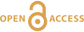Generation of oxidising fluids by comminution of fault rocks
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





-
Article views:93Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
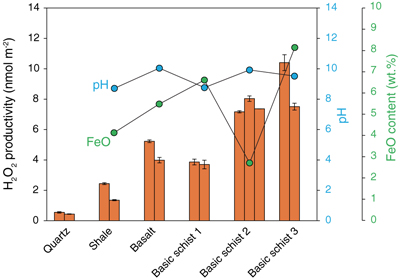 Figure 1 H2O2 productivity of the analysed samples. Error bars for H2O2 productivity were estimated from the equation for the standard addition method (Larsen et al., 1973). The pH of the solution after the experiment (during the duplicate analyses) and the concentration of FeO in the rock samples are also shown. | 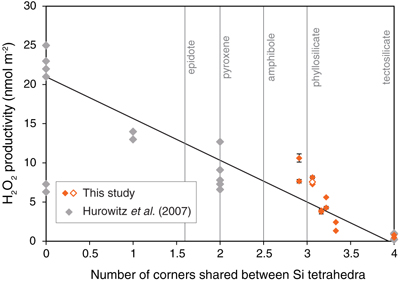 Figure 2 H2O2 productivity vs. number of corners shared between silica tetrahedra (weighted mean) for the analysed rocks. The open symbol is data for the experiment using 0.01 M NaCl solution. Data and regression line from Hurowitz et al. (2007) are also shown. |
| Figure 1 | Figure 2 |
top
Introduction
Frictional slip on a fault zone causes comminution and wearing of fault rocks, producing highly reactive fine particles (Sammis et al., 1986
Sammis, C.G., Osborne, R.H., Anderson, J.L., Banerdt, M., White, P. (1986) Self-similar cataclasis in the formation of fault gouge. Pure and Applied Geophysics 124, 53–78.
; Scholz, 2002Scholz, C.H. (2002) The mechanics of earthquake and faulting. Second Edition, Cambridge University Press, Cambridge.
). Radical species such as Si· and SiO· are commonly created on such particles of silicate minerals owing to homolytic rupture of Si–O covalent bonds (Hochstrasser and Antonini, 1972Hochstrasser, G., Antonini, J.F. (1972) Surface states of pristine silica surfaces I. ESR studies of Es’ dangling bonds and of CO2-adsorbed radicals. Surface Science 32, 644–664.
; Narayanasamy and Kubicki, 2005Narayanasamy, J., Kubicki, J.D. (2005) Mechanism of Hydroxyl Radical Generation from a Silica Surface: Molecular Orbital Calculations. The Journal of Physical Chemistry B 109, 21796–21807.
). Hydrogen (H2) is a typical product that is generated by reaction between Si· and water molecules within a fault zone. The production of H2 by the interaction of fluids and comminuted rocks has been demonstrated by laboratory experiments (Kita et al., 1982Kita, I., Matsuo, S., Wakita, H. (1982) H2 generation by reaction between H2O and crushed rock: An experimental study on H2 degassing from the active fault zone. Journal of Geophysical Research: Solid Earth 87, 10789–10795.
; Kameda et al., 2003Kameda, J., Saruwatari, K., Tanaka, H. (2003) H2 generation in wet grinding of granite and single-crystal powders and implications for H2 concentration on active faults. Geophysical Research Letters 30, 2063, doi: 10.1029/2003GL018252.
; Hirose et al., 2011Hirose, T., Kawagucci, S., Suzuki, K. (2011) Mechanoradical H2 generation during simulated faulting: Implications for an earthquake-driven subsurface biosphere. Geophysical Research Letters 38, L17303, doi: 10.1029/2011GL048850.
) and that associated with seismic activity has been observed by field survey (Wakita et al., 1980Wakita, H., Nakamura, Y., Kita, I., Fujii, N., Notsu, K. (1980) Hydrogen release: New indicator of fault activity. Science 210, 188–190, doi: 10.1126/science.210.4466.188.
; Ito et al., 1999Ito, T., Nagamine, K., Yamamoto, K., Adachi, M., Kawabe, I. (1999) Preseismic hydrogen gas anomalies caused by stress-corrosion process preceding earthquakes. Geophysical Research Letters 26, 2009–2012.
). In addition, numerous studies have demonstrated that pulverisation of pure powders of minerals forming igneous rocks can produce oxidising compounds such as hydrogen peroxide (H2O2), superoxide (O2·−), and hydroxy (·OH) radicals (Schoonen et al., 2006Schoonen, M.A.A., Cohn, C.A., Roemer, E., Laffers, R., Simon, S.R., O’Riordan, T. (2006) Mineral-induced formation of reactive oxygen species. Reviews in Mineralogy and Geochemistry 64, 179–221.
; Hurowitz et al., 2007Hurowitz, J.A., Tosca, N.J., McLennan, S.M., Schoonen, M.A.A. (2007) Production of hydrogen peroxide in Martian and lunar soils. Earth and Planetary Science Letters 255, 41–52, doi: 10.1016/j.epsl.2006.12.004.
; Hendrix et al., 2019Hendrix, D.A., Port, S.T., Hurowitz, J.A., Schoonen, M.A. (2019) Measurement of OH* generation by pulverized minerals using electron spin resonance spectroscopy and implications for the reactivity of planetary regolith. GeoHealth 3, 28–42, doi: 10.1029/2018GH000175.
