Enrichment mechanism of trace elements in pyrite under methane seepage
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:152Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
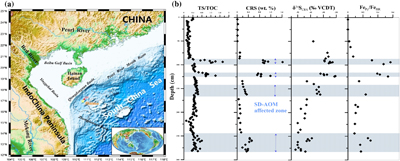 Figure 1 (a) Map showing the study region; the Q6 core located in the Qiongdongnan Basin of the SCS (modified from Miao et al., 2021a,b). (b) Down core variations of TS/TOC, CRS, δ34SCRS, and FePy/FeHR in sediments (based on Miao et al., 2021b). The blue horizontal bars indicate methane seepage (based on Miao et al., 2021a,b). TS/TOC = total sulfur/total organic carbon; CRS = chromium reducible sulfur; δ34SCRS = sulfur isotope of chromium reducible sulfur; Fepy = pyrite Fe; FeHR = highly reactive Fe. | 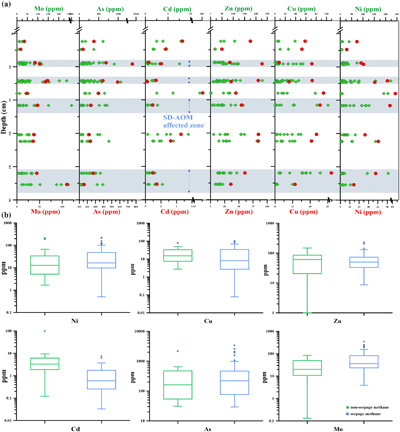 Figure 2 (a) Down core variations of the trace element contents (Cd, Ni, Cu, Zn, As, and Mo) in pyrite samples. The grey shaded bars indicate methane seeps; each green circle represents a single pyrite analysis; each red circle represents the average pyrite analysis. (b) Box plots of the average values of Ni, Cu, Zn, Cd, As, and Mo contents in pyrites. | 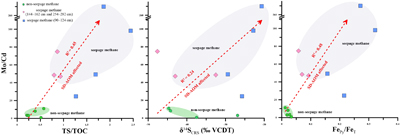 Figure 3 Scatter diagram of Mo/Cd vs. TS/TOC, δ34SCRS and FePy/FeT (based on Miao et al., 2021a,b). TS/TOC = total sulfur/total organic carbon; CRS = chromium-reducible sulfur; δ34SCRS = sulfur isotope of chromium-reducible sulfur; Fepy = pyrite Fe; FeT = total Fe. |
| Figure 1 | Figure 2 | Figure 3 |
top
Introduction
Authigenic pyrite is considered to be the most important sulfide mineral because it is more stable than other iron sulfides and is the main sink of sulfur (Berner, 1984
Berner, R.A. (1984) Sedimentary pyrite formation: An update. Geochimica et Cosmochimica Acta 48, 605–615. https://doi.org/10.1016/0016-7037(84)90089-9
). Its formation is often related to organoclastic sulfate reduction (OSR), using organic matter and seawater sulfate as substrate and yielding hydrogen sulfide (H2S) (Berner, 1984Berner, R.A. (1984) Sedimentary pyrite formation: An update. Geochimica et Cosmochimica Acta 48, 605–615. https://doi.org/10.1016/0016-7037(84)90089-9
). However, OSR is different (e.g., reaction rates) in various sedimentary environments (Berner, 1978Berner, R.A. (1978) Sulfate reduction and the rate of deposition of marine sediments. Earth and Planetary Science Letters 37, 492–498. https://doi.org/10.1016/0012-821X(78)90065-1
). This will eventually lead to corresponding changes in the morphology and geochemical characteristics of pyrite (Berner, 1978Berner, R.A. (1978) Sulfate reduction and the rate of deposition of marine sediments. Earth and Planetary Science Letters 37, 492–498. https://doi.org/10.1016/0012-821X(78)90065-1
, 1984Berner, R.A. (1984) Sedimentary pyrite formation: An update. Geochimica et Cosmochimica Acta 48, 605–615. https://doi.org/10.1016/0016-7037(84)90089-9
; Huerta-Diaz and Morse, 1992Huerta-Diaz, M.A., Morse, J.W. (1992) Pyritization of trace metals in anoxic marine sediments. Geochimica et Cosmochimica Acta 56, 2681–2702. https://doi.org/10.1016/0016-7037(92)90353-K
; Wilkin et al., 1996Wilkin, R.T., Barnes, H.L., Brantley, S.L. (1996) The size distribution of framboidal pyrite in modern sediments: an indicator of redox conditions. Geochimica et Cosmochimica Acta 60, 3897–3912. https://doi.org/10.1016/0016-7037(96)00209-8
). In recent years, many indicators have been used to study sedimentary environment evolution, such as the sulfur and iron isotopic compositions of pyrite (Lin et al., 2016Lin, Z., Sun, X., Peckmann, J., Lu, Y., Xu, L., Strauss, H., Zhou, H., Gong, J., Lu, H., Teichert, B.M.A. (2016) How sulfate-driven anaerobic oxidation of methane affects the sulfur isotopic composition of pyrite: A SIMS study from the South China Sea. Chemical Geology 440, 26–41. https://doi.org/10.1016/j.chemgeo.2016.07.007
), the grain size of framboidal pyrite (Wilkin et al., 1996Wilkin, R.T., Barnes, H.L., Brantley, S.L. (1996) The size distribution of framboidal pyrite in modern sediments: an indicator of redox conditions. Geochimica et Cosmochimica Acta 60, 3897–3912. https://doi.org/10.1016/0016-7037(96)00209-8
), and iron speciation (Slotznick et al., 2018Slotznick, S.P., Eiler, J.M., Fischer, W.W. (2018) The Effects of Metamorphism on Iron Mineralogy and the Iron Speciation Redox Proxy. Geochimica et Cosmochimica Acta 224, 96–115. https://doi.org/10.1016/j.gca.2017.12.003
). Trace elements can also adsorb onto pyrite, an important sink in many geochemical cycles that has great potential for reconstructing palaeoenvironments (Morse and Luther, 1999Morse, J.W., Luther, G. (1999) Chemical influences on trace metal-sulfide interactions in anoxic sediments. Geochimica et Cosmochimica Acta 63, 3373–3378. https://doi.org/10.1016/S0016-7037(99)00258-6
; Berner et al., 2013Berner, Z.A., Puchelt, H., Noeltner, T., Kramar, U. (2013) Pyrite geochemistry in the Toarcian Posidonia shale of south-west Germany: evidence for contrasting trace-element patterns of diagenetic and syngenetic pyrites. Sedimentology 60, 548–573. https://doi.org/10.1111/j.1365-3091.2012.01350.x
; Mukherjee and Large, 2020Mukherjee, I., Large, R.R. (2020) Co-evolution of trace elements and life in Precambrian oceans: The pyrite edition. Geology 48, 1018–1022. https://doi.org/10.1130/G47890.1
; Large et al., 2022Large, R.R., Mukherjee, I., Danyushevsky, L., Gregory, D., Steadman, J., Corkrey, R. (2022) Sedimentary pyrite proxy for atmospheric oxygen; evaluation of strengths and limitations. Earth-Science Reviews 227, 103941. https://doi.org/10.1016/j.earscirev.2022.103941
). Initially, research on trace elements in pyrite mainly focused on defining ore genetic types, determining the source of ore forming materials, and inverting the evolution of ore forming fluids (Clark et al., 2004Clark, C., Grguric, B., Schmidt Mumm, A. (2004) Genetic implications of pyrite chemistry from the Palaeoproterozoic Olary Domain and overlying Neoproterozoic Adelaidean sequences, northeastern South Australia. Ore Geology Reviews 25, 237–257. https://doi.org/10.1016/j.oregeorev.2004.04.003
; Large et al., 2009Large, R.R., Danyushevsky, L., Hollit, C., Maslennikov, V., Meffre, S., Gilbert, S., Bull, S., Scott, R., Emsbo, P., Thomas, H., Singh, B., Foster, J. (2009) Gold and trace element zonation in pyrite using a laser imaging technique: Implications for the timing of gold in orogenic and carlin-style sediment-hosted deposits. Economic Geology 104, 635–668. https://doi.org/10.2113/gsecongeo.104.5.635
; Ulrich et al., 2011Ulrich, T., Long, D.G.F., Kamber, B.S., Whitehouse, M.J. (2011) In situ trace element and sulfur isotope analysis of pyrite in a Paleoproterozoic gold placer deposit, Pardo and Clement Townships, Ontario, Canada. Economic Geology 106, 667–686. https://doi.org/10.2113/econgeo.106.4.667
