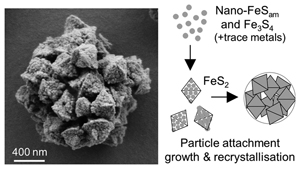
Inferred pyrite growth via the particle attachment pathway in the presence of trace metals
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:788Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
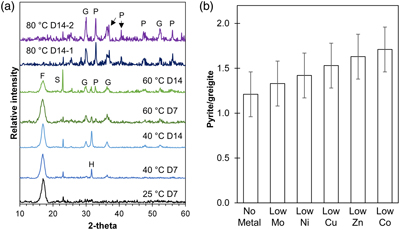 Figure 1 (a) XRD patterns showing the progressive transformation from FeSam (F) to greigite (G) and pyrite (P) with increasing temperatures (25, 40, 60 and 80 °C) and time (after 7 and 14 days). Residual sulphur (S) and halite (H) were also detected. Samples from two replicate bottles of experiments at 80 °C after 14 days (80 °C D14-1 or D14-2) indicate high reproducibility. (b) Relative intensity of pyrite/greigite signals as determined from thin-film XRD after 14 days of incubation at 80 °C. | 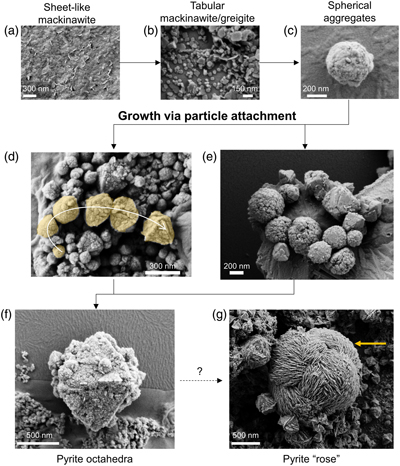 Figure 2 Representative SEM micrographs suggestive of growth via particle attachment. (a–c) Transformation of mackinawite to tabular greigite to spherical aggregates of tabular particles. (d) The arrow denotes a potential transformation from spherical aggregates to pyrite octahedra. (e) Close association between spherical aggregates (microframboids) that are recrystallising to form pyrite octahedra. (f) Colloidal pyrite octahedra (centre) with a rough surface and porous structure. (g) Micrometre-sized pyrite “rose” (yellow arrow) surrounded by smaller pyrite octahedra. The transformation mechanism may be related to skeletal growth. | 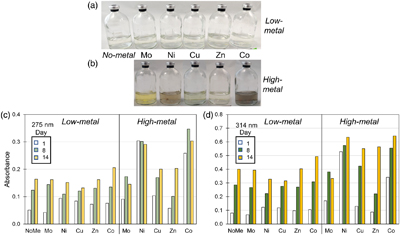 Figure 3 (a–b) Pictures of Fe-free setups after 8 days of incubation at 80 °C. Yellow tinge indicates the presence of polysulphides. Precipitates (grey or colourless) are observed in some high-metal setups. (c–d) Bar charts of the polysulphide peak intensities at (c) 275 nm and (d) 314 nm at Day 1, 8 and 14. Polysulphide intensities increase with time and with higher metal concentrations. | 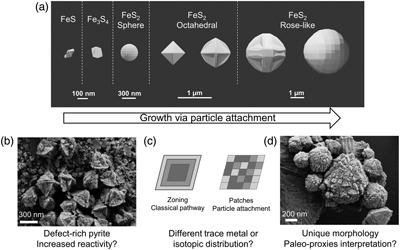 Figure 4 (a) Schematic of pyrite formation via particle attachment, showing skeletal growth and twinning between octahedral and rose-like particles (generated in Blender 3.2.2) and (b–d) the potential implications to the environment. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Pyrite is a widespread mineral that is involved in a variety of biogeochemical processes with implications for interpreting Earth’s past, present and future (Huang et al., 2017
Huang, F., Gao, W., Gao, S., Meng, L., Zhang, Z., Yan, Y., Ren, Y., Li, Y., Liu, K., Xing, M. and Wang, Y. (2017) Morphology evolution of nano-micron pyrite: A review. Journal of Nanoscience and Nanotechnology 17, 5980–5995. https://doi.org/10.1166/jnn.2017.14430
). Natural pyrite typically adopts either a euhedral or framboidal (raspberry-like) morphology. Euhedral pyrite is proposed to form via slow growth on pre-existing pyrite, while framboids are proposed to form under fast nucleation conditions in close association with Fe sulphide precursors, such as mackinawite (FeS) and greigite (Fe3S4) (Raiswell, 1982Raiswell, R. (1982) Pyrite texture, isotopic composition and the availability of iron. American Journal of Science 282, 1244–1263. https://doi.org/10.2475/ajs.282.8.1244
; Butler and Rickard, 2000Butler, I.B., Rickard, D. (2000) Framboidal pyrite formation via the oxidation of iron (II) monosulfide by hydrogen sulphide. Geochimica et Cosmochimica Acta 64, 2665–2672. https://doi.org/10.1016/S0016-7037(00)00387-2
). In the geological record, high abundances of framboids have been interpreted as indicators of euxinic conditions in water columns (Wilkin et al., 1996Wilkin, R.T., Barnes, H.L., Brantley, S.L. (1996) The size distribution of framboidal pyrite in modern sediments: An indicator of redox conditions. Geochimica et Cosmochimica Acta 60, 3897–3912. https://doi.org/10.1016/0016-7037(96)00209-8
; Rickard, 2019Rickard, D. (2019) Sedimentary pyrite framboid size-frequency distributions: A meta-analysis. Palaeogeography, Palaeoclimatology, Palaeoecology 522, 62–75. https://doi.org/10.1016/j.palaeo.2019.03.010
). The striking morphology of framboids and their association with organic matter have led to their interpretation as biosignatures, despite the various reports of framboid synthesis via abiotic pathways (Ohfuji and Rickard, 2005Ohfuji, H., Rickard, D. (2005) Experimental syntheses of framboids - A review. Earth-Science Reviews 71, 147–170. https://doi.org/10.1016/j.earscirev.2005.02.001
).The continuum model for pyrite growth (Sawlowicz, 1993
Sawlowicz, Z. (1993) Pyrite framboids and their development: a new conceptual mechanism. Geologische Rundschau 82, 148–156. https://doi.org/10.1007/BF00563277
) has received increasing support from growing textural, geochemical and isotopic evidence (Lin et al., 2016Lin, Z., Sun, X., Peckmann, J., Lu, Y., Xu, L., Strauss, H., Zhou, H., Gong, J., Lu, H. and Teichert, B.M. (2016) How sulfate-driven anaerobic oxidation of methane affects the sulfur isotopic composition of pyrite: A SIMS study from the South China Sea. Chemical Geology 440, 26–41. https://doi.org/10.1016/j.chemgeo.2016.07.007
, 2017Lin, Z., Sun, X., Lu, Y., Strauss, H., Xu, L., Gong, J., Teichert, B.M., Lu, R., Lu, H., Sun, W. and Peckmann, J. (2017) The enrichment of heavy iron isotopes in authigenic pyrite as a possible indicator of sulfate-driven anaerobic oxidation of methane: Insights from the South China Sea. Chemical Geology 449, 15–29. https://doi.org/10.1016/j.chemgeo.2016.11.032
; Liu et al., 2022Liu, K., Huang, F., Gao, S., Zhang, Z., Ren, Y., An, B. (2022) Morphology of framboidal pyrite and its textural evolution: Evidence from the Logatchev area, Mid-Atlantic Ridge. Ore Geology Reviews 141. https://doi.org/10.1016/j.oregeorev.2021.104630
