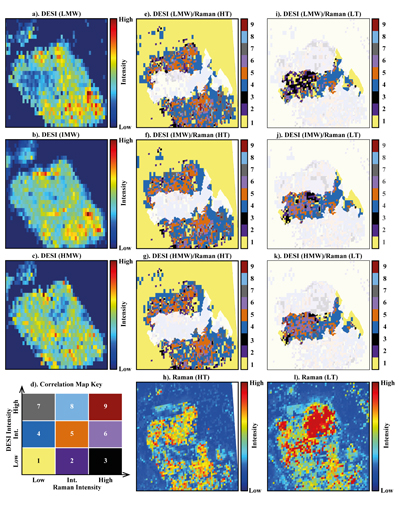 Figure 3 A compilation of DESI, Raman and correlation maps for selected alkylpyridine homologues. (a-c) DESI intensity maps for mass 136.11205, 178.15891 and 220.20580, respectively. Highest normalised intensity is 2.17 x 10-3, 5.44 x 10-2 and 2.58 x 10-3, respectively. (d) A schematic outlining the criteria for the generation of DESI vs. Raman correlation maps. (e-g) Correlation maps showing the similarity between a given DESI and high topography (HT) Raman map. (h) A Raman intensity map focused on high topography, for the interlayer/adsorbed water band (3000-3800 cm-1). (i-k) Correlation maps showing the similarity between a given DESI and low topography (LT) Raman map. (l) As for h except focused on low topography. Note that for the correlation maps a partially transparent white mask has been used to highlight the high topography (top 40 µm) or low topography (below top 40 µm). | 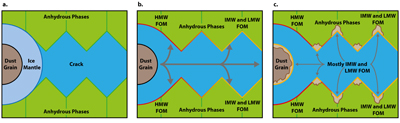 Figure 4 FOM distribution during aqueous alteration. (a) FOM and water ice are accreted by the parent body. (b) The ice melts and HMW FOM (red) adsorbs onto phases close to the site of accretion, but LMW and IMW FOM (light orange) are transported further into the matrix, generating a geochromatographic effect (red to light orange). (c) With the generation of phyllosilicates, adsorption of the remaining organic matter (LMW and IMW), as well as any previously adsorbed, occurs on these new charged mineral surfaces. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
CM2 carbonaceous chondrites, such as Murchison, demonstrate significant aqueous alteration recorded by their hydrous phases. Phyllosilicates replaced anhydrous minerals, such as olivine and pyroxene, originally accreted by the meteorite parent body/bodies (McSween, 1987
McSween, H.Y. (1987) Aqueous alteration in carbonaceous chondrites: Mass balance constraints on matrix mineralogy. Geochimica et Cosmochimica Acta 51, 2469–2477.
; Brearley, 2006Brearley, A.J. (2006) The action of water. In: Lauretta, D.S., McSween Jr., H.Y. (Eds.) Meteorites and the Early Solar System II. University of Arizona Press, Tucson, 587–624.
). Carbonaceous chondrites also contain abundant free and macromolecular organic matter (Pizzarello and Shock, 2010Pizzarello, S., Shock, E. (2010) The organic composition of carbonaceous meteorites: the evolutionary story ahead of biochemistry. Cold Spring Harbor Perspectives in Biology 2, 1–19.
; Sephton, 2013Sephton, M.A. (2013) Organic Geochemistry of Meteorites. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry: Second Edition. Elsevier, Oxford, 1–31.
) (FOM and MOM, respectively). Much of the meteoritic organic matter is thought to originate from within the interstellar medium (Sandford, 1996Sandford, S.A. (1996) The inventory of interstellar materials available for the formation of the solar system. Meteoritics and Planetary Science 31, 449–476.
; Kerridge, 1999Kerridge, J.F. (1999) Formation and processing of organics in the early solar system. Space Science Reviews 90, 275–288.
) and/or protosolar nebular (Ciesla and Sandford, 2012Ciesla, F.J., Sandford, S.A. (2012) Organic Synthesis via Irradiation and Warming of Ice Grains in the Solar Nebula. Science 336, 452–454.
; Bekaert et al., 2018Bekaert, D. V, Derenne, S., Tissandier, L., Marrocchi, Y., Anquetil, C., Marty, B., Charnoz, S. (2018) High-temperature Ionization-induced Synthesis of Biologically Relevant Molecules in the Protosolar Nebula. The Astrophysical Journal 859, 142.
). Nevertheless, aqueous alteration has been indicated in the origin of some FOM compounds (Peltzer et al., 1984Peltzer, E.T., Bada, J.L., Schlesinger, G., Miller, S.L. (1984) The chemical conditions on the parent body of the murchison meteorite: Some conclusions based on amino, hydroxy and dicarboxylic acids. Advances in Space Research 4, 69–74.
; Sephton et al., 1998Sephton, M.A., Pillinger, C.T., Gilmour, I. (1998) δ13C of free and macromolecular aromatic structures in the murchison meteorite. Geochimica et Cosmochimica Acta 62, 1821–1828.
). Subsequently, experiments have indicated the alteration of accreted organic matter could yield present day organic matter fractions and compounds within carbonaceous chondrites (Kebukawa et al., 2017Kebukawa, Y., Chan, Q.H.S., Tachibana, S., Kobayashi, K., Zolensky, M.E. (2017) One-pot synthesis of amino acid precursors with insoluble organic matter in planetesimals with aqueous activity. Science Advances 3, e1602093.
; Vinogradoff et al., 2018Vinogradoff, V., Bernard, S., Le Guillou, C., Remusat, L. (2018) Evolution of interstellar organic compounds under asteroidal hydrothermal conditions. Icarus 305, 358–370.
).Whilst many previous studies have examined the role of aqueous alteration on the mineral and organic phases, fewer have focussed on how aqueous alteration generates the relationships between these two phases. Phyllosilicates and carbonates are known to be associated with organic matter and are also known products of water-anhydrous mineral interaction. Although other interpretations exist, carbonates have been indicated as the products of organic matter oxidation (Alexander et al., 2015
Alexander, C.M.O., Bowden, R., Fogel, M.L., Howard, K.T. (2015) Carbonate abundances and isotopic compositions in chondrites. Meteoritics & Planetary Science 50, 810–833.
