An arsenic-driven pump for invisible gold in hydrothermal systems
Affiliations | Corresponding Author | Cite as | Funding information- Share this article





-
Article views:3,558Cumulative count of HTML views and PDF downloads.
- Download Citation
- Rights & Permissions
top
Abstract
Figures
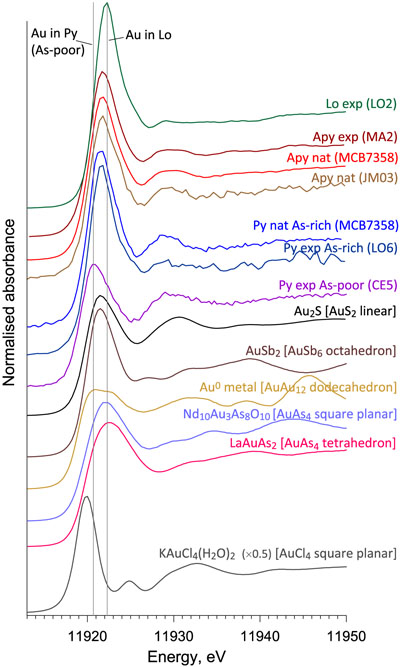 Figure 1 Gold L3-edge HERFD XANES spectra (offset vertically) of representative Au-bearing natural (nat) and experimental (exp) pyrite (Py), arsenopyrite (Apy) and löllingite (Lo) samples, and selected reference compounds with indicated Au first shell atomic coordination (see Supplementary Information for details). | 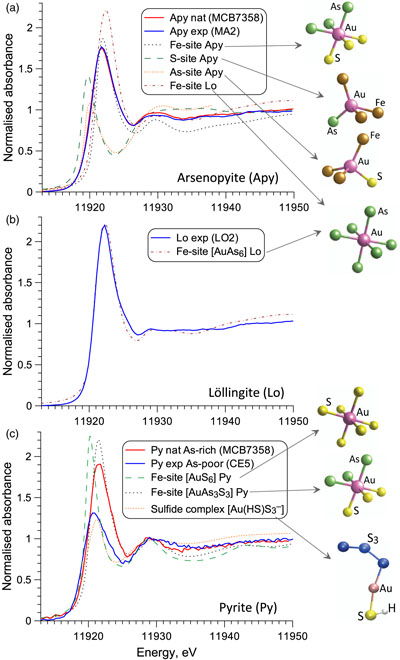 Figure 2 Comparison of Au L3-edge HERFD XANES spectra of representative samples with quantum chemistry simulated spectra of Au in different structural sites as pictured by the displayed atomic clusters. The spectra of (a) Apy, (b) Lo, and (c) As-rich Py are consistent with Au in an As-enriched [Au(As,S)6] octahedral site, whereas Au in As-poor Py (c) is in [AuS2] moieties similar to those in exemplified AuI-(poly)sulfide complexes. | 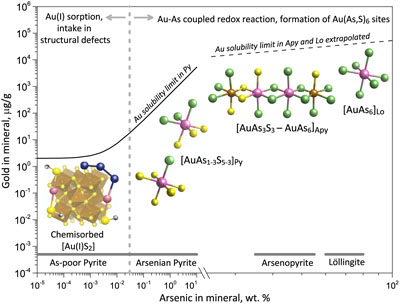 Figure 3 Structural model for chemically bound Au in pyrite, arsenopyrite and löllingite (not to scale). The Au coordination is shown by ball-and-stick atomic clusters (Au = pink, S = yellow, As = green, Fe = brown, S3 = blue, H = grey). Horizontal gray bars indicate the typical range of As contents in each mineral. Empirical Au solubility limit in arsenian pyrite (solid curve; Reich et al., 2005) was extrapolated to Apy and Lo (dashed curve). Note a fundamental transition in the Au incorporation mechanism (vertical dashed line, indicative position), from chemisorption as AuI-polysulfide complexes at low As content in pyrite to coupled Au-As redox reactions driving Au entry in As-enriched Fe crystallographic sites of the three minerals. |  Figure 4 Gold solubility in arsenopyrite (Apy) and löllingite (Lo) in equilibrium with hydrothermal fluid and native gold, predicted as a function of (a) log fO2 (in bars, relative to the nickel-nickel oxide O2 buffer, NNO) at typical H2S and As(OH)3 fluid phase concentrations for the indicated major types of gold deposits, and (b) As and S fluid phase content at fO2 of NNO–0.5 within the Apy stability domain (Perfetti et al., 2008) in metamorphic gold deposits (circles; Table S-11 for compilation). Also plotted are equilibrium Au(HS)2− concentrations in fluid (blue curve), Au Apy/Fluid partition coefficient (D(Au)Apy/Fluid, mass units ratio, same y scale, red curve), and Apy-Lo, Apy-pyrrhotite (Po), and Apy-pyrite (Py) equilibrium lines. |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
top
Introduction
Pyrite, arsenopyrite and löllingite are the key minerals in hydrothermal systems capable of concentrating gold by up to 106 times its mean crustal and mantle abundance (∼1 ng/g). A large part of gold hosted by these minerals is “invisible” or “refractory” (i.e. optically undetectable) occurring both as metal nanoparticles (Au0) and chemically bound Au, the latter being often the dominant gold state and commonly associated with arsenic on the (sub)micron scale (e.g., Cathelineau et al., 1989
Cathelineau, M., Boiron, M.-C., Holliger, P., Marion, P., Denis, M. (1989) Gold in arsenopyrites: Crystal chemistry, location and state, physical and chemical conditions of deposition. Economic Geology Monograph 6, 328–341.
; Cook and Chryssoulis, 1990Cook, N.J., Chryssoulis, S.L. (1990) Concentrations of “invisible” gold in the common sulfides. Canadian Mineralogist 28, 1–16.
; Reich et al., 2005Reich, M., Kesler, S.E., Utsunomiya, S., Palenik, C.S., Chryssoulis, S.L., Ewing, R.C. (2005) Solubility of gold in arsenian pyrite. Geochimica et Cosmochimica Acta 69, 2781–2796.
). Thus, the redox and structural state of chemically bound Au and its link with As may define a deposit’s economic potential (e.g., Kusebauch et al., 2019Kusebauch, C., Gleeson, S.A., Oelze, M. (2019) Coupled partitioning of Au and As into pyrite controls the formation of giant Au deposits. Science Advances 5, eaav5891.
), determine the type and cost of Au recovery from ore (e.g., Adams, 2005Adams, M.D. (Ed.) (2005) Advances in Gold Ore Processing. Elsevier Science, Amsterdam.
) and, more generally, affect the gold distribution at the Earth’s crust scale (e.g., Large et al., 2011Large, R.R., Bull, S.W., Maslennikov, V.V. (2011) A carbonaceous sedimentary source-rock model for Carlin-type and orogenic gold deposits. Economic Geology 106, 331–358.
). Despite significant advances in micro/nanoanalytical techniques over the past 20 years, the fundamental causes of the Au-As relationship and processes that could drive gold, the most chemically inert metal of the Periodic Table, to such levels of enrichment in a host mineral yet remain enigmatic. Existing interpretations vary from the formation of aqueous Au-As complexes or Au0 electrochemical deposition and Au(HS)2− chemisorption on arsenian pyrite and arsenopyrite surfaces or precipitation of gold sulfide phases, to Au entering Fe, S or As crystallographic sites, along with other cation substitutions or structural vacancies, and with the formal oxidation state of chemically bound Au spanning from –1 to +3 and coordination from 2 to 6 (e.g., Arehart et al., 1993Arehart, G.B., Chryssoulis, S.L., Kesler, S.E. (1993) Gold and arsenic in iron sulfides from sediment-hosted disseminated gold deposits: Implication for depositional processes. Economic Geology 88, 171−185.
; Möller and Kersten, 1994Möller, P., Kersten, G. (1994) Electrochemical accumulation of visible gold on pyrite and arsenopyrite surfaces. Mineralium Deposita 29, 404–413.
; Simon et al., 1999Simon, G., Huang, H., Penner-Hahn, J.E., Kesler, S.E., Kao, L.-S. (1999) Oxidation state of gold and arsenic in gold-bearing arsenian pyrite. American Mineralogist 84, 1071–1079.
; Deditius et al., 2014Deditius, A.P., Reich, M., Kesler, S.E., Utsunomiya, S., Walshe, J., Chryssoulis, S.L., Ewing, R.C. (2014) The coupled geochemistry of Au and As in pyrite from hydrothermal deposits. Geochimica et Cosmochimica Acta 140, 644–670.
