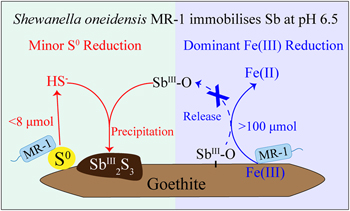
Iron(III) reducing bacteria immobilise antimonite by respiring elemental sulfur
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:106Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
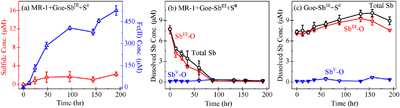 Figure 1 (a) Changes in dissolved sulfide (red) and Fe(II) (blue). (b) Total dissolved Sb (black), SbIII-O (red), and SbV-O (blue) during incubation in MR-1+Goe-SbIII+S0 at pH 6.5. (c) Changes in dissolved Sb species in abiotic control Goe-SbIII+S0. Fe(II) and sulfide were not detected in Goe-SbIII+S0. | 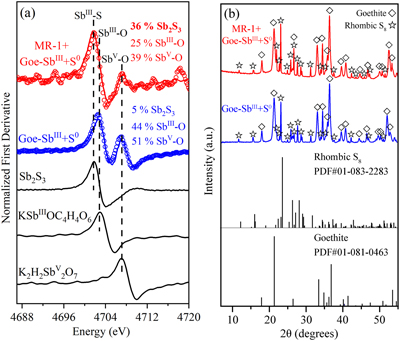 Figure 2 (a) Observed (circles) and linear combination fitting (lines) for the first derivative of normalised Sb LI-edge XANES for samples at the end of incubation. Spectra for standard references are also shown for comparison. The results of linear combination fitting analysis are shown in Table S-4. (b) Synchrotron X-ray powder diffraction pattern recorded from the solid in MR-1+Goe-SbIII+S0 and Goe-SbIII+S0 at the end of incubation. | 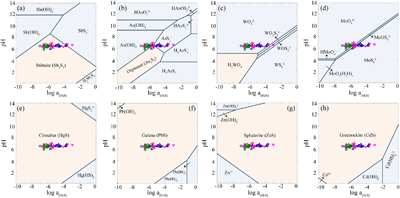 Figure 3 Species of (a) Sb, (b) As, (c) W, (d) Mo, (e) Hg, (f) Pb, (g) Zn and (h) Cd in the reaction with different log activity (H2S) and pH values in the presence of goethite at 25 °C. The activities of these metal(loid)s were set as 10 μM. The red, blue, green and magenta symbols represent the sulfide concentrations in mildly acidic paddy, wetland, groundwater and lake/river systems, respectively. The data are from references in Table S-7. |
| Figure 1 | Figure 2 | Figure 3 |
top
Introduction
Iron oxides are natural scavengers for toxic metal(loid)s and iron reducing bacteria (IRB) mobilise these metal(loid)s by facilitating the reductive dissolution of iron oxides (Kappler et al., 2021
Kappler, A., Bryce, C., Mansor, M., Lueder, U., Byrne, J.M., Swanner, E.D. (2021) An evolving view on biogeochemical cycling of iron. Nature Reviews Microbiology 19, 2679–2698. https://doi.org/10.1038/s41579-020-00502-7
). For example, elevated arsenic (As) (Kontny et al., 2021Kontny, A., Schneider, M., Eiche, E., Stopelli, E., Glodowska, M., Rathi, B., Göttlicher, J., Byrne, J.M., Kappler, A., Berg, M., Thi, D.V., Trang, P.T.K., Viet, P.H., Neumann, T. (2021) Iron mineral transformations and their impact on As (im)mobilization at redox interfaces in As-contaminated aquifers. Geochimica et Cosmochimica Acta 296, 189–209. https://doi.org/10.1016/j.gca.2020.12.029
), antimony (Sb) (Hockmann et al., 2014Hockmann, K., Lenz, M., Tandy, S., Nachtegaal, M., Janousch, M., Schulin, R. (2014) Release of antimony from contaminated soil induced by redox changes. Journal of Hazardous Materials 275, 215–221. https://doi.org/10.1016/j.jhazmat.2014.04.065
), mercury (Hg) (Wang et al., 2021Wang, J., Shaheen, S.M., Jing, M., Anderson, C.W.N., Swertz, A.-C., Wang, S.-L., Feng, X., Rinklebe, J. (2021) Mobilization, methylation, and demethylation of mercury in a paddy soil under systematic redox changes. Environmental Science & Technology 55, 10133–10141. https://doi.org/10.1021/acs.est.0c07321
) and cadmium (Cd) (Zhou et al., 2020Zhou, Z., Muehe, E.M., Tomaszewski, E., Lezama Pacheco, J., Kappler, A., Byrne, J.M. (2020) Effect of natural organic matter on the fate of cadmium during microbial ferrihydrite reduction. Environmental Science & Technology 54, 9445–9453. https://doi.org/10.1021/acs.est.0c03062
) in waters usually associate with increasing Fe(II) concentrations in the presence of IRB. In fact, many IRB, such as Shewanella and Geobacter spp., have an underappreciated ability: elemental sulfur (S0) respiration (Flynn et al., 2014Flynn, T.M., O’Loughlin, E.J., Mishra, B., DiChristina, T.J., Kemner, K.M. (2014) Sulfur-mediated electron shuttling during bacterial iron reduction. Science 344, 1039–1042. https://doi.org/10.1126/science.1252066
). Accordingly, the biogenic sulfide (HS–) may either immobilise metal(loid)s via sulfide precipitates or mobilise them by forming soluble thio-species (Planer-Friedrich et al., 2020Planer-Friedrich, B., Forberg, J., Lohmayer, R., Kerl, C.F., Boeing, F., Kaasalainen, H., Stefánsson, A. (2020) Relative abundance of thiolated species of As, Mo, W, and Sb in hot springs of Yellowstone National Park and Iceland. Environmental Science & Technology 54, 4295–4304. https://doi.org/10.1021/acs.est.0c00668
; Ye et al., 2020Ye, L., Meng, X., Jing, C. (2020) Influence of sulfur on the mobility of arsenic and antimony during oxic-anoxic cycles: Differences and competition. Geochimica et Cosmochimica Acta 288, 51–67. https://doi.org/10.1016/j.gca.2020.08.007
