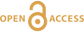Abiotic formation of organic biomorphs under diagenetic conditions
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





-
Article views:143Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract

Figures
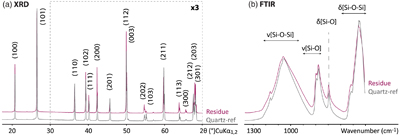 Figure 1 XRD and FTIR results. (a) Powder XRD patterns of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days) and of the α-quartz reference (intensity increased ×3 from 30 to 70° 2θ). (b) ATR-FTIR spectra of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days) and of the α-quartz reference. | 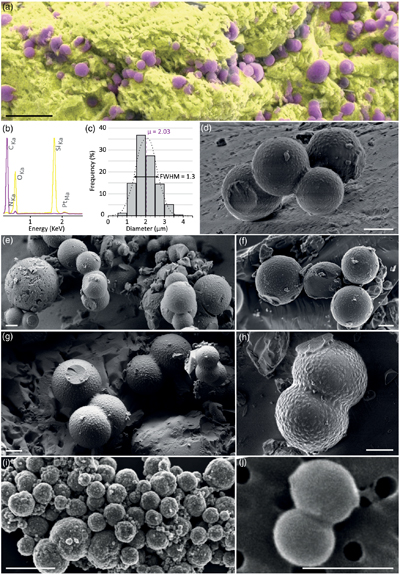 Figure 2 SEM investigations of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days). (a) EDX map of the residue and (b) corresponding EDX spectra. Quartz appears in yellow and the spheroidal organic biomorphs appear in purple. (c) Bar chart showing the size distribution of the spheroidal organic biomorphs produced during the experiments (FWHM: full width at half maximum). (e-h) SEM images (secondary electrons) of the spheroidal organic biomorphs produced during the experiments. (f, g) SEM images (secondary electrons) of Thermococcus prieurii cells (courtesy of Aurore Gorlas). Scale bars: (a) 10 μm, (d-j) 1 μm. |  Figure 3 Solid state 13C NMR and ATR-FTIR results. (a) Solid state 13C CP MAS NMR spectra of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days) and of the RNA reference. Spectra are normalised to the quantity of carbon. (b) ATR-FTIR spectra of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days) and of the RNA reference. Spectra are normalised to the total carbon content. Note that the intensity of the signals were increased ×3 for clarity from ∼3035 to ∼2775 cm−1. | 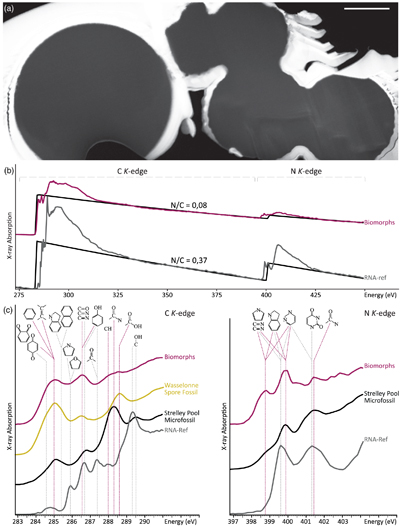 Figure 4 STEM and STXM-XANES results. (a) Transmission electron microscopy image (STEM mode) of the FIB section extracted from spheroidal organic biomorphs. Scale bar: 1μm. (b) X-Ray absorption spectra of the organic biomorphs and of the RNA reference, with their corresponding N/C values. (c) C- and N-XANES spectra of the organic biomorphs and of the RNA reference compared to spectra of organic microfossils from Wasselonne (Bernard et al., 2009) and Strelley Pool (Alleon et al., 2018). All spectra are normalised to C and N quantities. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Letter
We still do not know when, where and how life started to exist on Earth. As the unique source of direct information about past life, the ancient fossil record may provide answers. Yet, although the ancient fossil record may contain fundamentally important ‘biogeochemical’ signals, its quality is far from perfect, making it not that easy to decode (Brasier et al., 2006
Brasier, M., McLoughlin, N., Green, O., Wacey, D. (2006) A fresh look at the fossil evidence for early Archaean cellular life. Philosophical Transactions of the Royal Society B: Biological Sciences 361, 887–902.
; Javaux, 2019Javaux, E.J. (2019) Challenges in evidencing the earliest traces of life. Nature 572, 451–460.
). Archean palaeontology only relies on degraded signals difficult to interpret, as illustrated by the number of controversies having so far hindered the search for the most ancient traces of life on Earth (Schopf, 1975Schopf, J.W. (1975) Precambrian Paleobiology: Problems and Perspectives. Annual Review of Earth and Planetary Sciences 3, 213–249.
; Javaux, 2019Javaux, E.J. (2019) Challenges in evidencing the earliest traces of life. Nature 572, 451–460.
).A main difficulty is the lack of a univocal criterion to rely on when discussing the biogenicity of putative remains of life in ancient rocks: neither the carbon isotopic compositions nor the morphologies should be seen as unambiguous biosignatures (Craig, 1954
Craig, H. (1954) Geochemical implications of the isotopic composition of carbon in ancient rocks. Geochimica et Cosmochimica Acta 6, 186–196.
; Horita, 2005Horita, J. (2005) Some perspectives on isotope biosignatures for early life. Chemical Geology 218, 171–186.
; Cosmidis and Templeton, 2016Cosmidis, J., Templeton, A.S. (2016) Self-Assembly of biomorphic carbon/sulfur microstructures in sulfidic environments. Nature Communications 7, 12812.
; Garcia-Ruiz et al., 2020Garcia-Ruiz, J.M., van Zuilen, M.A., Bach, W. (2020) Mineral self-organization on a lifeless planet. Physics of Life Reviews 34–35, 62–82.
). In fact, mineral biomorphs may easily be produced experimentally via self-assembly processes (Garcia-Ruiz et al., 2003Garcia-Ruiz, J.M., Hyde, S.T., Carnerup, A.M., Christy, A.G., Van Kranendonk, M.J., Welham, N.J. (2003) Self-Assembled Silica-Carbonate Structures and Detection of Ancient Microfossils. Science 302, 1194–1197.
) and may exhibit high levels of complexity (Garcia-Ruiz et al., 2009Garcia-Ruiz, J.M., Melero-Garcia, E., Hyde, S.T. (2009) Morphogenesis of Self-Assembled Nanocrystalline Materials of Barium Carbonate and Silica. Science 323, 362–365.
; Noorduin et al., 2013Noorduin, W.L., Grinthal, A., Mahadevan, L., Aizenberg, J. (2013) Rationally Designed Complex, Hierarchical Microarchitectures. Science 340, 832–837.
; Rouillard et al., 2018Rouillard, J., García-Ruiz, J.-M., Gong, J., van Zuilen, M.A. (2018) A morphogram for silica-witherite biomorphs and its application to microfossil identification in the early earth rock record. Geobiology 16, 279–296.
). Worse still, Cosmidis and Templeton (2016)Cosmidis, J., Templeton, A.S. (2016) Self-Assembly of biomorphic carbon/sulfur microstructures in sulfidic environments. Nature Communications 7, 12812.
recently demonstrated that carbon-sulfur biomorphs could also be produced.Chemical information may help to identify remains of life (Benzerara et al., 2006
Benzerara, K., Menguy, N., Lopez-Garcia, P., Yoon, T.-H., Kazmierczak, J., Tyliszczak, T., Guyot, F., Brown, G.E. (2006) Nanoscale detection of organic signatures in carbonate microbialites. Proceedings of the National Academy of Sciences 103, 9440–9445.
; Bernard et al., 2007Bernard, S., Benzerara, K., Beyssac, O., Menguy, N., Guyot, F., Brownjr, G., Goffe, B. (2007) Exceptional preservation of fossil plant spores in high-pressure metamorphic rocks. Earth and Planetary Science Letters 262, 257–272.
; Alleon et al., 2018Alleon, J., Bernard, S., Le Guillou, C., Beyssac, O., Sugitani, K., Robert, F. (2018) Chemical nature of the 3.4 Ga Strelley Pool microfossils. Geochemical Perspectives Letters 7, 37–42.
; Loron et al., 2019Loron, C.C., François, C., Rainbird, R.H., Turner, E.C., Borensztajn, S., Javaux, E.J. (2019) Early fungi from the Proterozoic era in Arctic Canada. Nature 570, 232–235.
), but abiotic processes may lead to the formation of disordered carbonaceous materials difficult to distinguish from biogenic ones (Pasteris and Wopenka, 2003Pasteris, J.D., Wopenka, B. (2003) Necessary, but Not Sufficient: Raman Identification of Disordered Carbon as a Signature of Ancient Life. Astrobiology 3, 727–738.
