Lanthanum anomalies as fingerprints of methanotrophy
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:439Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract

Figures
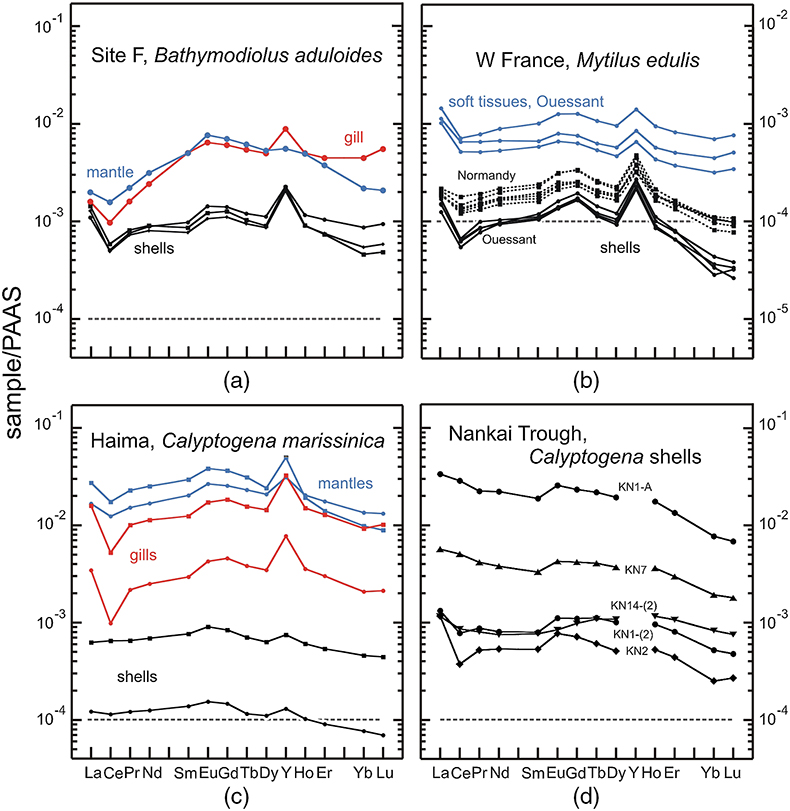
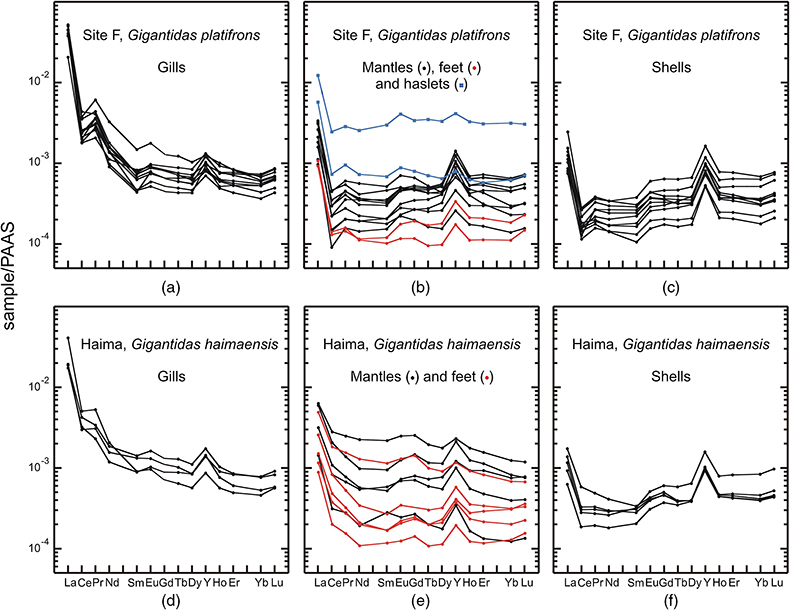
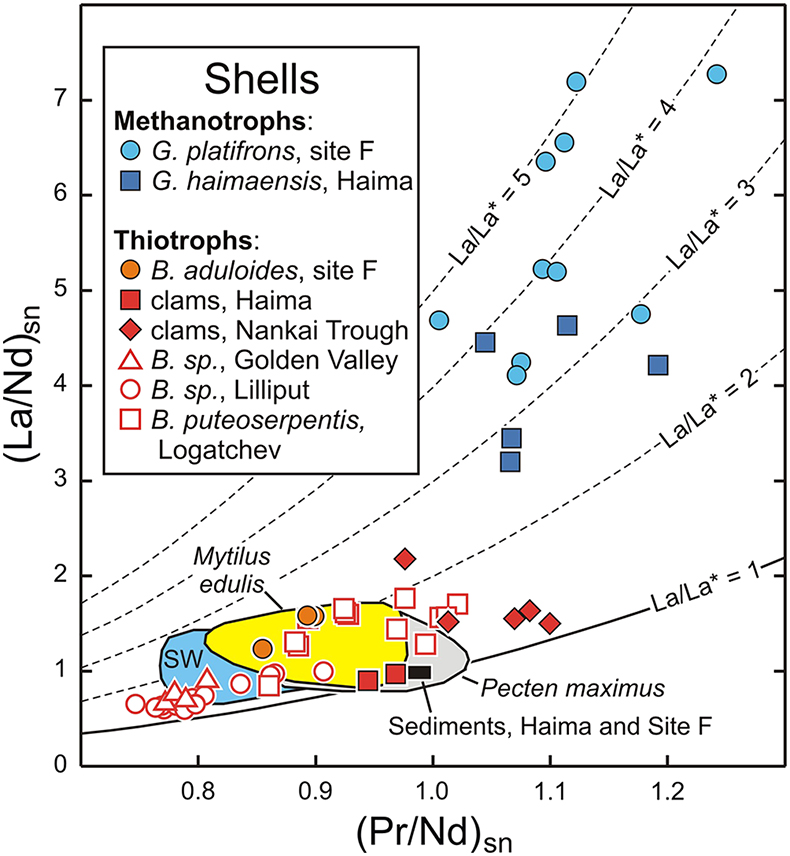
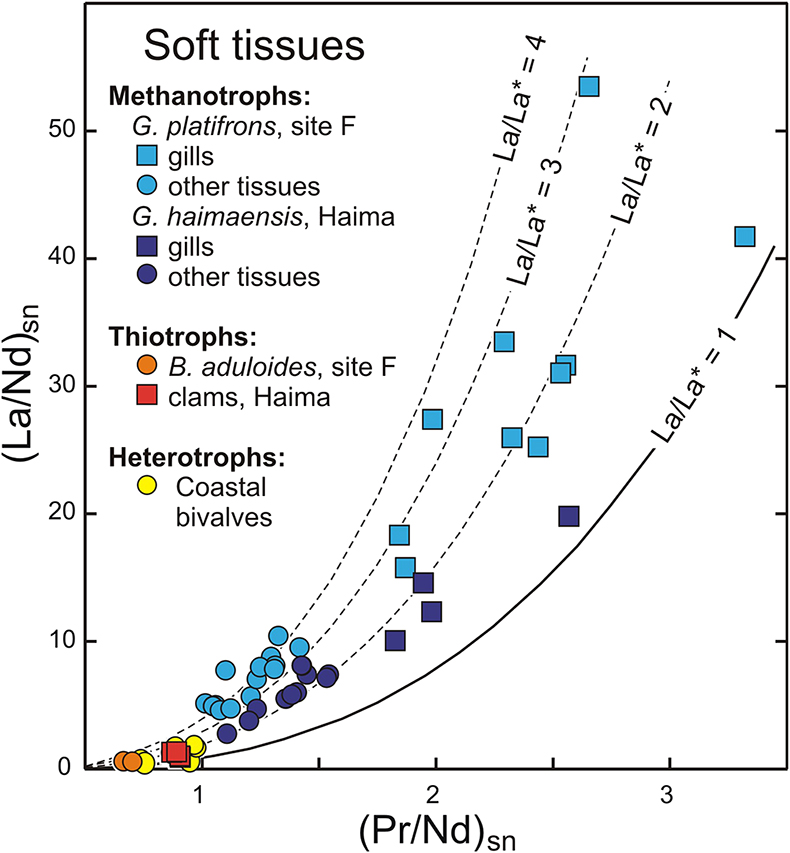
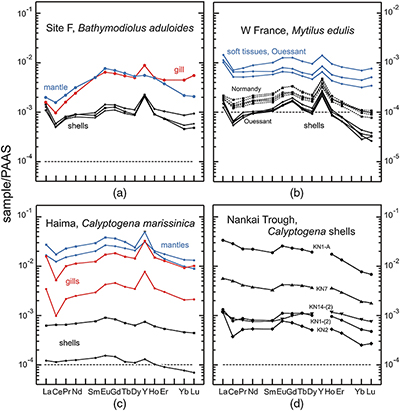 Figure 1 REE + Y patterns normalised to Post Archean Australian Shale (PAAS; Pourmand et al., 2012) for thiotrophic shellfish from cold seeps and for heterotrophic Mytilus edulis from France. The grey dashed line corresponds to the 10−4 x PAAS level. Notice that the Bathymodiolus shells are ten times more REE richer than the Mytilus ones. | 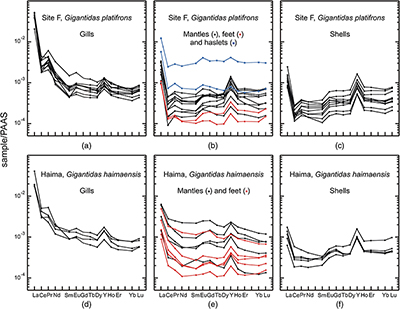 Figure 2 REE + Y patterns normalised to PAAS (Pourmand et al., 2012) for methanotrophic mussels from South China Sea cold seeps. | 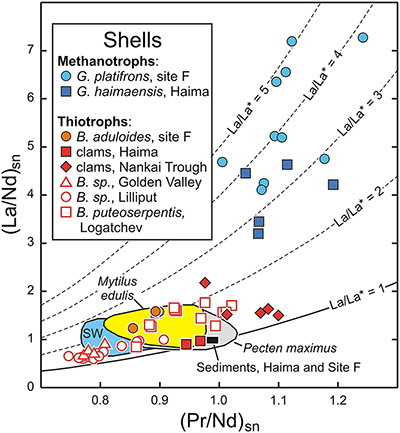 Figure 3 (La/Nd)sn vs. (Pr/Nd)sn plot for the shells from various shellfish from cold seeps (this work), from Atlantic hydrothermal sites (Golden Valley, Lilliput and Logatchev; Bau et al., 2010), heterotrophic shellfish (Mytilus edulis and Pecten maximus; Bau et al., 2010; Ponnurangam et al., 2016; Le Goff et al., 2019 and this work). Fields for seawater (SW; Alibo and Nozaki, 2000) and sediments from Haima and Site F (this work) are shown for comparison. | 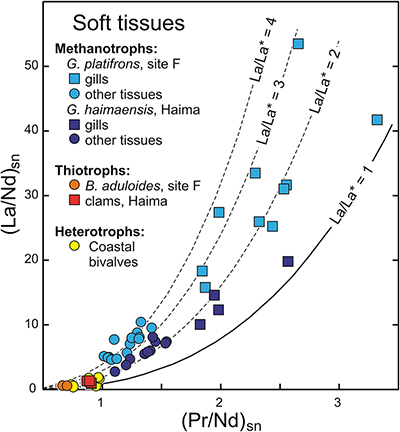 Figure 4 (La/Nd)sn vs. (Pr/Nd)sn plot for the soft tissues prepared from various