River chemistry reveals a large decrease in dolomite abundance across the Phanerozoic
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:1,314Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
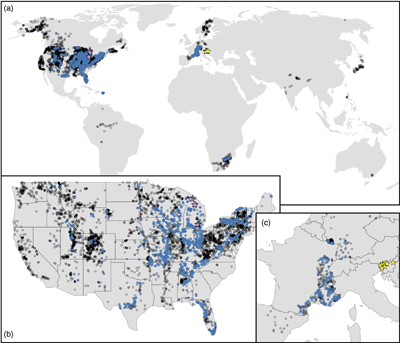 Figure 1 (a) Map showing locations of river stations plotted in Figure 2. All localities are included in Figure 2a, whereas blue dots are carbonate-draining rivers included in Figure 2b or c. In (b) and (c), inset maps of the contiguous U.S.A. and western Europe, respectively, are shown. Also plotted are rivers in Michigan (purple stars; Williams et al., 2007) and Slovenia (yellow stars; Szramek et al., 2011) draining catchments with ∼30–70 % carbonate outcrop. | 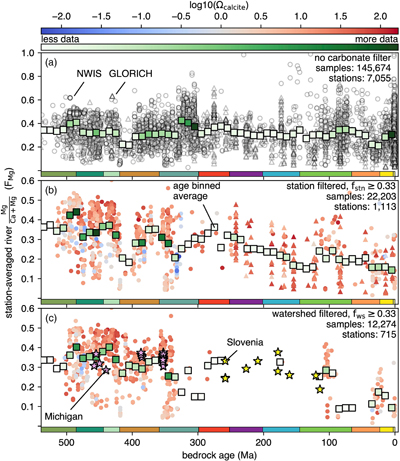 Figure 2 Station-averaged river Mg/(Ca + Mg) ratios (FMg) vs. bedrock age from the NWIS (circles) and GLORICH (triangles) databases, and 10 Myr binned means (squares, colour coded by the amount of data averaged in each bin, scaled to each time series). In (a), all rivers are included, whereas (b) and (c) only show rivers that drain carbonate bedrock, have relatively low Na and K, and have a ratio of HCO3− to (Mg2+ + Ca2+) between 1 and 2 (see Results). In (b), fstn value is used to filter for carbonate containing bedrock, and in (c), fws is used. In (b) and (c), values of FMg are colour coded by station-averaged calcite saturation (Ω; see Discussion). Also plotted in (c) are FMg from rivers in Michigan (purple stars; Williams et al., 2007) and Slovenia (yellow stars; Szramek et al., 2011) that drain carbonate dominated catchments, which are not included in the age binned averages. | 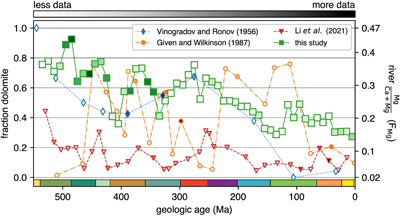 Figure 3 Using the age binned values from Figure 2b, river FMg (right y axis) is used to predict mole fraction dolomite in carbonate bedrock (left axis; see Discussion), and plotted vs. bedrock age. Fraction dolomite based on rock sample geochemistry from the Russian platform (Vinogradov and Ronov, 1956) and North America (Given and Wilkinson, 1987) and on a compilation of unit thicknesses of dolostones and limestones in carbonate sequences (Li et al., 2021) are also shown. All records are colour coded by the relative amount of data averaged into each point, scaled to each time series. |
| Figure 1 | Figure 2 | Figure 3 |
top
Introduction
A truism in sedimentary geology is that dolomite, (Ca0.5,Mg0.5)CO3, is more common in ancient carbonates compared to younger sequences (Daly, 1909
Daly, R.A. (1909) First calcareous fossils and the evolution of the limestones. Geological Society of America Bulletin 20, 153–170. https://doi.org/10.1130/GSAB-20-153
). Dolomite is also a rare precipitate from the modern ocean, despite seawater being highly dolomite supersaturated (Land, 1998Land, L.S. (1998) Failure to Precipitate Dolomite at 25 °C from Dilute Solution Despite 1000-Fold Oversaturation after 32 Years. Aquatic Geochemistry 4, 361–368. https://doi.org/10.1023/A:1009688315854
). These two observations form the core of “the dolomite problem” (e.g., Holland and Zimmermann, 2000Holland, H.D., Zimmermann, H. (2000) The dolomite problem revisited. International Geology Review 42, 481–490. https://doi.org/10.1080/00206810009465093
; Petrash et al., 2017Petrash, D.A., Bialik, O.M., Bontognali, T.R., Vasconcelos, C., Roberts, J.A., McKenzie, J.A., Konhauser, K.O. (2017) Microbially catalyzed dolomite formation: From near-surface to burial. Earth-Science Reviews 171, 558–582. https://doi.org/10.1029/2018GC007467
). While much research addresses the origin of dolomite, there is still no consensus on how its abundance varies with geologic age. Some time series show a monotonic decrease in the dolomite to calcite ratio from the beginning of the Phanerozoic towards today (Vinogradov and Ronov, 1956Vinogradov, A., Ronov, A. (1956) Compositions of the sedimentary rocks of the Russian Platform in relation to the history of its tectonic movements. Geokhimiya 6, 533–559.
; Schmoker et al., 1985Schmoker, J.W., Krystinik, K.B., Halley, R.B. (1985) Selected characteristics of limestone and dolomite reservoirs in the United States. AAPG Bulletin 69, 733–741. https://doi.org/10.1306/AD4627F9-16F7-11D7-8645000102C1865D
). Others are stationary and show large oscillations around a Phanerozoic mean (Given and Wilkinson, 1987Given, R.K., Wilkinson, B.H. (1987) Dolomite abundance and stratigraphic age; constraints on rates and mechanisms of Phanerozoic dolostone formation. Journal of Sedimentary Research 57, 1068–1078. https://doi.org/10.1306/212F8CF1-2B24-11D7-8648000102C1865D
; Li et al., 2021Li, M., Wignall, P.B., Dai, X., Hu, M., Song, H. (2021) Phanerozoic variation in dolomite abundance linked to oceanic anoxia. Geology 49, 698–702. https://doi.org/10.1130/G48502.1
). Better constraints on dolomite abundance across Earth history are crucial for understanding its broader implications. If older rocks systematically contain more dolomite than younger ones, then carbonate weathering delivers more Mg to the oceans than can be balanced by carbonate burial, requiring an additional sink(s). The dolomite problem thus affects the cycling of Ca-Mg-C in the Earth system, with important implications for global climate (e.g., Arvidson et al., 2011Arvidson, R.S., Guidry, M.W., Mackenzie, F.T. (2011) Dolomite controls on Phanerozoic seawater chemistry. Aquatic Geochemistry 17, 735–747. https://doi.org/10.1007/s10498-011-9130-7
).Previous dolomite abundance records have used Mg/Ca ratios in carbonate samples (Vinogradov and Ronov, 1956
Vinogradov, A., Ronov, A. (1956) Compositions of the sedimentary rocks of the Russian Platform in relation to the history of its tectonic movements. Geokhimiya 6, 533–559.
