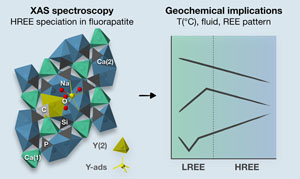
Sorption model for yttrium in fluorapatite: Geochemical implications
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:160Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
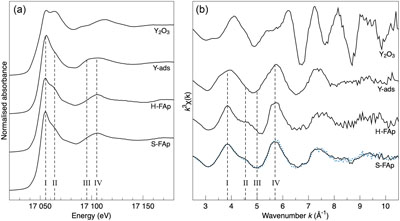 Figure 1 X-ray absorption spectra collected at the Y K-edge for Y2O3 and Y-ads FAp model compounds, and H-FAp and S-FAp samples. (a) Normalised XANES spectra, (b) k3-weighted EXAFS spectra. Roman numerals (I to IV) refer to characteristic spectral features. Blue dotted line represents the linear combination fit (LCF) result based on Y-ads and H-FAp components, suggesting a mixture of 59 % Y substituted at the Ca(2) site and 41 % of adsorbed Y for S-FAp. | 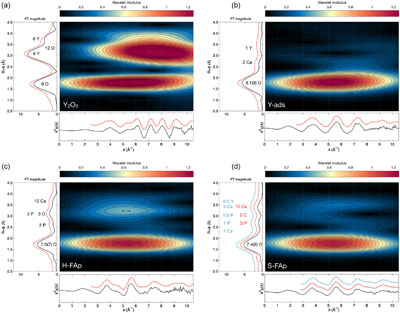 Figure 2 Wavelet transform analysis of EXAFS spectra with corresponding k3-weighted EXAFS spectra and Fourier transform (black solid lines). Results of multi-shell fits are shown in both the k-, and R-spaces (shifted dotted lines), (a) Y2O3, (b) Y-ads, (c) H-FAp, (d) S-FAp with the mixed “Y(2) + Y-ads” model in blue and the “Y(2) + carbonate” model in red. | 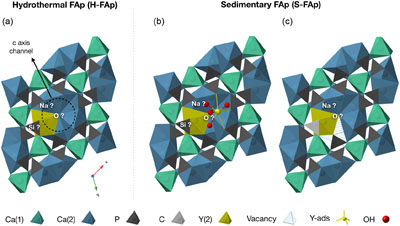 Figure 3 Fluorapatite crystal structure projected along c axis showing yttrium speciation models, (a) H-FAp: substitution at the Ca(2) site, (b) S-FAp: mixture of substitution at the Ca(2) site together with an inner shell adsorption at the c axis channel, (c) S-FAp: substitution at the Ca(2) site with concomitant C replacing P and Ca vacancy. Si, Na and O labels indicate potential coupled substitutions required to compensate the excess of electronic charge due to Y3+-Ca2+ substitution. | 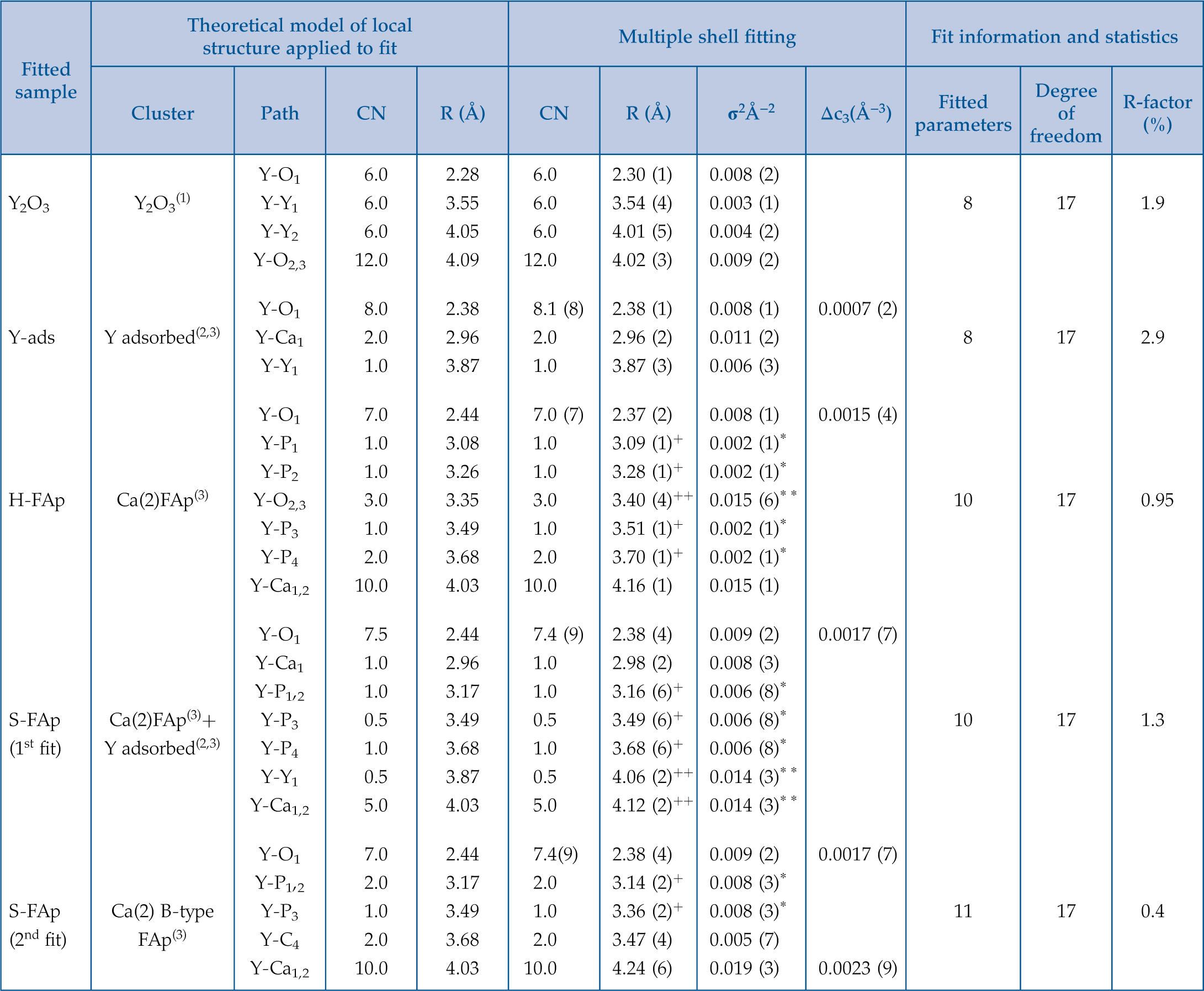 Table 1 EXAFS fitting results for selected Y-standards and natural FAp samples and corresponding theoretical structural model. CN stands for the coordination number, R for the radial distance, σ2 for the Debye-Waller factor and Δc3 for the anharmonic parameter. The estimated parameters have uncertainty values in brackets, while fixed parameters do not. Linked estimated parameters share one of these superscript symbols: +, ++, *, **. Theoretical structures references: (1) Faucher and Pannetier (1980); (2) Ni et al. (1995); (3) Hughes et al. (1990). |
| Figure 1 | Figure 2 | Figure 3 | Table 1 |
top
Introduction
Fluorapatite (FAp; Ca5(PO4)3F) is an accessory mineral that hosts significant amounts of rare earth elements (REE, including yttrium) from ∼200 to 20,000 ppm (Emsbo et al., 2015
Emsbo, P., McLaughlin, P.I., Breit, G.N., du Bray, E.A., Koenig, A.E. (2015) Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Research 27, 776–785. https://doi.org/10.1016/j.gr.2014.10.008
), where Y exhibits similar behaviour to heavy rare earth elements (HREE, from Gd to Lu) and is therefore associated with them. For Earth Sciences, REE normalised patterns in FAp are proxies to i) reconstruct partitioning models and determine partition coefficients (Blundy and Wood, 2003Blundy, J., Wood, B. (2003) Partitioning of trace elements between crystals and melts. Earth and Planetary Science Letters 210, 383–397. https://doi.org/10.1016/S0012-821X(03)00129-8
), ii) identify the source fluids, their chemical composition and their REE complexation (Mackie and Young, 1973Mackie, P.E., Young, R.A. (1973) Location of Nd dopant in fluorapatite, Ca5(PO4)3F: Nd. Journal of Applied Crystallography 6, 26–31. https://doi.org/10.1107/S0021889873008009
; Krneta et al., 2018Krneta, S., Ciobanu, C.L., Cook, N.J., Ehrig, K.J. (2018) Numerical Modeling of REE Fractionation Patterns in Fluorapatite from the Olympic Dam Deposit (South Australia). Minerals 8, 342. https://doi.org/10.3390/min8080342
), iii) characterise the deposition/crystallisation conditions such as temperature, pH, redox (Chen et al., 2002Chen, N., Pan, Y., Weil, J.A. (2002) Electron paramagnetic resonance spectroscopic study of synthetic fluorapatite: Part I. Local structural environment and substitution mechanism of Gd3+ at the Ca2 site. American Mineralogist 87, 37–46. https://doi.org/10.2138/am-2002-0105
; Kocsis et al., 2016Kocsis, L., Gheerbrant, E., Mouflih, M., Cappetta, H., Ulianov, A., Chiaradia, M., Bardet, N. (2016) Gradual changes in upwelled seawater conditions (redox, pH) from the late Cretaceous through early Paleogene at the northwest coast of Africa: Negative Ce anomaly trend recorded in fossil bio-apatite. Chemical Geology 421, 44–54. https://doi.org/10.1016/j.chemgeo.2015.12.001
), and iv) reveal the potential late diagenetic or hydrothermal alterations (Reynard et al., 1999Reynard, B., Lécuyer, C., Grandjean, P. (1999) Crystal-chemical controls on rare-earth element concentrations in fossil biogenic apatites and implications for paleoenvironmental reconstructions. Chemical Geology 155, 233–241. https://doi.org/10.1016/S0009-2541(98)00169-7
; Cherniak, 2000Cherniak, D.J. (2000) Rare earth element diffusion in apatite. Geochimica et Cosmochimica Acta 64, 3871–3885. https://doi.org/10.1016/S0016-7037(00)00467-1
). The crystal chemistry of REE in FAp also controls its partitioning and normalised patterns (Blundy and Wood, 2003Blundy, J., Wood, B. (2003) Partitioning of trace elements between crystals and melts. Earth and Planetary Science Letters 210, 383–397. https://doi.org/10.1016/S0012-821X(03)00129-8
). In addition, it can potentially provide valuable insight into the crystallisation conditions such as temperature (Khudolozhkin et al., 1973Khudolozhkin, V.O., Urusov, V.S., Tobelko, K.I., Vernadskiy, V.I. (1973) Dependence of structural ordering of rare earth atoms in the isomorphous series apatite-britholite (abukumalite) on composition and temperature. Geochemical International 10, 1171–1177.
