Isotope evidence for the coupled iron and carbon cycles 1.4 billion years ago
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




Article views:111Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
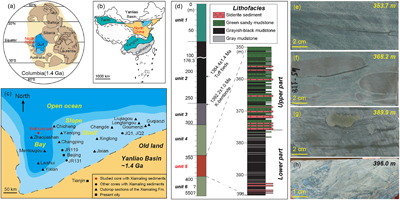 Figure 1 Location of the (a) 1.4 Ga and (b) present North China Craton, modified from Zhang et al. (2021). (c) Paleogeography of the Yanliao Basin 1.4 Ga, modified from Lyu et al. (2021). (d) Stratigraphy of iron formation defined as unit 5 of the Xiamaling Formation. Photos of laminar (e) and nodular (f–h) siderites from the core in the Xiahuayuan region. | 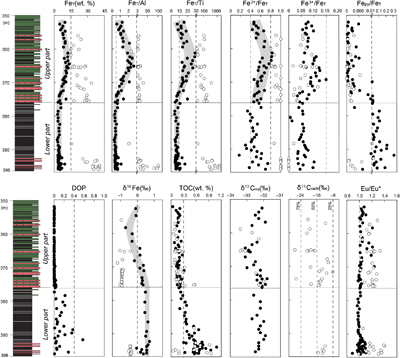 Figure 2 Geochemical profiles of the unit 5 of the Xiamaling Formation. Degree of pyritisation (DOP) was calculated by Fepy/(Fe2++Fe3++Fepy). Data of the FeT, total organic carbon (TOC), Eu/Eu*, carbon isotopes of carbonate (δ13Ccarb) and kerogen (δ13Corg) with a depth range of 390−350 m were from Canfield et al. (2018). Note the abscissas breaks of FeT/Al, FeT/Ti, Fepy/FeT are at 3, 30 and 0.01, respectively. Cycle and solid points represent IF (FeT > 15 %) and clastic (FeT < 15 %) samples, respectively. Dashed lines in the δ13Ccarb panel represent the proportions of organic-sourced bicarbonate of measured samples, with an initial δ13Corg of −32 ‰ and an initial δ13C of bicarbonate in water column of 0 ‰. | 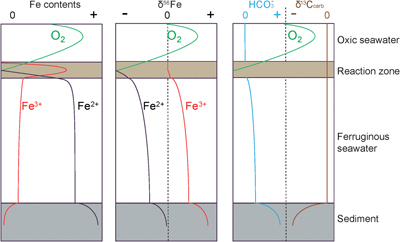 Figure 3 Genetic model of the coupled Fe and C cycles and isotope fractionations in the water column and sediment. | 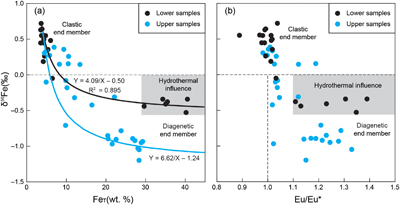 Figure 4 Correlation diagrams of δ56Fe with (a) FeT and (b) Eu/Eu*. The black and blue lines in (a) are the optimal mixing lines of the lower and upper parts, respectively, with a clastic end member and a diagenetic end member. Grey areas mean the Fe cycle and δ56Fe values were influenced by the hydrothermal fluids. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Most marine massive Fe deposits formed 3.0−1.8 Ga, with a billion-year hiatus until a brief return around 0.8 Ga (Konhauser et al., 2017
Konhauser, K.O., Planavsky, N.J., Hardisty, D.S., Robbins, L.J., Warchola, T.J., Haugaard, R., Lalonde, S.V., Partin, C.A., Oonk, P.B.H., Tsikos, H., Lyons, T.W., Bekker, A., Johnson, C.M. (2017) Iron formations: A global record of Neoarchaean to Palaeoproterozoic environmental history. Earth-Science Reviews 172, 140–177. https://doi.org/10.1016/j.earscirev.2017.06.012
). During this gap period, the iron formation (IF) shortage was thought to be a result of significantly decreased Fe2+ concentrations in the ocean, which was either substantially oxidised (Holland, 1990Holland, H.D. (1990) Origins of breathable air. Nature 347, 17–17. https://doi.org/10.1038/347017a0
) or sulfurised (Canfield, 1998Canfield, D.E. (1998) A new model for Proterozoic ocean chemistry. Nature 396, 450–453. https://doi.org/10.1038/24839
). Recent studies provide increasing evidence that ferruginous water was still widespread in the deep ocean until 0.58 Ga (Canfield et al., 2008Canfield, D.E., Poulton, S.W., Knoll, A.H., Narbonne, G.M., Ross, G., Goldberg, T., Strauss, H. (2008) Ferruginous conditions dominated later neoproterozoic deep-water chemistry. Science 321, 949–952. https://doi.org/10.1126/science.1154499
). Although atmospheric oxygen levels are still controversial in the time window of 1.8−0.8 Ga (Planavsky et al., 2014Planavsky, N.J., Reinhard, C.T., Wang, X.L., Thomson, D., McGoldrick, P., Rainbird, R.H., Johnson, T., Fischer, W.W., Lyons, T.W. (2014) Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 346, 635–638. https://doi.org/10.1126/science.1258410
; Zhang et al., 2016Zhang, S., Wang, X., Wang, H., Bjerrum, C.J., Hammarlund, E.U., Costa, M.M., Connelly, J.N., Zhang, B., Su, J., Canfield, D.E. (2016) Sufficient oxygen for animal respiration 1,400 million years ago. Proceedings of the National Academy of Sciences 113, 1731–1736. https://doi.org/10.1073/pnas.1523449113
, 2021Zhang, S., Wang, H., Wang, X., Ye, Y. (2021) The Mesoproterozoic Oxygenation Event. Science China: Earth Science 64, 2043–2068. https://doi.org/10.1007/s11430-020-9825-x
; Canfield et al., 2021Canfield, D.E., van Zuilen, M.A., Nabhan, S., Bjerrum, C.J., Zhang, S.C., Wang, H.J., Wang, X.M. (2021) Petrographic carbon in ancient sediments constrains Proterozoic Era atmospheric oxygen levels. Proceedings of the National Academy of Sciences 118, e2101544118. https://doi.org/10.1073/pnas.2101544118
), it is believed that oxygen was deficient compared to the last 0.7 Ga.Thus, although IF deposition is limited from 1.8−0.8 Ga, there are recent reports of significant ferrous carbonate deposition generating siderite-dominated IFs, such as the ∼1.40 Ga Xiamaling (XML) IF in North China (Canfield et al., 2018
Canfield, D.E., Zhang, S.C., Wang, H.J., Wang, X.M., Zhao, W.Z., Su, J., Bjerrum, C.J., Haxen, E.R., Hammarlund, E.U. (2018) A Mesoproterozoic Iron Formation. Proceedings of the National Academy of Sciences 115, E3895–E3904. https://doi.org/10.1073/pnas.1720529115
; Tang etal., 2018Tang, D., Shi, X., Jiang, G., Wu, T., Ma, J., Zhou, X. (2018) Stratiform siderites from the Mesoproterozoic Xiamaling Formation in North China: Genesis and environmental implications. Gondwana Research 58, 1–15. https://doi.org/10.1016/j.gr.2018.01.013
) and the ∼1.33 Ga Jingtieshan IF in Qilian (Yang et al., 2018Yang, X., Zhang, Z., Santosh, M., Duan, S., Liang, T. (2018) Anoxic to suboxic Mesoproterozoic ocean: Evidence from iron isotope and geochemistry of siderite in the Banded Iron Formations from North Qilian, NW China. Precambrian Research 307, 115–124. https://doi.org/10.1016/j.precamres.2018.01.007
