Zirconium isotopic composition of the mantle through time
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:392Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
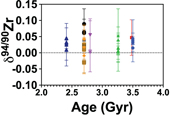
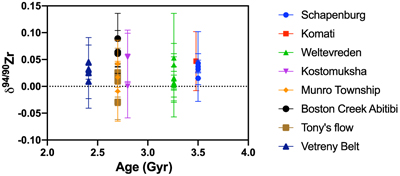 Figure 1 Zirconium isotopic composition of komatiites as a function of their crystallisation ages. The δ94/90Zr values are constant through 1 Ga and in various locations indicating that the terrestrial mantle had a constant δ94/90Zr value through time. The Weltevreden komatiites have unusual Ca isotope signatures consistent with magma ocean crystallisation processes. They do not have a different Zr isotopic composition from other komatiites. Errors as 2 s.d. of replicated measurements (typically 4). | 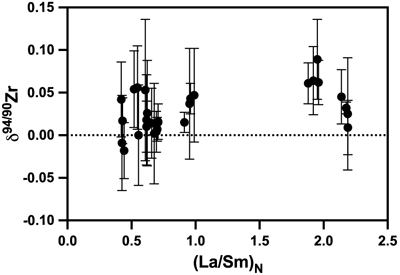 Figure 2 Zirconium isotopic composition of the komatiites as a function of the La/Sm ratio normalised to primitive mantle composition [(La/Sm)N]. Variations in (La/Sm)N are interpreted to reflect prior melt extraction from komatiite source mantles. The absence of variation suggests that prior melt extraction did not fractionate Zr isotopes and that the source of the komatiites reflects the composition of the Archean mantle. Errors as 2 s.d. of the replicated measurements (typically 4). | 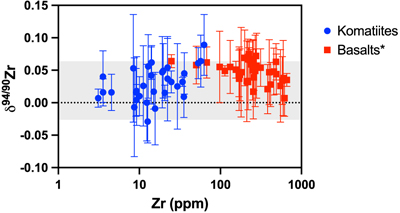 Figure 3 Zirconium isotopic composition of komatiites (this study) and basalts (literature) as a function of the Zr content of the samples. While Zr content varies over two orders of magnitude, the δ94/90Zr are consistent with an average value of 0.040 ± 0.044 (2 s.d., n = 72), which best reflects the composition of Earth’s mantle (grey band). Errors as 2 s.d. of replicate measurements (typically 4). Data from Inglis et al. (2019). |
| Figure 1 | Figure 2 | Figure 3 |
top
Introduction
Zirconium is a refractory element (Tc = 1741K; Lodders, 2003
Lodders, K. (2003) Solar System abundances and condensation temperatures of the elements. Astrophysical Journal 591, 1220–1247.
), and its abundance and stable isotopic composition is, a priori, robust to evaporation processes that occurred during planetary formation. This makes Zr distinct from more volatile elements, like Zn (Moynier et al., 2017Moynier, F., Vance, D., Fujii, T., Savage, P. (2017) The isotope geochemistry of copper and zinc. In: Teng, F.-Z., Watkins, J., Dauphas, N. (Eds.) Reviews in Mineralogy & Geochemistry 82, 543–600.
) or even Si and Mg (Pringle et al., 2014Pringle, E.A., Moynier, F., Savage, P.S., Badro, J., Barrat, J.A. (2014) Silicon isotopes in angrites and volatile loss in planetesimals. Proceedings of the National Academy of Science USA 111, 17029–32.
; Hin et al., 2017Hin, R.C., Coath, C.D., Carter, P.J., Nimmo, F., Lai, Y.J., Pogge von Strandmann, P.A.E., Willbold, M., Leinhardt, Z.M., Walter, M.J., Elliott, T. (2017) Magnesium isotope evidence that accretional vapour loss shapes planetary compositions. Nature 549, 511–515.
). As with other high field strength elements, Zr is lithophile and incompatible (Woodhead et al., 1993Woodhead, J., Eggins, S., Gamble, J. (1993) High field strength and transition element systematics in island arc and back-arc basin basalts: evidence for multi-phase melt extraction and a depleted mantle wedge. Earth and Planetary Science Letters 114, 491–504.
; Johnson, 1998Johnson, K.T. (1998) Experimental determination of partition coefficients for rare earth and high-field-strength elements between clinopyroxene, garnet, and basaltic melt at high pressures. Contributions to Mineralogy and Petrology 133, 60–68.
) and is enriched in the continental crust (Rudnick and Gao, 2003Rudnick, R.L., Gao, S. (2003) Composition of the Continental Crust. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry 3, 1–64.
). Zirconium isotopes fractionate during magmatic differentiation. For example, the most evolved lavas from Hekla volcano, Iceland, have a 94Zr/90Zr ratio 0.5 ‰ higher than less evolved lavas. This is interpreted to reflect differences in coordination of zirconium between zircon and melt (Inglis et al., 2019Inglis, E., Moynier, F., Creech, J., Deng, Z., Day, J.M.D., Teng, F.-Z., Bizzarro, M., Jackson, M.J., Savage, P. (2019) Isotopic fractionation of zirconium during magmatic differentiation and the stable isotope composition of the silicate Earth. Geochimica et Cosmochimica Acta 250, 311–323.
). Because of the resistance of zircons to physical and chemical abrasion and the availability of other isotope tracers (e.g., O, Hf) and elements within them (e.g., rare earth elements), they have found widespread application in the study of Earth’s crust through time (Condie, 2005Condie, K.C. (2005) High field strength element ratios in Archean basalts: a window to evolving sources of mantle plumes? Lithos 79, 491–504.
). The isotopic fractionation of Zr between granites and basalts (Inglis et al., 2018Inglis, E.C., Creech, J.B., Deng, Z., Moynier, F. (2018) High-precision zirconium stable isotope measurements of geological reference materials as measured by double-spike MC-ICPMS. Chemical Geology 493, 544–552.
) suggests that Zr isotopes could be used as tracers within zircons, and of crustal recycling in Earth’s mantle, especially since Zr is a fluid immobile element and should be efficiently recycled into the mantle. Presently, the Zr isotopic composition of the mantle is estimated by the analysis of relatively low degree basaltic partial melts (Inglis et al., 2019Inglis, E., Moynier, F., Creech, J., Deng, Z., Day, J.M.D., Teng, F.-Z., Bizzarro, M., Jackson, M.J., Savage, P. (2019) Isotopic fractionation of zirconium during magmatic differentiation and the stable isotope composition of the silicate Earth. Geochimica et Cosmochimica Acta 250, 311–323.
).Komatiites are ultramafic volcanic rocks with >18 wt. % MgO. They are formed by partial melting in hot mantle plumes, mostly during the Archean (e.g., Arndt et al., 2008
Arndt, N., Lesher, M., Barnes, S. (2008) Komatiite. Cambridge University Press. Cambridge, New York, Melbourne.
). Since komatiites are mantle-derived high degree partial melts (>30 %) (Herzberg, 1992Herzberg, C. (1992) Depth and degree of melting of komatiite. Journal of Geophysical Research 97, 4521–4540.
), and Zr is moderately incompatible, almost all Zr from the mantle source is efficiently extracted into komatiitic melts. As such, komatiitic melts must faithfully reflect the Zr isotopic composition of their mantle source. Similar logic has been used to estimate the composition of the mantle through time of several other elements, including Ca (Amsellem et al., 2019Amsellem, E., Moynier, F., Puchtel, I.S. (2019) Evolution of the Ca isotopic composition of the mantle. Geochimica et Cosmochimica Acta 258, 195–206.
), Ga (Kato et al., 2017Kato, C., Moynier, F., Foriel, J., Teng, F., Puchtel, I.S. (2017) The gallium isotopic composition of the bulk silicate Earth. Chemical Geology 448, 164–172.
