The direct observation and interpretation of gas hydrate decomposition with ocean depth
Affiliations | Corresponding Author | Cite as | Funding information- Share this article




-
Article views:246Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
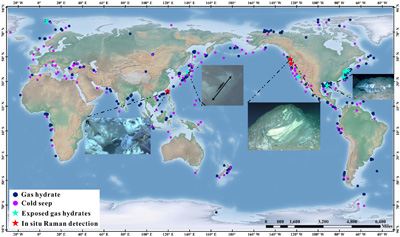 Figure 1 Global map of gas hydrates (Kvenvolden, 1988; Waite et al., 2020) and cold seeps (Mazurenko and Soloviev, 2003; Suess, 2014). Cyan stars represent the locations of exposed gas hydrate occurrence (MacDonald et al., 2003; Sassen et al., 2004; Pohlman et al., 2005; Roberts et al, 2006; Hester et al., 2007; Case et al., 2017). Red stars indicate locations where in situ Raman investigations of exposed hydrates have been performed (Hester et al., 2007; Zhang et al., 2017a). | 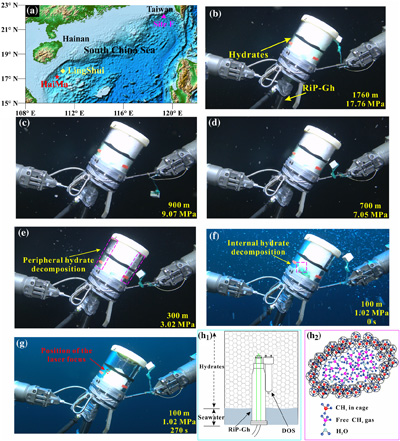 Figure 2 (a) Map showing the locations of the Haima, Lingshui and Site F cold seep vents. (b–g) Images showing hydrate collection and monitoring controlled by the ROV manipulator in Lingshui in situ experiment. (h1) Schematic diagram of the RiP-Gh and DOS probes touching and being inserted into the EGHs for dynamic monitoring. (h2) Schematic diagram of the hydrate film formed in the gas bubbles. | 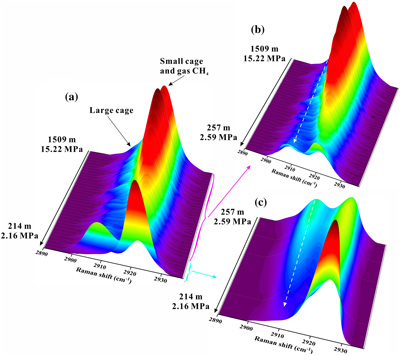 Figure 3 In situ Raman spectra for EGHs during their ascent towards the surface in the Haima cold seep. (a) Overall ascent over the pressure range of 15.22–2.16 MPa (1509–214 m). (b) The continuous growth stage at a Raman intensity of 2907 cm−1, and the continuous decrease at 2917 cm−1. (c) Hydrate decomposition stage. | 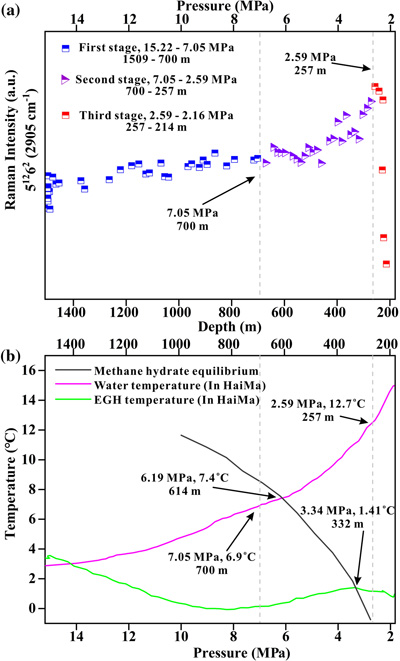 Figure 4 (a) Variation in the large cage Raman intensity with pressure during EGH ascent. (b) Temperature-pressure conditions present in the ambient seawater and the interior of the hydrate during EGH ascent. The black curve represents the phase equilibrium conditions for methane hydrate in seawater (S = 35 ‰) (Dickens and Quinby-Hunt, 1994). |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Exposed gas hydrates (EGHs) have been frequently reported in shallow surface sediments, and are common beneath authigenic carbonate rocks and empty mussel shells in active cold seeps (Fig. 1) (Sassen et al., 2004
Sassen, R., Roberts, H.H., Carney, R., Milkov, A.V., DeFreitas, D.A., Lanoil, B., Zhang, C. (2004) Free hydrocarbon gas, gas hydrate, and authigenic minerals in chemosynthetic communities of the northern Gulf of Mexico continental slope: relation to microbial processes. Chemical Geology 205, 195–217. https://doi.org/10.1016/j.chemgeo.2003.12.032
; Pohlman et al., 2005Pohlman, J.W., Canuel, E.A., Chapman, N.R., Spence, G.D., Whiticar, M.J., Coffin, R.B. (2005) The origin of thermogenic gas hydrates on the northern Cascadia Margin as inferred from isotopic (13C/12C and D/H) and molecular composition of hydrate and vent gas. Organic Geochemistry 36, 703–716. https://doi.org/10.1016/j.orggeochem.2005.01.011
; Hester et al., 2007Hester, K.C., Dunk, R.M., Walz, P.M., Peltzer, E.T., Sloan, E.D., Brewer, P.G. (2007) Direct measurements of multi-component hydrates on the seafloor: Pathways to growth. Fluid Phase Equilibria 261, 396–406. https://doi.org/10.1016/j.fluid.2007.07.053
). EGHs are formed by the accumulation of rising methane bubbles, which are rapidly covered with hydrate film. These EGHs contain a large amount of free methane gas and have a soft and loose structure, representing the initial state of hydrate formation (Zhang et al., 2017aZhang, X., Du, Z., Luan, Z., Wang, X., Xi, S., Wang, B., Li, L., Lian, C., Yan, J. (2017a) In Situ Raman Detection of Gas Hydrates Exposed on the Seafloor of the South China Sea. Geochemistry, Geophysics, Geosystems 18, 3700–3713. https://doi.org/10.1002/2017gc006987
; Du et al., 2018Du, Z., Zhang, X., Xi, S., Li, L., Luan, Z., Lian, C., Wang, B., Yan, J. (2018) In situ Raman spectroscopy study of synthetic gas hydrate formed by cold seep flow in the South China Sea. Journal of Asian Earth Sciences 168, 197–206. https://doi.org/10.1016/j.jseaes.2018.02.003
). Disturbances such as submarine turbidity currents, processes related to climate change, and geological activity can cause the ascent of EGHs due to destabilisation (Rehder et al., 2009Rehder, G., Leifer, I., Brewer, P.G., Friederich, G., Peltzer, E.T. (2009) Controls on methane bubble dissolution inside and outside the hydrate stability field from open ocean field experiments and numerical modeling. Marine Chemistry 114, 19–30. https://doi.org/10.1016/j.marchem.2009.03.004
). Rising decomposition may occur when EGHs are destabilised, but thermodynamic conditions in the deep ocean environment have the potential to convert the gas back to hydrates (Zheng et al., 2020Zheng, J., Chong, Z.R., Qureshi, M.F., Linga, P. (2020) Carbon Dioxide Sequestration via Gas Hydrates: A Potential Pathway toward Decarbonization. Energy & Fuels 34, 10529–10546. https://doi.org/10.1021/acs.energyfuels.0c02309
). The complex marine environment makes it difficult to predict the fate of EGHs in the natural state after destabilisation occurs and they escape into seawater. Previous research has shown that methane fluid from cold seep vents can be dissolved into the water column or consumed by methanotrophic microorganisms (Thornton et al., 2016Thornton, B.F., Geibel, M.C., Crill, P.M., Humborg, C., Mörth, C.-M. (2016) Methane fluxes from the sea to the atmosphere across the Siberian shelf seas. Geophysical Research Letters 43, 5869–5877. https://doi.org/10.1002/2016gl068977
; Egger et al., 2018Egger, M., Riedinger, N., Mogollón, J.M., Jørgensen, B.B. (2018) Global diffusive fluxes of methane in marine sediments. Nature Geoscience 11, 421–425. https://doi.org/10.1038/s41561-018-0122-8
). It is unknown whether EGHs destabilised into seawater can improve the survival ability of methane gas, and the depths to which EGHs can carry methane gas after a destabilised ascent remain uncertain.
