Martian core composition from experimental high-pressure metal-silicate phase equilibria
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:195Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
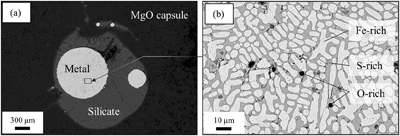 Figure 1 (a) BSE (backscattered electron) image of one of the recovered samples (ELMO 445, P = 2 GPa and T = 1873 K). The contrast is linked to changes in atomic number, with light areas corresponding to heavy (high-Z) phases (e.g., iron) and dark phases to lighter (low-Z) material (e.g., silicate). (b) BSE image of the metallic phase. The dendritic pattern results from the exsolution of two different metallic melts as the system cools during quench (the light-coloured phase is an iron-rich melt, and the darker phase is a sulfur-rich melt). The oxygen-rich blobs were lost while polishing and the observed dark spots correspond to their print in the structure. | 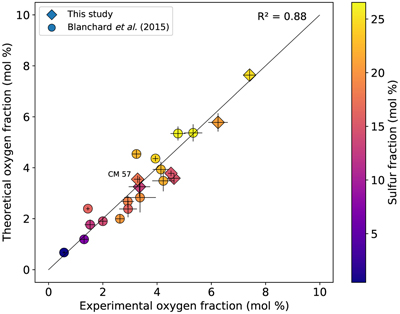 Figure 2 Predicted oxygen concentration (mol %) in the metallic phase versus measured oxygen concentration (mol %). Symbol colour is matched to the sulfur content (mol %) of each experiment, according to the colour bar to the right. The theoretical predictions (y-axis) fall close to the real values (x-axis) including CM 57 that equilibrated at a higher pressure (12 GPa). The vertical errors were set equal to the horizontal ones (1σ standard error) for simplification. | 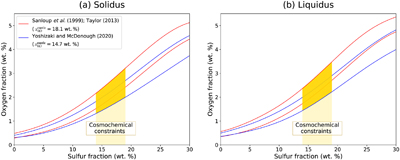 Figure 3 Predicted amount of oxygen in the Martian core for an FeO-rich (Sanloup et al., 1999; Taylor, 2013) (red curves) and an FeO-poor mantle (Yoshizaki and McDonough, 2020) (blue curves). The curves were obtained by fitting the output data (Table S-7) with either a 4th order (solidus) or a 6th order (liquidus) polynomial (See Supplementary Information). For each of these mantle compositions, the associated amount of oxygen in the core is bracketed between a minimum and a maximum defined by the two curves of the same colour. They reflect the range of possible magma ocean depths at which the core can form. Both (a) solidus and (b) liquidus geotherms were tested. Cosmochemical constraints predict <14−19 wt. % S in the Martian core (Steenstra and van Westrenen, 2018; Brennan et al., 2020) (yellow shaded area) that could lead up to 3.5 wt. % O in the core. |
| Figure 1 | Figure 2 | Figure 3 |
top
Introduction
Like the Earth, Mars’s core is essentially made of Fe + Ni alloyed with lighter elements (S, O, Si, C, H). Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998
Bertka, C.M., Fei, Y. (1998) Implications of Mars Pathfinder Data for the Accretion History of the Terrestrial Planets. Science 281, 1838–1840. https://doi.org/10.1126/science.281.5384.1838
; Yoshizaki and McDonough, 2020Yoshizaki, T., McDonough, W.F. (2020) The composition of Mars. Geochimica et Cosmochimica Acta 273, 137–162. https://doi.org/10.1016/j.gca.2020.01.011
), the composition of Martian meteorites (Dreibus and Wanke, 1985Dreibus, G., Wanke, H. (1985) Mars, a volatile-rich planet. Meteoritics 20, 367–381.
; McSween, 1994McSween Jr., H.Y. (1994) What we have learned about Mars from SNC meteorites. Meteoritics 29, 757–779. https://doi.org/10.1111/j.1945-5100.1994.tb01092.x
; Lodders and Fegley, 1997Lodders, K., Fegley Jr., B. (1997) An Oxygen Isotope Model for the Composition of Mars. Icarus 126, 373–394. https://doi.org/10.1006/icar.1996.5653
; Sanloup et al., 1999Sanloup, C., Jambon, A., Gillet, P. (1999) A simple chondritic model of Mars. Physics of the Earth and Planetary Interiors 112, 43–54. https://doi.org/10.1016/S0031-9201(98)00175-7
; Taylor, 2013Taylor, G.J. (2013) The bulk composition of Mars. Geochemistry 73, 401–420. https://doi.org/10.1016/j.chemer.2013.09.006
; Righter, 2017Righter, K. (2017) The Martian Meteorite Compendium. https://curator.jsc.nasa.gov/antmet/mmc/
), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011Rivoldini, A., Van Hoolst, T., Verhoeven, O., Mocquet, A., Dehant, V. (2011) Geodesy constraints on the interior structure and composition of Mars. Icarus 213, 451–472. https://doi.org/10.1016/j.icarus.2011.03.024
; Khan et al., 2018Khan, A., Liebske, C., Rozel, A., Rivoldini, A., Nimmo, F., Connolly, J.A.D., Plesa, A.-C., Giardini, D. (2018) A Geophysical Perspective on the Bulk Composition of Mars. Journal of Geophysical Research: Planets 123, 575–611. https://doi.org/10.1002/2017JE005371
; Stähler et al., 2021Stähler, S.C., Khan, A., Banerdt, W.B., Lognonné, P., Giardini, D., Ceylan, S., Drilleau, M., Duran, A.C., Garcia, R.F., Huang, Q., Kim, D., Lekic, V., Samuel, H., Schimmel, M., Schmerr, N., Sollberger, D., Stutzmann, E., Xu, Z., Antonangeli, D., Charalambous, C., Davis, P.M., Irving, J.C.E., Kawamura, T., Knapmeyer, M., Maguire, R., Marusiak, A.G., Panning, M.P., Perrin, C., Plesa, A.-C., Rivoldini, A., Schmelzbach, C., Zenhäusern, G., Beucler, E., Clinton, J., Dahmen, N., van Driel, M., Gudkova, T., Horleston, A., Pike, W.T., Plasman, M., Smrekar, S. (2021) Seismic detection of the martian core. Science 373, 443–448. https://doi.org/10.1126/science.abi7730
) and high-pressure, high-temperature experimental work (Fei et al., 1995Fei, Y., Prewitt, C.T., Mao, H.-K., Bertka, C.M. (1995) Structure and Density of FeS at High Pressure and High Temperature and the Internal Structure of Mars. Science 268, 1892–1894. https://doi.org/10.1126/science.268.5219.1892
; Morard et al., 2007Morard, G., Sanloup, C., Fiquet, G., Mezouar, M., Rey, N., Poloni, R., Beck, P. (2007) Structure of eutectic Fe–FeS melts to pressures up to 17 GPa: Implications for planetary cores. Earth and Planetary Science Letters 263, 128–139. https://doi.org/10.1016/j.epsl.2007.09.009
, 2018Morard, G., Bouchet, J., Rivoldini, A., Antonangeli, D., Roberge, M., Boulard, E., Denoeud, A., Mezouar, M. (2018) Liquid properties in the Fe-FeS system under moderate pressure: Tool box to model small planetary cores. American Mineralogist 103, 1770–1779. https://doi.org/10.2138/am-2018-6405
