Bacterial use of siderophores increases olivine dissolution rates by nearly an order of magnitude
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:539Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract

Figures
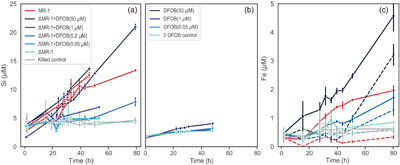 Figure 1 Dissolved Si measured in (a) biotic and (b) abiotic experiments, and (c) dissolved and total Fe in the biotic experiments (mean ± s.d.). Colours in all figures correspond to the same concentration of exogenous DFOB. Dashed lines in (c) represent dissolved concentrations, while solid lines are total concentrations. | 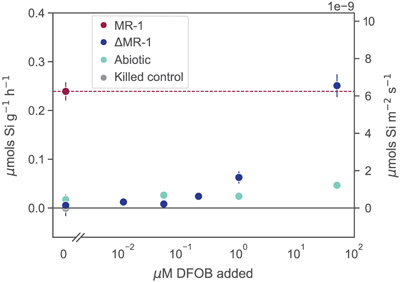 Figure 2 Olivine dissolution rates (based on Si release) as a function of DFOB addition. Dissolution rates in biotic treatments (ΔMR-1) exceed abiotic treatments at the same DFOB addition for DFOB > 1 μM. At 50 μM DFOB addition, biotic dissolution rates exceed abiotic rates by 8 fold. Dashed line represents dissolution rate for MR-1, with no exogenous siderophore addition. | 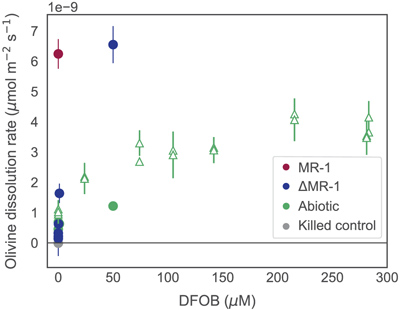 Figure 3 Olivine dissolution rates as a function of DFOB addition. Filled circles are from this study; hollow triangles are from Torres et al. (2019), adjusted to pH 7.2 as per the abiotic rate equations in Rimstidt et al. (2012). At 50 μM DFOB addition, our abiotic rate is reasonably characterised by (and slightly lower than) the abiotic adsorption isotherm. In contrast, the MR-1 and ΔMR-1 rates are significantly above the isotherm. |
| Figure 1 | Figure 2 | Figure 3 |
top
Introduction
The chemical weathering of silicate minerals is an important control on global biogeochemical cycling and climate via the release of metal cations and alkalinity (White and Brantley, 1995
White, A.F., Brantley, S.L. (1995) Chemical Weathering Rates of Silicate Minerals. Reviews in Mineralogy v. 31, De Gruyter, Berlin, 583 p. https://doi.org/10.1515/9781501509650
). Improved understanding of the environmental controls on silicate dissolution is thus important for characterising the climate-weathering feedback (Berner et al., 1983Berner, R.A., Lasaga, A.C., Garrels, R.M. (1983) The Carbonate-Silicate Geochemical Cycle and Its Effect on Atmospheric Carbon Dioxide Over the Past 100 Million Years. American Journal of Science 283, 641–683. https://doi.org/10.2475/ajs.283.7.641
), and for informing engineered “enhanced weathering” efforts to mitigate climate change (Hartmann et al., 2013Hartmann, J., West, A.J., Renforth, P., Köhler, P., De La Rocha, C.L., Wolf‐Gladrow, D.A., Dürr, H.H., Scheffran, J. (2013) Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Reviews of Geophysics 51, 113–149. https://doi.org/10.1002/rog.20004
). In this regard, olivine ((Mg2+,Fe2+)2SiO4) and olivine-rich rocks (such as basalt) are often studied as model silicates due to their high solubility, abundance, and resulting large contribution to earth’s weathering flux (Dessert et al., 2003Dessert, C., Dupré, B., Gaillardet, J., François, L.M., Allegre, C.J. (2003) Basalt weathering laws and the impact of basalt weathering on the global carbon cycle. Chemical Geology 202, 257–273. https://doi.org/10.1016/j.chemgeo.2002.10.001
; Hartmann et al., 2009Hartmann, J., Jansen, N., Dürr, H.H., Kempe, S., Köhler, P. (2009) Global CO2-consumption by chemical weathering: What is the contribution of highly active weathering regions? Global and Planetary Change 69, 185–194. https://doi.org/10.1016/j.gloplacha.2009.07.007
).At Earth’s surface, olivine dissolution kinetics can vary by orders of magnitude as a function of both abiotic and biotic environmental factors. The effect of abiotic conditions such as temperature, solution pH, and solution chemistry are relatively well constrained (Rimstidt et al., 2012
Rimstidt, J.D., Brantley, S.L., Olsen, A.A. (2012) Systematic review of forsterite dissolution rate data. Geochimica et Cosmochimica Acta 99, 159–178. https://doi.org/10.1016/j.gca.2012.09.019
; Oelkers et al., 2018Oelkers, E.H., Declercq, J., Saldi, G.D., Gislason, S.R., Schott, J. (2018) Olivine dissolution rates: A critical review. Chemical Geology 500, 1–19. https://doi.org/10.1016/j.chemgeo.2018.10.008
). The effects of biology on dissolution rates, on the other hand, are less clear. While the net effect of biology on mineral dissolution is typically assumed to be positive, observed biotic effects range from inhibition to enhancement (Schwartzman and Volk, 1989Schwartzman, D.W., Volk, T. (1989) Biotic enhancement of weathering and the habitability of Earth. Nature 340, 457–460. https://doi.org/10.1038/340457a0
).Low molecular weight, multi-dentate ligands known as siderophores may be important drivers of microbially enhanced olivine dissolution. Siderophores are secreted by many bacteria, fungi, and grasses in response to Fe limitation. While they are structurally diverse, siderophores typically contain multiple metal-binding ligands that collectively result in exceptionally high binding affinity with Fe3+ (log Kf > 30), preventing its loss from solution via Fe oxide precipitation (Hider and Kong, 2010
Hider, R.C., Kong, X. (2010) Chemistry and biology of siderophores. Natural Product Reports 27, 637–657. https://doi.org/10.1039/b906679a
). Significant research has shown that siderophores can abiotically enhance dissolution rates for a range of Fe3+ and Al3+-containing minerals, including Fe oxides (Cheah et al., 2003Cheah, S.-F., Kraemer, S.M., Cervini-Silva, J., Sposito, G. (2003) Steady-state dissolution kinetics of goethite in the presence of desferrioxamine B and oxalate ligands: implications for the microbial acquisition of iron. Chemical Geology 198, 63–75. https://doi.org/10.1016/S0009-2541(02)00421-7
; Kraemer, 2004Kraemer, S.M. (2004) Iron oxide dissolution and solubility in the presence of siderophores. Aquatic Sciences 66, 3–18. https://doi.org/10.1007/s00027-003-0690-5
; Reichard et al., 2007Reichard, P.U., Kretzschmar, R., Kraemer, S.M. (2007) Dissolution mechanisms of goethite in the presence of siderophores and organic acids. Geochimica et Cosmochimica Acta 71, 5635–5650. https://doi.org/10.1016/j.gca.2006.12.022
), Fe containing phyllosilicates (Rosenberg and Maurice, 2003Rosenberg, D.R., Maurice, P.A. (2003) Siderophore adsorption to and dissolution of kaolinite at pH 3 to 7 and 22°C. Geochimica et Cosmochimica Acta 67, 223–229. https://doi.org/10.1016/S0016-7037(02)01082-7
; Haack et al., 2008Haack, E.A., Johnston, C.T., Maurice, P.A. (2008) Mechanisms of siderophore sorption to smectite and siderophore-enhanced release of structural Fe3+. Geochimica et Cosmochimica Acta 72, 3381–3397. https://doi.org/10.1016/j.gca.2008.03.027
; Shirvani and Nourbakhsh, 2010Shirvani, M., Nourbakhsh, F. (2010) Desferrioxamine-B adsorption to and iron dissolution from palygorskite and sepiolite. Applied Clay Science 48, 393–397. https://doi.org/10.1016/j.clay.2010.01.012
; Ferret et al., 2014Ferret, C., Sterckeman, T., Cornu, J.-Y., Gangloff, S., Schalk, I.J., Geoffroy, V.A. (2014) Siderophore‐promoted dissolution of smectite by fluorescent Pseudomonas. Environmental Microbiology Reports 6, 459–467. https://doi.org/10.1111/1758-2229.12146
; Bray et al., 2015Bray, A.W., Oelkers, E.H., Bonneville, S., Wolff-Boenisch, D., Potts, N.J., Fones, G., Benning, L.G. (2015) The effect of pH, grain size, and organic ligands on biotite weathering rates. Geochimica et Cosmochimica Acta 164, 127–145. https://doi.org/10.1016/j.gca.2015.04.048