; Helz, 2021Helz, G.R. (2021) Dissolved molybdenum asymptotes in sulfidic waters. Geochemical Perspectives Letters 19, 23–26. https://doi.org/10.7185/geochemlet.2129
). Previous studies suggested that S0 is widely distributed in sediments and waters, and its concentration can be as high as 60 mM as summarised in Table S-1. S0 usually co-exists with Fe(III) oxides in the environment where FeS oxidation occurs (Burton et al., 2009Burton, E.D., Bush, R.T., Sullivan, L.A., Hocking, R.K., Mitchell, D.R.G., Johnston, S.G., Fitzpatrick, R.W., Raven, M., McClure, S., Jang, L.Y. (2009) Iron-monosulfide oxidation in natural sediments: Resolving microbially mediated S transformations using XANES, electron microscopy, and selective extractions. Environmental Science & Technology 43, 3128–3134. https://doi.org/10.1021/es8036548
) or where microbial sulfate reduction occurs in the presence of Fe(III) oxides (Burton et al., 2011Burton, E.D., Bush, R.T., Johnston, S.G., Sullivan, L.A., Keene, A.F. (2011) Sulfur biogeochemical cycling and novel Fe-S mineralization pathways in a tidally re-flooded wetland. Geochimica et Cosmochimica Acta 75, 3434–3451. https://doi.org/10.1016/j.gca.2011.03.020
). Thus, rather than Fe(III) reduction alone, IRB influence the mobility of metal(loid)s via coupled Fe(III) and S0 reduction.The extents of Fe(III) and S0 reduction reveal their contribution in regulating the fate of metal(loid)s. Our recent work found that IRB prefer to reduce S0 under mildly alkaline conditions, and the biogenic sulfide greatly enhanced Sb release by the formation of thioantimonates (Ye et al., 2022
Ye, L., Zhong, W., Zhang, M., Jing, C. (2022) New mobilization pathway of antimonite: thiolation and oxidation by dissimilatory metal-reducing bacteria via elemental sulfur respiration. Environmental Science & Technology 56, 652–659. https://doi.org/10.1021/acs.est.1c05206
). In contrast, in the acidic environments, IRB prefer to respire Fe(III) rather than S0 to conserve more energy (Flynn et al., 2014Flynn, T.M., O’Loughlin, E.J., Mishra, B., DiChristina, T.J., Kemner, K.M. (2014) Sulfur-mediated electron shuttling during bacterial iron reduction. Science 344, 1039–1042. https://doi.org/10.1126/science.1252066
). The effect of S0 respiration by IRB is previously neglected and probably masked by appreciable Fe(III) reduction on the fate of metal(loid)s. Thus, prevailing Fe(III) reduction (Cummings et al., 2000Cummings, D.E., March, A.W., Bostick, B., Spring, S., Caccavo, F., Fendorf, S., Rosenzweig, R.F. (2000) Evidence for microbial Fe(III) reduction in anoxic, mining-impacted lake sediments (Lake Coeur d’Alene, Idaho). Applied and Environmental Microbiology 66, 154–162. https://doi.org/10.1128/AEM.66.1.154-162.2000
) is commonly considered to drive the mobility of metal(loid)s in mildly acidic sediments (Hockmann et al., 2015Hockmann, K., Tandy, S., Lenz, M., Reiser, R., Conesa, H.M., Keller, M., Studer, B., Schulin, R. (2015) Antimony retention and release from drained and waterlogged shooting range soil under field conditions. Chemosphere 134, 536–543. https://doi.org/10.1016/j.chemosphere.2014.12.020
), such as mining areas and acidic wetlands (Karimian et al., 2018Karimian, N., Johnston, S.G., Burton, E.D. (2018) Iron and sulfur cycling in acid sulfate soil wetlands under dynamic redox conditions: A review. Chemosphere 197, 803–816. https://doi.org/10.1016/j.chemosphere.2018.01.096
).Contrary to the previous preconception, our study revealed that S0 respiration by IRB even to a marginal extent can greatly shape the fate of metal(loid)s under mildly acidic conditions. In detail, antimony (Sb), an emerging contaminant, is chosen as an example of metal(loid)s. Antimonite-adsorbed goethite was incubated with Shewanella oneidensis MR-1, a typical Fe(III) reducing bacterium, in the presence of S0 at pH 6.5. Our incubation and characterisation results suggest that although Fe(III) was the dominant electron acceptor and Fe(III) reduction was over ten times greater than S0 reduction, Sb release was mainly inhibited by biogenic sulfide through the formation of Sb2S3 precipitates. The thermodynamic calculation further indicates that the strong effect of marginal S0 reduction can be extended from Sb to other metal(loid)s. This study breaks the stereotype that IRB influence the mobility of metal(loid)s via Fe(III) reduction alone, and highlights the importance of S0 respiration.
