Experimental evidence for light Ba isotopes favouring aqueous fluids over silicate melts
Affiliations | Corresponding Author | Cite as | Funding information- Share this article

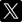



-
Article views:153Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
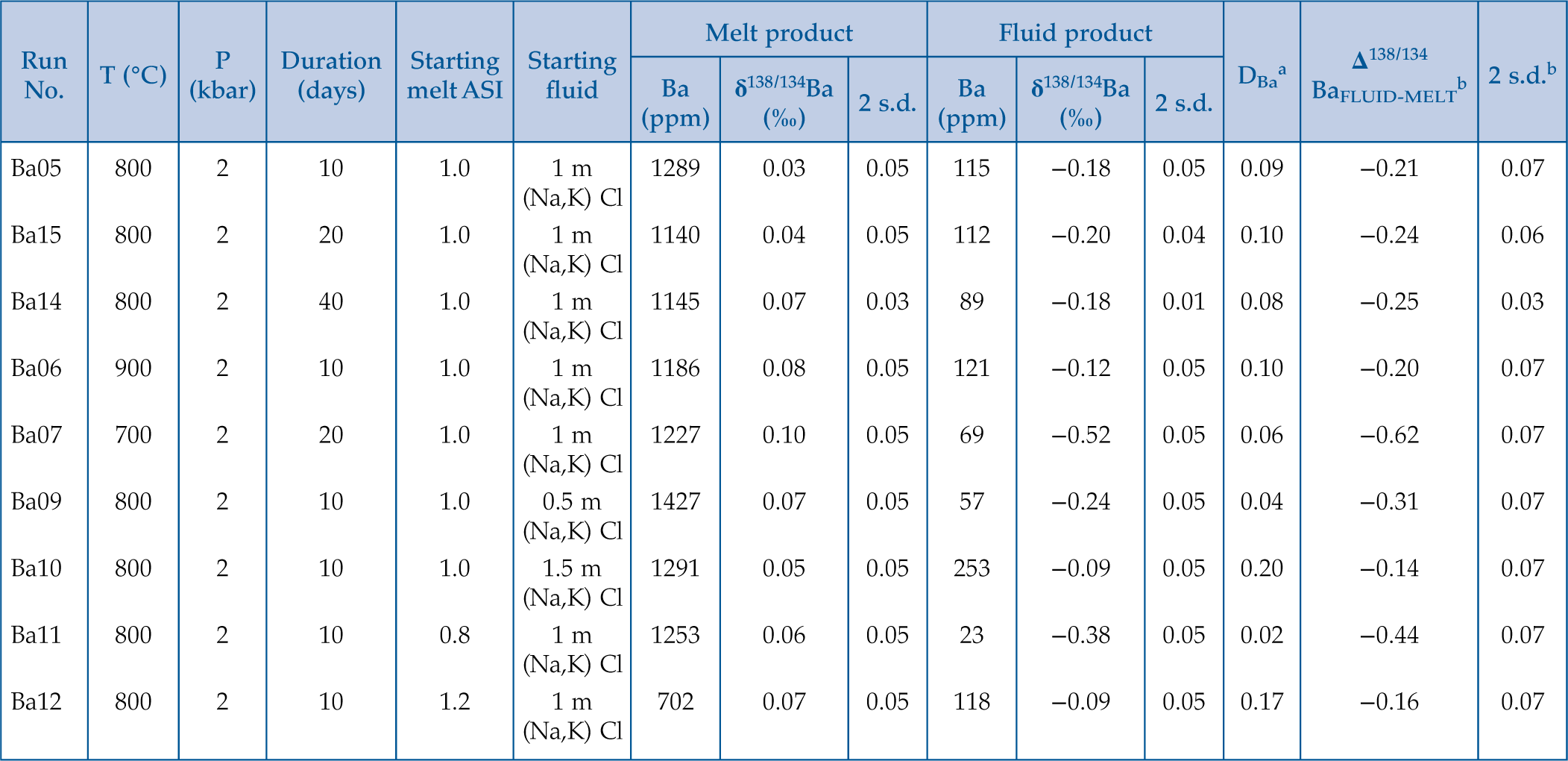 Table 1 Summary of experimental conditions and results. | 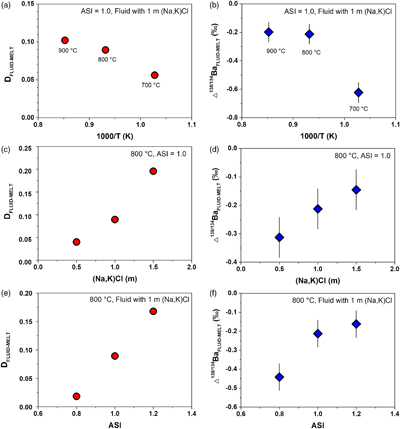 Figure 1 Partitioning coefficient (DFLUID-MELT) and equilibrium isotope fractionation (Δ138/134BaFLUID-MELT) of Ba between aqueous fluid and silicate melt as a function of temperature from 700 to 900 °C (a,b), salinity of the fluid (c,d), and ASI of the melt (e,f). Error bars in b, d, and f represent 2 s.d. uncertainties (see Table 1). | 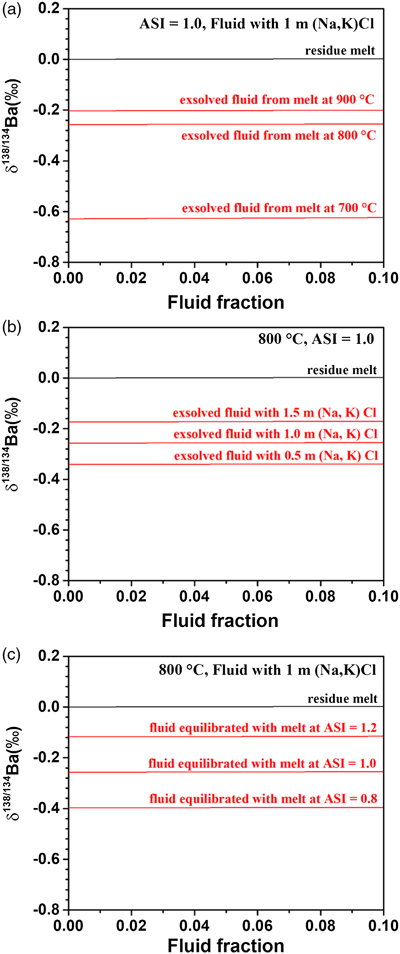 Figure 2 The modelled δ138/134Ba values of residue melt and exsolved fluid as a function of fluid fraction, using the Rayleigh fractionation model. The Ba isotopic composition of the initial fluid-saturated melt is set as 0 ‰, which is the average δ138/134Ba of the upper continental crust (Nan et al., 2018). Three factors affecting the Ba isotope fractionation between the melt and fluid are considered: (a) temperature, (b) salinity of the fluid, and (c) ASI of the melt. Based on Eq. S-1 in the Supplementary Information, the fluid phase only removes less than 2 % of the total Ba from the hydrous magma system, even if the fluid exsolution fraction is as high as 0.1. See text for the details. | 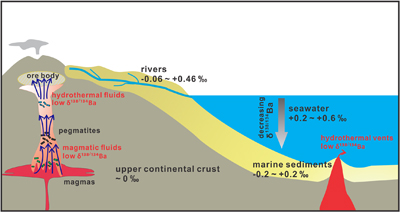 Figure 3 A schematic diagram illustrating the global δ138/134Ba isotopic characteristics of the upper continental crust, marine sediments, seawater and rivers as well as the relevant hydrothermal fluids and vents. The magmatic fluids, hydrothermal fluids and vents are expected to have low δ138/134Ba according to the negative Δ138/134BaFLUID-MELT values (from −0.62 ‰ to −0.14 ‰) obtained in this study. Data sources: Horner et al. (2015); Cao et al. (2016); Bates et al. (2017); Hsieh and Henderson (2017); Bridgestock et al. (2018, 2019); Nan et al. (2018); Nielsen et al. (2018); Crockford et al. (2019); Gou et al. (2020). |
| Table 1 | Figure 1 | Figure 2 | Figure 3 |
top
Introduction
As an alkaline earth metal, Ba is a large ion lithophile element (LILE) and fluid mobile element. It has seven stable isotopes, i.e. 130Ba (0.11 %), 132Ba (0.10 %), 134Ba (2.42 %), 135Ba (6.59 %), 136Ba (7.85 %), 137Ba (11.23 %) and 138Ba (71.70 %) (Eugster et al., 1969
Eugster, O., Tera, F., Wasserburg, G.J. (1969) Isotopic analyses of barium in meteorites and in terrestrial samples. Journal of Geophysical Research 74, 3897–3908.