). In this model, pyrite of different morphologies and sizes reflects cyclic growth stages of small euhedral particles aggregating to form framboids that recrystallise over time into a larger euhedral particle. This continuum model mirrors the particle attachment pathway in that mineral growth occurs via aggregation and recrystallisation of smaller particles. This pathway explains defects in crystal structures, distributions of trace metals and isotopes, and unusual particle morphologies in nature (De Yoreo et al., 2015De Yoreo, J.J., Gilbert, P.U., Sommerdijk, N.A., Penn, R.L., Whitelam, S., Joester, D., Zhang, H., Rimer, J.D., Navrotsky, A., Banfield, J.F. and Wallace, A.F. (2015) Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 349, aaa6760. https://doi.org/10.1126/science.aaa6760
). This pathway has been demonstrated for pyrite synthesised at >100 °C (Hunger and Benning, 2007Hunger, S., Benning, L.G. (2007) Greigite: A true intermediate on the polysulfide pathway to pyrite. Geochemical Transactions 8, 1–20. https://doi.org/10.1186/1467-4866-8-1
; Li et al., 2011Li, W., Döblinger, M., Vaneski, A., Rogach, A.L., Jäckel, F., Feldmann, J. (2011) Pyrite nanocrystals: Shape-controlled synthesis and tunable optical properties via reversible self-assembly. Journal of Materials Chemistry 21, 17946–17952. https://doi.org/10.1039/c1jm13336e
; Gong et al., 2013Gong, M., Kirkeminde, A., Ren, S. (2013) Symmetry-defying iron pyrite (FeS2) nanocrystals through oriented attachment. Scientific Reports 3, 1–6. https://doi.org/10.1038/srep02092
), but not at lower temperatures.Recent studies have investigated how trace metals impact pyrite formation rates (Table S-1). Comparatively, the effects of trace metals on pyrite morphology are under-constrained. Here, we tested the influence of five bioessential trace metals (Co, Cu, Mo, Ni, Zn) on pyrite formation. These bioessential trace metals are common impurities in pyrite and play key roles in Earth’s biogeochemical evolution (Robbins et al., 2016
Robbins, L.J., Lalonde, S.V., Planavsky, N.J., Partin, C.A., Reinhard, C.T., Kendall, B., Scott, C., Hardisty, D.S., Gill, B.C., Alessi, D.S. and Dupont, C.L. (2016) Trace elements at the intersection of marine biological and geochemical evolution. Earth-Science Reviews 163, 323–348. https://doi.org/10.1016/j.earscirev.2016.10.013
). Their effects on pyrite formation need to be constrained in order to disentangle factors that can affect the utility of pyrite morphologies as environmental proxies and biosignatures.top
Fe Sulphide Transformation Sequence
Iron sulphides were synthesised in the presence of 3 mM Fe2+, 6 mM Na2S and 30 mM elemental sulphur (S0) in 50 mM HEPES buffer (pH 7). Two sets of experiments were performed and termed Set-1 (97 % N2, 3 % H2 headspace) and Set-2 (100 % N2), respectively (details in SI Methods; Table S-2). In both sets, the addition of Na2S to Fe2+ led to the immediate formation of fine black precipitates identified as a disordered mackinawite-like phase (FeSam) based on a single broad reflection with d-spacings of 5.2–5.3 Å via X-ray diffraction (XRD) (Fig. 1a; Table S-3), and characteristic sheet-like aggregate structures under scanning electron microscopy (SEM) (Fig. 2a) (Csákberényi-Malasics et al., 2012
Csákberényi-Malasics, D., Rodriguez-Blanco, J.D., Kis, V.K., Rečnik, A., Benning, L.G., Pósfai, M. (2012) Structural properties and transformations of precipitated FeS. Chemical Geology 294–295, 249–258. https://doi.org/10.1016/j.chemgeo.2011.12.009
).
Figure 1 (a) XRD patterns showing the progressive transformation from FeSam (F) to greigite (G) and pyrite (P) with increasing temperatures (25, 40, 60 and 80 °C) and time (after 7 and 14 days). Residual sulphur (S) and halite (H) were also detected. Samples from two replicate bottles of experiments at 80 °C after 14 days (80 °C D14-1 or D14-2) indicate high reproducibility. (b) Relative intensity of pyrite/greigite signals as determined from thin-film XRD after 14 days of incubation at 80 °C.

Figure 2 Representative SEM micrographs suggestive of growth via particle attachment. (a–c) Transformation of mackinawite to tabular greigite to spherical aggregates of tabular particles. (d) The arrow denotes a potential transformation from spherical aggregates to pyrite octahedra. (e) Close association between spherical aggregates (microframboids) that are recrystallising to form pyrite octahedra. (f) Colloidal pyrite octahedra (centre) with a rough surface and porous structure. (g) Micrometre-sized pyrite “rose” (yellow arrow) surrounded by smaller pyrite octahedra. The transformation mechanism may be related to skeletal growth.
Despite following the same methods, Set-1 and Set-2 experiments demonstrated differences in greigite contents, pyrite formation rates and the extent of pyritisation. In Set-1 experiments, FeSam transformed to greigite and eventually to pyrite with increasing time (up to 14 days) and temperature (25–80 °C). FeSam was no longer detectable by XRD after 14 days of incubation at 80 °C, but greigite was still not fully transformed to pyrite (Fig. 1a). Comparatively, in Set-2 experiments, FeSam was completely transformed to pyrite within 3–7 days of incubation at 80 °C, with no greigite detected. Nonetheless, greigite was likely present at low relative abundances because minerals attracted to hand magnets were observed (Table S-3). These magnetic minerals were associated with black coatings around S0 particles.