top
Materials and Methods
The experiments were performed at pH 6.5 in the dark, and the experimental setup and analytical techniques were similar to our previous study (Ye et al., 2022
Ye, L., Zhong, W., Zhang, M., Jing, C. (2022) New mobilization pathway of antimonite: thiolation and oxidation by dissimilatory metal-reducing bacteria via elemental sulfur respiration. Environmental Science & Technology 56, 652–659. https://doi.org/10.1021/acs.est.1c05206
). Dissolved Fe(II), sulfide, antimonite, antimonate and thioantimonates were analysed periodically during the incubation. To extend the effect of marginal S0 reduction from Sb to other metal(loid)s, reaction path models were established to calculate the species of Sb, arsenic (As), tungsten (W), molybdenum (Mo), mercury (Hg), lead (Pb), zinc (Zn), cadmium (Cd) and copper (Cu) as a function of pH and the activity of sulfide in the presence of goethite. More details of the methods are shown in the Supplementary Information (SI).top
Results and Discussion
Prevalent Fe(III) reduction versus minor S0 reduction. During the incubation with Shewanella oneidensis MR-1 at pH 6.5 (Fig. S-2), the electron donor was formate and the electron acceptor was either goethite or S0. To determine the dominant electron acceptor in our experiments, dissolved Fe(II) and sulfide (HS–) were measured (Fig. 1) and the electrons accepted by goethite or S0 were calculated (Table S-2). In the abiotic control without MR-1 (Goe-SbIII+S0), Fe(II) and sulfide were not detected (Fig. 1). In the presence of MR-1 (MR-1+Goe-SbIII+S0), dissolved Fe(II) concentrations reached up to 527 ± 27 μM whereas sulfide was below 3 μM during the 192 hr incubation (Fig. 1a). Such a low sulfide concentration (<3 μM) would have a negligible effect on goethite reduction (Poulton et al., 2004
Poulton, S.W., Krom, M.D., Raiswell, R. (2004) A revised scheme for the reactivity of iron (oxyhydr)oxide minerals towards dissolved sulfide. Geochimica et Cosmochimica Acta 68, 3703–3715. https://doi.org/10.1016/j.gca.2004.03.012
). At the end of incubation, 2.7 ± 0.5 μmol/g Fe(II) were adsorbed on solids and 26.6 ± 3.4 μmol/g sulfide were precipitated (Table S-2). Overall, goethite accepted 106.1 ± 5.3 μmol electrons from MR-1 while S0 accepted 14.4 ± 1.6 μmol. The order of magnitude difference in electrons suggested that goethite was the dominant electron acceptor at pH 6.5.
Figure 1 (a) Changes in dissolved sulfide (red) and Fe(II) (blue). (b) Total dissolved Sb (black), SbIII-O (red), and SbV-O (blue) during incubation in MR-1+Goe-SbIII+S0 at pH 6.5. (c) Changes in dissolved Sb species in abiotic control Goe-SbIII+S0. Fe(II) and sulfide were not detected in Goe-SbIII+S0.
Consistent with the prevailing Fe(III) reduction, the thermodynamic calculations show that the free energy gain (ΔGr) with goethite as the electron acceptor is higher than that with S0 (Fig. S-3), as detailed in SI. Previous studies also suggested that microbial Fe(III) reduction is the central pathway in acidic environments under anoxic conditions, and IRB are the dominant bacteria therein (Sun et al., 2015
Sun, W., Xiao, T., Sun, M., Dong, Y., Ning, Z., Xiao, E., Tang, S., Li, J. (2015) Diversity of the sediment microbial community in the Aha watershed (southwest China) in response to acid mine drainage pollution gradients. Applied and Environmental Microbiology 81, 4874–4884. https://doi.org/10.1128/AEM.00935-15
). Inconsistent with the redox ladder concept (Peiffer et al., 2021Peiffer, S., Kappler, A., Haderlein, S.B., Schmidt, C., Byrne, J.M., Kleindienst, S., Vogt, C., Richnow, H.H., Obst, M., Angenent, L.T., Bryce, C., McCammon, C., Planer-Friedrich, B. (2021) A biogeochemical–hydrological framework for the role of redox-active compounds in aquatic systems. Nature Geoscience 14, 264–272. https://doi.org/10.1038/s41561-021-00742-z
), minor S0 reduction still occurred although in a lower ΔGr than goethite. However, no research has paid attention to the marginal S0 reduction by IRB in acidic environments.Minor S0 reduction immobilises Sb. As a primary pathway, Fe(III) reduction has been reported to enhance Sb release due to a depletion of sorption sites (Hockmann et al., 2014
Hockmann, K., Lenz, M., Tandy, S., Nachtegaal, M., Janousch, M., Schulin, R. (2014) Release of antimony from contaminated soil induced by redox changes. Journal of Hazardous Materials 275, 215–221. https://doi.org/10.1016/j.jhazmat.2014.04.065
); however, the concentration of total dissolved Sb decreased from 9.07 ± 0.48 μM to 0.19 ± 0.07 μM in MR-1+Goe-SbIII+S0 (Fig. 1b). In the abiotic control (Goe-SbIII+S0; Fig. 1c), the Sb concentration slightly increased from 7.33 ± 0.27 μM to 8.95 ± 0.57 μM during the incubation. The dissolved Sb was mainly SbIII-O, and no thiolated Sb species was detected by IC-ICP-MS (Fig. 1b-c). The decrease in dissolved Sb in MR-1+Goe-SbIII+S0 was consistent with the loss of SbIII-O (Fig. 1b).The loss of dissolved SbIII-O in MR-1+Goe-SbIII+S0 was due to the formation of Sb2S3 as evidenced by Sb LI-edge XANES analysis (Figs. 2a, S-4 and Table S-4). In detail, the first derivative peak at 4703 eV in Goe-SbIII+S0 was attributed to SbIII-O, in accordance with the initial SbIII-O adsorbed on goethite (Fig. 2a). In the presence of MR-1, SbIII-O was transformed to Sb2S3 at 4702 eV (Fig. 2a). Linear combination fitting (LCF) of XANES spectra further confirmed that 36 ± 4 % Sb2S3 were formed in MR-1+Goe-SbIII+S0, whereas the proportion of Sb2S3 in Goe-SbIII+S0 (5 ± 2 %) was negligible (Table S-4). In addition, approximately 51 ± 1 % and 39 ± 2 % SbIII-O were oxidised to antimonate (SbV-O) in Goe-SbIII+S0 and MR-1+Goe-SbIII+S0, respectively (Fig. 2a and Table S-4). The abiotic oxidation was ascribed to the electron transfer between SbIII-O and goethite (Yin et al., 2021
Yin, X.L., Zhang, G.Q., Su, R., Zeng, X.F., Yan, Z.L., Zhang, D.N., Ma, X., Lei, L., Lin, J.R., Wang, S.F., Jia, Y.F. (2021) Oxidation and incorporation of adsorbed antimonite during iron(II)-catalyzed recrystallization of ferrihydrite. Science of The Total Environment 778, 146424. https://doi.org/10.1016/j.scitotenv.2021.146424
), as detailed in SI .
Figure 2 (a) Observed (circles) and linear combination fitting (lines) for the first derivative of normalised Sb LI-edge XANES for samples at the end of incubation. Spectra for standard references are also shown for comparison. The results of linear combination fitting analysis are shown in Table S-4. (b) Synchrotron X-ray powder diffraction pattern recorded from the solid in MR-1+Goe-SbIII+S0 and Goe-SbIII+S0 at the end of incubation.