). Due to the high incompatibility of Ba during mantle melting (DSOLID-MELT = 0.00012; Workman and Hart, 2005Workman, R.K., Hart, S.R. (2005) Major and trace element composition of the depleted MORB mantle (DMM). Earth and Planetary Science Letters 231, 53–72.
), it is strongly enriched in the continental crust relative to the mantle. The average concentration of Ba of the upper continental crust (628 ppm; Rudnick and Gao, 2003Rudnick, R., Gao, S. (2003) Composition of the continental crust. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry, Volume 3: The Crust, Pergamon, Oxford, 1–64.
) is remarkably higher than that of the average mantle (∼7 ppm; Sun and McDonough, 1989Sun, S.-S., McDonough, W. (1989) Chemical and isotopic systematics of oceanic basalts: implications for mantle composition and processes. Geological Society, London, Special Publications 42, 313–345.
). Recently, significant Ba isotopic variations (δ138/134Ba = −0.62 ‰ to +0.15 ‰; Nan et al., 2018Nan, X.-Y., Yu, H.-M., Rudnick, R.L., Gaschnig, R.M., Xu, J., Li, W.-Y., Zhang, Q., Jin, Z.-D., Li, X.-H., Huang, F. (2018) Barium isotopic composition of the upper continental crust. Geochimica et Cosmochimica Acta 233, 33–49.
) have been observed in granites (Fig. S-1), which may result from assimilation of crustal materials, fractional crystallisation, and/or fluid exsolution. Barium is also strongly enriched in seafloor hydrothermal fluids (1.6 to 100 μmol/kg) compared to seawater (0.14 μmol/kg; Tivey, 2007Tivey, M.K. (2007) Generation of seafloor hydrothermal vent fluids and associated mineral deposits. Oceanography 20, 50–65.
). The input of Ba from seafloor hydrothermal activities has been suggested to affect the Ba isotopic composition of the deep ocean (Hsieh and Henderson, 2017Hsieh, Y.-T., Henderson, G.M. (2017) Barium stable isotopes in the global ocean: Tracer of Ba inputs and utilization. Earth and Planetary Science Letters 473, 269–278.
). Therefore, Ba isotopes may provide a potential tool for constraining differentiation of granitic magma and the oceanic budget of Ba.Fluid exsolution from melts is common in igneous processes and plays an important role in the formation of magmatic-hydrothermal ore deposits (e.g., Hedenquist and Lowenstern, 1994
Hedenquist, J.W., Lowenstern, J.B. (1994) The role of magmas in the formation of hydrothermal ore deposits. Nature 370, 519.
). Significant stable isotope fractionation of Zn, B and Cu isotopes during fluid exsolution has been demonstrated in previous studies, with corresponding geological applications (e.g., Telus et al., 2012Telus, M., Dauphas, N., Moynier, F., Tissot, F.L.H., Teng, F.-Z., Nabelek, P.I., Craddock, P.R., Groat, L.A. (2012) Iron, zinc, magnesium and uranium isotopic fractionation during continental crust differentiation: The tale from migmatites, granitoids, and pegmatites. Geochimica et Cosmochimica Acta 97, 247–265.
; Maner and London, 2018Maner, J.L., London, D. (2018) Fractionation of the isotopes of boron between granitic melt and aqueous solution at 700 °C and 800 °C (200 MPa). Chemical Geology 489, 16–27.
; Guo et al., 2020Guo, H., Xia, Y., Bai, R., Zhang, X., Huang, F. (2020) Experiments on Cu isotope fractionation between chlorine-bearing fluid and silicate magma: implications for fluid exsolution and porphyry Cu deposits. National Science Review 7, 1319–1330.
). Barium isotopes may also be useful in tracing fluid activities, and the knowledge about the fractionation behaviour of Ba isotopes during magmatic-hydrothermal processes is a necessary prerequisite. However, there is still no study on the key parameter of the equilibrium Ba isotope fractionation factor between aqueous fluids and silicate melts (α138/134BaFLUID-MELT).Here, for the first time, we experimentally measured α138/134BaFLUID-MELT between aqueous fluids and silicate melts with different chemical compositions at 700–900 °C and 200 MPa. The results show that, in equilibrium, the fluids are isotopically lighter than the melts. This finding promises important applications of Ba isotopes in tracing hydrothermal fluid activities during magma differentiation, magmatic-hydrothermal mineralisation, and seafloor hydrothermal activity.
top
Methods
Phase equilibrium experiments within cold seal pressure vessels have been widely used to determine the partitioning coefficients of elements (e.g., Borchert et al., 2010
Borchert, M., Wilke, M., Schmidt, C., Cauzid, J., Tucoulou, R. (2010) Partitioning of Ba, La, Yb and Y between haplogranitic melts and aqueous solutions: An experimental study. Chemical Geology 276, 225–240.
). Recently, this method has been successfully applied to obtain the equilibrium Cu isotope fractionation factors between aqueous fluids and silicate melts (Guo et al., 2020Guo, H., Xia, Y., Bai, R., Zhang, X., Huang, F. (2020) Experiments on Cu isotope fractionation between chlorine-bearing fluid and silicate magma: implications for fluid exsolution and porphyry Cu deposits. National Science Review 7, 1319–1330.
). In this study, similar experiments were performed at 700–900 °C and 200 MPa in rapid quenched cold seal pressure vessels (the capsule design is shown in Fig. S-2a). Besides temperature, chemical compositions of the fluid and melt will also affect the equilibrium Ba isotope fractionation factors, so a series of Ba-doped haplogranitic glasses from peralkaline to peraluminous (with ASI ranging from 0.8 to 1.2), and 0.5–1.5 mol/L NaCl + KCl (Na∶K = 1∶1 in moles) bearing fluids were used as starting materials. Approximately 1∶1 fluid and glass in mass were loaded into gold capsules, and were subsequently equilibrated in the cold seal pressure vessels for 10–40 days. After rapid quenching the experiments, the fluid and glass (quenched melt) phases were carefully and completely separated, and then transferred into solutions respectively. No other mineral phases were observed in the quenched glasses (Fig. S-2b). Afterwards, the Ba concentrations and Ba isotopic compositions of the solutions prepared from the run products were measured by ICP-MS and MC-ICP-MS, respectively. Additional details about the experimental and analytical methods are given in the Supplementary Information.top
Results and Discussion
The experimental conditions and results are listed in Table 1. The attainment of equilibrium Ba partitioning and isotope fractionation between fluids and melts was evidenced by the time series experiments. Experiments with the duration of 10, 20 and 40 days show similar results for both DFLUID-MELT and Δ138/134BaFLUID-MELT (Fig. S-3). In addition, our experimental durations are longer than or comparable to those of previous studies that have achieved equilibrium partitioning of Ba between fluids and melts (e.g., 5–12 days at 750–950 °C and 200 MPa; Borchert et al., 2010
Borchert, M., Wilke, M., Schmidt, C., Cauzid, J., Tucoulou, R. (2010) Partitioning of Ba, La, Yb and Y between haplogranitic melts and aqueous solutions: An experimental study. Chemical Geology 276, 225–240.