Samples containing greigite and pyrite from Set-1 experiments were analysed using SEM, which revealed morphologies classified into four categories: tabular (<150 nm), spherical aggregates (100–250 nm), octahedral (300–1500 nm) and rose-like particles (1000–3000 nm) (Fig. 2). Treatment with 6 M HCl led to dissolution of the tabular particles, which we interpret as HCl-soluble greigite given its morphological similarity to previous lab-synthesised greigite (Csákberényi-Malasics et al., 2012
Csákberényi-Malasics, D., Rodriguez-Blanco, J.D., Kis, V.K., Rečnik, A., Benning, L.G., Pósfai, M. (2012) Structural properties and transformations of precipitated FeS. Chemical Geology 294–295, 249–258. https://doi.org/10.1016/j.chemgeo.2011.12.009
; Mansor et al., 2019Mansor, M., Berti, D., Hochella Jr, M.F., Murayama, M., Xu, J. (2019) Phase, morphology, elemental composition and formation mechanisms of biogenic and abiogenic Fe-Cu-sulfide nanoparticles: A comparative study on their occurrences under anoxic conditions. American Mineralogist 104, 703–717. https://doi.org/10.2138/am-2019-6848
). The other particles (spherical aggregates, octahedra and “roses”) were interpreted as pyrite since they did not dissolve in HCl (Voelz et al., 2019Voelz, J.L., Johnson, N.W., Chun, C.L., Arnold, W.A., Penn, R.L. (2019) Quantitative dissolution of environmentally accessible iron residing in iron-rich minerals: A review. ACS Earth and Space Chemistry 3, 1371–1392. https://doi.org/10.1021/acsearthspacechem.9b00012
). In Set-2 experiments, where greigite was not detected by XRD, tabular particles were rarely observed whilst other particles were common. Occasionally, acicular particles (100–5000 nm length) were also observed from day 3 onwards and identified via energy dispersive X-ray spectroscopy (EDS) to be rich in Fe and oxygen, suggestive of Fe(III) oxyhydroxides (Fig. S-1).Overall, Set-1 and Set-2 experiments exhibited similar transformation sequences of FeSam to greigite to pyrite with increasing time and temperature, consistent with previous studies (Hunger and Benning, 2007
Hunger, S., Benning, L.G. (2007) Greigite: A true intermediate on the polysulfide pathway to pyrite. Geochemical Transactions 8, 1–20. https://doi.org/10.1186/1467-4866-8-1
; Mansor and Fantle, 2019Mansor, M., Fantle, M.S. (2019) A novel framework for interpreting pyrite-based Fe isotope records of the past. Geochimica et Cosmochimica Acta 253, 39–62. https://doi.org/10.1016/j.gca.2019.03.017
). Alternative pathways without a greigite intermediate are possible (see Sanden et al., 2021Sanden, S.A., Szilagyi, R.K., Li, Y., Kitadai, N., Webb, S.M., Yano, T., Nakamura, R., Hara, M., McGlynn, S.E. (2021) Electrochemically induced metal- vs. ligand-based redox changes in mackinawite: identification of a Fe3+- and polysulfide-containing intermediate. Dalton Transactions 50, 11763–11774. https://doi.org/10.1039/D1DT01684A
) but seem unlikely in our experiments. We suggest that the differences in pyrite formation rates (∼10 × faster in Set-1) are caused by the headspace composition (3 % H2 vs. pure N2). The lack of H2 in Set-2 experiments likely led to a more oxidising condition, which accelerated pyrite formation, consistent with the detection of trace Fe(III) oxyhydroxides (SI Discussion). Differences in headspace gas composition should be considered for experimental studies on pyrite.top
Influence of Trace Metals on Pyrite Formation
Prior to Na2S addition, trace metals (Co, Cu, Mo, Ni, Zn) were added to Set-1 and Set-2 experiments to obtain metal:Fe ratios of 1:105 and 1:102, respectively, to determine their effects on pyrite formation. These ratios represent the broad range of environments (e.g., low temperature sediments, acid mine drainage, hydrothermal vents) in which natural pyrite can form (Von Damm et al., 1985
Von Damm, K.L., Edmond, J.M., Grant, B., Measures, C.I., Walden, B., Weiss, R.F. (1985) Chemistry of submarine hydrothermal solutions at 21°N, East Pacific Rise. Geochimica et Cosmochimica Acta 49, 2197–2220. https://doi.org/10.1016/0016-7037(85)90222-4
; Shaw et al., 1990Shaw, T.J., Gieskes, J.M., Jahnke, R.A. (1990) Early diagenesis in differing depositional environments: The response of transition metals in pore water. Geochimica et Cosmochimica Acta 54, 1233–1246. https://doi.org/10.1016/0016-7037(90)90149-F
; Allman et al., 2021Allman, C.J., Gómez-Ortiz, D., Burke, A., Amils, R., Rodriguez, N., Fernández-Remolar, D. (2021) Hydrogeochemical variability of the acidic springs in the Rio Tinto headwaters. Water 13, 2861. https://doi.org/10.3390/w13202861
).From the low-metal setups (1∶105 ratio), XRD analysis suggested that all trace metals accelerated pyrite formation after 14 days of incubation at 80 °C. The ratio of pyrite/greigite increased in the following order: no-metal < Mo < Ni < Cu < Zn < Co, although it must be noted that the ratios overlap within error (Fig. 1b). We were unable to determine if any accelerating effects occurred in the high-metal setups (1∶102) given the unexpectedly rapid pyrite formation within Set-2 experiments. In both experimental sets, stronger magnetism was observed in the presence of Mo compared to other metals.
Trace metals were proposed to influence pyrite formation via either: (1) formation of metal-rich nanoparticles that serve as nuclei, (2) complexation or redox reactions that affect polysulphides reactivity and formation, and S(-II) and Fe(II) oxidation, or (3) stabilisation of FeS precursors via coprecipitation or adsorption (Table S-1). To test the first two possibilities, we repeated the no-metal, low-metal (30 nM metals) and high-metal (30 μM) setups with the omission of Fe and monitored the formation of nanoparticles and polysulphides at 80 °C.
In the no-metal and low-metal setups, no precipitates formed and a slight yellow tinge indicative of polysulphides was observed (Fig. 3a). The polysulphide spectra obtained by UV-VIS spectroscopy generally increased in intensity with time with two peaks observed at 275 and 314 nm. At day 1, higher polysulphide peaks were observed in the presence of Co, Cu, Ni and Zn relative to the no-metal setup (Fig. 3c–d; S-2). After day 8, however, polysulphides were elevated only in the presence of Co, while the other metals showed no increase or even a slight decrease compared to the no-metal setup. High polysulphides with Co correlated with increased pyrite formation rate (Fig. 1b), suggesting that the interaction between this metal and polysulphides may play a role in accelerating pyrite formation.

Figure 3 (a–b) Pictures of Fe-free setups after 8 days of incubation at 80 °C. Yellow tinge indicates the presence of polysulphides. Precipitates (grey or colourless) are observed in some high-metal setups. (c–d) Bar charts of the polysulphide peak intensities at (c) 275 nm and (d) 314 nm at Day 1, 8 and 14. Polysulphide intensities increase with time and with higher metal concentrations.