The mass balance calculations show that 1.52 ± 0.62 μmol SbIII-O were precipitated in the presence of MR-1 at the end of incubation, but 4.30 ± 0.48 μmol Sb as Sb2S3 were detected by XANES analysis. The much higher amount of Sb as Sb2S3 than the loss of dissolved SbIII-O suggests that not only dissolved, but also adsorbed SbIII-O, reacted with biogenic sulfide to form Sb2S3 precipitates. Consistent with this observation, the precipitation of Sb2S3 may readily occur in mildly acidic environments, such as paddy soils, wetland, groundwater and lake/river systems where sulfide was commonly less than 10 μM (Figs. 3a, S-6a). Actually, if sulfide or pH slightly increased, instead of Sb2S3, soluble thioantimony (Sb-S) would be prevalent (Figs. 3a, S-6a), resulting in enhanced Sb release (Ye et al., 2022
Ye, L., Zhong, W., Zhang, M., Jing, C. (2022) New mobilization pathway of antimonite: thiolation and oxidation by dissimilatory metal-reducing bacteria via elemental sulfur respiration. Environmental Science & Technology 56, 652–659. https://doi.org/10.1021/acs.est.1c05206
).
Figure 3 Species of (a) Sb, (b) As, (c) W, (d) Mo, (e) Hg, (f) Pb, (g) Zn and (h) Cd in the reaction with different log activity (H2S) and pH values in the presence of goethite at 25 °C. The activities of these metal(loid)s were set as 10 μM. The red, blue, green and magenta symbols represent the sulfide concentrations in mildly acidic paddy, wetland, groundwater and lake/river systems, respectively. The data are from references in Table S-7.
Unlike our results, a previous study ascribed the immobilisation of Sb to incorporation of SbV-O into the newly formed secondary iron phases (i.e. feroxyhyte and goethite) (Burton et al., 2019
Burton, E.D., Hockmann, K., Karimian, N., Johnston, S.G. (2019) Antimony mobility in reducing environments: The effect of microbial iron(III)-reduction and associated secondary mineralization. Geochimica et Cosmochimica Acta 245, 278–289. https://doi.org/10.1016/j.gca.2018.11.005
). However, that pathway is not suitable in our system because the phase transformation of goethite was not significant. In detail, Fe XANES LCF analysis suggest that 99 ± 0 % of Fe phases remained as goethite and negligible iron sulfides (1 ± 1 % of total Fe) were formed at the end of incubation (Fig. S-5 and Table S-5). S K-edge XANES also confirmed the negligible formation of iron sulfides (4 ± 1 % of total S; Fig. S-5 and Table S-6). The newly formed iron sulfide may co-precipitate with or re-adsorb Sb, but its low content (1 ± 1 % of total Fe) limited its contribution to Sb mobility (detailed in SI). In addition, synchrotron X-ray diffraction (SXRD) did not resolve new Fe minerals other than goethite and rhombic S8 in both Goe-SbIII+S0 and MR-1+Goe-SbIII+S0 samples at the end of incubation (Fig. 2b).A recent study demonstrated that SbV-O can readily incorporate into the goethite structure during Fe(II) catalysed recrystallisation without phase transformation (Burton et al., 2020
Burton, E.D., Hockmann, K., Karimian, N. (2020) Antimony sorption to goethite: Effects of Fe(II)-catalyzed recrystallization. ACS Earth and Space Chemistry 4, 476–487. https://doi.org/10.1021/acsearthspacechem.0c00013
). This phenomenon, which involves relatively rapid interactions between goethite and Fe(II), may have contributed to the immobilisation of Sb under our experimental conditions. However, an assessment of the quantitative contribution of Fe(II)-induced goethite recrystallisation to Sb immobilisation in our experiment is beyond the scope of the present study. Despite this possible uncertainty, it is clear from our results that a substantial amount of Sb was certainly immobilised via Sb2S3 precipitation due to the IRB-driven S0 reduction.Strong impacts from weak S0 reduction on metal(loid)s mobility. Since environmental acidification is inevitable with increasing CO2 emission (Terhaar et al., 2020
Terhaar, J., Kwiatkowski, L., Bopp, L. (2020) Emergent constraint on Arctic Ocean acidification in the twenty-first century. Nature 582, 379–383. https://doi.org/10.1038/s41586-020-2360-3
), the effect of marginal S0 reduction under mildly acidic conditions should be paid more attention. This effect can be extended to other metal(loid)s including arsenic, tungsten, molybdenum, mercury, lead, zinc and cadmium. To justify the proposition, reaction path modelling was performed to predict the speciation change of these metal(loid)s at 10 μM (Fig. 3) and 1 μM (Fig. S-6) as a function of pH and the activity of sulfide. As shown in our thermodynamic calculations (Figs. 3b-d, S-6b-d), soluble thiolated species such as thioarsenic (As-S), thiotungsten (W-S), and thiomolybdenum (Mo-S) would occur in multiple low sulfide (<0.1 mM) environments. Consistent with our results, As-S has been detected in paddy soil porewaters when sulfide <10 μM at pH 6.5, and the As-S formation was promoted by S0 (Wang et al., 2020Wang, J.J., Kerl, C.F., Hu, P.J., Martin, M., Mu, T.T., Bruggenwirth, L., Wu, G.M., Said-Pullicino, D., Romani, M., Wu, L.H., Planer-Friedrich, B. (2020) Thiolated arsenic species observed in rice paddy pore waters. Nature Geoscience 13, 282–287. https://doi.org/10.1038/s41561-020-0533-1
). In general, the formation of As-S and W-S may enhance their mobility (Mohajerin et al., 2014Mohajerin, T.J., Helz, G.R., White, C.D., Johannesson, K.H. (2014) Tungsten speciation in sulfidic waters: Determination of thiotungstate formation constants and modeling their distribution in natural waters. Geochimica et Cosmochimica Acta 144, 157–172. https://doi.org/10.1016/j.gca.2014.08.037
; Ye et al., 2020Ye, L., Meng, X., Jing, C. (2020) Influence of sulfur on the mobility of arsenic and antimony during oxic-anoxic cycles: Differences and competition. Geochimica et Cosmochimica Acta 288, 51–67. https://doi.org/10.1016/j.gca.2020.08.007
