Clumped isotope temperature calibration for calcite: Bridging theory and experimentation
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





Article views:126Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
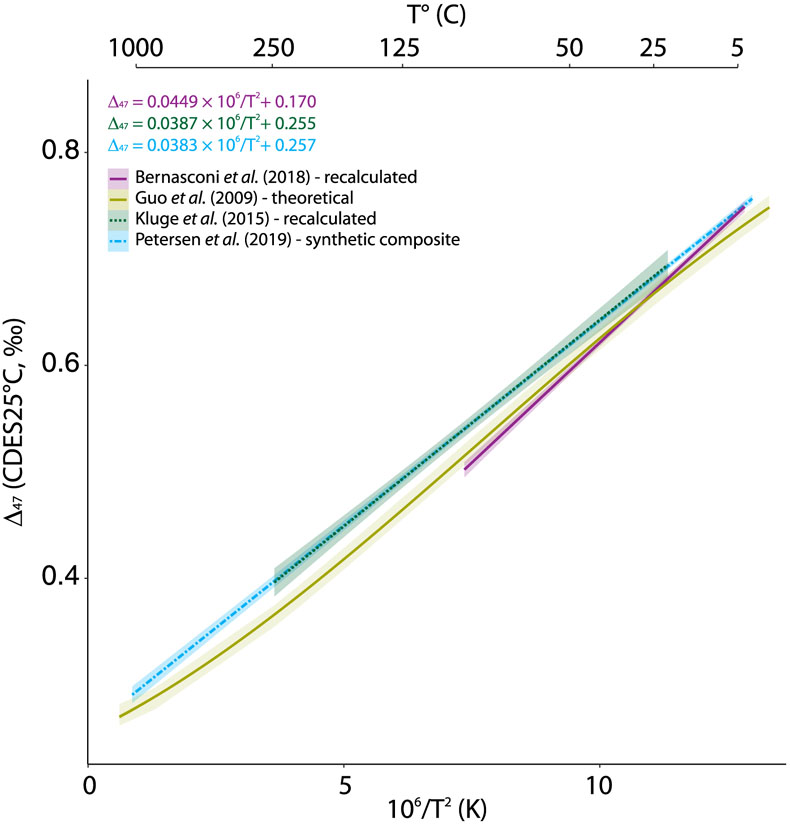
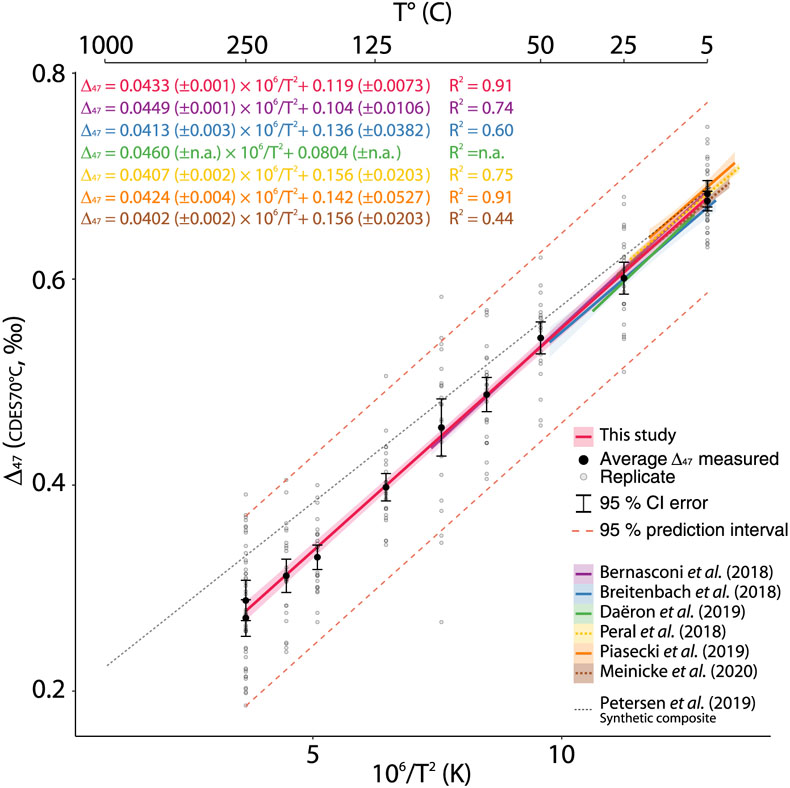
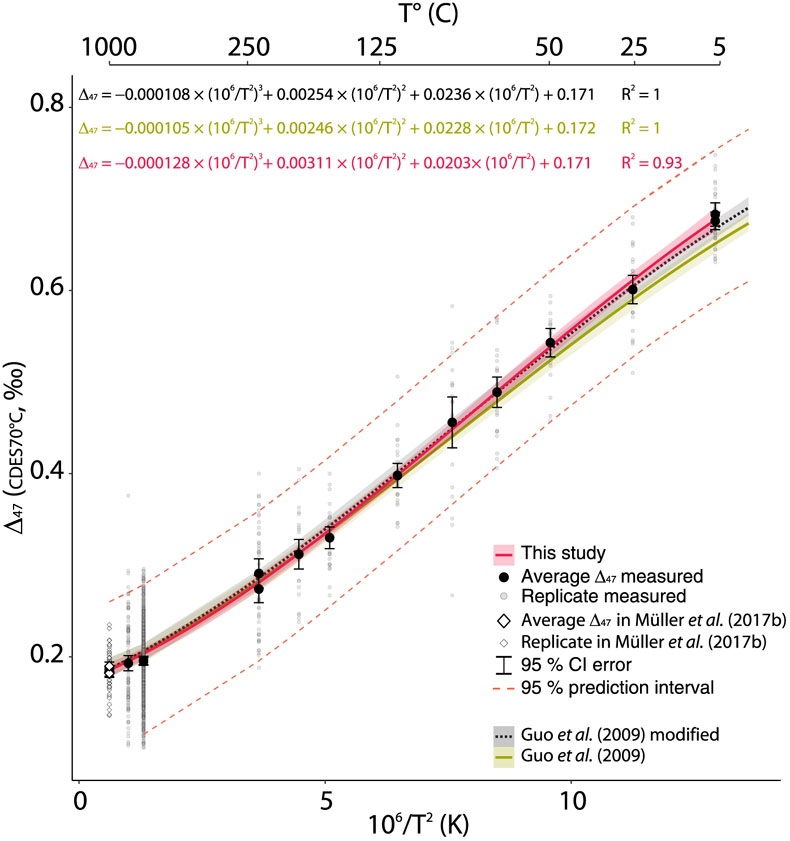
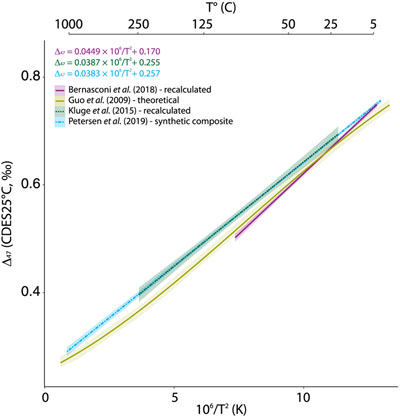 Figure 1 Δ47–T linear relationships in the CDES25°C for the empirical and theoretical calibrations (see text for details). Lines represent the regression models based on all replicates, and shaded envelopes show their 95 % confidence intervals (CI). For more information on the reprocessing of these different datasets see Supplementary Information S-6. | 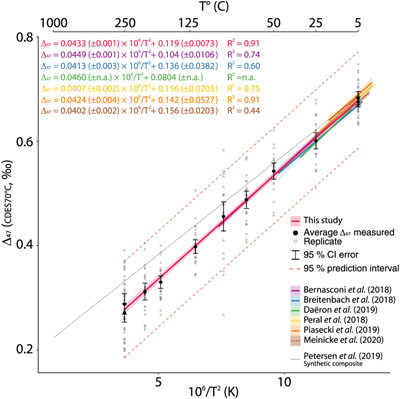 Figure 2 Δ47–T linear relationship in the CDES70°C for the studies fully referenced to ETH carbonate standards; lines represent each study’s linear models, with shaded envelopes representing their respective 95 % CI. For more information on the reprocessing of the different datasets, see Supplementary Information S-6. | 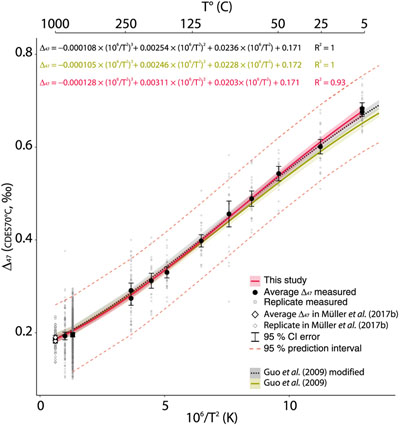 Figure 3 Δ47–T third-order polynomial relationship (CDES70°C) for this study, the theoretical, thermodynamic based relationship in Guo et al. (2009) and a modified version of this equation taking into account the dependence of the Δ*47–63 on the reactant Δ63 (35 ppm increase in Δ*47–63 per 1 ‰ increase in Δ63). ETH1 and 2 (i.e. 600 °C) are illustrated for visual comparison but are not included in the regression model. |
| Figure 1 | Figure 2 | Figure 3 |
top
Introduction
Clumped isotope measurements on CO2 extracted through acidification of carbonates has evolved over the past 15 years as a tool to probe directly the temperature (T) and the parent fluid oxygen isotope composition (δ18O) of precipitates (Schauble et al., 2006
Schauble, E.A., Ghosh, P., Eiler, J.M. (2006) Preferential formation of 13C–18O bonds in carbonate minerals, estimated using first-principles lattice dynamics. Geochimica et Cosmochimica Acta 70, 2510–2529.