In the high-metal setups, grey or colourless precipitates were formed with all trace metals except for Mo. The yellow polysulphide tinge was evident in the presence of Mo, Cu and Zn but was obscured by the presence of colloidal nanoparticles in the presence of Ni and Co (Fig. 3b). Polysulphide intensities increased with higher trace metal concentrations with the exception of Mo. High Mo concentration induced an additional peak at 470 nm corresponding to tetrathiomolybdate (MoS42−) (Erickson and Helz, 2000
Erickson, B.E., Helz, G.R. (2000) Molybdenum(VI) speciation in sulfidic waters: Stability and lability of thiomolybdates. Geochimica et Cosmochimica Acta 64, 1149–1158. https://doi.org/10.1016/S0016-7037(99)00423-8
). The highest polysulphide intensities at day 14 were observed in the presence of high Co concentration, followed by Ni, Cu and Zn (Fig. 3c–d). The amount of polysulphide formed was likely influenced by varying availability of H2S and S0 after metal sulphide precipitation. All the metals tested in this study were proposed to form polysulphide complexes (Rickard and Luther, 2006Rickard, D., Luther, G.W. (2006) Metal sulfide complexes and clusters. Reviews in Mineralogy and Geochemistry 61, 421–504. https://doi.org/10.2138/rmg.2006.61.8
), with Co known to enhance polysulphide conversions in lithium-sulphur batteries (Liu et al., 2021Liu, W., Luo, C., Zhang, S., Zhang, B., Ma, J., Wang, X., Liu, W., Li, Z., Yang, Q.H. and Lv, W. (2021) Cobalt-doping of molybdenum disulfide for enhanced catalytic polysulfide conversion in lithium-sulfur batteries. ACS Nano 15, 7491–7499. https://doi.org/10.1021/acsnano.1c00896
). The interactions between these metals and polysulphides and their impact on biogeochemistry are currently poorly known.The tentative accelerating effects of Mo and Ni on pyrite formation observed in this study are consistent with previous studies (Table S-1). Mo is also known to promote greigite formation (Mansor and Fantle, 2019
Mansor, M., Fantle, M.S. (2019) A novel framework for interpreting pyrite-based Fe isotope records of the past. Geochimica et Cosmochimica Acta 253, 39–62. https://doi.org/10.1016/j.gca.2019.03.017
; Miller et al., 2020Miller, N., Dougherty, M., Du, R., Sauers, T., Yan, C., Pines, J.E., Meyers, K.L., Dang, Y.M., Nagle, E., Ni, Z. and Pungsrisai, T. (2020) Adsorption of tetrathiomolybdate to iron sulfides and its impact on iron sulfide transformations. ACS Earth and Space Chemistry 4, 2246–2260. https://doi.org/10.1021/acsearthspacechem.0c00176
), which could explain the lower pyrite/greigite ratio observed in the presence of Mo compared to other trace metals. In contrast, other studies demonstrated that Co and Ni (Swanner et al., 2019Swanner, E.D., Webb, S.M., Kappler, A. (2019) Fate of cobalt and nickel in mackinawite during diagenetic pyrite formation. American Mineralogist 104, 917–928. https://doi.org/10.2138/am-2019-6834
) and Mo (Baya et al., 2022Baya, C., Le Pape, P., Baptiste, B., Menguy, N., Delbes, L., Morand, M., Rouelle, M., Aubry, E., Ona-Nguema, G., Noël, V. and Juillot, F. (2022) A methodological framework to study the behavior and kinetic influence of V, Mn, Co, Ni, Cu, Zn, As, Se and Mo during pyrite formation via the polysulfide pathway at ambient temperature. Chemical Geology 613. https://doi.org/10.1016/j.chemgeo.2022.121139
) inhibited pyrite formation. Differences in metal:Fe ratios and synthesis conditions (e.g., pH and formation pathways) between studies likely led to differences in how specific trace metals affect pyrite formation (SI Discussion). Nevertheless, our results clearly show that trace metals influence polysulphide chemistry and form metal-rich nuclei that may affect pyrite formation.Despite differences in precipitation rates, the presence of trace metals had little influence on pyrite morphologies (Fig. S-3). Spherical aggregates, octahedral and rose-like particles interpreted as pyrite were present in all samples with no systematic correlation with precipitation rate. Therefore, we conclude that pyrite morphologies were unaffected by trace metal loading. Other factors, such as aging time, S/Fe ratio, organic matter and biological activities, should be experimentally studied to determine their influence on pyrite morphologies and how these affect subsequent geological interpretations.
top
Particle Attachment Pathway
Detailed SEM analyses revealed two striking features: (1) particles existed in a continuum of size and shape, and (2) many of the larger particles had rough surface textures that indicate growth via aggregation of smaller particles with varying degrees of recrystallisation, similar in appearance to mesocrystals (Sturm and Cölfen, 2016
Sturm, E. V., Cölfen, H. (2016) Mesocrystals: Structural and morphogenetic aspects. Chemical Society Reviews 45, 5821–5833. https://doi.org/10.1039/C6CS00208K
). Similar surface features have been observed before and interpreted as screw dislocations (Wang and Morse, 1996Wang, Q., Morse, J.W. (1996) Pyrite formation under conditions approximating those in anoxic sediments I. Pathway and morphology. Marine Chemistry 52, 99–121. https://doi.org/10.1016/0304-4203(95)00082-8
; Butler and Rickard, 2000Butler, I.B., Rickard, D. (2000) Framboidal pyrite formation via the oxidation of iron (II) monosulfide by hydrogen sulphide. Geochimica et Cosmochimica Acta 64, 2665–2672. https://doi.org/10.1016/S0016-7037(00)00387-2
). We instead interpret these combined features imaged across multiple experiments as evidence for the particle attachment pathway (Fig. 2, 4) and propose the following transformation sequences. Initially, nanometer-scale FeSam precipitated and transformed to tabular greigite particles (<150 nm). The tabular particles, perhaps in combination with nano-scale FeSam particles, acted as primary units that attached together, forming larger aggregates (<100–250 nm). The aggregates tended to become less rounded and showed signs of recrystallisation towards particles with sharp edges, eventually forming octahedral particles (300–1500 nm). Pyrite containing multiple layers of flat particles and twinned developed into rose-like particles (1000–3000 nm). This morphology was the rarest type observed, and it was more common in Set-2 compared to Set-1 experiments. The developmental link between rose-like particles to other smaller particles was less clear. We propose that as particle attachment proceeded on octahedral pyrite, preferential stabilisation of the {111} faces are amplified, leading to a skeletal structure (Fig. 4), similar to those observed previously for ZnS (Xu et al., 2016Xu, J., Murayama, M., Roco, C.M., Veeramani, H., Michel, F.M., Rimstidt, J.D., Winkler, C., Hochella, M.F. (2016) Highly-defective nanocrystals of ZnS formed via dissimilatory bacterial sulfate reduction: A comparative study with their abiogenic analogues. Geochimica et Cosmochimica Acta 180, 1–14. https://doi.org/10.1016/j.gca.2016.02.007
). The skeletal crystals continue to grow driven by higher attachment rates along the edges (Salas et al., 2021Salas, P., Ruprecht, P., Hernández, L., Rabbia, O. (2021) Out-of-sequence skeletal growth causing oscillatory zoning in arc olivines. Nature Communications 12. https://doi.org/10.1038/s41467-021-24275-6
) until they develop into a rose-like structure.
Figure 4 (a) Schematic of pyrite formation via particle attachment, showing skeletal growth and twinning between octahedral and rose-like particles (generated in Blender 3.2.2) and (b–d) the potential implications to the environment.