), which are termed “reactive oxygen species” or “ROS”. ROS are formed by human activities such as mining, which produces fine particles of rocks and minerals (Schoonen et al., 2006Schoonen, M.A.A., Cohn, C.A., Roemer, E., Laffers, R., Simon, S.R., O’Riordan, T. (2006) Mineral-induced formation of reactive oxygen species. Reviews in Mineralogy and Geochemistry 64, 179–221.
). ROS are also formed due to reaction with reactive secondary minerals and photochemistry in soils and sediments (e.g., Georgiou et al., 2015Georgiou, C.D., Sun, H.J., McKay, C.P., Grintzalis, K., Papapostolou, I., Zisimopoulos, D., Panagiotidis, K., Zhang, G., Koutsopoulou, E., Christidis, G.E., Margiolaki, I. (2015) Evidence for photochemical production of reactive oxygen species in desert soils. Nature Communications 6, 7100, doi: 10.1038/ncomms8100.
). Because fault activity causes comminution of fault rocks, it can be assumed that ROS are also produced within fault zones. Moreover, such ROS may modify the chemical and even physical state of the fault zone. For example, some lithologies such as shales have been shown to undergo a variety of degradation on interaction with H2O2 (formation of cracks and voids, decomposition and dissolution of constituent materials, etc.), which results in modification of their hydromechanical properties at varying rates depending on temperature (Chen et al., 2017Chen, Q., Kang, Y., You, L., Yang, P., Zhang, X., Cheng, Q. (2017) Change in composition and pore structure of Longmaxi black shale during oxidative dissolution. International Journal of Coal Geology 172, 95–111.
; Zhou et al., 2018Zhou, Y., Lei, Y., Cheng, W., Yu, W. (2018) Enhance low temperature oxidization of shale gas recovery using hydrogen peroxide. Journal of Petroleum Science and Engineering 164, 523–530.
; Yu et al., 2019Yu, W.G., Lei, J., Wang, T., Chen, W. (2019) H2O2-enhanced shale gas recovery under different thermal conditions. Energies 12, 2127, doi: 10.3390/en12112127.
). Thus, the production and diffusion of oxidising compounds by a seismic event can potentially degrade surrounding fault zones and stimulate subsequent fault activity.In this study, we examine the production of H2O2 by reaction between water and ground rock samples at ambient conditions. The samples are sedimentary, metamorphic, and igneous rocks that may constitute plate boundaries at various depths in subduction zones (Hacker et al., 2003
Hacker, B.R., Peacock, S.M., Abers, G.A., Holloway, S.D. (2003) Subduction factory 2. Are intermediate-depth earthquakes in subducting slabs linked to metamorphic dehydration reactions? Journal of Geophysical Research: Solid Earth 108, 2030, doi: 10.1029/2001JB001127.
; Kimura et al., 2012Kimura, G., Yamaguchi, A., Hojo, M., Kitamura, Y., Kameda, J., Ujiie, K., Hamada, Y., Hamahashi, M., Hina, S. (2012) Tectonic mélange as fault rock of subduction plate boundary. Tectonophysics 568, 25–38.
; Kameda et al., 2017Kameda, J., Inoue, S., Tanikawa, W., Yamaguchi, A., Hamada, Y., Hashimoto, Y., Kimura, G. (2017) Alteration and dehydration of subducting oceanic crust within subduction zones: implications for decollement step-down and plate-boundary seismogenesis. Earth Planets and Space 69, 52, doi: 10.1186/s40623-017-0635-1.
). Our results show that oxidising fluid can be easily produced throughout the plate boundary zone after an earthquake. On the basis of our findings, we discuss the importance of the chemical aspects of fluids on fault behaviours.top
Samples and Experimental Methods
We analysed five natural rock samples: shale and oceanic basalt from the Cretaceous accretionary complex (Shimanto belt, Japan), and three basic schist samples from the high pressure Sanbagawa metamorphic belt, Japan. The basic schist samples (basic schist 1, basic schist 2, and basic schist 3) were taken from the chlorite, garnet, and albite–biotite zones of the Sanbagawa belt, respectively, which correspond to pressure-temperature (P-T) conditions ranging from greenschist to epidote-amphibolite facies (∼300 to ∼550 °C; Enami et al., 1994
Enami, M., Wallis, S.R., Banno, Y. (1994) Paragenesis of sodic pyroxene-bearing quartz schists: implications for P-T history of the Sanbagawa belt. Contributions to Mineralogy and Petrology 116, 182–198.
). For comparison, quartz sand (Wako Purer Chemicals) was also used for the experiments.Whole rock major element concentrations of the rock samples were determined by an X-ray fluorescence spectrometer (XRF; Rigaku 25X Primus IV) using the conventional glass bead method. The Fe2+/ΣFe ratios were determined by wet chemical analyses. Mineral compositions were analysed by X-ray diffraction analysis (XRD; MAC Science MX-Labo), and the obtained XRD patterns were used for quantitative analysis by RockJock software (Eberl, 2003
Eberl, D.D. (2003) User’s guide to RockJock-A program for determining quantitative mineralogy from powder X-ray diffraction data. USGS Open-file Report 03–78.