). As research has advanced, scientists have found that the composition of trace elements in diagenetic pyrite is greatly affected by the geochemical characteristics of pore water in the sediments (Huerta-Diaz and Morse, 1992Huerta-Diaz, M.A., Morse, J.W. (1992) Pyritization of trace metals in anoxic marine sediments. Geochimica et Cosmochimica Acta 56, 2681–2702. https://doi.org/10.1016/0016-7037(92)90353-K
; Berner et al., 2013Berner, Z.A., Puchelt, H., Noeltner, T., Kramar, U. (2013) Pyrite geochemistry in the Toarcian Posidonia shale of south-west Germany: evidence for contrasting trace-element patterns of diagenetic and syngenetic pyrites. Sedimentology 60, 548–573. https://doi.org/10.1111/j.1365-3091.2012.01350.x
; Large et al., 2014Large, R.R., Halpin, J.A., Danyushevsky, L.V., Maslennikov, V.V., Bull, S.W., Long, J.A., Gregory, D.D., Lounejeva, E., Lyons, T.W., Sack, P.J., McGoldrick, P.J., Calver, C.R. (2014) Trace element content of sedimentary pyrite as a new proxy for deep-time ocean atmosphere evolution. Earth and Planetary Science Letters 389, 209–220. https://doi.org/10.1016/j.epsl.2013.12.020
, 2022Large, R.R., Mukherjee, I., Danyushevsky, L., Gregory, D., Steadman, J., Corkrey, R. (2022) Sedimentary pyrite proxy for atmospheric oxygen; evaluation of strengths and limitations. Earth-Science Reviews 227, 103941. https://doi.org/10.1016/j.earscirev.2022.103941
). When the concentration of H2S in the environment increases, those of redox sensitive elements (such as Mo) in pyrite will also increase (Huerta-Diaz and Morse, 1992Huerta-Diaz, M.A., Morse, J.W. (1992) Pyritization of trace metals in anoxic marine sediments. Geochimica et Cosmochimica Acta 56, 2681–2702. https://doi.org/10.1016/0016-7037(92)90353-K
; Berner et al., 2013Berner, Z.A., Puchelt, H., Noeltner, T., Kramar, U. (2013) Pyrite geochemistry in the Toarcian Posidonia shale of south-west Germany: evidence for contrasting trace-element patterns of diagenetic and syngenetic pyrites. Sedimentology 60, 548–573. https://doi.org/10.1111/j.1365-3091.2012.01350.x
). Therefore, trace elements in diagenetic pyrite within sediments can be used to record the geochemical information of original sedimentary environments and have great potential for palaeoenvironmental reconstruction.However, there are some unique sedimentary environments where additional sources of H2S play an important role in pyrite formation, such as hydrothermal environments (Large et al., 2014
Large, R.R., Halpin, J.A., Danyushevsky, L.V., Maslennikov, V.V., Bull, S.W., Long, J.A., Gregory, D.D., Lounejeva, E., Lyons, T.W., Sack, P.J., McGoldrick, P.J., Calver, C.R. (2014) Trace element content of sedimentary pyrite as a new proxy for deep-time ocean atmosphere evolution. Earth and Planetary Science Letters 389, 209–220. https://doi.org/10.1016/j.epsl.2013.12.020
) and oil seepage areas (Steadman et al., 2021Steadman, J.A., Large, R.R., Olin, P.H., Danyushevsky, L.V., Meffre, S., Huston, D., Fabris, A., Lisitsin, V., Wells, T. (2021) Pyrite trace element behavior in magmatic-hydrothermal environments: An LA-ICPMS imaging study. Ore Geology Reviews 128, 103878. https://doi.org/10.1016/j.oregeorev.2020.103878
). Cold seep environments are also important sedimentary environments for the dissociation of seabed natural gas hydrate. Both methane released from cold seeps and the sulfate released from seawater undergo sulfate driven anaerobic oxidation of methane (SD-AOM) in the sulfate-methane transition zone (SMTZ) (Boetius et al., 2000Boetius, A., Ravenschlag, K., Schubert, C.J., Rickert, D., Widdel, F., Gieseke, A., Amann, R., Jørgensen, B., Witte, U., Pfannkuche, O. (2000) A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407, 623–626. https://doi.org/10.1038/35036572
). This releases a large amount of H2S and provides the constituents necessary to form pyrite (Lin et al., 2016Lin, Z., Sun, X., Peckmann, J., Lu, Y., Xu, L., Strauss, H., Zhou, H., Gong, J., Lu, H., Teichert, B.M.A. (2016) How sulfate-driven anaerobic oxidation of methane affects the sulfur isotopic composition of pyrite: A SIMS study from the South China Sea. Chemical Geology 440, 26–41. https://doi.org/10.1016/j.chemgeo.2016.07.007
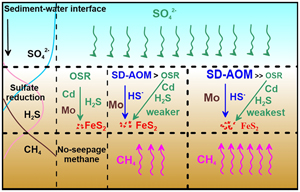
). Thus, pyrite formation in normal marine sedimentary environments and cold seep environments is controlled by different geochemical processes (OSR vs. SD-AOM). Based on previous studies of trace elements in authigenic carbonate and sediments in methane seepage, we found a remarkable dependency: when Mo is enriched, Cd, Ni, Cu, and Zn are not enriched (Sato et al., 2012Sato, H., Hayashi, K., Ogawa, Y., Kawamura, K. (2012) Geochemistry of deep sea sediments at cold seep sites in the Nankai Trough: insights into the effect of anaerobic oxidation of methane. Marine Geology 323, 47–55. https://doi.org/10.1016/j.margeo.2012.07.013
; Chen et al., 2016Chen, F., Hu, Y., Feng, D., Zhang, X., Cheng, S., Cao, J., Lu, H., Chen, D. (2016) Evidence of intense methane seepages from molybdenum enrichments in gas hydrate-bearing sediments of the northern South China Sea. Chemical Geology 443, 173–181. https://doi.org/10.1016/j.chemgeo.2016.09.029
). We speculate that pyrite generated in methane seepage environments and in normal marine sedimentary environments should have obvious differences in their geochemical elemental composition.To test this hypothesis, here we used pyrite samples from the Haima seep sedimentary area to compare the trace element contents in pyrite obtained from the SMTZ and in pyrite obtained from normal (non-seepage) sedimentary environments. This provides a new perspective for identifying methane seepage and provides a new reference for discussing the relationship between pyrite elements and sedimentary environments.
top
Materials and Methods
The Q6 core was taken by the Guangzhou Marine Geological Survey in 2019 using the R/V Haiyang-6 (Fig. 1a). Based on previous geochemical analysis results (Miao et al., 2021a
Miao, X., Feng, X., Li, J., Lin, L. (2021a) Tracing the paleo-methane seepage activity over the past 20,000 years in the sediments of Qiongdongnan Basin, northwestern South China Sea. Chemical Geology 559, 119956. https://doi.org/10.1016/j.chemgeo.2020.119956
,bMiao, X., Feng, X., Liu, X., Li, J., Wei, J. (2021b) Effects of methane seepage activity on the morphology and geochemistry of authigenic pyrite. Marine and Petroleum Geology 133, 105231. https://doi.org/10.1016/j.marpetgeo.2021.105231
), we identified several palaeo-methane seeps using TS/TOC, sulfur isotopes, and iron speciation (Fig. 1b). To test their elemental compositions, we selected pyrite from different Q6 layers and then conducted laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) to analyse pyrite obtained from the methane seepage environments and from normal sedimentary environments. For more detailed information, please refer to the Supplementary Information.