To our knowledge, this is the first time that pyrite formation via particle attachment has been described at <100 °C, leading to micro-framboid formation. In situ real-time microscopy observations will be crucial to confirm and describe the exact steps of this pathway. Studies aimed at investigating the subsequent effects on the reactivity and stability of pyrite grains, as well as the distribution of isotopes and trace metals, will help to constrain the potential implications to environmental proxies and biosignature interpretation (Fig. 4).
top
Acknowledgements
This study was supported by the DFG (SPP 1833, Emmy Noether Programme, 1450/3-2, DU 1450/7-1, JPD; INST 37/1027-1 FUGG, AK) and the German Excellence Strategy of the Federal and State Governments (EXC2124, 390838134; Tuebingen Structural Microscopy Core Facility; AK, MM, SF, JS). Part of the discussion is based upon the multiple transition metal sulfide work supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences program under Award Number DE-SC0023251.
Editor: Liane Benning
top
References
Allman, C.J., Gómez-Ortiz, D., Burke, A., Amils, R., Rodriguez, N., Fernández-Remolar, D. (2021) Hydrogeochemical variability of the acidic springs in the Rio Tinto headwaters. Water 13, 2861. https://doi.org/10.3390/w13202861
 Show in context
Show in context These ratios represent the broad range of environments (e.g., low temperature sediments, acid mine drainage, hydrothermal vents) in which natural pyrite can form (Von Damm et al., 1985; Shaw et al., 1990; Allman et al., 2021).
View in article
Baya, C., Le Pape, P., Baptiste, B., Menguy, N., Delbes, L., Morand, M., Rouelle, M., Aubry, E., Ona-Nguema, G., Noël, V. and Juillot, F. (2022) A methodological framework to study the behavior and kinetic influence of V, Mn, Co, Ni, Cu, Zn, As, Se and Mo during pyrite formation via the polysulfide pathway at ambient temperature. Chemical Geology 613. https://doi.org/10.1016/j.chemgeo.2022.121139
 Show in context
Show in context In contrast, other studies demonstrated that Co and Ni (Swanner et al., 2019) and Mo (Baya et al., 2022) inhibited pyrite formation. Differences in metal:Fe ratios and synthesis conditions (e.g., pH and formation pathways) between studies likely led to differences in how specific trace metals affect pyrite formation (SI Discussion).
View in article
Butler, I.B., Rickard, D. (2000) Framboidal pyrite formation via the oxidation of iron (II) monosulfide by hydrogen sulphide. Geochimica et Cosmochimica Acta 64, 2665–2672. https://doi.org/10.1016/S0016-7037(00)00387-2
 Show in context
Show in context Euhedral pyrite is proposed to form via slow growth on pre-existing pyrite, while framboids are proposed to form under fast nucleation conditions in close association with Fe sulphide precursors, such as mackinawite (FeS) and greigite (Fe3S4) (Raiswell, 1982; Butler and Rickard, 2000).
View in article
Similar surface features have been observed before and interpreted as screw dislocations (Wang and Morse, 1996; Butler and Rickard, 2000).
View in article
Csákberényi-Malasics, D., Rodriguez-Blanco, J.D., Kis, V.K., Rečnik, A., Benning, L.G., Pósfai, M. (2012) Structural properties and transformations of precipitated FeS. Chemical Geology 294–295, 249–258. https://doi.org/10.1016/j.chemgeo.2011.12.009
 Show in context
Show in context In both sets, the addition of Na2S to Fe2+ led to the immediate formation of fine black precipitates identified as a disordered mackinawite-like phase (FeSam) based on a single broad reflection with d-spacings of 5.2–5.3 Å via X-ray diffraction (XRD) (Fig. 1a; Table S-3), and characteristic sheet-like aggregate structures under scanning electron microscopy (SEM) (Fig. 2a) (Csákberényi-Malasics et al., 2012).
View in article
Treatment with 6 M HCl led to dissolution of the tabular particles, which we interpret as HCl-soluble greigite given its morphological similarity to previous lab-synthesised greigite (Csákberényi-Malasics et al., 2012; Mansor et al., 2019).
View in article
De Yoreo, J.J., Gilbert, P.U., Sommerdijk, N.A., Penn, R.L., Whitelam, S., Joester, D., Zhang, H., Rimer, J.D., Navrotsky, A., Banfield, J.F. and Wallace, A.F. (2015) Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 349, aaa6760. https://doi.org/10.1126/science.aaa6760
 Show in context
Show in context This pathway explains defects in crystal structures, distributions of trace metals and isotopes, and unusual particle morphologies in nature (De Yoreo et al., 2015).
View in article
Erickson, B.E., Helz, G.R. (2000) Molybdenum(VI) speciation in sulfidic waters: Stability and lability of thiomolybdates. Geochimica et Cosmochimica Acta 64, 1149–1158. https://doi.org/10.1016/S0016-7037(99)00423-8
 Show in context
Show in context Polysulphide intensities increased with higher trace metal concentrations with the exception of Mo. High Mo concentration induced an additional peak at 470 nm corresponding to tetrathiomolybdate (MoS42−) (Erickson and Helz, 2000).
View in article
Gong, M., Kirkeminde, A., Ren, S. (2013) Symmetry-defying iron pyrite (FeS2) nanocrystals through oriented attachment. Scientific Reports 3, 1–6. https://doi.org/10.1038/srep02092
 Show in context
Show in context This pathway has been demonstrated for pyrite synthesised at >100 °C (Hunger and Benning, 2007; Li et al., 2011; Gong et al., 2013), but not at lower temperatures.
View in article
Huang, F., Gao, W., Gao, S., Meng, L., Zhang, Z., Yan, Y., Ren, Y., Li, Y., Liu, K., Xing, M. and Wang, Y. (2017) Morphology evolution of nano-micron pyrite: A review. Journal of Nanoscience and Nanotechnology 17, 5980–5995. https://doi.org/10.1166/jnn.2017.14430
 Show in context
Show in context Pyrite is a widespread mineral that is involved in a variety of biogeochemical processes with implications for interpreting Earth’s past, present and future (Huang et al., 2017).
View in article
Hunger, S., Benning, L.G. (2007) Greigite: A true intermediate on the polysulfide pathway to pyrite. Geochemical Transactions 8, 1–20. https://doi.org/10.1186/1467-4866-8-1
 Show in context
Show in context This pathway has been demonstrated for pyrite synthesised at >100 °C (Hunger and Benning, 2007; Li et al., 2011; Gong et al., 2013), but not at lower temperatures.
View in article
Overall, Set-1 and Set-2 experiments exhibited similar transformation sequences of FeSam to greigite to pyrite with increasing time and temperature, consistent with previous studies (Hunger and Benning, 2007; Mansor and Fantle, 2019).
View in article
Li, W., Döblinger, M., Vaneski, A., Rogach, A.L., Jäckel, F., Feldmann, J. (2011) Pyrite nanocrystals: Shape-controlled synthesis and tunable optical properties via reversible self-assembly. Journal of Materials Chemistry 21, 17946–17952. https://doi.org/10.1039/c1jm13336e
 Show in context
Show in context This pathway has been demonstrated for pyrite synthesised at >100 °C (Hunger and Benning, 2007; Li et al., 2011; Gong et al., 2013), but not at lower temperatures.