; Given and Wilkinson, 1987Given, R.K., Wilkinson, B.H. (1987) Dolomite abundance and stratigraphic age; constraints on rates and mechanisms of Phanerozoic dolostone formation. Journal of Sedimentary Research 57, 1068–1078. https://doi.org/10.1306/212F8CF1-2B24-11D7-8648000102C1865D
), or descriptions of limestone and dolostone in sedimentary sequences (Schmoker et al., 1985Schmoker, J.W., Krystinik, K.B., Halley, R.B. (1985) Selected characteristics of limestone and dolomite reservoirs in the United States. AAPG Bulletin 69, 733–741. https://doi.org/10.1306/AD4627F9-16F7-11D7-8645000102C1865D
; Li et al., 2021Li, M., Wignall, P.B., Dai, X., Hu, M., Song, H. (2021) Phanerozoic variation in dolomite abundance linked to oceanic anoxia. Geology 49, 698–702. https://doi.org/10.1130/G48502.1
). A major challenge to these methods is that the samples or units must be representative of a much larger volume of carbonate rock. Description based syntheses have the added challenge of converting qualitative field descriptions to quantitative percentages of carbonate mineralogies. To circumvent these problems, we measure dolomite abundance in the rock record using dissolved Mg2+ and Ca2+ concentrations in rivers. Owing to its greater efficiency compared to silicate weathering, carbonate weathering globally delivers ∼84 % of the total riverine flux of Mg2+ and Ca2+ (Gaillardet et al., 1999Gaillardet, J., Dupré, B., Louvat, P., Allegre, C. (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chemical Geology 159, 3–30. https://doi.org/10.1016/S0009-2541(99)00031-5
). Therefore, it is reasonable to expect that rivers draining carbonate bedrock would have a similar Mg/Ca to average carbonate in the catchment, and detailed studies of watersheds with ∼30–70 % carbonate outcrop in Slovenia (Szramek et al., 2011Szramek, K., Walter, L.M., Kanduc, T., Ogrinc, N. (2011) Dolomite versus calcite weathering in hydrogeochemically diverse watersheds established on bedded carbonates (Sava and Soča Rivers, Slovenia). Aquatic Geochemistry 17, 357–396. https://doi.org/10.1007/s10498-011-9125-4
) and Michigan (Williams et al., 2007Williams, E.L., Szramek, K.J., Jin, L., Ku, T.C., Walter, L.M. (2007) The carbonate system geochemistry of shallow groundwater–surface water systems in temperate glaciated watersheds (Michigan, USA): Significance of open-system dolomite weathering. Geological Society of America Bulletin 119, 515–528. https://doi.org/10.1130/B25967.1
) support this assertion. In our compiled record, in catchments that drain carbonate-bearing bedrock, the average Mg/(Ca + Mg) molar ratio of rivers (referred to here as FMg) declines monotonically with decreasing bedrock age. This signal is interpreted as providing the most robust published record of the decrease in dolomite to calcite ratio over the Phanerozoic.top
Methods
Measurements of dissolved Ca2+, Mg2+, Na+, K+, Cl−, pH, CO2(aq) and alkalinity on filtered river water samples were compiled from the USGS National Water Information System (NWIS) and the Global River Chemistry (GLORICH; Hartmann et al., 2014
Hartmann, J., Lauerwald, R., Moosdorf, N. (2014) A brief overview of the GLObal RIver CHemistry Database, GLORICH. Procedia Earth and Planetary Science 10, 23–27. https://doi.org/10.1016/j.proeps.2014.08.005
) databases. Geologic maps stored in Macrostrat (Peters et al., 2018Peters, S.E., Husson, J.M., Czaplewski, J. (2018) Macrostrat: a platform for geological data integration and deep-time Earth crust research. Geochemistry, Geophysics, Geosystems 19, 1393–1409. https://doi.org/10.1029/2018GC007467
) were used to constrain the bedrock geology that directly underlies each stream measurement station (Supplementary Information). An upper and lower bedrock age was assigned based on the chronostratigraphic age bin assigned to the underlying geologic unit. The bedrock for each river sampling site was designated a ‘station fraction carbonate’ value (fstn), calculated as the number of listed carbonate lithologies divided by the total number of listed lithologies. For example, if a measurement station sits on a geologic unit that is described as “limestone, sandstone and siltstone,” its fstn value would be 0.33. In all, the total area of the carbonate containing map units that underlie stream stations is 3.39 x 106 km2, which is 10 % of the total continental area estimated to be covered by carbonate or mixed carbonate-siliciclastic sediments (33.4 x 106 km2; Hartmann and Moosdorf, 2012Hartmann, J., Moosdorf, N. (2012) The new global lithological map database GLiM: A representation of rock properties at the Earth surface. Geochemistry, Geophysics, Geosystems 13, Q12004. https://doi.org/10.1029/2012GC004370
).As a test whether this surface lithology determination was robust, we used Macrostrat to describe the bedrock geology of entire watersheds that contain NWIS stations. This approach utilised the USGS National Watershed Boundary Dataset (WBD) that demarcates the U.S.A. into separate watersheds (Supplementary Information). Using geological map data, an area-weighted ‘watershed fraction carbonate’ value (fws) was calculated for each watershed that contains a NWIS station. For example, if a watershed is composed of two mapped units of equal area (one igneous, and one carbonate-siliciclastic), its fws would be 0.25. An upper and lower age for the watershed’s bedrock was determined by the area-weighted average of the upper and lower ages of the carbonate containing map units. Both fstn and fws are derived from qualitative text descriptions from 174 different geologic maps (Supplementary Information). Therefore, these ‘fraction carbonate’ parameters do not quantify carbonate abundance in bedrock precisely, but simply provide a filter that we can use to separate the dataset into carbonate-richer and carbonate-poorer subsets, to test the role this difference plays in controlling the resulting river chemistry.
top
Results
Population averages and standard deviations of FMg for each station were calculated to avoid overweighting of sites with multiple measurements. Station-averaged FMg was then included if 1σ is less than 10 % to ensure reproducibility of FMg at the station level. Locations of these NWIS and GLORICH stations are shown in Figure 1.

Figure 1 (a) Map showing locations of river stations plotted in Figure 2. All localities are included in Figure 2a, whereas blue dots are carbonate-draining rivers included in Figure 2b or c. In (b) and (c), inset maps of the contiguous U.S.A. and western Europe, respectively, are shown. Also plotted are rivers in Michigan (purple stars; Williams et al., 2007
Williams, E.L., Szramek, K.J., Jin, L., Ku, T.C., Walter, L.M. (2007) The carbonate system geochemistry of shallow groundwater–surface water systems in temperate glaciated watersheds (Michigan, USA): Significance of open-system dolomite weathering. Geological Society of America Bulletin 119, 515–528. https://doi.org/10.1130/B25967.1
) and Slovenia (yellow stars; Szramek et al., 2011Szramek, K., Walter, L.M., Kanduc, T., Ogrinc, N. (2011) Dolomite versus calcite weathering in hydrogeochemically diverse watersheds established on bedded carbonates (Sava and Soča Rivers, Slovenia). Aquatic Geochemistry 17, 357–396. https://doi.org/10.1007/s10498-011-9125-4
) draining catchments with ∼30–70 % carbonate outcrop.Bedrock age vs. station-averaged FMg shows no secular trend (Fig. 2a), a result more clearly seen when the data are shown as age binned averages. Before binning, the dataset was sampled via 10,000 Monte Carlo trials, wherein each station’s FMg is drawn from a normal distribution defined by its mean and standard deviation, and bedrock age is drawn from a uniform distribution defined by each station’s top and bottom age. Average FMg was then calculated on this re-sampled dataset for evenly spaced 10 million year (Myr) bins that span the Phanerozoic. These age binned FMg values have a mean value of 0.32, similar to the mean of all station averages (0.33).