).The grinding experiments were carried out by using a planetary-type ball mill apparatus (P6; Fritsch). An amount of 3 g of each powder was loaded into a zirconia mill pot and was processed at 250–300 rpm for 5 min. The freshly ground powders with the post-grinding grain size of ∼0.5 μm (estimated from the surface area data described below, assuming uniform spherical particles) were then mixed with pure water (Direct-Q 3UV, Merck; pH = 5.84) in a Teflon beaker at room temperature (∼22 °C), and settled for 10–20 min. The H2O2 concentrations of the filtered solutions were then measured with the Scopoletin-Horseradish peroxidase (HRP) Fluorometric method using a fluorometer (RF-5300PC; Shimazu). The post-experiment pH of the solutions was also measured. The specific surface areas of the processed powders were measured by the Brunauer-Emmett-Teller (BET) method using an automated sorption analyser (Autosorb, Quantachrome Instruments). More details on the samples and experimental methods are described in the Supplementary Information.
top
Results
Our experiments demonstrated that all of the studied samples were able to produce H2O2 by mechanical activation and subsequent immersion in water. Figure 1 shows the H2O2 productivity of each sample (nmol m−2), which is calculated from the H2O2 concentration of the reacting fluid (the background H2O2 concentration of 0.060 μmol L−1 was subtracted from the measured concentration) normalised to the total surface area of the ground powders, as well as pH of the reacted solution and Fe2+ content in the solid samples (Tables S-1, S-2). The two analysed quartz samples have values of 0.55 and 0.44 nmol m−2, which are slightly higher than previous results (0.2–0.3 nmol m−2; Hurowitz et al., 2007
Hurowitz, J.A., Tosca, N.J., McLennan, S.M., Schoonen, M.A.A. (2007) Production of hydrogen peroxide in Martian and lunar soils. Earth and Planetary Science Letters 255, 41–52, doi: 10.1016/j.epsl.2006.12.004.
). Factors such as different temperatures, pH of the matrix, reaction time, hydration state of the samples, etc. could explain the differences. The H2O2 productivities of the natural rock samples are generally one order of magnitude greater than those of quartz, ranging from 1.3 nmol m−2 (shale) to 10.4 nmol m−2 (basic schist 3) with a mean value of ∼5.4 nmol m−2. No obvious correlation was found between the H2O2 productivities and pH or Fe2+ content.
Figure 1 H2O2 productivity of the analysed samples. Error bars for H2O2 productivity were estimated from the equation for the standard addition method (Larsen et al., 1973
Larsen, I.L., Hartmann, N.A., Wagner, J.J. (1973) Estimating precision for the method of standard additions. Analytical Chemistry 45, 1511–1513.
). The pH of the solution after the experiment (during the duplicate analyses) and the concentration of FeO in the rock samples are also shown.top
Discussion
Although the actual mechanism of H2O2 production from pulverised mineral surfaces is not completely understood, Hendrix et al. (2019)
Hendrix, D.A., Port, S.T., Hurowitz, J.A., Schoonen, M.A. (2019) Measurement of OH* generation by pulverized minerals using electron spin resonance spectroscopy and implications for the reactivity of planetary regolith. GeoHealth 3, 28–42, doi: 10.1029/2018GH000175.
argued that Si−O· on a newly created mineral surface reacts with H2O to generate H2O2 as follows:Eq. 1

Hurowitz et al. (2007
Hurowitz, J.A., Tosca, N.J., McLennan, S.M., Schoonen, M.A.A. (2007) Production of hydrogen peroxide in Martian and lunar soils. Earth and Planetary Science Letters 255, 41–52, doi: 10.1016/j.epsl.2006.12.004.
) conducted pulverisation experiments using various pure mineral types and found that H2O2 productivity depends on the crystallographic nature of silicate minerals, with productivity increasing with a decreasing number of corners shared between Si (Al) tetrahedra (here termed the “c” number; Fig. 2). For example, tectosilicate minerals such as quartz and plagioclase have c = 4 and show the lowest H2O2 productivity of <1.0 nmol m−2 of other types of silicate minerals with c < 4 (Hurowitz et al., 2007Hurowitz, J.A., Tosca, N.J., McLennan, S.M., Schoonen, M.A.A. (2007) Production of hydrogen peroxide in Martian and lunar soils. Earth and Planetary Science Letters 255, 41–52, doi: 10.1016/j.epsl.2006.12.004.
; Fig. 2). As the present work used rock samples that included different types of silicate minerals, the weighted mean of c of each sample was estimated by XRD analysis (Table S-3). Figure 2 presents the relationship between H2O2 productivity and c of the analysed rock samples, and shows that the data are broadly consistent with the results of Hurowitz et al. (2007)Hurowitz, J.A., Tosca, N.J., McLennan, S.M., Schoonen, M.A.A. (2007) Production of hydrogen peroxide in Martian and lunar soils. Earth and Planetary Science Letters 255, 41–52, doi: 10.1016/j.epsl.2006.12.004.
. Our results reveal that H2O2 can be generated by activation of natural rocks irrespective of their type (i.e. sedimentary, metamorphic, and igneous rocks), and the amount generated is likely to depend on the mineral composition of the rock.
Figure 2 H2O2 productivity vs. number of corners shared between silica tetrahedra (weighted mean) for the analysed rocks. The open symbol is data for the experiment using 0.01 M NaCl solution. Data and regression line from Hurowitz et al. (2007)
Hurowitz, J.A., Tosca, N.J., McLennan, S.M., Schoonen, M.A.A. (2007) Production of hydrogen peroxide in Martian and lunar soils. Earth and Planetary Science Letters 255, 41–52, doi: 10.1016/j.epsl.2006.12.004.
are also shown.Stability of H2O2 in fault zones following slip. Since fault movement causes comminution of fault rocks, H2O2 is expected to be produced by the reaction with porewater after an earthquake event. Furthermore, larger slip events may also lead to greater amount of comminution (Scholz, 2002
Scholz, C.H. (2002) The mechanics of earthquake and faulting. Second Edition, Cambridge University Press, Cambridge.
