Nitrogen isotope fractionation during magma ocean degassing: tracing the composition of early Earth’s atmosphere
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:2,855Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
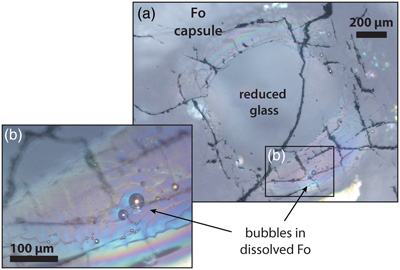 Figure 1 Optical microscope photograph of sample V125-PB8N, which contains 13,342 ± 841 ppm N and lost 15 % of its initial N content by degassing. The reduced glasses contain aligned microscopic bubbles, likely produced during quenching. Large bubbles, produced by degassing at high pressure and high temperature, are located within a rim of dissolved forsterite (Fo) at the border between the glass and the forsterite capsule. The dissolved forsterite contains negligible amounts of N (2 ± 2 ppm). | 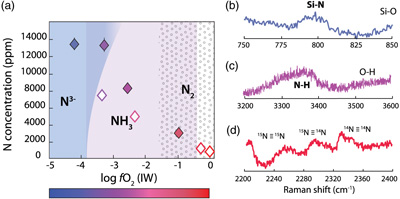 Figure 2 (a) N concentrations measured by SIMS in reduced glasses synthesised at variable fO2 conditions, as represented by the colour scale. Open symbols represent samples that did not reach saturation. Raman spectra show (b) Si-N vibrations in sample V125-PB8N and also observed in sample V150-PB8N6Si, (c) N-H vibrations in sample V148-PB4N and also observed in all samples between IW−4 and IW−1 (pink shaded area in (a)), and (d) N2 vibrations in sample V141-PB1N, corrected for air contamination, and also observed in samples V152-PB05N and V129-PB2N. | 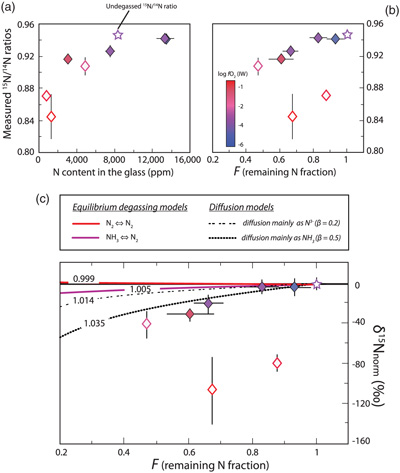 Figure 3 (a) 15N/14N ratios as a function of N content in glass, and (b) 15N/14N ratios, and (c) δ15N values normalised to the initial value (see text) in reduced glasses as a function of F, the fraction of N remaining. F was calculated by dividing the measured N content in the glass by the estimated N content added to the starting material. Because all starting compositions were prepared from the same initial N-rich powder by variable mixes with a N-free powder, the initial 15N/14N ratios were the same in all starting materials. The degassing models are described in the text. | 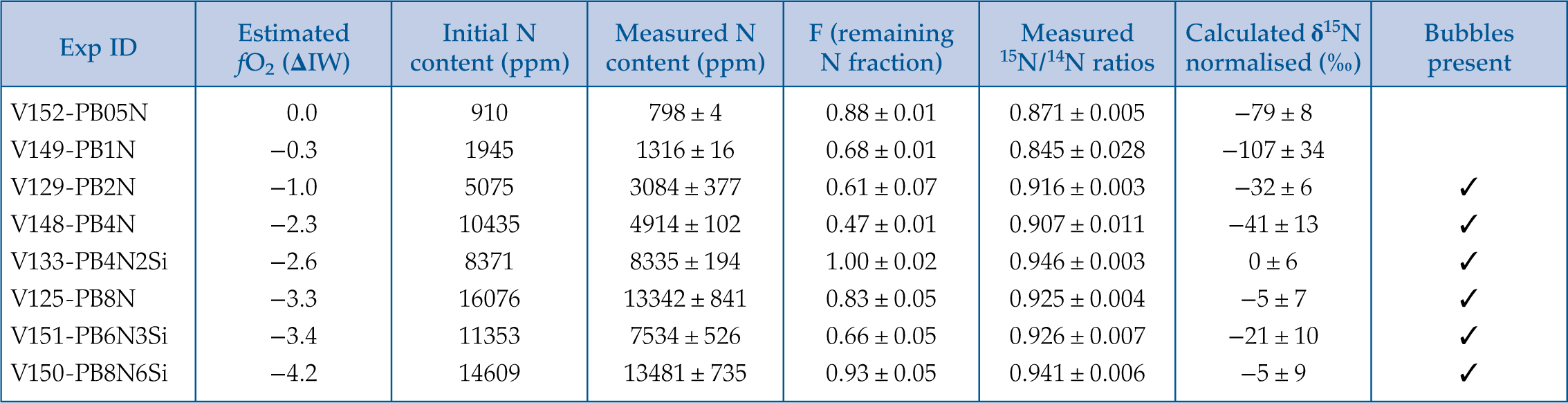 Table 1 Experimental results on nitrogen contents and isotope fractionation in silicate glasses. |
| Figure 1 | Figure 2 | Figure 3 | Table 1 |
top
Introduction
The accretion of reduced building blocks during Earth’s formation released enough energy to at least partially melt the Earth, forming one or more magma oceans (Elkins-Tanton, 2012
Elkins-Tanton, L.T. (2012) Magma oceans in the inner solar system. Annual Review of Earth and Planetary Sciences 40, 113–139.
). The evolving nitrogen abundance in the degassing magma ocean, and thus in Earth’s early atmosphere, was fundamental to the development of habitable conditions and the maintenance of the terrestrial atmosphere and biosphere (Goldblatt et al., 2009Goldblatt, C., Claire, M.W., Lenton, T.M., Matthews, A.J., Watson, A.J., Zahnle, K.J. (2009) Nitrogen-enhanced greenhouse warming on early Earth. Nature Geosciences 2, 891–896.
). Yet, the composition of Earth’s early atmosphere remains debated: it is thought to have been either i) neutral to oxidising and composed of H2O, CO2, and N2 if it resembled modern volcanic gases (Kasting, 1993Kasting, J.F. (1993) Earth’s early atmosphere. Science 259, 920–926.
), or ii) reducing and CH4- and NH3-rich if it degassed from the reduced materials that formed the Earth (Zahnle et al., 2020Zahnle, K.J., Lupu, R., Catling, D.C., Wogan, N. (2020) Creation and evolution of impact-generated reduced atmospheres of early Earth. Planetary Science Journal 1, 11.
), based on geochemical evidence that magma ocean degassing contributed to the formation of the Hadean atmosphere (see review by Gaillard et al., 2021Gaillard, F., Bouhifd, M.A., Füri, E., Malavergne, V., Marrocchi, Y., Noack, L., Ortenzi, G., Roskosz, M., Vulpius, S. (2021) The Diverse Planetary Ingassing/Outgassing Paths Produced over Billions of Years of Magmatic Activity. Space Science Reviews 217, 1–54.
). Therefore, reconstructing nitrogen degassing during Earth’s magma ocean stage(s) is critical to constraining the composition of the early atmosphere.Nitrogen is often considered to be an inert molecule (N2) and expected to behave somewhat like noble gases. However, recent works have shown that nitrogen is not always chemically inert and occurs as various species (N3−, NH3, NH2−, NH2− and N2) in silicate melts (Mikhail and Sverjensky, 2014
Mikhail, S., Sverjensky, D.A. (2014) Nitrogen speciation in upper mantle fluids and the origin of Earth’s nitrogen-rich atmosphere. Nature Geosciences 7, 816–819.
; Dalou et al., 2019aDalou, C., Hirschmann, M.M., Jacobsen, S.D., Le Losq, C. (2019a) Raman spectroscopy study of C-O-H-N speciation in reduced basaltic glasses: Implications for reduced planetary mantles. Geochimica et Cosmochimica Acta 265, 32–47.