), and hornblende (Kalinowski et al., 2000Kalinowski, B.E., Liermann, L.J., Givens, S., Brantley, S.L. (2000) Rates of bacteria-promoted solubilization of Fe from minerals: a review of problems and approaches. Chemical Geology 169, 357–370. https://doi.org/10.1016/S0009-2541(00)00214-X
; Liermann et al., 2000Liermann, L.J., Kalinowski, B.E., Brantley, S.L., Ferry, J.G. (2000) Role of bacterial siderophores in dissolution of hornblende. Geochimica et Cosmochimica Acta 64, 587–602. https://doi.org/10.1016/S0016-7037(99)00288-4
; Buss et al., 2007Buss, H.L., Lüttge, A., Brantley, S.L. (2007) Etch pit formation on iron silicate surfaces during siderophore-promoted dissolution. Chemical Geology 240, 326–342. https://doi.org/10.1016/j.chemgeo.2007.03.003
). Olivine, in contrast, contains Fe almost exclusively in the +2 oxidation state, which is more weakly bound by siderophores (e.g., for the siderophore deferoxamine B, log Kf = 30 for Fe3+ vs. 10 for Fe2+) (Dhungana and Crumbliss, 2005Dhungana, S., Crumbliss, A.L. (2005) Coordination Chemistry and Redox Processes in Siderophore-Mediated Iron Transport. Geomicrobiology Journal 22, 87–98. https://doi.org/10.1080/01490450590945870
). Nonetheless, recent research has shown that siderophores also increase olivine dissolution rates by nearly an order of magnitude (Torres et al., 2019Torres, M.A., Dong, S., Nealson, K.H., West, A.J. (2019) The kinetics of siderophore‐mediated olivine dissolution. Geobiology 17, 401–416. https://doi.org/10.1111/gbi.12332
). An important distinction, however, is that these studies use purified siderophores at high micromolar concentrations, whereas environmental concentrations are consistently much lower (pico- to nanomolar) (Kraemer, 2004Kraemer, S.M. (2004) Iron oxide dissolution and solubility in the presence of siderophores. Aquatic Sciences 66, 3–18. https://doi.org/10.1007/s00027-003-0690-5
). Thus, there remains a disconnect between our understanding of catalyst driven and biology driven mineral dissolution, and uncertainty as to whether siderophores are ultimately important drivers of dissolution in the environment (Brantley et al., 2006Brantley, S.L., Ruebush, S., Jang, J.-H., Tien, M. (2006) Analysis of (Bio)Geochemical Kinetics of Fe(III) Oxides. In: Maurice, P.A., Warren, L.A., Bain, D.C. (Eds.) Methods for Study of Microbe-Mineral Interactions. Clay Minerals Society Workshop Lectures v. 14, Clay Mineral Society, Chantilly, VA, 80–116. https://doi.org/10.1346/CMS-WLS-14.3
).This study attempts to clarify whether siderophores enhance olivine dissolution in biotic conditions (i.e. when actively utilised by microbes), and if so, if they are a requisite component of biotically enhanced dissolution. Specifically, we use sub-micromolar concentrations of siderophores — which do not significantly increase dissolution abiotically — to assess whether their active use by microbes increases mineral dissolution rates. While previous studies have found that siderophores are indeed critical for microbes to access or use mineral bound Fe, they have not linked this dependency quantitatively to mineral dissolution rates (Dehner et al., 2010
Dehner, C.A., Awaya, J.D., Maurice, P.A., DuBois, J.L. (2010) Roles of Siderophores, Oxalate, and Ascorbate in Mobilization of Iron from Hematite by the Aerobic Bacterium Pseudomonas mendocina. Applied and Environmental Microbiology 76, 2041–2048. https://doi.org/10.1128/AEM.02349-09
; Ferret et al., 2014Ferret, C., Sterckeman, T., Cornu, J.-Y., Gangloff, S., Schalk, I.J., Geoffroy, V.A. (2014) Siderophore‐promoted dissolution of smectite by fluorescent Pseudomonas. Environmental Microbiology Reports 6, 459–467. https://doi.org/10.1111/1758-2229.12146
; Van Den Berghe et al., 2021Van Den Berghe, M., Merino, N., Nealson, K.H., West, A.J. (2021) Silicate minerals as a direct source of limiting nutrients: Siderophore synthesis and uptake promote ferric iron bioavailability from olivine and microbial growth. Geobiology 19, 618–630. https://doi.org/10.1111/gbi.12457
). Here, we focus specifically on this link between microbial use of siderophores and rates of mineral dissolution. To do so, we measured olivine dissolution rates in the presence of the bacteria Shewanella oneidensis (MR-1) wild type, and in the presence of a gene-deletion mutant of MR-1 incapable of producing siderophores (ΔMR-1) plus varying concentrations of the exogenous siderophore, deferoxamine B (DFOB). We furthermore compare these dissolution rates to abiotic rates with DFOB alone. While MR-1 is often studied for dissimilatory metal reduction, it was used in this study only due to availability of the siderophore gene-deletion mutant; dissimilatory Fe reduction was not a concern, as olivine bound Fe is almost exclusively in the +2 oxidation state (Van Den Berghe et al., 2021Van Den Berghe, M., Merino, N., Nealson, K.H., West, A.J. (2021) Silicate minerals as a direct source of limiting nutrients: Siderophore synthesis and uptake promote ferric iron bioavailability from olivine and microbial growth. Geobiology 19, 618–630. https://doi.org/10.1111/gbi.12457
).top
Siderophores are Required for Biotically Enhanced Olivine Dissolution
In the absence of DFOB, ΔMR-1 could not access olivine bound Fe at rates fast enough to support growth, and correspondingly did not enhance mineral dissolution above well established abiotic rates (Figs. 1–3, S-1, Table S-1) (Rimstidt et al., 2012
Rimstidt, J.D., Brantley, S.L., Olsen, A.A. (2012) Systematic review of forsterite dissolution rate data. Geochimica et Cosmochimica Acta 99, 159–178. https://doi.org/10.1016/j.gca.2012.09.019
). S. oneidensis has the capacity to take up Fe through alternative pathways not involving siderophores — including direct uptake of Fe2+ and/or ligand bound Fe3+ transport — so a dependence on siderophores for microbial growth is not obvious a priori (Liu et al., 2018Liu, L., Li, S., Wang, S., Dong, Z., Gao, H. (2018) Complex Iron Uptake by the Putrebactin-Mediated and Feo Systems in Shewanella oneidensis. Applied and Environmental Microbiology 84, e01752–18. https://doi.org/10.1128/AEM.01752-18
). Furthermore, alternative microbial processes, such as decreased pH or production of low molecular weight organic acids could also facilitate mineral dissolution to feed microbial growth (Wogelius and Walther, 1991Wogelius, R.A., Walther, J.V. (1991) Olivine dissolution at 25°C: Effects of pH, CO2, and organic acids. Geochimica et Cosmochimica Acta 55, 943–954. https://doi.org/10.1016/0016-7037(91)90153-V
). However, these processes may be insufficient to drive dissolution alone: ligand facilitated dissolution has been shown to be significant only at high ligand concentrations (typically >1 mM), which may not be achieved by slow growing microbes (Reichard et al., 2007Reichard, P.U., Kretzschmar, R., Kraemer, S.M. (2007) Dissolution mechanisms of goethite in the presence of siderophores and organic acids. Geochimica et Cosmochimica Acta 71, 5635–5650. https://doi.org/10.1016/j.gca.2006.12.022
), and pH gradients in biofilms may not be severe enough to facilitate dissolution (Liermann et al., 2000Liermann, L.J., Kalinowski, B.E., Brantley, S.L., Ferry, J.G. (2000) Role of bacterial siderophores in dissolution of hornblende. Geochimica et Cosmochimica Acta 64, 587–602. https://doi.org/10.1016/S0016-7037(99)00288-4
).
