Equilibrium olivine-melt Mg isotopic fractionation explains high δ26Mg values in arc lavas
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:2,298Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
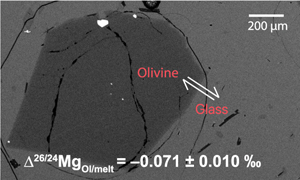
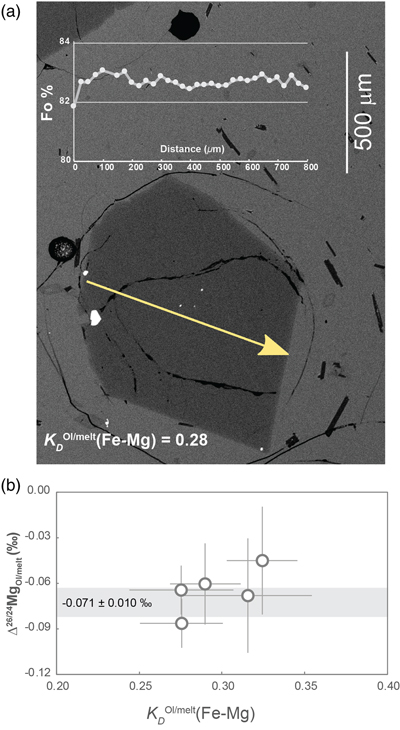 Figure 1 (a) Backscattered electron microscope image of an olivine-glass pair (PN3-10), together with electron microprobe traverse of olivine Fo content. (b) Measured Δ26/24MgOl/melt, corrected to a common temperature (1438 K), plotted against Fe-Mg distribution coefficients. | 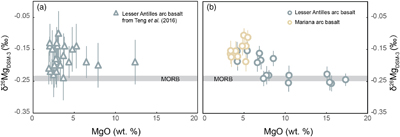 Figure 2 δ26Mg against MgO for (a) Lesser Antilles lavas from Teng et al. (2016) and (b) Lesser Antilles and Mariana lavas from this study. MORB reference value is −0.24 ± 0.01 ‰ (2 s.e.); see Supplementary Information section 3.4. | 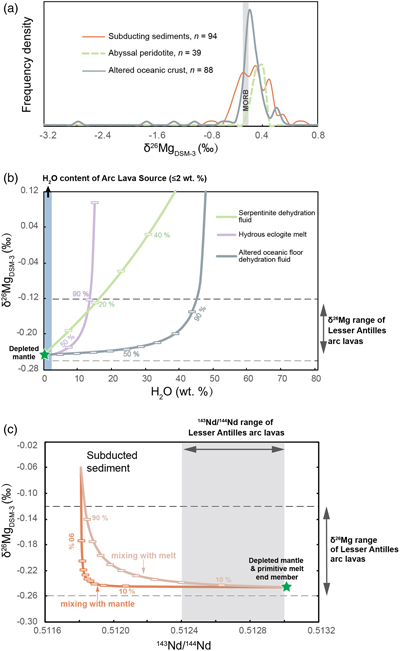 Figure 3 (a) Frequency density plot of δ26Mg of subducted materials. (b) Modelled δ26Mg against H2O from mixing depleted mantle with dehydration fluids and hydrous melt. (c) Modelled δ26Mg against 143Nd/144Nd from mixing depleted mantle with subducted sediments (See SI section 3.5 for more details). | 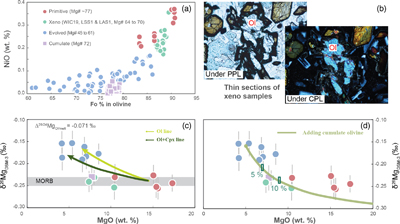 Figure 4 (a) Electron microprobe analyses of Lesser Antilles olivines plotted in groups according to bulk Mg# of host magmas. (b) Thin-section images of disequilibrium olivines in “xeno” group samples. (c) Modelled δ26Mg evolution during two main differentiation paths. (d) Modelled effect on δ26Mg of cumulate olivine addition to an “evolved” sample composition. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
A burgeoning literature in the mass-dependent variability of major rock forming elements in magmatic samples have the potential to provide novel constraints on source mineralogy and melting processes (e.g., Teng et al., 2017
Teng, F.-Z., Watkins, J.M., Dauphas, N. (2017) Non-Traditional Stable Isotopes. Reviews in Mineralogy and Geochemistry 82. The Mineralogical Society of America. https://doi.org/10.1515/9783110545630
; Soderman et al., 2022Soderman, C.R., Shorttle, O., Matthews, S., Williams, H.M. (2022) Global trends in novel stable isotopes in basalts: Theory and observations. Geochimica et Cosmochimica Acta 318, 388–414. https://doi.org/10.1016/j.gca.2021.12.008
), but in many cases the key parameter of isotopic fractionation, i.e. the fractionation factor between solid and melt, is insufficiently well constrained to make the most of the observations. In large part, mineral-melt fractionation factors have been determined by the magnitude (or absence) of isotopic variability in sample suites that show well behaved differentiation trends. Although valuable, this strategy convolves the natural complexity of magmatic fractionation with the determination of fractionation factors. A more direct method is to measure the isotope ratios of coexisting equilibrated mineral-melt pairs. This poses the difficulty of obtaining precise measurements on small samples that are demonstrably in equilibrium.As the third most abundant element in the silicate Earth, there is much interest in the Mg isotopic systematics of magmatic rocks for improving our understanding of igneous processes and broader planetary evolution (e.g., Teng et al., 2010
Teng, F.-Z., Li, W.-Y., Ke, S., Marty, B., Dauphas, N., Huang, S., Wu, F.-Y., Pourmand, A. (2010) Magnesium isotopic composition of the Earth and chondrites. Geochimica et Cosmochimica Acta 74, 4150–4166. https://doi.org/10.1016/j.gca.2010.04.019
; Hin et al., 2017Hin, R.C., Coath, C.D., Carter, P.J., Nimmo, F., Lai, Y.-J., Pogge von Strandmann, P.A.E., Willbold, M., Leinhardt, Z.M., Walter, M.J., Elliott, T. (2017) Magnesium isotope evidence that accretional vapour loss shapes planetary compositions. Nature 549, 511–515. https://doi.org/10.1038/nature23899
; Teng, 2017Teng, F.-Z. (2017) Magnesium Isotope Geochemistry. Reviews in Mineralogy and Geochemistry 82, 219–287. https://doi.org/10.2138/rmg.2017.82.7
). In interpreting the relatively small isotopic variations in Mg, it is critical to determine a precise fractionation factor between olivine, the major mineral host of Mg, and melt. This parameter is expressed as Δ26/24MgOl/melt, defined as δ26MgOl − δ26Mgmelt, where δ26Mg is the relative difference in 26Mg/24Mg between sample and DSM-3 reference standard. Once determined, Δ26/24MgOl/melt can be used in conjunction with inter-mineral fractionation factors to model Mg isotopic variability in magmatic processes. While inter-mineral fractionations can be determined observationally or by ab initio methods, numerical modelling of isotopic exchange between mineral and melt structure is not straight-forward, which has motivated our empirical approach.There have been two previous attempts to measure Δ26/24MgOl/melt. The absence of systematic Mg isotopic variation in a suite of whole rocks with variable MgO contents from Kilauea Iki (Teng et al., 2007