; Mosenfelder et al., 2019Mosenfelder, J.L., Von Der Handt, A., Füri, E., Dalou, C., Hervig, R.L., Rossman, G.R., Hirschmann, M.M. (2019) Nitrogen incorporation in silicates and metals: Results from SIMS, EPMA, FTIR, and laser-extraction mass spectrometry. American Mineralogist 104, 31–46.
; Boulliung et al., 2020Boulliung, J., Füri, E., Dalou, C., Tissandier, L., Zimmermann, L., Marrocchi, Y. (2020) Oxygen fugacity and melt composition controls on nitrogen solubility in silicate melts. Geochimica et Cosmochimica Acta 284, 120–133.
; Grewal et al., 2020Grewal, D.S., Dasgupta, R., Farnell, A. (2020) The speciation of carbon, nitrogen, and water in magma oceans and its effect on volatile partitioning between major reservoirs of the Solar System rocky bodies. Geochimica et Cosmochimica Acta 280, 281–301.
) and fluids (Li and Keppler, 2014Li, Y., Keppler, H. (2014) Nitrogen speciation in mantle and crustal fluids. Geochimica et Cosmochimica Acta 129, 13–32.
). The speciation and solubility of nitrogen primarily depend on oxygen fugacity (Libourel et al., 2003Libourel, G., Marty, B., Humbert, F. (2003) Nitrogen solubility in basaltic melt. Part I. Effect of oxygen fugacity. Geochimica et Cosmochimica Acta 67, 4123–4135.
; Boulliung et al., 2020Boulliung, J., Füri, E., Dalou, C., Tissandier, L., Zimmermann, L., Marrocchi, Y. (2020) Oxygen fugacity and melt composition controls on nitrogen solubility in silicate melts. Geochimica et Cosmochimica Acta 284, 120–133.
; Bernadou et al., 2021Bernadou, F., Gaillard, F., Füri, E., Marrocchi, Y., Slodczyk, A. (2021) Nitrogen solubility in basaltic silicate melt-Implications for degassing processes. Chemical Geology 573, 120192.
), which increased during the evolution of Earth’s magma ocean (Frost et al., 2008Frost, D.J., Mann, U., Asahara, Y., Rubie, D.C. (2008) The redox state of the mantle during and just after core formation. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 366, 4315–4337.
). Thus, the rate of nitrogen degassing and the reactions involved must have evolved similarly over geological time.Nitrogen isotopic compositions, conventionally normalised to the present atmospheric value and reported as
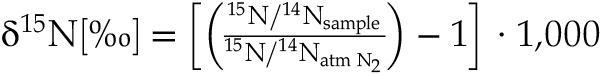 , are useful for reconstructing Earth’s N degassing history. For instance, the range of δ15N values observed in Archean diamonds corresponds to that in present day diamonds and mid-ocean ridge basalts (MORBs), implying that the mantle δ15N value has not evolved since the Archean (Cartigny and Marty, 2013
, are useful for reconstructing Earth’s N degassing history. For instance, the range of δ15N values observed in Archean diamonds corresponds to that in present day diamonds and mid-ocean ridge basalts (MORBs), implying that the mantle δ15N value has not evolved since the Archean (Cartigny and Marty, 2013Cartigny, P., Marty, B. (2013) Nitrogen isotopes and mantle geodynamics: The emergence of life and the atmosphere–crust–mantle connection. Elements 9, 359–366.
). This is consistent with the observation that diffusion controlled N2 degassing from the present day mantle, which is expected to preferentially segregate 14N into the atmosphere following Graham’s law (i.e. the rate of diffusion/effusion of a gas is inversely proportional to the square root of its molecular weight; Javoy et al., 1986Javoy, M., Pineau, F., Delorme, H. (1986) Carbon and nitrogen isotopes in the mantle. Chemical Geology 57, 41–62.
), is limited to isotopic fractionations of 1–1.5 ‰ (Marty and Dauphas, 2003Marty, B., Dauphas, N. (2003) The nitrogen record of crust–mantle interaction and mantle convection from Archean to present. Earth and Planetary Sciences Letters 206, 397–410.
). However, Rayleigh models of equilibrium MORB degassing (Javoy et al., 1986Javoy, M., Pineau, F., Delorme, H. (1986) Carbon and nitrogen isotopes in the mantle. Chemical Geology 57, 41–62.
; Cartigny et al., 2001Cartigny, P., Jendrzejewski, N., Pineau, F., Petit, E., Javoy, M. (2001) Volatile (C, N, Ar) variability in MORB and the respective roles of mantle source heterogeneity and degassing: the case of the Southwest Indian Ridge. Earth and Planetary Science Letters 194, 241–257.
) only consider the reaction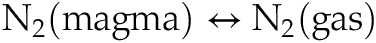 . This reaction has been experimentally determined at oxygen fugacity conditions (fO2) > IW (Libourel et al., 2003
. This reaction has been experimentally determined at oxygen fugacity conditions (fO2) > IW (Libourel et al., 2003Libourel, G., Marty, B., Humbert, F. (2003) Nitrogen solubility in basaltic melt. Part I. Effect of oxygen fugacity. Geochimica et Cosmochimica Acta 67, 4123–4135.
; Boulliung et al., 2020Boulliung, J., Füri, E., Dalou, C., Tissandier, L., Zimmermann, L., Marrocchi, Y. (2020) Oxygen fugacity and melt composition controls on nitrogen solubility in silicate melts. Geochimica et Cosmochimica Acta 284, 120–133.
), which applies to present day conditions of upper mantle degassing. However, because the integrated fO2 during core formation was closer to IW–2 (Frost et al., 2008Frost, D.J., Mann, U., Asahara, Y., Rubie, D.C. (2008) The redox state of the mantle during and just after core formation. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 366, 4315–4337.
), this reaction does not hold for the more reduced N species present in the magma ocean. Larger N isotopic fractionations are expected under reducing conditions at which N does not behave inertly. Therefore, quantifying N isotopic fractionations under conditions at which N dissolves in magmas as nitride or N-H complexes is prerequisite to quantitatively modelling the evolution of N concentrations and isotopic compositions during the formation of the Hadean atmosphere.top
Degassing Experiments and Results
To establish the effect of fO2 on the N degassing rate and identify N degassing reactions that may have occurred in the Earth’s magma ocean, we determined the N concentration, speciation, and isotopic fractionation of a basaltic komatiite (used as magma ocean analogue melt) during degassing at 1.5 GPa and 1550 °C in pure forsterite capsules. Degassing experiments were performed using a series of Fe-free primitive basalts in which we explored fO2 conditions ranging from IW to IW−4.2 (Table 1). These fO2 conditions were not determined in the Fe-free experiments, but only estimated afterwards from the IW reaction in equivalent Fe-bearing runs (see Supplementary Information). The initial 15N/14N ratio of the starting material was 0.946 ± 0.003, as determined in sample V133-PB4N2Si (Table 1), in which the initial N content was equal, within errors, to that measured after the experiment. Nitrogen was saturated in five experiments, as evidenced by bubbles in the quenched glass and/or surrounding forsterite (Fig. 1, Table 1).