Figure 1 Dissolved Si measured in (a) biotic and (b) abiotic experiments, and (c) dissolved and total Fe in the biotic experiments (mean ± s.d.). Colours in all figures correspond to the same concentration of exogenous DFOB. Dashed lines in (c) represent dissolved concentrations, while solid lines are total concentrations.

Figure 2 Olivine dissolution rates (based on Si release) as a function of DFOB addition. Dissolution rates in biotic treatments (ΔMR-1) exceed abiotic treatments at the same DFOB addition for DFOB > 1 μM. At 50 μM DFOB addition, biotic dissolution rates exceed abiotic rates by 8 fold. Dashed line represents dissolution rate for MR-1, with no exogenous siderophore addition.

Figure 3 Olivine dissolution rates as a function of DFOB addition. Filled circles are from this study; hollow triangles are from Torres et al. (2019)
Torres, M.A., Dong, S., Nealson, K.H., West, A.J. (2019) The kinetics of siderophore‐mediated olivine dissolution. Geobiology 17, 401–416. https://doi.org/10.1111/gbi.12332
, adjusted to pH 7.2 as per the abiotic rate equations in Rimstidt et al. (2012)Rimstidt, J.D., Brantley, S.L., Olsen, A.A. (2012) Systematic review of forsterite dissolution rate data. Geochimica et Cosmochimica Acta 99, 159–178. https://doi.org/10.1016/j.gca.2012.09.019
. At 50 μM DFOB addition, our abiotic rate is reasonably characterised by (and slightly lower than) the abiotic adsorption isotherm. In contrast, the MR-1 and ΔMR-1 rates are significantly above the isotherm.Similar studies using mutant Pseudomonas sp. grown in the presence of Fe3+ oxides and clays also found reduced growth rates in the absence of siderophores (Dehner et al., 2010
Dehner, C.A., Awaya, J.D., Maurice, P.A., DuBois, J.L. (2010) Roles of Siderophores, Oxalate, and Ascorbate in Mobilization of Iron from Hematite by the Aerobic Bacterium Pseudomonas mendocina. Applied and Environmental Microbiology 76, 2041–2048. https://doi.org/10.1128/AEM.02349-09
; Kuhn et al., 2013Kuhn, K.M., DuBois, J.L., Maurice, P.A. (2013) Strategies of aerobic microbial Fe acquisition from Fe-bearing montmorillonite clay. Geochimica et Cosmochimica Acta 117, 191–202. https://doi.org/10.1016/j.gca.2013.04.028
; Ferret et al., 2014Ferret, C., Sterckeman, T., Cornu, J.-Y., Gangloff, S., Schalk, I.J., Geoffroy, V.A. (2014) Siderophore‐promoted dissolution of smectite by fluorescent Pseudomonas. Environmental Microbiology Reports 6, 459–467. https://doi.org/10.1111/1758-2229.12146
). In contrast to our study, however, microbial growth was detectable without siderophores, indicating that alternative dissolution facilitating processes may be sufficient to facilitate some amount of microbial growth on Fe oxides and clays. The insignificant growth and dissolution in our ΔMR-1 (0 DFOB) treatment, on the other hand, indicates that siderophores are required to access olivine bound Fe. This difference suggests that Fe2+ bound within the olivine structure may be more difficult for microbes to access without siderophores than Fe3+ in oxides or clays.top
Biotic Dissolution Rates exceed Abiotic Rates at the same Siderophore Concentration
It has been established that siderophores, independent of live culture, stimulate the dissolution of many Fe containing minerals, including olivine (Torres et al., 2019
Torres, M.A., Dong, S., Nealson, K.H., West, A.J. (2019) The kinetics of siderophore‐mediated olivine dissolution. Geobiology 17, 401–416. https://doi.org/10.1111/gbi.12332
). We show here, however, that live bacteria enhance this dissolution even further, beyond the rates expected for siderophore associated dissolution alone (Fig. 2, Table S-1). In all ΔMR-1 treatments where microbial growth was detectable (DFOB > 0.2 μM), dissolution rates exceeded those in abiotic conditions at the same DFOB concentration: at 1 μM DFOB addition, dissolution with ΔMR-1 exceeded the abiotic rate by three fold, and at 50 μM DFOB addition, this increased to eight fold. The enhanced dissolution observed in the biotic treatments implies that S. oneidensis does not simply benefit from passive use of siderophores, but rather, magnifies their efficiency.top
Possible Mechanisms for Magnification of Siderophore Effects
One possible mechanism explaining higher rates in biotic vs. abiotic conditions is simply the microbial recycling of siderophores. In abiotic conditions, siderophores in solution can become saturated with chelated Fe. Active microbial populations, however, can transfer Fe to biomass, thus freeing siderophores for reuse. Our total vs. dissolved Fe data (Fig. 1c) support this mechanism, as Fe is almost entirely shunted into biomass, with no accumulation of chelated Fe in solution for the first 40 h of the ΔMR-1 experiments, and for the entirety of the MR-1 experiment. Even at 50 μM DFOB addition, which exceeds the concentration needed for maximum growth of ΔMR-1 (approximately 5 μM; Van Den Berghe et al., 2021
Van Den Berghe, M., Merino, N., Nealson, K.H., West, A.J. (2021) Silicate minerals as a direct source of limiting nutrients: Siderophore synthesis and uptake promote ferric iron bioavailability from olivine and microbial growth. Geobiology 19, 618–630. https://doi.org/10.1111/gbi.12457
), chelated Fe does not accumulate in solution until well after stationary phase is reached, suggesting highly efficient siderophore use and shuttling of siderophore bound Fe into biomass.However, mineral dissolution does not necessarily scale with siderophore concentration, but rather is known to scale with siderophores adsorbed to the mineral surface. As a result, dissolution rates plateau according to an adsorption isotherm. For DFOB, this has been well documented on olivine, Fe oxides and Fe containing phyllosilicates (Cheah et al., 2003
Cheah, S.-F., Kraemer, S.M., Cervini-Silva, J., Sposito, G. (2003) Steady-state dissolution kinetics of goethite in the presence of desferrioxamine B and oxalate ligands: implications for the microbial acquisition of iron. Chemical Geology 198, 63–75. https://doi.org/10.1016/S0009-2541(02)00421-7
; Kraemer, 2004Kraemer, S.M. (2004) Iron oxide dissolution and solubility in the presence of siderophores. Aquatic Sciences 66, 3–18. https://doi.org/10.1007/s00027-003-0690-5
; Haack et al., 2008Haack, E.A., Johnston, C.T., Maurice, P.A. (2008) Mechanisms of siderophore sorption to smectite and siderophore-enhanced release of structural Fe3+. Geochimica et Cosmochimica Acta 72, 3381–3397. https://doi.org/10.1016/j.gca.2008.03.027
; Torres et al., 2019Torres, M.A., Dong, S., Nealson, K.H., West, A.J. (2019) The kinetics of siderophore‐mediated olivine dissolution. Geobiology 17, 401–416. https://doi.org/10.1111/gbi.12332
). Considering the relationship between olivine dissolution and DFOB concentration published by Torres et al. (2019)Torres, M.A., Dong, S., Nealson, K.H., West, A.J. (2019) The kinetics of siderophore‐mediated olivine dissolution. Geobiology 17, 401–416. https://doi.org/10.1111/gbi.12332