; Hirose et al., 2012Hirose, T., Mizoguchi, K., Shimamoto, T. (2012) Wear processes in rocks at slow to high slip rates. Journal of Structural Geology 38, 102–116, doi: 10.1016/j.jsg.2011.12.007.
), which would produce more H2O2. In a natural fault zone environment, generated H2O2 reacts with Fe-bearing mineral surfaces or Fe2+ in porewater to produce more oxidative ·OH, as follows (i.e. the Fenton reaction):Eq. 2

In addition, H2O2 decomposes to two ·OH at high temperature conditions. The rate coefficient of the decomposition reaction is described as k = 6.4 × 105 exp(−71 kJ mol−1 / RT) s−1 (Takagi and Ishigure, 1985
Takagi, J., Ishigure, K. (1985) Thermal decomposition of hydrogen peroxide and its effect on reactor water monitoring of boiling water reactors. Nuclear Science and Engineering 89, 177.
). This suggests that the half-life of H2O2 (i.e. ln(2)/k) is 158 min at 100 °C but is only ∼1 min at 200 °C. In the supercritical condition (T > 374 °C), the activation energy of the decomposition reaction can be higher (182 kJ mol−1; Croiset et al., 2004Croiset, E., Rice, S.F., Hanush, R.G. (2004) Hydrogen peroxide decomposition in supercritical water. Reactors, Kinetics, and Catalysis 43, 2343–2352.
), but the half-life is again very short (e.g., several seconds at 400 °C). Thus, it is presumed that H2O2 quickly decomposes to ·OH after an earthquake, and fluid containing these oxidative compounds may diffuse along the fault zone.Influence of oxidising fluid generation on surrounding fault zones. In general, an earthquake does not occur in isolation but occurs as part of a sequence controlled by physical processes such as stress perturbation (Scholz, 2002
Scholz, C.H. (2002) The mechanics of earthquake and faulting. Second Edition, Cambridge University Press, Cambridge.
). Porewater diffusion is usually invoked as a reason for aftershock sequence (e.g., Nur and Booker, 1972Nur, A., Booker, J.R. (1972) Aftershocks caused by pore fluid flow? Science 175, 885–887.
), but only the physical aspect of water (i.e. fluid pressure) has been argued in these models. Our study indicates the importance of chemical interaction between the fluid and surrounding fault zones. Specifically, generation of highly oxidising fluid can potentially promote corrosion of nearby fault zones and stimulate further fault activity. Such oxidising fluid is thought to interact with Fe-bearing minerals as well as organic compounds (Chen et al., 2017Chen, Q., Kang, Y., You, L., Yang, P., Zhang, X., Cheng, Q. (2017) Change in composition and pore structure of Longmaxi black shale during oxidative dissolution. International Journal of Coal Geology 172, 95–111.
; Zhou et al., 2018Zhou, Y., Lei, Y., Cheng, W., Yu, W. (2018) Enhance low temperature oxidization of shale gas recovery using hydrogen peroxide. Journal of Petroleum Science and Engineering 164, 523–530.
). ·OH oxidises even graphite (Wang and Zhang, 2018Wang, X., Zhang, L. (2018) Green and facile production of high-quality graphene from graphite by the combination of hydroxyl radical and electrical exfoliation. RSC Advances 8, 40621.
), a common product of the thermal maturation of organic compounds in metamorphic rocks, and may therefore degrade fault zones containing such minerals and materials. In natural examples, degradation or consumption of graphite has been observed in some fault zones (Nishiyama et al., 1990Nishiyama, T. (1990) CO2-metasomatism of a metabasite block in a serpentine mélange from the Nishisonogi metamorphic rocks, southwest Japan. Contributions to Mineralogy and Petrology 104, 35–46.
; Kretz, 1996Kretz, R. (1996) Graphite deformation in marble and mylonitic marble, Grenville Province, Canadian Shield. Journal of Metamorphic Geology 14, 399–412.
; Nakamura et al., 2015Nakamura, Y., Oohashi, K., Toyoshima, T., Satish-Kumar, M., Akai, J. (2015) Strain-induced amorphization of graphite in fault zones of the Hidaka metamorphic belt, Hokkaido, Japan. Journal of Structural Geology 72, 142–161.
), and the involvement of oxidising fluids in these processes has been suggested (Nishiyama, 1990Nishiyama, T. (1990) CO2-metasomatism of a metabasite block in a serpentine mélange from the Nishisonogi metamorphic rocks, southwest Japan. Contributions to Mineralogy and Petrology 104, 35–46.
; Okamoto et al., 2021Okamoto, A., Oyanagi, R., Yoshida, K., Uno, M., Shimizu, H., Satish-Kumar, M. (2021) Rupture of wet mantle wedge by self-promoting carbonation. Communications Earth & Environment 2, 151, doi: 10.1038/s43247-021-00224-5.
). In addition to mechanical processes, oxidative corrosion could promote such graphite degradation. However, it has not been fully clarified how the redox state of a fault temporally and spatially changes during the seismic cycle. For a more quantitative consideration, the kinetics of the reaction between oxidising fluid and various types of fault rocks and the lifetime of oxidising state of the fluid need to be clarified in future work, as well as how such fluid can affect the shear strength and frictional properties of the rock.top
Acknowledgments
We are grateful to Norifumi Abo (Central Institute of Isotope Science, Hokkaido University) for technical assistance. We also acknowledge two anonymous reviewers and editor Satish Myneni for their constructive comments, which greatly improved the manuscript. The analysers (Quantachrome Autosorb) are registered in the Open Facility system managed by the Global Facility Center, Creative Research Institution, Hokkaido University.