; Terasaki et al., 2019Terasaki, H., Rivoldini, A., Shimoyama, Y., Nishida, K., Urakawa, S., Maki, M., Kurokawa, F., Takubo, Y., Shibazaki, Y., Sakamaki, T., Machida, A., Higo, Y., Uesugi, K., Takeuchi, A., Watanuki, T., Kondo, T. (2019) Pressure and Composition Effects on Sound Velocity and Density of Core‐Forming Liquids: Implication to Core Compositions of Terrestrial Planets. Journal of Geophysical Research: Planets 124, 2272–2293. https://doi.org/10.1029/2019JE005936
) all argue for a sulfur-rich Martian core. This has led studies on Mars’s core to focus on sulfur as an alloying element, with estimates ranging from 3.5 wt. % (Morgan and Anders, 1979Morgan, J.W., Anders, E. (1979) Chemical composition of Mars. Geochimica et Cosmochimica Acta 43, 1601–1610. https://doi.org/10.1016/0016-7037(79)90180-7
) up to 36 wt. % S (Zharkov and Gudkova, 2005Zharkov, V.N., Gudkova, T.V. (2005) Construction of Martian Interior Model. Solar System Research 39, 343–373. https://doi.org/10.1007/s11208-005-0049-7
).According to recent results on the elastic properties of Fe–Ni–S liquids, 30 wt. % S is required to fit the core density estimate (6000 ± 300 kg/m3) if S is the only light element (Terasaki et al., 2019
Terasaki, H., Rivoldini, A., Shimoyama, Y., Nishida, K., Urakawa, S., Maki, M., Kurokawa, F., Takubo, Y., Shibazaki, Y., Sakamaki, T., Machida, A., Higo, Y., Uesugi, K., Takeuchi, A., Watanuki, T., Kondo, T. (2019) Pressure and Composition Effects on Sound Velocity and Density of Core‐Forming Liquids: Implication to Core Compositions of Terrestrial Planets. Journal of Geophysical Research: Planets 124, 2272–2293. https://doi.org/10.1029/2019JE005936
; Stähler et al., 2021Stähler, S.C., Khan, A., Banerdt, W.B., Lognonné, P., Giardini, D., Ceylan, S., Drilleau, M., Duran, A.C., Garcia, R.F., Huang, Q., Kim, D., Lekic, V., Samuel, H., Schimmel, M., Schmerr, N., Sollberger, D., Stutzmann, E., Xu, Z., Antonangeli, D., Charalambous, C., Davis, P.M., Irving, J.C.E., Kawamura, T., Knapmeyer, M., Maguire, R., Marusiak, A.G., Panning, M.P., Perrin, C., Plesa, A.-C., Rivoldini, A., Schmelzbach, C., Zenhäusern, G., Beucler, E., Clinton, J., Dahmen, N., van Driel, M., Gudkova, T., Horleston, A., Pike, W.T., Plasman, M., Smrekar, S. (2021) Seismic detection of the martian core. Science 373, 443–448. https://doi.org/10.1126/science.abi7730
). Paradoxically, such a high S-fraction exceeds the maximum sulfur concentration (∼18–19 wt. %) that satisfies cosmochemical observables (Steenstra and van Westrenen, 2018Steenstra, E.S., van Westrenen, W. (2018) A synthesis of geochemical constraints on the inventory of light elements in the core of Mars. Icarus 315, 69–78. https://doi.org/10.1016/j.icarus.2018.06.023
; Brennan et al., 2020Brennan, M.C., Fischer, R.A., Irving, J.C.E. (2020) Core formation and geophysical properties of Mars. Earth and Planetary Science Letters 530, 115923. https://doi.org/10.1016/j.epsl.2019.115923
). Alternatively, the dissolution of at least another light element can reduce the density to the current Martian estimate without impeding on those observables. For instance, Stähler et al. (2021)Stähler, S.C., Khan, A., Banerdt, W.B., Lognonné, P., Giardini, D., Ceylan, S., Drilleau, M., Duran, A.C., Garcia, R.F., Huang, Q., Kim, D., Lekic, V., Samuel, H., Schimmel, M., Schmerr, N., Sollberger, D., Stutzmann, E., Xu, Z., Antonangeli, D., Charalambous, C., Davis, P.M., Irving, J.C.E., Kawamura, T., Knapmeyer, M., Maguire, R., Marusiak, A.G., Panning, M.P., Perrin, C., Plesa, A.-C., Rivoldini, A., Schmelzbach, C., Zenhäusern, G., Beucler, E., Clinton, J., Dahmen, N., van Driel, M., Gudkova, T., Horleston, A., Pike, W.T., Plasman, M., Smrekar, S. (2021) Seismic detection of the martian core. Science 373, 443–448. https://doi.org/10.1126/science.abi7730
suggest 5 wt. % O in addition to 10 to 15 wt. % S to satisfy the density requirement, but they do not provide a mechanism to account for its dissolution in iron. Indeed, oxygen is known to be soluble in liquid iron only at high pressures and temperatures (Ohtani et al., 1984Ohtani, E., Ringwood, A.E., Hibberson, W. (1984) Composition of the core, II. Effect of high pressure on solubility of FeO in molten iron. Earth and Planetary Science Letters 71, 94–103. https://doi.org/10.1016/0012-821X(84)90055-4
; Ricolleau et al., 2011Ricolleau, A., Fei, Y., Corgne, A., Siebert, J., Badro, J. (2011) Oxygen and silicon contents of Earth’s core from high pressure metal–silicate partitioning experiments. Earth and Planetary Science Letters 310, 409–421. https://doi.org/10.1016/j.epsl.2011.08.004
; Siebert et al., 2013Siebert, J., Badro, J., Antonangeli, D., Ryerson, F.J. (2013) Terrestrial Accretion Under Oxidizing Conditions. Science 339, 1194–1197. https://doi.org/10.1126/science.1227923
; Badro et al., 2015Badro, J., Brodholt, J.P., Piet, H., Siebert, J., Ryerson, F.J. (2015) Core formation and core composition from coupled geochemical and geophysical constraints. Proceedings of the National Academy of Sciences 112, 12310–12314. https://doi.org/10.1073/pnas.1505672112
) which are not prevalent on smaller planets, such as Mars. Yet, another way to incorporate oxygen in the Martian core is through chemical interaction with sulfur in an Fe-liquid, that can drive its dissolution in the metal at lower pressures and temperatures (Tsuno et al., 2011Tsuno, K., Frost, D.J., Rubie, D.C. (2011) The effects of nickel and sulphur on the core–mantle partitioning of oxygen in Earth and Mars. Physics of the Earth and Planetary Interiors 185, 1–12. https://doi.org/10.1016/j.pepi.2010.11.009
).Here, we quantified the amount of oxygen that can be dissolved in the Martian core by taking into account oxygen-sulfur interaction. High-pressure, high-temperature metal-silicate equilibration experiments were carried out on Martian-like compositions and at Martian P and T conditions. The amount of S in the metal varied from 8 to 15 wt. %, and its effect on oxygen dissolution in the core was measured. Using thermodynamic modelling, we parametrised the oxygen-sulfur interaction from the experimental data and tested the robustness of the model to validate its predictive potential. We then applied our model to constrain the composition of the Martian core using multi-stage core formation modelling.