Figure 1 Global map of gas hydrates (Kvenvolden, 1988
Kvenvolden, K.A. (1988) Methane hydrate — A major reservoir of carbon in the shallow geosphere? Chemical Geology 71, 41–51. https://doi.org/10.1016/0009-2541(88)90104-0
; Waite et al., 2020Waite, W.F., Ruppel, C.D., Boze, L.-G., Lorenson, T.D., Buczkowski, B.J., McMullen, K.Y., Kvenvolden, K.A. (2020) Preliminary global database of known and inferred gas hydrate locations. U.S. Geological Survey data release. https://doi.org/10.5066/P9LLFVJM
) and cold seeps (Mazurenko and Soloviev, 2003Mazurenko, L.L., Soloviev, V.A. (2003) Worldwide distribution of deep-water fluid venting and potential occurrences of gas hydrate accumulations. Geo-Marine Letters 23, 162–176. https://doi.org/10.1007/s00367-003-0146-x
; Suess, 2014Suess, E. (2014) Marine cold seeps and their manifestations: geological control, biogeochemical criteria and environmental conditions. International Journal of Earth Sciences 103, 1889–1916. https://doi.org/10.1007/s00531-014-1010-0
). Cyan stars represent the locations of exposed gas hydrate occurrence (MacDonald et al., 2003MacDonald, I.R., Sager, W.W., Peccini, M.B. (2003) Gas hydrate and chemosynthetic biota in mounded bathymetry at mid-slope hydrocarbon seeps: Northern Gulf of Mexico. Marine Geology 198, 133–158. https://doi.org/10.1016/s0025-3227(03)00098-7
; Sassen et al., 2004Sassen, R., Roberts, H.H., Carney, R., Milkov, A.V., DeFreitas, D.A., Lanoil, B., Zhang, C. (2004) Free hydrocarbon gas, gas hydrate, and authigenic minerals in chemosynthetic communities of the northern Gulf of Mexico continental slope: relation to microbial processes. Chemical Geology 205, 195–217. https://doi.org/10.1016/j.chemgeo.2003.12.032
; Pohlman et al., 2005Pohlman, J.W., Canuel, E.A., Chapman, N.R., Spence, G.D., Whiticar, M.J., Coffin, R.B. (2005) The origin of thermogenic gas hydrates on the northern Cascadia Margin as inferred from isotopic (13C/12C and D/H) and molecular composition of hydrate and vent gas. Organic Geochemistry 36, 703–716. https://doi.org/10.1016/j.orggeochem.2005.01.011
; Roberts et al, 2006Roberts, H.H., Hardage, B.A., Shedd, W.W., Hunt Jr, J. (2006) Seafloor reflectivity—An important seismic property for interpreting fluid/gas expulsion geology and the presence of gas hydrate. The Leading Edge 25, 620–628. https://doi.org/10.1190/1.2202667
; Hester et al., 2007Hester, K.C., Dunk, R.M., Walz, P.M., Peltzer, E.T., Sloan, E.D., Brewer, P.G. (2007) Direct measurements of multi-component hydrates on the seafloor: Pathways to growth. Fluid Phase Equilibria 261, 396–406. https://doi.org/10.1016/j.fluid.2007.07.053
; Case et al., 2017Case, D.H., Ijiri, A., Morono, Y., Tavormina, P., Orphan, V.J., Inagaki, F. (2017) Aerobic and Anaerobic Methanotrophic Communities Associated with Methane Hydrates Exposed on the Seafloor: A High-Pressure Sampling and Stable Isotope-Incubation Experiment. Frontiers in Microbiology 8, 2569. https://doi.org/10.3389/fmicb.2017.02569
). Red stars indicate locations where in situ Raman investigations of exposed hydrates have been performed (Hester et al., 2007Hester, K.C., Dunk, R.M., Walz, P.M., Peltzer, E.T., Sloan, E.D., Brewer, P.G. (2007) Direct measurements of multi-component hydrates on the seafloor: Pathways to growth. Fluid Phase Equilibria 261, 396–406. https://doi.org/10.1016/j.fluid.2007.07.053
; Zhang et al., 2017aZhang, X., Du, Z., Luan, Z., Wang, X., Xi, S., Wang, B., Li, L., Lian, C., Yan, J. (2017a) In Situ Raman Detection of Gas Hydrates Exposed on the Seafloor of the South China Sea. Geochemistry, Geophysics, Geosystems 18, 3700–3713. https://doi.org/10.1002/2017gc006987
).The investigation of the hydrate evolution after their initial formation has been conducted via laboratory simulations in recent decades (Zhong et al., 2016
Zhong, J.-R., Zeng, X.-Y., Zhou, F.-H., Ran, Q.-D., Sun, C.-Y., Zhong, R.-Q., Yang, L.-Y., Chen, G.-J., Koh, C.A. (2016) Self-preservation and structural transition of gas hydrates during dissociation below the ice point: an in situ study using Raman spectroscopy. Scientific Reports 6, 38855. https://doi.org/10.1038/srep38855
; Lei et al., 2019Lei, L., Seol, Y., Myshakin, E.M. (2019) Methane Hydrate Film Thickening in Porous Media. Geophysical Research Letters 46, 11091–11099. https://doi.org/10.1029/2019gl084450
). The morphology, thickening pattern, and growth resistance of hydrate film have been explored in detail (Zeng et al., 2019Zeng, X.-Y., Wu, G., Zhong, J.-R., Chen, D.-Y., Sun, C.-Y., Chen, G.-J. (2019) Three-Scale in Situ Investigation on the Film Morphology and Mass Transfer Channels during the Thickening Growth of Hydrates on Gas Bubble. Crystal Growth & Design 19, 3158–3165. https://doi.org/10.1021/acs.cgd.8b01847
; Qureshi et al., 2022aQureshi, M.F., Dhamu, V., Usadi, A., Barckholtz, T.A., Mhadeshwar, A.B., Linga, P. (2022a) CO2 Hydrate Formation Kinetics and Morphology Observations Using High-Pressure Liquid CO2 Applicable to Sequestration. Energy & Fuels 36, 10627–10641. https://doi.org/10.1021/acs.energyfuels.1c03840
; Dhamu et al., 2023Dhamu, V., Qureshi, M.F., Abubakar, S., Usadi, A., Barckholtz, T.A., Mhadeshwar, A.B., Linga, P. (2023) Investigating High-Pressure Liquid CO2 Hydrate Formation, Dissociation Kinetics, and Morphology in Brine and Freshwater Static Systems. Energy & Fuels 37, 8406–8420. https://doi.org/10.1021/acs.energyfuels.3c01089
). Research conducted on hydrates in laboratories has typically been limited to steady-state systems at specific temperature and pressure conditions. Laboratory techniques are unavailable for use in real and complex ocean environments (Brewer et al., 1998Brewer, P.G., Orr Jr., F.M., Friederich, G., Kvenvolden, K.A., Orange, D.L. (1998) Gas Hydrate Formation in the Deep Sea: In Situ Experiments with Controlled Release of Methane, Natural Gas, and Carbon Dioxide. Energy & Fuels 12, 183–188. https://doi.org/10.1021/ef970172q
; Warzinski et al., 2014Warzinski, R.P., Lynn, R., Haljasmaa, I., Leifer, I., Shaffer, F., Anderson, B.J., Levine, J.S. (2014) Dynamic morphology of gas hydrate on a methane bubble in water: Observations and new insights for hydrate film models. Geophysical Research Letters 41, 6841–6847. https://doi.org/10.1002/2014gl061665
). Compared to freshwater environments in the laboratory, EGHs in the marine environment are more unstable due to factors such as salinity and currents (Qureshi et al., 2022bQureshi, M.F., Khandelwal, H., Usadi, A., Barckholtz, T.A., Mhadeshwar, A.B., Linga, P. (2022b) CO2 hydrate stability in oceanic sediments under brine conditions. Energy 256, 124625. https://doi.org/10.1016/j.energy.2022.124625
, 2022cQureshi, M.F., Zheng, J., Khandelwal, H., Venkataraman, P., Usadi, A., Barckholtz, T.A., Mhadeshwar, A.B., Linga, P. (2022c) Laboratory demonstration of the stability of CO2 hydrates in deep-oceanic sediments. Chemical Engineering Journal 432, 134290. https://doi.org/10.1016/j.cej.2021.134290
). With the development of underwater vehicle technology and the increase in our understanding of cold seep regions, in situ technologies that can more accurately analyse natural gas hydrates have gradually matured. Initially, Brewer et al. (1998)Brewer, P.G., Orr Jr., F.M., Friederich, G., Kvenvolden, K.A., Orange, D.L. (1998) Gas Hydrate Formation in the Deep Sea: In Situ Experiments with Controlled Release of Methane, Natural Gas, and Carbon Dioxide. Energy & Fuels 12, 183–188. https://doi.org/10.1021/ef970172q
