Fluid-melt Mo isotope fractionation: implications for the δ98/95Mo of the upper crust
Affiliations | Corresponding Authors | Cite as | Funding information- Share this article




-
Article views:363Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
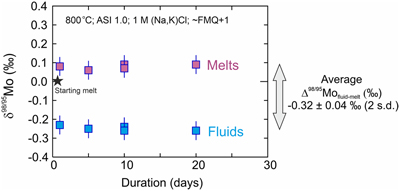 Figure 1 Mo isotopic composition (δ98/95Mo) of fluids and melts versus time. All experiments are identical except for their duration (days). Two experiments were performed for the 10 day duration. The average Δ98/95Mofluid-melt (‰) for the 5 experiments is also shown. | 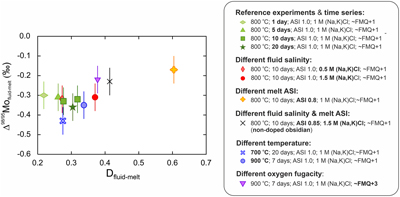 Figure 2 Mo isotope fractionation value between fluid and melt (Δ98/95Mofluid-melt) versus the partition coefficient (Dfluid-melt = Cfluid/Cmelt) for each experiment. | 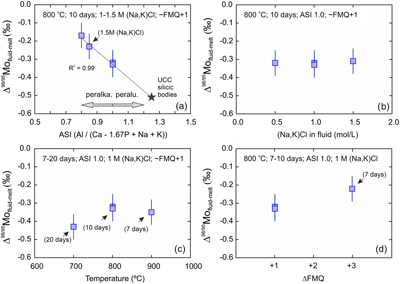 Figure 3 Evolution of the Mo isotope fractionation value between fluids and melts (Δ98/95Mofluid-melt) at different experimental (a) melt aluminium saturation indexes (ASI = Al/(Ca − 1.67P + Na + K)), (b) fluid salinities, (c) temperatures and (d) oxygen fugacities (fO2 relative to the fayalite-magnetite-quartz buffer, FMQ). Abbreviations: peralka., peralkaline; peralu., peraluminous. | 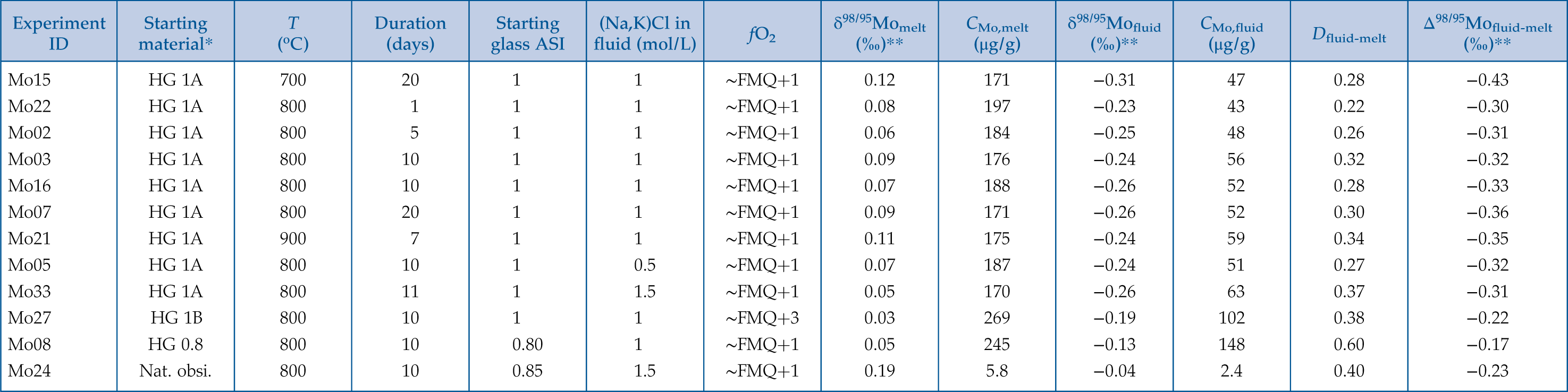 Table 1 Experimental conditions and results. |
| Figure 1 | Figure 2 | Figure 3 | Table 1 |
top
Introduction
The Mo stable isotopic system is a very promising tool to explore both the chemical evolution of the silicate Earth (e.g., McCoy-West et al., 2019
McCoy-West, A.J., Chowdhury, P., Burton, K.W., Sossi, P., Nowell, G.M., Fitton, J.G., Kerr, A.C., Cawood, P.A., Williams, H.M. (2019) Extensive crustal extraction in Earth’s early history inferred from molybdenum isotopes. Nature Geoscience 12, 946–951. https://doi.org/10.1038/s41561-019-0451-2
) and the palaeo-redox conditions of oceans (e.g., Wille et al., 2007Wille, M., Kramers, J.D., Nägler, T.F., Beukes, N.J., Schröder, S., Meisel, Th., Lacassie, J.P., Voegelin, A.R. (2007) Evidence for a gradual rise of oxygen between 2.6 and 2.5 Ga from Mo isotopes and Re-PGE signatures in shales. Geochimica et Cosmochimica Acta 71, 2417–2435. https://doi.org/10.1016/j.gca.2007.02.019
). Mass balance models associated with both types of applications strongly rely on the Mo isotopic composition (δ98/95Mo = 1000 × [(98Mo/95Mosample)/(98Mo/95Mostandard) − 1]) of the continental crust (CC), especially its upper layer (UCC), because it is highly enriched in Mo and in direct contact with the hydrosphere. While an estimate for the Mo stable isotope composition of the UCC created prior to the Great Oxidation Event (GOE; ∼2.4–2.2 Ga) exists (δ98/95Mo = +0.03 ‰; Greaney et al., 2020Greaney, A.T., Rudnick, R.L., Romaniello, S.J., Johnson, A.C., Gaschnig, R.M., Anbar, A.D. (2020) Molybdenum isotope fractionation in glacial diamictites tracks the onset of oxidative weathering of the continental crust. Earth and Planetary Science Letters 534, 116083. https://doi.org/10.1016/j.epsl.2020.116083
), constraints on post-GOE UCC δ98/95Mo are scarce and conflicting. The difficulty in constraining the δ98/95Mo of the UCC after the GOE is a consequence of the redox sensitivity of Mo and its fluid-mobility under oxidising conditions. These properties, for instance, prevent the usage of fine grained clastic sedimentary rocks for that purpose (e.g., Greaney et al., 2020Greaney, A.T., Rudnick, R.L., Romaniello, S.J., Johnson, A.C., Gaschnig, R.M., Anbar, A.D. (2020) Molybdenum isotope fractionation in glacial diamictites tracks the onset of oxidative weathering of the continental crust. Earth and Planetary Science Letters 534, 116083. https://doi.org/10.1016/j.epsl.2020.116083
). One approach to constrain the δ98/95Mo of modern UCC has been to use the signatures of molybdenites (MoS2), mostly derived from magmatic-hydrothermal fluids, as proxies for exposed rocks. It was initially thought that isotopic fractionation would be minor in high temperature systems and that, MoS2 could therefore represent the isotopic composition of their crustal source rocks (e.g., Barling et al., 2001Barling, J., Arnold, G.L., Anbar, A.D. (2001) Natural mass-dependent variations in the isotopic composition of molybdenum. Earth and Planetary Science Letters 193, 447–457. https://doi.org/10.1016/S0012-821X(01)00514-3
). As studies multiplied, it became clear that the controls on Mo isotopes in hydrothermal systems were more complex than initially thought (e.g., Hannah et al., 2007Hannah, J.L., Stein, H.J., Wieser, M.E., de Laeter, J.R., Varner, M.D. (2007) Molybdenum isotope variations in molybdenite: Vapor transport and Rayleigh fractionation of Mo. Geology 35, 703–706. https://doi.org/10.1130/G23538A.1
). Nevertheless, because these controls were inferred to result in progressively heavier MoS2, a global average for MoS2 δ98/95Mo was suggested to represent a maximum value for Phanerozoic UCC (Greber et al., 2014Greber, N.D., Pettke, T., Nägler, T.F. (2014) Magmatic–hydrothermal molybdenum isotope fractionation and its relevance to the igneous crustal signature. Lithos 190–191, 104–110. https://doi.org/10.1016/j.lithos.2013.11.006
). This, however, is at odds with a recent Phanerozoic UCC composition derived from igneous rock compositions (δ98/95Mo = +0.14 ± 0.07 ‰; Yang et al., 2017Yang, J., Barling, J., Siebert, C., Fietzke, J., Stephens, E., Halliday, A.N. (2017) The molybdenum isotopic compositions of I-, S- and A-type granitic suites. Geochimica et Cosmochimica Acta 205, 168–186. https://doi.org/10.1016/j.gca.2017.01.027
), since the latter is visibly heavier than the most recent MoS2 δ98/95Mo averages (+0.04 ‰ in Breillat et al., 2016Breillat, N., Guerrot, C., Marcoux, E., Négrel, Ph. (2016) A new global database of δ98Mo in molybdenites: A literature review and new data. Journal of Geochemical Exploration 161, 1–15. https://doi.org/10.1016/j.gexplo.2015.07.019
; −0.04 ‰ in Willbold and Elliott, 2017Willbold, M., Elliott, T. (2017) Molybdenum isotope variations in magmatic rocks. Chemical Geology 449, 253–268. https://doi.org/10.1016/j.chemgeo.2016.12.011