). This is done by analysing the excess abundance of 13C–18O bonds in the crystal lattice relative to a stochastic distribution (Δ47). This measurement has a direct link to the formation temperature of the carbonate crystal (Schauble et al., 2006Schauble, E.A., Ghosh, P., Eiler, J.M. (2006) Preferential formation of 13C–18O bonds in carbonate minerals, estimated using first-principles lattice dynamics. Geochimica et Cosmochimica Acta 70, 2510–2529.
) and, therefore, avoids needing to making potentially incorrect assumptions on the isotopic composition of the precipitating fluid as necessary when using the carbonate-water δ18O geothermometer (Urey, 1947Urey, H.C. (1947) The thermodynamic properties of isotopic substances. Journal of the Chemical Society (Resumed). The Royal Society of Chemistry 562–581.
). The pioneering work of Ghosh et al. (2006)Ghosh, P., Adkins, J., Affek, H., Balta, B., Guo, W., Schauble, E.A., Schrag, D., Eiler, J.M. (2006) 13C–18O bonds in carbonate minerals: A new kind of paleothermometer. Geochimica et Cosmochimica Acta 70, 1439–1456.
reported the first experimental calibration of the Δ47–T relationship between 1 and 50 °C. The use of ab initio thermodynamic models (Schauble et al., 2006Schauble, E.A., Ghosh, P., Eiler, J.M. (2006) Preferential formation of 13C–18O bonds in carbonate minerals, estimated using first-principles lattice dynamics. Geochimica et Cosmochimica Acta 70, 2510–2529.
; Guo et al., 2009Guo, W., Mosenfelder, J.L., Goddard, W.A., Eiler, J.M. (2009) Isotopic fractionations associated with phosphoric acid digestion of carbonate minerals: Insights from first-principles theoretical modeling and clumped isotope measurements. Geochimica et Cosmochimica Acta 73, 7203–7225.
) allowed the calculation of the 13C–18O bond ordering in the carbonate crystal lattice as a function of formation temperature, theoretically grounding this geothermometer.Since its inception, this Δ47–T calibration has been reproduced in different laboratories by measuring both natural (e.g., Kele et al., 2015
Kele, S., Breitenbach, S.F.M., Capezzuoli, E., Meckler, A.N., Ziegler, M., Millan, I.M., Kluge, T., Deák, J., Hanselmann, K., John, C.M., Yan, H., Liu, Z., Bernasconi, S.M. (2015) Temperature dependence of oxygen- and clumped isotope fractionation in carbonates: A study of travertines and tufas in the 6-95°C temperature range. Geochimica et Cosmochimica Acta 168, 172–192.
; Breitenbach et al., 2018Breitenbach, S.F.M., Mleneck-Vautravers, M.J., Grauel, A.-L., Lo, L., Bernasconi, S.M., Müller, I.A., Rolfe, J., Gázquez, F., Greaves, M., Hodell, D.A. (2018) Coupled Mg/Ca and clumped isotope analyses of foraminifera provide consistent water temperatures. Geochimica et Cosmochimica Acta 236, 283–296.
; Peral et al., 2018Peral, M., Daëron, M., Blamart, D., Bassinot, F., Dewilde, F., Smialkowski, N., Isguder, G., Bonnin, J., Jorissen, F., Kissel, C., Michel, E., Vázquez Riveiros, N., Waelbroeck, C. (2018) Updated calibration of the clumped isotope thermometer in planktonic and benthic foraminifera. Geochimica et Cosmochimica Acta 239, 1–16.
; Daëron et al., 2019Daëron, M., Drysdale, R.N., Peral, M., Huyghe, D., Blamart, D., Coplen, T.B., Lartaud, F., Zanchetta, G. (2019) Most Earth-surface calcites precipitate out of isotopic equilibrium. Nature Communications 10, 429.
) and synthetic (e.g., Dennis and Schrag, 2010Dennis, K.J., Schrag, D.P. (2010) Clumped isotope thermometry of carbonatites as an indicator of diagenetic alteration. Geochimica et Cosmochimica Acta 74, 4110–4122.
; Passey and Henkes, 2012Passey, B.H., Henkes, G.A. (2012) Carbonate clumped isotope bond reordering and geospeedometry. Earth and Planetary Science Letters 351–352, 223–236.
; Defliese et al., 2015Defliese, W.F., Hren, M.T., Lohmann, K.C. (2015) Compositional and temperature effects of phosphoric acid fractionation on Δ47 analysis and implications for discrepant calibrations. Chemical Geology 396, 51–60.
; Kluge et al., 2015Kluge, T., John, C.M., Jourdan, A.L., Davis, S., Crawshaw, J. (2015) Laboratory calibration of the calcium carbonate clumped isotope thermometer in the 25-250°C temperature range. Geochimica et Cosmochimica Acta 157, 213–227.
) calcites. Improvements of the T–calibration have been based on refining the preparation and analytical techniques (e.g., sample preparation automatisation, sample size reduction), establishing an absolute scale (carbon dioxide equilibrium scale at 25 °C acid digestion temperature; CDES25°C; Dennis et al., 2011Dennis, K.J., Affek, H.P., Passey, B.H., Schrag, D.P., Eiler, J.M. (2011) Defining an absolute reference frame for “clumped” isotope studies of CO2. Geochimica et Cosmochimica Acta 75, 7117–7131.
), increasing the number of replicates per anchor points, and abating the analytical artefacts inherent to clumped isotopic measurements. While these advances have considerably reduced the differences observed between the T–calibrations produced in independent laboratories, significant differences still remain. More recently, the clumped isotope community has put effort into understanding the causes of the remaining inter-laboratory differences. Practitioners have also promoted the principle of similar treatment for standards and unknown samples as a mean of improving inter-laboratory comparisons (Bernasconi et al., 2018Bernasconi, S.M., Müller, I.A., Bergmann, K.D., Breitenbach, S.F.M., Fernandez, A., Hodell, D.A., Jaggi, M., Meckler, A.N., Millan, I., Ziegler, M. (2018) Reducing Uncertainties in Carbonate Clumped Isotope Analysis Through Consistent Carbonate-Based Standardization. Geochemistry, Geophysics, Geosystems 19, 2895–2914.
). However, a lack of temperature calibration points above 100 °C and large differences at higher temperatures persist, weakening the application of this thermometer to high temperature geological systems. More importantly, none of the published T–calibrations can reconcile theory and experimentation and the non-linearity of the theoretical Δ47–T mathematical relationship (Guo et al., 2009Guo, W., Mosenfelder, J.L., Goddard, W.A., Eiler, J.M. (2009) Isotopic fractionations associated with phosphoric acid digestion of carbonate minerals: Insights from first-principles theoretical modeling and clumped isotope measurements. Geochimica et Cosmochimica Acta 73, 7203–7225.
) also observed experimentally for dolomite (Müller et al., 2019Müller, I.A., Rodriguez-Blanco, J.D., Storck, J.-C., do Nascimento, G.S., Bontognali, T.R.R., Vasconcelos, C., Benning, L.G., Bernasconi, S.M. (2019) Calibration of the oxygen and clumped isotope thermometers for (proto-)dolomite based on synthetic and natural carbonates. Chemical Geology 525, 1–17.
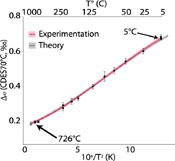
) has not been reproduced empirically at high temperatures for calcite (Fig. 1).
Figure 1 Δ47–T linear relationships in the CDES25°C for the empirical and theoretical calibrations (see text for details). Lines represent the regression models based on all replicates, and shaded envelopes show their 95 % confidence intervals (CI). For more information on the reprocessing of these different datasets see Supplementary Information S-6.
Apart from the challenges inherent to comparing T–calibrations directly based upon different standardisation strategies, the differences (i.e. slope p values <1 × 10–4 and intercept p values <1 × 10–4; Supplementary Information S-1, Table S-1) observed between the latest composite (Petersen et al., 2019
Petersen, S.V., Defliese, W.F., Saenger, C., Daëron, M., Huntington, K.W., John, C.M., Kelson, J.R., Bernasconi, S.M., Coleman, A.S., Kluge, T., Olack, G.A., Schauer, A.J., Bajnai, D., Bonifacie, M., Breitenbach, S.F.M., Fiebig, J., Fernandez, A.B., Henkes, G.A., Hodell, D., Katz, A., Kele, S., Lohmann, K.C., Passey, B.H., Peral, M.Y., Petrizzo, D.A., Rosenheim, B.E., Tripati, A., Venturelli, R., Young, E.D., Winkelstern, I.Z. (2019) Effects of Improved 17O Correction on Inter‐Laboratory Agreement in Clumped Isotope Calibrations, Estimates of Mineral‐Specific Offsets, and Temperature Dependence of Acid Digestion Fractionation. Geochemistry, Geophysics, Geosystems 20, 3495–3519.