made detailed recordings of the formation and growth patterns of gas hydrates as a function of depth, using a remotely operated vehicle (ROV). Hester et al. (2009)Hester, K.C., Peltzer, E.T., Walz, P.M., Dunk, R.M., Sloan, E.D., Brewer, P.G. (2009) A natural hydrate dissolution experiment on complex multi-component hydrates on the sea floor. Geochimica et Cosmochimica Acta 73, 6747–6756. https://doi.org/10.1016/j.gca.2009.08.007
concluded that mass-transfer is the rate-controlling mechanism for the dissolution of EGHs and recorded the in situ decomposition of EGHs located in the Barkley Canyon area, off Vancouver Island, Canada. Recently, Du et al. (2018)Du, Z., Zhang, X., Xi, S., Li, L., Luan, Z., Lian, C., Wang, B., Yan, J. (2018) In situ Raman spectroscopy study of synthetic gas hydrate formed by cold seep flow in the South China Sea. Journal of Asian Earth Sciences 168, 197–206. https://doi.org/10.1016/j.jseaes.2018.02.003
characterised the structural features and evolution process of EGHs using Raman spectroscopy in the cold seep area of the South China Sea. A series of investigations of hydrate formation processes and their natural evolution have been performed through in situ experiments in recent decades. One similarity among all these in situ experiments was that they were conducted in stationary environments, and there were no records obtained of EGHs as they rose to the surface. Thus, the evolution of EGHs during their rise is not yet clear.In this study, we performed in situ experiments on the ascent decomposition of EGHs in the Haima, Lingshui, and Site F cold seep areas in the South China Sea (SCS) (Fig. 2a). The EGH samples were formed directly using gas-rich fluids that erupted from cold seep vents (Video S-1). We monitored the morphological changes that occurred during the EGH ascent using a camera mounted on the remotely operated vehicle (ROV) “Faxian” (Fig. 2b–g). A Raman insertion probe for gas hydrates (RiP-Gh), previously developed by Zhang et al. (2017b)
Zhang, X., Du, Z., Zheng, R., Luan, Z., Qi, F., Cheng, K., Wang, B., Ye, W., Liu, X., Lian, C., Chen, C., Guo, J., Li, Y., Yan, J. (2017b) Development of a new deep-sea hybrid Raman insertion probe and its application to the geochemistry of hydrothermal vent and cold seep fluids. Deep Sea Research Part I: Oceanographic Research Papers 123, 1–12. https://doi.org/10.1016/j.dsr.2017.02.005
, and a dissolved oxygen sensor (DOS, JFE RINKO I ARO-USB) were used to capture the kinetic and thermodynamic behaviour of EGHs during their ascent (analytical methods are provided in detail in the Supplementary Information). Here, we report data from the in situ ascent of EGHs in a natural environment and analyse the corresponding impact on the upper water layers.
Figure 2 (a) Map showing the locations of the Haima, Lingshui and Site F cold seep vents. (b–g) Images showing hydrate collection and monitoring controlled by the ROV manipulator in Lingshui in situ experiment. (h1) Schematic diagram of the RiP-Gh and DOS probes touching and being inserted into the EGHs for dynamic monitoring. (h2) Schematic diagram of the hydrate film formed in the gas bubbles.
top
In Situ Raman Spectra of Exposed Gas Hydrate Samples
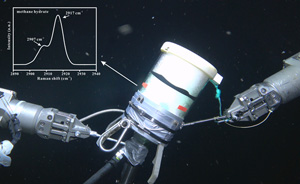
The Raman spectral data indicated that the gaseous component of the cold seep fluids consisted primarily of methane gas (Fig. S-1). When conducting the in situ experiments using cold seep vent fluids in the seafloor, we found that the formation of hydrates occurred nearly instantaneously (Video S-1). Methane gas bubbles were rapidly wrapped with a hydrate film during their ascent, which provided separation between the internal gas and external water (Fig. 2h). Multiple bubbles carrying hydrate films accumulated in cells to form a honeycomb structure in the EGH sample (Video S-1, Fig. 2h1). After a stationary period, an RiP-Gh probe was inserted into the EGH sample at approximately 20 cm for in situ Raman spectral observation (Videos S-2 to S-4). Notably, the time that hydrates remained on the seafloor after formation varied in each in situ experiment. Hydrate samples remained on the seafloor for half an hour at the Haima cold seep, 2.6 hours at the Lingshui cold seep and 48 hours at the Site F cold seep (Table S-2). The different stationary periods of hydrates make our experiments more generalisable.
The Raman spectra of EGHs indicated two apparent Raman peaks occurring at 2907 cm−1 and 2917 cm−1. Both Raman spectra and the guest molecule species indicated that the EGHs in our in situ experiments were all type I structures. Notably, the peak intensity at 2907 cm−1 was less than that at 2917 cm−1 (Fig. S-2). The Raman peak located at 2917 cm−1 consisted of a composite peak that was influenced by both a small cage vibration and the gaseous CH4 vibration.
top
Stage Changes in the Rising Process of Exposed Gas Hydrates
In the Haima in situ experiments, the entire monitoring time of the rising EGHs was 0.79 h. There were three significant stage changes during the rising of the EGHs towards the surface (Video S-2). The first stage occurred at pressures of 15.22–7.05 MPa (1509–700 m). There were no significant changes in the morphology of the EGHs during this stage (Fig. S-3). However, the 2917 cm−1 composite peak intensity exhibited a decreasing trend, which was associated with the free CH4 gas escaping as the hydrate migrated towards the surface (Fig. 3b). In normal circumstances, the further growth of the hydrate can only occur via the diffusion of water molecules through the hydrate film, which occurs slowly (Dhamu et al., 2023
Dhamu, V., Qureshi, M.F., Abubakar, S., Usadi, A., Barckholtz, T.A., Mhadeshwar, A.B., Linga, P. (2023) Investigating High-Pressure Liquid CO2 Hydrate Formation, Dissociation Kinetics, and Morphology in Brine and Freshwater Static Systems. Energy & Fuels 37, 8406–8420. https://doi.org/10.1021/acs.energyfuels.3c01089
). However, during the ROV ascent, the hydrate film can rupture due to the decrease in pressure exerted by the seawater. Consequently, the gas inside the EGH samples will escape, leading to an accelerated exchange with water molecules and other surrounding media. In contrast, the peak intensity that occurred at 2907 cm−1 showed a weaker enhancement trend (Fig. 3b). Simultaneously, the large cage vibration after Gaussian fitting also shows an enhanced trend (Fig. 4a). This indicates the growth of the EGH samples at this stage. By comparing the hydrate stability curves, we found that the internal environment of the EGHs was consistent with the stable existence of hydrates (Fig. 4b).
Figure 3 In situ Raman spectra for EGHs during their ascent towards the surface in the Haima cold seep. (a) Overall ascent over the pressure range of 15.22–2.16 MPa (1509–214 m). (b) The continuous growth stage at a Raman intensity of 2907 cm−1, and the continuous decrease at 2917 cm−1. (c) Hydrate decomposition stage.

