The key role of bedrock composition in the formation of carbonates on Mars
Affiliations | Corresponding Author | Cite as | Funding information- Share this article

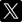



Article views:217Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures and Tables
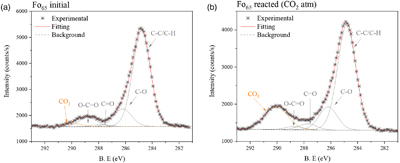 Figure 1 Fitting model of the C1s orbital for Fo65 sample, (a) initially, and (b) after reaction (under CO2 atmosphere) (see SI for spectra deconvolution). | 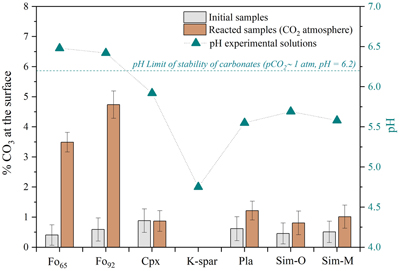 Figure 2 Amount of carbonate in the samples (bars) and pH value (triangles) of the solutions at the end of the experiment (see SI for % calculations). The error bars show the uncertainty of the carbon estimation associated with the survey quantification (standard deviation in Fig. S-2). The pH0 = 3.6, corresponding to the pure water equilibrated with the pCO2 = 1 bar. The dashed line marks the pH threshold of carbonates stability for pCO2 = 1 bar (Bullock and Moore, 2007). | 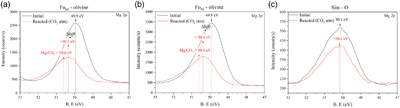 Figure 3 Mg 2p high resolution XPS spectra before (black colour) and after reaction under a CO2 atmosphere (red colour) of (a) Fo65-olivine, (b) Fo92-olivine, and (c) simulant sample with Fo65 (Sim-O). The solid arrows (black colour) mark the position of the main peak of the unreacted samples whereas dashed arrows (red colour) mark the position of the MgCO3 peak. | 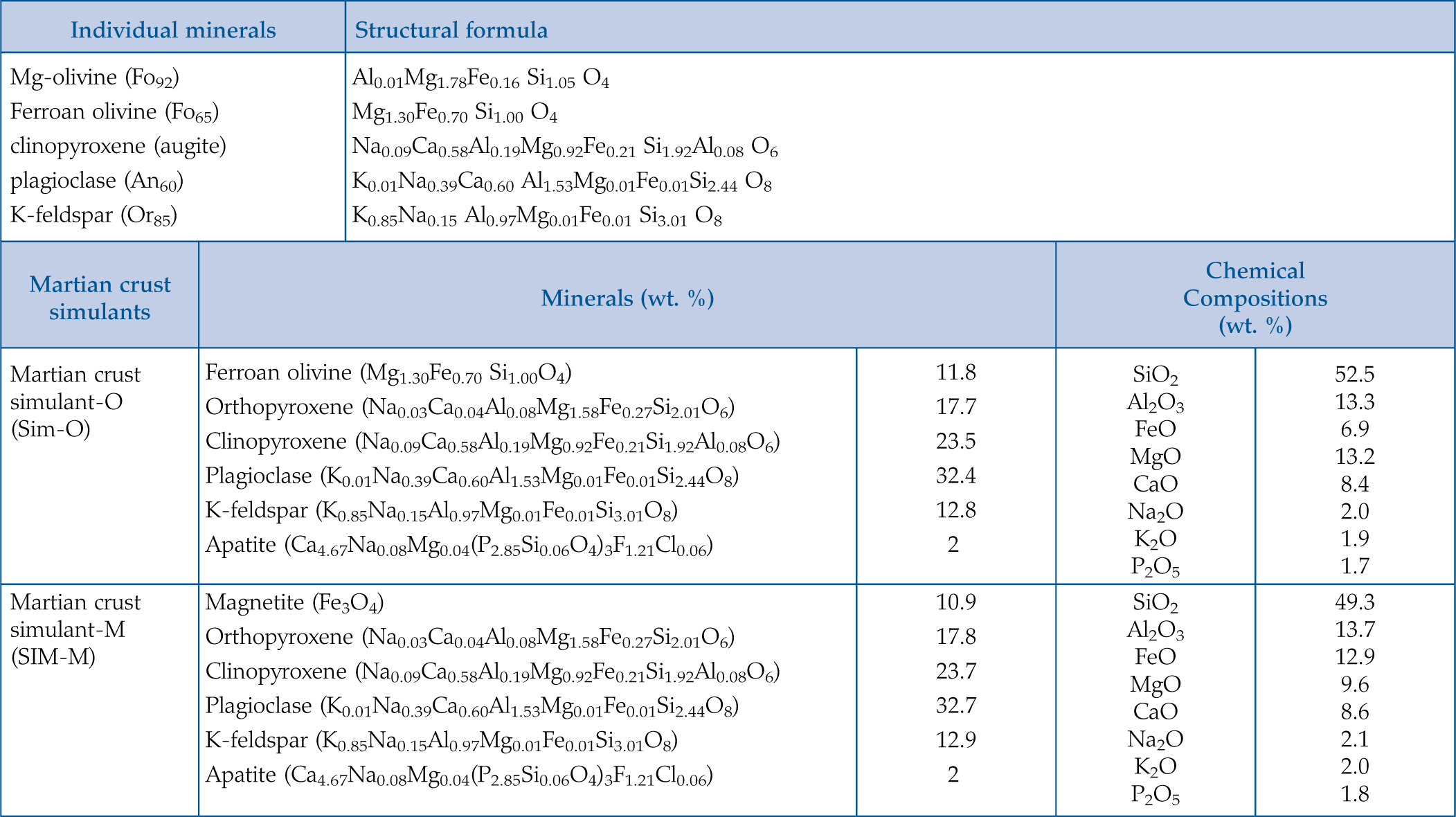 Table 1 Summary of the samples analysed in this study. The bulk chemical composition of martian simulants samples was estimated according to the proportion of the minerals used (Baron et al., 2019). |
| Figure 1 | Figure 2 | Figure 3 | Table 1 |
top
Introduction
Carbonates on Mars are reported from orbital, in situ, and martian meteorite studies. Apart from minor carbonates (<5 wt. %) identified within more recent polar terrains (Boynton et al., 2009
Boynton, W.V., Ming, D.W., Kounaves, S.P., Young, S.M.M., Arvidson, R.E., Hecht, M.H., Hoffman, J., Niles, P.B., Hamara, D.K., Quinn, R.C., Smith, P.H., Sutter, B., Catling, D.C., Morris, R.V. (2009) Evidence for Calcium Carbonate at the Mars Phoenix Landing Site. Science 325, 61–64. https://doi.org/10.1126/science.1172768
) and the carbonate compounds identified in three nakhlite meteorites (Bridges et al., 2019Bridges, J.C., Hicks, L.J., Treiman, A.H. (2019) Chapter 5 - Carbonates on Mars. In: Filiberto, J., Schwenzer, S.P. (Eds.) Volatiles in the Martian Crust. Elsevier, Amsterdam (Netherlands), Oxford (UK), Cambridge (USA), 89–118. https://doi.org/10.1016/B978-0-12-804191-8.00005-2
), these carbonates are inferred to have formed during the Noachian epoch (i.e. >3.6 Ga). Indeed, the largest known carbonate-bearing unit, at Nili Fossae, is inferred to be pre-Hesperian in age (∼3.8 Ga; Mandon et al., 2020Mandon, L., Quantin-Nataf, C., Thollot, P., Mangold, N., Lozac’h, L., Dromart, G., Beck, P., Dehouck, E., Breton, S., Millot, C., Volat, M. (2020) Refining the age, emplacement and alteration scenarios of the olivine-rich unit in the Nili Fossae region, Mars. Icarus 336, 113436. https://doi.org/10.1016/j.icarus.2019.113436
). Hypotheses for the formation of the Nili Fossae carbonates include both local hydrothermalism (Mangold et al., 2007Mangold, N., Poulet, F., Mustard, J.F., Bibring, J.-P., Gondet, B., Langevin, Y., Ansan, V., Masson, P., Fassett, C., Head III, J.W., Hoffmann, H., Neukum, G. (2007) Mineralogy of the Nili Fossae region with OMEGA/Mars Express data: 2. Aqueous alteration of the crust. Journal of Geophysical Research: Planets 112, E08S04. https://doi.org/10.1029/2006JE002835
; Ehlmann et al., 2009Ehlmann, B.L., Mustard, J.F., Swayze, G.A., Clark, R.N., Bishop, J.L., Poulet, F., Des Marais, D.J., Roach, L.H., Milliken, R.E., Wray, J.J., Barnouin-Jha, O., Murchie, S.L. (2009) Identification of hydrated silicate minerals on Mars using MRO-CRISM: Geologic context near Nili Fossae and implications for aqueous alteration. Journal of Geophysical Research: Planets 114, E00D08. https://doi.org/10.1029/2009JE003339
), and regional weathering (Ehlmann et al., 2009Ehlmann, B.L., Mustard, J.F., Swayze, G.A., Clark, R.N., Bishop, J.L., Poulet, F., Des Marais, D.J., Roach, L.H., Milliken, R.E., Wray, J.J., Barnouin-Jha, O., Murchie, S.L. (2009) Identification of hydrated silicate minerals on Mars using MRO-CRISM: Geologic context near Nili Fossae and implications for aqueous alteration. Journal of Geophysical Research: Planets 114, E00D08. https://doi.org/10.1029/2009JE003339
), which is consistent with the persistence of fluvial activity in the region (Mangold et al., 2007Mangold, N., Poulet, F., Mustard, J.F., Bibring, J.-P., Gondet, B., Langevin, Y., Ansan, V., Masson, P., Fassett, C., Head III, J.W., Hoffmann, H., Neukum, G. (2007) Mineralogy of the Nili Fossae region with OMEGA/Mars Express data: 2. Aqueous alteration of the crust. Journal of Geophysical Research: Planets 112, E08S04. https://doi.org/10.1029/2006JE002835
) and the inferred lacustrine precipitation of carbonates in the Jezero Crater (Horgan et al., 2020Horgan, B.H.N., Anderson, R.B., Dromart, G., Amador, E.S., Rice, M.S. (2020) The mineral diversity of Jezero crater: Evidence for possible lacustrine carbonates on Mars. Icarus 339, 113526. https://doi.org/10.1016/j.icarus.2019.113526
). Similarly, the carbonate concretions in the ALH84001 meteorite, which formed at ∼3.9 Ga, originated from cool water (18 ± 4 °C) that had interacted with atmospheric CO2 (Halevy et al., 2011Halevy, I., Fischer, W.W., Eiler, J.M. (2011) Carbonates in the Martian meteorite Allan Hills 84001 formed at 18 ± 4 °C in a near-surface aqueous environment. Proceedings of the National Academy of Sciences 108, 16895–16899. https://doi.org/10.1073/pnas.1109444108
). These observations indicate that carbonate minerals likely formed at, or near, Mars’ surface during its early history, coinciding with the development of fluvial valleys and deep weathering profiles. These findings suggest that Mars experienced a warmer and wetter climate in its early history, likely sustained by a thicker CO2 atmosphere. Indeed, the MAVEN’s observations show a significant loss of Mars’s atmosphere (<0.8 bar CO2) in its early history (Jakosky et al., 2018Jakosky, B.M., Brain, D., Chaffin, M., Curry, S., Deighan, J., Grebowsky, J., Halekas, J., Leblanc, F., Lillis, R., Luhmann, J.G., Andersson, L., Andre, N., Andrews, D., Baird, D., Baker, D., Bell, J., Benna, M., Bhattacharyya, D., Bougher, S., Bowers, C., Chamberlin, P., Chaufray, J.Y., Clarke, J., Collinson, G., Combi, M., Connerney, J., Connour, K., Correira, J., Crabb, K., Crary, F., Cravens, T., Crismani, M., Delory, G., Dewey, R., DiBraccio, G., Dong, C., Dong, Y., Dunn, P., Egan, H., Elrod, M., England, S., Eparvier, F., Ergun, R., Eriksson, A., Esman, T., Espley, J., Evans, S., Fallows, K., Fang, X., Fillingim, M., Flynn, C., Fogle, A., Fowler, C., Fox, J., Fujimoto, M., Garnier, P., Girazian, Z., Groeller, H., Gruesbeck, J., Hamil, O., Hanley, K.G., Hara, T., Harada, Y., Hermann, J., Holmberg, M., Holsclaw, G., Houston, S., Inui, S., Jain, S., Jolitz, R., Kotova, A., Kuroda, T., Larson, D., Lee, Y., Lee, C., Lefevre, F., Lentz, C., Lo, D., Lugo, R., Ma, Y.J., Mahaffy, P., Marquette, M.L., Matsumoto, Y., Mayyasi, M., Mazelle, C., McClintock, W., McFadden, J., Medvedev, A., Mendillo, M., Meziane, K., Milby, Z., Mitchell, D., Modolo, R., Montmessin, F., Nagy, A., Nakagawa, H., Narvaez, C., Olsen, K., Pawlowski, D., Peterson, W., et al. (2018) Loss of the Martian atmosphere to space: Present-day loss rates determined from MAVEN observations and integrated loss through time. Icarus 315, 146–157. https://doi.org/10.1016/j.icarus.2018.05.030