; Pan and Fleet, 2002Pan, Y., Fleet, M.E. (2002) Compositions of the Apatite-Group Minerals: Substitution Mechanisms and Controlling Factors. Reviews in Mineralogy and Geochemistry 48, 13–49. https://doi.org/10.2138/rmg.2002.48.2
and references therein), fluids (Mackie and Young, 1973Mackie, P.E., Young, R.A. (1973) Location of Nd dopant in fluorapatite, Ca5(PO4)3F: Nd. Journal of Applied Crystallography 6, 26–31. https://doi.org/10.1107/S0021889873008009
) or diffusion (Cherniak, 2000Cherniak, D.J. (2000) Rare earth element diffusion in apatite. Geochimica et Cosmochimica Acta 64, 3871–3885. https://doi.org/10.1016/S0016-7037(00)00467-1
). However, determining the sorption models of REE is not systematically well constrained and is at the heart of many studies since FAp can potentially integrate REE in two distinct crystallographic Ca sites, namely the 9 fold coordinated Ca(1) and the 7 fold coordinated Ca(2) sites (Hughes et al., 1991Hughes, J.M., Cameron, M., Mariano, A.N. (1991) Rare-earth-element ordering and structural variations in natural rare-earth-bearing apatites. American Mineralogist 76, 1165–1173. http://www.minsocam.org/ammin/AM76/AM76_1165.pdf
) (Fig. S-1a, b), and can also show nano-crystallinity typically observed in sedimentary deposits (Aubineau et al., 2022Aubineau, J., Parat, F., Elghali, A., Raji, O., Addou, A., Bonnet, C., Muñoz, M., Mauguin, O., Baron, F., Jouti, M.B., Yazamid, O.K., Bodinier, J.-L. (2022) Highly variable content of fluorapatite-hosted CO32− in the Upper Cretaceous/ Paleogene phosphorites (Morocco) and implications for paleodepositional conditions. Chemical Geology 597, 120818. https://doi.org/10.1016/j.chemgeo.2022.120818
) that may favour adsorption mechanisms and apparent no-fractionation behaviour (Reynard et al., 1999Reynard, B., Lécuyer, C., Grandjean, P. (1999) Crystal-chemical controls on rare-earth element concentrations in fossil biogenic apatites and implications for paleoenvironmental reconstructions. Chemical Geology 155, 233–241. https://doi.org/10.1016/S0009-2541(98)00169-7
). In order to maintain electroneutrality, each of the above sorption models may involve coupled substitutions such as (Pan and Fleet, 2002Pan, Y., Fleet, M.E. (2002) Compositions of the Apatite-Group Minerals: Substitution Mechanisms and Controlling Factors. Reviews in Mineralogy and Geochemistry 48, 13–49. https://doi.org/10.2138/rmg.2002.48.2
and references therein):Eq. 1
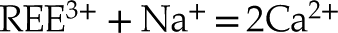
Eq. 2
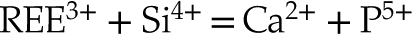
Eq. 3
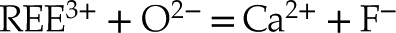
Eq. 4
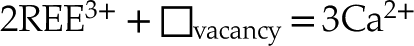
In a study based on ligand type, Urusov and Khudolozhkin (1974)
Urusov, V.S., Khudolozhkin, V.O. (1974) An energy analysis of cation ordering in apatite. Geochemistry International 11, 1048–1053.
suggested that light (L)REE preferentially occupy the more covalent Ca(l) position, while HREE display preference for the more ionic Ca(2) position. Conversely, X-ray diffraction structure refinements on synthetic REE-doped FAp suggest that LREE are favoured in Ca(2) while HREE are favoured in Ca(1) (Hughes et al., 1991Hughes, J.M., Cameron, M., Mariano, A.N. (1991) Rare-earth-element ordering and structural variations in natural rare-earth-bearing apatites. American Mineralogist 76, 1165–1173. http://www.minsocam.org/ammin/AM76/AM76_1165.pdf
; Fleet and Pan, 1995Fleet, M.E., Pan, Y. (1995) Site preference of rare earth elements in fluorapatite. American Mineralogist 80, 329–335. https://doi.org/10.2138/am-1995-3-414
). Moreover, Borisov and Klevtśova (1963)Borisov, S.V., Klevtśova, R.F. (1963) The crystal structure of TR-Sr-apatite. Journal of Structural Chemistry 4, 575–577. https://doi.org/10.1007/BF00747639
have demonstrated that substitutions of both LREE and HREE for Ca occurred only at the Ca(2) site which was confirmed by EPR (Electron Paramagnetic Resonance) spectroscopy (Chen et al., 2002Chen, N., Pan, Y., Weil, J.A. (2002) Electron paramagnetic resonance spectroscopic study of synthetic fluorapatite: Part I. Local structural environment and substitution mechanism of Gd3+ at the Ca2 site. American Mineralogist 87, 37–46. https://doi.org/10.2138/am-2002-0105
). In natural marine environments, Kashiwabara et al. (2018)Kashiwabara, T., Toda, R., Nakamura, K., Yasukawa, K., Fujinaga, K., Kubo, S., Nozaki, T., Takahashi, Y., Suzuki, K., Kato, Y. (2018) Synchrotron X-ray spectroscopic perspective on the formation mechanism of REY-rich muds in the Pacific Ocean. Geochimica et Cosmochimica Acta 240, 274–292. https://doi.org/10.1016/j.gca.2018.08.013
proposed that deep sea muds mostly trap REE by adsorption onto FAp crystals. Similarly, Reynard et al. (1999)Reynard, B., Lécuyer, C., Grandjean, P. (1999) Crystal-chemical controls on rare-earth element concentrations in fossil biogenic apatites and implications for paleoenvironmental reconstructions. Chemical Geology 155, 233–241. https://doi.org/10.1016/S0009-2541(98)00169-7
conclude that the REE adsorption is likely to occur in the case of sedimentary/biogenic FAp.To contribute to the understanding of the crystal chemistry of REE, and more specifically HREE in FAp and geochemical implications that follows, we propose a direct characterisation of the yttrium speciation (i.e. fixation modes, coupled substitutions and crystallographic preferences) using X-ray absorption spectroscopy (XAS) at the Y K-edge. XANES (X-ray absorption near edge structure) and EXAFS (extended absorption near edge structure) spectra are analysed by quantitative refinements, linear combinations and wavelet analysis (Muñoz et al., 2003
Muñoz, M., Argoul, P., Farges, F. (2003) Continuous Cauchy wavelet transform analyses of EXAFS spectra: A qualitative approach. American Mineralogist 88, 694–700. https://doi.org/10.2138/am-2003-0423
). This study focuses on two representative and genetically contrasting natural FAp: i) of hydrothermal origin (H-FAp) from Durango, Mexico, and ii) of sedimentary origin (S-FAp) from Moroccan phosphorites, and mainly composed of B-type carbonated FAp (i.e. FAp showing carbonate-phosphate substitutions).top
Yttrium Speciation in Model Compounds
Two model compounds, Y2O3 and synthetic Y-adsorbed FAp (Y-ads), were studied and used for qualitative and quantitative analysis of spectroscopic data. Their normalised XANES and EXAFS spectra show contrasting spectral signatures (Fig. 1a and b, respectively). For both compounds, the wavelet analysis of the EXAFS spectra (Fig. 2a and b, respectively) shows a maximum of the 1st neighbours’ amplitude (i.e. (R + Φ) = 1.75 Å) localised around 5.5 Å−1. For Y2O3, the second and third shells are significantly shifted at higher k (wavenumber) values due to the presence of yttrium (Fig. 2a). However, the presence of oxygen in these shells moderates the shift of the amplitude term (maximum ∼7.5 Å−1), showing a strong asymmetric shape due to the distribution of Y, O and Y at distances of 3.53(4), 4.02(3) and 4.01(5) Å, respectively (Table 1).

Figure 1 X-ray absorption spectra collected at the Y K-edge for Y2O3 and Y-ads FAp model compounds, and H-FAp and S-FAp samples. (a) Normalised XANES spectra, (b) k3-weighted EXAFS spectra. Roman numerals (I to IV) refer to characteristic spectral features. Blue dotted line represents the linear combination fit (LCF) result based on Y-ads and H-FAp components, suggesting a mixture of 59 % Y substituted at the Ca(2) site and 41 % of adsorbed Y for S-FAp.