; Yesiltas and Kebukawa, 2016Yesiltas, M., Kebukawa, Y. (2016) Associations of organic matter with minerals in Tagish Lake meteorite via high spatial resolution synchrotron-based FTIR microspectroscopy. Meteoritics and Planetary Science 51, 584–595.
; Chan et al., 2017Chan, Q.H.S., Zolensky, M.E., Bodnar, R.J., Farley, C., Cheung, J.C.H. (2017) Investigation of organo-carbonate associations in carbonaceous chondrites by Raman spectroscopy. Geochimica et Cosmochimica Acta 201, 392–409.
) and phyllosilicates are thought to protect or catalyse the synthesis/alteration of organic matter (Pearson et al., 2007Pearson, V.K., Kearsley, A.T., Sephton, M.A., Gilmour, I. (2007) The labelling of meteoritic organic material using osmium tetroxide vapour impregnation. Planetary and Space Science 55, 1310–1318.
; Kebukawa et al., 2010Kebukawa, Y., Nakashima, S., Ishikawa, M., Aizawa, K., Inoue, T., Nakamura-messenger, K., Zolensky, M.E. (2010) Spatial distribution of organic matter in the Bells CM2 chondrite using near-field infrared microspectroscopy. Meteoritics & Planetary Science 405, 394–405.
; Le Guillou et al., 2014Le Guillou, C., Bernard, S., Brearley, A.J., Remusat, L. (2014) Evolution of organic matter in Orgueil, Murchison and Renazzo during parent body aqueous alteration: In situ investigations. Geochimica et Cosmochimica Acta 131, 368–392.
; Yesiltas and Kebukawa, 2016Yesiltas, M., Kebukawa, Y. (2016) Associations of organic matter with minerals in Tagish Lake meteorite via high spatial resolution synchrotron-based FTIR microspectroscopy. Meteoritics and Planetary Science 51, 584–595.
).Desorption electrospray ionisation-orbitrap-mass spectrometry (DESI-OT-MS) has been used previously to study Murchison (CM2) and Murray (CM2) chondrites (Hashiguchi and Naraoka, 2018
Hashiguchi, M., Naraoka, H. (2018) High-mass resolution molecular imaging of organic compounds on the surface of Murchison meteorite. Meteoritics & Planetary Science 17, 1–17.
; Naraoka and Hashiguchi, 2018Naraoka, H., Hashiguchi, M. (2018) In situ organic compound analysis on a meteorite surface by desorption electrospray ionization coupled with an Orbitrap mass spectrometer. Rapid Communications in Mass Spectrometry 32, 959–964.
). 2D DESI-OT-MS maps of alkylimidazole, alkylpyridine and CnH2nNO+ homologues were reported. It was found that for a given mass the distribution of the homologues was different and this was interpreted as a result of chromatographic separation through water-matrix interaction. Nevertheless, despite the use of scanning electron microscopy (SEM), no definitive relationship between mineral phases and the organic compounds could be established. The difference in the resolution of DESI-OT-MS (~50-200 µm) and SEM data (sub-µm) was suggested as the likely reason for the lack of a mineral-organic relationship.Raman spectroscopy can reveal the spatial distribution of both organic and mineral phases. Meteoritic organic matter was revealed to be a useful indicator of thermal alteration and was employed as a metamorphic thermometer (Bonal et al., 2006
Bonal, L., Quirico, E., Bourot-Denise, M., Montagnac, G. (2006) Determination of the petrologic type of CV3 chondrites by Raman spectroscopy of included organic matter. Geochimica et Cosmochimica Acta 70, 1849–1863.
; Huss et al., 2006Huss, G.R., Rubin, A.E., Grossman, J.N. (2006) Thermal Metamorphism in Chondrites. In: Lauretta, D., McSween, H.Y. (Eds.) Meteorites and the early solar system II. University of Arizona Press, Tuscon, 567–586.
; Busemann et al., 2007Busemann, H., Alexander, C.M.O., Nittler, L.R. (2007) Characterization of insoluble organic matter in primitive meteorites by microRaman spectroscopy. Meteoritics & Planetary Science 42, 1387–1416.
). Previous Raman spectroscopic studies have also identified olivine, pyroxene, Fe-oxides, Fe-oxyhydroxides and phyllosilicates in carbonaceous chondrites and enabled 2D mapping (El Amri et al., 2005El Amri, C., Maurel, M.-C., Sagon, G., Baron, M.-H. (2005) The micro-distribution of carbonaceous matter in the Murchison meteorite as investigated by Raman imaging. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 61, 2049–2056.
; Kong and Wang, 2010Kong, W.G., Wang, A. (2010) Planetary Laser Raman Spectroscopy for Surface Exploration on C/D-Type Asteroids - A Case Study. 41st Lunar and Planetary Science Conference, 2730–2731.
; Wang et al., 2015Wang, A., Korotev, R.L., Jolliff, B.L., Ling, Z. (2015) Raman imaging of extraterrestrial materials. Planetary and Space Science 112, 23–34.
). However, no previous studies have combined DESI-OT-MS and Raman mapping to probe carbonaceous chondrites.top
Results and Discussion
In order to highlight relationships between FOM, mineral phases and textures, we imaged the sample using SEM. Two chondrules were identified from the SEM image (Fig. 1a), a large central chondrule (CC) and a smaller one to the top left (TLC). Energy dispersive X-ray (EDX) analysis indicates the CC is composed of forsteritic olivine (Fo99), whilst the TLC is a mixture of forsteritic olivine (Fo94) and low Ca pyroxene (Wo1En92) (Table S-1). Considering the texture, mineral assemblages and chemical composition of the chondrules, the chondrules can be classified as type 1 PO and POP, respectively (Scott and Krot, 2013
Scott, E.R.D., Krot, A.N. (2013) Chondrites and Their Components. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry: Second Edition. Elsevier, Oxford, 65–137.