; Pokrovski et al., 2019Pokrovski, G.S., Kokh, M.A., Proux, O., Hazemann, J.-L., Bazarkina, E.F., Testemale, D., Escoda, C., Boiron, M.-C., Blanchard, M., Ajgouy, T., Gouy, S., de Parseval, P., Thibaut, M. (2019) The nature and partitioning of invisible gold in the pyrite-fluid system. Ore Geology Reviews 109, 545–563.
; Merkulova et al., 2019Merkulova, M., Mathon, O., Glatzel, P., Rovezzi, M., Batanova, V., Marion, P., Boiron, M.-C., Manceau, A. (2019) Revealing the chemical form of “invisible” gold in natural arsenian pyrite and arsenopyrite with high energy-resolution X-ray absorption spectroscopy: ACS Earth Space Chemistry 3, 1905–1914.
; Filimonova et al., 2020Filimonova, O.N., Tagirov, B.R., Trigub, A.L., Nickolsky, M.S., Rovezzi, M., Belogub, E.V., Reukov, V.L., Vikentiev, I.A. (2020) The state of Au and As in pyrite studied by X-ray absorption spectroscopy of natural minerals and synthetic phases. Ore Geology Reviews 121, 103475.
).In an attempt to elucidate fundamental factors controlling the nature of invisible Au in arsenian pyrite and Fe sulfarsenides and the role played by As in Au intake, we used high energy resolution fluorescence detection X-ray absorption spectroscopy (HERFD-XAS), which is the most direct method to provide information about a trace element redox state, chemical bonding, and coordination at the atomic scale (e.g., Proux et al., 2017
Proux, O., Lahera, E., Del Net, W., Kieffer, I., Rovezzi, M., Testemale, D., Irar, M., Thomas, S., Aguilar-Tapia, A., Bazarkina, E.F., Prat, A., Tella, M., Auffan, M., Rose, J., Hazemann, J.-L. (2017) High energy resolution fluorescence detected X-ray absorption spectroscopy: a new powerful structural tool in environmental biogeochemistry sciences. Journal of Environmental Quality 46, 1146–1157.
). Both X-ray absorption near-edge (XANES) and extended X-ray absorption fine structure (EXAFS) spectra were acquired on a set of thoroughly characterised Au-bearing pyrite and arsenopyrite samples from major metamorphic and sedimentary-hosted gold deposits and their synthetic analogues (including löllingite) prepared in controlled laboratory experiments (Supplementary Information). The obtained spectroscopic and fluid-mineral partitioning data were interpreted using quantum chemistry and thermodynamic approaches to generate a new structural and physico-chemical model that reveals the fundamental role played by arsenic in gold incorporation in pyrite and Fe sulfarsenides.top
Atomic State of Gold from Spectroscopic Data
XANES spectra of chemically bound gold in all natural and synthetic arsenopyrite, löllingite and As-rich pyrite (≥1 wt. % As) samples examined here significantly differ from those of metallic Au0 and other reference compounds in which Au is coordinated by 2 to 4 As/S/Cl atoms or by 6 Sb/Te atoms, implying a different Au local environment in sulfarsenides (Figs. 1, S-6). In contrast, spectra of As-poor pyrites (<0.1 wt. % As) display distinctly lower energy and amplitudes that indicate a lower Au coordination and/or different electronic configuration than in more As-enriched samples. Quantum chemistry simulated XANES spectra of various Au positions in the mineral structure (Supplementary Information) show that Au enters (Au,Fe)(As,S)6 octahedral sites variably enriched in As (Fig. 2). For löllingite, the measured spectra are perfectly matched by a simulated one for the AuAs6 site. For arsenopyrite, the spectra are consistent with AuAs3S3-AuAs6 units, rather than simple Au-to-Fe substitution in a stoichiometric FeAs3S3 site (Fig. S-8). These interpretations are fully supported by EXAFS analyses that yield 6 ± 1 As atoms at 2.53 ± 0.01 Å bound to Au in löllingite, and 4.5 ± 0.5 As at 2.51 ± 0.02 Å plus 1.5 ± 0.5 S atoms at 2.42 ± 0.05 Å in arsenopyrite (Table S-4). The remarkable similarity of bound Au atomic environment among all natural and synthetic arsenopyrite samples from various geological settings and experimental conditions demonstrates the universality of the Au substitution mechanism. For arsenian pyrites studied here, both XANES and EXAFS data (Figs. 2, S-9) demonstrate Au to be in an As-enriched Fe site, AuAs3S3. In contrast, for As-poor pyrite, spectra indicate quasi-linear AuS2 geometries, which are typical of AuI aqueous (poly)sulfide species and most thiol and sulfide solids (Pokrovski et al., 2015
Pokrovski, G.S., Kokh, M.A., Guillaume, D., Borisova, A.Y., Gisquet, P., Hazemann, J.-L., Lahera, E., Del Net, W., Proux, O., Testemale, D., Haigis, V., Jonchière, R., Seitsonen, A.P., Ferlat, G., Vuilleumier, R., Saitta, A.M., Boiron, M.-C., Dubessy, J. (2015) Sulfur radical species form gold deposits on Earth. Proceedings of the National Academy of Sciences 112, 13484–13489.
, 2019Pokrovski, G.S., Kokh, M.A., Proux, O., Hazemann, J.-L., Bazarkina, E.F., Testemale, D., Escoda, C., Boiron, M.-C., Blanchard, M., Ajgouy, T., Gouy, S., de Parseval, P., Thibaut, M. (2019) The nature and partitioning of invisible gold in the pyrite-fluid system. Ore Geology Reviews 109, 545–563.
). The difference in Au atomic environment between As-poor pyrite on one hand and arsenian pyrite, arsenopyrite and löllingite on the other hand is strong evidence that As directly impacts the mode of Au incorporation.
Figure 1 Gold L3-edge HERFD XANES spectra (offset vertically) of representative Au-bearing natural (nat) and experimental (exp) pyrite (Py), arsenopyrite (Apy) and löllingite (Lo) samples, and selected reference compounds with indicated Au first shell atomic coordination (see Supplementary Information for details).

Figure 2 Comparison of Au L3-edge HERFD XANES spectra of representative samples with quantum chemistry simulated spectra of Au in different structural sites as pictured by the displayed atomic clusters. The spectra of (a) Apy, (b) Lo, and (c) As-rich Py are consistent with Au in an As-enriched [Au(As,S)6] octahedral site, whereas Au in As-poor Py (c) is in [AuS2] moieties similar to those in exemplified AuI-(poly)sulfide complexes.
top
Structural Model for Chemically Bound Gold in Pyrite and Iron Sulfarsenides
Our spectroscopic results combined with available data are consistent with the structural model shown in Figure 3. The degree of Au enrichment in pyrite generally depends on As content, being the lowest in As-poor pyrite and increasing with As content, as demonstrated by the large body of micro-to-nano scale analyses of Au and As concentrations in the mineral (Reich et al., 2005
Reich, M., Kesler, S.E., Utsunomiya, S., Palenik, C.S., Chryssoulis, S.L., Ewing, R.C. (2005) Solubility of gold in arsenian pyrite. Geochimica et Cosmochimica Acta 69, 2781–2796.
; Deditius et al., 2014Deditius, A.P., Reich, M., Kesler, S.E., Utsunomiya, S., Walshe, J., Chryssoulis, S.L., Ewing, R.C. (2014) The coupled geochemistry of Au and As in pyrite from hydrothermal deposits. Geochimica et Cosmochimica Acta 140, 644–670.
), and also directly evidenced by our and recent experiments (Kusebauch et al., 2019Kusebauch, C., Gleeson, S.A., Oelze, M. (2019) Coupled partitioning of Au and As into pyrite controls the formation of giant Au deposits. Science Advances 5, eaav5891.
). In As-poor pyrite, Au is dominantly surface-chemisorbed as AuI (poly)sulfide clusters that may be partly incorporated into structural defects and dislocations depending on crystal growth kinetics (e.g., Wu et al., 2019Wu, Y.-F., Fougerouse, D., Evans, K., Reddy, S.M., Saxey, D.W., Guagliardo, P., Li, J.-W. (2019) Gold, arsenic, and copper zoning in pyrite: A record of fluid chemistry and growth kinetics. Geology 47, 641–644.
), and partly expulsed as Au0 nanoparticles due to the very limited capacity of pyrite to intake Au in the absence of As (Pokrovski et al., 2019Pokrovski, G.S., Kokh, M.A., Proux, O., Hazemann, J.-L., Bazarkina, E.F., Testemale, D., Escoda, C., Boiron, M.-C., Blanchard, M., Ajgouy, T., Gouy, S., de Parseval, P., Thibaut, M. (2019) The nature and partitioning of invisible gold in the pyrite-fluid system. Ore Geology Reviews 109, 545–563.
