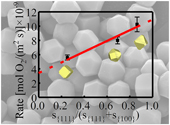
 | Morphology dominated rapid oxidation of framboidal pyrite Abstract: The rapid oxidation of framboidal pyrite is conventionally attributed to its fine grain size. However, the effect of the crystal facets of the microcrystals in the framboids on the oxidation process has been overlooked. We synthesised pyrite monocrystals of microscopic size with both {100} and {111} facets, which are two major forms of framboidal pyrite crystals, in order to examine the oxidation behaviour of pyrite framboids. The results showed that the oxidation rate of microcrystals with a greater proportion of {111} facets was approximately 2 times higher than that of those with a greater proportion of {100} facets although the latter’s size was smaller. Such a difference makes framboidal pyrite with {111} facets more sensitive to oxidative weathering in geochemical cycles than other forms of pyrite. These findings emphasise the role of crystal anisotropy in controlling the oxidation of framboidal pyrite, thereby suggesting that the shape controlled oxidation of pyrite is a potential indicator of the local redox conditions of the palaeoenvironment where it occurred. |
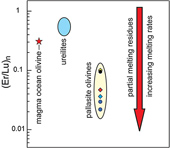 | Olivines in main-group pallasites: magma-ocean cumulates or partial melting residues? Abstract: Main-group pallasites (MGPs) are meteorites mainly composed of Fe-Ni metal and olivines, the latter being considered as one of the largest sampling of extraterrestrial mantle material available for study on Earth. We analysed the rare earth element (REE) concentrations of olivines from six MGPs to understand better the processes of formation of their parent mantle. All the investigated samples display very low REE abundances, and enrichments in both light REEs and heavy REEs. We interpret the light REE enrichments as a fingerprint of terrestrial contamination. The least contaminated olivines have higher heavy REE enrichments than those inferred for olivines directly crystallised in a magma ocean. Such enrichments in heavy REEs are possible if the mantle of the MGPs parent body is a residue of partial melting from a chondritic source. Alternatively, re-melting of magma ocean cumulates would explain both the homogeneity of the Δ17O values of MGPs, and the heavy REE enrichments of the olivines. |
 | Abiotic formation of organic biomorphs under diagenetic conditions Abstract: The most ancient fossil record contains fundamentally important information on both the diversity and disparity of ancient life. Yet this ancient record is not that easy to decode, due to difficulties mainly pertaining to the impact of the geological history. Thus, the convergence of multiple lines of evidence is seen as necessary to build a robust demonstration of the biogenicity of putative traces of life. Yet, we experimentally show here that abiotic organic cell-like microstructures meeting all the criteria of biogenicity may form in cherts under classical conditions of diagenesis. These organic biomorphs produced from a mixture of RNA and quartz in water exposed to temperature and pressure conditions (200 °C, ∼15 bars) exhibit morphological, chemical and isotopic signatures typical of organic microfossils. The results of this study exemplify the pitfalls that Archean palaeontologists may encounter when searching for traces of life in ancient rocks. |
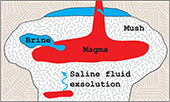 | Chlorine isotope ratios record magmatic brine assimilation during rhyolite genesis Abstract: Magmatic volatile phases within crustal silicic magma domains influence key volcanic processes such as the build up to eruptions and formation of magmatic-hydrothermal ore deposits. However, the extent and nature of fluid-melt interaction in such environments is poorly understood, as geochemical signals in volcanic rocks originating from pre-eruptive volatile processes are commonly overprinted by syn-eruptive degassing. Here, we use δ37Cl as a conservative tracer of brine-melt interaction on a broad suite of silicic volcanic rocks from Iceland. We find that the δ37Cl values of silicic rocks are systematically shifted to more negative values compared to associated basalts and intermediate rocks by up to 2.9 ‰. These large shifts cannot be explained by well known processes inherent to silicic magma genesis, including crustal assimilation, mineral-melt fractionation and syn-eruptive degassing. Instead, we show that low δ37Cl values in silicic rocks can be attributed to assimilation of magmatic brines that are formed and stored in long lived crustal magma mushes. Our results indicate that magmatic brine assimilation is a fundamental, but previously unrecognised part of rhyolite genesis. |
 | Aqueous concentration of CO2 in carbon-saturated fluids as a highly sensitive oxybarometer Abstract: The CO2 content of aqueous fluids in equilibrium with carbon can be used to retrieve their oxygen fugacity if pressure and temperature are known. Applicable to both natural and experimental systems, we present a new oxybarometer based on the aqueous concentration of CO2 in fluids saturated with either graphite or glass-like carbon, suitable to retrieve their oxygen fugacity. The method was experimentally tested by measuring by mass spectrometry the CO2 content in aqueous fluids coexisting with glass-like carbon buffered externally with Ni-NiO, employing ordered and disordered forms of NiO characterised by small differences in free energy (<5 kJ/mol). Considering analytical uncertainties on CO2 measurements, fO2 values can be resolved with an accuracy of about 0.01 log units, which is one order of magnitude lower than uncertainties affecting conventional solid state redox sensors. The CO2-in-fluid oxybarometer is the first available parameterisation of the fO2 dependency on pressure, temperature and CO2 content of aqueous fluids and can be used for fluids containing >1 mol. % CO2 beneath the graphite-diamond transition. |