Figure 1 (a) Map showing the study region; the Q6 core located in the Qiongdongnan Basin of the SCS (modified from Miao et al., 2021a
Miao, X., Feng, X., Li, J., Lin, L. (2021a) Tracing the paleo-methane seepage activity over the past 20,000 years in the sediments of Qiongdongnan Basin, northwestern South China Sea. Chemical Geology 559, 119956. https://doi.org/10.1016/j.chemgeo.2020.119956
,bMiao, X., Feng, X., Liu, X., Li, J., Wei, J. (2021b) Effects of methane seepage activity on the morphology and geochemistry of authigenic pyrite. Marine and Petroleum Geology 133, 105231. https://doi.org/10.1016/j.marpetgeo.2021.105231
). (b) Down core variations of TS/TOC, CRS, δ34SCRS, and FePy/FeHR in sediments (based on Miao et al., 2021bMiao, X., Feng, X., Liu, X., Li, J., Wei, J. (2021b) Effects of methane seepage activity on the morphology and geochemistry of authigenic pyrite. Marine and Petroleum Geology 133, 105231. https://doi.org/10.1016/j.marpetgeo.2021.105231
). The blue horizontal bars indicate methane seepage (based on Miao et al., 2021aMiao, X., Feng, X., Li, J., Lin, L. (2021a) Tracing the paleo-methane seepage activity over the past 20,000 years in the sediments of Qiongdongnan Basin, northwestern South China Sea. Chemical Geology 559, 119956. https://doi.org/10.1016/j.chemgeo.2020.119956
,bMiao, X., Feng, X., Liu, X., Li, J., Wei, J. (2021b) Effects of methane seepage activity on the morphology and geochemistry of authigenic pyrite. Marine and Petroleum Geology 133, 105231. https://doi.org/10.1016/j.marpetgeo.2021.105231
). TS/TOC = total sulfur/total organic carbon; CRS = chromium reducible sulfur; δ34SCRS = sulfur isotope of chromium reducible sulfur; Fepy = pyrite Fe; FeHR = highly reactive Fe.top
Results
Trace element contents of the pyrites are shown in Figure 2. In Q6, the Cd content of pyrite taken from the methane seepage was lower than that of pyrite from the normal sedimentary environment. The corresponding average value (and its range) was 1.02 ppm (0.03–7.54 ppm). In a normal sedimentary environment, the average Cd contents were higher, with the corresponding average value (and its range) being 8.5 ppm (0.1–99.5 ppm). At the same time, the Mo content was significantly higher in pyrite from the methane seepage, with the average value (and its range) equal to 61.9 ppm (3.9–350.5 ppm), while, in a normal sedimentary environment, the Mo content was significantly lower, with the average value (and its range) equal to 28.1 ppm (0.1–84.5 ppm). It is shown that the Mo/Cd ratios of pyrite in the methane seepage are mostly above the line of Mo/Cd = 16.02, whereas they lie below this line for pyrites in the non-methane seepage (Fig. S-5).

Figure 2 (a) Down core variations of the trace element contents (Cd, Ni, Cu, Zn, As, and Mo) in pyrite samples. The grey shaded bars indicate methane seeps; each green circle represents a single pyrite analysis; each red circle represents the average pyrite analysis. (b) Box plots of the average values of Ni, Cu, Zn, Cd, As, and Mo contents in pyrites.
There is little difference in trace element contents of As, Ni, Cu and Zn in pyrite between the methane seepages (406.0 ppm, 29.2–3396.0 ppm; 36.3 ppm, 0.5–222.4 ppm; 18.9 ppm, 0.1–103.1 ppm; 57.4 ppm, 8.6–228.2 ppm, respectively) and the normal sedimentary environment (243.8 ppm, 30.7–759.0 ppm; 37.8 ppm, 1.7–210.0 ppm; 21. 9 ppm, 2.8–78.3 ppm; 67.7 ppm, 1.0–205.1 ppm, respectively).
top
Discussion
Geochemical composition of trace elements in pyrite: methane vs. non-methane seepage. During early diagenesis, trace elements are captured by metastable substances in the sediment (such as labile organic matter and metastable precursors of Fe and Mn oxides). Thus, the remineralisation of labile organic matter and reductive dissolution of Fe and Mn oxides were the primary sources of trace elements in pore water in the marine sediment (Smrzka et al., 2019
Smrzka, D., Zwicker, J., Bach, W., Feng, D., Himmler, T., Chen, D., Peckmann, J. (2019) The behavior of trace elements in seawater, sedimentary pore water, and their in-corporation into carbonate minerals: A review. Facies 65, 1–47. https://doi.org/10.1007/s10347-019-0581-4
, 2020Smrzka, D., Feng, D., Himmler, T., Zwicker, J., Hu, Y., Monien, P., Tribovillard, N., Chen, D., Peckmann, J. (2020) Trace elements in methane-seep carbonates: Potentials, limitations, and perspectives. Earth-Science Reviews 208, 103263. https://doi.org/10.1016/j.earscirev.2020.103263
). The Cd is mainly derived from the remineralisation of labile organic matter. The Mo is mainly derived from reductive dissolution of Fe and Mn oxides, and will be fixed into the sediment when H2S exists (Smrzka et al., 2019Smrzka, D., Zwicker, J., Bach, W., Feng, D., Himmler, T., Chen, D., Peckmann, J. (2019) The behavior of trace elements in seawater, sedimentary pore water, and their in-corporation into carbonate minerals: A review. Facies 65, 1–47. https://doi.org/10.1007/s10347-019-0581-4
, 2020Smrzka, D., Feng, D., Himmler, T., Zwicker, J., Hu, Y., Monien, P., Tribovillard, N., Chen, D., Peckmann, J. (2020) Trace elements in methane-seep carbonates: Potentials, limitations, and perspectives. Earth-Science Reviews 208, 103263. https://doi.org/10.1016/j.earscirev.2020.103263
). In analysing the trace elements in the diagenetic pyrite, it was found that the content of Cd in pyrite generated during methane seepage was low, while the content of Mo was high (Fig. 2). This may be controlled by different biogeochemical processes for pyrite formation. In non-seepage, the formation of pyrite is mainly controlled by OSR (Berner, 1984Berner, R.A. (1984) Sedimentary pyrite formation: An update. Geochimica et Cosmochimica Acta 48, 605–615. https://doi.org/10.1016/0016-7037(84)90089-9
), while SD-AOM plays a leading role in methane seepage (Lin et al., 2016Lin, Z., Sun, X., Peckmann, J., Lu, Y., Xu, L., Strauss, H., Zhou, H., Gong, J., Lu, H., Teichert, B.M.A. (2016) How sulfate-driven anaerobic oxidation of methane affects the sulfur isotopic composition of pyrite: A SIMS study from the South China Sea. Chemical Geology 440, 26–41. https://doi.org/10.1016/j.chemgeo.2016.07.007
).SD-AOM and OSR have significantly different influences on the distribution and behaviour of trace elements: (1) methane itself does not carry trace elements, while the organic matter contains trace elements such as Cd (Smrzka et al., 2019
Smrzka, D., Zwicker, J., Bach, W., Feng, D., Himmler, T., Chen, D., Peckmann, J. (2019) The behavior of trace elements in seawater, sedimentary pore water, and their in-corporation into carbonate minerals: A review. Facies 65, 1–47. https://doi.org/10.1007/s10347-019-0581-4
, 2020Smrzka, D., Feng, D., Himmler, T., Zwicker, J., Hu, Y., Monien, P., Tribovillard, N., Chen, D., Peckmann, J. (2020) Trace elements in methane-seep carbonates: Potentials, limitations, and perspectives. Earth-Science Reviews 208, 103263. https://doi.org/10.1016/j.earscirev.2020.103263
); (2) the concentration of dissolved sulfide produced by SD-AOM is higher than that produced by OSR, which changes the behaviour of some trace elements, such as Mo (Smrzka et al., 2019Smrzka, D., Zwicker, J., Bach, W., Feng, D., Himmler, T., Chen, D., Peckmann, J. (2019) The behavior of trace elements in seawater, sedimentary pore water, and their in-corporation into carbonate minerals: A review. Facies 65, 1–47. https://doi.org/10.1007/s10347-019-0581-4
, 2020Smrzka, D., Feng, D., Himmler, T., Zwicker, J., Hu, Y., Monien, P., Tribovillard, N., Chen, D., Peckmann, J. (2020) Trace elements in methane-seep carbonates: Potentials, limitations, and perspectives. Earth-Science Reviews 208, 103263. https://doi.org/10.1016/j.earscirev.2020.103263
). These processes may eventually change the corresponding elements in pyrite. In non-seepage sedimentary environments, the OSR re-released the absorbed trace elements into the pore water during early diagenesis (Berner et al., 2013Berner, Z.A., Puchelt, H., Noeltner, T., Kramar, U. (2013) Pyrite geochemistry in the Toarcian Posidonia shale of south-west Germany: evidence for contrasting trace-element patterns of diagenetic and syngenetic pyrites. Sedimentology 60, 548–573. https://doi.org/10.1111/j.1365-3091.2012.01350.x
; Smrzka et al., 2019Smrzka, D., Zwicker, J., Bach, W., Feng, D., Himmler, T., Chen, D., Peckmann, J. (2019) The behavior of trace elements in seawater, sedimentary pore water, and their in-corporation into carbonate minerals: A review. Facies 65, 1–47. https://doi.org/10.1007/s10347-019-0581-4
, 2020Smrzka, D., Feng, D., Himmler, T., Zwicker, J., Hu, Y., Monien, P., Tribovillard, N., Chen, D., Peckmann, J. (2020) Trace elements in methane-seep carbonates: Potentials, limitations, and perspectives. Earth-Science Reviews 208, 103263. https://doi.org/10.1016/j.earscirev.2020.103263