top
Methods
We conducted piston cylinder (PC) and multi-anvil press (MAP) experiments in MgO capsules between 2 and 12 GPa. The starting materials are made of an 18 wt. % FeO ultramafic silicate, representative of the Martian mantle, and an Fe–S metallic alloy, representative of the Martian core. Different sulfur fractions were tested, by varying the ratios of Fe and FeS in the metal, to cover a range of plausible sulfur concentrations going from 8 to 15 wt. %. The silicate and metal were then mixed, compressed, heated between 1673 and 2473 K, and equilibrated between 2 and 10 minutes. Textural observations and chemical analyses were conducted with a Zeiss Auriga 40 field emission scanning electron microscope equipped with a Bruker EDX spectrometer. Measurements were carried out using a focused beam raster and the resulting averages were obtained by statistical analyses of such scanned regions. We ensured that all the samples fully melted and reached equilibrium from their chemical homogeneity and texture that displays a large metallic blob in a silicate matrix (Fig. S-1). More details on the experimental procedure are provided in the supplementary material as well the detailed chemical composition of the starting materials (Tables S-1 and S-2) and recovered samples (Table S-3).
top
Results
As seen on the backscattered electron (BSE) image of a typical run (Fig. 1), the metallic phase systematically undergoes exsolution to iron-rich and sulfur-rich phases during quench, as previously reported (Blanchard et al., 2015
Blanchard, I., Badro, J., Siebert, J., Ryerson, F.J. (2015) Composition of the core from gallium metal–silicate partitioning experiments. Earth and Planetary Science Letters 427, 191–201. https://doi.org/10.1016/j.epsl.2015.06.063
). It also includes a small fraction of oxide phases that show up as dark blobs in the brighter metal (Fig. 1b). These blobs are almost always located in the sulfur-rich zones (darker grey areas in Fig. 1b) which already suggests an affinity between S and O. This is confirmed by chemical analysis of the metal as plotted in Figure S-2 alongside similar data from Blanchard et al. (2015)Blanchard, I., Badro, J., Siebert, J., Ryerson, F.J. (2015) Composition of the core from gallium metal–silicate partitioning experiments. Earth and Planetary Science Letters 427, 191–201. https://doi.org/10.1016/j.epsl.2015.06.063
. The composition of the whole metallic phase was averaged to yield that of the homogeneous high-temperature melt, and we observe a positive correlation between the sulfur and oxygen concentrations, despite the fact that experiments were not performed at the same T and P.
Figure 1 (a) BSE (backscattered electron) image of one of the recovered samples (ELMO 445, P = 2 GPa and T = 1873 K). The contrast is linked to changes in atomic number, with light areas corresponding to heavy (high-Z) phases (e.g., iron) and dark phases to lighter (low-Z) material (e.g., silicate). (b) BSE image of the metallic phase. The dendritic pattern results from the exsolution of two different metallic melts as the system cools during quench (the light-coloured phase is an iron-rich melt, and the darker phase is a sulfur-rich melt). The oxygen-rich blobs were lost while polishing and the observed dark spots correspond to their print in the structure.
Combining the data of Blanchard et al. (2015)
Blanchard, I., Badro, J., Siebert, J., Ryerson, F.J. (2015) Composition of the core from gallium metal–silicate partitioning experiments. Earth and Planetary Science Letters 427, 191–201. https://doi.org/10.1016/j.epsl.2015.06.063
with our dataset, we further parametrised the O-S interaction to evaluate the potential oxygen concentration in an S-rich Martian core. Starting from the dissolution reaction of oxygen into metal, FeOsilicate = Femetal + Ometal, we imposed the minimisation of the standard state Gibbs free energy of reaction ΔrG°P,T at thermodynamic equilibrium to obtain the expression that we fitted (the thermodynamic framework is detailed in the supplementary material):Eq. 1
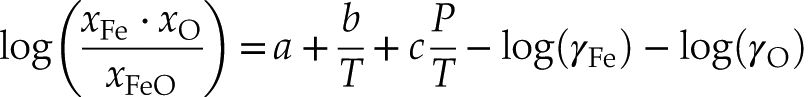
P, T and the xi (i = O, Fe, FeO) respectively refer to the pressure, temperature and molar concentrations of O and Fe in the metal and FeO in the silicate. log(γFe) and log(γO) are the activity coefficients of Fe and O in the metallic melt, given in the supplementary material (Eqs. S-13 and S-14). They depend on the molar concentrations of the solutes (S and O) in the metal and on the interaction parameters between them (εOS, εOO and εSS). P, T and the xi (here i refers to O, S, Fe, FeO) are all known and the O-O and S-S interaction parameters were taken from Badro et al. (2015)
Badro, J., Brodholt, J.P., Piet, H., Siebert, J., Ryerson, F.J. (2015) Core formation and core composition from coupled geochemical and geophysical constraints. Proceedings of the National Academy of Sciences 112, 12310–12314. https://doi.org/10.1073/pnas.1505672112
. The unknowns are a, b, c and εOS, the thermodynamic interaction parameter between O and S in iron metal. The εOS parameter describes how S affects the metal-silicate partitioning of O, a negative value meaning that the presence of S favours the presence of O in the metallic alloy, and therefore enhances its solubility, all other things being equal.Using Equation 1, we performed a multivariate linear regression on the experimental data in order to estimate the thermodynamic parameters controlling oxygen dissolution in metal, i.e. a, b, c and εOS. The first fit returned a c parameter that was statistically irrelevant (c = −12 ± 24 K GPa−1 with an associated p-value of 0.63), showing no influence of pressure on the oxygen dissolution over the pressure range being considered (from 2 to 12 GPa). This pressure term was removed for the second fit (R2 > 0.97) for which we obtained a = 1.9 ± 0.2, b = (−9.3 ± 0.3) × 103 K and a negative value of −6.2 ± 0.6 for εOS. The latter strongly deviates from the value of −17 found in the metallurgy literature (Steelmaking Data Sourcebook, 1988
Steelmaking Data Sourcebook (1988) The Japan Society for the Promotion of Science: The 19th Committee on Steelmaking. Gordon and Breach Science Publishers, New York.
) determined at room pressure and that cannot reproduce the data well as seen on Figure S-4 .The robustness and predictive potential of our thermodynamic model was further investigated by calculating the expected oxygen concentration for each experimental run (i.e. for a given xS, xFe, xFeO and T) and comparing it to the measured value. Removing the pressure term in Equation 1 and rearranging the expression gives:
Eq. 2
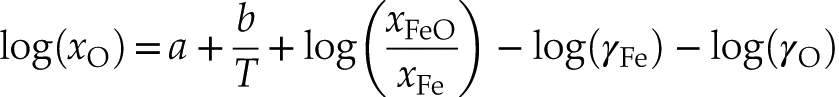
a, b and εOS were set equal to those from the second fit (reported in Table S-6) while T, xS, xFeO and xFe correspond to the experimental values. The results are plotted in Figure 2, and show an excellent agreement between our predictions and the data as the points do not scatter from the theoretical ideal 1:1 line (R2 = 0.88), putting the model on robust grounds.