; De Gregorio et al., 2011De Gregorio, B.T., Sharp, T.G., Rushdi, A.I., Simoneit, B.R.T. (2011) Bugs or Gunk? Nanoscale Methods for Assessing the Biogenicity of Ancient Microfossils and Organic Matter. In: Golding, S.D., Glikson, M. (Eds.) Earliest Life on Earth: Habitats, Environments and Methods of Detection. Springer Netherlands, Dordrecht, 239–289.
). Collectively, because none of the criteria commonly used to discuss biogenicity are sufficient in themselves, many authors have emphasised the need for gathering multiple lines of evidence to demonstrate convincingly the biological origin of any putative remain of life in ancient cherts (e.g., Westall, 2005Westall, F. (2005) Early Life on Earth: The Ancient Fossil Record. In: Ehrenfreund, P., Irvine, W.M., Owen, T., Becker, L., Blank, J., Brucato, J.R., Colangeli, L., Derenne, S., Dutrey, A., Despois, D., Lazcano, A., Robert, F. (Eds.) Astrobiology: Future Perspectives. Springer Netherlands, Dordrecht, 287–316.
; Wacey, 2009Wacey, D. (Ed.) (2009) Early Life on Earth. Springer Netherlands, Dordrecht.
; Bernard and Papineau, 2014Bernard, S., Papineau, D. (2014) Graphitic Carbons and Biosignatures. Elements 10, 435–440.
; Javaux, 2019Javaux, E.J. (2019) Challenges in evidencing the earliest traces of life. Nature 572, 451–460.
). Yet, as illustrated by the present study, purely abiotic microstructures may fulfill not only a couple but many (if not all) of the commonly used criteria of biogenicity, i.e. morphological, chemical and isotopic criteria, even the most conservative ones (cf. below).Here, we exposed RNA (i.e. the most emblematic organic molecule of the prebiotic RNA World; Higgs and Lehman, 2015
Higgs, P.G., Lehman, N. (2015) The RNA World: molecular cooperation at the origins of life. Nature Reviews Genetics 16, 7–17.
) to thermal conditions typical of diagenesis (200 °C), in pure bi-distilled water under an autogenic pressure of 15 bars, in the presence of quartz (i.e. the main mineral of Archean cherts; Perry and Lefticariu, 2007Perry, E.C., Lefticariu, L. (2007) Formation and Geochemistry of Precambrian Cherts. Treatise on Geochemistry 7, 1–21.
) and under an argon atmosphere for 20 days. We conducted additional experiments under the same conditions with RNA in the absence of quartz and with quartz in the absence of RNA to serve as controls. The water insoluble experimental residues were characterised using X-ray diffraction (XRD), isotopic ratio mass spectrometry (IRMS), solid-state 13C cross polarization magic-angle spinning nuclear magnetic resonance (13C CP MAS NMR) and Fourier transform infrared (FTIR) spectroscopies. Additional characterisation was conducted using advanced microscopy and spectroscopy tools including scanning electron microscopy (SEM) coupled with energy dispersive X-ray spectroscopy (EDXS), scanning transmission electron microscopy (STEM) and scanning transmission X-ray microscopy (STXM) coupled with X-ray absorption near edge structure (XANES) spectroscopy.Quartz was relatively unaffected by the experimental conditions. The main peaks of the XRD pattern of the experimental residue are those of α-quartz (i.e. 4.47 Å (100), 3.40 Å (101) and 2.57 Å (110); Fig. 1a). Likewise, the FTIR spectrum of the residue exhibits absorption bands typical of α-quartz (Fig. 1b): Si-O-Si and Si-O bending vibrations at 455 cm−1, 514 cm−1 and 694 cm−1, Si-O stretching vibrations at 775 and 794 cm−1 and Si-O-Si stretching vibrations at 1052 and 1160 cm−1 (Fig. 1b; Anbalagan et al., 2010
Anbalagan, G., Prabakaran, A., Gunasekaran, S. (2010) Spectroscopic characterization of Indian standard sand. Journal of Applied Spectroscopy 77, 86–94.
). Nevertheless, SEM images reveal the presence of dissolution pits at the surface of quartz grains, indicating that a certain fraction of quartz dissolved during the experiment (Fig. 2a).
Figure 1 XRD and FTIR results. (a) Powder XRD patterns of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days) and of the α-quartz reference (intensity increased ×3 from 30 to 70° 2θ). (b) ATR-FTIR spectra of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days) and of the α-quartz reference.

Figure 2 SEM investigations of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days). (a) EDX map of the residue and (b) corresponding EDX spectra. Quartz appears in yellow and the spheroidal organic biomorphs appear in purple. (c) Bar chart showing the size distribution of the spheroidal organic biomorphs produced during the experiments (FWHM: full width at half maximum). (e-h) SEM images (secondary electrons) of the spheroidal organic biomorphs produced during the experiments. (f, g) SEM images (secondary electrons) of Thermococcus prieurii cells (courtesy of Aurore Gorlas). Scale bars: (a) 10 μm, (d-j) 1 μm.
Most importantly, SEM images show the presence, at the surface of the quartz grains, of newly formed spheroidal carbonaceous microstructures (Fig. 2), resembling micro-organisms such as Staphylococcus or Thermococcales (Fig. 2i,j). Arranged in clusters, these spheroidal organic biomorphs exhibit a rather restricted range of diameters of about ∼0.5 to ∼5 μm (μ = 2.03 μm; Fig. 2c). Most display a rough surface resembling the ultrastructure of living cells, and many are connected together as if they were microbes encompassing cell division (Fig. 2). These spheroidal organic biomorphs exhibit N/C values of 0.1 (vs. 0.4 for RNA), δ13C values of −19.35 ± 0.04 ‰ (vs. −22.62 ± 0.04 ‰ for RNA) and δ15N values of −9.95 ± 0.09 ‰ (vs. −12.11‰ ± 0.09 ‰ for RNA), i.e. values not that different from those expected for organic microfossils (e.g., Craig, 1954
Craig, H. (1954) Geochemical implications of the isotopic composition of carbon in ancient rocks. Geochimica et Cosmochimica Acta 6, 186–196.
; Mojzsis et al., 1996Mojzsis, S.J., Arrhenius, G., McKeegan, K.D., Harrison, T.M., Nutman, A.P., Friend, C.R.L. (1996) Evidence for life on Earth before 3,800 million years ago. Nature 384, 55–59.
; Horita, 2005Horita, J. (2005) Some perspectives on isotope biosignatures for early life. Chemical Geology 218, 171–186.
).While the NMR spectrum of RNA is dominated by the signals of ribose (between 60 and 105 ppm) and nucleobases (between 140 and 170 ppm), the NMR spectrum of these spheroidal organic biomorphs indicates the presence of aliphatic, aromatic and heterocyclic carbons (signals from 0 to 50 ppm, 100 to 130, and 130 to 150 ppm respectively) and amide and ketone groups (features at ∼170 and 200 ppm) (Fig. 3a; Jacquemot et al., 2019
Jacquemot, P., Viennet, J.C., Bernard, S., Le Guillou, C., Rigaud, B., Delbes, L., Georgelin, T., Jaber, M. (2019) The degradation of organic compounds impacts the crystallization of clay minerals and vice versa. Scientific Reports 9, 20251.
). This is in line with the FTIR spectrum showing C-H bending vibrations at 1367 cm−1, aromatic C=C stretching vibrations at 1442 cm−1 and CH3/CH2 stretching vibrations from 2850 to 2980 cm−1 (Fig. 3b). The additional features at 1594 cm−1 and 1675 cm−1 highlight the presence of N-H bonds in amides or amines, as well as C=N bounds in imines or C=O bounds in ketones (Fig. 3b; Li et al., 2014Li, J., Bernard, S., Benzerara, K., Beyssac, O., Allard, T., Cosmidis, J., Moussou, J. (2014) Impact of biomineralization on the preservation of microorganisms during fossilization: An experimental perspective. Earth and Planetary Science Letters 400, 113–122.
; Bernard et al., 2015Bernard, S., Benzerara, K., Beyssac, O., Balan, E., Brown Jr., G.E. (2015) Evolution of the macromolecular structure of sporopollenin during thermal degradation. Heliyon 1, e00034.
).