shellfish from cold seeps, and various heterotrophic coastal bivalves (Akagi and Edanami, 2016 and this work). |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Until recently, REEs were assumed to have no manifest biological function. The discovery of a REE dependent enzyme involved in the metabolism of aerobic methanotrophic bacteria has radically changed this view (Pol et al., 2014
Pol, A., Barends, T.R.M., Dietl, A., Khadem, A.F., Eygensteyn, J., Jetten, M.S.M., Op den Camp, H.J.M. (2014) Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environmental Microbiology 16, 255–264.
; Semrau et al., 2018Semrau, J.D., DiSpirito, A.A., Gu, W., Yoon, S. (2018) Metals and Methanotrophy. Applied and Environmental Microbiology 84, e02289–17.
; Cotruvo, 2019Cotruvo, Jr., J.A. (2019) The chemistry of lanthanides in biology: recent discoveries, emerging principles, and technological applications. ACS Central Science 5, 1496–1506.
), showing that these elements could be essential for microbial life. Methanotrophic bacteria first convert methane to methanol. This first step of aerobic methane oxidation is followed by the degradation of methanol into formaldehyde using methanol dehydrogenase enzymes. These enzymes are either Ca dependent (MxaF type) or light REE dependent (XoxF type) (Skovran et al., 2011Skovran, E., Palmer, A.D., Rountree, A.M., Good, N.M., Lidstrom, M.E. (2011) XoxF is required for expression of methanol dehydrogenase in Methylobacterium extorquens AM1. Journal of Bacteriology 193, 6032–6038.