Figure 4 (a) Variation in the large cage Raman intensity with pressure during EGH ascent. (b) Temperature-pressure conditions present in the ambient seawater and the interior of the hydrate during EGH ascent. The black curve represents the phase equilibrium conditions for methane hydrate in seawater (S = 35 ‰) (Dickens and Quinby-Hunt, 1994
Dickens, G.R., Quinby-Hunt, M.S. (1994) Methane hydrate stability in seawater. Geophysical Research Letters 21, 2115–2118. https://doi.org/10.1029/94GL01858
).The second stage occurs at pressures of 7.05–2.59 MPa (700–257 m) during the ascent. At this stage, the composite peak at 2917 cm−1 also shows a decreasing trend (Fig. 3b), indicating that the gas escapes. Raman spectral data suggest that the gas escapes throughout the experiment (Fig. 3a). The same process was also observed in the in situ experiments conducted at Lingshui and Site F (Figs. S-5 and S-6). The hydrate samples started decomposing from the periphery boundary at a pressure of approximately 7.05 MPa (700 m), when the ambient temperature of the seawater was 6.9 °C (Fig. 4a). The hydrate stability curve (Dickens and Quinby-Hunt, 1994
Dickens, G.R., Quinby-Hunt, M.S. (1994) Methane hydrate stability in seawater. Geophysical Research Letters 21, 2115–2118. https://doi.org/10.1029/94GL01858
) shows that the theoretical depth at which the decomposition of the EGHs begins is at the pressure of 6.19 MPa (614 m) (Fig. 4b). Here, the depth where decomposition of the EGH sample occurred was advanced during the ascent. Noticeably, although the EGH sample started to decompose from the periphery boundary, there was also a continued increase in the Raman intensity of the large cage inside of the EGHs (Fig. 4a). This suggests that there exists a continued growth inside the EGHs. Moreover, the temperature and pressure conditions inside the EGHs at this stage were consistent with the stable existence of hydrates (Fig. 4b). We monitored the continued hydrate growth process up to a pressure of 2.59 MPa (257 m) (Fig. 4a).The third stage occurs at pressures of 2.59–2.16 MPa (257–214 m) during the ascent. At this stage, the peak intensity at 2907 cm−1 and the large cage vibration (2905 cm−1) abruptly decreased, indicating that the inside of the EGHs sample started to decompose (Figs. 3c and 4a). At a pressure of 2.16 MPa (214 m), the Raman signal of the hydrate disappeared, and only gaseous CH4 remained (Fig. 3c). One interesting point we observed is that internal hydrate decomposition started at 2.59 MPa (257 m) during this stage. However, the hydrate equilibrium curve shows that the temperature and pressure inside the EGHs did not satisfy the conditions for the stable existence of EGHs as early as 3.34 MPa (332 m) (Fig. 4b). This indicates that the peripheral hydrate decomposition causes a hysteresis effect on the internal hydrate decomposition. Notably, at a pressure of 2.16 MPa (214 m), the complete decomposition of EGHs was recorded by the RiP-Gh probe. The residue of the EGHs, however, was still present above the sample cell.
The above data and discussions are based on the experiment conducted at the Haima cold seep vent. The in situ experiments conducted at Site F (Video S-4) and the Lingshui cold seep (Video S-3) vent locations also showed similar results to those at the Haima cold seep vent. The monitoring time for the rising process of EGHs in the Site F cold seep area was 0.61 h, while in the Lingshui cold seep area the monitoring time was 1.34 h. There were also three distinct stage changes during the EGH ascent (Figs. S-3 to S-7). During the in situ ascent experiment performed at Site F, the first stage occurred at pressures of 11.09–7.05 MPa (1100–700 m), the second stage occurred at pressures of 7.05–0.65 MPa (700–65 m), and the third stage occurred at pressures of 0.65–0.28 MPa (65–27 m) (Fig. S-7b). The first stage of the in situ experiment conducted at the Lingshui cold seep vent occurred at pressures of 17.76–7.05 MPa (1760–700 m) during the ascent process, and the second stage occurred at pressures of 7.05–1.01 MPa (700–100 m) (Fig. S-7a). The ROV did not continue to ascend in the third stage during the in situ experiment performed at the Lingshui cold seep vent. The ROV instead remained stationary at a water depth of 100 m (1.01 MPa), and we monitored the hydrate decomposition process of the third stage over time (Fig. S-7a). The third stage of the in situ experiment for the Lingshui cold seep vent lasted approximately 270 s (Fig. S-7a).
top
Specific Temperature Variations Inside Hydrates
The temperature of the EGHs showed a distinct trend from that of seawater. This appeared inconsistent with both the commonly believed exothermic and absorbed nature of hydrate formation and decomposition. In the first stage, the internal temperature of the hydrate samples dropped by 2.7 °C when the ambient temperature-pressure conditions fit the zone of hydrate stability (Fig. 4b). The ambient temperature of the seawater increased by 4 °C. At this stage, the hydrate periphery had not yet begun to dissipate, thus protecting the internal temperature of the gas hydrate from the influence of the ambient temperature of the seawater. The decrease in internal temperature was attributed to the dissolution of EGH samples. Various factors, such as the high methane chemical potential in the sample cell, the presence of oxygen, and the unsaturated methane concentration in the seawater, are likely reasons contributing to the dissolution of the EGHs.
However, when the EGH samples decomposed at the periphery boundary during the second stage, the internal temperature increased by 1.02 °C (Fig. 4b), and the temperature of the ambient seawater increased by 5.7 °C. During this stage, the inner environment of the hydrate was gradually exposed due to the periphery of the EGHs decomposing. The influence of seawater ambient temperature on the internal area of the hydrates intensified. The increase in the EGH inner temperature was likely influenced by the wide variation in the ambient temperature of the seawater. In the third stage, an approximately 1.5 °C increase in ambient temperature occurred at water depths of 257–214 m. Since the third stage only spanned 43 m, the influence of the ambient temperature of the seawater on the internal hydrates was small. The EGH internal temperature decreased by 0.31 °C at this stage due to the hydrate decomposition and endothermic processes.
top
New Insights into the Vertical Spreading of Bubbles in Cold Seep Areas
The depth of the location at which the Raman signal disappeared during the third stage represents the complete EGH decomposition. We estimated the variation in the hydrate volume with depth in the sample cells (Fig. S-8). All experiments showed that the gas hydrate was still retained above the sample cell at the end of the third stage. The rate of gas hydrate decomposition during the third stage was the fastest. The gas hydrate decomposition rates of the third stage in our three in situ experiments were estimated to be approximately 7.1 ± 0.32 cm3/m at the Haima cold seep vent site, 21.8 ± 0.31 cm3/m at the Site F, and 3.7 ± 0.22 cm3/s at the Lingshui site (Fig. S-8). Even considering the rapid decomposition rate that occurred at the third stage, the ROV retained some of the hydrate when it rose to the water surface. The EGHs can significantly enhance the survival of methane bubbles. The EGHs easily form in the cold seep areas on the seafloor, in shallow surface sediments, beneath authigenic carbonate rocks, or even in an empty mussel shell (Fig. S-9). Given the abundance of active cold seep vents worldwide, the potential environmental impact caused by the ascent and subsequent decomposition of EGHs carrying methane bubbles cannot be ignored.
top
Conclusions
Here, we provide the first record of the destabilising ascent of EGHs. The evolutional patterns of EGHs throughout the water depth scale were established, including the gas escape processes, and the specific temperature variations. Notably, the gas escape process occurred throughout all depths in the experiment. At water pressures > 7.05 MPa (depths > 700 m), the first stage of the evolution of EGHs was observed, during which the morphology of EGHs remained stable. Pressures of 7.05–2.59 MPa (depths of 700–257 m) corresponded to the second stage of EGH ascent, during which peripheral hydrate decomposition and internal hydrate growth coexisted. The decomposition of the peripheral hydrates will cause a hysteresis effect on the decomposition of the hydrates. The third stage (2.59–2.16 MPa, 257–214 m) was the period of internal hydrate decomposition. From our in situ experiments, it appears that the presence of EGHs results in methane gas influencing the overlying water column or even the atmospheric environment. It can also provide in situ data for gas hydrate exploitation to avoid construction-induced block hydrate uplift, which may increase greenhouse gas emissions.
top
Acknowledgements
This work was supported by the National Natural Science Foundation of China (52001303, 92058206, 42221005, 41822604, 42049582), Strategic Priority Research Program, CAS (XDB42040302), Young Taishan Scholars Program (tsqn201909158), and Key Project of Ocean Research Center, Chinese Academy of Sciences (COMS2020J03).