). The pronounced change in the Au solubility pattern with increasing As content above 0.01–0.1 wt. % As (Fig. 3), as documented in many natural studies, implies a fundamental change in the gold intake mechanism to increased Au partitioning into As-enriched Fe(As,S)6 sites compared to the pyrite FeS6 crystallographic site, as evidenced by the direct detection of As in the Au nearest atomic shell (Figs. 2, S-9). The number of As atoms bound to Au (n) may display large variability, from below detection limit (n < 1) to n ≥ 3, as reported in recent XAS studies of some natural and synthetic pyrites with >1 wt. % As (Merkulova et al., 2019Merkulova, M., Mathon, O., Glatzel, P., Rovezzi, M., Batanova, V., Marion, P., Boiron, M.-C., Manceau, A. (2019) Revealing the chemical form of “invisible” gold in natural arsenian pyrite and arsenopyrite with high energy-resolution X-ray absorption spectroscopy: ACS Earth Space Chemistry 3, 1905–1914.
; Filimonova et al., 2020Filimonova, O.N., Tagirov, B.R., Trigub, A.L., Nickolsky, M.S., Rovezzi, M., Belogub, E.V., Reukov, V.L., Vikentiev, I.A. (2020) The state of Au and As in pyrite studied by X-ray absorption spectroscopy of natural minerals and synthetic phases. Ore Geology Reviews 121, 103475.
). Thus, Au in arsenian pyrite is likely to exhibit a range of Au(AsnS6-n) environments, from S-rich (n ≤ 1) to As-rich (n ≥ 3). This trend is followed by the continued enhancement of Au binding to As in arsenopyrite and löllingite (n > 4; Fig. 3). Formation of Au chemical bonds with As−I, which is the dominant As redox state in the three minerals in most settings, provides a fundamental explanation for the systematically higher invisible Au contents analysed at the micron scale in arsenopyrite and löllingite compared to coexisting pyrite in many hydrothermal deposits (Tables S-3, S-11), as well as for experimentally measured very high Au partition coefficients between iron sulfarsenides and aqueous fluid (>105; Table S-8).
Figure 3 Structural model for chemically bound Au in pyrite, arsenopyrite and löllingite (not to scale). The Au coordination is shown by ball-and-stick atomic clusters (Au = pink, S = yellow, As = green, Fe = brown, S3 = blue, H = grey). Horizontal gray bars indicate the typical range of As contents in each mineral. Empirical Au solubility limit in arsenian pyrite (solid curve; Reich et al., 2005
Reich, M., Kesler, S.E., Utsunomiya, S., Palenik, C.S., Chryssoulis, S.L., Ewing, R.C. (2005) Solubility of gold in arsenian pyrite. Geochimica et Cosmochimica Acta 69, 2781–2796.
) was extrapolated to Apy and Lo (dashed curve). Note a fundamental transition in the Au incorporation mechanism (vertical dashed line, indicative position), from chemisorption as AuI-polysulfide complexes at low As content in pyrite to coupled Au-As redox reactions driving Au entry in As-enriched Fe crystallographic sites of the three minerals.The available XAS data on natural pyrite and arsenopyrite samples do not reveal atoms other than As or S in the Au nearest shell that might accompany Au incorporation. Thus, direct AuII to FeII substitution favoured by As−I would be a universal mechanism that does not require specific structural vacancies or charge compensations from other minor elements in different redox states (CuI, SbIII, FeIII, AsIII). Although the notion of redox state for strongly covalent bonds such as Au-As or Au-S has no strict sense (Cabri et al., 2000
Cabri, L.J., Newville, M., Gordon, R.A., Crozier, E.D., Sutton, S.R., McMahon, G., Jiang, D.-T. (2000) Chemical speciation of gold in arsenopyrite. Canadian Mineralogist 38, 1265−1281.
), the formal AuII state would be consistent with the three key features of gold coordination chemistry: i) the great majority of formally AuI and AuIII compounds have either 2 (linear) or 4 (square/tetrahedral) Au coordination (Cotton and Wilkinson, 1988Cotton, A.F., Wilkinson, G. (1988) Advanced Inorganic Chemistry. Fifth Edition, Wiley, New York.
) poorly compatible with an octahedral site, ii) complexes of AuII coordinated by 4 to 6 ligands (S, P, Se), and often paralleled by [Au⋯Au] clustering as found in arsenopyrite in this study (Fig. S-8), are well known in chemistry (Laguna and Laguna, 1999Laguna, A., Laguna, M. (1999) Coordination chemistry of gold(II) complexes. Coordination Chemistry Reviews 193–195, 837–856.
), iii) gold in many arsenide and chalcogenide compounds (AuAs2, AuSb2, AuTe2, AuSe) has a formal oxidation state of AuII and a coordination of 6 (ICSD, 2020ICSD (2020) Inorganic Crystal Structure Database. ICSD, FIZ Karlsruhe.
George, L.L., Cook, N.J., Ciobanu, C.L. (2017) Minor and trace elements in natural tetrahedrite-tennantite: Effects of element partitioning among base metal sulphides. Minerals 7, 17.
, 2018George, L.L., Cook, N.J., Crowe, B.B.P., Ciobanu, C.L. (2018) Trace elements in hydrothermal chalcopyrite. Mineralogical Magazine 82, 59–88.
).top
Thermodynamic Model for Gold Solubility in Arsenopyrite and Löllingite
Gold incorporation in iron sulfarsenides may thus be interpreted as a coupled redox reaction between the dominant AuI/AsIII redox states in the hydrothermal fluid and AuII/As−I in the mineral. Because Au in arsenopyrite occurs in a combination of arsenopyrite and löllingite-type sites (Fig. 3), Au solubility and partitioning may be formally approximated by a hypothetical sulfarsenide end member AuAsS-AuAs2 (equivalent to AuAs1.5S0.5) “dissolved” in arsenopyrite:
Eq. 1
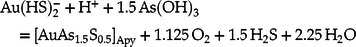
Eq. 2

where [AuAs1.5S0.5]Apy is the mole fraction of the Au-sulfarsenide end member in the arsenopyrite solid solution, and Au(HS)2− and As(OH)3 are the activities of the major Au and As aqueous species in the fluid (Perfetti et al., 2008
Perfetti, E., Pokrovski, G.S., Ballerat-Busserolles, K., Majer, V., Gibert, F. (2008) Densities and heat capacities of aqueous arsenious and arsenic acid solutions to 350 °C and 300 bar, and revised thermodynamic properties of As(OH)30(aq), AsO(OH)30(aq) and iron sulfarsenide minerals. Geochimica et Cosmochimica Acta 72, 713–731.
; Pokrovski et al., 2015Pokrovski, G.S., Kokh, M.A., Guillaume, D., Borisova, A.Y., Gisquet, P., Hazemann, J.-L., Lahera, E., Del Net, W., Proux, O., Testemale, D., Haigis, V., Jonchière, R., Seitsonen, A.P., Ferlat, G., Vuilleumier, R., Saitta, A.M., Boiron, M.-C., Dubessy, J. (2015) Sulfur radical species form gold deposits on Earth. Proceedings of the National Academy of Sciences 112, 13484–13489.
). The thermodynamic constants of reactions (Eq. 1) and (Eq. 2), derived from experimental mineral-fluid partition coefficients at 450 °C and 700 bar are log10K1 = −18.2 ± 0.3 and log10K2 = −22.4 ± 0.3, respectively (1 s.d.; Table S-9). An analogous model of the AuAs2-FeAs2 solid solution is valid for löllingite (Table S-10). The generated constants enable, for the first time, direct predictions of Au mineral-fluid partitioning and solubility in sulfarsenides as a function of the key hydrothermal fluid parameters such as As/S content, redox and pH (Fig. 4). Applying similar quantitative models to pyrite would require more systematic experimental and natural data to account for the large range in the Au(AsnS6-n) site stoichiometry (Fig. 3). The empirical gold solubility limit reported for arsenian pyrite is likely to be defined by reactions analogous to (Eq. 1) and (Eq. 2), thereby reflecting the natural variability limits of the fluid parameters.
Figure 4 Gold solubility in arsenopyrite (Apy) and löllingite (Lo) in equilibrium with hydrothermal fluid and native gold, predicted as a function of (a) log fO2 (in bars, relative to the nickel-nickel oxide O2 buffer, NNO) at typical H2S and As(OH)3 fluid phase concentrations for the indicated major types of gold deposits, and (b) As and S fluid phase content at fO2 of NNO–0.5 within the Apy stability domain (Perfetti et al., 2008
Perfetti, E., Pokrovski, G.S., Ballerat-Busserolles, K., Majer, V., Gibert, F. (2008) Densities and heat capacities of aqueous arsenious and arsenic acid solutions to 350 °C and 300 bar, and revised thermodynamic properties of As(OH)30(aq), AsO(OH)30(aq) and iron sulfarsenide minerals. Geochimica et Cosmochimica Acta 72, 713–731.