). However, the latter XoxF type seems to be more frequently used by marine bacteria (Ramachandran et al., 2015Ramachandran, A., Walsh, D.A. (2015) Investigation of XoxF methanol dehydrogenases reveals new methylotrophic bacteria in pelagic marine and freshwater ecosystems. FEMS Microbiology Ecology 91, fiv105.
; Taubert et al., 2015Taubert, M., Grob, C., Howat, A.M., Burns, O.J., Dixon, J.L., Chen, Y., Colin Murrell, J. (2015) XoxF encoding an alternative methanol dehydrogenase is widespread in coastal marine environments. Environmental Microbiology 17, 3937–3948.
), hence suggesting that REEs could play a previously unsuspected and important role in the development of marine ecosystems relying on aerobic methanotrophic symbioses.At ocean margins, areas of active methane seepage at the seafloor (or cold seeps) typically host abundant macrofaunal communities, which derive the vast majority of their nutrition from symbiotic chemotrophic microbes hosted in their gills. The dominant biogeochemical reaction at cold seeps is the anaerobic oxidation of methane (AOM) typically coupled with sulphate reduction, a process that is mediated by a consortium of anaerobic methanotrophic archaea and sulphate reducing bacteria (Boetius et al., 2000
Boetius, A., Ravenschlag, K., Schubert, C.J., Rickert, D., Widdel, F., Gieseke, A., Amann, R., Barker Jørgensen, B., Witte, U., Pfannkuche, O. (2000) A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407, 623–626.
). While a substantial fraction of the macrofauna at methane seeps relies on anaerobic microbial symbionts (mostly using dissolved sulphide, such as tubeworms and clams), mussels are instead commonly associated with aerobic methanotrophic symbionts. In this study, we analysed a series of well characterised shellfish samples from two active seepage sites (Haima and Site F) located in the South China Sea (Feng et al., 2015Feng, D., Cheng, M., Kiel, S., Qiu, J.W., Yang, Q., Zhou, H., Peng, Y., Chen, D. (2015) Using Bathymodiolus tissue stable carbon, nitrogen and sulfur isotopes to infer biogeochemical process at a cold seep in the South China Sea. Deep-Sea Research I 104, 52–59.
, 2018Feng, D., Peckmann, J., Li, N., Kield, S., Qiu, J.W., Liang, Q., Carney, R.S., Peng, Y., Tao, J., Chen, D. (2018) The stable isotope fingerprint of chemosymbiosis in the shell organic matrix of seep-dwelling bivalves. Chemical Geology 479, 241–250.
; Liang et al., 2017Liang, Q., Hu, Y., Feng, D., Peckmann, J., Chen, L., Yang, S., Liang, J., Tao, J., Chen, D. (2017) Authigenic carbonates from two newly discovered active cold seeps on the northwestern slope of the South China Sea: Constraints on fluids sources, formation environments and seepage dynamics. Deep Sea Research I 124, 31–41.
). Our sampling includes both methanotrophic (Gigantidas platifrons and Gigantidas haimaensis) and thiotrophic mussels (Bathymodiolus aduloides), in addition to other bivalves (clams) associated with sulphur oxidising bacteria (Calyptogena marissinica). For comparison, additional thiotrophic clams from the Nankai Trough (Fiala-Médioni et al., 1993Fiala-Médioni, A., Boulègue, J., Ohta, S., Felbeck, H., Mariotti, A. (1993) Source of energy sustaining the Calyptogena populations from deep trenches in subduction zones off Japan. Deep-Sea Research 1, 40, 6,1241–1258.
), and a series of heterotrophic blue mussels (Mytilus edulis) devoid of any chemotrophic symbionts from coastal waters of France were also analysed (Table S-1). Methods are described in Supplementary Information.top
Results and Discussion
The abundances of REEs in shellfish samples are low, but highly variable, in both shells and soft tissues (Figs. 1, 2; Tables S-2–8). Most samples display positive yttrium and negative cerium anomalies respectively, which represent conspicuous features of seawater and marine-derived materials such as biogenic carbonates. A striking feature of our results is that methanotrophic mussels display shale normalised distribution patterns that strongly differ from the other studied shellfish samples. Compared to the thiotrophic shellfish samples, the shells and soft tissues (feet, mantles) of methanotrophic mussels are characterised by often large positive lanthanum anomalies (La/La* = 2.0–5.9; Figs. 2–4, see Supplementary Information for the calculations of the anomalies). Furthermore, their gills exhibit spectacular enrichment in light REEs (Figs. 2, 4).

Figure 1 REE + Y patterns normalised to Post Archean Australian Shale (PAAS; Pourmand et al., 2012
Pourmand, A., Dauphas, N., Ireland, T.J. (2012) A novel extraction chromatography and MC-ICP-MS technique for rapid analysis of REE, Sc and Y: Revising CI-chondrite and Post-Archean Australian Shale (PAAS) abundances. Chemical Geology 291, 38–54.
) for thiotrophic shellfish from cold seeps and for heterotrophic Mytilus edulis from France. The grey dashed line corresponds to the 10−4 x PAAS level. Notice that the Bathymodiolus shells are ten times more REE richer than the Mytilus ones.
Figure 2 REE + Y patterns normalised to PAAS (Pourmand et al., 2012
Pourmand, A., Dauphas, N., Ireland, T.J. (2012) A novel extraction chromatography and MC-ICP-MS technique for rapid analysis of REE, Sc and Y: Revising CI-chondrite and Post-Archean Australian Shale (PAAS) abundances. Chemical Geology 291, 38–54.
) for methanotrophic mussels from South China Sea cold seeps.To a large extent, the shale normalised distribution patterns of REEs in the studied shellfish samples are controlled by variable source contributions to the different organs or tissues. In the case of heterotrophic shellfish from coastal areas, the REEs contained in the soft tissues are mainly derived from suspended particles with a reduced contribution from seawater (Akagi and Edanami, 2017
Akagi, T., Edanami, K. (2017) Sources of rare earth elements in shells and soft-tissues of bivalves from Tokyo Bay. Marine Chemistry 194, 55–62.
). Shells result from the activity of the mantle epithelium inside specific internal liquid (Wheeler, 1992Wheeler, A.P. (1992) Mechanisms of molluscan shell formation. In: Bonucci, E. (Ed.) Calcification in biological systems. CRC press, Boca Raton, FL, 179–226.
). Consequently, their REE distribution patterns directly reflect the composition of the mantle, with additional potential inputs from the fluids associated with carbonate secretion. In any case, the corresponding REE patterns differ significantly from typical seawater signatures, more closely resembling those of the soft tissues, dominated by inputs from filtered suspended particles.Overall, heterotrophic (Bau et al., 2010
Bau, M., Balan, S., Schmidt, K., Koschinsky, A. (2010) Rare earth elements in mussel shells of the Mytilidae family as tracers for hidden and fossil high-temperature hydrothermal systems. Earth and Planetary Science Letters 299, 310–316.