— which was derived at experimental conditions similar to ours (30 °C, pH 7.5) — dissolution rates plateau at >100 μM DFOB (Fig. 3). However, our measured dissolution rates for MR-1 and ΔMR-1 with only 50 μM DFOB addition are well above the bounds of the isotherm, suggesting that siderophore recycling alone does not fully explain biotic dissolution rates. Some other biotic mechanism likely facilitates increased siderophore efficiency. Siderophore adsorption to mineral surfaces is thought to be limited by both steric hindrance and charge repulsion (Cocozza et al., 2002Cocozza, C., Tsao, C.C.G., Cheah, S.-F., Kraemer, S.M., Raymond, K.N., Miano, T.M., Sposito, G. (2002) Temperature dependence of goethite dissolution promoted by trihydroxamate siderophores. Geochimica et Cosmochimica Acta 66, 431–438. https://doi.org/10.1016/S0016-7037(01)00780-3
), so microbial secretion of surfactants or negatively charged ligands could increase adsorption efficiency (Carrasco et al., 2007Carrasco, N., Kretzschmar, R., Pesch, M.-L., Kraemer, S.M. (2007) Low Concentrations of Surfactants Enhance Siderophore-Promoted Dissolution of Goethite. Environmental Science & Technology 41, 3633–3638. https://doi.org/10.1021/es062897r
). Alternatively, small ligands like oxalate can interact synergistically with siderophores by liberating surface bound Fe when the saturation state of surrounding solution is lowered by siderophore mediated chelation of dissolved Fe (Cheah et al., 2003Cheah, S.-F., Kraemer, S.M., Cervini-Silva, J., Sposito, G. (2003) Steady-state dissolution kinetics of goethite in the presence of desferrioxamine B and oxalate ligands: implications for the microbial acquisition of iron. Chemical Geology 198, 63–75. https://doi.org/10.1016/S0009-2541(02)00421-7
; Reichard et al., 2007Reichard, P.U., Kretzschmar, R., Kraemer, S.M. (2007) Dissolution mechanisms of goethite in the presence of siderophores and organic acids. Geochimica et Cosmochimica Acta 71, 5635–5650. https://doi.org/10.1016/j.gca.2006.12.022
). Of course, it is possible that a combination of these mechanisms collectively magnify siderophore efficiency.top
Biotically Enhanced Dissolution persists with DFOB Addition, but Slows with Wild Type
When comparing the dissolution trends of MR-1 wild type and ΔMR-1 + DFOB (50 μM) treatments, three key observations stand out. First, dissolution rates are similar, despite ΔMR-1 achieving only 50 % of the wild type planktonic cell density (Figs. S-1, S-2). Second, dissolution in the wild type treatment slows after stationary phase is reached, whereas dissolution rates are constant in the ΔMR-1 treatments (Fig. 1a). And third, dissolved Fe begins to accumulate in the ΔMR-1 treatments during stationary phase, whereas dissolved Fe is completely absent in the wild type treatments (Fig. 1c). The fact that dissolution slows in the presence of MR-1 wild type can be explained by down regulation of siderophore production in response to Fe availability, a well established biofeedback mechanism in siderophore producers. In contrast, the unabated dissolution and accumulation of dissolved Fe in ΔMR-1 + DFOB experiments indicate that exogenous siderophores stimulate dissolution in excess of bacterial nutritional needs. Because dissolution continues at rates much higher than the abiotic DFOB rate, it can be inferred that the mechanism responsible for siderophore “magnification” is not limited by the same processes that down regulate siderophore production upon reaching stationary phase. Note that by the end of the experiment, dissolved Fe in the ΔMR-1 + DFOB (50 μM) treatment is only 3 μM. If not limited by other mechanisms, enhanced dissolution would likely continue until siderophores become saturated with Fe, i.e. up to 50 μM (Reichard et al., 2007
Reichard, P.U., Kretzschmar, R., Kraemer, S.M. (2007) Dissolution mechanisms of goethite in the presence of siderophores and organic acids. Geochimica et Cosmochimica Acta 71, 5635–5650. https://doi.org/10.1016/j.gca.2006.12.022
).The fact that dissolved Fe does not accumulate in the MR-1 wild type treatment suggests that this bacterium uses siderophores extremely efficiently, shunting all chelated Fe into biomass. Considering the stoichiometry of total metal release, however, total Fe recovery in MR-1 was only 60 % of expected (based on Si release) at the end of the experiment, indicating that some dissolved Fe may escape chelation and be lost to secondary mineral precipitation (Fig. S-4). In contrast, the ΔMR-1 + DFOB treatments had full Fe recovery. The reduced Fe recovery and lack of dissolved Fe in MR-1 wild type experiments together suggest that siderophore production is low and/or localised at the microbe-mineral interface, with little siderophore loss to solution. Furthermore, a conundrum persists in that our previous research confirmed the presence of siderophores in MR-1 solution (using chrome-azurol S), and an experiment with ΔMR-1 fed filtrate from MR-1 exhibited normal growth patterns (Van Den Berghe et al., 2021
Van Den Berghe, M., Merino, N., Nealson, K.H., West, A.J. (2021) Silicate minerals as a direct source of limiting nutrients: Siderophore synthesis and uptake promote ferric iron bioavailability from olivine and microbial growth. Geobiology 19, 618–630. https://doi.org/10.1111/gbi.12457
). It is possible that extremely low concentrations of Shewanella’s native siderophore putrebactin are sufficient to maintain the bacteria’s growth and mineral dissolution needs, and that structural differences between putrebactin (a cyclic dihydroxamate) and DFOB (a tris-hydroxamate) allow for more efficient use in the former. The fact that MR-1 grows to stationary phase cell densities double those of ΔMR-1, yet dissolution rates are lower, further suggests that MR-1 uses available (chelated) Fe more efficiently with putrebactin.top
Conclusions and Further Considerations
In conclusion, Shewanella does not use siderophores passively to enhance dissolution, but rather increases their efficiency to rapidly access mineral bound Fe. The observed biotically enhanced dissolution rates may not persist long term in natural conditions as they require the active biosynthesis of siderophores, which likely peak during exponential growth. Thus, significantly enhanced dissolution in the environment may be characteristic of non-stationary conditions, e.g., when populations experience Fe limitation or other environmental perturbation (Reichard et al., 2007
Reichard, P.U., Kretzschmar, R., Kraemer, S.M. (2007) Dissolution mechanisms of goethite in the presence of siderophores and organic acids. Geochimica et Cosmochimica Acta 71, 5635–5650. https://doi.org/10.1016/j.gca.2006.12.022
). On the other hand, the continued high dissolution rates observed in the ΔMR-1 + DFOB treatments suggest that dissolution could persist in environments in which siderophores are present in excess of a given microbial group’s growth needs, e.g., when supplied by other bacteria, fungi, or plants. Similarly, the addition of exogenous siderophores, and/or microbes with altered genetic capabilities, may help maintain high dissolution rates in engineered conditions. These findings may be useful for engineering biotically mediated enhanced weathering systems, a nascent but potentially useful approach to increasing mineral dissolution rates. If dissolution rates can be maintained at 10× above abiotic rates, with minimal energy inputs, such geobiological approaches may be efficient methods for effective carbon dioxide sequestration.top
Acknowledgements
This research was funded by the National Science Foundation, Division of Earth Sciences (EAR-1324929) and by the University of Southern California. We are also grateful to two anonymous reviewers for their helpful feedback.