), the fully carbonate-based standardised (Kele et al., 2015Kele, S., Breitenbach, S.F.M., Capezzuoli, E., Meckler, A.N., Ziegler, M., Millan, I.M., Kluge, T., Deák, J., Hanselmann, K., John, C.M., Yan, H., Liu, Z., Bernasconi, S.M. (2015) Temperature dependence of oxygen- and clumped isotope fractionation in carbonates: A study of travertines and tufas in the 6-95°C temperature range. Geochimica et Cosmochimica Acta 168, 172–192.
) recalculated by Bernasconi et al. (2018)Bernasconi, S.M., Müller, I.A., Bergmann, K.D., Breitenbach, S.F.M., Fernandez, A., Hodell, D.A., Jaggi, M., Meckler, A.N., Millan, I., Ziegler, M. (2018) Reducing Uncertainties in Carbonate Clumped Isotope Analysis Through Consistent Carbonate-Based Standardization. Geochemistry, Geophysics, Geosystems 19, 2895–2914.
, and the theoretical T–calibrations (Guo et al., 2009Guo, W., Mosenfelder, J.L., Goddard, W.A., Eiler, J.M. (2009) Isotopic fractionations associated with phosphoric acid digestion of carbonate minerals: Insights from first-principles theoretical modeling and clumped isotope measurements. Geochimica et Cosmochimica Acta 73, 7203–7225.
) could be due to the strong influence of the high temperature calcite calibration points (Passey and Henkes, 2012Passey, B.H., Henkes, G.A. (2012) Carbonate clumped isotope bond reordering and geospeedometry. Earth and Planetary Science Letters 351–352, 223–236.
; Kluge et al., 2015Kluge, T., John, C.M., Jourdan, A.L., Davis, S., Crawshaw, J. (2015) Laboratory calibration of the calcium carbonate clumped isotope thermometer in the 25-250°C temperature range. Geochimica et Cosmochimica Acta 157, 213–227.
) used in the data compilation effort of Petersen et al. (2019)Petersen, S.V., Defliese, W.F., Saenger, C., Daëron, M., Huntington, K.W., John, C.M., Kelson, J.R., Bernasconi, S.M., Coleman, A.S., Kluge, T., Olack, G.A., Schauer, A.J., Bajnai, D., Bonifacie, M., Breitenbach, S.F.M., Fiebig, J., Fernandez, A.B., Henkes, G.A., Hodell, D., Katz, A., Kele, S., Lohmann, K.C., Passey, B.H., Peral, M.Y., Petrizzo, D.A., Rosenheim, B.E., Tripati, A., Venturelli, R., Young, E.D., Winkelstern, I.Z. (2019) Effects of Improved 17O Correction on Inter‐Laboratory Agreement in Clumped Isotope Calibrations, Estimates of Mineral‐Specific Offsets, and Temperature Dependence of Acid Digestion Fractionation. Geochemistry, Geophysics, Geosystems 20, 3495–3519.
(Fig. 1). Some additional effects might have influenced the high temperature precipitates from Kluge et al. (2015)Kluge, T., John, C.M., Jourdan, A.L., Davis, S., Crawshaw, J. (2015) Laboratory calibration of the calcium carbonate clumped isotope thermometer in the 25-250°C temperature range. Geochimica et Cosmochimica Acta 157, 213–227.
(Supplementary Information S-2, Fig. S-1) and the re-ordering experiments in Passey and Henkes (2012)Passey, B.H., Henkes, G.A. (2012) Carbonate clumped isotope bond reordering and geospeedometry. Earth and Planetary Science Letters 351–352, 223–236.
(e.g., incomplete solid state re-ordering), potentially influencing the slope of this composite Δ47–T relationship (Petersen et al., 2019Petersen, S.V., Defliese, W.F., Saenger, C., Daëron, M., Huntington, K.W., John, C.M., Kelson, J.R., Bernasconi, S.M., Coleman, A.S., Kluge, T., Olack, G.A., Schauer, A.J., Bajnai, D., Bonifacie, M., Breitenbach, S.F.M., Fiebig, J., Fernandez, A.B., Henkes, G.A., Hodell, D., Katz, A., Kele, S., Lohmann, K.C., Passey, B.H., Peral, M.Y., Petrizzo, D.A., Rosenheim, B.E., Tripati, A., Venturelli, R., Young, E.D., Winkelstern, I.Z. (2019) Effects of Improved 17O Correction on Inter‐Laboratory Agreement in Clumped Isotope Calibrations, Estimates of Mineral‐Specific Offsets, and Temperature Dependence of Acid Digestion Fractionation. Geochemistry, Geophysics, Geosystems 20, 3495–3519.
).This study attempts to bridge the gap between the fully stochastic isotope distribution reached at high temperature and the large number of low to medium temperature points produced since the onset of this new geochemical tool. We choose to work solely on calcites to avoid potential biases due to specific acid fractionation factors (AFF) when using different carbonates (i.e. aragonite, dolomite, siderite) as theoretically predicted (Guo et al., 2009
Guo, W., Mosenfelder, J.L., Goddard, W.A., Eiler, J.M. (2009) Isotopic fractionations associated with phosphoric acid digestion of carbonate minerals: Insights from first-principles theoretical modeling and clumped isotope measurements. Geochimica et Cosmochimica Acta 73, 7203–7225.
) and shown by recent experimental work (Müller et al., 2017aMüller, I.A., Violay, M.E.S., Storck, J.C., Fernandez, A., van Dijk, J., Madonna, C., Bernasconi, S.M. (2017a) Clumped isotope fractionation during phosphoric acid digestion of carbonates at 70 °C. Chemical Geology 449, 1–14.
; van Dijk et al., 2019van Dijk, J., Fernandez, A., Storck, J.C., White, T.S., Lever, M., Müller, I.A., Bishop, S., Seifert, R.F., Driese, S.G., Krylov, A., Ludvigson, G.A., Turchyn, A.V., Lin, C.Y., Wittkop, C., Bernasconi, S.M. (2019) Experimental calibration of clumped isotopes in siderite between 8.5 and 62 °C and its application as paleo-thermometer in paleosols. Geochimica et Cosmochimica Acta 254, 1–20.
) at the temperature of acid digestion used in this study (70 °C). We also took advantage of the growing number of laboratories that adopted a fully carbonate-based standardisation of Δ47 measurements – identical to the treatment used here – to evaluate further the improvement brought by this standardisation scheme to inter-laboratory comparison.top
Methodology
Synthetic precipitates. Precipitation of synthetic calcites was performed using high pressure and temperature reaction cells (70–250 °C) and passive diffusion techniques (5–70 °C) (Supplementary Information S-3, S-4, Table S-2, Fig. S-2) (Dennis and Schrag, 2010
Dennis, K.J., Schrag, D.P. (2010) Clumped isotope thermometry of carbonatites as an indicator of diagenetic alteration. Geochimica et Cosmochimica Acta 74, 4110–4122.
; Defliese et al., 2015Defliese, W.F., Hren, M.T., Lohmann, K.C. (2015) Compositional and temperature effects of phosphoric acid fractionation on Δ47 analysis and implications for discrepant calibrations. Chemical Geology 396, 51–60.
). Briefly, solutions of NaHCO3 and CaCl2 were equilibrated separately at the target temperature for 24 to 144 hr prior to mixing. The solutions were then left to precipitate for at least 24 hr. We carried out near-equilibrium precipitation by keeping the calcite saturation index below 2.5 and verifying the δ18O values of the precipitates relative to the δ18O of the water used (−10.9 ‰; see Fig. S-1, Table S-2, Supplementary Information S-2, S-3). The production of the heated calcite (i.e. CM γ-5; 726 °C) is described in Schmid and Bernasconi (2010)Schmid, T.W., Bernasconi, S.M. (2010) An automated method for ‘clumped-isotope’ measurements on small carbonate samples. Rapid Communications in Mass Spectrometry 24, 1955–1963.
.Measurements. Synthetic precipitates were analysed at the Delta-lab (Geological Survey of Canada) using a MAT 253 (Thermo Scientific, Bremen, Germany) equipped with six shielded faraday cups (m/z = 44–49) and an additional half-mass detector at m/z = 47.5 for live background monitoring. The preparation of carbonate was performed with a KIEL-IV carbonate device (Thermo Scientific, Bremen, Germany) modified according to Schmid and Bernasconi (2010)
Schmid, T.W., Bernasconi, S.M. (2010) An automated method for ‘clumped-isotope’ measurements on small carbonate samples. Rapid Communications in Mass Spectrometry 24, 1955–1963.