Teng, F.-Z., Wadhwa, M., Helz, R.T. (2007) Investigation of magnesium isotope fractionation during basalt differentiation: Implications for a chondritic composition of the terrestrial mantle. Earth and Planetary Science Letters 261, 84–92. https://doi.org/10.1016/j.epsl.2007.06.004
) is often cited as evidence for the absence of Mg isotope fractionation during crystallisation. Yet, strictly, this study placed a maximum bound on Mg isotope fractionation during differentiation, namely |Δ26/24MgOl/melt| ≤ 0.07 ‰. Schiller et al. (2017)Schiller, M., Dallas, J.A., Creech, J., Bizzarro, M., Baker, J.A. (2017) Tracking the formation of magma oceans in the Solar System using stable magnesium isotopes. Geochemical Perspectives Letters 3, 22–31. https://doi.org/10.7185/geochemlet.1703
analysed the Mg isotopic compositions of olivines and rapidly cooled groundmass (dominantly intergrown plagioclase and clinopyroxene) from an angrite meteorite (NWA1670). These authors also reprocessed Mg isotope measurements of olivine and groundmass from Teng et al. (2011)Teng, F.-Z., Dauphas, N., Helz, R.T., Gao, S., Huang, S. (2011) Diffusion-driven magnesium and iron isotope fractionation in Hawaiian olivine. Earth and Planetary Science Letters 308, 317–324. https://doi.org/10.1016/j.epsl.2011.06.003
to calculate a fractionation factor. Yet, the latter had been originally used to illustrate the effects of diffusive fractionation of Mg isotopes in the chemical potential gradient of zoned minerals. Although Schiller et al. (2017)Schiller, M., Dallas, J.A., Creech, J., Bizzarro, M., Baker, J.A. (2017) Tracking the formation of magma oceans in the Solar System using stable magnesium isotopes. Geochemical Perspectives Letters 3, 22–31. https://doi.org/10.7185/geochemlet.1703
reported those samples closest to elemental Fe-Mg equilibrium, these samples evidently do not constitute an equilibrium assemblage necessary for reliable determination of a fractionation factor. Equally, the bulk olivine phenocrysts in NWA1670 are not in equilibrium with the groundmass, given their Mg/(Mg + Fe) decrease from ∼0.9 in their core to ∼0.6 in their rim (Jambon et al., 2008Jambon, A., Boudouma, O., Fonteilles, M., Le Guillou, C., Badia, D., Barrat, J.A. (2008) Petrology and mineralogy of the angrite Northwest Africa 1670. Meteoritics & Planetary Science 43, 1783–1795. https://doi.org/10.1111/j.1945-5100.2008.tb00643.x
).Here, we employ the high precision attainable using critical mixture double-spiking (Coath et al., 2017
Coath, C.D., Elliott, T., Hin, R.C. (2017) Double-spike inversion for three-isotope systems. Chemical Geology 451, 78–89. https://doi.org/10.1016/j.chemgeo.2016.12.025
; Hin et al., 2017Hin, R.C., Coath, C.D., Carter, P.J., Nimmo, F., Lai, Y.-J., Pogge von Strandmann, P.A.E., Willbold, M., Leinhardt, Z.M., Walter, M.J., Elliott, T. (2017) Magnesium isotope evidence that accretional vapour loss shapes planetary compositions. Nature 549, 511–515. https://doi.org/10.1038/nature23899
) to determine Δ26/24MgOl/melt for five carefully selected, equilibrated olivine-glass pairs from ocean island and mid-ocean ridge basalts. Using this value, we then explore the subtly elevated δ26Mg in arc lavas (Teng et al., 2016Teng, F.-Z., Hu, Y., Chauvel, C. (2016) Magnesium isotope geochemistry in arc volcanism. Proceedings of the National Academy of Sciences 113, 7082–7087. https://doi.org/10.1073/pnas.1518456113
) with new analyses of a suite of samples from the Lesser Antilles, as well as a set of archetypical mafic samples from the Mariana arc.top
Olivine-melt fractionation factor
We selected samples with petrographic, equilibrium olivine textures in naturally quenched glass from Kilauea (Hawaii), submarine eruptions from Pitcairn and mid-ocean ridge basalts from the Pacific and Indian oceans (for details, see section 1.1 of the Supplementary Information, SI). Prior work on the Hawaii and Pitcairn samples (Jeffcoate et al., 2007
Jeffcoate, A.B., Elliott, T., Kasemann, S.A., Ionov, D., Cooper, K., Brooker, R. (2007) Li isotope fractionation in peridotites and mafic melts. Geochimica et Cosmochimica Acta 71, 202–218. https://doi.org/10.1016/j.gca.2006.06.1611
) showed an absence of Li isotopic fractionation across the olivine phenocrysts, documenting an absence of late-stage diffusive fractionation. From these potentially suitable samples we then selected individual olivine crystals with a variability in Fo < 1 % across the full cross-sectional electron microprobe profile of the crystal (Fig. 1a). We rejected any olivine which had an olivine-glass Fe-Mg exchange coefficient outside the range of equilibrium values (Ulmer, 1989Ulmer, P. (1989) The dependence of the Fe2+-Mg cation-partitioning between olivine and basaltic liquid on pressure, temperature and composition. Contributions to Mineralogy and Petrology 101, 261–273. https://doi.org/10.1007/BF00375311
), namely KDOl/melt(Fe-Mg) from 0.28 to 0.32 (Fig. 1b).
Figure 1 (a) Backscattered electron microscope image of an olivine-glass pair (PN3-10), together with electron microprobe traverse of olivine Fo content. (b) Measured Δ26/24MgOl/melt, corrected to a common temperature (1438 K), plotted against Fe-Mg distribution coefficients.