Editor: Andreas Kappler
top
References
Berner, R.A., Lasaga, A.C., Garrels, R.M. (1983) The Carbonate-Silicate Geochemical Cycle and Its Effect on Atmospheric Carbon Dioxide Over the Past 100 Million Years. American Journal of Science 283, 641–683. https://doi.org/10.2475/ajs.283.7.641
 Show in context
Show in context Improved understanding of the environmental controls on silicate dissolution is thus important for characterising the climate-weathering feedback (Berner et al., 1983), and for informing engineered “enhanced weathering” efforts to mitigate climate change (Hartmann et al., 2013).
View in article
Brantley, S.L., Ruebush, S., Jang, J.-H., Tien, M. (2006) Analysis of (Bio)Geochemical Kinetics of Fe(III) Oxides. In: Maurice, P.A., Warren, L.A., Bain, D.C. (Eds.) Methods for Study of Microbe-Mineral Interactions. Clay Minerals Society Workshop Lectures v. 14, Clay Mineral Society, Chantilly, VA, 80–116. https://doi.org/10.1346/CMS-WLS-14.3
 Show in context
Show in context Thus, there remains a disconnect between our understanding of catalyst driven and biology driven mineral dissolution, and uncertainty as to whether siderophores are ultimately important drivers of dissolution in the environment (Brantley et al., 2006).
View in article
Bray, A.W., Oelkers, E.H., Bonneville, S., Wolff-Boenisch, D., Potts, N.J., Fones, G., Benning, L.G. (2015) The effect of pH, grain size, and organic ligands on biotite weathering rates. Geochimica et Cosmochimica Acta 164, 127–145. https://doi.org/10.1016/j.gca.2015.04.048
 Show in context
Show in context Significant research has shown that siderophores can abiotically enhance dissolution rates for a range of Fe3+ and Al3+-containing minerals, including Fe oxides (Cheah et al., 2003; Kraemer, 2004; Reichard et al., 2007), Fe containing phyllosilicates (Rosenberg and Maurice, 2003; Haack et al., 2008; Shirvani and Nourbakhsh, 2010; Ferret et al., 2014; Bray et al., 2015), and hornblende (Kalinowski et al., 2000; Liermann et al., 2000; Buss et al., 2007).
View in article
Olivine dissolution rates as a function of DFOB addition. Filled circles are from this study; hollow triangles are from Torres et al. (2019), adjusted to pH 7.2 as per the abiotic rate equations in Rimstidt et al. (2012).
View in article
Buss, H.L., Lüttge, A., Brantley, S.L. (2007) Etch pit formation on iron silicate surfaces during siderophore-promoted dissolution. Chemical Geology 240, 326–342. https://doi.org/10.1016/j.chemgeo.2007.03.003
 Show in context
Show in context Significant research has shown that siderophores can abiotically enhance dissolution rates for a range of Fe3+ and Al3+-containing minerals, including Fe oxides (Cheah et al., 2003; Kraemer, 2004; Reichard et al., 2007), Fe containing phyllosilicates (Rosenberg and Maurice, 2003; Haack et al., 2008; Shirvani and Nourbakhsh, 2010; Ferret et al., 2014; Bray et al., 2015), and hornblende (Kalinowski et al., 2000; Liermann et al., 2000; Buss et al., 2007).
View in article
Carrasco, N., Kretzschmar, R., Pesch, M.-L., Kraemer, S.M. (2007) Low Concentrations of Surfactants Enhance Siderophore-Promoted Dissolution of Goethite. Environmental Science & Technology 41, 3633–3638. https://doi.org/10.1021/es062897r
 Show in context
Show in context Siderophore adsorption to mineral surfaces is thought to be limited by both steric hindrance and charge repulsion (Cocozza et al., 2002), so microbial secretion of surfactants or negatively charged ligands could increase adsorption efficiency (Carrasco et al., 2007).
View in article
Cheah, S.-F., Kraemer, S.M., Cervini-Silva, J., Sposito, G. (2003) Steady-state dissolution kinetics of goethite in the presence of desferrioxamine B and oxalate ligands: implications for the microbial acquisition of iron. Chemical Geology 198, 63–75. https://doi.org/10.1016/S0009-2541(02)00421-7
 Show in context
Show in context Significant research has shown that siderophores can abiotically enhance dissolution rates for a range of Fe3+ and Al3+-containing minerals, including Fe oxides (Cheah et al., 2003; Kraemer, 2004; Reichard et al., 2007), Fe containing phyllosilicates (Rosenberg and Maurice, 2003; Haack et al., 2008; Shirvani and Nourbakhsh, 2010; Ferret et al., 2014; Bray et al., 2015), and hornblende (Kalinowski et al., 2000; Liermann et al., 2000; Buss et al., 2007).
View in article
As a result, dissolution rates plateau according to an adsorption isotherm. For DFOB, this has been well documented on olivine, Fe oxides and Fe containing phyllosilicates (Cheah et al., 2003; Kraemer, 2004; Haack et al., 2008; Torres et al., 2019).
View in article
Alternatively, small ligands like oxalate can interact synergistically with siderophores by liberating surface bound Fe when the saturation state of surrounding solution is lowered by siderophore mediated chelation of dissolved Fe (Cheah et al., 2003; Reichard et al., 2007).
View in article
Cocozza, C., Tsao, C.C.G., Cheah, S.-F., Kraemer, S.M., Raymond, K.N., Miano, T.M., Sposito, G. (2002) Temperature dependence of goethite dissolution promoted by trihydroxamate siderophores. Geochimica et Cosmochimica Acta 66, 431–438. https://doi.org/10.1016/S0016-7037(01)00780-3
 Show in context
Show in context Siderophore adsorption to mineral surfaces is thought to be limited by both steric hindrance and charge repulsion (Cocozza et al., 2002), so microbial secretion of surfactants or negatively charged ligands could increase adsorption efficiency (Carrasco et al., 2007).
View in article
Dehner, C.A., Awaya, J.D., Maurice, P.A., DuBois, J.L. (2010) Roles of Siderophores, Oxalate, and Ascorbate in Mobilization of Iron from Hematite by the Aerobic Bacterium Pseudomonas mendocina. Applied and Environmental Microbiology 76, 2041–2048. https://doi.org/10.1128/AEM.02349-09
 Show in context
Show in context While previous studies have found that siderophores are indeed critical for microbes to access or use mineral bound Fe, they have not linked this dependency quantitatively to mineral dissolution rates (Dehner et al., 2010; Ferret et al., 2014; Van Den Berghe et al., 2021).
View in article
Similar studies using mutant Pseudomonas sp. grown in the presence of Fe3+ oxides and clays also found reduced growth rates in the absence of siderophores (Dehner et al., 2010; Kuhn et al., 2013; Ferret et al., 2014).
View in article
Dessert, C., Dupré, B., Gaillardet, J., François, L.M., Allegre, C.J. (2003) Basalt weathering laws and the impact of basalt weathering on the global carbon cycle. Chemical Geology 202, 257–273. https://doi.org/10.1016/j.chemgeo.2002.10.001
 Show in context
Show in context In this regard, olivine ((Mg2+,Fe2+)2SiO4) and olivine-rich rocks (such as basalt) are often studied as model silicates due to their high solubility, abundance, and resulting large contribution to earth’s weathering flux (Dessert et al., 2003; Hartmann et al., 2009).