Table 1 Experimental results on nitrogen contents and isotope fractionation in silicate glasses.
| Exp ID | Estimated fO2 (ΔIW) | Initial N content (ppm) | Measured N content (ppm) | F (remaining N fraction) | Measured 15N/14N ratios | Calculated δ15N normalised (‰) | Bubbles present |
| V152-PB05N | 0.0 | 910 | 798 ± 4 | 0.88 ± 0.01 | 0.871 ± 0.005 | −79 ± 8 | |
| V149-PB1N | −0.3 | 1945 | 1316 ± 16 | 0.68 ± 0.01 | 0.845 ± 0.028 | −107 ± 34 | |
| V129-PB2N | −1.0 | 5075 | 3084 ± 377 | 0.61 ± 0.07 | 0.916 ± 0.003 | −32 ± 6 | ✓ |
| V148-PB4N | −2.3 | 10435 | 4914 ± 102 | 0.47 ± 0.01 | 0.907 ± 0.011 | −41 ± 13 | ✓ |
| V133-PB4N2Si | −2.6 | 8371 | 8335 ± 194 | 1.00 ± 0.02 | 0.946 ± 0.003 | 0 ± 6 | ✓ |
| V125-PB8N | −3.3 | 16076 | 13342 ± 841 | 0.83 ± 0.05 | 0.925 ± 0.004 | −5 ± 7 | ✓ |
| V151-PB6N3Si | −3.4 | 11353 | 7534 ± 526 | 0.66 ± 0.05 | 0.926 ± 0.007 | −21 ± 10 | ✓ |
| V150-PB8N6Si | −4.2 | 14609 | 13481 ± 735 | 0.93 ± 0.05 | 0.941 ± 0.006 | −5 ± 9 | ✓ |

Figure 1 Optical microscope photograph of sample V125-PB8N, which contains 13,342 ± 841 ppm N and lost 15 % of its initial N content by degassing. The reduced glasses contain aligned microscopic bubbles, likely produced during quenching. Large bubbles, produced by degassing at high pressure and high temperature, are located within a rim of dissolved forsterite (Fo) at the border between the glass and the forsterite capsule. The dissolved forsterite contains negligible amounts of N (2 ± 2 ppm).
The major element compositions of the silicate glasses were measured by electron microprobe (Table S-1). Nitrogen concentrations and 15N/14N ratios were determined by secondary ion mass spectrometry (SIMS) using the CAMECA IMS 1280-HR2 at the CRPG (Table 1). On each sample, four to six spot analyses of 14N16O− and 15N16O− were performed (see Supplementary Information). Measured N concentrations were homogeneous (i.e. with standard deviations below 10 %). N concentrations at 1.5 GPa and 1550 °C decreased from 13,481 ± 735 ppm (at IW−4.2) to 798 ± 4 ppm (at IW; Fig. 2a). In N saturated samples (as attested by the presence of bubbles), N concentrations decreased from 13,481 ± 735 ppm (at IW−4.2) to 3,084 ± 377 ppm (at IW–1). This decrease of N solubility with increasing fO2 is consistent with observations in silicate melts at 1 atm (Libourel et al., 2003
Libourel, G., Marty, B., Humbert, F. (2003) Nitrogen solubility in basaltic melt. Part I. Effect of oxygen fugacity. Geochimica et Cosmochimica Acta 67, 4123–4135.
; Boulliung et al., 2020Boulliung, J., Füri, E., Dalou, C., Tissandier, L., Zimmermann, L., Marrocchi, Y. (2020) Oxygen fugacity and melt composition controls on nitrogen solubility in silicate melts. Geochimica et Cosmochimica Acta 284, 120–133.
) and modelled at pressures up to 1 GPa (Bernadou et al., 2021Bernadou, F., Gaillard, F., Füri, E., Marrocchi, Y., Slodczyk, A. (2021) Nitrogen solubility in basaltic silicate melt-Implications for degassing processes. Chemical Geology 573, 120192.
). Yet, the N solubilities observed herein are up to three orders of magnitude higher than those in a similar melt composition and at similar temperatures at 1 atm (Boulliung et al., 2020Boulliung, J., Füri, E., Dalou, C., Tissandier, L., Zimmermann, L., Marrocchi, Y. (2020) Oxygen fugacity and melt composition controls on nitrogen solubility in silicate melts. Geochimica et Cosmochimica Acta 284, 120–133.
) and one order of magnitude higher than those at 0.08 GPa (Bernardou et al., 2021), consistent with the effect of pressure on N solubility described by Bernardou et al. (2021). The variation of N solubilities with fO2 is directly related to changes of N speciation in the silicate melts, which were determined by Raman spectroscopy (see Supplementary Information). Around IW−4 and below, N is very soluble because it is dissolved as nitride, forming Si-N bonds with the silicate network; indeed, in very N-rich samples (>1 wt. % N), Si-N vibrations around 800 cm−1 were observed (Fig. 2b). In less reduced samples (fO2 > IW−4), N is mostly dissolved as N-H complexes (Fig. 2c), and above IW−2, N is also dissolved as molecular N2 (Fig. 2d). Consistent with previous studies (Mosenfelder et al., 2019Mosenfelder, J.L., Von Der Handt, A., Füri, E., Dalou, C., Hervig, R.L., Rossman, G.R., Hirschmann, M.M. (2019) Nitrogen incorporation in silicates and metals: Results from SIMS, EPMA, FTIR, and laser-extraction mass spectrometry. American Mineralogist 104, 31–46.
; Grewal et al., 2020Grewal, D.S., Dasgupta, R., Farnell, A. (2020) The speciation of carbon, nitrogen, and water in magma oceans and its effect on volatile partitioning between major reservoirs of the Solar System rocky bodies. Geochimica et Cosmochimica Acta 280, 281–301.
), N speciation in the silicate glasses controls N solubility variations with fO2 conditions. Depending on initial N content and fO2, the fraction of N degassed varied from 7 to 53 %. Samples V152-PB0.5N and V149-PB1N did not show any evidence of degassing, i.e. no bubbles were observed in the glass or the forsterite rim, but lost 12 and 32 % of their initial N, respectively, likely due to diffusion through cracks formed in the forsterite capsule during compression.
Figure 2 (a) N concentrations measured by SIMS in reduced glasses synthesised at variable fO2 conditions, as represented by the colour scale. Open symbols represent samples that did not reach saturation. Raman spectra show (b) Si-N vibrations in sample V125-PB8N and also observed in sample V150-PB8N6Si, (c) N-H vibrations in sample V148-PB4N and also observed in all samples between IW−4 and IW−1 (pink shaded area in (a)), and (d) N2 vibrations in sample V141-PB1N, corrected for air contamination, and also observed in samples V152-PB05N and V129-PB2N.
The use of 15N spiked samples permits us to obtain precise 15N/14N ratios in the silicate (with low uncertainties of 0.1 ‰ on average). With increasing fraction of degassed N, 15N/14N ratios decreased from 0.946 ± 0.003 in the undegassed sample to 0.907 ± 0.011 in the most degassed sample (53 % of initial N lost). The 15N/14N ratios were normalised to the initial 15N/14N ratio of the undegassed glass V133-PB4N2Si (0.946 ± 0.003) and expressed as δ15Nnorm as:
Eq. 1
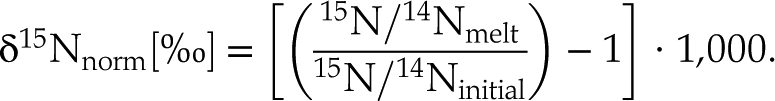
These normalised values are not calculated relative to the atmospheric N isotopic composition (15N/14Nair = 0.003676) and cannot be directly compared to the δ15N values of natural samples.
top
Degassing Models of N Isotopic Fractionation under Reduced Conditions
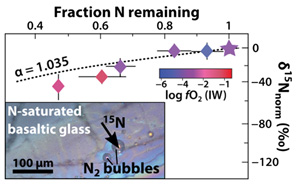
The normalised N isotopic composition of the glasses decreases from 0 ‰ in the undegassed glass to −41 ± 13 ‰ in the most degassed glass (Fig. 3). Although this δ15N variation is opposite to that observed during MORB degassing (Cartigny et al., 2001
Cartigny, P., Jendrzejewski, N., Pineau, F., Petit, E., Javoy, M. (2001) Volatile (C, N, Ar) variability in MORB and the respective roles of mantle source heterogeneity and degassing: the case of the Southwest Indian Ridge. Earth and Planetary Science Letters 194, 241–257.