; Ponnurangam et al., 2016Ponnurangam, A., Bau, M., Brenner, M., Kochinsky, A. (2016) Mussel shells of Mytilus edulis as bioarchives of the distribution of rare earth elements and yttrium in seawater and the potential impact of pH and temperature on their partitioning behavior. Biogeosciences 13, 751–760.
; Akagi and Edanami, 2017Akagi, T., Edanami, K. (2017) Sources of rare earth elements in shells and soft-tissues of bivalves from Tokyo Bay. Marine Chemistry 194, 55–62.
; Le Goff et al., 2019Le Goff, S, Barrat, J.A., Chauvaud, L., Paulet, Y.M., Gueguen, B., Ben Salem, D. (2019) Compound-specific recording of gadolinium pollution in coastal waters by great scallops. Scientific Reports 9, 8015.
) and thiotrophic shellfish both display comparable REE features. The shells of thiotrophic mussels Bathymodiolus aduloides and of coastal mussels Mytilus edulis (Bau et al., 2010Bau, M., Balan, S., Schmidt, K., Koschinsky, A. (2010) Rare earth elements in mussel shells of the Mytilidae family as tracers for hidden and fossil high-temperature hydrothermal systems. Earth and Planetary Science Letters 299, 310–316.
; Ponnurangam et al., 2016Ponnurangam, A., Bau, M., Brenner, M., Kochinsky, A. (2016) Mussel shells of Mytilus edulis as bioarchives of the distribution of rare earth elements and yttrium in seawater and the potential impact of pH and temperature on their partitioning behavior. Biogeosciences 13, 751–760.
and this work, Table S-7) exhibit very similar REE patterns, albeit being characterised by different REE abundances (Fig. 1). Gill and mantle samples also display similar patterns, both characterised by light REE depletions [(La/Sm)sn = 0.32–0.40] and moderately positive La anomalies (La/La* = 1.83–2.26). In agreement with previous studies (Akagi and Edanami, 2017Akagi, T., Edanami, K. (2017) Sources of rare earth elements in shells and soft-tissues of bivalves from Tokyo Bay. Marine Chemistry 194, 55–62.
), soft tissues are enriched in REEs compared to shells. Like previous mussels, the REE patterns of thiotrophic Calyptogena display mixed features between those of terrigenous sediments and seawater. Importantly, all these samples plot within the range of La/La* values for seawater (Fig. 3). The same conclusion also applies to the dominantly thiotrophic shellfish collected at hydrothermal fields along oceanic ridges (Bau et al., 2010Bau, M., Balan, S., Schmidt, K., Koschinsky, A. (2010) Rare earth elements in mussel shells of the Mytilidae family as tracers for hidden and fossil high-temperature hydrothermal systems. Earth and Planetary Science Letters 299, 310–316.
). Their shells exhibit patterns largely influenced by the composition of hydrothermal fluids but never show any particular lanthanum excesses, suggesting that these animals and their symbionts do not require light REEs (Fig. 3).
Figure 3 (La/Nd)sn vs. (Pr/Nd)sn plot for the shells from various shellfish from cold seeps (this work), from Atlantic hydrothermal sites (Golden Valley, Lilliput and Logatchev; Bau et al., 2010
Bau, M., Balan, S., Schmidt, K., Koschinsky, A. (2010) Rare earth elements in mussel shells of the Mytilidae family as tracers for hidden and fossil high-temperature hydrothermal systems. Earth and Planetary Science Letters 299, 310–316.
), heterotrophic shellfish (Mytilus edulis and Pecten maximus; Bau et al., 2010Bau, M., Balan, S., Schmidt, K., Koschinsky, A. (2010) Rare earth elements in mussel shells of the Mytilidae family as tracers for hidden and fossil high-temperature hydrothermal systems. Earth and Planetary Science Letters 299, 310–316.
; Ponnurangam et al., 2016Ponnurangam, A., Bau, M., Brenner, M., Kochinsky, A. (2016) Mussel shells of Mytilus edulis as bioarchives of the distribution of rare earth elements and yttrium in seawater and the potential impact of pH and temperature on their partitioning behavior. Biogeosciences 13, 751–760.
; Le Goff et al., 2019Le Goff, S, Barrat, J.A., Chauvaud, L., Paulet, Y.M., Gueguen, B., Ben Salem, D. (2019) Compound-specific recording of gadolinium pollution in coastal waters by great scallops. Scientific Reports 9, 8015.
and this work). Fields for seawater (SW; Alibo and Nozaki, 2000Alibo, D.S., Nozaki, Y. (2000) Dissolved rare earth elements in the South China Sea: geochemical characterization of the water masses. Journal of Geophysical Research 105, C12, 28771–28783.
) and sediments from Haima and Site F (this work) are shown for comparison.
Figure 4 (La/Nd)sn vs. (Pr/Nd)sn plot for the soft tissues prepared from various shellfish from cold seeps, and various heterotrophic coastal bivalves (Akagi and Edanami, 2016 and this work).
The situation for methanotrophic shellfish is clearly different (Figs. 3, 4). Their gills show exceptional enrichments in light REEs, which, to the best of our knowledge, have never been reported for any natural sediments, seafloor rocks or authigenic phases. Other soft tissues and shells also exhibit significant lanthanum enrichments, as well as marked lanthanum anomalies, with La/La* ratios (up to 5) that largely exceed the highest values measured in South China Sea water (La/La* = 2.5; Alibo and Nozaki, 2000
Alibo, D.S., Nozaki, Y. (2000) Dissolved rare earth elements in the South China Sea: geochemical characterization of the water masses. Journal of Geophysical Research 105, C12, 28771–28783.
). To date, the exchange and recycling of REEs between the different organs of the studied molluscs has never been investigated. However, the exceptional La anomalies reported here cannot be explained by the contribution from any hypothetical fluid or sediment sources. If this was the case, thiotrophic clams and mussels sampled from the same sites would have also displayed similar La enrichments. Therefore, the specific REE features observed in the methanotrophic shellfish samples are best explained by biological processes. It is very important to note that the gills show the highest enrichments in light REEs and lanthanum abundances, because this is precisely where the methanotrophic symbionts are hosted in these mussels (Barry et al., 2002Barry, J.P., Buck, K.R., Kochevar, R.K., Nelson, D.C., Fujiwara, Y., Goffredi, S.K., Hashimoto, J. (2002) Methane-based symbiosis in a mussel, Bathymodiolus platifrons, from cold seeps in Sagami Bay, Japan. Invertebrate Biology 121, 47–54.