Figure 2 Station-averaged river Mg/(Ca + Mg) ratios (FMg) vs. bedrock age from the NWIS (circles) and GLORICH (triangles) databases, and 10 Myr binned means (squares, colour coded by the amount of data averaged in each bin, scaled to each time series). In (a), all rivers are included, whereas (b) and (c) only show rivers that drain carbonate bedrock, have relatively low Na and K, and have a ratio of HCO3− to (Mg2+ + Ca2+) between 1 and 2 (see Results). In (b), fstn value is used to filter for carbonate containing bedrock, and in (c), fws is used. In (b) and (c), values of FMg are colour coded by station-averaged calcite saturation (Ω; see Discussion). Also plotted in (c) are FMg from rivers in Michigan (purple stars; Williams et al., 2007
Williams, E.L., Szramek, K.J., Jin, L., Ku, T.C., Walter, L.M. (2007) The carbonate system geochemistry of shallow groundwater–surface water systems in temperate glaciated watersheds (Michigan, USA): Significance of open-system dolomite weathering. Geological Society of America Bulletin 119, 515–528. https://doi.org/10.1130/B25967.1
) and Slovenia (yellow stars; Szramek et al., 2011Szramek, K., Walter, L.M., Kanduc, T., Ogrinc, N. (2011) Dolomite versus calcite weathering in hydrogeochemically diverse watersheds established on bedded carbonates (Sava and Soča Rivers, Slovenia). Aquatic Geochemistry 17, 357–396. https://doi.org/10.1007/s10498-011-9125-4
) that drain carbonate dominated catchments, which are not included in the age binned averages.When data are only included from rivers that drain carbonate containing bedrock, the time series look dramatically different (Fig. 2b, c). We took three steps to identify these rivers (blue dots; Fig. 1). First, to reduce the influence of silicate weathering, samples with molar ratios of K/(Ca + Mg) and Na/(Ca + Mg) above 0.061 and 0.36, respectively, were excluded. These upper limits are based upon the K+ and Na+ content of NWIS stream samples from 134 watersheds with fws ≥ 0.9, where carbonate weathering is expected to dominate (Fig. S-1). While these selected upper limits are somewhat arbitrary, there is no correlation between FMg and K+ or Na+ (Fig. S-2); therefore, picking different limits would not change the overall distribution of FMg.
Second, river water samples were only included if their ratio of HCO3− to (Mg2+ + Ca2+) is between 1 and 2, which are the predicted end member ratios for calcite and dolomite weathering via sulfuric acid and carbonic acid, respectively (Szramek et al., 2011
Szramek, K., Walter, L.M., Kanduc, T., Ogrinc, N. (2011) Dolomite versus calcite weathering in hydrogeochemically diverse watersheds established on bedded carbonates (Sava and Soča Rivers, Slovenia). Aquatic Geochemistry 17, 357–396. https://doi.org/10.1007/s10498-011-9125-4
). Therefore, a HCO3/(Ca + Mg) ratio within this range is evidence that the Mg2+ and Ca2+ within a sample are derived from dissolved carbonate minerals. For each water sample, we calculated [HCO3−] and calcite saturation state, Ω, using the Python toolbox PyCO2SYS 1.8.1 (Humphreys et al., 2022Humphreys, M.P., Lewis, E.R., Sharp, J.D., Pierrot, D. (2022) PyCO2SYS v1.8: marine carbonate system calculations in Python. Geoscientific Model Development 15, 15–43. https://doi.org/10.5194/gmd-15-15-2022
), using either measured pH and CO2(aq) (148,621 samples) or measured pH and alkalinity (58,983 samples) to solve the carbonate system. Of these samples, 80 % have a HCO3/(Ca + Mg) ratio consistent with a carbonate weathering source.Third, and lastly, the relative number of described carbonate lithologies found in geologic maps were used to select rivers that drain carbonate containing bedrock, determined either at the station level (fstn ≥ 0.33; Fig. 2b) or at the watershed level (fws ≥ 0.33; Fig. 2c). These cut offs are similar to the low end of carbonate richness in watersheds that were studied in detail for carbonate and dolomite weathering (Williams et al., 2007
Williams, E.L., Szramek, K.J., Jin, L., Ku, T.C., Walter, L.M. (2007) The carbonate system geochemistry of shallow groundwater–surface water systems in temperate glaciated watersheds (Michigan, USA): Significance of open-system dolomite weathering. Geological Society of America Bulletin 119, 515–528. https://doi.org/10.1130/B25967.1
; Szramek et al., 2011Szramek, K., Walter, L.M., Kanduc, T., Ogrinc, N. (2011) Dolomite versus calcite weathering in hydrogeochemically diverse watersheds established on bedded carbonates (Sava and Soča Rivers, Slovenia). Aquatic Geochemistry 17, 357–396. https://doi.org/10.1007/s10498-011-9125-4
), although our results are not sensitive to the exact cut off used (see below).In the fstn filtered subset, a clear secular change in FMg is observed, with a decrease from ∼0.44 to 0.14 from the beginning to end of the Phanerozoic. To test whether the approach to filtering the data using the fraction of carbonate rock in weathered bedrock impacts the result, a range of fstn limits were used, along with the K/(Ca + Mg), Na/(Ca + Mg) and HCO3/(Ca + Mg) filtering. These experiments produce similar time series with similar Phanerozoic declines in FMg (Figs. S-3, S-4). Comparable results are found if using the same filtering parameters except excluding the HCO3/(Ca + Mg) filter (Fig. S-5). These sensitivity tests suggest that our results are robust to the uncertainties in how the fraction of carbonate in the bedrock is used to filter the river chemistry. A similar decline in FMg over the Phanerozoic is seen with the fws filtered subset, although this subset is only from NWIS, and lacks data between 260 and 120 Ma (Fig. 2c). Watershed bedrock age and average FMg (per sampling site) were also calculated for the well characterised, carbonate weathering Slovenian and Michigan watersheds (Williams et al., 2007
Williams, E.L., Szramek, K.J., Jin, L., Ku, T.C., Walter, L.M. (2007) The carbonate system geochemistry of shallow groundwater–surface water systems in temperate glaciated watersheds (Michigan, USA): Significance of open-system dolomite weathering. Geological Society of America Bulletin 119, 515–528. https://doi.org/10.1130/B25967.1
; Szramek et al., 2011Szramek, K., Walter, L.M., Kanduc, T., Ogrinc, N. (2011) Dolomite versus calcite weathering in hydrogeochemically diverse watersheds established on bedded carbonates (Sava and Soča Rivers, Slovenia). Aquatic Geochemistry 17, 357–396. https://doi.org/10.1007/s10498-011-9125-4
). These data are not included in the age binned FMg values in Figure 2c, but they follow the same trend. In summary, regardless of the exact filtering approach used, the observation that Mg/(Ca + Mg) ratio of river water declines steadily with decreasing bedrock age is a clear and reproducible signal from these data.top
Discussion
Where carbonate weathering is the predominant source of dissolved Ca2+ and Mg2+, the most important control on river FMg is the relative abundance of calcite and dolomite in the catchment’s bedrock (Williams et al., 2007
Williams, E.L., Szramek, K.J., Jin, L., Ku, T.C., Walter, L.M. (2007) The carbonate system geochemistry of shallow groundwater–surface water systems in temperate glaciated watersheds (Michigan, USA): Significance of open-system dolomite weathering. Geological Society of America Bulletin 119, 515–528. https://doi.org/10.1130/B25967.1
; Szramek et al., 2011Szramek, K., Walter, L.M., Kanduc, T., Ogrinc, N. (2011) Dolomite versus calcite weathering in hydrogeochemically diverse watersheds established on bedded carbonates (Sava and Soča Rivers, Slovenia). Aquatic Geochemistry 17, 357–396. https://doi.org/10.1007/s10498-011-9125-4
). Factors that can alter FMg after weathering include calcite precipitation (increase FMg through Ca2+ removal) or pollution from CaCl2 in road salts (decrease FMg through Ca2+ addition). No discernible relationship exists between Ω and FMg (Fig. S-6 and colour coding in Fig. 2b, c) or between Cl/(Ca + Mg) and FMg (Fig. S-7) and there is no obvious reason why either process should correlate with bedrock age. Thus, we consider the systematic decline in FMg across the Phanerozoic (Fig. 2b, c) to reflect systematic changes in bedrock carbonate mineralogy. A simple mixing model is used to convert the fstn filtered FMg record (squares in Fig. 2b) to mole fraction dolomite in carbonate bedrock (Fig. 3). This model assumes that carbonates are composed of either dolomite (47.4 mol % MgCO3, the average for Phanerozoic dolomites from Manche and Kaczmarek, 2021Manche, C.J., Kaczmarek, S.E. (2021) A global study of dolomite stoichiometry and cation ordering through the Phanerozoic. Journal of Sedimentary Research 91, 520–546. https://doi.org/10.2110/jsr.2020.204
) or low-Mg calcite (2 mol % MgCO3; Morse and Mackenzie, 1990Morse, J.W., Mackenzie, F.T. (1990) Geochemistry of Sedimentary Carbonates. Developments in Sedimentology, 48, Elsevier, Amsterdam.