). Clearly, current constraints on Phanerozoic UCC do not converge, and deriving a robust estimate will require a better understanding of magmatic-hydrothermal systems.One geological process having the potential to create the discrepancy described above is Mo isotopic fractionation during fluid exsolution in silicic systems. The dominant igneous rock types in the UCC are plutonic silicic rocks, and these lithologies were shown to be depleted in Mo (20–90 % depletion; mean 60 %) compared to fluid immobile elements of similar incompatibility (e.g., Ce and Pr; Greaney et al., 2018
Greaney, A.T., Rudnick, R.L., Gaschnig, R.M., Whalen, J.B., Luais, B., Clemens, J.D. (2018) Geochemistry of molybdenum in the continental crust. Geochimica et Cosmochimica Acta 238, 36–54. https://doi.org/10.1016/j.gca.2018.06.039
). In some plutonic suites, correlations between Mo and fluid soluble elements exist, suggesting fluid exsolution as a dominant control over the Mo depletions observed (Greaney et al., 2018Greaney, A.T., Rudnick, R.L., Gaschnig, R.M., Whalen, J.B., Luais, B., Clemens, J.D. (2018) Geochemistry of molybdenum in the continental crust. Geochimica et Cosmochimica Acta 238, 36–54. https://doi.org/10.1016/j.gca.2018.06.039
). Significant Mo partitioning in magmatic fluids is also supported by a large number of experimental (e.g., Candela and Holland, 1984Candela, P.A., Holland, H.D. (1984) The partitioning of copper and molybdenum between silicate melts and aqueous fluids. Geochimica et Cosmochimica Acta 48, 373–380. https://doi.org/10.1016/0016-7037(84)90257-6
) and empirical (e.g., Zajacz et al., 2008Zajacz, Z., Halter, W.E., Pettke, T., Guillong, M. (2008) Determination of fluid/melt partition coefficients by LA-ICPMS analysis of co-existing fluid and silicate melt inclusions: Controls on element partitioning. Geochimica et Cosmochimica Acta 72, 2169–2197. https://doi.org/10.1016/j.gca.2008.01.034
) studies. Whether isotopic fractionation is associated with this process remains unclear, with only one empirical study suggesting a possible enrichment of heavy Mo isotopes in fluids in a Mo porphyry deposit (Questa, USA; Greber et al., 2014Greber, N.D., Pettke, T., Nägler, T.F. (2014) Magmatic–hydrothermal molybdenum isotope fractionation and its relevance to the igneous crustal signature. Lithos 190–191, 104–110. https://doi.org/10.1016/j.lithos.2013.11.006
). This is based on the very low Mo and light δ98/95Mo of a porphyry rhyolite dike compared to the molybdenites in the nearby and contemporaneous Questa mineralisation. However, the porphyry dike is altered and may not derive from the same magma as that from which the fluids of the mineralisation are derived (Greber et al., 2014Greber, N.D., Pettke, T., Nägler, T.F. (2014) Magmatic–hydrothermal molybdenum isotope fractionation and its relevance to the igneous crustal signature. Lithos 190–191, 104–110. https://doi.org/10.1016/j.lithos.2013.11.006
and references therein). Furthermore, theoretical constraints suggest that, in the occurrence of isotopic fractionation, a preferential enrichment of light Mo isotopes in the fluids should occur. This is because Mo coordination in silicate melts is tetrahedral, while both tetrahedral and octahedral coordination have been inferred for Mo in magmatic-hydrothermal fluids (e.g., Borg et al., 2012Borg, S., Liu, W., Etschmann, B., Tian, Y., Brugger, J. (2012) An XAS study of molybdenum speciation in hydrothermal chloride solutions from 25–385 °C and 600 bar. Geochimica et Cosmochimica Acta 92, 292–307. https://doi.org/10.1016/j.gca.2012.06.001
). Given that most MoS2 measured thus far have crystallised from fluids exsolved from silicic magmas or melts highly enriched in those fluids (Breillat et al., 2016Breillat, N., Guerrot, C., Marcoux, E., Négrel, Ph. (2016) A new global database of δ98Mo in molybdenites: A literature review and new data. Journal of Geochemical Exploration 161, 1–15. https://doi.org/10.1016/j.gexplo.2015.07.019
), a preferential enrichment in light Mo isotopes in the fluids could explain the lighter δ98/95Mo of MoS2 averages, compared to average silicic rocks (δ98/95Mo = +0.16 ‰ in Yang et al., 2017Yang, J., Barling, J., Siebert, C., Fietzke, J., Stephens, E., Halliday, A.N. (2017) The molybdenum isotopic compositions of I-, S- and A-type granitic suites. Geochimica et Cosmochimica Acta 205, 168–186. https://doi.org/10.1016/j.gca.2017.01.027
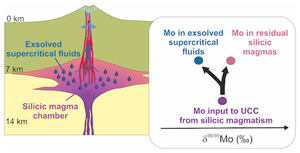
) and the associated discrepancies in UCC constraints. It is therefore the aim of this contribution to establish the first experimental constraints of the fluid/melt equilibrium fractionation value of Mo stable isotopes (Δ98/95Mofluid-melt = δ98/95Mofluid − δ98/95Momelt) at temperatures, fluid salinities, melt compositions and oxygen fugacities relevant to supercritical fluid exsolution in upper crustal silicic magmatic systems.top
Methods
Twelve experiments were conducted at McGill University and Institut des Sciences de la Terre d’Orléans (ISTO) to simulate the equilibration of exsolved fluids with upper crustal felsic magmas. Conditions for each experiment are shown in Table 1. Cold seal pressure vessels were used, except for two experiments (Mo21 and Mo27) requiring the usage of internally heated pressure vessels (IHPV) to allow for higher temperature (900 °C) or higher oxygen fugacity (∼FMQ+3). Powders of Mo-doped haplogranitic glasses (n = 11) or non-doped natural obsidian (n = 1) were loaded into gold capsules together with Mo-free fluids in similar proportions (1:1) (see Supplementary Information for details on the experimental approach). The experiments were equilibrated at 200 MPa, a pressure relevant for most long lived magma chambers (e.g., Huber et al., 2019
Huber, C., Townsend, M., Degruyter, W., Bachmann, O. (2019) Optimal depth of subvolcanic magma chamber growth controlled by volatiles and crust rheology. Nature Geoscience 12, 762–768. https://doi.org/10.1038/s41561-019-0415-6
). At such conditions, a single magmatic fluid phase with low to intermediate salinity (e.g., 1–5 wt. % NaCl in Rusk et al., 2004Rusk, B.G., Reed, M.H., Dilles, J.H., Klemm, L.M., Heinrich, C.A. (2004) Compositions of magmatic hydrothermal fluids determined by LA-ICP-MS of fluid inclusions from the porphyry copper–molybdenum deposit at Butte, MT. Chemical Geology 210, 173–199. https://doi.org/10.1016/j.chemgeo.2004.06.011
), a supercritical fluid, exsolves from magmas (Burnham, 1979Burnham, C.W. (1979) Magmas and hydrothermal fluids. In: Barnes, H.L. (Ed.) Geochemistry of Hydrothermal Ore Deposits. Second Edition, Wiley, New York, 71–136.