). Therefore, the observational evidence from both the surface and the upper atmosphere points toward extensive interactions between the early Martian crust, an active hydrosphere, and a thicker CO2 atmosphere.Climatic conditions in this early Mars could have induced abundant carbonate precipitation in near surface environments. However, orbiting near-infrared spectrometers primarily detect phyllosilicates and sulphates among alteration minerals, whereas carbonate minerals are relatively rare (Carter et al., 2023
Carter, J., Riu, L., Poulet, F., Bibring, J.-P., Langevin, Y., Gondet, B. (2023) A Mars orbital catalog of aqueous alteration signatures (MOCAAS). Icarus 389, 115164. https://doi.org/10.1016/j.icarus.2022.115164
). Keep in mind that the identification of minerals by remote sensing spectra can be obscured by dust. The scarcity of large carbonate reservoirs could be explained by an acidic period in the Hesperian age that prevented their formation and dissolved those previously formed. However, the extension and age of Nili Fossae carbonates contradict such a hypothesis.The overall composition of Mars’ bedrock is commonly inferred to be basaltic and olivine-rich (olivine > 20 wt. %) (e.g., McSween et al., 2006
McSween, H.Y., Wyatt, M.B., Gellert, R., Bell III, J.F., Morris, R.V., Herkenhoff, K.E., Crumpler, L.S., Milam, K.A., Stockstill, K.R., Tornabene, L.L., Arvidson, R.E., Bartlett, P., Blaney, D., Cabrol, N.A., Christensen, P.R., Clark, B.C., Crisp, J.A., Des Marais, D.J., Economou, T., Farmer, J.D., Farrand, W., Ghosh, A., Golombek, M., Gorevan, S., Greeley, R., Hamilton, V.E., Johnson, J.R., Joliff, B.L., Klingelhöfer, G., Knudson, A.T., McLennan, S., Ming, D., Moersch, J.E., Rieder, R., Ruff, S.W., Schröder, C., de Souza Jr., P.A., Squyres, S.W., Wänke, H., Wang, A., Yen, A., Zipfel, J. (2006) Characterization and petrologic interpretation of olivine-rich basalts at Gusev Crater, Mars. Journal of Geophysical Research: Planets 111, E02S10. https://doi.org/10.1029/2005JE002477
). However, recent analyses of igneous rocks from the ancient bedrock surrounding Gale Crater (Mangold et al., 2016Mangold, N., Thompson, L.M., Forni, O., Williams, A.J., Fabre, C., Le Deit, L., Wiens, R.C., Williams, R., Anderson, R.B., Blaney, D.L., Calef, F., Cousin, A., Clegg, S.M., Dromart, G., Dietrich, W.E., Edgett, K.S., Fisk, M.R., Gasnault, O., Gellert, R., Grotzinger, J.P., Kah, L., Le Mouélic, S., McLennan, S.M., Maurice, S., Meslin, P.-Y., Newsom, H.E., Palucis, M.C., Rapin, W., Sautter, V., Siebach, K.L., Stack, K., Sumner, D., Yingst, A. (2016) Composition of conglomerates analyzed by the Curiosity rover: Implications for Gale Crater crust and sediment sources. Journal of Geophysical Research: Planets 121, 353–387. https://doi.org/10.1002/2015JE004977
) and of ancient Martian meteorites (Hewins et al., 2017Hewins, R.H., Zanda, B., Humayun, M., Nemchin, A., Lorand, J.-P., Pont, S., Deldicque, D., Bellucci, J.J., Beck, P., Leroux, H., Marinova, M., Remusat, L., Göpel, C., Lewin, E., Grange, M., Kennedy, A., Whitehouse, M.J. (2017) Regolith breccia Northwest Africa 7533: Mineralogy and petrology with implications for early Mars. Meteoritics & Planetary Science 52, 89–124. https://doi.org/10.1111/maps.12740
) suggest that felsic and alkali-rich rocks are more abundant in the Mars’ ancient crust than initially presumed (Sautter et al., 2016Sautter, V., Toplis, M.J., Beck, P., Mangold, N., Wiens, R., Pinet, P., Cousin, A., Maurice, S., LeDeit, L., Hewins, R., Gasnault, O., Quantin, C., Forni, O., Newsom, H., Meslin, P.-Y., Wray, J., Bridges, N., Payré, V., Rapin, W., Le Mouélic, S. (2016) Magmatic complexity on early Mars as seen through a combination of orbital, in-situ and meteorite data. Lithos 254–255, 36–52. https://doi.org/10.1016/j.lithos.2016.02.023
). Therefore, most carbonates >3.6 Ga old may have been formed from the alteration of this ancient crust, and not from the post-Noachian basaltic plains. In this study, we investigate the role of the bedrock compositions on the formation of carbonate by aqueous alteration. In prior experiments simulating early Mars conditions (pCO2 = 1, T = 45 °C), moderate (Gaudin et al., 2018Gaudin, A., Dehouck, E., Grauby, O., Mangold, N. (2018) Formation of clay minerals on Mars: Insights from long-term experimental weathering of olivine. Icarus 311, 210–223. https://doi.org/10.1016/j.icarus.2018.01.029
) to minor magnesium carbonates (Dehouck et al., 2014Dehouck, E., Gaudin, A., Mangold, N., Lajaunie, L., Dauzères, A., Grauby, O., Le Menn, E. (2014) Weathering of olivine under CO2 atmosphere: A martian perspective. Geochimica et Cosmochimica Acta 135, 170–189. https://doi.org/10.1016/j.gca.2014.03.032
) were identified from forsteritic-olivine (Fo90) weathering. However, Fourier transform infrared spectroscopy (FTIR) showed no carbonate detection (>∼0.8 wt. %) in less magnesian olivines under similar geochemical conditions (pCO2 from 0.1 to 1 bar, T = 25 °C; Kissick et al., 2021Kissick, L.E., Mather, T.A., Tosca, N.J. (2021) Unravelling surface and subsurface carbon sinks within the early Martian crust. Earth and Planetary Science Letters 557, 116663. https://doi.org/10.1016/j.epsl.2020.116663
). These weathering experiments focused on olivine minerals alone. Here, we experimentally investigate the reactions of a range of potential crustal compositions (e.g., from ultramafic to more felsic composition) on carbonate formation under a thick CO2 atmosphere (1 bar). The initial materials cover various individual silicate minerals including two types of olivine (Fo92 and Fo65) to assess differences between the Mg-rich forsterite with the more Fe-rich forsterite identified at Nili Fossae (Brown et al., 2020Brown, A.J., Viviano, C.E., Goudge, T.A. (2020) Olivine-Carbonate Mineralogy of the Jezero Crater Region. Journal of Geophysical Research: Planets 125, e2019JE006011. https://doi.org/10.1029/2019JE006011
), pyroxenes, and feldspars. We also analyse two synthetic ancient crust samples based on the chemical composition of the NWA 7533 and NWA 7034 martian regolith breccias (e.g., Hewins et al., 2017Hewins, R.H., Zanda, B., Humayun, M., Nemchin, A., Lorand, J.-P., Pont, S., Deldicque, D., Bellucci, J.J., Beck, P., Leroux, H., Marinova, M., Remusat, L., Göpel, C., Lewin, E., Grange, M., Kennedy, A., Whitehouse, M.J. (2017) Regolith breccia Northwest Africa 7533: Mineralogy and petrology with implications for early Mars. Meteoritics & Planetary Science 52, 89–124. https://doi.org/10.1111/maps.12740