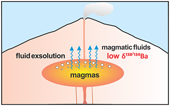
). Therefore, the experimental results summarised in Table 1 represent the equilibrium values.Table 1 Summary of experimental conditions and results.
| Run No. | T (°C) | P (kbar) | Duration (days) | Starting melt ASI | Starting fluid | Melt product | Fluid product | DBaa | Δ138/134BaFLUID-MELTb | 2 s.d.b | ||||
| Ba (ppm) | δ138/134Ba (‰) | 2 s.d. | Ba (ppm) | δ138/134Ba (‰) | 2 s.d. | |||||||||
| Ba05 | 800 | 2 | 10 | 1.0 | 1 m (Na,K) Cl | 1289 | 0.03 | 0.05 | 115 | −0.18 | 0.05 | 0.09 | −0.21 | 0.07 |
| Ba15 | 800 | 2 | 20 | 1.0 | 1 m (Na,K) Cl | 1140 | 0.04 | 0.05 | 112 | −0.20 | 0.04 | 0.10 | −0.24 | 0.06 |
| Ba14 | 800 | 2 | 40 | 1.0 | 1 m (Na,K) Cl | 1145 | 0.07 | 0.03 | 89 | −0.18 | 0.01 | 0.08 | −0.25 | 0.03 |
| Ba06 | 900 | 2 | 10 | 1.0 | 1 m (Na,K) Cl | 1186 | 0.08 | 0.05 | 121 | −0.12 | 0.05 | 0.10 | −0.20 | 0.07 |
| Ba07 | 700 | 2 | 20 | 1.0 | 1 m (Na,K) Cl | 1227 | 0.10 | 0.05 | 69 | −0.52 | 0.05 | 0.06 | −0.62 | 0.07 |
| Ba09 | 800 | 2 | 10 | 1.0 | 0.5 m (Na,K) Cl | 1427 | 0.07 | 0.05 | 57 | −0.24 | 0.05 | 0.04 | −0.31 | 0.07 |
| Ba10 | 800 | 2 | 10 | 1.0 | 1.5 m (Na,K) Cl | 1291 | 0.05 | 0.05 | 253 | −0.09 | 0.05 | 0.20 | −0.14 | 0.07 |
| Ba11 | 800 | 2 | 10 | 0.8 | 1 m (Na,K) Cl | 1253 | 0.06 | 0.05 | 23 | −0.38 | 0.05 | 0.02 | −0.44 | 0.07 |
| Ba12 | 800 | 2 | 10 | 1.2 | 1 m (Na,K) Cl | 702 | 0.07 | 0.05 | 118 | −0.09 | 0.05 | 0.17 | −0.16 | 0.07 |
aDBa is the partitioning coefficient between fluid and melt, which is calculated from the concentration of Ba in the fluid product and that in the melt product.
bΔ138/134BaFLUID-MELT = δ138/134BaFluid − δ138/134BaMelt, and the uncertainties (2 s.d.) are calculated by error propagation.
Figure 1 shows that DFLUID-MELT ranges from 0.02 to 0.20, while Δ138/134BaFLUID-MELT ranges from −0.62 ‰ to −0.14 ‰, both of which change with the temperature and display good positive correlations with salinity of the fluid and ASI of the melt. Specifically, at the fixed melt ASI = 1.0 and fluid salinity of 1 mol/L (Na, K)Cl, DFLUID-MELT increases from 0.06 to 0.10, and Δ138/134BaFLUID-MELT increases from −0.62 ± 0.07 ‰ to −0.20 ± 0.07 ‰ with temperature increasing from 700 to 900 °C (Table 1, Fig. 1a,b). Both fluid salinity and melt ASI are important factors that affect DFLUID-MELT and Δ138/134BaFLUID-MELT. For example, at 800 °C and the melt ASI of 1.0, increasing the (Na, K)Cl concentration from 0.5 to 1.5 mol/L in the starting fluids enhances DFLUID-MELT from 0.04 to 0.20, and Δ138/134BaFLUID-MELT from −0.31 ± 0.07 ‰ to −0.14 ± 0.07 ‰, respectively (Table 1, Fig. 1c,d). Similarly, at 800 °C and the fluid with 1 mol/L (Na, K)Cl, increasing the ASI from 0.8 to 1.2 in the starting haplogranitic glasses enhances DFLUID-MELT from 0.02 to 0.17, and Δ138/134BaFLUID-MELT from −0.44 ± 0.07 ‰ to −0.16 ± 0.07 ‰, respectively (Table 1, Fig. 1e,f).

Figure 1 Partitioning coefficient (DFLUID-MELT) and equilibrium isotope fractionation (Δ138/134BaFLUID-MELT) of Ba between aqueous fluid and silicate melt as a function of temperature from 700 to 900 °C (a,b), salinity of the fluid (c,d), and ASI of the melt (e,f). Error bars in b, d, and f represent 2 s.d. uncertainties (see Table 1).
The correlations of DFLUID-MELT and Δ138/134BaFLUID-MELT with temperature, fluid salinity and melt ASI can be expressed by numerical regression equations. Since both DFLUID-MELT and Δ138/134BaFLUID-MELT have a linear function with fluid salinity and melt ASI (Fig. 1c–f), as well as DFLUID-MELT is generally expressed as a function of 1/T while Δ138/134BaFLUID-MELT as a function of 1/T and 1/T2, the regression equations can be described as
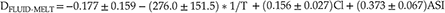
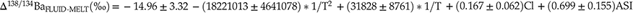
where T is temperature in Kelvin and Cl is the (Na, K)Cl molarity concentration of fluid. The DFLUID-MELT and Δ138/134BaFLUID-MELT values predicted by the Eqs. 1 and 2 are consistent with the experimentally measured data (Fig. S-4), indicating that these two equations can provide reliable estimations.
Our work demonstrates for the first time that, in equilibrium conditions, aqueous fluids are enriched in light Ba isotopes compared to the coexisting silicate melts. This observation provides direct constraints on the behaviour of Ba isotopes during fluid exsolution from silicate melts. Based on the DFLUID-MELT and Δ138/134BaFLUID-MELT obtained here (Eqs. 1 and 2), we use the Rayleigh fractionation model to simulate the Ba isotopic compositions of the exsolved aqueous fluid and residual silicate melt in natural conditions (modelling details are available in the Supplementary Information).
Figure 2 shows that the modelled δ138/134Ba of the residual melt and exsolved fluid are related to the fluid fraction, temperature, ASI of the melt, and salinity of the fluid. The most obvious result is that δ138/134Baresidual melt is similar to that of the initial melt (set as 0 ‰ in the model), regardless of the fraction of fluid exsolution, temperature, salinity of the fluid, and ASI of the melt. The reason for the nearly unchanged Ba isotopic composition of the residual melt after fluid segregation is that Ba is incompatible in aqueous fluids relative to silicate melts (Borchert et al., 2010
Borchert, M., Wilke, M., Schmidt, C., Cauzid, J., Tucoulou, R. (2010) Partitioning of Ba, La, Yb and Y between haplogranitic melts and aqueous solutions: An experimental study. Chemical Geology 276, 225–240.
