Vivianite-parasymplesite solid solution: A sink for arsenic in ferruginous environments?
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




Article views:387Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
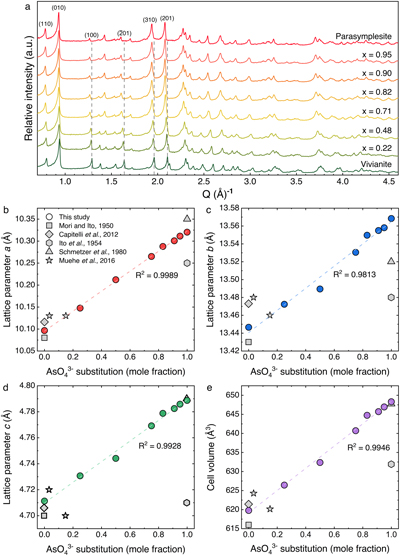 Figure 1 (a) XRD patterns of As-substituted vivianites (x = mole fraction of substituted AsO43−) aged for 24 hr, plotted in Q-space (Q = 2π/dhkl). Dashed grey lines indicate displacement in selected lattice planes in vivianite due to AsO43− substitution for PO43−. Full XRD patterns can be found in Figure S-2. Variations in lattice parameter values (b–d) and cell volumes (e) of our As-vivianites (coloured circles) compared to literature (Mori and Ito, 1950; Ito et al., 1954; Schmetzer et al., 1980; Capitelli et al., 2012; Muehe et al., 2016) shown as grey symbols. Dashed lines represent linear relationships as a function of As(V) substitution. | 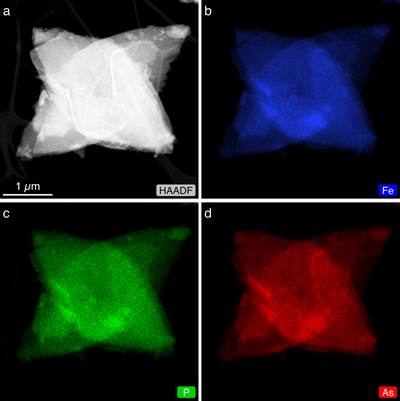 Figure 2 (a) HAADF-STEM image of As-substituted vivianite (x = 0.48) and corresponding EDX maps: (b) Fe (blue); (c) P (green); and (d) As (red). | 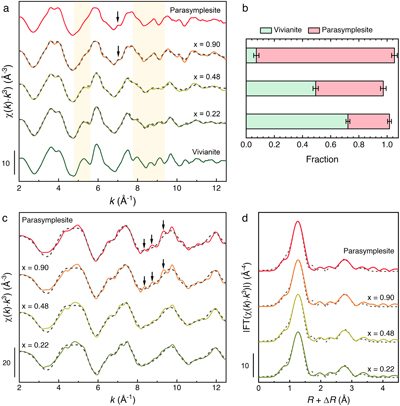 Figure 3 (a) Fe K-edge k3-weighted EXAFS spectra of As-vivianites. Dashed grey lines denote (b) LCF of Fe K-edge EXAFS spectra of mineral end members (i.e. vivianite, parasymplesite). (c) As K-edge k3-weighted EXAFS spectra of As vivianites and (d) corresponding Fourier transform magnitude. Dashed grey lines denote shell-by-shell fits. | 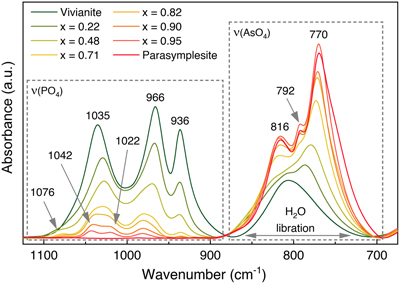 Figure 4 FTIR spectra of As(V)-substituted vivianites showing stretching regions for phosphate [ν(PO4)] and arsenate [ν(AsO4)], and H2O libration vibrations. Full spectra and band assignments can be found in Figures S-10 and Table S-8, respectively. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Phosphorus is an essential nutrient that controls primary productivity in terrestrial and aquatic systems. Retention of phosphorus is strongly dependent on biomass uptake, mineral co-precipitation, or sorption onto mineral surfaces (Filippelli, 2002
Filippelli, G.M. (2002) The Global Phosphorus Cycle. In: Kohn, M.J., Rakovan, J., Hughes, J.M. (Eds.) Phosphates: Geochemical, Geobiological, and Materials Importance. Reviews in Mineralogy and Geochemistry 48, 391–425. https://doi.org/10.2138/rmg.2002.48.10
). In anoxic soils and sediments, reductive dissolution of Fe(III) (oxyhydr)oxides results in the release of dissolved phosphate (PO43−) and Fe2+ (Patrick and Khalid, 1974Patrick Jr., W.H., Khalid, R.A. (1974) Phosphate Release and Sorption by Soils and Sediments: Effect of Aerobic and Anaerobic Conditions. Science 186, 53–55. https://doi.org/10.1126/science.186.4158.53
), accompanied by increase to near neutral pH due to H+ consumption (Walpersdorf et al., 2013Walpersdorf, E., Bender Koch, C., Heiberg, L., O’Connell, D.W., Kjaergaard, C., Bruun Hansen, H.C. (2013) Does vivianite control phosphate solubility in anoxic meadow soils? Geoderma 193–194, 189–199. https://doi.org/10.1016/j.geoderma.2012.10.003
). Under anoxic, non-sulfidic (i.e. ferruginuous) conditions, high dissolved Fe2+ and PO43− may lead to vivianite [FeII3(PO4)2 · 8 H2O] (Heiberg et al., 2012Heiberg, L., Koch, C.B., Kjaergaard, C., Jensen, H.S., Hansen, H.C.B. (2012) Vivianite Precipitation and Phosphate Sorption following Iron Reduction in Anoxic Soils. Journal of Environmental Quality 41, 938–949. https://doi.org/10.2134/jeq2011.0067
) precipitation at near neutral pH. Vivianite can also form in aquatic ferruginous environments such as marine and lacustrine sediments and lakes (Egger et al., 2015Egger, M., Jilbert, T., Behrends, T., Rivard, C., Slomp, C.P. (2015) Vivianite is a major sink for phosphorus in methanogenic coastal surface sediments. Geochimica et Cosmochimica Acta 169, 217–235. https://doi.org/10.1016/j.gca.2015.09.012
; Vuillemin et al., 2020Vuillemin, A., Friese, A., Wirth, R., Schuessler, J.A., Schleicher, A.M., Kemnitz, H., Lücke, A., Bauer, K.W., Nomosatryo, S., von Blanckenburg, F., Simister, R., Ordoñez, L.G., Ariztegui, D., Henny, C., Russell, J.M., Bijaksana, S., Vogel, H., Crowe, S.A., Kallmeyer, J., the Towuti Drilling Project Science team (2020) Vivianite formation in ferruginous sediments from Lake Towuti, Indonesia. Biogeosciences 17, 1955–1973. https://doi.org/10.5194/bg-17-1955-2020
), and has been suggested as a potential phosphate sink in early Earth oceans (Hao et al., 2020Hao, J., Knoll, A.H., Huang, F., Schieber, J., Hazen, R.M., Daniel, I. (2020) Cycling phosphorus on the Archean Earth: Part II. Phosphorus limitation on primary production in Archean ecosystems. Geochimica et Cosmochimica Acta 280, 360–377. https://doi.org/10.1016/j.gca.2020.04.005
), affecting trace element cycling and nutrient availability.Vivianite is the Fe(II) phosphate end member of the vivianite mineral group (monoclinic C2/m), represented by the general formula M3(YO4)2 · 8 H2O, where M refers to divalent cation(s) (e.g., Fe(II), Mg, Mn) and Y can be either P or As. Vivianite is a poorly soluble mineral (pKsp ≈ 35.8 ± 0.08 at 25 °C; Al-Borno and Tomson, 1994
Al-Borno, A., Tomson, M.B. (1994) The temperature dependence of the solubility product constant of vivianite. Geochimica et Cosmochimica Acta 58, 5373–5378. https://doi.org/10.1016/0016-7037(94)90236-4
) that is stable under reducing and circum-neutral pH (6–9) conditions (Nriagu, 1972Nriagu, J.O. (1972) Stability of vivianite and ion-pair formation in the system Fe3(PO4)2-H3PO4-H2O. Geochimica et Cosmochimica Acta 36, 459–470. https://doi.org/10.1016/0016-7037(72)90035-X
). Its structure has two crystallographically and chemically distinct Fe sites coordinated with phosphate (Fig. S-1): (i) a single octahedral Fe1 site [FeO2(H2O)4)], and (ii) two edge sharing octahedral Fe2 sites [Fe2O6(H2O)4] (Mori and Ito, 1950Mori, H., Ito, T. (1950) The Structure of Vivianite and Symplesite. Acta Crystallographica 3, 1–6. https://doi.org/10.1107/S0365110X5000001X
; Bartl, 1989Bartl, H. (1989) Water of crystallization and its hydrogen-bonded crosslinking in vivianite Fe3(PO4)2 · 8H2O; a neutron diffraction investigation. Fresenius’ Zeitschrift für analytische Chemie 333, 401–403. https://doi.org/10.1007/BF00572335
). These single and double edge sharing Fe octahedra are connected by tetrahedral phosphate to form layers in (010), interconnected by hydrogen bonds between lattice water molecules.Isomorphic substitution of Fe2+ by divalent cations (e.g., Mg, Mn) occurs frequently in natural vivianites, and although rarely documented, anions like arsenate (AsO43−) can also replace PO43− in the crystal structure (Muehe et al., 2016
Muehe, E.M., Morin, G., Scheer, L., Le Pape, P., Esteve, I., Daus, B., Kappler, A. (2016) Arsenic(V) Incorporation in Vivianite during Microbial Reduction of Arsenic(V)-Bearing Biogenic Fe(III) (Oxyhydr)oxides. Environmental Science & Technology 50, 2281–2291. https://doi.org/10.1021/acs.est.5b04625
). Anion substitution is possible because of chemical and structural similarities between phosphate and arsenate (i.e. geometry, size, pKa). Full substitution of phosphate for arsenate results in a new phase, parasymplesite [FeII3(AsO4)2 · 8 H2O]. Contrary to vivianite, parasymplesite is usually found in oxidised zones of As-rich hydrothermal mineral deposits (Anthony et al., 2000Anthony, J.W., Bideaux, R.A., Bladh, K.W., Nichols, M.C. (2000) Handbook of Mineralogy Volume IV: Arsenates, Phosphates and Vanadates. Mineral Data Publishing, Tucson, Arizona, USA.