). We therefore set the range of salinity of our experimental fluids accordingly (0.5–1.5 M (Na,K)Cl). The effects of changing temperature, melt aluminium saturation index (ASI) and oxygen fugacity (fO2) on Δ98/95Mofluid-melt were also assessed over ranges relevant for upper crustal silicic magma chambers (see Table 1). In similar experiments, chemical equilibrium is typically reached within a maximum of 10 days (e.g., Jiang et al., 2021Jiang, Z., Shang, L., Guo, H., Wang, X.-s., Chen, C., Zhou, Y. (2021) An experimental investigation into the partition of Mo between aqueous fluids and felsic melts: Implications for the genesis of porphyry Mo ore deposits. Ore Geology Reviews 134, 104144. https://doi.org/10.1016/j.oregeorev.2021.104144
and references therein). Time scales for chemical and isotopic equilibrium were therefore assessed via a series of identical experiments performed at 800 °C with durations ranging from 1 to 20 days.Table 1 Experimental conditions and results.
ID | Starting material* | T (oC) | Duration (days) | Starting glass ASI | fO2 | (‰)** | (μg/g) | (‰)** | (μg/g) | (‰)** | ||
| Mo15 | HG 1A | 700 | 20 | 1 | 1 | ∼FMQ+1 | 0.12 | 171 | −0.31 | 47 | 0.28 | −0.43 |
| Mo22 | HG 1A | 800 | 1 | 1 | 1 | ∼FMQ+1 | 0.08 | 197 | −0.23 | 43 | 0.22 | −0.30 |
| Mo02 | HG 1A | 800 | 5 | 1 | 1 | ∼FMQ+1 | 0.06 | 184 | −0.25 | 48 | 0.26 | −0.31 |
| Mo03 | HG 1A | 800 | 10 | 1 | 1 | ∼FMQ+1 | 0.09 | 176 | −0.24 | 56 | 0.32 | −0.32 |
| Mo16 | HG 1A | 800 | 10 | 1 | 1 | ∼FMQ+1 | 0.07 | 188 | −0.26 | 52 | 0.28 | −0.33 |
| Mo07 | HG 1A | 800 | 20 | 1 | 1 | ∼FMQ+1 | 0.09 | 171 | −0.26 | 52 | 0.30 | −0.36 |
| Mo21 | HG 1A | 900 | 7 | 1 | 1 | ∼FMQ+1 | 0.11 | 175 | −0.24 | 59 | 0.34 | −0.35 |
| Mo05 | HG 1A | 800 | 10 | 1 | 0.5 | ∼FMQ+1 | 0.07 | 187 | −0.24 | 51 | 0.27 | −0.32 |
| Mo33 | HG 1A | 800 | 11 | 1 | 1.5 | ∼FMQ+1 | 0.05 | 170 | −0.26 | 63 | 0.37 | −0.31 |
| Mo27 | HG 1B | 800 | 10 | 1 | 1 | ∼FMQ+3 | 0.03 | 269 | −0.19 | 102 | 0.38 | −0.22 |
| Mo08 | HG 0.8 | 800 | 10 | 0.80 | 1 | ∼FMQ+1 | 0.05 | 245 | −0.13 | 148 | 0.60 | −0.17 |
| Mo24 | Nat. obsi. | 800 | 10 | 0.85 | 1.5 | ∼FMQ+1 | 0.19 | 5.8 | −0.04 | 2.4 | 0.40 | −0.23 |
Abbreviations: ASI, aluminum saturation index; fO2, oxygen fugacity relative to the fayalite-magnetite-quartz buffer (FMQ).
*HG 1A: haplogranite with ASI = 1, 254 μg/g Mo and δ98/95Mo = −0.01 ± 0.05 ‰. HG 1B: haplogranite with ASI = 1, 383 μg/g Mo and δ98/95Mo = −0.01 ± 0.05 ‰. HG 0.8: haplogranite with ASI = 0.8, 421 μg/g Mo and δ98/95Mo = −0.01 ± 0.05 ‰. HG 1A, HG 1B and HG 0.8 contain 78.7 wt. % SiO2. Nat. obsi.: natural obsidian with ASI = 0.85, 76.7 wt. % SiO2, 8.8 μg/g Mo and δ98/95Mo = 0.13 ± 0.05 ‰.
**Error on δ98/95Mo is 0.05 ‰; error on Δ98/95Mofluid-melt is propagated (0.07 ‰).
Mo isotope compositions and Mo concentrations of starting glasses, final (quenched) glasses and fluids were measured using double spike MC-ICP-MS at the University of Göttingen. The detailed description of the analytical approach is shown in the Supplementary Information. The uncertainty presented for the δ98/95Mo of each sample corresponds to twice the standard deviation of the replicate analyses of the Japan Geological Survey reference materials (±0.05 ‰).
top
Results
δ98/95Mo values and Mo concentrations of starting materials and experimental results are presented in Table 1, together with the calculated Mo partition coefficients between fluid and melt (Dfluid-melt = CMo,fluid /CMo,melt) and the Δ98/95Mofluid-melt for each pair. The Dfluid-melt and Δ98/95Mofluid-melt of identical experiments produced at different durations (the time series experiments; Figs. 1, 2) suggest that both chemical and isotopic equilibrium were reached between 5 and 10 days at 800 °C. This is consistent with chemical equilibrium time scales observed in similar experimental studies (e.g., Jiang et al., 2021
Jiang, Z., Shang, L., Guo, H., Wang, X.-s., Chen, C., Zhou, Y. (2021) An experimental investigation into the partition of Mo between aqueous fluids and felsic melts: Implications for the genesis of porphyry Mo ore deposits. Ore Geology Reviews 134, 104144. https://doi.org/10.1016/j.oregeorev.2021.104144
and references therein). Therefore, with the exception of the 1 and 5 days experiments, all presented experimental data represent equilibrium values.
Figure 1 Mo isotopic composition (δ98/95Mo) of fluids and melts versus time. All experiments are identical except for their duration (days). Two experiments were performed for the 10 day duration. The average Δ98/95Mofluid-melt (‰) for the 5 experiments is also shown.

Figure 2 Mo isotope fractionation value between fluid and melt (Δ98/95Mofluid-melt) versus the partition coefficient (Dfluid-melt = Cfluid/Cmelt) for each experiment.