. The sample is first acidified for 500 s with 3 drops of 104 % phosphoric acid prepared following the method of Burman et al. (2005)Burman, J., Gustafsson, O., Segl, M., Schmitz, B. (2005) A simplified method of preparing phosphoric acid for stable isotope analyses of carbonates. Rapid Communications in Mass Spectrometry 19, 3086–3088.
. Evolved CO2 and H2O are trapped continuously at liquid N2 (LN2) temperature followed by a 60 s of non-condensable gas pumping. The CO2 is then released at −100 °C and cryopumped through a Porapak trap surrounded by Ag wool held at −14 °C into a micro-volume at LN2 temperature. The purified CO2 is then released into the ion source of the mass spectrometer through an inert capillary. δ18O, δ13C, and δ47 data were acquired simultaneously using the long integration dual inlet (LIDI) method (Müller et al., 2017bMüller, I.A., Fernandez, A., Radke, J., van Dijk, J., Bowen, D., Schwieters, J., Bernasconi, S.M. (2017b) Carbonate clumped isotope analyses with the long-integration dual-inlet (LIDI) workflow: scratching at the lower sample weight boundaries. Rapid Communications in Mass Spectrometry 31, 1057–1066.
).Each measurement comprises 70 cycles of 10 s integrations of the unknown gas followed by a similar integration strategy of the working gas (δ13CVPDB = –4.5‰, δ18OVPDB = –14.05 ‰). Each synthetic calcite measurement was replicated at least 20 times (80–100 μg aliquots) in order to achieve good precision for the evaluation of the Δ47–T relationship. Error estimation is reported as 95 % confidence interval (CI) of all replicates.
Data processing. Raw beam intensities were imported into Easotope (John and Bowen, 2016
John, C.M., Bowen, D. (2016) Community software for challenging isotope analysis: First applications of ‘Easotope’ to clumped isotopes. Rapid Communications in Mass Spectrometry 30, 2285–2300.
) for calculation of the Δ47. The IUPAC parameter set (Brand et al., 2010Brand, W.A., Assonov, S.S., Coplen, T.B. (2010) Correction for the 17O interference in δ13C measurements when analyzing CO2 with stable isotope mass spectrometry (IUPAC Technical Report). Pure and Applied Chemistry 82, 1719–1733.
) was used to correct for 17O interferences (Daëron et al., 2016Daëron, M., Blamart, D., Peral, M., Affek, H.P. (2016) Absolute isotopic abundance ratios and the accuracy of Δ47 measurements. Chemical Geology 442, 83–96.
; Schauer et al., 2016Schauer, A.J., Kelson, J., Saenger, C., Huntington, K.W. (2016) Choice of 17O correction affects clumped isotope (Δ47) values of CO2 measured with mass spectrometry. Rapid Communications in Mass Spectrometry 30, 2607–2616.
). We adopted a fully carbonate-based calibration scheme by interspersing 50 % of carbonate standards developed at ETH-Zurich (Meckler et al., 2014Meckler, A.N., Ziegler, M., Millán, M.I., Breitenbach, S.F.M., Bernasconi, S.M. (2014) Long-term performance of the Kiel carbonate device with a new correction scheme for clumped isotope measurements. Rapid Communications in Mass Spectrometry 28, 1705–1715.
) in every analytical session, and by keeping similar average sample and standard sizes. The recalculated values (IUPAC parameter) of the different standards (ETH1-4) were used (Bernasconi et al., 2018Bernasconi, S.M., Müller, I.A., Bergmann, K.D., Breitenbach, S.F.M., Fernandez, A., Hodell, D.A., Jaggi, M., Meckler, A.N., Millan, I., Ziegler, M. (2018) Reducing Uncertainties in Carbonate Clumped Isotope Analysis Through Consistent Carbonate-Based Standardization. Geochemistry, Geophysics, Geosystems 19, 2895–2914.
) with an external long term standard deviation of 37 ppm. ETH-1 and ETH-2 were used to correct for non-linearity effects in the ion source, ETH-1, 2 and 3 aided with constructing the empirical transfer function (ETF) for translation of the measurements to the CDES, and ETH-4 was treated as an unknown for data acquisition and processing monitoring. The calibration was made on a running window of 56 standards before and after each unknown to correct for drift in ion source stability. Minor negative background correction was performed using the pressure baseline correction (Bernasconi et al., 2013Bernasconi, S.M., Hu, B., Wacker, U., Fiebig, J., Breitenbach, S.F.M., Rutz, T. (2013) Background effects on Faraday collectors in gas-source mass spectrometry and implications for clumped isotope measurements. Rapid Communications in Mass Spectrometry 27, 603–612.
) after every 46 replicates using high voltage scans made from 5 to 25 V intensities (m/z = 44), in 5 V increments, to produce a correction function that can be applied to the decaying beam intensity inherent to the LIDI method of measurement. Discussion on the effect of linearities on the ETH carbonate standardisation scheme can be found in the Supplementary Information S-5.top
Results and Discussion
Following the mentioned methodologies, we produced a linear T–Δ47 calibration over the temperature range of 5 to 250 °C (Fig. 2).

Figure 2 Δ47–T linear relationship in the CDES70°C for the studies fully referenced to ETH carbonate standards; lines represent each study’s linear models, with shaded envelopes representing their respective 95 % CI. For more information on the reprocessing of the different datasets, see Supplementary Information S-6.
To evaluate the efficiency of the ETH carbonate standardisation scheme in improving inter-laboratory comparisons, we compiled the T–calibrations from this study and recently published Δ47–T relationships standardised with ETH carbonates (Bernasconi et al., 2018
Bernasconi, S.M., Müller, I.A., Bergmann, K.D., Breitenbach, S.F.M., Fernandez, A., Hodell, D.A., Jaggi, M., Meckler, A.N., Millan, I., Ziegler, M. (2018) Reducing Uncertainties in Carbonate Clumped Isotope Analysis Through Consistent Carbonate-Based Standardization. Geochemistry, Geophysics, Geosystems 19, 2895–2914.
; Breitenbach et al., 2018Breitenbach, S.F.M., Mleneck-Vautravers, M.J., Grauel, A.-L., Lo, L., Bernasconi, S.M., Müller, I.A., Rolfe, J., Gázquez, F., Greaves, M., Hodell, D.A. (2018) Coupled Mg/Ca and clumped isotope analyses of foraminifera provide consistent water temperatures. Geochimica et Cosmochimica Acta 236, 283–296.
; Peral et al., 2018Peral, M., Daëron, M., Blamart, D., Bassinot, F., Dewilde, F., Smialkowski, N., Isguder, G., Bonnin, J., Jorissen, F., Kissel, C., Michel, E., Vázquez Riveiros, N., Waelbroeck, C. (2018) Updated calibration of the clumped isotope thermometer in planktonic and benthic foraminifera. Geochimica et Cosmochimica Acta 239, 1–16.
; Daëron et al., 2019Daëron, M., Drysdale, R.N., Peral, M., Huyghe, D., Blamart, D., Coplen, T.B., Lartaud, F., Zanchetta, G. (2019) Most Earth-surface calcites precipitate out of isotopic equilibrium. Nature Communications 10, 429.
; Piasecki et al., 2019Piasecki, A., Bernasconi, S.M., Grauel, A.-L., Hannisdal, B., Ho, S.L., Marchitto, T.M., Meinicke, N., Tisserand, A., Meckler, N. (2019) Application of Clumped Isotope Thermometry to Benthic Foraminifera. Geochemistry, Geophysics, Geosystems 20, 2090.
; Meinicke et al., 2020Meinicke, N., Ho, S.L., Hannisdal, B., Nürnberg, D., Tripati, A., Schiebel, R., Meckler, A.N. (2020) A robust calibration of the clumped isotopes to temperature relationship for foraminifers. Geochimica et Cosmochimica Acta 270, 160–183.
; Fig. 2) in the CDES70°C. This approach avoids the potential propagation of errors due to the use of somewhat loosely constrained AFF conversions. An analysis of covariance on the different slopes and y intercepts of the Δ47–T relationships reveals strong similarities at the 95 % CI (i.e. slope and y intercept p values > 0.05; Table S-3). This exercise confirms that the proposition to use a fully carbonate-based calibration scheme (Bernasconi et al., 2018Bernasconi, S.M., Müller, I.A., Bergmann, K.D., Breitenbach, S.F.M., Fernandez, A., Hodell, D.A., Jaggi, M., Meckler, A.N., Millan, I., Ziegler, M. (2018) Reducing Uncertainties in Carbonate Clumped Isotope Analysis Through Consistent Carbonate-Based Standardization. Geochemistry, Geophysics, Geosystems 19, 2895–2914.