). Johnston and Singer (2007)Johnston, R.B., Singer, P.C. (2007) Solubility of Symplesite (Ferrous Arsenate): Implications for Reduced Groundwaters and Other Geochemical Environments. Soil Science Society of America Journal 71, 101–107. https://doi.org/10.2136/sssaj2006.0023
, however, predicted that parasymplesite can form in near neutral pH, reduced As-impacted groundwater (e.g., Bangladesh), yet it has so far not been reported. As(V)-substituted vivianite (∼15 mol %; hereafter referred as As-vivianite) have been observed during microbial reduction of As(V)-bearing Fe(III) (oxyhydr)oxides (Muehe et al., 2016Muehe, E.M., Morin, G., Scheer, L., Le Pape, P., Esteve, I., Daus, B., Kappler, A. (2016) Arsenic(V) Incorporation in Vivianite during Microbial Reduction of Arsenic(V)-Bearing Biogenic Fe(III) (Oxyhydr)oxides. Environmental Science & Technology 50, 2281–2291. https://doi.org/10.1021/acs.est.5b04625
), and such high As contents suggest a possible vivianite-parasymplesite solid solution. Thus, it is possible that As-vivianites are unrecognised possible host minerals and sinks for As in many anoxic environments.To test this, we synthesised As-vivianites (Fe3[(PO4)1−x(AsO4)x]2 · 8 H2O), with increasing degrees of AsO43− substitution (0.22 ≤ x ≤ 0.95), under anoxic and near neutral pH conditions. Our results will help us better understand As dynamics in oxygen limited, Fe2+-rich environments. We show that a continuous solid solution between vivianite and parasymplesite exist, and document how the structure, morphology and bonding environment of As-vivianite changes with increasing As(V) substitution. We also discuss how such a solid solution mineral system can be important in modern and ancient ferruginous, or contaminated environmental settings.
top
Materials and Methods
Co-precipitation experiments were conducted at room temperature in acid cleaned 120 mL perfluoroalkoxy (PFA) jars inside a vinyl-walled glovebox (97 % N2, 3 % H2). Stock solutions were prepared using deoxygenated ultrapure water (resistivity ∼18.2 MΩ·cm), obtained by purging with argon at 90 °C for ∼4 hr. Aliquots from stock solutions of 20 mM Na2HAsO4 and 20 mM Na2HPO4 (pH ∼ 7) were mixed with ultrapure water to achieve desired AsO43− mole fractions of 0.25, 0.50, 0.75, 0.83, 0.91 and 0.95 ([AsO43− + PO43−] = 8 mM). These were mixed with an aliquot of 0.58 M FeIISO4 stock solution to achieve an [Fe2+]/[AsO43− + PO43−] ratio of 1.5 and pH ≈ 5. Precipitation was triggered by dropwise addition of 1 M NaOH, while stirring until pH 7.1 ± 0.2 was reached. Mineral suspensions were aged for 1 and 24 hr under constant stirring. End members, vivianite (no AsO43−) and parasymplesite (no PO43−) were also synthesised under similar conditions. Solids were collected by filtration, washed with deoxygenated ultrapure water, and subsequently dried, ground and stored in the glovebox until characterisation. The solutions and solids were characterised using inductively coupled plasma optical emission spectrometry (ICP-OES), powder X-ray diffraction (XRD), scanning and transmission electron microscopy (S/TEM), synchrotron-based X-ray absorption spectroscopy (XAS) and Fourier transform infrared (FTIR) spectroscopy. Detailed information on all characterisation methods and geochemical modelling can be found in Supplementary Text S-2.
top
Results and Discussion
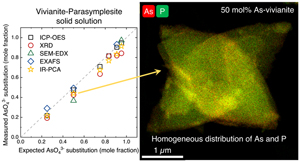
Structure and composition of As-vivianites. The precipitates after 1 and 24 hr of aging were highly crystalline (Figs. 1a, S-2). All Bragg reflections could be assigned to vivianite or parasymplesite with no observable peak splitting, indicating homogeneity of the precipitates. No other crystalline Fe-P or Fe-As phases were present, and the similarity in the backgrounds in all patterns also suggest the lack of any amorphous phase contribution. The XRD results agree with our geochemical calculations (see saturation indices in Table S-2), which showed that vivianite and parasymplesite are the only thermodynamically stable phases in our system, irrespective of initial P:As ratios. The Eh-pH diagram of the Fe-P-As-H2O system (Fig. S-4a) also clearly showed the overlapping predominance fields of vivianite and parasymplesite, and is well supported by the calculated mineral stability of the vivianite-parasymplesite solid solution (Fig. S-4b). Following the changes in concentration in the reacting solutions, it is clear that after 1 hr of aging, As and P removal was between 95 to 99 %, while Fe was less efficiently removed (80 to 96 %), revealing that the precipitated solids scavenged the phosphate and arsenate at a similar extent (Table S-3). The similarities and lack of differences between the 1 hr and aged solid phases led us to continue with the solid characterisations only on the 24 hr solids.

Figure 1 (a) XRD patterns of As-substituted vivianites (x = mole fraction of substituted AsO43−) aged for 24 hr, plotted in Q-space (Q = 2π/dhkl). Dashed grey lines indicate displacement in selected lattice planes in vivianite due to AsO43− substitution for PO43−. Full XRD patterns can be found in Figure S-2. Variations in lattice parameter values (b–d) and cell volumes (e) of our As-vivianites (coloured circles) compared to literature (Mori and Ito, 1950
Mori, H., Ito, T. (1950) The Structure of Vivianite and Symplesite. Acta Crystallographica 3, 1–6. https://doi.org/10.1107/S0365110X5000001X
; Ito et al., 1954Ito, T.-i., Minato, H., Sakurai, K.'i. (1954) Parasymplesite, a New Mineral Potymorphous with Symplesite. Proceedings of the Japan Academy 30, 318–324. https://doi.org/10.2183/pjab1945.30.318
; Schmetzer et al., 1980Schmetzer, K., Tremmel, G., Bartelke, W. (1980) Eine Paragenese seltener Minerale aus Bou-Azzer, Marokko: Parasymplesit, Symplesit, Schneiderhöhnit, Karibibit. Neues Jahrbuch für Mineralogie - Abhandlungen 138, 94–108.