View in article
Dhungana, S., Crumbliss, A.L. (2005) Coordination Chemistry and Redox Processes in Siderophore-Mediated Iron Transport. Geomicrobiology Journal 22, 87–98. https://doi.org/10.1080/01490450590945870
 Show in context
Show in context Olivine, in contrast, contains Fe almost exclusively in the +2 oxidation state, which is more weakly bound by siderophores (e.g., for the siderophore deferoxamine B, log Kf = 30 for Fe3+ vs. 10 for Fe2+) (Dhungana and Crumbliss, 2005).
View in article
Ferret, C., Sterckeman, T., Cornu, J.-Y., Gangloff, S., Schalk, I.J., Geoffroy, V.A. (2014) Siderophore‐promoted dissolution of smectite by fluorescent Pseudomonas. Environmental Microbiology Reports 6, 459–467. https://doi.org/10.1111/1758-2229.12146
 Show in context
Show in context Significant research has shown that siderophores can abiotically enhance dissolution rates for a range of Fe3+ and Al3+-containing minerals, including Fe oxides (Cheah et al., 2003; Kraemer, 2004; Reichard et al., 2007), Fe containing phyllosilicates (Rosenberg and Maurice, 2003; Haack et al., 2008; Shirvani and Nourbakhsh, 2010; Ferret et al., 2014; Bray et al., 2015), and hornblende (Kalinowski et al., 2000; Liermann et al., 2000; Buss et al., 2007).
View in article
While previous studies have found that siderophores are indeed critical for microbes to access or use mineral bound Fe, they have not linked this dependency quantitatively to mineral dissolution rates (Dehner et al., 2010; Ferret et al., 2014; Van Den Berghe et al., 2021).
View in article
Similar studies using mutant Pseudomonas sp. grown in the presence of Fe3+ oxides and clays also found reduced growth rates in the absence of siderophores (Dehner et al., 2010; Kuhn et al., 2013; Ferret et al., 2014).
View in article
Haack, E.A., Johnston, C.T., Maurice, P.A. (2008) Mechanisms of siderophore sorption to smectite and siderophore-enhanced release of structural Fe3+. Geochimica et Cosmochimica Acta 72, 3381–3397. https://doi.org/10.1016/j.gca.2008.03.027
 Show in context
Show in context Significant research has shown that siderophores can abiotically enhance dissolution rates for a range of Fe3+ and Al3+-containing minerals, including Fe oxides (Cheah et al., 2003; Kraemer, 2004; Reichard et al., 2007), Fe containing phyllosilicates (Rosenberg and Maurice, 2003; Haack et al., 2008; Shirvani and Nourbakhsh, 2010; Ferret et al., 2014; Bray et al., 2015), and hornblende (Kalinowski et al., 2000; Liermann et al., 2000; Buss et al., 2007).
View in article
As a result, dissolution rates plateau according to an adsorption isotherm. For DFOB, this has been well documented on olivine, Fe oxides and Fe containing phyllosilicates (Cheah et al., 2003; Kraemer, 2004; Haack et al., 2008; Torres et al., 2019).
View in article
Hartmann, J., Jansen, N., Dürr, H.H., Kempe, S., Köhler, P. (2009) Global CO2-consumption by chemical weathering: What is the contribution of highly active weathering regions? Global and Planetary Change 69, 185–194. https://doi.org/10.1016/j.gloplacha.2009.07.007
 Show in context
Show in context In this regard, olivine ((Mg2+,Fe2+)2SiO4) and olivine-rich rocks (such as basalt) are often studied as model silicates due to their high solubility, abundance, and resulting large contribution to earth’s weathering flux (Dessert et al., 2003; Hartmann et al., 2009).
View in article
Hartmann, J., West, A.J., Renforth, P., Köhler, P., De La Rocha, C.L., Wolf‐Gladrow, D.A., Dürr, H.H., Scheffran, J. (2013) Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Reviews of Geophysics 51, 113–149. https://doi.org/10.1002/rog.20004
 Show in context
Show in context Improved understanding of the environmental controls on silicate dissolution is thus important for characterising the climate-weathering feedback (Berner et al., 1983), and for informing engineered “enhanced weathering” efforts to mitigate climate change (Hartmann et al., 2013).
View in article
Hider, R.C., Kong, X. (2010) Chemistry and biology of siderophores. Natural Product Reports 27, 637–657. https://doi.org/10.1039/b906679a
 Show in context
Show in context While they are structurally diverse, siderophores typically contain multiple metal-binding ligands that collectively result in exceptionally high binding affinity with Fe3+ (log Kf > 30), preventing its loss from solution via Fe oxide precipitation (Hider and Kong, 2010).
View in article
Kalinowski, B.E., Liermann, L.J., Givens, S., Brantley, S.L. (2000) Rates of bacteria-promoted solubilization of Fe from minerals: a review of problems and approaches. Chemical Geology 169, 357–370. https://doi.org/10.1016/S0009-2541(00)00214-X
 Show in context
Show in context Significant research has shown that siderophores can abiotically enhance dissolution rates for a range of Fe3+ and Al3+-containing minerals, including Fe oxides (Cheah et al., 2003; Kraemer, 2004; Reichard et al., 2007), Fe containing phyllosilicates (Rosenberg and Maurice, 2003; Haack et al., 2008; Shirvani and Nourbakhsh, 2010; Ferret et al., 2014; Bray et al., 2015), and hornblende (Kalinowski et al., 2000; Liermann et al., 2000; Buss et al., 2007).
View in article
Kraemer, S.M. (2004) Iron oxide dissolution and solubility in the presence of siderophores. Aquatic Sciences 66, 3–18. https://doi.org/10.1007/s00027-003-0690-5
 Show in context
Show in context Significant research has shown that siderophores can abiotically enhance dissolution rates for a range of Fe3+ and Al3+-containing minerals, including Fe oxides (Cheah et al., 2003; Kraemer, 2004; Reichard et al., 2007), Fe containing phyllosilicates (Rosenberg and Maurice, 2003; Haack et al., 2008; Shirvani and Nourbakhsh, 2010; Ferret et al., 2014; Bray et al., 2015), and hornblende (Kalinowski et al., 2000; Liermann et al., 2000; Buss et al., 2007).
View in article
An important distinction, however, is that these studies use purified siderophores at high micromolar concentrations, whereas environmental concentrations are consistently much lower (pico- to nanomolar) (Kraemer, 2004).
View in article
As a result, dissolution rates plateau according to an adsorption isotherm. For DFOB, this has been well documented on olivine, Fe oxides and Fe containing phyllosilicates (Cheah et al., 2003; Kraemer, 2004; Haack et al., 2008; Torres et al., 2019).
View in article
Kuhn, K.M., DuBois, J.L., Maurice, P.A. (2013) Strategies of aerobic microbial Fe acquisition from Fe-bearing montmorillonite clay. Geochimica et Cosmochimica Acta 117, 191–202. https://doi.org/10.1016/j.gca.2013.04.028
 Show in context
Show in context Similar studies using mutant Pseudomonas sp. grown in the presence of Fe3+ oxides and clays also found reduced growth rates in the absence of siderophores (Dehner et al., 2010; Kuhn et al., 2013; Ferret et al., 2014).
