Early precipitated micropyrite in microbialites: A time capsule of microbial sulfur cycling
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:167Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
1δ34S = ((34S/32S)sample/(34S/32S)reference − 1) × 1000 in ‰, with Vienna Canyon Diablo Troilite as the reference.
Figures
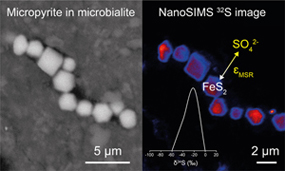
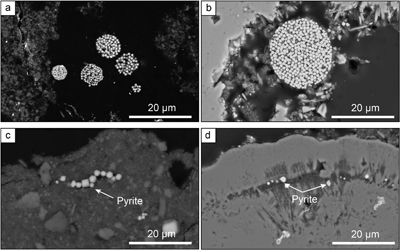 Figure 1 Secondary Electron microscopy pictures of (a, b) framboidal pyrites and (c, d) micropyrites from (a, c) Cayo Coco Lagoon and (b, d) Atexcac. Framboidal pyrites are located at the surface of the mineralised microbialite (in dark) while micropyrites are entombed within aragonite (in light grey) or Mg rich silicate (dark grey). | 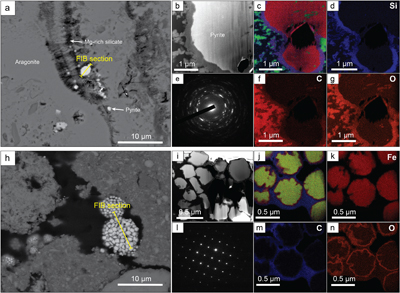 Figure 2 (a) SEM picture of micropyrites. Location where FIB section was extracted is shown by the yellow line, (b) TEM picture of the pyrite crystal and (e) its associated powder-like diffraction pattern, (c) false colour STEM EDXS image (Si in blue, Ca in green, Fe in red) and (d, f, g) Si, C and O images of the submicrometric pyrites, respectively. (h) SEM picture of framboidal pyrite with FIB section location (yellow line), (i) TEM image and (l) associated single crystal diffraction pattern along the [112] zone axis of pyrite, (j) false colour STEM EDXS image of pyrite crystallites (Fe in red, S in green, C in blue) and (k, m, n) Fe, C and O images, respectively. | 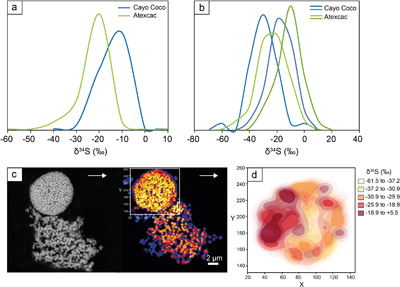 Figure 3 (a) δ34S probability density function of all framboidal pyrites from Atexcac and Cayo Coco uncertainties of analyses ranges from 0.4 to 4 ‰, (b) δ34S probability density function of four individual framboidal pyrites containing up to 100 pyrite crystallites, (c) SEM and corresponding NanoSIMS 32S image of one framboidal pyrite; the arrow indicates the top of the mat, and (d) δ34S values reconstructed for individual pyrite crystallites showing strong variations in S isotope composition across the framboidal pyrite. | 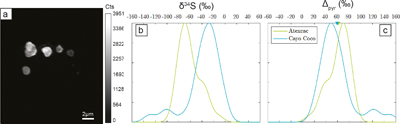 Figure 4 (a) NanoSIMS 32S image of submicrometric pyrites, (b) δ34S probability density function, taking account of the range of uncertainties from 1 ‰ to 8 ‰ of micropyrites from Cayo Coco and Atexcac, (c) Δpyr distribution calculated for both environments. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Sulfate-reducing bacteria, i.e. microorganisms that use sulfate as a terminal electron acceptor, are ubiquitous in Earth environments where they play a major role both in S and C biogeochemical cycles (e.g., Jørgensen et al., 2019
Jørgensen, B.B., Findlay, A.J., Pallerin, A. (2019) The Biogeochemical Sulfur Cycle of Marine Sediments. Frontiers in Microbiology 10, 849. https://doi.org/10.3389/fmicb.2019.00849
). Microbial sulfate reduction (MSR) reduces sulfate to dissolved S species, such as HS− and H2S, and discriminates against heavy sulfur isotopes. The resulting sulfide δ34S values are relatively light and can be as much as −70 ‰ relative to sulfate (Jørgensen et al., 2019Jørgensen, B.B., Findlay, A.J., Pallerin, A. (2019) The Biogeochemical Sulfur Cycle of Marine Sediments. Frontiers in Microbiology 10, 849. https://doi.org/10.3389/fmicb.2019.00849
). The fractionation induced by this metabolic activity (34ɛmic hereafter) depends on the sulfate concentration, identity of the electron donor, bioavailable carbon (content and chemical form) and, perhaps most importantly, the cell-specific sulfate reduction rates (csSRR; Bradley et al., 2016Bradley, A.S., Leavitt, W.D., Schmidt, M., Knoll, A.H., Girguis, P.R., Johnston, D.T. (2016) Patterns of sulfur isotope fractionation during microbial sulfate reduction. Geobiology 14, 91–101. https://doi.org/10.1111/gbi.12149
). In modern environments, MSR can be identified by rate measurements with radiotracers or genomic and proteomic approaches. However, since genetic markers are not preserved in the geological record, the recognition of MSR in palaeoenvironments mostly relies on the sulfur isotopic compositions of sedimentary sulfide and sulfate minerals (Visscher et al., 2000Visscher, P.T., Reid, R.P., Bebout, B.M. (2000) Microscale observations of sulfate reduction: Correlation of microbial activity with lithified micritic laminae in modern marine stromatolites. Geology 28, 919–922. https://doi.org/10.1130/0091-7613(2000)28<919:MOOSRC>2.0.CO;2
; Fike et al., 2008Fike, D.A., Gammon, C.L., Ziebis, W., Orphan, V.J. (2008) Micron-scale mapping of sulfur cycling across the oxycline of a cyanobacterial mat: a paired nanoSIMS and CARD-FISH approach. The ISME Journal 2, 749–759. https://doi.org/10.1038/ismej.2008.39
).MSR plays a key role in carbonate mineralisation, especially identified in microbialites and microbial mats (Visscher et al., 2000
Visscher, P.T., Reid, R.P., Bebout, B.M. (2000) Microscale observations of sulfate reduction: Correlation of microbial activity with lithified micritic laminae in modern marine stromatolites. Geology 28, 919–922. https://doi.org/10.1130/0091-7613(2000)28<919:MOOSRC>2.0.CO;2
). Microbial mats are stratified microbial communities whose metabolic activities produce geochemical gradients and drive elemental cycling (Canfield and Des Marais, 1993Canfield, D.E., Des Marais, D.J. (1993) Biogeochemical cycles of carbon, sulfur, and free oxygen in a microbial mat. Geochimica et Cosmochimica Acta 57, 3971–3984. https://doi.org/10.1016/0016-7037(93)90347-Y
; Paerl and Pinckney, 1996Paerl, H.W., Pinckney, J.L. (1996) A mini-review of microbial consortia: their roles in aquatic production and biogeochemical cycling. Microbial Ecology 31, 225–247. https://doi.org/10.1007/BF00171569
). In the geological record, such deposits (often referred to as stromatolites) are considered among the oldest trace of life on Earth (Allwood et al., 2009Allwood, A.C., Grotzinger, J.P., Knoll, A.H., Burch, I.W., Anderson, M.S., Coleman, M.L., Kanik, I. (2009) Controls on development and diversity of Early Archean stromatolites. Proceedings of the National Academy of Sciences 106, 9548–9555. https://doi.org/10.1073/pnas.0903323106
). Some Archaean stromatolites contain carbonaceous laminae that have been interpreted as fossil microbial mats or biofilms based on textural evidence (Awramik, 1992Awramik, S.M. (1992) The history and significance of stromatolite. In: Schidlowski, M., Golubic, S., Kimberley, M.M., McKirdy, D.M., Trudinger, P.A. (Eds.) Early organic evolution. Springer, Berlin, 435–449. https://doi.org/10.1007/978-3-642-76884-2_34
; Lepot, 2020Lepot, K. (2020) Signatures of early microbial life from the Archean (4 to 2.5 Ga) eon. Earth-Science Reviews 209, 103296. https://doi.org/10.1016/j.earscirev.2020.103296
). Interestingly, determining the precise nature of the fossil microbial community is challenging because these organosedimentary rocks resulted from a complex balance between microbial activities, sedimentation and intermittent lithification (Reid et al., 2000Reid, R.P., Visscher, P.T., Decho, A.W., Stolz, J.F., Bebout, B.M., Dupraz, C., Macintyre, I.G., Paerl, H.W., Pinckney, J.L., Prufert-Bebout, J., Steppe, T.F., DesMarais, D.J. (2000) The role of microbes in accretion, lamination and early lithification of modern marine stromatolites. Nature 406, 989–992. https://doi.org/10.1038/35023158
). In addition, the biosignatures preserved in fossil biofilms are ambiguous, especially after diagenesis and post-depositional history (Javaux, 2019Javaux, E.J. (2019) Challenges in evidencing the earliest traces of life. Nature 572, 451–460. https://doi.org/10.1038/s41586-019-1436-4
; Alleon et al., 2021Alleon, J., Bernard, S., Olivier, N., Thomazo, C., Marin-Carbonne, J. (2021) Inherited geochemical diversity of 3.4 Ga organic films from the Buck Reef Chert, South Africa. Communications Earth & Environment 2, 6. https://doi.org/10.1038/s43247-020-00066-7
). The oldest evidence for MSR in the Archaean geological record are sulfur isotopic signatures from deep marine sediments (Kamber and Whitehouse, 2007Kamber, B.S., Whitehouse, M.J. (2007) Micro-scale sulphur isotope evidence for sulphur cycling in the late Archean shallow ocean. Geobiology 5, 5–17. https://doi.org/10.1111/j.1472-4669.2006.00091.x
; Shen et al., 2009Shen, Y., Farquhar, J., Masterson, A., Kaufman, A.J., Buick, R. (2009) Evaluating the role of microbial sulfate reduction in the early Archean using quadruple isotope systematics. Earth and Planetary Science Letters 279, 383–391. https://doi.org/10.1016/j.epsl.2009.01.018