Figure 3 Solid state 13C NMR and ATR-FTIR results. (a) Solid state 13C CP MAS NMR spectra of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days) and of the RNA reference. Spectra are normalised to the quantity of carbon. (b) ATR-FTIR spectra of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days) and of the RNA reference. Spectra are normalised to the total carbon content. Note that the intensity of the signals were increased ×3 for clarity from ∼3035 to ∼2775 cm−1.
In contrast to RNA which C-XANES spectrum exhibits a number of well defined absorption features attributed to nucleobases and ribose (Fig. 4; Viennet et al., 2019
Viennet, J.-C., Bernard, S., Le Guillou, C., Jacquemot, P., Balan, E., Delbes, L., Rigaud, B., Georgelin, T., Jaber, M. (2019) Experimental clues for detecting biosignatures on Mars. Geochemical Perspectives Letters 12, 28–33.
, 2020Viennet, J.-C., Bernard, S., Le Guillou, C., Jacquemot, P., Delbes, L., Balan, E., Jaber, M. (2020) Influence of the nature of the gas phase on the degradation of RNA during fossilization processes. Applied Clay Science 191, 105616.
), the spheroidal organic biomorphs display a XANES spectrum with large features attributed to (hetero)quinones and olefinic or aromatic carbons (284.8–285.5 eV), imines, nitriles, ketones and/or phenols (286.4 eV) and amide groups (288.2 eV) (Fig. 4; Le Guillou et al., 2018Le Guillou, C., Bernard, S., De la Pena, F., Le Brech, Y. (2018) XANES-Based Quantification of Carbon Functional Group Concentrations. Analytical Chemistry 90, 8379–8386.
), i.e. a spectrum not that different from those expected for microfossils. The N-XANES spectrum confirms the presence of imine/nitrile (peaks at 398.3 and 399.4 eV) and amide functions (feature at 401.5 eV) (Fig. 4; Alleon et al., 2017Alleon, J., Bernard, S., Le Guillou, C., Daval, D., Skouri-Panet, F., Kuga, M., Robert, F. (2017) Organic molecular heterogeneities can withstand diagenesis. Scientific Reports 7, 1508.
). Control experiments revealed that the presence of quartz has no influence on the properties of the produced spheroidal organic biomorphs (Fig. S-1).
Figure 4 STEM and STXM-XANES results. (a) Transmission electron microscopy image (STEM mode) of the FIB section extracted from spheroidal organic biomorphs. Scale bar: 1μm. (b) X-Ray absorption spectra of the organic biomorphs and of the RNA reference, with their corresponding N/C values. (c) C- and N-XANES spectra of the organic biomorphs and of the RNA reference compared to spectra of organic microfossils from Wasselonne (Bernard et al., 2009
Bernard, S., Benzerara, K., Beyssac, O., Brown, G.E., Stamm, L.G., Duringer, P. (2009) Ultrastructural and chemical study of modern and fossil sporoderms by Scanning Transmission X-ray Microscopy (STXM). Review of Palaeobotany and Palynology 156, 248–261.
) and Strelley Pool (Alleon et al., 2018Alleon, J., Bernard, S., Le Guillou, C., Beyssac, O., Sugitani, K., Robert, F. (2018) Chemical nature of the 3.4 Ga Strelley Pool microfossils. Geochemical Perspectives Letters 7, 37–42.
). All spectra are normalised to C and N quantities.By analogy with the production of hydrothermal carbon spheres (a.k.a. hydrochars), it can be assumed that the formation of these spheroidal organic biomorphs resulted from a cascade of reactions involving hydrolysis, dehydration, aromatisation and condensation (LaMer, 1952
LaMer, V.K. (1952) Nucleation in Phase Transitions. Industrial & Engineering Chemistry 44, 1270–1277.
; Sevilla and Fuertes, 2009aSevilla, M., Fuertes, A.B. (2009a) Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chemistry – A European Journal 15, 4195–4203.
,bSevilla, M., Fuertes, A.B. (2009b) The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 47, 2281–2289.
). The entire process should not be seen as a suite of consecutive reactions, but rather as a parallel network of different reaction paths (Funke and Ziegler, 2010Funke, A., Ziegler, F. (2010) Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels, Bioproducts and Biorefining 4, 160–177.
; Hu et al., 2010Hu, B., Wang, K., Wu, L., Yu, S.-H., Antonietti, M., Titirici, M.-M. (2010) Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Advanced Materials 22, 813–828.
). The hydrolysis of RNA likely produced organic acids having accelerated dehydration and fragmentation processes (i.e. ring opening and bond breaking), forming soluble by-products such as furfural-like compounds (Sevilla and Fuertes, 2009aSevilla, M., Fuertes, A.B. (2009a) Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chemistry – A European Journal 15, 4195–4203.
,bSevilla, M., Fuertes, A.B. (2009b) The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 47, 2281–2289.
). These by-products likely underwent aromatisation and condensation (possibly via intermolecular dehydration), leading to the production of aromatic clusters (Sevilla and Fuertes, 2009aSevilla, M., Fuertes, A.B. (2009a) Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chemistry – A European Journal 15, 4195–4203.
,bSevilla, M., Fuertes, A.B. (2009b) The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 47, 2281–2289.
). Burst nucleation processes likely took place when the concentration of aromatic clusters reached the critical supersaturation point, the nuclei growing by diffusion to the surface of the chemical species present in the solution, eventually forming the spheroidal organic biomorphs (Sevilla and Fuertes, 2009aSevilla, M., Fuertes, A.B. (2009a) Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chemistry – A European Journal 15, 4195–4203.
,bSevilla, M., Fuertes, A.B. (2009b) The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 47, 2281–2289.
). According to the LaMer model (LaMer, 1952LaMer, V.K. (1952) Nucleation in Phase Transitions. Industrial & Engineering Chemistry 44, 1270–1277.
), the structure of these spheroidal organic biomorphs should be composed of an aromatic-rich hydrophobic core and a hydrophilic surface containing a larger concentration of reactive oxygen-rich functional groups, as observed for hydrochars (Baccile et al., 2009Baccile, N., Laurent, G., Babonneau, F., Fayon, F., Titirici, M.-M., Antonietti, M. (2009) Structural Characterization of Hydrothermal Carbon Spheres by Advanced Solid-State MAS 13 C NMR Investigations. The Journal of Physical Chemistry C 113, 9644–9654.
; Sevilla and Fuertes, 2009aSevilla, M., Fuertes, A.B. (2009a) Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chemistry – A European Journal 15, 4195–4203.
,bSevilla, M., Fuertes, A.B. (2009b) The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 47, 2281–2289.
; Higgins et al., 2020Higgins, L.J.R., Brown, A.P., Harrington, J.P., Ross, A.B., Kaulich, B., Mishra, B. (2020) Evidence for a core-shell structure of hydrothermal carbon. Carbon 161, 423–431.
). Yet, spatially resolved STXM investigations reveal that the spheroidal organic biomorphs produced here are quite homogeneous chemically, at least at the submicrometre scale, with rather equivalent concentrations of aromatic, ketone and amide groups inside their core and at their surface.Collectively, in contrast to mobile hydrocarbon microspheres that can be encountered in the geological record (Wanger et al., 2012
Wanger, G., Moser, D., Hay, M., Myneni, S., Onstott, T.C., Southam, G. (2012) Mobile hydrocarbon microspheres from >2-billion-year-old carbon-bearing seams in the South African deep subsurface. Geobiology 10, 496–505.
), the spheroidal organic biomorphs produced here exhibit all the morphological and geochemical features typical of organic microfossils (size, morphology, ultrastructure, chemistry, isotopic signatures). Worse still, it has been shown that, if exposed to pressure and temperature conditions typical of the geological history undergone by ancient cherts, such spheroidal organic biomorphs may evolve into double shell hollow spheres (Hu et al., 2010Hu, B., Wang, K., Wu, L., Yu, S.-H., Antonietti, M., Titirici, M.-M. (2010) Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Advanced Materials 22, 813–828.
; Li et al., 2016Li, S., Pasc, A., Fierro, V., Celzard, A. (2016) Hollow carbon spheres, synthesis and applications – a review. Journal of Materials Chemistry A 4, 12686–12713.
). In other words, because they meet all the criteria commonly used to discuss biogenicity, even the most conservative ones (Brasier et al., 2006Brasier, M., McLoughlin, N., Green, O., Wacey, D. (2006) A fresh look at the fossil evidence for early Archaean cellular life. Philosophical Transactions of the Royal Society B: Biological Sciences 361, 887–902.