) in metamorphic gold deposits (circles; Table S-11 for compilation). Also plotted are equilibrium Au(HS)2− concentrations in fluid (blue curve), Au Apy/Fluid partition coefficient (D(Au)Apy/Fluid, mass units ratio, same y scale, red curve), and Apy-Lo, Apy-pyrrhotite (Po), and Apy-pyrite (Py) equilibrium lines.top
Implications
Our new findings help to clarify one of the oldest enigmas of geochemistry about the unique role played by arsenic in Au fate in hydrothermal systems. Our results demonstrate that both arsenopyrite and löllingite are capable of accommodating large concentrations of bound Au (100s to 1000s μg/g) even from Au-poor fluids (<0.1 μg/g Au) in relatively reduced metamorphic and ultramafic rock settings (fO2 < NNO; Fig. 4a), whereas in more oxidised orogenic- and porphyry-related systems little Au is incorporated, in full agreement with natural observations (e.g., Deditius et al., 2014
Deditius, A.P., Reich, M., Kesler, S.E., Utsunomiya, S., Walshe, J., Chryssoulis, S.L., Ewing, R.C. (2014) The coupled geochemistry of Au and As in pyrite from hydrothermal deposits. Geochimica et Cosmochimica Acta 140, 644–670.
). Our quantitative predictions are currently limited at 450 °C, but lower temperatures may further favour Au-As redox reactions and Au intake in sulfarsenides, consistent with enhanced equilibrium As partitioning into pyrite (Xing et al., 2019Xing, Y., Brugger, J., Tomkins, A., Shvarov, Y.V. (2019) Arsenic evolution as a tool for understanding formation of pyritic gold ores. Geology 47, 335–338.
). Surface sorption and mineral growth rate factors may also contribute to Au scavenging (e.g., Wu et al., 2019Wu, Y.-F., Fougerouse, D., Evans, K., Reddy, S.M., Saxey, D.W., Guagliardo, P., Li, J.-W. (2019) Gold, arsenic, and copper zoning in pyrite: A record of fluid chemistry and growth kinetics. Geology 47, 641–644.
), but the fundamental arsenic-driven redox control will equally hold. Changes in redox, pH and dissolved As/S contents upon fluid evolution adequately account for the large variability of Au content widely documented in different mineral generations within individual deposits and across mineralised domains in different deposit types (Fig. 4b). Gold intake by and release from sulfarsenides driven by variations in fluid composition appear to be key factors for hydrothermal gold deposit formation. For example, in giant Carlin-type deposits, these factors may have driven focused coupled Au and As-pyrite deposition (Kusebauch et al., 2019Kusebauch, C., Gleeson, S.A., Oelze, M. (2019) Coupled partitioning of Au and As into pyrite controls the formation of giant Au deposits. Science Advances 5, eaav5891.
). In orogenic-type deposits, first order Au “pumping” from the fluid by pyrite and arsenopyrite, followed by large scale Au liberation from those minerals during further metamorphism, may have provided a gold source and strongly influenced metal endowment, timing of mineralisation, and spatial distribution (Large et al., 2011Large, R.R., Bull, S.W., Maslennikov, V.V. (2011) A carbonaceous sedimentary source-rock model for Carlin-type and orogenic gold deposits. Economic Geology 106, 331–358.
; Velásquez et al., 2014Velásquez, G., Béziat, D., Salvi, S., Siebenaller, L., Borisova, A.Y., Pokrovski, G.S., de Parseval, P. (2014) Formation and deformation of pyrite and implications for gold mineralization at the El Callao mining district, Venezuela. Economic Geology 109, 457–486.
; Fougerouse et al., 2016Fougerouse, D., Micklethwaite, S., Tomkins, A.G., Mei, Y., Kilburn, M., Guagliardo, P., Fisher, L.A., Halfpenny, A., Gee, M., Paterson, D., Howard, D.L. (2016) Gold remobilisation and formation of high grade ore shoots driven by dissolution-reprecipitation replacement and Ni substitution into auriferous arsenopyrite. Geochimica et Cosmochimica Acta 178, 143–159.
). Future advances of in situ spectroscopy will offer more systematic quantification of invisible gold along with other valuable trace metals hidden in iron sulfarsenide minerals, thereby enabling a better understanding of trace element geochemical cycles and improving resource assessment, exploration and recovery.top
Acknowledgments
This work was funded by the French National Research Agency (RadicalS ANR-16-CE31-0017, SOUMET ANR-2011-Blanc-SIMI-5-6-009), the Institut des Sciences de l’Univers of the Centre National de la Recherche Scientifique, INSU-CNRS (CESSUR-OrPy-AsOrPy), the Institut Carnot ISIFoR (OrPet), and the West African Exploration Initiative (WAXI-P934A). We acknowledge the European Synchrotron Radiation Facility (ESRF) for providing access to beam time and infrastructure, and AMIRA International for support in field-related aspects. The FAME-UHD project is supported by the French Grand Emprunt EquipEx (EcoX ANR-10-EQPX-27-01), the CEA-CNRS CRG consortium and the INSU-CNRS. DFT calculations were performed using HPC resources from CALMIP (2020-P1037). We thank A.-M. Cousin for invaluable help with figure layout, S. Foulon for tube welding, A. Manceau and B. Tagirov for sharing XAS data and references, and Y. Joly for advice on XANES modeling. Comments by Editor S. Redfern and an anonymous referee greatly improved this article.
Editor: Simon Redfern
top
References
Adams, M.D. (Ed.) (2005) Advances in Gold Ore Processing. Elsevier Science, Amsterdam.
 Show in context
Show in context Thus, the redox and structural state of chemically bound Au and its link with As may define a deposit’s economic potential (e.g., Kusebauch et al., 2019), determine the type and cost of Au recovery from ore (e.g., Adams, 2005) and, more generally, affect the gold distribution at the Earth’s crust scale (e.g., Large et al., 2011).
View in article
Arehart, G.B., Chryssoulis, S.L., Kesler, S.E. (1993) Gold and arsenic in iron sulfides from sediment-hosted disseminated gold deposits: Implication for depositional processes. Economic Geology 88, 171−185.
 Show in context
Show in context Existing interpretations vary from the formation of aqueous Au-As complexes or Au0 electrochemical deposition and Au(HS)2− chemisorption on arsenian pyrite and arsenopyrite surfaces or precipitation of gold sulfide phases, to Au entering Fe, S or As crystallographic sites, along with other cation substitutions or structural vacancies, and with the formal oxidation state of chemically bound Au spanning from –1 to +3 and coordination from 2 to 6 (e.g., Arehart et al., 1993; Möller and Kersten, 1994; Simon et al., 1999; Deditius et al., 2014; Pokrovski et al., 2019; Merkulova et al., 2019; Filimonova et al., 2020).
View in article
Cabri, L.J., Newville, M., Gordon, R.A., Crozier, E.D., Sutton, S.R., McMahon, G., Jiang, D.-T. (2000) Chemical speciation of gold in arsenopyrite. Canadian Mineralogist 38, 1265−1281.
 Show in context
Show in context Although the notion of redox state for strongly covalent bonds such as Au-As or Au-S has no strict sense (Cabri et al., 2000), the formal AuII state would be consistent with the three key features of gold coordination chemistry: i) the great majority of formally AuI and AuIII compounds have either 2 (linear) or 4 (square/tetrahedral) Au coordination (Cotton and Wilkinson, 1988) poorly compatible with an octahedral site, ii) complexes of AuII coordinated by 4 to 6 ligands (S, P, Se), and often paralleled by [Au⋯Au] clustering as found in arsenopyrite in this study (Fig. S-8), are well known in chemistry (Laguna and Laguna, 1999), iii) gold in many arsenide and chalcogenide compounds (AuAs2, AuSb2, AuTe2, AuSe) has a formal oxidation state of AuII and a coordination of 6 (ICSD, 2020).
View in article
Cathelineau, M., Boiron, M.-C., Holliger, P., Marion, P., Denis, M. (1989) Gold in arsenopyrites: Crystal chemistry, location and state, physical and chemical conditions of deposition. Economic Geology Monograph 6, 328–341.