Editor: Eric Oelkers
top
References
Brewer, P.G., Orr Jr., F.M., Friederich, G., Kvenvolden, K.A., Orange, D.L. (1998) Gas Hydrate Formation in the Deep Sea: In Situ Experiments with Controlled Release of Methane, Natural Gas, and Carbon Dioxide. Energy & Fuels 12, 183–188. https://doi.org/10.1021/ef970172q
 Show in context
Show in context Research conducted on hydrates in laboratories has typically been limited to steady-state systems at specific temperature and pressure conditions. Laboratory techniques are unavailable for use in real and complex ocean environments (Brewer et al., 1998; Warzinski et al., 2014).
View in article
Initially, Brewer et al. (1998) made detailed recordings of the formation and growth patterns of gas hydrates as a function of depth, using a remotely operated vehicle (ROV).
View in article
Case, D.H., Ijiri, A., Morono, Y., Tavormina, P., Orphan, V.J., Inagaki, F. (2017) Aerobic and Anaerobic Methanotrophic Communities Associated with Methane Hydrates Exposed on the Seafloor: A High-Pressure Sampling and Stable Isotope-Incubation Experiment. Frontiers in Microbiology 8, 2569. https://doi.org/10.3389/fmicb.2017.02569
 Show in context
Show in context Cyan stars represent the locations of exposed gas hydrate occurrence (MacDonald et al., 2003; Sassen et al., 2004; Pohlman et al., 2005; Roberts et al, 2006; Hester et al., 2007; Case et al., 2017).
View in article
Dhamu, V., Qureshi, M.F., Abubakar, S., Usadi, A., Barckholtz, T.A., Mhadeshwar, A.B., Linga, P. (2023) Investigating High-Pressure Liquid CO2 Hydrate Formation, Dissociation Kinetics, and Morphology in Brine and Freshwater Static Systems. Energy & Fuels 37, 8406–8420. https://doi.org/10.1021/acs.energyfuels.3c01089
 Show in context
Show in context The morphology, thickening pattern, and growth resistance of hydrate film have been explored in detail (Zeng et al., 2019; Qureshi et al., 2022a; Dhamu et al., 2023).
View in article
In normal circumstances, the further growth of the hydrate can only occur via the diffusion of water molecules through the hydrate film, which occurs slowly (Dhamu et al., 2023).
View in article
Dickens, G.R., Quinby-Hunt, M.S. (1994) Methane hydrate stability in seawater. Geophysical Research Letters 21, 2115–2118. https://doi.org/10.1029/94GL01858
 Show in context
Show in context The black curve represents the phase equilibrium conditions for methane hydrate in seawater (S = 35 ‰) (Dickens and Quinby-Hunt, 1994).
View in article
The hydrate stability curve (Dickens and Quinby-Hunt, 1994) shows that the theoretical depth at which the decomposition of the EGHs begins is at the pressure of 6.19 MPa (614 m) (Fig. 4b).
View in article
Du, Z., Zhang, X., Xi, S., Li, L., Luan, Z., Lian, C., Wang, B., Yan, J. (2018) In situ Raman spectroscopy study of synthetic gas hydrate formed by cold seep flow in the South China Sea. Journal of Asian Earth Sciences 168, 197–206. https://doi.org/10.1016/j.jseaes.2018.02.003
 Show in context
Show in context These EGHs contain a large amount of free methane gas and have a soft and loose structure, representing the initial state of hydrate formation (Zhang et al., 2017a; Du et al., 2018).
View in article
Recently, Du et al. (2018) characterised the structural features and evolution process of EGHs using Raman spectroscopy in the cold seep area of the South China Sea.
View in article
Egger, M., Riedinger, N., Mogollón, J.M., Jørgensen, B.B. (2018) Global diffusive fluxes of methane in marine sediments. Nature Geoscience 11, 421–425. https://doi.org/10.1038/s41561-018-0122-8
 Show in context
Show in context Previous research has shown that methane fluid from cold seep vents can be dissolved into the water column or consumed by methanotrophic microorganisms (Thornton et al., 2016; Egger et al., 2018).
View in article
Hester, K.C., Dunk, R.M., Walz, P.M., Peltzer, E.T., Sloan, E.D., Brewer, P.G. (2007) Direct measurements of multi-component hydrates on the seafloor: Pathways to growth. Fluid Phase Equilibria 261, 396–406. https://doi.org/10.1016/j.fluid.2007.07.053
 Show in context
Show in context Exposed gas hydrates (EGHs) have been frequently reported in shallow surface sediments, and are common beneath authigenic carbonate rocks and empty mussel shells in active cold seeps (Fig. 1) (Sassen et al., 2004; Pohlman et al., 2005; Hester et al., 2007).
View in article
Cyan stars represent the locations of exposed gas hydrate occurrence (MacDonald et al., 2003; Sassen et al., 2004; Pohlman et al., 2005; Roberts et al, 2006; Hester et al., 2007; Case et al., 2017).
View in article
Red stars indicate locations where in situ Raman investigations of exposed hydrates have been performed (Hester et al., 2007; Zhang et al., 2017a).
View in article
Hester, K.C., Peltzer, E.T., Walz, P.M., Dunk, R.M., Sloan, E.D., Brewer, P.G. (2009) A natural hydrate dissolution experiment on complex multi-component hydrates on the sea floor. Geochimica et Cosmochimica Acta 73, 6747–6756. https://doi.org/10.1016/j.gca.2009.08.007
 Show in context
Show in context Hester et al. (2009) concluded that mass-transfer is the rate-controlling mechanism for the dissolution of EGHs and recorded the in situ decomposition of EGHs located in the Barkley Canyon area, off Vancouver Island, Canada.
View in article
Kvenvolden, K.A. (1988) Methane hydrate — A major reservoir of carbon in the shallow geosphere? Chemical Geology 71, 41–51. https://doi.org/10.1016/0009-2541(88)90104-0
 Show in context
Show in context Global map of gas hydrates (Kvenvolden, 1988; Waite et al., 2020) and cold seeps (Mazurenko and Soloviev, 2003; Suess, 2014).
View in article
Lei, L., Seol, Y., Myshakin, E.M. (2019) Methane Hydrate Film Thickening in Porous Media. Geophysical Research Letters 46, 11091–11099. https://doi.org/10.1029/2019gl084450
 Show in context
Show in context The investigation of the hydrate evolution after their initial formation has been conducted via laboratory simulations in recent decades (Zhong et al., 2016; Lei et al., 2019).
View in article
MacDonald, I.R., Sager, W.W., Peccini, M.B. (2003) Gas hydrate and chemosynthetic biota in mounded bathymetry at mid-slope hydrocarbon seeps: Northern Gulf of Mexico. Marine Geology 198, 133–158. https://doi.org/10.1016/s0025-3227(03)00098-7
 Show in context
Show in context Cyan stars represent the locations of exposed gas hydrate occurrence (MacDonald et al., 2003; Sassen et al., 2004; Pohlman et al., 2005; Roberts et al, 2006; Hester et al., 2007; Case et al., 2017).
View in article
Mazurenko, L.L., Soloviev, V.A. (2003) Worldwide distribution of deep-water fluid venting and potential occurrences of gas hydrate accumulations. Geo-Marine Letters 23, 162–176. https://doi.org/10.1007/s00367-003-0146-x
 Show in context
Show in context Global map of gas hydrates (Kvenvolden, 1988; Waite et al., 2020) and cold seeps (Mazurenko and Soloviev, 2003; Suess, 2014).