) and the more alkali-rich composition of the early Mars crust identified in conglomerates in Gale Crater (Mangold et al., 2016Mangold, N., Thompson, L.M., Forni, O., Williams, A.J., Fabre, C., Le Deit, L., Wiens, R.C., Williams, R., Anderson, R.B., Blaney, D.L., Calef, F., Cousin, A., Clegg, S.M., Dromart, G., Dietrich, W.E., Edgett, K.S., Fisk, M.R., Gasnault, O., Gellert, R., Grotzinger, J.P., Kah, L., Le Mouélic, S., McLennan, S.M., Maurice, S., Meslin, P.-Y., Newsom, H.E., Palucis, M.C., Rapin, W., Sautter, V., Siebach, K.L., Stack, K., Sumner, D., Yingst, A. (2016) Composition of conglomerates analyzed by the Curiosity rover: Implications for Gale Crater crust and sediment sources. Journal of Geophysical Research: Planets 121, 353–387. https://doi.org/10.1002/2015JE004977
) (Table 1). To explore the influence of olivine concentration, we added Fo65 (∼11.8 wt. %) to the crust simulant-O (Sim-O), whereas the simulant-M (Sim-M), contains magnetite but no olivine. The description of the experimental set up (Fig. S-1) and the chemical proprieties of the experimental solutions (Fig. S-7) have been reported by Baron et al. (2019)Baron, F., Gaudin, A., Lorand, J.-P., Mangold, N. (2019) New Constraints on Early Mars Weathering Conditions From an Experimental Approach on Crust Simulants. Journal of Geophysical Research: Planets 124, 1783–1801. https://doi.org/10.1029/2019JE005920
. Here, we investigate the potential carbonate occurrences in the altered solids. Aiming to analyse the surface alteration of the samples, we used X-ray Photoelectron Spectroscopy (XPS) (sampling depth between 3–10 nm) in addition to the more conventional FTIR.Table 1 Summary of the samples analysed in this study. The bulk chemical composition of martian simulants samples was estimated according to the proportion of the minerals used (Baron et al., 2019
Baron, F., Gaudin, A., Lorand, J.-P., Mangold, N. (2019) New Constraints on Early Mars Weathering Conditions From an Experimental Approach on Crust Simulants. Journal of Geophysical Research: Planets 124, 1783–1801. https://doi.org/10.1029/2019JE005920
).| Individual minerals | Structural formula |
| Mg-olivine (Fo92) | Al0.01Mg1.78Fe0.16 Si1.05 O4 |
| Ferroan olivine (Fo65) | Mg1.30Fe0.70 Si1.00 O4 |
| clinopyroxene (augite) | Na0.09Ca0.58Al0.19Mg0.92Fe0.21 Si1.92Al0.08 O6 |
| plagioclase (An60) | K0.01Na0.39Ca0.60 Al1.53Mg0.01Fe0.01Si2.44 O8 |
| K-feldspar (Or85) | K0.85Na0.15 Al0.97Mg0.01Fe0.01 Si3.01 O8 |
| Martian crust simulants | Minerals (wt. %) | Chemical Compositions (wt. %) | ||
| Martian crust simulant-O (Sim-O) | Ferroan olivine (Mg1.30Fe0.70 Si1.00O4) | 11.8 | SiO2 Al2O3 FeO MgO CaO Na2O K2O P2O5 | 52.5 13.3 6.9 13.2 8.4 2.0 1.9 1.7 |
| Orthopyroxene (Na0.03Ca0.04Al0.08Mg1.58Fe0.27Si2.01O6) | 17.7 | |||
| 23.5 | ||||
| 32.4 | ||||
| K-feldspar (K0.85Na0.15Al0.97Mg0.01Fe0.01Si3.01O8) | 12.8 | |||
| Apatite (Ca4.67Na0.08Mg0.04(P2.85Si0.06O4)3F1.21Cl0.06) | 2 | |||
| Martian crust simulant-M (SIM-M) | Magnetite (Fe3O4) | 10.9 | SiO2 Al2O3 FeO MgO CaO Na2O K2O P2O5 | 49.3 13.7 12.9 9.6 8.6 2.1 2.0 1.8 |
| Orthopyroxene (Na0.03Ca0.04Al0.08Mg1.58Fe0.27Si2.01O6) | 17.8 | |||
| Clinopyroxene (Na0.09Ca0.58Al0.19Mg0.92Fe0.21Si1.92Al0.08O6) | 23.7 | |||
| Plagioclase (K0.01Na0.39Ca0.60Al1.53Mg0.01Fe0.01Si2.44O8) | 32.7 | |||
| K-feldspar (K0.85Na0.15Al0.97Mg0.01Fe0.01Si3.01O8) | 12.9 | |||
| Apatite (Ca4.67Na0.08Mg0.04(P2.85Si0.06O4)3F1.21Cl0.06) | 2 | |||
top
Results
We estimate the atomic percentages of the elements on the samples’ surfaces by analysing XPS survey spectra (Fig. S-2). Then, we fit the binding energy of the core level C1s feature to identify the specific carbonate species (Fig. 1). In this energy range, all samples exhibit the usual contribution of ubiquitous adventitious carbon resulting from hydrocarbon physisorption onto surfaces. The primary peak of this adventitious carbon, denoted as C-H, occurs at 284.8 eV, while the carbonate peak (CO3) emerges around ∼289.7 eV, depending on the ionic character of the metal-carbon bond (e.g., MgCO3 ∼290 eV and CaCO3/FeCO3 ∼289.5 eV). All the unreacted samples (i.e. fresh material before reaction) except potassium feldspar (K-spar), show minor contribution from carbonate. However, only the reacted olivine-bearing samples (Fo92 and Fo65) show a substantial increase of a well resolved carbonate peak around 290 eV, which represents Mg-CO3 groups (Fig. 1, extended Fig. S-3 and Table S-1).

Figure 1 Fitting model of the C1s orbital for Fo65 sample, (a) initially, and (b) after reaction (under CO2 atmosphere) (see SI for spectra deconvolution).
Figure 2 depicts the amount of carbonate at mineral surfaces before and after reactions together with the final pH values of the solutions. High pH values result from the weathering of mafic minerals (i.e. olivine and pyroxene), which neutralise the acidity derived from a CO2-rich atmosphere (pH0 ∼ 3.6); the solutions with lower pH values reflect the hydrolysis of felsic minerals (i.e. feldspars). Solutions from the martian simulants also have low pHs because they contain abundant feldspars. Only the olivine samples (Fo92 and Fo65) formed substantial proportions of carbonates during reaction, and only their solutions had pH > 6.2, the threshold for carbonates formation under a pCO2 = 1 bar atmosphere (Bullock and Moore, 2007
Bullock, M.A., Moore, J.M. (2007) Atmospheric conditions on early Mars and the missing layered carbonates. Geophysical Research Letters 34, L19201. https://doi.org/10.1029/2007GL030688
). Mg-rich olivine (Fo92) produced higher carbonate formation than Fe-rich olivine (Fo65). Conversely, potassium feldspar (K-spar) lacked carbonates and yielded the lowest pH solution (pH ∼ 4.75). Simulant samples (Sim-O and Sim-M) did not show either a significant increase in carbonate compounds, nor appreciable differences between them, despite the presence of olivine in Sim-O (Fo65 ∼ 11.8 wt. %) and their differences in the iron and magnesium content.
Figure 2 Amount of carbonate in the samples (bars) and pH value (triangles) of the solutions at the end of the experiment (see SI for % calculations). The error bars show the uncertainty of the carbon estimation associated with the survey quantification (standard deviation in Fig. S-2). The pH0 = 3.6, corresponding to the pure water equilibrated with the pCO2 = 1 bar. The dashed line marks the pH threshold of carbonates stability for pCO2 = 1 bar (Bullock and Moore, 2007
Bullock, M.A., Moore, J.M. (2007) Atmospheric conditions on early Mars and the missing layered carbonates. Geophysical Research Letters 34, L19201. https://doi.org/10.1029/2007GL030688
).Figure 3 shows the magnesium high resolution XPS spectra (Mg2p orbital) of the olivine and Sim-O samples. When comparing initial and reacted samples, we only identified a change in the magnesium peak of olivine samples. This change manifests as a shift towards higher binding energies and an asymmetric broadening, a feature not observed in Sim-O. This change suggests the presence of MgCO3 (∼50.6 eV), consistent with the carbonate peak position identified in their C1s spectra (i.e. 290 eV ∼MgCO3).

Figure 3 Mg 2p high resolution XPS spectra before (black colour) and after reaction under a CO2 atmosphere (red colour) of (a) Fo65-olivine, (b) Fo92-olivine, and (c) simulant sample with Fo65 (Sim-O). The solid arrows (black colour) mark the position of the main peak of the unreacted samples whereas dashed arrows (red colour) mark the position of the MgCO3 peak.