), which increased the content of Cd related to organics and affected their contents in pyrite. On the contrary, in the methane seepage, large amounts of methane gas generated by hydrate dissociation led to sulfate reduction (Lin et al., 2016Lin, Z., Sun, X., Peckmann, J., Lu, Y., Xu, L., Strauss, H., Zhou, H., Gong, J., Lu, H., Teichert, B.M.A. (2016) How sulfate-driven anaerobic oxidation of methane affects the sulfur isotopic composition of pyrite: A SIMS study from the South China Sea. Chemical Geology 440, 26–41. https://doi.org/10.1016/j.chemgeo.2016.07.007
). It has been previously shown that the sulfate reduction rate in methane seeps can be several orders of magnitude higher than that in normal marine sedimentary environments (Aharon and Fu, 2000Aharon, P., Fu, B. (2000) Microbial sulfate reduction rates and sulfur and oxygen isotope fractionations at oil and gas seeps in deepwater Gulf of Mexico. Geochimica et Cosmochimica Acta 64, 233–246. https://doi.org/10.1016/S0016-7037(99)00292-6
). In addition, SD-AOM consumes less energy and is more likely to react with sulfate (Dickens, 2001Dickens, G.R. (2001) Sulfate profiles and barium fronts in sediment on the Blake Ridge: Present and past methane fluxes through a large gas hydrate reservoir. Geochimica et Cosmochimica Acta 65, 529–543. https://doi.org/10.1016/S0016-7037(00)00556-1
). Therefore, we believe that SD-AOM first consumes a large amount of sulfate, which inhibits OSR. This process also delays the release of the absorbed trace elements from organics, which results in the lower Cd contents in the pore water. Moreover, methane itself does not carry trace elements (Smrzka et al., 2020Smrzka, D., Feng, D., Himmler, T., Zwicker, J., Hu, Y., Monien, P., Tribovillard, N., Chen, D., Peckmann, J. (2020) Trace elements in methane-seep carbonates: Potentials, limitations, and perspectives. Earth-Science Reviews 208, 103263. https://doi.org/10.1016/j.earscirev.2020.103263
). SD-AOM should not participate directly in trace element cycling because none of the compounds involved in the reaction are trace element carriers (Smrzka et al., 2019Smrzka, D., Zwicker, J., Bach, W., Feng, D., Himmler, T., Chen, D., Peckmann, J. (2019) The behavior of trace elements in seawater, sedimentary pore water, and their in-corporation into carbonate minerals: A review. Facies 65, 1–47. https://doi.org/10.1007/s10347-019-0581-4
, 2020Smrzka, D., Feng, D., Himmler, T., Zwicker, J., Hu, Y., Monien, P., Tribovillard, N., Chen, D., Peckmann, J. (2020) Trace elements in methane-seep carbonates: Potentials, limitations, and perspectives. Earth-Science Reviews 208, 103263. https://doi.org/10.1016/j.earscirev.2020.103263
). Thus, their dissociation does not affect trace element contents in the pore water. Meanwhile, under methane seepage, the source of the trace metals in pore water is the reductive dissolution of Fe and Mn oxides. These reactions are typically unrelated to the organic matter present in the same system (Smrzka et al., 2019Smrzka, D., Zwicker, J., Bach, W., Feng, D., Himmler, T., Chen, D., Peckmann, J. (2019) The behavior of trace elements in seawater, sedimentary pore water, and their in-corporation into carbonate minerals: A review. Facies 65, 1–47. https://doi.org/10.1007/s10347-019-0581-4
, 2020Smrzka, D., Feng, D., Himmler, T., Zwicker, J., Hu, Y., Monien, P., Tribovillard, N., Chen, D., Peckmann, J. (2020) Trace elements in methane-seep carbonates: Potentials, limitations, and perspectives. Earth-Science Reviews 208, 103263. https://doi.org/10.1016/j.earscirev.2020.103263
). Thus, organic matter Cd contents were lower in the pyrite produced under methane seepage than in that produced under a normal sedimentary environment.In the normal marine sedimentary environment, H2S production is limited (Lin et al., 2016
Lin, Z., Sun, X., Peckmann, J., Lu, Y., Xu, L., Strauss, H., Zhou, H., Gong, J., Lu, H., Teichert, B.M.A. (2016) How sulfate-driven anaerobic oxidation of methane affects the sulfur isotopic composition of pyrite: A SIMS study from the South China Sea. Chemical Geology 440, 26–41. https://doi.org/10.1016/j.chemgeo.2016.07.007
) due to OSR being relatively slow. However, SD-AOM can accelerate sulfate reduction, yielding more dissolved sulfide, which leads to sulfide reduction in that local micro-environment (Chen et al., 2016Chen, F., Hu, Y., Feng, D., Zhang, X., Cheng, S., Cao, J., Lu, H., Chen, D. (2016) Evidence of intense methane seepages from molybdenum enrichments in gas hydrate-bearing sediments of the northern South China Sea. Chemical Geology 443, 173–181. https://doi.org/10.1016/j.chemgeo.2016.09.029
; Lin et al., 2016Lin, Z., Sun, X., Peckmann, J., Lu, Y., Xu, L., Strauss, H., Zhou, H., Gong, J., Lu, H., Teichert, B.M.A. (2016) How sulfate-driven anaerobic oxidation of methane affects the sulfur isotopic composition of pyrite: A SIMS study from the South China Sea. Chemical Geology 440, 26–41. https://doi.org/10.1016/j.chemgeo.2016.07.007
). On the one hand, this sulfidic environment will accelerate the dissolution of Fe and Mn oxides and release more Mo into the pore water (Eroglu et al., 2020Eroglu, S., Scholz, F., Frank, M., Siebert, C. (2020) Influence of particulate versus diffusive molybdenum supply mechanisms on the molybdenum isotope composition of continental margin sediments. Geochimica et Cosmochimica Acta 273, 51–69. https://doi.org/10.1016/j.gca.2020.01.009
). On the other hand, due to the high H2S content, Mo will be removed from the pore water onto pyrite more efficiently (Smrzka et al., 2020Smrzka, D., Feng, D., Himmler, T., Zwicker, J., Hu, Y., Monien, P., Tribovillard, N., Chen, D., Peckmann, J. (2020) Trace elements in methane-seep carbonates: Potentials, limitations, and perspectives. Earth-Science Reviews 208, 103263. https://doi.org/10.1016/j.earscirev.2020.103263
). Our previous work showed that the iron (oxy) hydroxides contents were very low in the SMTZ of Q6, which indicates their substantial dissolution (Miao et al., 2021bMiao, X., Feng, X., Liu, X., Li, J., Wei, J. (2021b) Effects of methane seepage activity on the morphology and geochemistry of authigenic pyrite. Marine and Petroleum Geology 133, 105231. https://doi.org/10.1016/j.marpetgeo.2021.105231
). In addition, enrichment factors of Mo are positively correlated with Fe/Al (R2 = 0.24), and R2 can even exceed 0.9 at 90–98 cm (R2 = 0.97) and 118–124 cm (R2 = 0.97) depths, indicating that the reductive dissolution of Fe and Mn oxides plays an important role in the enrichment of Mo (Fig. S-6; Miao et al., 2021aMiao, X., Feng, X., Li, J., Lin, L. (2021a) Tracing the paleo-methane seepage activity over the past 20,000 years in the sediments of Qiongdongnan Basin, northwestern South China Sea. Chemical Geology 559, 119956. https://doi.org/10.1016/j.chemgeo.2020.119956
). All these results proved that the Mo enrichment in the pyrite under the methane seepage was mainly affected by SD-AOM. However, OSR does not disappear in the SMTZ. We found that the correlations between FePy and TOC in the SMTZ are poor (R2 = 0.12; Fig. S-7). This indicates that the distribution and importance of OSR-derived pyrite in methane-rich, but organic poor, sediments is limited.In addition, the Ni, Cu, and Zn contents of pyrite in the two environments were not significantly different (Fig. 2). This may be because, of these and similar elements, Cd is the most sensitive to organic matter, and its content in sediment pore water is strongly affected by the presence of organic matter (Smrzka et al., 2019
Smrzka, D., Zwicker, J., Bach, W., Feng, D., Himmler, T., Chen, D., Peckmann, J. (2019) The behavior of trace elements in seawater, sedimentary pore water, and their in-corporation into carbonate minerals: A review. Facies 65, 1–47. https://doi.org/10.1007/s10347-019-0581-4
, 2020Smrzka, D., Feng, D., Himmler, T., Zwicker, J., Hu, Y., Monien, P., Tribovillard, N., Chen, D., Peckmann, J. (2020) Trace elements in methane-seep carbonates: Potentials, limitations, and perspectives. Earth-Science Reviews 208, 103263. https://doi.org/10.1016/j.earscirev.2020.103263
). However, Ni, Cu, Zn, and other elements may combine with H2S to different degrees to transform the corresponding sulfides into pyrite (Morse and Luther, 1999Morse, J.W., Luther, G. (1999) Chemical influences on trace metal-sulfide interactions in anoxic sediments. Geochimica et Cosmochimica Acta 63, 3373–3378. https://doi.org/10.1016/S0016-7037(99)00258-6