; and this study), leading to limited fraction of Ba transferred from melts to fluids. For example, based on Eq. S-1 of the Supplementary Information, the fluid phase only removes less than 2 % of the total Ba from the hydrous melt even if the fluid exsolution fraction is as high as 0.1. On the contrary, the exsolved fluids display significantly lower δ138/134Ba, down to −0.63 ‰, than the residual melts (Fig. 2). The δ138/134Baexsolved fluid depends strongly on temperature (Fig. 2a), and also on salinity of the fluid and ASI of the melt (Fig. 2b,c), i.e. δ138/134Baexsolved fluid increases with increasing temperature, the (Na, K)Cl concentration and ASI.
Figure 2 The modelled δ138/134Ba values of residue melt and exsolved fluid as a function of fluid fraction, using the Rayleigh fractionation model. The Ba isotopic composition of the initial fluid-saturated melt is set as 0 ‰, which is the average δ138/134Ba of the upper continental crust (Nan et al., 2018
Nan, X.-Y., Yu, H.-M., Rudnick, R.L., Gaschnig, R.M., Xu, J., Li, W.-Y., Zhang, Q., Jin, Z.-D., Li, X.-H., Huang, F. (2018) Barium isotopic composition of the upper continental crust. Geochimica et Cosmochimica Acta 233, 33–49.
). Three factors affecting the Ba isotope fractionation between the melt and fluid are considered: (a) temperature, (b) salinity of the fluid, and (c) ASI of the melt. Based on Eq. S-1 in the Supplementary Information, the fluid phase only removes less than 2 % of the total Ba from the hydrous magma system, even if the fluid exsolution fraction is as high as 0.1. See text for the details.top
Implications
Aqueous fluids could be derived from cooling of the hydrous crustal melts, when they rise up to the depth where the temperature decreases to the solidus. Our experimental results and models demonstrate that fluid exsolution from granitic melts cannot significantly affect the Ba isotopic composition of the residual melt (Fig. 2). Thus, our work supports the suggestion of Nan et al. (2018)
Nan, X.-Y., Yu, H.-M., Rudnick, R.L., Gaschnig, R.M., Xu, J., Li, W.-Y., Zhang, Q., Jin, Z.-D., Li, X.-H., Huang, F. (2018) Barium isotopic composition of the upper continental crust. Geochimica et Cosmochimica Acta 233, 33–49.
that the light Ba isotopic compositions observed in some granites (Fig. S-1) probably result from fractional crystallisation of Ba-bearing minerals with heavy Ba isotopes rather than from fluid exsolution. More data from natural samples and experimental determination of the equilibrium Ba isotope fractionation factors between magmatic minerals and silicate melts are needed for further interpretation. In any event, the behaviour of Ba isotopes during fluid exsolution revealed in this study is essential for understanding the Ba isotopic signatures of granites.The light Ba isotopic compositions of the aqueous fluids derived from magmas also have implications for tracing the process of extracting metal elements from magmas into fluids, which is an initial process (generally occurring at T > 600 °C) related to many kinds of magmatic-hydrothermal ore deposits. The hydrogen and oxygen isotopic data have been applied to discriminate the origin of hydrothermal fluids (e.g., Sheppard, 1986
Sheppard, S.M.F. (1986) Characterization and isotopic variations in natural waters. Reviews in Mineralogy and Geochemistry 16, 165–183.
), as the magmatic H2O (δD = −80 ∼ −40 ‰, δ18O =∼5–10 ‰) has distinct H–O isotopic compositions from the Global Meteoric Water Line (GMWL, δD = 8δ18O + 10; Craig, 1961Craig, H. (1961) Isotopic variations in meteoric waters. Science 133, 1702–1703.
). However, since oxygen is a major component in both fluids and rocks, hydrothermal alteration of rocks may cause meteoric waters to have similar δ18O values to those of magmatic fluids. By contrast, Ba isotopes may serve as an effective tracer of magmatic fluids because hydrothermal fluids that exsolved from melts will be enriched in light Ba isotopes (Fig. 2), whereas meteoric waters with dissolved Ba are enriched in heavy Ba isotopes (e.g., δ138/134Ba ranging from +0.17 ‰ to +0.46 ‰; Gou et al., 2020Gou, L.-F., Jin, Z., Galy, A., Gong, Y.-Z., Nan, X.-Y., Jin, C., Wang, X.-D., Bouchez, J., Cai, H.-M., Chen, J.-B., Yu, H.-M., Huang, F. (2020) Seasonal riverine barium isotopic variation in the middle Yellow River: Sources and fractionation. Earth and Planetary Science Letters 531, 115990.
). For example, alkaline intrusions particularly have the capacity to form rare earth element and other metal deposits (e.g., Verplanck et al., 2014Verplanck, P.L., Van Gosen, B.S., Seal, R.R., McCafferty, A.E. (2014) A deposit model for carbonatite and peralkaline intrusion-related rare earth element deposits. U.S. Geological Survey Scientific Investigations Report, 2010-5070-J.
). According to Fig. 2c, the exsolved fluids from the alkaline intrusion systems (e.g., ASI = 0.8) are expected to display significantly low δ138/134Ba (Fig. 3). In addition, pegmatites reacted with such exsolved fluids and volatiles with the potential for rare earth element mineralisation may also be characterised by light Ba isotopic compositions (Fig. 3). Therefore, Ba isotopes may be a new monitor for hydrothermal activities associated with metal mineralisation at shallow crustal levels. Such hypotheses can be tested by further measurements of the Ba isotopic compositions of pegmatite and hydrothermal deposit samples.
Figure 3 A schematic diagram illustrating the global δ138/134Ba isotopic characteristics of the upper continental crust, marine sediments, seawater and rivers as well as the relevant hydrothermal fluids and vents. The magmatic fluids, hydrothermal fluids and vents are expected to have low δ138/134Ba according to the negative Δ138/134BaFLUID-MELT values (from −0.62 ‰ to −0.14 ‰) obtained in this study. Data sources: Horner et al. (2015)
Horner, T.J., Kinsley, C.W., Nielsen, S.G. (2015) Barium-isotopic fractionation in seawater mediated by barite cycling and oceanic circulation. Earth and Planetary Science Letters 430, 511–522.
; Cao et al. (2016)Cao, Z., Siebert, C., Hathorne, E.C., Dai, M., Frank, M. (2016) Constraining the oceanic barium cycle with stable barium isotopes. Earth and Planetary Science Letters 434, 1–9.
; Bates et al. (2017)Bates, S.L., Hendry, K.R., Pryer, H.V., Kinsley, C.W., Pyle, K.M., Woodward, E.M.S., Horner, T.J. (2017) Barium isotopes reveal role of ocean circulation on barium cycling in the Atlantic. Geochimica et Cosmochimica Acta 204, 286–299.
; Hsieh and Henderson (2017)Hsieh, Y.-T., Henderson, G.M. (2017) Barium stable isotopes in the global ocean: Tracer of Ba inputs and utilization. Earth and Planetary Science Letters 473, 269–278.
; Bridgestock et al. (2018Bridgestock, L., Hsieh, Y.-T., Porcelli, D., Homoky, W.B., Bryan, A., Henderson, G.M. (2018) Controls on the barium isotope compositions of marine sediments. Earth and Planetary Science Letters 481, 101–110.
, 2019Bridgestock, L., Hsieh, Y.-T., Porcelli, D., Henderson, G.M. (2019) Increased export production during recovery from the Paleocene–Eocene thermal maximum constrained by sedimentary Ba isotopes. Earth and Planetary Science Letters 510, 53–63.
); Nan et al. (2018)Nan, X.-Y., Yu, H.-M., Rudnick, R.L., Gaschnig, R.M., Xu, J., Li, W.-Y., Zhang, Q., Jin, Z.-D., Li, X.-H., Huang, F. (2018) Barium isotopic composition of the upper continental crust. Geochimica et Cosmochimica Acta 233, 33–49.