; Capitelli et al., 2012Capitelli, F., Chita, G., Ghiara, M.R., Rossi, M. (2012) Crystal-chemical investigation of Fe3(PO4)2 · 8 H2O vivianite minerals. Zeitschrift für Kristallographie - Crystalline Materials 227, 92–101. https://doi.org/10.1524/zkri.2012.1442
; Muehe et al., 2016Muehe, E.M., Morin, G., Scheer, L., Le Pape, P., Esteve, I., Daus, B., Kappler, A. (2016) Arsenic(V) Incorporation in Vivianite during Microbial Reduction of Arsenic(V)-Bearing Biogenic Fe(III) (Oxyhydr)oxides. Environmental Science & Technology 50, 2281–2291. https://doi.org/10.1021/acs.est.5b04625
) shown as grey symbols. Dashed lines represent linear relationships as a function of As(V) substitution.The gradual transition from a phosphate to an arsenate structure could be quantified from the XRD patterns, which showed slow structural transition from vivianite to parasymplesite with increasing AsO43− mole fraction from 0.22 to 0.95 (Table S-4). These structural changes are evidenced by the displacement of the (100), (
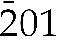 ), (310) and (201) vivianite reflections (dashed lines in Figs. 1a and S-2). As seen from the vivianite crystal structure model (Fig. S-1), these reflections correspond to lattice planes intersecting the interlinked Fe octahedra via PO43− or AsO43− tetrahedra, which expand at a higher degree of substitution because of the larger thermochemical radius of AsO43− (2.48 Å) compared to PO43− (2.38 Å) tetrahedra (Frausto da Silva and Williams, 2001
), (310) and (201) vivianite reflections (dashed lines in Figs. 1a and S-2). As seen from the vivianite crystal structure model (Fig. S-1), these reflections correspond to lattice planes intersecting the interlinked Fe octahedra via PO43− or AsO43− tetrahedra, which expand at a higher degree of substitution because of the larger thermochemical radius of AsO43− (2.48 Å) compared to PO43− (2.38 Å) tetrahedra (Frausto da Silva and Williams, 2001Frausto da Silva, J.J.R., Williams, R.J.P. (2001) The Biological Chemistry of the Elements: The Inorganic Chemistry of Life. Oxford University Press, Oxford.
). This is also reflected in the larger lattice parameters and associated cell volumes of As-vivianites (Fig. 1b–e) derived from Rietveld refinements (Table S-5, Fig. S-3), which increased linearly and suggest an isomorphous substitution following Vegard’s law (Vegard, 1921Vegard, L. (1921) Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Zeitschrift für Physik 5, 17–26. https://doi.org/10.1007/BF01349680
). Lattice parameters a, b and c increased by 1 to 2 %, while the unit cell volume expanded up to 5 % compared to parasymplesite. The lattice parameters of natural vivianites, rarely occurring parasymplesite (only two refined structures exist in literature), and biogenic As-vivianites are also in agreement with the values obtained in this study (grey coloured symbols in Fig. 1b–e); although variations arising from natural heterogeneity (e.g., partial substitution of Fe(II) sites by divalent cations, structural Fe(II) oxidation) exist in natural mineral specimens.Morphology of As-vivianites and local distribution of P and As. The synthesised As vivianites (Figs. 2a, S-5a,b) exhibited large platy crystals (between 5–10 μm in length and 2–5 μm in width) elongated along the a axis direction and often radiating from the centre, similar to As-free vivianite (Fig. S-5c). In addition, all crystals in the vivianite-parasymplesite solid solution series were extremely thin, ranging from 50 to 100 nm, indicating slow growth of (010).

Figure 2 (a) HAADF-STEM image of As-substituted vivianite (x = 0.48) and corresponding EDX maps: (b) Fe (blue); (c) P (green); and (d) As (red).
Mapping the elemental distribution of P and As in As-vivianites (e.g., x = 0.48; Fig. 2b–d) by high angular dark field (HAADF) STEM imaging coupled with energy dispersive X-ray (EDX) mapping showed homogeneously distributed P and As in the crystals and no chemical zonation, even at higher magnification (Fig. S-6).
Further increasing the AsO43− substitution even to x = 0.95, still resulted in plate-like crystals (Fig. S-5b). This is very different to the morphology of synthetic parasymplesite (i.e. As end member), which has a needle or lath-type crystal habit (0.5–5 μm in length and 0.5–2 μm in width; Fig. S-5d), indicating preferred crystal growth along the c axis. It is worth noting that synthetic As-vivianites had uneven crystal edges compared to synthetic As-free vivianite (Fig. S-5), and that vivianite and all As-vivianites, were extremely beam sensitive, degrading rapidly upon continuous exposure to electrons during imaging (e.g., Fig. S-7).
Local bonding environment and speciation of Fe and As. Distinct features in the Fe K-edge EXAFS spectra clearly showed the gradual transition of Fe local bonding environment (yellow bands in Fig. 3a) with increasing AsO43− substitution (x = 0.22, 0.48, 0.90). For example, the characteristic, prominent shoulders in the first and second oscillations at 5.2 and 8.0 Å−1 in vivianite slowly decreased in intensity as AsO43− substitution increased. Intense oscillations between 8.4 and 10 Å−1 also dampened at 48 mol % AsO43− substitution, and were replaced by a single oscillation at 90 mol % substitution as the Fe local bonding environment became more parasymplesite-like. The subtle beat feature found in parasymplesite at 7 Å−1 only appeared at 90 mol % As(V) substitution (arrows in Fig. 3a). We could not perform shell-by-shell fits on the Fe K-edge EXAFS of As-vivianites because of the lack of spatial resolution required to fit multiple atomic pair paths (i.e. Fe-As, Fe-P) in the second shell. However, linear combination fitting (LCF) results of the Fe K-edge EXAFS spectra (Fig. 3b, Table S-6), in combination with our XRD Rietveld refinement, suggest that the Fe local bonding environment gradually changed from a vivianite-like to parasymplesite-like environment as As(V) substitution in the solids increased.

Figure 3 (a) Fe K-edge k3-weighted EXAFS spectra of As-vivianites. Dashed grey lines denote (b) LCF of Fe K-edge EXAFS spectra of mineral end members (i.e. vivianite, parasymplesite). (c) As K-edge k3-weighted EXAFS spectra of As vivianites and (d) corresponding Fourier transform magnitude. Dashed grey lines denote shell-by-shell fits.
The As K-edge EXAFS spectrum of parasymplesite (topmost spectra; Fig. 3c) has pronounced shoulders in the first and second oscillations at 4.3 and 6.5 Å−1, two beat features between 8 and 9 Å−1 and a peak splitting in the third oscillation (arrows at x = 0.90 in Fig. 3c). These characteristic pronounced shoulders of parasymplesite can be seen in all As-vivianites, irrespective of degree of AsO43− substitution. However, the two beat features and peak splitting in the third oscillation (arrows in Fig. 3c) only become apparent at high AsO43− substitution (x ≥ 0.90). Nonetheless, shell-by-shell fitting (Fig. 3d, Table S-7) showed similarities in As local bonding environment in all As-vivianites and parasymplesite, even at low AsO43− substitution (x = 0.22). In general, the first neighbour contribution to the EXAFS fit correspond to the As-O atomic correlation for tetrahedral AsO43− (RAs-O = 1.69 Å); the second neighbour contribution arises from As-Fe atomic pairs of two corner-sharing linkages between AsO43− tetrahedron and two Fe octahedra (RAs-Fe1 ≈ 3.30 Å, RAs-Fe2 ≈ 3.47 Å; Fig. S-8). These fit-derived interatomic distances are in excellent agreement with crystallographic values for parasymplesite (Mori and Ito, 1950
Mori, H., Ito, T. (1950) The Structure of Vivianite and Symplesite. Acta Crystallographica 3, 1–6. https://doi.org/10.1107/S0365110X5000001X
), and are consistent with EXAFS-derived Fe-As distances in parasymplesite (Jönsson and Sherman, 2008Jönsson, J., Sherman, D.M. (2008) Sorption of As(III) and As(V) to siderite, green rust (fougerite) and magnetite: Implications for arsenic release in anoxic groundwaters. Chemical Geology 255, 173–181. https://doi.org/10.1016/j.chemgeo.2008.06.036
).More importantly, As(V) was not reduced by aqueous or structural Fe(II) during As-vivianite crystallisation. This is evident from the positions of the Fe and As K-edge X-ray absorption near edge structure (XANES) maxima of As-vivianites (x = 0.22, 0.48, 0.90) centred at 7,127 and 11,874 eV, respectively (Fig. S-9). These positions match the reference vivianite and parasymplesite, as well as other vivianites reported in literature (Miot et al., 2009
Miot, J., Benzerara, K., Morin, G., Bernard, S., Beyssac, O., Larquet, E., Kappler, A., Guyot, F. (2009) Transformation of vivianite by anaerobic nitrate-reducing iron-oxidizing bacteria. Geobiology 7, 373–384. https://doi.org/10.1111/j.1472-4669.2009.00203.x
; Muehe et al., 2016Muehe, E.M., Morin, G., Scheer, L., Le Pape, P., Esteve, I., Daus, B., Kappler, A. (2016) Arsenic(V) Incorporation in Vivianite during Microbial Reduction of Arsenic(V)-Bearing Biogenic Fe(III) (Oxyhydr)oxides. Environmental Science & Technology 50, 2281–2291. https://doi.org/10.1021/acs.est.5b04625
).Our As K-edge EXAFS results are also supported by the FTIR data (Figs. 4, S-10), which confirmed increased AsO43− substitution through gradually increasing intensities of ν(AsO4) bands between ∼885 and 700 cm−1 at the expense of ν(PO4) bands between ∼1120 and 885 cm−1 (Table S-8). The shoulder at ∼792 cm−1 [ν(AsO4)] only appeared in the spectra of As-vivianite with >71 mol % substitution. At even higher AsO43− substitution, a new band appeared at ∼1076 cm−1 and the 1035 cm−1 band split into two component bands at 1042 and 1022 cm−1, representing ν(PO4) antisymmetric stretching modes (Makreski et al., 2015
Makreski, P., Stefov, S., Pejov, L., Jovanovski, G. (2015) Theoretical and experimental study of the vibrational spectra of (para)symplesite and hornesite. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 144, 155–162. https://doi.org/10.1016/j.saa.2015.01.108
). Substitution of AsO43− in vivianite also affects the bonding environment of structural water; however, interpretation of the ν(OH) and δ(HOH) regions can be complicated (see further discussion in Supplementary Information S-2.5).