 Show in context
Show in context A large part of gold hosted by these minerals is “invisible” or “refractory” (i.e. optically undetectable) occurring both as metal nanoparticles (Au0) and chemically bound Au, the latter being often the dominant gold state and commonly associated with arsenic on the (sub)micron scale (e.g., Cathelineau et al., 1989; Cook and Chryssoulis, 1990; Reich et al., 2005).
View in article
Cook, N.J., Chryssoulis, S.L. (1990) Concentrations of “invisible” gold in the common sulfides. Canadian Mineralogist 28, 1–16.
 Show in context
Show in context A large part of gold hosted by these minerals is “invisible” or “refractory” (i.e. optically undetectable) occurring both as metal nanoparticles (Au0) and chemically bound Au, the latter being often the dominant gold state and commonly associated with arsenic on the (sub)micron scale (e.g., Cathelineau et al., 1989; Cook and Chryssoulis, 1990; Reich et al., 2005).
View in article
Cotton, A.F., Wilkinson, G. (1988) Advanced Inorganic Chemistry. Fifth Edition, Wiley, New York.
 Show in context
Show in context Although the notion of redox state for strongly covalent bonds such as Au-As or Au-S has no strict sense (Cabri et al., 2000), the formal AuII state would be consistent with the three key features of gold coordination chemistry: i) the great majority of formally AuI and AuIII compounds have either 2 (linear) or 4 (square/tetrahedral) Au coordination (Cotton and Wilkinson, 1988) poorly compatible with an octahedral site, ii) complexes of AuII coordinated by 4 to 6 ligands (S, P, Se), and often paralleled by [Au⋯Au] clustering as found in arsenopyrite in this study (Fig. S-8), are well known in chemistry (Laguna and Laguna, 1999), iii) gold in many arsenide and chalcogenide compounds (AuAs2, AuSb2, AuTe2, AuSe) has a formal oxidation state of AuII and a coordination of 6 (ICSD, 2020).
View in article
Deditius, A.P., Reich, M., Kesler, S.E., Utsunomiya, S., Walshe, J., Chryssoulis, S.L., Ewing, R.C. (2014) The coupled geochemistry of Au and As in pyrite from hydrothermal deposits. Geochimica et Cosmochimica Acta 140, 644–670.
 Show in context
Show in context The degree of Au enrichment in pyrite generally depends on As content, being the lowest in As-poor pyrite and increasing with As content, as demonstrated by the large body of micro-to-nano scale analyses of Au and As concentrations in the mineral (Reich et al., 2005; Deditius et al., 2014), and also directly evidenced by our and recent experiments (Kusebauch et al., 2019).
View in article
Our results demonstrate that both arsenopyrite and löllingite are capable of accommodating large concentrations of bound Au (100s to 1000s μg/g) even from Au-poor fluids (<0.1 μg/g Au) in relatively reduced metamorphic and ultramafic rock settings (fO2 < NNO; Fig. 4a), whereas in more oxidised orogenic- and porphyry-related systems little Au is incorporated, in full agreement with natural observations (e.g., Deditius et al., 2014).
View in article
Existing interpretations vary from the formation of aqueous Au-As complexes or Au0 electrochemical deposition and Au(HS)2− chemisorption on arsenian pyrite and arsenopyrite surfaces or precipitation of gold sulfide phases, to Au entering Fe, S or As crystallographic sites, along with other cation substitutions or structural vacancies, and with the formal oxidation state of chemically bound Au spanning from –1 to +3 and coordination from 2 to 6 (e.g., Arehart et al., 1993; Möller and Kersten, 1994; Simon et al., 1999; Deditius et al., 2014; Pokrovski et al., 2019; Merkulova et al., 2019; Filimonova et al., 2020).
View in article
Filimonova, O.N., Tagirov, B.R., Trigub, A.L., Nickolsky, M.S., Rovezzi, M., Belogub, E.V., Reukov, V.L., Vikentiev, I.A. (2020) The state of Au and As in pyrite studied by X-ray absorption spectroscopy of natural minerals and synthetic phases. Ore Geology Reviews 121, 103475.
 Show in context
Show in context The number of As atoms bound to Au (n) may display large variability, from below detection limit (n < 1) to n ≥ 3, as reported in recent XAS studies of some natural and synthetic pyrites with >1 wt. % As (Merkulova et al., 2019; Filimonova et al., 2020).
View in article
Existing interpretations vary from the formation of aqueous Au-As complexes or Au0 electrochemical deposition and Au(HS)2− chemisorption on arsenian pyrite and arsenopyrite surfaces or precipitation of gold sulfide phases, to Au entering Fe, S or As crystallographic sites, along with other cation substitutions or structural vacancies, and with the formal oxidation state of chemically bound Au spanning from –1 to +3 and coordination from 2 to 6 (e.g., Arehart et al., 1993; Möller and Kersten, 1994; Simon et al., 1999; Deditius et al., 2014; Pokrovski et al., 2019; Merkulova et al., 2019; Filimonova et al., 2020).
View in article
Fougerouse, D., Micklethwaite, S., Tomkins, A.G., Mei, Y., Kilburn, M., Guagliardo, P., Fisher, L.A., Halfpenny, A., Gee, M., Paterson, D., Howard, D.L. (2016) Gold remobilisation and formation of high grade ore shoots driven by dissolution-reprecipitation replacement and Ni substitution into auriferous arsenopyrite. Geochimica et Cosmochimica Acta 178, 143–159.
 Show in context
Show in context In orogenic-type deposits, first order Au “pumping” from the fluid by pyrite and arsenopyrite, followed by large scale Au liberation from those minerals during further metamorphism, may have provided a gold source and strongly influenced metal endowment, timing of mineralisation, and spatial distribution (Large et al., 2011; Velásquez et al., 2014; Fougerouse et al., 2016).
View in article
George, L.L., Cook, N.J., Ciobanu, C.L. (2017) Minor and trace elements in natural tetrahedrite-tennantite: Effects of element partitioning among base metal sulphides. Minerals 7, 17.
 Show in context
Show in context These key features distinguish these minerals from other hydrothermal sulfides such as pyrrhotite, chalcopyrite, bornite, or sulfosalts that host very little invisible Au (e.g., George et al., 2017, 2018).
View in article
George, L.L., Cook, N.J., Crowe, B.B.P., Ciobanu, C.L. (2018) Trace elements in hydrothermal chalcopyrite. Mineralogical Magazine 82, 59–88.
 Show in context
Show in context These key features distinguish these minerals from other hydrothermal sulfides such as pyrrhotite, chalcopyrite, bornite, or sulfosalts that host very little invisible Au (e.g., George et al., 2017, 2018).
View in article
ICSD (2020) Inorganic Crystal Structure Database. ICSD, FIZ Karlsruhe. https://icsd.products.fiz-karlsruhe.de, accessed 2 April 2021.
 Show in context
Show in context Although the notion of redox state for strongly covalent bonds such as Au-As or Au-S has no strict sense (Cabri et al., 2000), the formal AuII state would be consistent with the three key features of gold coordination chemistry: i) the great majority of formally AuI and AuIII compounds have either 2 (linear) or 4 (square/tetrahedral) Au coordination (Cotton and Wilkinson, 1988) poorly compatible with an octahedral site, ii) complexes of AuII coordinated by 4 to 6 ligands (S, P, Se), and often paralleled by [Au⋯Au] clustering as found in arsenopyrite in this study (Fig. S-8), are well known in chemistry (Laguna and Laguna, 1999), iii) gold in many arsenide and chalcogenide compounds (AuAs2, AuSb2, AuTe2, AuSe) has a formal oxidation state of AuII and a coordination of 6 (ICSD, 2020).
View in article
Kusebauch, C., Gleeson, S.A., Oelze, M. (2019) Coupled partitioning of Au and As into pyrite controls the formation of giant Au deposits. Science Advances 5, eaav5891.
 Show in context
Show in context Thus, the redox and structural state of chemically bound Au and its link with As may define a deposit’s economic potential (e.g., Kusebauch et al., 2019), determine the type and cost of Au recovery from ore (e.g., Adams, 2005) and, more generally, affect the gold distribution at the Earth’s crust scale (e.g., Large et al., 2011).
View in article
For example, in giant Carlin-type deposits, these factors may have driven focused coupled Au and As-pyrite deposition (Kusebauch et al., 2019).
View in article
The degree of Au enrichment in pyrite generally depends on As content, being the lowest in As-poor pyrite and increasing with As content, as demonstrated by the large body of micro-to-nano scale analyses of Au and As concentrations in the mineral (Reich et al., 2005; Deditius et al., 2014), and also directly evidenced by our and recent experiments (Kusebauch et al., 2019).
