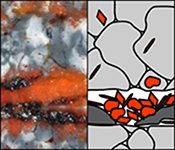
 | Colloidal origin of microbands in banded iron formations Abstract: Precambrian banded iron formations record the composition of Earth’s atmosphere and hydrosphere during the global rise of oxygen. It has been suggested that the banded texture of these rocks points to fluctuations in ocean chemistry although this remains a subject of debate. Here we show, by petrographic and electron microscopy of Palaeoproterozoic banded iron formations from the Hamersley Province, NW Australia, that not all iron oxide microbands represent primary sedimentary layers. Some iron oxide laminae are derived from abundant hematite particles that were originally encapsulated in chert layers and subsequently liberated by removal of quartz during post-depositional deformation by dissolution–precipitation creep. The liberated hematite particles progressively accumulated in layer-parallel aggregates forming microbands, with new hematite crystals forming via non-classical crystallisation pathways during diagenesis and metamorphism. Therefore, microbands do not necessarily correspond to fluctuations in the depositional environment. |
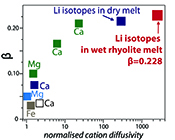 | Diffusive fractionation of Li isotopes in wet, highly silicic melts Abstract: The discovery of large lithium isotopic gradients in geologic media has motivated recent work examining the kinetic fractionation of Li isotopes in silicate materials. Here, piston-cylinder experiments were used to determine Li diffusivities in rhyolitic melts containing ~6 wt. % H2O at 1 GPa pressure and 790-875 ºC. Lithium transport in wet rhyolitic melt is almost an order of magnitude faster than diffusion in dry obsidian glass over the investigated temperature range. Li isotope profiles collected by ion microprobe show that the kinetic exponent β = 0.228 for diffusive fractionation of Li isotopes in wet rhyolite. This value is very close to β = 0.215 determined by Richter et al. (2003) for Li isotope diffusion in a dry basalt-rhyolite couple at 1350 °C. The similarity of the two values indicates little or no dependence of βLi in silicate melts on either temperature or melt composition. The new data confirm a very high potential for diffusive fractionation of 6Li from 7Li and can be confidently used to model deviations in δ7Li to determine the time-temperature histories of natural rhyolite samples. |
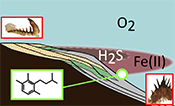 | Oxygen minimum zones in the early Cambrian ocean Abstract: The relationship between the evolution of early animal communities and oceanic oxygen levels remains unclear. In particular, uncertainty persists in reconstructions of redox conditions during the pivotal early Cambrian (541-510 million years ago, Ma), where conflicting datasets from deeper marine settings suggest either ocean anoxia or fully oxygenated conditions. By coupling geochemical palaeoredox proxies with a record of organic-walled fossils from exceptionally well-defined successions of the early Cambrian Baltic Basin, we provide evidence for the early establishment of modern-type oxygen minimum zones (OMZs). Both inner- and outer-shelf environments were pervasively oxygenated, whereas mid-depth settings were characterised by spatially oscillating anoxia. As such, conflicting redox signatures recovered from individual sites most likely derive from sampling bias, whereby anoxic conditions represent mid-shelf environments with higher productivity. This picture of a spatially restricted anoxic wedge contrasts with prevailing models of globally stratified oceans, offering a more nuanced and realistic account of the Proterozoic-Phanerozoic ocean transition. |
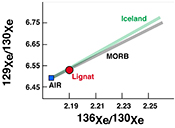 | The xenon isotopic signature of the mantle beneath Massif Central Abstract: The origin of the Central European Volcanic Province, which includes the Massif Central and the Eifel regions, is currently debated. Several different causes have been proposed to account for the volcanism observed in the area. Namely, both the presence of one or more mantle plumes under Europe, and the upwelling and melting of upper mantle related to the formation of the Alps, have been suggested as possible drivers of volcanism. In order to distinguish between these possibilities, we have analysed noble gases in the Lignat Spring to constrain the nature of the mantle source below the Massif Central. The gas has a 3He/4He ratio of 5.51 Ra, whereas its neon isotopic signature is identical to that of MORB source. The gas has an 40Ar/36Ar ratio of 1113 ± 3, far in excess of the atmospheric ratio. The xenon isotopic pattern is explained by 95 % atmospheric contamination of a MORB-like gas. The noble gases clearly show that the mantle beneath Massif Central has a geochemical signature similar to MORB source mantle, with the exception of helium, which more closely corresponds to SCLM signatures, and thus removes the need for the presence of a mantle plume in the region. |
 | Atmospheric helium isotopic ratio from 1910 to 2016 recorded in stainless steel containers Abstract: The atmospheric helium isotope composition (RA= 3He/4Heair = 1.39 × 10-6) could have varied over recent times due to anthropogenic activities. In order to check this possibility, we conducted high-precision helium isotope measurements of air trapped in various stainless steel containers from France (pétanque balls, a float carburettor; 1910–2016) and Cape Grim, Tasmania (archived air tanks; 1978, 1988). We used a double collector mass spectrometer at the Centre de Recherches Pétrographiques et Géochimiques (CRPG, Nancy, France). We found a similar composition between the French and Cape Grim air samples. The temporal variation estimated from all samples including data previously published is not significant, with a trend of +0.002 ± 0.024 ‰/yr over 106 years (2σ). We suspect that the release of radiogenic 4He by fossil fuel exploitation could have been at least partly offset by the production of 3He (via the decay of 3H) from nuclear tests. This study supports the suitability of atmospheric helium as an inter-laboratory isotope standard. |