; Nielsen et al. (2018)Nielsen, S.G., Horner, T.J., Pryer, H.V., Blusztajn, J., Shu, Y., Kurz, M.D., Le Roux, V. (2018) Barium isotope evidence for pervasive sediment recycling in the upper mantle. Science Advances 4, eaas8675.
; Crockford et al. (2019)Crockford, P.W., Wing, B.A., Paytan A., Hodgskiss, M.S.W., Mayfield, K.K., Hayles, J.A., Middleton, J.E., Ahm, A.-S.C., Johnston, D.T., Caxito, F., Uhlein, G., Halverson, G.P., Eickmann, B., Torres, M., Horner, T.J. (2019) Barium-isotopic constraints on the origin of post-Marinoan barites. Earth and Planetary Science Letters 519, 234–244.
; Gou et al. (2020)Gou, L.-F., Jin, Z., Galy, A., Gong, Y.-Z., Nan, X.-Y., Jin, C., Wang, X.-D., Bouchez, J., Cai, H.-M., Chen, J.-B., Yu, H.-M., Huang, F. (2020) Seasonal riverine barium isotopic variation in the middle Yellow River: Sources and fractionation. Earth and Planetary Science Letters 531, 115990.
.Supposing that basaltic melt has a similar structure to granitic melt, the exsolved fluids from seafloor volcanoes are also expected to have low δ138/134Ba (<0 ‰), which will contribute to the hydrosphere (Fig. 3). The vertical ocean profiles have heterogeneous Ba isotopic compositions with the deep ocean being enriched in light Ba isotopes (∼ +0.2 ‰) relative to the surface ocean (∼ +0.6 ‰; Fig. 3). The light Ba isotopic compositions of the deep ocean have been suggested to result from dissolution of isotopically light barite during its sinking (e.g., Horner et al., 2015
Horner, T.J., Kinsley, C.W., Nielsen, S.G. (2015) Barium-isotopic fractionation in seawater mediated by barite cycling and oceanic circulation. Earth and Planetary Science Letters 430, 511–522.
; Bates et al., 2017Bates, S.L., Hendry, K.R., Pryer, H.V., Kinsley, C.W., Pyle, K.M., Woodward, E.M.S., Horner, T.J. (2017) Barium isotopes reveal role of ocean circulation on barium cycling in the Atlantic. Geochimica et Cosmochimica Acta 204, 286–299.
; Hsieh and Henderson, 2017Hsieh, Y.-T., Henderson, G.M. (2017) Barium stable isotopes in the global ocean: Tracer of Ba inputs and utilization. Earth and Planetary Science Letters 473, 269–278.
; Bridgestock et al., 2018Bridgestock, L., Hsieh, Y.-T., Porcelli, D., Homoky, W.B., Bryan, A., Henderson, G.M. (2018) Controls on the barium isotope compositions of marine sediments. Earth and Planetary Science Letters 481, 101–110.
). Our results imply that, besides the effect of barite, fluid derived from the seafloor hydrothermal system may be another important source of isotopically light Ba to the deep ocean (Fig. 3), which should be taken into account in future studies on the global oceanic budget of Ba isotopes (the relevant Ba fluxes and isotopic compositions are shown in Fig. S-5). Collectively, our experimental results and models suggest that Ba isotopes could be a novel tracer for hydrothermal fluids in the shallow crust and at the seafloor, on the premise that more Ba isotopic data of granites, felsic intrusion-related hydrothermal deposits and seafloor hydrothermal vents will be accumulated.top
Acknowledgements
We are grateful to Hui-Min Yu, Ying-Zeng Gong and Lan-Lan Tian for discussions. Heye Freymuth and an anonymous reviewer, as well as Editor Helen Williams for reviewing and improving the manuscript are thanked. This work is financially supported by the National Natural Science Foundation of China (42073004, 41803003), and the Open Research Fund of the State Key Laboratory of Ore Deposit Geochemistry of China (201805).
Editor: Helen Williams
top
References
Bates, S.L., Hendry, K.R., Pryer, H.V., Kinsley, C.W., Pyle, K.M., Woodward, E.M.S., Horner, T.J. (2017) Barium isotopes reveal role of ocean circulation on barium cycling in the Atlantic. Geochimica et Cosmochimica Acta 204, 286–299.
 Show in context
Show in context Data sources: Horner et al. (2015); Cao et al. (2016); Bates et al. (2017); Hsieh and Henderson (2017); Bridgestock et al. (2018, 2019); Nan et al. (2018); Nielsen et al. (2018); Crockford et al. (2019); Gou et al. (2020).
View in article
The light Ba isotopic compositions of the deep ocean have been suggested to result from dissolution of isotopically light barite during its sinking (e.g., Horner et al., 2015; Bates et al., 2017; Hsieh and Henderson, 2017; Bridgestock et al., 2018).
View in article
Borchert, M., Wilke, M., Schmidt, C., Cauzid, J., Tucoulou, R. (2010) Partitioning of Ba, La, Yb and Y between haplogranitic melts and aqueous solutions: An experimental study. Chemical Geology 276, 225–240.
 Show in context
Show in context Phase equilibrium experiments within cold seal pressure vessels have been widely used to determine the partitioning coefficients of elements (e.g., Borchert et al., 2010).
View in article
In addition, our experimental durations are longer than or comparable to those of previous studies that have achieved equilibrium partitioning of Ba between fluids and melts (e.g., 5–12 days at 750–950 °C and 200 MPa; Borchert et al., 2010).
View in article
The reason for the nearly unchanged Ba isotopic composition of the residual melt after fluid segregation is that Ba is incompatible in aqueous fluids relative to silicate melts (Borchert et al., 2010; and this study), leading to limited fraction of Ba transferred from melts to fluids
View in article
Bridgestock, L., Hsieh, Y.-T., Porcelli, D., Homoky, W.B., Bryan, A., Henderson, G.M. (2018) Controls on the barium isotope compositions of marine sediments. Earth and Planetary Science Letters 481, 101–110.
 Show in context
Show in context Data sources: Horner et al. (2015); Cao et al. (2016); Bates et al. (2017); Hsieh and Henderson (2017); Bridgestock et al. (2018, 2019); Nan et al. (2018); Nielsen et al. (2018); Crockford et al. (2019); Gou et al. (2020).
View in article
The light Ba isotopic compositions of the deep ocean have been suggested to result from dissolution of isotopically light barite during its sinking (e.g., Horner et al., 2015; Bates et al., 2017; Hsieh and Henderson, 2017; Bridgestock et al., 2018).
View in article
Bridgestock, L., Hsieh, Y.-T., Porcelli, D., Henderson, G.M. (2019) Increased export production during recovery from the Paleocene–Eocene thermal maximum constrained by sedimentary Ba isotopes. Earth and Planetary Science Letters 510, 53–63.
 Show in context
Show in context Data sources: Horner et al. (2015); Cao et al. (2016); Bates et al. (2017); Hsieh and Henderson (2017); Bridgestock et al. (2018, 2019); Nan et al. (2018); Nielsen et al. (2018); Crockford et al. (2019); Gou et al. (2020).
View in article
Cao, Z., Siebert, C., Hathorne, E.C., Dai, M., Frank, M. (2016) Constraining the oceanic barium cycle with stable barium isotopes. Earth and Planetary Science Letters 434, 1–9.
 Show in context
Show in context Data sources: Horner et al. (2015); Cao et al. (2016); Bates et al. (2017); Hsieh and Henderson (2017); Bridgestock et al. (2018, 2019); Nan et al. (2018); Nielsen et al. (2018); Crockford et al. (2019); Gou et al. (2020).