), it is consistent with glasses becoming isotopically lighter with increased degassing as observed for H, C, and S in natural settings (Supplementary Information). An open system degassing model, in which the exsolved vapour is immediately removed from contact with the melt during degassing, is suitable with the presence of gas bubbles within the dissolved forsterite rims (Fig. 1):Eq. 2
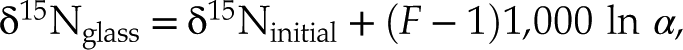
where δ15Ninitial is the “normalised initial” isotopic composition of the melt (here 0 ‰; sample V133-PB4N2Si), F is the fraction of N remaining in the melt, and α is the isotopic fractionation factor. In this case, open system degassing occurs in a finite N budget, conforming to a Rayleigh process (Equation 2).

Figure 3 (a) 15N/14N ratios as a function of N content in glass, and (b) 15N/14N ratios, and (c) δ15N values normalised to the initial value (see text) in reduced glasses as a function of F, the fraction of N remaining. F was calculated by dividing the measured N content in the glass by the estimated N content added to the starting material. Because all starting compositions were prepared from the same initial N-rich powder by variable mixes with a N-free powder, the initial 15N/14N ratios were the same in all starting materials. The degassing models are described in the text.
In bubbles for which the gas pressure was sufficient to obtain a Raman signal above the noise, N was observed only as N2, even in very reduced samples in which N was dissolved as N-H complexes (V148-PB4N) and/or nitrides (V125-PB8N). Therefore, we propose that at IW to IW−1, the N degassing reaction is:
Eq. 3
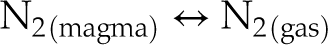
Had the least reduced samples (V152-PB0.5N and V149-PB1N) not been affected by diffusion through cracks, they would be expected to degas following Equation 3, since N2 is the only N species dissolved in those melts. In contrast, under more reduced conditions, N degassed as (e.g., Dalou et al., 2019a
Dalou, C., Hirschmann, M.M., Jacobsen, S.D., Le Losq, C. (2019a) Raman spectroscopy study of C-O-H-N speciation in reduced basaltic glasses: Implications for reduced planetary mantles. Geochimica et Cosmochimica Acta 265, 32–47.
):Eq. 4
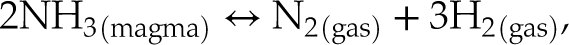
Eq. 5
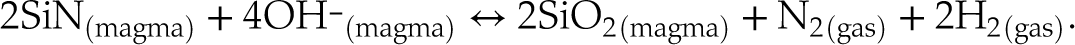
The fugacities of the relevant N species cannot be calculated at present due to a lack of thermodynamic data (see Supplementary Information).
To understand how N speciation can affect gas-melt N isotopic fractionation, we derived the basic equation for equilibrium isotopic fractionation for stable isotopes (Young et al., 2015
Young, E.D., Manning, C.E., Schauble, E.A., Shahar, A., Macris, C.A., Lazar, C., Jordan, M. (2015) High-temperature equilibrium isotope fractionation of non-traditional stable isotopes: Experiments, theory, and applications. Chemical Geology 395, 176–195.
):Eq. 6
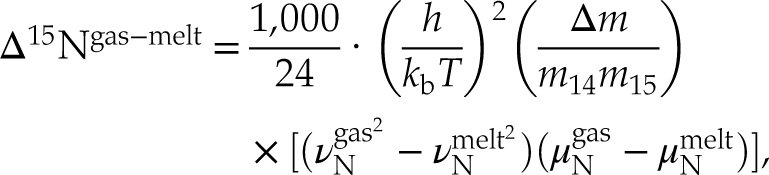
Eq. 7
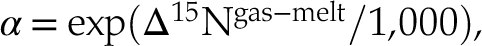
where h is Planck’s constant (J.K−1), kb is the Boltzmann constant (J.Hz−1), T is temperature (K), vgas and vmelt denote the Raman vibration modes (Hz) of N species in the gas and silicate phases, respectively, and μN is the reduced mass of the N-bearing molecule. Using this expression, we calculated that the N isotopic fractionation factor at 1550 °C is 0.999 for degassing by Equation 3, and, under more reduced conditions, 1.005 for Equation 4, both in agreement with Hanschmann (1981)
Hanschmann, G. (1981) Quantum chemical calculations of isotope effects in nitrogen-containing molecules. ZFI-Mitteilungen 41, 19–39.
, and 0.998 for Equation 5. These equilibrium fractionation factors cannot explain the variation of δ15Nnorm values observed in the degassed samples; the high isotopic fractionation undergone by these degassed melts rather suggests a kinetic process. Indeed, such large kinetic nitrogen isotopic effects have also been observed in mantle-derived samples (Yokochi et al., 2009Yokochi, R., Marty, B., Chazot, G., Burnard, P. (2009) Nitrogen in peridotite xenoliths: Lithophile behavior and magmatic isotope fractionation. Geochimica et Cosmochimica Acta 73, 4843–4861.
). However, kinetic fractionation via evaporation results in increasingly heavy isotopic values (Saal and Hauri, 2021Saal, A.E., Hauri, E.H. (2021) Large sulfur isotope fractionation in lunar volcanic glasses reveals the magmatic differentiation and degassing of the Moon. Science Advances 7, eabe4641.
), opposite to our observations (Fig. 3c). The observed large isotopic fractionation may thus be explained by a variable diffusion process depending on N speciation, and therefore on fO2 conditions. The magnitude of diffusive N isotopic fractionation during degassing is similar to that observed in natural high pressure-high temperature peridotitic systems (>25 ‰; Yokochi et al., 2009Yokochi, R., Marty, B., Chazot, G., Burnard, P. (2009) Nitrogen in peridotite xenoliths: Lithophile behavior and magmatic isotope fractionation. Geochimica et Cosmochimica Acta 73, 4843–4861.
; see Supplementary Information). Such a fractionation effect can be estimated from the ratio of the diffusion coefficients (D) of 14N and 15N, expressed as a function of their mass (m):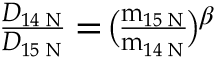 , where β is an empirical parameter with values between 0 and 0.5 that positively correlates with the diffusivity of the ion in silicate melts (Van Orman and Krawczynski, 2015
, where β is an empirical parameter with values between 0 and 0.5 that positively correlates with the diffusivity of the ion in silicate melts (Van Orman and Krawczynski, 2015Van Orman, J.A., Krawczynski, M.J. (2015) Theoretical constraints on the isotope effect for diffusion in minerals. Geochimica et Cosmochimica Acta 164, 365–381.
). Hence, N2 and NH3, which diffuse rapidly through silicate melts relative to Si, have a large isotopic effect (high β), whereas N3−, which is bonded to Si, diffuses slowly (Boulliung et al., 2021Boulliung, J., Dalou, C., Tissandier, L., Füri, E., Marrocchi, Y. (2021) Nitrogen diffusion in silicate melts under reducing conditions. American Mineralogist 106, 662–666.
) and has a smaller isotopic effect (low β). Good fits to our data are β = 0.2 for N diffusing as N3− and β = 0.5 for N as NH3 (Fig. 3c). A simple diffusion process cannot explain the extremely large fractionation in undegassed samples (V152-PB0.5N and V149-PB1N), unless the diffusion of 14N and 15N is decoupled, with 15N favouring the stiffest bonds (N2) and diffusing significantly faster than 14N, favouring weaker N-H bonds in NH3. It is possible that N2 diffusion in these melts is faster than degassing and controls the isotopic fractionation mechanism in these undegassed samples.top
Evolution of the N Composition of Earth’s Primitive Atmosphere
Figure 3 shows that fO2 conditions determine N solubility and, therefore, the fraction of N degassed and the isotopic fractionation trends. Our results suggest that the upper layer of Earth’s magma ocean degassed N as N2, regardless of fO2 conditions, but that the intensity of N2 degassing increased as the magma ocean became less reduced. This is in agreement with the recent model of Sossi et al. (2020)
Sossi, P.A., Burnham, A.D., Badro, J., Lanzirotti, A., Newville, M., O’Neill, H.S.C. (2020) Redox state of Earth’s magma ocean and its Venus-like early atmosphere. Science Advances 6, eabd1387.