). Siderite deposits are also found in the ∼1.45 Ga Sherwin ironstone in Australia (Planavsky et al., 2014Planavsky, N.J., Reinhard, C.T., Wang, X.L., Thomson, D., McGoldrick, P., Rainbird, R.H., Johnson, T., Fischer, W.W., Lyons, T.W. (2014) Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 346, 635–638. https://doi.org/10.1126/science.1258410
). To further explore the mechanisms of siderite-dominated IF deposition and the associated marine Fe and C cycles, we analysed the contents and isotopic compositions of Fe and C of the XML IF. Combined with the reported data in Canfield et al. (2018)Canfield, D.E., Zhang, S.C., Wang, H.J., Wang, X.M., Zhao, W.Z., Su, J., Bjerrum, C.J., Haxen, E.R., Hammarlund, E.U. (2018) A Mesoproterozoic Iron Formation. Proceedings of the National Academy of Sciences 115, E3895–E3904. https://doi.org/10.1073/pnas.1720529115
, our results show evidence for hydrothermal Fe2+ input during the deposition of XML IF, where the Fe and C cycles were tightly coupled.top
Geological Background and Samples
Samples were obtained from a core in the Xiahuayuan region located in the Yanliao Basin, which was an offshore basin in North China during the breakup of the Columbia supercontinent (Fig. 1a–c). The XML IF is found over 45 m of stratigraphic thickness and is defined as unit 5, which can be further divided into the lower part with greyish-black mudstone and the upper part with green sandy mudstone (Fig. 1d). Centimetre- to decimetre-sized laminar and nodular siderites are embedded in the clastic rocks (Fig. 1e–h), indicating a pore-water diagenetic environment. Detailed information of the geological background is in the Supplementary Information (SI).

Figure 1 Location of the (a) 1.4 Ga and (b) present North China Craton, modified from Zhang et al. (2021)
Zhang, S., Wang, H., Wang, X., Ye, Y. (2021) The Mesoproterozoic Oxygenation Event. Science China: Earth Science 64, 2043–2068. https://doi.org/10.1007/s11430-020-9825-x
. (c) Paleogeography of the Yanliao Basin 1.4 Ga, modified from Lyu et al. (2021)Lyu, D., Deng, Y., Wang, H., Zhang, F., Ren, R., Gao, Z., Zhou, C., Luo, Z., Wang, X., Bi, L., Zhang, S., Canfield, D.E. (2021) Using cyclostratigraphic evidence to define the unconformity caused by the Mesoproterozoic Qinyu Uplift in the North China Craton. Journal of Asian Earth Sciences 206, 104608. https://doi.org/10.1016/j.jseaes.2020.104608
. (d) Stratigraphy of iron formation defined as unit 5 of the Xiamaling Formation. Photos of laminar (e) and nodular (f–h) siderites from the core in the Xiahuayuan region.top
Methods and Results
As the normal Fe extraction protocol in Poulton and Canfield (2005)
Poulton, S.W., Canfield, D.E. (2005) Development of a sequential extraction procedure for iron: implications for iron partitioning in continentally derived particulates. Chemical Geology 214, 209–221. https://doi.org/10.1016/j.chemgeo.2004.09.003
proved ineffective at removing siderite from our IF samples, an acid extraction method was used. We used ferrozine to determine the contents of acid-soluble Fe2+, and used ferrozine with the addition of hydroxylamine to determine the sum of Fe2+ and Fe3+. On separate samples, the Cr-reduction method was used to quantify the Fe bound in pyrite (Fepy). Iron isotope values (δ56Fe) from whole rock samples were determined and expressed as the 56Fe/54Fe ratio relative to IRMM-014. Contents of total organic carbon (TOC), elements and main diagenetic minerals of whole rock samples were also determined. The isotopic composition of carbonate (δ13Ccarb) of the IF samples and the isotopic composition of organic carbon (δ13Corg) of the clastic samples were determined and expressed relative to VPDB. Detailed methods are in the SI.Geochemical data of the IF samples (defined as samples with total Fe (FeT) > 15 %) and the interbedded clastic rocks are illustrated in Figure 2 and Table S-1. As has been observed in the upper part (discussed in Canfield et al., 2018
Canfield, D.E., Zhang, S.C., Wang, H.J., Wang, X.M., Zhao, W.Z., Su, J., Bjerrum, C.J., Haxen, E.R., Hammarlund, E.U. (2018) A Mesoproterozoic Iron Formation. Proceedings of the National Academy of Sciences 115, E3895–E3904. https://doi.org/10.1073/pnas.1720529115
), siderite is the dominant diagenetic mineral of the lower IF samples (Table S-1). The lower IF samples have extremely high FeT/Al ratios (mostly >15) and FeT/Ti ratios (mostly >200), high Fe2+/FeT ratios (>0.8), extremely low Fe3+/FeT ratios (<0.02) and Fepy/FeT ratios (<0.01), and negative δ56Fe values (from −0.53 ‰ to −0.34 ‰). In contrast, the interbedded clastic rocks of the lower IF have low FeT/Al ratios (∼0.46) and FeT/Ti ratios (mostly from 4 to 10), medium Fe2+/FeT ratios (from 0.17 to 0.74), low Fe3+/FeT ratios (<0.1), medium Fepy/FeT ratios (from 0.01 to 0.3), and positive δ56Fe values (mostly from +0.19 ‰ to +0.72 ‰). The upper IF samples also have high FeT/Al ratios (mostly from 3 to 15) and FeT/Ti ratios (mostly from 30 to 200), high Fe2+/FeT ratios (>0.8), low Fe3+/FeT ratios (<0.15), extremely low Fepy/FeT ratios (<0.01), and more negative δ56Fe values (from −1.20 ‰ to −0.32 ‰) than those of the lower IF samples. The interbedded clastic rocks of the upper IF have medium FeT/Al ratios (from 0.5 to 2.7) and FeT/Ti ratios (mostly from 10 to 30), medium Fe2+/FeT ratios (from 0.46 to 0.83), low Fe3+/FeT ratios (<0.15), extremely low Fepy/FeT ratios (<0.01), and fluctuating δ56Fe values (from −0.71 ‰ to +0.38 ‰). The sedimentary rocks of the lower part (including the IF samples) have higher TOC values (mostly from 0.3 % to 1.0 %) than those of the upper part (mostly <0.3 %). The δ13Corg values of most samples were between −33 ‰ and −32 ‰, while the δ13Ccarb values of the IF samples fluctuated from −27 ‰ to −8 ‰.