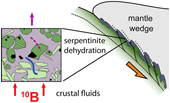 | Metamorphic olivine records external fluid infiltration during serpentinite dehydration Abstract: We used boron (B) isotope systematics of co-existing olivine and serpentine to study deep fluid flow in subduction zones. Metamorphic olivine produced by serpentine dehydration at sub-arc conditions from high pressure ophiolites in the Western Alps contains significant concentrations of B (2–30 μg/g) with a high δ11B values (+9 to +28 ‰), whilst co-existing serpentine has 2–50 μg/g B with δ11B = +6 to +24 ‰. Boron isotope fractionation between olivine and its precursor serpentine (Δ11Bol-srp = δ11Bol – δ11Bsrp) is highly variable, which indicates significant isotopic disequilibrium between these minerals. Importantly, samples with B-enriched olivine have low Δ11Bol-srp (down to −9 ‰), evidence that olivine grew in the presence of a mixture of serpentine-derived fluids and external fluids with δ11B of ca. +6 to +15 ‰. The composition of these external fluids is consistent with those from subducting sediments and altered oceanic crust at 50–80 km depth, and at least 15–45 % fluid addition. Our work shows that large scale slab fluid infiltration and fluid-mobile element transport accompanies serpentinite dehydration in subduction zones. |
 | Quantifying magmatic volatiles by Raman microtomography of glass inclusion-hosted bubbles Abstract: We present a novel application of Raman microtomography for quantitative characterisation of glass inclusion-hosted bubbles, which allows for the simultaneous identification and volumetric quantification of mineral and fluid phases filling the bubble. The combination of Raman microtomography with synchrotron XRF mapping and scanning electron microscopy provides a complete compositional and textural characterisation of the bubble. In the studied samples, minerals are systematically present on the walls of the bubbles: dominantly carbonates in samples from continental intraplate and hotspot volcanic provinces, and sulfates in the sample from subduction-related settings. Along with fluid CO2, carbonates sequester 65 to 84 % of the CO2 originally dissolved in the melt, while 18 to 60 % of the sulfur contained in the inclusion is stored in sulfides and/or sulfates. Thus, the total melt inclusion CO2 and S contents can be underestimated (by up to ∼ 40 % and 60 %, respectively) if minerals in the bubbles are neglected. This study highlights the importance of 3D mapping of shrinkage bubbles hosted in glass inclusions for a better assessment of the bulk pre-eruptive contents of volatiles in magmas. |
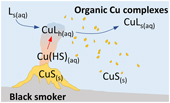 | Hydrothermal vents and organic ligands sustained the Precambrian copper budget Abstract: The bioavailability of metals in the early ocean is a key parameter for understanding the evolution and expansion of Earth’s biosphere. Theoretical work suggested extremely low Zn and Cu levels in Precambrian seawater, but these predictions are not supported by recent geochemical data. One explanation for this discrepancy is a strong hydrothermal influx of metals and/or stabilisation in solution by organic ligands. Here new models are constructed to test this hypothesis for the solubility of Cu. The results show that hydrothermal vents constituted the major source of Cu to the Archean ocean, but higher ocean temperatures or higher levels of organic matter may have been needed to stabilise dissolved Cu in seawater. From the Proterozoic onwards, rivers contributed most of the marine Cu budget and concentrations were probably close to the modern range, even if the residence time of Cu in seawater was shorter than today. Biological Cu limitation was thus probably lifted in the Proterozoic, but the origin of Cu toxicity for cyanobacteria likely emerged in the Archean. The results provide a new interpretive framework for geochemical records. |
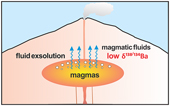 | Experimental evidence for light Ba isotopes favouring aqueous fluids over silicate melts Abstract: Barium (Ba) is a fluid mobile element and enriched in the Earth’s crust, which has potential implications for constraining fluid activities during magmatic-hydrothermal processes. However, the behaviour of Ba and its isotopes during fluid exsolution from magma is poorly known. Here we present an experimental study on determining the Ba partition coefficient (DFLUID-MELT) and equilibrium isotope fractionation factor (α138/134BaFLUID-MELT) between aqueous fluids and silicate melts with different chemical compositions at 700–900 °C and 200 MPa using cold seal pressure vessels. The results show that DFLUID-MELT ranges from 0.02 to 0.20, while Δ138/134BaFLUID-MELT [≈1000 × (α − 1)] ranges from −0.62 ‰ to −0.14 ‰. Both DFLUID-MELT and Δ138/134BaFLUID-MELT positively correlate with temperature, the salinity of fluid and alumina saturation index (ASI) of melt. The finding that light Ba isotopes are enriched in aqueous fluids relative to silicate melts suggests that the fluid exsolution process cannot explain the observed light Ba isotopic compositions of some granites. Moreover, the experimentally determined α138/134BaFLUID-MELT is useful for tracing fluid activities in felsic intrusion-related hydrothermal deposits and in seafloor hydrothermal systems. |
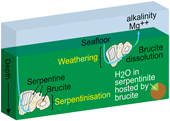 | Brucite formation and dissolution in oceanic serpentinite Abstract: Brucite is an important, albeit elusive, hydrous mineral formed during serpentinisation, a vector of Mg from the mantle to seawater, and possibly a significant host of water in oceanic serpentinite. However, the abundance of brucite has not been quantified in oceanic serpentinite and its fate and related chemical fluxes remain uncertain. We used thermal analysis and confocal Raman spectroscopy to determine the abundance and distribution of brucite in serpentinite recovered by seafloor drilling (n = 48) and dredging (n = 22). Almost all (90 %) of the drilled serpentinite samples contained brucite. The brucite contents increased with increasing extent of serpentinisation and constituted up to 15.6 wt. % of the altered rock. In contrast, dredged serpentinites were devoid of brucite and lost 4.0 wt. % MgO on average, which translates to an estimated average annual flux of 1.3 × 1010 mole Mg and about 2 × 1010 mole alkalinity during seafloor weathering of serpentinite globally. Our data suggest that, on average, brucite stores ∼20 % of the water in unweathered serpentinite, making brucite one of the largest water carriers in slow and ultra-slow spreading oceanic lithosphere. |
<< Previous issueNext issue >>