At equilibrium, Dfluid-melt range from 0.27 to 0.60 and overlap the literature range for similar experiments (e.g., compilation in Fang and Audétat, 2022
Fang, J., Audétat, A. (2022) The effects of pressure, fO2, fS2 and melt composition on the fluid–melt partitioning of Mo: Implications for the Mo-mineralization potential of upper crustal granitic magmas. Geochimica et Cosmochimica Acta 336, 1–14. https://doi.org/10.1016/j.gca.2022.08.016
). Δ98/95Mofluid-melt range from −0.43 ± 0.07 ‰ to −0.17 ± 0.07 ‰ (Fig. 2) and therefore indicate the preferential incorporation of light Mo isotopes in fluids under all investigated conditions, and whether or not the starting glass was doped. The data set shows a clear control of the melt ASI over Δ98/95Mofluid-melt (linear regression with R2 = 0.99; Fig. 3a) with the greatest isotopic differences observed in the samples with the least peralkaline compositions. No effect of fluid salinity is observed (Fig. 3b). Apparent systematics suggest that Δ98/95Mofluid-melt could be larger at lower temperatures and lower oxygen fugacities (Fig. 3c, d), but this is not resolvable in this data set, and further experimental investigation will be required to test these possible correlations.
Figure 3 Evolution of the Mo isotope fractionation value between fluids and melts (Δ98/95Mofluid-melt) at different experimental (a) melt aluminium saturation indexes (ASI = Al/(Ca − 1.67P + Na + K)), (b) fluid salinities, (c) temperatures and (d) oxygen fugacities (fO2 relative to the fayalite-magnetite-quartz buffer, FMQ). Abbreviations: peralka., peralkaline; peralu., peraluminous.
top
Interpretation and Discussion of Experimental Results
The experimental data suggest that silicic melts with geologically realistic ASI will preferentially lose light Mo isotopes to exsolved supercritical fluids. In theory, both a difference in coordination and valence state of Mo between fluid and melt could induce the isotopic fractionation observed, i.e. higher coordination and/or lower valence state of Mo in the fluid than in the melt (e.g., Urey, 1947
Urey, H.C. (1947) The Thermodynamic Properties of Isotopic Substances. Journal of the Chemical Society (Resumed) 1947, 562–581. https://doi.org/10.1039/jr9470000562
). However, thermodynamic and empirical evidence suggests that the valence state of Mo should be similar in silicate melts and associated magmatic-hydrothermal fluids, i.e. hexavalent (e.g., Kaufmann et al., 2021Kaufmann, A.K.C., Pettke, T., Wille, M. (2021) Molybdenum isotope fractionation at upper-crustal magmatic-hydrothermal conditions. Chemical Geology 578, 120319. https://doi.org/10.1016/j.chemgeo.2021.120319
; Willbold and Elliott, 2017Willbold, M., Elliott, T. (2017) Molybdenum isotope variations in magmatic rocks. Chemical Geology 449, 253–268. https://doi.org/10.1016/j.chemgeo.2016.12.011
and references therein). Therefore, the most likely driver behind the Δ98/95Mofluid-melt observed in our experiments is higher Mo coordination in the fluid compared to the melt. In silicate melts, Mo dominantly occurs as tetrahedral molybdate species (MoO42−). On the other hand, in high temperature fluids, the coordination of Mo remains debated. Some studies suggested that Mo dominantly occurs as Na-K molybdate, monochloride or thio-molybdate at modest salinity, and as Mo-oxy-hydroxy complexes at low salinity (Zhang et al., 2012Zhang, L., Audétat, A., Dolejš, D. (2012) Solubility of molybdenite (MoS2) in aqueous fluids at 600–800 °C, 200 MPa: A synthetic fluid inclusion study. Geochimica et Cosmochimica Acta 77, 175–185. https://doi.org/10.1016/j.gca.2011.11.015
; Tattitch and Blundy, 2017Tattitch, B.C., Blundy, J.D. (2017) Cu-Mo partitioning between felsic melts and saline-aqueous fluids as a function of XNaCleq, fO2, and fS2. American Mineralogist 102, 1987–2006. https://doi.org/10.2138/am-2017-5998
). In such cases, Mo would be tetrahedrally coordinated, and no isotopic fractionation should be observed between fluids and melts, which is inconsistent with our experimental results. However, others have suggested the occurrence of species involving octahedrally coordinated Mo. For instance, Ulrich and Mavrogenes (2008)Ulrich, T., Mavrogenes, J. (2008) An experimental study of the solubility of molybdenum in H2O and KCl–H2O solutions from 500 °C to 800 °C, and 150 to 300 MPa. Geochimica et Cosmochimica Acta 72, 2316–2330. https://doi.org/10.1016/j.gca.2008.02.014
suggested the presence of a chloro-oxo Mo (VI) complex in high salinity (>20 % KCl) fluids (at 490 °C and ≥150 MPa). Borg et al. (2012)Borg, S., Liu, W., Etschmann, B., Tian, Y., Brugger, J. (2012) An XAS study of molybdenum speciation in hydrothermal chloride solutions from 25–385 °C and 600 bar. Geochimica et Cosmochimica Acta 92, 292–307. https://doi.org/10.1016/j.gca.2012.06.001
, based on experiments performed at lower temperatures and pressures (up to 385 °C, 60 MPa), found that octahedral species were becoming predominant in increasingly acidic solutions (pH < 5), with species such as molybdic acid and chloro-oxo Mo complexes. They also showed that increasing temperature favoured the formation of oxo-chloro complexes and suggested that these could be responsible for Mo transport in less acidic solutions at higher temperature (e.g., 700 °C), as proposed by Ulrich and Mavrogenes (2008)Ulrich, T., Mavrogenes, J. (2008) An experimental study of the solubility of molybdenum in H2O and KCl–H2O solutions from 500 °C to 800 °C, and 150 to 300 MPa. Geochimica et Cosmochimica Acta 72, 2316–2330. https://doi.org/10.1016/j.gca.2008.02.014
. Based on the lack of correlation between Δ98/95Mofluid-melt and starting fluid salinity in our experiments, a control of Mo isotopes by chloro-oxo Mo complexes alone seems unlikely. Hence, at least one other species in which Mo coordination is greater than tetrahedral, perhaps molybdic acid, is required in the fluids to explain the systematics observed in our experiments. Finally, the negative correlation between Δ98/95Mofluid-melt and ASI suggests that the latter exerts a strong influence on the coordination of Mo.top
Implications for the Composition of the Upper Continental Crust
Most large and long lived silicic magma chambers are located at upper crustal levels corresponding to lithostatic pressures of ∼200 MPa (Huber et al., 2019
Huber, C., Townsend, M., Degruyter, W., Bachmann, O. (2019) Optimal depth of subvolcanic magma chamber growth controlled by volatiles and crust rheology. Nature Geoscience 12, 762–768. https://doi.org/10.1038/s41561-019-0415-6
). There, significant amounts of exsolved supercritical fluids accumulate and equilibrate with silicic magmas. Slow fluid exsolution associated with the cooling and crystallisation of the melt is punctuated by repetitive fast exsolution events associated with decompression of incoming recharging melt (e.g., Edmonds and Woods, 2018Edmonds, M., Woods, A.W. (2018) Exsolved volatiles in magma reservoirs. Journal of Volcanology and Geothermal Research 368, 13–30. https://doi.org/10.1016/j.jvolgeores.2018.10.018
). Our data indicate that the extraction of these exsolved fluids likely results in the removal of light Mo from magma bodies, including precursor bodies of the silicic plutonic rocks making ∼50 % of the UCC (e.g., Wedepohl, 1995Wedepohl, K.H. (1995) The composition of the continental crust. Geochimica et Cosmochimica Acta 59, 1217–1232. https://doi.org/10.1016/0016-7037(95)00038-2
). The Mo depletion of these plutons (e.g., Greaney et al., 2018Greaney, A.T., Rudnick, R.L., Gaschnig, R.M., Whalen, J.B., Luais, B., Clemens, J.D. (2018) Geochemistry of molybdenum in the continental crust. Geochimica et Cosmochimica Acta 238, 36–54. https://doi.org/10.1016/j.gca.2018.06.039