Figure 2 Geochemical profiles of the unit 5 of the Xiamaling Formation. Degree of pyritisation (DOP) was calculated by Fepy/(Fe2++Fe3++Fepy). Data of the FeT, total organic carbon (TOC), Eu/Eu*, carbon isotopes of carbonate (δ13Ccarb) and kerogen (δ13Corg) with a depth range of 390−350 m were from Canfield et al. (2018)
Canfield, D.E., Zhang, S.C., Wang, H.J., Wang, X.M., Zhao, W.Z., Su, J., Bjerrum, C.J., Haxen, E.R., Hammarlund, E.U. (2018) A Mesoproterozoic Iron Formation. Proceedings of the National Academy of Sciences 115, E3895–E3904. https://doi.org/10.1073/pnas.1720529115
. Note the abscissas breaks of FeT/Al, FeT/Ti, Fepy/FeT are at 3, 30 and 0.01, respectively. Cycle and solid points represent IF (FeT > 15 %) and clastic (FeT < 15 %) samples, respectively. Dashed lines in the δ13Ccarb panel represent the proportions of organic-sourced bicarbonate of measured samples, with an initial δ13Corg of −32 ‰ and an initial δ13C of bicarbonate in water column of 0 ‰.top
Depositional Redox Environments
We could not use standard Fe speciation protocols on the siderite-rich samples, but high FeT/Al ratios (Lyons and Severmann, 2006
Lyons, T.W., Severmann, S. (2006) A critical look at iron paleoredox proxies: New insights from modern euxinic marine basins. Geochimica et Cosmochimica Acta 70, 5698–5722. https://doi.org/10.1016/j.gca.2006.08.021
) and high FeT/Ti ratios (Dauphas et al., 2004Dauphas, N., Van Zuilen, M., Wadhwa, M., Davis, A.M, Marty, B.J., Philip, E. (2004) Clues from Fe isotope variations on the origin of early Archean BIFs from Greenland. Science 306, 2077–2080. https://doi.org/10.1126/science.1104639
), combined with high Fe2+/FeT ratios (>0.8) and extremely low Fepy/FeT ratios (<0.01) confirmed ferruginous conditions during the IF deposition, consistent with the previous redox-sensitive element proxies from Canfield et al. (2018)Canfield, D.E., Zhang, S.C., Wang, H.J., Wang, X.M., Zhao, W.Z., Su, J., Bjerrum, C.J., Haxen, E.R., Hammarlund, E.U. (2018) A Mesoproterozoic Iron Formation. Proceedings of the National Academy of Sciences 115, E3895–E3904. https://doi.org/10.1073/pnas.1720529115
and Tang et al. (2018)Tang, D., Shi, X., Jiang, G., Wu, T., Ma, J., Zhou, X. (2018) Stratiform siderites from the Mesoproterozoic Xiamaling Formation in North China: Genesis and environmental implications. Gondwana Research 58, 1–15. https://doi.org/10.1016/j.gr.2018.01.013
. As we did not use the standard Fe speciation protocol, water column redox conditions in the clastic rocks could only be evaluated where the FeT/Al and FeT/Ti ratios well exceeded the crustal average values (∼0.5 and ∼10, respectively, Rudnick and Gao, 2003Rudnick, R.L., Gao, S. (2003) Composition of the continental crust. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry. Elsevier−Pergamon, Oxford, UK, 1–64. https://doi.org/10.1016/B0-08-043751-6/03016-4
). For the clastic rocks of the upper part, high FeT/Al ratios (0.5−2.7), high FeT/Ti ratios (10−30) and extremely low sulfurised Fe ratios (Fepy/FeT < 0.01) indicate ferruginous deposition conditions. For clastic rocks of the lower part, the FeT/Al and FeT/Ti ratios hover around the crustal average values, and the sulfurised Fe ratios range from 0.01 to 0.3, so the redox conditions during deposition are still uncertain. However, a euxinic depositional environment can be excluded, as it needs a degree of iron pyritisation (DOP) of at least 0.4 (Raiswell and Canfield, 2012Raiswell, R., Canfield, D.E (2012) The Iron Biogeochemical Cycle Past and Present. Geochemical Perspectives 1, 1–220. https://doi.org/10.7185/geochempersp.1.1
).top
Sources of the Iron
Positive Eu anomalies (Eu/Eu* ratios 1.0−1.4) for all IF samples (Fig. 2) indicate a hydrothermal impact on the water chemistry during the IF formation, and hydrothermal Fe2+ may have been the most important source of Fe2+ to the Precambrian ocean (Johnson et al., 2020
Johnson, C., Beard, B., Weyer, S. (2020) Iron Geochemistry: An Isotopic Perspective. Springer Nature Press, Switzerland AG. https://doi.org/10.1007/978-3-030-33828-2
). During the deposition of the XML IF, a hydrothermal Fe2+ source may be associated with the breakup of the supercontinent Columbia (Tang et al., 2018Tang, D., Shi, X., Jiang, G., Wu, T., Ma, J., Zhou, X. (2018) Stratiform siderites from the Mesoproterozoic Xiamaling Formation in North China: Genesis and environmental implications. Gondwana Research 58, 1–15. https://doi.org/10.1016/j.gr.2018.01.013
). However, the positive Eu anomalies of the XML IF are rather muted when compared with the ∼1.3 Ga Jingtieshan IF (Eu/Eu* ratios 4.4–6.5) (Yang et al., 2018Yang, X., Zhang, Z., Santosh, M., Duan, S., Liang, T. (2018) Anoxic to suboxic Mesoproterozoic ocean: Evidence from iron isotope and geochemistry of siderite in the Banded Iron Formations from North Qilian, NW China. Precambrian Research 307, 115–124. https://doi.org/10.1016/j.precamres.2018.01.007