) and stromatolites (Shen and Buick, 2004Shen, Y., Buick, R. (2004). The antiquity of microbial sulfate reduction. Earth-Science Reviews 64, 243–272. https://doi.org/10.1016/S0012-8252(03)00054-0
). In modern microbialites, numerous studies have reported dynamic MSR activity based on H2S labelling (Visscher et al., 2000Visscher, P.T., Reid, R.P., Bebout, B.M. (2000) Microscale observations of sulfate reduction: Correlation of microbial activity with lithified micritic laminae in modern marine stromatolites. Geology 28, 919–922. https://doi.org/10.1130/0091-7613(2000)28<919:MOOSRC>2.0.CO;2
; Fike et al., 2008Fike, D.A., Gammon, C.L., Ziebis, W., Orphan, V.J. (2008) Micron-scale mapping of sulfur cycling across the oxycline of a cyanobacterial mat: a paired nanoSIMS and CARD-FISH approach. The ISME Journal 2, 749–759. https://doi.org/10.1038/ismej.2008.39
; Pace et al., 2018Pace, A., Bourillot, R., Bouton, A., Vennin, E., Braissant, O., Dupraz, C., Duteil, T., Bundeleva, I., Patrier, P., Galaup, S., Yokoyama, Y., Franceschi, M., Virgone, A., Visscher, P.T. (2018) Formation of stromatolite lamina at the interface of oxygenic-anoxygenic photosynthesis. Geobiology 16, 378–398. https://doi.org/10.1111/gbi.12281
; Gomes et al., 2021Gomes, M.L., Klatt, J.M., Dick, G.J., Grim, S.L., Rico, K.I., Medina, M., Ziebis, W., Kinsman-Costello, L., Sheldon, N.D., Fike, D.A. (2021) Sedimentary pyrite sulfur isotope compositions preserve signatures of the surface microbial mat environment in sediments underlying low-oxygen cyanobacterial mats. Geobiology 20, 60–78. https://doi.org/10.1111/gbi.12466
), but only a few studies have investigated sulfur isotope signatures of individual pyrite grains (Gomes et al., 2021Gomes, M.L., Klatt, J.M., Dick, G.J., Grim, S.L., Rico, K.I., Medina, M., Ziebis, W., Kinsman-Costello, L., Sheldon, N.D., Fike, D.A. (2021) Sedimentary pyrite sulfur isotope compositions preserve signatures of the surface microbial mat environment in sediments underlying low-oxygen cyanobacterial mats. Geobiology 20, 60–78. https://doi.org/10.1111/gbi.12466
).The primary S isotopic signatures of pyrites (FeS2) are often modified by fluid circulation during metasomatism or metamorphism (Marin-Carbonne et al., 2020
Marin-Carbonne, J., Busigny, V., Miot, J., Rollion-Bard, C., Muller, E., Drabon, N., Jacob, D., Pont, S., Robyr, M., Bontognali, T.R.R., François, C., Reynaud, S., Van Zuilen, M., Philippot, P. (2020) In Situ Fe and S isotope analyses in pyrite from the 3.2 Ga Mendon Formation (Barberton Greenstone Belt, South Africa): Evidence for early microbial iron reduction. Geobiology 18, 306–325. https://doi.org/10.1111/gbi.12385
; Slotznick et al., 2022Slotznick, S.P., Johnson, J.E., Rasmussen, B., Raub, T.D., Webb, S.M., Zi, J.-W., Kirschvink, J.L., Fischer, W.W. (2022) Reexamination of 2.5-Ga “whiff” of oxygen interval points to anoxic ocean before GOE. Science Advances 8, eabj7190. https://doi.org/10.1126/sciadv.abj7190
), occurring millions or billions of years after sediment deposition. While late diagenesis can modify both pyrite crystallinity and S isotope composition (Williford et al., 2011Williford, K.H., Van Kranendonk, M.J., Ushikubo, T., Kozdon, R., Valley, J.W. (2011) Constraining atmospheric oxygen and seawater sulfate concentrations during Paleoproterozoic glaciation: In situ sulfur three-isotope microanalysis of pyrite from the Turee Creek Group, Western Australia. Geochimica et Cosmochimica Acta 75, 5686–5705. https://doi.org/10.1016/j.gca.2011.07.010
; Gomes et al., 2018Gomes, M.L., Fike, D.A., Bergmann, K.D., Jones, C., Knoll, A.H. (2018) Environmental insights from high-resolution (SIMS) sulfur isotope analyses of sulfides in Proterozoic microbialites with diverse mat textures. Geobiology 16, 17–34. https://doi.org/10.1111/gbi.12265
; Marin-Carbonne et al., 2020Marin-Carbonne, J., Busigny, V., Miot, J., Rollion-Bard, C., Muller, E., Drabon, N., Jacob, D., Pont, S., Robyr, M., Bontognali, T.R.R., François, C., Reynaud, S., Van Zuilen, M., Philippot, P. (2020) In Situ Fe and S isotope analyses in pyrite from the 3.2 Ga Mendon Formation (Barberton Greenstone Belt, South Africa): Evidence for early microbial iron reduction. Geobiology 18, 306–325. https://doi.org/10.1111/gbi.12385
), early diagenesis in microbial mats is thought to have a limited effect on the S isotopic composition of pyrite, meaning that microbialitic pyrites may preserve ‘pristine’ isotopic signatures. However, the observation of large isotopic differences of about ∼30 ‰ (Raven et al., 2016Raven, M.R., Sessions, A.L., Fischer, W.W., Adkins, J.F. (2016) Sedimentary pyrite δ34S differs from porewater sulfide in Santa Barbara Basin: Proposed role of organic sulfur. Geochimica et Cosmochimica Acta 186, 120–134. https://doi.org/10.1016/j.gca.2016.04.037
) between pore water sulfur species (SO42− and H2S) and pyrite shows that other S-bearing pools, such as organic matter, should be considered in order to quantitatively and isotopically describe sulfur cycling in microbialites. Pyrite often precipitates at the microbial mat surface (Gomes et al., 2021Gomes, M.L., Klatt, J.M., Dick, G.J., Grim, S.L., Rico, K.I., Medina, M., Ziebis, W., Kinsman-Costello, L., Sheldon, N.D., Fike, D.A. (2021) Sedimentary pyrite sulfur isotope compositions preserve signatures of the surface microbial mat environment in sediments underlying low-oxygen cyanobacterial mats. Geobiology 20, 60–78. https://doi.org/10.1111/gbi.12466
) and its isotopic composition is more representative of the local setting rather than global environmental conditions, e.g., water column (Lang et al., 2020Lang, X., Tang, W., Ma, H., Shen, B. (2020) Local environmental variation obscures the interpretation of pyrite sulfur isotope records. Earth and Planetary Science Letters 533, 116056. https://doi.org/10.1016/j.epsl.2019.116056
; Pasquier et al., 2021Pasquier, V., Bryant, R.N., Fike, D.A., Halevy, I. (2021) Strong local, not global, controls on marine pyrite sulfur isotopes. Science Advances 7, eabb7403. https://doi.org/10.1126/sciadv.abb7403
). Decoding pyrite S isotopes at the microscale in sedimentary rocks is required to better understand how local conditions may affect the isotopic composition of microbialite pyrites. Here, we focus our investigation on two geographically independent modern microbial mats, which have not yet undergone (complete) lithification, and/or metasomatism.top
Syngenetic Microbialitic Pyrites
We studied two samples from 1) the Atexcac Lake, a monomictic volcanic crater lake (Mexico; Zeyen et al., 2021
Zeyen, N., Benzerara, K., Beyssac, O., Daval, D., Muller, E., Thomazo, C., Tavera, R., López-García, P., Moreira, D., Duprat, E. (2021) Integrative analysis of the mineralogical and chemical composition of modern microbialites from ten Mexican lakes: What do we learn about their formation. Geochimica et Cosmochimica Acta 305, 148–184. https://doi.org/10.1016/j.gca.2021.04.030
) and 2) Cayo Coco Lake, a shallow hypersaline lagoon in Cuba (Pace et al., 2018Pace, A., Bourillot, R., Bouton, A., Vennin, E., Braissant, O., Dupraz, C., Duteil, T., Bundeleva, I., Patrier, P., Galaup, S., Yokoyama, Y., Franceschi, M., Virgone, A., Visscher, P.T. (2018) Formation of stromatolite lamina at the interface of oxygenic-anoxygenic photosynthesis. Geobiology 16, 378–398. https://doi.org/10.1111/gbi.12281
; Bouton et al., 2020Bouton, A., Vennin, E., Thomazo, C., Mathieu, O., Garcia, F., Jaubert, M., Visscher, P. (2020) Microbial Origin of the Organic Matter Preserved in the Cayo Coco Lagoonal Network, Cuba. Minerals 10, 143. https://doi.org/10.3390/min10020143
). These two depositional settings exhibit contrasting water column sulfate concentrations of 2.1 and 62 mM for Lake Atexcac and Cayo Coco, respectively (Figs. S-1 and S-2, SI). Both samples were produced by mineralising microbial mats and contained authigenic aragonite, Mg-rich calcite, dolomite, authigenic hydrated Mg-silicates/silica such as kerolite, and detrital phases such as feldspars and illite (Figs S-1 and S-2, SI). In each locality, pyrite morphologies fall into two different categories (Fig. 1): framboidal pyrites, ranging from 3 to 15 μm, and mono-crystal pyrites of a few micrometres (>3 μm), hereafter called micropyrites (Figs. 1 and 2, SI). Transmission electron microscopy analyses revealed an early origin of the micropyrite grains (SI). Considering both the alignment of the micropyrites within the organic lamination and their crystallinity, micropyrites are likely formed during an early lithification stage (SI).