). Aragonite and high-Mg calcite, both important constituents of modern shallow water carbonate sediments, are assumed to undergo open system recrystallisation to low-Mg calcite during early diagenesis and lithification (Morse and Mackenzie, 1990Morse, J.W., Mackenzie, F.T. (1990) Geochemistry of Sedimentary Carbonates. Developments in Sedimentology, 48, Elsevier, Amsterdam.
).
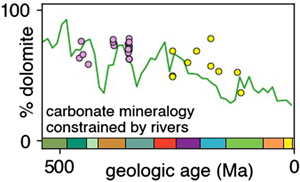
Figure 3 Using the age binned values from Figure 2b, river FMg (right y axis) is used to predict mole fraction dolomite in carbonate bedrock (left axis; see Discussion), and plotted vs. bedrock age. Fraction dolomite based on rock sample geochemistry from the Russian platform (Vinogradov and Ronov, 1956
Vinogradov, A., Ronov, A. (1956) Compositions of the sedimentary rocks of the Russian Platform in relation to the history of its tectonic movements. Geokhimiya 6, 533–559.
) and North America (Given and Wilkinson, 1987Given, R.K., Wilkinson, B.H. (1987) Dolomite abundance and stratigraphic age; constraints on rates and mechanisms of Phanerozoic dolostone formation. Journal of Sedimentary Research 57, 1068–1078. https://doi.org/10.1306/212F8CF1-2B24-11D7-8648000102C1865D
) and on a compilation of unit thicknesses of dolostones and limestones in carbonate sequences (Li et al., 2021Li, M., Wignall, P.B., Dai, X., Hu, M., Song, H. (2021) Phanerozoic variation in dolomite abundance linked to oceanic anoxia. Geology 49, 698–702. https://doi.org/10.1130/G48502.1
) are also shown. All records are colour coded by the relative amount of data averaged into each point, scaled to each time series.River-derived dolomite abundances are compared with previous Phanerozoic compilations of dolomite fraction in Figure 3. The record of Vinogradov and Ronov (1956)
Vinogradov, A., Ronov, A. (1956) Compositions of the sedimentary rocks of the Russian Platform in relation to the history of its tectonic movements. Geokhimiya 6, 533–559.
is from a region not covered by the river data (the Russian platform) and is the largest previously published dataset (8,847 sample analyses from 198 formations). Dolomite abundances from this record are similar to, or slightly lower than, ours for most of the Phanerozoic, but show a much lower fraction of dolomite in the Cretaceous and Cenozoic. The Russian dataset is much sparser in these time periods, containing only 5 % of the studied formations and 3 % of samples. By contrast, 15 % of Phanerozoic river stations, representing 10 % of the river sample analyses, are of these ages. In contrast to its similarity to the Russian time series, river-derived fraction dolomite (Fig. 3) is strikingly different to the records from both Given and Wilkinson (1987)Given, R.K., Wilkinson, B.H. (1987) Dolomite abundance and stratigraphic age; constraints on rates and mechanisms of Phanerozoic dolostone formation. Journal of Sedimentary Research 57, 1068–1078. https://doi.org/10.1306/212F8CF1-2B24-11D7-8648000102C1865D
and Li et al. (2021)Li, M., Wignall, P.B., Dai, X., Hu, M., Song, H. (2021) Phanerozoic variation in dolomite abundance linked to oceanic anoxia. Geology 49, 698–702. https://doi.org/10.1130/G48502.1
. The lack of systematic fluctuations in dolomite fraction in the river record, unlike those proposed by these authors, and much higher early Phanerozoic dolomite abundance than suggested by Li et al. (2021)Li, M., Wignall, P.B., Dai, X., Hu, M., Song, H. (2021) Phanerozoic variation in dolomite abundance linked to oceanic anoxia. Geology 49, 698–702. https://doi.org/10.1130/G48502.1
, raises questions about their models of sea level (Given and Wilkinson, 1987Given, R.K., Wilkinson, B.H. (1987) Dolomite abundance and stratigraphic age; constraints on rates and mechanisms of Phanerozoic dolostone formation. Journal of Sedimentary Research 57, 1068–1078. https://doi.org/10.1306/212F8CF1-2B24-11D7-8648000102C1865D
) or ocean redox (Li et al., 2021Li, M., Wignall, P.B., Dai, X., Hu, M., Song, H. (2021) Phanerozoic variation in dolomite abundance linked to oceanic anoxia. Geology 49, 698–702. https://doi.org/10.1130/G48502.1
) controlling dolomite formation.The record of the fraction of dolomite in carbonate successions determined here is based on those carbonates exposed on the continents and hence is largely composed of shelf carbonates. However, there was shift of a substantial amount of carbonate deposition from continental margins to the abyss in the mid-Mesozoic (Wilkinson and Walker, 1989
Wilkinson, B.H., Walker, J.C. (1989) Phanerozoic cycling of sedimentary carbonate. American Journal of Science 289, 525–548. https://doi.org/10.2475/ajs.289.4.525
). Because abyssal carbonates are dominantly calcite, this shift means that the change in the dolomite fraction recorded by any study of continental rocks (including this one) must provide an underestimate of the global change in the fraction of dolomite in carbonate rocks formed since the mid-Mesozoic.If the average Mg/Ca of carbonates has dropped systematically over the Phanerozoic, this has important implications for the coupled Ca-Mg-C-cycles in seawater (Arvidson et al., 2011
Arvidson, R.S., Guidry, M.W., Mackenzie, F.T. (2011) Dolomite controls on Phanerozoic seawater chemistry. Aquatic Geochemistry 17, 735–747. https://doi.org/10.1007/s10498-011-9130-7
). First, it is generally thought that the Mg/Ca of seawater has oscillated over the Phanerozoic on a 100 Myr timescale with no long term secular change (e.g., Lowenstein et al., 2001Lowenstein, T.K., Timofeeff, M.N., Brennan, S.T., Hardie, L.A., Demicco, R.V (2001) Oscillations in Phanerozoic seawater chemistry: evidence from fluid inclusions. Science 294, 1086–1088. https://doi.org/10.1126/science.1064280
; Weldeghebriel et al., 2022Weldeghebriel, M.F., Lowenstein, T.K., García-Veigas, J., Cendo´n, D.I. (2022) [Ca2+] and [SO42-] in Phanerozoic and terminal Proterozoic seawater from fluid inclusions in halite: The significance of Ca-SO4 crossover points. Earth and Planetary Science Letters 594, 117–712. https://doi.org/10.1016/j.epsl.2022.117712
), and it has been suggested that this might have been controlled by variations in the fraction of dolomite buried (e.g., Holland et al., 1996Holland, H.D., Horita, J., Seyfried, W.E. (1996) On the secular variations in the composition of Phanerozoic marine potash evaporites. Geology 24, 993–996. https://doi.org/10.1130/0091-7613(1996)024<0993:OTSVIT>2.3.CO;2
). However, there is no simple relationship between the dolomite/calcite ratio in carbonates and seawater Mg/Ca, suggesting that if the proposed oscillations in seawater Mg/Ca are correct, these were not driven by changes in the fraction of dolomite buried on continental shelves.Second, the change in the fraction of dolomite as a function of carbonate sediment age means that carbonate weathering must be a source of Mg, and sink of Ca, from seawater, assuming the alkalinity released by carbonate weathering has been consumed by carbonate precipitation. For example, given a rate of change in the Mg/(Ca + Mg) of continental carbonates of ∼0.04 per 100 Myr (Fig. 3), and a half-life of carbonates in the continental crust of ∼250 Myr (Wilkinson and Walker, 1989
Wilkinson, B.H., Walker, J.C. (1989) Phanerozoic cycling of sedimentary carbonate. American Journal of Science 289, 525–548. https://doi.org/10.2475/ajs.289.4.525
), the average carbonate precipitated has a Mg/(Ca + Mg) ∼0.1 lower than the average carbonate dissolved during contemporaneous weathering. If carbonate weathering feeds 50 % of the ∼20 Tmol yr−1 of carbonate burial (Wilkinson and Walker, 1989Wilkinson, B.H., Walker, J.C. (1989) Phanerozoic cycling of sedimentary carbonate. American Journal of Science 289, 525–548. https://doi.org/10.2475/ajs.289.4.525
), a very conservative estimate, carbonate weathering is equivalent to a flux of 1 Tmol yr−1 of Mg into seawater. This suggestion should be testable using the Mg isotopic composition of seawater, given the strong fractionation of Mg isotopes between dolomite and Mg silicates, once robust records of the evolution of the Mg isotopic composition of seawater for the entire Phanerozoic are available.The nature of the sink(s) for the Mg flux from dolomite weathering has important implications for the carbon cycle. The most likely sink is clay uptake, either in oceanic crust or in sediments. In the first scenario, the Mg released by dolomite dissolution may be largely exchanged for Ca in high or low temperature hydrothermal systems on the seafloor. In this model, the Ca could then be precipitated out of the ocean with the carbon released by dolomite dissolution as calcite, thus roughly balancing the carbon/alkalinity cycle. Alternatively, Garrels and Berner (1983)
Garrels, R.M., Berner, R.A. (1983) The Global Carbonate-Silicate Sedimentary System — Some Feedback Relations. In: Westbroek, P., de Jong, E.W. (Eds.) Biomineralization and Biological Metal Accumulation: Biological and Geological Perspectives. Springer, Netherlands, 73–87.