), the abiotic spheroidal organic biomorphs described here would logically be recognised as truly biogenic organic microfossils if they were found in ancient cherts.The results of the present study exemplify the pitfalls that Archean palaeontologists may encounter when searching for traces of life in ancient rocks (e.g., Schopf, 1975
Schopf, J.W. (1975) Precambrian Paleobiology: Problems and Perspectives. Annual Review of Earth and Planetary Sciences 3, 213–249.
; Javaux, 2019Javaux, E.J. (2019) Challenges in evidencing the earliest traces of life. Nature 572, 451–460.
). It is clear that if new strategies are not adopted, ambiguities and controversies will persist. Advanced spatially resolved spectroscopy techniques may provide some clues regarding the molecular structure of putative organic microfossils (e.g., Brasier et al., 2015Brasier, M.D., Antcliffe, J., Saunders, M., Wacey, D. (2015) Changing the picture of Earth’s earliest fossils (3.5–1.9 Ga) with new approaches and new discoveries. Proceedings of the National Academy of Sciences 112, 4859–4864.
; Alleon and Summons, 2019Alleon, J., Summons, R.E. (2019) Organic geochemical approaches to understanding early life. Free Radical Biology and Medicine 140, 103–112.
), but this is not sufficient. Because unambiguously determining the exact nature of putative organic microfossils requires information on their original chemical nature, only laboratory experiments may provide the necessary constraints to eventually decode the most ancient fossil record (e.g., Javaux, 2019Javaux, E.J. (2019) Challenges in evidencing the earliest traces of life. Nature 572, 451–460.
).top
Author Contributions
IC, PJ and SB designed the present study. IC and PJ performed the NMR analyses. IC, PJ and SB performed the SEM analyses. IC and JCV performed the EA-IRMS analyses, the XRD analyses and the FTIR analyses. IC, JCV and SB performed the STXM analyses. All authors contributed to the interpretation of the data and discussed their implications. IC, JVC and SB wrote the manuscript, with critical inputs from PJ and MJ.
top
Acknowledgements
We acknowledge the support of the MNHN and of the IMPMC spectroscopy platform. We thank Elisabeth Malassis (IMPMC) for administrative support, Aurore Gorlas (I2BC) for SEM images of Thermococcus prieurii cells, Imène Esteve (IMPMC) for her help with SEM, Etienne Balan (IMPMC) for his help with FTIR, Denis Fiorillo (AASPE) for his help with EA-IRMS, Baptiste Rigaud (IMPC) for his help with NMR, David Troadec (IEMN) for the preparation of FIB sections, Corentin Le Guillou (UMET) for the TEM image and Sufal Swaraj (Soleil) for the expert support of the HERMES STXM beamline at SOLEIL. The SEM facility at IMPMC is supported by Region Ile de France grant SESAME Number I-07-593/R, INSU-CNRS, INP-CNRS and UPMC-Paris 6, and by the Agence Nationale de la Recherche (ANR) grant number ANR-07-BLAN-0124-01. The TEM facility at the CCM (Lille University) is supported by the Chevreul Institute, the European FEDER, and Région Nord-Pas-de-Calais. The HERMES beamline (SOLEIL) is supported by the CNRS, the CEA, the Region Ile de France, the Departmental Council of Essonne and the Region Centre.
Editor: Satish Myneni
top
References
Anbalagan, G., Prabakaran, A., Gunasekaran, S. (2010) Spectroscopic characterization of Indian standard sand. Journal of Applied Spectroscopy 77, 86–94.
 Show in context
Show in context Likewise, the FTIR spectrum of the residue exhibits absorption bands typical of α-quartz (Fig. 1b): Si-O-Si and Si-O bending vibrations at 455 cm−1, 514 cm−1 and 694 cm−1, Si-O stretching vibrations at 775 and 794 cm−1 and Si-O-Si stretching vibrations at 1052 and 1160 cm−1 (Fig. 1b; Anbalagan et al., 2010).
View in article
Alleon, J., Bernard, S., Le Guillou, C., Daval, D., Skouri-Panet, F., Kuga, M., Robert, F. (2017) Organic molecular heterogeneities can withstand diagenesis. Scientific Reports 7, 1508.
 Show in context
Show in context The N-XANES spectrum confirms the presence of imine/nitrile (peaks at 398.3 and 399.4 eV) and amide functions (feature at 401.5 eV) (Fig. 4; Alleon et al., 2017).
View in article
Alleon, J., Bernard, S., Le Guillou, C., Beyssac, O., Sugitani, K., Robert, F. (2018) Chemical nature of the 3.4 Ga Strelley Pool microfossils. Geochemical Perspectives Letters 7, 37–42.
 Show in context
Show in context Chemical information may help to identify remains of life (Benzerara et al., 2006; Bernard et al., 2007; Alleon et al., 2018; Loron et al., 2019), but abiotic processes may lead to the formation of disordered carbonaceous materials difficult to distinguish from biogenic ones (Pasteris and Wopenka, 2003; De Gregorio et al., 2011).
View in article
(c) C- and N-XANES spectra of the organic biomorphs and of the RNA reference compared to spectra of organic microfossils from Wasselonne (Bernard et al., 2009) and Strelley Pool (Alleon et al., 2018).
View in article
Alleon, J., Summons, R.E. (2019) Organic geochemical approaches to understanding early life. Free Radical Biology and Medicine 140, 103–112.
 Show in context
Show in context Advanced spatially resolved spectroscopy techniques may provide some clues regarding the molecular structure of putative organic microfossils (e.g., Brasier et al., 2015; Alleon and Summons, 2019), but this is not sufficient.
View in article
Baccile, N., Laurent, G., Babonneau, F., Fayon, F., Titirici, M.-M., Antonietti, M. (2009) Structural Characterization of Hydrothermal Carbon Spheres by Advanced Solid-State MAS 13 C NMR Investigations. The Journal of Physical Chemistry C 113, 9644–9654.
 Show in context
Show in context According to the LaMer model (LaMer, 1952), the structure of these spheroidal organic biomorphs should be composed of an aromatic-rich hydrophobic core and a hydrophilic surface containing a larger concentration of reactive oxygen-rich functional groups, as observed for hydrochars (Baccile et al., 2009; Sevilla and Fuertes, 2009a,b; Higgins et al., 2020).
View in article
Benzerara, K., Menguy, N., Lopez-Garcia, P., Yoon, T.-H., Kazmierczak, J., Tyliszczak, T., Guyot, F., Brown, G.E. (2006) Nanoscale detection of organic signatures in carbonate microbialites. Proceedings of the National Academy of Sciences 103, 9440–9445.
 Show in context
Show in context Chemical information may help to identify remains of life (Benzerara et al., 2006; Bernard et al., 2007; Alleon et al., 2018; Loron et al., 2019), but abiotic processes may lead to the formation of disordered carbonaceous materials difficult to distinguish from biogenic ones (Pasteris and Wopenka, 2003; De Gregorio et al., 2011).
View in article
Bernard, S., Papineau, D. (2014) Graphitic Carbons and Biosignatures. Elements 10, 435–440.
 Show in context
Show in context Collectively, because none of the criteria commonly used to discuss biogenicity are sufficient in themselves, many authors have emphasised the need for gathering multiple lines of evidence to demonstrate convincingly the biological origin of any putative remain of life in ancient cherts (e.g., Westall, 2005; Wacey, 2009; Bernard and Papineau, 2014; Javaux, 2019).
View in article
Bernard, S., Benzerara, K., Beyssac, O., Menguy, N., Guyot, F., Brownjr, G., Goffe, B. (2007) Exceptional preservation of fossil plant spores in high-pressure metamorphic rocks. Earth and Planetary Science Letters 262, 257–272.
 Show in context
Show in context Chemical information may help to identify remains of life (Benzerara et al., 2006; Bernard et al., 2007; Alleon et al., 2018; Loron et al., 2019), but abiotic processes may lead to the formation of disordered carbonaceous materials difficult to distinguish from biogenic ones (Pasteris and Wopenka, 2003; De Gregorio et al., 2011).
View in article
Bernard, S., Benzerara, K., Beyssac, O., Brown, G.E., Stamm, L.G., Duringer, P. (2009) Ultrastructural and chemical study of modern and fossil sporoderms by Scanning Transmission X-ray Microscopy (STXM). Review of Palaeobotany and Palynology 156, 248–261.
 Show in context
Show in context (c) C- and N-XANES spectra of the organic biomorphs and of the RNA reference compared to spectra of organic microfossils from Wasselonne (Bernard et al., 2009) and Strelley Pool (Alleon et al., 2018).
View in article
Bernard, S., Benzerara, K., Beyssac, O., Balan, E., Brown Jr., G.E. (2015) Evolution of the macromolecular structure of sporopollenin during thermal degradation. Heliyon 1, e00034.