Figure 2 Predicted oxygen concentration (mol %) in the metallic phase versus measured oxygen concentration (mol %). Symbol colour is matched to the sulfur content (mol %) of each experiment, according to the colour bar to the right. The theoretical predictions (y-axis) fall close to the real values (x-axis) including CM 57 that equilibrated at a higher pressure (12 GPa). The vertical errors were set equal to the horizontal ones (1σ standard error) for simplification.
top
Core Composition Modelling
We incorporated our parametrisation of the oxygen-sulfur interaction in a multi-stage core formation model (Badro et al., 2015
Badro, J., Brodholt, J.P., Piet, H., Siebert, J., Ryerson, F.J. (2015) Core formation and core composition from coupled geochemical and geophysical constraints. Proceedings of the National Academy of Sciences 112, 12310–12314. https://doi.org/10.1073/pnas.1505672112
) that was adapted for Mars (See Supplementary Information). The model tracks the oxygen and silicon concentrations in the core as well as the final pressure at the base of the magma ocean (output parameters) for a given sulfur content in the core, iron oxide fraction in the mantle and geotherm (input parameters). The outcome of our models is detailed in Table S-7 and further summarised in Figure 3 below where we plotted the oxygen concentration in the Martian core as a function of its sulfur content for a solidus (Fig. 3a) and a liquidus (Fig. 3b) magma ocean geotherm. The blue and red curves correspond to the computed oxygen fractions in the Martian core, either assuming an iron-rich (Sanloup et al., 1999Sanloup, C., Jambon, A., Gillet, P. (1999) A simple chondritic model of Mars. Physics of the Earth and Planetary Interiors 112, 43–54. https://doi.org/10.1016/S0031-9201(98)00175-7
; Taylor, 2013Taylor, G.J. (2013) The bulk composition of Mars. Geochemistry 73, 401–420. https://doi.org/10.1016/j.chemer.2013.09.006
) or an iron-poor (Yoshizaki and McDonough, 2020Yoshizaki, T., McDonough, W.F. (2020) The composition of Mars. Geochimica et Cosmochimica Acta 273, 137–162. https://doi.org/10.1016/j.gca.2020.01.011
) Martian mantle. The range of oxygen concentrations in the core for a given mantle composition is therefore bracketed by these two curves for each mantle composition model.
Figure 3 Predicted amount of oxygen in the Martian core for an FeO-rich (Sanloup et al., 1999
Sanloup, C., Jambon, A., Gillet, P. (1999) A simple chondritic model of Mars. Physics of the Earth and Planetary Interiors 112, 43–54. https://doi.org/10.1016/S0031-9201(98)00175-7
; Taylor, 2013Taylor, G.J. (2013) The bulk composition of Mars. Geochemistry 73, 401–420. https://doi.org/10.1016/j.chemer.2013.09.006
) (red curves) and an FeO-poor mantle (Yoshizaki and McDonough, 2020Yoshizaki, T., McDonough, W.F. (2020) The composition of Mars. Geochimica et Cosmochimica Acta 273, 137–162. https://doi.org/10.1016/j.gca.2020.01.011
) (blue curves). The curves were obtained by fitting the output data (Table S-7) with either a 4th order (solidus) or a 6th order (liquidus) polynomial (See Supplementary Information). For each of these mantle compositions, the associated amount of oxygen in the core is bracketed between a minimum and a maximum defined by the two curves of the same colour. They reflect the range of possible magma ocean depths at which the core can form. Both (a) solidus and (b) liquidus geotherms were tested. Cosmochemical constraints predict <14−19 wt. % S in the Martian core (Steenstra and van Westrenen, 2018Steenstra, E.S., van Westrenen, W. (2018) A synthesis of geochemical constraints on the inventory of light elements in the core of Mars. Icarus 315, 69–78. https://doi.org/10.1016/j.icarus.2018.06.023
; Brennan et al., 2020Brennan, M.C., Fischer, R.A., Irving, J.C.E. (2020) Core formation and geophysical properties of Mars. Earth and Planetary Science Letters 530, 115923. https://doi.org/10.1016/j.epsl.2019.115923
) (yellow shaded area) that could lead up to 3.5 wt. % O in the core.As expected, the liquidus allows for dissolution of more oxygen than the solidus. Yet, this effect is of second order since the difference between the two is 0.4 wt. % O at most. The choice of mantle composition also influences the final results since the FeO-rich mantle of Taylor (2013)
Taylor, G.J. (2013) The bulk composition of Mars. Geochemistry 73, 401–420. https://doi.org/10.1016/j.chemer.2013.09.006
results in greater oxygen concentrations in the core and favours a shallow magma ocean, while the model of Yoshizaki and McDonough (2020)Yoshizaki, T., McDonough, W.F. (2020) The composition of Mars. Geochimica et Cosmochimica Acta 273, 137–162. https://doi.org/10.1016/j.gca.2020.01.011
yields less oxygen in the core and requires a deeper magma ocean that can even reach the CMB for an S-poor core. We additionally observe that the Si content in the core remains negligible (≤0.07 wt. %) during the whole differentiation process (as seen in Fig. S-6f and further observed for all the simulations), confirming previous results by Gessmann et al. (2001)Gessmann, C.K., Wood, B., Rubie, D.C., Kilburn, M.R. (2001) Solubility of silicon in liquid metal at high pressure: Implications for the composition of the Earth’s core. Earth and Planetary Science Letters 184, 367–376. https://doi.org/10.1016/S0012-821X(00)00325-3
, who found that Martian equilibrium conditions cannot dissolve a substantial amount of Si in the metal. Likewise, core formation modelling by Brennan et al. (2020)Brennan, M.C., Fischer, R.A., Irving, J.C.E. (2020) Core formation and geophysical properties of Mars. Earth and Planetary Science Letters 530, 115923. https://doi.org/10.1016/j.epsl.2019.115923
only gives a little fraction of Si in the Martian core, but also leads to less than 1 wt. % O in an S-rich core. This is a result of the fact that their modelling did not take into account O-S interaction during metal-silicate differentiation, thus severely underestimating the final amount of oxygen in Mars’s core. Running our models with εOS = 0 (equivalent to no O-S interaction) gives a core with less than 0.6 wt. % O, consistent with their findings. This stresses the importance of taking into account all thermodynamic aspects of metal-silicate interactions when modelling systems that contain large amounts of light elements, such as Mars’s core.The maximum core density of Mars (6300 kg/m3) necessitates on average 22–29 wt. % S (Figure S11-1 of Stähler et al., 2021
Stähler, S.C., Khan, A., Banerdt, W.B., Lognonné, P., Giardini, D., Ceylan, S., Drilleau, M., Duran, A.C., Garcia, R.F., Huang, Q., Kim, D., Lekic, V., Samuel, H., Schimmel, M., Schmerr, N., Sollberger, D., Stutzmann, E., Xu, Z., Antonangeli, D., Charalambous, C., Davis, P.M., Irving, J.C.E., Kawamura, T., Knapmeyer, M., Maguire, R., Marusiak, A.G., Panning, M.P., Perrin, C., Plesa, A.-C., Rivoldini, A., Schmelzbach, C., Zenhäusern, G., Beucler, E., Clinton, J., Dahmen, N., van Driel, M., Gudkova, T., Horleston, A., Pike, W.T., Plasman, M., Smrekar, S. (2021) Seismic detection of the martian core. Science 373, 443–448. https://doi.org/10.1126/science.abi7730