View in article
Laguna, A., Laguna, M. (1999) Coordination chemistry of gold(II) complexes. Coordination Chemistry Reviews 193–195, 837–856.
 Show in context
Show in context Although the notion of redox state for strongly covalent bonds such as Au-As or Au-S has no strict sense (Cabri et al., 2000), the formal AuII state would be consistent with the three key features of gold coordination chemistry: i) the great majority of formally AuI and AuIII compounds have either 2 (linear) or 4 (square/tetrahedral) Au coordination (Cotton and Wilkinson, 1988) poorly compatible with an octahedral site, ii) complexes of AuII coordinated by 4 to 6 ligands (S, P, Se), and often paralleled by [Au⋯Au] clustering as found in arsenopyrite in this study (Fig. S-8), are well known in chemistry (Laguna and Laguna, 1999), iii) gold in many arsenide and chalcogenide compounds (AuAs2, AuSb2, AuTe2, AuSe) has a formal oxidation state of AuII and a coordination of 6 (ICSD, 2020).
View in article
Large, R.R., Bull, S.W., Maslennikov, V.V. (2011) A carbonaceous sedimentary source-rock model for Carlin-type and orogenic gold deposits. Economic Geology 106, 331–358.
 Show in context
Show in context In orogenic-type deposits, first order Au “pumping” from the fluid by pyrite and arsenopyrite, followed by large scale Au liberation from those minerals during further metamorphism, may have provided a gold source and strongly influenced metal endowment, timing of mineralisation, and spatial distribution (Large et al., 2011; Velásquez et al., 2014; Fougerouse et al., 2016).
View in article
Thus, the redox and structural state of chemically bound Au and its link with As may define a deposit’s economic potential (e.g., Kusebauch et al., 2019), determine the type and cost of Au recovery from ore (e.g., Adams, 2005) and, more generally, affect the gold distribution at the Earth’s crust scale (e.g., Large et al., 2011).
View in article
Merkulova, M., Mathon, O., Glatzel, P., Rovezzi, M., Batanova, V., Marion, P., Boiron, M.-C., Manceau, A. (2019) Revealing the chemical form of “invisible” gold in natural arsenian pyrite and arsenopyrite with high energy-resolution X-ray absorption spectroscopy: ACS Earth Space Chemistry 3, 1905–1914.
 Show in context
Show in context The number of As atoms bound to Au (n) may display large variability, from below detection limit (n < 1) to n ≥ 3, as reported in recent XAS studies of some natural and synthetic pyrites with >1 wt. % As (Merkulova et al., 2019; Filimonova et al., 2020).
View in article
Existing interpretations vary from the formation of aqueous Au-As complexes or Au0 electrochemical deposition and Au(HS)2− chemisorption on arsenian pyrite and arsenopyrite surfaces or precipitation of gold sulfide phases, to Au entering Fe, S or As crystallographic sites, along with other cation substitutions or structural vacancies, and with the formal oxidation state of chemically bound Au spanning from –1 to +3 and coordination from 2 to 6 (e.g., Arehart et al., 1993; Möller and Kersten, 1994; Simon et al., 1999; Deditius et al., 2014; Pokrovski et al., 2019; Merkulova et al., 2019; Filimonova et al., 2020).
View in article
Möller, P., Kersten, G. (1994) Electrochemical accumulation of visible gold on pyrite and arsenopyrite surfaces. Mineralium Deposita 29, 404–413.
 Show in context
Show in context Existing interpretations vary from the formation of aqueous Au-As complexes or Au0 electrochemical deposition and Au(HS)2− chemisorption on arsenian pyrite and arsenopyrite surfaces or precipitation of gold sulfide phases, to Au entering Fe, S or As crystallographic sites, along with other cation substitutions or structural vacancies, and with the formal oxidation state of chemically bound Au spanning from –1 to +3 and coordination from 2 to 6 (e.g., Arehart et al., 1993; Möller and Kersten, 1994; Simon et al., 1999; Deditius et al., 2014; Pokrovski et al., 2019; Merkulova et al., 2019; Filimonova et al., 2020).
View in article
Perfetti, E., Pokrovski, G.S., Ballerat-Busserolles, K., Majer, V., Gibert, F. (2008) Densities and heat capacities of aqueous arsenious and arsenic acid solutions to 350 °C and 300 bar, and revised thermodynamic properties of As(OH)30(aq), AsO(OH)30(aq) and iron sulfarsenide minerals. Geochimica et Cosmochimica Acta 72, 713–731.
 Show in context
Show in context Gold solubility in arsenopyrite (Apy) and löllingite (Lo) in equilibrium with hydrothermal fluid and native gold, predicted as a function of (a) log fO2 (in bars, relative to the nickel-nickel oxide O2 buffer, NNO) at typical H2S and As(OH)3 fluid phase concentrations for the indicated major types of gold deposits, and (b) As and S fluid phase content at fO2 of NNO–0.5 within the Apy stability domain (Perfetti et al., 2008) in metamorphic gold deposits (circles; Table S-11 for compilation).
View in article
Because Au in arsenopyrite occurs in a combination of arsenopyrite and löllingite-type sites (Fig. 3), Au solubility and partitioning may be formally approximated by a hypothetical sulfarsenide end member AuAsS-AuAs2 (equivalent to AuAs1.5S0.5) “dissolved” in arsenopyrite: 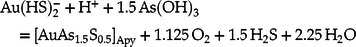 Eq. 1
Eq. 1 Eq. 2
Eq. 2
where [AuAs1.5S0.5]Apy is the mole fraction of the Au-sulfarsenide end member in the arsenopyrite solid solution, and Au(HS)2− and As(OH)3 are the activities of the major Au and As aqueous species in the fluid (Perfetti et al., 2008; Pokrovski et al., 2015).
View in article
Pokrovski, G.S., Kokh, M.A., Guillaume, D., Borisova, A.Y., Gisquet, P., Hazemann, J.-L., Lahera, E., Del Net, W., Proux, O., Testemale, D., Haigis, V., Jonchière, R., Seitsonen, A.P., Ferlat, G., Vuilleumier, R., Saitta, A.M., Boiron, M.-C., Dubessy, J. (2015) Sulfur radical species form gold deposits on Earth. Proceedings of the National Academy of Sciences 112, 13484–13489.
 Show in context
Show in context In contrast, for As-poor pyrite, spectra indicate quasi-linear AuS2 geometries, which are typical of AuI aqueous (poly)sulfide species and most thiol and sulfide solids (Pokrovski et al., 2015, 2019).
View in article
Because Au in arsenopyrite occurs in a combination of arsenopyrite and löllingite-type sites (Fig. 3), Au solubility and partitioning may be formally approximated by a hypothetical sulfarsenide end member AuAsS-AuAs2 (equivalent to AuAs1.5S0.5) “dissolved” in arsenopyrite: 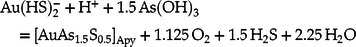 Eq. 1
Eq. 1 Eq. 2
Eq. 2
where [AuAs1.5S0.5]Apy is the mole fraction of the Au-sulfarsenide end member in the arsenopyrite solid solution, and Au(HS)2− and As(OH)3 are the activities of the major Au and As aqueous species in the fluid (Perfetti et al., 2008; Pokrovski et al., 2015).
View in article
Pokrovski, G.S., Kokh, M.A., Proux, O., Hazemann, J.-L., Bazarkina, E.F., Testemale, D., Escoda, C., Boiron, M.-C., Blanchard, M., Ajgouy, T., Gouy, S., de Parseval, P., Thibaut, M. (2019) The nature and partitioning of invisible gold in the pyrite-fluid system. Ore Geology Reviews 109, 545–563.
 Show in context
Show in context In contrast, for As-poor pyrite, spectra indicate quasi-linear AuS2 geometries, which are typical of AuI aqueous (poly)sulfide species and most thiol and sulfide solids (Pokrovski et al., 2015, 2019).
View in article
In As-poor pyrite, Au is dominantly surface-chemisorbed as AuI (poly)sulfide clusters that may be partly incorporated into structural defects and dislocations depending on crystal growth kinetics (e.g., Wu et al., 2019), and partly expulsed as Au0 nanoparticles due to the very limited capacity of pyrite to intake Au in the absence of As (Pokrovski et al., 2019).
View in article
Existing interpretations vary from the formation of aqueous Au-As complexes or Au0 electrochemical deposition and Au(HS)2− chemisorption on arsenian pyrite and arsenopyrite surfaces or precipitation of gold sulfide phases, to Au entering Fe, S or As crystallographic sites, along with other cation substitutions or structural vacancies, and with the formal oxidation state of chemically bound Au spanning from –1 to +3 and coordination from 2 to 6 (e.g., Arehart et al., 1993; Möller and Kersten, 1994; Simon et al., 1999; Deditius et al., 2014; Pokrovski et al., 2019; Merkulova et al., 2019; Filimonova et al., 2020).