), together with the large number of experimental constraints, suggests that a significant Mo fraction must have been removed from their precursor melts via exsolved fluids. Since UCC silicic plutonic rocks are dominantly peraluminous (ASI ≈ 1.25; Wedepohl, 1995Wedepohl, K.H. (1995) The composition of the continental crust. Geochimica et Cosmochimica Acta 59, 1217–1232. https://doi.org/10.1016/0016-7037(95)00038-2
), our experiments suggest that resolvable Δ98/95Mofluid-melt likely applied during the devolatilisation of their precursor melt. In other words, the δ98/95Mo of UCC silicic plutons should be heavier than their precursor, un-degassed melts. Based on our Dfluid-melt, the difference between undegassed and degassed silicic magmas might, however, not be large. For instance, using the highest Dfluid-melt in our experiments and assuming an extreme scenario whereby a hydrous melt with 10 wt. % volatiles undergoes fluid exsolution with a Δ98/95Mofluid-melt of −0.51 ‰ (extrapolation of the linear regression to ASI = 1.25 in Fig. 3a), would result in a residual melt that is only 0.03 ‰ heavier than the undegassed melt (see Supplementary Information for calculation). This is smaller than our analytical uncertainty. However, the range of published Dfluid-melt for similar experiments is large and includes significantly higher values (e.g., compilation in Fang and Audétat, 2022Fang, J., Audétat, A. (2022) The effects of pressure, fO2, fS2 and melt composition on the fluid–melt partitioning of Mo: Implications for the Mo-mineralization potential of upper crustal granitic magmas. Geochimica et Cosmochimica Acta 336, 1–14. https://doi.org/10.1016/j.gca.2022.08.016
). It is therefore best to consider the average δ98/95Mo of UCC silicic plutonic rocks (Yang et al., 2017Yang, J., Barling, J., Siebert, C., Fietzke, J., Stephens, E., Halliday, A.N. (2017) The molybdenum isotopic compositions of I-, S- and A-type granitic suites. Geochimica et Cosmochimica Acta 205, 168–186. https://doi.org/10.1016/j.gca.2017.01.027
) as a maximum value for the signature of the total Mo contribution to the UCC from undegassed silicic magmatism. In turn, UCC δ98/95Mo estimates derived from igneous rocks should also be viewed as maxima.Based on our experiments, the average δ98/95Mo of UCC silicic rocks should also be clearly heavier than global δ98/95Mo average for UCC MoS2, since MoS2 measured thus far are from magmatic-hydrothermal systems. There, MoS2 crystallises from both brines and low salinity vapours that unmix from supercritical fluids once they reach a miscibility gap in the NaCl-H2O system during ascent in the shallowest part of magmatic-hydrothermal complexes (typically <140 MPa and 400–700 °C; e.g., Bodnar et al., 1985
Bodnar, R.J., Burnham, C.W., Sterner, S.M. (1985) Synthetic fluid inclusions in natural quartz. III. Determination of phase equilibrium properties in the system H2O-NaCl to 1000°C and 1500 bars. Geochimica et Cosmochimica Acta 49, 1861–1873. https://doi.org/10.1016/0016-7037(85)90081-X
). The average UCC silicic rock composition (δ98/95Mo = +0.16 ‰) of Yang et al. (2017)Yang, J., Barling, J., Siebert, C., Fietzke, J., Stephens, E., Halliday, A.N. (2017) The molybdenum isotopic compositions of I-, S- and A-type granitic suites. Geochimica et Cosmochimica Acta 205, 168–186. https://doi.org/10.1016/j.gca.2017.01.027
is indeed ∼0.18 ‰ and ∼0.12 ‰ heavier than the two most extensive and recent MoS2 global averages of Willbold and Elliott (2017)Willbold, M., Elliott, T. (2017) Molybdenum isotope variations in magmatic rocks. Chemical Geology 449, 253–268. https://doi.org/10.1016/j.chemgeo.2016.12.011
and Breillat et al. (2016)Breillat, N., Guerrot, C., Marcoux, E., Négrel, Ph. (2016) A new global database of δ98Mo in molybdenites: A literature review and new data. Journal of Geochemical Exploration 161, 1–15. https://doi.org/10.1016/j.gexplo.2015.07.019
(−0.04 ‰ and +0.04 ‰, respectively), in agreement with the direction of isotopic fractionation in the experiments. The isotopic difference between UCC silicic rocks and UCC magmatic-hydrothermal MoS2 averages is however smaller than suggested by the experimental Δ98/95Mofluid-melt values. In the experiments, equilibrated melts are up to 0.4 ‰ heavier than associated supercritical fluids, and while this will need to be confirmed in future experiments, the negative correlation between Δ98/95Mofluid-melt and ASI suggests that even greater values could apply during exsolution from more peraluminous melts, such as those of the plutons dominating the UCC. While other factors are possible, this smaller isotopic difference could simply be the consequence of the timing of fluid exsolution relative to mineral fractionation in silicic magma chambers. Most recent studies suggest that long lived silicic magma reservoirs are largely crystallised between recharge events (e.g., Schmitt et al., 2010Schmitt, A.K., Stockli, D.F., Lindsay, J.M., Robertson, R., Lovera, O.M., Kislitsyn, R. (2010) Episodic growth and homogenization of plutonic roots in arc volcanoes from combined U–Th and (U–Th)/He zircon dating. Earth and Planetary Science Letters 295, 91–103. https://doi.org/10.1016/j.epsl.2010.03.028
). Hence, over the lifespan of a silicic magma body, most exsolved fluids will equilibrate with interstitial melts of crystal mushes. These interstitial melts are expected to be enriched in Mo, since it is an incompatible element. More importantly, based on the Mo coordination (octahedral) in all minerals capable of carrying significant amount of Mo in the mush (Ti-bearing oxides, biotite, amphibole, K feldspar; Greaney et al., 2018Greaney, A.T., Rudnick, R.L., Gaschnig, R.M., Whalen, J.B., Luais, B., Clemens, J.D. (2018) Geochemistry of molybdenum in the continental crust. Geochimica et Cosmochimica Acta 238, 36–54. https://doi.org/10.1016/j.gca.2018.06.039
), interstitial melts (in which Mo is tetrahedral) are most likely isotopically heavier than the bulk mush. Exsolved fluids in equilibrium with these melts should therefore be heavier than hypothetical fluids in equilibrium with bulk mushes or fully crystallised equivalents. This would explain the smaller isotopic difference between UCC silicic rocks and UCC MoS2 averages, than expected based on the experiments herein.Overall, our results provide a solution for the discrepancy between the UCC δ98/95Mo estimate derived from exposed igneous UCC rocks and constraints obtained from average UCC MoS2. They also stress the lack of straight forward assessment of the UCC δ98/95Mo via MoS2 and suggest that UCC δ98/95Mo derived from igneous rock compositions should be considered as a maximum value.
top
Acknowledgements
We thank Horst Marschall for the editorial handling of the manuscript and Alex McCoy-West and an anonymous reviewer for constructive reviews. This work was supported by the German Research Foundation grants BE 6670/1-1 and BE 6670/2-1 (RB) and Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan) CUG230610 (HG).
Editor: Horst R. Marschall
top
References
Barling, J., Arnold, G.L., Anbar, A.D. (2001) Natural mass-dependent variations in the isotopic composition of molybdenum. Earth and Planetary Science Letters 193, 447–457. https://doi.org/10.1016/S0012-821X(01)00514-3
 Show in context
Show in context It was initially thought that isotopic fractionation would be minor in high temperature systems and that, MoS2 could therefore represent the isotopic composition of their crustal source rocks (e.g., Barling et al., 2001).
View in article
Bodnar, R.J., Burnham, C.W., Sterner, S.M. (1985) Synthetic fluid inclusions in natural quartz. III. Determination of phase equilibrium properties in the system H2O-NaCl to 1000°C and 1500 bars. Geochimica et Cosmochimica Acta 49, 1861–1873. https://doi.org/10.1016/0016-7037(85)90081-X
 Show in context
Show in context There, MoS2 crystallises from both brines and low salinity vapours that unmix from supercritical fluids once they reach a miscibility gap in the NaCl-H2O system during ascent in the shallowest part of magmatic-hydrothermal complexes (typically <140 MPa and 400–700 °C; e.g., Bodnar et al., 1985).