We analysed micro-drilled spots (cones of 100 μm depth and largest diameter) in these olivines and hand-picked coexisting glass and processed the samples for Mg isotope analysis by critical mixture double-spiking as reported in Hin et al. (2017)
Hin, R.C., Coath, C.D., Carter, P.J., Nimmo, F., Lai, Y.-J., Pogge von Strandmann, P.A.E., Willbold, M., Leinhardt, Z.M., Walter, M.J., Elliott, T. (2017) Magnesium isotope evidence that accretional vapour loss shapes planetary compositions. Nature 549, 511–515. https://doi.org/10.1038/nature23899
. This method yields a long-term reproducibility of 0.027 ‰ (δ26Mg, 2 s.d.) based on repeated BHVO-2 analyses (see SI section 2.3). We used the pooled δ26Mg of each phase to yield Mg isotope differences between olivine and melt (i.e. glass) for each of the five basalt samples (Table S-1). Olivine thermometry (Putirka, 2005Putirka, K.D. (2005) Mantle potential temperatures at Hawaii, Iceland, and the mid‐ocean ridge system, as inferred from olivine phenocrysts: Evidence for thermally driven mantle plumes. Geochemistry, Geophysics, Geosystems 6, Q05L08. https://doi.org/10.1029/2005GC000915
) indicates that equilibration temperatures of the five samples varied between 1379 and 1481 K (see SI section 1.2). Using a 1/T2 scaling, we corrected the isotope differences to a single, average temperature of 1438 K, yielding Δ26/24MgOl/melt between −0.045 ± 0.036 ‰ and −0.086 ± 0.016 ‰ (Fig. 1b, Table S-2). These values are consistent with each other and yield a weighted mean of Δ26/24MgOl/melt = −0.071 ± 0.010 ‰ or Δ26/24MgOl/melt = (−1.46 ± 0.26) × 105/T2 (T in Kelvin). For the first time, we thus show that olivine in equilibrium with melt is significantly enriched in light Mg isotopes and we recommend that our olivine-melt fractionation factor should be considered when modelling Mg isotope fractionation in magmatic process. We also tried to experimentally determine Δ26/24MgOl/melt but this attempt unfortunately failed, most likely due to thermal diffusion (see SI section 1.3).The Hawaiian and Pitcairn samples have water contents of ∼0.1–0.4 wt. % (e.g., Hauri, 2002
Hauri, E. (2002) SIMS analysis of volatiles in silicate glasses, 2: isotopes and abundances in Hawaiian melt inclusions. Chemical Geology 183, 115–141. https://doi.org/10.1016/S0009-2541(01)00374-6
) and ∼1.2 wt. % (Aubaud et al., 2006Aubaud, C., Pineau, F., Hekinian, R., Javoy, M. (2006) Carbon and hydrogen isotope constraints on degassing of CO2 and H2O in submarine lavas from the Pitcairn hotspot (South Pacific). Geophysics Research Letters 33, L02308. https://doi.org/10.1029/2005GL024907
), respectively. MORB samples generally have low water contents around 0.1 to 0.2 wt. % (e.g., Sobolev et al., 1996Sobolev, A.V., Chaussidon, M. (1996) H2O concentrations in primary melts from supra-subduction zones and mid-ocean ridges: Implications for H2O storage and recycling in the mantle. Earth and Planetary Science Letters 137, 45–55. https://doi.org/10.1016/0012-821X(95)00203-O
). Water decreases the Mg-O coordination number of silicate melt (Mookherjee et al., 2008Mookherjee, M., Stixrude, L., Karki, B. (2008) Hydrous silicate melt at high pressure. Nature 452, 983–986. https://doi.org/10.1038/nature06918
), which may lead to a preference for heavier isotopes. Nonetheless, the Δ26/24MgOl/melt determined from Hawaiian, Pitcairn and the three MORB olivine-glass pairs are all within the analytical error (Fig. 1b). The absence of a discernible effect on the fractionation factor for water contents up to 1.2 wt. % leads us to assume that water has a limited effect on Δ26/24MgOl/melt.top
Elevated δ26Mg in arc lavas
Teng et al. (2016)
Teng, F.-Z., Hu, Y., Chauvel, C. (2016) Magnesium isotope geochemistry in arc volcanism. Proceedings of the National Academy of Sciences 113, 7082–7087. https://doi.org/10.1073/pnas.1518456113
analysed arc lavas from Martinique, Lesser Antilles, and reported δ26Mg slightly higher than MORB (Fig. 2a), which they attributed to subduction zone processes. As discussed by Teng et al. (2016)Teng, F.-Z., Hu, Y., Chauvel, C. (2016) Magnesium isotope geochemistry in arc volcanism. Proceedings of the National Academy of Sciences 113, 7082–7087. https://doi.org/10.1073/pnas.1518456113
, however, it is not clear that subduction zone components have the leverage to sufficiently perturb the δ26Mg of the mantle wedge, given mixing constraints from other elemental and isotopic tracers. To further explore this intriguing phenomenon, we have made high precision, critical mixture double-spiked analyses of Lesser Antilles samples from a wider range of islands, including rare primitive lavas from the southern arc. We also analysed a well characterised set of samples from the Mariana arc (see SI sections 3.2, 3.3). Our new measurements show the Lesser Antilles lavas have MORB-like δ26Mg in the most MgO-rich samples, while more evolved lavas have δ26Mg up to 0.12 ‰ higher (Figs. 2b, S-5). All the Mariana arc lavas plot together with the less mafic Lesser Antilles samples (Fig. 2b, Table S-5). To better understand the causes of the higher δ26Mg in some arc lavas, we initially concentrate on the Lesser Antilles example, for which we have samples with a wider compositional range.