View in article
Lin, Z., Sun, X., Peckmann, J., Lu, Y., Xu, L., Strauss, H., Zhou, H., Gong, J., Lu, H. and Teichert, B.M. (2016) How sulfate-driven anaerobic oxidation of methane affects the sulfur isotopic composition of pyrite: A SIMS study from the South China Sea. Chemical Geology 440, 26–41. https://doi.org/10.1016/j.chemgeo.2016.07.007
 Show in context
Show in context The continuum model for pyrite growth (Sawlowicz, 1993) has received increasing support from growing textural, geochemical and isotopic evidence (Lin et al., 2016, 2017; Liu et al., 2022).
View in article
Lin, Z., Sun, X., Lu, Y., Strauss, H., Xu, L., Gong, J., Teichert, B.M., Lu, R., Lu, H., Sun, W. and Peckmann, J. (2017) The enrichment of heavy iron isotopes in authigenic pyrite as a possible indicator of sulfate-driven anaerobic oxidation of methane: Insights from the South China Sea. Chemical Geology 449, 15–29. https://doi.org/10.1016/j.chemgeo.2016.11.032
 Show in context
Show in context The continuum model for pyrite growth (Sawlowicz, 1993) has received increasing support from growing textural, geochemical and isotopic evidence (Lin et al., 2016, 2017; Liu et al., 2022).
View in article
Liu, K., Huang, F., Gao, S., Zhang, Z., Ren, Y., An, B. (2022) Morphology of framboidal pyrite and its textural evolution: Evidence from the Logatchev area, Mid-Atlantic Ridge. Ore Geology Reviews 141. https://doi.org/10.1016/j.oregeorev.2021.104630
 Show in context
Show in context The continuum model for pyrite growth (Sawlowicz, 1993) has received increasing support from growing textural, geochemical and isotopic evidence (Lin et al., 2016, 2017; Liu et al., 2022).
View in article
Liu, W., Luo, C., Zhang, S., Zhang, B., Ma, J., Wang, X., Liu, W., Li, Z., Yang, Q.H. and Lv, W. (2021) Cobalt-doping of molybdenum disulfide for enhanced catalytic polysulfide conversion in lithium-sulfur batteries. ACS Nano 15, 7491–7499. https://doi.org/10.1021/acsnano.1c00896
 Show in context
Show in context The amount of polysulphide formed was likely influenced by varying availability of H2S and S0 after metal sulphide precipitation. All the metals tested in this study were proposed to form polysulphide complexes (Rickard and Luther, 2006), with Co known to enhance polysulphide conversions in lithium-sulphur batteries (Liu et al., 2021).
View in article
Mansor, M., Fantle, M.S. (2019) A novel framework for interpreting pyrite-based Fe isotope records of the past. Geochimica et Cosmochimica Acta 253, 39–62. https://doi.org/10.1016/j.gca.2019.03.017
 Show in context
Show in context Overall, Set-1 and Set-2 experiments exhibited similar transformation sequences of FeSam to greigite to pyrite with increasing time and temperature, consistent with previous studies (Hunger and Benning, 2007; Mansor and Fantle, 2019).
View in article
Mo is also known to promote greigite formation (Mansor and Fantle, 2019; Miller et al., 2020), which could explain the lower pyrite/greigite ratio observed in the presence of Mo compared to other trace metals.
View in article
Mansor, M., Berti, D., Hochella Jr, M.F., Murayama, M., Xu, J. (2019) Phase, morphology, elemental composition and formation mechanisms of biogenic and abiogenic Fe-Cu-sulfide nanoparticles: A comparative study on their occurrences under anoxic conditions. American Mineralogist 104, 703–717. https://doi.org/10.2138/am-2019-6848
 Show in context
Show in context Treatment with 6 M HCl led to dissolution of the tabular particles, which we interpret as HCl-soluble greigite given its morphological similarity to previous lab-synthesised greigite (Csákberényi-Malasics et al., 2012; Mansor et al., 2019).
View in article
Miller, N., Dougherty, M., Du, R., Sauers, T., Yan, C., Pines, J.E., Meyers, K.L., Dang, Y.M., Nagle, E., Ni, Z. and Pungsrisai, T. (2020) Adsorption of tetrathiomolybdate to iron sulfides and its impact on iron sulfide transformations. ACS Earth and Space Chemistry 4, 2246–2260. https://doi.org/10.1021/acsearthspacechem.0c00176
 Show in context
Show in context Mo is also known to promote greigite formation (Mansor and Fantle, 2019; Miller et al., 2020), which could explain the lower pyrite/greigite ratio observed in the presence of Mo compared to other trace metals.
View in article
Ohfuji, H., Rickard, D. (2005) Experimental syntheses of framboids - A review. Earth-Science Reviews 71, 147–170. https://doi.org/10.1016/j.earscirev.2005.02.001
 Show in context
Show in context The striking morphology of framboids and their association with organic matter have led to their interpretation as biosignatures, despite the various reports of framboid synthesis via abiotic pathways (Ohfuji and Rickard, 2005).
View in article
Raiswell, R. (1982) Pyrite texture, isotopic composition and the availability of iron. American Journal of Science 282, 1244–1263. https://doi.org/10.2475/ajs.282.8.1244
 Show in context
Show in context Euhedral pyrite is proposed to form via slow growth on pre-existing pyrite, while framboids are proposed to form under fast nucleation conditions in close association with Fe sulphide precursors, such as mackinawite (FeS) and greigite (Fe3S4) (Raiswell, 1982; Butler and Rickard, 2000).
View in article
Rickard, D. (2019) Sedimentary pyrite framboid size-frequency distributions: A meta-analysis. Palaeogeography, Palaeoclimatology, Palaeoecology 522, 62–75. https://doi.org/10.1016/j.palaeo.2019.03.010
 Show in context
Show in context In the geological record, high abundances of framboids have been interpreted as indicators of euxinic conditions in water columns (Wilkin et al., 1996; Rickard, 2019).
View in article
Rickard, D., Luther, G.W. (2006) Metal sulfide complexes and clusters. Reviews in Mineralogy and Geochemistry 61, 421–504. https://doi.org/10.2138/rmg.2006.61.8
 Show in context
Show in context The amount of polysulphide formed was likely influenced by varying availability of H2S and S0 after metal sulphide precipitation. All the metals tested in this study were proposed to form polysulphide complexes (Rickard and Luther, 2006), with Co known to enhance polysulphide conversions in lithium-sulphur batteries (Liu et al., 2021).
View in article
Robbins, L.J., Lalonde, S.V., Planavsky, N.J., Partin, C.A., Reinhard, C.T., Kendall, B., Scott, C., Hardisty, D.S., Gill, B.C., Alessi, D.S. and Dupont, C.L. (2016) Trace elements at the intersection of marine biological and geochemical evolution. Earth-Science Reviews 163, 323–348. https://doi.org/10.1016/j.earscirev.2016.10.013
 Show in context
Show in context These bioessential trace metals are common impurities in pyrite and play key roles in Earth’s biogeochemical evolution (Robbins et al., 2016).