View in article
Craig, H. (1961) Isotopic variations in meteoric waters. Science 133, 1702–1703.
 Show in context
Show in context The hydrogen and oxygen isotopic data have been applied to discriminate the origin of hydrothermal fluids (e.g., Sheppard, 1986), as the magmatic H2O (δD = −80 ∼ −40 ‰, δ18O =∼5–10 ‰) has distinct H–O isotopic compositions from the Global Meteoric Water Line (GMWL, δD = 8δ18O + 10; Craig, 1961).
View in article
Crockford, P.W., Wing, B.A., Paytan A., Hodgskiss, M.S.W., Mayfield, K.K., Hayles, J.A., Middleton, J.E., Ahm, A.-S.C., Johnston, D.T., Caxito, F., Uhlein, G., Halverson, G.P., Eickmann, B., Torres, M., Horner, T.J. (2019) Barium-isotopic constraints on the origin of post-Marinoan barites. Earth and Planetary Science Letters 519, 234–244.
 Show in context
Show in context Data sources: Horner et al. (2015); Cao et al. (2016); Bates et al. (2017); Hsieh and Henderson (2017); Bridgestock et al. (2018, 2019); Nan et al. (2018); Nielsen et al. (2018); Crockford et al. (2019); Gou et al. (2020).
View in article
Eugster, O., Tera, F., Wasserburg, G.J. (1969) Isotopic analyses of barium in meteorites and in terrestrial samples. Journal of Geophysical Research 74, 3897–3908.
 Show in context
Show in context It has seven stable isotopes, i.e. 130Ba (0.11 %), 132Ba (0.10 %), 134Ba (2.42 %), 135Ba (6.59 %), 136Ba (7.85 %), 137Ba (11.23 %) and 138Ba (71.70 %) (Eugster et al., 1969).
View in article
Gou, L.-F., Jin, Z., Galy, A., Gong, Y.-Z., Nan, X.-Y., Jin, C., Wang, X.-D., Bouchez, J., Cai, H.-M., Chen, J.-B., Yu, H.-M., Huang, F. (2020) Seasonal riverine barium isotopic variation in the middle Yellow River: Sources and fractionation. Earth and Planetary Science Letters 531, 115990.
 Show in context
Show in context By contrast, Ba isotopes may serve as an effective tracer of magmatic fluids because hydrothermal fluids that exsolved from melts will be enriched in light Ba isotopes (Fig. 2), whereas meteoric waters with dissolved Ba are enriched in heavy Ba isotopes (e.g., δ138/134Ba ranging from +0.17 ‰ to +0.46 ‰; Gou et al., 2020).
View in article
Data sources: Horner et al. (2015); Cao et al. (2016); Bates et al. (2017); Hsieh and Henderson (2017); Bridgestock et al. (2018, 2019); Nan et al. (2018); Nielsen et al. (2018); Crockford et al. (2019); Gou et al. (2020).
View in article
Guo, H., Xia, Y., Bai, R., Zhang, X., Huang, F. (2020) Experiments on Cu isotope fractionation between chlorine-bearing fluid and silicate magma: implications for fluid exsolution and porphyry Cu deposits. National Science Review 7, 1319–1330.
 Show in context
Show in context Significant stable isotope fractionation of Zn, B and Cu isotopes during fluid exsolution has been demonstrated in previous studies, with corresponding geological applications (e.g., Telus et al., 2012; Maner and London, 2018; Guo et al., 2020).
View in article
Recently, this method has been successfully applied to obtain the equilibrium Cu isotope fractionation factors between aqueous fluids and silicate melts (Guo et al., 2020).
View in article
Hedenquist, J.W., Lowenstern, J.B. (1994) The role of magmas in the formation of hydrothermal ore deposits. Nature 370, 519.
 Show in context
Show in context Fluid exsolution from melts is common in igneous processes and plays an important role in the formation of magmatic-hydrothermal ore deposits (e.g., Hedenquist and Lowenstern, 1994).
View in article
Horner, T.J., Kinsley, C.W., Nielsen, S.G. (2015) Barium-isotopic fractionation in seawater mediated by barite cycling and oceanic circulation. Earth and Planetary Science Letters 430, 511–522.
 Show in context
Show in context Data sources: Horner et al. (2015); Cao et al. (2016); Bates et al. (2017); Hsieh and Henderson (2017); Bridgestock et al. (2018, 2019); Nan et al. (2018); Nielsen et al. (2018); Crockford et al. (2019); Gou et al. (2020).
View in article
The light Ba isotopic compositions of the deep ocean have been suggested to result from dissolution of isotopically light barite during its sinking (e.g., Horner et al., 2015; Bates et al., 2017; Hsieh and Henderson, 2017; Bridgestock et al., 2018).
View in article
Hsieh, Y.-T., Henderson, G.M. (2017) Barium stable isotopes in the global ocean: Tracer of Ba inputs and utilization. Earth and Planetary Science Letters 473, 269–278.
 Show in context
Show in context The input of Ba from seafloor hydrothermal activities has been suggested to affect the Ba isotopic composition of the deep ocean (Hsieh and Henderson, 2017).
View in article
Data sources: Horner et al. (2015); Cao et al. (2016); Bates et al. (2017); Hsieh and Henderson (2017); Bridgestock et al. (2018, 2019); Nan et al. (2018); Nielsen et al. (2018); Crockford et al. (2019); Gou et al. (2020).
View in article
The light Ba isotopic compositions of the deep ocean have been suggested to result from dissolution of isotopically light barite during its sinking (e.g., Horner et al., 2015; Bates et al., 2017; Hsieh and Henderson, 2017; Bridgestock et al., 2018).
View in article
Maner, J.L., London, D. (2018) Fractionation of the isotopes of boron between granitic melt and aqueous solution at 700 °C and 800 °C (200 MPa). Chemical Geology 489, 16–27.
 Show in context
Show in context Significant stable isotope fractionation of Zn, B and Cu isotopes during fluid exsolution has been demonstrated in previous studies, with corresponding geological applications (e.g., Telus et al., 2012; Maner and London, 2018; Guo et al., 2020).
View in article
Nan, X.-Y., Yu, H.-M., Rudnick, R.L., Gaschnig, R.M., Xu, J., Li, W.-Y., Zhang, Q., Jin, Z.-D., Li, X.-H., Huang, F. (2018) Barium isotopic composition of the upper continental crust. Geochimica et Cosmochimica Acta 233, 33–49.
 Show in context
Show in context Recently, significant Ba isotopic variations (δ138/134Ba = −0.62 ‰ to +0.15 ‰; Nan et al., 2018) have been observed in granites (Fig. S-1), which may result from assimilation of crustal materials, fractional crystallisation, and/or fluid exsolution.
View in article
Thus, our work supports the suggestion of Nan et al. (2018) that the light Ba isotopic compositions observed in some granites (Fig. S-1) probably result from fractional crystallisation of Ba-bearing minerals with heavy Ba isotopes rather than from fluid exsolution
View in article
The Ba isotopic composition of the initial fluid-saturated melt is set as 0 ‰, which is the average δ138/134Ba of the upper continental crust (Nan et al., 2018).
View in article
Data sources: Horner et al. (2015); Cao et al. (2016); Bates et al. (2017); Hsieh and Henderson (2017); Bridgestock et al. (2018, 2019); Nan et al. (2018); Nielsen et al. (2018); Crockford et al. (2019); Gou et al. (2020).
View in article
Nielsen, S.G., Horner, T.J., Pryer, H.V., Blusztajn, J., Shu, Y., Kurz, M.D., Le Roux, V. (2018) Barium isotope evidence for pervasive sediment recycling in the upper mantle. Science Advances 4, eaas8675.