 Show in context
Show in context The additional features at 1594 cm−1 and 1675 cm−1 highlight the presence of N-H bonds in amides or amines, as well as C=N bounds in imines or C=O bounds in ketones (Fig. 3b; Li et al., 2014; Bernard et al., 2015).
View in article
Brasier, M., McLoughlin, N., Green, O., Wacey, D. (2006) A fresh look at the fossil evidence for early Archaean cellular life. Philosophical Transactions of the Royal Society B: Biological Sciences 361, 887–902.
 Show in context
Show in context Yet, although the ancient fossil record may contain fundamentally important ‘biogeochemical’ signals, its quality is far from perfect, making it not that easy to decode (Brasier et al., 2006; Javaux, 2019).
View in article
In other words, because they meet all the criteria commonly used to discuss biogenicity, even the most conservative ones (Brasier et al., 2006), the abiotic spheroidal organic biomorphs described here would logically be recognised as truly biogenic organic microfossils if they were found in ancient cherts.
View in article
Brasier, M.D., Antcliffe, J., Saunders, M., Wacey, D. (2015) Changing the picture of Earth’s earliest fossils (3.5–1.9 Ga) with new approaches and new discoveries. Proceedings of the National Academy of Sciences 112, 4859–4864.
 Show in context
Show in context Advanced spatially resolved spectroscopy techniques may provide some clues regarding the molecular structure of putative organic microfossils (e.g., Brasier et al., 2015; Alleon and Summons, 2019), but this is not sufficient.
View in article
Cosmidis, J., Templeton, A.S. (2016) Self-Assembly of biomorphic carbon/sulfur microstructures in sulfidic environments. Nature Communications 7, 12812.
 Show in context
Show in context A main difficulty is the lack of a univocal criterion to rely on when discussing the biogenicity of putative remains of life in ancient rocks: neither the carbon isotopic compositions nor the morphologies should be seen as unambiguous biosignatures (Craig, 1954; Horita, 2005; Cosmidis and Templeton, 2016; Garcia-Ruiz et al., 2020).
View in article
Worse still, Cosmidis and Templeton (2016) recently demonstrated that carbon-sulfur biomorphs could also be produced.
View in article
Craig, H. (1954) Geochemical implications of the isotopic composition of carbon in ancient rocks. Geochimica et Cosmochimica Acta 6, 186–196.
 Show in context
Show in context A main difficulty is the lack of a univocal criterion to rely on when discussing the biogenicity of putative remains of life in ancient rocks: neither the carbon isotopic compositions nor the morphologies should be seen as unambiguous biosignatures (Craig, 1954; Horita, 2005; Cosmidis and Templeton, 2016; Garcia-Ruiz et al., 2020).
View in article
These spheroidal organic biomorphs exhibit N/C values of 0.1 (vs. 0.4 for RNA), δ13C values of −19.35 ± 0.04 ‰ (vs. −22.62 ± 0.04 ‰ for RNA) and δ15N values of −9.95 ± 0.09 ‰ (vs. −12.11‰ ± 0.09 ‰ for RNA), i.e. values not that different from those expected for organic microfossils (e.g., Craig, 1954; Mojzsis et al., 1996; Horita, 2005).
View in article
De Gregorio, B.T., Sharp, T.G., Rushdi, A.I., Simoneit, B.R.T. (2011) Bugs or Gunk? Nanoscale Methods for Assessing the Biogenicity of Ancient Microfossils and Organic Matter. In: Golding, S.D., Glikson, M. (Eds.) Earliest Life on Earth: Habitats, Environments and Methods of Detection. Springer Netherlands, Dordrecht, 239–289.
 Show in context
Show in context Chemical information may help to identify remains of life (Benzerara et al., 2006; Bernard et al., 2007; Alleon et al., 2018; Loron et al., 2019), but abiotic processes may lead to the formation of disordered carbonaceous materials difficult to distinguish from biogenic ones (Pasteris and Wopenka, 2003; De Gregorio et al., 2011).
View in article
Funke, A., Ziegler, F. (2010) Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels, Bioproducts and Biorefining 4, 160–177.
 Show in context
Show in context The entire process should not be seen as a suite of consecutive reactions, but rather as a parallel network of different reaction paths (Funke and Ziegler, 2010; Hu et al., 2010).
View in article
Garcia-Ruiz, J.M., Hyde, S.T., Carnerup, A.M., Christy, A.G., Van Kranendonk, M.J., Welham, N.J. (2003) Self-Assembled Silica-Carbonate Structures and Detection of Ancient Microfossils. Science 302, 1194–1197.
 Show in context
Show in context In fact, mineral biomorphs may easily be produced experimentally via self-assembly processes (Garcia-Ruiz et al., 2003) and may exhibit high levels of complexity (Garcia-Ruiz et al., 2009; Noorduin et al., 2013; Rouillard et al., 2018).
View in article
Garcia-Ruiz, J.M., Melero-Garcia, E., Hyde, S.T. (2009) Morphogenesis of Self-Assembled Nanocrystalline Materials of Barium Carbonate and Silica. Science 323, 362–365.
 Show in context
Show in context In fact, mineral biomorphs may easily be produced experimentally via self-assembly processes (Garcia-Ruiz et al., 2003) and may exhibit high levels of complexity (Garcia-Ruiz et al., 2009; Noorduin et al., 2013; Rouillard et al., 2018).
View in article
Garcia-Ruiz, J.M., van Zuilen, M.A., Bach, W. (2020) Mineral self-organization on a lifeless planet. Physics of Life Reviews 34–35, 62–82.
 Show in context
Show in context A main difficulty is the lack of a univocal criterion to rely on when discussing the biogenicity of putative remains of life in ancient rocks: neither the carbon isotopic compositions nor the morphologies should be seen as unambiguous biosignatures (Craig, 1954; Horita, 2005; Cosmidis and Templeton, 2016; Garcia-Ruiz et al., 2020).
View in article
Higgins, L.J.R., Brown, A.P., Harrington, J.P., Ross, A.B., Kaulich, B., Mishra, B. (2020) Evidence for a core-shell structure of hydrothermal carbon. Carbon 161, 423–431.
 Show in context
Show in context According to the LaMer model (LaMer, 1952), the structure of these spheroidal organic biomorphs should be composed of an aromatic-rich hydrophobic core and a hydrophilic surface containing a larger concentration of reactive oxygen-rich functional groups, as observed for hydrochars (Baccile et al., 2009; Sevilla and Fuertes, 2009a,b; Higgins et al., 2020).
View in article
Higgs, P.G., Lehman, N. (2015) The RNA World: molecular cooperation at the origins of life. Nature Reviews Genetics 16, 7–17.
 Show in context
Show in context Here, we exposed RNA (i.e. the most emblematic organic molecule of the prebiotic RNA World; Higgs and Lehman, 2015) to thermal conditions typical of diagenesis (200 °C), in pure bi-distilled water under an autogenic pressure of 15 bars, in the presence of quartz (i.e. the main mineral of Archean cherts; Perry and Lefticariu, 2007) and under an argon atmosphere for 20 days.
View in article
Horita, J. (2005) Some perspectives on isotope biosignatures for early life. Chemical Geology 218, 171–186.
 Show in context
Show in context A main difficulty is the lack of a univocal criterion to rely on when discussing the biogenicity of putative remains of life in ancient rocks: neither the carbon isotopic compositions nor the morphologies should be seen as unambiguous biosignatures (Craig, 1954; Horita, 2005; Cosmidis and Templeton, 2016; Garcia-Ruiz et al., 2020).
View in article
These spheroidal organic biomorphs exhibit N/C values of 0.1 (vs. 0.4 for RNA), δ13C values of −19.35 ± 0.04 ‰ (vs. −22.62 ± 0.04 ‰ for RNA) and δ15N values of −9.95 ± 0.09 ‰ (vs. −12.11‰ ± 0.09 ‰ for RNA), i.e. values not that different from those expected for organic microfossils (e.g., Craig, 1954; Mojzsis et al., 1996; Horita, 2005).
View in article
Hu, B., Wang, K., Wu, L., Yu, S.-H., Antonietti, M., Titirici, M.-M. (2010) Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Advanced Materials 22, 813–828.
 Show in context
Show in context The entire process should not be seen as a suite of consecutive reactions, but rather as a parallel network of different reaction paths (Funke and Ziegler, 2010; Hu et al., 2010).