View in article
Salas, P., Ruprecht, P., Hernández, L., Rabbia, O. (2021) Out-of-sequence skeletal growth causing oscillatory zoning in arc olivines. Nature Communications 12. https://doi.org/10.1038/s41467-021-24275-6
 Show in context
Show in context The skeletal crystals continue to grow driven by higher attachment rates along the edges (Salas et al., 2021) until they develop into a rose-like structure.
View in article
Sanden, S.A., Szilagyi, R.K., Li, Y., Kitadai, N., Webb, S.M., Yano, T., Nakamura, R., Hara, M., McGlynn, S.E. (2021) Electrochemically induced metal- vs. ligand-based redox changes in mackinawite: identification of a Fe3+- and polysulfide-containing intermediate. Dalton Transactions 50, 11763–11774. https://doi.org/10.1039/D1DT01684A
 Show in context
Show in context Alternative pathways without a greigite intermediate are possible (see Sanden et al., 2021) but seem unlikely in our experiments. We suggest that the differences in pyrite formation rates (∼10 × faster in Set-1) are caused by the headspace composition (3 % H2 vs. pure N2).
View in article
Sawlowicz, Z. (1993) Pyrite framboids and their development: a new conceptual mechanism. Geologische Rundschau 82, 148–156. https://doi.org/10.1007/BF00563277
 Show in context
Show in context The continuum model for pyrite growth (Sawlowicz, 1993) has received increasing support from growing textural, geochemical and isotopic evidence (Lin et al., 2016, 2017; Liu et al., 2022).
View in article
Shaw, T.J., Gieskes, J.M., Jahnke, R.A. (1990) Early diagenesis in differing depositional environments: The response of transition metals in pore water. Geochimica et Cosmochimica Acta 54, 1233–1246. https://doi.org/10.1016/0016-7037(90)90149-F
 Show in context
Show in context These ratios represent the broad range of environments (e.g., low temperature sediments, acid mine drainage, hydrothermal vents) in which natural pyrite can form (Von Damm et al., 1985; Shaw et al., 1990; Allman et al., 2021).
View in article
Sturm, E. V., Cölfen, H. (2016) Mesocrystals: Structural and morphogenetic aspects. Chemical Society Reviews 45, 5821–5833. https://doi.org/10.1039/C6CS00208K
 Show in context
Show in context Detailed SEM analyses revealed two striking features: (1) particles existed in a continuum of size and shape, and (2) many of the larger particles had rough surface textures that indicate growth via aggregation of smaller particles with varying degrees of recrystallisation, similar in appearance to mesocrystals (Sturm and Cölfen, 2016).
View in article
Swanner, E.D., Webb, S.M., Kappler, A. (2019) Fate of cobalt and nickel in mackinawite during diagenetic pyrite formation. American Mineralogist 104, 917–928. https://doi.org/10.2138/am-2019-6834
 Show in context
Show in context In contrast, other studies demonstrated that Co and Ni (Swanner et al., 2019) and Mo (Baya et al., 2022) inhibited pyrite formation. Differences in metal:Fe ratios and synthesis conditions (e.g., pH and formation pathways) between studies likely led to differences in how specific trace metals affect pyrite formation (SI Discussion).
View in article
Voelz, J.L., Johnson, N.W., Chun, C.L., Arnold, W.A., Penn, R.L. (2019) Quantitative dissolution of environmentally accessible iron residing in iron-rich minerals: A review. ACS Earth and Space Chemistry 3, 1371–1392. https://doi.org/10.1021/acsearthspacechem.9b00012
 Show in context
Show in context The other particles (spherical aggregates, octahedra and “roses”) were interpreted as pyrite since they did not dissolve in HCl (Voelz et al., 2019).
View in article
Von Damm, K.L., Edmond, J.M., Grant, B., Measures, C.I., Walden, B., Weiss, R.F. (1985) Chemistry of submarine hydrothermal solutions at 21°N, East Pacific Rise. Geochimica et Cosmochimica Acta 49, 2197–2220. https://doi.org/10.1016/0016-7037(85)90222-4
 Show in context
Show in context These ratios represent the broad range of environments (e.g., low temperature sediments, acid mine drainage, hydrothermal vents) in which natural pyrite can form (Von Damm et al., 1985; Shaw et al., 1990; Allman et al., 2021).
View in article
Wang, Q., Morse, J.W. (1996) Pyrite formation under conditions approximating those in anoxic sediments I. Pathway and morphology. Marine Chemistry 52, 99–121. https://doi.org/10.1016/0304-4203(95)00082-8
 Show in context
Show in context Similar surface features have been observed before and interpreted as screw dislocations (Wang and Morse, 1996; Butler and Rickard, 2000).
View in article
Wilkin, R.T., Barnes, H.L., Brantley, S.L. (1996) The size distribution of framboidal pyrite in modern sediments: An indicator of redox conditions. Geochimica et Cosmochimica Acta 60, 3897–3912. https://doi.org/10.1016/0016-7037(96)00209-8
 Show in context
Show in context In the geological record, high abundances of framboids have been interpreted as indicators of euxinic conditions in water columns (Wilkin et al., 1996; Rickard, 2019).
View in article
Xu, J., Murayama, M., Roco, C.M., Veeramani, H., Michel, F.M., Rimstidt, J.D., Winkler, C., Hochella, M.F. (2016) Highly-defective nanocrystals of ZnS formed via dissimilatory bacterial sulfate reduction: A comparative study with their abiogenic analogues. Geochimica et Cosmochimica Acta 180, 1–14. https://doi.org/10.1016/j.gca.2016.02.007
 Show in context
Show in context We propose that as particle attachment proceeded on octahedral pyrite, preferential stabilisation of the {111} faces are amplified, leading to a skeletal structure (Fig. 4), similar to those observed previously for ZnS (Xu et al., 2016).
View in article
top
Supplementary Information
The Supplementary Information includes:
Download the Supplementary Information (PDF)
Figures
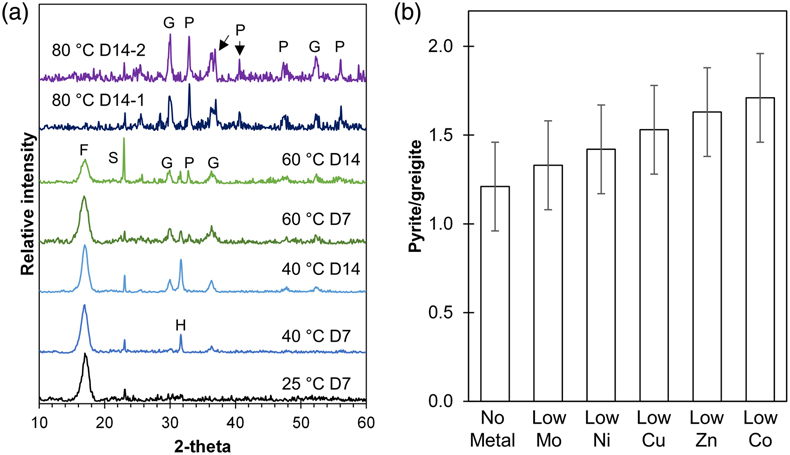
Figure 1 (a) XRD patterns showing the progressive transformation from FeSam (F) to greigite (G) and pyrite (P) with increasing temperatures (25, 40, 60 and 80 °C) and time (after 7 and 14 days). Residual sulphur (S) and halite (H) were also detected. Samples from two replicate bottles of experiments at 80 °C after 14 days (80 °C D14-1 or D14-2) indicate high reproducibility. (b) Relative intensity of pyrite/greigite signals as determined from thin-film XRD after 14 days of incubation at 80 °C.