). Despite significant aqueous alteration of CM2 chondrites on their parent body/bodies, the chondrules represent mostly unaltered mineral phases.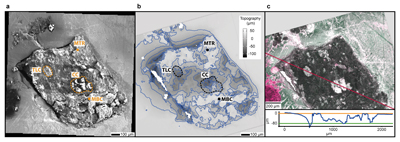
Figure 1 (a) An SEM image indicating the positions of chondrules (orange dashed lines) and matrix areas where EDX analyses were performed. (b) A topographic image of the sample surface obtained using a laser profiler. (c) An optical microscope image and topographic profile across the sample (red line).

Figure 2 A compilation of DESI, Raman and correlation maps for selected alkylimidazole homologues. (a-c) DESI intensity maps for mass 139.12293, 181.16978 and 237.23225, respectively. Highest normalised intensity is 8.79 x 10-3, 1.22 x 10-2 and 3.84 x 10-3, respectively. (d) A schematic outlining the criteria for the generation of DESI vs. Raman correlation maps. (e-g) Correlation maps showing the similarity between a given DESI and high topography (HT) Raman map. (h) A Raman intensity map focused on high topography, for the interlayer/adsorbed water band (3000-3800 cm-1). (i-k) Correlation maps showing the similarity between a given DESI and low topography (LT) Raman map. (l) As for h except focused on low topography. Note that for the correlation maps a partially transparent white mask has been used to highlight the high topography (top 40 µm) or low topography (below top 40 µm).
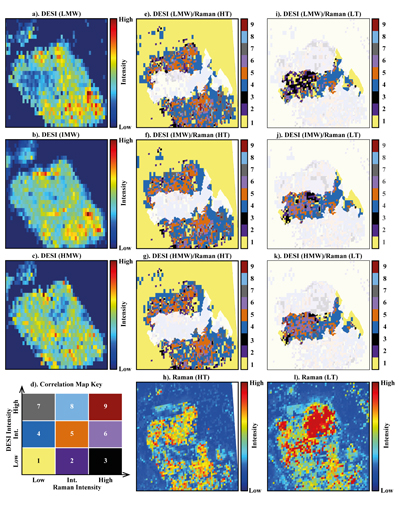
Figure 3 A compilation of DESI, Raman and correlation maps for selected alkylpyridine homologues. (a-c) DESI intensity maps for mass 136.11205, 178.15891 and 220.20580, respectively. Highest normalised intensity is 2.17 x 10-3, 5.44 x 10-2 and 2.58 x 10-3, respectively. (d) A schematic outlining the criteria for the generation of DESI vs. Raman correlation maps. (e-g) Correlation maps showing the similarity between a given DESI and high topography (HT) Raman map. (h) A Raman intensity map focused on high topography, for the interlayer/adsorbed water band (3000-3800 cm-1). (i-k) Correlation maps showing the similarity between a given DESI and low topography (LT) Raman map. (l) As for h except focused on low topography. Note that for the correlation maps a partially transparent white mask has been used to highlight the high topography (top 40 µm) or low topography (below top 40 µm).
To assess the contribution of topographic effects, a full assessment of the sample surface topography was undertaken using a laser profiler (Fig. 1b). High intensity areas are found in relation to both high and low topography in the DESI-OT-MS maps. Therefore, the observed intensity distributions cannot be explained by topography. Furthermore, Raman maps focussed on high and low topography (Figs. 2 and 3) were used to mitigate topographic effects, when comparing areas related to high adsorbed/interlayer water and DESI-OT-MS intensity. A more comprehensive and technical assessment of topography is available in the methods section.
Similar to previous studies (Hashiguchi and Naraoka, 2018
Hashiguchi, M., Naraoka, H. (2018) High-mass resolution molecular imaging of organic compounds on the surface of Murchison meteorite. Meteoritics & Planetary Science 17, 1–17.
; Naraoka and Hashiguchi, 2018Naraoka, H., Hashiguchi, M. (2018) In situ organic compound analysis on a meteorite surface by desorption electrospray ionization coupled with an Orbitrap mass spectrometer. Rapid Communications in Mass Spectrometry 32, 959–964.
), a disparity in the intensity distribution between low and intermediate and high molecular weight alkylimidazole homologues was observed (Fig. S-5). The correlation maps for low and intermediate molecular weight (LMW and IMW, respectively) alkylimidazoles (Fig. 2) record a widespread distribution of intermediate and high DESI/low Raman correlation, whereas the high molecular weight (HMW) maps show predominantly an intermediate/low Raman correlation. Such a relationship is compatible with a geochromatographic effect associated with the anhydrous unaltered phases. Thus, HMW homologues may have been adsorbed close to the accretion source, whilst IMW and LMW homologues were adsorbed further away.The alkylpyridine homologues did not record a similar relationship, instead they show a relatively consistent intensity distribution between HMW, IMW and LMW homologues (Figs. 3 and S-6). It is possible that all homologues were deposited close to or at the site of accretion, potentially because they are less soluble or insoluble in aqueous solutions when compared to alkylimidazoles. Unfortunately, information concerning the solubility of the homologues studied here is very limited and standards were not available.
No studies have yet confirmed a relationship between specific FOM homologues and mineral phases such as phyllosilicates. Raman spectroscopy revealed an intense interlayer/adsorbed water band between 3000-3800 cm-1 in Murchison. Both our Raman data (Fig. S-7) and those of previous studies link this water band to phyllosilicates (Kong and Wang, 2010
Kong, W.G., Wang, A. (2010) Planetary Laser Raman Spectroscopy for Surface Exploration on C/D-Type Asteroids - A Case Study. 41st Lunar and Planetary Science Conference, 2730–2731.
; Wang et al., 2015Wang, A., Korotev, R.L., Jolliff, B.L., Ling, Z. (2015) Raman imaging of extraterrestrial materials. Planetary and Space Science 112, 23–34.