We also examined FTIR spectra for signatures of carbonate minerals. The bulk samples (i.e. whole size fractions) showed no features assignable to carbonate minerals, because of their low degree of alteration. However, the finest size fractions (<1 μm), did show absorptions from the strongest vibrational IR band for carbonates (i.e. the asymmetric stretching mode [v3(CO3)]) in several samples (Fig. S-4). The intensity of this band in reacted olivine samples suggests that carbonate formed during reaction, in agreement with the XPS results (Fig. 2). FEG-SEM images also suggest the presence of small particles of carbonate minerals, inferred by their crystal shape, on olivine samples (Fig. S-5). Note, however, that we cannot directly use the carbonate IR band intensity as a measure of carbonate abundances because it is only apparent in the size fraction <1 μm. The spectra enlargement (2000–600 cm−1) of olivine samples shows that v3(CO3) feature is split into two barely resolved peaks, which match well with a hydrated magnesium carbonate (e.g., hydromagnesite; Fig. S-6). The saturation indices of carbonates calculated from the chemistry of experimental solutions (Figs. S-7, S-8) (Baron et al., 2019
Baron, F., Gaudin, A., Lorand, J.-P., Mangold, N. (2019) New Constraints on Early Mars Weathering Conditions From an Experimental Approach on Crust Simulants. Journal of Geophysical Research: Planets 124, 1783–1801. https://doi.org/10.1029/2019JE005920
) are also consistent with the solid products identified in this study. However, the use of this variable to predict mineral formation should be employed with caution (see geochemical modelling and carbonates in SI).top
Discussion
Our weathering experiments under early Mars-like conditions show that, among potential materials of the early Mars crust, only olivine (Fo92 and Fo65) reacts to form significant proportions of carbonate minerals (Fig. 1). Carbonation resulting from ultramafic rock reaction has been extensively studied as a CO2(g) capture strategy on Earth (i.e. high T and/or high P conditions) because it can neutralise the acidity imposed from CO2 dissolution (Snæbjörnsdóttir et al., 2020
Snæbjörnsdóttir, S.Ó., Sigfússon, B., Marieni, C., Goldberg, D., Gislason, S.R., Oelkers, E.H. (2020) Carbon dioxide storage through mineral carbonation. Nature Reviews Earth & Environment 1, 90–102. https://doi.org/10.1038/s43017-019-0011-8
). The pH values of our post-reaction solutions show that under early Mars-like conditions, this neutralisation is particularly effective in olivine samples (Fig. 2), where we identify the formation of Mg carbonates (Figs. 1, 3 and Figs. S-3, S-6). The formation of magnesite is usually linked to elevated temperatures (T > 50 °C) due to the high hydration energy of Mg2+. However, hydrated Mg carbonates can form in solutions with a high concentration of Mg2+ under near ambient temperatures (Gaudin et al., 2018Gaudin, A., Dehouck, E., Grauby, O., Mangold, N. (2018) Formation of clay minerals on Mars: Insights from long-term experimental weathering of olivine. Icarus 311, 210–223. https://doi.org/10.1016/j.icarus.2018.01.029
), as shown by our XPS and FTIR spectra (Figs. 1, 3 and Figs. S-3, S-6). Then, this hydrated precursor can turn into anhydrous magnesite as occurs in low temperature evaporitic environments on Earth (e.g., Scheller et al., 2021Scheller, E.L., Swindle, C., Grotzinger, J., Barnhart, H., Bhattacharjee, S., Ehlmann, B.L., Farley, K., Fischer, W.W., Greenberger, R., Ingalls, M., Martin, P.E., Osorio-Rodriguez, D., Smith, B.P. (2021) Formation of Magnesium Carbonates on Earth and Implications for Mars. Journal of Geophysical Research: Planets 126, e2021JE006828. https://doi.org/10.1029/2021JE006828
). This geological scenario could explain the formation of carbonates identified (from orbit) along the margins of the Jezero (playa) lake (Horgan et al., 2020Horgan, B.H.N., Anderson, R.B., Dromart, G., Amador, E.S., Rice, M.S. (2020) The mineral diversity of Jezero crater: Evidence for possible lacustrine carbonates on Mars. Icarus 339, 113526. https://doi.org/10.1016/j.icarus.2019.113526
), setting aside the specific interferences that the development of passivation layers may cause on olivine carbonation rates (Oelkers et al., 2018Oelkers, E., Declercq, J., Saldi, G., Gislason, S. (2018) Olivine dissolution rates: A critical review. Chemical Geology 500, 1–19. https://doi.org/10.1016/j.chemgeo.2018.10.008
and references therein). Interestingly, we do not identify siderite, not even in the iron-rich forsterite (Fo65) in agreement with Baron et al. (2019)Baron, F., Gaudin, A., Lorand, J.-P., Mangold, N. (2019) New Constraints on Early Mars Weathering Conditions From an Experimental Approach on Crust Simulants. Journal of Geophysical Research: Planets 124, 1783–1801. https://doi.org/10.1029/2019JE005920
, who showed that olivine reaction solutions were oxidising, hindering siderite formation. These results seem in disagreement with the iron-rich carbonate minerals observed in the nakhlite Martian meteorites (Nakhla, Lafayette, and Governador Valadares). However, they are inferred to have formed through high temperature hydrothermal subsurface processes induced by an impact event (Bridges and Schwenzer, 2012Bridges, J.C., Schwenzer, S.P. (2012) The nakhlite hydrothermal brine on Mars. Earth and Planetary Science Letters 359–360, 117–123. https://doi.org/10.1016/j.epsl.2012.09.044
), contrasting with our experimental conditions (i.e. water equilibrated with a pCO2 = 1 bar atmosphere, T = 45 °C). Moreover, the lower carbonation observed on the Fo65 compared to the Fo92 sample (Fig. 2) suggests that the Fe enrichment in martian olivine limits the quantity of carbonate but does not prevent its formation, in agreement with the results of Brown et al. (2020)Brown, A.J., Viviano, C.E., Goudge, T.A. (2020) Olivine-Carbonate Mineralogy of the Jezero Crater Region. Journal of Geophysical Research: Planets 125, e2019JE006011. https://doi.org/10.1029/2019JE006011
who identify Fo40-66 in the olivine-carbonate lithology of Nili Fossae region. This effect could be due to the development of an Fe-rich silica layer on the olivine surface that limits the carbonatation reaction (Oelkers et al., 2018Oelkers, E., Declercq, J., Saldi, G., Gislason, S. (2018) Olivine dissolution rates: A critical review. Chemical Geology 500, 1–19. https://doi.org/10.1016/j.chemgeo.2018.10.008
). Interestingly, in Kissick et al. (2021)Kissick, L.E., Mather, T.A., Tosca, N.J. (2021) Unravelling surface and subsurface carbon sinks within the early Martian crust. Earth and Planetary Science Letters 557, 116663. https://doi.org/10.1016/j.epsl.2020.116663
, no carbonates were detected from aqueous alteration of fayalite-forsterite mixtures, but this could be due to the FTIR detection limit. We do not identify either a carbonate signal when analysing the whole fraction of the sample with this technique.An important question is why carbonates do not form in the crustal simulant samples. As noted by Baron et al. (2019)
Baron, F., Gaudin, A., Lorand, J.-P., Mangold, N. (2019) New Constraints on Early Mars Weathering Conditions From an Experimental Approach on Crust Simulants. Journal of Geophysical Research: Planets 124, 1783–1801. https://doi.org/10.1029/2019JE005920
, our experiments demonstrate that the mineralogy of the reactants significantly influences solution properties, particularly pH, subsequent reaction pathways, and the formation of secondary products. In a dense CO2 atmosphere on early Mars (i.e. pCO2 = 1 bar), carbonate minerals are not stable at pH < 6.2 (Bullock and Moore, 2007Bullock, M.A., Moore, J.M. (2007) Atmospheric conditions on early Mars and the missing layered carbonates. Geophysical Research Letters 34, L19201. https://doi.org/10.1029/2007GL030688
) and the simulant samples yield solutions with pH values below this threshold (Fig. 2); the pH values of the simulant solutions are intermediate between those of pure olivine and pure feldspar samples. In our experiments, dissolution-precipitation reactions are likely coupled, and both martian simulants contain high percentages of feldspars that generally form Al-OH-rich secondary minerals (e.g., amorphous Al-OH precursors, gibbsite or kaolinite) as alteration products (Zhu and Lu, 2009Zhu, C., Lu, P. (2009) Alkali feldspar dissolution and secondary mineral precipitation in batch systems: 3. Saturation states of product minerals and reaction paths. Geochimica et Cosmochimica Acta 73, 3171–3200. https://doi.org/10.1016/j.gca.2009.03.015
). The insoluble nature of the Al3+ under weathering conditions favours precipitation of such phases and therefore the production of H+ by the hydrolysis of this cation (e.g., Al3+ + 3H2O = Al(OH)3(s) + 3H+). As we do not identify Al3+ in simulant solutions (Fig. S-7), we hypothesise that the incorporation of OH in these secondary products helps to hold the pH at slightly acidic values. Comparing the fluid chemistry of forsterite and simulant solutions (Fig. S-7) is evident that forsterite solutions hold the highest Mg2+ concentration from the incongruent dissolution of forsterite, favouring the formation of Mg-carbonates.These findings suggest that the formation of carbonates by surface weathering on early Mars depends on the host rock composition, as suggested by Kissick et al. (2021)
Kissick, L.E., Mather, T.A., Tosca, N.J. (2021) Unravelling surface and subsurface carbon sinks within the early Martian crust. Earth and Planetary Science Letters 557, 116663. https://doi.org/10.1016/j.epsl.2020.116663
. Accordingly, surface carbonate deposits might only have formed from olivine-rich rock (i.e. olivine > 20 wt. %), like in Nile Fossae region, but not from more felsic rocks. Regardless, this scenario does not preclude the formation of carbonates by migration of meteoric water and/or diffusion which would induce a pH increase with depth, groundwater circulation, or hydrothermal processes. Indeed, Thorpe et al. (2022)Thorpe, M.T., Bristow, T.F., Rampe, E.B., Tosca, N.J., Grotzinger, J.P., Bennett, K.A., Achilles, C.N., Blake, D.F., Chipera, S.J., Downs, G., Downs, R.T., Morrison, S.M., Tu, V., Castle, N., Craig, P., Marais, D.J.D., Hazen, R.M., Ming, D.W., Morris, R.V., Treiman, A.H., Vaniman, D.T., Yen, A.S., Vasavada, A.R., Dehouck, E., Bridges, J.C., Berger, J., McAdam, A., Peretyazhko, T., Siebach, K.L., Bryk, A.B., Fox, V.K., Fedo, C.M. (2022) Mars Science Laboratory CheMin Data From the Glen Torridon Region and the Significance of Lake-Groundwater Interactions in Interpreting Mineralogy and Sedimentary History. Journal of Geophysical Research: Planets 127, e2021JE007099. https://doi.org/10.1029/2021JE007099
recently identified Fe-rich carbonate in Glen Torridon (Gale Crater) and inferred that it likely formed in a subsurface mixing zone between lacustrine water and deep groundwater.Geochemical models for basalt dissolution under a CO2-rich early Mars atmosphere generally predict more abundant carbonate formation than observed on the martian surface. However, these models usually assume olivine-rich basalts as starting protoliths (i.e. olivine > 20 wt. %) and equilibrium conditions. Consequently, they do not address kinetic barrier effects (Kissick et al., 2021
Kissick, L.E., Mather, T.A., Tosca, N.J. (2021) Unravelling surface and subsurface carbon sinks within the early Martian crust. Earth and Planetary Science Letters 557, 116663. https://doi.org/10.1016/j.epsl.2020.116663