), if it is the sole light element. This exceeds the range (14–19 wt. % S) allowed by cosmochemical constraints (Steenstra and van Westrenen, 2018Steenstra, E.S., van Westrenen, W. (2018) A synthesis of geochemical constraints on the inventory of light elements in the core of Mars. Icarus 315, 69–78. https://doi.org/10.1016/j.icarus.2018.06.023
; Brennan et al., 2020Brennan, M.C., Fischer, R.A., Irving, J.C.E. (2020) Core formation and geophysical properties of Mars. Earth and Planetary Science Letters 530, 115923. https://doi.org/10.1016/j.epsl.2019.115923
). Alternatively, a core with 19 wt. % S (consistent with cosmochemical constraints) would contain as much as 3.5 wt. % O (Fig. 3). Because 1 wt. % O has the same effect on density as 1.3 wt. % S (Stähler et al., 2021Stähler, S.C., Khan, A., Banerdt, W.B., Lognonné, P., Giardini, D., Ceylan, S., Drilleau, M., Duran, A.C., Garcia, R.F., Huang, Q., Kim, D., Lekic, V., Samuel, H., Schimmel, M., Schmerr, N., Sollberger, D., Stutzmann, E., Xu, Z., Antonangeli, D., Charalambous, C., Davis, P.M., Irving, J.C.E., Kawamura, T., Knapmeyer, M., Maguire, R., Marusiak, A.G., Panning, M.P., Perrin, C., Plesa, A.-C., Rivoldini, A., Schmelzbach, C., Zenhäusern, G., Beucler, E., Clinton, J., Dahmen, N., van Driel, M., Gudkova, T., Horleston, A., Pike, W.T., Plasman, M., Smrekar, S. (2021) Seismic detection of the martian core. Science 373, 443–448. https://doi.org/10.1126/science.abi7730
), such a core would have the same density as a S-only core containing 23.6 wt. % S. As the Martian core is likely lighter than this, we see that an Fe–O–S core would still require a high fraction of S (>19 wt. %) that is non-permissible in cosmochemistry.top
Conclusions
Our work predicts the possible dissolution of up to 3.5 wt. % O in the Martian core alongside 19 wt. % S that would fit within the upper core density estimate and be in agreement with cosmochemical constraints. While the dissolution of oxygen in the terrestrial core was driven by the very high pressures (∼55 GPa) and high temperatures (3300–3400 K) that prevailed at the base of the magma ocean (Siebert et al., 2012
Siebert, J., Badro, J., Antonangeli, D., Ryerson, F.J. (2012). Metal–silicate partitioning of Ni and Co in a deep magma ocean. Earth and Planetary Science Letters 321–322, 189–197. https://doi.org/10.1016/j.epsl.2012.01.013
; Badro et al., 2015Badro, J., Brodholt, J.P., Piet, H., Siebert, J., Ryerson, F.J. (2015) Core formation and core composition from coupled geochemical and geophysical constraints. Proceedings of the National Academy of Sciences 112, 12310–12314. https://doi.org/10.1073/pnas.1505672112
; Fischer et al., 2015Fischer, R.A., Nakajima, Y., Campbell, A.J., Frost, D.J., Harries, D., Langenhorst, F., Miyajima, N., Pollok, K., Rubie, D.C. (2015) High pressure metal–silicate partitioning of Ni, Co, V, Cr, Si, and O. Geochimica et Cosmochimica Acta 167, 177–194. https://doi.org/10.1016/j.gca.2015.06.026
), the amount of oxygen in the Martian core only results from its interaction with sulfur. This unavoidable concomitance of S and O in the Martian core should be taken into account in future Martian compositional models.top
Acknowledgements
We thank two anonymous reviewers whose comments helped to substantially improve the paper. We thank Attilio Rivoldini for fruitful discussions as well as Kurt Leinenweber, Julien Siebert, and Edith Kubik for the experimental and analytical help. We also thank Pierre-Olivier Foucault for designing the graphical abstract. We acknowledge the financial support of the UnivEarthS Labex program at Université Paris Cité (ANR-10-LABX-0023 and ANR-11-IDEX-0005-02), and IdEx Université Paris Cité (ANR-18-IDEX-0001). This work has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 101019965— SEPtiM). Parts of this work were supported by IPGP multidisciplinary program PARI, and by Paris–IdF region SESAME Grant no. 12015908.
Editor: Anat Shahar
top
References
Badro, J., Brodholt, J.P., Piet, H., Siebert, J., Ryerson, F.J. (2015) Core formation and core composition from coupled geochemical and geophysical constraints. Proceedings of the National Academy of Sciences 112, 12310–12314. https://doi.org/10.1073/pnas.1505672112
 Show in context
Show in context We incorporated our parametrisation of the oxygen-sulfur interaction in a multi-stage core formation model (Badro et al., 2015) that was adapted for Mars (See Supplementary Information).
View in article
While the dissolution of oxygen in the terrestrial core was driven by the very high pressures (∼55 GPa) and high temperatures (3300–3400 K) that prevailed at the base of the magma ocean (Siebert et al., 2012; Badro et al., 2015; Fischer et al., 2015), the amount of oxygen in the Martian core only results from its interaction with sulfur.
View in article
Indeed, oxygen is known to be soluble in liquid iron only at high pressures and temperatures (Ohtani et al., 1984; Ricolleau et al., 2011; Siebert et al., 2013; Badro et al., 2015) which are not prevalent on smaller planets, such as Mars.
View in article
Bertka, C.M., Fei, Y. (1998) Implications of Mars Pathfinder Data for the Accretion History of the Terrestrial Planets. Science 281, 1838–1840. https://doi.org/10.1126/science.281.5384.1838
 Show in context
Show in context Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
Blanchard, I., Badro, J., Siebert, J., Ryerson, F.J. (2015) Composition of the core from gallium metal–silicate partitioning experiments. Earth and Planetary Science Letters 427, 191–201. https://doi.org/10.1016/j.epsl.2015.06.063
 Show in context
Show in context As seen on the backscattered electron (BSE) image of a typical run (Fig. 1), the metallic phase systematically undergoes exsolution to iron-rich and sulfur-rich phases during quench, as previously reported (Blanchard et al., 2015).
View in article
These blobs are almost always located in the sulfur-rich zones (darker grey areas in Fig. 1b) which already suggests an affinity between S and O. This is confirmed by chemical analysis of the metal as plotted in Figure S-2 alongside similar data from Blanchard et al. (2015).
View in article
Combining the data of Blanchard et al. (2015) with our dataset, we further parametrised the O-S interaction to evaluate the potential oxygen concentration in an S-rich Martian core.
View in article
Brennan, M.C., Fischer, R.A., Irving, J.C.E. (2020) Core formation and geophysical properties of Mars. Earth and Planetary Science Letters 530, 115923. https://doi.org/10.1016/j.epsl.2019.115923
 Show in context
Show in context Paradoxically, such a high S-fraction exceeds the maximum sulfur concentration (∼18–19 wt. %) that satisfies cosmochemical observables (Steenstra and van Westrenen, 2018; Brennan et al., 2020).
View in article
We additionally observe that the Si content in the core remains negligible (≤0.07 wt. %) during the whole differentiation process (as seen in Fig. S-6f and further observed for all the simulations), confirming previous results by Gessmann et al. (2001), who found that Martian equilibrium conditions cannot dissolve a substantial amount of Si in the metal. Likewise, core formation modelling by Brennan et al. (2020) only gives a little fraction of Si in the Martian core, but also leads to less than 1 wt. % O in an S-rich core.