Figure 2 Wavelet transform analysis of EXAFS spectra with corresponding k3-weighted EXAFS spectra and Fourier transform (black solid lines). Results of multi-shell fits are shown in both the k-, and R-spaces (shifted dotted lines), (a) Y2O3, (b) Y-ads, (c) H-FAp, (d) S-FAp with the mixed “Y(2) + Y-ads” model in blue and the “Y(2) + carbonate” model in red.
Table 1 EXAFS fitting results for selected Y-standards and natural FAp samples and corresponding theoretical structural model. CN stands for the coordination number, R for the radial distance, σ2 for the Debye-Waller factor and Δc3 for the anharmonic parameter. The estimated parameters have uncertainty values in brackets, while fixed parameters do not. Linked estimated parameters share one of these superscript symbols: +, ++, *, **. Theoretical structures references: (1) Faucher and Pannetier (1980)
Faucher, M., Pannetier, J. (1980) Refinement of the Y2O3 structure at 77 K. Acta Crystallographica Section B 36, 3209–3211. https://doi.org/10.1107/S0567740880011351
; (2) Ni et al. (1995)Ni, Y., Hughes, J.M., Mariano, A.N. (1995) Crystal chemistry of the monazite and xenotime structures. American Mineralogist 80, 21–26. https://doi.org/10.2138/am-1995-1-203
; (3) Hughes et al. (1990)Hughes, J.M., Cameron, M., Crowley, K.D. (1990) Crystal structures of natural ternary apatites: Solid solution in the Ca5(PO4)3X (X = F, OH, Cl) system. American Mineralogist 75, 295–304. http://www.minsocam.org/ammin/AM75/AM75_295.pdf
.| Fitted sample | Theoretical model of local structure applied to fit | Multiple shell fitting | Fit information and statistics | ||||||||
| Cluster | Path | CN | R (Å) | CN | R (Å) | σ2 (Å−2) | Δc3 (Å−3) | Fitted parameters | Degree of freedom | R-factor (%) | |
| Y-O1 | 6.0 | 2.28 | 6.0 | 2.30 (1) | 0.008 (2) | ||||||
| Y2O3 | Y2O3(1) | Y-Y1 | 6.0 | 3.55 | 6.0 | 3.54 (4) | 0.003 (1) | 8 | 17 | 1.9 | |
| Y-Y2 | 6.0 | 4.05 | 6.0 | 4.01 (5) | 0.004 (2) | ||||||
| Y-O2,3 | 12.0 | 4.09 | 12.0 | 4.02 (3) | 0.009 (2) | ||||||
| Y-O1 | 8.0 | 2.38 | 8.1 (8) | 2.38 (1) | 0.008 (1) | 0.0007 (2) | |||||
| Y-ads | Y adsorbed(2,3) | Y-Ca1 | 2.0 | 2.96 | 2.0 | 2.96 (2) | 0.011 (2) | 8 | 17 | 2.9 | |
| Y-Y1 | 1.0 | 3.87 | 1.0 | 3.87 (3) | 0.006 (3) | ||||||
| Y-O1 | 7.0 | 2.44 | 7.0 (7) | 2.37 (2) | 0.008 (1) | 0.0015 (4) | |||||
| Y-P1 | 1.0 | 3.08 | 1.0 | 3.09 (1)+ | 0.002 (1)* | ||||||
| Y-P2 | 1.0 | 3.26 | 1.0 | 3.28 (1)+ | 0.002 (1)* | ||||||
| H-FAp | Ca(2)FAp(3) | Y-O2,3 | 3.0 | 3.35 | 3.0 | 3.40 (4)++ | 0.015 (6)* * | 10 | 17 | 0.95 | |
| Y-P3 | 1.0 | 3.49 | 1.0 | 3.51 (1)+ | 0.002 (1)* | ||||||
| Y-P4 | 2.0 | 3.68 | 2.0 | 3.70 (1)+ | 0.002 (1)* | ||||||
| Y-Ca1,2 | 10.0 | 4.03 | 10.0 | 4.16 (1) | 0.015 (1) | ||||||
| Y-O1 | 7.5 | 2.44 | 7.4 (9) | 2.38 (4) | 0.009 (2) | 0.0017 (7) | |||||
| Y-Ca1 | 1.0 | 2.96 | 1.0 | 2.98 (2) | 0.008 (3) | ||||||
| Y-P1,2 | 1.0 | 3.17 | 1.0 | 3.16 (6)+ | 0.006 (8)* | ||||||
| S-FAp | Ca(2)FAp(3)+ | Y-P3 | 0.5 | 3.49 | 0.5 | 3.49 (6)+ | 0.006 (8)* | 10 | 17 | 1.3 | |
| (1st fit) | Y adsorbed(2,3) | Y-P4 | 1.0 | 3.68 | 1.0 | 3.68 (6)+ | 0.006 (8)* | ||||
| Y-Y1 | 0.5 | 3.87 | 0.5 | 4.06 (2)++ | 0.014 (3)* * | ||||||
| Y-Ca1,2 | 5.0 | 4.03 | 5.0 | 4.12 (2)++ | 0.014 (3)* * | ||||||
| Y-O1 | 7.0 | 2.44 | 7.4(9) | 2.38 (4) | 0.009 (2) | 0.0017 (7) | |||||
| Y-P1,2 | 2.0 | 3.17 | 2.0 | 3.14 (2)+ | 0.008 (3)* | ||||||
| S-FAp | Ca(2) B-type | Y-P3 | 1.0 | 3.49 | 1.0 | 3.36 (2)+ | 0.008 (3)* | 11 | 17 | 0.4 | |
| (2nd fit) | FAp(3) | Y-C4 | 2.0 | 3.68 | 2.0 | 3.47 (4) | 0.005 (7) | ||||
| Y-Ca1,2 | 10.0 | 4.03 | 10.0 | 4.24 (6) | 0.019 (3) | 0.0023 (9) | |||||
Regarding Y-ads, the EXAFS shell fitting reveals the presence of 8.1(8) oxygen 1st neighbours located at 2.38(1) Å (Fig. 2b, Table 1), which is consistent for Y-adsorbed species (Ragnarsdottir et al., 1998
Ragnarsdottir, K.V., Oelkers, E.H., Sherman, D.M., Collins, C.R. (1998) Aqueous speciation of yttrium at temperatures from 25 to 340°C at Psat: an in situ EXAFS study. Chemical Geology 151, 29–39. https://doi.org/10.1016/S0009-2541(98)00068-0
). Additionally, the best fit is obtained with two Ca as 2nd neighbours at 2.98(2) Å, suggesting a tridendate ionic bonding with the FAp crystal surface in the c axis channel. Note that in such a configuration (Fig. 3b), a typical O–Y–O angle of about 73°, characteristic of the regular 8 fold polyhedron, is preserved (Ni et al., 1995Ni, Y., Hughes, J.M., Mariano, A.N. (1995) Crystal chemistry of the monazite and xenotime structures. American Mineralogist 80, 21–26. https://doi.org/10.2138/am-1995-1-203
). The 3rd shell observed on the wavelet modulus (Fig. 2b) shows a contribution at high k values (∼8.5 Å−1), which is in agreement with the presence of one Y fitted at 3.87(3) Å (Table 1). The latter probably results from Y pairs adsorbed on a single c axis channel, consistent with the formation of Y polyatomic species or Y polymerisation in water (Ragnarsdottir et al., 1998Ragnarsdottir, K.V., Oelkers, E.H., Sherman, D.M., Collins, C.R. (1998) Aqueous speciation of yttrium at temperatures from 25 to 340°C at Psat: an in situ EXAFS study. Chemical Geology 151, 29–39. https://doi.org/10.1016/S0009-2541(98)00068-0
).
Figure 3 Fluorapatite crystal structure projected along c axis showing yttrium speciation models, (a) H-FAp: substitution at the Ca(2) site, (b) S-FAp: mixture of substitution at the Ca(2) site together with an inner shell adsorption at the c axis channel, (c) S-FAp: substitution at the Ca(2) site with concomitant C replacing P and Ca vacancy. Si, Na and O labels indicate potential coupled substitutions required to compensate the excess of electronic charge due to Y3+-Ca2+ substitution.
top
Yttrium Speciation in Hydrothermal Fluorapatite
The shell fitting performed on the EXAFS spectrum of hydrothermal FAp (H-FAp) reveals the presence of 7.0(7) oxygen located at 2.37(2) Å (Table 1), confirming that Y integrates the FAp crystal lattice within the 7 fold coordinated Ca(2) site by a substitution mechanism (hereafter referred to as Y(2); Fig. 3a). The fitted next nearest neighbour environment around Y, which consists of 5 P distributed between 3.09(1) and 3.70(1) Å, and 10 Ca at an average distance of 4.16(1) Å (Table 1), shows consistency with the theoretical Ca(2) atomic landscape (Tables 1, S-1b). In contrast, the radial distribution function around Ca(1) shows an intense peak in the Fourier transform (FT) magnitude (see ab initio EXAFS calculations and corresponding FT in Fig. S-2), due to the presence of 2 Ca located as close as 3.42 and 3.45 Å (Table S-1a). This feature appears strongly incompatible with the yttrium atomic landscape observed and successfully fitted in H-FAp (Fig. 2c, Table 1).