suggested that the net effect of dolomite dissolution and calcite precipitation is CO2 release, through reactions such as: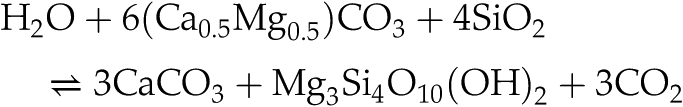
in which Mg released by dolomite weathering reacts with Si to form authigenic Mg phyllosilicates, and the carbon released by dolomite weathering remains in the ocean-atmosphere system. Based on the Mg flux estimates given above, dolomite weathering could lead to the release of 1 Tmol yr−1 of CO2 into the ocean-atmosphere system, which is equivalent to 10–25 % of solid Earth degassing (e.g., Berner, 2004
Berner, R.A. (2004) The Phanerozoic Carbon Cycle. Oxford University Press, New York.
). While both sinks for the Mg released by dolomite weathering may have been important over the Phanerozoic, the potential for this process to play a non-trivial role in the global carbon cycle deserves further consideration.top
Acknowledgements
We would like to thank two anonymous reviewers, whose comments improved the manuscript, and Gavin Foster for his work as editor.
Editor: Gavin Foster
top
References
Arvidson, R.S., Guidry, M.W., Mackenzie, F.T. (2011) Dolomite controls on Phanerozoic seawater chemistry. Aquatic Geochemistry 17, 735–747. https://doi.org/10.1007/s10498-011-9130-7
 Show in context
Show in context The dolomite problem thus affects the cycling of Ca-Mg-C in the Earth system, with important implications for global climate (e.g., Arvidson et al., 2011).
View in article
If the average Mg/Ca of carbonates has dropped systematically over the Phanerozoic, this has important implications for the coupled Ca-Mg-C-cycles in seawater (Arvidson et al., 2011).
View in article
Berner, R.A. (2004) The Phanerozoic Carbon Cycle. Oxford University Press, New York.
 Show in context
Show in context Based on the Mg flux estimates given above, dolomite weathering could lead to the release of 1 Tmol yr−1 of CO2 into the ocean-atmosphere system, which is equivalent to 10–25 % of solid Earth degassing (e.g., Berner, 2004).
View in article
Daly, R.A. (1909) First calcareous fossils and the evolution of the limestones. Geological Society of America Bulletin 20, 153–170. https://doi.org/10.1130/GSAB-20-153
 Show in context
Show in context A truism in sedimentary geology is that dolomite, (Ca0.5,Mg0.5)CO3, is more common in ancient carbonates compared to younger sequences (Daly, 1909).
View in article
Gaillardet, J., Dupré, B., Louvat, P., Allegre, C. (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chemical Geology 159, 3–30. https://doi.org/10.1016/S0009-2541(99)00031-5
 Show in context
Show in context Owing to its greater efficiency compared to silicate weathering, carbonate weathering globally delivers ∼84 % of the total riverine flux of Mg2+ and Ca2+ (Gaillardet et al., 1999).
View in article
Garrels, R.M., Berner, R.A. (1983) The Global Carbonate-Silicate Sedimentary System — Some Feedback Relations. In: Westbroek, P., de Jong, E.W. (Eds.) Biomineralization and Biological Metal Accumulation: Biological and Geological Perspectives. Springer, Netherlands, 73–87.
 Show in context
Show in context Alternatively, Garrels and Berner (1983) suggested that the net effect of dolomite dissolution and calcite precipitation is CO2 release, through reactions such as:
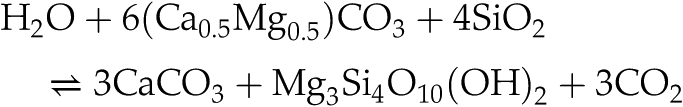
in which Mg released by dolomite weathering reacts with Si to form authigenic Mg phyllosilicates, and the carbon released by dolomite weathering remains in the ocean-atmosphere system.
View in article
Given, R.K., Wilkinson, B.H. (1987) Dolomite abundance and stratigraphic age; constraints on rates and mechanisms of Phanerozoic dolostone formation. Journal of Sedimentary Research 57, 1068–1078. https://doi.org/10.1306/212F8CF1-2B24-11D7-8648000102C1865D
 Show in context
Show in context Others are stationary and show large oscillations around a Phanerozoic mean (Given and Wilkinson, 1987; Li et al., 2021).
View in article
Previous dolomite abundance records have used Mg/Ca ratios in carbonate samples (Vinogradov and Ronov, 1956; Given and Wilkinson, 1987), or descriptions of limestone and dolostone in sedimentary sequences (Schmoker et al., 1985; Li et al., 2021).
View in article
Fraction dolomite based on rock sample geochemistry from the Russian platform (Vinogradov and Ronov, 1956) and North America (Given and Wilkinson, 1987) and on a compilation of unit thicknesses of dolostones and limestones in carbonate sequences (Li et al., 2021) are also shown.
View in article
In contrast to its similarity to the Russian time series, river-derived fraction dolomite (Fig. 3) is strikingly different to the records from both Given and Wilkinson (1987) and Li et al. (2021).
View in article
The lack of systematic fluctuations in dolomite fraction in the river record, unlike those proposed by these authors, and much higher early Phanerozoic dolomite abundance than suggested by Li et al. (2021), raises questions about their models of sea level (Given and Wilkinson, 1987) or ocean redox (Li et al., 2021) controlling dolomite formation.
View in article
Hartmann, J., Moosdorf, N. (2012) The new global lithological map database GLiM: A representation of rock properties at the Earth surface. Geochemistry, Geophysics, Geosystems 13, Q12004. https://doi.org/10.1029/2012GC004370
 Show in context
Show in context In all, the total area of the carbonate containing map units that underlie stream stations is 3.39 x 106 km2, which is 10 % of the total continental area estimated to be covered by carbonate or mixed carbonate-siliciclastic sediments (33.4 x 106 km2; Hartmann and Moosdorf, 2012).