) improves the comparison of data measured in different laboratories with various treatment, analytical methods and different types of calcite formation while avoiding the effects of the uncertainty in the AFF. Hence, this supports producing the following composite linear Δ47–T relationship with T in K (Fig. 2).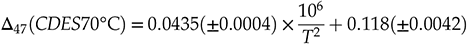
We additionally plotted the composite calibration from Petersen et al. (2019)
Petersen, S.V., Defliese, W.F., Saenger, C., Daëron, M., Huntington, K.W., John, C.M., Kelson, J.R., Bernasconi, S.M., Coleman, A.S., Kluge, T., Olack, G.A., Schauer, A.J., Bajnai, D., Bonifacie, M., Breitenbach, S.F.M., Fiebig, J., Fernandez, A.B., Henkes, G.A., Hodell, D., Katz, A., Kele, S., Lohmann, K.C., Passey, B.H., Peral, M.Y., Petrizzo, D.A., Rosenheim, B.E., Tripati, A., Venturelli, R., Young, E.D., Winkelstern, I.Z. (2019) Effects of Improved 17O Correction on Inter‐Laboratory Agreement in Clumped Isotope Calibrations, Estimates of Mineral‐Specific Offsets, and Temperature Dependence of Acid Digestion Fractionation. Geochemistry, Geophysics, Geosystems 20, 3495–3519.
for qualitative comparison (Fig. 2). While partially overlapping within the 95 % CI error estimates of the low temperature calcite precipitates synthesised in this study, differences in Δ47 of up to 50 ppm are observed between the two calibrations at temperatures above 90 °C. These discrepancies at ca. 200 °C translate into temperature estimates differences of around 50 °C, which can influence the interpretation of high temperature systems when using the clumped isotope geothermometer.In an attempt to investigate the theoretical non-linear nature of the Δ47–T relationship, we expanded the studied temperature range to the equivalent stochastic 13C–18O bonds distribution by adding measurements of the heated calcite CM γ-5 (726 °C) (Schmid and Bernasconi, 2010
Schmid, T.W., Bernasconi, S.M. (2010) An automated method for ‘clumped-isotope’ measurements on small carbonate samples. Rapid Communications in Mass Spectrometry 24, 1955–1963.
) and the data for the three stochastic calcites (1000 °C) reported in Müller et al. (2017) (Fig. 3, Supplementary Information S-6).
Figure 3 Δ47–T third-order polynomial relationship (CDES70°C) for this study, the theoretical, thermodynamic based relationship in Guo et al. (2009)
Guo, W., Mosenfelder, J.L., Goddard, W.A., Eiler, J.M. (2009) Isotopic fractionations associated with phosphoric acid digestion of carbonate minerals: Insights from first-principles theoretical modeling and clumped isotope measurements. Geochimica et Cosmochimica Acta 73, 7203–7225.
and a modified version of this equation taking into account the dependence of the Δ*47–63 on the reactant Δ63 (35 ppm increase in Δ*47–63 per 1 ‰ increase in Δ63). ETH1 and 2 (i.e. 600 °C) are illustrated for visual comparison but are not included in the regression model.The polynomial relationship from Guo et al. (2009)
Guo, W., Mosenfelder, J.L., Goddard, W.A., Eiler, J.M. (2009) Isotopic fractionations associated with phosphoric acid digestion of carbonate minerals: Insights from first-principles theoretical modeling and clumped isotope measurements. Geochimica et Cosmochimica Acta 73, 7203–7225.
is projected into the CDES70°C frame by replacing the Δ*47–63 (= 0.268 ‰ for a 25 °C acid digestion) with a 70 °C Δ*47–63 (= 0.184 ‰) determined experimentally by Müller et al. (2017b)Müller, I.A., Fernandez, A., Radke, J., van Dijk, J., Bowen, D., Schwieters, J., Bernasconi, S.M. (2017b) Carbonate clumped isotope analyses with the long-integration dual-inlet (LIDI) workflow: scratching at the lower sample weight boundaries. Rapid Communications in Mass Spectrometry 31, 1057–1066.
and updated to the IUPAC parameters (Supplementary Information S-6, S-7). While the theoretical Δ47–T polynomial relationship (Guo et al., 2009Guo, W., Mosenfelder, J.L., Goddard, W.A., Eiler, J.M. (2009) Isotopic fractionations associated with phosphoric acid digestion of carbonate minerals: Insights from first-principles theoretical modeling and clumped isotope measurements. Geochimica et Cosmochimica Acta 73, 7203–7225.
) fits relatively well with our empirical calibration curve at high temperature (i.e. within the 95 % CI), our curve lies above the theoretical line and outside the 95 % CI at low temperature. When we modify the theoretical curve by integrating the dependency of the Δ*47–63 to the Δ63 of the reactant carbonates (Guo et al., 2009Guo, W., Mosenfelder, J.L., Goddard, W.A., Eiler, J.M. (2009) Isotopic fractionations associated with phosphoric acid digestion of carbonate minerals: Insights from first-principles theoretical modeling and clumped isotope measurements. Geochimica et Cosmochimica Acta 73, 7203–7225.
), a significant fit between the two polynomial curves is observed (i.e. p value = 0.96; Supplementary Information S-1), reconciling the experimentation with theory at the 95 % CI over the full temperature spectrum (Fig. 3). This, in addition to the significant similarities of all Δ47–T linear relationships (Fig. 2), further supports using the carbonate standardisation scheme for inter-laboratory comparisons (Bernasconi et al., 2018Bernasconi, S.M., Müller, I.A., Bergmann, K.D., Breitenbach, S.F.M., Fernandez, A., Hodell, D.A., Jaggi, M., Meckler, A.N., Millan, I., Ziegler, M. (2018) Reducing Uncertainties in Carbonate Clumped Isotope Analysis Through Consistent Carbonate-Based Standardization. Geochemistry, Geophysics, Geosystems 19, 2895–2914.
; Peral et al., 2018Peral, M., Daëron, M., Blamart, D., Bassinot, F., Dewilde, F., Smialkowski, N., Isguder, G., Bonnin, J., Jorissen, F., Kissel, C., Michel, E., Vázquez Riveiros, N., Waelbroeck, C. (2018) Updated calibration of the clumped isotope thermometer in planktonic and benthic foraminifera. Geochimica et Cosmochimica Acta 239, 1–16.
; Meinicke et al., 2020Meinicke, N., Ho, S.L., Hannisdal, B., Nürnberg, D., Tripati, A., Schiebel, R., Meckler, A.N. (2020) A robust calibration of the clumped isotopes to temperature relationship for foraminifers. Geochimica et Cosmochimica Acta 270, 160–183.
). Moreover, this study provides an empirical T–calibration agreeing with theory over the broadest temperature range to date. This new T–calibration, which can be applied by any laboratory using a carbonate-based standardisation of their measurements on calcite, may also serve for investigating geological contexts of calcite formation at high temperature.top
Acknowledgements
This research was funded by the Lands and Minerals Sector of Natural Resources Canada under the joint framework of the Targeted Geosciences Initiatives and the Geo-mapping for Energy and Minerals programs. Marc Luzincourt is thanked for his assistance on Δ47 measurements in the Delta-lab and Pierre Masselot for statistical support. We thank Will Defliese, Nami Kitchen, Ury Ryb and John Eiler for advice and discussions that helped setting up this clumped isotope facility, Magali Bonifacie for discussions that lead to the preparation of this manuscript and Jason Ahad for providing comments that greatly improved this manuscript. Joep van Dijk as well as two anonymous reviewers and the editor Eric Oelkers are thanked for their comments that contributed to improve this manuscript. Natural Resources Canada Contribution No. 20190528.
Data Statement: Originally processed data are included in the references cited. The data acquired for this study has been archived in the EarthChem, ClumpDB database under the following doi: 10.26022/IEDA/111559.
Editor: Eric H. Oelkers
top
References
Bernasconi, S.M., Hu, B., Wacker, U., Fiebig, J., Breitenbach, S.F.M., Rutz, T. (2013) Background effects on Faraday collectors in gas-source mass spectrometry and implications for clumped isotope measurements. Rapid Communications in Mass Spectrometry 27, 603–612.
 Show in context
Show in contextMinor negative background correction was performed using the pressure baseline correction (Bernasconi et al., 2013) after every 46 replicates using high voltage scans made from 5 to 25 V intensities (m/z = 44), in 5 V increments, to produce a correction function that can be applied to the decaying beam intensity inherent to the LIDI method of measurement.
View in article
Bernasconi, S.M., Müller, I.A., Bergmann, K.D., Breitenbach, S.F.M., Fernandez, A., Hodell, D.A., Jaggi, M., Meckler, A.N., Millan, I., Ziegler, M. (2018) Reducing Uncertainties in Carbonate Clumped Isotope Analysis Through Consistent Carbonate-Based Standardization. Geochemistry, Geophysics, Geosystems 19, 2895–2914.
 Show in context
Show in contextPractitioners have also promoted the principle of similar treatment for standards and unknown samples as a mean of improving inter-laboratory comparisons (Bernasconi et al., 2018).
View in article
Apart from the challenges inherent to comparing T–calibrations directly based upon different standardisation strategies, the differences (i.e. slope p values <1 × 10–4 and intercept p values <1 × 10–4; Supplementary Information S-1, Table S-1) observed between the latest composite (Petersen et al., 2019), the fully carbonate-based standardised (Kele et al., 2015) recalculated by Bernasconi et al. (2018), and the theoretical T–calibrations (Guo et al., 2009) could be due to the strong influence of the high temperature calcite calibration points (Passey and Henkes, 2012; Kluge et al., 2015) used in the data compilation effort of Petersen et al. (2019) (Fig. 1).