Editor: Satish Myneni
top
References
Chen, Q., Kang, Y., You, L., Yang, P., Zhang, X., Cheng, Q. (2017) Change in composition and pore structure of Longmaxi black shale during oxidative dissolution. International Journal of Coal Geology 172, 95–111.
 Show in context
Show in context For example, some lithologies such as shales have been shown to undergo a variety of degradation on interaction with H2O2 (formation of cracks and voids, decomposition and dissolution of constituent materials, etc.), which results in modification of their hydromechanical properties at varying rates depending on temperature (Chen et al., 2017; Zhou et al., 2018; Yu et al., 2019).
View in article
Such oxidising fluid is thought to interact with Fe-bearing minerals as well as organic compounds (Chen et al., 2017; Zhou et al., 2018).
View in article
Croiset, E., Rice, S.F., Hanush, R.G. (2004) Hydrogen peroxide decomposition in supercritical water. Reactors, Kinetics, and Catalysis 43, 2343–2352.
 Show in context
Show in context In the supercritical condition (T > 374 °C), the activation energy of the decomposition reaction can be higher (182 kJ mol−1; Croiset et al., 2004), but the half-life is again very short (e.g., several seconds at 400 °C).
View in article
Eberl, D.D. (2003) User’s guide to RockJock-A program for determining quantitative mineralogy from powder X-ray diffraction data. USGS Open-file Report 03–78.
 Show in context
Show in context Mineral compositions were analysed by X-ray diffraction analysis (XRD; MAC Science MX-Labo), and the obtained XRD patterns were used for quantitative analysis by RockJock software (Eberl, 2003).
View in article
Enami, M., Wallis, S.R., Banno, Y. (1994) Paragenesis of sodic pyroxene-bearing quartz schists: implications for P-T history of the Sanbagawa belt. Contributions to Mineralogy and Petrology 116, 182–198.
 Show in context
Show in context The basic schist samples (basic schist 1, basic schist 2, and basic schist 3) were taken from the chlorite, garnet, and albite–biotite zones of the Sanbagawa belt, respectively, which correspond to pressure-temperature (P-T) conditions ranging from greenschist to epidote-amphibolite facies (∼300 to ∼550 °C; Enami et al., 1994).
View in article
Georgiou, C.D., Sun, H.J., McKay, C.P., Grintzalis, K., Papapostolou, I., Zisimopoulos, D., Panagiotidis, K., Zhang, G., Koutsopoulou, E., Christidis, G.E., Margiolaki, I. (2015) Evidence for photochemical production of reactive oxygen species in desert soils. Nature Communications 6, 7100, doi: 10.1038/ncomms8100.
 Show in context
Show in context ROS are also formed due to reaction with reactive secondary minerals and photochemistry in soils and sediments (e.g., Georgiou et al., 2015).
View in article
Hacker, B.R., Peacock, S.M., Abers, G.A., Holloway, S.D. (2003) Subduction factory 2. Are intermediate-depth earthquakes in subducting slabs linked to metamorphic dehydration reactions? Journal of Geophysical Research: Solid Earth 108, 2030, doi: 10.1029/2001JB001127.
 Show in context
Show in context The samples are sedimentary, metamorphic, and igneous rocks that may constitute plate boundaries at various depths in subduction zones (Hacker et al., 2003; Kimura et al., 2012; Kameda et al., 2017).
View in article
Hendrix, D.A., Port, S.T., Hurowitz, J.A., Schoonen, M.A. (2019) Measurement of OH* generation by pulverized minerals using electron spin resonance spectroscopy and implications for the reactivity of planetary regolith. GeoHealth 3, 28–42, doi: 10.1029/2018GH000175.
 Show in context
Show in context Although the actual mechanism of H2O2 production from pulverised mineral surfaces is not completely understood, Hendrix et al. (2019) argued that Si−O· on a newly created mineral surface reacts with H2O to generate H2O2 as follows:
 Eq. 1
Eq. 1
View in article
In addition, numerous studies have demonstrated that pulverisation of pure powders of minerals forming igneous rocks can produce oxidising compounds such as hydrogen peroxide (H2O2), superoxide (O2·−), and hydroxy (·OH) radicals (Schoonen et al., 2006; Hurowitz et al., 2007; Hendrix et al., 2019), which are termed “reactive oxygen species” or “ROS”.
View in article
Hirose, T., Kawagucci, S., Suzuki, K. (2011) Mechanoradical H2 generation during simulated faulting: Implications for an earthquake-driven subsurface biosphere. Geophysical Research Letters 38, L17303, doi: 10.1029/2011GL048850.
 Show in context
Show in context The production of H2 by the interaction of fluids and comminuted rocks has been demonstrated by laboratory experiments (Kita et al., 1982; Kameda et al., 2003; Hirose et al., 2011) and that associated with seismic activity has been observed by field survey (Wakita et al., 1980; Ito et al., 1999).
View in article
Hirose, T., Mizoguchi, K., Shimamoto, T. (2012) Wear processes in rocks at slow to high slip rates. Journal of Structural Geology 38, 102–116, doi: 10.1016/j.jsg.2011.12.007.
 Show in context
Show in context Furthermore, larger slip events may also lead to greater amount of comminution (Scholz, 2002; Hirose et al., 2012), which would produce more H2O2.