 Show in context
Show in context Data sources: Horner et al. (2015); Cao et al. (2016); Bates et al. (2017); Hsieh and Henderson (2017); Bridgestock et al. (2018, 2019); Nan et al. (2018); Nielsen et al. (2018); Crockford et al. (2019); Gou et al. (2020).
View in article
Rudnick, R., Gao, S. (2003) Composition of the continental crust. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry, Volume 3: The Crust, Pergamon, Oxford, 1–64.
 Show in context
Show in context The average concentration of Ba of the upper continental crust (628 ppm; Rudnick and Gao, 2003) is remarkably higher than that of the average mantle (∼7 ppm; Sun and McDonough, 1989).
View in article
Sheppard, S.M.F. (1986) Characterization and isotopic variations in natural waters. Reviews in Mineralogy and Geochemistry 16, 165–183.
 Show in context
Show in context The hydrogen and oxygen isotopic data have been applied to discriminate the origin of hydrothermal fluids (e.g., Sheppard, 1986), as the magmatic H2O (δD = −80 ∼ −40 ‰, δ18O =∼5–10 ‰) has distinct H–O isotopic compositions from the Global Meteoric Water Line (GMWL, δD = 8δ18O + 10; Craig, 1961).
View in article
Sun, S.-S., McDonough, W. (1989) Chemical and isotopic systematics of oceanic basalts: implications for mantle composition and processes. Geological Society, London, Special Publications 42, 313–345.
 Show in context
Show in context The average concentration of Ba of the upper continental crust (628 ppm; Rudnick and Gao, 2003) is remarkably higher than that of the average mantle (∼7 ppm; Sun and McDonough, 1989).
View in article
Tivey, M.K. (2007) Generation of seafloor hydrothermal vent fluids and associated mineral deposits. Oceanography 20, 50–65.
 Show in context
Show in context Barium is also strongly enriched in seafloor hydrothermal fluids (1.6 to 100 μmol/kg) compared to seawater (0.14 μmol/kg; Tivey, 2007).
View in article
Telus, M., Dauphas, N., Moynier, F., Tissot, F.L.H., Teng, F.-Z., Nabelek, P.I., Craddock, P.R., Groat, L.A. (2012) Iron, zinc, magnesium and uranium isotopic fractionation during continental crust differentiation: The tale from migmatites, granitoids, and pegmatites. Geochimica et Cosmochimica Acta 97, 247–265.
 Show in context
Show in context Significant stable isotope fractionation of Zn, B and Cu isotopes during fluid exsolution has been demonstrated in previous studies, with corresponding geological applications (e.g., Telus et al., 2012; Maner and London, 2018; Guo et al., 2020).
View in article
Verplanck, P.L., Van Gosen, B.S., Seal, R.R., McCafferty, A.E. (2014) A deposit model for carbonatite and peralkaline intrusion-related rare earth element deposits. U.S. Geological Survey Scientific Investigations Report, 2010-5070-J.
 Show in context
Show in context For example, alkaline intrusions particularly have the capacity to form rare earth element and other metal deposits (e.g., Verplanck et al., 2014).
View in article
Workman, R.K., Hart, S.R. (2005) Major and trace element composition of the depleted MORB mantle (DMM). Earth and Planetary Science Letters 231, 53–72.
 Show in context
Show in context Due to the high incompatibility of Ba during mantle melting (DSOLID-MELT = 0.00012; Workman and Hart, 2005), it is strongly enriched in the continental crust relative to the mantle
View in article
top
Supplementary Information
The Supplementary Information includes:
- Starting Materials and Encapsulation
- High Temperature and Pressure experiments
- Run Product Separation and Dissolution
- Barium Concentration Analyses
- Barium Isotope Analyses
- Rayleigh Fractionation Model
- Figures S-1 to S-5
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures
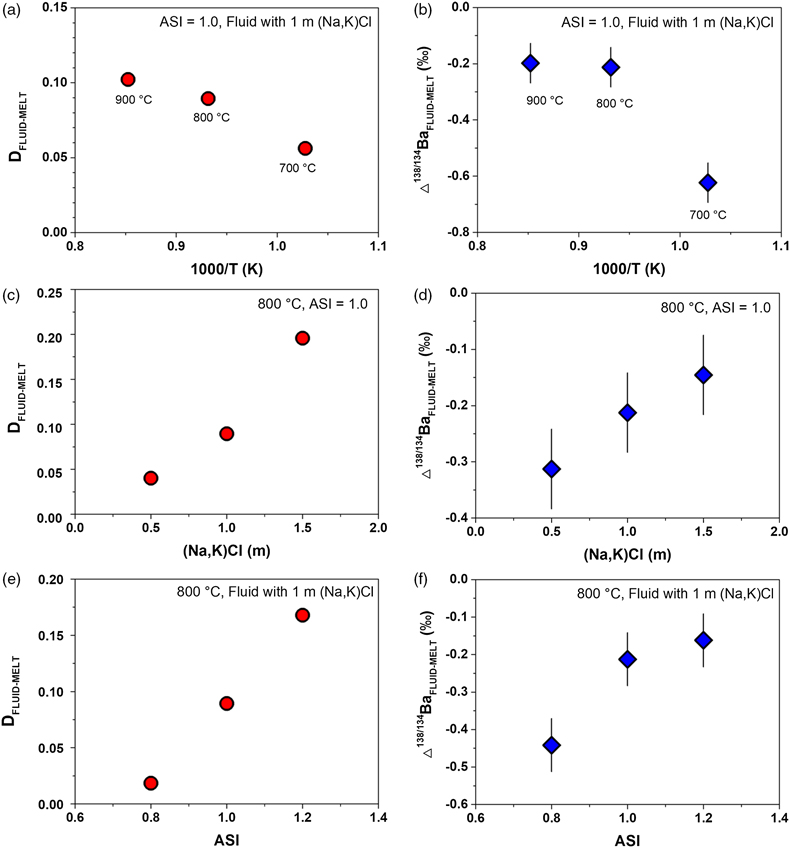
Figure 1 Partitioning coefficient (DFLUID-MELT) and equilibrium isotope fractionation (Δ138/134BaFLUID-MELT) of Ba between aqueous fluid and silicate melt as a function of temperature from 700 to 900 °C (a,b), salinity of the fluid (c,d), and ASI of the melt (e,f). Error bars in b, d, and f represent 2 s.d. uncertainties (see Table 1).