Figure 4 FTIR spectra of As(V)-substituted vivianites showing stretching regions for phosphate [ν(PO4)] and arsenate [ν(AsO4)], and H2O libration vibrations. Full spectra and band assignments can be found in Figures S-10 and Table S-8, respectively.
Naturally, the question arises whether As(V) is adsorbed to vivianite surfaces (cf. Thinnappan et al., 2008
Thinnappan, V., Merrifield, C.M., Islam, F.S., Polya, D.A., Wincott, P., Wogelius, R.A. (2008) A combined experimental study of vivianite and As (V) reactivity in the pH range 2–11. Applied Geochemistry 23, 3187–3204. https://doi.org/10.1016/j.apgeochem.2008.07.001
), as in the case of other Fe(II)-bearing minerals. The As-Fe atomic distance for As(V) adsorbed as bidentate binuclear (2C) and monodentate mononuclear (1V) inner sphere surface complexes are RAs-Fe of ∼3.4 and ∼3.5 Å, respectively (Jönsson and Sherman, 2008Jönsson, J., Sherman, D.M. (2008) Sorption of As(III) and As(V) to siderite, green rust (fougerite) and magnetite: Implications for arsenic release in anoxic groundwaters. Chemical Geology 255, 173–181. https://doi.org/10.1016/j.chemgeo.2008.06.036
; Perez et al., 2020Perez, J.P.H., Freeman, H.M., Brown, A.P., van Genuchten, C.M., Dideriksen, K., S’Ari, M., Tobler, D.J., Benning, L.G. (2020) Direct Visualization of Arsenic Binding on Green Rust Sulfate. Environmental Science & Technology 54, 3297–3305. https://doi.org/10.1021/acs.est.9b07092
), similar to our fit-derived distances for As-vivianites. We attempted to fit these two As-Fe paths to the second neighbour contribution of As-vivianites individually, but the fit yielded unrealistic CN values (i.e. negative, or extremely high CN values). To distinguish potentially adsorbed As(V) species in our As-vivianites, we reacted As(V) ([As]initial = 4 mM) with pure vivianite ([Fe]vivianite = 12 mM) at pH ∼ 7 for 24 hr, comparable to the 48 mol % substituted As-vivianite. Structurally incorporated As would have resulted in shifts in vivianite reflections (cf. Fig. 1), but the XRD pattern of As-adsorbed vivianite was equivalent to the pure vivianite (Fig. S-11a). In addition, the ν(AsO4) band in the FTIR spectrum (Fig. S-11b) of As-adsorbed vivianite only exhibited a very weak shoulder feature at ∼780 cm−1 instead of the characteristic sharp bands seen in As-vivianites and parasymplesite (cf. Fig. 4). More importantly, adsorbed As(V) would not systematically decrease the intensities of ν(PO4) bands in As-vivianites. SEM-EDX maps of As-adsorbed vivianite (Fig. S-12) also showed clear differences in As signal intensities and distribution compared to 48 mol % substituted As-vivianite (Fig. S-13). Overall, these results can be interpreted as arsenic being primarily structurally incorporated in As-vivianites, with only minor contributions from adsorbed species, if at all present.top
Geochemical Implications
In this study, we explored the structural incorporation of As(V) in vivianite under anoxic conditions. Our complementary results (Fig. S-14) document and cross correlate that AsO43− can systematically substitute for PO43− in the vivianite crystal structure, thereby forming a continuous solid solution. We have showed that up to 95 mol % (∼24 wt. %) of As(V) can be incorporated (Table S-4), with a very minor fraction of potentially adsorbed species. Sequestration of As(V) in vivianite via partial substitution for PO43− under reduced conditions is favourable because most Fe(II)-bearing minerals have far lower As uptake, and sorption mechanisms are more susceptible to remobilisation upon desorption. Compared to interactions with other common Fe(II)-bearing minerals in ferruginous environments, where arsenic is primarily adsorbed and only reaches ∼15 wt. % in magnetite (Yean et al., 2005
Yean, S., Cong, L., Yavuz, C.T., Mayo, J.T., Yu, W.W., Kan, A.T., Colvin, V.L., Tomson, M.B. (2005) Effect of magnetite particle size on adsorption and desorption of arsenite and arsenate. Journal of Materials Research 20, 3255–3264. https://doi.org/10.1557/jmr.2005.0403
) or ∼9 wt. % in green rust (Perez et al., 2019Perez, J.P.H., Freeman, H.M., Schuessler, J.A., Benning, L.G. (2019) The interfacial reactivity of arsenic species with green rust sulfate (GRSO4). Science of The Total Environment 648, 1161–1170. https://doi.org/10.1016/j.scitotenv.2018.08.163
), our current results show that arsenic incorporation into vivianite could be stable sinks for As(V) removal in ferruginous settings. For example, vivianite found in anoxic, non-sulfidic environments could incorporate major and trace elements (Vuillemin et al., 2020Vuillemin, A., Friese, A., Wirth, R., Schuessler, J.A., Schleicher, A.M., Kemnitz, H., Lücke, A., Bauer, K.W., Nomosatryo, S., von Blanckenburg, F., Simister, R., Ordoñez, L.G., Ariztegui, D., Henny, C., Russell, J.M., Bijaksana, S., Vogel, H., Crowe, S.A., Kallmeyer, J., the Towuti Drilling Project Science team (2020) Vivianite formation in ferruginous sediments from Lake Towuti, Indonesia. Biogeosciences 17, 1955–1973. https://doi.org/10.5194/bg-17-1955-2020
; Kubeneck et al., 2023Kubeneck, L.J., ThomasArrigo, L.K., Rothwell, K.A., Kaegi, R., Kretzschmar, R. (2023) Competitive incorporation of Mn and Mg in vivianite at varying salinity and effects on crystal structure and morphology. Geochimica et Cosmochimica Acta 346, 231–244. https://doi.org/10.1016/j.gca.2023.01.029
). Therefore, As-vivianites may be present, yet so far undocumented in contaminated, anoxic settings such as in Bangladesh where vivianite and parasymplesite are both saturated (Johnston and Singer, 2007Johnston, R.B., Singer, P.C. (2007) Solubility of Symplesite (Ferrous Arsenate): Implications for Reduced Groundwaters and Other Geochemical Environments. Soil Science Society of America Journal 71, 101–107. https://doi.org/10.2136/sssaj2006.0023
). However, since naturally occurring As-vivianites have yet to be found, their stability as a sink for arsenic under environmentally relevant conditions needs to be evaluated in further studies. In addition to the potential importance of As-vivianites in contaminated settings, our findings have implications for phosphorus (and arsenic) cycling since vivianite has been suggested as a P sink in Precambrian oceans (Hao et al., 2020Hao, J., Knoll, A.H., Huang, F., Schieber, J., Hazen, R.M., Daniel, I. (2020) Cycling phosphorus on the Archean Earth: Part II. Phosphorus limitation on primary production in Archean ecosystems. Geochimica et Cosmochimica Acta 280, 360–377. https://doi.org/10.1016/j.gca.2020.04.005
), and modern lake analogues (Xiong et al., 2019Xiong, Y., Guilbaud, R., Peacock, C.L., Cox, R.P., Canfield, D.E., Krom, M.D., Poulton, S.W. (2019) Phosphorus cycling in Lake Cadagno, Switzerland: A low sulfate euxinic ocean analogue. Geochimica et Cosmochimica Acta 251, 116–135. https://doi.org/10.1016/j.gca.2019.02.011