View in article
Hartmann, J., Lauerwald, R., Moosdorf, N. (2014) A brief overview of the GLObal RIver CHemistry Database, GLORICH. Procedia Earth and Planetary Science 10, 23–27. https://doi.org/10.1016/j.proeps.2014.08.005
 Show in context
Show in context Measurements of dissolved Ca2+, Mg2+, Na+, K+, Cl−, pH, CO2(aq) and alkalinity on filtered river water samples were compiled from the USGS National Water Information System (NWIS) and the Global River Chemistry (GLORICH; Hartmann et al., 2014) databases.
View in article
Holland, H.D., Horita, J., Seyfried, W.E. (1996) On the secular variations in the composition of Phanerozoic marine potash evaporites. Geology 24, 993–996. https://doi.org/10.1130/0091-7613(1996)024<0993:OTSVIT>2.3.CO;2
 Show in context
Show in context First, it is generally thought that the Mg/Ca of seawater has oscillated over the Phanerozoic on a 100 Myr timescale with no long term secular change (e.g., Lowenstein et al., 2001; Weldeghebriel et al., 2022), and it has been suggested that this might have been controlled by variations in the fraction of dolomite buried (e.g., Holland et al., 1996).
View in article
Holland, H.D., Zimmermann, H. (2000) The dolomite problem revisited. International Geology Review 42, 481–490. https://doi.org/10.1080/00206810009465093
 Show in context
Show in context These two observations form the core of “the dolomite problem” (e.g., Holland and Zimmermann, 2000; Petrash et al., 2017).
View in article
Humphreys, M.P., Lewis, E.R., Sharp, J.D., Pierrot, D. (2022) PyCO2SYS v1.8: marine carbonate system calculations in Python. Geoscientific Model Development 15, 15–43. https://doi.org/10.5194/gmd-15-15-2022
 Show in context
Show in context For each water sample, we calculated [HCO3−] and calcite saturation state, Ω, using the Python toolbox PyCO2SYS 1.8.1 (Humphreys et al., 2022), using either measured pH and CO2(aq) (148,621 samples) or measured pH and alkalinity (58,983 samples) to solve the carbonate system. Of these samples, 80 % have a HCO3/(Ca + Mg) ratio consistent with a carbonate weathering source.
View in article
Land, L.S. (1998) Failure to Precipitate Dolomite at 25 °C from Dilute Solution Despite 1000-Fold Oversaturation after 32 Years. Aquatic Geochemistry 4, 361–368. https://doi.org/10.1023/A:1009688315854
 Show in context
Show in context Dolomite is also a rare precipitate from the modern ocean, despite seawater being highly dolomite supersaturated (Land, 1998).
View in article
Li, M., Wignall, P.B., Dai, X., Hu, M., Song, H. (2021) Phanerozoic variation in dolomite abundance linked to oceanic anoxia. Geology 49, 698–702. https://doi.org/10.1130/G48502.1
 Show in context
Show in context Others are stationary and show large oscillations around a Phanerozoic mean (Given and Wilkinson, 1987; Li et al., 2021).
View in article
Previous dolomite abundance records have used Mg/Ca ratios in carbonate samples (Vinogradov and Ronov, 1956; Given and Wilkinson, 1987), or descriptions of limestone and dolostone in sedimentary sequences (Schmoker et al., 1985; Li et al., 2021).
View in article
Fraction dolomite based on rock sample geochemistry from the Russian platform (Vinogradov and Ronov, 1956) and North America (Given and Wilkinson, 1987) and on a compilation of unit thicknesses of dolostones and limestones in carbonate sequences (Li et al., 2021) are also shown.
View in article
In contrast to its similarity to the Russian time series, river-derived fraction dolomite (Fig. 3) is strikingly different to the records from both Given and Wilkinson (1987) and Li et al. (2021).
View in article
The lack of systematic fluctuations in dolomite fraction in the river record, unlike those proposed by these authors, and much higher early Phanerozoic dolomite abundance than suggested by Li et al. (2021), raises questions about their models of sea level (Given and Wilkinson, 1987) or ocean redox (Li et al., 2021) controlling dolomite formation.
View in article
Lowenstein, T.K., Timofeeff, M.N., Brennan, S.T., Hardie, L.A., Demicco, R.V (2001) Oscillations in Phanerozoic seawater chemistry: evidence from fluid inclusions. Science 294, 1086–1088. https://doi.org/10.1126/science.1064280
 Show in context
Show in context First, it is generally thought that the Mg/Ca of seawater has oscillated over the Phanerozoic on a 100 Myr timescale with no long term secular change (e.g., Lowenstein et al., 2001; Weldeghebriel et al., 2022), and it has been suggested that this might have been controlled by variations in the fraction of dolomite buried (e.g., Holland et al., 1996).
View in article
Manche, C.J., Kaczmarek, S.E. (2021) A global study of dolomite stoichiometry and cation ordering through the Phanerozoic. Journal of Sedimentary Research 91, 520–546. https://doi.org/10.2110/jsr.2020.204
 Show in context
Show in context This model assumes that carbonates are composed of either dolomite (47.4 mol % MgCO3, the average for Phanerozoic dolomites from Manche and Kaczmarek, 2021) or low-Mg calcite (2 mol % MgCO3; Morse and Mackenzie, 1990).
View in article
Morse, J.W., Mackenzie, F.T. (1990) Geochemistry of Sedimentary Carbonates. Developments in Sedimentology, 48, Elsevier, Amsterdam.
 Show in context
Show in context This model assumes that carbonates are composed of either dolomite (47.4 mol % MgCO3, the average for Phanerozoic dolomites from Manche and Kaczmarek, 2021) or low-Mg calcite (2 mol % MgCO3; Morse and Mackenzie, 1990).
View in article
Aragonite and high-Mg calcite, both important constituents of modern shallow water carbonate sediments, are assumed to undergo open system recrystallisation to low-Mg calcite during early diagenesis and lithification (Morse and Mackenzie, 1990).
View in article
Peters, S.E., Husson, J.M., Czaplewski, J. (2018) Macrostrat: a platform for geological data integration and deep-time Earth crust research. Geochemistry, Geophysics, Geosystems 19, 1393–1409. https://doi.org/10.1029/2018GC007467
 Show in context
Show in context Geologic maps stored in Macrostrat (Peters et al., 2018) were used to constrain the bedrock geology that directly underlies each stream measurement station (Supplementary Information).
View in article
Petrash, D.A., Bialik, O.M., Bontognali, T.R., Vasconcelos, C., Roberts, J.A., McKenzie, J.A., Konhauser, K.O. (2017) Microbially catalyzed dolomite formation: From near-surface to burial. Earth-Science Reviews 171, 558–582. https://doi.org/10.1029/2018GC007467
 Show in context
Show in context These two observations form the core of “the dolomite problem” (e.g., Holland and Zimmermann, 2000; Petrash et al., 2017).
View in article
Schmoker, J.W., Krystinik, K.B., Halley, R.B. (1985) Selected characteristics of limestone and dolomite reservoirs in the United States. AAPG Bulletin 69, 733–741. https://doi.org/10.1306/AD4627F9-16F7-11D7-8645000102C1865D
 Show in context
Show in context Some time series show a monotonic decrease in the dolomite to calcite ratio from the beginning of the Phanerozoic towards today (Vinogradov and Ronov, 1956; Schmoker et al., 1985).
View in article
Previous dolomite abundance records have used Mg/Ca ratios in carbonate samples (Vinogradov and Ronov, 1956; Given and Wilkinson, 1987), or descriptions of limestone and dolostone in sedimentary sequences (Schmoker et al., 1985; Li et al., 2021).
View in article
Szramek, K., Walter, L.M., Kanduc, T., Ogrinc, N. (2011) Dolomite versus calcite weathering in hydrogeochemically diverse watersheds established on bedded carbonates (Sava and Soča Rivers, Slovenia). Aquatic Geochemistry 17, 357–396. https://doi.org/10.1007/s10498-011-9125-4
 Show in context
Show in context Therefore, it is reasonable to expect that rivers draining carbonate bedrock would have a similar Mg/Ca to average carbonate in the catchment, and detailed studies of watersheds with ∼30–70 % carbonate outcrop in Slovenia (Szramek et al., 2011) and Michigan (Williams et al., 2007) support this assertion.