 | Th/U and U series systematics of saprolite: importance for the oceanic 234U excess Abstract: The presence of excess 234U in seawater is a compelling argument for active delivery of solutes from the continents to the oceans. Previous studies found, however, that the complementary 234U deficit on the continents is surprisingly modest, which would require protracted U loss from a large continental weathering pool. Our new compilation and statistical analysis of the published data, coupled with a mass balance calculation demonstrates that the apparent small 234U deficit in the continental weathering pool implied by previous studies is insufficient to balance the observed oceanic excess. Our new data for a saprolite weathering profile developed on Deccan basalt reveal a very strong overall loss of U (elevated Th/U) with a strong 234U deficit attributable to chemical weathering. The U and 234U deficits reported here from a geologically recent saprolite confirm the importance of the early stages of chemical weathering at the weathering front in the supply of nutrients to the oceans. Thus, as much as half the oceanic 234U inventory is likely sourced from a thin active saprolite zone. |
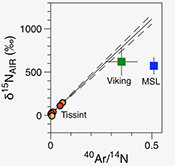 | Noble gases and nitrogen in Tissint reveal the composition of the Mars atmosphere Abstract: Comparative planetology is crucial to unravel the origin and evolution of volatile elements on terrestrial planets. We report precise measurements of the elemental and isotopic composition of nitrogen and noble gases in the Martian meteorite Tissint. Ar-N2 correlations confirm discrepancies between results from Viking and Martian meteorites and those from the Mars Science Laboratory (MSL) mission. The Martian atmospheric 40Ar/36Ar ratio is estimated to be 1714 ± 170 (1σ), lower than the value determined by Viking but in agreement with, and with higher precision than, data from MSL. We confirm a solar wind-like origin for Martian Kr and Xe. Excesses on light Kr isotopes are lower than those measured by MSL. Cosmogenic excesses in the Xe isotopic spectrum could have been produced in space during exposure of the Tissint parent body to cosmic rays. |
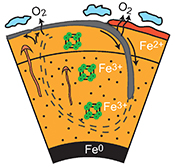 | Large oxygen excess in the primitive mantle could be the source of the Great Oxygenation Event Abstract: Before the Archean to Proterozoic Transition (APT) the tectonic regime was dominated by microplates floating on a low viscosity mantle. Such a regime restricted chemical exchange between the shallow and deeper mantle reservoirs. After the APT, a more global convection regime led to deep subduction of slabs. We propose that the improved vertical mixing of the mantle favoured the release to the Earth’s surface of an oxygen excess initially trapped in the deep mantle. This excess built up when the primordial lower mantle was left with a high Fe3+/(Fe2++Fe3+) ratio (#Fe3+), after metallic iron segregated down into the core. Our synchrotron-based in situ experiments suggest a primordial Fe3+excess of ~20 % for the mantle iron. By comparison with the #Fe3+ of the present mantle, this Fe3+excess would correspond to 500–1000 times the O2 content in the Earth’s atmosphere. The tectonic transition greatly facilitated the ascent of oxidised lower mantle material towards the Earth’s surface, inducing a continuous arrival of O2 at the Earth’s surface and into the atmosphere. |
| Reply to comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Le Merrer and Colombani, 2017 Reply to comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Le Merrer and Colombani, 2017 The comment by Le Merrer and Colombani (2017) focuses on the mechanisms that could account for our experiments, in which we observed the detachment of micrometre scale calcite grains from the surface of micritic limestone during contact with a reactive fluid (Levenson and Emmanuel, 2017). They discuss some of the forces acting on the grains and imply that our observations are likely to be artefacts of the experimental method. Furthermore, they suggest that because our measured calcite dissolution rates do not match exactly the dependence on ionic strength predicted by Colombani (2016), an “unidentified phenomenon” could be at play in our experiments. While Le Merrer and Colombani (2017) raise some valid points, we think that an alternative interpretation is possible. We are pleased to be able to discuss these aspects of our paper in greater detail. | |
 | Comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Levenson and Emmanuel, 2017 Comment on “Repulsion between calcite crystals and grain detachment during water-rock interaction” by Levenson and Emmanuel, 2017: Levenson and Emmanuel suggested recently that the mechanism of carbonate rock weathering in fluids is not limited to nanoscale processes but that chemico-mechanical processes also take place at the micrometre scale, such as grain detachment from the material surface. This phenomenon was first observed in flowing liquids (Levenson and Emmanuel, 2016). In this case, the removal of the grain was understood to be a consequence both of mineral dissolution at grain boundaries and shear stress imposed by the fluid on the grain. Unexpectedly, this grain removal process has been subsequently observed in quiescent liquids. From these experiments, Levenson and Emmanuel (2017) showed atomic force microscopy (AFM) pictures where grains unambiguously disappeared from the surface, even when the rock was left in a solution at rest. The expulsion of the grains was interpreted to result from dissolution and from repulsive forces between the grain surface and the underlying surface. Based on AFM measurements, such repulsion is believed to be caused by interactions between the Debye layers, as well as hydration of the strongly hydrophilic calcite surfaces (Røyne et al., 2015). |
<< Previous issueNext issue >>