Figure 1 Secondary Electron microscopy pictures of (a, b) framboidal pyrites and (c, d) micropyrites from (a, c) Cayo Coco Lagoon and (b, d) Atexcac. Framboidal pyrites are located at the surface of the mineralised microbialite (in dark) while micropyrites are entombed within aragonite (in light grey) or Mg rich silicate (dark grey).

Figure 2 (a) SEM picture of micropyrites. Location where FIB section was extracted is shown by the yellow line, (b) TEM picture of the pyrite crystal and (e) its associated powder-like diffraction pattern, (c) false colour STEM EDXS image (Si in blue, Ca in green, Fe in red) and (d, f, g) Si, C and O images of the submicrometric pyrites, respectively. (h) SEM picture of framboidal pyrite with FIB section location (yellow line), (i) TEM image and (l) associated single crystal diffraction pattern along the [112] zone axis of pyrite, (j) false colour STEM EDXS image of pyrite crystallites (Fe in red, S in green, C in blue) and (k, m, n) Fe, C and O images, respectively.
top
NanoSIMS S Isotope Composition of Pyrites
The S isotope compositions of 66 framboidal pyrites and 55 individual micropyrites were measured by NanoSIMS with a reproducibility better than 2 ‰ (2σ, see SI). Framboidal pyrites display a ∼20–30 ‰ range in δ34S values with an average of −26.1 ± 7 ‰ and −26.4 ± 9 ‰ (2 s.d.) for Atexcac and Cayo Coco, respectively (Figs. 3 and 4). We have extracted S isotope composition of individual crystallites from four framboids (Fig. 3, SI). All framboidal pyrites (n = 4) show a large internal δ34S variability (∼40 ‰, Fig. 3) characterised by a gradient from ∼+8.5 ± 1.5 ‰ to more 34S-depleted values ranging from −42 to −69 ‰. Micropyrites also show large S isotope heterogeneities with δ 34S values ranging from −86 to −17 ‰ with an average value of −61.4 ± 17 ‰ for Atexcac, and from −53 to −21 ‰ with an average value of −34.5 ± 29 ‰ in Cayo Coco (Fig. 4).

Figure 3 (a) δ34S probability density function of all framboidal pyrites from Atexcac and Cayo Coco uncertainties of analyses ranges from 0.4 to 4 ‰, (b) δ34S probability density function of four individual framboidal pyrites containing up to 100 pyrite crystallites, (c) SEM and corresponding NanoSIMS 32S image of one framboidal pyrite; the arrow indicates the top of the mat, and (d) δ34S values reconstructed for individual pyrite crystallites showing strong variations in S isotope composition across the framboidal pyrite.

Figure 4 (a) NanoSIMS 32S image of submicrometric pyrites, (b) δ34S probability density function, taking account of the range of uncertainties from 1 ‰ to 8 ‰ of micropyrites from Cayo Coco and Atexcac, (c) Δpyr distribution calculated for both environments.
top
Framboidal Pyrites Record a Mixing of Reduction and Oxidation Processes
Framboidal pyrites display a large range of δ 34S values but also an internal isotope variation across the length scale of individual framboidal grains (Fig. 3), best explained by a combination of MSR and partial sulfide oxidation (Fig. 3; Pellerin et al., 2019
Pellerin, A., Antler, G., Holm, S.A., Findlay, A.J., Crockford, P.W., Turchyn, A.V., Jørgensen, B.B., Finster, K. (2019) Large sulfur isotope fractionation by bacterial sulfide oxidation. Science Advances 5, eaaw1480. https://doi.org/10.1126/sciadv.aaw1480
). As framboidal pyrites are mostly observed at the surface of the mat, S isotope variations reflect the mixing of in situ production, upward diffusion of sulfide in the mat and its subsequent reoxidation at the mat surface. The fractionation required to produce such an isotopic gradient is well above abiotic sulfide oxidation (i.e. ∼+5 ‰; Fry et al., 1988Fry, B., Ruf, W., Gest, H., Hayes, J.M. (1988) Sulfur isotope effects associated with oxidation of sulfide by O2 in aqueous solution. Chemical Geology: Isotope Geoscience section 73, 205–210. https://doi.org/10.1016/0168-9622(88)90001-2
), yet can also be consistent with microbial sulfide oxidation in high pH environments (Pellerin et al., 2019Pellerin, A., Antler, G., Holm, S.A., Findlay, A.J., Crockford, P.W., Turchyn, A.V., Jørgensen, B.B., Finster, K. (2019) Large sulfur isotope fractionation by bacterial sulfide oxidation. Science Advances 5, eaaw1480. https://doi.org/10.1126/sciadv.aaw1480
). Both sites are characterised by high pH (pH > 8, see SI), which is known to promote large isotope fractionation during sulfide oxidation (Pellerin et al., 2019Pellerin, A., Antler, G., Holm, S.A., Findlay, A.J., Crockford, P.W., Turchyn, A.V., Jørgensen, B.B., Finster, K. (2019) Large sulfur isotope fractionation by bacterial sulfide oxidation. Science Advances 5, eaaw1480. https://doi.org/10.1126/sciadv.aaw1480
). Consequently, part of the observed range of δ 34S values may be attributed to local variation of S speciation associated with pH. As such, the internal gradient may be the result of microbially mediated surface H2S oxidation. Alternatively, the internal isotope gradient across the framboidal pyrites (Fig. 2, SI) can be due to Rayleigh isotope fractionation, as even under oxidising (abundant sulfate) conditions, consumption can occur faster than diffusive replenishment (Goldhaber and Kaplan, 1980Goldhaber, M.B., Kaplan, I.R. (1980) Mechanisms of sulfur incorporation and isotope fractionation during early diagenesis in sediments of the Gulf of California. Marine Chemistry 9, 95–143. https://doi.org/10.1016/0304-4203(80)90063-8
). Rather than reflecting water column conditions, the S isotope composition of framboidal pyrites appears to be strongly influenced by local redox conditions (i.e. at the microbial mat scale).top
Microbialitic Micropyrite Preserve Primary Isotopic Microbial Fractionation Signatures
The presence of Mg silicate rich rims (SI) suggests that micropyrites were probably formed very early during lithification (Fig. 2). Moreover, the small crystal size of micropyrites composed of nanocrystals with different orientations has been highlighted as a possible biogenic signature (Picard et al., 2018
Picard, A., Gartman, A., Clarke, D.R., Girguis, P.R. (2018) Sulfate-reducing bacteria influence the nucleation and growth of mackinawite and greigite. Geochimica et Cosmochimica Acta 220, 367–384. https://doi.org/10.1016/j.gca.2017.10.006
). The δ34S values of dissolved sulfate are +0.52 ‰ in Atexcac and are assumed to be close to seawater composition (+21 ‰) for Cayo Coco (SI). Considering these hugely contrasting isotopic compositions of sulfate, micropyrites display surprisingly similar Δpyr values (i.e. Δpyr = δ34SSO4 − δ34Spyr) of 61.4‰ ± 18.3‰ and 59.1‰ ± 29.6‰ for Atexcac and Cayo Coco, respectively (Fig. 4). These Δpyr values are consistent with near thermodynamic equilibrium fractionation as observed in i) MSR batch culture experiments characterised by low growth rate and csSRR (Leavitt et al., 2013Leavitt, W.D., Halevy, I., Bradley, A.S., Johnston, D.T. (2013) Influence of sulfate reduction rates on the Phanerozoic sulfur isotope record. Proceedings of the National Academy of Sciences 110, 11244–11249. https://doi.org/10.1073/pnas.1218874110
; Bradley et al., 2016Bradley, A.S., Leavitt, W.D., Schmidt, M., Knoll, A.H., Girguis, P.R., Johnston, D.T. (2016) Patterns of sulfur isotope fractionation during microbial sulfate reduction. Geobiology 14, 91–101. https://doi.org/10.1111/gbi.12149
) and ii) natural environments (e.g., Cadagno Lake; Canfield et al., 2010Canfield, D.E., Farquhar, J., Zerkle, A.L. (2010) High isotope fractionations during sulfate reduction in a low-sulfate euxinic ocean analog. Geology 38, 415–418. https://doi.org/10.1130/G30723.1