View in article
The recalculated values (IUPAC parameter) of the different standards (ETH1-4) were used (Bernasconi et al., 2018) with an external long term standard deviation of 37 ppm.
View in article
This exercise confirms that the proposition to use a fully carbonate-based calibration scheme (Bernasconi et al., 2018) improves the comparison of data measured in different laboratories with various treatment, analytical methods and different types of calcite formation while avoiding the effects of the uncertainty in the AFF.
View in article
To evaluate the efficiency of the ETH carbonate standardisation scheme in improving inter-laboratory comparisons, we compiled the T–calibrations from this study and recently published Δ47–T relationships standardised with ETH carbonates (Bernasconi et al., 2018; Breitenbach et al., 2018; Peral et al., 2018; Daëron et al., 2019; Piasecki et al., 2019; Meinicke et al., 2020; Fig. 2) in the CDES70°C.
View in article
This, in addition to the significant similarities of all Δ47–T linear relationships (Fig. 2), further supports using the carbonate standardisation scheme for inter-laboratory comparisons (Bernasconi et al., 2018; Peral et al., 2018; Meinicke et al., 2020).
View in article
Brand, W.A., Assonov, S.S., Coplen, T.B. (2010) Correction for the 17O interference in δ13C measurements when analyzing CO2 with stable isotope mass spectrometry (IUPAC Technical Report). Pure and Applied Chemistry 82, 1719–1733.
 Show in context
Show in context The IUPAC parameter set (Brand et al., 2010) was used to correct for 17O interferences (Daëron et al., 2016; Schauer et al., 2016).
View in article
Breitenbach, S.F.M., Mleneck-Vautravers, M.J., Grauel, A.-L., Lo, L., Bernasconi, S.M., Müller, I.A., Rolfe, J., Gázquez, F., Greaves, M., Hodell, D.A. (2018) Coupled Mg/Ca and clumped isotope analyses of foraminifera provide consistent water temperatures. Geochimica et Cosmochimica Acta 236, 283–296.
 Show in context
Show in context Since its inception, this Δ47–T calibration has been reproduced in different laboratories by measuring both natural (e.g., Kele et al., 2015; Breitenbach et al., 2018; Peral et al., 2018; Daëron et al., 2019) and synthetic (e.g., Dennis and Schrag, 2010; Passey and Henkes, 2012; Defliese et al., 2015; Kluge et al., 2015) calcites.
View in article
To evaluate the efficiency of the ETH carbonate standardisation scheme in improving inter-laboratory comparisons, we compiled the T–calibrations from this study and recently published Δ47–T relationships standardised with ETH carbonates (Bernasconi et al., 2018; Breitenbach et al., 2018; Peral et al., 2018; Daëron et al., 2019; Piasecki et al., 2019; Meinicke et al., 2020; Fig. 2) in the CDES70°C.
View in article
Burman, J., Gustafsson, O., Segl, M., Schmitz, B. (2005) A simplified method of preparing phosphoric acid for stable isotope analyses of carbonates. Rapid Communications in Mass Spectrometry 19, 3086–3088.
 Show in context
Show in context The sample is first acidified for 500 s with 3 drops of 104 % phosphoric acid prepared following the method of Burman et al. (2005).
View in article
Daëron, M., Blamart, D., Peral, M., Affek, H.P. (2016) Absolute isotopic abundance ratios and the accuracy of Δ47 measurements. Chemical Geology 442, 83–96.
 Show in context
Show in context The IUPAC parameter set (Brand et al., 2010) was used to correct for 17O interferences (Daëron et al., 2016; Schauer et al., 2016).
View in article
Daëron, M., Drysdale, R.N., Peral, M., Huyghe, D., Blamart, D., Coplen, T.B., Lartaud, F., Zanchetta, G. (2019) Most Earth-surface calcites precipitate out of isotopic equilibrium. Nature Communications 10, 429.
 Show in context
Show in context Since its inception, this Δ47–T calibration has been reproduced in different laboratories by measuring both natural (e.g., Kele et al., 2015; Breitenbach et al., 2018; Peral et al., 2018; Daëron et al., 2019) and synthetic (e.g., Dennis and Schrag, 2010; Passey and Henkes, 2012; Defliese et al., 2015; Kluge et al., 2015) calcites.
View in article
To evaluate the efficiency of the ETH carbonate standardisation scheme in improving inter-laboratory comparisons, we compiled the T–calibrations from this study and recently published Δ47–T relationships standardised with ETH carbonates (Bernasconi et al., 2018; Breitenbach et al., 2018; Peral et al., 2018; Daëron et al., 2019; Piasecki et al., 2019; Meinicke et al., 2020; Fig. 2) in the CDES70°C.
View in article
Defliese, W.F., Hren, M.T., Lohmann, K.C. (2015) Compositional and temperature effects of phosphoric acid fractionation on Δ47 analysis and implications for discrepant calibrations. Chemical Geology 396, 51–60.
 Show in context
Show in context Since its inception, this Δ47–T calibration has been reproduced in different laboratories by measuring both natural (e.g., Kele et al., 2015; Breitenbach et al., 2018; Peral et al., 2018; Daëron et al., 2019) and synthetic (e.g., Dennis and Schrag, 2010; Passey and Henkes, 2012; Defliese et al., 2015; Kluge et al., 2015) calcites.
View in article
Precipitation of synthetic calcites was performed using high pressure and temperature reaction cells (70–250 °C) and passive diffusion techniques (5–70 °C) (Supplementary Information S-3, S-4, Table S-2, Fig. S-2) (Dennis and Schrag, 2010; Defliese et al., 2015).
View in article
Dennis, K.J., Schrag, D.P. (2010) Clumped isotope thermometry of carbonatites as an indicator of diagenetic alteration. Geochimica et Cosmochimica Acta 74, 4110–4122.
 Show in context
Show in context Since its inception, this Δ47–T calibration has been reproduced in different laboratories by measuring both natural (e.g., Kele et al., 2015; Breitenbach et al., 2018; Peral et al., 2018; Daëron et al., 2019) and synthetic (e.g., Dennis and Schrag, 2010; Passey and Henkes, 2012; Defliese et al., 2015; Kluge et al., 2015) calcites.
View in article
Precipitation of synthetic calcites was performed using high pressure and temperature reaction cells (70–250 °C) and passive diffusion techniques (5–70 °C) (Supplementary Information S-3, S-4, Table S-2, Fig. S-2) (Dennis and Schrag, 2010; Defliese et al., 2015).
View in article
Dennis, K.J., Affek, H.P., Passey, B.H., Schrag, D.P., Eiler, J.M. (2011) Defining an absolute reference frame for “clumped” isotope studies of CO2. Geochimica et Cosmochimica Acta 75, 7117–7131.
 Show in context
Show in context Improvements of the T–calibration have been based on refining the preparation and analytical techniques (e.g., sample preparation automatisation, sample size reduction), establishing an absolute scale (carbon dioxide equilibrium scale at 25 °C acid digestion temperature; CDES25°C; Dennis et al., 2011), increasing the number of replicates per anchor points, and abating the analytical artefacts inherent to clumped isotopic measurements. While these advances have considerably reduced the differences observed between the T–calibrations produced in independent laboratories, significant differences still remain.
View in article
Ghosh, P., Adkins, J., Affek, H., Balta, B., Guo, W., Schauble, E.A., Schrag, D., Eiler, J.M. (2006) 13C–18O bonds in carbonate minerals: A new kind of paleothermometer. Geochimica et Cosmochimica Acta 70, 1439–1456.
 Show in context
Show in context The pioneering work of Ghosh et al. (2006) reported the first experimental calibration of the Δ47–T relationship between 1 and 50 °C.
View in article
Guo, W., Mosenfelder, J.L., Goddard, W.A., Eiler, J.M. (2009) Isotopic fractionations associated with phosphoric acid digestion of carbonate minerals: Insights from first-principles theoretical modeling and clumped isotope measurements. Geochimica et Cosmochimica Acta 73, 7203–7225.
 Show in context
Show in context The use of ab initio thermodynamic models (Schauble et al., 2006; Guo et al., 2009) allowed the calculation of the 13C–18O bond ordering in the carbonate crystal lattice as a function of formation temperature, theoretically grounding this geothermometer.
View in article
More importantly, none of the published T–calibrations can reconcile theory and experimentation and the non-linearity of the theoretical Δ47–T mathematical relationship (Guo et al., 2009) also observed experimentally for dolomite (Müller et al., 2019) has not been reproduced empirically at high temperatures for calcite (Fig. 1).
View in article
Apart from the challenges inherent to comparing T–calibrations directly based upon different standardisation strategies, the differences (i.e. slope p values <1 × 10–4 and intercept p values <1 × 10–4; Supplementary Information S-1, Table S-1) observed between the latest composite (Petersen et al., 2019), the fully carbonate-based standardised (Kele et al., 2015) recalculated by Bernasconi et al. (2018), and the theoretical T–calibrations (Guo et al., 2009) could be due to the strong influence of the high temperature calcite calibration points (Passey and Henkes, 2012; Kluge et al., 2015) used in the data compilation effort of Petersen et al. (2019) (Fig. 1).