), and Ti (Greber et al., 2017Greber, N.D., Dauphas, N., Puchtel, I.S., Hofmann, B.A., Arndt, N.T. (2017) Titanium stable isotopic variations in chondrites, achondrites and lunar rocks. Geochimica et Cosmochimica Acta 213, 534–552.
; Deng et al., 2018Deng, Z., Moynier, F., Sossi, P., Chaussidon, M. (2018) Bridging the depleted MORB mantle and the continental crust using titanium isotopes. Geochemical Perspectives Letters 9, 11–15.
).Komatiites span a range of ages and, thus, provide the potential to investigate initial mantle composition and, in particular, the processes of crystallisation of an early terrestrial magma ocean (Puchtel et al., 2013
Puchtel, I., Blichert-Toft, J., Touboul, M., Walker, R., Byerly, G., Nisbet, E., Anhaeusser, C. (2013) Insights into early Earth from Barberton komatiites: evidence from lithophile isotope and trace element systematics. Geochimica et Cosmochimica Acta 108, 63–90.
, 2016bPuchtel, I.S., Blichert-Toft, J., Touboul, M., Horan, M.F., Walker, R.J. (2016b) The coupled 182W-142Nd record of early terrestrial mantle differentiation. Geochemistry, Geophysics, Geosystems 17, 2168–2193.
; Byerly et al., 2017Byerly, B.L., Kareem, K., Bao, H., Byerly, G.R. (2017) Early Earth mantle heterogeneity revealed by light oxygen isotopes of Archaean komatiites. Nature Geoscience 10, 871–875.
). For example, some komatiites from the Weltevreden formation in the Barberton Greenstone Belt, South Africa, have unusual Ca isotopic compositions compared with other komatiites and are interpreted as a record of mantle source heterogeneities induced by magma ocean crystallisation (Amsellem et al., 2019Amsellem, E., Moynier, F., Puchtel, I.S. (2019) Evolution of the Ca isotopic composition of the mantle. Geochimica et Cosmochimica Acta 258, 195–206.
). Zirconium is useful to search for traces of magma ocean crystallisation processes because it is compatible in bridgmanite (Dbridgmanite-melt > 1; Corgne et al., 2005Corgne, A., Liebske, C., Wood, B.J., Rubie, D.C., Frost, D.J. (2005) Silicate perovskite-melt partitioning of trace elements and geochemical signature of a deep perovskitic reservoir. Geochimica et Cosmochimica Acta 69, 485–496.
) and has different coordination numbers (CN) between bridgmanite (CN = 8, where it substitutes for Mg; Smyth and Bish, 1988Smyth, J.R., Bish, D.L. (1988) Crystal structures and cation sites of the rock-forming minerals. Allen & Unwin, Boston.
), and silicate melt (CN = 6; Farges et al., 1991Farges, F., Ponader, C.W., Brown Jr, G.E. (1991) Structural environments of incompatible elements in silicate glass/melt systems: I. Zirconium at trace levels. Geochimica et Cosmochimica Acta 55, 1563–1574.
). Since heavier isotopes tend to concentrate in the phases where it makes tighter bonds it is likely that crystallisation of bridgmanite from a melt would leave the residual liquid enriched in the heavier isotopes compared to bulk mantle.top
Samples and Methods
Thirty one komatiite samples (olivine- and pyroxene-spinifex-textured and olivine cumulates) were studied (Table S-1). The komatiite samples are from Fennoscandia (Victoria’s Lava Lake, Vetreny Belt; Kostomuksha Greenstone Belt), South Africa (Komati, Weltevreden, and Schapenburg Formations of the Barberton Greenstone Belt), Zimbabwe (Belingwe Greenstone Belt) and Canada (Boston Creek Flow, Abitibi Greenstone Belt and Munro Township). All samples have previously been studied for trace and highly siderophile element abundances, 186,187Os, 142,143Nd, 182W isotope systematics (Puchtel et al., 1998
Puchtel, I., Hofmann, A., Mezger, K., Jochum, K., Shchipansky, A., Samsonov, A. (1998) Oceanic plateau model for continental crustal growth in the Archaean: a case study from the Kostomuksha greenstone belt, NW Baltic Shield. Earth and Planetary Science Letters 155, 57–74.
, 2001Puchtel, I.S., Brügmann, G.E., Hofmann, A.W. (2001) 187Os-enriched domain in an Archean mantle plume: evidence from 2.8 Ga komatiites of the Kostomuksha greenstone belt, NW Baltic Shield. Earth and Planetary Science Letters 186, 513–526.
, 2005Puchtel, I.S., Brandon, A.D., Humayun, M., Walker, R.J. (2005) Evidence for the early differentiation of the core from Pt–Re–Os isotope systematics of 2.8-Ga komatiites. Earth and Planetary Science Letters 237, 118–134.
, 2009Puchtel, I., Walker, R., Brandon, A., Nisbet, E. (2009) Pt–Re–Os and Sm–Nd isotope and HSE and REE systematics of the 2.7Ga Belingwe and Abitibi komatiites. Geochimica et Cosmochimica Acta 73, 6367–6389.
, 2013Puchtel, I., Blichert-Toft, J., Touboul, M., Walker, R., Byerly, G., Nisbet, E., Anhaeusser, C. (2013) Insights into early Earth from Barberton komatiites: evidence from lithophile isotope and trace element systematics. Geochimica et Cosmochimica Acta 108, 63–90.
, 2016aPuchtel, I., Touboul, M., Blichert-Toft, J., Walker, R., Brandon, A., Nicklas, R., Kulikov, V., Samsonov, A. (2016a) Lithophile and siderophile element systematics of Earth’s mantle at the Archean–Proterozoic boundary: evidence from 2.4Ga komatiites. Geochimica et Cosmochimica Acta 180, 227–255.
,bPuchtel, I.S., Blichert-Toft, J., Touboul, M., Horan, M.F., Walker, R.J. (2016b) The coupled 182W-142Nd record of early terrestrial mantle differentiation. Geochemistry, Geophysics, Geosystems 17, 2168–2193.
), and for Ca stable isotopes (Amsellem et al., 2019Amsellem, E., Moynier, F., Puchtel, I.S. (2019) Evolution of the Ca isotopic composition of the mantle. Geochimica et Cosmochimica Acta 258, 195–206.
). Two widely available reference materials (BHVO-2 and AGV-2) for which Zr isotopic compositions have been reported previously (Inglis et al., 2018Inglis, E.C., Creech, J.B., Deng, Z., Moynier, F. (2018) High-precision zirconium stable isotope measurements of geological reference materials as measured by double-spike MC-ICPMS. Chemical Geology 493, 544–552.
; Tian et al., 2020Tian, S., Inglis, E., Creech, J., Zhang, W., Wang, Z., Hu, Z., Liu, Y., Moynier, F. (2020) The zirconium stable isotope compositions of 22 geological reference materials, 4 zircons and 3 standard solutions. Chemical Geology, doi: 10.1016/j.chemgeo.2020.119791.
) were also analysed.Given that komatiites have much lower Zr content than any previously analysed samples, we performed several full replicates (weighing, spike addition, dissolution, chemical separation, mass spectrometry analyses) (see Table S-1). These samples are noted “R” in Table S-1. The Zr isotopic composition was measured using a ThermoFischer Neptune plus MC-ICP-MS at the IPGP as described in Tian et al. (2020)
Tian, S., Inglis, E., Creech, J., Zhang, W., Wang, Z., Hu, Z., Liu, Y., Moynier, F. (2020) The zirconium stable isotope compositions of 22 geological reference materials, 4 zircons and 3 standard solutions. Chemical Geology, doi: 10.1016/j.chemgeo.2020.119791.