View in article
Pohlman, J.W., Canuel, E.A., Chapman, N.R., Spence, G.D., Whiticar, M.J., Coffin, R.B. (2005) The origin of thermogenic gas hydrates on the northern Cascadia Margin as inferred from isotopic (13C/12C and D/H) and molecular composition of hydrate and vent gas. Organic Geochemistry 36, 703–716. https://doi.org/10.1016/j.orggeochem.2005.01.011
 Show in context
Show in context Exposed gas hydrates (EGHs) have been frequently reported in shallow surface sediments, and are common beneath authigenic carbonate rocks and empty mussel shells in active cold seeps (Fig. 1) (Sassen et al., 2004; Pohlman et al., 2005; Hester et al., 2007).
View in article
Cyan stars represent the locations of exposed gas hydrate occurrence (MacDonald et al., 2003; Sassen et al., 2004; Pohlman et al., 2005; Roberts et al, 2006; Hester et al., 2007; Case et al., 2017).
View in article
Qureshi, M.F., Dhamu, V., Usadi, A., Barckholtz, T.A., Mhadeshwar, A.B., Linga, P. (2022a) CO2 Hydrate Formation Kinetics and Morphology Observations Using High-Pressure Liquid CO2 Applicable to Sequestration. Energy & Fuels 36, 10627–10641. https://doi.org/10.1021/acs.energyfuels.1c03840
 Show in context
Show in context The morphology, thickening pattern, and growth resistance of hydrate film have been explored in detail (Zeng et al., 2019; Qureshi et al., 2022a; Dhamu et al., 2023).
View in article
Qureshi, M.F., Khandelwal, H., Usadi, A., Barckholtz, T.A., Mhadeshwar, A.B., Linga, P. (2022b) CO2 hydrate stability in oceanic sediments under brine conditions. Energy 256, 124625. https://doi.org/10.1016/j.energy.2022.124625
 Show in context
Show in context Compared to freshwater environments in the laboratory, EGHs in the marine environment are more unstable due to factors such as salinity and currents (Qureshi et al., 2022b, 2022c).
View in article
Qureshi, M.F., Zheng, J., Khandelwal, H., Venkataraman, P., Usadi, A., Barckholtz, T.A., Mhadeshwar, A.B., Linga, P. (2022c) Laboratory demonstration of the stability of CO2 hydrates in deep-oceanic sediments. Chemical Engineering Journal 432, 134290. https://doi.org/10.1016/j.cej.2021.134290
 Show in context
Show in context Compared to freshwater environments in the laboratory, EGHs in the marine environment are more unstable due to factors such as salinity and currents (Qureshi et al., 2022b, 2022c).
View in article
Rehder, G., Leifer, I., Brewer, P.G., Friederich, G., Peltzer, E.T. (2009) Controls on methane bubble dissolution inside and outside the hydrate stability field from open ocean field experiments and numerical modeling. Marine Chemistry 114, 19–30. https://doi.org/10.1016/j.marchem.2009.03.004
 Show in context
Show in context Disturbances such as submarine turbidity currents, processes related to climate change, and geological activity can cause the ascent of EGHs due to destabilisation (Rehder et al., 2009).
View in article
Roberts, H.H., Hardage, B.A., Shedd, W.W., Hunt Jr, J. (2006) Seafloor reflectivity—An important seismic property for interpreting fluid/gas expulsion geology and the presence of gas hydrate. The Leading Edge 25, 620–628. https://doi.org/10.1190/1.2202667
 Show in context
Show in context Cyan stars represent the locations of exposed gas hydrate occurrence (MacDonald et al., 2003; Sassen et al., 2004; Pohlman et al., 2005; Roberts et al, 2006; Hester et al., 2007; Case et al., 2017).
View in article
Sassen, R., Roberts, H.H., Carney, R., Milkov, A.V., DeFreitas, D.A., Lanoil, B., Zhang, C. (2004) Free hydrocarbon gas, gas hydrate, and authigenic minerals in chemosynthetic communities of the northern Gulf of Mexico continental slope: relation to microbial processes. Chemical Geology 205, 195–217. https://doi.org/10.1016/j.chemgeo.2003.12.032
 Show in context
Show in context Exposed gas hydrates (EGHs) have been frequently reported in shallow surface sediments, and are common beneath authigenic carbonate rocks and empty mussel shells in active cold seeps (Fig. 1) (Sassen et al., 2004; Pohlman et al., 2005; Hester et al., 2007).
View in article
Cyan stars represent the locations of exposed gas hydrate occurrence (MacDonald et al., 2003; Sassen et al., 2004; Pohlman et al., 2005; Roberts et al, 2006; Hester et al., 2007; Case et al., 2017).
View in article
Suess, E. (2014) Marine cold seeps and their manifestations: geological control, biogeochemical criteria and environmental conditions. International Journal of Earth Sciences 103, 1889–1916. https://doi.org/10.1007/s00531-014-1010-0
 Show in context
Show in context Global map of gas hydrates (Kvenvolden, 1988; Waite et al., 2020) and cold seeps (Mazurenko and Soloviev, 2003; Suess, 2014).
View in article
Thornton, B.F., Geibel, M.C., Crill, P.M., Humborg, C., Mörth, C.-M. (2016) Methane fluxes from the sea to the atmosphere across the Siberian shelf seas. Geophysical Research Letters 43, 5869–5877. https://doi.org/10.1002/2016gl068977
 Show in context
Show in context Previous research has shown that methane fluid from cold seep vents can be dissolved into the water column or consumed by methanotrophic microorganisms (Thornton et al., 2016; Egger et al., 2018).
View in article
Waite, W.F., Ruppel, C.D., Boze, L.-G., Lorenson, T.D., Buczkowski, B.J., McMullen, K.Y., Kvenvolden, K.A. (2020) Preliminary global database of known and inferred gas hydrate locations. U.S. Geological Survey data release. https://doi.org/10.5066/P9LLFVJM
 Show in context
Show in context Global map of gas hydrates (Kvenvolden, 1988; Waite et al., 2020) and cold seeps (Mazurenko and Soloviev, 2003; Suess, 2014).
View in article
Warzinski, R.P., Lynn, R., Haljasmaa, I., Leifer, I., Shaffer, F., Anderson, B.J., Levine, J.S. (2014) Dynamic morphology of gas hydrate on a methane bubble in water: Observations and new insights for hydrate film models. Geophysical Research Letters 41, 6841–6847. https://doi.org/10.1002/2014gl061665
 Show in context
Show in context Research conducted on hydrates in laboratories has typically been limited to steady-state systems at specific temperature and pressure conditions. Laboratory techniques are unavailable for use in real and complex ocean environments (Brewer et al., 1998; Warzinski et al., 2014).
View in article
Zeng, X.-Y., Wu, G., Zhong, J.-R., Chen, D.-Y., Sun, C.-Y., Chen, G.-J. (2019) Three-Scale in Situ Investigation on the Film Morphology and Mass Transfer Channels during the Thickening Growth of Hydrates on Gas Bubble. Crystal Growth & Design 19, 3158–3165. https://doi.org/10.1021/acs.cgd.8b01847
 Show in context
Show in context The morphology, thickening pattern, and growth resistance of hydrate film have been explored in detail (Zeng et al., 2019; Qureshi et al., 2022a; Dhamu et al., 2023).
View in article
Zhang, X., Du, Z., Luan, Z., Wang, X., Xi, S., Wang, B., Li, L., Lian, C., Yan, J. (2017a) In Situ Raman Detection of Gas Hydrates Exposed on the Seafloor of the South China Sea. Geochemistry, Geophysics, Geosystems 18, 3700–3713. https://doi.org/10.1002/2017gc006987
 Show in context
Show in context These EGHs contain a large amount of free methane gas and have a soft and loose structure, representing the initial state of hydrate formation (Zhang et al., 2017a; Du et al., 2018).
View in article
Red stars indicate locations where in situ Raman investigations of exposed hydrates have been performed (Hester et al., 2007; Zhang et al., 2017a).
View in article
Zhang, X., Du, Z., Zheng, R., Luan, Z., Qi, F., Cheng, K., Wang, B., Ye, W., Liu, X., Lian, C., Chen, C., Guo, J., Li, Y., Yan, J. (2017b) Development of a new deep-sea hybrid Raman insertion probe and its application to the geochemistry of hydrothermal vent and cold seep fluids. Deep Sea Research Part I: Oceanographic Research Papers 123, 1–12. https://doi.org/10.1016/j.dsr.2017.02.005
 Show in context
Show in context A Raman insertion probe for gas hydrates (RiP-Gh), previously developed by Zhang et al. (2017b), and a dissolved oxygen sensor (DOS, JFE RINKO I ARO-USB) were used to capture the kinetic and thermodynamic behaviour of EGHs during their ascent (analytical methods are provided in detail in the Supplementary Information).