). In the correlation maps for the alkylimidazoles (Fig. 2e-g.), there are almost no low/low, intermediate or high relationships observed for DESI/Raman. However, the LMW and IMW alkylimidazoles demonstrate widespread clusters of high/high and high/intermediate correlation for DESI/Raman correlation maps. Therefore, where there are large abundances of phyllosilicates, there are either intermediate or high levels of the alkylimidazole homologues. Furthermore, there are more clusters of high/high and high/intermediate correlation within the LMW and IMW homologue correlation maps, compared to those for the HMW maps, indicating a stronger relationship between these homologues and phyllosilicates. Conversely, the alkylpyridine homologues show mainly an intermediate/low correlation for high topography DESI/Raman (Fig. 2e-g.), with clusters of intermediate/intermediate correlation. Furthermore, the distribution of the clusters is similar between each map, suggesting that a similar interaction with phyllosilicates occurred for the alkylpyridine homologues.The results reported here suggest a more complex picture (Fig. 4) than those of previous studies (Hashiguchi and Naraoka, 2018
Hashiguchi, M., Naraoka, H. (2018) High-mass resolution molecular imaging of organic compounds on the surface of Murchison meteorite. Meteoritics & Planetary Science 17, 1–17.
; Naraoka and Hashiguchi, 2018Naraoka, H., Hashiguchi, M. (2018) In situ organic compound analysis on a meteorite surface by desorption electrospray ionization coupled with an Orbitrap mass spectrometer. Rapid Communications in Mass Spectrometry 32, 959–964.
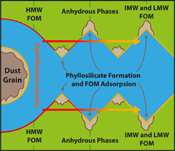
). Alkylimidazole and alkylpyridine homologues contained within ice mantles could be introduced at the time of accretion. Aqueous fluids generated from accreted ice then mobilise the alkylimidazole homologues. HMW homologues are relatively hydrophobic due to their large chain lengths or numbers of substitutions (Naraoka et al., 2017Naraoka, H., Yamashita, Y., Yamaguchi, M., Orthous-Daunay, F.R. (2017) Molecular Evolution of N-Containing Cyclic Compounds in the Parent Body of the Murchison Meteorite. ACS Earth and Space Chemistry 1, 540–550.
) and may have thus been transported only a short distance (Fig. 4a). Conversely, the LMW and IMW alkylimidazole homologues were transported further into the matrix of Murchison (Fig. 4b) and generated the spatial disparity between these homologues and their HMW counterparts. Subsequently, phyllosilicates form due to the alteration of anhydrous phases (Fig. 4c). Phyllosilicates have a larger surface area and surface charge, which allows the previously adsorbed homologues and LMW and IMW homologues in solution to be adsorbed on these phases. Such a process could give rise to the high/high and high/intermediate correlation clusters, from the LMW and IMW correlation maps of the alkylimidazole homologues. Indeed, organic adsorption onto phyllosilicate surfaces has been observed in marine clays (Kennedy et al., 2002Kennedy, M.J., Pevear, D.R., Hill, R.J. (2002) Mineral Surface Control of Organic Carbon in Black Shale. Science 295, 657–660.
) and has been proposed to protect organic matter from oxidising fluids on meteorite parent bodies (Yesiltas and Kebukawa, 2016Yesiltas, M., Kebukawa, Y. (2016) Associations of organic matter with minerals in Tagish Lake meteorite via high spatial resolution synchrotron-based FTIR microspectroscopy. Meteoritics and Planetary Science 51, 584–595.
).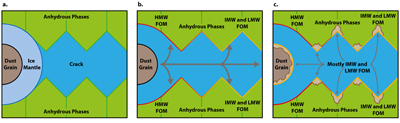
Figure 4 FOM distribution during aqueous alteration. (a) FOM and water ice are accreted by the parent body. (b) The ice melts and HMW FOM (red) adsorbs onto phases close to the site of accretion, but LMW and IMW FOM (light orange) are transported further into the matrix, generating a geochromatographic effect (red to light orange). (c) With the generation of phyllosilicates, adsorption of the remaining organic matter (LMW and IMW), as well as any previously adsorbed, occurs on these new charged mineral surfaces.
In conclusion, aqueous alteration affects homologue series in different ways, which may be dependent on their affinity for aqueous rich fluids. For alkylimidazoles, aqueous alteration concentrates LMW and IMW FOM within and upon phyllosilicate phases. The organic compounds can then be protected from subsequent alteration and delivered to rocky planets over the course of the solar system’s history. Many FOM components are important to living organisms, such as amino acids, sugars and nucleobases. The availability of these compounds early in the Earth’s history could have aided the evolution of life.
top
Acknowledgements
We are greatly indebted to PML members for their assistance maintaining the laboratory. We would also like to thank Dr. Kengo Nojima, Tottori Institute of Industrial Technology, for providing the laser profiler information for the Murchison sample discussed within this manuscript. This work was supported by Ministry of Education, Culture and Sports, Science and Technology (MEXT) of Japan.
Editor: Liane G. Benning
top
References
Alexander, C.M.O., Bowden, R., Fogel, M.L., Howard, K.T. (2015) Carbonate abundances and isotopic compositions in chondrites. Meteoritics & Planetary Science 50, 810–833.
 Show in context
Show in context Although other interpretations exist, carbonates have been indicated as the products of organic matter oxidation (Alexander et al., 2015; Yesiltas and Kebukawa, 2016; Chan et al., 2017) and phyllosilicates are thought to protect or catalyse the synthesis/alteration of organic matter (Pearson et al., 2007; Kebukawa et al., 2010; Le Guillou et al., 2014; Yesiltas and Kebukawa, 2016).
View in article
Bekaert, D. V, Derenne, S., Tissandier, L., Marrocchi, Y., Anquetil, C., Marty, B., Charnoz, S. (2018) High-temperature Ionization-induced Synthesis of Biologically Relevant Molecules in the Protosolar Nebula. The Astrophysical Journal 859, 142.