; Scheller et al., 2021Scheller, E.L., Swindle, C., Grotzinger, J., Barnhart, H., Bhattacharjee, S., Ehlmann, B.L., Farley, K., Fischer, W.W., Greenberger, R., Ingalls, M., Martin, P.E., Osorio-Rodriguez, D., Smith, B.P. (2021) Formation of Magnesium Carbonates on Earth and Implications for Mars. Journal of Geophysical Research: Planets 126, e2021JE006828. https://doi.org/10.1029/2021JE006828
), which may result in an overestimation of the carbonate abundance (see geochemical modelling and carbonates in SI).Carbonate occurrences detected on Mars are commonly associated with olivine-bearing lithologies (olivine > 20 % in volume) (Wray et al., 2016
Wray, J.J., Murchie, S.L., Bishop, J.L., Ehlmann, B.L., Milliken, R.E., Wilhelm, M.B., Seelos, K.D., Chojnacki, M. (2016) Orbital evidence for more widespread carbonate-bearing rocks on Mars. Journal of Geophysical Research: Planets 121, 652–677. https://doi.org/10.1002/2015JE004972
), consistent with our experimental results. The Comanche outcrops in the Columbia Hills (Gusev Crater) contain a mineral assemblage of olivine and carbonates. These carbonates, of inferred Noachian age, are abundant (16 to 34 wt. %) and intimately associated with olivine (Fo68) and amorphous silicate (Morris et al., 2010Morris, R.V., Ruff, S.W., Gellert, R., Ming, D.W., Arvidson, R.E., Clark, B.C., Golden, D.C., Siebach, K., Klingelhöfer, G., Schröder, C., Fleischer, I., Yen, A.S., Squyres, S.W. (2010) Identification of Carbonate-Rich Outcrops on Mars by the Spirit Rover. Science 329, 421–424. https://doi.org/10.1126/science.1189667
). Orbital reflectance spectra of the Capri Chasma region also show phyllosilicates, carbonates, and a host rock potentially rich in olivine (Jain and Chauhan, 2015Jain, N., Chauhan, P. (2015) Study of phyllosilicates and carbonates from the Capri Chasma region of Valles Marineris on Mars based on Mars Reconnaissance Orbiter-Compact Reconnaissance Imaging Spectrometer for Mars (MRO-CRISM) observations. Icarus 250, 7–17. https://doi.org/10.1016/j.icarus.2014.11.018
), like the carbonate-olivine association observed at Nili Fossae (Ehlmann et al., 2009Ehlmann, B.L., Mustard, J.F., Swayze, G.A., Clark, R.N., Bishop, J.L., Poulet, F., Des Marais, D.J., Roach, L.H., Milliken, R.E., Wray, J.J., Barnouin-Jha, O., Murchie, S.L. (2009) Identification of hydrated silicate minerals on Mars using MRO-CRISM: Geologic context near Nili Fossae and implications for aqueous alteration. Journal of Geophysical Research: Planets 114, E00D08. https://doi.org/10.1029/2009JE003339
). In Jezero Crater, carbonates detected from orbit are associated with olivine-bearing rocks and mapped as the same geological unit identified across Nili Fossae (Mandon et al., 2020Mandon, L., Quantin-Nataf, C., Thollot, P., Mangold, N., Lozac’h, L., Dromart, G., Beck, P., Dehouck, E., Breton, S., Millot, C., Volat, M. (2020) Refining the age, emplacement and alteration scenarios of the olivine-rich unit in the Nili Fossae region, Mars. Icarus 336, 113436. https://doi.org/10.1016/j.icarus.2019.113436
). These alteration units share similarities with the mineralogical associations described in the oldest carbonates identified in ALH 84001, likely formed by a low temperature weathering fluid equilibrated with CO2 atmosphere (Halevy et al., 2011Halevy, I., Fischer, W.W., Eiler, J.M. (2011) Carbonates in the Martian meteorite Allan Hills 84001 formed at 18 ± 4 °C in a near-surface aqueous environment. Proceedings of the National Academy of Sciences 108, 16895–16899. https://doi.org/10.1073/pnas.1109444108
).Recent results show that the ancient Mars crust was more alkali-rich and felsic than previously thought (Sautter et al., 2016
Sautter, V., Toplis, M.J., Beck, P., Mangold, N., Wiens, R., Pinet, P., Cousin, A., Maurice, S., LeDeit, L., Hewins, R., Gasnault, O., Quantin, C., Forni, O., Newsom, H., Meslin, P.-Y., Wray, J., Bridges, N., Payré, V., Rapin, W., Le Mouélic, S. (2016) Magmatic complexity on early Mars as seen through a combination of orbital, in-situ and meteorite data. Lithos 254–255, 36–52. https://doi.org/10.1016/j.lithos.2016.02.023
), as indicated by the abundance of felsic igneous rocks (trachytic, alkali feldspar-rich) found in Gale Crater as float and pebbles sourced from Noachian age crust around the crater (Mangold et al., 2016Mangold, N., Thompson, L.M., Forni, O., Williams, A.J., Fabre, C., Le Deit, L., Wiens, R.C., Williams, R., Anderson, R.B., Blaney, D.L., Calef, F., Cousin, A., Clegg, S.M., Dromart, G., Dietrich, W.E., Edgett, K.S., Fisk, M.R., Gasnault, O., Gellert, R., Grotzinger, J.P., Kah, L., Le Mouélic, S., McLennan, S.M., Maurice, S., Meslin, P.-Y., Newsom, H.E., Palucis, M.C., Rapin, W., Sautter, V., Siebach, K.L., Stack, K., Sumner, D., Yingst, A. (2016) Composition of conglomerates analyzed by the Curiosity rover: Implications for Gale Crater crust and sediment sources. Journal of Geophysical Research: Planets 121, 353–387. https://doi.org/10.1002/2015JE004977
). Likewise, the ancient meteorite breccia NWA 7034 (and pairs) is rich in feldspars and lacks primary olivine (Hewins et al., 2017Hewins, R.H., Zanda, B., Humayun, M., Nemchin, A., Lorand, J.-P., Pont, S., Deldicque, D., Bellucci, J.J., Beck, P., Leroux, H., Marinova, M., Remusat, L., Göpel, C., Lewin, E., Grange, M., Kennedy, A., Whitehouse, M.J. (2017) Regolith breccia Northwest Africa 7533: Mineralogy and petrology with implications for early Mars. Meteoritics & Planetary Science 52, 89–124. https://doi.org/10.1111/maps.12740
). Regarding the olivine distribution through Mars, olivine-rich regions such as Nili Fossae (i.e. olivine > 20 wt. %) are rare in Noachian highlands (e.g., Ody et al., 2013Ody, A., Poulet, F., Bibring, J.-P., Loizeau, D., Carter, J., Gondet, B., Langevin, Y. (2013) Global investigation of olivine on Mars: Insights into crust and mantle compositions. Journal of Geophysical Research: Planets 118, 234–262. https://doi.org/10.1029/2012JE004149
). Our results show that a more felsic and alkali-rich crustal composition does not produce carbonates by weathering under early Mars-like conditions, i.e., pCO2 = 1 bar, moderate temperature. Our results are likewise consistent with the limited occurrence of carbonates (<3.2 wt. %) detected by CheMin in the Glen Torridon area of Gale Crater (Thorpe et al., 2022Thorpe, M.T., Bristow, T.F., Rampe, E.B., Tosca, N.J., Grotzinger, J.P., Bennett, K.A., Achilles, C.N., Blake, D.F., Chipera, S.J., Downs, G., Downs, R.T., Morrison, S.M., Tu, V., Castle, N., Craig, P., Marais, D.J.D., Hazen, R.M., Ming, D.W., Morris, R.V., Treiman, A.H., Vaniman, D.T., Yen, A.S., Vasavada, A.R., Dehouck, E., Bridges, J.C., Berger, J., McAdam, A., Peretyazhko, T., Siebach, K.L., Bryk, A.B., Fox, V.K., Fedo, C.M. (2022) Mars Science Laboratory CheMin Data From the Glen Torridon Region and the Significance of Lake-Groundwater Interactions in Interpreting Mineralogy and Sedimentary History. Journal of Geophysical Research: Planets 127, e2021JE007099. https://doi.org/10.1029/2021JE007099
) and the absence of carbonates in other crater locations (Bristow et al., 2017Bristow, T.F., Haberle, R.M., Blake, D.F., Des Marais, D.J., Eigenbrode, J.L., Fairén, A.G., Grotzinger, J.P., Stack, K.M., Mischna, M.A., Rampe, E.B., Siebach, K.L., Sutter, B., Vaniman, D.T., Vasavada, A.R. (2017) Low Hesperian PCO2 constrained from in situ mineralogical analysis at Gale Crater, Mars. Proceedings of the National Academy of Sciences 114, 2166–2170. https://doi.org/10.1073/pnas.1616649114
), despite significant diagenetic alteration in the mudstones (i.e. 20 to 30 wt. % of phyllosilicates). However, the sedimentary record in Gale reveals a rich spectrum of alteration stages, which may encompass processes such as the formation-dissolution of pre-existing carbonates and the later formation of clay minerals, as observed in Nakhlites. Our results also help to illustrate why martian carbonates did not form abundant surface deposits, thus, serving as a limited storage for atmospheric CO2 (Edwards and Ehlmann, 2015Edwards, C.S., Ehlmann, B.L. (2015) Carbon sequestration on Mars. Geology 43, 863–866. https://doi.org/10.1130/G36983.1
). Considering that there have been no detections of substantial CO2 reservoirs at Mars’ surface, MAVEN observations propose that gas loss to space may have driven Martian climate change (Jakosky et al., 2018Jakosky, B.M., Brain, D., Chaffin, M., Curry, S., Deighan, J., Grebowsky, J., Halekas, J., Leblanc, F., Lillis, R., Luhmann, J.G., Andersson, L., Andre, N., Andrews, D., Baird, D., Baker, D., Bell, J., Benna, M., Bhattacharyya, D., Bougher, S., Bowers, C., Chamberlin, P., Chaufray, J.Y., Clarke, J., Collinson, G., Combi, M., Connerney, J., Connour, K., Correira, J., Crabb, K., Crary, F., Cravens, T., Crismani, M., Delory, G., Dewey, R., DiBraccio, G., Dong, C., Dong, Y., Dunn, P., Egan, H., Elrod, M., England, S., Eparvier, F., Ergun, R., Eriksson, A., Esman, T., Espley, J., Evans, S., Fallows, K., Fang, X., Fillingim, M., Flynn, C., Fogle, A., Fowler, C., Fox, J., Fujimoto, M., Garnier, P., Girazian, Z., Groeller, H., Gruesbeck, J., Hamil, O., Hanley, K.G., Hara, T., Harada, Y., Hermann, J., Holmberg, M., Holsclaw, G., Houston, S., Inui, S., Jain, S., Jolitz, R., Kotova, A., Kuroda, T., Larson, D., Lee, Y., Lee, C., Lefevre, F., Lentz, C., Lo, D., Lugo, R., Ma, Y.J., Mahaffy, P., Marquette, M.L., Matsumoto, Y., Mayyasi, M., Mazelle, C., McClintock, W., McFadden, J., Medvedev, A., Mendillo, M., Meziane, K., Milby, Z., Mitchell, D., Modolo, R., Montmessin, F., Nagy, A., Nakagawa, H., Narvaez, C., Olsen, K., Pawlowski, D., Peterson, W., et al. (2018) Loss of the Martian atmosphere to space: Present-day loss rates determined from MAVEN observations and integrated loss through time. Icarus 315, 146–157. https://doi.org/10.1016/j.icarus.2018.05.030
).The weathering experiments presented here suggest that (1) Fo65 and Fo92 samples induce the formation of Mg carbonate (no evidence of siderite is here detected), and (2) the inhibiting effect of a more felsic composition in the early Mars crust could have on carbonate formation. As a corollary, we suggest that the sparse distribution of surface carbonates under a warmer and thicker CO2 atmosphere can be associated with the compositional diversity of the magmatic rocks in the ancient martian crust. Therefore, their scarcity does not have to be evidence of a cold and dried early Mars.
top
Acknowledgements
We thank reviewers Mike Thorpe, Allan Treiman and one anonymous reviewer whose suggestions helped improve and clarify this manuscript. We thank Erwan Le Menn for his assistance during the experiment set up. This research was supported by the project “Mars-Prime” (ANR-16-CE31-0012) from the Agence Nationale de la Recherche. CG-L was supported for the Postdoctoral fellowship ED481B-2019-068 (Xunta de Galicia) and by the project PID2020-119412RJ-I00 from MICINN Spain.
Editor: Francis McCubbin
top
References
Baron, F., Gaudin, A., Lorand, J.-P., Mangold, N. (2019) New Constraints on Early Mars Weathering Conditions From an Experimental Approach on Crust Simulants. Journal of Geophysical Research: Planets 124, 1783–1801. https://doi.org/10.1029/2019JE005920
 Show in context
Show in contextThe description of the experimental set up (Fig. S-1) and the chemical proprieties of the experimental solutions (Fig. S-7) have been reported by Baron et al. (2019).