). High 34ɛmic has been observed in sulfate reducing strains only partially oxidising their carbon substrate and is sometimes associated with the degradation of carbohydrate components, including exopolymeric substances (EPS) (Sim et al., 2011Sim, M.S., Ono, S., Donovan, K., Templer, S.P., Bosak, T. (2011) Effect of electron donors on the fractionation of sulfur isotopes by a marine Desulfovibrio sp. Geochimica et Cosmochimica Acta 75, 4244–4259. https://doi.org/10.1016/j.gca.2011.05.021
), which are abundant in microbialite-forming mats. Atexcac waters have a high dissolved organic carbon content (over 15 times that of the modern ocean) which can sustain MSR activity, while Cayo Coco harbours conspicuous suspended EPS-rich organic slimes (Bouton et al., 2016Bouton, A., Vennin, E., Pace, A., Bourillot, R., Dupraz, C., Thomazo, C., Brayard, A., Désaubliaux, G., Visscher, P.T. (2016) External controls on the distribution, fabrics and mineralization of modern microbial mats in a coastal hypersaline lagoon, Cayo Coco (Cuba). Sedimentology 63, 972–1016. https://doi.org/10.1111/sed.12246
). Despite abundant sulfate (at Cayo Coco) and organic matter, csSRR in these mats are intriguingly low and contrast with previous occurrences of high SRR in surface microbial mats (Canfield and Des Marais, 1993Canfield, D.E., Des Marais, D.J. (1993) Biogeochemical cycles of carbon, sulfur, and free oxygen in a microbial mat. Geochimica et Cosmochimica Acta 57, 3971–3984. https://doi.org/10.1016/0016-7037(93)90347-Y
). Low csSRR and high S isotope fractionations in both lakes could be explained by the refractory nature of this organic matter (Bouton et al., 2020Bouton, A., Vennin, E., Thomazo, C., Mathieu, O., Garcia, F., Jaubert, M., Visscher, P. (2020) Microbial Origin of the Organic Matter Preserved in the Cayo Coco Lagoonal Network, Cuba. Minerals 10, 143. https://doi.org/10.3390/min10020143
; Gomes et al., 2021Gomes, M.L., Klatt, J.M., Dick, G.J., Grim, S.L., Rico, K.I., Medina, M., Ziebis, W., Kinsman-Costello, L., Sheldon, N.D., Fike, D.A. (2021) Sedimentary pyrite sulfur isotope compositions preserve signatures of the surface microbial mat environment in sediments underlying low-oxygen cyanobacterial mats. Geobiology 20, 60–78. https://doi.org/10.1111/gbi.12466
). At the microbial mat scale, strong gradients of sulfate reduction within layered mats (Visscher et al., 2000Visscher, P.T., Reid, R.P., Bebout, B.M. (2000) Microscale observations of sulfate reduction: Correlation of microbial activity with lithified micritic laminae in modern marine stromatolites. Geology 28, 919–922. https://doi.org/10.1130/0091-7613(2000)28<919:MOOSRC>2.0.CO;2
; Fike et al., 2009Fike, D.A., Finke, N., Zha, J., Blake, G., Hoehler, T.M., Orphan, V.J. (2009) The effect of sulfate concentration on (sub)millimeter-scale sulfide δ34S in hypersaline cyanobacterial mats over the diurnal cycle. Geochimica et Cosmochimica Acta 73, 6187–6204. https://doi.org/10.1016/j.gca.2009.07.006
; Pace et al., 2018Pace, A., Bourillot, R., Bouton, A., Vennin, E., Braissant, O., Dupraz, C., Duteil, T., Bundeleva, I., Patrier, P., Galaup, S., Yokoyama, Y., Franceschi, M., Virgone, A., Visscher, P.T. (2018) Formation of stromatolite lamina at the interface of oxygenic-anoxygenic photosynthesis. Geobiology 16, 378–398. https://doi.org/10.1111/gbi.12281
) have been attributed to small scale variations in csSRR and/or localised MSR micro-niches (Fike et al., 2009Fike, D.A., Finke, N., Zha, J., Blake, G., Hoehler, T.M., Orphan, V.J. (2009) The effect of sulfate concentration on (sub)millimeter-scale sulfide δ34S in hypersaline cyanobacterial mats over the diurnal cycle. Geochimica et Cosmochimica Acta 73, 6187–6204. https://doi.org/10.1016/j.gca.2009.07.006
; Gomes et al., 2021Gomes, M.L., Klatt, J.M., Dick, G.J., Grim, S.L., Rico, K.I., Medina, M., Ziebis, W., Kinsman-Costello, L., Sheldon, N.D., Fike, D.A. (2021) Sedimentary pyrite sulfur isotope compositions preserve signatures of the surface microbial mat environment in sediments underlying low-oxygen cyanobacterial mats. Geobiology 20, 60–78. https://doi.org/10.1111/gbi.12466
). The observed laminations, which contain micropyrites, likely reflect local high density microbe spots, which can result from a more pronounced local distillation of δ34S (Pasquier et al., 2021Pasquier, V., Bryant, R.N., Fike, D.A., Halevy, I. (2021) Strong local, not global, controls on marine pyrite sulfur isotopes. Science Advances 7, eabb7403. https://doi.org/10.1126/sciadv.abb7403
). Alternatively, the composition of microbial consortia may affect the range of csSRR at the microbial mat scale (Bradley et al., 2016Bradley, A.S., Leavitt, W.D., Schmidt, M., Knoll, A.H., Girguis, P.R., Johnston, D.T. (2016) Patterns of sulfur isotope fractionation during microbial sulfate reduction. Geobiology 14, 91–101. https://doi.org/10.1111/gbi.12149
), with guild diversity having opposite effects on trophic group functions, thus modulating csSRR (Bell et al., 2005Bell, T., Newman, J.A., Silverman, B.W., Turner, S.L., Lilley, A.K. (2005) The contribution of species richness and composition to bacterial services. Nature 436, 1157–1160. https://doi.org/10.1038/nature03891
; Peter et al., 2011Peter, H., Beier, S., Bertilsson, S., Lindström, E.S., Langenheder, S., Tranvik, L.J. (2011) Function-specific response to depletion of microbial diversity. The ISME Journal 5, 351–361. https://doi.org/10.1038/ismej.2010.119
).top
Conclusions
Here, we have shown that the S isotope composition of framboids and micropyrites reflects sulfur cycling at the scale of the mat environment. While S isotope signatures in microbialite micropyrites are primarily controlled by MSR, they can also be influenced by oxidative sulfur cycling in high pH environments. Notably, microbialites growing at different dissolved sulfate concentrations and in marine versus lacustrine environments display similar micropyrite morphologies and comparable Δpyr. Such observations demonstrate that microbialites have the potential to record the isotopic fractionation associated with MSR irrespective of the depositional environment and sulfate level. Consequently, we propose that microbialite micropyrites can be used as a mineral signature for reconstructing past Earth surface and microbial environments, as already suggested for Archaean stromatolites (Marin-Carbonne et al., 2018
Marin-Carbonne, J., Remusat, L., Sforna, M.C., Thomazo, C., Cartigny, P., Philippot, P. (2018) Sulfur isotope’s signal of nanopyrites enclosed in 2.7 Ga stromatolitic organic remains reveal microbial sulfate reduction. Geobiology 16, 121–138. https://doi.org/10.1111/gbi.12275
). In addition, this study clearly shows that caution should be used in reconstructing past environmental parameters, such as water body sulfate levels, from Δpyr. Finally, the respective influence of different electron donors, sulfate concentration, and non-actualistic microbial communities on the csSRR and associated sedimentary pyrites δ34S remains to be explored in order to deepen our understanding of the evolutionary trajectory of biogeochemical sulfur cycling on Earth.top
Author contributions
JMC, LR, SB and CT designed the study, KB, EV, CT and AB collected samples in the field. JMC, LR, MND and SE conducted the NanoSIMS analyses, CT and RH conducted the bulk S isotope analyses. JMC, MND, JA, AB, NZ and KB conducted the microscope observations. All authors have contributed to the data interpretation. JMC wrote the manuscript with important contributions of all co-authors.