View in article
We choose to work solely on calcites to avoid potential biases due to specific acid fractionation factors (AFF) when using different carbonates (i.e. aragonite, dolomite, siderite) as theoretically predicted (Guo et al., 2009) and shown by recent experimental work (Müller et al., 2017a; van Dijk et al., 2019) at the temperature of acid digestion used in this study (70 °C).
View in article
Δ47–T third-order polynomial relationship (CDES70°C) for this study, the theoretical, thermodynamic based relationship in Guo et al. (2009) and a modified version of this equation taking into account the dependence of the Δ*47–63 on the reactant Δ63 (35 ppm increase in Δ*47–63 per 1 ‰ increase in Δ63).
View in article
While the theoretical Δ47–T polynomial relationship (Guo et al., 2009) fits relatively well with our empirical calibration curve at high temperature (i.e. within the 95 % CI), our curve lies above the theoretical line and outside the 95 % CI at low temperature.
View in article
When we modify the theoretical curve by integrating the dependency of the Δ*47–63 to the Δ63 of the reactant carbonates (Guo et al., 2009), a significant fit between the two polynomial curves is observed (i.e. p value = 0.96; Supplementary Information S-1), reconciling the experimentation with theory at the 95 % CI over the full temperature spectrum (Fig. 3).
View in article
The polynomial relationship from Guo et al. (2009) is projected into the CDES70°C frame by replacing the Δ*47–63 (= 0.268 ‰ for a 25 °C acid digestion) with a 70 °C Δ*47–63 (= 0.184 ‰) determined experimentally by Müller et al. (2017b) and updated to the IUPAC parameters (Supplementary Information S-6, S-7).
View in article
John, C.M., Bowen, D. (2016) Community software for challenging isotope analysis: First applications of ‘Easotope’ to clumped isotopes. Rapid Communications in Mass Spectrometry 30, 2285–2300.
 Show in context
Show in context Raw beam intensities were imported into Easotope (John and Bowen, 2016) for calculation of the Δ47.
View in article
Kele, S., Breitenbach, S.F.M., Capezzuoli, E., Meckler, A.N., Ziegler, M., Millan, I.M., Kluge, T., Deák, J., Hanselmann, K., John, C.M., Yan, H., Liu, Z., Bernasconi, S.M. (2015) Temperature dependence of oxygen- and clumped isotope fractionation in carbonates: A study of travertines and tufas in the 6-95°C temperature range. Geochimica et Cosmochimica Acta 168, 172–192.
 Show in context
Show in context Since its inception, this Δ47–T calibration has been reproduced in different laboratories by measuring both natural (e.g., Kele et al., 2015; Breitenbach et al., 2018; Peral et al., 2018; Daëron et al., 2019) and synthetic (e.g., Dennis and Schrag, 2010; Passey and Henkes, 2012; Defliese et al., 2015; Kluge et al., 2015) calcites.
View in article
Apart from the challenges inherent to comparing T–calibrations directly based upon different standardisation strategies, the differences (i.e. slope p values <1 × 10–4 and intercept p values <1 × 10–4; Supplementary Information S-1, Table S-1) observed between the latest composite (Petersen et al., 2019), the fully carbonate-based standardised (Kele et al., 2015) recalculated by Bernasconi et al. (2018), and the theoretical T–calibrations (Guo et al., 2009) could be due to the strong influence of the high temperature calcite calibration points (Passey and Henkes, 2012; Kluge et al., 2015) used in the data compilation effort of Petersen et al. (2019) (Fig. 1).
View in article
Kluge, T., John, C.M., Jourdan, A.L., Davis, S., Crawshaw, J. (2015) Laboratory calibration of the calcium carbonate clumped isotope thermometer in the 25-250°C temperature range. Geochimica et Cosmochimica Acta 157, 213–227.
 Show in context
Show in context Since its inception, this Δ47–T calibration has been reproduced in different laboratories by measuring both natural (e.g., Kele et al., 2015; Breitenbach et al., 2018; Peral et al., 2018; Daëron et al., 2019) and synthetic (e.g., Dennis and Schrag, 2010; Passey and Henkes, 2012; Defliese et al., 2015; Kluge et al., 2015) calcites.
View in article
Apart from the challenges inherent to comparing T–calibrations directly based upon different standardisation strategies, the differences (i.e. slope p values <1 × 10–4 and intercept p values <1 × 10–4; Supplementary Information S-1, Table S-1) observed between the latest composite (Petersen et al., 2019), the fully carbonate-based standardised (Kele et al., 2015) recalculated by Bernasconi et al. (2018), and the theoretical T–calibrations (Guo et al., 2009) could be due to the strong influence of the high temperature calcite calibration points (Passey and Henkes, 2012; Kluge et al., 2015) used in the data compilation effort of Petersen et al. (2019) (Fig. 1).
View in article
Some additional effects might have influenced the high temperature precipitates from Kluge et al. (2015) (Supplementary Information S-2, Fig. S-1) and the re-ordering experiments in Passey and Henkes (2012) (e.g., incomplete solid state re-ordering), potentially influencing the slope of this composite Δ47–T relationship (Petersen et al., 2019).
View in article
Meckler, A.N., Ziegler, M., Millán, M.I., Breitenbach, S.F.M., Bernasconi, S.M. (2014) Long-term performance of the Kiel carbonate device with a new correction scheme for clumped isotope measurements. Rapid Communications in Mass Spectrometry 28, 1705–1715.
 Show in context
Show in context We adopted a fully carbonate-based calibration scheme by interspersing 50 % of carbonate standards developed at ETH-Zurich (Meckler et al., 2014) in every analytical session, and by keeping similar average sample and standard sizes.
View in article
Meinicke, N., Ho, S.L., Hannisdal, B., Nürnberg, D., Tripati, A., Schiebel, R., Meckler, A.N. (2020) A robust calibration of the clumped isotopes to temperature relationship for foraminifers. Geochimica et Cosmochimica Acta 270, 160–183.
 Show in context
Show in context To evaluate the efficiency of the ETH carbonate standardisation scheme in improving inter-laboratory comparisons, we compiled the T–calibrations from this study and recently published Δ47–T relationships standardised with ETH carbonates (Bernasconi et al., 2018; Breitenbach et al., 2018; Peral et al., 2018; Daëron et al., 2019; Piasecki et al., 2019; Meinicke et al., 2020; Fig. 2) in the CDES70°C.
View in article
This, in addition to the significant similarities of all Δ47–T linear relationships (Fig. 2), further supports using the carbonate standardisation scheme for inter-laboratory comparisons (Bernasconi et al., 2018; Peral et al., 2018; Meinicke et al., 2020).
View in article
Müller, I.A., Violay, M.E.S., Storck, J.C., Fernandez, A., van Dijk, J., Madonna, C., Bernasconi, S.M. (2017a) Clumped isotope fractionation during phosphoric acid digestion of carbonates at 70 °C. Chemical Geology 449, 1–14.
 Show in context
Show in context We choose to work solely on calcites to avoid potential biases due to specific acid fractionation factors (AFF) when using different carbonates (i.e. aragonite, dolomite, siderite) as theoretically predicted (Guo et al., 2009) and shown by recent experimental work (Müller et al., 2017a; van Dijk et al., 2019) at the temperature of acid digestion used in this study (70 °C).
View in article
Müller, I.A., Fernandez, A., Radke, J., van Dijk, J., Bowen, D., Schwieters, J., Bernasconi, S.M. (2017b) Carbonate clumped isotope analyses with the long-integration dual-inlet (LIDI) workflow: scratching at the lower sample weight boundaries. Rapid Communications in Mass Spectrometry 31, 1057–1066.
 Show in context
Show in context δ18O, δ13C, and δ47 data were acquired simultaneously using the long integration dual inlet (LIDI) method (Müller et al., 2017b).
View in article
The polynomial relationship from Guo et al. (2009) is projected into the CDES70°C frame by replacing the Δ*47–63 (= 0.268 ‰ for a 25 °C acid digestion) with a 70 °C Δ*47–63 (= 0.184 ‰) determined experimentally by Müller et al. (2017b) and updated to the IUPAC parameters (Supplementary Information S-6, S-7).
View in article
Müller, I.A., Rodriguez-Blanco, J.D., Storck, J.-C., do Nascimento, G.S., Bontognali, T.R.R., Vasconcelos, C., Benning, L.G., Bernasconi, S.M. (2019) Calibration of the oxygen and clumped isotope thermometers for (proto-)dolomite based on synthetic and natural carbonates. Chemical Geology 525, 1–17.
 Show in context
Show in context More importantly, none of the published T–calibrations can reconcile theory and experimentation and the non-linearity of the theoretical Δ47–T mathematical relationship (Guo et al., 2009) also observed experimentally for dolomite (Müller et al., 2019) has not been reproduced empirically at high temperatures for calcite (Fig. 1).
View in article
Passey, B.H., Henkes, G.A. (2012) Carbonate clumped isotope bond reordering and geospeedometry. Earth and Planetary Science Letters 351–352, 223–236.