, which predicts that Earth’s prebiotic atmosphere was dominated by CO2 and N2 gases.Whether N was dissolved in the magma ocean as nitride, ammonia, or both, N loss depleted the surface or upper parts of the magma ocean in 15N relative to the atmosphere. This could have produced the currently observed isotopic differences between the 15N-depleted mantle (δ15N = –5 ± 2 ‰ in diamonds and MORBs) and the 15N-enriched surface (δ15N = +6 ‰ in sediments and δ15N = 0 ‰, by definition, in the atmosphere) (Cartigny and Marty, 2013
Cartigny, P., Marty, B. (2013) Nitrogen isotopes and mantle geodynamics: The emergence of life and the atmosphere–crust–mantle connection. Elements 9, 359–366.
). Other explanations of the N isotopic distribution among terrestrial reservoirs include i) recycling (subduction) of surficial materials, and ii) core–mantle differentiation (Li et al., 2016Li, Y.F., Marty, B., Shcheka, S., Zimmermann, L., Keppler, H. (2016) Nitrogen isotope fractionation during terrestrial core-mantle separation. Geochemical Perspectives Letters 2, 138–147.
; Dalou et al., 2019bDalou, C., Füri, E., Deligny, C., Piani, L., Caumon, M.C., Laumonier, M., Boulliung, J., Edén, M. (2019b) Redox control on nitrogen isotope fractionation during planetary core formation. Proceedings of the National Academy of Sciences 116, 14485–14494.
) However, Labidi et al. (2020)Labidi, J., Barry, P.H., Bekaert, D.V., Broadley, M.W., Marty, B., Giunta, T., Warr, O., Sherwood Lollar, B., Fisher, T.B., Avice, G., Caracausi, A., Ballentine, C.J., Halldórsson, S.A., Stefánsson, A., Kurz, M.D., Kohl, I.E., Young, E.D. (2020) Hydrothermal 15N15N abundances constrain the origins of mantle nitrogen. Nature 580, 367–371.
recently showed that N recycling is inefficient. How core–mantle differentiation would have affected the upper layer of the magma ocean and thus the present day depleted mantle, would have depended on the depth of the magma ocean and the efficiency of post-magma ocean convection to homogenise the δ15N value of the mantle; thus this process remains disputable.top
Acknowledgements
We thank Johan Villeneuve for his assistance and support during SIMS analyses, Jean-Luc Devidal for performing microprobe analyses on silicate glasses, and Marie-Camille Caumon for her help with Raman spectroscopy analyses. We are grateful to Robert Dennen for English editing. We thank reviewers Paolo Sossi and Sami Mikhail for their comments that greatly improved the manuscript, and editor Helen Williams for handling the manuscript. This work was supported by the European Research Council under the European Union’s Horizon 2020 research and innovation program (Grant Agreement no. 715028). This is CRPG contribution 2812.
Editor: Helen Williams
top
References
Bernadou, F., Gaillard, F., Füri, E., Marrocchi, Y., Slodczyk, A. (2021) Nitrogen solubility in basaltic silicate melt-Implications for degassing processes. Chemical Geology 573, 120192. https://doi.org/10.1016/j.chemgeo.2021.120192
 Show in context
Show in context The speciation and solubility of nitrogen primarily depend on oxygen fugacity (Libourel et al., 2003; Boulliung et al., 2020; Bernadou et al., 2021), which increased during the evolution of Earth’s magma ocean (Frost et al., 2008).
View in article
This decrease of N solubility with increasing fO2 is consistent with observations in silicate melts at 1 atm (Libourel et al., 2003; Boulliung et al., 2020) and modelled at pressures up to 1 GPa (Bernadou et al., 2021).
View in article
Boulliung, J., Füri, E., Dalou, C., Tissandier, L., Zimmermann, L., Marrocchi, Y. (2020) Oxygen fugacity and melt composition controls on nitrogen solubility in silicate melts. Geochimica et Cosmochimica Acta 284, 120–133. https://doi.org/10.1016/j.gca.2020.06.020
 Show in context
Show in context The speciation and solubility of nitrogen primarily depend on oxygen fugacity (Libourel et al., 2003; Boulliung et al., 2020; Bernadou et al., 2021), which increased during the evolution of Earth’s magma ocean (Frost et al., 2008).
View in article
This reaction has been experimentally determined at oxygen fugacity conditions (fO2) > IW (Libourel et al., 2003; Boulliung et al., 2020), which applies to present day conditions of upper mantle degassing.
View in article
This decrease of N solubility with increasing fO2 is consistent with observations in silicate melts at 1 atm (Libourel et al., 2003; Boulliung et al., 2020) and modelled at pressures up to 1 GPa (Bernadou et al., 2021).
View in article
Yet, the N solubilities observed herein are up to three orders of magnitude higher than those in a similar melt composition and at similar temperatures at 1 atm (Boulliung et al., 2020) and one order of magnitude higher than those at 0.08 GPa (Bernardou et al., 2021), consistent with the effect of pressure on N solubility described by Bernardou et al. (2021).
View in article
However, recent works have shown that nitrogen is not always chemically inert and occurs as various species (N3−, NH3, NH2−, NH2− and N2) in silicate melts (Mikhail and Sverjensky, 2014; Dalou et al., 2019a; Mosenfelder et al., 2019; Boulliung et al., 2020; Grewal et al., 2020) and fluids (Li and Keppler, 2014).
View in article
Boulliung, J., Dalou, C., Tissandier, L., Füri, E., Marrocchi, Y. (2021) Nitrogen diffusion in silicate melts under reducing conditions. American Mineralogist 106, 662–666. https://doi.org/10.2138/am-2021-7799CCBYNCND
 Show in context
Show in context Hence, N2 and NH3, which diffuse rapidly through silicate melts relative to Si, have a large isotopic effect (high β), whereas N3−, which is bonded to Si, diffuses slowly (Boulliung et al., 2021) and has a smaller isotopic effect (low β).
View in article
Cartigny, P., Marty, B. (2013) Nitrogen isotopes and mantle geodynamics: The emergence of life and the atmosphere–crust–mantle connection. Elements 9, 359–366. https://doi.org/10.2113/gselements.9.5.359
 Show in context
Show in context For instance, the range of δ15N values observed in Archean diamonds corresponds to that in present day diamonds and mid-ocean ridge basalts (MORBs), implying that the mantle δ15N value has not evolved since the Archean (Cartigny and Marty, 2013).
View in article
This could have produced the currently observed isotopic differences between the 15N-depleted mantle (δ15N = –5 ± 2 ‰ in diamonds and MORBs) and the 15N-enriched surface (δ15N = +6 ‰ in sediments and δ15N = 0 ‰, by definition, in the atmosphere) (Cartigny and Marty, 2013).
View in article
Cartigny, P., Jendrzejewski, N., Pineau, F., Petit, E., Javoy, M. (2001) Volatile (C, N, Ar) variability in MORB and the respective roles of mantle source heterogeneity and degassing: the case of the Southwest Indian Ridge. Earth and Planetary Science Letters 194, 241–257. https://doi.org/10.1016/S0012-821X(01)00540-4
 Show in context
Show in context Although this δ15N variation is opposite to that observed during MORB degassing (Cartigny et al., 2001), it is consistent with glasses becoming isotopically lighter with increased degassing as observed for H, C, and S in natural settings (Supplementary Information).