. The protocol utilised for Zr stable isotope measurements was adapted from Inglis et al. (2018)Inglis, E.C., Creech, J.B., Deng, Z., Moynier, F. (2018) High-precision zirconium stable isotope measurements of geological reference materials as measured by double-spike MC-ICPMS. Chemical Geology 493, 544–552.
and is reported in Table S-2. The size of columns utilised had to be scaled up and the chemical purification method modified due to the high Ca/Zr of komatiites, and the associated risk of Zr coprecipitation with Mg, Ca fluorides (Tanaka et al., 2003Tanaka, R., Makishima, A., Kitagawa, H., Nakamura, E. (2003) Suppression of Zr, Nb, Hf and Ta coprecipitation in fluoride compounds for determination in Ca-rich materials. Journal of Analytical Atomic Spectrometry 18, 1458–1463.
). To do this, the column procedure from Inglis et al. (2018)Inglis, E.C., Creech, J.B., Deng, Z., Moynier, F. (2018) High-precision zirconium stable isotope measurements of geological reference materials as measured by double-spike MC-ICPMS. Chemical Geology 493, 544–552.
was reversed. The details of the analytical methods can be found in the Supplementary Information.top
Results
The Zr isotopic compositions are reported in Table S-1 as:
Eq. 1
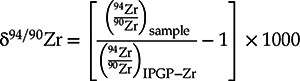
There is presently no international Zr isotopic standard commercially available and the majority of the Zr isotopic data for rock samples have been reported against the IPGP-Zr standard (calibrated against a variety of geostandards; Inglis et al., 2018
Inglis, E.C., Creech, J.B., Deng, Z., Moynier, F. (2018) High-precision zirconium stable isotope measurements of geological reference materials as measured by double-spike MC-ICPMS. Chemical Geology 493, 544–552.
; Feng et al., 2020Feng, L., Hu, W., Jiao, Y., Zhou, L., Zhang, W., Hu, Z., Liu, Y. (2020) High-precision stable zirconium isotope ratio measurements by double spike thermal ionization mass spectrometry. Journal of Analytical Atomic Spectrometry 35, 736–745.
; Guo et al., 2020Guo, J.L., Wang, Z., Zhang, W., Moynier, F., Cui, D., Hu, Z., Ducea, M. (2020) Significant Zr isotope variations in single zircon grains recording magma evolution history. Proceedings of the National Academy of Sciences, doi: 10.1073/pnas.2002053117.
; Tian et al., 2020Tian, S., Inglis, E., Creech, J., Zhang, W., Wang, Z., Hu, Z., Liu, Y., Moynier, F. (2020) The zirconium stable isotope compositions of 22 geological reference materials, 4 zircons and 3 standard solutions. Chemical Geology, doi: 10.1016/j.chemgeo.2020.119791.
). Alternatively, three other standards have been used: zircon GJ-1 for in situ analyses (Zhang et al., 2019Zhang, W., Wang, Z., Moynier, F., Inglis, E., Tian, S., Li, M., Liu, Y., Hu, Z. (2019) Determination of Zr isotopic ratios in zircons using laser-ablation multiple-collector inductively coupled-plasma mass-spectrometry. Journal of Analytical Atomic Spectrometry 34, 1800–1809.
), an elemental Zr standard, NIST SRM3169 (Feng et al., 2020Feng, L., Hu, W., Jiao, Y., Zhou, L., Zhang, W., Hu, Z., Liu, Y. (2020) High-precision stable zirconium isotope ratio measurements by double spike thermal ionization mass spectrometry. Journal of Analytical Atomic Spectrometry 35, 736–745.
) and a standard in development by NIST (Ibañez-Mejia and Tissot, 2019Ibañez-Mejia, M., Tissot, F.L. (2019) Extreme Zr stable isotope fractionation during magmatic fractional crystallization. Science Advances 5, eaax8648.
; Tompkins et al., 2020Tompkins, H., Zieman, L., Ibanez-Meija, M., Tissot, F. (2020) Zirconium stable isotope analysis of zircon by MCICP-MS:methods and application to evaluating intra-crystalline zonation in a zircon megacryst. Journal of Analytical Atomic Spectrometry, doi: 10.1039/c9ja00315k.
) for bulk measurements. These three standards have been calibrated against the IPGP-Zr standard (Tian et al., 2020Tian, S., Inglis, E., Creech, J., Zhang, W., Wang, Z., Hu, Z., Liu, Y., Moynier, F. (2020) The zirconium stable isotope compositions of 22 geological reference materials, 4 zircons and 3 standard solutions. Chemical Geology, doi: 10.1016/j.chemgeo.2020.119791.
).The δ94/90Zr values of BHVO-2 (0.033 ± 0.025 ‰) and AGV-2 (0.017 ± 0.065 ‰) are consistent with the previous measurements obtained using a different chemical protocol (δ94/90Zr = 0.044 ± 0.044 ‰ and 0.044 ±0.050 ‰, respectively), (Inglis et al., 2018
Inglis, E.C., Creech, J.B., Deng, Z., Moynier, F. (2018) High-precision zirconium stable isotope measurements of geological reference materials as measured by double-spike MC-ICPMS. Chemical Geology 493, 544–552.
) and the values reported in Tian et al. (2020)Tian, S., Inglis, E., Creech, J., Zhang, W., Wang, Z., Hu, Z., Liu, Y., Moynier, F. (2020) The zirconium stable isotope compositions of 22 geological reference materials, 4 zircons and 3 standard solutions. Chemical Geology, doi: 10.1016/j.chemgeo.2020.119791.
(0.045 ± 0.025 ‰ and 0.035 ± 0.037 ‰, respectively). Reproducibility of replicate analyses is consistent within the internal error defined by multiple replicate measurements of the same sample solutions. For example, the two replicates of SCH2.6 return a value of δ94/90Zr = 0.043 ± 0.018 ‰ (2 s.d.). In general, the Zr isotopic compositions of komatiites are relatively invariant, and show no correlation with age (Fig. 1) or the degree of prior melt removal (Fig. 2). The δ94/90Zr values of all the komatiites range from −0.018 to +0.089, with an average value of 0.030 ± 0.04 ‰ (2 s.d., n = 31).
Figure 1 Zirconium isotopic composition of komatiites as a function of their crystallisation ages. The δ94/90Zr values are constant through 1 Ga and in various locations indicating that the terrestrial mantle had a constant δ94/90Zr value through time. The Weltevreden komatiites have unusual Ca isotope signatures consistent with magma ocean crystallisation processes. They do not have a different Zr isotopic composition from other komatiites. Errors as 2 s.d. of replicated measurements (typically 4).

Figure 2 Zirconium isotopic composition of the komatiites as a function of the La/Sm ratio normalised to primitive mantle composition [(La/Sm)N]. Variations in (La/Sm)N are interpreted to reflect prior melt extraction from komatiite source mantles. The absence of variation suggests that prior melt extraction did not fractionate Zr isotopes and that the source of the komatiites reflects the composition of the Archean mantle. Errors as 2 s.d. of the replicated measurements (typically 4).
top
Discussion
No measurable effects on Zr during magma ocean crystallisation. Based on Os, Nd, Hf, O and Ca isotopic compositions of some komatiites like Weltevreden it has been suggested that they recorded the fractionation of minerals stable under magma ocean conditions (Puchtel et al., 2013
Puchtel, I., Blichert-Toft, J., Touboul, M., Walker, R., Byerly, G., Nisbet, E., Anhaeusser, C. (2013) Insights into early Earth from Barberton komatiites: evidence from lithophile isotope and trace element systematics. Geochimica et Cosmochimica Acta 108, 63–90.
; Byerly et al., 2017Byerly, B.L., Kareem, K., Bao, H., Byerly, G.R. (2017) Early Earth mantle heterogeneity revealed by light oxygen isotopes of Archaean komatiites. Nature Geoscience 10, 871–875.