The lack of a high k contribution in the next nearest environment around Y eliminates the hypothesis of a coupled substitution involving a second heavy trivalent cation such as REE plus a vacancy (i.e. Eq. 4). Thus, the charge compensation is likely to meet the assumptions presented in Equations 1, 2 and 3, although these cannot be formally verified since the contrast in atomic number and coordination number (CN) between, respectively, Na and Ca, Si and P, and O and F are too subtle to be distinguished.
top
Yttrium Speciation in Sedimentary Fluorapatite
The fit of the S-FAp EXAFS spectrum provides, for the 1st atomic shell, an average coordination of 7.4(9) (Table 1), which is slightly higher than that obtained for H-FAp, but within the margin of error. We also note on the wavelet modulus of S-FAp (Fig. 2d), a major difference in the 3rd neighbour shell, at (R + Φ) ≈ 3.25 Å (Fig. 2c), where a clear contribution arising from 3 P and 3 O (at 3.39 and 3.67 Å, respectively) is observed for H-FAp, but absent for S-FAp. To interpret these discrepancies, we propose two distinct structural models that were successfully fitted (Table 1, Fig. 2).
The first one (blue dotted lines in Fig. 2d) involves a mixture of two speciation models: a Y(2) substitution and an inner shell Y adsorption, the latter tending to decrease the intensity of the contribution located at (R + Φ) ≈ 3.25 Å. Note that a mixture with Y(1) results in a failed fit and is therefore excluded given the absence of its typical contribution of Ca located at ∼3.43 Å (Figs. S-1a, S-2b, Table S-1a; (R + Φ) ≈ 3 Å in Fig. 2c and d). Additionally, different features of the XANES and EXAFS spectra tend to corroborate such a scenario (Fig. 1). Among them, the maximum of the white line (feature I in Fig. 1a) is significantly higher for Y-ads compared to H-FAp, while it shows an intermediate value for S-FAp. In accordance, the linear combination fit (LCF) of the EXAFS spectrum (Fig. 1b) also suggests a mixture of Y(2) and Y-ads.
The second model (red dotted lines in Fig. 2d) rather implies Y(2) together with two concomitant carbonate groups (CO3)2− replacing two phosphate groups (PO4)3− in the 3rd atomic shell. The presence of carbon in the neighbouring of yttrium causes the decrease in intensity of the (R + Φ) ≈ 3.25 Å contribution; light elements (C) having lower back scattering amplitudes than heavier elements (P). This model is in perfect agreement with i) the nature of S-FAp, which is identified as B-type carbonate-FAp (Figs. S-5, S-6), and ii) the location of structural carbonate groups, as determined by high resolution transmission electron microscopy on B-type carbonate-FAp (Kis et al., 2019
Kis, V.K., Czigány, Z., Dallos, Z., Nagy, D., Dódony, I. (2019) HRTEM study of individual bone apatite nanocrystals reveals symmetry reduction with respect to P63/m apatite. Materials Science and Engineering: C 104, 109966. https://doi.org/10.1016/j.msec.2019.109966
). Such a “Y(2) + carbonate” model implies a significant distortion of the crystal lattice (Table 1) and a co-location of C and REE, which is consistent with the results of Liao et al. (2019)Liao, J., Suna, X., Li, D., Sae, R., Lub, Y., Lin, Z., Xub, L., Zhanf, R., Pang, Y., Xuh, H. (2019) New insights into nanostructure and geochemistry of bioapatite in REE-rich deep-sea sediments: LA-ICP-MS, TEM, and Z-contrast imaging studies. Chemical Geology 512, 58–68. https://doi.org/10.1016/j.chemgeo.2019.02.039
in analogous samples. In this case, the excess of charge of Y3+ could be balanced according to the following equations:Eq. 5
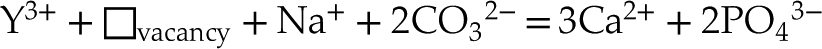
Eq. 6
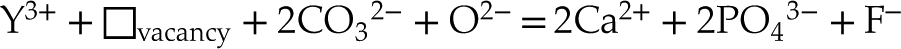
where the vacancies likely refer to Ca(2) sites, and sodium, fluorine or carbonates are most often in excess relative to rare earth elements. However, it is not possible to formally distinguish these two hypotheses using the approach proposed here, and further in situ characterisation at the micrometric scale is required.
top
Geochemical Implications
In accordance with the crystal lattice strain partitioning model, it is proposed that the partition coefficient of REE in magmatic-hydrothermal FAp originates from the crystallographic preference of HREE for the Ca(1) site, and LREE for the Ca(2) site (Hughes et al., 1991
Hughes, J.M., Cameron, M., Mariano, A.N. (1991) Rare-earth-element ordering and structural variations in natural rare-earth-bearing apatites. American Mineralogist 76, 1165–1173. http://www.minsocam.org/ammin/AM76/AM76_1165.pdf
). However, in the case of Durango FAp, we clearly point out that Y substitutes for Ca in the Ca(2) site. This could suggest that a partition model involving the Ca(1) site for HREE is not appropriate for the Durango FAp, where a HREE-depleted normalised pattern (Fleet and Pan, 1995Fleet, M.E., Pan, Y. (1995) Site preference of rare earth elements in fluorapatite. American Mineralogist 80, 329–335. https://doi.org/10.2138/am-1995-3-414
) could rather reflect the progressive ionic incompatibility of REE for the Ca(2) site along the lanthanide series. These assumptions should be taken with caution given that the Durango FAp does not prevail for all other FAp, and given that REE patterns may vary significantly depending on the fluid conditions (Krneta et al., 2018Krneta, S., Ciobanu, C.L., Cook, N.J., Ehrig, K.J. (2018) Numerical Modeling of REE Fractionation Patterns in Fluorapatite from the Olympic Dam Deposit (South Australia). Minerals 8, 342. https://doi.org/10.3390/min8080342
). Indeed, it is possible that the Durango H-FAp undergoes geochemical processes that favour Ca(2), as diffusion of REE in the Ca(2) site through the c axis channel may occur during thermal events (Cherniak, 2000Cherniak, D.J. (2000) Rare earth element diffusion in apatite. Geochimica et Cosmochimica Acta 64, 3871–3885. https://doi.org/10.1016/S0016-7037(00)00467-1
). Furthermore, the different complexation modes in solution play a role in the distribution of REE between Ca(2) and Ca(1) sites involving Na or Si coupled substitutions (Mackie and Young, 1973Mackie, P.E., Young, R.A. (1973) Location of Nd dopant in fluorapatite, Ca5(PO4)3F: Nd. Journal of Applied Crystallography 6, 26–31. https://doi.org/10.1107/S0021889873008009
). For example, the preference of REE for Ca(2) decreases with increasing Si content or temperature in the 700–1200 °C range (Khudolozhkin et al., 1973Khudolozhkin, V.O., Urusov, V.S., Tobelko, K.I., Vernadskiy, V.I. (1973) Dependence of structural ordering of rare earth atoms in the isomorphous series apatite-britholite (abukumalite) on composition and temperature. Geochemical International 10, 1171–1177.
; Pan and Fleet, 2002Pan, Y., Fleet, M.E. (2002) Compositions of the Apatite-Group Minerals: Substitution Mechanisms and Controlling Factors. Reviews in Mineralogy and Geochemistry 48, 13–49. https://doi.org/10.2138/rmg.2002.48.2
and references therein). This could confirm that the Durango FAp was more likely formed at a medium temperature with saline fluids, in agreement with the literature (Gleason et al., 2000Gleason, J.D., Marikos, M.A., Barton, M.D., Johnson, D.A. (2000) Neodymium isotopic study of rare earth element sources and mobility in hydrothermal Fe oxide (Fe-P-REE) systems. Geochimica et Cosmochimica Acta 64, 1059–1068. https://doi.org/10.1016/S0016-7037(99)00325-7
and references therein).In this perspective, systematic in situ X-ray absorption studies of REE on other natural FAp could provide more information on the hydrothermal-magmatic fluids or be used as a geothermometer, which could lead to a major re-interpretation of geochemical processes and crystallisation conditions (Krneta et al., 2018
Krneta, S., Ciobanu, C.L., Cook, N.J., Ehrig, K.J. (2018) Numerical Modeling of REE Fractionation Patterns in Fluorapatite from the Olympic Dam Deposit (South Australia). Minerals 8, 342. https://doi.org/10.3390/min8080342
). This could also make it possible to decipher the effect of extrinsic (e.g., temperature, source fluids) and intrinsic (e.g., crystal-chemical) variables on REE patterns (Rakovan et al., 2001Rakovan, J., Newville, M., Sutton, S. (2001) Evidence of heterovalent europium in zoned Llallgua apatite using wavelength dispersive XANES. American Mineralogist 86, 697–700. https://doi.org/10.2138/am-2001-5-610
; Borst et al., 2020Borst, A.M., Finch, A.A., Friis, H., Horsburgh, N.J., Gamaletsos, P.N., Goettlicher, J., Steininger, R., Geraki, K. (2020) Structural state of rare earth elements in eudialyte-group minerals. Mineralogical Magazine 84, 19–34. https://doi.org/10.1180/mgm.2019.50
).For sedimentary FAp, the assumptions for Y speciation, which include either mixed “Y(2) + Y-ads” speciation or the “Y(2) + carbonates” speciation, are both compatible with an adsorption-diffusion-substitution process (Koeppenkastrop and de Carlo, 1992
Koeppenkastrop, D., de Carlo, E.H. (1992) Sorption of rare-earth elements from seawater onto synthetic mineral particles: An experimental approach. Chemical Geology 95, 251–263. https://doi.org/10.1016/0009-2541(92)90015-W