View in article
This exceeds the range (14–19 wt. % S) allowed by cosmochemical constraints (Steenstra and van Westrenen, 2018; Brennan et al., 2020).
View in article
They reflect the range of possible magma ocean depths at which the core can form. Both (a) solidus and (b) liquidus geotherms were tested. Cosmochemical constraints predict <14−19 wt. % S in the Martian core (Steenstra and van Westrenen, 2018; Brennan et al., 2020) (yellow shaded area) that could lead up to 3.5 wt. % O in the core.
View in article
Dreibus, G., Wanke, H. (1985) Mars, a volatile-rich planet. Meteoritics 20, 367–381.
 Show in context
Show in context Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
Fei, Y., Prewitt, C.T., Mao, H.-K., Bertka, C.M. (1995) Structure and Density of FeS at High Pressure and High Temperature and the Internal Structure of Mars. Science 268, 1892–1894. https://doi.org/10.1126/science.268.5219.1892
 Show in context
Show in context Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
Fischer, R.A., Nakajima, Y., Campbell, A.J., Frost, D.J., Harries, D., Langenhorst, F., Miyajima, N., Pollok, K., Rubie, D.C. (2015) High pressure metal–silicate partitioning of Ni, Co, V, Cr, Si, and O. Geochimica et Cosmochimica Acta 167, 177–194. https://doi.org/10.1016/j.gca.2015.06.026
 Show in context
Show in context While the dissolution of oxygen in the terrestrial core was driven by the very high pressures (∼55 GPa) and high temperatures (3300–3400 K) that prevailed at the base of the magma ocean (Siebert et al., 2012; Badro et al., 2015; Fischer et al., 2015), the amount of oxygen in the Martian core only results from its interaction with sulfur.
View in article
Gessmann, C.K., Wood, B., Rubie, D.C., Kilburn, M.R. (2001) Solubility of silicon in liquid metal at high pressure: Implications for the composition of the Earth’s core. Earth and Planetary Science Letters 184, 367–376. https://doi.org/10.1016/S0012-821X(00)00325-3
 Show in context
Show in context We additionally observe that the Si content in the core remains negligible (≤0.07 wt. %) during the whole differentiation process (as seen in Fig. S-6f and further observed for all the simulations), confirming previous results by Gessmann et al. (2001), who found that Martian equilibrium conditions cannot dissolve a substantial amount of Si in the metal. Likewise, core formation modelling by Brennan et al. (2020) only gives a little fraction of Si in the Martian core, but also leads to less than 1 wt. % O in an S-rich core.
View in article
Khan, A., Liebske, C., Rozel, A., Rivoldini, A., Nimmo, F., Connolly, J.A.D., Plesa, A.-C., Giardini, D. (2018) A Geophysical Perspective on the Bulk Composition of Mars. Journal of Geophysical Research: Planets 123, 575–611. https://doi.org/10.1002/2017JE005371
 Show in context
Show in context Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
Lodders, K., Fegley Jr., B. (1997) An Oxygen Isotope Model for the Composition of Mars. Icarus 126, 373–394. https://doi.org/10.1006/icar.1996.5653
 Show in context
Show in context Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
McSween Jr., H.Y. (1994) What we have learned about Mars from SNC meteorites. Meteoritics 29, 757–779. https://doi.org/10.1111/j.1945-5100.1994.tb01092.x
 Show in context
Show in context Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
Morard, G., Sanloup, C., Fiquet, G., Mezouar, M., Rey, N., Poloni, R., Beck, P. (2007) Structure of eutectic Fe–FeS melts to pressures up to 17 GPa: Implications for planetary cores. Earth and Planetary Science Letters 263, 128–139. https://doi.org/10.1016/j.epsl.2007.09.009
 Show in context
Show in context Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
Morard, G., Bouchet, J., Rivoldini, A., Antonangeli, D., Roberge, M., Boulard, E., Denoeud, A., Mezouar, M. (2018) Liquid properties in the Fe-FeS system under moderate pressure: Tool box to model small planetary cores. American Mineralogist 103, 1770–1779. https://doi.org/10.2138/am-2018-6405
 Show in context
Show in context Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
Morgan, J.W., Anders, E. (1979) Chemical composition of Mars. Geochimica et Cosmochimica Acta 43, 1601–1610. https://doi.org/10.1016/0016-7037(79)90180-7
 Show in context
Show in context This has led studies on Mars’s core to focus on sulfur as an alloying element, with estimates ranging from 3.5 wt. % (Morgan and Anders, 1979) up to 36 wt. % S (Zharkov and Gudkova, 2005).
View in article
Ohtani, E., Ringwood, A.E., Hibberson, W. (1984) Composition of the core, II. Effect of high pressure on solubility of FeO in molten iron. Earth and Planetary Science Letters 71, 94–103. https://doi.org/10.1016/0012-821X(84)90055-4
 Show in context
Show in context Indeed, oxygen is known to be soluble in liquid iron only at high pressures and temperatures (Ohtani et al., 1984; Ricolleau et al., 2011; Siebert et al., 2013; Badro et al., 2015) which are not prevalent on smaller planets, such as Mars.
View in article
Ricolleau, A., Fei, Y., Corgne, A., Siebert, J., Badro, J. (2011) Oxygen and silicon contents of Earth’s core from high pressure metal–silicate partitioning experiments. Earth and Planetary Science Letters 310, 409–421. https://doi.org/10.1016/j.epsl.2011.08.004
 Show in context
Show in context Indeed, oxygen is known to be soluble in liquid iron only at high pressures and temperatures (Ohtani et al., 1984; Ricolleau et al., 2011; Siebert et al., 2013; Badro et al., 2015) which are not prevalent on smaller planets, such as Mars.
View in article
Righter, K. (2017) The Martian Meteorite Compendium. https://curator.jsc.nasa.gov/antmet/mmc/
 Show in context
Show in context Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
Rivoldini, A., Van Hoolst, T., Verhoeven, O., Mocquet, A., Dehant, V. (2011) Geodesy constraints on the interior structure and composition of Mars. Icarus 213, 451–472. https://doi.org/10.1016/j.icarus.2011.03.024
 Show in context
Show in context Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
Sanloup, C., Jambon, A., Gillet, P. (1999) A simple chondritic model of Mars. Physics of the Earth and Planetary Interiors 112, 43–54. https://doi.org/10.1016/S0031-9201(98)00175-7
 Show in context
Show in context The blue and red curves correspond to the computed oxygen fractions in the Martian core, either assuming an iron-rich (Sanloup et al., 1999; Taylor, 2013) or an iron-poor (Yoshizaki and McDonough, 2020) Martian mantle.
View in article
Predicted amount of oxygen in the Martian core for an FeO-rich (Sanloup et al., 1999; Taylor, 2013) (red curves) and an FeO-poor mantle (Yoshizaki and McDonough, 2020) (blue curves).
View in article
Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
Siebert, J., Badro, J., Antonangeli, D., Ryerson, F.J. (2012). Metal–silicate partitioning of Ni and Co in a deep magma ocean. Earth and Planetary Science Letters 321–322, 189–197. https://doi.org/10.1016/j.epsl.2012.01.013
 Show in context
Show in context While the dissolution of oxygen in the terrestrial core was driven by the very high pressures (∼55 GPa) and high temperatures (3300–3400 K) that prevailed at the base of the magma ocean (Siebert et al., 2012; Badro et al., 2015; Fischer et al., 2015), the amount of oxygen in the Martian core only results from its interaction with sulfur.