View in article
Borg, S., Liu, W., Etschmann, B., Tian, Y., Brugger, J. (2012) An XAS study of molybdenum speciation in hydrothermal chloride solutions from 25–385 °C and 600 bar. Geochimica et Cosmochimica Acta 92, 292–307. https://doi.org/10.1016/j.gca.2012.06.001
 Show in context
Show in context This is because Mo coordination in silicate melts is tetrahedral, while both tetrahedral and octahedral coordination have been inferred for Mo in magmatic-hydrothermal fluids (e.g., Borg et al., 2012).
View in article
Borg et al. (2012), based on experiments performed at lower temperatures and pressures (up to 385 °C, 60 MPa), found that octahedral species were becoming predominant in increasingly acidic solutions (pH < 5), with species such as molybdic acid and chloro-oxo Mo complexes.
View in article
Breillat, N., Guerrot, C., Marcoux, E., Négrel, Ph. (2016) A new global database of δ98Mo in molybdenites: A literature review and new data. Journal of Geochemical Exploration 161, 1–15. https://doi.org/10.1016/j.gexplo.2015.07.019
 Show in context
Show in context This, however, is at odds with a recent Phanerozoic UCC composition derived from igneous rock compositions (δ98/95Mo = +0.14 ± 0.07 ‰; Yang et al., 2017), since the latter is visibly heavier than the most recent MoS2 δ98/95Mo averages (+0.04 ‰ in Breillat et al., 2016; −0.04 ‰ in Willbold and Elliott, 2017).
View in article
Given that most MoS2 measured thus far have crystallised from fluids exsolved from silicic magmas or melts highly enriched in those fluids (Breillat et al., 2016), a preferential enrichment in light Mo isotopes in the fluids could explain the lighter δ98/95Mo of MoS2 averages, compared to average silicic rocks (δ98/95Mo = +0.16 ‰ in Yang et al., 2017) and the associated discrepancies in UCC constraints.
View in article
The average UCC silicic rock composition (δ98/95Mo = +0.16 ‰) of Yang et al. (2017) is indeed ∼0.18 ‰ and ∼0.12 ‰ heavier than the two most extensive and recent MoS2 global averages of Willbold and Elliott (2017) and Breillat et al. (2016) (−0.04 ‰ and +0.04 ‰, respectively), in agreement with the direction of isotopic fractionation in the experiments.
View in article
Burnham, C.W. (1979) Magmas and hydrothermal fluids. In: Barnes, H.L. (Ed.) Geochemistry of Hydrothermal Ore Deposits. Second Edition, Wiley, New York, 71–136.
 Show in context
Show in context At such conditions, a single magmatic fluid phase with low to intermediate salinity (e.g., 1–5 wt. % NaCl in Rusk et al., 2004), a supercritical fluid, exsolves from magmas (Burnham, 1979).
View in article
Candela, P.A., Holland, H.D. (1984) The partitioning of copper and molybdenum between silicate melts and aqueous fluids. Geochimica et Cosmochimica Acta 48, 373–380. https://doi.org/10.1016/0016-7037(84)90257-6
 Show in context
Show in context Significant Mo partitioning in magmatic fluids is also supported by a large number of experimental (e.g., Candela and Holland, 1984) and empirical (e.g., Zajacz et al., 2008) studies.
View in article
Edmonds, M., Woods, A.W. (2018) Exsolved volatiles in magma reservoirs. Journal of Volcanology and Geothermal Research 368, 13–30. https://doi.org/10.1016/j.jvolgeores.2018.10.018
 Show in context
Show in context Slow fluid exsolution associated with the cooling and crystallisation of the melt is punctuated by repetitive fast exsolution events associated with decompression of incoming recharging melt (e.g., Edmonds and Woods, 2018).
View in article
Fang, J., Audétat, A. (2022) The effects of pressure, fO2, fS2 and melt composition on the fluid–melt partitioning of Mo: Implications for the Mo-mineralization potential of upper crustal granitic magmas. Geochimica et Cosmochimica Acta 336, 1–14. https://doi.org/10.1016/j.gca.2022.08.016
 Show in context
Show in context At equilibrium, Dfluid-melt range from 0.27 to 0.60 and overlap the literature range for similar experiments (e.g., compilation in Fang and Audétat, 2022).
View in article
However, the range of published Dfluid-melt for similar experiments is large and includes significantly higher values (e.g., compilation in Fang and Audétat, 2022).
View in article
Greaney, A.T., Rudnick, R.L., Gaschnig, R.M., Whalen, J.B., Luais, B., Clemens, J.D. (2018) Geochemistry of molybdenum in the continental crust. Geochimica et Cosmochimica Acta 238, 36–54. https://doi.org/10.1016/j.gca.2018.06.039
 Show in context
Show in context The dominant igneous rock types in the UCC are plutonic silicic rocks, and these lithologies were shown to be depleted in Mo (20–90 % depletion; mean 60 %) compared to fluid immobile elements of similar incompatibility (e.g., Ce and Pr; Greaney et al., 2018).
View in article
In some plutonic suites, correlations between Mo and fluid soluble elements exist, suggesting fluid exsolution as a dominant control over the Mo depletions observed (Greaney et al., 2018).
View in article
The Mo depletion of these plutons (e.g., Greaney et al., 2018), together with the large number of experimental constraints, suggests that a significant Mo fraction must have been removed from their precursor melts via exsolved fluids.
View in article
More importantly, based on the Mo coordination (octahedral) in all minerals capable of carrying significant amount of Mo in the mush (Ti-bearing oxides, biotite, amphibole, K feldspar; Greaney et al., 2018), interstitial melts (in which Mo is tetrahedral) are most likely isotopically heavier than the bulk mush.
View in article
Greaney, A.T., Rudnick, R.L., Romaniello, S.J., Johnson, A.C., Gaschnig, R.M., Anbar, A.D. (2020) Molybdenum isotope fractionation in glacial diamictites tracks the onset of oxidative weathering of the continental crust. Earth and Planetary Science Letters 534, 116083. https://doi.org/10.1016/j.epsl.2020.116083
 Show in context
Show in context While an estimate for the Mo stable isotope composition of the UCC created prior to the Great Oxidation Event (GOE; ∼2.4–2.2 Ga) exists (δ98/95Mo = +0.03 ‰; Greaney et al., 2020), constraints on post-GOE UCC δ98/95Mo are scarce and conflicting.
View in article
These properties, for instance, prevent the usage of fine grained clastic sedimentary rocks for that purpose (e.g., Greaney et al., 2020).
View in article
Greber, N.D., Pettke, T., Nägler, T.F. (2014) Magmatic–hydrothermal molybdenum isotope fractionation and its relevance to the igneous crustal signature. Lithos 190–191, 104–110. https://doi.org/10.1016/j.lithos.2013.11.006
 Show in context
Show in context Nevertheless, because these controls were inferred to result in progressively heavier MoS2, a global average for MoS2 δ98/95Mo was suggested to represent a maximum value for Phanerozoic UCC (Greber et al., 2014).
View in article
Whether isotopic fractionation is associated with this process remains unclear, with only one empirical study suggesting a possible enrichment of heavy Mo isotopes in fluids in a Mo porphyry deposit (Questa, USA; Greber et al., 2014).
View in article
However, the porphyry dike is altered and may not derive from the same magma as that from which the fluids of the mineralisation are derived (Greber et al., 2014 and references therein).
View in article
Hannah, J.L., Stein, H.J., Wieser, M.E., de Laeter, J.R., Varner, M.D. (2007) Molybdenum isotope variations in molybdenite: Vapor transport and Rayleigh fractionation of Mo. Geology 35, 703–706. https://doi.org/10.1130/G23538A.1
 Show in context
Show in context As studies multiplied, it became clear that the controls on Mo isotopes in hydrothermal systems were more complex than initially thought (e.g., Hannah et al., 2007).