), so the XML IF may be rather distal from the hydrothermal source.Another source of Fe2+ to seawater is released from clastic particulates depositing in anoxic waters on the shelf (Raiswell and Canfield, 2012
Raiswell, R., Canfield, D.E (2012) The Iron Biogeochemical Cycle Past and Present. Geochemical Perspectives 1, 1–220. https://doi.org/10.7185/geochempersp.1.1
). Indeed, a benthic clastic source of dissolved Fe2+ likely supplies most Fe that accumulates as reactive Fe in anoxic depositional environments today, as revealed through sequential sediment extractions (Raiswell and Canfield, 2012Raiswell, R., Canfield, D.E (2012) The Iron Biogeochemical Cycle Past and Present. Geochemical Perspectives 1, 1–220. https://doi.org/10.7185/geochempersp.1.1
).top
Iron Isotope Fractionation Models
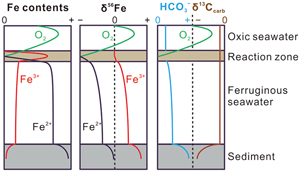
Formation of the XML IF has been suggested to result from three steps (Canfield et al., 2018
Canfield, D.E., Zhang, S.C., Wang, H.J., Wang, X.M., Zhao, W.Z., Su, J., Bjerrum, C.J., Haxen, E.R., Hammarlund, E.U. (2018) A Mesoproterozoic Iron Formation. Proceedings of the National Academy of Sciences 115, E3895–E3904. https://doi.org/10.1073/pnas.1720529115
), including: 1) oxidation of seawater Fe2+ to Fe-oxyhydroxides (Fe(OH)3), likely at the chemocline of the ancient marine basin, 2) reduction of Fe(OH)3 to diagenetic Fe2+ in the water column and sediments, and 3) crystallisation of Fe2+ as siderite. Whole rock δ56Fe values would be affected by the initial δ56Fe of seawater Fe2+ and the net isotopic fractionation between products and reactants (Δ56Feproduct-reactant) in the above steps. These steps are presented in Figure 3 and are reviewed in more detail in Johnson et al. (2020)Johnson, C., Beard, B., Weyer, S. (2020) Iron Geochemistry: An Isotopic Perspective. Springer Nature Press, Switzerland AG. https://doi.org/10.1007/978-3-030-33828-2
as well as the SI.
Figure 3 Genetic model of the coupled Fe and C cycles and isotope fractionations in the water column and sediment.
To briefly summarise, the oxidation of Fe2+ generates 56Fe-enriched Fe(OH)3 (Fe3+) compared to the Fe2+ source. With the subsequent reduction of this Fe3+, and generation of diagenetic Fe2+, both the Fe2+ product and the residual Fe3+ will be progressively 56Fe-enriched. Due to the Δ56FeFe(II)-Fe(III) of diagenetic Fe2+ and residual Fe3+, whole rock δ56Fe values in clastic rocks with residual Fe3+ are generally heavier than the Fe2+ in siderite alone. For the higher molar ratio of Fe2+ and bicarbonate (HCO3−) as products during step 2 (4∶1) than as reactants in step 3 (1∶1), excessive diagenetic Fe2+ diffuse upwards and/or insufficient HCO3− diffuse downwards during step 3. Because of the different isotopic trends of diagenetic Fe2+ and HCO3−, the δ56Fe and δ13Ccarb of siderite depend on the position of its precipitation. The genetic model shown in Figure 3 is illustrative only, not quantitative, but the trends in δ56Fe are similar to those reproduced in the open system diffusion/reaction model (Johnson et al., 2020
Johnson, C., Beard, B., Weyer, S. (2020) Iron Geochemistry: An Isotopic Perspective. Springer Nature Press, Switzerland AG. https://doi.org/10.1007/978-3-030-33828-2
).top
Dynamic Iron Cycle in the 1.4 Ga Marine Environment
The δ56Fe values of both lower and upper IF samples are significantly reduced compared to those of the clastic rocks in the same intervals (Fig. 2). With an average Fe content in the upper crust of 3.6 % (Rudnick and Gao, 2003
Rudnick, R.L., Gao, S. (2003) Composition of the continental crust. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry. Elsevier−Pergamon, Oxford, UK, 1–64. https://doi.org/10.1016/B0-08-043751-6/03016-4
), and for siderite of 48 %, rocks in the lower part are consistent with the mixing of a clastic end-member with a δ56Fe value of +0.6 ‰ and a diagenetic siderite end member with a δ56Fe value of −0.4 ‰ (R2 = 0.895, Fig. 4a), which is close to the measured δ56Fe values (from −0.53 ‰ to −0.34 ‰) of IF samples. However, for the upper part, the optimal δ56Fe value of the diagenetic siderite was −1.1 ‰, with a δ56Fe value of the clastic end member also set to +0.6 ‰ (Fig. 4a). Thus, the circumstances of IF formation in the lower and upper parts were clearly different. Details of the calculation method are in the SI.
Figure 4 Correlation diagrams of δ56Fe with (a) FeT and (b) Eu/Eu*. The black and blue lines in (a) are the optimal mixing lines of the lower and upper parts, respectively, with a clastic end member and a diagenetic end member. Grey areas mean the Fe cycle and δ56Fe values were influenced by the hydrothermal fluids.