 Show in context
Show in context Much of the meteoritic organic matter is thought to originate from within the interstellar medium (Sandford, 1996; Kerridge, 1999) and/or protosolar nebular (Ciesla and Sandford, 2012; Bekaert et al., 2018).
View in article
Bonal, L., Quirico, E., Bourot-Denise, M., Montagnac, G. (2006) Determination of the petrologic type of CV3 chondrites by Raman spectroscopy of included organic matter. Geochimica et Cosmochimica Acta 70, 1849–1863.
 Show in context
Show in context Meteoritic organic matter was revealed to be a useful indicator of thermal alteration and was employed as a metamorphic thermometer (Bonal et al., 2006; Huss et al., 2006; Busemann et al., 2007).
View in article
Brearley, A.J. (2006) The action of water. In: Lauretta, D.S., McSween Jr., H.Y. (Eds.) Meteorites and the Early Solar System II. University of Arizona Press, Tucson, 587–624.
 Show in context
Show in context Phyllosilicates replaced anhydrous minerals, such as olivine and pyroxene, originally accreted by the meteorite parent body/bodies (McSween, 1987; Brearley, 2006).
View in article
Busemann, H., Alexander, C.M.O., Nittler, L.R. (2007) Characterization of insoluble organic matter in primitive meteorites by microRaman spectroscopy. Meteoritics & Planetary Science 42, 1387–1416.
 Show in context
Show in context Meteoritic organic matter was revealed to be a useful indicator of thermal alteration and was employed as a metamorphic thermometer (Bonal et al., 2006; Huss et al., 2006; Busemann et al., 2007).
View in article
Chan, Q.H.S., Zolensky, M.E., Bodnar, R.J., Farley, C., Cheung, J.C.H. (2017) Investigation of organo-carbonate associations in carbonaceous chondrites by Raman spectroscopy. Geochimica et Cosmochimica Acta 201, 392–409.
 Show in context
Show in context Although other interpretations exist, carbonates have been indicated as the products of organic matter oxidation (Alexander et al., 2015; Yesiltas and Kebukawa, 2016; Chan et al., 2017) and phyllosilicates are thought to protect or catalyse the synthesis/alteration of organic matter (Pearson et al., 2007; Kebukawa et al., 2010; Le Guillou et al., 2014; Yesiltas and Kebukawa, 2016).
View in article
Ciesla, F.J., Sandford, S.A. (2012) Organic Synthesis via Irradiation and Warming of Ice Grains in the Solar Nebula. Science 336, 452–454.
 Show in context
Show in context Much of the meteoritic organic matter is thought to originate from within the interstellar medium (Sandford, 1996; Kerridge, 1999) and/or protosolar nebular (Ciesla and Sandford, 2012; Bekaert et al., 2018).
View in article
El Amri, C., Maurel, M.-C., Sagon, G., Baron, M.-H. (2005) The micro-distribution of carbonaceous matter in the Murchison meteorite as investigated by Raman imaging. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 61, 2049–2056.
 Show in context
Show in context Previous Raman spectroscopic studies have also identified olivine, pyroxene, Fe-oxides, Fe-oxyhydroxides and phyllosilicates in carbonaceous chondrites and enabled 2D mapping (El Amri et al., 2005; Kong and Wang, 2010; Wang et al., 2015).
View in article
Hashiguchi, M., Naraoka, H. (2018) High-mass resolution molecular imaging of organic compounds on the surface of Murchison meteorite. Meteoritics & Planetary Science 17, 1–17.
 Show in context
Show in context Desorption electrospray ionisation-orbitrap-mass spectrometry (DESI-OT-MS) has been used previously to study Murchison (CM2) and Murray (CM2) chondrites (Hashiguchi and Naraoka, 2018; Naraoka and Hashiguchi, 2018).
View in article
Similar to previous studies (Hashiguchi and Naraoka, 2018; Naraoka and Hashiguchi, 2018), a disparity in the intensity distribution between low and intermediate and high molecular weight alkylimidazole homologues was observed (Fig. S-5).
View in article
The results reported here suggest a more complex picture (Fig. 4) than those of previous studies (Hashiguchi and Naraoka, 2018; Naraoka and Hashiguchi, 2018).
View in article
Huss, G.R., Rubin, A.E., Grossman, J.N. (2006) Thermal Metamorphism in Chondrites. In: Lauretta, D., McSween, H.Y. (Eds.) Meteorites and the early solar system II. University of Arizona Press, Tuscon, 567–586.
 Show in context
Show in context Meteoritic organic matter was revealed to be a useful indicator of thermal alteration and was employed as a metamorphic thermometer (Bonal et al., 2006; Huss et al., 2006; Busemann et al., 2007).
View in article
Kebukawa, Y., Nakashima, S., Ishikawa, M., Aizawa, K., Inoue, T., Nakamura-messenger, K., Zolensky, M.E. (2010) Spatial distribution of organic matter in the Bells CM2 chondrite using near-field infrared microspectroscopy. Meteoritics & Planetary Science 405, 394–405.
 Show in context
Show in context Although other interpretations exist, carbonates have been indicated as the products of organic matter oxidation (Alexander et al., 2015; Yesiltas and Kebukawa, 2016; Chan et al., 2017) and phyllosilicates are thought to protect or catalyse the synthesis/alteration of organic matter (Pearson et al., 2007; Kebukawa et al., 2010; Le Guillou et al., 2014; Yesiltas and Kebukawa, 2016).
View in article
Kebukawa, Y., Chan, Q.H.S., Tachibana, S., Kobayashi, K., Zolensky, M.E. (2017) One-pot synthesis of amino acid precursors with insoluble organic matter in planetesimals with aqueous activity. Science Advances 3, e1602093.
 Show in context
Show in context Subsequently, experiments have indicated the alteration of accreted organic matter could yield present day organic matter fractions and compounds within carbonaceous chondrites (Kebukawa et al., 2017; Vinogradoff et al., 2018).
View in article
Kennedy, M.J., Pevear, D.R., Hill, R.J. (2002) Mineral Surface Control of Organic Carbon in Black Shale. Science 295, 657–660.