; Amsellem et al., 2019Amsellem, E., Moynier, F., Puchtel, I.S. (2019) Evolution of the Ca isotopic composition of the mantle. Geochimica et Cosmochimica Acta 258, 195–206.
). The source of these komatiites is enriched in the heavier isotopes of Ca, consistent with the extraction of light Ca in Ca perovskite into the lower mantle and the preservation of mantle heterogeneity until at least the extraction of the komatiites at 3.26 Ga (Amsellem et al., 2019Amsellem, E., Moynier, F., Puchtel, I.S. (2019) Evolution of the Ca isotopic composition of the mantle. Geochimica et Cosmochimica Acta 258, 195–206.
). Given the compatibility of Zr in bridgmanite and the CN differences between bridgmanite and silicate melt outlined previously, crystallisation of bridgmanite could fractionate Zr isotopes. The Weltevreden komatiites do not have anomalous Zr isotopic compositions (average δ94/90Zr value for Weltevreden is 0.019 ± 0.036 ‰, compared to 0.032 ± 0.054 ‰ for other komatiites; t test, p value =0.19) and, therefore, cannot validate the hypothesis. Assuming magma ocean fractionation effects in the source of Weltevreden komatiites, the absence of Zr isotopic variations likely reflects limited bridgmanite-melt fractionation, because the difference in CN at high temperatures was insufficient to induce isotopic fractionation resolvable within analytical uncertainty.Evolution of the Zr isotopic composition of Earth’s mantle. The similarity (t test, p value = 0.56) of Zr isotope composition between spinifex-textured and chilled margin komatiites (δ94/90Zr = 0.027 ± 0.044 ‰, n = 14) and olivine cumulates (δ94/90Zr = 0.033 ± 0.054 ‰, n = 17; Table S-1) indicates that there was limited isotopic fractionation during komatiite crystallisation. This is illustrated by the absence of correlation between δ94/90Zr and Zr content (Fig. 3, R2 = 0.2). Since komatiites are formed at temperatures >2000 K (Arndt et al., 2008
Arndt, N., Lesher, M., Barnes, S. (2008) Komatiite. Cambridge University Press. Cambridge, New York, Melbourne.
), isotopic fractionation during partial melting is minimised, and, therefore, they must represent the composition of their source. The sources of komatiites had been variably depleted by prior melt extractions, as evidenced from variations in the ratios of the light and heavy REE. This is exemplified by the normalised La/Sm ratio to the primitive mantle composition, (La/Sm)N, that deviates from one. Prior melt extraction would also have extracted Zr from the source and, potentially, fractionated Zr isotopes. The komatiites analysed here encompass a large range of (La/Sm)N values, from 0.44 to 2.2 (Puchtel et al., 1998Puchtel, I., Hofmann, A., Mezger, K., Jochum, K., Shchipansky, A., Samsonov, A. (1998) Oceanic plateau model for continental crustal growth in the Archaean: a case study from the Kostomuksha greenstone belt, NW Baltic Shield. Earth and Planetary Science Letters 155, 57–74.
, 2001Puchtel, I.S., Brügmann, G.E., Hofmann, A.W. (2001) 187Os-enriched domain in an Archean mantle plume: evidence from 2.8 Ga komatiites of the Kostomuksha greenstone belt, NW Baltic Shield. Earth and Planetary Science Letters 186, 513–526.
, 2005Puchtel, I.S., Brandon, A.D., Humayun, M., Walker, R.J. (2005) Evidence for the early differentiation of the core from Pt–Re–Os isotope systematics of 2.8-Ga komatiites. Earth and Planetary Science Letters 237, 118–134.
; 2009Puchtel, I., Walker, R., Brandon, A., Nisbet, E. (2009) Pt–Re–Os and Sm–Nd isotope and HSE and REE systematics of the 2.7Ga Belingwe and Abitibi komatiites. Geochimica et Cosmochimica Acta 73, 6367–6389.
, 2013Puchtel, I., Blichert-Toft, J., Touboul, M., Walker, R., Byerly, G., Nisbet, E., Anhaeusser, C. (2013) Insights into early Earth from Barberton komatiites: evidence from lithophile isotope and trace element systematics. Geochimica et Cosmochimica Acta 108, 63–90.
, 2016aPuchtel, I., Touboul, M., Blichert-Toft, J., Walker, R., Brandon, A., Nicklas, R., Kulikov, V., Samsonov, A. (2016a) Lithophile and siderophile element systematics of Earth’s mantle at the Archean–Proterozoic boundary: evidence from 2.4Ga komatiites. Geochimica et Cosmochimica Acta 180, 227–255.
,bPuchtel, I.S., Blichert-Toft, J., Touboul, M., Horan, M.F., Walker, R.J. (2016b) The coupled 182W-142Nd record of early terrestrial mantle differentiation. Geochemistry, Geophysics, Geosystems 17, 2168–2193.
). The absence of correlation (R2 = 0.2) between the δ94/90Zr values and (La/Sm)N (Fig. 2) indicates that the mantle sources of komatiites were not isotopically fractionated by prior melt extractions. The absence of temporal δ94/90Zr variations from 3.5 to 2.41 Ga (Fig. 1, R2 = 0.004) provides further evidence that prior melt extraction did not change the Zr isotopic composition of the komatiite mantle sources. Therefore, the average δ94/90Zr value of komatiites should reflect the composition of their mantle source. The average Zr isotopic composition of komatiites (δ94/90Zr = 0.030 ± 0.049 ‰) overlaps with the mantle’s estimate based on young basaltic rocks (δ94/90Zr = 0.048 ± 0.032 ‰, Inglis et al., 2019Inglis, E., Moynier, F., Creech, J., Deng, Z., Day, J.M.D., Teng, F.-Z., Bizzarro, M., Jackson, M.J., Savage, P. (2019) Isotopic fractionation of zirconium during magmatic differentiation and the stable isotope composition of the silicate Earth. Geochimica et Cosmochimica Acta 250, 311–323.
). When averaged together with the previously analysed basalts (Inglis et al., 2019Inglis, E., Moynier, F., Creech, J., Deng, Z., Day, J.M.D., Teng, F.-Z., Bizzarro, M., Jackson, M.J., Savage, P. (2019) Isotopic fractionation of zirconium during magmatic differentiation and the stable isotope composition of the silicate Earth. Geochimica et Cosmochimica Acta 250, 311–323.
), a conservative estimate for the δ94/90Zr for the Earth’s mantle of 0.040 ± 0.044 ‰ (2 s.d., n = 72) is calculated (grey band in Fig. 3). However, it should be noted that basalts are slightly lighter than komatiites (t test, p value = 0.001), which may indicate that low degree partial melt could be enriched in the heavier isotopes of Zr as previously suggested to explain the enrichments in light Zr isotopes in the mantle source of N-MORBs (Inglis et al., 2019Inglis, E., Moynier, F., Creech, J., Deng, Z., Day, J.M.D., Teng, F.-Z., Bizzarro, M., Jackson, M.J., Savage, P. (2019) Isotopic fractionation of zirconium during magmatic differentiation and the stable isotope composition of the silicate Earth. Geochimica et Cosmochimica Acta 250, 311–323.
). Future analyses of mantle peridotites would allow further assessment of this issue.
Figure 3 Zirconium isotopic composition of komatiites (this study) and basalts (literature) as a function of the Zr content of the samples. While Zr content varies over two orders of magnitude, the δ94/90Zr are consistent with an average value of 0.040 ± 0.044 (2 s.d., n = 72), which best reflects the composition of Earth’s mantle (grey band). Errors as 2 s.d. of replicate measurements (typically 4). Data from Inglis et al. (2019)
Inglis, E., Moynier, F., Creech, J., Deng, Z., Day, J.M.D., Teng, F.-Z., Bizzarro, M., Jackson, M.J., Savage, P. (2019) Isotopic fractionation of zirconium during magmatic differentiation and the stable isotope composition of the silicate Earth. Geochimica et Cosmochimica Acta 250, 311–323.