Moreover, the δ13Ccarb values of IF samples are much more 13C-depleted than the Mesoproterozoic seawater HCO3− (∼0 ‰, Saltzman and Thomas, 2012
Saltzman, M.R., Thomas, E. (2012) Carbon Isotope Stratigraphy. In: Gradstein, F.M., Ogg, J.G., Ogg, M.S.G. (Eds.) The Geologic Time Scale. Elsevier, Amsterdam, The Netherlands, 207–232. https://doi.org/10.1016/B978-0-444-59425-9.00011-1
), supporting the idea that the siderites most likely precipitated in diagenetic sediment environments with varied and limited exchange with the overlying waters. The δ13Ccarb values of siderites from the lower part are from −15 ‰ to −12 ‰, and thus more 13C-enriched than most samples from the upper part (Fig. 2). This difference in δ13Ccarb values would suggest that the siderite from the lower part precipitated closer to the water–sediment interface, with a greater seawater HCO3− contribution, when compared to the siderites from the upper part (Fig. 3). Following this logic, if we assume that the patterns of water column δ56Fe values remained similar during all of the XML IF formation, the siderites from the lower part should be more 56Fe-depleted than those from the upper part (Fig. 3). However, the elevated δ56Fe values (from −1.1 ‰ to −0.4 ‰) contradict this hypothesis and indicate more 56Fe-enriched water in the Yanliao Basin during the IF formation in the lower part.We propose that the lower part of the XML IF received an enhanced input of hydrothermal fluids with δ56Fe values of 0 ‰. The hydrothermal fluids with elevated Fe2+ concentrations (German et al., 1990
German, C.R., Klinkhammer, G.P., Edmond, J.M., Mitra, A., Elderfield, H. (1990) Hydrothermal scavenging of rare-earth elements in the ocean. Nature 345, 516–518. https://doi.org/10.1038/345516a0
) generated higher Fe2+ concentrations and elevated δ56Fe values in the bottom waters of the Yanliao Basin. Higher Fe2+ concentrations would have resulted in siderite precipitation with lower HCO3− concentrations and closer to the sediment–water interface, consistent with heavier δ13Ccarb values of siderites from the lower part. Higher Fe2+ concentrations could have also elevated the rates of Fe-associated primary production, leading to higher values of TOC (Fig. 2).Generally, the positive Eu anomalies of the XML IF can be taken to indicate hydrothermal contributions. We also note that the positive Eu anomalies are relatively small and of the same magnitude in both the upper and lower IFs. Overall, the positive Eu anomalies in the IFs (Fig. 4b) do support a hydrothermal source of Fe2+ to the Yanliao Basin. This conclusion is consistent with the enhanced hydrothermal Fe2+ supply that we argue impacted both the δ56Fe and δ13Ccarb values of the lower XML IF.
top
Implications on Marine Iron and Carbon Cycles
The XML IF was formed within a ferruginous marine basin with an active iron cycle that was intimately coupled to the C cycle. However, there are differences in the dynamics of the Fe and C cycles in the upper and lower parts of the sedimentary sequence where the IFs are formed. In particular, an enhanced hydrothermal Fe2+ contribution elevated the concentrations of Fe2+ in the water column and the rates of Fe associated with the C cycle in the lower IF, and should be a key controlling factor in regulating the rates of Fe associated with marine primary production during the siderite-dominated XML IF formation.
top
Acknowledgements
We thank Zhihong Li, Heidi Jensen and Iben Rosendahl for the experimental analysis. The research is financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA14010101), National Key Research and Development Program of China (2017YFC0603101), National Natural Science Foundation of China (41872125, 42102146) and Villum Foundation (16518).
Editor: Claudine Stirling
top
References
Canfield, D.E. (1998) A new model for Proterozoic ocean chemistry. Nature 396, 450–453. https://doi.org/10.1038/24839
 Show in context
Show in context During this gap period, the iron formation (IF) shortage was thought to be a result of significantly decreased Fe2+ concentrations in the ocean, which was either substantially oxidised (Holland, 1990) or sulfurised (Canfield, 1998).
View in article
Canfield, D.E., Poulton, S.W., Knoll, A.H., Narbonne, G.M., Ross, G., Goldberg, T., Strauss, H. (2008) Ferruginous conditions dominated later neoproterozoic deep-water chemistry. Science 321, 949–952. https://doi.org/10.1126/science.1154499
 Show in context
Show in context Recent studies provide increasing evidence that ferruginous water was still widespread in the deep ocean until 0.58 Ga (Canfield et al., 2008).
View in article
Canfield, D.E., Zhang, S.C., Wang, H.J., Wang, X.M., Zhao, W.Z., Su, J., Bjerrum, C.J., Haxen, E.R., Hammarlund, E.U. (2018) A Mesoproterozoic Iron Formation. Proceedings of the National Academy of Sciences 115, E3895–E3904. https://doi.org/10.1073/pnas.1720529115
 Show in context
Show in context Combined with the reported data in Canfield et al. (2018), our results show evidence for hydrothermal Fe2+ input during the deposition of XML IF, where the Fe and C cycles were tightly coupled.
View in article
As has been observed in the upper part (discussed in Canfield et al., 2018), siderite is the dominant diagenetic mineral of the lower IF samples (Table S-1).
View in article
Data of the FeT, total organic carbon (TOC), Eu/Eu*, carbon isotopes of carbonate (δ13Ccarb) and kerogen (δ13Corg) with a depth range of 390−350 m were from Canfield et al. (2018).
View in article
Thus, although IF deposition is limited from 1.8−0.8 Ga, there are recent reports of significant ferrous carbonate deposition generating siderite-dominated IFs, such as the ∼1.40 Ga Xiamaling (XML) IF in North China (Canfield et al., 2018; Tang etal., 2018) and the ∼1.33 Ga Jingtieshan IF in Qilian (Yang et al., 2018).
View in article
Formation of the XML IF has been suggested to result from three steps (Canfield et al., 2018), including: 1) oxidation of seawater Fe2+ to Fe-oxyhydroxides (Fe(OH)3), likely at the chemocline of the ancient marine basin, 2) reduction of Fe(OH)3 to diagenetic Fe2+ in the water column and sediments, and 3) crystallisation of Fe2+ as siderite.
View in article
We could not use standard Fe speciation protocols on the siderite-rich samples, but high FeT/Al ratios (Lyons and Severmann, 2006) and high FeT/Ti ratios (Dauphas et al., 2004), combined with high Fe2+/FeT ratios (>0.8) and extremely low Fepy/FeT ratios (<0.01) confirmed ferruginous conditions during the IF deposition, consistent with the previous redox-sensitive element proxies from Canfield et al. (2018) and Tang et al. (2018).
View in article
Canfield, D.E., van Zuilen, M.A., Nabhan, S., Bjerrum, C.J., Zhang, S.C., Wang, H.J., Wang, X.M. (2021) Petrographic carbon in ancient sediments constrains Proterozoic Era atmospheric oxygen levels. Proceedings of the National Academy of Sciences 118, e2101544118. https://doi.org/10.1073/pnas.2101544118
 Show in context
Show in context Although atmospheric oxygen levels are still controversial in the time window of 1.8−0.8 Ga (Planavsky et al., 2014; Zhang et al., 2016, 2021; Canfield et al., 2021), it is believed that oxygen was deficient compared to the last 0.7 Ga.