). In contrast, Huerta-Diaz and Morse (1992)Huerta-Diaz, M.A., Morse, J.W. (1992) Pyritization of trace metals in anoxic marine sediments. Geochimica et Cosmochimica Acta 56, 2681–2702. https://doi.org/10.1016/0016-7037(92)90353-K
found that cadmium was largely unaffected by the presence of sulfides in the pore water. Therefore, we believe that Mo/Cd ratios in diagenetic pyrite can indicate the presence of methane seeps. In Figure S-4, this conclusion is confirmed. The Mo/Cd ratio of pyrite in methane seepage is significantly higher than that in non-methane seepage (Mo/Cd > 16.02). However, no similar phenomenon was found for other elements (Fig. S-5).Trace elements of pyrite indicate methane seepage. To further verify the effectiveness of this indicator, the Mo/Cd ratios were compared and analysed alongside the indicators used to represent methane seepage. It was found that the Mo/Cd ratios of pyrite increased with increasing TS/TOC, δ34SCRS, and FePy/FeT (Fig. 3). In non-methane seepage, Mo/Cd, TS/TOC, δ34SCRS, and FePy/FeT are located in the low value region. On the contrary, these proxies are in the high value area in methane seepage. This indicates that SD-AOM does have a great influence on the Mo/Cd ratio of pyrite. It is indicated that, compared with normal marine environment, intense methane activity can not only accelerate pyrite formation and reduce sulfur isotope fractionation, but also change the surrounding pore water environment (inhibiting OSR and changing the redox environment). Moreover, we found that the Mo/Cd ratio of pyrite at 90–124 cm depth is higher than those at 144–162 cm and 254–282 cm depths, and corresponds to a higher TS/TOC ratio and other indicators (Fig. 3). This is inferred to be related to the intensity of methane seepage. At 90–124 cm depth, the TS/TOC ratios and δ34SCRS values are higher (Figs. 1 and 3), indicating a higher pyrite content and faster sulfate reduction rate (Miao et al., 2021b
Miao, X., Feng, X., Liu, X., Li, J., Wei, J. (2021b) Effects of methane seepage activity on the morphology and geochemistry of authigenic pyrite. Marine and Petroleum Geology 133, 105231. https://doi.org/10.1016/j.marpetgeo.2021.105231
), respectively. Meanwhile, iron composition data show that the pore water environment in this formation is more inclined to euxinic environments (Fig. S-8, Slotznick et al., 2018Slotznick, S.P., Eiler, J.M., Fischer, W.W. (2018) The Effects of Metamorphism on Iron Mineralogy and the Iron Speciation Redox Proxy. Geochimica et Cosmochimica Acta 224, 96–115. https://doi.org/10.1016/j.gca.2017.12.003
). This phenomena indicates that the methane seepage is most intensive in this layer, which further inhibits OSR. This is followed by a further reduction in Cd content in the pore water. The Cd content of pyrite in the 90–124 cm layer is the lowest, with most measurements lower than the detection limit of the instrument (Table S-2), which eventually leads to the largest difference in Mo and Cd content in pyrite. Therefore, we believe that the chemical composition of diagenetic pyrite has great potential to identify methane seepage.
Figure 3 Scatter diagram of Mo/Cd vs. TS/TOC, δ34SCRS and FePy/FeT (based on Miao et al., 2021a
Miao, X., Feng, X., Li, J., Lin, L. (2021a) Tracing the paleo-methane seepage activity over the past 20,000 years in the sediments of Qiongdongnan Basin, northwestern South China Sea. Chemical Geology 559, 119956. https://doi.org/10.1016/j.chemgeo.2020.119956
,bMiao, X., Feng, X., Liu, X., Li, J., Wei, J. (2021b) Effects of methane seepage activity on the morphology and geochemistry of authigenic pyrite. Marine and Petroleum Geology 133, 105231. https://doi.org/10.1016/j.marpetgeo.2021.105231
). TS/TOC = total sulfur/total organic carbon; CRS = chromium-reducible sulfur; δ34SCRS = sulfur isotope of chromium-reducible sulfur; Fepy = pyrite Fe; FeT = total Fe.top
Acknowledgements
We would like to thank the researchers for the help in the experimental work. And we are also very grateful to Professor J. Peckmann (Universität Hamburg) for his guidance in the writing of this article. This study was supported by the Fundamental Research Funds for the Central Universities (202061007), Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) (GML2019ZD0201), the National Natural Science Foundation of China (41976053), the National Key R&D Program of China (2017YFC0306703).
Editor: Claudine Stirling
top
References
Aharon, P., Fu, B. (2000) Microbial sulfate reduction rates and sulfur and oxygen isotope fractionations at oil and gas seeps in deepwater Gulf of Mexico. Geochimica et Cosmochimica Acta 64, 233–246. https://doi.org/10.1016/S0016-7037(99)00292-6
 Show in context
Show in context It has been previously shown that the sulfate reduction rate in methane seeps can be several orders of magnitude higher than that in normal marine sedimentary environments (Aharon and Fu, 2000).
View in article
Berner, R.A. (1978) Sulfate reduction and the rate of deposition of marine sediments. Earth and Planetary Science Letters 37, 492–498. https://doi.org/10.1016/0012-821X(78)90065-1
 Show in context
Show in context However, OSR is different (e.g., reaction rates) in various sedimentary environments (Berner, 1978).
View in article
This will eventually lead to corresponding changes in the morphology and geochemical characteristics of pyrite (Berner, 1978, 1984; Huerta-Diaz and Morse, 1992; Wilkin et al., 1996).
View in article
Berner, R.A. (1984) Sedimentary pyrite formation: An update. Geochimica et Cosmochimica Acta 48, 605–615. https://doi.org/10.1016/0016-7037(84)90089-9
 Show in context
Show in context Authigenic pyrite is considered to be the most important sulfide mineral because it is more stable than other iron sulfides and is the main sink of sulfur (Berner, 1984).
View in article
Its formation is often related to organoclastic sulfate reduction (OSR), using organic matter and seawater sulfate as substrate and yielding hydrogen sulfide (H2S) (Berner, 1984).
View in article
In non-seepage, the formation of pyrite is mainly controlled by OSR (Berner, 1984), while SD-AOM plays a leading role in methane seepage (Lin et al., 2016).
View in article
This will eventually lead to corresponding changes in the morphology and geochemical characteristics of pyrite (Berner, 1978, 1984; Huerta-Diaz and Morse, 1992; Wilkin et al., 1996).
View in article
Berner, Z.A., Puchelt, H., Noeltner, T., Kramar, U. (2013) Pyrite geochemistry in the Toarcian Posidonia shale of south-west Germany: evidence for contrasting trace-element patterns of diagenetic and syngenetic pyrites. Sedimentology 60, 548–573. https://doi.org/10.1111/j.1365-3091.2012.01350.x
 Show in context
Show in context Trace elements can also adsorb onto pyrite, an important sink in many geochemical cycles that has great potential for reconstructing palaeoenvironments (Morse and Luther, 1999; Berner et al., 2013; Mukherjee and Large, 2020; Large et al., 2022).
View in article
As research has advanced, scientists have found that the composition of trace elements in diagenetic pyrite is greatly affected by the geochemical characteristics of pore water in the sediments (Huerta-Diaz and Morse, 1992; Berner et al., 2013; Large et al., 2014, 2022).
View in article
In non-seepage sedimentary environments, the OSR re-released the absorbed trace elements into the pore water during early diagenesis (Berner et al., 2013; Smrzka et al., 2019, 2020), which increased the content of Cd related to organics and affected their contents in pyrite.
View in article
When the concentration of H2S in the environment increases, those of redox sensitive elements (such as Mo) in pyrite will also increase (Huerta-Diaz and Morse, 1992; Berner et al., 2013).
View in article
Boetius, A., Ravenschlag, K., Schubert, C.J., Rickert, D., Widdel, F., Gieseke, A., Amann, R., Jørgensen, B., Witte, U., Pfannkuche, O. (2000) A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407, 623–626. https://doi.org/10.1038/35036572
 Show in context
Show in context Both methane released from cold seeps and the sulfate released from seawater undergo sulfate driven anaerobic oxidation of methane (SD-AOM) in the sulfate-methane transition zone (SMTZ) (Boetius et al., 2000).
View in article
Chen, F., Hu, Y., Feng, D., Zhang, X., Cheng, S., Cao, J., Lu, H., Chen, D. (2016) Evidence of intense methane seepages from molybdenum enrichments in gas hydrate-bearing sediments of the northern South China Sea. Chemical Geology 443, 173–181. https://doi.org/10.1016/j.chemgeo.2016.09.029
 Show in context
Show in context However, SD-AOM can accelerate sulfate reduction, yielding more dissolved sulfide, which leads to sulfide reduction in that local micro-environment (Chen et al., 2016; Lin et al., 2016).