View in article
Hochstrasser, G., Antonini, J.F. (1972) Surface states of pristine silica surfaces I. ESR studies of Es’ dangling bonds and of CO2-adsorbed radicals. Surface Science 32, 644–664.
 Show in context
Show in context Radical species such as Si· and SiO· are commonly created on such particles of silicate minerals owing to homolytic rupture of Si–O covalent bonds (Hochstrasser and Antonini, 1972; Narayanasamy and Kubicki, 2005).
View in article
Hurowitz, J.A., Tosca, N.J., McLennan, S.M., Schoonen, M.A.A. (2007) Production of hydrogen peroxide in Martian and lunar soils. Earth and Planetary Science Letters 255, 41–52, doi: 10.1016/j.epsl.2006.12.004.
 Show in context
Show in context Hurowitz et al. (2007) conducted pulverisation experiments using various pure mineral types and found that H2O2 productivity depends on the crystallographic nature of silicate minerals, with productivity increasing with a decreasing number of corners shared between Si (Al) tetrahedra (here termed the “c” number; Fig. 2).
View in article
For example, tectosilicate minerals such as quartz and plagioclase have c = 4 and show the lowest H2O2 productivity of <1.0 nmol m−2 of other types of silicate minerals with c < 4 (Hurowitz et al., 2007; Fig. 2).
View in article
Figure 2 presents the relationship between H2O2 productivity and c of the analysed rock samples, and shows that the data are broadly consistent with the results of Hurowitz et al. (2007).
View in article
Data and regression line from Hurowitz et al. (2007) are also shown.
View in article
In addition, numerous studies have demonstrated that pulverisation of pure powders of minerals forming igneous rocks can produce oxidising compounds such as hydrogen peroxide (H2O2), superoxide (O2·−), and hydroxy (·OH) radicals (Schoonen et al., 2006; Hurowitz et al., 2007; Hendrix et al., 2019), which are termed “reactive oxygen species” or “ROS”.
View in article
Ito, T., Nagamine, K., Yamamoto, K., Adachi, M., Kawabe, I. (1999) Preseismic hydrogen gas anomalies caused by stress-corrosion process preceding earthquakes. Geophysical Research Letters 26, 2009–2012.
 Show in context
Show in context The production of H2 by the interaction of fluids and comminuted rocks has been demonstrated by laboratory experiments (Kita et al., 1982; Kameda et al., 2003; Hirose et al., 2011) and that associated with seismic activity has been observed by field survey (Wakita et al., 1980; Ito et al., 1999).
View in article
Kameda, J., Saruwatari, K., Tanaka, H. (2003) H2 generation in wet grinding of granite and single-crystal powders and implications for H2 concentration on active faults. Geophysical Research Letters 30, 2063, doi: 10.1029/2003GL018252.
 Show in context
Show in context The production of H2 by the interaction of fluids and comminuted rocks has been demonstrated by laboratory experiments (Kita et al., 1982; Kameda et al., 2003; Hirose et al., 2011) and that associated with seismic activity has been observed by field survey (Wakita et al., 1980; Ito et al., 1999).
View in article
Kameda, J., Inoue, S., Tanikawa, W., Yamaguchi, A., Hamada, Y., Hashimoto, Y., Kimura, G. (2017) Alteration and dehydration of subducting oceanic crust within subduction zones: implications for decollement step-down and plate-boundary seismogenesis. Earth Planets and Space 69, 52, doi: 10.1186/s40623-017-0635-1.
 Show in context
Show in context The samples are sedimentary, metamorphic, and igneous rocks that may constitute plate boundaries at various depths in subduction zones (Hacker et al., 2003; Kimura et al., 2012; Kameda et al., 2017).
View in article
Kimura, G., Yamaguchi, A., Hojo, M., Kitamura, Y., Kameda, J., Ujiie, K., Hamada, Y., Hamahashi, M., Hina, S. (2012) Tectonic mélange as fault rock of subduction plate boundary. Tectonophysics 568, 25–38.
 Show in context
Show in context The samples are sedimentary, metamorphic, and igneous rocks that may constitute plate boundaries at various depths in subduction zones (Hacker et al., 2003; Kimura et al., 2012; Kameda et al., 2017).
View in article
Kita, I., Matsuo, S., Wakita, H. (1982) H2 generation by reaction between H2O and crushed rock: An experimental study on H2 degassing from the active fault zone. Journal of Geophysical Research: Solid Earth 87, 10789–10795.
 Show in context
Show in context The production of H2 by the interaction of fluids and comminuted rocks has been demonstrated by laboratory experiments (Kita et al., 1982; Kameda et al., 2003; Hirose et al., 2011) and that associated with seismic activity has been observed by field survey (Wakita et al., 1980; Ito et al., 1999).
View in article
Kretz, R. (1996) Graphite deformation in marble and mylonitic marble, Grenville Province, Canadian Shield. Journal of Metamorphic Geology 14, 399–412.
 Show in context
Show in context In natural examples, degradation or consumption of graphite has been observed in some fault zones (Nishiyama et al., 1990; Kretz, 1996; Nakamura et al., 2015), and the involvement of oxidising fluids in these processes has been suggested (Nishiyama, 1990; Okamoto et al., 2021).
View in article
Larsen, I.L., Hartmann, N.A., Wagner, J.J. (1973) Estimating precision for the method of standard additions. Analytical Chemistry 45, 1511–1513.