View in article
Dauphas, N., Van Zuilen, M., Wadhwa, M., Davis, A.M, Marty, B.J., Philip, E. (2004) Clues from Fe isotope variations on the origin of early Archean BIFs from Greenland. Science 306, 2077–2080. https://doi.org/10.1126/science.1104639
 Show in context
Show in context We could not use standard Fe speciation protocols on the siderite-rich samples, but high FeT/Al ratios (Lyons and Severmann, 2006) and high FeT/Ti ratios (Dauphas et al., 2004), combined with high Fe2+/FeT ratios (>0.8) and extremely low Fepy/FeT ratios (<0.01) confirmed ferruginous conditions during the IF deposition, consistent with the previous redox-sensitive element proxies from Canfield et al. (2018) and Tang et al. (2018).
View in article
German, C.R., Klinkhammer, G.P., Edmond, J.M., Mitra, A., Elderfield, H. (1990) Hydrothermal scavenging of rare-earth elements in the ocean. Nature 345, 516–518. https://doi.org/10.1038/345516a0
 Show in context
Show in context The hydrothermal fluids with elevated Fe2+ concentrations (German et al., 1990) generated higher Fe2+ concentrations and elevated δ56Fe values in the bottom waters of the Yanliao Basin.
View in article
Holland, H.D. (1990) Origins of breathable air. Nature 347, 17–17. https://doi.org/10.1038/347017a0
 Show in context
Show in context During this gap period, the iron formation (IF) shortage was thought to be a result of significantly decreased Fe2+ concentrations in the ocean, which was either substantially oxidised (Holland, 1990) or sulfurised (Canfield, 1998).
View in article
Johnson, C., Beard, B., Weyer, S. (2020) Iron Geochemistry: An Isotopic Perspective. Springer Nature Press, Switzerland AG. https://doi.org/10.1007/978-3-030-33828-2
 Show in context
Show in context Positive Eu anomalies (Eu/Eu* ratios 1.0−1.4) for all IF samples (Fig. 2) indicate a hydrothermal impact on the water chemistry during the IF formation, and hydrothermal Fe2+ may have been the most important source of Fe2+ to the Precambrian ocean (Johnson et al., 2020).
View in article
These steps are presented in Figure 3 and are reviewed in more detail in Johnson et al. (2020) as well as the SI.
View in article
The genetic model shown in Figure 3 is illustrative only, not quantitative, but the trends in δ56Fe are similar to those reproduced in the open system diffusion/reaction model (Johnson et al., 2020).
View in article
Konhauser, K.O., Planavsky, N.J., Hardisty, D.S., Robbins, L.J., Warchola, T.J., Haugaard, R., Lalonde, S.V., Partin, C.A., Oonk, P.B.H., Tsikos, H., Lyons, T.W., Bekker, A., Johnson, C.M. (2017) Iron formations: A global record of Neoarchaean to Palaeoproterozoic environmental history. Earth-Science Reviews 172, 140–177. https://doi.org/10.1016/j.earscirev.2017.06.012
 Show in context
Show in context Most marine massive Fe deposits formed 3.0−1.8 Ga, with a billion-year hiatus until a brief return around 0.8 Ga (Konhauser et al., 2017).
View in article
Lyons, T.W., Severmann, S. (2006) A critical look at iron paleoredox proxies: New insights from modern euxinic marine basins. Geochimica et Cosmochimica Acta 70, 5698–5722. https://doi.org/10.1016/j.gca.2006.08.021
 Show in context
Show in context We could not use standard Fe speciation protocols on the siderite-rich samples, but high FeT/Al ratios (Lyons and Severmann, 2006) and high FeT/Ti ratios (Dauphas et al., 2004), combined with high Fe2+/FeT ratios (>0.8) and extremely low Fepy/FeT ratios (<0.01) confirmed ferruginous conditions during the IF deposition, consistent with the previous redox-sensitive element proxies from Canfield et al. (2018) and Tang et al. (2018).
View in article
Lyu, D., Deng, Y., Wang, H., Zhang, F., Ren, R., Gao, Z., Zhou, C., Luo, Z., Wang, X., Bi, L., Zhang, S., Canfield, D.E. (2021) Using cyclostratigraphic evidence to define the unconformity caused by the Mesoproterozoic Qinyu Uplift in the North China Craton. Journal of Asian Earth Sciences 206, 104608. https://doi.org/10.1016/j.jseaes.2020.104608
 Show in context
Show in context (c) Paleogeography of the Yanliao Basin 1.4 Ga, modified from Lyu et al. (2021).
View in article
Planavsky, N.J., Reinhard, C.T., Wang, X.L., Thomson, D., McGoldrick, P., Rainbird, R.H., Johnson, T., Fischer, W.W., Lyons, T.W. (2014) Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 346, 635–638. https://doi.org/10.1126/science.1258410
 Show in context
Show in context Siderite deposits are also found in the ∼1.45 Ga Sherwin ironstone in Australia (Planavsky et al., 2014).
View in article
Although atmospheric oxygen levels are still controversial in the time window of 1.8−0.8 Ga (Planavsky et al., 2014; Zhang et al., 2016, 2021; Canfield et al., 2021), it is believed that oxygen was deficient compared to the last 0.7 Ga.
View in article
Poulton, S.W., Canfield, D.E. (2005) Development of a sequential extraction procedure for iron: implications for iron partitioning in continentally derived particulates. Chemical Geology 214, 209–221. https://doi.org/10.1016/j.chemgeo.2004.09.003
 Show in context
Show in context As the normal Fe extraction protocol in Poulton and Canfield (2005) proved ineffective at removing siderite from our IF samples, an acid extraction method was used.
View in article
Raiswell, R., Canfield, D.E (2012) The Iron Biogeochemical Cycle Past and Present. Geochemical Perspectives 1, 1–220. https://doi.org/10.7185/geochempersp.1.1
 Show in context
Show in context However, a euxinic depositional environment can be excluded, as it needs a degree of iron pyritisation (DOP) of at least 0.4 (Raiswell and Canfield, 2012).
View in article
Another source of Fe2+ to seawater is released from clastic particulates depositing in anoxic waters on the shelf (Raiswell and Canfield, 2012).
View in article
Indeed, a benthic clastic source of dissolved Fe2+ likely supplies most Fe that accumulates as reactive Fe in anoxic depositional environments today, as revealed through sequential sediment extractions (Raiswell and Canfield, 2012).