top
Competing interests
Authors declare no competing interests.
top
Data and materials availability
All data is available in the main text or the supplementary materials.
top
Acknowledgements
This research was supported through the European Research council (ERC) under the European Union’s Horizon H2020 research and innovation programme (STROMATA grant agreement 759289). The NanoSIMS facility at the Museum National d’Histoire Naturelle in Paris was established by funds from the CNRS, region ile de France, Ministère délégué à l’enseignement et à la recherche and the Museum National d’Histoire Naturelle. We thank Kevin McKeegan and Jasmine Berg for their proofreading of the manuscript and for fruitful discussions.
Editor: Tanja Bosak
top
References
Alleon, J., Bernard, S., Olivier, N., Thomazo, C., Marin-Carbonne, J. (2021) Inherited geochemical diversity of 3.4 Ga organic films from the Buck Reef Chert, South Africa. Communications Earth & Environment 2, 6. https://doi.org/10.1038/s43247-020-00066-7
 Show in context
Show in context In addition, the biosignatures preserved in fossil biofilms are ambiguous, especially after diagenesis and post-depositional history (Javaux, 2019; Alleon et al., 2021).
View in article
Allwood, A.C., Grotzinger, J.P., Knoll, A.H., Burch, I.W., Anderson, M.S., Coleman, M.L., Kanik, I. (2009) Controls on development and diversity of Early Archean stromatolites. Proceedings of the National Academy of Sciences 106, 9548–9555. https://doi.org/10.1073/pnas.0903323106
 Show in context
Show in context In the geological record, such deposits (often referred to as stromatolites) are considered among the oldest trace of life on Earth (Allwood et al., 2009).
View in article
Awramik, S.M. (1992) The history and significance of stromatolite. In: Schidlowski, M., Golubic, S., Kimberley, M.M., McKirdy, D.M., Trudinger, P.A. (Eds.) Early organic evolution. Springer, Berlin, 435–449. https://doi.org/10.1007/978-3-642-76884-2_34
 Show in context
Show in context Some Archaean stromatolites contain carbonaceous laminae that have been interpreted as fossil microbial mats or biofilms based on textural evidence (Awramik, 1992; Lepot, 2020).
View in article
Bell, T., Newman, J.A., Silverman, B.W., Turner, S.L., Lilley, A.K. (2005) The contribution of species richness and composition to bacterial services. Nature 436, 1157–1160. https://doi.org/10.1038/nature03891
 Show in context
Show in context Alternatively, the composition of microbial consortia may affect the range of csSRR at the microbial mat scale (Bradley et al., 2016), with guild diversity having opposite effects on trophic group functions, thus modulating csSRR (Bell et al., 2005; Peter et al., 2011).
View in article
Bouton, A., Vennin, E., Pace, A., Bourillot, R., Dupraz, C., Thomazo, C., Brayard, A., Désaubliaux, G., Visscher, P.T. (2016) External controls on the distribution, fabrics and mineralization of modern microbial mats in a coastal hypersaline lagoon, Cayo Coco (Cuba). Sedimentology 63, 972–1016. https://doi.org/10.1111/sed.12246
 Show in context
Show in context Atexcac waters have a high dissolved organic carbon content (over 15 times that of the modern ocean) which can sustain MSR activity, while Cayo Coco harbours conspicuous suspended EPS-rich organic slimes (Bouton et al., 2016).
View in article
Bouton, A., Vennin, E., Thomazo, C., Mathieu, O., Garcia, F., Jaubert, M., Visscher, P. (2020) Microbial Origin of the Organic Matter Preserved in the Cayo Coco Lagoonal Network, Cuba. Minerals 10, 143. https://doi.org/10.3390/min10020143
 Show in context
Show in context Low csSRR and high S isotope fractionations in both lakes could be explained by the refractory nature of this organic matter (Bouton et al., 2020; Gomes et al., 2021).
View in article
We studied two samples from 1) the Atexcac Lake, a monomictic volcanic crater lake (Mexico; Zeyen et al., 2021) and 2) Cayo Coco Lake, a shallow hypersaline lagoon in Cuba (Pace et al., 2018; Bouton et al., 2020).
View in article
Bradley, A.S., Leavitt, W.D., Schmidt, M., Knoll, A.H., Girguis, P.R., Johnston, D.T. (2016) Patterns of sulfur isotope fractionation during microbial sulfate reduction. Geobiology 14, 91–101. https://doi.org/10.1111/gbi.12149
 Show in context
Show in context The fractionation induced by this metabolic activity (34ɛmic hereafter) depends on the sulfate concentration, identity of the electron donor, bioavailable carbon (content and chemical form) and, perhaps most importantly, the cell-specific sulfate reduction rates (csSRR; Bradley et al., 2016).
View in article
Alternatively, the composition of microbial consortia may affect the range of csSRR at the microbial mat scale (Bradley et al., 2016), with guild diversity having opposite effects on trophic group functions, thus modulating csSRR (Bell et al., 2005; Peter et al., 2011).
View in article
These Δpyr values are consistent with near thermodynamic equilibrium fractionation as observed in i) MSR batch culture experiments characterised by low growth rate and csSRR (Leavitt et al., 2013; Bradley et al., 2016) and ii) natural environments (e.g., Cadagno Lake; Canfield et al., 2010).
View in article
Canfield, D.E., Des Marais, D.J. (1993) Biogeochemical cycles of carbon, sulfur, and free oxygen in a microbial mat. Geochimica et Cosmochimica Acta 57, 3971–3984. https://doi.org/10.1016/0016-7037(93)90347-Y
 Show in context
Show in context Despite abundant sulfate (at Cayo Coco) and organic matter, csSRR in these mats are intriguingly low and contrast with previous occurrences of high SRR in surface microbial mats (Canfield and Des Marais, 1993).
View in article
Microbial mats are stratified microbial communities whose metabolic activities produce geochemical gradients and drive elemental cycling (Canfield and Des Marais, 1993; Paerl and Pinckney, 1996).
View in article
Canfield, D.E., Farquhar, J., Zerkle, A.L. (2010) High isotope fractionations during sulfate reduction in a low-sulfate euxinic ocean analog. Geology 38, 415–418. https://doi.org/10.1130/G30723.1
 Show in context
Show in context These Δpyr values are consistent with near thermodynamic equilibrium fractionation as observed in i) MSR batch culture experiments characterised by low growth rate and csSRR (Leavitt et al., 2013; Bradley et al., 2016) and ii) natural environments (e.g., Cadagno Lake; Canfield et al., 2010).
View in article
Fike, D.A., Finke, N., Zha, J., Blake, G., Hoehler, T.M., Orphan, V.J. (2009) The effect of sulfate concentration on (sub)millimeter-scale sulfide δ34S in hypersaline cyanobacterial mats over the diurnal cycle. Geochimica et Cosmochimica Acta 73, 6187–6204. https://doi.org/10.1016/j.gca.2009.07.006
 Show in context
Show in context At the microbial mat scale, strong gradients of sulfate reduction within layered mats (Visscher et al., 2000; Fike et al., 2009; Pace et al., 2018) have been attributed to small scale variations in csSRR and/or localised MSR micro-niches (Fike et al., 2009; Gomes et al., 2021).
View in article
At the microbial mat scale, strong gradients of sulfate reduction within layered mats (Visscher et al., 2000; Fike et al., 2009; Pace et al., 2018) have been attributed to small scale variations in csSRR and/or localised MSR micro-niches (Fike et al., 2009; Gomes et al., 2021).