View in article
Proux, O., Lahera, E., Del Net, W., Kieffer, I., Rovezzi, M., Testemale, D., Irar, M., Thomas, S., Aguilar-Tapia, A., Bazarkina, E.F., Prat, A., Tella, M., Auffan, M., Rose, J., Hazemann, J.-L. (2017) High energy resolution fluorescence detected X-ray absorption spectroscopy: a new powerful structural tool in environmental biogeochemistry sciences. Journal of Environmental Quality 46, 1146–1157.
 Show in context
Show in context In an attempt to elucidate fundamental factors controlling the nature of invisible Au in arsenian pyrite and Fe sulfarsenides and the role played by As in Au intake, we used high energy resolution fluorescence detection X-ray absorption spectroscopy (HERFD-XAS), which is the most direct method to provide information about a trace element redox state, chemical bonding, and coordination at the atomic scale (e.g., Proux et al., 2017).
View in article
Reich, M., Kesler, S.E., Utsunomiya, S., Palenik, C.S., Chryssoulis, S.L., Ewing, R.C. (2005) Solubility of gold in arsenian pyrite. Geochimica et Cosmochimica Acta 69, 2781–2796.
 Show in context
Show in context The degree of Au enrichment in pyrite generally depends on As content, being the lowest in As-poor pyrite and increasing with As content, as demonstrated by the large body of micro-to-nano scale analyses of Au and As concentrations in the mineral (Reich et al., 2005; Deditius et al., 2014), and also directly evidenced by our and recent experiments (Kusebauch et al., 2019).
View in article
Horizontal gray bars indicate the typical range of As contents in each mineral. Empirical Au solubility limit in arsenian pyrite (solid curve; Reich et al., 2005) was extrapolated to Apy and Lo (dashed curve).
View in article
A large part of gold hosted by these minerals is “invisible” or “refractory” (i.e. optically undetectable) occurring both as metal nanoparticles (Au0) and chemically bound Au, the latter being often the dominant gold state and commonly associated with arsenic on the (sub)micron scale (e.g., Cathelineau et al., 1989; Cook and Chryssoulis, 1990; Reich et al., 2005).
View in article
Simon, G., Huang, H., Penner-Hahn, J.E., Kesler, S.E., Kao, L.-S. (1999) Oxidation state of gold and arsenic in gold-bearing arsenian pyrite. American Mineralogist 84, 1071–1079.
 Show in context
Show in context Existing interpretations vary from the formation of aqueous Au-As complexes or Au0 electrochemical deposition and Au(HS)2− chemisorption on arsenian pyrite and arsenopyrite surfaces or precipitation of gold sulfide phases, to Au entering Fe, S or As crystallographic sites, along with other cation substitutions or structural vacancies, and with the formal oxidation state of chemically bound Au spanning from –1 to +3 and coordination from 2 to 6 (e.g., Arehart et al., 1993; Möller and Kersten, 1994; Simon et al., 1999; Deditius et al., 2014; Pokrovski et al., 2019; Merkulova et al., 2019; Filimonova et al., 2020).
View in article
Velásquez, G., Béziat, D., Salvi, S., Siebenaller, L., Borisova, A.Y., Pokrovski, G.S., de Parseval, P. (2014) Formation and deformation of pyrite and implications for gold mineralization at the El Callao mining district, Venezuela. Economic Geology 109, 457–486.
 Show in context
Show in context In orogenic-type deposits, first order Au “pumping” from the fluid by pyrite and arsenopyrite, followed by large scale Au liberation from those minerals during further metamorphism, may have provided a gold source and strongly influenced metal endowment, timing of mineralisation, and spatial distribution (Large et al., 2011; Velásquez et al., 2014; Fougerouse et al., 2016).
View in article
Wu, Y.-F., Fougerouse, D., Evans, K., Reddy, S.M., Saxey, D.W., Guagliardo, P., Li, J.-W. (2019) Gold, arsenic, and copper zoning in pyrite: A record of fluid chemistry and growth kinetics. Geology 47, 641–644.
 Show in context
Show in context Surface sorption and mineral growth rate factors may also contribute to Au scavenging (e.g., Wu et al., 2019), but the fundamental arsenic-driven redox control will equally hold.
View in article
In As-poor pyrite, Au is dominantly surface-chemisorbed as AuI (poly)sulfide clusters that may be partly incorporated into structural defects and dislocations depending on crystal growth kinetics (e.g., Wu et al., 2019), and partly expulsed as Au0 nanoparticles due to the very limited capacity of pyrite to intake Au in the absence of As (Pokrovski et al., 2019).
View in article
Xing, Y., Brugger, J., Tomkins, A., Shvarov, Y.V. (2019) Arsenic evolution as a tool for understanding formation of pyritic gold ores. Geology 47, 335–338.
 Show in context
Show in context Our quantitative predictions are currently limited at 450 °C, but lower temperatures may further favour Au-As redox reactions and Au intake in sulfarsenides, consistent with enhanced equilibrium As partitioning into pyrite (Xing et al., 2019).
View in article
top
Supplementary Information
The Supplementary Information includes:
Download the Supplementary Information (PDF).
Figures
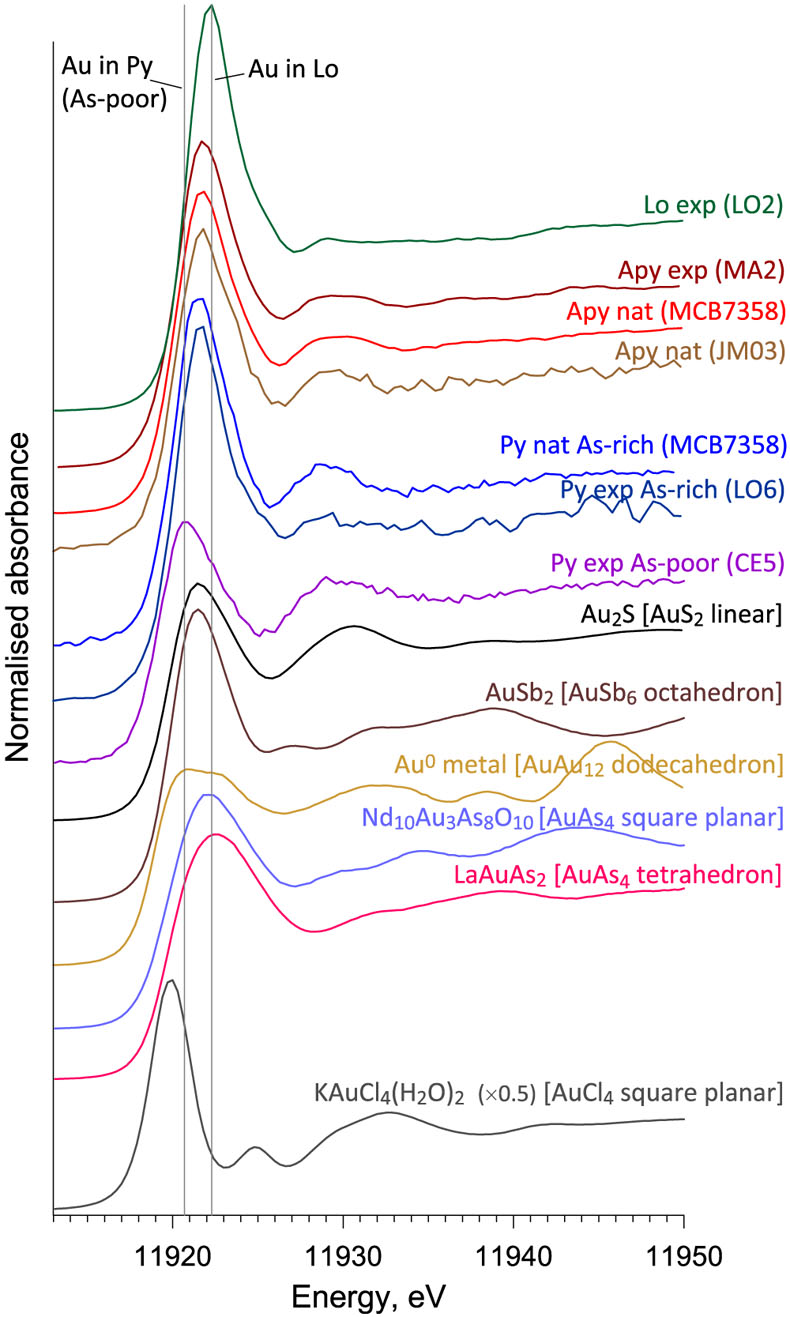
Figure 1 Gold L3-edge HERFD XANES spectra (offset vertically) of representative Au-bearing natural (nat) and experimental (exp) pyrite (Py), arsenopyrite (Apy) and löllingite (Lo) samples, and selected reference compounds with indicated Au first shell atomic coordination (see Supplementary Information for details).