View in article
Also plotted are rivers in Michigan (purple stars; Williams et al., 2007) and Slovenia (yellow stars; Szramek et al., 2011) draining catchments with ∼30–70 % carbonate outcrop.
View in article
Also plotted in (c) are FMg from rivers in Michigan (purple stars; Williams et al., 2007) and Slovenia (yellow stars; Szramek et al., 2011) that drain carbonate dominated catchments, which are not included in the age binned averages.
View in article
Second, river water samples were only included if their ratio of HCO3− to (Mg2+ + Ca2+) is between 1 and 2, which are the predicted end member ratios for calcite and dolomite weathering via sulfuric acid and carbonic acid, respectively (Szramek et al., 2011).
View in article
These cut offs are similar to the low end of carbonate richness in watersheds that were studied in detail for carbonate and dolomite weathering (Williams et al., 2007; Szramek et al., 2011), although our results are not sensitive to the exact cut off used (see below).
View in article
Watershed bedrock age and average FMg (per sampling site) were also calculated for the well characterised, carbonate weathering Slovenian and Michigan watersheds (Williams et al., 2007; Szramek et al., 2011).
View in article
Where carbonate weathering is the predominant source of dissolved Ca2+ and Mg2+, the most important control on river FMg is the relative abundance of calcite and dolomite in the catchment’s bedrock (Williams et al., 2007; Szramek et al., 2011).
View in article
Vinogradov, A., Ronov, A. (1956) Compositions of the sedimentary rocks of the Russian Platform in relation to the history of its tectonic movements. Geokhimiya 6, 533–559.
 Show in context
Show in context Some time series show a monotonic decrease in the dolomite to calcite ratio from the beginning of the Phanerozoic towards today (Vinogradov and Ronov, 1956; Schmoker et al., 1985).
View in article
Previous dolomite abundance records have used Mg/Ca ratios in carbonate samples (Vinogradov and Ronov, 1956; Given and Wilkinson, 1987), or descriptions of limestone and dolostone in sedimentary sequences (Schmoker et al., 1985; Li et al., 2021).
View in article
Fraction dolomite based on rock sample geochemistry from the Russian platform (Vinogradov and Ronov, 1956) and North America (Given and Wilkinson, 1987) and on a compilation of unit thicknesses of dolostones and limestones in carbonate sequences (Li et al., 2021) are also shown.
View in article
The record of Vinogradov and Ronov (1956) is from a region not covered by the river data (the Russian platform) and is the largest previously published dataset (8,847 sample analyses from 198 formations).
View in article
Weldeghebriel, M.F., Lowenstein, T.K., García-Veigas, J., Cendo´n, D.I. (2022) [Ca2+] and [SO42-] in Phanerozoic and terminal Proterozoic seawater from fluid inclusions in halite: The significance of Ca-SO4 crossover points. Earth and Planetary Science Letters 594, 117–712. https://doi.org/10.1016/j.epsl.2022.117712
 Show in context
Show in context First, it is generally thought that the Mg/Ca of seawater has oscillated over the Phanerozoic on a 100 Myr timescale with no long term secular change (e.g., Lowenstein et al., 2001; Weldeghebriel et al., 2022), and it has been suggested that this might have been controlled by variations in the fraction of dolomite buried (e.g., Holland et al., 1996).
View in article
Wilkinson, B.H., Walker, J.C. (1989) Phanerozoic cycling of sedimentary carbonate. American Journal of Science 289, 525–548. https://doi.org/10.2475/ajs.289.4.525
 Show in context
Show in context However, there was shift of a substantial amount of carbonate deposition from continental margins to the abyss in the mid-Mesozoic (Wilkinson and Walker, 1989).
View in article
For example, given a rate of change in the Mg/(Ca + Mg) of continental carbonates of ∼0.04 per 100 Myr (Fig. 3), and a half-life of carbonates in the continental crust of ∼250 Myr (Wilkinson and Walker, 1989), the average carbonate precipitated has a Mg/(Ca + Mg) ∼0.1 lower than the average carbonate dissolved during contemporaneous weathering.
View in article
If carbonate weathering feeds 50 % of the ∼20 Tmol yr−1 of carbonate burial (Wilkinson and Walker, 1989), a very conservative estimate, carbonate weathering is equivalent to a flux of 1 Tmol yr−1 of Mg into seawater.
View in article
Williams, E.L., Szramek, K.J., Jin, L., Ku, T.C., Walter, L.M. (2007) The carbonate system geochemistry of shallow groundwater–surface water systems in temperate glaciated watersheds (Michigan, USA): Significance of open-system dolomite weathering. Geological Society of America Bulletin 119, 515–528. https://doi.org/10.1130/B25967.1
 Show in context
Show in context Therefore, it is reasonable to expect that rivers draining carbonate bedrock would have a similar Mg/Ca to average carbonate in the catchment, and detailed studies of watersheds with ∼30–70 % carbonate outcrop in Slovenia (Szramek et al., 2011) and Michigan (Williams et al., 2007) support this assertion.
View in article
Also plotted are rivers in Michigan (purple stars; Williams et al., 2007) and Slovenia (yellow stars; Szramek et al., 2011) draining catchments with ∼30–70 % carbonate outcrop.
View in article
Also plotted in (c) are FMg from rivers in Michigan (purple stars; Williams et al., 2007) and Slovenia (yellow stars; Szramek et al., 2011) that drain carbonate dominated catchments, which are not included in the age binned averages.
View in article
These cut offs are similar to the low end of carbonate richness in watersheds that were studied in detail for carbonate and dolomite weathering (Williams et al., 2007; Szramek et al., 2011), although our results are not sensitive to the exact cut off used (see below).
View in article
Watershed bedrock age and average FMg (per sampling site) were also calculated for the well characterised, carbonate weathering Slovenian and Michigan watersheds (Williams et al., 2007; Szramek et al., 2011).
View in article
Where carbonate weathering is the predominant source of dissolved Ca2+ and Mg2+, the most important control on river FMg is the relative abundance of calcite and dolomite in the catchment’s bedrock (Williams et al., 2007; Szramek et al., 2011).