), whereas Mo-S is less mobile than Mo-O due to its higher affinity for sulfide minerals and natural organic matter (Smedley and Kinniburgh, 2017Smedley, P.L., Kinniburgh, D.G. (2017) Molybdenum in natural waters: A review of occurrence, distributions and controls. Applied Geochemistry 84, 387–432. https://doi.org/10.1016/j.apgeochem.2017.05.008
).On the other hand, S0 reduction leads to precipitation of sulfide minerals, such as cinnabar (HgS), galena (PbS), sphalerite (ZnS), and greenockite (CdS) (Figs. 3e-h, S-6e-h). These minerals immobilise metal(loid)s due to minor S0 reduction (∼10 μM), resulting in decoupling of Fe(II) and metal(loid)s release to the aqueous phase during the microbial Fe(III) reduction. However, most previous studies attributed the decoupling of Fe(II) and metal(loid)s release to re-adsorption on, or incorporation into, the newly formed iron minerals (Hockmann et al., 2020
Hockmann, K., Planer-Friedrich, B., Johnston, S.G., Peiffer, S., Burton, E.D. (2020) Antimony mobility in sulfidic systems: Coupling with sulfide-induced iron oxide transformations. Geochimica et Cosmochimica Acta 282, 276–296. https://doi.org/10.1016/j.gca.2020.05.024
). This study provides a new perspective that IRB shape the mobility and transformation of metal(loid)s via S0 respiration. Even under mildly acidic conditions where Fe(III) reduction is prevailing, the underappreciated and marginal S0 reduction plays a vital role in the mobility of metal(loid)s.top
Author Contributions
LY conceived the study, performed the experiments, interpreted the data and wrote the first draft; CJ contributed to the work design, data interpretation and manuscript revision.
top
Competing interests:
The authors declare no competing financial interest.
top
Acknowledgements
We acknowledge the financial support of the National Natural Science Foundation of China (41877378 and 41425016), the China Postdoctoral Science Foundation (2021M691924), and the Natural Science Foundation of Shandong Province, China (ZR2020ZD34, ZR2021QD054).
Editor: Andreas Kappler
top
References
Burton, E.D., Bush, R.T., Sullivan, L.A., Hocking, R.K., Mitchell, D.R.G., Johnston, S.G., Fitzpatrick, R.W., Raven, M., McClure, S., Jang, L.Y. (2009) Iron-monosulfide oxidation in natural sediments: Resolving microbially mediated S transformations using XANES, electron microscopy, and selective extractions. Environmental Science & Technology 43, 3128–3134. https://doi.org/10.1021/es8036548
 Show in context
Show in context Previous studies suggested that S0 is widely distributed in sediments and waters, and its concentration can be as high as 60 mM as summarised in Table S-1. S0 usually co-exists with Fe(III) oxides in the environment where FeS oxidation occurs (Burton et al., 2009) or where microbial sulfate reduction occurs in the presence of Fe(III) oxides (Burton et al., 2011).
View in article
Burton, E.D., Bush, R.T., Johnston, S.G., Sullivan, L.A., Keene, A.F. (2011) Sulfur biogeochemical cycling and novel Fe-S mineralization pathways in a tidally re-flooded wetland. Geochimica et Cosmochimica Acta 75, 3434–3451. https://doi.org/10.1016/j.gca.2011.03.020
 Show in context
Show in context Previous studies suggested that S0 is widely distributed in sediments and waters, and its concentration can be as high as 60 mM as summarised in Table S-1. S0 usually co-exists with Fe(III) oxides in the environment where FeS oxidation occurs (Burton et al., 2009) or where microbial sulfate reduction occurs in the presence of Fe(III) oxides (Burton et al., 2011).
View in article
Burton, E.D., Hockmann, K., Karimian, N., Johnston, S.G. (2019) Antimony mobility in reducing environments: The effect of microbial iron(III)-reduction and associated secondary mineralization. Geochimica et Cosmochimica Acta 245, 278–289. https://doi.org/10.1016/j.gca.2018.11.005
 Show in context
Show in context Unlike our results, a previous study ascribed the immobilisation of Sb to incorporation of SbV-O into the newly formed secondary iron phases (i.e. feroxyhyte and goethite) (Burton et al., 2019).
View in article
Burton, E.D., Hockmann, K., Karimian, N. (2020) Antimony sorption to goethite: Effects of Fe(II)-catalyzed recrystallization. ACS Earth and Space Chemistry 4, 476–487. https://doi.org/10.1021/acsearthspacechem.0c00013
 Show in context
Show in context A recent study demonstrated that SbV-O can readily incorporate into the goethite structure during Fe(II) catalysed recrystallisation without phase transformation (Burton et al., 2020).
View in article
Cummings, D.E., March, A.W., Bostick, B., Spring, S., Caccavo, F., Fendorf, S., Rosenzweig, R.F. (2000) Evidence for microbial Fe(III) reduction in anoxic, mining-impacted lake sediments (Lake Coeur d’Alene, Idaho). Applied and Environmental Microbiology 66, 154–162. https://doi.org/10.1128/AEM.66.1.154-162.2000
 Show in context
Show in context Thus, prevailing Fe(III) reduction (Cummings et al., 2000) is commonly considered to drive the mobility of metal(loid)s in mildly acidic sediments (Hockmann et al., 2015), such as mining areas and acidic wetlands (Karimian et al., 2018).
View in article
Flynn, T.M., O’Loughlin, E.J., Mishra, B., DiChristina, T.J., Kemner, K.M. (2014) Sulfur-mediated electron shuttling during bacterial iron reduction. Science 344, 1039–1042. https://doi.org/10.1126/science.1252066
 Show in context
Show in context In fact, many IRB, such as Shewanella and Geobacter spp., have an underappreciated ability: elemental sulfur (S0) respiration (Flynn et al., 2014).
View in article
In contrast, in the acidic environments, IRB prefer to respire Fe(III) rather than S0 to conserve more energy (Flynn et al., 2014).