View in article
Huber, C., Townsend, M., Degruyter, W., Bachmann, O. (2019) Optimal depth of subvolcanic magma chamber growth controlled by volatiles and crust rheology. Nature Geoscience 12, 762–768. https://doi.org/10.1038/s41561-019-0415-6
 Show in context
Show in context The experiments were equilibrated at 200 MPa, a pressure relevant for most long lived magma chambers (e.g., Huber et al., 2019).
View in article
Most large and long lived silicic magma chambers are located at upper crustal levels corresponding to lithostatic pressures of ∼200 MPa (Huber et al., 2019).
View in article
Jiang, Z., Shang, L., Guo, H., Wang, X.-s., Chen, C., Zhou, Y. (2021) An experimental investigation into the partition of Mo between aqueous fluids and felsic melts: Implications for the genesis of porphyry Mo ore deposits. Ore Geology Reviews 134, 104144. https://doi.org/10.1016/j.oregeorev.2021.104144
 Show in context
Show in context In similar experiments, chemical equilibrium is typically reached within a maximum of 10 days (e.g., Jiang et al., 2021 and references therein).
View in article
This is consistent with chemical equilibrium time scales observed in similar experimental studies (e.g., Jiang et al., 2021 and references therein).
View in article
Kaufmann, A.K.C., Pettke, T., Wille, M. (2021) Molybdenum isotope fractionation at upper-crustal magmatic-hydrothermal conditions. Chemical Geology 578, 120319. https://doi.org/10.1016/j.chemgeo.2021.120319
 Show in context
Show in context However, thermodynamic and empirical evidence suggests that the valence state of Mo should be similar in silicate melts and associated magmatic-hydrothermal fluids, i.e. hexavalent (e.g., Kaufmann et al., 2021; Willbold and Elliott, 2017 and references therein).
View in article
McCoy-West, A.J., Chowdhury, P., Burton, K.W., Sossi, P., Nowell, G.M., Fitton, J.G., Kerr, A.C., Cawood, P.A., Williams, H.M. (2019) Extensive crustal extraction in Earth’s early history inferred from molybdenum isotopes. Nature Geoscience 12, 946–951. https://doi.org/10.1038/s41561-019-0451-2
 Show in context
Show in context The Mo stable isotopic system is a very promising tool to explore both the chemical evolution of the silicate Earth (e.g., McCoy-West et al., 2019) and the palaeo-redox conditions of oceans (e.g., Wille et al., 2007).
View in article
Rusk, B.G., Reed, M.H., Dilles, J.H., Klemm, L.M., Heinrich, C.A. (2004) Compositions of magmatic hydrothermal fluids determined by LA-ICP-MS of fluid inclusions from the porphyry copper–molybdenum deposit at Butte, MT. Chemical Geology 210, 173–199. https://doi.org/10.1016/j.chemgeo.2004.06.011
 Show in context
Show in context At such conditions, a single magmatic fluid phase with low to intermediate salinity (e.g., 1–5 wt. % NaCl in Rusk et al., 2004), a supercritical fluid, exsolves from magmas (Burnham, 1979).
View in article
Schmitt, A.K., Stockli, D.F., Lindsay, J.M., Robertson, R., Lovera, O.M., Kislitsyn, R. (2010) Episodic growth and homogenization of plutonic roots in arc volcanoes from combined U–Th and (U–Th)/He zircon dating. Earth and Planetary Science Letters 295, 91–103. https://doi.org/10.1016/j.epsl.2010.03.028
 Show in context
Show in context Most recent studies suggest that long lived silicic magma reservoirs are largely crystallised between recharge events (e.g., Schmitt et al., 2010).
View in article
Tattitch, B.C., Blundy, J.D. (2017) Cu-Mo partitioning between felsic melts and saline-aqueous fluids as a function of XNaCleq, fO2, and fS2. American Mineralogist 102, 1987–2006. https://doi.org/10.2138/am-2017-5998
 Show in context
Show in context Some studies suggested that Mo dominantly occurs as Na-K molybdate, monochloride or thio-molybdate at modest salinity, and as Mo-oxy-hydroxy complexes at low salinity (Zhang et al., 2012; Tattitch and Blundy, 2017).
View in article
Ulrich, T., Mavrogenes, J. (2008) An experimental study of the solubility of molybdenum in H2O and KCl–H2O solutions from 500 °C to 800 °C, and 150 to 300 MPa. Geochimica et Cosmochimica Acta 72, 2316–2330. https://doi.org/10.1016/j.gca.2008.02.014
 Show in context
Show in context For instance, Ulrich and Mavrogenes (2008) suggested the presence of a chloro-oxo Mo (VI) complex in high salinity (>20 % KCl) fluids (at 490 °C and ≥150 MPa).
View in article
They also showed that increasing temperature favoured the formation of oxo-chloro complexes and suggested that these could be responsible for Mo transport in less acidic solutions at higher temperature (e.g., 700 °C), as proposed by Ulrich and Mavrogenes (2008).
View in article
Urey, H.C. (1947) The Thermodynamic Properties of Isotopic Substances. Journal of the Chemical Society (Resumed) 1947, 562–581. https://doi.org/10.1039/jr9470000562
 Show in context
Show in context In theory, both a difference in coordination and valence state of Mo between fluid and melt could induce the isotopic fractionation observed, i.e. higher coordination and/or lower valence state of Mo in the fluid than in the melt (e.g., Urey, 1947).
View in article
Wedepohl, K.H. (1995) The composition of the continental crust. Geochimica et Cosmochimica Acta 59, 1217–1232. https://doi.org/10.1016/0016-7037(95)00038-2
 Show in context
Show in context Our data indicate that the extraction of these exsolved fluids likely results in the removal of light Mo from magma bodies, including precursor bodies of the silicic plutonic rocks making ∼50 % of the UCC (e.g., Wedepohl, 1995).
View in article
Since UCC silicic plutonic rocks are dominantly peraluminous (ASI ≈ 1.25; Wedepohl, 1995), our experiments suggest that resolvable Δ98/95Mofluid-melt likely applied during the devolatilisation of their precursor melt.
View in article
Willbold, M., Elliott, T. (2017) Molybdenum isotope variations in magmatic rocks. Chemical Geology 449, 253–268. https://doi.org/10.1016/j.chemgeo.2016.12.011
 Show in context
Show in context This, however, is at odds with a recent Phanerozoic UCC composition derived from igneous rock compositions (δ98/95Mo = +0.14 ± 0.07 ‰; Yang et al., 2017), since the latter is visibly heavier than the most recent MoS2 δ98/95Mo averages (+0.04 ‰ in Breillat et al., 2016; −0.04 ‰ in Willbold and Elliott, 2017).
View in article
However, thermodynamic and empirical evidence suggests that the valence state of Mo should be similar in silicate melts and associated magmatic-hydrothermal fluids, i.e. hexavalent (e.g., Kaufmann et al., 2021; Willbold and Elliott, 2017 and references therein).
View in article
The average UCC silicic rock composition (δ98/95Mo = +0.16 ‰) of Yang et al. (2017) is indeed ∼0.18 ‰ and ∼0.12 ‰ heavier than the two most extensive and recent MoS2 global averages of Willbold and Elliott (2017) and Breillat et al. (2016) (−0.04 ‰ and +0.04 ‰, respectively), in agreement with the direction of isotopic fractionation in the experiments.