 Show in context
Show in context Error bars for H2O2 productivity were estimated from the equation for the standard addition method (Larsen et al., 1973).
View in article
Nakamura, Y., Oohashi, K., Toyoshima, T., Satish-Kumar, M., Akai, J. (2015) Strain-induced amorphization of graphite in fault zones of the Hidaka metamorphic belt, Hokkaido, Japan. Journal of Structural Geology 72, 142–161.
 Show in context
Show in context In natural examples, degradation or consumption of graphite has been observed in some fault zones (Nishiyama et al., 1990; Kretz, 1996; Nakamura et al., 2015), and the involvement of oxidising fluids in these processes has been suggested (Nishiyama, 1990; Okamoto et al., 2021).
View in article
Narayanasamy, J., Kubicki, J.D. (2005) Mechanism of Hydroxyl Radical Generation from a Silica Surface: Molecular Orbital Calculations. The Journal of Physical Chemistry B 109, 21796–21807.
 Show in context
Show in context Radical species such as Si· and SiO· are commonly created on such particles of silicate minerals owing to homolytic rupture of Si–O covalent bonds (Hochstrasser and Antonini, 1972; Narayanasamy and Kubicki, 2005).
View in article
Nishiyama, T. (1990) CO2-metasomatism of a metabasite block in a serpentine mélange from the Nishisonogi metamorphic rocks, southwest Japan. Contributions to Mineralogy and Petrology 104, 35–46.
 Show in context
Show in context In natural examples, degradation or consumption of graphite has been observed in some fault zones (Nishiyama et al., 1990; Kretz, 1996; Nakamura et al., 2015), and the involvement of oxidising fluids in these processes has been suggested (Nishiyama, 1990; Okamoto et al., 2021).
View in article
Nur, A., Booker, J.R. (1972) Aftershocks caused by pore fluid flow? Science 175, 885–887.
 Show in context
Show in context Porewater diffusion is usually invoked as a reason for aftershock sequence (e.g., Nur and Booker, 1972), but only the physical aspect of water (i.e. fluid pressure) has been argued in these models.
View in article
Okamoto, A., Oyanagi, R., Yoshida, K., Uno, M., Shimizu, H., Satish-Kumar, M. (2021) Rupture of wet mantle wedge by self-promoting carbonation. Communications Earth & Environment 2, 151, doi: 10.1038/s43247-021-00224-5.
 Show in context
Show in context In natural examples, degradation or consumption of graphite has been observed in some fault zones (Nishiyama et al., 1990; Kretz, 1996; Nakamura et al., 2015), and the involvement of oxidising fluids in these processes has been suggested (Nishiyama, 1990; Okamoto et al., 2021).
View in article
Sammis, C.G., Osborne, R.H., Anderson, J.L., Banerdt, M., White, P. (1986) Self-similar cataclasis in the formation of fault gouge. Pure and Applied Geophysics 124, 53–78.
 Show in context
Show in context Frictional slip on a fault zone causes comminution and wearing of fault rocks, producing highly reactive fine particles (Sammis et al., 1986; Scholz, 2002).
View in article
Scholz, C.H. (2002) The mechanics of earthquake and faulting. Second Edition, Cambridge University Press, Cambridge.
 Show in context
Show in context Furthermore, larger slip events may also lead to greater amount of comminution (Scholz, 2002; Hirose et al., 2012), which would produce more H2O2.
View in article
In general, an earthquake does not occur in isolation but occurs as part of a sequence controlled by physical processes such as stress perturbation (Scholz, 2002).
View in article
Frictional slip on a fault zone causes comminution and wearing of fault rocks, producing highly reactive fine particles (Sammis et al., 1986; Scholz, 2002).
View in article
Schoonen, M.A.A., Cohn, C.A., Roemer, E., Laffers, R., Simon, S.R., O’Riordan, T. (2006) Mineral-induced formation of reactive oxygen species. Reviews in Mineralogy and Geochemistry 64, 179–221.
 Show in context
Show in context ROS are formed by human activities such as mining, which produces fine particles of rocks and minerals (Schoonen et al., 2006).
View in article
In addition, numerous studies have demonstrated that pulverisation of pure powders of minerals forming igneous rocks can produce oxidising compounds such as hydrogen peroxide (H2O2), superoxide (O2·−), and hydroxy (·OH) radicals (Schoonen et al., 2006; Hurowitz et al., 2007; Hendrix et al., 2019), which are termed “reactive oxygen species” or “ROS”.
View in article
Takagi, J., Ishigure, K. (1985) Thermal decomposition of hydrogen peroxide and its effect on reactor water monitoring of boiling water reactors. Nuclear Science and Engineering 89, 177.
 Show in context
Show in context The rate coefficient of the decomposition reaction is described as k = 6.4 × 105 exp(−71 kJ mol−1 / RT) s−1 (Takagi and Ishigure, 1985).
View in article
Wakita, H., Nakamura, Y., Kita, I., Fujii, N., Notsu, K. (1980) Hydrogen release: New indicator of fault activity. Science 210, 188–190, doi: 10.1126/science.210.4466.188.
 Show in context
Show in context The production of H2 by the interaction of fluids and comminuted rocks has been demonstrated by laboratory experiments (Kita et al., 1982; Kameda et al., 2003; Hirose et al., 2011) and that associated with seismic activity has been observed by field survey (Wakita et al., 1980; Ito et al., 1999).
View in article
Wang, X., Zhang, L. (2018) Green and facile production of high-quality graphene from graphite by the combination of hydroxyl radical and electrical exfoliation. RSC Advances 8, 40621.