View in article
Siebert, J., Badro, J., Antonangeli, D., Ryerson, F.J. (2013) Terrestrial Accretion Under Oxidizing Conditions. Science 339, 1194–1197. https://doi.org/10.1126/science.1227923
 Show in context
Show in context Indeed, oxygen is known to be soluble in liquid iron only at high pressures and temperatures (Ohtani et al., 1984; Ricolleau et al., 2011; Siebert et al., 2013; Badro et al., 2015) which are not prevalent on smaller planets, such as Mars.
View in article
Stähler, S.C., Khan, A., Banerdt, W.B., Lognonné, P., Giardini, D., Ceylan, S., Drilleau, M., Duran, A.C., Garcia, R.F., Huang, Q., Kim, D., Lekic, V., Samuel, H., Schimmel, M., Schmerr, N., Sollberger, D., Stutzmann, E., Xu, Z., Antonangeli, D., Charalambous, C., Davis, P.M., Irving, J.C.E., Kawamura, T., Knapmeyer, M., Maguire, R., Marusiak, A.G., Panning, M.P., Perrin, C., Plesa, A.-C., Rivoldini, A., Schmelzbach, C., Zenhäusern, G., Beucler, E., Clinton, J., Dahmen, N., van Driel, M., Gudkova, T., Horleston, A., Pike, W.T., Plasman, M., Smrekar, S. (2021) Seismic detection of the martian core. Science 373, 443–448. https://doi.org/10.1126/science.abi7730
 Show in context
Show in context Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
For instance, Stähler et al. (2021) suggest 5 wt. % O in addition to 10 to 15 wt. % S to satisfy the density requirement, but they do not provide a mechanism to account for its dissolution in iron.
View in article
The maximum core density of Mars (6300 kg/m3) necessitates on average 22–29 wt. % S (Figure S11-1 of Stähler et al., 2021), if it is the sole light element.
View in article
Because 1 wt. % O has the same effect on density as 1.3 wt. % S (Stähler et al., 2021), such a core would have the same density as a S-only core containing 23.6 wt. % S.
View in article
According to recent results on the elastic properties of Fe–Ni–S liquids, 30 wt. % S is required to fit the core density estimate (6000 ± 300 kg/m3) if S is the only light element (Terasaki et al., 2019; Stähler et al., 2021).
View in article
Steelmaking Data Sourcebook (1988) The Japan Society for the Promotion of Science: The 19th Committee on Steelmaking. Gordon and Breach Science Publishers, New York.
 Show in context
Show in context The latter strongly deviates from the value of −17 found in the metallurgy literature (Steelmaking Data Sourcebook, 1988) determined at room pressure and that cannot reproduce the data well as seen on Figure S-4
View in article
Steenstra, E.S., van Westrenen, W. (2018) A synthesis of geochemical constraints on the inventory of light elements in the core of Mars. Icarus 315, 69–78. https://doi.org/10.1016/j.icarus.2018.06.023
 Show in context
Show in context Paradoxically, such a high S-fraction exceeds the maximum sulfur concentration (∼18–19 wt. %) that satisfies cosmochemical observables (Steenstra and van Westrenen, 2018; Brennan et al., 2020).
View in article
This exceeds the range (14–19 wt. % S) allowed by cosmochemical constraints (Steenstra and van Westrenen, 2018; Brennan et al., 2020).
View in article
They reflect the range of possible magma ocean depths at which the core can form. Both (a) solidus and (b) liquidus geotherms were tested. Cosmochemical constraints predict <14−19 wt. % S in the Martian core (Steenstra and van Westrenen, 2018; Brennan et al., 2020) (yellow shaded area) that could lead up to 3.5 wt. % O in the core.
View in article
Taylor, G.J. (2013) The bulk composition of Mars. Geochemistry 73, 401–420. https://doi.org/10.1016/j.chemer.2013.09.006
 Show in context
Show in context The blue and red curves correspond to the computed oxygen fractions in the Martian core, either assuming an iron-rich (Sanloup et al., 1999; Taylor, 2013) or an iron-poor (Yoshizaki and McDonough, 2020) Martian mantle.
View in article
Predicted amount of oxygen in the Martian core for an FeO-rich (Sanloup et al., 1999; Taylor, 2013) (red curves) and an FeO-poor mantle (Yoshizaki and McDonough, 2020) (blue curves).
View in article
The choice of mantle composition also influences the final results since the FeO-rich mantle of Taylor (2013) results in greater oxygen concentrations in the core and favours a shallow magma ocean, while the model of Yoshizaki and McDonough (2020) yields less oxygen in the core and requires a deeper magma ocean that can even reach the CMB for an S-poor core.
View in article
Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
Terasaki, H., Rivoldini, A., Shimoyama, Y., Nishida, K., Urakawa, S., Maki, M., Kurokawa, F., Takubo, Y., Shibazaki, Y., Sakamaki, T., Machida, A., Higo, Y., Uesugi, K., Takeuchi, A., Watanuki, T., Kondo, T. (2019) Pressure and Composition Effects on Sound Velocity and Density of Core‐Forming Liquids: Implication to Core Compositions of Terrestrial Planets. Journal of Geophysical Research: Planets 124, 2272–2293. https://doi.org/10.1029/2019JE005936
 Show in context
Show in context According to recent results on the elastic properties of Fe–Ni–S liquids, 30 wt. % S is required to fit the core density estimate (6000 ± 300 kg/m3) if S is the only light element (Terasaki et al., 2019; Stähler et al., 2021).
View in article
Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
Tsuno, K., Frost, D.J., Rubie, D.C. (2011) The effects of nickel and sulphur on the core–mantle partitioning of oxygen in Earth and Mars. Physics of the Earth and Planetary Interiors 185, 1–12. https://doi.org/10.1016/j.pepi.2010.11.009
 Show in context
Show in context Yet, another way to incorporate oxygen in the Martian core is through chemical interaction with sulfur in an Fe-liquid, that can drive its dissolution in the metal at lower pressures and temperatures (Tsuno et al., 2011).
View in article
Yoshizaki, T., McDonough, W.F. (2020) The composition of Mars. Geochimica et Cosmochimica Acta 273, 137–162. https://doi.org/10.1016/j.gca.2020.01.011
 Show in context
Show in context Although Mars’s accretion and its bulk chemical composition are still uncertain (Bertka and Fei, 1998; Yoshizaki and McDonough, 2020), the composition of Martian meteorites (Dreibus and Wanke, 1985; McSween, 1994; Lodders and Fegley, 1997; Sanloup et al., 1999; Taylor, 2013; Righter, 2017), the geophysical and geodetic data collected by spacecrafts and landers (Rivoldini et al., 2011; Khan et al., 2018; Stähler et al., 2021) and high-pressure, high-temperature experimental work (Fei et al., 1995; Morard et al., 2007, 2018; Terasaki et al., 2019) all argue for a sulfur-rich Martian core.
View in article
The blue and red curves correspond to the computed oxygen fractions in the Martian core, either assuming an iron-rich (Sanloup et al., 1999; Taylor, 2013) or an iron-poor (Yoshizaki and McDonough, 2020) Martian mantle.