Figure 2 δ26Mg against MgO for (a) Lesser Antilles lavas from Teng et al. (2016)
Teng, F.-Z., Hu, Y., Chauvel, C. (2016) Magnesium isotope geochemistry in arc volcanism. Proceedings of the National Academy of Sciences 113, 7082–7087. https://doi.org/10.1073/pnas.1518456113
and (b) Lesser Antilles and Mariana lavas from this study. MORB reference value is −0.24 ± 0.01 ‰ (2 s.e.); see Supplementary Information section 3.4.First, we reconsider the possible role of subduction components (Fig. 3a). The results of binary mixing calculations (see SI section 3.5 for details) between the sub-arc mantle and potential subduction inputs are illustrated in Figure 3b, c. Fluids released from subducted oceanic crust and serpentinite are naturally water-rich, over 50 wt. % and 80 wt. % H2O respectively, while the MgO concentrations of these fluids are generally low, less than 1 wt. % in oceanic crust dehydration fluid and about 6 wt. % in serpentinite dehydration fluid (Manning, 2004
Manning, C.E. (2004) The chemistry of subduction-zone fluids. Earth and Planetary Science Letters 223, 1–16. https://doi.org/10.1016/j.epsl.2004.04.030
; Kessel et al., 2005Kessel, R., Schmidt, M.W., Ulmer, P., Pettke, T. (2005) Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120–180 km depth. Nature 437, 724–727. https://doi.org/10.1038/nature03971
; Scambelluri et al., 2015Scambelluri, M., Pettke, T., Cannao, E. (2015) Fluid-related inclusions in Alpine high-pressure peridotite reveal trace element recycling during subduction-zone dehydration of serpentinized mantle (Cima di Gagnone, Swiss Alps). Earth and Planetary Science Letters 429, 45–59. https://doi.org/10.1016/j.epsl.2015.07.060
). Hydrous melts generated from eclogite are again enriched in water (over 15 wt. %) but also low in Mg (less than 2.5 wt. %) (Gervasoni et al., 2017Gervasoni, F., Klemme, S., Rohrbach, A., Griltzner, T., Berndt, J. (2017) Experimental constraints on mantle metasomatism caused by silicate and carbonate melts. Lithos 282–283, 173–186. https://doi.org/10.1016/j.lithos.2017.03.004
). The amount of these slab-derived fluid phases added to the mantle wedge is constrained by a maximum 2 wt. % H2O in the mantle source (assuming at most 10 wt. % H2O in primitive arc magmas and 20 % partial melting degree). As a result, the Mg isotopic perturbation of the arc lava source is inappreciably influenced by plausible contributions of slab-derived fluids (Fig. 3b). The subducting sediments are isotopically heavier than MORB (Fig. 3a), but a contribution over 90 wt. % sediment would be required in the source of the Lesser Antilles lavas to reproduce the highest δ26Mg. Such an amount of sediment is equally inconsistent with the 143Nd/144Nd of the arc lavas (Fig. 3c).
Figure 3 (a) Frequency density plot of δ26Mg of subducted materials. (b) Modelled δ26Mg against H2O from mixing depleted mantle with dehydration fluids and hydrous melt. (c) Modelled δ26Mg against 143Nd/144Nd from mixing depleted mantle with subducted sediments (See SI section 3.5 for more details).
Our high precision analyses allow some structure to be discerned in the variability of δ26Mg in the Lesser Antilles samples, which can have been caused by neither weathering nor variable melting depths (see SI section 3.6). The most striking feature is a systematic relationship between δ26Mg and MgO content (Fig. 2b), which implies a role for magmatic differentiation in fractionating Mg isotopes. When dealing with the effects of differentiation in bulk samples, however, it is important to evaluate crystal accumulation. This is especially marked for Mg given the high MgO contents of olivine. Therefore, we analysed the compositions of olivine crystals in our Lesser Antilles samples. Three samples (WIC19, LSS1 & LAS1) have unexpectedly Fo-rich, xenocrystic olivines compared with their bulk Mg# (Fig. 4a, SI section 3.7). Moreover, disequilibrium textures between olivine crystals and groundmass are observed in the thin sections of these samples (Fig. 4b).

Figure 4 (a) Electron microprobe analyses of Lesser Antilles olivines plotted in groups according to bulk Mg# of host magmas. (b) Thin-section images of disequilibrium olivines in “xeno” group samples. (c) Modelled δ26Mg evolution during two main differentiation paths. (d) Modelled effect on δ26Mg of cumulate olivine addition to an “evolved” sample composition.
We have divided Lesser Antilles arc samples into four groups (Fig. 4a, c, d). Five samples with high MgO are dubbed “primitive”, as detailed petrological experiments have identified such compositions are plausibly in equilibrium with the mantle wedge (see SI section 3.2). They have δ26Mg values within error of MORB (Fig. 4c). Seven samples are grouped as “evolved” and display elevated δ26Mg values. The three samples that contain Fo-rich olivine xenocrysts are named “xeno” and these also have MORB-like δ26Mg values. A single troctolite sample, LAE3, is labelled “cumulate”. The Mariana samples are all geochemically similar to the “evolved” group and also have elevated δ26Mg values.
Unlike in the Marianas, the magma differentiation paths of Lesser Antilles arc lavas are well constrained (see SI section 3.8). Two liquid lines of descent have been identified: one involves co-crystallisation of olivine and clinopyroxene at high pressure, while at low pressures, olivine is the only liquidus phase (e.g., Stamper et al., 2014
Stamper, C.C., Melekhova, E., Blundy, J.D., Arculus, R.J., Humphreys, M.C.S., Brooker, R.A. (2014) Oxidised phase relations of a primitive basalt from Grenada, Lesser Antilles. Contributions to Mineralogy and Petrology 167, 954. https://doi.org/10.1007/s00410-013-0954-6
). We model the variations in δ26Mg that result from these two differentiation trends using our new Δ26/24MgOl/melt and Δ26/24MgCpx/melt (derived by combining Δ26/24MgOl/melt with literature Δ26/24MgCpx/Ol, SI section 3.9). Both 20 % olivine fractionation (Ol line) and the co-crystallisation of 20 % olivine and 20 % clinopyroxene (Ol + Cpx line) reproduce the elevated δ26Mg of many evolved lavas (Fig. 4c), as a result of olivine crystallisation. The lower δ26Mg data of the “xeno” samples are well reproduced by olivine accumulation (Fig. 4d) in more evolved samples. The composition of the troctolite cumulate (LAE3) is consistent with its crystallisation from a melt with elevated δ26Mg ≈ −0.16 ‰ given our Δ26/24MgOl/melt. We infer that the relatively low MgO and high δ26Mg of the Mariana samples (Fig. 2b) reflect a differentiation process similar to that experienced by the Lesser Antilles “evolved” samples.In conclusion, we have replicated elevated δ26Mg in Lesser Antilles arc lavas and further shown this to be a common characteristic in Mariana arc lavas. However, our more precise analyses reveal that the elevated δ26Mg is only evident in more evolved, basaltic andesite compositions. Using the equilibrium Δ26/24MgOl/melt we determined, we can model the increase in δ26Mg from MORB-like values in primitive arc lavas as a natural consequence of magmatic differentiation. This illustrates the importance of a well determined solid-melt fractionation factor in interpreting subtle differences in stable isotope ratios.
top
Acknowledgements
Carolyn Taylor and Stuart Kearns are thanked for their help in the lab. We also thank Richard Robertson and Bernard Bourdon for providing Lesser Antilles and Pitcairn samples respectively. This work was funded by NERC grant NE/L007428/1 and ERC Grant 885531 NONUNE. X.-N. Liu was supported by a CSC scholarship.