View in article
Zheng, J., Chong, Z.R., Qureshi, M.F., Linga, P. (2020) Carbon Dioxide Sequestration via Gas Hydrates: A Potential Pathway toward Decarbonization. Energy & Fuels 34, 10529–10546. https://doi.org/10.1021/acs.energyfuels.0c02309
 Show in context
Show in context Rising decomposition may occur when EGHs are destabilised, but thermodynamic conditions in the deep ocean environment have the potential to convert the gas back to hydrates (Zheng et al., 2020).
View in article
Zhong, J.-R., Zeng, X.-Y., Zhou, F.-H., Ran, Q.-D., Sun, C.-Y., Zhong, R.-Q., Yang, L.-Y., Chen, G.-J., Koh, C.A. (2016) Self-preservation and structural transition of gas hydrates during dissociation below the ice point: an in situ study using Raman spectroscopy. Scientific Reports 6, 38855. https://doi.org/10.1038/srep38855
 Show in context
Show in context The investigation of the hydrate evolution after their initial formation has been conducted via laboratory simulations in recent decades (Zhong et al., 2016; Lei et al., 2019).
View in article
top
Supplementary Information
The Supplementary Information includes:
Download the Supplementary Information (PDF)
Download Video S-1 (mp4)
Download Video S-2 (mp4)
Download Video S-3 (mp4)
Download Video S-4 (mp4)
Figures
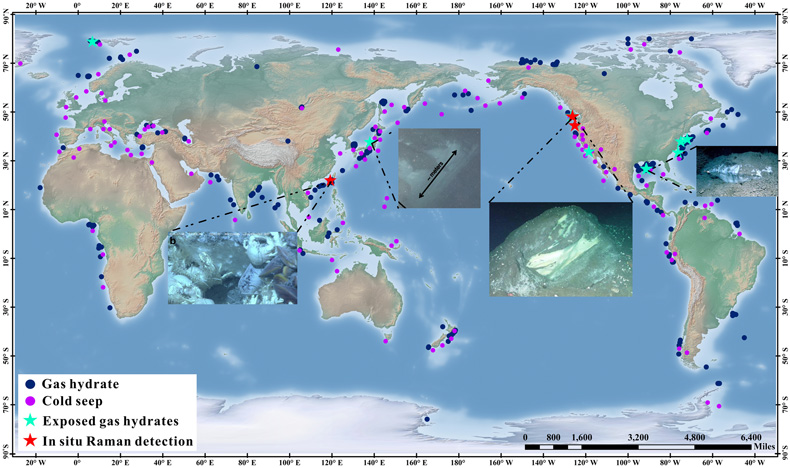
Figure 1 Global map of gas hydrates (Kvenvolden, 1988
Kvenvolden, K.A. (1988) Methane hydrate — A major reservoir of carbon in the shallow geosphere? Chemical Geology 71, 41–51. https://doi.org/10.1016/0009-2541(88)90104-0
; Waite et al., 2020Waite, W.F., Ruppel, C.D., Boze, L.-G., Lorenson, T.D., Buczkowski, B.J., McMullen, K.Y., Kvenvolden, K.A. (2020) Preliminary global database of known and inferred gas hydrate locations. U.S. Geological Survey data release. https://doi.org/10.5066/P9LLFVJM
) and cold seeps (Mazurenko and Soloviev, 2003Mazurenko, L.L., Soloviev, V.A. (2003) Worldwide distribution of deep-water fluid venting and potential occurrences of gas hydrate accumulations. Geo-Marine Letters 23, 162–176. https://doi.org/10.1007/s00367-003-0146-x
; Suess, 2014Suess, E. (2014) Marine cold seeps and their manifestations: geological control, biogeochemical criteria and environmental conditions. International Journal of Earth Sciences 103, 1889–1916. https://doi.org/10.1007/s00531-014-1010-0
). Cyan stars represent the locations of exposed gas hydrate occurrence (MacDonald et al., 2003MacDonald, I.R., Sager, W.W., Peccini, M.B. (2003) Gas hydrate and chemosynthetic biota in mounded bathymetry at mid-slope hydrocarbon seeps: Northern Gulf of Mexico. Marine Geology 198, 133–158. https://doi.org/10.1016/s0025-3227(03)00098-7
; Sassen et al., 2004Sassen, R., Roberts, H.H., Carney, R., Milkov, A.V., DeFreitas, D.A., Lanoil, B., Zhang, C. (2004) Free hydrocarbon gas, gas hydrate, and authigenic minerals in chemosynthetic communities of the northern Gulf of Mexico continental slope: relation to microbial processes. Chemical Geology 205, 195–217. https://doi.org/10.1016/j.chemgeo.2003.12.032
; Pohlman et al., 2005Pohlman, J.W., Canuel, E.A., Chapman, N.R., Spence, G.D., Whiticar, M.J., Coffin, R.B. (2005) The origin of thermogenic gas hydrates on the northern Cascadia Margin as inferred from isotopic (13C/12C and D/H) and molecular composition of hydrate and vent gas. Organic Geochemistry 36, 703–716. https://doi.org/10.1016/j.orggeochem.2005.01.011
; Roberts et al, 2006Roberts, H.H., Hardage, B.A., Shedd, W.W., Hunt Jr, J. (2006) Seafloor reflectivity—An important seismic property for interpreting fluid/gas expulsion geology and the presence of gas hydrate. The Leading Edge 25, 620–628. https://doi.org/10.1190/1.2202667
; Hester et al., 2007Hester, K.C., Dunk, R.M., Walz, P.M., Peltzer, E.T., Sloan, E.D., Brewer, P.G. (2007) Direct measurements of multi-component hydrates on the seafloor: Pathways to growth. Fluid Phase Equilibria 261, 396–406. https://doi.org/10.1016/j.fluid.2007.07.053
; Case et al., 2017Case, D.H., Ijiri, A., Morono, Y., Tavormina, P., Orphan, V.J., Inagaki, F. (2017) Aerobic and Anaerobic Methanotrophic Communities Associated with Methane Hydrates Exposed on the Seafloor: A High-Pressure Sampling and Stable Isotope-Incubation Experiment. Frontiers in Microbiology 8, 2569. https://doi.org/10.3389/fmicb.2017.02569
). Red stars indicate locations where in situ Raman investigations of exposed hydrates have been performed (Hester et al., 2007Hester, K.C., Dunk, R.M., Walz, P.M., Peltzer, E.T., Sloan, E.D., Brewer, P.G. (2007) Direct measurements of multi-component hydrates on the seafloor: Pathways to growth. Fluid Phase Equilibria 261, 396–406. https://doi.org/10.1016/j.fluid.2007.07.053
; Zhang et al., 2017aZhang, X., Du, Z., Luan, Z., Wang, X., Xi, S., Wang, B., Li, L., Lian, C., Yan, J. (2017a) In Situ Raman Detection of Gas Hydrates Exposed on the Seafloor of the South China Sea. Geochemistry, Geophysics, Geosystems 18, 3700–3713. https://doi.org/10.1002/2017gc006987
).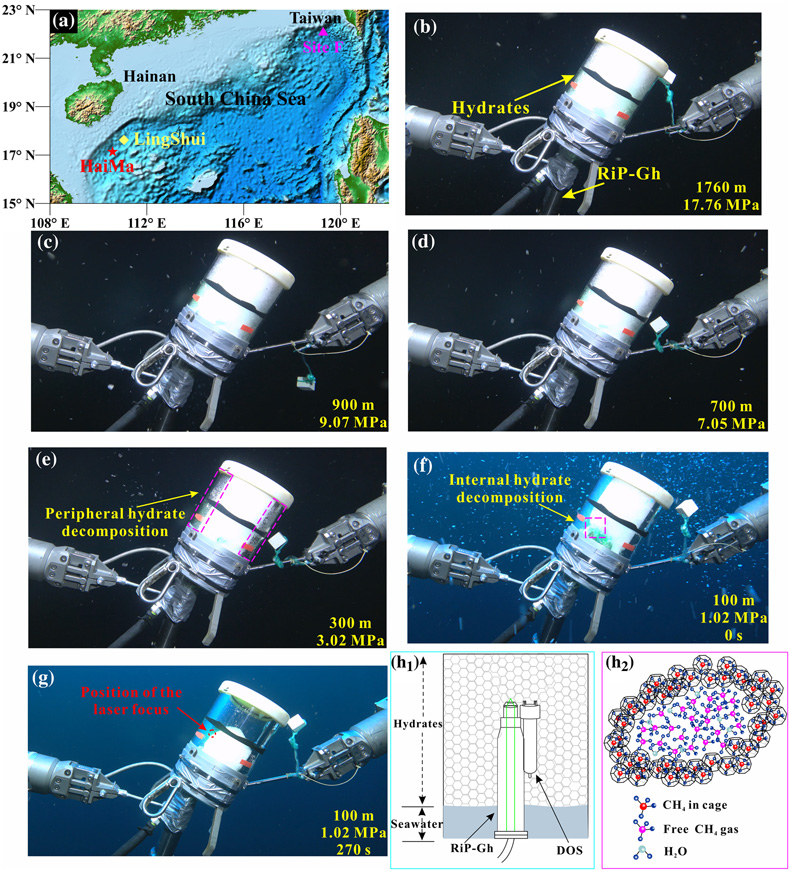
Figure 2 (a) Map showing the locations of the Haima, Lingshui and Site F cold seep vents. (b–g) Images showing hydrate collection and monitoring controlled by the ROV manipulator in Lingshui in situ experiment. (h1) Schematic diagram of the RiP-Gh and DOS probes touching and being inserted into the EGHs for dynamic monitoring. (h2) Schematic diagram of the hydrate film formed in the gas bubbles.