.top
Acknowledgements
We thank three anonymous reviewers and editor A. Shahar for their comments. SYT thanks support from CSC. FM acknowledges an ERC grant #637503. ISP thanks E. Nisbet, G. Byerly, and C. Anhaeusser for invaluable contributions to his komatiite collection.
Editor: Anat Shahar
top
References
Amsellem, E., Moynier, F., Puchtel, I.S. (2019) Evolution of the Ca isotopic composition of the mantle. Geochimica et Cosmochimica Acta 258, 195–206.
 Show in context
Show in context Similar logic has been used to estimate the composition of the mantle through time of several other elements, including Ca (Amsellem et al., 2019), Ga (Kato et al., 2017), and Ti (Greber et al., 2017; Deng et al., 2018).
View in article
For example, some komatiites from the Weltevreden formation in the Barberton Greenstone Belt, South Africa, have unusual Ca isotopic compositions compared with other komatiites and are interpreted as a record of mantle source heterogeneities induced by magma ocean crystallisation (Amsellem et al., 2019).
View in article
All samples have previously been studied for trace and highly siderophile element abundances, 186,187Os, 142,143Nd, 182W isotope systematics (Puchtel et al., 1998, 2001, 2005, 2009, 2013, 2016a,b), and for Ca stable isotopes (Amsellem et al., 2019).
View in article
Based on Os, Nd, Hf, O and Ca isotopic compositions of some komatiites like Weltevreden it has been suggested that they recorded the fractionation of minerals stable under magma ocean conditions (Puchtel et al., 2013; Byerly et al., 2017; Amsellem et al., 2019).
View in article
The source of these komatiites is enriched in the heavier isotopes of Ca, consistent with the extraction of light Ca in Ca perovskite into the lower mantle and the preservation of mantle heterogeneity until at least the extraction of the komatiites at 3.26 Ga (Amsellem et al., 2019).
View in article
Arndt, N., Lesher, M., Barnes, S. (2008) Komatiite. Cambridge University Press. Cambridge, New York, Melbourne.
 Show in context
Show in context They are formed by partial melting in hot mantle plumes, mostly during the Archean (e.g., Arndt et al., 2008).
View in article
Since komatiites are formed at temperatures >2000 K (Arndt et al., 2008), isotopic fractionation during partial melting is minimised, and, therefore, they must represent the composition of their source. The sources of komatiites had been variably depleted by prior melt extractions, as evidenced from variations in the ratios of the light and heavy REE. This is exemplified by the normalised La/Sm ratio to the primitive mantle composition, (La/Sm)N, that deviates from one. Prior melt extraction would also have extracted Zr from the source and, potentially, fractionated Zr isotopes.
View in article
Byerly, B.L., Kareem, K., Bao, H., Byerly, G.R. (2017) Early Earth mantle heterogeneity revealed by light oxygen isotopes of Archaean komatiites. Nature Geoscience 10, 871–875.
 Show in context
Show in context Komatiites span a range of ages and, thus, provide the potential to investigate initial mantle composition and, in particular, the processes of crystallisation of an early terrestrial magma ocean (Puchtel et al., 2013, 2016b; Byerly et al., 2017).
View in article
Based on Os, Nd, Hf, O and Ca isotopic compositions of some komatiites like Weltevreden it has been suggested that they recorded the fractionation of minerals stable under magma ocean conditions (Puchtel et al., 2013; Byerly et al., 2017; Amsellem et al., 2019).
View in article
Condie, K.C. (2005) High field strength element ratios in Archean basalts: a window to evolving sources of mantle plumes? Lithos 79, 491–504.
 Show in context
Show in context Because of the resistance of zircons to physical and chemical abrasion and the availability of other isotope tracers (e.g., O, Hf) and elements within them (e.g., rare earth elements), they have found widespread application in the study of Earth’s crust through time (Condie, 2005).
View in article
Corgne, A., Liebske, C., Wood, B.J., Rubie, D.C., Frost, D.J. (2005) Silicate perovskite-melt partitioning of trace elements and geochemical signature of a deep perovskitic reservoir. Geochimica et Cosmochimica Acta 69, 485–496.
 Show in context
Show in context Zirconium is useful to search for traces of magma ocean crystallisation processes because it is compatible in bridgmanite (Dbridgmanite-melt > 1; Corgne et al., 2005) and has different coordination numbers (CN) between bridgmanite (CN = 8, where it substitutes for Mg; Smyth and Bish, 1988), and silicate melt (CN = 6; Farges et al., 1991).
View in article
Deng, Z., Moynier, F., Sossi, P., Chaussidon, M. (2018) Bridging the depleted MORB mantle and the continental crust using titanium isotopes. Geochemical Perspectives Letters 9, 11–15.
 Show in context
Show in context Similar logic has been used to estimate the composition of the mantle through time of several other elements, including Ca (Amsellem et al., 2019), Ga (Kato et al., 2017), and Ti (Greber et al., 2017; Deng et al., 2018).
View in article
Farges, F., Ponader, C.W., Brown Jr, G.E. (1991) Structural environments of incompatible elements in silicate glass/melt systems: I. Zirconium at trace levels. Geochimica et Cosmochimica Acta 55, 1563–1574.
 Show in context
Show in context Zirconium is useful to search for traces of magma ocean crystallisation processes because it is compatible in bridgmanite (Dbridgmanite-melt > 1; Corgne et al., 2005) and has different coordination numbers (CN) between bridgmanite (CN = 8, where it substitutes for Mg; Smyth and Bish, 1988), and silicate melt (CN = 6; Farges et al., 1991).
View in article
Feng, L., Hu, W., Jiao, Y., Zhou, L., Zhang, W., Hu, Z., Liu, Y. (2020) High-precision stable zirconium isotope ratio measurements by double spike thermal ionization mass spectrometry. Journal of Analytical Atomic Spectrometry 35, 736–745.
 Show in context
Show in context There is presently no international Zr isotopic standard commercially available and the majority of the Zr isotopic data for rock samples have been reported against the IPGP-Zr standard (calibrated against a variety of geostandards; Inglis et al., 2018; Feng et al., 2020; Guo et al., 2020; Tian et al., 2020).
View in article
Alternatively, three other standards have been used: zircon GJ-1 for in situ analyses (Zhang et al., 2019), an elemental Zr standard, NIST SRM3169 (Feng et al., 2020) and a standard in development by NIST (Ibañez-Mejia and Tissot, 2019; Tompkins et al., 2020) for bulk measurements.
View in article
Greber, N.D., Dauphas, N., Puchtel, I.S., Hofmann, B.A., Arndt, N.T. (2017) Titanium stable isotopic variations in chondrites, achondrites and lunar rocks. Geochimica et Cosmochimica Acta 213, 534–552.
 Show in context
Show in context Similar logic has been used to estimate the composition of the mantle through time of several other elements, including Ca (Amsellem et al., 2019), Ga (Kato et al., 2017), and Ti (Greber et al., 2017; Deng et al., 2018).
View in article
Guo, J.L., Wang, Z., Zhang, W., Moynier, F., Cui, D., Hu, Z., Ducea, M. (2020) Significant Zr isotope variations in single zircon grains recording magma evolution history. Proceedings of the National Academy of Sciences, doi: 10.1073/pnas.2002053117.
 Show in context
Show in context There is presently no international Zr isotopic standard commercially available and the majority of the Zr isotopic data for rock samples have been reported against the IPGP-Zr standard (calibrated against a variety of geostandards; Inglis et al., 2018; Feng et al., 2020; Guo et al., 2020; Tian et al., 2020).