View in article
However, Rayleigh models of equilibrium MORB degassing (Javoy et al., 1986; Cartigny et al., 2001) only consider the reaction 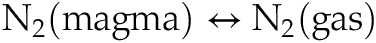
View in article
Dalou, C., Hirschmann, M.M., Jacobsen, S.D., Le Losq, C. (2019a) Raman spectroscopy study of C-O-H-N speciation in reduced basaltic glasses: Implications for reduced planetary mantles. Geochimica et Cosmochimica Acta 265, 32–47. https://doi.org/10.1016/j.gca.2019.08.029
 Show in context
Show in context In contrast, under more reduced conditions, N degassed as (e.g., Dalou et al., 2019a):
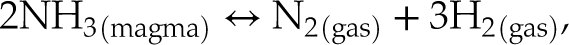 Eq. 4
Eq. 4
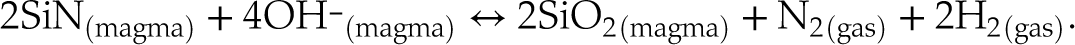 Eq. 5
Eq. 5
View in article
However, recent works have shown that nitrogen is not always chemically inert and occurs as various species (N3−, NH3, NH2−, NH2− and N2) in silicate melts (Mikhail and Sverjensky, 2014; Dalou et al., 2019a; Mosenfelder et al., 2019; Boulliung et al., 2020; Grewal et al., 2020) and fluids (Li and Keppler, 2014).
View in article
Dalou, C., Füri, E., Deligny, C., Piani, L., Caumon, M.C., Laumonier, M., Boulliung, J., Edén, M. (2019b) Redox control on nitrogen isotope fractionation during planetary core formation. Proceedings of the National Academy of Sciences 116, 14485–14494. https://doi.org/10.1073/pnas.1820719116
 Show in context
Show in context Other explanations of the N isotopic distribution among terrestrial reservoirs include i) recycling (subduction) of surficial materials, and ii) core–mantle differentiation (Li et al., 2016; Dalou et al., 2019b) However, Labidi et al. (2020) recently showed that N recycling is inefficient.
View in article
Elkins-Tanton, L.T. (2012) Magma oceans in the inner solar system. Annual Review of Earth and Planetary Sciences 40, 113–139. https://doi.org/10.1146/annurev-earth-042711-105503
 Show in context
Show in context The accretion of reduced building blocks during Earth’s formation released enough energy to at least partially melt the Earth, forming one or more magma oceans (Elkins-Tanton, 2012).
View in article
Frost, D.J., Mann, U., Asahara, Y., Rubie, D.C. (2008) The redox state of the mantle during and just after core formation. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 366, 4315–4337. https://doi.org/10.1098/rsta.2008.0147
 Show in context
Show in context However, because the integrated fO2 during core formation was closer to IW–2 (Frost et al., 2008), this reaction does not hold for the more reduced N species present in the magma ocean.
View in article
The speciation and solubility of nitrogen primarily depend on oxygen fugacity (Libourel et al., 2003; Boulliung et al., 2020; Bernadou et al., 2021), which increased during the evolution of Earth’s magma ocean (Frost et al., 2008).
View in article
Gaillard, F., Bouhifd, M.A., Füri, E., Malavergne, V., Marrocchi, Y., Noack, L., Ortenzi, G., Roskosz, M., Vulpius, S. (2021) The Diverse Planetary Ingassing/Outgassing Paths Produced over Billions of Years of Magmatic Activity. Space Science Reviews 217, 1–54. https://doi.org/10.1007/s11214-021-00802-1
 Show in context
Show in context Yet, the composition of Earth’s early atmosphere remains debated: it is thought to have been either i) neutral to oxidising and composed of H2O, CO2, and N2 if it resembled modern volcanic gases (Kasting, 1993), or ii) reducing and CH4- and NH3-rich if it degassed from the reduced materials that formed the Earth (Zahnle et al., 2020), based on geochemical evidence that magma ocean degassing contributed to the formation of the Hadean atmosphere (see review by Gaillard et al., 2021).
View in article
Goldblatt, C., Claire, M.W., Lenton, T.M., Matthews, A.J., Watson, A.J., Zahnle, K.J. (2009) Nitrogen-enhanced greenhouse warming on early Earth. Nature Geosciences 2, 891–896. https://doi.org/10.1038/ngeo692
 Show in context
Show in context The evolving nitrogen abundance in the degassing magma ocean, and thus in Earth’s early atmosphere, was fundamental to the development of habitable conditions and the maintenance of the terrestrial atmosphere and biosphere (Goldblatt et al., 2009).
View in article
Grewal, D.S., Dasgupta, R., Farnell, A. (2020) The speciation of carbon, nitrogen, and water in magma oceans and its effect on volatile partitioning between major reservoirs of the Solar System rocky bodies. Geochimica et Cosmochimica Acta 280, 281–301. https://doi.org/10.1016/j.gca.2020.04.023
 Show in context
Show in context Consistent with previous studies (Mosenfelder et al., 2019; Grewal et al., 2020), N speciation in the silicate glasses controls N solubility variations with fO2 conditions.
View in article
However, recent works have shown that nitrogen is not always chemically inert and occurs as various species (N3−, NH3, NH2−, NH2− and N2) in silicate melts (Mikhail and Sverjensky, 2014; Dalou et al., 2019a; Mosenfelder et al., 2019; Boulliung et al., 2020; Grewal et al., 2020) and fluids (Li and Keppler, 2014).
View in article
Hanschmann, G. (1981) Quantum chemical calculations of isotope effects in nitrogen-containing molecules. ZFI-Mitteilungen 41, 19–39.
 Show in context
Show in context Using this expression, we calculated that the N isotopic fractionation factor at 1550 °C is 0.999 for degassing by Equation 3, and, under more reduced conditions, 1.005 for Equation 4, both in agreement with Hanschmann (1981), and 0.998 for Equation 5.
View in article
Javoy, M., Pineau, F., Delorme, H. (1986) Carbon and nitrogen isotopes in the mantle. Chemical Geology 57, 41–62. https://doi.org/10.1016/0009-2541(86)90093-8
 Show in context
Show in context This is consistent with the observation that diffusion controlled N2 degassing from the present day mantle, which is expected to preferentially segregate 14N into the atmosphere following Graham’s law (i.e. the rate of diffusion/effusion of a gas is inversely proportional to the square root of its molecular weight; Javoy et al., 1986), is limited to isotopic fractionations of 1–1.5 ‰ (Marty and Dauphas, 2003).
View in article
However, Rayleigh models of equilibrium MORB degassing (Javoy et al., 1986; Cartigny et al., 2001) only consider the reaction 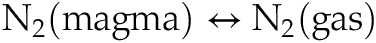
View in article
Kasting, J.F. (1993) Earth’s early atmosphere. Science 259, 920–926. https://doi.org/10.1126/science.11536547
 Show in context
Show in context Yet, the composition of Earth’s early atmosphere remains debated: it is thought to have been either i) neutral to oxidising and composed of H2O, CO2, and N2 if it resembled modern volcanic gases (Kasting, 1993), or ii) reducing and CH4- and NH3-rich if it degassed from the reduced materials that formed the Earth (Zahnle et al., 2020), based on geochemical evidence that magma ocean degassing contributed to the formation of the Hadean atmosphere (see review by Gaillard et al., 2021).
View in article
Labidi, J., Barry, P.H., Bekaert, D.V., Broadley, M.W., Marty, B., Giunta, T., Warr, O., Sherwood Lollar, B., Fisher, T.B., Avice, G., Caracausi, A., Ballentine, C.J., Halldórsson, S.A., Stefánsson, A., Kurz, M.D., Kohl, I.E., Young, E.D. (2020) Hydrothermal 15N15N abundances constrain the origins of mantle nitrogen. Nature 580, 367–371. https://doi.org/10.1038/s41586-020-2173-4
 Show in context
Show in context Other explanations of the N isotopic distribution among terrestrial reservoirs include i) recycling (subduction) of surficial materials, and ii) core–mantle differentiation (Li et al., 2016; Dalou et al., 2019b) However, Labidi et al. (2020) recently showed that N recycling is inefficient.