 Show in context
Show in context Indeed, organic adsorption onto phyllosilicate surfaces has been observed in marine clays (Kennedy et al., 2002) and has been proposed to protect organic matter from oxidising fluids on meteorite parent bodies (Yesiltas and Kebukawa, 2016).
View in article
Kerridge, J.F. (1999) Formation and processing of organics in the early solar system. Space Science Reviews 90, 275–288.
 Show in context
Show in context Much of the meteoritic organic matter is thought to originate from within the interstellar medium (Sandford, 1996; Kerridge, 1999) and/or protosolar nebular (Ciesla and Sandford, 2012; Bekaert et al., 2018).
View in article
Kong, W.G., Wang, A. (2010) Planetary Laser Raman Spectroscopy for Surface Exploration on C/D-Type Asteroids - A Case Study. 41st Lunar and Planetary Science Conference, 2730–2731.
 Show in context
Show in context Previous Raman spectroscopic studies have also identified olivine, pyroxene, Fe-oxides, Fe-oxyhydroxides and phyllosilicates in carbonaceous chondrites and enabled 2D mapping (El Amri et al., 2005; Kong and Wang, 2010; Wang et al., 2015).
View in article
Both our Raman data (Fig. S-7) and those of previous studies link this water band to phyllosilicates (Kong and Wang, 2010; Wang et al., 2015).
View in article
Le Guillou, C., Bernard, S., Brearley, A.J., Remusat, L. (2014) Evolution of organic matter in Orgueil, Murchison and Renazzo during parent body aqueous alteration: In situ investigations. Geochimica et Cosmochimica Acta 131, 368–392.
 Show in context
Show in context Although other interpretations exist, carbonates have been indicated as the products of organic matter oxidation (Alexander et al., 2015; Yesiltas and Kebukawa, 2016; Chan et al., 2017) and phyllosilicates are thought to protect or catalyse the synthesis/alteration of organic matter (Pearson et al., 2007; Kebukawa et al., 2010; Le Guillou et al., 2014; Yesiltas and Kebukawa, 2016).
View in article
McSween, H.Y. (1987) Aqueous alteration in carbonaceous chondrites: Mass balance constraints on matrix mineralogy. Geochimica et Cosmochimica Acta 51, 2469–2477.
 Show in context
Show in context Phyllosilicates replaced anhydrous minerals, such as olivine and pyroxene, originally accreted by the meteorite parent body/bodies (McSween, 1987; Brearley, 2006).
View in article
Naraoka, H., Hashiguchi, M. (2018) In situ organic compound analysis on a meteorite surface by desorption electrospray ionization coupled with an Orbitrap mass spectrometer. Rapid Communications in Mass Spectrometry 32, 959–964.
 Show in context
Show in context Desorption electrospray ionisation-orbitrap-mass spectrometry (DESI-OT-MS) has been used previously to study Murchison (CM2) and Murray (CM2) chondrites (Hashiguchi and Naraoka, 2018; Naraoka and Hashiguchi, 2018).
View in article
Similar to previous studies (Hashiguchi and Naraoka, 2018; Naraoka and Hashiguchi, 2018), a disparity in the intensity distribution between low and intermediate and high molecular weight alkylimidazole homologues was observed (Fig. S-5).
View in article
The results reported here suggest a more complex picture (Fig. 4) than those of previous studies (Hashiguchi and Naraoka, 2018; Naraoka and Hashiguchi, 2018).
View in article
Naraoka, H., Yamashita, Y., Yamaguchi, M., Orthous-Daunay, F.R. (2017) Molecular Evolution of N-Containing Cyclic Compounds in the Parent Body of the Murchison Meteorite. ACS Earth and Space Chemistry 1, 540–550.
 Show in context
Show in context HMW homologues are relatively hydrophobic due to their large chain lengths or numbers of substitutions (Naraoka et al., 2017) and may have thus been transported only a short distance (Fig 4a).
View in article
Pearson, V.K., Kearsley, A.T., Sephton, M.A., Gilmour, I. (2007) The labelling of meteoritic organic material using osmium tetroxide vapour impregnation. Planetary and Space Science 55, 1310–1318.
 Show in context
Show in context Although other interpretations exist, carbonates have been indicated as the products of organic matter oxidation (Alexander et al., 2015; Yesiltas and Kebukawa, 2016; Chan et al., 2017) and phyllosilicates are thought to protect or catalyse the synthesis/alteration of organic matter (Pearson et al., 2007; Kebukawa et al., 2010; Le Guillou et al., 2014; Yesiltas and Kebukawa, 2016).
View in article
Peltzer, E.T., Bada, J.L., Schlesinger, G., Miller, S.L. (1984) The chemical conditions on the parent body of the murchison meteorite: Some conclusions based on amino, hydroxy and dicarboxylic acids. Advances in Space Research 4, 69–74.
 Show in context
Show in context Nevertheless, aqueous alteration has been indicated in the origin of some FOM compounds (Peltzer et al., 1984; Sephton et al., 1998).
View in article
Pizzarello, S., Shock, E. (2010) The organic composition of carbonaceous meteorites: the evolutionary story ahead of biochemistry. Cold Spring Harbor Perspectives in Biology 2, 1–19.
 Show in context
Show in context Carbonaceous chondrites also contain abundant free and macromolecular organic matter (Pizzarello and Shock, 2010; Sephton, 2013) (FOM and MOM, respectively).
View in article
Sandford, S.A. (1996) The inventory of interstellar materials available for the formation of the solar system. Meteoritics and Planetary Science 31, 449–476.
 Show in context
Show in context Much of the meteoritic organic matter is thought to originate from within the interstellar medium (Sandford, 1996; Kerridge, 1999) and/or protosolar nebular (Ciesla and Sandford, 2012; Bekaert et al., 2018).
View in article
Scott, E.R.D., Krot, A.N. (2013) Chondrites and Their Components. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry: Second Edition. Elsevier, Oxford, 65–137.