View in article
Helz, G.R. (2021) Dissolved molybdenum asymptotes in sulfidic waters. Geochemical Perspectives Letters 19, 23–26. https://doi.org/10.7185/geochemlet.2129
 Show in context
Show in context Accordingly, the biogenic sulfide (HS–) may either immobilise metal(loid)s via sulfide precipitates or mobilise them by forming soluble thio-species (Planer-Friedrich et al., 2020; Ye et al., 2020; Helz, 2021).
View in article
Hockmann, K., Lenz, M., Tandy, S., Nachtegaal, M., Janousch, M., Schulin, R. (2014) Release of antimony from contaminated soil induced by redox changes. Journal of Hazardous Materials 275, 215–221. https://doi.org/10.1016/j.jhazmat.2014.04.065
 Show in context
Show in context As a primary pathway, Fe(III) reduction has been reported to enhance Sb release due to a depletion of sorption sites (Hockmann et al., 2014); however, the concentration of total dissolved Sb decreased from 9.07 ± 0.48 μM to 0.19 ± 0.07 μM in MR-1+Goe-SbIII+S0 (Fig. 1b). In the abiotic control (Goe-SbIII+S0; Fig. 1c), the Sb concentration slightly increased from 7.33 ± 0.27 μM to 8.95 ± 0.57 μM during the incubation. The dissolved Sb was mainly SbIII-O, and no thiolated Sb species was detected by IC-ICP-MS (Fig. 1b-c).
View in article
For example, elevated arsenic (As) (Kontny et al., 2021), antimony (Sb) (Hockmann et al., 2014), mercury (Hg) (Wang et al., 2021) and cadmium (Cd) (Zhou et al., 2020) in waters usually associate with increasing Fe(II) concentrations in the presence of IRB.
View in article
Hockmann, K., Tandy, S., Lenz, M., Reiser, R., Conesa, H.M., Keller, M., Studer, B., Schulin, R. (2015) Antimony retention and release from drained and waterlogged shooting range soil under field conditions. Chemosphere 134, 536–543. https://doi.org/10.1016/j.chemosphere.2014.12.020
 Show in context
Show in context Thus, prevailing Fe(III) reduction (Cummings et al., 2000) is commonly considered to drive the mobility of metal(loid)s in mildly acidic sediments (Hockmann et al., 2015), such as mining areas and acidic wetlands (Karimian et al., 2018).
View in article
Hockmann, K., Planer-Friedrich, B., Johnston, S.G., Peiffer, S., Burton, E.D. (2020) Antimony mobility in sulfidic systems: Coupling with sulfide-induced iron oxide transformations. Geochimica et Cosmochimica Acta 282, 276–296. https://doi.org/10.1016/j.gca.2020.05.024
 Show in context
Show in context However, most previous studies attributed the decoupling of Fe(II) and metal(loid)s release to re-adsorption on, or incorporation into, the newly formed iron minerals (Hockmann et al., 2020).
View in article
Kappler, A., Bryce, C., Mansor, M., Lueder, U., Byrne, J.M., Swanner, E.D. (2021) An evolving view on biogeochemical cycling of iron. Nature Reviews Microbiology 19, 2679–2698. https://doi.org/10.1038/s41579-020-00502-7
 Show in context
Show in context Iron oxides are natural scavengers for toxic metal(loid)s and iron reducing bacteria (IRB) mobilise these metal(loid)s by facilitating the reductive dissolution of iron oxides (Kappler et al., 2021).
View in article
Karimian, N., Johnston, S.G., Burton, E.D. (2018) Iron and sulfur cycling in acid sulfate soil wetlands under dynamic redox conditions: A review. Chemosphere 197, 803–816. https://doi.org/10.1016/j.chemosphere.2018.01.096
 Show in context
Show in context Thus, prevailing Fe(III) reduction (Cummings et al., 2000) is commonly considered to drive the mobility of metal(loid)s in mildly acidic sediments (Hockmann et al., 2015), such as mining areas and acidic wetlands (Karimian et al., 2018).
View in article
Kontny, A., Schneider, M., Eiche, E., Stopelli, E., Glodowska, M., Rathi, B., Göttlicher, J., Byrne, J.M., Kappler, A., Berg, M., Thi, D.V., Trang, P.T.K., Viet, P.H., Neumann, T. (2021) Iron mineral transformations and their impact on As (im)mobilization at redox interfaces in As-contaminated aquifers. Geochimica et Cosmochimica Acta 296, 189–209. https://doi.org/10.1016/j.gca.2020.12.029
 Show in context
Show in context For example, elevated arsenic (As) (Kontny et al., 2021), antimony (Sb) (Hockmann et al., 2014), mercury (Hg) (Wang et al., 2021) and cadmium (Cd) (Zhou et al., 2020) in waters usually associate with increasing Fe(II) concentrations in the presence of IRB.
View in article
Mohajerin, T.J., Helz, G.R., White, C.D., Johannesson, K.H. (2014) Tungsten speciation in sulfidic waters: Determination of thiotungstate formation constants and modeling their distribution in natural waters. Geochimica et Cosmochimica Acta 144, 157–172. https://doi.org/10.1016/j.gca.2014.08.037
 Show in context
Show in context In general, the formation of As-S and W-S may enhance their mobility (Mohajerin et al., 2014; Ye et al., 2020), whereas Mo-S is less mobile than Mo-O due to its higher affinity for sulfide minerals and natural organic matter (Smedley and Kinniburgh, 2017).
View in article
Peiffer, S., Kappler, A., Haderlein, S.B., Schmidt, C., Byrne, J.M., Kleindienst, S., Vogt, C., Richnow, H.H., Obst, M., Angenent, L.T., Bryce, C., McCammon, C., Planer-Friedrich, B. (2021) A biogeochemical–hydrological framework for the role of redox-active compounds in aquatic systems. Nature Geoscience 14, 264–272. https://doi.org/10.1038/s41561-021-00742-z
 Show in context
Show in context Previous studies also suggested that microbial Fe(III) reduction is the central pathway in acidic environments under anoxic conditions, and IRB are the dominant bacteria therein (Sun et al., 2015). Inconsistent with the redox ladder concept (Peiffer et al., 2021), minor S0 reduction still occurred although in a lower ΔGr than goethite.