View in article
Rudnick, R.L., Gao, S. (2003) Composition of the continental crust. In: Holland, H.D., Turekian, K.K. (Eds.) Treatise on Geochemistry. Elsevier−Pergamon, Oxford, UK, 1–64. https://doi.org/10.1016/B0-08-043751-6/03016-4
 Show in context
Show in context As we did not use the standard Fe speciation protocol, water column redox conditions in the clastic rocks could only be evaluated where the FeT/Al and FeT/Ti ratios well exceeded the crustal average values (∼0.5 and ∼10, respectively, Rudnick and Gao, 2003)
View in article
With an average Fe content in the upper crust of 3.6 % (Rudnick and Gao, 2003), and for siderite of 48 %, rocks in the lower part are consistent with the mixing of a clastic end-member with a δ56Fe value of +0.6 ‰ and a diagenetic siderite end member with a δ56Fe value of −0.4 ‰ (R2 = 0.895, Fig. 4a), which is close to the measured δ56Fe values (from −0.53 ‰ to −0.34 ‰) of IF samples.
View in article
Saltzman, M.R., Thomas, E. (2012) Carbon Isotope Stratigraphy. In: Gradstein, F.M., Ogg, J.G., Ogg, M.S.G. (Eds.) The Geologic Time Scale. Elsevier, Amsterdam, The Netherlands, 207–232. https://doi.org/10.1016/B978-0-444-59425-9.00011-1
 Show in context
Show in context Moreover, the δ13Ccarb values of IF samples are much more 13C-depleted than the Mesoproterozoic seawater HCO3− (∼0 ‰, Saltzman and Thomas, 2012), supporting the idea that the siderites most likely precipitated in diagenetic sediment environments with varied and limited exchange with the overlying waters.
View in article
Tang, D., Shi, X., Jiang, G., Wu, T., Ma, J., Zhou, X. (2018) Stratiform siderites from the Mesoproterozoic Xiamaling Formation in North China: Genesis and environmental implications. Gondwana Research 58, 1–15. https://doi.org/10.1016/j.gr.2018.01.013
 Show in context
Show in context Thus, although IF deposition is limited from 1.8−0.8 Ga, there are recent reports of significant ferrous carbonate deposition generating siderite-dominated IFs, such as the ∼1.40 Ga Xiamaling (XML) IF in North China (Canfield et al., 2018; Tang etal., 2018) and the ∼1.33 Ga Jingtieshan IF in Qilian (Yang et al., 2018).
View in article
During the deposition of the XML IF, a hydrothermal Fe2+ source may be associated with the breakup of the supercontinent Columbia (Tang et al., 2018).
View in article
We could not use standard Fe speciation protocols on the siderite-rich samples, but high FeT/Al ratios (Lyons and Severmann, 2006) and high FeT/Ti ratios (Dauphas et al., 2004), combined with high Fe2+/FeT ratios (>0.8) and extremely low Fepy/FeT ratios (<0.01) confirmed ferruginous conditions during the IF deposition, consistent with the previous redox-sensitive element proxies from Canfield et al. (2018) and Tang et al. (2018).
View in article
Yang, X., Zhang, Z., Santosh, M., Duan, S., Liang, T. (2018) Anoxic to suboxic Mesoproterozoic ocean: Evidence from iron isotope and geochemistry of siderite in the Banded Iron Formations from North Qilian, NW China. Precambrian Research 307, 115–124. https://doi.org/10.1016/j.precamres.2018.01.007
 Show in context
Show in context However, the positive Eu anomalies of the XML IF are rather muted when compared with the ∼1.3 Ga Jingtieshan IF (Eu/Eu* ratios 4.4–6.5) (Yang et al., 2018), so the XML IF may be rather distal from the hydrothermal source.
View in article
Thus, although IF deposition is limited from 1.8−0.8 Ga, there are recent reports of significant ferrous carbonate deposition generating siderite-dominated IFs, such as the ∼1.40 Ga Xiamaling (XML) IF in North China (Canfield et al., 2018; Tang etal., 2018) and the ∼1.33 Ga Jingtieshan IF in Qilian (Yang et al., 2018).
View in article
Zhang, S., Wang, X., Wang, H., Bjerrum, C.J., Hammarlund, E.U., Costa, M.M., Connelly, J.N., Zhang, B., Su, J., Canfield, D.E. (2016) Sufficient oxygen for animal respiration 1,400 million years ago. Proceedings of the National Academy of Sciences 113, 1731–1736. https://doi.org/10.1073/pnas.1523449113
 Show in context
Show in context Although atmospheric oxygen levels are still controversial in the time window of 1.8−0.8 Ga (Planavsky et al., 2014; Zhang et al., 2016, 2021; Canfield et al., 2021), it is believed that oxygen was deficient compared to the last 0.7 Ga.
View in article
Zhang, S., Wang, H., Wang, X., Ye, Y. (2021) The Mesoproterozoic Oxygenation Event. Science China: Earth Science 64, 2043–2068. https://doi.org/10.1007/s11430-020-9825-x
 Show in context
Show in context Location of the (a) 1.4 Ga and (b) present North China Craton, modified from Zhang et al. (2021).
View in article
Although atmospheric oxygen levels are still controversial in the time window of 1.8−0.8 Ga (Planavsky et al., 2014; Zhang et al., 2016, 2021; Canfield et al., 2021), it is believed that oxygen was deficient compared to the last 0.7 Ga.
View in article
top
Supplementary Information
The Supplementary Information includes:
Download Table S-1 (Excel).
Download the Supplementary Information (PDF).