View in article
Liermann, L.J., Kalinowski, B.E., Brantley, S.L., Ferry, J.G. (2000) Role of bacterial siderophores in dissolution of hornblende. Geochimica et Cosmochimica Acta 64, 587–602. https://doi.org/10.1016/S0016-7037(99)00288-4
 Show in context
Show in context Significant research has shown that siderophores can abiotically enhance dissolution rates for a range of Fe3+ and Al3+-containing minerals, including Fe oxides (Cheah et al., 2003; Kraemer, 2004; Reichard et al., 2007), Fe containing phyllosilicates (Rosenberg and Maurice, 2003; Haack et al., 2008; Shirvani and Nourbakhsh, 2010; Ferret et al., 2014; Bray et al., 2015), and hornblende (Kalinowski et al., 2000; Liermann et al., 2000; Buss et al., 2007).
View in article
However, these processes may be insufficient to drive dissolution alone: ligand facilitated dissolution has been shown to be significant only at high ligand concentrations (typically >1 mM), which may not be achieved by slow growing microbes (Reichard et al., 2007), and pH gradients in biofilms may not be severe enough to facilitate dissolution (Liermann et al., 2000).
View in article
Liu, L., Li, S., Wang, S., Dong, Z., Gao, H. (2018) Complex Iron Uptake by the Putrebactin-Mediated and Feo Systems in Shewanella oneidensis. Applied and Environmental Microbiology 84, e01752–18. https://doi.org/10.1128/AEM.01752-18
 Show in context
Show in context S. oneidensis has the capacity to take up Fe through alternative pathways not involving siderophores — including direct uptake of Fe2+ and/or ligand bound Fe3+ transport — so a dependence on siderophores for microbial growth is not obvious a priori (Liu et al., 2018).
View in article
Oelkers, E.H., Declercq, J., Saldi, G.D., Gislason, S.R., Schott, J. (2018) Olivine dissolution rates: A critical review. Chemical Geology 500, 1–19. https://doi.org/10.1016/j.chemgeo.2018.10.008
 Show in context
Show in context The effect of abiotic conditions such as temperature, solution pH, and solution chemistry are relatively well constrained (Rimstidt et al., 2012; Oelkers et al., 2018).
View in article
Reichard, P.U., Kretzschmar, R., Kraemer, S.M. (2007) Dissolution mechanisms of goethite in the presence of siderophores and organic acids. Geochimica et Cosmochimica Acta 71, 5635–5650. https://doi.org/10.1016/j.gca.2006.12.022
 Show in context
Show in context Significant research has shown that siderophores can abiotically enhance dissolution rates for a range of Fe3+ and Al3+-containing minerals, including Fe oxides (Cheah et al., 2003; Kraemer, 2004; Reichard et al., 2007), Fe containing phyllosilicates (Rosenberg and Maurice, 2003; Haack et al., 2008; Shirvani and Nourbakhsh, 2010; Ferret et al., 2014; Bray et al., 2015), and hornblende (Kalinowski et al., 2000; Liermann et al., 2000; Buss et al., 2007).
View in article
However, these processes may be insufficient to drive dissolution alone: ligand facilitated dissolution has been shown to be significant only at high ligand concentrations (typically >1 mM), which may not be achieved by slow growing microbes (Reichard et al., 2007), and pH gradients in biofilms may not be severe enough to facilitate dissolution (Liermann et al., 2000).
View in article
Alternatively, small ligands like oxalate can interact synergistically with siderophores by liberating surface bound Fe when the saturation state of surrounding solution is lowered by siderophore mediated chelation of dissolved Fe (Cheah et al., 2003; Reichard et al., 2007).
View in article
If not limited by other mechanisms, enhanced dissolution would likely continue until siderophores become saturated with Fe, i.e. up to 50 μM (Reichard et al., 2007).
View in article
Thus, significantly enhanced dissolution in the environment may be characteristic of non-stationary conditions, e.g., when populations experience Fe limitation or other environmental perturbation (Reichard et al., 2007).
View in article
Rimstidt, J.D., Brantley, S.L., Olsen, A.A. (2012) Systematic review of forsterite dissolution rate data. Geochimica et Cosmochimica Acta 99, 159–178. https://doi.org/10.1016/j.gca.2012.09.019
 Show in context
Show in context The effect of abiotic conditions such as temperature, solution pH, and solution chemistry are relatively well constrained (Rimstidt et al., 2012; Oelkers et al., 2018).
View in article
In the absence of DFOB, ΔMR-1 could not access olivine bound Fe at rates fast enough to support growth, and correspondingly did not enhance mineral dissolution above well established abiotic rates (Figs. 1–3, S-1, Table S-1) (Rimstidt et al., 2012).
View in article
Rosenberg, D.R., Maurice, P.A. (2003) Siderophore adsorption to and dissolution of kaolinite at pH 3 to 7 and 22°C. Geochimica et Cosmochimica Acta 67, 223–229. https://doi.org/10.1016/S0016-7037(02)01082-7
 Show in context
Show in context Significant research has shown that siderophores can abiotically enhance dissolution rates for a range of Fe3+ and Al3+-containing minerals, including Fe oxides (Cheah et al., 2003; Kraemer, 2004; Reichard et al., 2007), Fe containing phyllosilicates (Rosenberg and Maurice, 2003; Haack et al., 2008; Shirvani and Nourbakhsh, 2010; Ferret et al., 2014; Bray et al., 2015), and hornblende (Kalinowski et al., 2000; Liermann et al., 2000; Buss et al., 2007).
View in article
Schwartzman, D.W., Volk, T. (1989) Biotic enhancement of weathering and the habitability of Earth. Nature 340, 457–460. https://doi.org/10.1038/340457a0
 Show in context
Show in context While the net effect of biology on mineral dissolution is typically assumed to be positive, observed biotic effects range from inhibition to enhancement (Schwartzman and Volk, 1989).
View in article
Shirvani, M., Nourbakhsh, F. (2010) Desferrioxamine-B adsorption to and iron dissolution from palygorskite and sepiolite. Applied Clay Science 48, 393–397. https://doi.org/10.1016/j.clay.2010.01.012
 Show in context
Show in context Significant research has shown that siderophores can abiotically enhance dissolution rates for a range of Fe3+ and Al3+-containing minerals, including Fe oxides (Cheah et al., 2003; Kraemer, 2004; Reichard et al., 2007), Fe containing phyllosilicates (Rosenberg and Maurice, 2003; Haack et al., 2008; Shirvani and Nourbakhsh, 2010; Ferret et al., 2014; Bray et al., 2015), and hornblende (Kalinowski et al., 2000; Liermann et al., 2000; Buss et al., 2007).
View in article
Torres, M.A., Dong, S., Nealson, K.H., West, A.J. (2019) The kinetics of siderophore‐mediated olivine dissolution. Geobiology 17, 401–416. https://doi.org/10.1111/gbi.12332
 Show in context
Show in context Nonetheless, recent research has shown that siderophores also increase olivine dissolution rates by nearly an order of magnitude (Torres et al., 2019).
View in article
It has been established that siderophores, independent of live culture, stimulate the dissolution of many Fe containing minerals, including olivine (Torres et al., 2019).
View in article
Olivine dissolution rates as a function of DFOB addition. Filled circles are from this study; hollow triangles are from Torres et al. (2019), adjusted to pH 7.2 as per the abiotic rate equations in Rimstidt et al. (2012).
View in article
As a result, dissolution rates plateau according to an adsorption isotherm. For DFOB, this has been well documented on olivine, Fe oxides and Fe containing phyllosilicates (Cheah et al., 2003; Kraemer, 2004; Haack et al., 2008; Torres et al., 2019).
View in article
Considering the relationship between olivine dissolution and DFOB concentration published by Torres et al. (2019) — which was derived at experimental conditions similar to ours (30 °C, pH 7.5) — dissolution rates plateau at >100 μM DFOB (Fig. 3).