View in article
The bulk chemical composition of martian simulants samples was estimated according to the proportion of the minerals used (Baron et al., 2019).
View in article
The saturation indices of carbonates calculated from the chemistry of experimental solutions (Figs. S-7, S-8) (Baron et al., 2019) are also consistent with the solid products identified in this study.
View in article
Interestingly, we do not identify siderite, not even in the iron-rich forsterite (Fo65) in agreement with Baron et al. (2019), who showed that olivine reaction solutions were oxidising, hindering siderite formation.
View in article
An important question is why carbonates do not form in the crustal simulant samples. As noted by Baron et al. (2019), our experiments demonstrate that the mineralogy of the reactants significantly influences solution properties, particularly pH, subsequent reaction pathways, and the formation of secondary products.
View in article
Boynton, W.V., Ming, D.W., Kounaves, S.P., Young, S.M.M., Arvidson, R.E., Hecht, M.H., Hoffman, J., Niles, P.B., Hamara, D.K., Quinn, R.C., Smith, P.H., Sutter, B., Catling, D.C., Morris, R.V. (2009) Evidence for Calcium Carbonate at the Mars Phoenix Landing Site. Science 325, 61–64. https://doi.org/10.1126/science.1172768
 Show in context
Show in contextApart from minor carbonates (<5 wt. %) identified within more recent polar terrains (Boynton et al., 2009) and the carbonate compounds identified in three nakhlite meteorites (Bridges et al., 2019), these carbonates are inferred to have formed during the Noachian epoch (i.e. >3.6 Ga).
View in article
Bridges, J.C., Schwenzer, S.P. (2012) The nakhlite hydrothermal brine on Mars. Earth and Planetary Science Letters 359–360, 117–123. https://doi.org/10.1016/j.epsl.2012.09.044
 Show in context
Show in contextHowever, they are inferred to have formed through high temperature hydrothermal subsurface processes induced by an impact event (Bridges and Schwenzer, 2012), contrasting with our experimental conditions (i.e. water equilibrated with a pCO2 = 1 bar atmosphere, T = 45 °C).
View in article
Bridges, J.C., Hicks, L.J., Treiman, A.H. (2019) Chapter 5 - Carbonates on Mars. In: Filiberto, J., Schwenzer, S.P. (Eds.) Volatiles in the Martian Crust. Elsevier, Amsterdam (Netherlands), Oxford (UK), Cambridge (USA), 89–118. https://doi.org/10.1016/B978-0-12-804191-8.00005-2
 Show in context
Show in contextApart from minor carbonates (<5 wt. %) identified within more recent polar terrains (Boynton et al., 2009) and the carbonate compounds identified in three nakhlite meteorites (Bridges et al., 2019), these carbonates are inferred to have formed during the Noachian epoch (i.e. >3.6 Ga).
View in article
Bristow, T.F., Haberle, R.M., Blake, D.F., Des Marais, D.J., Eigenbrode, J.L., Fairén, A.G., Grotzinger, J.P., Stack, K.M., Mischna, M.A., Rampe, E.B., Siebach, K.L., Sutter, B., Vaniman, D.T., Vasavada, A.R. (2017) Low Hesperian PCO2 constrained from in situ mineralogical analysis at Gale Crater, Mars. Proceedings of the National Academy of Sciences 114, 2166–2170. https://doi.org/10.1073/pnas.1616649114
 Show in context
Show in contextOur results are likewise consistent with the limited occurrence of carbonates (<3.2 wt. %) detected by CheMin in the Glen Torridon area of Gale Crater (Thorpe et al., 2022) and the absence of carbonates in other crater locations (Bristow et al., 2017), despite significant diagenetic alteration in the mudstones (i.e. 20 to 30 wt. % of phyllosilicates).
View in article
Brown, A.J., Viviano, C.E., Goudge, T.A. (2020) Olivine-Carbonate Mineralogy of the Jezero Crater Region. Journal of Geophysical Research: Planets 125, e2019JE006011. https://doi.org/10.1029/2019JE006011
 Show in context
Show in contextMoreover, the lower carbonation observed on the Fo65 compared to the Fo92 sample (Fig. 2) suggests that the Fe enrichment in martian olivine limits the quantity of carbonate but does not prevent its formation, in agreement with the results of Brown et al. (2020) who identify Fo40-66 in the olivine-carbonate lithology of Nili Fossae region.
View in article
Bullock, M.A., Moore, J.M. (2007) Atmospheric conditions on early Mars and the missing layered carbonates. Geophysical Research Letters 34, L19201. https://doi.org/10.1029/2007GL030688
 Show in context
Show in contextOnly the olivine samples (Fo92 and Fo65) formed substantial proportions of carbonates during reaction, and only their solutions had pH > 6.2, the threshold for carbonates formation under a pCO2 = 1 bar atmosphere (Bullock and Moore, 2007).
View in article
The dashed line marks the pH threshold of carbonates stability for pCO2 = 1 bar (Bullock and Moore, 2007).
View in article
In a dense CO2 atmosphere on early Mars (i.e. pCO2 = 1 bar), carbonate minerals are not stable at pH < 6.2 (Bullock and Moore, 2007) and the simulant samples yield solutions with pH values below this threshold (Fig. 2); the pH values of the simulant solutions are intermediate between those of pure olivine and pure feldspar samples.
View in article
Carter, J., Riu, L., Poulet, F., Bibring, J.-P., Langevin, Y., Gondet, B. (2023) A Mars orbital catalog of aqueous alteration signatures (MOCAAS). Icarus 389, 115164. https://doi.org/10.1016/j.icarus.2022.115164
 Show in context
Show in contextHowever, orbiting near-infrared spectrometers primarily detect phyllosilicates and sulphates among alteration minerals, whereas carbonate minerals are relatively rare (Carter et al., 2023).
View in article
Dehouck, E., Gaudin, A., Mangold, N., Lajaunie, L., Dauzères, A., Grauby, O., Le Menn, E. (2014) Weathering of olivine under CO2 atmosphere: A martian perspective. Geochimica et Cosmochimica Acta 135, 170–189. https://doi.org/10.1016/j.gca.2014.03.032
 Show in context
Show in contextIn prior experiments simulating early Mars conditions (pCO2 = 1, T = 45 °C), moderate (Gaudin et al., 2018) to minor magnesium carbonates (Dehouck et al., 2014) were identified from forsteritic-olivine (Fo90) weathering.
View in article
Edwards, C.S., Ehlmann, B.L. (2015) Carbon sequestration on Mars. Geology 43, 863–866. https://doi.org/10.1130/G36983.1
 Show in context
Show in contextOur results also help to illustrate why martian carbonates did not form abundant surface deposits, thus, serving as a limited storage for atmospheric CO2 (Edwards and Ehlmann, 2015).
View in article
Ehlmann, B.L., Mustard, J.F., Swayze, G.A., Clark, R.N., Bishop, J.L., Poulet, F., Des Marais, D.J., Roach, L.H., Milliken, R.E., Wray, J.J., Barnouin-Jha, O., Murchie, S.L. (2009) Identification of hydrated silicate minerals on Mars using MRO-CRISM: Geologic context near Nili Fossae and implications for aqueous alteration. Journal of Geophysical Research: Planets 114, E00D08. https://doi.org/10.1029/2009JE003339
 Show in context
Show in contextHypotheses for the formation of the Nili Fossae carbonates include both local hydrothermalism (Mangold et al., 2007; Ehlmann et al., 2009), and regional weathering (Ehlmann et al., 2009), which is consistent with the persistence of fluvial activity in the region (Mangold et al., 2007) and the inferred lacustrine precipitation of carbonates in the Jezero Crater (Horgan et al., 2020).
View in article
Orbital reflectance spectra of the Capri Chasma region also show phyllosilicates, carbonates, and a host rock potentially rich in olivine (Jain and Chauhan, 2015), like the carbonate-olivine association observed at Nili Fossae (Ehlmann et al., 2009).
View in article
Gaudin, A., Dehouck, E., Grauby, O., Mangold, N. (2018) Formation of clay minerals on Mars: Insights from long-term experimental weathering of olivine. Icarus 311, 210–223. https://doi.org/10.1016/j.icarus.2018.01.029
 Show in context
Show in contextIn prior experiments simulating early Mars conditions (pCO2 = 1, T = 45 °C), moderate (Gaudin et al., 2018) to minor magnesium carbonates (Dehouck et al., 2014) were identified from forsteritic-olivine (Fo90) weathering.
View in article
However, hydrated Mg carbonates can form in solutions with a high concentration of Mg2+ under near ambient temperatures (Gaudin et al., 2018), as shown by our XPS and FTIR spectra (Figs. 1, 3 and Figs. S-3, S-6).
View in article
Halevy, I., Fischer, W.W., Eiler, J.M. (2011) Carbonates in the Martian meteorite Allan Hills 84001 formed at 18 ± 4 °C in a near-surface aqueous environment. Proceedings of the National Academy of Sciences 108, 16895–16899. https://doi.org/10.1073/pnas.1109444108
 Show in context
Show in contextSimilarly, the carbonate concretions in the ALH84001 meteorite, which formed at ∼3.9 Ga, originated from cool water (18 ± 4 °C) that had interacted with atmospheric CO2 (Halevy et al., 2011).
View in article
These alteration units share similarities with the mineralogical associations described in the oldest carbonates identified in ALH 84001, likely formed by a low temperature weathering fluid equilibrated with CO2 atmosphere (Halevy et al., 2011).
View in article
Hewins, R.H., Zanda, B., Humayun, M., Nemchin, A., Lorand, J.-P., Pont, S., Deldicque, D., Bellucci, J.J., Beck, P., Leroux, H., Marinova, M., Remusat, L., Göpel, C., Lewin, E., Grange, M., Kennedy, A., Whitehouse, M.J. (2017) Regolith breccia Northwest Africa 7533: Mineralogy and petrology with implications for early Mars. Meteoritics & Planetary Science 52, 89–124. https://doi.org/10.1111/maps.12740
 Show in context
Show in contextHowever, recent analyses of igneous rocks from the ancient bedrock surrounding Gale Crater (Mangold et al., 2016) and of ancient Martian meteorites (Hewins et al., 2017) suggest that felsic and alkali-rich rocks are more abundant in the Mars’ ancient crust than initially presumed (Sautter et al., 2016).
View in article
We also analyse two synthetic ancient crust samples based on the chemical composition of the NWA 7533 and NWA 7034 martian regolith breccias (e.g., Hewins et al., 2017) and the more alkali-rich composition of the early Mars crust identified in conglomerates in Gale Crater (Mangold et al., 2016) (Table 1).
View in article
Likewise, the ancient meteorite breccia NWA 7034 (and pairs) is rich in feldspars and lacks primary olivine (Hewins et al., 2017).