View in article
Fike, D.A., Gammon, C.L., Ziebis, W., Orphan, V.J. (2008) Micron-scale mapping of sulfur cycling across the oxycline of a cyanobacterial mat: a paired nanoSIMS and CARD-FISH approach. The ISME Journal 2, 749–759. https://doi.org/10.1038/ismej.2008.39
 Show in context
Show in context However, since genetic markers are not preserved in the geological record, the recognition of MSR in palaeoenvironments mostly relies on the sulfur isotopic compositions of sedimentary sulfide and sulfate minerals (Visscher et al., 2000; Fike et al., 2008).
View in article
In modern microbialites, numerous studies have reported dynamic MSR activity based on H2S labelling (Visscher et al., 2000; Fike et al., 2008; Pace et al., 2018; Gomes et al., 2021), but only a few studies have investigated sulfur isotope signatures of individual pyrite grains (Gomes et al., 2021).
View in article
Fry, B., Ruf, W., Gest, H., Hayes, J.M. (1988) Sulfur isotope effects associated with oxidation of sulfide by O2 in aqueous solution. Chemical Geology: Isotope Geoscience section 73, 205–210. https://doi.org/10.1016/0168-9622(88)90001-2
 Show in context
Show in context The fractionation required to produce such an isotopic gradient is well above abiotic sulfide oxidation (i.e. ∼+5 ‰; Fry et al., 1988), yet can also be consistent with microbial sulfide oxidation in high pH environments (Pellerin et al., 2019).
View in article
Goldhaber, M.B., Kaplan, I.R. (1980) Mechanisms of sulfur incorporation and isotope fractionation during early diagenesis in sediments of the Gulf of California. Marine Chemistry 9, 95–143. https://doi.org/10.1016/0304-4203(80)90063-8
 Show in context
Show in context Alternatively, the internal isotope gradient across the framboidal pyrites (Fig. 2, SI) can be due to Rayleigh isotope fractionation, as even under oxidising (abundant sulfate) conditions, consumption can occur faster than diffusive replenishment (Goldhaber and Kaplan, 1980).
View in article
Gomes, M.L., Fike, D.A., Bergmann, K.D., Jones, C., Knoll, A.H. (2018) Environmental insights from high-resolution (SIMS) sulfur isotope analyses of sulfides in Proterozoic microbialites with diverse mat textures. Geobiology 16, 17–34. https://doi.org/10.1111/gbi.12265
 Show in context
Show in context While late diagenesis can modify both pyrite crystallinity and S isotope composition (Williford et al., 2011; Gomes et al., 2018; Marin-Carbonne et al., 2020), early diagenesis in microbial mats is thought to have a limited effect on the S isotopic composition of pyrite, meaning that microbialitic pyrites may preserve ‘pristine’ isotopic signatures.
View in article
Gomes, M.L., Klatt, J.M., Dick, G.J., Grim, S.L., Rico, K.I., Medina, M., Ziebis, W., Kinsman-Costello, L., Sheldon, N.D., Fike, D.A. (2021) Sedimentary pyrite sulfur isotope compositions preserve signatures of the surface microbial mat environment in sediments underlying low-oxygen cyanobacterial mats. Geobiology 20, 60–78. https://doi.org/10.1111/gbi.12466
 Show in context
Show in context Pyrite often precipitates at the microbial mat surface (Gomes et al., 2021) and its isotopic composition is more representative of the local setting rather than global environmental conditions, e.g., water column (Lang et al., 2020; Pasquier et al., 2021).
View in article
In modern microbialites, numerous studies have reported dynamic MSR activity based on H2S labelling (Visscher et al., 2000; Fike et al., 2008; Pace et al., 2018; Gomes et al., 2021), but only a few studies have investigated sulfur isotope signatures of individual pyrite grains (Gomes et al., 2021).
View in article
At the microbial mat scale, strong gradients of sulfate reduction within layered mats (Visscher et al., 2000; Fike et al., 2009; Pace et al., 2018) have been attributed to small scale variations in csSRR and/or localised MSR micro-niches (Fike et al., 2009; Gomes et al., 2021).
View in article
Javaux, E.J. (2019) Challenges in evidencing the earliest traces of life. Nature 572, 451–460. https://doi.org/10.1038/s41586-019-1436-4
 Show in context
Show in context In addition, the biosignatures preserved in fossil biofilms are ambiguous, especially after diagenesis and post-depositional history (Javaux, 2019; Alleon et al., 2021).
View in article
Jørgensen, B.B., Findlay, A.J., Pallerin, A. (2019) The Biogeochemical Sulfur Cycle of Marine Sediments. Frontiers in Microbiology 10, 849. https://doi.org/10.3389/fmicb.2019.00849
 Show in context
Show in context Sulfate-reducing bacteria, i.e. microorganisms that use sulfate as a terminal electron acceptor, are ubiquitous in Earth environments where they play a major role both in S and C biogeochemical cycles (e.g., Jørgensen et al., 2019).
View in article
The resulting sulfide δ34S values are relatively light and can be as much as −70 ‰ relative to sulfate (Jørgensen et al., 2019).
View in article
Kamber, B.S., Whitehouse, M.J. (2007) Micro-scale sulphur isotope evidence for sulphur cycling in the late Archean shallow ocean. Geobiology 5, 5–17. https://doi.org/10.1111/j.1472-4669.2006.00091.x
 Show in context
Show in context The oldest evidence for MSR in the Archaean geological record are sulfur isotopic signatures from deep marine sediments (Kamber and Whitehouse, 2007; Shen et al., 2009) and stromatolites (Shen and Buick, 2004).
View in article
Lang, X., Tang, W., Ma, H., Shen, B. (2020) Local environmental variation obscures the interpretation of pyrite sulfur isotope records. Earth and Planetary Science Letters 533, 116056. https://doi.org/10.1016/j.epsl.2019.116056
 Show in context
Show in context Pyrite often precipitates at the microbial mat surface (Gomes et al., 2021) and its isotopic composition is more representative of the local setting rather than global environmental conditions, e.g., water column (Lang et al., 2020; Pasquier et al., 2021).
View in article
Leavitt, W.D., Halevy, I., Bradley, A.S., Johnston, D.T. (2013) Influence of sulfate reduction rates on the Phanerozoic sulfur isotope record. Proceedings of the National Academy of Sciences 110, 11244–11249. https://doi.org/10.1073/pnas.1218874110
 Show in context
Show in context These Δpyr values are consistent with near thermodynamic equilibrium fractionation as observed in i) MSR batch culture experiments characterised by low growth rate and csSRR (Leavitt et al., 2013; Bradley et al., 2016) and ii) natural environments (e.g., Cadagno Lake; Canfield et al., 2010).
View in article
Lepot, K. (2020) Signatures of early microbial life from the Archean (4 to 2.5 Ga) eon. Earth-Science Reviews 209, 103296. https://doi.org/10.1016/j.earscirev.2020.103296
 Show in context
Show in context Some Archaean stromatolites contain carbonaceous laminae that have been interpreted as fossil microbial mats or biofilms based on textural evidence (Awramik, 1992; Lepot, 2020).
View in article
Marin-Carbonne, J., Busigny, V., Miot, J., Rollion-Bard, C., Muller, E., Drabon, N., Jacob, D., Pont, S., Robyr, M., Bontognali, T.R.R., François, C., Reynaud, S., Van Zuilen, M., Philippot, P. (2020) In Situ Fe and S isotope analyses in pyrite from the 3.2 Ga Mendon Formation (Barberton Greenstone Belt, South Africa): Evidence for early microbial iron reduction. Geobiology 18, 306–325. https://doi.org/10.1111/gbi.12385
 Show in context
Show in context The primary S isotopic signatures of pyrites (FeS2) are often modified by fluid circulation during metasomatism or metamorphism (Marin-Carbonne et al., 2020; Slotznick et al., 2022), occurring millions or billions of years after sediment deposition.
View in article
While late diagenesis can modify both pyrite crystallinity and S isotope composition (Williford et al., 2011; Gomes et al., 2018; Marin-Carbonne et al., 2020), early diagenesis in microbial mats is thought to have a limited effect on the S isotopic composition of pyrite, meaning that microbialitic pyrites may preserve ‘pristine’ isotopic signatures.
View in article
Marin-Carbonne, J., Remusat, L., Sforna, M.C., Thomazo, C., Cartigny, P., Philippot, P. (2018) Sulfur isotope’s signal of nanopyrites enclosed in 2.7 Ga stromatolitic organic remains reveal microbial sulfate reduction. Geobiology 16, 121–138. https://doi.org/10.1111/gbi.12275
 Show in context
Show in context Consequently, we propose that microbialite micropyrites can be used as a mineral signature for reconstructing past Earth surface and microbial environments, as already suggested for Archaean stromatolites (Marin-Carbonne et al., 2018).