 Show in context
Show in context Considering the texture, mineral assemblages and chemical composition of the chondrules, the chondrules can be classified as type 1 PO and POP, respectively (Scott and Krot, 2013).
View in article
Sephton, M.A. (2013) Organic Geochemistry of Meteorites. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry: Second Edition. Elsevier, Oxford, 1–31.
 Show in context
Show in context Carbonaceous chondrites also contain abundant free and macromolecular organic matter (Pizzarello and Shock, 2010; Sephton, 2013) (FOM and MOM, respectively).
View in article
Sephton, M.A., Pillinger, C.T., Gilmour, I. (1998) δ13C of free and macromolecular aromatic structures in the murchison meteorite. Geochimica et Cosmochimica Acta 62, 1821–1828.
 Show in context
Show in context Nevertheless, aqueous alteration has been indicated in the origin of some FOM compounds (Peltzer et al., 1984; Sephton et al., 1998).
View in article
Vinogradoff, V., Bernard, S., Le Guillou, C., Remusat, L. (2018) Evolution of interstellar organic compounds under asteroidal hydrothermal conditions. Icarus 305, 358–370.
 Show in context
Show in context Subsequently, experiments have indicated the alteration of accreted organic matter could yield present day organic matter fractions and compounds within carbonaceous chondrites (Kebukawa et al., 2017; Vinogradoff et al., 2018).
View in article
Wang, A., Korotev, R.L., Jolliff, B.L., Ling, Z. (2015) Raman imaging of extraterrestrial materials. Planetary and Space Science 112, 23–34.
 Show in context
Show in context Previous Raman spectroscopic studies have also identified olivine, pyroxene, Fe-oxides, Fe-oxyhydroxides and phyllosilicates in carbonaceous chondrites and enabled 2D mapping (El Amri et al., 2005; Kong and Wang, 2010; Wang et al., 2015).
View in article
Both our Raman data (Fig. S-7) and those of previous studies link this water band to phyllosilicates (Kong and Wang, 2010; Wang et al., 2015).
View in article
Yesiltas, M., Kebukawa, Y. (2016) Associations of organic matter with minerals in Tagish Lake meteorite via high spatial resolution synchrotron-based FTIR microspectroscopy. Meteoritics and Planetary Science 51, 584–595.
 Show in context
Show in context Although other interpretations exist, carbonates have been indicated as the products of organic matter oxidation (Alexander et al., 2015; Yesiltas and Kebukawa, 2016; Chan et al., 2017) and phyllosilicates are thought to protect or catalyse the synthesis/alteration of organic matter (Pearson et al., 2007; Kebukawa et al., 2010; Le Guillou et al., 2014; Yesiltas and Kebukawa, 2016).
View in article
Indeed, organic adsorption onto phyllosilicate surfaces has been observed in marine clays (Kennedy et al., 2002) and has been proposed to protect organic matter from oxidising fluids on meteorite parent bodies (Yesiltas and Kebukawa, 2016).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Materials and Methodology
- Tables S-1 and S-2
- Figures S-1 to S-8
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures and Tables
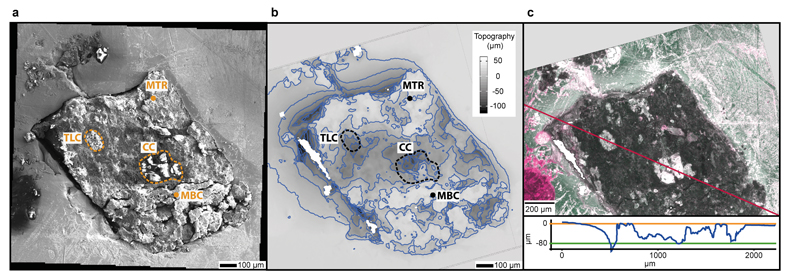
Figure 1 (a) An SEM image indicating the positions of chondrules (orange dashed lines) and matrix areas where EDX analyses were performed. (b) A topographic image of the sample surface obtained using a laser profiler. (c) An optical microscope image and topographic profile across the sample (red line).
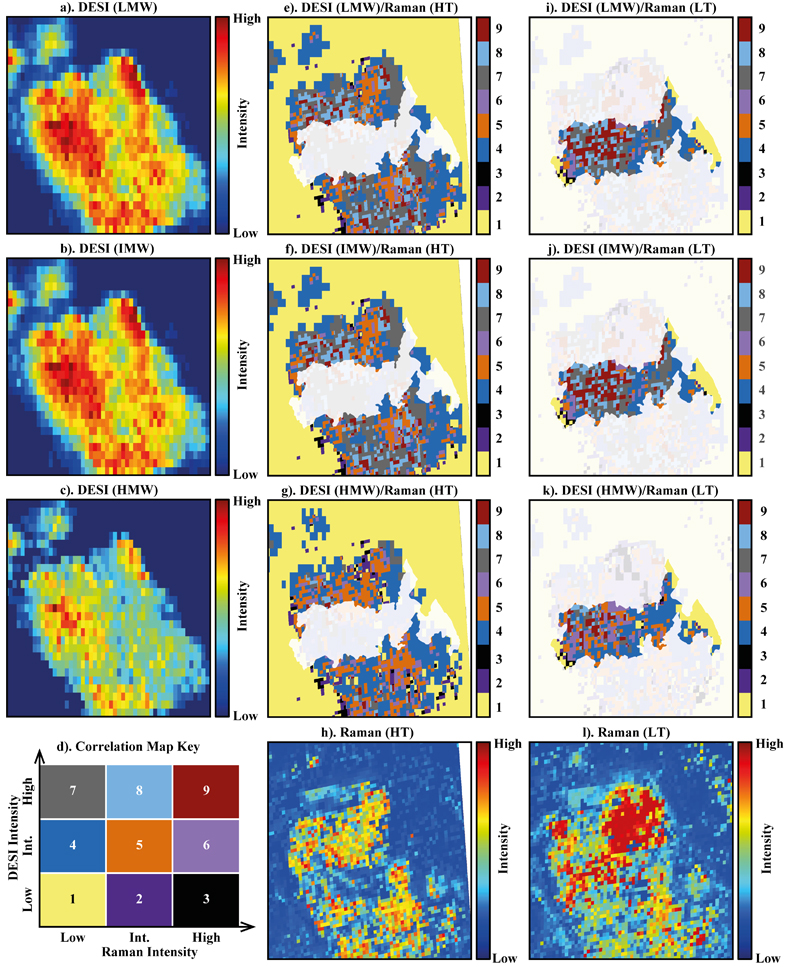
Figure 2 A compilation of DESI, Raman and correlation maps for selected alkylimidazole homologues. (a-c) DESI intensity maps for mass 139.12293, 181.16978 and 237.23225, respectively. Highest normalised intensity is 8.79 x 10-3, 1.22 x 10-2 and 3.84 x 10-3, respectively. (d) A schematic outlining the criteria for the generation of DESI vs. Raman correlation maps. (e-g) Correlation maps showing the similarity between a given DESI and high topography (HT) Raman map. (h) A Raman intensity map focused on high topography, for the interlayer/adsorbed water band (3000-3800 cm-1). (i-k) Correlation maps showing the similarity between a given DESI and low topography (LT) Raman map. (l) As for h except focused on low topography. Note that for the correlation maps a partially transparent white mask has been used to highlight the high topography (top 40 µm) or low topography (below top 40 µm).