View in article
Based on previous studies of trace elements in authigenic carbonate and sediments in methane seepage, we found a remarkable dependency: when Mo is enriched, Cd, Ni, Cu, and Zn are not enriched (Sato et al., 2012; Chen et al., 2016).
View in article
Clark, C., Grguric, B., Schmidt Mumm, A. (2004) Genetic implications of pyrite chemistry from the Palaeoproterozoic Olary Domain and overlying Neoproterozoic Adelaidean sequences, northeastern South Australia. Ore Geology Reviews 25, 237–257. https://doi.org/10.1016/j.oregeorev.2004.04.003
 Show in context
Show in context Initially, research on trace elements in pyrite mainly focused on defining ore genetic types, determining the source of ore forming materials, and inverting the evolution of ore forming fluids (Clark et al., 2004; Large et al., 2009; Ulrich et al., 2011).
View in article
Dickens, G.R. (2001) Sulfate profiles and barium fronts in sediment on the Blake Ridge: Present and past methane fluxes through a large gas hydrate reservoir. Geochimica et Cosmochimica Acta 65, 529–543. https://doi.org/10.1016/S0016-7037(00)00556-1
 Show in context
Show in context In addition, SD-AOM consumes less energy and is more likely to react with sulfate (Dickens, 2001).
View in article
Eroglu, S., Scholz, F., Frank, M., Siebert, C. (2020) Influence of particulate versus diffusive molybdenum supply mechanisms on the molybdenum isotope composition of continental margin sediments. Geochimica et Cosmochimica Acta 273, 51–69. https://doi.org/10.1016/j.gca.2020.01.009
 Show in context
Show in context On the one hand, this sulfidic environment will accelerate the dissolution of Fe and Mn oxides and release more Mo into the pore water (Eroglu et al., 2020).
View in article
Huerta-Diaz, M.A., Morse, J.W. (1992) Pyritization of trace metals in anoxic marine sediments. Geochimica et Cosmochimica Acta 56, 2681–2702. https://doi.org/10.1016/0016-7037(92)90353-K
 Show in context
Show in context As research has advanced, scientists have found that the composition of trace elements in diagenetic pyrite is greatly affected by the geochemical characteristics of pore water in the sediments (Huerta-Diaz and Morse, 1992; Berner et al., 2013; Large et al., 2014, 2022).
View in article
When the concentration of H2S in the environment increases, those of redox sensitive elements (such as Mo) in pyrite will also increase (Huerta-Diaz and Morse, 1992; Berner et al., 2013).
View in article
In contrast, Huerta-Diaz and Morse (1992) found that cadmium was largely unaffected by the presence of sulfides in the pore water.
View in article
This will eventually lead to corresponding changes in the morphology and geochemical characteristics of pyrite (Berner, 1978, 1984; Huerta-Diaz and Morse, 1992; Wilkin et al., 1996).
View in article
Large, R.R., Danyushevsky, L., Hollit, C., Maslennikov, V., Meffre, S., Gilbert, S., Bull, S., Scott, R., Emsbo, P., Thomas, H., Singh, B., Foster, J. (2009) Gold and trace element zonation in pyrite using a laser imaging technique: Implications for the timing of gold in orogenic and carlin-style sediment-hosted deposits. Economic Geology 104, 635–668. https://doi.org/10.2113/gsecongeo.104.5.635
 Show in context
Show in context Initially, research on trace elements in pyrite mainly focused on defining ore genetic types, determining the source of ore forming materials, and inverting the evolution of ore forming fluids (Clark et al., 2004; Large et al., 2009; Ulrich et al., 2011).
View in article
Large, R.R., Halpin, J.A., Danyushevsky, L.V., Maslennikov, V.V., Bull, S.W., Long, J.A., Gregory, D.D., Lounejeva, E., Lyons, T.W., Sack, P.J., McGoldrick, P.J., Calver, C.R. (2014) Trace element content of sedimentary pyrite as a new proxy for deep-time ocean atmosphere evolution. Earth and Planetary Science Letters 389, 209–220. https://doi.org/10.1016/j.epsl.2013.12.020
 Show in context
Show in context However, there are some unique sedimentary environments where additional sources of H2S play an important role in pyrite formation, such as hydrothermal environments (Large et al., 2014) and oil seepage areas (Steadman et al., 2021).
View in article
As research has advanced, scientists have found that the composition of trace elements in diagenetic pyrite is greatly affected by the geochemical characteristics of pore water in the sediments (Huerta-Diaz and Morse, 1992; Berner et al., 2013; Large et al., 2014, 2022).
View in article
Large, R.R., Mukherjee, I., Danyushevsky, L., Gregory, D., Steadman, J., Corkrey, R. (2022) Sedimentary pyrite proxy for atmospheric oxygen; evaluation of strengths and limitations. Earth-Science Reviews 227, 103941. https://doi.org/10.1016/j.earscirev.2022.103941
 Show in context
Show in context Trace elements can also adsorb onto pyrite, an important sink in many geochemical cycles that has great potential for reconstructing palaeoenvironments (Morse and Luther, 1999; Berner et al., 2013; Mukherjee and Large, 2020; Large et al., 2022).
View in article
As research has advanced, scientists have found that the composition of trace elements in diagenetic pyrite is greatly affected by the geochemical characteristics of pore water in the sediments (Huerta-Diaz and Morse, 1992; Berner et al., 2013; Large et al., 2014, 2022).
View in article
Lin, Z., Sun, X., Peckmann, J., Lu, Y., Xu, L., Strauss, H., Zhou, H., Gong, J., Lu, H., Teichert, B.M.A. (2016) How sulfate-driven anaerobic oxidation of methane affects the sulfur isotopic composition of pyrite: A SIMS study from the South China Sea. Chemical Geology 440, 26–41. https://doi.org/10.1016/j.chemgeo.2016.07.007
 Show in context
Show in context This releases a large amount of H2S and provides the constituents necessary to form pyrite (Lin et al., 2016).
View in article
In recent years, many indicators have been used to study sedimentary environment evolution, such as the sulfur and iron isotopic compositions of pyrite (Lin et al., 2016), the grain size of framboidal pyrite (Wilkin et al., 1996), and iron speciation (Slotznick et al., 2018).
View in article
On the contrary, in the methane seepage, large amounts of methane gas generated by hydrate dissociation led to sulfate reduction (Lin et al., 2016).
View in article
In the normal marine sedimentary environment, H2S production is limited (Lin et al., 2016) due to OSR being relatively slow.
View in article
In non-seepage, the formation of pyrite is mainly controlled by OSR (Berner, 1984), while SD-AOM plays a leading role in methane seepage (Lin et al., 2016).
View in article
However, SD-AOM can accelerate sulfate reduction, yielding more dissolved sulfide, which leads to sulfide reduction in that local micro-environment (Chen et al., 2016; Lin et al., 2016).
View in article
Miao, X., Feng, X., Li, J., Lin, L. (2021a) Tracing the paleo-methane seepage activity over the past 20,000 years in the sediments of Qiongdongnan Basin, northwestern South China Sea. Chemical Geology 559, 119956. https://doi.org/10.1016/j.chemgeo.2020.119956
 Show in context
Show in context (a) Map showing the study region; the Q6 core located in the Qiongdongnan Basin of the SCS (modified from Miao et al., 2021a).
View in article
Based on previous geochemical analysis results (Miao et al., 2021a,b), we identified several palaeo-methane seeps using TS/TOC, sulfur isotopes, and iron speciation (Fig. 1b).
View in article
In addition, enrichment factors of Mo are positively correlated with Fe/Al (R2 = 0.24), and R2 can even exceed 0.9 at 90–98 cm (R2 = 0.97) and 118–124 cm (R2 = 0.97) depths, indicating that the reductive dissolution of Fe and Mn oxides plays an important role in the enrichment of Mo (
View in article
The blue horizontal bars indicate methane seepage (based on Miao et al., 2021a,b). TS/TOC = total sulfur/total organic carbon; CRS = chromium reducible sulfur; δ34SCRS = sulfur isotope of chromium reducible sulfur; Fepy = pyrite Fe; FeHR = highly reactive Fe.
View in article
Scatter diagram of Mo/Cd vs. TS/TOC, δ34SCRS and FePy/FeT (based on Miao et al., 2021a,b).
View in article
Miao, X., Feng, X., Liu, X., Li, J., Wei, J. (2021b) Effects of methane seepage activity on the morphology and geochemistry of authigenic pyrite. Marine and Petroleum Geology 133, 105231. https://doi.org/10.1016/j.marpetgeo.2021.105231
 Show in context
Show in context (b) Down core variations of TS/TOC, CRS, δ34SCRS, and FePy/FeHR in sediments (based on Miao et al., 2021b).