View in article
Planer-Friedrich, B., Forberg, J., Lohmayer, R., Kerl, C.F., Boeing, F., Kaasalainen, H., Stefánsson, A. (2020) Relative abundance of thiolated species of As, Mo, W, and Sb in hot springs of Yellowstone National Park and Iceland. Environmental Science & Technology 54, 4295–4304. https://doi.org/10.1021/acs.est.0c00668
 Show in context
Show in context Accordingly, the biogenic sulfide (HS–) may either immobilise metal(loid)s via sulfide precipitates or mobilise them by forming soluble thio-species (Planer-Friedrich et al., 2020; Ye et al., 2020; Helz, 2021).
View in article
Poulton, S.W., Krom, M.D., Raiswell, R. (2004) A revised scheme for the reactivity of iron (oxyhydr)oxide minerals towards dissolved sulfide. Geochimica et Cosmochimica Acta 68, 3703–3715. https://doi.org/10.1016/j.gca.2004.03.012
 Show in context
Show in context Such a low sulfide concentration (<3 μM) would have a negligible effect on goethite reduction (Poulton et al., 2004).
View in article
Smedley, P.L., Kinniburgh, D.G. (2017) Molybdenum in natural waters: A review of occurrence, distributions and controls. Applied Geochemistry 84, 387–432. https://doi.org/10.1016/j.apgeochem.2017.05.008
 Show in context
Show in context In general, the formation of As-S and W-S may enhance their mobility (Mohajerin et al., 2014; Ye et al., 2020), whereas Mo-S is less mobile than Mo-O due to its higher affinity for sulfide minerals and natural organic matter (Smedley and Kinniburgh, 2017).
View in article
Sun, W., Xiao, T., Sun, M., Dong, Y., Ning, Z., Xiao, E., Tang, S., Li, J. (2015) Diversity of the sediment microbial community in the Aha watershed (southwest China) in response to acid mine drainage pollution gradients. Applied and Environmental Microbiology 81, 4874–4884. https://doi.org/10.1128/AEM.00935-15
 Show in context
Show in context Previous studies also suggested that microbial Fe(III) reduction is the central pathway in acidic environments under anoxic conditions, and IRB are the dominant bacteria therein (Sun et al., 2015). Inconsistent with the redox ladder concept (Peiffer et al., 2021), minor S0 reduction still occurred although in a lower ΔGr than goethite.
View in article
Terhaar, J., Kwiatkowski, L., Bopp, L. (2020) Emergent constraint on Arctic Ocean acidification in the twenty-first century. Nature 582, 379–383. https://doi.org/10.1038/s41586-020-2360-3
 Show in context
Show in context Since environmental acidification is inevitable with increasing CO2 emission (Terhaar et al., 2020), the effect of marginal S0 reduction under mildly acidic conditions should be paid more attention.
View in article
Wang, J., Shaheen, S.M., Jing, M., Anderson, C.W.N., Swertz, A.-C., Wang, S.-L., Feng, X., Rinklebe, J. (2021) Mobilization, methylation, and demethylation of mercury in a paddy soil under systematic redox changes. Environmental Science & Technology 55, 10133–10141. https://doi.org/10.1021/acs.est.0c07321
 Show in context
Show in context For example, elevated arsenic (As) (Kontny et al., 2021), antimony (Sb) (Hockmann et al., 2014), mercury (Hg) (Wang et al., 2021) and cadmium (Cd) (Zhou et al., 2020) in waters usually associate with increasing Fe(II) concentrations in the presence of IRB.
View in article
Wang, J.J., Kerl, C.F., Hu, P.J., Martin, M., Mu, T.T., Bruggenwirth, L., Wu, G.M., Said-Pullicino, D., Romani, M., Wu, L.H., Planer-Friedrich, B. (2020) Thiolated arsenic species observed in rice paddy pore waters. Nature Geoscience 13, 282–287. https://doi.org/10.1038/s41561-020-0533-1
 Show in context
Show in context As shown in our thermodynamic calculations (Figs. 3b-d, S-6b-d), soluble thiolated species such as thioarsenic (As-S), thiotungsten (W-S), and thiomolybdenum (Mo-S) would occur in multiple low sulfide (<0.1 mM) environments. Consistent with our results, As-S has been detected in paddy soil porewaters when sulfide <10 μM at pH 6.5, and the As-S formation was promoted by S0 (Wang et al., 2020).
View in article
Ye, L., Meng, X., Jing, C. (2020) Influence of sulfur on the mobility of arsenic and antimony during oxic-anoxic cycles: Differences and competition. Geochimica et Cosmochimica Acta 288, 51–67. https://doi.org/10.1016/j.gca.2020.08.007
 Show in context
Show in context Accordingly, the biogenic sulfide (HS–) may either immobilise metal(loid)s via sulfide precipitates or mobilise them by forming soluble thio-species (Planer-Friedrich et al., 2020; Ye et al., 2020; Helz, 2021).
View in article
In general, the formation of As-S and W-S may enhance their mobility (Mohajerin et al., 2014; Ye et al., 2020), whereas Mo-S is less mobile than Mo-O due to its higher affinity for sulfide minerals and natural organic matter (Smedley and Kinniburgh, 2017).
View in article
Ye, L., Zhong, W., Zhang, M., Jing, C. (2022) New mobilization pathway of antimonite: thiolation and oxidation by dissimilatory metal-reducing bacteria via elemental sulfur respiration. Environmental Science & Technology 56, 652–659. https://doi.org/10.1021/acs.est.1c05206
 Show in context
Show in context Our recent work found that IRB prefer to reduce S0 under mildly alkaline conditions, and the biogenic sulfide greatly enhanced Sb release by the formation of thioantimonates (Ye et al., 2022).
View in article
The experiments were performed at pH 6.5 in the dark, and the experimental setup and analytical techniques were similar to our previous study (Ye et al., 2022).
View in article
Actually, if sulfide or pH slightly increased, instead of Sb2S3, soluble thioantimony (Sb-S) would be prevalent (Figs. 3a, S-6a), resulting in enhanced Sb release (Ye et al., 2022).