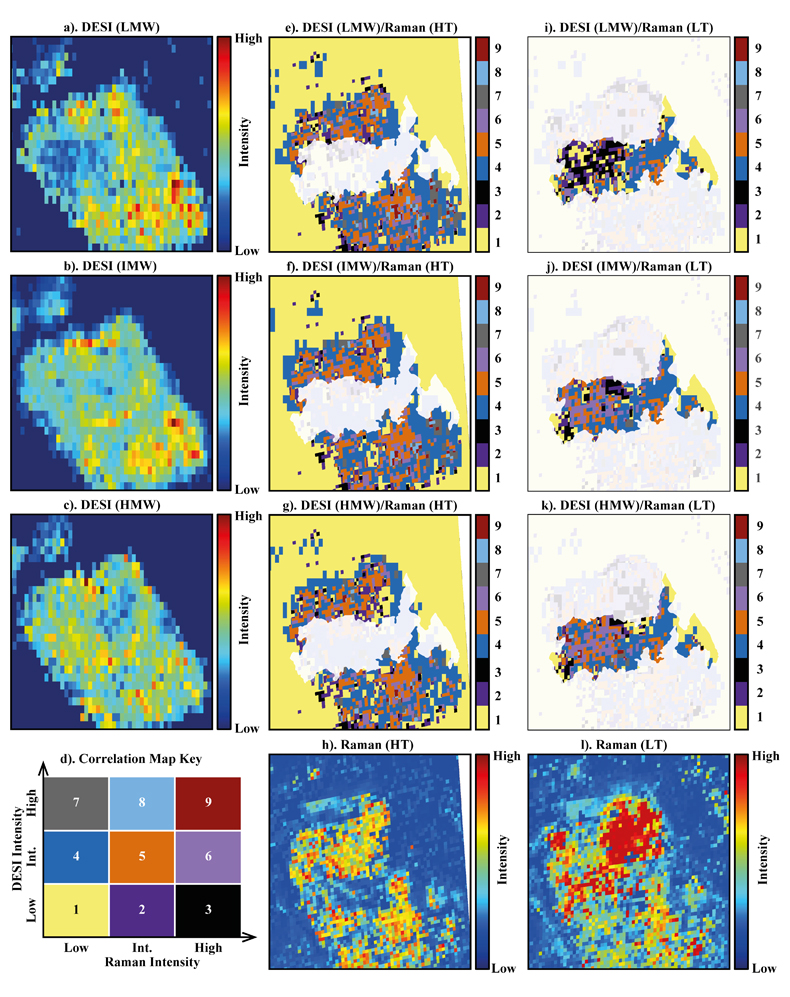
Figure 3 A compilation of DESI, Raman and correlation maps for selected alkylpyridine homologues. (a-c) DESI intensity maps for mass 136.11205, 178.15891 and 220.20580, respectively. Highest normalised intensity is 2.17 x 10-3, 5.44 x 10-2 and 2.58 x 10-3, respectively. (d) A schematic outlining the criteria for the generation of DESI vs. Raman correlation maps. (e-g) Correlation maps showing the similarity between a given DESI and high topography (HT) Raman map. (h) A Raman intensity map focused on high topography, for the interlayer/adsorbed water band (3000-3800 cm-1). (i-k) Correlation maps showing the similarity between a given DESI and low topography (LT) Raman map. (l) As for h except focused on low topography. Note that for the correlation maps a partially transparent white mask has been used to highlight the high topography (top 40 µm) or low topography (below top 40 µm).
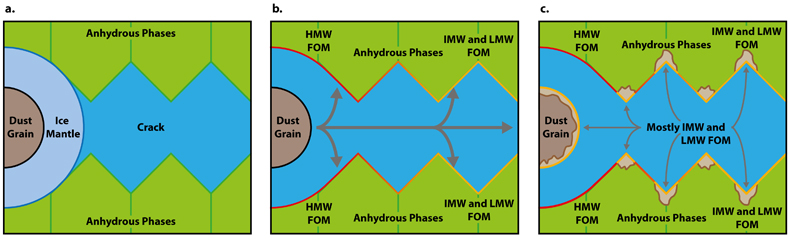
Figure 4 FOM distribution during aqueous alteration. (a) FOM and water ice are accreted by the parent body. (b) The ice melts and HMW FOM (red) adsorbs onto phases close to the site of accretion, but LMW and IMW FOM (light orange) are transported further into the matrix, generating a geochromatographic effect (red to light orange). (c) With the generation of phyllosilicates, adsorption of the remaining organic matter (LMW and IMW), as well as any previously adsorbed, occurs on these new charged mineral surfaces.