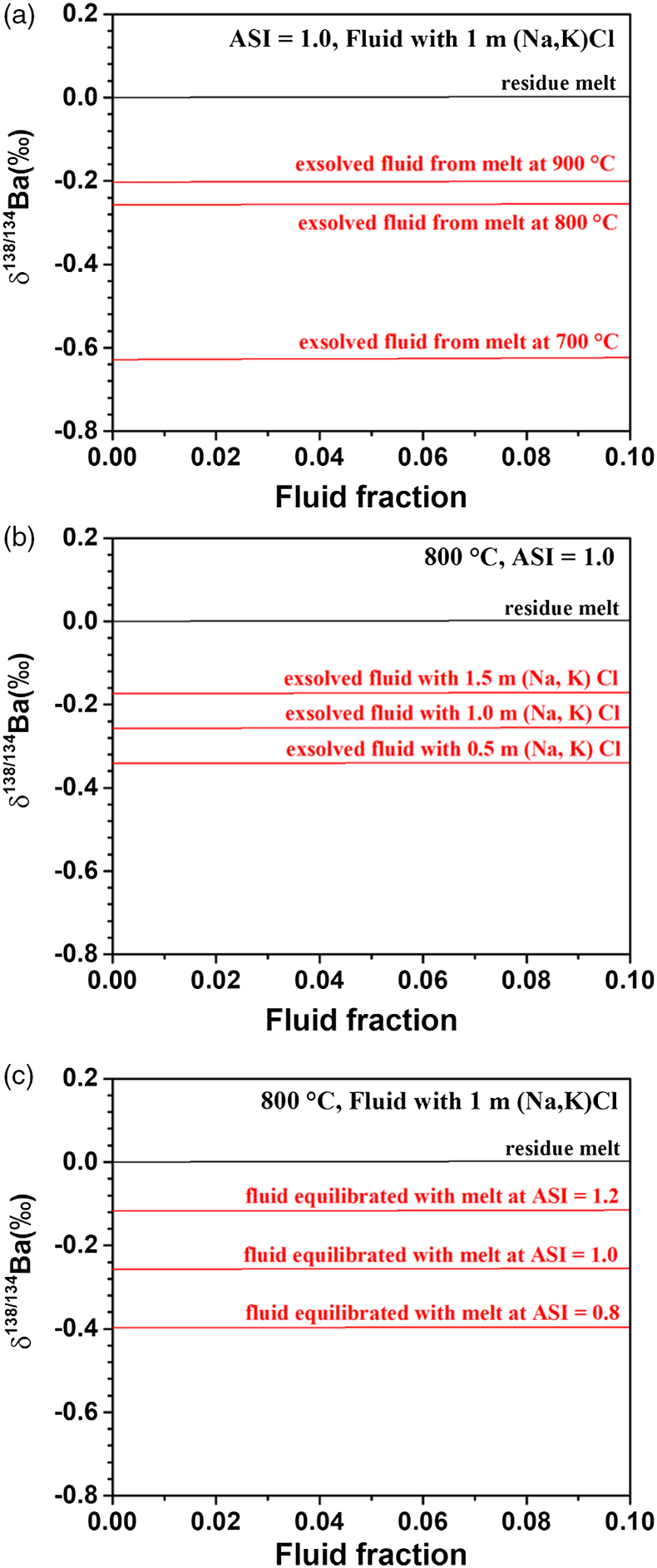
Figure 2 The modelled δ138/134Ba values of residue melt and exsolved fluid as a function of fluid fraction, using the Rayleigh fractionation model. The Ba isotopic composition of the initial fluid-saturated melt is set as 0 ‰, which is the average δ138/134Ba of the upper continental crust (Nan et al., 2018
Nan, X.-Y., Yu, H.-M., Rudnick, R.L., Gaschnig, R.M., Xu, J., Li, W.-Y., Zhang, Q., Jin, Z.-D., Li, X.-H., Huang, F. (2018) Barium isotopic composition of the upper continental crust. Geochimica et Cosmochimica Acta 233, 33–49.
). Three factors affecting the Ba isotope fractionation between the melt and fluid are considered: (a) temperature, (b) salinity of the fluid, and (c) ASI of the melt. Based on Eq. S-1 in the Supplementary Information, the fluid phase only removes less than 2 % of the total Ba from the hydrous magma system, even if the fluid exsolution fraction is as high as 0.1. See text for the details.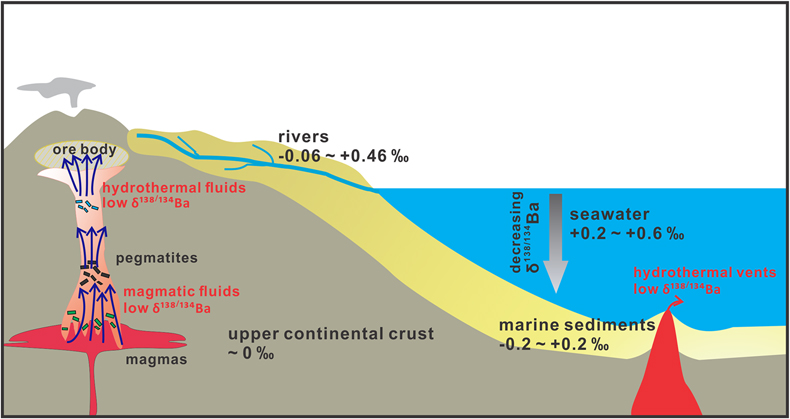
Figure 3 A schematic diagram illustrating the global δ138/134Ba isotopic characteristics of the upper continental crust, marine sediments, seawater and rivers as well as the relevant hydrothermal fluids and vents. The magmatic fluids, hydrothermal fluids and vents are expected to have low δ138/134Ba according to the negative Δ138/134BaFLUID-MELT values (from −0.62 ‰ to −0.14 ‰) obtained in this study. Data sources: Horner et al. (2015)
Horner, T.J., Kinsley, C.W., Nielsen, S.G. (2015) Barium-isotopic fractionation in seawater mediated by barite cycling and oceanic circulation. Earth and Planetary Science Letters 430, 511–522.
; Cao et al. (2016)Cao, Z., Siebert, C., Hathorne, E.C., Dai, M., Frank, M. (2016) Constraining the oceanic barium cycle with stable barium isotopes. Earth and Planetary Science Letters 434, 1–9.
; Bates et al. (2017)Bates, S.L., Hendry, K.R., Pryer, H.V., Kinsley, C.W., Pyle, K.M., Woodward, E.M.S., Horner, T.J. (2017) Barium isotopes reveal role of ocean circulation on barium cycling in the Atlantic. Geochimica et Cosmochimica Acta 204, 286–299.
; Hsieh and Henderson (2017)Hsieh, Y.-T., Henderson, G.M. (2017) Barium stable isotopes in the global ocean: Tracer of Ba inputs and utilization. Earth and Planetary Science Letters 473, 269–278.
; Bridgestock et al. (2018Bridgestock, L., Hsieh, Y.-T., Porcelli, D., Homoky, W.B., Bryan, A., Henderson, G.M. (2018) Controls on the barium isotope compositions of marine sediments. Earth and Planetary Science Letters 481, 101–110.
, 2019Bridgestock, L., Hsieh, Y.-T., Porcelli, D., Henderson, G.M. (2019) Increased export production during recovery from the Paleocene–Eocene thermal maximum constrained by sedimentary Ba isotopes. Earth and Planetary Science Letters 510, 53–63.
); Nan et al. (2018)Nan, X.-Y., Yu, H.-M., Rudnick, R.L., Gaschnig, R.M., Xu, J., Li, W.-Y., Zhang, Q., Jin, Z.-D., Li, X.-H., Huang, F. (2018) Barium isotopic composition of the upper continental crust. Geochimica et Cosmochimica Acta 233, 33–49.
; Nielsen et al. (2018)Nielsen, S.G., Horner, T.J., Pryer, H.V., Blusztajn, J., Shu, Y., Kurz, M.D., Le Roux, V. (2018) Barium isotope evidence for pervasive sediment recycling in the upper mantle. Science Advances 4, eaas8675.
; Crockford et al. (2019)Crockford, P.W., Wing, B.A., Paytan A., Hodgskiss, M.S.W., Mayfield, K.K., Hayles, J.A., Middleton, J.E., Ahm, A.-S.C., Johnston, D.T., Caxito, F., Uhlein, G., Halverson, G.P., Eickmann, B., Torres, M., Horner, T.J. (2019) Barium-isotopic constraints on the origin of post-Marinoan barites. Earth and Planetary Science Letters 519, 234–244.
; Gou et al. (2020)Gou, L.-F., Jin, Z., Galy, A., Gong, Y.-Z., Nan, X.-Y., Jin, C., Wang, X.-D., Bouchez, J., Cai, H.-M., Chen, J.-B., Yu, H.-M., Huang, F. (2020) Seasonal riverine barium isotopic variation in the middle Yellow River: Sources and fractionation. Earth and Planetary Science Letters 531, 115990.
.