; Yu et al., 2019Yu, J., Wang, M., Liu, B., Yue, X., Li, C. (2019) Gill symbionts of the cold-seep mussel Bathymodiolus platifrons: Composition, environmental dependency and immune control. Fish and Shellfish Immunology 86, 246–252.
). Therefore, the enzymatic activity of the methanotrophic symbionts is the most plausible cause for the selective accumulation of light REEs in the Gigantidas gills, and the large lanthanum anomalies in the other soft tissues and recorded in the shells.An immediate question concerns the presumed source of the light REEs hosted in the mussel gills. Marine sediments are significantly enriched in REEs and contain about 10 million times more lanthanum than seawater, and as such could possibly contribute to the observed light REEs enrichment of the gills, via possibly an unrecognised process of remineralisation by the gill cells or by the symbionts. However, it is more likely that most of the light REEs contained in the gills are derived from the fluids filtered by the shellfish. Considering an average mass for Gigantidas gill of ∼0.7 g (on a dry basis), we can estimate that it contains about 1.9 μg/g lanthanum at Site F, and 1 μg/g at Haima. This corresponds to the quantity of lanthanum contained in about 275 litres of seawater at Site F (or 14.4 litres of pore waters; Himmler et al. 2013
Himmler, T., Haley, B.A., Torres, M.E., Klinkhammer, G.P., Bohrmann, G., Peckmann, J. (2013) Rare earth element geochemistry in cold-seep pore waters of Hydrate Ridge, northeast Pacific Ocean. Geo-Marine Letters 33, 369–379.
) and 145 litres of sea water at Haima (or 7.6 litres of pore waters). Whatever the exact composition of the involved fluids, these volumes are very small compared to the volume of water that a mussel can filter every day: ∼180 litres, estimated assuming a mussel with 3 g of soft tissues on a dry basis and a rate similar to that of Mytilus edulis (Blayne et al., 1989Blayne, B.L., Hawkins, A.J.S., Navarro, E., Iglesias, I.P. (1989) Effects of seston concentration on feeding, digestion and growth in the mussel Mytilus edulis. Marine Ecology Progress Series 55, 47–54.
). Based on these considerations, it is very likely that the methanotrophic symbionts hosted in gills can access the light REEs they need from the seawater filtered by the shellfish, without invoking more complex uptake processes. This raises the question as to whether methanotrophic activity at cold seeps could have an impact on the oceanic REE budget, at least in the water column surrounding the seepage sites. We can estimate that about 72 m3 of water is filtered every day by one square metre of a typical mussel bed with 400 animals. And if we limit our estimate to the amount of lanthanum contained only by gills, the same square metre at Site F contains as much lanthanum as 110 m3 of seawater. At present, we do not know what proportion of dissolved REEs in the fluids filtered by molluscs are actually taken up by the symbionts, nor what proportions of these are transferred to the organs of the shellfish. However, the biological activity (including not only the mussels that dominate the biomass, but all the other methanotrophs that use XoxF-type enzymes) must necessarily have an impact on the chemistry of seawater near cold seeps. The capability of methanotrophic bacteria to affect the local distribution of REEs in seawater has already been observed in the Gulf of Mexico. Following the Deepwater Horizon well blowout in 2010, the release of massive methane plumes in the water column was accompanied by substantial removal of light REEs, interpreted as the result of intense methanotrophic activity (Shiller et al., 2017Shiller, A.M., Chan, E.W., Joung D.J., Redmond, M.C., Kessler, J.D. (2017) Light rare earth element depletion during Deepwater Horizon blowout methanotrophy. Scientific Reports 7, 10389, doi:10.1038/s41598-017-11060-z.
).Our findings could have implications for the understanding of other lanthanum excesses previously identified in seawater and various marine precipitates, but remaining unexplained so far. However, this element was assumed to be more stable than other light REEs during complexation in seawater (de Baar et al., 1985
de Baar, H.J.W., Bacon, M.P., Brewer, P.G., Bruland, K.W. (1985) Rare earth elements in the Pacific and Atlantic Oceans. Geochimica et Cosmochimica Acta 49, 1943–1959.
) or could be released from suspended barite particles (Grenier et al., 2018Grenier, M, Garcia-Solsona, E., Lemaitre, N., Trull, T.W., Bouvier, V., Nonnotte, P., van Beek, P., Souhaut, M., Lacan, F., Jeandel, C. (2018) Differentiating lithogenic supplies, water mass transport, and biological processes on and off the Kerguelen Plateau using rare earth element concentrations and neodymium isotopic compositions. Frontiers in Marine Science 5, 426.
). In marine precipitates, lanthanum anomalies are common features that are usually inferred to be largely inherited from seawater (e.g., Bau and Dulski, 1996Bau, M., Dulski, P. (1996) Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Research 79, 37–55.
; Kamber and Webb, 2001Kamber, B.S., Webb, G.E. (2001) The geochemistry of late Archaean microbial carbonate: Implications for ocean chemistry and continental erosion history. Geochimica et Cosmochimica Acta 65, 2509–2525.
), but for which no clear explanation has been proposed. Our data demonstrate unambiguously that positive lanthanum anomalies can be generated by the enzymatic activity of methanotrophs, hence providing the possibility of promising applications in various fields of research. Importantly, in future studies, the presence of marked lanthanum anomalies or any other large light REE enrichments in fossil shells could be used as proxies for past methanotrophic activity. Methanotrophic organisms have been present since the Archean, but their detection in the geological record, and in the sedimentary archives of the early Earth oceans are still the subject of intense debate (Thomazo et al., 2009Thomazo C., Ader M., Farquhar J., Philippot P. (2009) Methanotrophs regulated atmospheric sulfur isotope anomalies during the MesoArchaean (Tumbiana Formation, Western Australia). Earth and Planetary Science Letters 279, 65–75.
; Knoll et al., 2016Knoll, A.H., Bergmann, K.D., Strauss, J.V. (2016) Life: the first two billion years. Philosophical Transactions of the Royal Society B B371, 20150493.
). In particular, lanthanum anomalies are frequent in ancient microbial carbonates and banded iron formations (Bau and Dulski, 1996Bau, M., Dulski, P. (1996) Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Research 79, 37–55.
; Kamber and Webb, 2001Kamber, B.S., Webb, G.E. (2001) The geochemistry of late Archaean microbial carbonate: Implications for ocean chemistry and continental erosion history. Geochimica et Cosmochimica Acta 65, 2509–2525.