; Vuillemin et al., 2020Vuillemin, A., Friese, A., Wirth, R., Schuessler, J.A., Schleicher, A.M., Kemnitz, H., Lücke, A., Bauer, K.W., Nomosatryo, S., von Blanckenburg, F., Simister, R., Ordoñez, L.G., Ariztegui, D., Henny, C., Russell, J.M., Bijaksana, S., Vogel, H., Crowe, S.A., Kallmeyer, J., the Towuti Drilling Project Science team (2020) Vivianite formation in ferruginous sediments from Lake Towuti, Indonesia. Biogeosciences 17, 1955–1973. https://doi.org/10.5194/bg-17-1955-2020
). Given the widespread occurrence of arsenate in these ancient oceans (Chi Fru et al., 2016Chi Fru, E., Arvestål, E., Callac, N., El Albani, A., Kilias, S., Argyraki, A., Jakobsson, M. (2016) Arsenic stress after the Proterozoic glaciations. Scientific Reports 5, 17789. https://doi.org/10.1038/srep17789
), As(V) substitution for P in vivianites would have reduced the bioavailability of toxic As species, and could have shaped how life emerged and adapted in the Precambrian oceans (Visscher et al., 2020Visscher, P.T., Gallagher, K.L., Bouton, A., Farias, M.E., Kurth, D., Sancho-Tomás, M., Philippot, P., Somogyi, A., Medjoubi, K., Vennin, E., Bourillot, R., Walter, M.R., Burns, B.P., Contreras, M., Dupraz, C. (2020) Modern arsenotrophic microbial mats provide an analogue for life in the anoxic Archean. Communications Earth & Environment 1, 24. https://doi.org/10.1038/s43247-020-00025-2
).top
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 Marie Skłodowska-Curie Innovative Training Network METAL-AID (
Editor: Juan Liu
top
References
Al-Borno, A., Tomson, M.B. (1994) The temperature dependence of the solubility product constant of vivianite. Geochimica et Cosmochimica Acta 58, 5373–5378. https://doi.org/10.1016/0016-7037(94)90236-4
 Show in context
Show in context Vivianite is a poorly soluble mineral (pKsp ≈ 35.8 ± 0.08 at 25 °C; Al-Borno and Tomson, 1994) that is stable under reducing and circum-neutral pH (6–9) conditions (Nriagu, 1972).
View in article
Anthony, J.W., Bideaux, R.A., Bladh, K.W., Nichols, M.C. (2000) Handbook of Mineralogy Volume IV: Arsenates, Phosphates and Vanadates. Mineral Data Publishing, Tucson, Arizona, USA.
 Show in context
Show in context Contrary to vivianite, parasymplesite is usually found in oxidised zones of As-rich hydrothermal mineral deposits (Anthony et al., 2000). Johnston and Singer (2007), however, predicted that parasymplesite can form in near neutral pH, reduced As-impacted groundwater (e.g., Bangladesh), yet it has so far not been reported.
View in article
Bartl, H. (1989) Water of crystallization and its hydrogen-bonded crosslinking in vivianite Fe3(PO4)2 · 8H2O; a neutron diffraction investigation. Fresenius’ Zeitschrift für analytische Chemie 333, 401–403. https://doi.org/10.1007/BF00572335
 Show in context
Show in context Its structure has two crystallographically and chemically distinct Fe sites coordinated with phosphate (Fig. S-1): (i) a single octahedral Fe1 site [FeO2(H2O)4)], and (ii) two edge sharing octahedral Fe2 sites [Fe2O6(H2O)4] (Mori and Ito, 1950; Bartl, 1989).
View in article
Capitelli, F., Chita, G., Ghiara, M.R., Rossi, M. (2012) Crystal-chemical investigation of Fe3(PO4)2 · 8 H2O vivianite minerals. Zeitschrift für Kristallographie - Crystalline Materials 227, 92–101. https://doi.org/10.1524/zkri.2012.1442
 Show in context
Show in context Variations in lattice parameter values (b–d) and cell volumes (e) of our As-vivianites (coloured circles) compared to literature (Mori and Ito, 1950; Ito et al., 1954; Schmetzer et al., 1980; Capitelli et al., 2012; Muehe et al., 2016) shown as grey symbols.
View in article
Chi Fru, E., Arvestål, E., Callac, N., El Albani, A., Kilias, S., Argyraki, A., Jakobsson, M. (2016) Arsenic stress after the Proterozoic glaciations. Scientific Reports 5, 17789. https://doi.org/10.1038/srep17789
 Show in context
Show in context Given the widespread occurrence of arsenate in these ancient oceans (Chi Fru et al., 2016), As(V) substitution for P in vivianites would have reduced the bioavailability of toxic As species, and could have shaped how life emerged and adapted in the Precambrian oceans (Visscher et al., 2020).
View in article
Egger, M., Jilbert, T., Behrends, T., Rivard, C., Slomp, C.P. (2015) Vivianite is a major sink for phosphorus in methanogenic coastal surface sediments. Geochimica et Cosmochimica Acta 169, 217–235. https://doi.org/10.1016/j.gca.2015.09.012
 Show in context
Show in context Vivianite can also form in aquatic ferruginous environments such as marine and lacustrine sediments and lakes (Egger et al., 2015; Vuillemin et al., 2020), and has been suggested as a potential phosphate sink in early Earth oceans (Hao et al., 2020), affecting trace element cycling and nutrient availability.
View in article
Filippelli, G.M. (2002) The Global Phosphorus Cycle. In: Kohn, M.J., Rakovan, J., Hughes, J.M. (Eds.) Phosphates: Geochemical, Geobiological, and Materials Importance. Reviews in Mineralogy and Geochemistry 48, 391–425. https://doi.org/10.2138/rmg.2002.48.10
 Show in context
Show in context Retention of phosphorus is strongly dependent on biomass uptake, mineral co-precipitation, or sorption onto mineral surfaces (Filippelli, 2002).
View in article
Frausto da Silva, J.J.R., Williams, R.J.P. (2001) The Biological Chemistry of the Elements: The Inorganic Chemistry of Life. Oxford University Press, Oxford.
 Show in context
Show in context These structural changes are evidenced by the displacement of the (100), (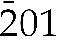 ), (310) and (201) vivianite reflections (dashed lines in Figs. 1a and S-2). As seen from the vivianite crystal structure model (Fig. S-1), these reflections correspond to lattice planes intersecting the interlinked Fe octahedra via PO43− or AsO43− tetrahedra, which expand at a higher degree of substitution because of the larger thermochemical radius of AsO43− (2.48 Å) compared to PO43− (2.38 Å) tetrahedra (Frausto da Silva and Williams, 2001).
), (310) and (201) vivianite reflections (dashed lines in Figs. 1a and S-2). As seen from the vivianite crystal structure model (Fig. S-1), these reflections correspond to lattice planes intersecting the interlinked Fe octahedra via PO43− or AsO43− tetrahedra, which expand at a higher degree of substitution because of the larger thermochemical radius of AsO43− (2.48 Å) compared to PO43− (2.38 Å) tetrahedra (Frausto da Silva and Williams, 2001).
View in article
Hao, J., Knoll, A.H., Huang, F., Schieber, J., Hazen, R.M., Daniel, I. (2020) Cycling phosphorus on the Archean Earth: Part II. Phosphorus limitation on primary production in Archean ecosystems. Geochimica et Cosmochimica Acta 280, 360–377. https://doi.org/10.1016/j.gca.2020.04.005
 Show in context
Show in context Vivianite can also form in aquatic ferruginous environments such as marine and lacustrine sediments and lakes (Egger et al., 2015; Vuillemin et al., 2020), and has been suggested as a potential phosphate sink in early Earth oceans (Hao et al., 2020), affecting trace element cycling and nutrient availability.