View in article
Van Den Berghe, M., Merino, N., Nealson, K.H., West, A.J. (2021) Silicate minerals as a direct source of limiting nutrients: Siderophore synthesis and uptake promote ferric iron bioavailability from olivine and microbial growth. Geobiology 19, 618–630. https://doi.org/10.1111/gbi.12457
 Show in context
Show in context While previous studies have found that siderophores are indeed critical for microbes to access or use mineral bound Fe, they have not linked this dependency quantitatively to mineral dissolution rates (Dehner et al., 2010; Ferret et al., 2014; Van Den Berghe et al., 2021).
View in article
While MR-1 is often studied for dissimilatory metal reduction, it was used in this study only due to availability of the siderophore gene-deletion mutant; dissimilatory Fe reduction was not a concern, as olivine bound Fe is almost exclusively in the +2 oxidation state (Van Den Berghe et al., 2021).
View in article
Even at 50 μM DFOB addition, which exceeds the concentration needed for maximum growth of ΔMR-1 (approximately 5 μM; Van Den Berghe et al., 2021), chelated Fe does not accumulate in solution until well after stationary phase is reached, suggesting highly efficient siderophore use and shuttling of siderophore bound Fe into biomass.
View in article
Furthermore, a conundrum persists in that our previous research confirmed the presence of siderophores in MR-1 solution (using chrome-azurol S), and an experiment with ΔMR-1 fed filtrate from MR-1 exhibited normal growth patterns (Van Den Berghe et al., 2021).
View in article
White, A.F., Brantley, S.L. (1995) Chemical Weathering Rates of Silicate Minerals. Reviews in Mineralogy v. 31, De Gruyter, Berlin, 583 p. https://doi.org/10.1515/9781501509650
 Show in context
Show in context The chemical weathering of silicate minerals is an important control on global biogeochemical cycling and climate via the release of metal cations and alkalinity (White and Brantley, 1995).
View in article
Wogelius, R.A., Walther, J.V. (1991) Olivine dissolution at 25°C: Effects of pH, CO2, and organic acids. Geochimica et Cosmochimica Acta 55, 943–954. https://doi.org/10.1016/0016-7037(91)90153-V
 Show in context
Show in context Furthermore, alternative microbial processes, such as decreased pH or production of low molecular weight organic acids could also facilitate mineral dissolution to feed microbial growth (Wogelius and Walther, 1991).
View in article
top
Supplementary Information
The Supplementary Information includes:
Download the Supplementary Information (PDF)
Figures
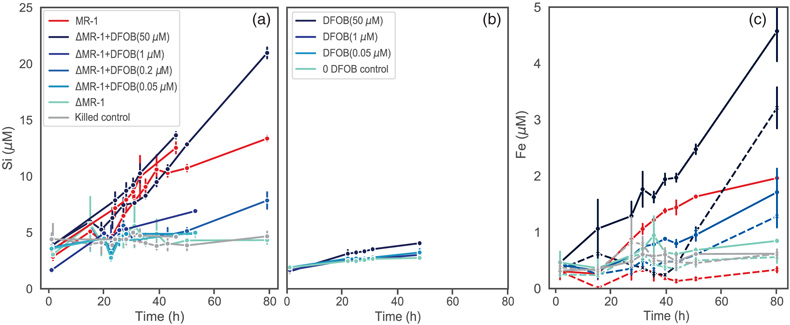
Figure 1 Dissolved Si measured in (a) biotic and (b) abiotic experiments, and (c) dissolved and total Fe in the biotic experiments (mean ± s.d.). Colours in all figures correspond to the same concentration of exogenous DFOB. Dashed lines in (c) represent dissolved concentrations, while solid lines are total concentrations.
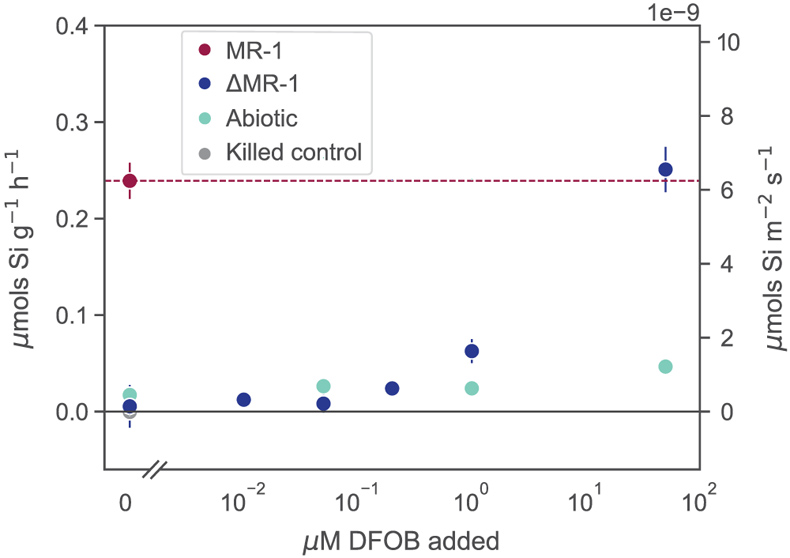
Figure 2 Olivine dissolution rates (based on Si release) as a function of DFOB addition. Dissolution rates in biotic treatments (ΔMR-1) exceed abiotic treatments at the same DFOB addition for DFOB > 1 μM. At 50 μM DFOB addition, biotic dissolution rates exceed abiotic rates by 8 fold. Dashed line represents dissolution rate for MR-1, with no exogenous siderophore addition.
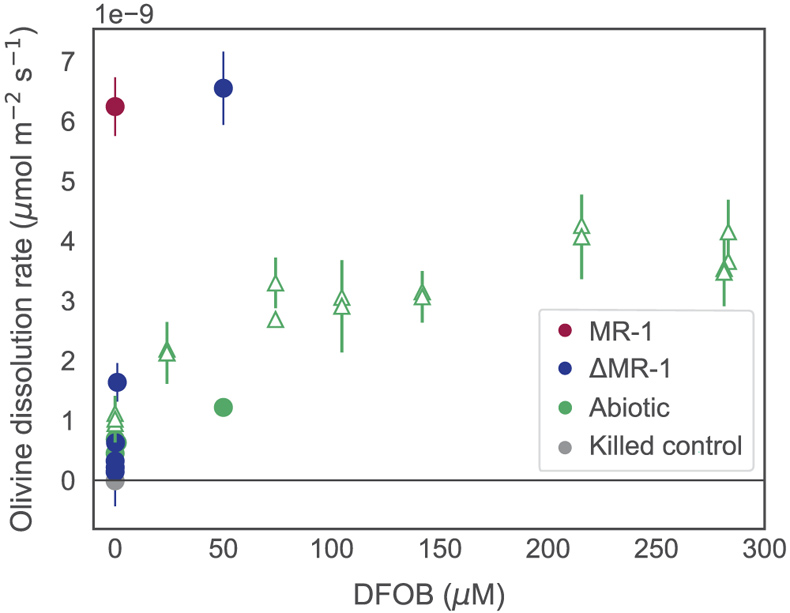
Figure 3 Olivine dissolution rates as a function of DFOB addition. Filled circles are from this study; hollow triangles are from Torres et al. (2019)
Torres, M.A., Dong, S., Nealson, K.H., West, A.J. (2019) The kinetics of siderophore‐mediated olivine dissolution. Geobiology 17, 401–416. https://doi.org/10.1111/gbi.12332
, adjusted to pH 7.2 as per the abiotic rate equations in Rimstidt et al. (2012)Rimstidt, J.D., Brantley, S.L., Olsen, A.A. (2012) Systematic review of forsterite dissolution rate data. Geochimica et Cosmochimica Acta 99, 159–178. https://doi.org/10.1016/j.gca.2012.09.019
. At 50 μM DFOB addition, our abiotic rate is reasonably characterised by (and slightly lower than) the abiotic adsorption isotherm. In contrast, the MR-1 and ΔMR-1 rates are significantly above the isotherm.