; Planavsky et al., 2010Planavsky, N., Bekker, A., Rouxel, O.J., Kamber, B., Hofmann, A., Knudsen A., Lyons, T.W. (2010) Rare Earth Element and yttrium compositions of Archean and Paleoproterozoic Fe formations revisited: new perspectives on the significance and mechanisms of deposition. Geochimica et Cosmochimica Acta 74, 6387–6405.
), which may point towards a putative REE dependent microbiological origin. Revisiting the REE geochemistry of these ancient rocks could provide fresh insights into the emergence and diversification of life.top
Acknowledgements
We thank Karim Benzerara for the editorial handling, and the anonymous reviewers for constructive comments. ICP-MS analyses were obtained with the help of Bleuenn Gueguen. We are grateful to Jacques Boulègue who provided us the samples from Nankai Trough, Samuel Le Goff for the mussels from Ouessant, Richard Greenwood (Open University) and Michel Rafini (UBO) for discussions and grammatical corrections. We express our sincere appreciation to the crews of the manned submersible Jiaolong, ROV Haima, ROV ROPOS, and the research vessels. This work was supported by the “Laboratoire d’Excellence” LabexMER (ANR-10-LABX-19) and co-funded by grants from the French Government under the programme “Investissements d’Avenir”, by the National Program on Global Change and Air-Sea Interaction (GASI-GEOGE-05-04), the National Key R&D Program of China (2017YFC0306702) and the NSF of China (Grants: 41773091, 41730528, and 41761134084). XW acknowledges the China Scholarship Council for supporting a research visit to IFREMER.
Editor: Karim Benzerara
top
References
Akagi, T., Edanami, K. (2017) Sources of rare earth elements in shells and soft-tissues of bivalves from Tokyo Bay. Marine Chemistry 194, 55–62.
 Show in context
Show in contextIn the case of heterotrophic shellfish from coastal areas, the REEs contained in the soft tissues are mainly derived from suspended particles with a reduced contribution from seawater (Akagi and Edanami, 2017).
View in article
Overall, heterotrophic (Bau et al., 2010; Ponnurangam et al., 2016; Akagi and Edanami, 2017; Le Goff et al., 2019) and thiotrophic shellfish both display comparable REE features.
View in article
In agreement with previous studies (Akagi and Edanami, 2017), soft tissues are enriched in REEs compared to shells. Like previous mussels, the REE patterns of thiotrophic Calyptogena display mixed features between those of terrigenous sediments and seawater.
View in article
Alibo, D.S., Nozaki, Y. (2000) Dissolved rare earth elements in the South China Sea: geochemical characterization of the water masses. Journal of Geophysical Research 105, C12, 28771–28783.
 Show in context
Show in contextFields for seawater (SW; Alibo and Nozaki, 2000) and sediments from Haima and Site F (this work) are shown for comparison.
View in article
Other soft tissues and shells also exhibit significant lanthanum enrichments, as well as marked lanthanum anomalies, with La/La* ratios (up to 5) that largely exceed the highest values measured in South China Sea water (La/La* = 2.5; Alibo and Nozaki, 2000).
View in article
de Baar, H.J.W., Bacon, M.P., Brewer, P.G., Bruland, K.W. (1985) Rare earth elements in the Pacific and Atlantic Oceans. Geochimica et Cosmochimica Acta 49, 1943–1959.
 Show in context
Show in contextHowever, this element was assumed to be more stable than other light REEs during complexation in seawater (de Baar et al., 1985) or could be released from suspended barite particles (Grenier et al., 2018).
View in article
Barry, J.P., Buck, K.R., Kochevar, R.K., Nelson, D.C., Fujiwara, Y., Goffredi, S.K., Hashimoto, J. (2002) Methane-based symbiosis in a mussel, Bathymodiolus platifrons, from cold seeps in Sagami Bay, Japan. Invertebrate Biology 121, 47–54.
 Show in context
Show in contextIt is very important to note that the gills show the highest enrichments in light REEs and lanthanum abundances, because this is precisely where the methanotrophic symbionts are hosted in these mussels (Barry et al., 2002; Yu et al., 2019).
View in article
Bau, M., Dulski, P. (1996) Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Research 79, 37–55.
 Show in context
Show in contextIn marine precipitates, lanthanum anomalies are common features that are usually inferred to be largely inherited from seawater (e.g., Bau and Dulski, 1996; Kamber and Webb, 2001), but for which no clear explanation has been proposed.
View in article
In particular, lanthanum anomalies are frequent in ancient microbial carbonates and banded iron formations (Bau and Dulski, 1996; Kamber and Webb, 2001; Planavsky et al., 2010), which may point towards a putative REE dependent microbiological origin.
View in article
Bau, M., Balan, S., Schmidt, K., Koschinsky, A. (2010) Rare earth elements in mussel shells of the Mytilidae family as tracers for hidden and fossil high-temperature hydrothermal systems. Earth and Planetary Science Letters 299, 310–316.
 Show in context
Show in contextOverall, heterotrophic (Bau et al., 2010; Ponnurangam et al., 2016; Akagi and Edanami, 2017; Le Goff et al., 2019) and thiotrophic shellfish both display comparable REE features.
View in article
The shells of thiotrophic mussels Bathymodiolus aduloides and of coastal mussels Mytilus edulis (Bau et al., 2010; Ponnurangam et al., 2016 and this work, Table S-7) exhibit very similar REE patterns, albeit being characterised by different REE abundances (Fig. 1).
View in article
The same conclusion also applies to the dominantly thiotrophic shellfish collected at hydrothermal fields along oceanic ridges (Bau et al., 2010).
View in article
(La/Nd)sn vs. (Pr/Nd)sn plot for the shells from various shellfish from cold seeps (this work), from Atlantic hydrothermal sites (Golden Valley, Lilliput and Logatchev; Bau et al., 2010), heterotrophic shellfish (Mytilus edulis and Pecten maximus; Bau et al., 2010; Ponnurangam et al., 2016; Le Goff et al., 2019 and this work).
View in article
Blayne, B.L., Hawkins, A.J.S., Navarro, E., Iglesias, I.P. (1989) Effects of seston concentration on feeding, digestion and growth in the mussel Mytilus edulis. Marine Ecology Progress Series 55, 47–54.
 Show in context
Show in contextWhatever the exact composition of the involved fluids, these volumes are very small compared to the volume of water that a mussel can filter every day: ∼180 litres, estimated assuming a mussel with 3 g of soft tissues on a dry basis and a rate similar to that of Mytilus edulis (Blayne et al., 1989).
View in article
Boetius, A., Ravenschlag, K., Schubert, C.J., Rickert, D., Widdel, F., Gieseke, A., Amann, R., Barker Jørgensen, B., Witte, U., Pfannkuche, O. (2000) A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407, 623–626.