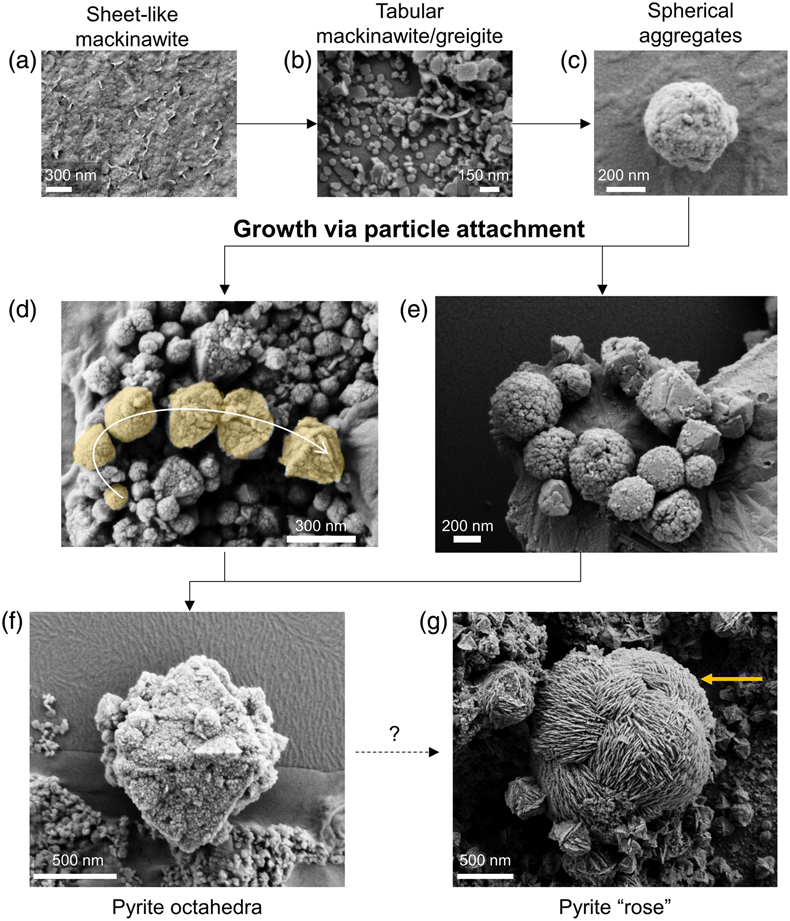
Figure 2 Representative SEM micrographs suggestive of growth via particle attachment. (a–c) Transformation of mackinawite to tabular greigite to spherical aggregates of tabular particles. (d) The arrow denotes a potential transformation from spherical aggregates to pyrite octahedra. (e) Close association between spherical aggregates (microframboids) that are recrystallising to form pyrite octahedra. (f) Colloidal pyrite octahedra (centre) with a rough surface and porous structure. (g) Micrometre-sized pyrite “rose” (yellow arrow) surrounded by smaller pyrite octahedra. The transformation mechanism may be related to skeletal growth.
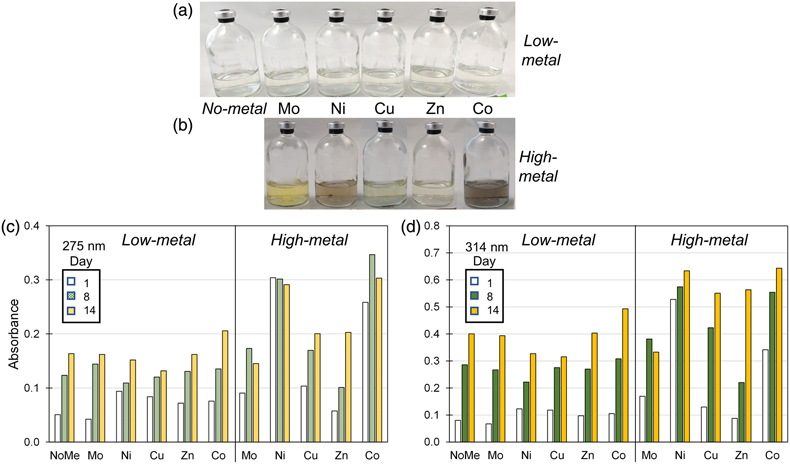
Figure 3 (a–b) Pictures of Fe-free setups after 8 days of incubation at 80 °C. Yellow tinge indicates the presence of polysulphides. Precipitates (grey or colourless) are observed in some high-metal setups. (c–d) Bar charts of the polysulphide peak intensities at (c) 275 nm and (d) 314 nm at Day 1, 8 and 14. Polysulphide intensities increase with time and with higher metal concentrations.
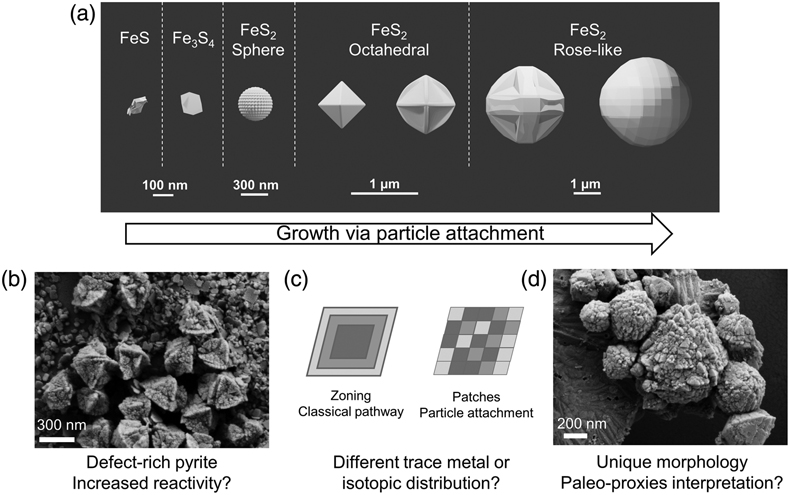
Figure 4 (a) Schematic of pyrite formation via particle attachment, showing skeletal growth and twinning between octahedral and rose-like particles (generated in Blender 3.2.2) and (b–d) the potential implications to the environment.