Editor: Ambre Luguet
top
References
Aubaud, C., Pineau, F., Hekinian, R., Javoy, M. (2006) Carbon and hydrogen isotope constraints on degassing of CO2 and H2O in submarine lavas from the Pitcairn hotspot (South Pacific). Geophysics Research Letters 33, L02308. https://doi.org/10.1029/2005GL024907
 Show in context
Show in context The Hawaiian and Pitcairn samples have water contents of ∼0.1–0.4 wt. % (e.g., Hauri, 2002) and ∼1.2 wt. % (Aubaud et al., 2006), respectively. MORB samples generally have low water contents around 0.1 to 0.2 wt. % (e.g., Sobolev et al., 1996).
View in article
Coath, C.D., Elliott, T., Hin, R.C. (2017) Double-spike inversion for three-isotope systems. Chemical Geology 451, 78–89. https://doi.org/10.1016/j.chemgeo.2016.12.025
 Show in context
Show in context Here, we employ the high precision attainable using critical mixture double-spiking (Coath et al., 2017; Hin et al., 2017) to determine Δ26/24MgOl/melt for five carefully selected, equilibrated olivine-glass pairs from ocean island and mid-ocean ridge basalts.
View in article
Gervasoni, F., Klemme, S., Rohrbach, A., Griltzner, T., Berndt, J. (2017) Experimental constraints on mantle metasomatism caused by silicate and carbonate melts. Lithos 282–283, 173–186. https://doi.org/10.1016/j.lithos.2017.03.004
 Show in context
Show in context Hydrous melts generated from eclogite are again enriched in water (over 15 wt. %) but also low in Mg (less than 2.5 wt. %) (Gervasoni et al., 2017).
View in article
Hauri, E. (2002) SIMS analysis of volatiles in silicate glasses, 2: isotopes and abundances in Hawaiian melt inclusions. Chemical Geology 183, 115–141. https://doi.org/10.1016/S0009-2541(01)00374-6
 Show in context
Show in context The Hawaiian and Pitcairn samples have water contents of ∼0.1–0.4 wt. % (e.g., Hauri, 2002) and ∼1.2 wt. % (Aubaud et al., 2006), respectively. MORB samples generally have low water contents around 0.1 to 0.2 wt. % (e.g., Sobolev et al., 1996).
View in article
Hin, R.C., Coath, C.D., Carter, P.J., Nimmo, F., Lai, Y.-J., Pogge von Strandmann, P.A.E., Willbold, M., Leinhardt, Z.M., Walter, M.J., Elliott, T. (2017) Magnesium isotope evidence that accretional vapour loss shapes planetary compositions. Nature 549, 511–515. https://doi.org/10.1038/nature23899
 Show in context
Show in context As the third most abundant element in the silicate Earth, there is much interest in the Mg isotopic systematics of magmatic rocks for improving our understanding of igneous processes and broader planetary evolution (e.g., Teng et al., 2010; Hin et al., 2017; Teng, 2017).
View in article
Here, we employ the high precision attainable using critical mixture double-spiking (Coath et al., 2017; Hin et al., 2017) to determine Δ26/24MgOl/melt for five carefully selected, equilibrated olivine-glass pairs from ocean island and mid-ocean ridge basalts.
View in article
We analysed micro-drilled spots (cones of 100 μm depth and largest diameter) in these olivines and hand-picked coexisting glass and processed the samples for Mg isotope analysis by critical mixture double-spiking as reported in Hin et al. (2017).
View in article
Jambon, A., Boudouma, O., Fonteilles, M., Le Guillou, C., Badia, D., Barrat, J.A. (2008) Petrology and mineralogy of the angrite Northwest Africa 1670. Meteoritics & Planetary Science 43, 1783–1795. https://doi.org/10.1111/j.1945-5100.2008.tb00643.x
 Show in context
Show in context Equally, the bulk olivine phenocrysts in NWA1670 are not in equilibrium with the groundmass, given their Mg/(Mg + Fe) decrease from ∼0.9 in their core to ∼0.6 in their rim (Jambon et al., 2008).
View in article
Jeffcoate, A.B., Elliott, T., Kasemann, S.A., Ionov, D., Cooper, K., Brooker, R. (2007) Li isotope fractionation in peridotites and mafic melts. Geochimica et Cosmochimica Acta 71, 202–218. https://doi.org/10.1016/j.gca.2006.06.1611
 Show in context
Show in context Prior work on the Hawaii and Pitcairn samples (Jeffcoate et al., 2007) showed an absence of Li isotopic fractionation across the olivine phenocrysts, documenting an absence of late-stage diffusive fractionation.
View in article
Kessel, R., Schmidt, M.W., Ulmer, P., Pettke, T. (2005) Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120–180 km depth. Nature 437, 724–727. https://doi.org/10.1038/nature03971
 Show in context
Show in context Fluids released from subducted oceanic crust and serpentinite are naturally water-rich, over 50 wt. % and 80 wt. % H2O respectively, while the MgO concentrations of these fluids are generally low, less than 1 wt. % in oceanic crust dehydration fluid and about 6 wt. % in serpentinite dehydration fluid (Manning, 2004; Kessel et al., 2005; Scambelluri et al., 2015).
View in article
Manning, C.E. (2004) The chemistry of subduction-zone fluids. Earth and Planetary Science Letters 223, 1–16. https://doi.org/10.1016/j.epsl.2004.04.030
 Show in context
Show in context Fluids released from subducted oceanic crust and serpentinite are naturally water-rich, over 50 wt. % and 80 wt. % H2O respectively, while the MgO concentrations of these fluids are generally low, less than 1 wt. % in oceanic crust dehydration fluid and about 6 wt. % in serpentinite dehydration fluid (Manning, 2004; Kessel et al., 2005; Scambelluri et al., 2015).