View in article
Herzberg, C. (1992) Depth and degree of melting of komatiite. Journal of Geophysical Research 97, 4521–4540.
 Show in context
Show in context Since komatiites are mantle-derived high degree partial melts (>30 %) (Herzberg, 1992), and Zr is moderately incompatible, almost all Zr from the mantle source is efficiently extracted into komatiitic melts.
View in article
Hin, R.C., Coath, C.D., Carter, P.J., Nimmo, F., Lai, Y.J., Pogge von Strandmann, P.A.E., Willbold, M., Leinhardt, Z.M., Walter, M.J., Elliott, T. (2017) Magnesium isotope evidence that accretional vapour loss shapes planetary compositions. Nature 549, 511–515.
 Show in context
Show in context This makes Zr distinct from more volatile elements, like Zn (Moynier et al., 2017) or even Si and Mg (Pringle et al., 2014; Hin et al., 2017).
View in article
Ibañez-Mejia, M., Tissot, F.L. (2019) Extreme Zr stable isotope fractionation during magmatic fractional crystallization. Science Advances 5, eaax8648.
 Show in context
Show in context Alternatively, three other standards have been used: zircon GJ-1 for in situ analyses (Zhang et al., 2019), an elemental Zr standard, NIST SRM3169 (Feng et al., 2020) and a standard in development by NIST (Ibañez-Mejia and Tissot, 2019; Tompkins et al., 2020) for bulk measurements.
View in article
Inglis, E.C., Creech, J.B., Deng, Z., Moynier, F. (2018) High-precision zirconium stable isotope measurements of geological reference materials as measured by double-spike MC-ICPMS. Chemical Geology 493, 544–552.
 Show in context
Show in context The isotopic fractionation of Zr between granites and basalts (Inglis et al., 2018) suggests that Zr isotopes could be used as tracers within zircons, and of crustal recycling in Earth’s mantle, especially since Zr is a fluid immobile element and should be efficiently recycled into the mantle.
View in article
Two widely available reference materials (BHVO-2 and AGV-2) for which Zr isotopic compositions have been reported previously (Inglis et al., 2018; Tian et al., 2020) were also analysed.
View in article
The protocol utilised for Zr stable isotope measurements was adapted from Inglis et al. (2018) and is reported in Table S-2.
View in article
To do this, the column procedure from Inglis et al. (2018) was reversed. The details of the analytical methods can be found in the Supplementary Information.
View in article
There is presently no international Zr isotopic standard commercially available and the majority of the Zr isotopic data for rock samples have been reported against the IPGP-Zr standard (calibrated against a variety of geostandards; Inglis et al., 2018; Feng et al., 2020; Guo et al., 2020; Tian et al., 2020).
View in article
The δ94/90Zr values of BHVO-2 (0.033 ± 0.025 ‰) and AGV-2 (0.017 ± 0.065 ‰) are consistent with the previous measurements obtained using a different chemical protocol (δ94/90Zr = 0.044 ± 0.044 ‰ and 0.044 ±0.050 ‰, respectively), (Inglis et al., 2018) and the values reported in Tian et al. (2020) (0.045 ± 0.025 ‰ and 0.035 ± 0.037 ‰, respectively).
View in article
Inglis, E., Moynier, F., Creech, J., Deng, Z., Day, J.M.D., Teng, F.-Z., Bizzarro, M., Jackson, M.J., Savage, P. (2019) Isotopic fractionation of zirconium during magmatic differentiation and the stable isotope composition of the silicate Earth. Geochimica et Cosmochimica Acta 250, 311–323.
 Show in context
Show in context This is interpreted to reflect differences in coordination of zirconium between zircon and melt (Inglis et al., 2019).
View in article
Presently, the Zr isotopic composition of the mantle is estimated by the analysis of relatively low degree basaltic partial melts (Inglis et al., 2019).
View in article
The average Zr isotopic composition of komatiites (δ94/90Zr = 0.030 ± 0.049 ‰) overlaps with the mantle’s estimate based on young basaltic rocks (δ94/90Zr = 0.048 ± 0.032 ‰, Inglis et al., 2019).
View in article
When averaged together with the previously analysed basalts (Inglis et al., 2019), a conservative estimate for the δ94/90Zr for the Earth’s mantle of 0.040 ± 0.044 ‰ (2 s.d., n = 72) is calculated (grey band in Fig. 3).
View in article
However, it should be noted that basalts are slightly lighter than komatiites (t test, p value = 0.001), which may indicate that low degree partial melt could be enriched in the heavier isotopes of Zr as previously suggested to explain the enrichments in light Zr isotopes in the mantle source of N-MORBs (Inglis et al., 2019). Future analyses of mantle peridotites would allow further assessment of this issue.
View in article
Data from Inglis et al. (2019).
View in article
Johnson, K.T. (1998) Experimental determination of partition coefficients for rare earth and high-field-strength elements between clinopyroxene, garnet, and basaltic melt at high pressures. Contributions to Mineralogy and Petrology 133, 60–68.
 Show in context
Show in context As with other high field strength elements, Zr is lithophile and incompatible (Woodhead et al., 1993; Johnson, 1998) and is enriched in the continental crust (Rudnick and Gao, 2003).
View in article
Kato, C., Moynier, F., Foriel, J., Teng, F., Puchtel, I.S. (2017) The gallium isotopic composition of the bulk silicate Earth. Chemical Geology 448, 164–172.
 Show in context
Show in context Similar logic has been used to estimate the composition of the mantle through time of several other elements, including Ca (Amsellem et al., 2019), Ga (Kato et al., 2017), and Ti (Greber et al., 2017; Deng et al., 2018).
View in article
Lodders, K. (2003) Solar System abundances and condensation temperatures of the elements. Astrophysical Journal 591, 1220–1247.
 Show in context
Show in context Zirconium is a refractory element (Tc = 1741K; Lodders, 2003), and its abundance and stable isotopic composition is, a priori, robust to evaporation processes that occurred during planetary formation.
View in article
Moynier, F., Vance, D., Fujii, T., Savage, P. (2017) The isotope geochemistry of copper and zinc. In: Teng, F.-Z., Watkins, J., Dauphas, N. (Eds.) Reviews in Mineralogy & Geochemistry 82, 543–600.
 Show in context
Show in context This makes Zr distinct from more volatile elements, like Zn (Moynier et al., 2017) or even Si and Mg (Pringle et al., 2014; Hin et al., 2017).
View in article
Pringle, E.A., Moynier, F., Savage, P.S., Badro, J., Barrat, J.A. (2014) Silicon isotopes in angrites and volatile loss in planetesimals. Proceedings of the National Academy of Science USA 111, 17029–32.
 Show in context
Show in context This makes Zr distinct from more volatile elements, like Zn (Moynier et al., 2017) or even Si and Mg (Pringle et al., 2014; Hin et al., 2017).
View in article
Puchtel, I., Hofmann, A., Mezger, K., Jochum, K., Shchipansky, A., Samsonov, A. (1998) Oceanic plateau model for continental crustal growth in the Archaean: a case study from the Kostomuksha greenstone belt, NW Baltic Shield. Earth and Planetary Science Letters 155, 57–74.
 Show in context
Show in context All samples have previously been studied for trace and highly siderophile element abundances, 186,187Os, 142,143Nd, 182W isotope systematics (Puchtel et al., 1998, 2001, 2005, 2009, 2013, 2016a,b), and for Ca stable isotopes (Amsellem et al., 2019).
View in article
The komatiites analysed here encompass a large range of (La/Sm)N values, from 0.44 to 2.2 (Puchtel et al., 1998, 2001, 2005; 2009, 2013, 2016a,b).