View in article
top
Supplementary Information
The Supplementary Information includes:
Download Tables S-1 to S-4 (xlsx)
Download the Supplementary Information (PDF)
Figures
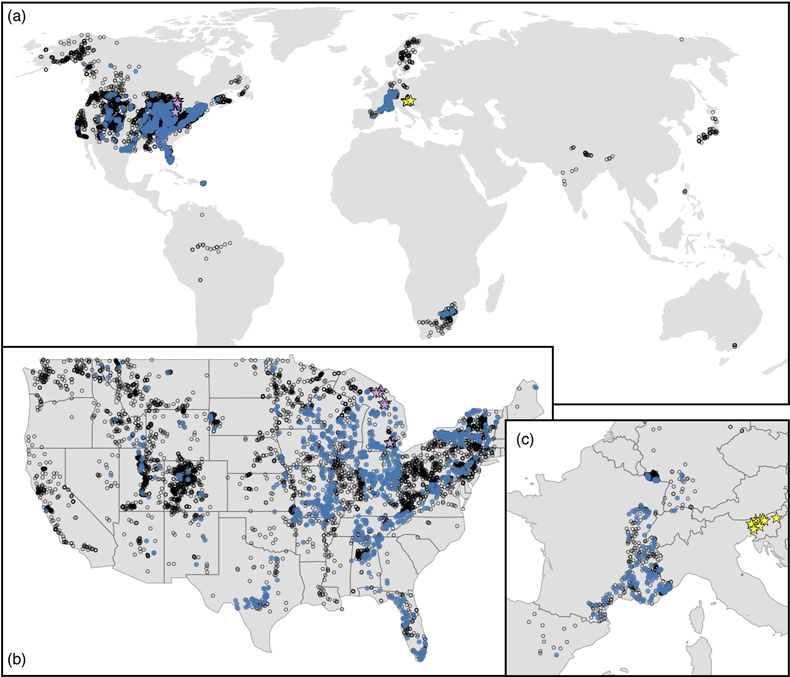
Figure 1 (a) Map showing locations of river stations plotted in Figure 2. All localities are included in Figure 2a, whereas blue dots are carbonate-draining rivers included in Figure 2b or c. In (b) and (c), inset maps of the contiguous U.S.A. and western Europe, respectively, are shown. Also plotted are rivers in Michigan (purple stars; Williams et al., 2007
Williams, E.L., Szramek, K.J., Jin, L., Ku, T.C., Walter, L.M. (2007) The carbonate system geochemistry of shallow groundwater–surface water systems in temperate glaciated watersheds (Michigan, USA): Significance of open-system dolomite weathering. Geological Society of America Bulletin 119, 515–528. https://doi.org/10.1130/B25967.1
) and Slovenia (yellow stars; Szramek et al., 2011Szramek, K., Walter, L.M., Kanduc, T., Ogrinc, N. (2011) Dolomite versus calcite weathering in hydrogeochemically diverse watersheds established on bedded carbonates (Sava and Soča Rivers, Slovenia). Aquatic Geochemistry 17, 357–396. https://doi.org/10.1007/s10498-011-9125-4
) draining catchments with ∼30–70 % carbonate outcrop.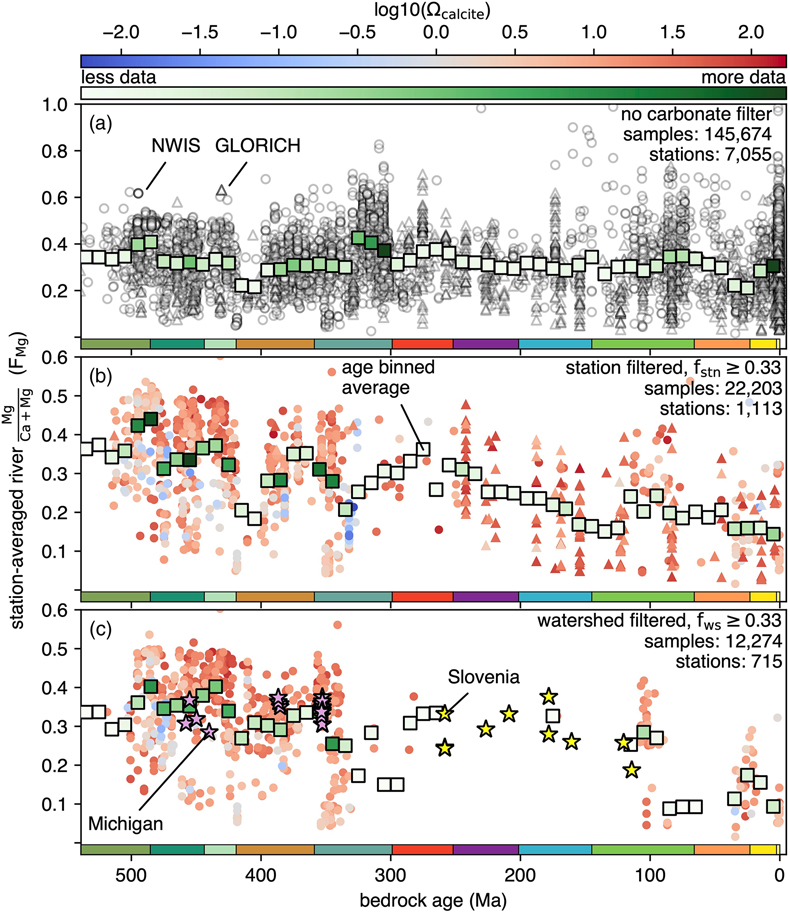
Figure 2 Station-averaged river Mg/(Ca + Mg) ratios (FMg) vs. bedrock age from the NWIS (circles) and GLORICH (triangles) databases, and 10 Myr binned means (squares, colour coded by the amount of data averaged in each bin, scaled to each time series). In (a), all rivers are included, whereas (b) and (c) only show rivers that drain carbonate bedrock, have relatively low Na and K, and have a ratio of HCO3− to (Mg2+ + Ca2+) between 1 and 2 (see Results). In (b), fstn value is used to filter for carbonate containing bedrock, and in (c), fws is used. In (b) and (c), values of FMg are colour coded by station-averaged calcite saturation (Ω; see Discussion). Also plotted in (c) are FMg from rivers in Michigan (purple stars; Williams et al., 2007
Williams, E.L., Szramek, K.J., Jin, L., Ku, T.C., Walter, L.M. (2007) The carbonate system geochemistry of shallow groundwater–surface water systems in temperate glaciated watersheds (Michigan, USA): Significance of open-system dolomite weathering. Geological Society of America Bulletin 119, 515–528. https://doi.org/10.1130/B25967.1
) and Slovenia (yellow stars; Szramek et al., 2011Szramek, K., Walter, L.M., Kanduc, T., Ogrinc, N. (2011) Dolomite versus calcite weathering in hydrogeochemically diverse watersheds established on bedded carbonates (Sava and Soča Rivers, Slovenia). Aquatic Geochemistry 17, 357–396. https://doi.org/10.1007/s10498-011-9125-4
) that drain carbonate dominated catchments, which are not included in the age binned averages.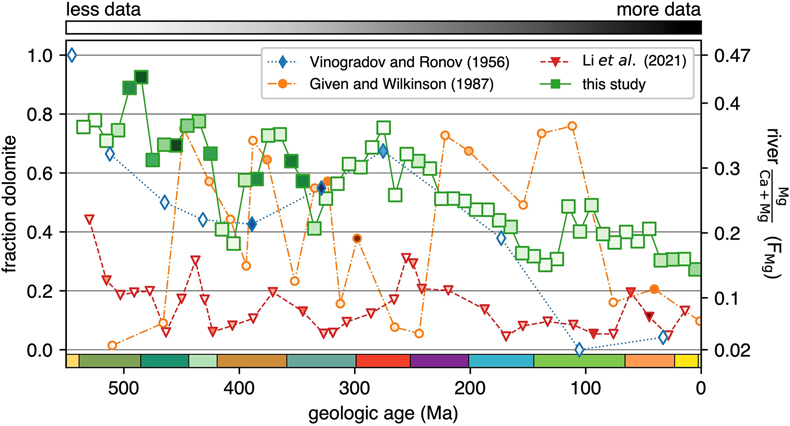
Figure 3 Using the age binned values from Figure 2b, river FMg (right y axis) is used to predict mole fraction dolomite in carbonate bedrock (left axis; see Discussion), and plotted vs. bedrock age. Fraction dolomite based on rock sample geochemistry from the Russian platform (Vinogradov and Ronov, 1956
Vinogradov, A., Ronov, A. (1956) Compositions of the sedimentary rocks of the Russian Platform in relation to the history of its tectonic movements. Geokhimiya 6, 533–559.
) and North America (Given and Wilkinson, 1987Given, R.K., Wilkinson, B.H. (1987) Dolomite abundance and stratigraphic age; constraints on rates and mechanisms of Phanerozoic dolostone formation. Journal of Sedimentary Research 57, 1068–1078. https://doi.org/10.1306/212F8CF1-2B24-11D7-8648000102C1865D
) and on a compilation of unit thicknesses of dolostones and limestones in carbonate sequences (Li et al., 2021Li, M., Wignall, P.B., Dai, X., Hu, M., Song, H. (2021) Phanerozoic variation in dolomite abundance linked to oceanic anoxia. Geology 49, 698–702. https://doi.org/10.1130/G48502.1
) are also shown. All records are colour coded by the relative amount of data averaged into each point, scaled to each time series.