View in article
Mookherjee, M., Stixrude, L., Karki, B. (2008) Hydrous silicate melt at high pressure. Nature 452, 983–986. https://doi.org/10.1038/nature06918
 Show in context
Show in context Water decreases the Mg-O coordination number of silicate melt (Mookherjee et al., 2008), which may lead to a preference for heavier isotopes.
View in article
Putirka, K.D. (2005) Mantle potential temperatures at Hawaii, Iceland, and the mid‐ocean ridge system, as inferred from olivine phenocrysts: Evidence for thermally driven mantle plumes. Geochemistry, Geophysics, Geosystems 6, Q05L08. https://doi.org/10.1029/2005GC000915
 Show in context
Show in context Olivine thermometry (Putirka, 2005) indicates that equilibration temperatures of the five samples varied between 1379 and 1481 K (see SI section 1.2).
View in article
Scambelluri, M., Pettke, T., Cannao, E. (2015) Fluid-related inclusions in Alpine high-pressure peridotite reveal trace element recycling during subduction-zone dehydration of serpentinized mantle (Cima di Gagnone, Swiss Alps). Earth and Planetary Science Letters 429, 45–59. https://doi.org/10.1016/j.epsl.2015.07.060
 Show in context
Show in context Fluids released from subducted oceanic crust and serpentinite are naturally water-rich, over 50 wt. % and 80 wt. % H2O respectively, while the MgO concentrations of these fluids are generally low, less than 1 wt. % in oceanic crust dehydration fluid and about 6 wt. % in serpentinite dehydration fluid (Manning, 2004; Kessel et al., 2005; Scambelluri et al., 2015).
View in article
Schiller, M., Dallas, J.A., Creech, J., Bizzarro, M., Baker, J.A. (2017) Tracking the formation of magma oceans in the Solar System using stable magnesium isotopes. Geochemical Perspectives Letters 3, 22–31. https://doi.org/10.7185/geochemlet.1703
 Show in context
Show in context Schiller et al. (2017) analysed the Mg isotopic compositions of olivines and rapidly cooled groundmass (dominantly intergrown plagioclase and clinopyroxene) from an angrite meteorite (NWA1670).
View in article
Although Schiller et al. (2017) reported those samples closest to elemental Fe-Mg equilibrium, these samples evidently do not constitute an equilibrium assemblage necessary for reliable determination of a fractionation factor.
View in article
Sobolev, A.V., Chaussidon, M. (1996) H2O concentrations in primary melts from supra-subduction zones and mid-ocean ridges: Implications for H2O storage and recycling in the mantle. Earth and Planetary Science Letters 137, 45–55. https://doi.org/10.1016/0012-821X(95)00203-O
 Show in context
Show in context The Hawaiian and Pitcairn samples have water contents of ∼0.1–0.4 wt. % (e.g., Hauri, 2002) and ∼1.2 wt. % (Aubaud et al., 2006), respectively. MORB samples generally have low water contents around 0.1 to 0.2 wt. % (e.g., Sobolev et al., 1996).
View in article
Soderman, C.R., Shorttle, O., Matthews, S., Williams, H.M. (2022) Global trends in novel stable isotopes in basalts: Theory and observations. Geochimica et Cosmochimica Acta 318, 388–414. https://doi.org/10.1016/j.gca.2021.12.008
 Show in context
Show in context A burgeoning literature in the mass-dependent variability of major rock forming elements in magmatic samples have the potential to provide novel constraints on source mineralogy and melting processes (e.g., Teng et al., 2017; Soderman et al., 2022), but in many cases the key parameter of isotopic fractionation, i.e. the fractionation factor between solid and melt, is insufficiently well constrained to make the most of the observations.
View in article
Stamper, C.C., Melekhova, E., Blundy, J.D., Arculus, R.J., Humphreys, M.C.S., Brooker, R.A. (2014) Oxidised phase relations of a primitive basalt from Grenada, Lesser Antilles. Contributions to Mineralogy and Petrology 167, 954. https://doi.org/10.1007/s00410-013-0954-6
 Show in context
Show in context Two liquid lines of descent have been identified: one involves co-crystallisation of olivine and clinopyroxene at high pressure, while at low pressures, olivine is the only liquidus phase (e.g., Stamper et al., 2014).
View in article
Teng, F.-Z. (2017) Magnesium Isotope Geochemistry. Reviews in Mineralogy and Geochemistry 82, 219–287. https://doi.org/10.2138/rmg.2017.82.7
 Show in context
Show in context As the third most abundant element in the silicate Earth, there is much interest in the Mg isotopic systematics of magmatic rocks for improving our understanding of igneous processes and broader planetary evolution (e.g., Teng et al., 2010; Hin et al., 2017; Teng, 2017).
View in article
Teng, F.-Z., Wadhwa, M., Helz, R.T. (2007) Investigation of magnesium isotope fractionation during basalt differentiation: Implications for a chondritic composition of the terrestrial mantle. Earth and Planetary Science Letters 261, 84–92. https://doi.org/10.1016/j.epsl.2007.06.004
 Show in context
Show in context The absence of systematic Mg isotopic variation in a suite of whole rocks with variable MgO contents from Kilauea Iki (Teng et al., 2007) is often cited as evidence for the absence of Mg isotope fractionation during crystallisation.
View in article
Teng, F.-Z., Li, W.-Y., Ke, S., Marty, B., Dauphas, N., Huang, S., Wu, F.-Y., Pourmand, A. (2010) Magnesium isotopic composition of the Earth and chondrites. Geochimica et Cosmochimica Acta 74, 4150–4166. https://doi.org/10.1016/j.gca.2010.04.019
 Show in context
Show in context As the third most abundant element in the silicate Earth, there is much interest in the Mg isotopic systematics of magmatic rocks for improving our understanding of igneous processes and broader planetary evolution (e.g., Teng et al., 2010; Hin et al., 2017; Teng, 2017).
View in article
Teng, F.-Z., Dauphas, N., Helz, R.T., Gao, S., Huang, S. (2011) Diffusion-driven magnesium and iron isotope fractionation in Hawaiian olivine. Earth and Planetary Science Letters 308, 317–324. https://doi.org/10.1016/j.epsl.2011.06.003
 Show in context
Show in context These authors also reprocessed Mg isotope measurements of olivine and groundmass from Teng et al. (2011) to calculate a fractionation factor.
View in article
Teng, F.-Z., Hu, Y., Chauvel, C. (2016) Magnesium isotope geochemistry in arc volcanism. Proceedings of the National Academy of Sciences 113, 7082–7087. https://doi.org/10.1073/pnas.1518456113
 Show in context
Show in context Using this value, we then explore the subtly elevated δ26Mg in arc lavas (Teng et al., 2016) with new analyses of a suite of samples from the Lesser Antilles, as well as a set of archetypical mafic samples from the Mariana arc.
View in article
Teng et al. (2016) analysed arc lavas from Martinique, Lesser Antilles, and reported δ26Mg slightly higher than MORB (Fig. 2a), which they attributed to subduction zone processes.
View in article
As discussed by Teng et al. (2016), however, it is not clear that subduction zone components have the leverage to sufficiently perturb the δ26Mg of the mantle wedge, given mixing constraints from other elemental and isotopic tracers.
View in article
δ26Mg against MgO for (a) Lesser Antilles lavas from Teng et al. (2016) and (b) Lesser Antilles and Mariana lavas from this study.
View in article
Teng, F.-Z., Watkins, J.M., Dauphas, N. (2017) Non-Traditional Stable Isotopes. Reviews in Mineralogy and Geochemistry 82. The Mineralogical Society of America. https://doi.org/10.1515/9783110545630
 Show in context
Show in context A burgeoning literature in the mass-dependent variability of major rock forming elements in magmatic samples have the potential to provide novel constraints on source mineralogy and melting processes (e.g., Teng et al., 2017; Soderman et al., 2022), but in many cases the key parameter of isotopic fractionation, i.e. the fractionation factor between solid and melt, is insufficiently well constrained to make the most of the observations.
View in article
Ulmer, P. (1989) The dependence of the Fe2+-Mg cation-partitioning between olivine and basaltic liquid on pressure, temperature and composition. Contributions to Mineralogy and Petrology 101, 261–273. https://doi.org/10.1007/BF00375311
 Show in context
Show in context We rejected any olivine which had an olivine-glass Fe-Mg exchange coefficient outside the range of equilibrium values (Ulmer, 1989), namely KDOl/melt(Fe-Mg) from 0.28 to 0.32 (Fig. 1b).
View in article
top
Supplementary Information
The Supplementary Information includes:
- 1. Olivine-Glass Pair Samples
- 2. Analytical Methods
- 3. Oceanic Arc Lava Samples
- Tables S-1 to S-7
- Figures S-1 to S-14
- Supplementary Information References
Download Table S-6 (Excel).
Download Table S-7 (Excel).
Download the Supplementary Information (PDF).
Figures
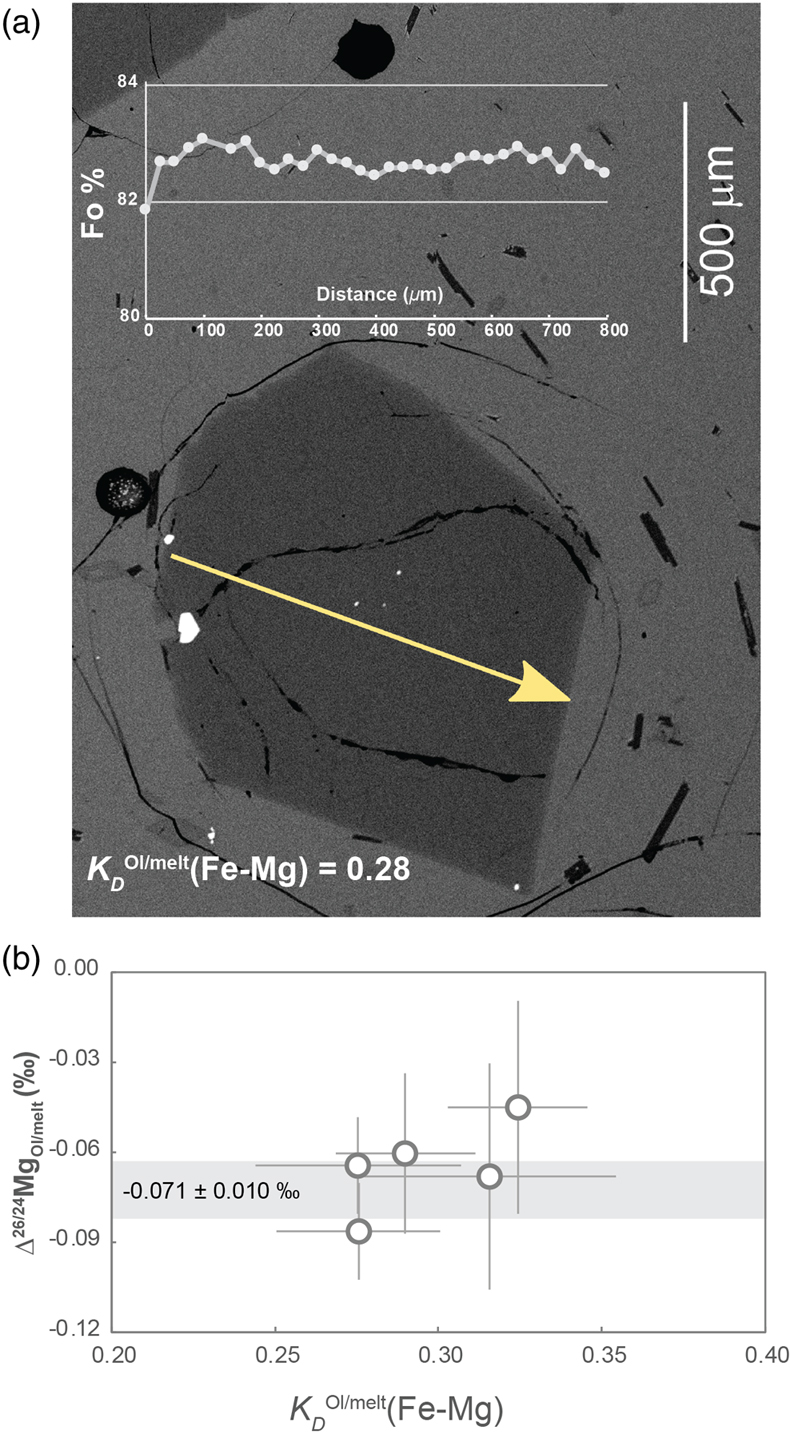
Figure 1 (a) Backscattered electron microscope image of an olivine-glass pair (PN3-10), together with electron microprobe traverse of olivine Fo content. (b) Measured Δ26/24MgOl/melt, corrected to a common temperature (1438 K), plotted against Fe-Mg distribution coefficients.
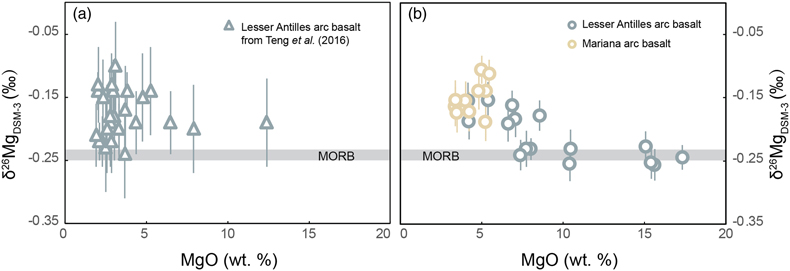
Figure 2 δ26Mg against MgO for (a) Lesser Antilles lavas from Teng et al. (2016)
Teng, F.-Z., Hu, Y., Chauvel, C. (2016) Magnesium isotope geochemistry in arc volcanism. Proceedings of the National Academy of Sciences 113, 7082–7087. https://doi.org/10.1073/pnas.1518456113
and (b) Lesser Antilles and Mariana lavas from this study. MORB reference value is −0.24 ± 0.01 ‰ (2 s.e.); see Supplementary Information section 3.4.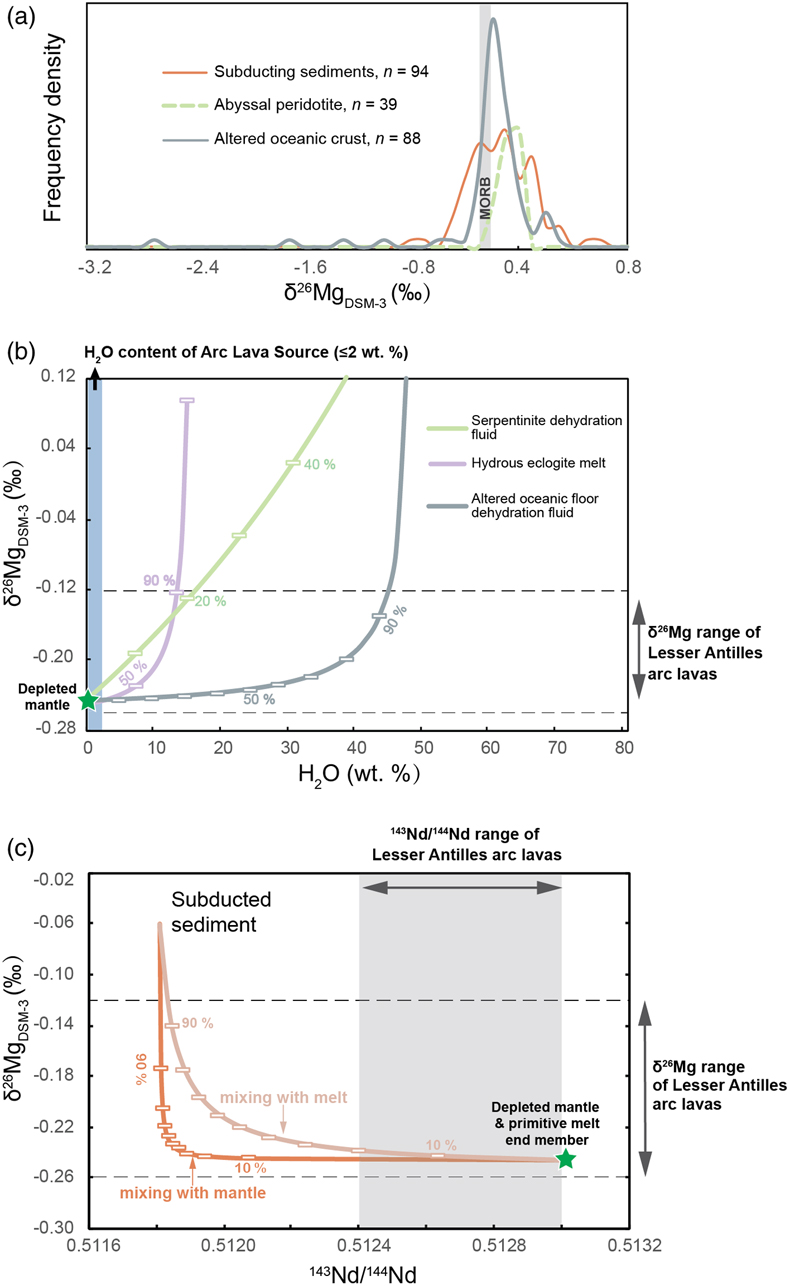
Figure 3 (a) Frequency density plot of δ26Mg of subducted materials. (b) Modelled δ26Mg against H2O from mixing depleted mantle with dehydration fluids and hydrous melt. (c) Modelled δ26Mg against 143Nd/144Nd from mixing depleted mantle with subducted sediments (See SI section 3.5 for more details).
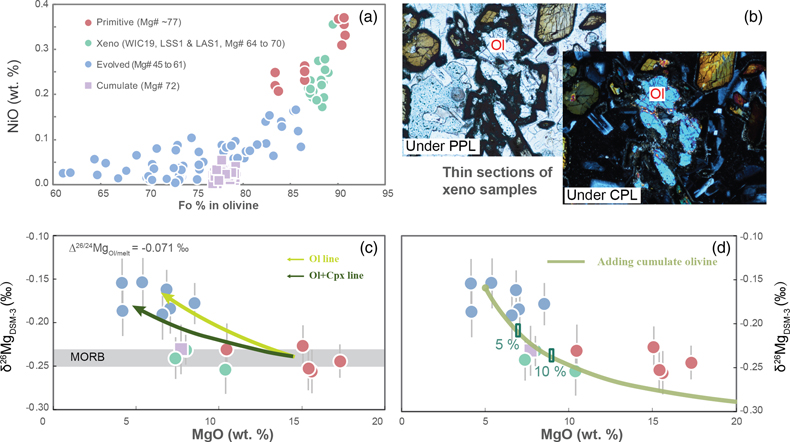
Figure 4 (a) Electron microprobe analyses of Lesser Antilles olivines plotted in groups according to bulk Mg# of host magmas. (b) Thin-section images of disequilibrium olivines in “xeno” group samples. (c) Modelled δ26Mg evolution during two main differentiation paths. (d) Modelled effect on δ26Mg of cumulate olivine addition to an “evolved” sample composition.