View in article
Puchtel, I.S., Brügmann, G.E., Hofmann, A.W. (2001) 187Os-enriched domain in an Archean mantle plume: evidence from 2.8 Ga komatiites of the Kostomuksha greenstone belt, NW Baltic Shield. Earth and Planetary Science Letters 186, 513–526.
 Show in context
Show in context All samples have previously been studied for trace and highly siderophile element abundances, 186,187Os, 142,143Nd, 182W isotope systematics (Puchtel et al., 1998, 2001, 2005, 2009, 2013, 2016a,b), and for Ca stable isotopes (Amsellem et al., 2019).
View in article
The komatiites analysed here encompass a large range of (La/Sm)N values, from 0.44 to 2.2 (Puchtel et al., 1998, 2001, 2005; 2009, 2013, 2016a,b).
View in article
Puchtel, I.S., Brandon, A.D., Humayun, M., Walker, R.J. (2005) Evidence for the early differentiation of the core from Pt–Re–Os isotope systematics of 2.8-Ga komatiites. Earth and Planetary Science Letters 237, 118–134.
 Show in context
Show in context All samples have previously been studied for trace and highly siderophile element abundances, 186,187Os, 142,143Nd, 182W isotope systematics (Puchtel et al., 1998, 2001, 2005, 2009, 2013, 2016a,b), and for Ca stable isotopes (Amsellem et al., 2019).
View in article
The komatiites analysed here encompass a large range of (La/Sm)N values, from 0.44 to 2.2 (Puchtel et al., 1998, 2001, 2005; 2009, 2013, 2016a,b).
View in article
Puchtel, I., Walker, R., Brandon, A., Nisbet, E. (2009) Pt–Re–Os and Sm–Nd isotope and HSE and REE systematics of the 2.7Ga Belingwe and Abitibi komatiites. Geochimica et Cosmochimica Acta 73, 6367–6389.
 Show in context
Show in context All samples have previously been studied for trace and highly siderophile element abundances, 186,187Os, 142,143Nd, 182W isotope systematics (Puchtel et al., 1998, 2001, 2005, 2009, 2013, 2016a,b), and for Ca stable isotopes (Amsellem et al., 2019).
View in article
The komatiites analysed here encompass a large range of (La/Sm)N values, from 0.44 to 2.2 (Puchtel et al., 1998, 2001, 2005; 2009, 2013, 2016a,b).
View in article
Puchtel, I., Blichert-Toft, J., Touboul, M., Walker, R., Byerly, G., Nisbet, E., Anhaeusser, C. (2013) Insights into early Earth from Barberton komatiites: evidence from lithophile isotope and trace element systematics. Geochimica et Cosmochimica Acta 108, 63–90.
 Show in context
Show in context Komatiites span a range of ages and, thus, provide the potential to investigate initial mantle composition and, in particular, the processes of crystallisation of an early terrestrial magma ocean (Puchtel et al., 2013, 2016b; Byerly et al., 2017).
View in article
All samples have previously been studied for trace and highly siderophile element abundances, 186,187Os, 142,143Nd, 182W isotope systematics (Puchtel et al., 1998, 2001, 2005, 2009, 2013, 2016a,b), and for Ca stable isotopes (Amsellem et al., 2019).
View in article
Based on Os, Nd, Hf, O and Ca isotopic compositions of some komatiites like Weltevreden it has been suggested that they recorded the fractionation of minerals stable under magma ocean conditions (Puchtel et al., 2013; Byerly et al., 2017; Amsellem et al., 2019).
View in article
The komatiites analysed here encompass a large range of (La/Sm)N values, from 0.44 to 2.2 (Puchtel et al., 1998, 2001, 2005; 2009, 2013, 2016a,b).
View in article
Puchtel, I., Touboul, M., Blichert-Toft, J., Walker, R., Brandon, A., Nicklas, R., Kulikov, V., Samsonov, A. (2016a) Lithophile and siderophile element systematics of Earth’s mantle at the Archean–Proterozoic boundary: evidence from 2.4Ga komatiites. Geochimica et Cosmochimica Acta 180, 227–255.
 Show in context
Show in context All samples have previously been studied for trace and highly siderophile element abundances, 186,187Os, 142,143Nd, 182W isotope systematics (Puchtel et al., 1998, 2001, 2005, 2009, 2013, 2016a,b), and for Ca stable isotopes (Amsellem et al., 2019).
View in article
The komatiites analysed here encompass a large range of (La/Sm)N values, from 0.44 to 2.2 (Puchtel et al., 1998, 2001, 2005; 2009, 2013, 2016a,b).
View in article
Puchtel, I.S., Blichert-Toft, J., Touboul, M., Horan, M.F., Walker, R.J. (2016b) The coupled 182W-142Nd record of early terrestrial mantle differentiation. Geochemistry, Geophysics, Geosystems 17, 2168–2193.
 Show in context
Show in context Komatiites span a range of ages and, thus, provide the potential to investigate initial mantle composition and, in particular, the processes of crystallisation of an early terrestrial magma ocean (Puchtel et al., 2013, 2016b; Byerly et al., 2017).
View in article
All samples have previously been studied for trace and highly siderophile element abundances, 186,187Os, 142,143Nd, 182W isotope systematics (Puchtel et al., 1998, 2001, 2005, 2009, 2013, 2016a,b), and for Ca stable isotopes (Amsellem et al., 2019).
View in article
The komatiites analysed here encompass a large range of (La/Sm)N values, from 0.44 to 2.2 (Puchtel et al., 1998, 2001, 2005; 2009, 2013, 2016a,b).
View in article
Rudnick, R.L., Gao, S. (2003) Composition of the Continental Crust. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry 3, 1–64.
 Show in context
Show in context As with other high field strength elements, Zr is lithophile and incompatible (Woodhead et al., 1993; Johnson, 1998) and is enriched in the continental crust (Rudnick and Gao, 2003).
View in article
Smyth, J.R., Bish, D.L. (1988) Crystal structures and cation sites of the rock-forming minerals. Allen & Unwin, Boston.
 Show in context
Show in context Zirconium is useful to search for traces of magma ocean crystallisation processes because it is compatible in bridgmanite (Dbridgmanite-melt > 1; Corgne et al., 2005) and has different coordination numbers (CN) between bridgmanite (CN = 8, where it substitutes for Mg; Smyth and Bish, 1988), and silicate melt (CN = 6; Farges et al., 1991).
View in article
Tanaka, R., Makishima, A., Kitagawa, H., Nakamura, E. (2003) Suppression of Zr, Nb, Hf and Ta coprecipitation in fluoride compounds for determination in Ca-rich materials. Journal of Analytical Atomic Spectrometry 18, 1458–1463.
 Show in context
Show in context The size of columns utilised had to be scaled up and the chemical purification method modified due to the high Ca/Zr of komatiites, and the associated risk of Zr coprecipitation with Mg, Ca fluorides (Tanaka et al., 2003).
View in article
Tian, S., Inglis, E., Creech, J., Zhang, W., Wang, Z., Hu, Z., Liu, Y., Moynier, F. (2020) The zirconium stable isotope compositions of 22 geological reference materials, 4 zircons and 3 standard solutions. Chemical Geology, doi: 10.1016/j.chemgeo.2020.119791.
 Show in context
Show in context Two widely available reference materials (BHVO-2 and AGV-2) for which Zr isotopic compositions have been reported previously (Inglis et al., 2018; Tian et al., 2020) were also analysed.
View in article
The Zr isotopic composition was measured using a ThermoFischer Neptune plus MC-ICP-MS at the IPGP as described in Tian et al. (2020).
View in article
There is presently no international Zr isotopic standard commercially available and the majority of the Zr isotopic data for rock samples have been reported against the IPGP-Zr standard (calibrated against a variety of geostandards; Inglis et al., 2018; Feng et al., 2020; Guo et al., 2020; Tian et al., 2020).