View in article
Based on previous geochemical analysis results (Miao et al., 2021a,b), we identified several palaeo-methane seeps using TS/TOC, sulfur isotopes, and iron speciation (Fig. 1b).
View in article
Our previous work showed that the iron (oxy) hydroxides contents were very low in the SMTZ of Q6, which indicates their substantial dissolution (Miao et al., 2021b).
View in article
At 90–124 cm depth, the TS/TOC ratios and δ34SCRS values are higher (Figs. 1 and 3), indicating a higher pyrite content and faster sulfate reduction rate (Miao et al., 2021b), respectively.
View in article
The blue horizontal bars indicate methane seepage (based on Miao et al., 2021a,b). TS/TOC = total sulfur/total organic carbon; CRS = chromium reducible sulfur; δ34SCRS = sulfur isotope of chromium reducible sulfur; Fepy = pyrite Fe; FeHR = highly reactive Fe.
View in article
Scatter diagram of Mo/Cd vs. TS/TOC, δ34SCRS and FePy/FeT (based on Miao et al., 2021a,b).
View in article
Morse, J.W., Luther, G. (1999) Chemical influences on trace metal-sulfide interactions in anoxic sediments. Geochimica et Cosmochimica Acta 63, 3373–3378. https://doi.org/10.1016/S0016-7037(99)00258-6
 Show in context
Show in context However, Ni, Cu, Zn, and other elements may combine with H2S to different degrees to transform the corresponding sulfides into pyrite (Morse and Luther, 1999).
View in article
Trace elements can also adsorb onto pyrite, an important sink in many geochemical cycles that has great potential for reconstructing palaeoenvironments (Morse and Luther, 1999; Berner et al., 2013; Mukherjee and Large, 2020; Large et al., 2022).
View in article
Mukherjee, I., Large, R.R. (2020) Co-evolution of trace elements and life in Precambrian oceans: The pyrite edition. Geology 48, 1018–1022. https://doi.org/10.1130/G47890.1
 Show in context
Show in context Trace elements can also adsorb onto pyrite, an important sink in many geochemical cycles that has great potential for reconstructing palaeoenvironments (Morse and Luther, 1999; Berner et al., 2013; Mukherjee and Large, 2020; Large et al., 2022).
View in article
Sato, H., Hayashi, K., Ogawa, Y., Kawamura, K. (2012) Geochemistry of deep sea sediments at cold seep sites in the Nankai Trough: insights into the effect of anaerobic oxidation of methane. Marine Geology 323, 47–55. https://doi.org/10.1016/j.margeo.2012.07.013
 Show in context
Show in context Based on previous studies of trace elements in authigenic carbonate and sediments in methane seepage, we found a remarkable dependency: when Mo is enriched, Cd, Ni, Cu, and Zn are not enriched (Sato et al., 2012; Chen et al., 2016).
View in article
Slotznick, S.P., Eiler, J.M., Fischer, W.W. (2018) The Effects of Metamorphism on Iron Mineralogy and the Iron Speciation Redox Proxy. Geochimica et Cosmochimica Acta 224, 96–115. https://doi.org/10.1016/j.gca.2017.12.003
 Show in context
Show in context Meanwhile, iron composition data show that the pore water environment in this formation is more inclined to euxinic environments (
View in article
In recent years, many indicators have been used to study sedimentary environment evolution, such as the sulfur and iron isotopic compositions of pyrite (Lin et al., 2016), the grain size of framboidal pyrite (Wilkin et al., 1996), and iron speciation (Slotznick et al., 2018).
View in article
Smrzka, D., Zwicker, J., Bach, W., Feng, D., Himmler, T., Chen, D., Peckmann, J. (2019) The behavior of trace elements in seawater, sedimentary pore water, and their in-corporation into carbonate minerals: A review. Facies 65, 1–47. https://doi.org/10.1007/s10347-019-0581-4
 Show in context
Show in context Thus, the remineralisation of labile organic matter and reductive dissolution of Fe and Mn oxides were the primary sources of trace elements in pore water in the marine sediment (Smrzka et al., 2019, 2020).
View in article
The Mo is mainly derived from reductive dissolution of Fe and Mn oxides, and will be fixed into the sediment when H2S exists (Smrzka et al., 2019, 2020).
View in article
SD-AOM and OSR have significantly different influences on the distribution and behaviour of trace elements: (1) methane itself does not carry trace elements, while the organic matter contains trace elements such as Cd (Smrzka et al., 2019, 2020); (2) the concentration of dissolved sulfide produced by SD-AOM is higher than that produced by OSR, which changes the behaviour of some trace elements, such as Mo (Smrzka et al., 2019, 2020).
View in article
In non-seepage sedimentary environments, the OSR re-released the absorbed trace elements into the pore water during early diagenesis (Berner et al., 2013; Smrzka et al., 2019, 2020), which increased the content of Cd related to organics and affected their contents in pyrite.
View in article
SD-AOM should not participate directly in trace element cycling because none of the compounds involved in the reaction are trace element carriers (Smrzka et al., 2019, 2020).
View in article
These reactions are typically unrelated to the organic matter present in the same system (Smrzka et al., 2019, 2020).
View in article
This may be because, of these and similar elements, Cd is the most sensitive to organic matter, and its content in sediment pore water is strongly affected by the presence of organic matter (Smrzka et al., 2019, 2020).
View in article
SD-AOM and OSR have significantly different influences on the distribution and behaviour of trace elements: (1) methane itself does not carry trace elements, while the organic matter contains trace elements such as Cd (Smrzka et al., 2019, 2020); (2) the concentration of dissolved sulfide produced by SD-AOM is higher than that produced by OSR, which changes the behaviour of some trace elements, such as Mo (Smrzka et al., 2019, 2020).
View in article
Smrzka, D., Feng, D., Himmler, T., Zwicker, J., Hu, Y., Monien, P., Tribovillard, N., Chen, D., Peckmann, J. (2020) Trace elements in methane-seep carbonates: Potentials, limitations, and perspectives. Earth-Science Reviews 208, 103263. https://doi.org/10.1016/j.earscirev.2020.103263
 Show in context
Show in context Thus, the remineralisation of labile organic matter and reductive dissolution of Fe and Mn oxides were the primary sources of trace elements in pore water in the marine sediment (Smrzka et al., 2019, 2020).
View in article
The Mo is mainly derived from reductive dissolution of Fe and Mn oxides, and will be fixed into the sediment when H2S exists (Smrzka et al., 2019, 2020).
View in article
SD-AOM and OSR have significantly different influences on the distribution and behaviour of trace elements: (1) methane itself does not carry trace elements, while the organic matter contains trace elements such as Cd (Smrzka et al., 2019, 2020); (2) the concentration of dissolved sulfide produced by SD-AOM is higher than that produced by OSR, which changes the behaviour of some trace elements, such as Mo (Smrzka et al., 2019, 2020).
View in article
In non-seepage sedimentary environments, the OSR re-released the absorbed trace elements into the pore water during early diagenesis (Berner et al., 2013; Smrzka et al., 2019, 2020), which increased the content of Cd related to organics and affected their contents in pyrite.
View in article
Moreover, methane itself does not carry trace elements (Smrzka et al., 2020).
View in article
SD-AOM should not participate directly in trace element cycling because none of the compounds involved in the reaction are trace element carriers (Smrzka et al., 2019, 2020).
View in article
These reactions are typically unrelated to the organic matter present in the same system (Smrzka et al., 2019, 2020).
View in article
On the other hand, due to the high H2S content, Mo will be removed from the pore water onto pyrite more efficiently (Smrzka et al., 2020).
View in article
This may be because, of these and similar elements, Cd is the most sensitive to organic matter, and its content in sediment pore water is strongly affected by the presence of organic matter (Smrzka et al., 2019, 2020).
View in article
SD-AOM and OSR have significantly different influences on the distribution and behaviour of trace elements: (1) methane itself does not carry trace elements, while the organic matter contains trace elements such as Cd (Smrzka et al., 2019, 2020); (2) the concentration of dissolved sulfide produced by SD-AOM is higher than that produced by OSR, which changes the behaviour of some trace elements, such as Mo (Smrzka et al., 2019, 2020).
View in article
Steadman, J.A., Large, R.R., Olin, P.H., Danyushevsky, L.V., Meffre, S., Huston, D., Fabris, A., Lisitsin, V., Wells, T. (2021) Pyrite trace element behavior in magmatic-hydrothermal environments: An LA-ICPMS imaging study. Ore Geology Reviews 128, 103878. https://doi.org/10.1016/j.oregeorev.2020.103878
 Show in context
Show in context However, there are some unique sedimentary environments where additional sources of H2S play an important role in pyrite formation, such as hydrothermal environments (Large et al., 2014) and oil seepage areas (Steadman et al., 2021).