View in article
Li, Y., Keppler, H. (2014) Nitrogen speciation in mantle and crustal fluids. Geochimica et Cosmochimica Acta 129, 13–32. https://doi.org/10.1016/j.gca.2013.12.031
 Show in context
Show in context However, recent works have shown that nitrogen is not always chemically inert and occurs as various species (N3−, NH3, NH2−, NH2− and N2) in silicate melts (Mikhail and Sverjensky, 2014; Dalou et al., 2019a; Mosenfelder et al., 2019; Boulliung et al., 2020; Grewal et al., 2020) and fluids (Li and Keppler, 2014).
View in article
Li, Y.F., Marty, B., Shcheka, S., Zimmermann, L., Keppler, H. (2016) Nitrogen isotope fractionation during terrestrial core-mantle separation. Geochemical Perspectives Letters 2, 138–147. https://doi.org/10.7185/geochemlet.1614
 Show in context
Show in context Other explanations of the N isotopic distribution among terrestrial reservoirs include i) recycling (subduction) of surficial materials, and ii) core–mantle differentiation (Li et al., 2016; Dalou et al., 2019b) However, Labidi et al. (2020) recently showed that N recycling is inefficient.
View in article
Libourel, G., Marty, B., Humbert, F. (2003) Nitrogen solubility in basaltic melt. Part I. Effect of oxygen fugacity. Geochimica et Cosmochimica Acta 67, 4123–4135. https://doi.org/10.1016/S0016-7037(03)00259-X
 Show in context
Show in context The speciation and solubility of nitrogen primarily depend on oxygen fugacity (Libourel et al., 2003; Boulliung et al., 2020; Bernadou et al., 2021), which increased during the evolution of Earth’s magma ocean (Frost et al., 2008).
View in article
This reaction has been experimentally determined at oxygen fugacity conditions (fO2) > IW (Libourel et al., 2003; Boulliung et al., 2020), which applies to present day conditions of upper mantle degassing.
View in article
This decrease of N solubility with increasing fO2 is consistent with observations in silicate melts at 1 atm (Libourel et al., 2003; Boulliung et al., 2020) and modelled at pressures up to 1 GPa (Bernadou et al., 2021).
View in article
Marty, B., Dauphas, N. (2003) The nitrogen record of crust–mantle interaction and mantle convection from Archean to present. Earth and Planetary Sciences Letters 206, 397–410. https://doi.org/10.1016/S0012-821X(02)01108-1
 Show in context
Show in context This is consistent with the observation that diffusion controlled N2 degassing from the present day mantle, which is expected to preferentially segregate 14N into the atmosphere following Graham’s law (i.e. the rate of diffusion/effusion of a gas is inversely proportional to the square root of its molecular weight; Javoy et al., 1986), is limited to isotopic fractionations of 1–1.5 ‰ (Marty and Dauphas, 2003).
View in article
Mikhail, S., Sverjensky, D.A. (2014) Nitrogen speciation in upper mantle fluids and the origin of Earth’s nitrogen-rich atmosphere. Nature Geosciences 7, 816–819. https://doi.org/10.1038/ngeo2271
 Show in context
Show in context However, recent works have shown that nitrogen is not always chemically inert and occurs as various species (N3−, NH3, NH2−, NH2− and N2) in silicate melts (Mikhail and Sverjensky, 2014; Dalou et al., 2019a; Mosenfelder et al., 2019; Boulliung et al., 2020; Grewal et al., 2020) and fluids (Li and Keppler, 2014).
View in article
Mosenfelder, J.L., Von Der Handt, A., Füri, E., Dalou, C., Hervig, R.L., Rossman, G.R., Hirschmann, M.M. (2019) Nitrogen incorporation in silicates and metals: Results from SIMS, EPMA, FTIR, and laser-extraction mass spectrometry. American Mineralogist 104, 31–46. https://doi.org/10.2138/am-2019-6533
 Show in context
Show in context Consistent with previous studies (Mosenfelder et al., 2019; Grewal et al., 2020), N speciation in the silicate glasses controls N solubility variations with fO2 conditions.
View in article
However, recent works have shown that nitrogen is not always chemically inert and occurs as various species (N3−, NH3, NH2−, NH2− and N2) in silicate melts (Mikhail and Sverjensky, 2014; Dalou et al., 2019a; Mosenfelder et al., 2019; Boulliung et al., 2020; Grewal et al., 2020) and fluids (Li and Keppler, 2014).
View in article
Van Orman, J.A., Krawczynski, M.J. (2015) Theoretical constraints on the isotope effect for diffusion in minerals. Geochimica et Cosmochimica Acta 164, 365–381. https://doi.org/10.1016/j.gca.2015.04.051
 Show in context
Show in context Such a fractionation effect can be estimated from the ratio of the diffusion coefficients (D) of 14N and 15N, expressed as a function of their mass (m): 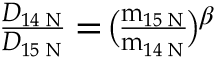 , where β is an empirical parameter with values between 0 and 0.5 that positively correlates with the diffusivity of the ion in silicate melts (Van Orman and Krawczynski, 2015).
, where β is an empirical parameter with values between 0 and 0.5 that positively correlates with the diffusivity of the ion in silicate melts (Van Orman and Krawczynski, 2015).
View in article
Saal, A.E., Hauri, E.H. (2021) Large sulfur isotope fractionation in lunar volcanic glasses reveals the magmatic differentiation and degassing of the Moon. Science Advances 7, eabe4641. https://doi.org/10.1126/sciadv.abe4641
 Show in context
Show in context However, kinetic fractionation via evaporation results in increasingly heavy isotopic values (Saal and Hauri, 2021), opposite to our observations (Fig. 3c).
View in article
Sossi, P.A., Burnham, A.D., Badro, J., Lanzirotti, A., Newville, M., O’Neill, H.S.C. (2020) Redox state of Earth’s magma ocean and its Venus-like early atmosphere. Science Advances 6, eabd1387. https://doi.org/10.1126/sciadv.abd1387
 Show in context
Show in context This is in agreement with the recent model of Sossi et al. (2020), which predicts that Earth’s prebiotic atmosphere was dominated by CO2 and N2 gases.
View in article
Yokochi, R., Marty, B., Chazot, G., Burnard, P. (2009) Nitrogen in peridotite xenoliths: Lithophile behavior and magmatic isotope fractionation. Geochimica et Cosmochimica Acta 73, 4843–4861. https://doi.org/10.1016/j.gca.2009.05.054
 Show in context
Show in context Indeed, such large kinetic nitrogen isotopic effects have also been observed in mantle-derived samples (Yokochi et al., 2009).
View in article
The magnitude of diffusive N isotopic fractionation during degassing is similar to that observed in natural high pressure-high temperature peridotitic systems (>25 ‰; Yokochi et al., 2009; see Supplementary Information).
View in article
Young, E.D., Manning, C.E., Schauble, E.A., Shahar, A., Macris, C.A., Lazar, C., Jordan, M. (2015) High-temperature equilibrium isotope fractionation of non-traditional stable isotopes: Experiments, theory, and applications. Chemical Geology 395, 176–195. https://doi.org/10.1016/j.chemgeo.2014.12.013
 Show in context
Show in context To understand how N speciation can affect gas-melt N isotopic fractionation, we derived the basic equation for equilibrium isotopic fractionation for stable isotopes (Young et al., 2015):
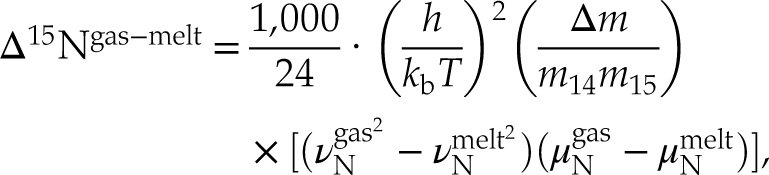 Eq. 6
Eq. 6
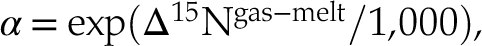 Eq. 7
Eq. 7
where h is Planck’s constant (J.K−1), kb is the Boltzmann constant (J.Hz−1), T is temperature (K), vgas and vmelt denote the Raman vibration modes (Hz) of N species in the gas and silicate phases, respectively, and μN is the reduced mass of the N-bearing molecule.