View in article
Yin, X.L., Zhang, G.Q., Su, R., Zeng, X.F., Yan, Z.L., Zhang, D.N., Ma, X., Lei, L., Lin, J.R., Wang, S.F., Jia, Y.F. (2021) Oxidation and incorporation of adsorbed antimonite during iron(II)-catalyzed recrystallization of ferrihydrite. Science of The Total Environment 778, 146424. https://doi.org/10.1016/j.scitotenv.2021.146424
 Show in context
Show in context The abiotic oxidation was ascribed to the electron transfer between SbIII-O and goethite (Yin et al., 2021), as detailed in SI.
View in article
Zhou, Z., Muehe, E.M., Tomaszewski, E., Lezama Pacheco, J., Kappler, A., Byrne, J.M. (2020) Effect of natural organic matter on the fate of cadmium during microbial ferrihydrite reduction. Environmental Science & Technology 54, 9445–9453. https://doi.org/10.1021/acs.est.0c03062
 Show in context
Show in context For example, elevated arsenic (As) (Kontny et al., 2021), antimony (Sb) (Hockmann et al., 2014), mercury (Hg) (Wang et al., 2021) and cadmium (Cd) (Zhou et al., 2020) in waters usually associate with increasing Fe(II) concentrations in the presence of IRB.
View in article
top
Supplementary Information
The Supplementary Information includes:
Download the Supplementary Information (PDF).
Figures
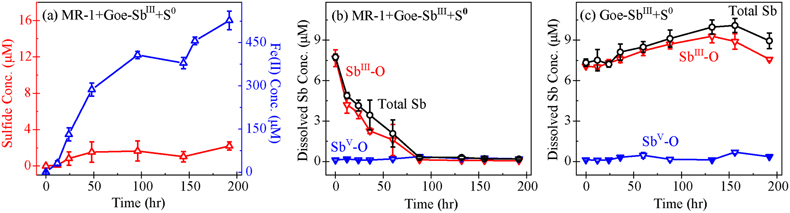
Figure 1 (a) Changes in dissolved sulfide (red) and Fe(II) (blue). (b) Total dissolved Sb (black), SbIII-O (red), and SbV-O (blue) during incubation in MR-1+Goe-SbIII+S0 at pH 6.5. (c) Changes in dissolved Sb species in abiotic control Goe-SbIII+S0. Fe(II) and sulfide were not detected in Goe-SbIII+S0.
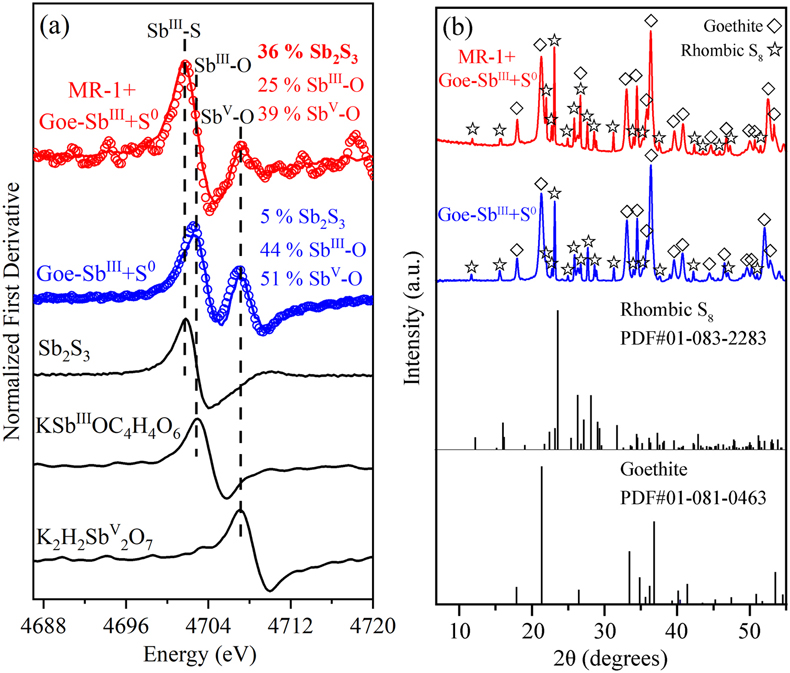
Figure 2 (a) Observed (circles) and linear combination fitting (lines) for the first derivative of normalised Sb LI-edge XANES for samples at the end of incubation. Spectra for standard references are also shown for comparison. The results of linear combination fitting analysis are shown in Table S-4. (b) Synchrotron X-ray powder diffraction pattern recorded from the solid in MR-1+Goe-SbIII+S0 and Goe-SbIII+S0 at the end of incubation.
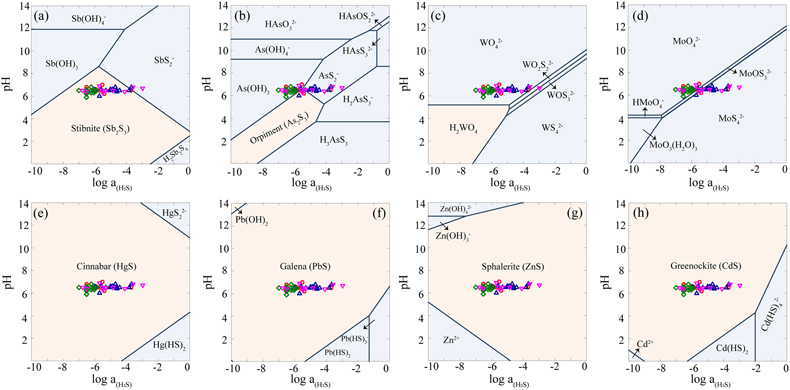
Figure 3 Species of (a) Sb, (b) As, (c) W, (d) Mo, (e) Hg, (f) Pb, (g) Zn and (h) Cd in the reaction with different log activity (H2S) and pH values in the presence of goethite at 25 °C. The activities of these metal(loid)s were set as 10 μM. The red, blue, green and magenta symbols represent the sulfide concentrations in mildly acidic paddy, wetland, groundwater and lake/river systems, respectively. The data are from references in Table S-7.