View in article
Ulrich, T., Long, D.G.F., Kamber, B.S., Whitehouse, M.J. (2011) In situ trace element and sulfur isotope analysis of pyrite in a Paleoproterozoic gold placer deposit, Pardo and Clement Townships, Ontario, Canada. Economic Geology 106, 667–686. https://doi.org/10.2113/econgeo.106.4.667
 Show in context
Show in context Initially, research on trace elements in pyrite mainly focused on defining ore genetic types, determining the source of ore forming materials, and inverting the evolution of ore forming fluids (Clark et al., 2004; Large et al., 2009; Ulrich et al., 2011).
View in article
Wilkin, R.T., Barnes, H.L., Brantley, S.L. (1996) The size distribution of framboidal pyrite in modern sediments: an indicator of redox conditions. Geochimica et Cosmochimica Acta 60, 3897–3912. https://doi.org/10.1016/0016-7037(96)00209-8
 Show in context
Show in context In recent years, many indicators have been used to study sedimentary environment evolution, such as the sulfur and iron isotopic compositions of pyrite (Lin et al., 2016), the grain size of framboidal pyrite (Wilkin et al., 1996), and iron speciation (Slotznick et al., 2018).
View in article
This will eventually lead to corresponding changes in the morphology and geochemical characteristics of pyrite (Berner, 1978, 1984; Huerta-Diaz and Morse, 1992; Wilkin et al., 1996).
View in article
top
Supplementary Information
The Supplementary Information includes:
Download the Supplementary Information (PDF).
Figures
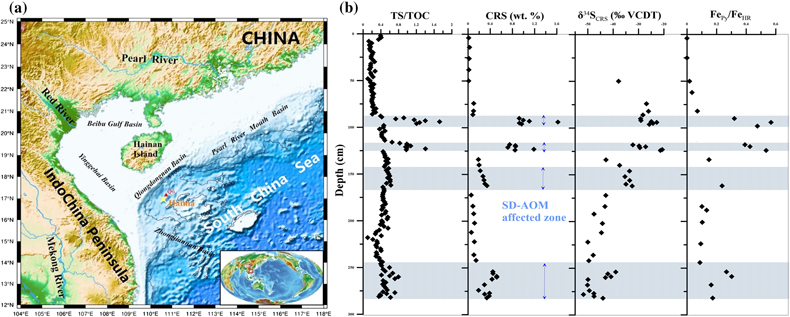
Figure 1 (a) Map showing the study region; the Q6 core located in the Qiongdongnan Basin of the SCS (modified from Miao et al., 2021a
Miao, X., Feng, X., Li, J., Lin, L. (2021a) Tracing the paleo-methane seepage activity over the past 20,000 years in the sediments of Qiongdongnan Basin, northwestern South China Sea. Chemical Geology 559, 119956. https://doi.org/10.1016/j.chemgeo.2020.119956
,bMiao, X., Feng, X., Liu, X., Li, J., Wei, J. (2021b) Effects of methane seepage activity on the morphology and geochemistry of authigenic pyrite. Marine and Petroleum Geology 133, 105231. https://doi.org/10.1016/j.marpetgeo.2021.105231
). (b) Down core variations of TS/TOC, CRS, δ34SCRS, and FePy/FeHR in sediments (based on Miao et al., 2021bMiao, X., Feng, X., Liu, X., Li, J., Wei, J. (2021b) Effects of methane seepage activity on the morphology and geochemistry of authigenic pyrite. Marine and Petroleum Geology 133, 105231. https://doi.org/10.1016/j.marpetgeo.2021.105231
). The blue horizontal bars indicate methane seepage (based on Miao et al., 2021aMiao, X., Feng, X., Li, J., Lin, L. (2021a) Tracing the paleo-methane seepage activity over the past 20,000 years in the sediments of Qiongdongnan Basin, northwestern South China Sea. Chemical Geology 559, 119956. https://doi.org/10.1016/j.chemgeo.2020.119956
,bMiao, X., Feng, X., Liu, X., Li, J., Wei, J. (2021b) Effects of methane seepage activity on the morphology and geochemistry of authigenic pyrite. Marine and Petroleum Geology 133, 105231. https://doi.org/10.1016/j.marpetgeo.2021.105231
). TS/TOC = total sulfur/total organic carbon; CRS = chromium reducible sulfur; δ34SCRS = sulfur isotope of chromium reducible sulfur; Fepy = pyrite Fe; FeHR = highly reactive Fe.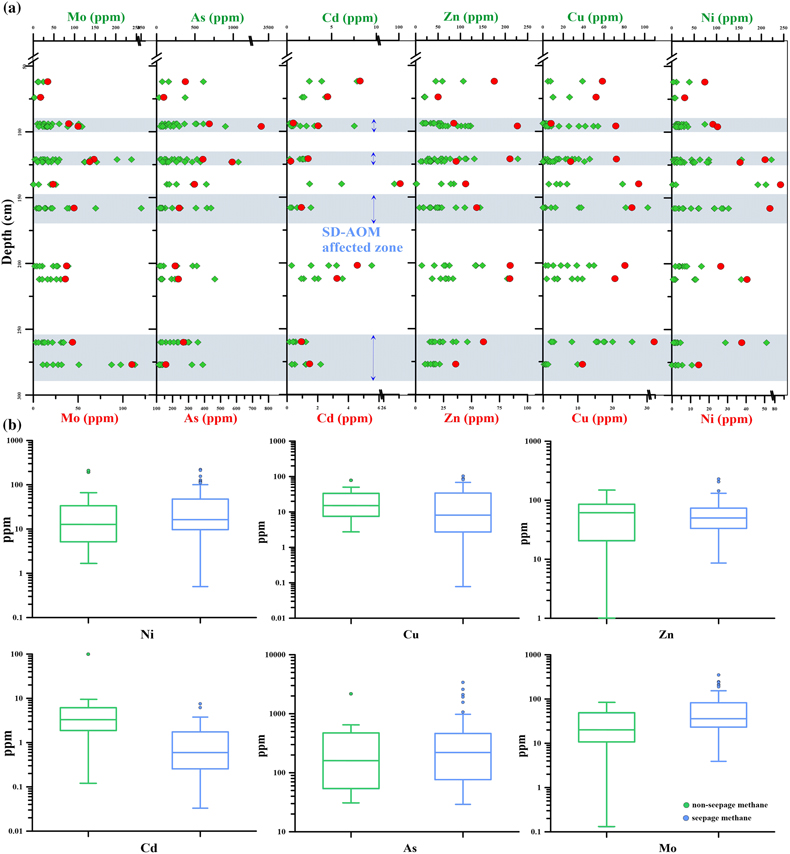
Figure 2 (a) Down core variations of the trace element contents (Cd, Ni, Cu, Zn, As, and Mo) in pyrite samples. The grey shaded bars indicate methane seeps; each green circle represents a single pyrite analysis; each red circle represents the average pyrite analysis. (b) Box plots of the average values of Ni, Cu, Zn, Cd, As, and Mo contents in pyrites.
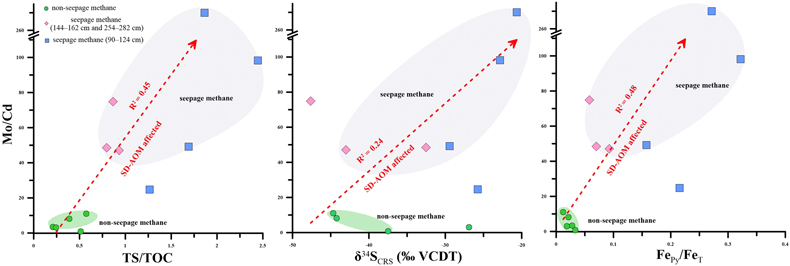
Figure 3 Scatter diagram of Mo/Cd vs. TS/TOC, δ34SCRS and FePy/FeT (based on Miao et al., 2021a
Miao, X., Feng, X., Li, J., Lin, L. (2021a) Tracing the paleo-methane seepage activity over the past 20,000 years in the sediments of Qiongdongnan Basin, northwestern South China Sea. Chemical Geology 559, 119956. https://doi.org/10.1016/j.chemgeo.2020.119956
,bMiao, X., Feng, X., Liu, X., Li, J., Wei, J. (2021b) Effects of methane seepage activity on the morphology and geochemistry of authigenic pyrite. Marine and Petroleum Geology 133, 105231. https://doi.org/10.1016/j.marpetgeo.2021.105231
). TS/TOC = total sulfur/total organic carbon; CRS = chromium-reducible sulfur; δ34SCRS = sulfur isotope of chromium-reducible sulfur; Fepy = pyrite Fe; FeT = total Fe.