View in article
Worse still, it has been shown that, if exposed to pressure and temperature conditions typical of the geological history undergone by ancient cherts, such spheroidal organic biomorphs may evolve into double shell hollow spheres (Hu et al., 2010; Li et al., 2016).
View in article
Jacquemot, P., Viennet, J.C., Bernard, S., Le Guillou, C., Rigaud, B., Delbes, L., Georgelin, T., Jaber, M. (2019) The degradation of organic compounds impacts the crystallization of clay minerals and vice versa. Scientific Reports 9, 20251.
 Show in context
Show in context While the NMR spectrum of RNA is dominated by the signals of ribose (between 60 and 105 ppm) and nucleobases (between 140 and 170 ppm), the NMR spectrum of these spheroidal organic biomorphs indicates the presence of aliphatic, aromatic and heterocyclic carbons (signals from 0 to 50 ppm, 100 to 130, and 130 to 150 ppm respectively) and amide and ketone groups (features at ∼170 and 200 ppm) (Fig. 3a; Jacquemot et al., 2019).
View in article
Javaux, E.J. (2019) Challenges in evidencing the earliest traces of life. Nature 572, 451–460.
 Show in context
Show in context Yet, although the ancient fossil record may contain fundamentally important ‘biogeochemical’ signals, its quality is far from perfect, making it not that easy to decode (Brasier et al., 2006; Javaux, 2019).
View in article
Archean palaeontology only relies on degraded signals difficult to interpret, as illustrated by the number of controversies having so far hindered the search for the most ancient traces of life on Earth (Schopf, 1975; Javaux, 2019).
View in article
Collectively, because none of the criteria commonly used to discuss biogenicity are sufficient in themselves, many authors have emphasised the need for gathering multiple lines of evidence to demonstrate convincingly the biological origin of any putative remain of life in ancient cherts (e.g., Westall, 2005; Wacey, 2009; Bernard and Papineau, 2014; Javaux, 2019).
View in article
The results of the present study exemplify the pitfalls that Archean palaeontologists may encounter when searching for traces of life in ancient rocks (e.g., Schopf, 1975; Javaux, 2019).
View in article
Because unambiguously determining the exact nature of putative organic microfossils requires information on their original chemical nature, only laboratory experiments may provide the necessary constraints to eventually decode the most ancient fossil record (e.g., Javaux, 2019).
View in article
LaMer, V.K. (1952) Nucleation in Phase Transitions. Industrial & Engineering Chemistry 44, 1270–1277.
 Show in context
Show in context By analogy with the production of hydrothermal carbon spheres (a.k.a. hydrochars), it can be assumed that the formation of these spheroidal organic biomorphs resulted from a cascade of reactions involving hydrolysis, dehydration, aromatisation and condensation (LaMer, 1952; Sevilla and Fuertes, 2009a,b).
View in article
According to the LaMer model (LaMer, 1952), the structure of these spheroidal organic biomorphs should be composed of an aromatic-rich hydrophobic core and a hydrophilic surface containing a larger concentration of reactive oxygen-rich functional groups, as observed for hydrochars (Baccile et al., 2009; Sevilla and Fuertes, 2009a,b; Higgins et al., 2020).
View in article
Le Guillou, C., Bernard, S., De la Pena, F., Le Brech, Y. (2018) XANES-Based Quantification of Carbon Functional Group Concentrations. Analytical Chemistry 90, 8379–8386.
 Show in context
Show in context In contrast to RNA which C-XANES spectrum exhibits a number of well defined absorption features attributed to nucleobases and ribose (Fig. 4; Viennet et al., 2019, 2020), the spheroidal organic biomorphs display a XANES spectrum with large features attributed to (hetero)quinones and olefinic or aromatic carbons (284.8–285.5 eV), imines, nitriles, ketones and/or phenols (286.4 eV) and amide groups (288.2 eV) (Fig. 4; Le Guillou et al., 2018), i.e. a spectrum not that different from those expected for microfossils.
View in article
Li, J., Bernard, S., Benzerara, K., Beyssac, O., Allard, T., Cosmidis, J., Moussou, J. (2014) Impact of biomineralization on the preservation of microorganisms during fossilization: An experimental perspective. Earth and Planetary Science Letters 400, 113–122.
 Show in context
Show in context The additional features at 1594 cm−1 and 1675 cm−1 highlight the presence of N-H bonds in amides or amines, as well as C=N bounds in imines or C=O bounds in ketones (Fig. 3b; Li et al., 2014; Bernard et al., 2015).
View in article
Li, S., Pasc, A., Fierro, V., Celzard, A. (2016) Hollow carbon spheres, synthesis and applications – a review. Journal of Materials Chemistry A 4, 12686–12713.
 Show in context
Show in context Worse still, it has been shown that, if exposed to pressure and temperature conditions typical of the geological history undergone by ancient cherts, such spheroidal organic biomorphs may evolve into double shell hollow spheres (Hu et al., 2010; Li et al., 2016).
View in article
Loron, C.C., François, C., Rainbird, R.H., Turner, E.C., Borensztajn, S., Javaux, E.J. (2019) Early fungi from the Proterozoic era in Arctic Canada. Nature 570, 232–235.
 Show in context
Show in context Chemical information may help to identify remains of life (Benzerara et al., 2006; Bernard et al., 2007; Alleon et al., 2018; Loron et al., 2019), but abiotic processes may lead to the formation of disordered carbonaceous materials difficult to distinguish from biogenic ones (Pasteris and Wopenka, 2003; De Gregorio et al., 2011).
View in article
Mojzsis, S.J., Arrhenius, G., McKeegan, K.D., Harrison, T.M., Nutman, A.P., Friend, C.R.L. (1996) Evidence for life on Earth before 3,800 million years ago. Nature 384, 55–59.
 Show in context
Show in context These spheroidal organic biomorphs exhibit N/C values of 0.1 (vs. 0.4 for RNA), δ13C values of −19.35 ± 0.04 ‰ (vs. −22.62 ± 0.04 ‰ for RNA) and δ15N values of −9.95 ± 0.09 ‰ (vs. −12.11‰ ± 0.09 ‰ for RNA), i.e. values not that different from those expected for organic microfossils (e.g., Craig, 1954; Mojzsis et al., 1996; Horita, 2005).
View in article
Noorduin, W.L., Grinthal, A., Mahadevan, L., Aizenberg, J. (2013) Rationally Designed Complex, Hierarchical Microarchitectures. Science 340, 832–837.
 Show in context
Show in context In fact, mineral biomorphs may easily be produced experimentally via self-assembly processes (Garcia-Ruiz et al., 2003) and may exhibit high levels of complexity (Garcia-Ruiz et al., 2009; Noorduin et al., 2013; Rouillard et al., 2018).
View in article
Pasteris, J.D., Wopenka, B. (2003) Necessary, but Not Sufficient: Raman Identification of Disordered Carbon as a Signature of Ancient Life. Astrobiology 3, 727–738.
 Show in context
Show in context Chemical information may help to identify remains of life (Benzerara et al., 2006; Bernard et al., 2007; Alleon et al., 2018; Loron et al., 2019), but abiotic processes may lead to the formation of disordered carbonaceous materials difficult to distinguish from biogenic ones (Pasteris and Wopenka, 2003; De Gregorio et al., 2011).
View in article
Perry, E.C., Lefticariu, L. (2007) Formation and Geochemistry of Precambrian Cherts. Treatise on Geochemistry 7, 1–21.
 Show in context
Show in context Here, we exposed RNA (i.e. the most emblematic organic molecule of the prebiotic RNA World; Higgs and Lehman, 2015) to thermal conditions typical of diagenesis (200 °C), in pure bi-distilled water under an autogenic pressure of 15 bars, in the presence of quartz (i.e. the main mineral of Archean cherts; Perry and Lefticariu, 2007) and under an argon atmosphere for 20 days.
View in article
Rouillard, J., García-Ruiz, J.-M., Gong, J., van Zuilen, M.A. (2018) A morphogram for silica-witherite biomorphs and its application to microfossil identification in the early earth rock record. Geobiology 16, 279–296.
 Show in context
Show in context In fact, mineral biomorphs may easily be produced experimentally via self-assembly processes (Garcia-Ruiz et al., 2003) and may exhibit high levels of complexity (Garcia-Ruiz et al., 2009; Noorduin et al., 2013; Rouillard et al., 2018).
View in article
Schopf, J.W. (1975) Precambrian Paleobiology: Problems and Perspectives. Annual Review of Earth and Planetary Sciences 3, 213–249.