View in article
Zahnle, K.J., Lupu, R., Catling, D.C., Wogan, N. (2020) Creation and evolution of impact-generated reduced atmospheres of early Earth. Planetary Science Journal 1, 11. https://doi.org/10.3847/PSJ/ab7e2c
 Show in context
Show in context Yet, the composition of Earth’s early atmosphere remains debated: it is thought to have been either i) neutral to oxidising and composed of H2O, CO2, and N2 if it resembled modern volcanic gases (Kasting, 1993), or ii) reducing and CH4- and NH3-rich if it degassed from the reduced materials that formed the Earth (Zahnle et al., 2020), based on geochemical evidence that magma ocean degassing contributed to the formation of the Hadean atmosphere (see review by Gaillard et al., 2021).
View in article
top
Supplementary Information
The Supplementary Information includes:
- 1. Methods
- 2. Supplementary Discussion
- Tables S-1 and S-2
- Figures S-1 to S-3
- Supplementary Information References
Download the Supplementary Information (PDF).
Figures
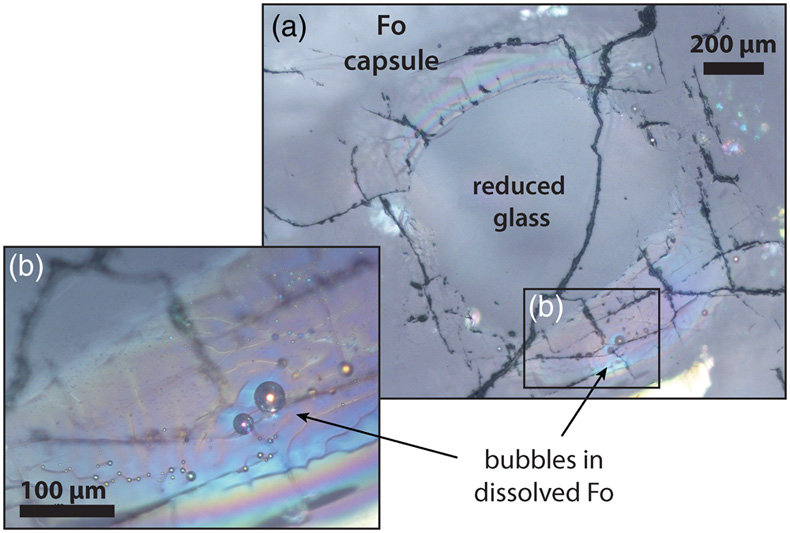
Figure 1 Optical microscope photograph of sample V125-PB8N, which contains 13,342 ± 841 ppm N and lost 15 % of its initial N content by degassing. The reduced glasses contain aligned microscopic bubbles, likely produced during quenching. Large bubbles, produced by degassing at high pressure and high temperature, are located within a rim of dissolved forsterite (Fo) at the border between the glass and the forsterite capsule. The dissolved forsterite contains negligible amounts of N (2 ± 2 ppm).
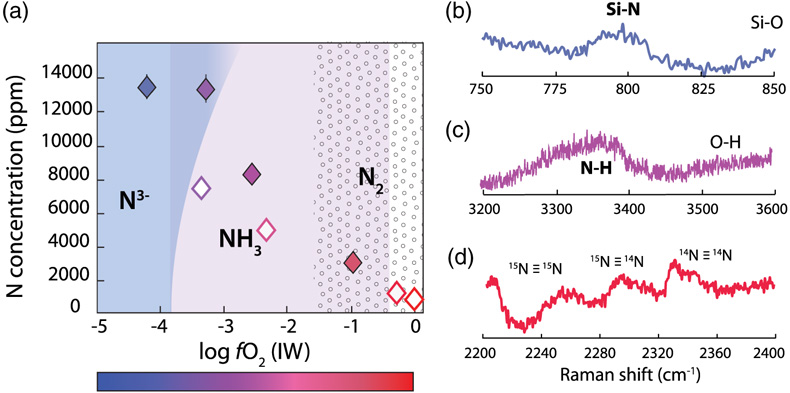
Figure 2 (a) N concentrations measured by SIMS in reduced glasses synthesised at variable fO2 conditions, as represented by the colour scale. Open symbols represent samples that did not reach saturation. Raman spectra show (b) Si-N vibrations in sample V125-PB8N and also observed in sample V150-PB8N6Si, (c) N-H vibrations in sample V148-PB4N and also observed in all samples between IW−4 and IW−1 (pink shaded area in (a)), and (d) N2 vibrations in sample V141-PB1N, corrected for air contamination, and also observed in samples V152-PB05N and V129-PB2N.
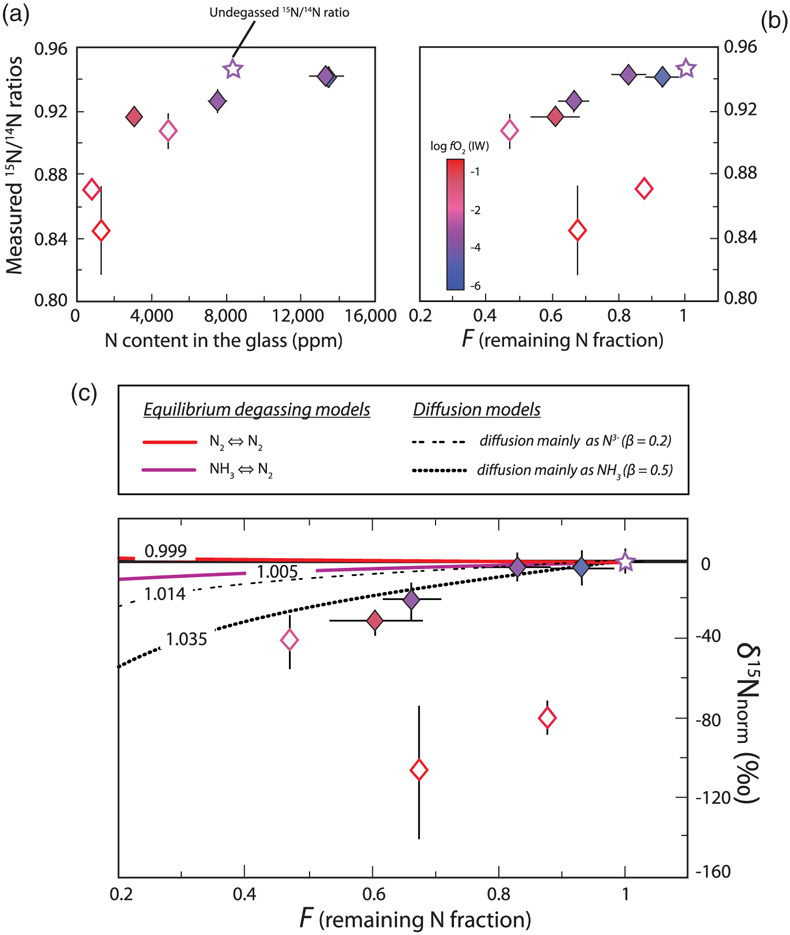
Figure 3 (a) 15N/14N ratios as a function of N content in glass, and (b) 15N/14N ratios, and (c) δ15N values normalised to the initial value (see text) in reduced glasses as a function of F, the fraction of N remaining. F was calculated by dividing the measured N content in the glass by the estimated N content added to the starting material. Because all starting compositions were prepared from the same initial N-rich powder by variable mixes with a N-free powder, the initial 15N/14N ratios were the same in all starting materials. The degassing models are described in the text.