View in article
Wille, M., Kramers, J.D., Nägler, T.F., Beukes, N.J., Schröder, S., Meisel, Th., Lacassie, J.P., Voegelin, A.R. (2007) Evidence for a gradual rise of oxygen between 2.6 and 2.5 Ga from Mo isotopes and Re-PGE signatures in shales. Geochimica et Cosmochimica Acta 71, 2417–2435. https://doi.org/10.1016/j.gca.2007.02.019
 Show in context
Show in context The Mo stable isotopic system is a very promising tool to explore both the chemical evolution of the silicate Earth (e.g., McCoy-West et al., 2019) and the palaeo-redox conditions of oceans (e.g., Wille et al., 2007).
View in article
Yang, J., Barling, J., Siebert, C., Fietzke, J., Stephens, E., Halliday, A.N. (2017) The molybdenum isotopic compositions of I-, S- and A-type granitic suites. Geochimica et Cosmochimica Acta 205, 168–186. https://doi.org/10.1016/j.gca.2017.01.027
 Show in context
Show in context This, however, is at odds with a recent Phanerozoic UCC composition derived from igneous rock compositions (δ98/95Mo = +0.14 ± 0.07 ‰; Yang et al., 2017), since the latter is visibly heavier than the most recent MoS2 δ98/95Mo averages (+0.04 ‰ in Breillat et al., 2016; −0.04 ‰ in Willbold and Elliott, 2017).
View in article
Given that most MoS2 measured thus far have crystallised from fluids exsolved from silicic magmas or melts highly enriched in those fluids (Breillat et al., 2016), a preferential enrichment in light Mo isotopes in the fluids could explain the lighter δ98/95Mo of MoS2 averages, compared to average silicic rocks (δ98/95Mo = +0.16 ‰ in Yang et al., 2017) and the associated discrepancies in UCC constraints.
View in article
It is therefore best to consider the average δ98/95Mo of UCC silicic plutonic rocks (Yang et al., 2017) as a maximum value for the signature of the total Mo contribution to the UCC from undegassed silicic magmatism.
View in article
The average UCC silicic rock composition (δ98/95Mo = +0.16 ‰) of Yang et al. (2017) is indeed ∼0.18 ‰ and ∼0.12 ‰ heavier than the two most extensive and recent MoS2 global averages of Willbold and Elliott (2017) and Breillat et al. (2016) (−0.04 ‰ and +0.04 ‰, respectively), in agreement with the direction of isotopic fractionation in the experiments.
View in article
Zajacz, Z., Halter, W.E., Pettke, T., Guillong, M. (2008) Determination of fluid/melt partition coefficients by LA-ICPMS analysis of co-existing fluid and silicate melt inclusions: Controls on element partitioning. Geochimica et Cosmochimica Acta 72, 2169–2197. https://doi.org/10.1016/j.gca.2008.01.034
 Show in context
Show in context Significant Mo partitioning in magmatic fluids is also supported by a large number of experimental (e.g., Candela and Holland, 1984) and empirical (e.g., Zajacz et al., 2008) studies.
View in article
Zhang, L., Audétat, A., Dolejš, D. (2012) Solubility of molybdenite (MoS2) in aqueous fluids at 600–800 °C, 200 MPa: A synthetic fluid inclusion study. Geochimica et Cosmochimica Acta 77, 175–185. https://doi.org/10.1016/j.gca.2011.11.015
 Show in context
Show in context Some studies suggested that Mo dominantly occurs as Na-K molybdate, monochloride or thio-molybdate at modest salinity, and as Mo-oxy-hydroxy complexes at low salinity (Zhang et al., 2012; Tattitch and Blundy, 2017).
View in article
top
Supplementary Information
The Supplementary Information includes:
Download the Supplementary Information (PDF)
Figures
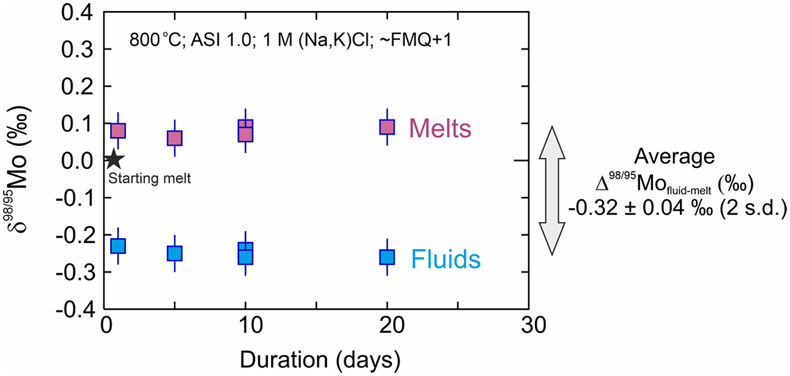
Figure 1 Mo isotopic composition (δ98/95Mo) of fluids and melts versus time. All experiments are identical except for their duration (days). Two experiments were performed for the 10 day duration. The average Δ98/95Mofluid-melt (‰) for the 5 experiments is also shown.
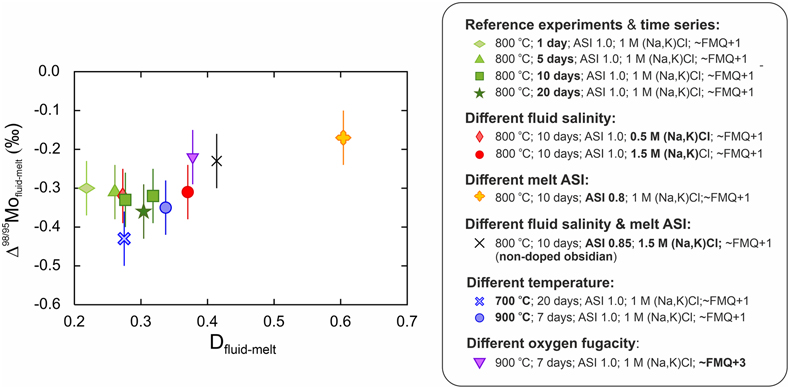
Figure 2 Mo isotope fractionation value between fluid and melt (Δ98/95Mofluid-melt) versus the partition coefficient (Dfluid-melt = Cfluid/Cmelt) for each experiment.
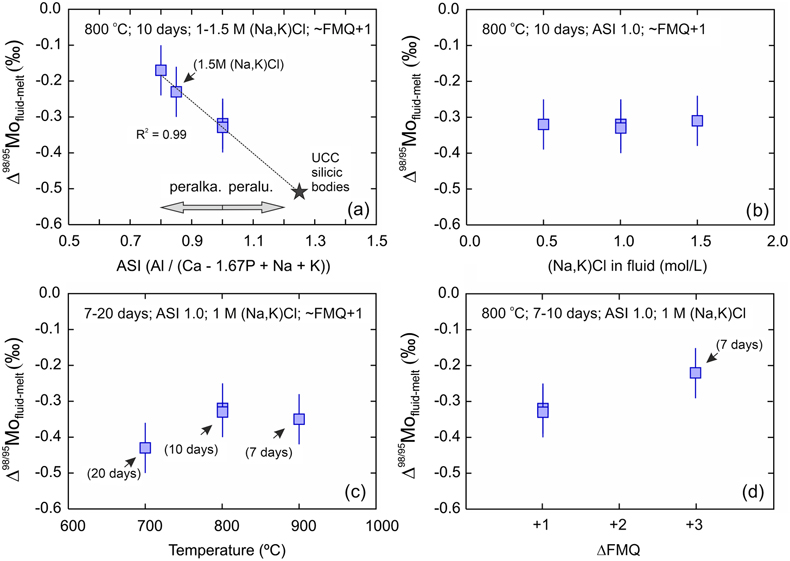
Figure 3 Evolution of the Mo isotope fractionation value between fluids and melts (Δ98/95Mofluid-melt) at different experimental (a) melt aluminium saturation indexes (ASI = Al/(Ca − 1.67P + Na + K)), (b) fluid salinities, (c) temperatures and (d) oxygen fugacities (fO2 relative to the fayalite-magnetite-quartz buffer, FMQ). Abbreviations: peralka., peralkaline; peralu., peraluminous.