 Show in context
Show in context Archean palaeontology only relies on degraded signals difficult to interpret, as illustrated by the number of controversies having so far hindered the search for the most ancient traces of life on Earth (Schopf, 1975; Javaux, 2019).
View in article
The results of the present study exemplify the pitfalls that Archean palaeontologists may encounter when searching for traces of life in ancient rocks (e.g., Schopf, 1975; Javaux, 2019).
View in article
Sevilla, M., Fuertes, A.B. (2009a) Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chemistry – A European Journal 15, 4195–4203.
 Show in context
Show in context By analogy with the production of hydrothermal carbon spheres (a.k.a. hydrochars), it can be assumed that the formation of these spheroidal organic biomorphs resulted from a cascade of reactions involving hydrolysis, dehydration, aromatisation and condensation (LaMer, 1952; Sevilla and Fuertes, 2009a,b).
View in article
The hydrolysis of RNA likely produced organic acids having accelerated dehydration and fragmentation processes (i.e. ring opening and bond breaking), forming soluble by-products such as furfural-like compounds (Sevilla and Fuertes, 2009a,b).
View in article
These by-products likely underwent aromatisation and condensation (possibly via intermolecular dehydration), leading to the production of aromatic clusters (Sevilla and Fuertes, 2009a,b).
View in article
Burst nucleation processes likely took place when the concentration of aromatic clusters reached the critical supersaturation point, the nuclei growing by diffusion to the surface of the chemical species present in the solution, eventually forming the spheroidal organic biomorphs (Sevilla and Fuertes, 2009a,b).
View in article
According to the LaMer model (LaMer, 1952), the structure of these spheroidal organic biomorphs should be composed of an aromatic-rich hydrophobic core and a hydrophilic surface containing a larger concentration of reactive oxygen-rich functional groups, as observed for hydrochars (Baccile et al., 2009; Sevilla and Fuertes, 2009a,b; Higgins et al., 2020).
View in article
Sevilla, M., Fuertes, A.B. (2009b) The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 47, 2281–2289.
 Show in context
Show in context By analogy with the production of hydrothermal carbon spheres (a.k.a. hydrochars), it can be assumed that the formation of these spheroidal organic biomorphs resulted from a cascade of reactions involving hydrolysis, dehydration, aromatisation and condensation (LaMer, 1952; Sevilla and Fuertes, 2009a,b).
View in article
The hydrolysis of RNA likely produced organic acids having accelerated dehydration and fragmentation processes (i.e. ring opening and bond breaking), forming soluble by-products such as furfural-like compounds (Sevilla and Fuertes, 2009a,b).
View in article
These by-products likely underwent aromatisation and condensation (possibly via intermolecular dehydration), leading to the production of aromatic clusters (Sevilla and Fuertes, 2009a,b).
View in article
Burst nucleation processes likely took place when the concentration of aromatic clusters reached the critical supersaturation point, the nuclei growing by diffusion to the surface of the chemical species present in the solution, eventually forming the spheroidal organic biomorphs (Sevilla and Fuertes, 2009a,b).
View in article
According to the LaMer model (LaMer, 1952), the structure of these spheroidal organic biomorphs should be composed of an aromatic-rich hydrophobic core and a hydrophilic surface containing a larger concentration of reactive oxygen-rich functional groups, as observed for hydrochars (Baccile et al., 2009; Sevilla and Fuertes, 2009a,b; Higgins et al., 2020).
View in article
Viennet, J.-C., Bernard, S., Le Guillou, C., Jacquemot, P., Balan, E., Delbes, L., Rigaud, B., Georgelin, T., Jaber, M. (2019) Experimental clues for detecting biosignatures on Mars. Geochemical Perspectives Letters 12, 28–33.
 Show in context
Show in context In contrast to RNA which C-XANES spectrum exhibits a number of well defined absorption features attributed to nucleobases and ribose (Fig. 4; Viennet et al., 2019, 2020), the spheroidal organic biomorphs display a XANES spectrum with large features attributed to (hetero)quinones and olefinic or aromatic carbons (284.8–285.5 eV), imines, nitriles, ketones and/or phenols (286.4 eV) and amide groups (288.2 eV) (Fig. 4; Le Guillou et al., 2018), i.e. a spectrum not that different from those expected for microfossils.
View in article
Viennet, J.-C., Bernard, S., Le Guillou, C., Jacquemot, P., Delbes, L., Balan, E., Jaber, M. (2020) Influence of the nature of the gas phase on the degradation of RNA during fossilization processes. Applied Clay Science 191, 105616.
 Show in context
Show in context In contrast to RNA which C-XANES spectrum exhibits a number of well defined absorption features attributed to nucleobases and ribose (Fig. 4; Viennet et al., 2019, 2020), the spheroidal organic biomorphs display a XANES spectrum with large features attributed to (hetero)quinones and olefinic or aromatic carbons (284.8–285.5 eV), imines, nitriles, ketones and/or phenols (286.4 eV) and amide groups (288.2 eV) (Fig. 4; Le Guillou et al., 2018), i.e. a spectrum not that different from those expected for microfossils.
View in article
Wacey, D. (Ed.) (2009) Early Life on Earth. Springer Netherlands, Dordrecht.
 Show in context
Show in context Collectively, because none of the criteria commonly used to discuss biogenicity are sufficient in themselves, many authors have emphasised the need for gathering multiple lines of evidence to demonstrate convincingly the biological origin of any putative remain of life in ancient cherts (e.g., Westall, 2005; Wacey, 2009; Bernard and Papineau, 2014; Javaux, 2019).
View in article
Wanger, G., Moser, D., Hay, M., Myneni, S., Onstott, T.C., Southam, G. (2012) Mobile hydrocarbon microspheres from >2-billion-year-old carbon-bearing seams in the South African deep subsurface. Geobiology 10, 496–505.
 Show in context
Show in context Collectively, in contrast to mobile hydrocarbon microspheres that can be encountered in the geological record (Wanger et al., 2012), the spheroidal organic biomorphs produced here exhibit all the morphological and geochemical features typical of organic microfossils (size, morphology, ultrastructure, chemistry, isotopic signatures).
View in article
Westall, F. (2005) Early Life on Earth: The Ancient Fossil Record. In: Ehrenfreund, P., Irvine, W.M., Owen, T., Becker, L., Blank, J., Brucato, J.R., Colangeli, L., Derenne, S., Dutrey, A., Despois, D., Lazcano, A., Robert, F. (Eds.) Astrobiology: Future Perspectives. Springer Netherlands, Dordrecht, 287–316.
 Show in context
Show in context Collectively, because none of the criteria commonly used to discuss biogenicity are sufficient in themselves, many authors have emphasised the need for gathering multiple lines of evidence to demonstrate convincingly the biological origin of any putative remain of life in ancient cherts (e.g., Westall, 2005; Wacey, 2009; Bernard and Papineau, 2014; Javaux, 2019).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Methods
- Figure S-1
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures
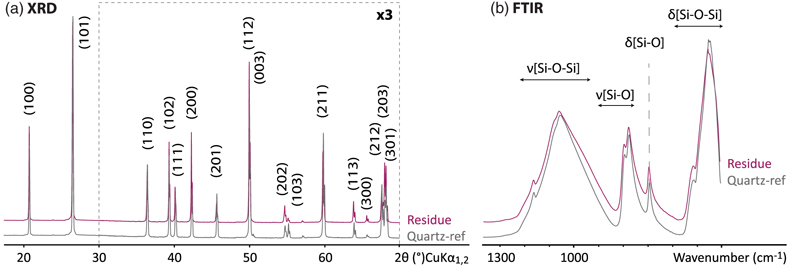
Figure 1 XRD and FTIR results. (a) Powder XRD patterns of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days) and of the α-quartz reference (intensity increased ×3 from 30 to 70° 2θ). (b) ATR-FTIR spectra of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days) and of the α-quartz reference.