View in article
In addition to the potential importance of As-vivianites in contaminated settings, our findings have implications for phosphorus (and arsenic) cycling since vivianite has been suggested as a P sink in Precambrian oceans (Hao et al., 2020), and modern lake analogues (Xiong et al., 2019; Vuillemin et al., 2020).
View in article
Heiberg, L., Koch, C.B., Kjaergaard, C., Jensen, H.S., Hansen, H.C.B. (2012) Vivianite Precipitation and Phosphate Sorption following Iron Reduction in Anoxic Soils. Journal of Environmental Quality 41, 938–949. https://doi.org/10.2134/jeq2011.0067
 Show in context
Show in context Under anoxic, non-sulfidic (i.e. ferruginuous) conditions, high dissolved Fe2+ and PO43− may lead to vivianite [FeII3(PO4)2 · 8 H2O] (Heiberg et al., 2012) precipitation at near neutral pH.
View in article
Ito, T.-i., Minato, H., Sakurai, K.'i. (1954) Parasymplesite, a New Mineral Potymorphous with Symplesite. Proceedings of the Japan Academy 30, 318–324. https://doi.org/10.2183/pjab1945.30.318
 Show in context
Show in context Variations in lattice parameter values (b–d) and cell volumes (e) of our As-vivianites (coloured circles) compared to literature (Mori and Ito, 1950; Ito et al., 1954; Schmetzer et al., 1980; Capitelli et al., 2012; Muehe et al., 2016) shown as grey symbols.
View in article
Johnston, R.B., Singer, P.C. (2007) Solubility of Symplesite (Ferrous Arsenate): Implications for Reduced Groundwaters and Other Geochemical Environments. Soil Science Society of America Journal 71, 101–107. https://doi.org/10.2136/sssaj2006.0023
 Show in context
Show in context Contrary to vivianite, parasymplesite is usually found in oxidised zones of As-rich hydrothermal mineral deposits (Anthony et al., 2000). Johnston and Singer (2007), however, predicted that parasymplesite can form in near neutral pH, reduced As-impacted groundwater (e.g., Bangladesh), yet it has so far not been reported.
View in article
Therefore, As-vivianites may be present, yet so far undocumented in contaminated, anoxic settings such as in Bangladesh where vivianite and parasymplesite are both saturated (Johnston and Singer, 2007).
View in article
Jönsson, J., Sherman, D.M. (2008) Sorption of As(III) and As(V) to siderite, green rust (fougerite) and magnetite: Implications for arsenic release in anoxic groundwaters. Chemical Geology 255, 173–181. https://doi.org/10.1016/j.chemgeo.2008.06.036
 Show in context
Show in context These fit-derived interatomic distances are in excellent agreement with crystallographic values for parasymplesite (Mori and Ito, 1950), and are consistent with EXAFS-derived Fe-As distances in parasymplesite (Jönsson and Sherman, 2008).
View in article
The As-Fe atomic distance for As(V) adsorbed as bidentate binuclear (2C) and monodentate mononuclear (1V) inner sphere surface complexes are RAs-Fe of ∼3.4 and ∼3.5 Å, respectively (Jönsson and Sherman, 2008; Perez et al., 2020), similar to our fit-derived distances for As-vivianites.
View in article
Kubeneck, L.J., ThomasArrigo, L.K., Rothwell, K.A., Kaegi, R., Kretzschmar, R. (2023) Competitive incorporation of Mn and Mg in vivianite at varying salinity and effects on crystal structure and morphology. Geochimica et Cosmochimica Acta 346, 231–244. https://doi.org/10.1016/j.gca.2023.01.029
 Show in context
Show in context For example, vivianite found in anoxic, non-sulfidic environments could incorporate major and trace elements (Vuillemin et al., 2020; Kubeneck et al., 2023).
View in article
Makreski, P., Stefov, S., Pejov, L., Jovanovski, G. (2015) Theoretical and experimental study of the vibrational spectra of (para)symplesite and hornesite. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 144, 155–162. https://doi.org/10.1016/j.saa.2015.01.108
 Show in context
Show in context At even higher AsO43− substitution, a new band appeared at ∼1076 cm−1 and the 1035 cm−1 band split into two component bands at 1042 and 1022 cm−1, representing ν(PO4) antisymmetric stretching modes (Makreski et al., 2015).
View in article
Miot, J., Benzerara, K., Morin, G., Bernard, S., Beyssac, O., Larquet, E., Kappler, A., Guyot, F. (2009) Transformation of vivianite by anaerobic nitrate-reducing iron-oxidizing bacteria. Geobiology 7, 373–384. https://doi.org/10.1111/j.1472-4669.2009.00203.x
 Show in context
Show in context These positions match the reference vivianite and parasymplesite, as well as other vivianites reported in literature (Miot et al., 2009; Muehe et al., 2016).
View in article
Mori, H., Ito, T. (1950) The Structure of Vivianite and Symplesite. Acta Crystallographica 3, 1–6. https://doi.org/10.1107/S0365110X5000001X
 Show in context
Show in context Its structure has two crystallographically and chemically distinct Fe sites coordinated with phosphate (Fig. S-1): (i) a single octahedral Fe1 site [FeO2(H2O)4)], and (ii) two edge sharing octahedral Fe2 sites [Fe2O6(H2O)4] (Mori and Ito, 1950; Bartl, 1989).
View in article
Variations in lattice parameter values (b–d) and cell volumes (e) of our As-vivianites (coloured circles) compared to literature (Mori and Ito, 1950; Ito et al., 1954; Schmetzer et al., 1980; Capitelli et al., 2012; Muehe et al., 2016) shown as grey symbols.
View in article
These fit-derived interatomic distances are in excellent agreement with crystallographic values for parasymplesite (Mori and Ito, 1950), and are consistent with EXAFS-derived Fe-As distances in parasymplesite (Jönsson and Sherman, 2008).
View in article
Muehe, E.M., Morin, G., Scheer, L., Le Pape, P., Esteve, I., Daus, B., Kappler, A. (2016) Arsenic(V) Incorporation in Vivianite during Microbial Reduction of Arsenic(V)-Bearing Biogenic Fe(III) (Oxyhydr)oxides. Environmental Science & Technology 50, 2281–2291. https://doi.org/10.1021/acs.est.5b04625
 Show in context
Show in context Isomorphic substitution of Fe2+ by divalent cations (e.g., Mg, Mn) occurs frequently in natural vivianites, and although rarely documented, anions like arsenate (AsO43−) can also replace PO43− in the crystal structure (Muehe et al., 2016).
View in article
As(V)-substituted vivianite (∼15 mol %; hereafter referred as As-vivianite) have been observed during microbial reduction of As(V)-bearing Fe(III) (oxyhydr)oxides (Muehe et al., 2016), and such high As contents suggest a possible vivianite-parasymplesite solid solution.
View in article
Variations in lattice parameter values (b–d) and cell volumes (e) of our As-vivianites (coloured circles) compared to literature (Mori and Ito, 1950; Ito et al., 1954; Schmetzer et al., 1980; Capitelli et al., 2012; Muehe et al., 2016) shown as grey symbols.
View in article
These positions match the reference vivianite and parasymplesite, as well as other vivianites reported in literature (Miot et al., 2009; Muehe et al., 2016).
View in article
Nriagu, J.O. (1972) Stability of vivianite and ion-pair formation in the system Fe3(PO4)2-H3PO4-H2O. Geochimica et Cosmochimica Acta 36, 459–470. https://doi.org/10.1016/0016-7037(72)90035-X
 Show in context
Show in context Vivianite is a poorly soluble mineral (pKsp ≈ 35.8 ± 0.08 at 25 °C; Al-Borno and Tomson, 1994) that is stable under reducing and circum-neutral pH (6–9) conditions (Nriagu, 1972).
View in article
Patrick Jr., W.H., Khalid, R.A. (1974) Phosphate Release and Sorption by Soils and Sediments: Effect of Aerobic and Anaerobic Conditions. Science 186, 53–55. https://doi.org/10.1126/science.186.4158.53
 Show in context
Show in context In anoxic soils and sediments, reductive dissolution of Fe(III) (oxyhydr)oxides results in the release of dissolved phosphate (PO43−) and Fe2+ (Patrick and Khalid, 1974), accompanied by increase to near neutral pH due to H+ consumption (Walpersdorf et al., 2013).
View in article
Perez, J.P.H., Freeman, H.M., Schuessler, J.A., Benning, L.G. (2019) The interfacial reactivity of arsenic species with green rust sulfate (GRSO4). Science of The Total Environment 648, 1161–1170. https://doi.org/10.1016/j.scitotenv.2018.08.163
 Show in context
Show in context Compared to interactions with other common Fe(II)-bearing minerals in ferruginous environments, where arsenic is primarily adsorbed and only reaches ∼15 wt. % in magnetite (Yean et al., 2005) or ∼9 wt. % in green rust (Perez et al., 2019), our current results show that arsenic incorporation into vivianite could be stable sinks for As(V) removal in ferruginous settings.
View in article
Perez, J.P.H., Freeman, H.M., Brown, A.P., van Genuchten, C.M., Dideriksen, K., S’Ari, M., Tobler, D.J., Benning, L.G. (2020) Direct Visualization of Arsenic Binding on Green Rust Sulfate. Environmental Science & Technology 54, 3297–3305. https://doi.org/10.1021/acs.est.9b07092
 Show in context
Show in context The As-Fe atomic distance for As(V) adsorbed as bidentate binuclear (2C) and monodentate mononuclear (1V) inner sphere surface complexes are RAs-Fe of ∼3.4 and ∼3.5 Å, respectively (Jönsson and Sherman, 2008; Perez et al., 2020), similar to our fit-derived distances for As-vivianites.
View in article
Schmetzer, K., Tremmel, G., Bartelke, W. (1980) Eine Paragenese seltener Minerale aus Bou-Azzer, Marokko: Parasymplesit, Symplesit, Schneiderhöhnit, Karibibit. Neues Jahrbuch für Mineralogie - Abhandlungen 138, 94–108.
 Show in context
Show in context Variations in lattice parameter values (b–d) and cell volumes (e) of our As-vivianites (coloured circles) compared to literature (Mori and Ito, 1950; Ito et al., 1954; Schmetzer et al., 1980; Capitelli et al., 2012; Muehe et al., 2016) shown as grey symbols.
View in article
Thinnappan, V., Merrifield, C.M., Islam, F.S., Polya, D.A., Wincott, P., Wogelius, R.A. (2008) A combined experimental study of vivianite and As (V) reactivity in the pH range 2–11. Applied Geochemistry 23, 3187–3204. https://doi.org/10.1016/j.apgeochem.2008.07.001
 Show in context
Show in context Naturally, the question arises whether As(V) is adsorbed to vivianite surfaces (cf. Thinnappan et al., 2008), as in the case of other Fe(II)-bearing minerals.
View in article
Vegard, L. (1921) Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Zeitschrift für Physik 5, 17–26. https://doi.org/10.1007/BF01349680
 Show in context
Show in context This is also reflected in the larger lattice parameters and associated cell volumes of As-vivianites (Fig. 1b–e) derived from Rietveld refinements (Table S-5, Fig. S-3), which increased linearly and suggest an isomorphous substitution following Vegard’s law (Vegard, 1921).
View in article
Visscher, P.T., Gallagher, K.L., Bouton, A., Farias, M.E., Kurth, D., Sancho-Tomás, M., Philippot, P., Somogyi, A., Medjoubi, K., Vennin, E., Bourillot, R., Walter, M.R., Burns, B.P., Contreras, M., Dupraz, C. (2020) Modern arsenotrophic microbial mats provide an analogue for life in the anoxic Archean. Communications Earth & Environment 1, 24. https://doi.org/10.1038/s43247-020-00025-2
 Show in context
Show in context Given the widespread occurrence of arsenate in these ancient oceans (Chi Fru et al., 2016), As(V) substitution for P in vivianites would have reduced the bioavailability of toxic As species, and could have shaped how life emerged and adapted in the Precambrian oceans (Visscher et al., 2020).
View in article
Vuillemin, A., Friese, A., Wirth, R., Schuessler, J.A., Schleicher, A.M., Kemnitz, H., Lücke, A., Bauer, K.W., Nomosatryo, S., von Blanckenburg, F., Simister, R., Ordoñez, L.G., Ariztegui, D., Henny, C., Russell, J.M., Bijaksana, S., Vogel, H., Crowe, S.A., Kallmeyer, J., the Towuti Drilling Project Science team (2020) Vivianite formation in ferruginous sediments from Lake Towuti, Indonesia. Biogeosciences 17, 1955–1973. https://doi.org/10.5194/bg-17-1955-2020
 Show in context
Show in context Vivianite can also form in aquatic ferruginous environments such as marine and lacustrine sediments and lakes (Egger et al., 2015; Vuillemin et al., 2020), and has been suggested as a potential phosphate sink in early Earth oceans (Hao et al., 2020), affecting trace element cycling and nutrient availability.
View in article
For example, vivianite found in anoxic, non-sulfidic environments could incorporate major and trace elements (Vuillemin et al., 2020; Kubeneck et al., 2023).
View in article
In addition to the potential importance of As-vivianites in contaminated settings, our findings have implications for phosphorus (and arsenic) cycling since vivianite has been suggested as a P sink in Precambrian oceans (Hao et al., 2020), and modern lake analogues (Xiong et al., 2019; Vuillemin et al., 2020).
View in article
Walpersdorf, E., Bender Koch, C., Heiberg, L., O’Connell, D.W., Kjaergaard, C., Bruun Hansen, H.C. (2013) Does vivianite control phosphate solubility in anoxic meadow soils? Geoderma 193–194, 189–199. https://doi.org/10.1016/j.geoderma.2012.10.003
 Show in context
Show in context In anoxic soils and sediments, reductive dissolution of Fe(III) (oxyhydr)oxides results in the release of dissolved phosphate (PO43−) and Fe2+ (Patrick and Khalid, 1974), accompanied by increase to near neutral pH due to H+ consumption (Walpersdorf et al., 2013).
View in article
Xiong, Y., Guilbaud, R., Peacock, C.L., Cox, R.P., Canfield, D.E., Krom, M.D., Poulton, S.W. (2019) Phosphorus cycling in Lake Cadagno, Switzerland: A low sulfate euxinic ocean analogue. Geochimica et Cosmochimica Acta 251, 116–135. https://doi.org/10.1016/j.gca.2019.02.011
 Show in context
Show in context In addition to the potential importance of As-vivianites in contaminated settings, our findings have implications for phosphorus (and arsenic) cycling since vivianite has been suggested as a P sink in Precambrian oceans (Hao et al., 2020), and modern lake analogues (Xiong et al., 2019; Vuillemin et al., 2020).
View in article
Yean, S., Cong, L., Yavuz, C.T., Mayo, J.T., Yu, W.W., Kan, A.T., Colvin, V.L., Tomson, M.B. (2005) Effect of magnetite particle size on adsorption and desorption of arsenite and arsenate. Journal of Materials Research 20, 3255–3264. https://doi.org/10.1557/jmr.2005.0403
 Show in context
Show in context Compared to interactions with other common Fe(II)-bearing minerals in ferruginous environments, where arsenic is primarily adsorbed and only reaches ∼15 wt. % in magnetite (Yean et al., 2005) or ∼9 wt. % in green rust (Perez et al., 2019), our current results show that arsenic incorporation into vivianite could be stable sinks for As(V) removal in ferruginous settings.
View in article
top
Supplementary Information
The Supplementary Information includes:
Download the Supplementary Information (PDF)
Figures
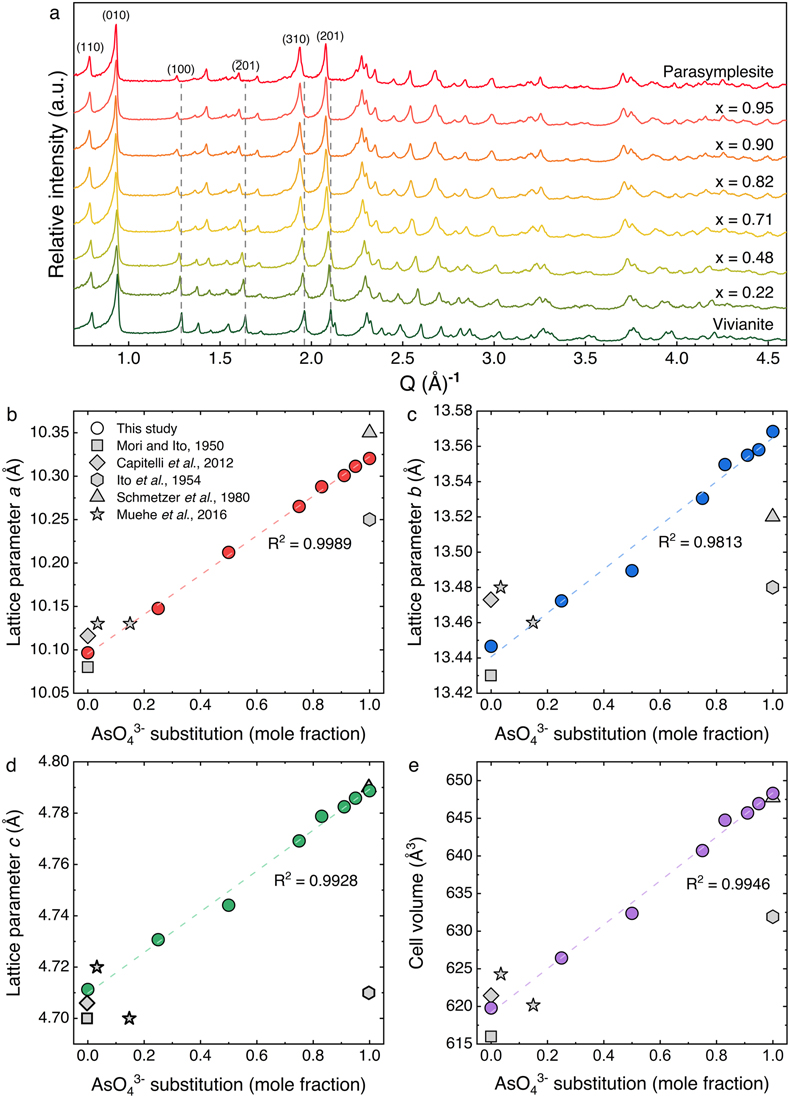
Figure 1 (a) XRD patterns of As-substituted vivianites (x = mole fraction of substituted AsO43−) aged for 24 hr, plotted in Q-space (Q = 2π/dhkl). Dashed grey lines indicate displacement in selected lattice planes in vivianite due to AsO43− substitution for PO43−. Full XRD patterns can be found in Figure S-2. Variations in lattice parameter values (b–d) and cell volumes (e) of our As-vivianites (coloured circles) compared to literature (Mori and Ito, 1950
Mori, H., Ito, T. (1950) The Structure of Vivianite and Symplesite. Acta Crystallographica 3, 1–6. https://doi.org/10.1107/S0365110X5000001X
; Ito et al., 1954Ito, T.-i., Minato, H., Sakurai, K.'i. (1954) Parasymplesite, a New Mineral Potymorphous with Symplesite. Proceedings of the Japan Academy 30, 318–324. https://doi.org/10.2183/pjab1945.30.318
; Schmetzer et al., 1980Schmetzer, K., Tremmel, G., Bartelke, W. (1980) Eine Paragenese seltener Minerale aus Bou-Azzer, Marokko: Parasymplesit, Symplesit, Schneiderhöhnit, Karibibit. Neues Jahrbuch für Mineralogie - Abhandlungen 138, 94–108.
; Capitelli et al., 2012Capitelli, F., Chita, G., Ghiara, M.R., Rossi, M. (2012) Crystal-chemical investigation of Fe3(PO4)2 · 8 H2O vivianite minerals. Zeitschrift für Kristallographie - Crystalline Materials 227, 92–101. https://doi.org/10.1524/zkri.2012.1442
; Muehe et al., 2016Muehe, E.M., Morin, G., Scheer, L., Le Pape, P., Esteve, I., Daus, B., Kappler, A. (2016) Arsenic(V) Incorporation in Vivianite during Microbial Reduction of Arsenic(V)-Bearing Biogenic Fe(III) (Oxyhydr)oxides. Environmental Science & Technology 50, 2281–2291. https://doi.org/10.1021/acs.est.5b04625
) shown as grey symbols. Dashed lines represent linear relationships as a function of As(V) substitution.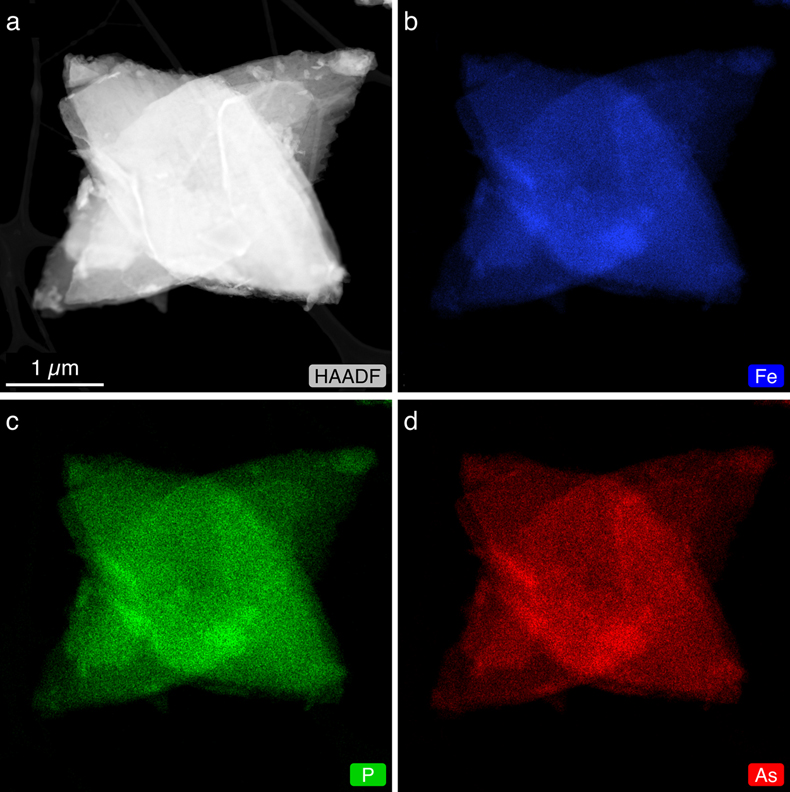
Figure 2 (a) HAADF-STEM image of As-substituted vivianite (x = 0.48) and corresponding EDX maps: (b) Fe (blue); (c) P (green); and (d) As (red).
Figure 3 (a) Fe K-edge k3-weighted EXAFS spectra of As-vivianites. Dashed grey lines denote (b) LCF of Fe K-edge EXAFS spectra of mineral end members (i.e. vivianite, parasymplesite). (c) As K-edge k3-weighted EXAFS spectra of As vivianites and (d) corresponding Fourier transform magnitude. Dashed grey lines denote shell-by-shell fits.
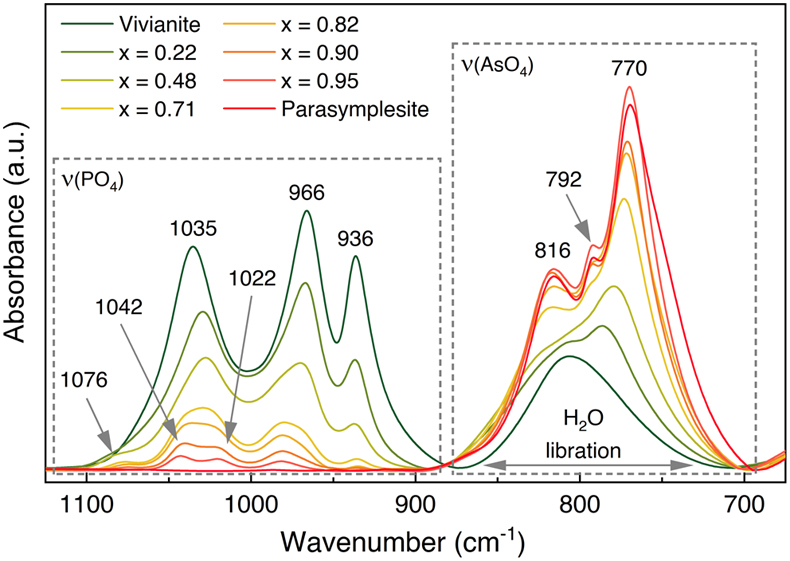
Figure 4 FTIR spectra of As(V)-substituted vivianites showing stretching regions for phosphate [ν(PO4)] and arsenate [ν(AsO4)], and H2O libration vibrations. Full spectra and band assignments can be found in Figures S-10 and Table S-8, respectively.