Figures
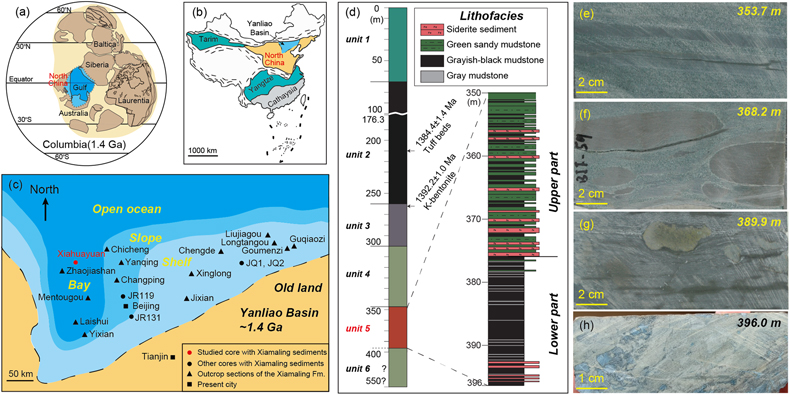
Figure 1 Location of the (a) 1.4 Ga and (b) present North China Craton, modified from Zhang et al. (2021)
Zhang, S., Wang, H., Wang, X., Ye, Y. (2021) The Mesoproterozoic Oxygenation Event. Science China: Earth Science 64, 2043–2068. https://doi.org/10.1007/s11430-020-9825-x
. (c) Paleogeography of the Yanliao Basin 1.4 Ga, modified from Lyu et al. (2021)Lyu, D., Deng, Y., Wang, H., Zhang, F., Ren, R., Gao, Z., Zhou, C., Luo, Z., Wang, X., Bi, L., Zhang, S., Canfield, D.E. (2021) Using cyclostratigraphic evidence to define the unconformity caused by the Mesoproterozoic Qinyu Uplift in the North China Craton. Journal of Asian Earth Sciences 206, 104608. https://doi.org/10.1016/j.jseaes.2020.104608
. (d) Stratigraphy of iron formation defined as unit 5 of the Xiamaling Formation. Photos of laminar (e) and nodular (f–h) siderites from the core in the Xiahuayuan region.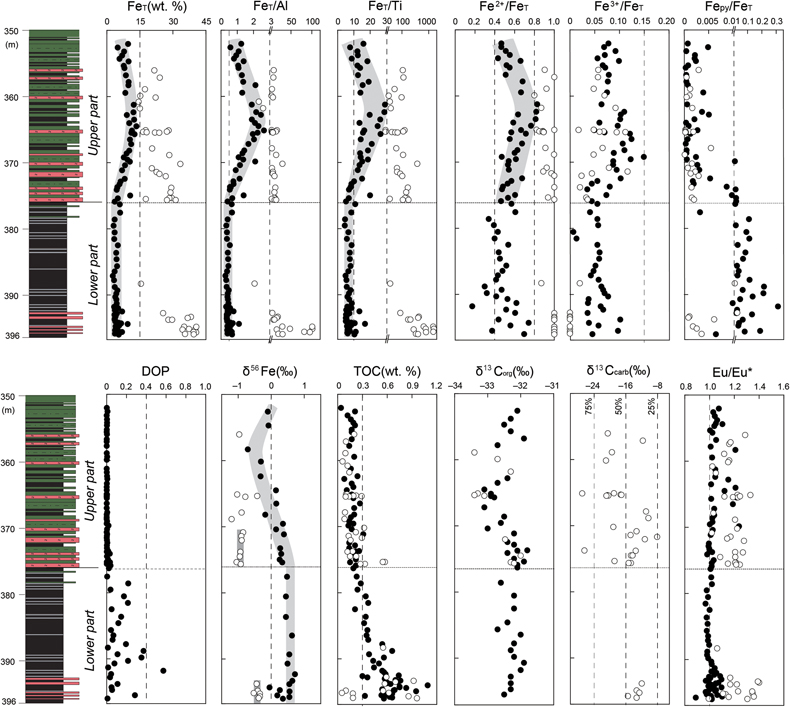
Figure 2 Geochemical profiles of the unit 5 of the Xiamaling Formation. Degree of pyritisation (DOP) was calculated by Fepy/(Fe2++Fe3++Fepy). Data of the FeT, total organic carbon (TOC), Eu/Eu*, carbon isotopes of carbonate (δ13Ccarb) and kerogen (δ13Corg) with a depth range of 390−350 m were from Canfield et al. (2018)
Canfield, D.E., Zhang, S.C., Wang, H.J., Wang, X.M., Zhao, W.Z., Su, J., Bjerrum, C.J., Haxen, E.R., Hammarlund, E.U. (2018) A Mesoproterozoic Iron Formation. Proceedings of the National Academy of Sciences 115, E3895–E3904. https://doi.org/10.1073/pnas.1720529115
. Note the abscissas breaks of FeT/Al, FeT/Ti, Fepy/FeT are at 3, 30 and 0.01, respectively. Cycle and solid points represent IF (FeT > 15 %) and clastic (FeT < 15 %) samples, respectively. Dashed lines in the δ13Ccarb panel represent the proportions of organic-sourced bicarbonate of measured samples, with an initial δ13Corg of −32 ‰ and an initial δ13C of bicarbonate in water column of 0 ‰.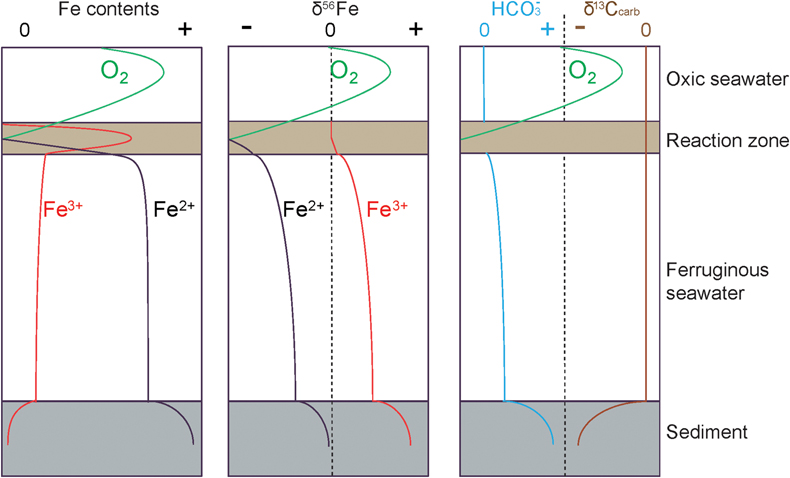
Figure 3 Genetic model of the coupled Fe and C cycles and isotope fractionations in the water column and sediment.
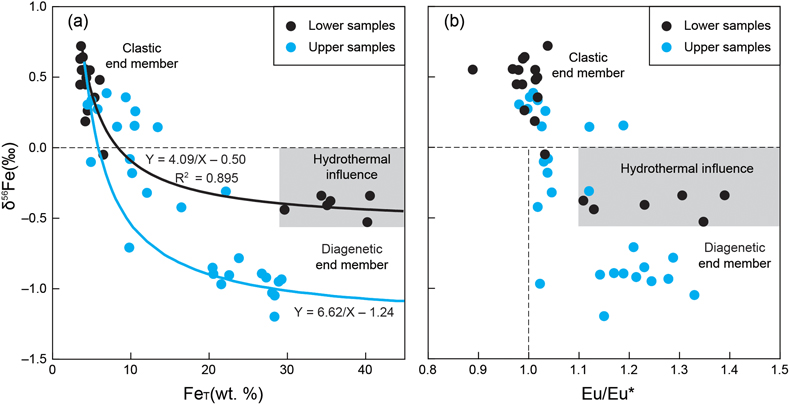
Figure 4 Correlation diagrams of δ56Fe with (a) FeT and (b) Eu/Eu*. The black and blue lines in (a) are the optimal mixing lines of the lower and upper parts, respectively, with a clastic end member and a diagenetic end member. Grey areas mean the Fe cycle and δ56Fe values were influenced by the hydrothermal fluids.