View in article
These three standards have been calibrated against the IPGP-Zr standard (Tian et al., 2020).
View in article
The δ94/90Zr values of BHVO-2 (0.033 ± 0.025 ‰) and AGV-2 (0.017 ± 0.065 ‰) are consistent with the previous measurements obtained using a different chemical protocol (δ94/90Zr = 0.044 ± 0.044 ‰ and 0.044 ±0.050 ‰, respectively), (Inglis et al., 2018) and the values reported in Tian et al. (2020) (0.045 ± 0.025 ‰ and 0.035 ± 0.037 ‰, respectively).
View in article
Tompkins, H., Zieman, L., Ibanez-Meija, M., Tissot, F. (2020) Zirconium stable isotope analysis of zircon by MCICP-MS:methods and application to evaluating intra-crystalline zonation in a zircon megacryst. Journal of Analytical Atomic Spectrometry, doi: 10.1039/c9ja00315k.
 Show in context
Show in context Alternatively, three other standards have been used: zircon GJ-1 for in situ analyses (Zhang et al., 2019), an elemental Zr standard, NIST SRM3169 (Feng et al., 2020) and a standard in development by NIST (Ibañez-Mejia and Tissot, 2019; Tompkins et al., 2020) for bulk measurements.
View in article
Woodhead, J., Eggins, S., Gamble, J. (1993) High field strength and transition element systematics in island arc and back-arc basin basalts: evidence for multi-phase melt extraction and a depleted mantle wedge. Earth and Planetary Science Letters 114, 491–504.
 Show in context
Show in context As with other high field strength elements, Zr is lithophile and incompatible (Woodhead et al., 1993; Johnson, 1998) and is enriched in the continental crust (Rudnick and Gao, 2003).
View in article
Zhang, W., Wang, Z., Moynier, F., Inglis, E., Tian, S., Li, M., Liu, Y., Hu, Z. (2019) Determination of Zr isotopic ratios in zircons using laser-ablation multiple-collector inductively coupled-plasma mass-spectrometry. Journal of Analytical Atomic Spectrometry 34, 1800–1809.
 Show in context
Show in context Alternatively, three other standards have been used: zircon GJ-1 for in situ analyses (Zhang et al., 2019), an elemental Zr standard, NIST SRM3169 (Feng et al., 2020) and a standard in development by NIST (Ibañez-Mejia and Tissot, 2019; Tompkins et al., 2020) for bulk measurements.
View in article
top
Supplementary Information
The Supplementary Information includes:
- Methods
- Tables S-1 to S-3
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures
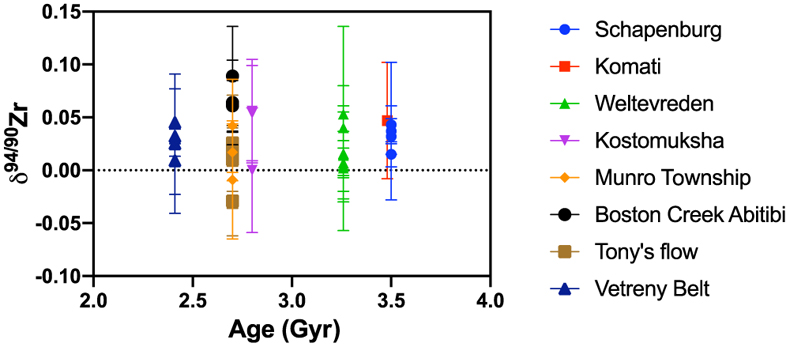
Figure 1 Zirconium isotopic composition of komatiites as a function of their crystallisation ages. The δ94/90Zr values are constant through 1 Ga and in various locations indicating that the terrestrial mantle had a constant δ94/90Zr value through time. The Weltevreden komatiites have unusual Ca isotope signatures consistent with magma ocean crystallisation processes. They do not have a different Zr isotopic composition from other komatiites. Errors as 2 s.d. of replicated measurements (typically 4).
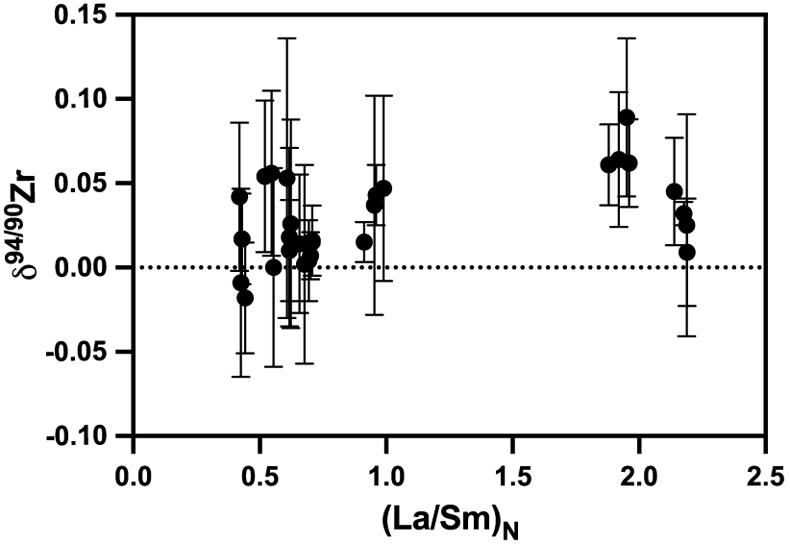
Figure 2 Zirconium isotopic composition of the komatiites as a function of the La/Sm ratio normalised to primitive mantle composition [(La/Sm)N]. Variations in (La/Sm)N are interpreted to reflect prior melt extraction from komatiite source mantles. The absence of variation suggests that prior melt extraction did not fractionate Zr isotopes and that the source of the komatiites reflects the composition of the Archean mantle. Errors as 2 s.d. of the replicated measurements (typically 4).
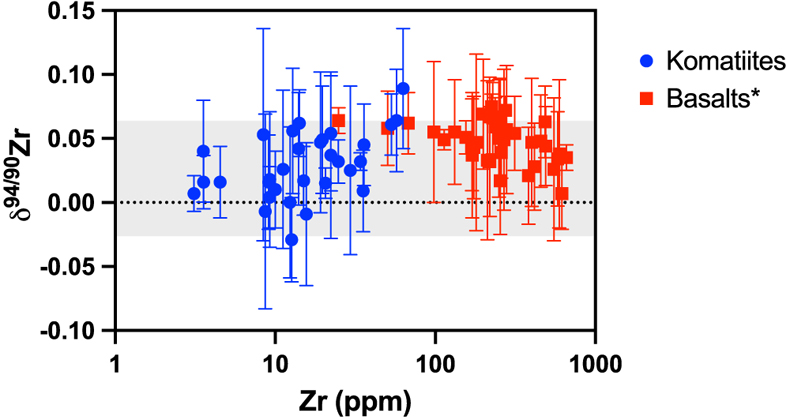
Figure 3 Zirconium isotopic composition of komatiites (this study) and basalts (literature) as a function of the Zr content of the samples. While Zr content varies over two orders of magnitude, the δ94/90Zr are consistent with an average value of 0.040 ± 0.044 (2 s.d., n = 72), which best reflects the composition of Earth’s mantle (grey band). Errors as 2 s.d. of replicate measurements (typically 4). Data from Inglis et al. (2019)
Inglis, E., Moynier, F., Creech, J., Deng, Z., Day, J.M.D., Teng, F.-Z., Bizzarro, M., Jackson, M.J., Savage, P. (2019) Isotopic fractionation of zirconium during magmatic differentiation and the stable isotope composition of the silicate Earth. Geochimica et Cosmochimica Acta 250, 311–323.
.