View in article
Horgan, B.H.N., Anderson, R.B., Dromart, G., Amador, E.S., Rice, M.S. (2020) The mineral diversity of Jezero crater: Evidence for possible lacustrine carbonates on Mars. Icarus 339, 113526. https://doi.org/10.1016/j.icarus.2019.113526
 Show in context
Show in contextHypotheses for the formation of the Nili Fossae carbonates include both local hydrothermalism (Mangold et al., 2007; Ehlmann et al., 2009), and regional weathering (Ehlmann et al., 2009), which is consistent with the persistence of fluvial activity in the region (Mangold et al., 2007) and the inferred lacustrine precipitation of carbonates in the Jezero Crater (Horgan et al., 2020).
View in article
This geological scenario could explain the formation of carbonates identified (from orbit) along the margins of the Jezero (playa) lake (Horgan et al., 2020), setting aside the specific interferences that the development of passivation layers may cause on olivine carbonation rates (Oelkers et al., 2018 and references therein).
View in article
Jain, N., Chauhan, P. (2015) Study of phyllosilicates and carbonates from the Capri Chasma region of Valles Marineris on Mars based on Mars Reconnaissance Orbiter-Compact Reconnaissance Imaging Spectrometer for Mars (MRO-CRISM) observations. Icarus 250, 7–17. https://doi.org/10.1016/j.icarus.2014.11.018
 Show in context
Show in contextOrbital reflectance spectra of the Capri Chasma region also show phyllosilicates, carbonates, and a host rock potentially rich in olivine (Jain and Chauhan, 2015), like the carbonate-olivine association observed at Nili Fossae (Ehlmann et al., 2009).
View in article
Jakosky, B.M., Brain, D., Chaffin, M., Curry, S., Deighan, J., Grebowsky, J., Halekas, J., Leblanc, F., Lillis, R., Luhmann, J.G., Andersson, L., Andre, N., Andrews, D., Baird, D., Baker, D., Bell, J., Benna, M., Bhattacharyya, D., Bougher, S., Bowers, C., Chamberlin, P., Chaufray, J.Y., Clarke, J., Collinson, G., Combi, M., Connerney, J., Connour, K., Correira, J., Crabb, K., Crary, F., Cravens, T., Crismani, M., Delory, G., Dewey, R., DiBraccio, G., Dong, C., Dong, Y., Dunn, P., Egan, H., Elrod, M., England, S., Eparvier, F., Ergun, R., Eriksson, A., Esman, T., Espley, J., Evans, S., Fallows, K., Fang, X., Fillingim, M., Flynn, C., Fogle, A., Fowler, C., Fox, J., Fujimoto, M., Garnier, P., Girazian, Z., Groeller, H., Gruesbeck, J., Hamil, O., Hanley, K.G., Hara, T., Harada, Y., Hermann, J., Holmberg, M., Holsclaw, G., Houston, S., Inui, S., Jain, S., Jolitz, R., Kotova, A., Kuroda, T., Larson, D., Lee, Y., Lee, C., Lefevre, F., Lentz, C., Lo, D., Lugo, R., Ma, Y.J., Mahaffy, P., Marquette, M.L., Matsumoto, Y., Mayyasi, M., Mazelle, C., McClintock, W., McFadden, J., Medvedev, A., Mendillo, M., Meziane, K., Milby, Z., Mitchell, D., Modolo, R., Montmessin, F., Nagy, A., Nakagawa, H., Narvaez, C., Olsen, K., Pawlowski, D., Peterson, W., et al. (2018) Loss of the Martian atmosphere to space: Present-day loss rates determined from MAVEN observations and integrated loss through time. Icarus 315, 146–157. https://doi.org/10.1016/j.icarus.2018.05.030
 Show in context
Show in contextIndeed, the MAVEN’s observations show a significant loss of Mars’s atmosphere (<0.8 bar CO2) in its early history (Jakosky et al., 2018).
View in article
Considering that there have been no detections of substantial CO2 reservoirs at Mars’ surface, MAVEN observations propose that gas loss to space may have driven Martian climate change (Jakosky et al., 2018).
View in article
Kissick, L.E., Mather, T.A., Tosca, N.J. (2021) Unravelling surface and subsurface carbon sinks within the early Martian crust. Earth and Planetary Science Letters 557, 116663. https://doi.org/10.1016/j.epsl.2020.116663
 Show in context
Show in contextHowever, Fourier transform infrared spectroscopy (FTIR) showed no carbonate detection (>∼0.8 wt. %) in less magnesian olivines under similar geochemical conditions (pCO2 from 0.1 to 1 bar, T = 25 °C; Kissick et al., 2021).
View in article
Interestingly, in Kissick et al. (2021), no carbonates were detected from aqueous alteration of fayalite-forsterite mixtures, but this could be due to the FTIR detection limit.
View in article
These findings suggest that the formation of carbonates by surface weathering on early Mars depends on the host rock composition, as suggested by Kissick et al. (2021).
View in article
However, these models usually assume olivine-rich basalts as starting protoliths (i.e. olivine > 20 wt. %) and equilibrium conditions. Consequently, they do not address kinetic barrier effects (Kissick et al., 2021; Scheller et al., 2021), which may result in an overestimation of the carbonate abundance (see geochemical modelling and carbonates in SI).
View in article
Mandon, L., Quantin-Nataf, C., Thollot, P., Mangold, N., Lozac’h, L., Dromart, G., Beck, P., Dehouck, E., Breton, S., Millot, C., Volat, M. (2020) Refining the age, emplacement and alteration scenarios of the olivine-rich unit in the Nili Fossae region, Mars. Icarus 336, 113436. https://doi.org/10.1016/j.icarus.2019.113436
 Show in context
Show in contextIndeed, the largest known carbonate-bearing unit, at Nili Fossae, is inferred to be pre-Hesperian in age (∼3.8 Ga; Mandon et al., 2020).
View in article
In Jezero Crater, carbonates detected from orbit are associated with olivine-bearing rocks and mapped as the same geological unit identified across Nili Fossae (Mandon et al., 2020).
View in article
Mangold, N., Poulet, F., Mustard, J.F., Bibring, J.-P., Gondet, B., Langevin, Y., Ansan, V., Masson, P., Fassett, C., Head III, J.W., Hoffmann, H., Neukum, G. (2007) Mineralogy of the Nili Fossae region with OMEGA/Mars Express data: 2. Aqueous alteration of the crust. Journal of Geophysical Research: Planets 112, E08S04. https://doi.org/10.1029/2006JE002835
 Show in context
Show in contextHypotheses for the formation of the Nili Fossae carbonates include both local hydrothermalism (Mangold et al., 2007; Ehlmann et al., 2009), and regional weathering (Ehlmann et al., 2009), which is consistent with the persistence of fluvial activity in the region (Mangold et al., 2007) and the inferred lacustrine precipitation of carbonates in the Jezero Crater (Horgan et al., 2020).
View in article
Mangold, N., Thompson, L.M., Forni, O., Williams, A.J., Fabre, C., Le Deit, L., Wiens, R.C., Williams, R., Anderson, R.B., Blaney, D.L., Calef, F., Cousin, A., Clegg, S.M., Dromart, G., Dietrich, W.E., Edgett, K.S., Fisk, M.R., Gasnault, O., Gellert, R., Grotzinger, J.P., Kah, L., Le Mouélic, S., McLennan, S.M., Maurice, S., Meslin, P.-Y., Newsom, H.E., Palucis, M.C., Rapin, W., Sautter, V., Siebach, K.L., Stack, K., Sumner, D., Yingst, A. (2016) Composition of conglomerates analyzed by the Curiosity rover: Implications for Gale Crater crust and sediment sources. Journal of Geophysical Research: Planets 121, 353–387. https://doi.org/10.1002/2015JE004977
 Show in context
Show in contextHowever, recent analyses of igneous rocks from the ancient bedrock surrounding Gale Crater (Mangold et al., 2016) and of ancient Martian meteorites (Hewins et al., 2017) suggest that felsic and alkali-rich rocks are more abundant in the Mars’ ancient crust than initially presumed (Sautter et al., 2016).
View in article
We also analyse two synthetic ancient crust samples based on the chemical composition of the NWA 7533 and NWA 7034 martian regolith breccias (e.g., Hewins et al., 2017) and the more alkali-rich composition of the early Mars crust identified in conglomerates in Gale Crater (Mangold et al., 2016) (Table 1).
View in article
Recent results show that the ancient Mars crust was more alkali-rich and felsic than previously thought (Sautter et al., 2016), as indicated by the abundance of felsic igneous rocks (trachytic, alkali feldspar-rich) found in Gale Crater as float and pebbles sourced from Noachian age crust around the crater (Mangold et al., 2016).
View in article
McSween, H.Y., Wyatt, M.B., Gellert, R., Bell III, J.F., Morris, R.V., Herkenhoff, K.E., Crumpler, L.S., Milam, K.A., Stockstill, K.R., Tornabene, L.L., Arvidson, R.E., Bartlett, P., Blaney, D., Cabrol, N.A., Christensen, P.R., Clark, B.C., Crisp, J.A., Des Marais, D.J., Economou, T., Farmer, J.D., Farrand, W., Ghosh, A., Golombek, M., Gorevan, S., Greeley, R., Hamilton, V.E., Johnson, J.R., Joliff, B.L., Klingelhöfer, G., Knudson, A.T., McLennan, S., Ming, D., Moersch, J.E., Rieder, R., Ruff, S.W., Schröder, C., de Souza Jr., P.A., Squyres, S.W., Wänke, H., Wang, A., Yen, A., Zipfel, J. (2006) Characterization and petrologic interpretation of olivine-rich basalts at Gusev Crater, Mars. Journal of Geophysical Research: Planets 111, E02S10. https://doi.org/10.1029/2005JE002477
 Show in context
Show in contextThe overall composition of Mars’ bedrock is commonly inferred to be basaltic and olivine-rich (olivine > 20 wt. %) (e.g., McSween et al., 2006).
View in article
Morris, R.V., Ruff, S.W., Gellert, R., Ming, D.W., Arvidson, R.E., Clark, B.C., Golden, D.C., Siebach, K., Klingelhöfer, G., Schröder, C., Fleischer, I., Yen, A.S., Squyres, S.W. (2010) Identification of Carbonate-Rich Outcrops on Mars by the Spirit Rover. Science 329, 421–424. https://doi.org/10.1126/science.1189667
 Show in context
Show in contextThese carbonates, of inferred Noachian age, are abundant (16 to 34 wt. %) and intimately associated with olivine (Fo68) and amorphous silicate (Morris et al., 2010).