View in article
Pace, A., Bourillot, R., Bouton, A., Vennin, E., Braissant, O., Dupraz, C., Duteil, T., Bundeleva, I., Patrier, P., Galaup, S., Yokoyama, Y., Franceschi, M., Virgone, A., Visscher, P.T. (2018) Formation of stromatolite lamina at the interface of oxygenic-anoxygenic photosynthesis. Geobiology 16, 378–398. https://doi.org/10.1111/gbi.12281
 Show in context
Show in context We studied two samples from 1) the Atexcac Lake, a monomictic volcanic crater lake (Mexico; Zeyen et al., 2021) and 2) Cayo Coco Lake, a shallow hypersaline lagoon in Cuba (Pace et al., 2018; Bouton et al., 2020).
View in article
In modern microbialites, numerous studies have reported dynamic MSR activity based on H2S labelling (Visscher et al., 2000; Fike et al., 2008; Pace et al., 2018; Gomes et al., 2021), but only a few studies have investigated sulfur isotope signatures of individual pyrite grains (Gomes et al., 2021).
View in article
At the microbial mat scale, strong gradients of sulfate reduction within layered mats (Visscher et al., 2000; Fike et al., 2009; Pace et al., 2018) have been attributed to small scale variations in csSRR and/or localised MSR micro-niches (Fike et al., 2009; Gomes et al., 2021).
View in article
Paerl, H.W., Pinckney, J.L. (1996) A mini-review of microbial consortia: their roles in aquatic production and biogeochemical cycling. Microbial Ecology 31, 225–247. https://doi.org/10.1007/BF00171569
 Show in context
Show in context Microbial mats are stratified microbial communities whose metabolic activities produce geochemical gradients and drive elemental cycling (Canfield and Des Marais, 1993; Paerl and Pinckney, 1996).
View in article
Pasquier, V., Bryant, R.N., Fike, D.A., Halevy, I. (2021) Strong local, not global, controls on marine pyrite sulfur isotopes. Science Advances 7, eabb7403. https://doi.org/10.1126/sciadv.abb7403
 Show in context
Show in context The observed laminations, which contain micropyrites, likely reflect local high density microbe spots, which can result from a more pronounced local distillation of δ34S (Pasquier et al., 2021).
View in article
Pyrite often precipitates at the microbial mat surface (Gomes et al., 2021) and its isotopic composition is more representative of the local setting rather than global environmental conditions, e.g., water column (Lang et al., 2020; Pasquier et al., 2021).
View in article
Pellerin, A., Antler, G., Holm, S.A., Findlay, A.J., Crockford, P.W., Turchyn, A.V., Jørgensen, B.B., Finster, K. (2019) Large sulfur isotope fractionation by bacterial sulfide oxidation. Science Advances 5, eaaw1480. https://doi.org/10.1126/sciadv.aaw1480
 Show in context
Show in context Framboidal pyrites display a large range of δ 34S values but also an internal isotope variation across the length scale of individual framboidal grains (Fig. 3), best explained by a combination of MSR and partial sulfide oxidation (Fig. 3; Pellerin et al., 2019).
View in article
Both sites are characterised by high pH (pH > 8, see SI), which is known to promote large isotope fractionation during sulfide oxidation (Pellerin et al., 2019).
View in article
The fractionation required to produce such an isotopic gradient is well above abiotic sulfide oxidation (i.e. ∼+5 ‰; Fry et al., 1988), yet can also be consistent with microbial sulfide oxidation in high pH environments (Pellerin et al., 2019).
View in article
Peter, H., Beier, S., Bertilsson, S., Lindström, E.S., Langenheder, S., Tranvik, L.J. (2011) Function-specific response to depletion of microbial diversity. The ISME Journal 5, 351–361. https://doi.org/10.1038/ismej.2010.119
 Show in context
Show in context Alternatively, the composition of microbial consortia may affect the range of csSRR at the microbial mat scale (Bradley et al., 2016), with guild diversity having opposite effects on trophic group functions, thus modulating csSRR (Bell et al., 2005; Peter et al., 2011).
View in article
Picard, A., Gartman, A., Clarke, D.R., Girguis, P.R. (2018) Sulfate-reducing bacteria influence the nucleation and growth of mackinawite and greigite. Geochimica et Cosmochimica Acta 220, 367–384. https://doi.org/10.1016/j.gca.2017.10.006
 Show in context
Show in context Moreover, the small crystal size of micropyrites composed of nanocrystals with different orientations has been highlighted as a possible biogenic signature (Picard et al., 2018).
View in article
Raven, M.R., Sessions, A.L., Fischer, W.W., Adkins, J.F. (2016) Sedimentary pyrite δ34S differs from porewater sulfide in Santa Barbara Basin: Proposed role of organic sulfur. Geochimica et Cosmochimica Acta 186, 120–134. https://doi.org/10.1016/j.gca.2016.04.037
 Show in context
Show in context However, the observation of large isotopic differences of about ∼30 ‰ (Raven et al., 2016) between pore water sulfur species (SO42− and H2S) and pyrite shows that other S-bearing pools, such as organic matter, should be considered in order to quantitatively and isotopically describe sulfur cycling in microbialites.
View in article
Reid, R.P., Visscher, P.T., Decho, A.W., Stolz, J.F., Bebout, B.M., Dupraz, C., Macintyre, I.G., Paerl, H.W., Pinckney, J.L., Prufert-Bebout, J., Steppe, T.F., DesMarais, D.J. (2000) The role of microbes in accretion, lamination and early lithification of modern marine stromatolites. Nature 406, 989–992. https://doi.org/10.1038/35023158
 Show in context
Show in context Interestingly, determining the precise nature of the fossil microbial community is challenging because these organosedimentary rocks resulted from a complex balance between microbial activities, sedimentation and intermittent lithification (Reid et al., 2000).
View in article
Shen, Y., Farquhar, J., Masterson, A., Kaufman, A.J., Buick, R. (2009) Evaluating the role of microbial sulfate reduction in the early Archean using quadruple isotope systematics. Earth and Planetary Science Letters 279, 383–391. https://doi.org/10.1016/j.epsl.2009.01.018
 Show in context
Show in context The oldest evidence for MSR in the Archaean geological record are sulfur isotopic signatures from deep marine sediments (Kamber and Whitehouse, 2007; Shen et al., 2009) and stromatolites (Shen and Buick, 2004).
View in article
Shen, Y., Buick, R. (2004). The antiquity of microbial sulfate reduction. Earth-Science Reviews 64, 243–272. https://doi.org/10.1016/S0012-8252(03)00054-0
 Show in context
Show in context The oldest evidence for MSR in the Archaean geological record are sulfur isotopic signatures from deep marine sediments (Kamber and Whitehouse, 2007; Shen et al., 2009) and stromatolites (Shen and Buick, 2004).
View in article
Sim, M.S., Ono, S., Donovan, K., Templer, S.P., Bosak, T. (2011) Effect of electron donors on the fractionation of sulfur isotopes by a marine Desulfovibrio sp. Geochimica et Cosmochimica Acta 75, 4244–4259. https://doi.org/10.1016/j.gca.2011.05.021
 Show in context
Show in context High 34ɛmic has been observed in sulfate reducing strains only partially oxidising their carbon substrate and is sometimes associated with the degradation of carbohydrate components, including exopolymeric substances (EPS) (Sim et al., 2011), which are abundant in microbialite-forming mats.
View in article
Slotznick, S.P., Johnson, J.E., Rasmussen, B., Raub, T.D., Webb, S.M., Zi, J.-W., Kirschvink, J.L., Fischer, W.W. (2022) Reexamination of 2.5-Ga “whiff” of oxygen interval points to anoxic ocean before GOE. Science Advances 8, eabj7190. https://doi.org/10.1126/sciadv.abj7190
 Show in context
Show in context The primary S isotopic signatures of pyrites (FeS2) are often modified by fluid circulation during metasomatism or metamorphism (Marin-Carbonne et al., 2020; Slotznick et al., 2022), occurring millions or billions of years after sediment deposition.
View in article
Visscher, P.T., Reid, R.P., Bebout, B.M. (2000) Microscale observations of sulfate reduction: Correlation of microbial activity with lithified micritic laminae in modern marine stromatolites. Geology 28, 919–922. https://doi.org/10.1130/0091-7613(2000)28<919:MOOSRC>2.0.CO;2
 Show in context
Show in context MSR plays a key role in carbonate mineralisation, especially identified in microbialites and microbial mats (Visscher et al., 2000).
View in article
However, since genetic markers are not preserved in the geological record, the recognition of MSR in palaeoenvironments mostly relies on the sulfur isotopic compositions of sedimentary sulfide and sulfate minerals (Visscher et al., 2000; Fike et al., 2008).
View in article
In modern microbialites, numerous studies have reported dynamic MSR activity based on H2S labelling (Visscher et al., 2000; Fike et al., 2008; Pace et al., 2018; Gomes et al., 2021), but only a few studies have investigated sulfur isotope signatures of individual pyrite grains (Gomes et al., 2021).