 Show in context
Show in context ·OH oxidises even graphite (Wang and Zhang, 2018), a common product of the thermal maturation of organic compounds in metamorphic rocks, and may therefore degrade fault zones containing such minerals and materials.
View in article
Yu, W.G., Lei, J., Wang, T., Chen, W. (2019) H2O2-enhanced shale gas recovery under different thermal conditions. Energies 12, 2127, doi: 10.3390/en12112127.
 Show in context
Show in context For example, some lithologies such as shales have been shown to undergo a variety of degradation on interaction with H2O2 (formation of cracks and voids, decomposition and dissolution of constituent materials, etc.), which results in modification of their hydromechanical properties at varying rates depending on temperature (Chen et al., 2017; Zhou et al., 2018; Yu et al., 2019).
View in article
Zhou, Y., Lei, Y., Cheng, W., Yu, W. (2018) Enhance low temperature oxidization of shale gas recovery using hydrogen peroxide. Journal of Petroleum Science and Engineering 164, 523–530.
 Show in context
Show in context For example, some lithologies such as shales have been shown to undergo a variety of degradation on interaction with H2O2 (formation of cracks and voids, decomposition and dissolution of constituent materials, etc.), which results in modification of their hydromechanical properties at varying rates depending on temperature (Chen et al., 2017; Zhou et al., 2018; Yu et al., 2019).
View in article
Such oxidising fluid is thought to interact with Fe-bearing minerals as well as organic compounds (Chen et al., 2017; Zhou et al., 2018).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Materials and Methods
- Tables S-1 to S-3
- Figure S-1
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures
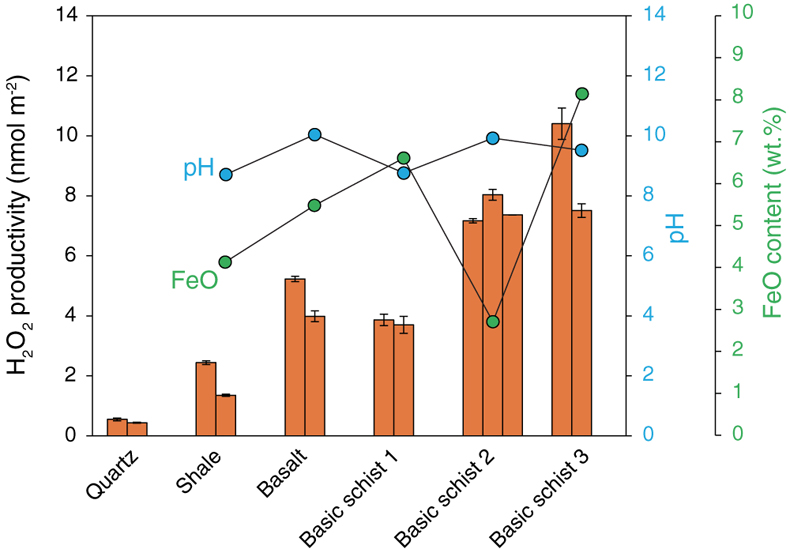
Figure 1 H2O2 productivity of the analysed samples. Error bars for H2O2 productivity were estimated from the equation for the standard addition method (Larsen et al., 1973
Larsen, I.L., Hartmann, N.A., Wagner, J.J. (1973) Estimating precision for the method of standard additions. Analytical Chemistry 45, 1511–1513.
). The pH of the solution after the experiment (during the duplicate analyses) and the concentration of FeO in the rock samples are also shown.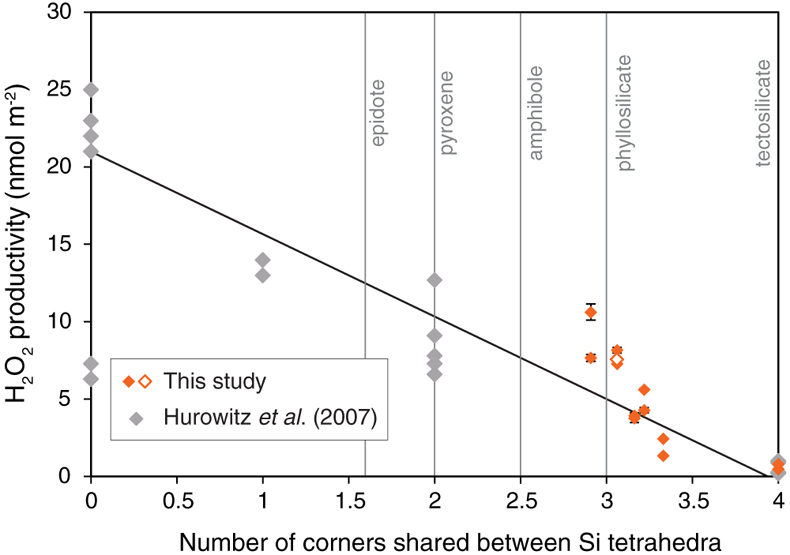
Figure 2 H2O2 productivity vs. number of corners shared between silica tetrahedra (weighted mean) for the analysed rocks. The open symbol is data for the experiment using 0.01 M NaCl solution. Data and regression line from Hurowitz et al. (2007)
Hurowitz, J.A., Tosca, N.J., McLennan, S.M., Schoonen, M.A.A. (2007) Production of hydrogen peroxide in Martian and lunar soils. Earth and Planetary Science Letters 255, 41–52, doi: 10.1016/j.epsl.2006.12.004.
are also shown.