View in article
The choice of mantle composition also influences the final results since the FeO-rich mantle of Taylor (2013) results in greater oxygen concentrations in the core and favours a shallow magma ocean, while the model of Yoshizaki and McDonough (2020) yields less oxygen in the core and requires a deeper magma ocean that can even reach the CMB for an S-poor core.
View in article
Predicted amount of oxygen in the Martian core for an FeO-rich (Sanloup et al., 1999; Taylor, 2013) (red curves) and an FeO-poor mantle (Yoshizaki and McDonough, 2020) (blue curves).
View in article
Zharkov, V.N., Gudkova, T.V. (2005) Construction of Martian Interior Model. Solar System Research 39, 343–373. https://doi.org/10.1007/s11208-005-0049-7
 Show in context
Show in context This has led studies on Mars’s core to focus on sulfur as an alloying element, with estimates ranging from 3.5 wt. % (Morgan and Anders, 1979) up to 36 wt. % S (Zharkov and Gudkova, 2005).
View in article
top
Supplementary Information
The Supplementary Information includes:
Download the Supplementary Information (PDF).
Figures
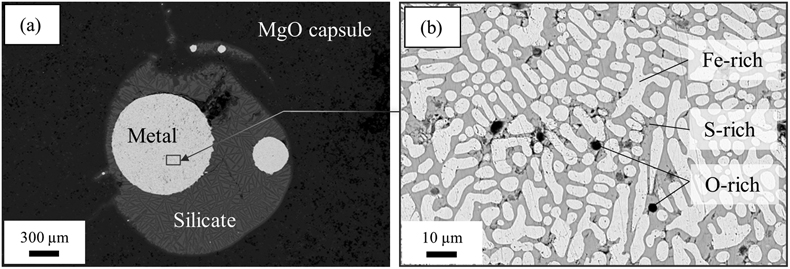
Figure 1 (a) BSE (backscattered electron) image of one of the recovered samples (ELMO 445, P = 2 GPa and T = 1873 K). The contrast is linked to changes in atomic number, with light areas corresponding to heavy (high-Z) phases (e.g., iron) and dark phases to lighter (low-Z) material (e.g., silicate). (b) BSE image of the metallic phase. The dendritic pattern results from the exsolution of two different metallic melts as the system cools during quench (the light-coloured phase is an iron-rich melt, and the darker phase is a sulfur-rich melt). The oxygen-rich blobs were lost while polishing and the observed dark spots correspond to their print in the structure.
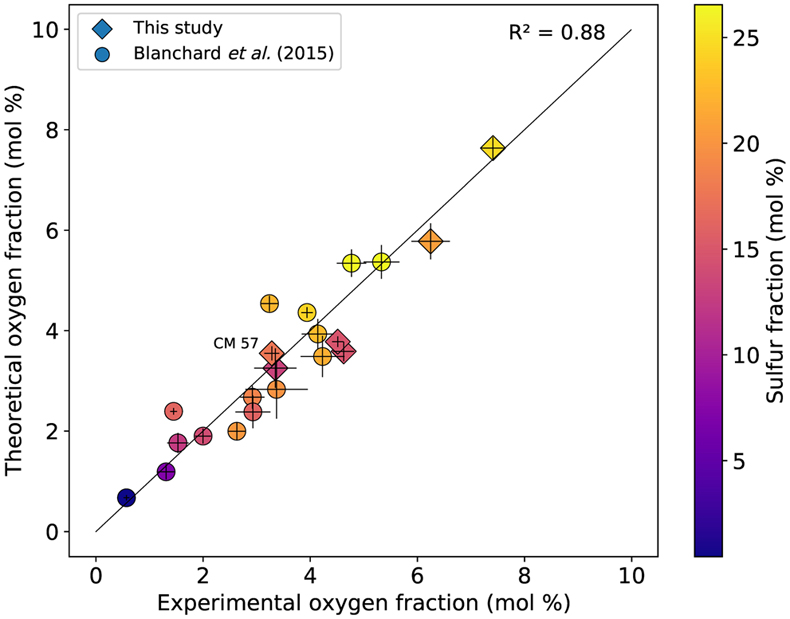
Figure 2 Predicted oxygen concentration (mol %) in the metallic phase versus measured oxygen concentration (mol %). Symbol colour is matched to the sulfur content (mol %) of each experiment, according to the colour bar to the right. The theoretical predictions (y-axis) fall close to the real values (x-axis) including CM 57 that equilibrated at a higher pressure (12 GPa). The vertical errors were set equal to the horizontal ones (1σ standard error) for simplification.
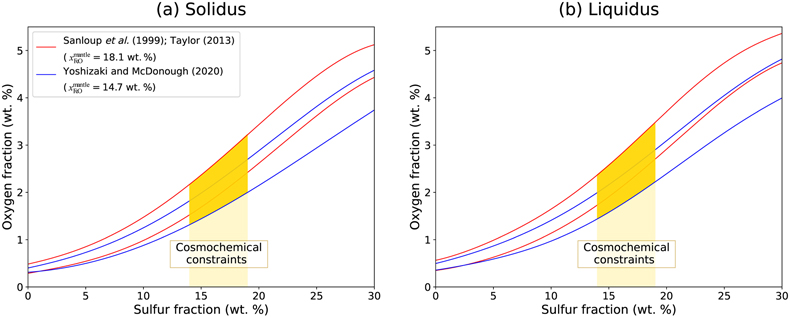
Figure 3 Predicted amount of oxygen in the Martian core for an FeO-rich (Sanloup et al., 1999
Sanloup, C., Jambon, A., Gillet, P. (1999) A simple chondritic model of Mars. Physics of the Earth and Planetary Interiors 112, 43–54. https://doi.org/10.1016/S0031-9201(98)00175-7
; Taylor, 2013Taylor, G.J. (2013) The bulk composition of Mars. Geochemistry 73, 401–420. https://doi.org/10.1016/j.chemer.2013.09.006
) (red curves) and an FeO-poor mantle (Yoshizaki and McDonough, 2020Yoshizaki, T., McDonough, W.F. (2020) The composition of Mars. Geochimica et Cosmochimica Acta 273, 137–162. https://doi.org/10.1016/j.gca.2020.01.011
) (blue curves). The curves were obtained by fitting the output data (Table S-7) with either a 4th order (solidus) or a 6th order (liquidus) polynomial (See Supplementary Information). For each of these mantle compositions, the associated amount of oxygen in the core is bracketed between a minimum and a maximum defined by the two curves of the same colour. They reflect the range of possible magma ocean depths at which the core can form. Both (a) solidus and (b) liquidus geotherms were tested. Cosmochemical constraints predict <14−19 wt. % S in the Martian core (Steenstra and van Westrenen, 2018Steenstra, E.S., van Westrenen, W. (2018) A synthesis of geochemical constraints on the inventory of light elements in the core of Mars. Icarus 315, 69–78. https://doi.org/10.1016/j.icarus.2018.06.023
; Brennan et al., 2020Brennan, M.C., Fischer, R.A., Irving, J.C.E. (2020) Core formation and geophysical properties of Mars. Earth and Planetary Science Letters 530, 115923. https://doi.org/10.1016/j.epsl.2019.115923
) (yellow shaded area) that could lead up to 3.5 wt. % O in the core.