View in article
Ody, A., Poulet, F., Bibring, J.-P., Loizeau, D., Carter, J., Gondet, B., Langevin, Y. (2013) Global investigation of olivine on Mars: Insights into crust and mantle compositions. Journal of Geophysical Research: Planets 118, 234–262. https://doi.org/10.1029/2012JE004149
 Show in context
Show in contextRegarding the olivine distribution through Mars, olivine-rich regions such as Nili Fossae (i.e. olivine > 20 wt. %) are rare in Noachian highlands (e.g., Ody et al., 2013).
View in article
Oelkers, E., Declercq, J., Saldi, G., Gislason, S. (2018) Olivine dissolution rates: A critical review. Chemical Geology 500, 1–19. https://doi.org/10.1016/j.chemgeo.2018.10.008
 Show in context
Show in contextThis geological scenario could explain the formation of carbonates identified (from orbit) along the margins of the Jezero (playa) lake (Horgan et al., 2020), setting aside the specific interferences that the development of passivation layers may cause on olivine carbonation rates (Oelkers et al., 2018 and references therein).
View in article
This effect could be due to the development of an Fe-rich silica layer on the olivine surface that limits the carbonatation reaction (Oelkers et al., 2018).
View in article
Sautter, V., Toplis, M.J., Beck, P., Mangold, N., Wiens, R., Pinet, P., Cousin, A., Maurice, S., LeDeit, L., Hewins, R., Gasnault, O., Quantin, C., Forni, O., Newsom, H., Meslin, P.-Y., Wray, J., Bridges, N., Payré, V., Rapin, W., Le Mouélic, S. (2016) Magmatic complexity on early Mars as seen through a combination of orbital, in-situ and meteorite data. Lithos 254–255, 36–52. https://doi.org/10.1016/j.lithos.2016.02.023
 Show in context
Show in contextHowever, recent analyses of igneous rocks from the ancient bedrock surrounding Gale Crater (Mangold et al., 2016) and of ancient Martian meteorites (Hewins et al., 2017) suggest that felsic and alkali-rich rocks are more abundant in the Mars’ ancient crust than initially presumed (Sautter et al., 2016).
View in article
Recent results show that the ancient Mars crust was more alkali-rich and felsic than previously thought (Sautter et al., 2016), as indicated by the abundance of felsic igneous rocks (trachytic, alkali feldspar-rich) found in Gale Crater as float and pebbles sourced from Noachian age crust around the crater (Mangold et al., 2016).
View in article
Scheller, E.L., Swindle, C., Grotzinger, J., Barnhart, H., Bhattacharjee, S., Ehlmann, B.L., Farley, K., Fischer, W.W., Greenberger, R., Ingalls, M., Martin, P.E., Osorio-Rodriguez, D., Smith, B.P. (2021) Formation of Magnesium Carbonates on Earth and Implications for Mars. Journal of Geophysical Research: Planets 126, e2021JE006828. https://doi.org/10.1029/2021JE006828
 Show in context
Show in contextThen, this hydrated precursor can turn into anhydrous magnesite as occurs in low temperature evaporitic environments on Earth (e.g., Scheller et al., 2021).
View in article
However, these models usually assume olivine-rich basalts as starting protoliths (i.e. olivine > 20 wt. %) and equilibrium conditions. Consequently, they do not address kinetic barrier effects (Kissick et al., 2021; Scheller et al., 2021), which may result in an overestimation of the carbonate abundance (see geochemical modelling and carbonates in SI).
View in article
Snæbjörnsdóttir, S.Ó., Sigfússon, B., Marieni, C., Goldberg, D., Gislason, S.R., Oelkers, E.H. (2020) Carbon dioxide storage through mineral carbonation. Nature Reviews Earth & Environment 1, 90–102. https://doi.org/10.1038/s43017-019-0011-8
 Show in context
Show in contextCarbonation resulting from ultramafic rock reaction has been extensively studied as a CO2(g) capture strategy on Earth (i.e. high T and/or high P conditions) because it can neutralise the acidity imposed from CO2 dissolution (Snæbjörnsdóttir et al., 2020).
View in article
Thorpe, M.T., Bristow, T.F., Rampe, E.B., Tosca, N.J., Grotzinger, J.P., Bennett, K.A., Achilles, C.N., Blake, D.F., Chipera, S.J., Downs, G., Downs, R.T., Morrison, S.M., Tu, V., Castle, N., Craig, P., Marais, D.J.D., Hazen, R.M., Ming, D.W., Morris, R.V., Treiman, A.H., Vaniman, D.T., Yen, A.S., Vasavada, A.R., Dehouck, E., Bridges, J.C., Berger, J., McAdam, A., Peretyazhko, T., Siebach, K.L., Bryk, A.B., Fox, V.K., Fedo, C.M. (2022) Mars Science Laboratory CheMin Data From the Glen Torridon Region and the Significance of Lake-Groundwater Interactions in Interpreting Mineralogy and Sedimentary History. Journal of Geophysical Research: Planets 127, e2021JE007099. https://doi.org/10.1029/2021JE007099
 Show in context
Show in contextIndeed, Thorpe et al. (2022) recently identified Fe-rich carbonate in Glen Torridon (Gale Crater) and inferred that it likely formed in a subsurface mixing zone between lacustrine water and deep groundwater.
View in article
Our results are likewise consistent with the limited occurrence of carbonates (<3.2 wt. %) detected by CheMin in the Glen Torridon area of Gale Crater (Thorpe et al., 2022) and the absence of carbonates in other crater locations (Bristow et al., 2017), despite significant diagenetic alteration in the mudstones (i.e. 20 to 30 wt. % of phyllosilicates).
View in article
Wray, J.J., Murchie, S.L., Bishop, J.L., Ehlmann, B.L., Milliken, R.E., Wilhelm, M.B., Seelos, K.D., Chojnacki, M. (2016) Orbital evidence for more widespread carbonate-bearing rocks on Mars. Journal of Geophysical Research: Planets 121, 652–677. https://doi.org/10.1002/2015JE004972
 Show in context
Show in contextCarbonate occurrences detected on Mars are commonly associated with olivine-bearing lithologies (olivine > 20 % in volume) (Wray et al., 2016), consistent with our experimental results.
View in article
Zhu, C., Lu, P. (2009) Alkali feldspar dissolution and secondary mineral precipitation in batch systems: 3. Saturation states of product minerals and reaction paths. Geochimica et Cosmochimica Acta 73, 3171–3200. https://doi.org/10.1016/j.gca.2009.03.015
 Show in context
Show in contextIn our experiments, dissolution-precipitation reactions are likely coupled, and both martian simulants contain high percentages of feldspars that generally form Al-OH-rich secondary minerals (e.g., amorphous Al-OH precursors, gibbsite or kaolinite) as alteration products (Zhu and Lu, 2009).
View in article
top
Supplementary Information
The Supplementary Information includes:
- Materials and Methods
- Table S-1
- Figures S-1 to S-8
- Geochemical Modelling and Carbonates
- Supplementary Information References
Download the Supplementary Information (PDF)
Figures
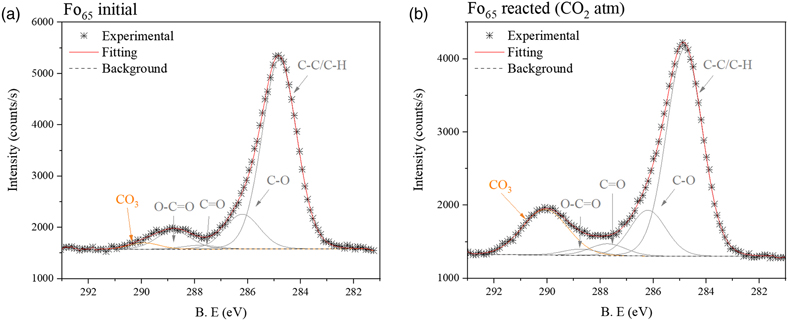
Figure 1 Fitting model of the C1s orbital for Fo65 sample, (a) initially, and (b) after reaction (under CO2 atmosphere) (see SI for spectra deconvolution).
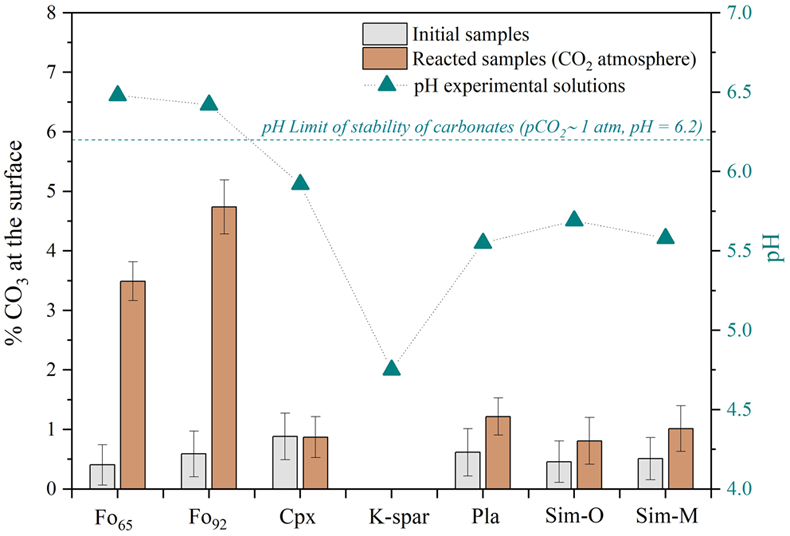
Figure 2 Amount of carbonate in the samples (bars) and pH value (triangles) of the solutions at the end of the experiment (see SI for % calculations). The error bars show the uncertainty of the carbon estimation associated with the survey quantification (standard deviation in Fig. S-2). The pH0 = 3.6, corresponding to the pure water equilibrated with the pCO2 = 1 bar. The dashed line marks the pH threshold of carbonates stability for pCO2 = 1 bar (Bullock and Moore, 2007
Bullock, M.A., Moore, J.M. (2007) Atmospheric conditions on early Mars and the missing layered carbonates. Geophysical Research Letters 34, L19201. https://doi.org/10.1029/2007GL030688
).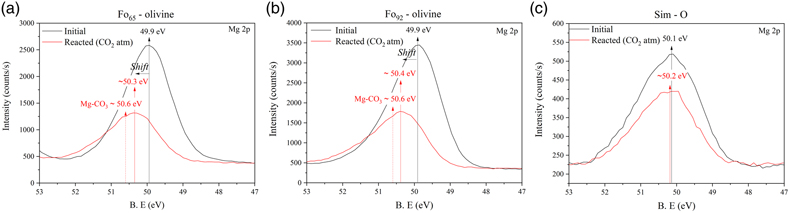
Figure 3 Mg 2p high resolution XPS spectra before (black colour) and after reaction under a CO2 atmosphere (red colour) of (a) Fo65-olivine, (b) Fo92-olivine, and (c) simulant sample with Fo65 (Sim-O). The solid arrows (black colour) mark the position of the main peak of the unreacted samples whereas dashed arrows (red colour) mark the position of the MgCO3 peak.