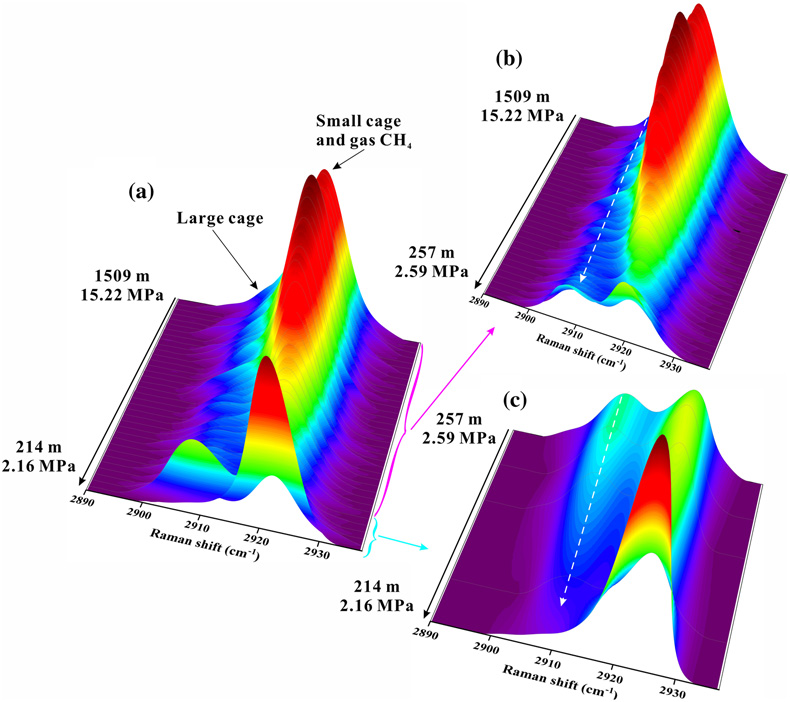
Figure 3 In situ Raman spectra for EGHs during their ascent towards the surface in the Haima cold seep. (a) Overall ascent over the pressure range of 15.22–2.16 MPa (1509–214 m). (b) The continuous growth stage at a Raman intensity of 2907 cm−1, and the continuous decrease at 2917 cm−1. (c) Hydrate decomposition stage.
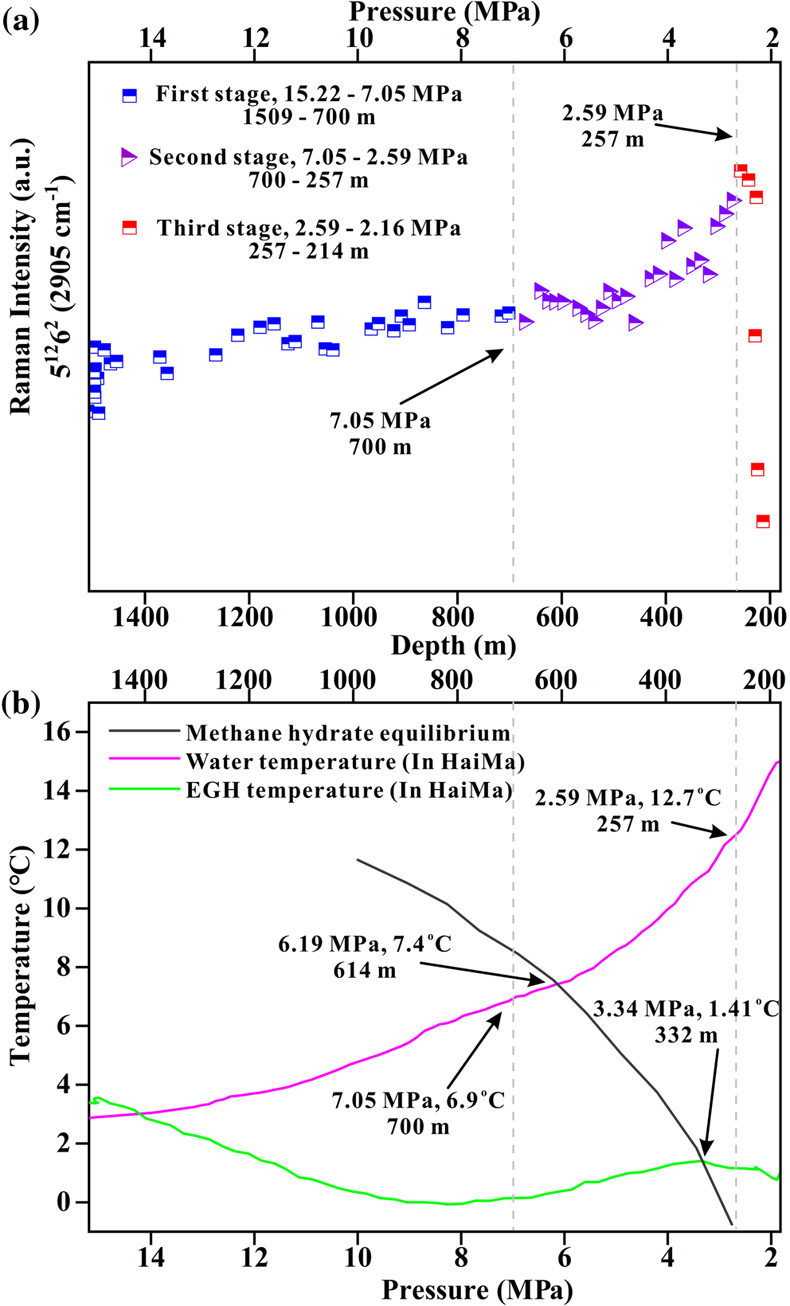
Figure 4 (a) Variation in the large cage Raman intensity with pressure during EGH ascent. (b) Temperature-pressure conditions present in the ambient seawater and the interior of the hydrate during EGH ascent. The black curve represents the phase equilibrium conditions for methane hydrate in seawater (S = 35 ‰) (Dickens and Quinby-Hunt, 1994
Dickens, G.R., Quinby-Hunt, M.S. (1994) Methane hydrate stability in seawater. Geophysical Research Letters 21, 2115–2118. https://doi.org/10.1029/94GL01858
).