 Show in context
Show in contextThe dominant biogeochemical reaction at cold seeps is the anaerobic oxidation of methane (AOM) typically coupled with sulphate reduction, a process that is mediated by a consortium of anaerobic methanotrophic archaea and sulphate reducing bacteria (Boetius et al., 2000).
View in article
Cotruvo, Jr., J.A. (2019) The chemistry of lanthanides in biology: recent discoveries, emerging principles, and technological applications. ACS Central Science 5, 1496–1506.
 Show in context
Show in contextThe discovery of a REE dependent enzyme involved in the metabolism of aerobic methanotrophic bacteria has radically changed this view (Pol et al., 2014; Semrau et al., 2018; Cotruvo, 2019), showing that these elements could be essential for microbial life. Methanotrophic bacteria first convert methane to methanol.
View in article
Feng, D., Cheng, M., Kiel, S., Qiu, J.W., Yang, Q., Zhou, H., Peng, Y., Chen, D. (2015) Using Bathymodiolus tissue stable carbon, nitrogen and sulfur isotopes to infer biogeochemical process at a cold seep in the South China Sea. Deep-Sea Research I 104, 52–59.
 Show in context
Show in contextIn this study, we analysed a series of well characterised shellfish samples from two active seepage sites (Haima and Site F) located in the South China Sea (Feng et al., 2015, 2018; Liang et al., 2017).
View in article
Feng, D., Peckmann, J., Li, N., Kield, S., Qiu, J.W., Liang, Q., Carney, R.S., Peng, Y., Tao, J., Chen, D. (2018) The stable isotope fingerprint of chemosymbiosis in the shell organic matrix of seep-dwelling bivalves. Chemical Geology 479, 241–250.
 Show in context
Show in contextIn this study, we analysed a series of well characterised shellfish samples from two active seepage sites (Haima and Site F) located in the South China Sea (Feng et al., 2015, 2018; Liang et al., 2017).
View in article
Fiala-Médioni, A., Boulègue, J., Ohta, S., Felbeck, H., Mariotti, A. (1993) Source of energy sustaining the Calyptogena populations from deep trenches in subduction zones off Japan. Deep-Sea Research 1, 40, 6,1241–1258.
 Show in context
Show in contextFor comparison, additional thiotrophic clams from the Nankai Trough (Fiala-Médioni et al., 1993), and a series of heterotrophic blue mussels (Mytilus edulis) devoid of any chemotrophic symbionts from coastal waters of France were also analysed (Table S-1).
View in article
Grenier, M, Garcia-Solsona, E., Lemaitre, N., Trull, T.W., Bouvier, V., Nonnotte, P., van Beek, P., Souhaut, M., Lacan, F., Jeandel, C. (2018) Differentiating lithogenic supplies, water mass transport, and biological processes on and off the Kerguelen Plateau using rare earth element concentrations and neodymium isotopic compositions. Frontiers in Marine Science 5, 426.
 Show in context
Show in contextHowever, this element was assumed to be more stable than other light REEs during complexation in seawater (de Baar et al., 1985) or could be released from suspended barite particles (Grenier et al., 2018).
View in article
Himmler, T., Haley, B.A., Torres, M.E., Klinkhammer, G.P., Bohrmann, G., Peckmann, J. (2013) Rare earth element geochemistry in cold-seep pore waters of Hydrate Ridge, northeast Pacific Ocean. Geo-Marine Letters 33, 369–379.
 Show in context
Show in contextThis corresponds to the quantity of lanthanum contained in about 275 litres of seawater at Site F (or 14.4 litres of pore waters; Himmler et al. 2013) and 145 litres of sea water at Haima (or 7.6 litres of pore waters).
View in article
Kamber, B.S., Webb, G.E. (2001) The geochemistry of late Archaean microbial carbonate: Implications for ocean chemistry and continental erosion history. Geochimica et Cosmochimica Acta 65, 2509–2525.
 Show in context
Show in contextIn marine precipitates, lanthanum anomalies are common features that are usually inferred to be largely inherited from seawater (e.g., Bau and Dulski, 1996; Kamber and Webb, 2001), but for which no clear explanation has been proposed.
View in article
In particular, lanthanum anomalies are frequent in ancient microbial carbonates and banded iron formations (Bau and Dulski, 1996; Kamber and Webb, 2001; Planavsky et al., 2010), which may point towards a putative REE dependent microbiological origin.
View in article
Knoll, A.H., Bergmann, K.D., Strauss, J.V. (2016) Life: the first two billion years. Philosophical Transactions of the Royal Society B B371, 20150493.
 Show in context
Show in contextMethanotrophic organisms have been present since the Archean, but their detection in the geological record, and in the sedimentary archives of the early Earth oceans are still the subject of intense debate (Thomazo et al., 2009; Knoll et al., 2016).
View in article
Le Goff, S, Barrat, J.A., Chauvaud, L., Paulet, Y.M., Gueguen, B., Ben Salem, D. (2019) Compound-specific recording of gadolinium pollution in coastal waters by great scallops. Scientific Reports 9, 8015.
 Show in context
Show in contextOverall, heterotrophic (Bau et al., 2010; Ponnurangam et al., 2016; Akagi and Edanami, 2017; Le Goff et al., 2019) and thiotrophic shellfish both display comparable REE features.
View in article
(La/Nd)sn vs. (Pr/Nd)sn plot for the shells from various shellfish from cold seeps (this work), from Atlantic hydrothermal sites (Golden Valley, Lilliput and Logatchev; Bau et al., 2010), heterotrophic shellfish (Mytilus edulis and Pecten maximus; Bau et al., 2010; Ponnurangam et al., 2016; Le Goff et al., 2019 and this work).
View in article
Liang, Q., Hu, Y., Feng, D., Peckmann, J., Chen, L., Yang, S., Liang, J., Tao, J., Chen, D. (2017) Authigenic carbonates from two newly discovered active cold seeps on the northwestern slope of the South China Sea: Constraints on fluids sources, formation environments and seepage dynamics. Deep Sea Research I 124, 31–41.
 Show in context
Show in contextIn this study, we analysed a series of well characterised shellfish samples from two active seepage sites (Haima and Site F) located in the South China Sea (Feng et al., 2015, 2018; Liang et al., 2017).
View in article
Planavsky, N., Bekker, A., Rouxel, O.J., Kamber, B., Hofmann, A., Knudsen A., Lyons, T.W. (2010) Rare Earth Element and yttrium compositions of Archean and Paleoproterozoic Fe formations revisited: new perspectives on the significance and mechanisms of deposition. Geochimica et Cosmochimica Acta 74, 6387–6405.