). Both models can explain why REE in carbonated-FAp show a normalised pattern without fractionation, characterised by HREE enrichment so-called “past seawater pattern” (Reynard et al., 1999Reynard, B., Lécuyer, C., Grandjean, P. (1999) Crystal-chemical controls on rare-earth element concentrations in fossil biogenic apatites and implications for paleoenvironmental reconstructions. Chemical Geology 155, 233–241. https://doi.org/10.1016/S0009-2541(98)00169-7
). The Y(2) + carbonate model is particularly relevant because REE are mainly complexed by carbonate groups in seawater, which also shows a HREE-enriched pattern (Schijf and Byrne, 2021Schijf, J., Byrne, R.H. (2021) Speciation of yttrium and the rare earth elements in seawater: Review of a 20-year analytical journey. Chemical Geology 584, 120479. https://doi.org/10.1016/j.chemgeo.2021.120479
). We thus assume that the conservative (i.e. without fractionation) REE uptake in FAp is likely to be promoted by carbonate complexation, where both REE and carbonates are trapped in the FAp lattice during early diagenesis by their direct uptake from the hydrated layer (Cazalbou et al., 2004Cazalbou, S., Eichert, D., Drouet, C., Combes, C., Rey, C. (2004) Minéralisations biologiques à base de phosphate de calcium. Comptes Rendus Palevol 3, 563–572. https://doi.org/10.1016/j.crpv.2004.07.003
). Since REE and carbonates are mainly enriched concomitantly in a strongly distorted crystal fringe located in the outer edges of FAp nanocrystallites (Liao et al., 2019Liao, J., Suna, X., Li, D., Sae, R., Lub, Y., Lin, Z., Xub, L., Zhanf, R., Pang, Y., Xuh, H. (2019) New insights into nanostructure and geochemistry of bioapatite in REE-rich deep-sea sediments: LA-ICP-MS, TEM, and Z-contrast imaging studies. Chemical Geology 512, 58–68. https://doi.org/10.1016/j.chemgeo.2019.02.039
; in agreement with our EXAFS results), the crystal chemistry effect on the partitioning is inhibited while the effect of the fluid complexation (i.e. REE carbonate ions in seawater or porewater) is predominantly recorded, which likely implies non-Henry’s Law behaviour for the REE partitioning in S-FAp (Pan et al., 2003Pan, Y., Dong, P., Chen, N. (2003) Non-Henry’s Law behavior of REE partitioning between fluorapatite and CaF2-rich melts: Controls of intrinsic vacancies and implications for natural apatites. Geochimica et Cosmochimica Acta 67, 1889–1900. https://doi.org/10.1016/S0016-7037(02)01285-1
).Therefore, in situ X-ray absorption studies of REE could be applied to marine geochemistry to estimate whether overprinting of REE FAp fractionation has occurred, and whether a reliable seawater-porewater signature is preserved. This is particularly valuable for examining temporally laminated structures containing FAp, such as phosphorite grains or some polymetallic nodules, used to unravel past seawater conditions and their temporal changes like pH, redox, or ocean circulation (Kocsis et al., 2016
Kocsis, L., Gheerbrant, E., Mouflih, M., Cappetta, H., Ulianov, A., Chiaradia, M., Bardet, N. (2016) Gradual changes in upwelled seawater conditions (redox, pH) from the late Cretaceous through early Paleogene at the northwest coast of Africa: Negative Ce anomaly trend recorded in fossil bio-apatite. Chemical Geology 421, 44–54. https://doi.org/10.1016/j.chemgeo.2015.12.001
).top
Conclusions
We studied the speciation of yttrium, a geochemical proxy for HREE, in hydrothermal and sedimentary FAp minerals using X-ray absorption spectroscopy. Analyses of Y K-edge XANES and EXAFS spectra, including ab initio calculations, shell fitting, wavelet analysis and linear combination show that:
- Y exclusively substitutes for Ca in the Ca(2) site in the hydrothermal Durango FAp;
- Y can be found either as a mixture of Ca(2) substituted and inner shell tridentate adsorbate in the c axis channel in sedimentary FAp, or as substitution for the Ca(2) site with coupled substitution of a carbonate group in the direct surrounding atomic environment in B-type carbonated FAp.
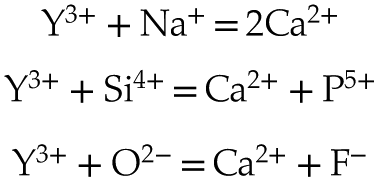
For the sedimentary B-type carbonated FAp, we suggest that the following substitutions are likely to occur:
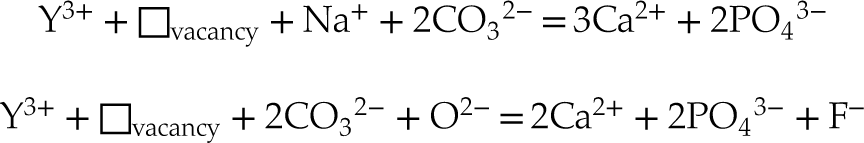
top
Acknowledgments
Work carried out within the framework of the scientific cooperation agreement between the Mohammed VI Polytechnic University, the University of Montpellier and the Centre National de la Recherche Scientifique [Specific Agreement n°UM190775 on “The multi-scale distribution of minor and trace elements in Moroccan phosphate basins”]. We also acknowledge the European Synchrotron Radiation Facility for provision of synchrotron radiation facilities. We thank Bernard Fraisse for his technical assistance with the XRD measurements at the RRXG platform (Réseau des Rayons X et Gamma) of University of Montpellier (Montpellier, France). We would also like to thank Valérie Magnin for her contribution to the micro-XRF data carried out at the ISTerre laboratory (Grenoble, France).
Editor: Satish Myneni
top
Author Contributions
JLB and AE participated in the sediment sampling strategy. VMR carried out the acquisition and processing of the LIBS data. JA provided FTIR measurements. CB carried out the XRF and XRD measurements. CB, MM and OM performed XANES and EXAFS acquisitions which were then processed by CB and MM. CB and MM wrote the manuscript. All authors participated to discussions.
top
References
Aubineau, J., Parat, F., Elghali, A., Raji, O., Addou, A., Bonnet, C., Muñoz, M., Mauguin, O., Baron, F., Jouti, M.B., Yazamid, O.K., Bodinier, J.-L. (2022) Highly variable content of fluorapatite-hosted CO32− in the Upper Cretaceous/ Paleogene phosphorites (Morocco) and implications for paleodepositional conditions. Chemical Geology 597, 120818. https://doi.org/10.1016/j.chemgeo.2022.120818
 Show in context
Show in context However, determining the sorption models of REE is not systematically well constrained and is at the heart of many studies since FAp can potentially integrate REE in two distinct crystallographic Ca sites, namely the 9 fold coordinated Ca(1) and the 7 fold coordinated Ca(2) sites (Hughes et al., 1991) (Fig. S-1a, b), and can also show nano-crystallinity typically observed in sedimentary deposits (Aubineau et al., 2022) that may favour adsorption mechanisms and apparent no-fractionation behaviour (Reynard et al., 1999).
View in article
Blundy, J., Wood, B. (2003) Partitioning of trace elements between crystals and melts. Earth and Planetary Science Letters 210, 383–397. https://doi.org/10.1016/S0012-821X(03)00129-8
 Show in context
Show in context The crystal chemistry of REE in FAp also controls its partitioning and normalised patterns (Blundy and Wood, 2003).
View in article
Fluorapatite (FAp; Ca5(PO4)3F) is an accessory mineral that hosts significant amounts of rare earth elements (REE, including yttrium) from ∼200 to 20,000 ppm (Emsbo et al., 2015), where Y exhibits similar behaviour to heavy rare earth elements (HREE, from Gd to Lu) and is therefore associated with them. For Earth Sciences, REE normalised patterns in FAp are proxies to i) reconstruct partitioning models and determine partition coefficients (Blundy and Wood, 2003), ii) identify the source fluids, their chemical composition and their REE complexation (Mackie and Young, 1973; Krneta et al., 2018), iii) characterise the deposition/crystallisation conditions such as temperature, pH, redox (Chen et al., 2002; Kocsis et al., 2016), and iv) reveal the potential late diagenetic or hydrothermal alterations (Reynard et al., 1999; Cherniak, 2000).
View in article
Borisov, S.V., Klevtśova, R.F. (1963) The crystal structure of TR-Sr-apatite. Journal of Structural Chemistry 4, 575–577. https://doi.org/10.1007/BF00747639
 Show in context
Show in context Moreover, Borisov and Klevtśova (1963) have demonstrated that substitutions of both LREE and HREE for Ca occurred only at the Ca(2) site which was confirmed by EPR (Electron Paramagnetic Resonance) spectroscopy (Chen et al., 2002).
View in article
Borst, A.M., Finch, A.A., Friis, H., Horsburgh, N.J., Gamaletsos, P.N., Goettlicher, J., Steininger, R., Geraki, K. (2020) Structural state of rare earth elements in eudialyte-group minerals. Mineralogical Magazine 84, 19–34. https://doi.org/10.1180/mgm.2019.50
 Show in context
Show in context This could also make it possible to decipher the effect of extrinsic (e.g., temperature, source fluids) and intrinsic (e.g., crystal-chemical) variables on REE patterns (Rakovan et al., 2001; Borst et al., 2020).
View in article
Cazalbou, S., Eichert, D., Drouet, C., Combes, C., Rey, C. (2004) Minéralisations biologiques à base de phosphate de calcium. Comptes Rendus Palevol 3, 563–572. https://doi.org/10.1016/j.crpv.2004.07.003
 Show in context
Show in context We thus assume that the conservative (i.e. without fractionation) REE uptake in FAp is likely to be promoted by carbonate complexation, where both REE and carbonates are trapped in the FAp lattice during early diagenesis by their direct uptake from the hydrated layer (Cazalbou et al., 2004).
View in article
Chen, N., Pan, Y., Weil, J.A. (2002) Electron paramagnetic resonance spectroscopic study of synthetic fluorapatite: Part I. Local structural environment and substitution mechanism of Gd3+ at the Ca2 site. American Mineralogist 87, 37–46. https://doi.org/10.2138/am-2002-0105
 Show in context
Show in context Moreover, Borisov and Klevtśova (1963) have demonstrated that substitutions of both LREE and HREE for Ca occurred only at the Ca(2) site which was confirmed by EPR (Electron Paramagnetic Resonance) spectroscopy (Chen et al., 2002).
View in article
Fluorapatite (FAp; Ca5(PO4)3F) is an accessory mineral that hosts significant amounts of rare earth elements (REE, including yttrium) from ∼200 to 20,000 ppm (Emsbo et al., 2015), where Y exhibits similar behaviour to heavy rare earth elements (HREE, from Gd to Lu) and is therefore associated with them. For Earth Sciences, REE normalised patterns in FAp are proxies to i) reconstruct partitioning models and determine partition coefficients (Blundy and Wood, 2003), ii) identify the source fluids, their chemical composition and their REE complexation (Mackie and Young, 1973; Krneta et al., 2018), iii) characterise the deposition/crystallisation conditions such as temperature, pH, redox (Chen et al., 2002; Kocsis et al., 2016), and iv) reveal the potential late diagenetic or hydrothermal alterations (Reynard et al., 1999; Cherniak, 2000).
View in article
Cherniak, D.J. (2000) Rare earth element diffusion in apatite. Geochimica et Cosmochimica Acta 64, 3871–3885. https://doi.org/10.1016/S0016-7037(00)00467-1
 Show in context
Show in context In addition, it can potentially provide valuable insight into the crystallisation conditions such as temperature (Khudolozhkin et al., 1973; Pan and Fleet, 2002 and references therein), fluids (Mackie and Young, 1973) or diffusion (Cherniak, 2000).
View in article
Indeed, it is possible that the Durango H-FAp undergoes geochemical processes that favour Ca(2), as diffusion of REE in the Ca(2) site through the c axis channel may occur during thermal events (Cherniak, 2000).
View in article
Fluorapatite (FAp; Ca5(PO4)3F) is an accessory mineral that hosts significant amounts of rare earth elements (REE, including yttrium) from ∼200 to 20,000 ppm (Emsbo et al., 2015), where Y exhibits similar behaviour to heavy rare earth elements (HREE, from Gd to Lu) and is therefore associated with them. For Earth Sciences, REE normalised patterns in FAp are proxies to i) reconstruct partitioning models and determine partition coefficients (Blundy and Wood, 2003), ii) identify the source fluids, their chemical composition and their REE complexation (Mackie and Young, 1973; Krneta et al., 2018), iii) characterise the deposition/crystallisation conditions such as temperature, pH, redox (Chen et al., 2002; Kocsis et al., 2016), and iv) reveal the potential late diagenetic or hydrothermal alterations (Reynard et al., 1999; Cherniak, 2000).
View in article
Emsbo, P., McLaughlin, P.I., Breit, G.N., du Bray, E.A., Koenig, A.E. (2015) Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Research 27, 776–785. https://doi.org/10.1016/j.gr.2014.10.008
 Show in context
Show in context Fluorapatite (FAp; Ca5(PO4)3F) is an accessory mineral that hosts significant amounts of rare earth elements (REE, including yttrium) from ∼200 to 20,000 ppm (Emsbo et al., 2015), where Y exhibits similar behaviour to heavy rare earth elements (HREE, from Gd to Lu) and is therefore associated with them. For Earth Sciences, REE normalised patterns in FAp are proxies to i) reconstruct partitioning models and determine partition coefficients (Blundy and Wood, 2003), ii) identify the source fluids, their chemical composition and their REE complexation (Mackie and Young, 1973; Krneta et al., 2018), iii) characterise the deposition/crystallisation conditions such as temperature, pH, redox (Chen et al., 2002; Kocsis et al., 2016), and iv) reveal the potential late diagenetic or hydrothermal alterations (Reynard et al., 1999; Cherniak, 2000).
View in article
Faucher, M., Pannetier, J. (1980) Refinement of the Y2O3 structure at 77 K. Acta Crystallographica Section B 36, 3209–3211. https://doi.org/10.1107/S0567740880011351
 Show in context
Show in context Theoretical structures references: (1) Faucher and Pannetier (1980); (2) Ni et al. (1995); (3) Hughes et al. (1990).
View in article
Fleet, M.E., Pan, Y. (1995) Site preference of rare earth elements in fluorapatite. American Mineralogist 80, 329–335. https://doi.org/10.2138/am-1995-3-414
 Show in context
Show in context This could suggest that a partition model involving the Ca(1) site for HREE is not appropriate for the Durango FAp, where a HREE-depleted normalised pattern (Fleet and Pan, 1995) could rather reflect the progressive ionic incompatibility of REE for the Ca(2) site along the lanthanide series.
View in article
Conversely, X-ray diffraction structure refinements on synthetic REE-doped FAp suggest that LREE are favoured in Ca(2) while HREE are favoured in Ca(1) (Hughes et al., 1991; Fleet and Pan, 1995).
View in article
Gleason, J.D., Marikos, M.A., Barton, M.D., Johnson, D.A. (2000) Neodymium isotopic study of rare earth element sources and mobility in hydrothermal Fe oxide (Fe-P-REE) systems. Geochimica et Cosmochimica Acta 64, 1059–1068. https://doi.org/10.1016/S0016-7037(99)00325-7
 Show in context
Show in context This could confirm that the Durango FAp was more likely formed at a medium temperature with saline fluids, in agreement with the literature (Gleason et al., 2000 and references therein).
View in article
Hughes, J.M., Cameron, M., Crowley, K.D. (1990) Crystal structures of natural ternary apatites: Solid solution in the Ca5(PO4)3X (X = F, OH, Cl) system. American Mineralogist 75, 295–304. http://www.minsocam.org/ammin/AM75/AM75_295.pdf
 Show in context
Show in context Theoretical structures references: (1) Faucher and Pannetier (1980); (2) Ni et al. (1995); (3) Hughes et al. (1990).
View in article
Hughes, J.M., Cameron, M., Mariano, A.N. (1991) Rare-earth-element ordering and structural variations in natural rare-earth-bearing apatites. American Mineralogist 76, 1165–1173. http://www.minsocam.org/ammin/AM76/AM76_1165.pdf
 Show in context
Show in context However, determining the sorption models of REE is not systematically well constrained and is at the heart of many studies since FAp can potentially integrate REE in two distinct crystallographic Ca sites, namely the 9 fold coordinated Ca(1) and the 7 fold coordinated Ca(2) sites (Hughes et al., 1991) (Fig. S-1a, b), and can also show nano-crystallinity typically observed in sedimentary deposits (Aubineau et al., 2022) that may favour adsorption mechanisms and apparent no-fractionation behaviour (Reynard et al., 1999).
View in article
In accordance with the crystal lattice strain partitioning model, it is proposed that the partition coefficient of REE in magmatic-hydrothermal FAp originates from the crystallographic preference of HREE for the Ca(1) site, and LREE for the Ca(2) site (Hughes et al., 1991).
View in article
Conversely, X-ray diffraction structure refinements on synthetic REE-doped FAp suggest that LREE are favoured in Ca(2) while HREE are favoured in Ca(1) (Hughes et al., 1991; Fleet and Pan, 1995).
View in article
Kashiwabara, T., Toda, R., Nakamura, K., Yasukawa, K., Fujinaga, K., Kubo, S., Nozaki, T., Takahashi, Y., Suzuki, K., Kato, Y. (2018) Synchrotron X-ray spectroscopic perspective on the formation mechanism of REY-rich muds in the Pacific Ocean. Geochimica et Cosmochimica Acta 240, 274–292. https://doi.org/10.1016/j.gca.2018.08.013
 Show in context
Show in context In natural marine environments, Kashiwabara et al. (2018) proposed that deep sea muds mostly trap REE by adsorption onto FAp crystals.
View in article
Khudolozhkin, V.O., Urusov, V.S., Tobelko, K.I., Vernadskiy, V.I. (1973) Dependence of structural ordering of rare earth atoms in the isomorphous series apatite-britholite (abukumalite) on composition and temperature. Geochemical International 10, 1171–1177.
 Show in context
Show in context In addition, it can potentially provide valuable insight into the crystallisation conditions such as temperature (Khudolozhkin et al., 1973; Pan and Fleet, 2002 and references therein), fluids (Mackie and Young, 1973) or diffusion (Cherniak, 2000).
View in article
For example, the preference of REE for Ca(2) decreases with increasing Si content or temperature in the 700–1200 °C range (Khudolozhkin et al., 1973; Pan and Fleet, 2002 and references therein).
View in article
Kis, V.K., Czigány, Z., Dallos, Z., Nagy, D., Dódony, I. (2019) HRTEM study of individual bone apatite nanocrystals reveals symmetry reduction with respect to P63/m apatite. Materials Science and Engineering: C 104, 109966. https://doi.org/10.1016/j.msec.2019.109966
 Show in context
Show in context This model is in perfect agreement with i) the nature of S-FAp, which is identified as B-type carbonate-FAp (Figs. S-5, S-6), and ii) the location of structural carbonate groups, as determined by high resolution transmission electron microscopy on B-type carbonate-FAp (Kis et al., 2019).
View in article
Kocsis, L., Gheerbrant, E., Mouflih, M., Cappetta, H., Ulianov, A., Chiaradia, M., Bardet, N. (2016) Gradual changes in upwelled seawater conditions (redox, pH) from the late Cretaceous through early Paleogene at the northwest coast of Africa: Negative Ce anomaly trend recorded in fossil bio-apatite. Chemical Geology 421, 44–54. https://doi.org/10.1016/j.chemgeo.2015.12.001
 Show in context
Show in context This is particularly valuable for examining temporally laminated structures containing FAp, such as phosphorite grains or some polymetallic nodules, used to unravel past seawater conditions and their temporal changes like pH, redox, or ocean circulation (Kocsis et al., 2016).
View in article
Fluorapatite (FAp; Ca5(PO4)3F) is an accessory mineral that hosts significant amounts of rare earth elements (REE, including yttrium) from ∼200 to 20,000 ppm (Emsbo et al., 2015), where Y exhibits similar behaviour to heavy rare earth elements (HREE, from Gd to Lu) and is therefore associated with them. For Earth Sciences, REE normalised patterns in FAp are proxies to i) reconstruct partitioning models and determine partition coefficients (Blundy and Wood, 2003), ii) identify the source fluids, their chemical composition and their REE complexation (Mackie and Young, 1973; Krneta et al., 2018), iii) characterise the deposition/crystallisation conditions such as temperature, pH, redox (Chen et al., 2002; Kocsis et al., 2016), and iv) reveal the potential late diagenetic or hydrothermal alterations (Reynard et al., 1999; Cherniak, 2000).
View in article
Koeppenkastrop, D., de Carlo, E.H. (1992) Sorption of rare-earth elements from seawater onto synthetic mineral particles: An experimental approach. Chemical Geology 95, 251–263. https://doi.org/10.1016/0009-2541(92)90015-W
 Show in context
Show in context For sedimentary FAp, the assumptions for Y speciation, which include either mixed “Y(2) + Y-ads” speciation or the “Y(2) + carbonates” speciation, are both compatible with an adsorption-diffusion-substitution process (Koeppenkastrop and de Carlo, 1992).
View in article
Krneta, S., Ciobanu, C.L., Cook, N.J., Ehrig, K.J. (2018) Numerical Modeling of REE Fractionation Patterns in Fluorapatite from the Olympic Dam Deposit (South Australia). Minerals 8, 342. https://doi.org/10.3390/min8080342
 Show in context
Show in context These assumptions should be taken with caution given that the Durango FAp does not prevail for all other FAp, and given that REE patterns may vary significantly depending on the fluid conditions (Krneta et al., 2018).
View in article
In this perspective, systematic in situ X-ray absorption studies of REE on other natural FAp could provide more information on the hydrothermal-magmatic fluids or be used as a geothermometer, which could lead to a major re-interpretation of geochemical processes and crystallisation conditions (Krneta et al., 2018).
View in article
Fluorapatite (FAp; Ca5(PO4)3F) is an accessory mineral that hosts significant amounts of rare earth elements (REE, including yttrium) from ∼200 to 20,000 ppm (Emsbo et al., 2015), where Y exhibits similar behaviour to heavy rare earth elements (HREE, from Gd to Lu) and is therefore associated with them. For Earth Sciences, REE normalised patterns in FAp are proxies to i) reconstruct partitioning models and determine partition coefficients (Blundy and Wood, 2003), ii) identify the source fluids, their chemical composition and their REE complexation (Mackie and Young, 1973; Krneta et al., 2018), iii) characterise the deposition/crystallisation conditions such as temperature, pH, redox (Chen et al., 2002; Kocsis et al., 2016), and iv) reveal the potential late diagenetic or hydrothermal alterations (Reynard et al., 1999; Cherniak, 2000).
View in article
Liao, J., Suna, X., Li, D., Sae, R., Lub, Y., Lin, Z., Xub, L., Zhanf, R., Pang, Y., Xuh, H. (2019) New insights into nanostructure and geochemistry of bioapatite in REE-rich deep-sea sediments: LA-ICP-MS, TEM, and Z-contrast imaging studies. Chemical Geology 512, 58–68. https://doi.org/10.1016/j.chemgeo.2019.02.039
 Show in context
Show in context Such a “Y(2) + carbonate” model implies a significant distortion of the crystal lattice (Table 1) and a co-location of C and REE, which is consistent with the results of Liao et al. (2019) in analogous samples.
View in article
Since REE and carbonates are mainly enriched concomitantly in a strongly distorted crystal fringe located in the outer edges of FAp nanocrystallites (Liao et al., 2019; in agreement with our EXAFS results), the crystal chemistry effect on the partitioning is inhibited while the effect of the fluid complexation (i.e. REE carbonate ions in seawater or porewater) is predominantly recorded, which likely implies non-Henry’s Law behaviour for the REE partitioning in S-FAp (Pan et al., 2003).
View in article
Mackie, P.E., Young, R.A. (1973) Location of Nd dopant in fluorapatite, Ca5(PO4)3F: Nd. Journal of Applied Crystallography 6, 26–31. https://doi.org/10.1107/S0021889873008009
 Show in context
Show in context Fluorapatite (FAp; Ca5(PO4)3F) is an accessory mineral that hosts significant amounts of rare earth elements (REE, including yttrium) from ∼200 to 20,000 ppm (Emsbo et al., 2015), where Y exhibits similar behaviour to heavy rare earth elements (HREE, from Gd to Lu) and is therefore associated with them. For Earth Sciences, REE normalised patterns in FAp are proxies to i) reconstruct partitioning models and determine partition coefficients (Blundy and Wood, 2003), ii) identify the source fluids, their chemical composition and their REE complexation (Mackie and Young, 1973; Krneta et al., 2018), iii) characterise the deposition/crystallisation conditions such as temperature, pH, redox (Chen et al., 2002; Kocsis et al., 2016), and iv) reveal the potential late diagenetic or hydrothermal alterations (Reynard et al., 1999; Cherniak, 2000).
View in article
Furthermore, the different complexation modes in solution play a role in the distribution of REE between Ca(2) and Ca(1) sites involving Na or Si coupled substitutions (Mackie and Young, 1973).
View in article
In addition, it can potentially provide valuable insight into the crystallisation conditions such as temperature (Khudolozhkin et al., 1973; Pan and Fleet, 2002 and references therein), fluids (Mackie and Young, 1973) or diffusion (Cherniak, 2000).
View in article
Muñoz, M., Argoul, P., Farges, F. (2003) Continuous Cauchy wavelet transform analyses of EXAFS spectra: A qualitative approach. American Mineralogist 88, 694–700. https://doi.org/10.2138/am-2003-0423
 Show in context
Show in context To contribute to the understanding of the crystal chemistry of REE, and more specifically HREE in FAp and geochemical implications that follows, we propose a direct characterisation of the yttrium speciation (i.e. fixation modes, coupled substitutions and crystallographic preferences) using X-ray absorption spectroscopy (XAS) at the Y K-edge. XANES (X-ray absorption near edge structure) and EXAFS (extended absorption near edge structure) spectra are analysed by quantitative refinements, linear combinations and wavelet analysis (Muñoz et al., 2003).
View in article
Ni, Y., Hughes, J.M., Mariano, A.N. (1995) Crystal chemistry of the monazite and xenotime structures. American Mineralogist 80, 21–26. https://doi.org/10.2138/am-1995-1-203
 Show in context
Show in context Theoretical structures references: (1) Faucher and Pannetier (1980); (2) Ni et al. (1995); (3) Hughes et al. (1990).
View in article
Note that in such a configuration (Fig. 3b), a typical O–Y–O angle of about 73°, characteristic of the regular 8 fold polyhedron, is preserved (Ni et al., 1995).
View in article
Pan, Y., Fleet, M.E. (2002) Compositions of the Apatite-Group Minerals: Substitution Mechanisms and Controlling Factors. Reviews in Mineralogy and Geochemistry 48, 13–49. https://doi.org/10.2138/rmg.2002.48.2
 Show in context
Show in context In order to maintain electroneutrality, each of the above sorption models may involve coupled substitutions such as (Pan and Fleet, 2002 and references therein).
View in article
In addition, it can potentially provide valuable insight into the crystallisation conditions such as temperature (Khudolozhkin et al., 1973; Pan and Fleet, 2002 and references therein), fluids (Mackie and Young, 1973) or diffusion (Cherniak, 2000).
View in article
For example, the preference of REE for Ca(2) decreases with increasing Si content or temperature in the 700–1200 °C range (Khudolozhkin et al., 1973; Pan and Fleet, 2002 and references therein).
View in article
Pan, Y., Dong, P., Chen, N. (2003) Non-Henry’s Law behavior of REE partitioning between fluorapatite and CaF2-rich melts: Controls of intrinsic vacancies and implications for natural apatites. Geochimica et Cosmochimica Acta 67, 1889–1900. https://doi.org/10.1016/S0016-7037(02)01285-1
 Show in context
Show in context Since REE and carbonates are mainly enriched concomitantly in a strongly distorted crystal fringe located in the outer edges of FAp nanocrystallites (Liao et al., 2019; in agreement with our EXAFS results), the crystal chemistry effect on the partitioning is inhibited while the effect of the fluid complexation (i.e. REE carbonate ions in seawater or porewater) is predominantly recorded, which likely implies non-Henry’s Law behaviour for the REE partitioning in S-FAp (Pan et al., 2003).
View in article
Ragnarsdottir, K.V., Oelkers, E.H., Sherman, D.M., Collins, C.R. (1998) Aqueous speciation of yttrium at temperatures from 25 to 340°C at Psat: an in situ EXAFS study. Chemical Geology 151, 29–39. https://doi.org/10.1016/S0009-2541(98)00068-0
 Show in context
Show in context Regarding Y-ads, the EXAFS shell fitting reveals the presence of 8.1(8) oxygen 1st neighbours located at 2.38(1) Å (Fig. 2b, Table 1), which is consistent for Y-adsorbed species (Ragnarsdottir et al., 1998).
View in article
The latter probably results from Y pairs adsorbed on a single c axis channel, consistent with the formation of Y polyatomic species or Y polymerisation in water (Ragnarsdottir et al., 1998).
View in article
Rakovan, J., Newville, M., Sutton, S. (2001) Evidence of heterovalent europium in zoned Llallgua apatite using wavelength dispersive XANES. American Mineralogist 86, 697–700. https://doi.org/10.2138/am-2001-5-610
 Show in context
Show in context This could also make it possible to decipher the effect of extrinsic (e.g., temperature, source fluids) and intrinsic (e.g., crystal-chemical) variables on REE patterns (Rakovan et al., 2001; Borst et al., 2020).
View in article
Reynard, B., Lécuyer, C., Grandjean, P. (1999) Crystal-chemical controls on rare-earth element concentrations in fossil biogenic apatites and implications for paleoenvironmental reconstructions. Chemical Geology 155, 233–241. https://doi.org/10.1016/S0009-2541(98)00169-7
 Show in context
Show in context However, determining the sorption models of REE is not systematically well constrained and is at the heart of many studies since FAp can potentially integrate REE in two distinct crystallographic Ca sites, namely the 9 fold coordinated Ca(1) and the 7 fold coordinated Ca(2) sites (Hughes et al., 1991) (Fig. S-1a, b), and can also show nano-crystallinity typically observed in sedimentary deposits (Aubineau et al., 2022) that may favour adsorption mechanisms and apparent no-fractionation behaviour (Reynard et al., 1999).
View in article
Similarly, Reynard et al. (1999) conclude that the REE adsorption is likely to occur in the case of sedimentary/biogenic FAp.
View in article
Both models can explain why REE in carbonated-FAp show a normalised pattern without fractionation, characterised by HREE enrichment so-called “past seawater pattern” (Reynard et al., 1999).
View in article
Fluorapatite (FAp; Ca5(PO4)3F) is an accessory mineral that hosts significant amounts of rare earth elements (REE, including yttrium) from ∼200 to 20,000 ppm (Emsbo et al., 2015), where Y exhibits similar behaviour to heavy rare earth elements (HREE, from Gd to Lu) and is therefore associated with them. For Earth Sciences, REE normalised patterns in FAp are proxies to i) reconstruct partitioning models and determine partition coefficients (Blundy and Wood, 2003), ii) identify the source fluids, their chemical composition and their REE complexation (Mackie and Young, 1973; Krneta et al., 2018), iii) characterise the deposition/crystallisation conditions such as temperature, pH, redox (Chen et al., 2002; Kocsis et al., 2016), and iv) reveal the potential late diagenetic or hydrothermal alterations (Reynard et al., 1999; Cherniak, 2000).
View in article
Schijf, J., Byrne, R.H. (2021) Speciation of yttrium and the rare earth elements in seawater: Review of a 20-year analytical journey. Chemical Geology 584, 120479. https://doi.org/10.1016/j.chemgeo.2021.120479
 Show in context
Show in context The Y(2) + carbonate model is particularly relevant because REE are mainly complexed by carbonate groups in seawater, which also shows a HREE-enriched pattern (Schijf and Byrne, 2021).
View in article
Urusov, V.S., Khudolozhkin, V.O. (1974) An energy analysis of cation ordering in apatite. Geochemistry International 11, 1048–1053.
 Show in context
Show in context In a study based on ligand type, Urusov and Khudolozhkin (1974) suggested that light (L)REE preferentially occupy the more covalent Ca(l) position, while HREE display preference for the more ionic Ca(2) position.
View in article
top
Supplementary Information
The Supplementary Information includes:
○ 1.1 Natural Fluorapatite Samples
○ 1.2 Model Compounds
○ 2.1. X-ray Fluorescence (XRF)
○ 2.2. X-ray Diffraction (XRD)
○ 2.3. Laser Induced Breakdown Spectroscopy (LIBS)
○ 2.4. Fourier Transform Infrared Spectroscopy (FTIR)
○ 2.5. X-ray Absorption Spectroscopy (XAS)
■ 2.5.1. XAS pellets
■ 2.5.2. Data acquisition
■ 2.5.3. XANES and EXAFS data reduction
■ 2.5.4. Wavelet analysis
■ 2.5.5. Linear combination fit (LCF)
■ 2.5.6. EXAFS and Fourier transform multi-shell fit
Download the Supplementary Information (PDF)
Figures
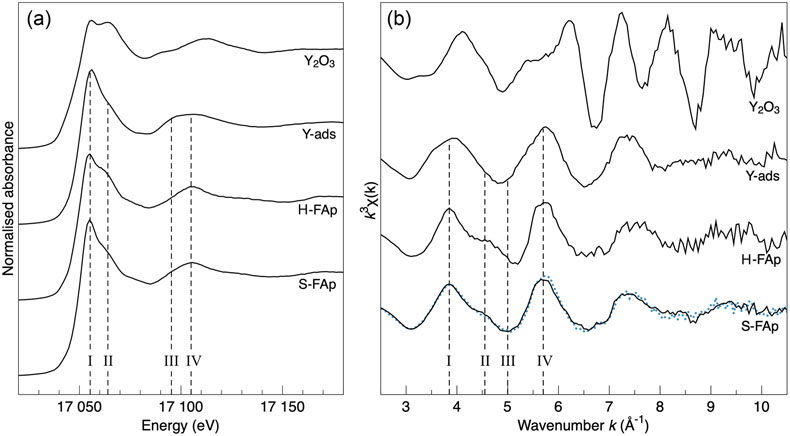
Figure 1 X-ray absorption spectra collected at the Y K-edge for Y2O3 and Y-ads FAp model compounds, and H-FAp and S-FAp samples. (a) Normalised XANES spectra, (b) k3-weighted EXAFS spectra. Roman numerals (I to IV) refer to characteristic spectral features. Blue dotted line represents the linear combination fit (LCF) result based on Y-ads and H-FAp components, suggesting a mixture of 59 % Y substituted at the Ca(2) site and 41 % of adsorbed Y for S-FAp.
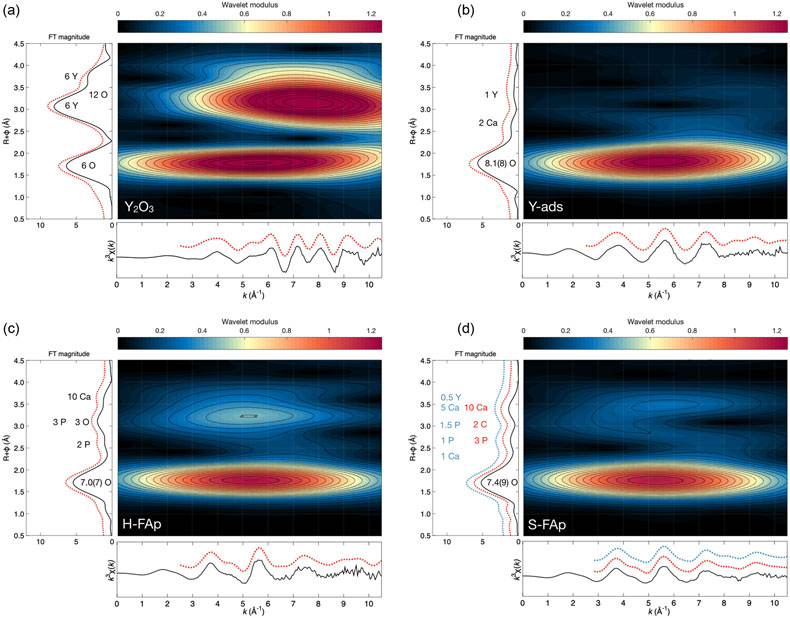
Figure 2 Wavelet transform analysis of EXAFS spectra with corresponding k3-weighted EXAFS spectra and Fourier transform (black solid lines). Results of multi-shell fits are shown in both the k-, and R-spaces (shifted dotted lines), (a) Y2O3, (b) Y-ads, (c) H-FAp, (d) S-FAp with the mixed “Y(2) + Y-ads” model in blue and the “Y(2) + carbonate” model in red.
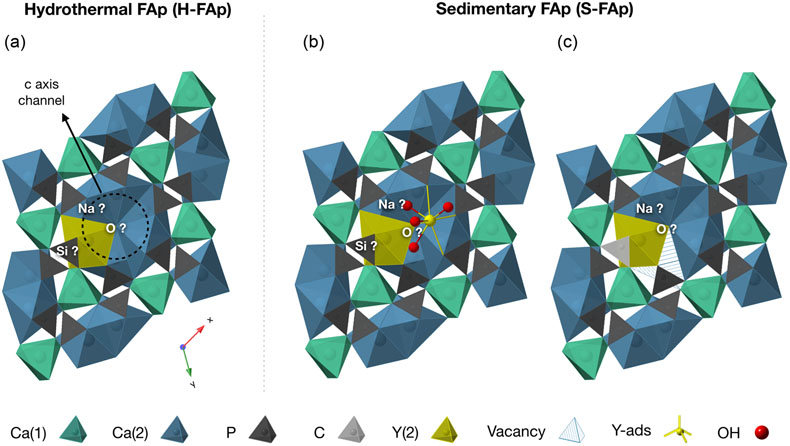
Figure 3 Fluorapatite crystal structure projected along c axis showing yttrium speciation models, (a) H-FAp: substitution at the Ca(2) site, (b) S-FAp: mixture of substitution at the Ca(2) site together with an inner shell adsorption at the c axis channel, (c) S-FAp: substitution at the Ca(2) site with concomitant C replacing P and Ca vacancy. Si, Na and O labels indicate potential coupled substitutions required to compensate the excess of electronic charge due to Y3+-Ca2+ substitution.