 Show in context
Show in context Apart from the challenges inherent to comparing T–calibrations directly based upon different standardisation strategies, the differences (i.e. slope p values <1 × 10–4 and intercept p values <1 × 10–4; Supplementary Information S-1, Table S-1) observed between the latest composite (Petersen et al., 2019), the fully carbonate-based standardised (Kele et al., 2015) recalculated by Bernasconi et al. (2018), and the theoretical T–calibrations (Guo et al., 2009) could be due to the strong influence of the high temperature calcite calibration points (Passey and Henkes, 2012; Kluge et al., 2015) used in the data compilation effort of Petersen et al. (2019) (Fig. 1).
View in article
Some additional effects might have influenced the high temperature precipitates from Kluge et al. (2015) (Supplementary Information S-2, Fig. S-1) and the re-ordering experiments in Passey and Henkes (2012) (e.g., incomplete solid state re-ordering), potentially influencing the slope of this composite Δ47–T relationship (Petersen et al., 2019).
View in article
Peral, M., Daëron, M., Blamart, D., Bassinot, F., Dewilde, F., Smialkowski, N., Isguder, G., Bonnin, J., Jorissen, F., Kissel, C., Michel, E., Vázquez Riveiros, N., Waelbroeck, C. (2018) Updated calibration of the clumped isotope thermometer in planktonic and benthic foraminifera. Geochimica et Cosmochimica Acta 239, 1–16.
 Show in context
Show in context To evaluate the efficiency of the ETH carbonate standardisation scheme in improving inter-laboratory comparisons, we compiled the T–calibrations from this study and recently published Δ47–T relationships standardised with ETH carbonates (Bernasconi et al., 2018; Breitenbach et al., 2018; Peral et al., 2018; Daëron et al., 2019; Piasecki et al., 2019; Meinicke et al., 2020; Fig. 2) in the CDES70°C.
View in article
This, in addition to the significant similarities of all Δ47–T linear relationships (Fig. 2), further supports using the carbonate standardisation scheme for inter-laboratory comparisons (Bernasconi et al., 2018; Peral et al., 2018; Meinicke et al., 2020).
View in article
Petersen, S.V., Defliese, W.F., Saenger, C., Daëron, M., Huntington, K.W., John, C.M., Kelson, J.R., Bernasconi, S.M., Coleman, A.S., Kluge, T., Olack, G.A., Schauer, A.J., Bajnai, D., Bonifacie, M., Breitenbach, S.F.M., Fiebig, J., Fernandez, A.B., Henkes, G.A., Hodell, D., Katz, A., Kele, S., Lohmann, K.C., Passey, B.H., Peral, M.Y., Petrizzo, D.A., Rosenheim, B.E., Tripati, A., Venturelli, R., Young, E.D., Winkelstern, I.Z. (2019) Effects of Improved 17O Correction on Inter‐Laboratory Agreement in Clumped Isotope Calibrations, Estimates of Mineral‐Specific Offsets, and Temperature Dependence of Acid Digestion Fractionation. Geochemistry, Geophysics, Geosystems 20, 3495–3519.
 Show in context
Show in context Apart from the challenges inherent to comparing T–calibrations directly based upon different standardisation strategies, the differences (i.e. slope p values <1 × 10–4 and intercept p values <1 × 10–4; Supplementary Information S-1, Table S-1) observed between the latest composite (Petersen et al., 2019), the fully carbonate-based standardised (Kele et al., 2015) recalculated by Bernasconi et al. (2018), and the theoretical T–calibrations (Guo et al., 2009) could be due to the strong influence of the high temperature calcite calibration points (Passey and Henkes, 2012; Kluge et al., 2015) used in the data compilation effort of Petersen et al. (2019) (Fig. 1).
View in article
Some additional effects might have influenced the high temperature precipitates from Kluge et al. (2015) (Supplementary Information S-2, Fig. S-1) and the re-ordering experiments in Passey and Henkes (2012) (e.g., incomplete solid state re-ordering), potentially influencing the slope of this composite Δ47–T relationship (Petersen et al., 2019).
View in article
We additionally plotted the composite calibration from Petersen et al. (2019) for qualitative comparison (Fig. 2).
View in article
Piasecki, A., Bernasconi, S.M., Grauel, A.-L., Hannisdal, B., Ho, S.L., Marchitto, T.M., Meinicke, N., Tisserand, A., Meckler, N. (2019) Application of Clumped Isotope Thermometry to Benthic Foraminifera. Geochemistry, Geophysics, Geosystems 20, 2090.
 Show in context
Show in context To evaluate the efficiency of the ETH carbonate standardisation scheme in improving inter-laboratory comparisons, we compiled the T–calibrations from this study and recently published Δ47–T relationships standardised with ETH carbonates (Bernasconi et al., 2018; Breitenbach et al., 2018; Peral et al., 2018; Daëron et al., 2019; Piasecki et al., 2019; Meinicke et al., 2020; Fig. 2) in the CDES70°C.
View in article
Schauble, E.A., Ghosh, P., Eiler, J.M. (2006) Preferential formation of 13C–18O bonds in carbonate minerals, estimated using first-principles lattice dynamics. Geochimica et Cosmochimica Acta 70, 2510–2529.
 Show in context
Show in context Clumped isotope measurements on CO2 extracted through acidification of carbonates has evolved over the past 15 years as a tool to probe directly the temperature (T) and the parent fluid oxygen isotope composition (δ18O) of precipitates (Schauble et al., 2006).
View in article
This measurement has a direct link to the formation temperature of the carbonate crystal (Schauble et al., 2006) and, therefore, avoids needing to making potentially incorrect assumptions on the isotopic composition of the precipitating fluid as necessary when using the carbonate-water δ18O geothermometer (Urey, 1947).
View in article
The use of ab initio thermodynamic models (Schauble et al., 2006; Guo et al., 2009) allowed the calculation of the 13C–18O bond ordering in the carbonate crystal lattice as a function of formation temperature, theoretically grounding this geothermometer.
View in article
Schauer, A.J., Kelson, J., Saenger, C., Huntington, K.W. (2016) Choice of 17O correction affects clumped isotope (Δ47) values of CO2 measured with mass spectrometry. Rapid Communications in Mass Spectrometry 30, 2607–2616.
 Show in context
Show in context The IUPAC parameter set (Brand et al., 2010) was used to correct for 17O interferences (Daëron et al., 2016; Schauer et al., 2016).
View in article
Schmid, T.W., Bernasconi, S.M. (2010) An automated method for ‘clumped-isotope’ measurements on small carbonate samples. Rapid Communications in Mass Spectrometry 24, 1955–1963.
 Show in context
Show in context The production of the heated calcite (i.e. CM γ-5; 726 °C) is described in Schmid and Bernasconi (2010).
View in article
The preparation of carbonate was performed with a KIEL-IV carbonate device (Thermo Scientific, Bremen, Germany) modified according to Schmid and Bernasconi (2010).
View in article
In an attempt to investigate the theoretical non-linear nature of the Δ47–T relationship, we expanded the studied temperature range to the equivalent stochastic 13C–18O bonds distribution by adding measurements of the heated calcite CM γ-5 (726 °C) (Schmid and Bernasconi, 2010) and the data for the three stochastic calcites (1000 °C) reported in Müller et al. (2017) (Fig. 3, Supplementary Information S-6).
View in article
Urey, H.C. (1947) The thermodynamic properties of isotopic substances. Journal of the Chemical Society (Resumed). The Royal Society of Chemistry 562–581.
 Show in context
Show in context This measurement has a direct link to the formation temperature of the carbonate crystal (Schauble et al., 2006) and, therefore, avoids needing to making potentially incorrect assumptions on the isotopic composition of the precipitating fluid as necessary when using the carbonate-water δ18O geothermometer (Urey, 1947).
View in article
van Dijk, J., Fernandez, A., Storck, J.C., White, T.S., Lever, M., Müller, I.A., Bishop, S., Seifert, R.F., Driese, S.G., Krylov, A., Ludvigson, G.A., Turchyn, A.V., Lin, C.Y., Wittkop, C., Bernasconi, S.M. (2019) Experimental calibration of clumped isotopes in siderite between 8.5 and 62 °C and its application as paleo-thermometer in paleosols. Geochimica et Cosmochimica Acta 254, 1–20.
 Show in context
Show in context We choose to work solely on calcites to avoid potential biases due to specific acid fractionation factors (AFF) when using different carbonates (i.e. aragonite, dolomite, siderite) as theoretically predicted (Guo et al., 2009) and shown by recent experimental work (Müller et al., 2017a; van Dijk et al., 2019) at the temperature of acid digestion used in this study (70 °C).
View in article
top
Supplementary Information
The Supplementary Information includes:
- S-1: Statistical Tests
- S-2: Carbonate-water Fractionation (αcc-w)
- S-3: Synthetic Calcite Precipitate
- S-4: No Bond Reordering
- S-5: 45R and 46R Linearity Effect on ETH Carbonates Standardisation
- S-6: Δ47–T Relationships Recalculation
- S-7: On the Signification of the Δ47-T Intercept
- Figures S-1 to S-3
- Tables S-1 to S-3
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures