Sun flare activity may solve unknown source of helium-3 in the atmosphere
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:486Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
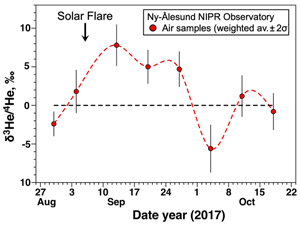
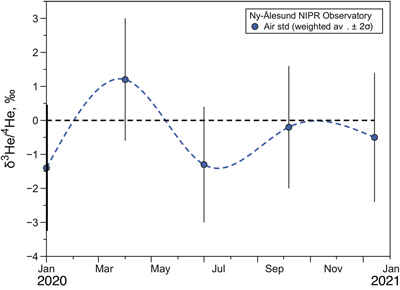 Figure 1 Temporal variation of atmospheric 3He/4He weighted averages in Ny-Ålesund, Svalbard, near the North Pole, from January 2020 to January 2021. The black dotted line and blue dotted curve show the standard atmospheric helium value and a spline curve approximating the time variability of the weighted average 3He/4He ratio for each sampling day, respectively. Bars indicate 2σ internal errors (see Supplementary Information for equations). | 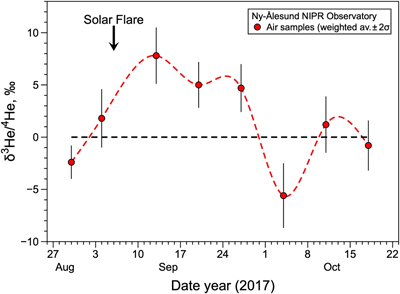 Figure 2 Temporal variation of atmospheric 3He/4He weighted averages in Ny-Ålesund, Svalbard, near the North Pole, around the time of the X9.3 solar flare event. The black dotted line and red dotted curve show the standard atmospheric helium value and a spline curve approximating the time variability of the weighted average 3He/4He ratio for each sampling day, respectively. Bars indicate 2σ internal errors (see Supplementary Information for equations). |
| Figure 1 | Figure 2 |
top
Introduction
Helium concentration in the atmosphere is determined by the balance between degassing from the solid Earth and the thermal and/or non-thermal escape from the upper atmosphere (MacDonald, 1963
MacDonald, G.J.F. (1963) The escape of helium from the Earth’s atmosphere. Reviews of Geophysics 1, 305–349. https://doi.org/10.1029/RG001i003p00305
). However, accelerated extraction and consumption of fossil fuels since the beginning of the 20th century may have altered this balance. Natural gas contains about 0.25 % helium (Zartman et al., 1961Zartman, R.E., Wasserburg, G.J., Reynolds, J.H. (1961) Helium, argon and carbon in some natural gases. Journal of Geophysical Research 66, 277–306. https://doi.org/10.1029/JZ066i001p00277
) with an isotopic ratio (Aldrich and Nier, 1948Aldrich, L.T., Nier, A.O. (1948) The occurrence of He-3 in natural sources of helium. Physical Review 74, 1590–1594. https://doi.org/10.1103/PhysRev.74.1590
) much smaller than that of the atmospheric value (1.382 × 10-6; Sano et al., 2013Sano, Y., Marty B., Burnard, P. (2013) Noble Gases in the Atmosphere. In: Burnard, P. (Ed.) The Noble Gases as Geochemical Tracers, Advances in Isotope Geochemistry. Springer-Verlag, Berlin, 17–31. https://doi.org/10.1007/978-3-642-28836-4_2
), because of the dominance of crustal 4He compared to 3He in fossil fuels. If a large amount of helium from extracted natural gas is added to the atmosphere, the 4He concentration in air should increase with time, while the isotope ratio 3He/4He should decrease. Oliver et al. (1984)Oliver, B.M., Bradley, J.G., Farrar, H. (1984) Helium concentration in the Earth’s lower atmosphere. Geochimica et Cosmochimica Acta 48, 1759–1767. https://doi.org/10.1016/0016-7037(84)90030-9
first reported a rough estimate of the 4He released in the atmosphere due to natural gas production (3–12 × 1016 cm3 STP between 1939 and 1981). Sano et al. (1989)Sano, Y., Wakita, H., Makide, Y., Tominaga, T. (1989) A ten year decrease in the atmospheric helium isotope ratio possibly caused by human activity. Geophysical Research Letters 16, 1371–1374. https://doi.org/10.1029/GL016i012p01371
measured a temporal decrease in the helium isotope ratio in the atmosphere of ∼1 x 10-9/year. Although there has been much debate since then, recent analyses of the 3He/4He ratio in sampled air of different epochs have concluded that the atmospheric 3He/4He ratio is rather constant (Lupton and Evans, 2004Lupton, J., Evans, L. (2004) The atmospheric helium isotope ratio: is it changing? Geophysical Research Letters 31, 1–4. https://doi.org/10.1029/2004GL020041
; Mabry et al., 2015Mabry, J., Lan, T., Boucher, C., Burnard, P.G., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the Cape Grim Air Archive. Earth and Planetary Science Letters 428, 134–138. https://doi.org/10.1016/j.epsl.2015.07.035
; Boucher et al., 2018Boucher, C., Marty, B., Zimmermann, L., Langenfelds, R. (2018) Atmospheric helium isotopic ratio from 1910 to 2016 recorded in stainless steel containers. Geochemical. Perspectives Letters 6, 23–27. https://doi.org/10.7185/geochemlet.1804
). Recently, Birner et al. (2022)Birner, B., Severinghaus, J., Paplawsky, B., Keeling, R.F. (2022) Increasing atmospheric helium from fossil fuel exploitation. Nature Geoscience 115, 346–348. https://doi.org/10.1038/s41561-022-00932-3
developed a method to measure helium concentrations with high precision (±0.07 ‰) and analysed atmospheric samples from the past 46 years. The results showed an increase in 4He concentration of 1.9 ‰ and these authors concluded that the increase was due to anthropogenic release of helium from natural gas. This apparent discrepancy – i.e. constant 3He/4He ratio but contemporary increase of anthropogenically-released 4He – suggests that an unknown source of 3He is compensating for the addition of crustal 4He (Birner et al., 2022Birner, B., Severinghaus, J., Paplawsky, B., Keeling, R.F. (2022) Increasing atmospheric helium from fossil fuel exploitation. Nature Geoscience 115, 346–348. https://doi.org/10.1038/s41561-022-00932-3
). This unknown source of 3He is here defined as the “missing helium-3”. Knowing the origin and fate of this source of helium is important, given that 3He is an important but scarce resource on Earth, necessary for the development and operation of nuclear fusion reactors and for cryogenic applications. Based on changes in the atmospheric helium isotope ratios measured in polar regions, the present study suggests that solar flares may be the source that supplies the 3He required to account for the near-constant 3He/4He atmospheric ratio.top
Samples
Atmospheric samples used in this study were collected at Ny-Ålesund (78°55'N, 11°56'E), Svalbard islands, near the North Pole, in the Norwegian-controlled International Observation Village. At Ny-Ålesund, atmospheric samples are usually collected weekly by collaborators at the Norwegian Polar Institute and transported to Japan approximately every two months. Each sample is collected using a diaphragm pump from an air intake on the roof of the Japanese base in Ny-Ålesund, passed through a water trap cooled to −78 °C, and pressurised into a pre-evacuated 800 ml stainless steel container. On September 6th, 2017, a massive solar explosion, or solar flare, occurred. The solar wind was expected to reach the Earth on September 8th (NASA, 2017
NASA (2017) Sun Erupts With Significant Flare. (Date of access: 29 November 2021) https://www.nasa.gov/feature/goddard/2017/active-region-on-sun-continues-to-emit-solar-flares
). Therefore, the dates of air sample collection were set for eight days about one week apart, from August 30th to October 18th, 2017. These samples were rapidly airlifted from Ny-Ålesund to Japan. At the end of October 2017, the atmospheric samples preserved in metal cylinders stored at the National Institute of Polar Research were transferred into lead glass reservoirs in Tachikawa City, Tokyo. The samples were brought to the Atmosphere and Ocean Research Institute of The University of Tokyo (Kashiwa, Chiba) and analysed for helium isotope ratios in May 2018.top
Analytical Procedures and Results
Noble gases contained in the samples were purified by an activated charcoal trap that was cooled to liquid nitrogen temperature together with a heated titanium getter. Finally, helium was separated and purified from neon in a cryogenic trap containing activated charcoal cooled to 40 K. Helium isotope ratios were measured with a double-collector noble gas mass spectrometer, GV® Helix SFT (Sano et al., 2008
Sano, Y., Tokutake, T., Takahata, N. (2008) Accurate measurement of atmospheric helium isotopes. Analytical Sciences 24, 521–525. https://doi.org/10.2116/analsci.24.521
). Measurements were performed 2–3 times for each individual sample and compared to a synthesised helium standard, HESJ (Matsuda et al., 2002Matsuda, J., Matsumoto, T., Sumino, H., Nagao, K., Yamamoto, J., Miura, Y., Kaneoka, I., Takahata, T., Sano, Y. (2002) The 3He/4He ratio of the new internal He Standard of Japan (HESJ). Geochemical Journal 36, 191–195. https://doi.org/10.2343/geochemj.36.191
) with an accepted 3He/4He value of 20.63 ± 0.10 Ra. The measured 3He/4He ratios were then normalised to the standard value of the atmosphere, 1.382 × 10-6 (Sano et al., 2013Sano, Y., Marty B., Burnard, P. (2013) Noble Gases in the Atmosphere. In: Burnard, P. (Ed.) The Noble Gases as Geochemical Tracers, Advances in Isotope Geochemistry. Springer-Verlag, Berlin, 17–31. https://doi.org/10.1007/978-3-642-28836-4_2
).In order to determine the atmospheric helium isotopic background at the Ny-Ålesund site, air samples were collected from January 2020 to January 2021, about three months apart, during a quiescent period of solar activity. These data are presented in Table S-1 and the 3He/4He variations are shown in Figure 1. The 3He/4He ratios vary from −3.0 ‰ to + 3.0 ‰, consistent with our laboratory air standard collected in the Kashiwa Park (Latitude, 35.8949; longitude, 139.9415) close to the campus of University of Tokyo, within experimental error. The weighted mean and internal error on 15 measurements are −0.47 ± 0.79 (see Supplementary Information for calculation), not distinguishable from the air value, assuming uncertainties. Therefore, there is no anomaly of the atmospheric helium isotopic ratio during the January 2020 to January 2021 period and the analytical system can be considered significantly stable.

Figure 1 Temporal variation of atmospheric 3He/4He weighted averages in Ny-Ålesund, Svalbard, near the North Pole, from January 2020 to January 2021. The black dotted line and blue dotted curve show the standard atmospheric helium value and a spline curve approximating the time variability of the weighted average 3He/4He ratio for each sampling day, respectively. Bars indicate 2σ internal errors (see Supplementary Information for equations).
The helium isotopic ratios of analysed Arctic air during a solar flare event are shown in Table S-2 and reported as δ values (deviation from the Kashiwa air standard expressed in part per thousand). Three runs were made on the same sample, with the second run being the most stable; for the first and third runs, some of the data were erroneous due to instrumental fluctuations and are not included in the discussion due to large uncertainty. The 3He/4He ratios of Arctic air varied from −5.6 ± 3.1 ‰ to +8.4 ± 3.4 ‰ (Table S-2). The 3He/4He ratios of 12 of the 18 samples agreed with the atmospheric values of Kashiwa air standard, within the error range. Of the six data showing anomalies, the five collected on September 13th, 20th, and 27th of 2017 showed a clear excess of 3He compared with the atmospheric ratio (Fig. 2). These data are noteworthy because they were taken immediately after the massive solar flare event of September 6th that registered X9.3 on the solar storm scale, which divides solar flares according to their strength (NASA, 2017
NASA (2017) Sun Erupts With Significant Flare. (Date of access: 29 November 2021) https://www.nasa.gov/feature/goddard/2017/active-region-on-sun-continues-to-emit-solar-flares
). The smallest events are A-class (near background levels), followed by B, C, M and X, with each letter class having a finer scale from 1 to 9, except class X which can go beyond it (with X28, detected in 2003, being the highest solar flare ever detected). Events equivalent and larger than X9.3 have occurred 27 times from 1975 to 2018 (NIICT, 2018NIICT (2018) Space Weather Forecast. (Date of access: 10 June 2018) https://web.archive.org/web/20180610090019/https://swc.nict.go.jp/forecast/majorflares.html
), so their occurrence is not significantly unusual. The other 3He/4He data are consistent with the standard atmospheric ratio, except for the data from the first run on October 4th, which shows a 3He deficiency for an unknown reason. We therefore propose that the source of the “missing helium-3” excess is the injection of solar wind-derived 3He into the atmosphere, enhanced by the solar flare as shown in Figure 2. This study illustrates for the first time the detection of a local excess of 3He in the lower atmosphere due to a solar flare.
Figure 2 Temporal variation of atmospheric 3He/4He weighted averages in Ny-Ålesund, Svalbard, near the North Pole, around the time of the X9.3 solar flare event. The black dotted line and red dotted curve show the standard atmospheric helium value and a spline curve approximating the time variability of the weighted average 3He/4He ratio for each sampling day, respectively. Bars indicate 2σ internal errors (see Supplementary Information for equations).
top
Discussion
The atmospheric 3He concentration should be 7.24 pptv, based on the helium concentration in the atmosphere of 5.24 ppmv (Gluckauf and Paneth, 1946
Gluckauf, E., Paneth, F.A. (1946) The helium content of atmospheric air. Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences 185, 89–98. https://doi.org/10.1098/rspa.1946.0006
) and a 3He/4He of 1.382 × 10-6 (Sano et al., 2013Sano, Y., Marty B., Burnard, P. (2013) Noble Gases in the Atmosphere. In: Burnard, P. (Ed.) The Noble Gases as Geochemical Tracers, Advances in Isotope Geochemistry. Springer-Verlag, Berlin, 17–31. https://doi.org/10.1007/978-3-642-28836-4_2
). A dominant source of atmospheric 3He is the Earth’s mantle through volcanic activity and degassing from mid-oceanic ridges (Clarke et al., 1969Clarke, W.B., Beg, M.A., Craig, H. (1969) Excess 3He in the sea: evidence for terrestrial primordial helium. Earth and Planetary Science Letters 6, 213–220. https://doi.org/10.1016/0012-821X(69)90093-4
) and subduction zones (Sano and Wakita, 1985Sano, Y., Wakita, H. (1985) Geographical distribution of 3He/4He ratios in Japan: Implications for arc tectonics and incipient magmatism. Journal of Geophysical Research 90, 8729–8741. https://doi.org/10.1029/JB090iB10p08729
). Another source is the interplanetary zone (Ozima and Podosek, 1983Ozima, M., Podosek, F.A. (1983) Noble gas geochemistry. Cambridge University Press, Cambridge.
). When an explosive event (solar flare) occurs in the sun, a plasma plume (solar wind) containing a large amount of 3He is generated and approaches the Earth in a path along the magnetic field lines. This 3He is captured as it passes through the Earth’s magnetosphere and is precipitating into the upper atmosphere of the polar regions. This air, locally mixed with air downward, may tentatively produce a high 3He/4He ratio. This mechanism is associated with the formation of auroras and is called Auroral Helium Precipitation (Buhler et al., 1976Buhler, F., Axford, W.I., Chivers, H.J.A., Marti, K. (1976) Helium Isotopes in an Aurora. Journal of Geophysical Research 81, 111–115. https://doi.org/10.1029/JA081i001p00111
), and it may explain excess 3He observed in this work. Other sources include the decay of tritium produced by nuclear weapons and nuclear reactors, but it is thought to be negligible in amount (Lupton and Evans, 2004Lupton, J., Evans, L. (2004) The atmospheric helium isotope ratio: is it changing? Geophysical Research Letters 31, 1–4. https://doi.org/10.1029/2004GL020041
). If the solar wind, enhanced by solar flares, were fed into the atmosphere by the auroral precipitation mechanism, it would increase the polar atmospheric helium isotope ratio, as shown in Figure 2. The helium would then be diluted by diffusion and the excess 3He would disappear soon after. Mixing of atmosphere along the latitude from east to west may take a few weeks by air transport, which is much faster than that of a few months along the longitude from north to south, generally observed by long-lived tracers (Jacob, 1999Jacob, D.J. (1999) Introduction to Atmospheric Chemistry. Princeton University Press, Princeton.
). In the present case, the lifetime of excess 3He may be short when compared with the mixing time. This discrepancy may be resolved by analysing more case studies of solar flare events. In addition to horizontal mixing of the atmosphere, vertical mixing must also be considered. Experiments using rockets have observed 3He of solar wind origin at an altitude of about 150 km (Axford et al., 1972Axford, W.I., Buhler, F., Chivers, H.J.A., Eberhardt, P., Geiss, J. (1972) Auroral Helium Precipitation. Journal of Geophysical Research 77, 6724–6730. https://doi.org/10.1029/JA077i034p06724
). However, there is no report on the mixing mechanism from the thermosphere to the ground surface. According to turbulent diffusion models, vertical mixing between the troposphere and the surface takes more than one month (Jacob, 1999Jacob, D.J. (1999) Introduction to Atmospheric Chemistry. Princeton University Press, Princeton.
). In our observations, the time difference between a solar flare observation and an increase of 3He/4He in the atmosphere is only a few days (Fig. 2). Thus, the change in helium is faster than the model mixing. It is beyond the scope of this paper to provide a physical explanation for the cause of the rate being too fast and the reason will be clarified in future studies. In addition, the surface region most strongly influenced by the stratosphere is found substantially south of the sampling location (Škerlak et al., 2014Škerlak, B., Sprenger, M., Wernli, H. (2014) A global climatology of stratosphere–troposphere exchange using the ERA-Interim data set from 1979 to 2011. Atmospheric Chemistry and Physics 14, 913–937. https://doi.org/10.5194/acp-14-913-2014
). The 3He signals originating from the stratosphere should therefore be larger at lower latitudes and we might underestimate the effect of auroral precipitation in Svalbard.To test the hypothesis of downward injection of solar 3He, a discussion on the mass balance of 3He is necessary. The observed increase in atmospheric helium concentration (Birner et al., 2022
Birner, B., Severinghaus, J., Paplawsky, B., Keeling, R.F. (2022) Increasing atmospheric helium from fossil fuel exploitation. Nature Geoscience 115, 346–348. https://doi.org/10.1038/s41561-022-00932-3
) suggests an anthropogenic supply of crustal 4He of 3.9 ± 0.3 × 1010 mol/year (2σ). Birner et al. (2022)Birner, B., Severinghaus, J., Paplawsky, B., Keeling, R.F. (2022) Increasing atmospheric helium from fossil fuel exploitation. Nature Geoscience 115, 346–348. https://doi.org/10.1038/s41561-022-00932-3
calculated that to compensate for the addition of anthropogenic 4He and to preserve a constant atmospheric 3He/4He ratio, the annual addition of 6.3 ± 2.5 × 104 mol of 3He is required. This is equivalent to an annual change in the atmospheric helium isotope ratio of +0.049 ± 0.020 ‰ (Birner et al., 2022Birner, B., Severinghaus, J., Paplawsky, B., Keeling, R.F. (2022) Increasing atmospheric helium from fossil fuel exploitation. Nature Geoscience 115, 346–348. https://doi.org/10.1038/s41561-022-00932-3
). However, this calculation requires that the 3He/4He of natural gases is 1.63 ± 0.68 × 10-6, which is much higher than the typical crustal ratio of 2 × 10-8, and it can be found only in hydrocarbons settled in extensional sedimentary basins where crustal and mantle helium can be found together (e.g., Ballentine et al., 1991Ballentine, C.J., O’Nions, R.K., Oxburgh, E.R., Horvarth, F., Deàk, J. (1991) Rare gas constraints on hydrocarbon accumulation, crustal degassing and groundwater flow in the Pannonian Basin. Earth and Planetary Science Letters 105, 229–246. https://doi.org/10.1016/0012-821X(91)90133-3
). Since natural gas and oil are often formed in marine sediments, we may assume that the helium isotope ratio of natural gas is 4.5 ± 3.5 × 10-7, the average value of marine sediment pore water measured in the ocean floor (Sano and Wakita, 1987Sano, Y., Wakita, H. (1987) Helium isotopes and heat flow on the ocean floor. Chemical Geology 66, 217–226. https://doi.org/10.1016/0168-9622(87)90043-1
) and consistent with early data in natural gases (Aldrich and Nier, 1948Aldrich, L.T., Nier, A.O. (1948) The occurrence of He-3 in natural sources of helium. Physical Review 74, 1590–1594. https://doi.org/10.1103/PhysRev.74.1590
). It leads to the required isotope ratio change of +0.028 ± 0.022 ‰/year, comparable with the lower figure estimated by Birner et al. (2022)Birner, B., Severinghaus, J., Paplawsky, B., Keeling, R.F. (2022) Increasing atmospheric helium from fossil fuel exploitation. Nature Geoscience 115, 346–348. https://doi.org/10.1038/s41561-022-00932-3
. Thus, the helium isotope variation to be adjusted and offset would be between 0.028 ± 0.020 ‰/year and 0.049 ± 0.020 ‰/year.The total amount of 3He injected by solar flares can be estimated based on the variation of the atmospheric helium isotope ratio, illustrated in Figure 2. The positive helium isotope ratio anomaly lasted from September 13th to September 27th with an average deviation of 5.5 ± 1.7 ‰ calculated from the six anomalous samples. The area of the Earth corresponding to the polar regions where auroral precipitation is effective is estimated to be about 3 % of the total area of the Earth (Buhler et al., 1976
Buhler, F., Axford, W.I., Chivers, H.J.A., Marti, K. (1976) Helium Isotopes in an Aurora. Journal of Geophysical Research 81, 111–115. https://doi.org/10.1029/JA081i001p00111
). If the 5.5 ‰ 3He excess is globally diffused and diluted, then the 3He/4He ratio of the entire atmosphere would increase by 0.165 ‰ by only one large event. Based on the observations, there were 27 events larger than X9.0 from 1975 to 2018 (NIICT, 2018NIICT (2018) Space Weather Forecast. (Date of access: 10 June 2018) https://web.archive.org/web/20180610090019/https://swc.nict.go.jp/forecast/majorflares.html
). This suggests almost one solar flare event per two years. If these events have similar effect on the atmosphere, the increase in the air helium isotope ratio would be 0.10 ± 0.03 ‰/year on average, which is much larger than the range of 0.028–0.049 (±0.020) ‰/year calculated and predicted above. Thus, large solar flare events could supply sufficient 3He in the atmosphere to compensate for the addition of anthropogenic 4He, even if diluted by the complex atmospheric circulation processes described above.If the increase in the helium isotope ratio due to the solar flare on September 6th is 0.165 ‰ on an annual basis, based on the atmospheric inventory of 3He, i.e. 1.5 × 1014 atoms/cm2 (Ozima and Podosek, 1983
Ozima, M., Podosek, F.A. (1983) Noble gas geochemistry. Cambridge University Press, Cambridge.
), it would result in a flux of 190 atoms/cm2sec. This value is about 40 times greater than the steady state auroral precipitation flux of 5 atoms/cm2sec (Buhler et al., 1976Buhler, F., Axford, W.I., Chivers, H.J.A., Marti, K. (1976) Helium Isotopes in an Aurora. Journal of Geophysical Research 81, 111–115. https://doi.org/10.1029/JA081i001p00111
). In other words, even if a solar flare of this scale were to occur once every two years, it would be 20 times larger than the normal auroral precipitation flux. Therefore, solar flares would be an important source controlling atmospheric 3He concentration, even if over estimated, compensating for its decrease caused by the injection of crustal 4He from natural gas extraction and consumption by humans.top
Conclusions
In order to maintain the 3He/4He ratio of the atmosphere constant despite an increasing contribution of crustal 4He during the last century caused by extraction of natural gas, the injection of solar flare 3He should also have increased during a comparable period of time. The record of solar activity based on archival proxies, such as the concentration of the cosmogenic isotopes 14C in tree rings (Solanki et al., 2004
Solanki, S.K., Usoskin, I.G., Kromer, B., Schüssler, M., Beer, J. (2004) Unusual activity of the Sun during recent decades compared to the previous 11,000 years. Nature 431, 1084–1087. https://doi.org/10.1038/nature02995
) or 10Be in ice cores (Beer, 2000Beer, J. (2000) Long-term indirect indices of solar variability. Space Science Reviews 94, 53–66. https://doi.org/10.1023/A:1026778013901
), may provide good examples, although other sources of cosmogenic isotope fluctuations are also possible (Heaton et al., 2021Heaton, T.J., Bard, E., Bronk-Ramsey, C., Butzin, M., Köhler, P., Muscheler, R., Reimer, P.J., Wacker, L. (2021) Radiocarbon: A key tracer for studying Earth’s dynamo, climate system, carbon cycle, and Sun. Science 374, eabd7096. https://doi.org/10.1126/science.abd7096
). Based on the precise measurements of air helium isotopes in a polar region, we found the first case of temporal variation and claim that sun flare activity may alter global mass balance of 3He in the atmosphere.top
Acknowledgements
We are grateful to the Norwegian Polar Institute’s staff for their careful air sampling at Ny-Ålesund. Thanks also for helpful comments from two anonymous reviewers.
Editor: Maud Boyet
top
References
Aldrich, L.T., Nier, A.O. (1948) The occurrence of He-3 in natural sources of helium. Physical Review 74, 1590–1594. https://doi.org/10.1103/PhysRev.74.1590
 Show in context
Show in context However, accelerated extraction and consumption of fossil fuels since the beginning of the 20th century may have altered this balance. Natural gas contains about 0.25 % helium (Zartman et al., 1961) with an isotopic ratio (Aldrich and Nier, 1948) much smaller than that of the atmospheric value (1.382 × 10-6; Sano et al., 2013), because of the dominance of crustal 4He compared to 3He in fossil fuels.
View in article
Since natural gas and oil are often formed in marine sediments, we may assume that the helium isotope ratio of natural gas is 4.5 ± 3.5 × 10-7, the average value of marine sediment pore water measured in the ocean floor (Sano and Wakita, 1987) and consistent with early data in natural gases (Aldrich and Nier, 1948).
View in article
Axford, W.I., Buhler, F., Chivers, H.J.A., Eberhardt, P., Geiss, J. (1972) Auroral Helium Precipitation. Journal of Geophysical Research 77, 6724–6730. https://doi.org/10.1029/JA077i034p06724
 Show in context
Show in context Experiments using rockets have observed 3He of solar wind origin at an altitude of about 150 km (Axford et al., 1972).
View in article
Ballentine, C.J., O’Nions, R.K., Oxburgh, E.R., Horvarth, F., Deàk, J. (1991) Rare gas constraints on hydrocarbon accumulation, crustal degassing and groundwater flow in the Pannonian Basin. Earth and Planetary Science Letters 105, 229–246. https://doi.org/10.1016/0012-821X(91)90133-3
 Show in context
Show in context However, this calculation requires that the 3He/4He of natural gases is 1.63 ± 0.68 × 10-6, which is much higher than the typical crustal ratio of 2 × 10-8, and it can be found only in hydrocarbons settled in extensional sedimentary basins where crustal and mantle helium can be found together (e.g., Ballentine et al., 1991).
View in article
Beer, J. (2000) Long-term indirect indices of solar variability. Space Science Reviews 94, 53–66. https://doi.org/10.1023/A:1026778013901
 Show in context
Show in context The record of solar activity based on archival proxies, such as the concentration of the cosmogenic isotopes 14C in tree rings (Solanki et al., 2004) or 10Be in ice cores (Beer, 2000), may provide good examples, although other sources of cosmogenic isotope fluctuations are also possible (Heaton et al., 2021).
View in article
Birner, B., Severinghaus, J., Paplawsky, B., Keeling, R.F. (2022) Increasing atmospheric helium from fossil fuel exploitation. Nature Geoscience 115, 346–348. https://doi.org/10.1038/s41561-022-00932-3
 Show in context
Show in context Recently, Birner et al. (2022) developed a method to measure helium concentrations with high precision (±0.07 ‰) and analysed atmospheric samples from the past 46 years.
View in article
This apparent discrepancy – i.e. constant 3He/4He ratio but contemporary increase of anthropogenically-released 4He – suggests that an unknown source of 3He is compensating for the addition of crustal 4He (Birner et al., 2022).
View in article
The observed increase in atmospheric helium concentration (Birner et al., 2022) suggests an anthropogenic supply of crustal 4He of 3.9 ± 0.3 × 1010 mol/year (2σ). Birner et al. (2022) calculated that to compensate for the addition of anthropogenic 4He and to preserve a constant atmospheric 3He/4He ratio, the annual addition of 6.3 ± 2.5 × 104 mol of 3He is required.
View in article
This is equivalent to an annual change in the atmospheric helium isotope ratio of +0.049 ± 0.020 ‰ (Birner et al., 2022).
View in article
It leads to the required isotope ratio change of +0.028 ± 0.022 ‰/year, comparable with the lower figure estimated by Birner et al. (2022).
View in article
Boucher, C., Marty, B., Zimmermann, L., Langenfelds, R. (2018) Atmospheric helium isotopic ratio from 1910 to 2016 recorded in stainless steel containers. Geochemical. Perspectives Letters 6, 23–27. https://doi.org/10.7185/geochemlet.1804
 Show in context
Show in context Although there has been much debate since then, recent analyses of the 3He/4He ratio in sampled air of different epochs have concluded that the atmospheric 3He/4He ratio is rather constant (Lupton and Evans, 2004; Mabry et al., 2015; Boucher et al., 2018).
View in article
Buhler, F., Axford, W.I., Chivers, H.J.A., Marti, K. (1976) Helium Isotopes in an Aurora. Journal of Geophysical Research 81, 111–115. https://doi.org/10.1029/JA081i001p00111
 Show in context
Show in context This mechanism is associated with the formation of auroras and is called Auroral Helium Precipitation (Buhler et al., 1976), and it may explain excess 3He observed in this work.
View in article
The area of the Earth corresponding to the polar regions where auroral precipitation is effective is estimated to be about 3 % of the total area of the Earth (Buhler et al., 1976).
View in article
This value is about 40 times greater than the steady state auroral precipitation flux of 5 atoms/cm2sec (Buhler et al., 1976).
View in article
Clarke, W.B., Beg, M.A., Craig, H. (1969) Excess 3He in the sea: evidence for terrestrial primordial helium. Earth and Planetary Science Letters 6, 213–220. https://doi.org/10.1016/0012-821X(69)90093-4
 Show in context
Show in context A dominant source of atmospheric 3He is the Earth’s mantle through volcanic activity and degassing from mid-oceanic ridges (Clarke et al., 1969) and subduction zones (Sano and Wakita, 1985).
View in article
Gluckauf, E., Paneth, F.A. (1946) The helium content of atmospheric air. Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences 185, 89–98. https://doi.org/10.1098/rspa.1946.0006
 Show in context
Show in context The atmospheric 3He concentration should be 7.24 pptv, based on the helium concentration in the atmosphere of 5.24 ppmv (Gluckauf and Paneth, 1946) and a 3He/4He of 1.382 × 10-6 (Sano et al., 2013).
View in article
Heaton, T.J., Bard, E., Bronk-Ramsey, C., Butzin, M., Köhler, P., Muscheler, R., Reimer, P.J., Wacker, L. (2021) Radiocarbon: A key tracer for studying Earth’s dynamo, climate system, carbon cycle, and Sun. Science 374, eabd7096. https://doi.org/10.1126/science.abd7096
 Show in context
Show in context The record of solar activity based on archival proxies, such as the concentration of the cosmogenic isotopes 14C in tree rings (Solanki et al., 2004) or 10Be in ice cores (Beer, 2000), may provide good examples, although other sources of cosmogenic isotope fluctuations are also possible (Heaton et al., 2021).
View in article
Jacob, D.J. (1999) Introduction to Atmospheric Chemistry. Princeton University Press, Princeton.
 Show in context
Show in context Mixing of atmosphere along the latitude from east to west may take a few weeks by air transport, which is much faster than that of a few months along the longitude from north to south, generally observed by long-lived tracers (Jacob, 1999).
View in article
According to turbulent diffusion models, vertical mixing between the troposphere and the surface takes more than one month (Jacob, 1999).
View in article
Lupton, J., Evans, L. (2004) The atmospheric helium isotope ratio: is it changing? Geophysical Research Letters 31, 1–4. https://doi.org/10.1029/2004GL020041
 Show in context
Show in context Although there has been much debate since then, recent analyses of the 3He/4He ratio in sampled air of different epochs have concluded that the atmospheric 3He/4He ratio is rather constant (Lupton and Evans, 2004; Mabry et al., 2015; Boucher et al., 2018).
View in article
Other sources include the decay of tritium produced by nuclear weapons and nuclear reactors, but it is thought to be negligible in amount (Lupton and Evans, 2004).
View in article
Mabry, J., Lan, T., Boucher, C., Burnard, P.G., Brennwald, M., Langenfelds, R., Marty, B. (2015) No evidence for change of the atmospheric helium isotope composition since 1978 from re-analysis of the Cape Grim Air Archive. Earth and Planetary Science Letters 428, 134–138. https://doi.org/10.1016/j.epsl.2015.07.035
 Show in context
Show in context Although there has been much debate since then, recent analyses of the 3He/4He ratio in sampled air of different epochs have concluded that the atmospheric 3He/4He ratio is rather constant (Lupton and Evans, 2004; Mabry et al., 2015; Boucher et al., 2018).
View in article
MacDonald, G.J.F. (1963) The escape of helium from the Earth’s atmosphere. Reviews of Geophysics 1, 305–349. https://doi.org/10.1029/RG001i003p00305
 Show in context
Show in context Helium concentration in the atmosphere is determined by the balance between degassing from the solid Earth and the thermal and/or non-thermal escape from the upper atmosphere (MacDonald, 1963).
View in article
Matsuda, J., Matsumoto, T., Sumino, H., Nagao, K., Yamamoto, J., Miura, Y., Kaneoka, I., Takahata, T., Sano, Y. (2002) The 3He/4He ratio of the new internal He Standard of Japan (HESJ). Geochemical Journal 36, 191–195. https://doi.org/10.2343/geochemj.36.191
 Show in context
Show in context Measurements were performed 2–3 times for each individual sample and compared to a synthesised helium standard, HESJ (Matsuda et al., 2002) with an accepted 3He/4He value of 20.63 ± 0.10 Ra.
View in article
NASA (2017) Sun Erupts With Significant Flare. (Date of access: 29 November 2021) https://www.nasa.gov/feature/goddard/2017/active-region-on-sun-continues-to-emit-solar-flares
 Show in context
Show in context The solar wind was expected to reach the Earth on September 8th (NASA, 2017).
View in article
These data are noteworthy because they were taken immediately after the massive solar flare event of September 6th that registered X9.3 on the solar storm scale, which divides solar flares according to their strength (NASA, 2017).
View in article
NIICT (2018) Space Weather Forecast. (Date of access: 10 June 2018) https://web.archive.org/web/20180610090019/https://swc.nict.go.jp/forecast/majorflares.html
 Show in context
Show in context Events equivalent and larger than X9.3 have occurred 27 times from 1975 to 2018 (NIICT, 2018), so their occurrence is not significantly unusual.
View in article
If the 5.5 ‰ 3He excess is globally diffused and diluted, then the 3He/4He ratio of the entire atmosphere would increase by 0.165 ‰ by only one large event. Based on the observations, there were 27 events larger than X9.0 from 1975 to 2018 (NIICT, 2018).
View in article
Oliver, B.M., Bradley, J.G., Farrar, H. (1984) Helium concentration in the Earth’s lower atmosphere. Geochimica et Cosmochimica Acta 48, 1759–1767. https://doi.org/10.1016/0016-7037(84)90030-9
 Show in context
Show in context Oliver et al. (1984) first reported a rough estimate of the 4He released in the atmosphere due to natural gas production (3–12 × 1016 cm3 STP between 1939 and 1981).
View in article
Ozima, M., Podosek, F.A. (1983) Noble gas geochemistry. Cambridge University Press, Cambridge.
 Show in context
Show in context Another source is the interplanetary zone (Ozima and Podosek, 1983).
View in article
If the increase in the helium isotope ratio due to the solar flare on September 6th is 0.165 ‰ on an annual basis, based on the atmospheric inventory of 3He, i.e. 1.5 × 1014 atoms/cm2 (Ozima and Podosek, 1983), it would result in a flux of 190 atoms/cm2sec.
View in article
Sano, Y., Wakita, H. (1985) Geographical distribution of 3He/4He ratios in Japan: Implications for arc tectonics and incipient magmatism. Journal of Geophysical Research 90, 8729–8741. https://doi.org/10.1029/JB090iB10p08729
 Show in context
Show in context A dominant source of atmospheric 3He is the Earth’s mantle through volcanic activity and degassing from mid-oceanic ridges (Clarke et al., 1969) and subduction zones (Sano and Wakita, 1985).
View in article
Sano, Y., Wakita, H. (1987) Helium isotopes and heat flow on the ocean floor. Chemical Geology 66, 217–226. https://doi.org/10.1016/0168-9622(87)90043-1
 Show in context
Show in context Since natural gas and oil are often formed in marine sediments, we may assume that the helium isotope ratio of natural gas is 4.5 ± 3.5 × 10-7, the average value of marine sediment pore water measured in the ocean floor (Sano and Wakita, 1987) and consistent with early data in natural gases (Aldrich and Nier, 1948).
View in article
Sano, Y., Wakita, H., Makide, Y., Tominaga, T. (1989) A ten year decrease in the atmospheric helium isotope ratio possibly caused by human activity. Geophysical Research Letters 16, 1371–1374. https://doi.org/10.1029/GL016i012p01371
 Show in context
Show in context Sano et al. (1989) measured a temporal decrease in the helium isotope ratio in the atmosphere of ∼1 x 10-9/year.
View in article
Sano, Y., Tokutake, T., Takahata, N. (2008) Accurate measurement of atmospheric helium isotopes. Analytical Sciences 24, 521–525. https://doi.org/10.2116/analsci.24.521
 Show in context
Show in context Helium isotope ratios were measured with a double-collector noble gas mass spectrometer, GV® Helix SFT (Sano et al., 2008).
View in article
Sano, Y., Marty B., Burnard, P. (2013) Noble Gases in the Atmosphere. In: Burnard, P. (Ed.) The Noble Gases as Geochemical Tracers, Advances in Isotope Geochemistry. Springer-Verlag, Berlin, 17–31. https://doi.org/10.1007/978-3-642-28836-4_2
 Show in context
Show in context
However, accelerated extraction and consumption of fossil fuels since the beginning of the 20th century may have altered this balance. Natural gas contains about 0.25 % helium (Zartman et al., 1961) with an isotopic ratio (Aldrich and Nier, 1948) much smaller than that of the atmospheric value (1.382 × 10-6; Sano et al., 2013), because of the dominance of crustal 4He compared to 3He in fossil fuels.
View in article
The measured 3He/4He ratios were then normalised to the standard value of the atmosphere, 1.382 × 10-6 (Sano et al., 2013).
View in article
The atmospheric 3He concentration should be 7.24 pptv, based on the helium concentration in the atmosphere of 5.24 ppmv (Gluckauf and Paneth, 1946) and a 3He/4He of 1.382 × 10-6 (Sano et al., 2013).
View in article
Škerlak, B., Sprenger, M., Wernli, H. (2014) A global climatology of stratosphere–troposphere exchange using the ERA-Interim data set from 1979 to 2011. Atmospheric Chemistry and Physics 14, 913–937. https://doi.org/10.5194/acp-14-913-2014
 Show in context
Show in context In addition, the surface region most strongly influenced by the stratosphere is found substantially south of the sampling location (Škerlak et al., 2014).
View in article
Solanki, S.K., Usoskin, I.G., Kromer, B., Schüssler, M., Beer, J. (2004) Unusual activity of the Sun during recent decades compared to the previous 11,000 years. Nature 431, 1084–1087. https://doi.org/10.1038/nature02995
 Show in context
Show in context The record of solar activity based on archival proxies, such as the concentration of the cosmogenic isotopes 14C in tree rings (Solanki et al., 2004) or 10Be in ice cores (Beer, 2000), may provide good examples, although other sources of cosmogenic isotope fluctuations are also possible (Heaton et al., 2021).
View in article
Zartman, R.E., Wasserburg, G.J., Reynolds, J.H. (1961) Helium, argon and carbon in some natural gases. Journal of Geophysical Research 66, 277–306. https://doi.org/10.1029/JZ066i001p00277
 Show in context
Show in context However, accelerated extraction and consumption of fossil fuels since the beginning of the 20th century may have altered this balance. Natural gas contains about 0.25 % helium (Zartman et al., 1961) with an isotopic ratio (Aldrich and Nier, 1948) much smaller than that of the atmospheric value (1.382 × 10-6; Sano et al., 2013), because of the dominance of crustal 4He compared to 3He in fossil fuels.
View in article
top
Supplementary Information
The Supplementary Information includes:
Download the Supplementary Information (PDF)
Figures
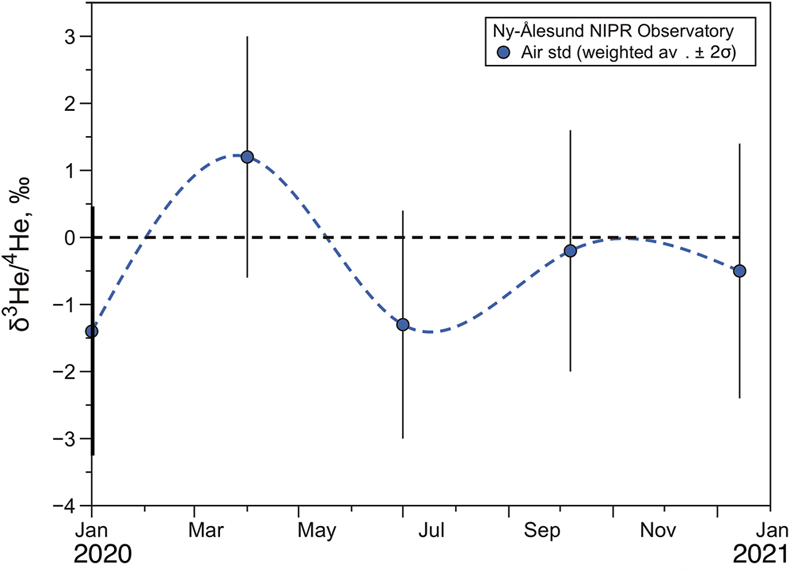
Figure 1 Temporal variation of atmospheric 3He/4He weighted averages in Ny-Ålesund, Svalbard, near the North Pole, from January 2020 to January 2021. The black dotted line and blue dotted curve show the standard atmospheric helium value and a spline curve approximating the time variability of the weighted average 3He/4He ratio for each sampling day, respectively. Bars indicate 2σ internal errors (see Supplementary Information for equations).
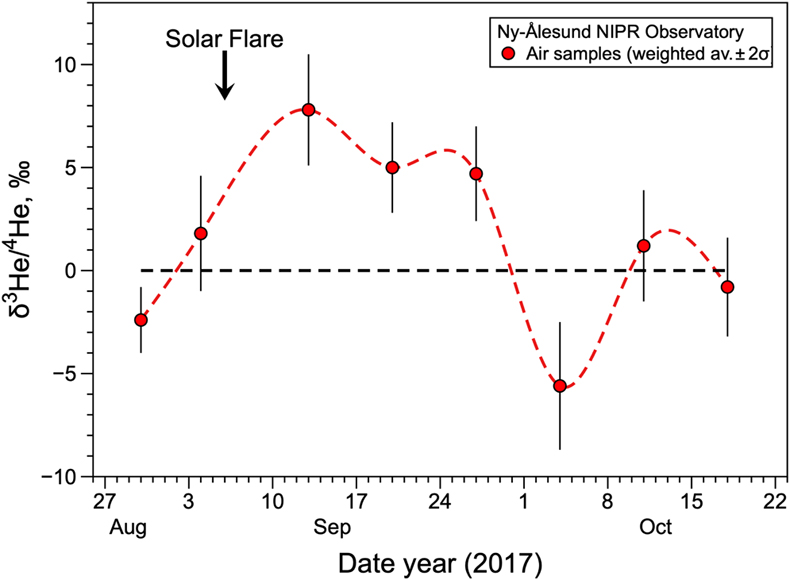
Figure 2 Temporal variation of atmospheric 3He/4He weighted averages in Ny-Ålesund, Svalbard, near the North Pole, around the time of the X9.3 solar flare event. The black dotted line and red dotted curve show the standard atmospheric helium value and a spline curve approximating the time variability of the weighted average 3He/4He ratio for each sampling day, respectively. Bars indicate 2σ internal errors (see Supplementary Information for equations).