 Show in context
Show in contextIn particular, lanthanum anomalies are frequent in ancient microbial carbonates and banded iron formations (Bau and Dulski, 1996; Kamber and Webb, 2001; Planavsky et al., 2010), which may point towards a putative REE dependent microbiological origin.
View in article
Pol, A., Barends, T.R.M., Dietl, A., Khadem, A.F., Eygensteyn, J., Jetten, M.S.M., Op den Camp, H.J.M. (2014) Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environmental Microbiology 16, 255–264.
 Show in context
Show in contextThe discovery of a REE dependent enzyme involved in the metabolism of aerobic methanotrophic bacteria has radically changed this view (Pol et al., 2014; Semrau et al., 2018; Cotruvo, 2019), showing that these elements could be essential for microbial life. Methanotrophic bacteria first convert methane to methanol.
View in article
Ponnurangam, A., Bau, M., Brenner, M., Kochinsky, A. (2016) Mussel shells of Mytilus edulis as bioarchives of the distribution of rare earth elements and yttrium in seawater and the potential impact of pH and temperature on their partitioning behavior. Biogeosciences 13, 751–760.
 Show in context
Show in contextOverall, heterotrophic (Bau et al., 2010; Ponnurangam et al., 2016; Akagi and Edanami, 2017; Le Goff et al., 2019) and thiotrophic shellfish both display comparable REE features.
View in article
The shells of thiotrophic mussels Bathymodiolus aduloides and of coastal mussels Mytilus edulis (Bau et al., 2010; Ponnurangam et al., 2016 and this work, Table S-7) exhibit very similar REE patterns, albeit being characterised by different REE abundances (Fig. 1).
View in article
(La/Nd)sn vs. (Pr/Nd)sn plot for the shells from various shellfish from cold seeps (this work), from Atlantic hydrothermal sites (Golden Valley, Lilliput and Logatchev; Bau et al., 2010), heterotrophic shellfish (Mytilus edulis and Pecten maximus; Bau et al., 2010; Ponnurangam et al., 2016; Le Goff et al., 2019 and this work).
View in article
Pourmand, A., Dauphas, N., Ireland, T.J. (2012) A novel extraction chromatography and MC-ICP-MS technique for rapid analysis of REE, Sc and Y: Revising CI-chondrite and Post-Archean Australian Shale (PAAS) abundances. Chemical Geology 291, 38–54.
 Show in context
Show in contextREE + Y patterns normalised to Post Archean Australian Shale (PAAS; Pourmand et al., 2012) for thiotrophic shellfish from cold seeps and for heterotrophic Mytilus edulis from France.
View in article
REE + Y patterns normalised to PAAS (Pourmand et al., 2012) for methanotrophic mussels from South China Sea cold seeps.
View in article
Ramachandran, A., Walsh, D.A. (2015) Investigation of XoxF methanol dehydrogenases reveals new methylotrophic bacteria in pelagic marine and freshwater ecosystems. FEMS Microbiology Ecology 91, fiv105.
 Show in context
Show in contextHowever, the latter XoxF type seems to be more frequently used by marine bacteria (Ramachandran et al., 2015; Taubert et al., 2015), hence suggesting that REEs could play a previously unsuspected and important role in the development of marine ecosystems relying on aerobic methanotrophic symbioses.
View in article
Semrau, J.D., DiSpirito, A.A., Gu, W., Yoon, S. (2018) Metals and Methanotrophy. Applied and Environmental Microbiology 84, e02289–17.
 Show in context
Show in contextThe discovery of a REE dependent enzyme involved in the metabolism of aerobic methanotrophic bacteria has radically changed this view (Pol et al., 2014; Semrau et al., 2018; Cotruvo, 2019), showing that these elements could be essential for microbial life. Methanotrophic bacteria first convert methane to methanol.
View in article
Shiller, A.M., Chan, E.W., Joung D.J., Redmond, M.C., Kessler, J.D. (2017) Light rare earth element depletion during Deepwater Horizon blowout methanotrophy. Scientific Reports 7, 10389, doi:10.1038/s41598-017-11060-z.
 Show in context
Show in contextFollowing the Deepwater Horizon well blowout in 2010, the release of massive methane plumes in the water column was accompanied by substantial removal of light REEs, interpreted as the result of intense methanotrophic activity (Shiller et al., 2017).
View in article
Skovran, E., Palmer, A.D., Rountree, A.M., Good, N.M., Lidstrom, M.E. (2011) XoxF is required for expression of methanol dehydrogenase in Methylobacterium extorquens AM1. Journal of Bacteriology 193, 6032–6038.
 Show in context
Show in contextThese enzymes are either Ca dependent (MxaF type) or light REE dependent (XoxF type) (Skovran et al., 2011).
View in article
Taubert, M., Grob, C., Howat, A.M., Burns, O.J., Dixon, J.L., Chen, Y., Colin Murrell, J. (2015) XoxF encoding an alternative methanol dehydrogenase is widespread in coastal marine environments. Environmental Microbiology 17, 3937–3948.
 Show in context
Show in contextHowever, the latter XoxF type seems to be more frequently used by marine bacteria (Ramachandran et al., 2015; Taubert et al., 2015), hence suggesting that REEs could play a previously unsuspected and important role in the development of marine ecosystems relying on aerobic methanotrophic symbioses.
View in article
Thomazo C., Ader M., Farquhar J., Philippot P. (2009) Methanotrophs regulated atmospheric sulfur isotope anomalies during the MesoArchaean (Tumbiana Formation, Western Australia). Earth and Planetary Science Letters 279, 65–75.
 Show in context
Show in contextMethanotrophic organisms have been present since the Archean, but their detection in the geological record, and in the sedimentary archives of the early Earth oceans are still the subject of intense debate (Thomazo et al., 2009; Knoll et al., 2016).
View in article
Wheeler, A.P. (1992) Mechanisms of molluscan shell formation. In: Bonucci, E. (Ed.) Calcification in biological systems. CRC press, Boca Raton, FL, 179–226.
 Show in context
Show in contextShells result from the activity of the mantle epithelium inside specific internal liquid (Wheeler, 1992).
View in article
Yu, J., Wang, M., Liu, B., Yue, X., Li, C. (2019) Gill symbionts of the cold-seep mussel Bathymodiolus platifrons: Composition, environmental dependency and immune control. Fish and Shellfish Immunology 86, 246–252.
 Show in context
Show in contextIt is very important to note that the gills show the highest enrichments in light REEs and lanthanum abundances, because this is precisely where the methanotrophic symbionts are hosted in these mussels (Barry et al., 2002; Yu et al., 2019).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Methods
- Tables S-1 to S-9
Download the Supplementary Information (PDF)
Figures