View in article
At the microbial mat scale, strong gradients of sulfate reduction within layered mats (Visscher et al., 2000; Fike et al., 2009; Pace et al., 2018) have been attributed to small scale variations in csSRR and/or localised MSR micro-niches (Fike et al., 2009; Gomes et al., 2021).
View in article
Williford, K.H., Van Kranendonk, M.J., Ushikubo, T., Kozdon, R., Valley, J.W. (2011) Constraining atmospheric oxygen and seawater sulfate concentrations during Paleoproterozoic glaciation: In situ sulfur three-isotope microanalysis of pyrite from the Turee Creek Group, Western Australia. Geochimica et Cosmochimica Acta 75, 5686–5705. https://doi.org/10.1016/j.gca.2011.07.010
 Show in context
Show in context While late diagenesis can modify both pyrite crystallinity and S isotope composition (Williford et al., 2011; Gomes et al., 2018; Marin-Carbonne et al., 2020), early diagenesis in microbial mats is thought to have a limited effect on the S isotopic composition of pyrite, meaning that microbialitic pyrites may preserve ‘pristine’ isotopic signatures.
View in article
Zeyen, N., Benzerara, K., Beyssac, O., Daval, D., Muller, E., Thomazo, C., Tavera, R., López-García, P., Moreira, D., Duprat, E. (2021) Integrative analysis of the mineralogical and chemical composition of modern microbialites from ten Mexican lakes: What do we learn about their formation. Geochimica et Cosmochimica Acta 305, 148–184. https://doi.org/10.1016/j.gca.2021.04.030
 Show in context
Show in context We studied two samples from 1) the Atexcac Lake, a monomictic volcanic crater lake (Mexico; Zeyen et al., 2021) and 2) Cayo Coco Lake, a shallow hypersaline lagoon in Cuba (Pace et al., 2018; Bouton et al., 2020).
View in article
top
Supplementary Information
The Supplementary Information includes:
Download Table S-5 (Excel).
Download the Supplementary Information (PDF).
Figures
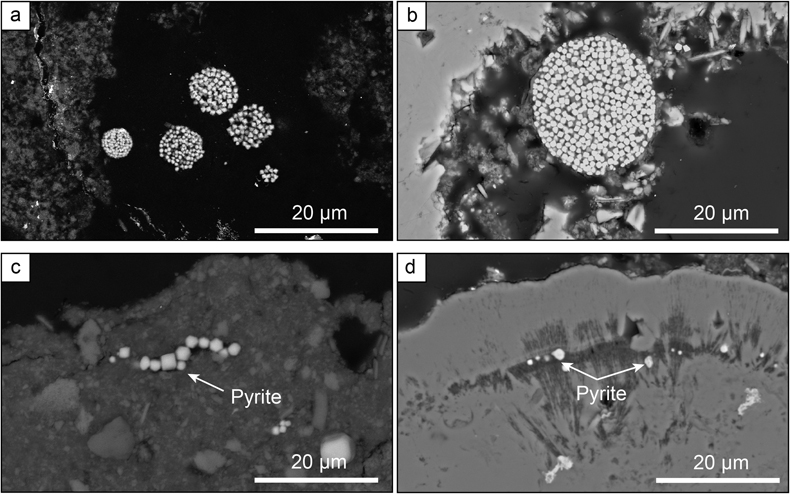
Figure 1 Secondary Electron microscopy pictures of (a, b) framboidal pyrites and (c, d) micropyrites from (a, c) Cayo Coco Lagoon and (b, d) Atexcac. Framboidal pyrites are located at the surface of the mineralised microbialite (in dark) while micropyrites are entombed within aragonite (in light grey) or Mg rich silicate (dark grey).
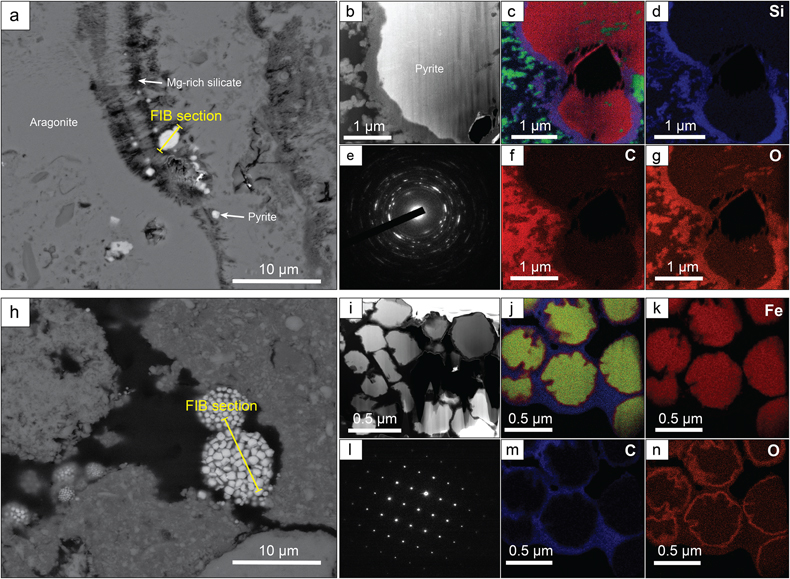
Figure 2 (a) SEM picture of micropyrites. Location where FIB section was extracted is shown by the yellow line, (b) TEM picture of the pyrite crystal and (e) its associated powder-like diffraction pattern, (c) false colour STEM EDXS image (Si in blue, Ca in green, Fe in red) and (d, f, g) Si, C and O images of the submicrometric pyrites, respectively. (h) SEM picture of framboidal pyrite with FIB section location (yellow line), (i) TEM image and (l) associated single crystal diffraction pattern along the [112] zone axis of pyrite, (j) false colour STEM EDXS image of pyrite crystallites (Fe in red, S in green, C in blue) and (k, m, n) Fe, C and O images, respectively.
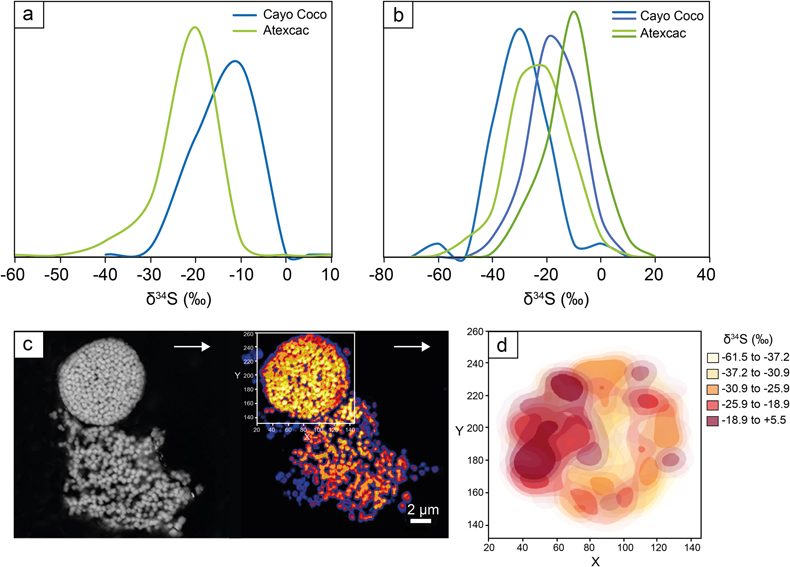
Figure 3 (a) δ34S probability density function of all framboidal pyrites from Atexcac and Cayo Coco uncertainties of analyses ranges from 0.4 to 4 ‰, (b) δ34S probability density function of four individual framboidal pyrites containing up to 100 pyrite crystallites, (c) SEM and corresponding NanoSIMS 32S image of one framboidal pyrite; the arrow indicates the top of the mat, and (d) δ34S values reconstructed for individual pyrite crystallites showing strong variations in S isotope composition across the framboidal pyrite.
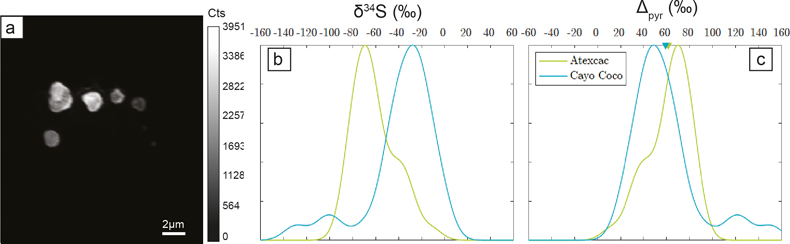
Figure 4 (a) NanoSIMS 32S image of submicrometric pyrites, (b) δ34S probability density function, taking account of the range of uncertainties from 1 ‰ to 8 ‰ of micropyrites from Cayo Coco and Atexcac, (c) Δpyr distribution calculated for both environments.