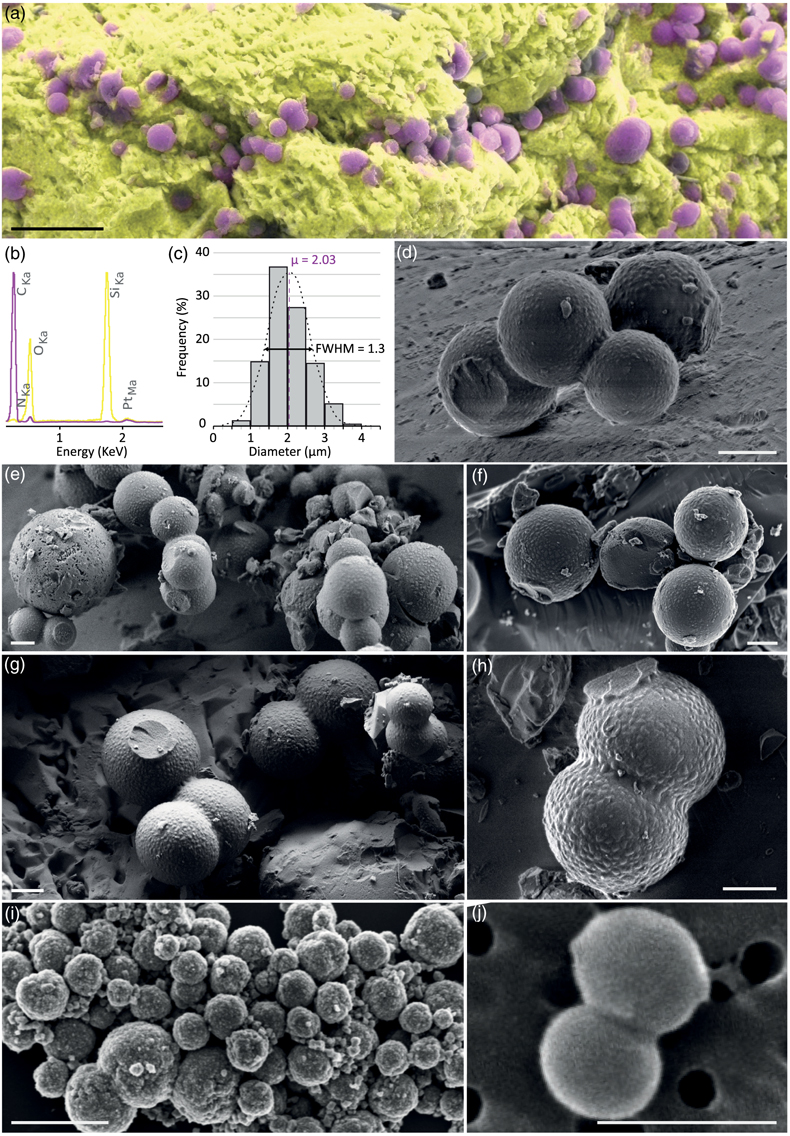
Figure 2 SEM investigations of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days). (a) EDX map of the residue and (b) corresponding EDX spectra. Quartz appears in yellow and the spheroidal organic biomorphs appear in purple. (c) Bar chart showing the size distribution of the spheroidal organic biomorphs produced during the experiments (FWHM: full width at half maximum). (e-h) SEM images (secondary electrons) of the spheroidal organic biomorphs produced during the experiments. (f, g) SEM images (secondary electrons) of Thermococcus prieurii cells (courtesy of Aurore Gorlas). Scale bars: (a) 10 μm, (d-j) 1 μm.
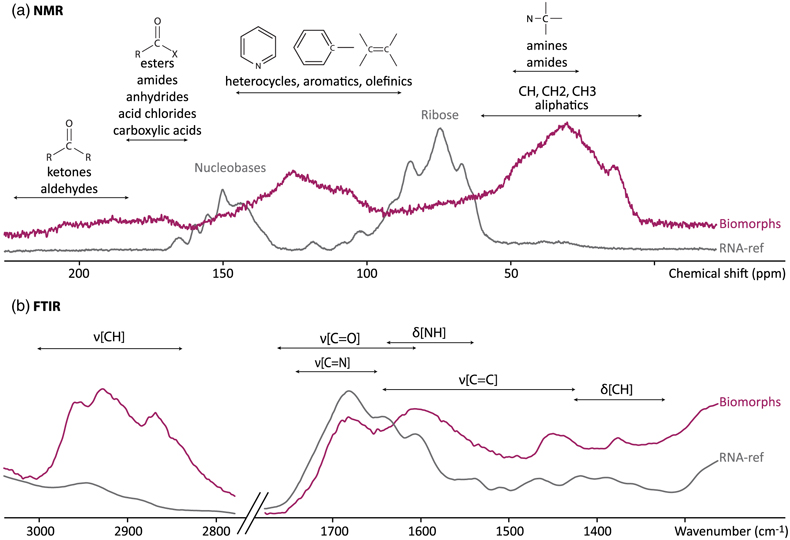
Figure 3 Solid state 13C NMR and ATR-FTIR results. (a) Solid state 13C CP MAS NMR spectra of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days) and of the RNA reference. Spectra are normalised to the quantity of carbon. (b) ATR-FTIR spectra of the experimental residue (α-quartz + RNA + H2O at 200 °C, 15 bars, 20 days) and of the RNA reference. Spectra are normalised to the total carbon content. Note that the intensity of the signals were increased ×3 for clarity from ∼3035 to ∼2775 cm−1.
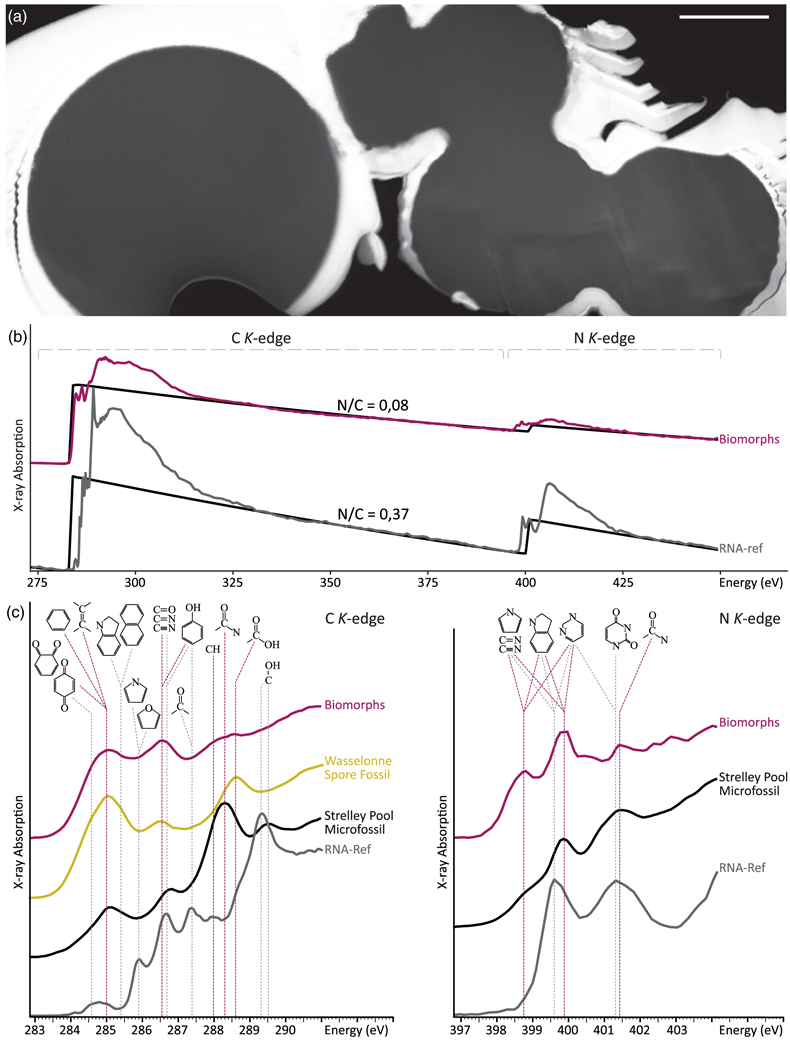
Figure 4 STEM and STXM-XANES results. (a) Transmission electron microscopy image (STEM mode) of the FIB section extracted from spheroidal organic biomorphs. Scale bar: 1μm. (b) X-Ray absorption spectra of the organic biomorphs and of the RNA reference, with their corresponding N/C values. (c) C- and N-XANES spectra of the organic biomorphs and of the RNA reference compared to spectra of organic microfossils from Wasselonne (Bernard et al., 2009
Bernard, S., Benzerara, K., Beyssac, O., Brown, G.E., Stamm, L.G., Duringer, P. (2009) Ultrastructural and chemical study of modern and fossil sporoderms by Scanning Transmission X-ray Microscopy (STXM). Review of Palaeobotany and Palynology 156, 248–261.
) and Strelley Pool (Alleon et al., 2018Alleon, J., Bernard, S., Le Guillou, C., Beyssac, O., Sugitani, K., Robert, F. (2018) Chemical nature of the 3.4 Ga Strelley Pool microfossils. Geochemical Perspectives Letters 7, 37–42.
). All spectra are normalised to C and N quantities.