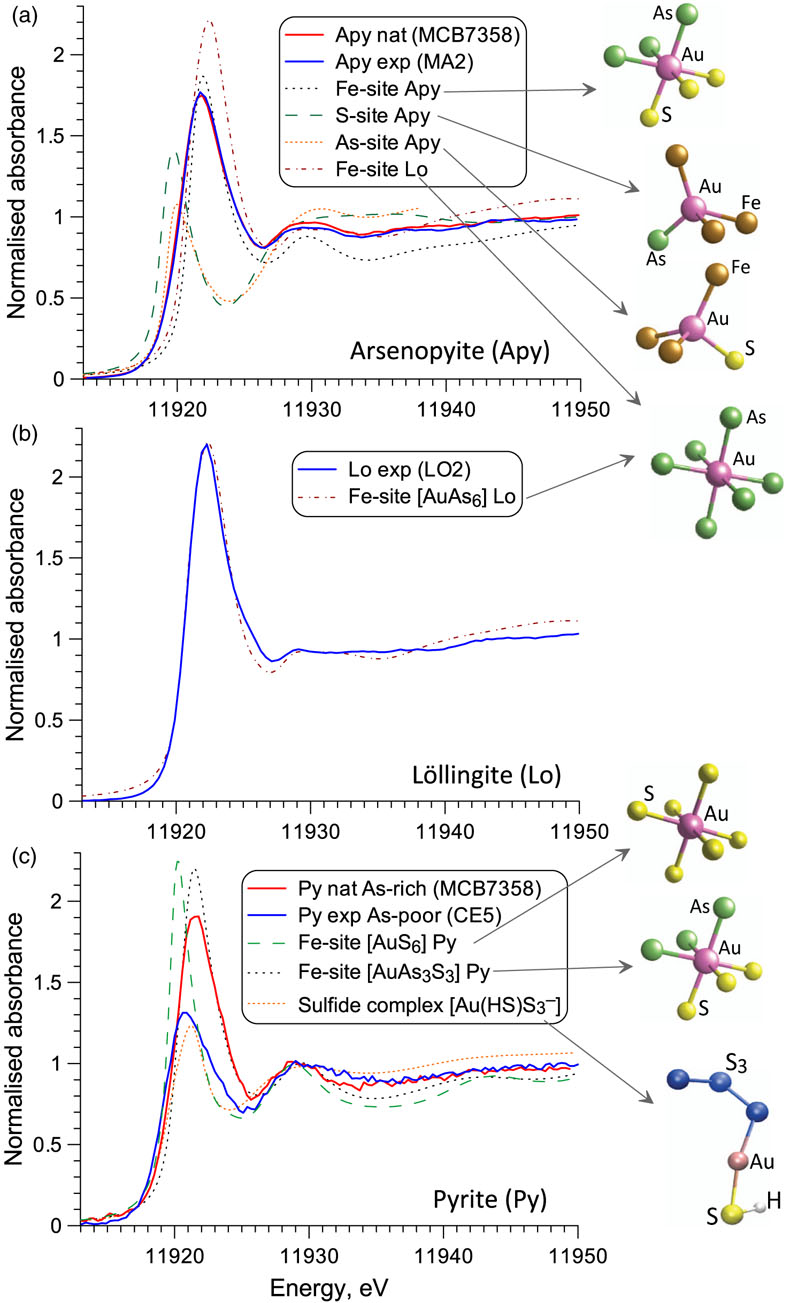
Figure 2 Comparison of Au L3-edge HERFD XANES spectra of representative samples with quantum chemistry simulated spectra of Au in different structural sites as pictured by the displayed atomic clusters. The spectra of (a) Apy, (b) Lo, and (c) As-rich Py are consistent with Au in an As-enriched [Au(As,S)6] octahedral site, whereas Au in As-poor Py (c) is in [AuS2] moieties similar to those in exemplified AuI-(poly)sulfide complexes.
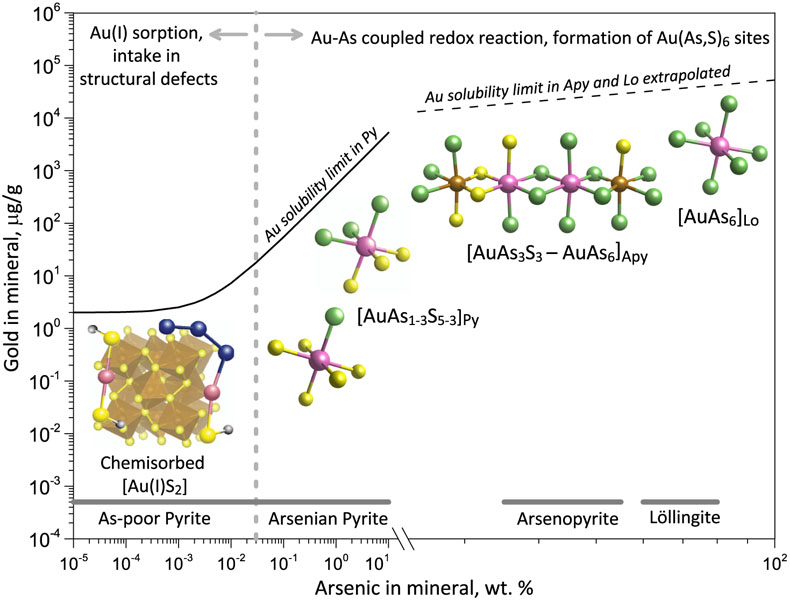
Figure 3 Structural model for chemically bound Au in pyrite, arsenopyrite and löllingite (not to scale). The Au coordination is shown by ball-and-stick atomic clusters (Au = pink, S = yellow, As = green, Fe = brown, S3 = blue, H = grey). Horizontal gray bars indicate the typical range of As contents in each mineral. Empirical Au solubility limit in arsenian pyrite (solid curve; Reich et al., 2005
Reich, M., Kesler, S.E., Utsunomiya, S., Palenik, C.S., Chryssoulis, S.L., Ewing, R.C. (2005) Solubility of gold in arsenian pyrite. Geochimica et Cosmochimica Acta 69, 2781–2796.
) was extrapolated to Apy and Lo (dashed curve). Note a fundamental transition in the Au incorporation mechanism (vertical dashed line, indicative position), from chemisorption as AuI-polysulfide complexes at low As content in pyrite to coupled Au-As redox reactions driving Au entry in As-enriched Fe crystallographic sites of the three minerals.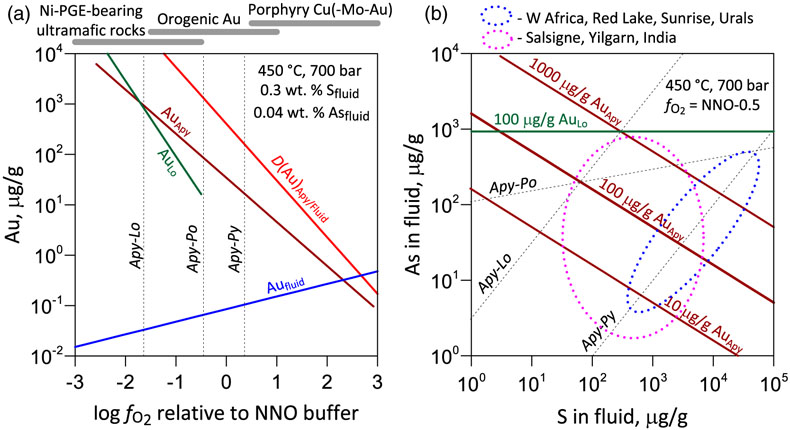
Figure 4 Gold solubility in arsenopyrite (Apy) and löllingite (Lo) in equilibrium with hydrothermal fluid and native gold, predicted as a function of (a) log fO2 (in bars, relative to the nickel-nickel oxide O2 buffer, NNO) at typical H2S and As(OH)3 fluid phase concentrations for the indicated major types of gold deposits, and (b) As and S fluid phase content at fO2 of NNO–0.5 within the Apy stability domain (Perfetti et al., 2008
Perfetti, E., Pokrovski, G.S., Ballerat-Busserolles, K., Majer, V., Gibert, F. (2008) Densities and heat capacities of aqueous arsenious and arsenic acid solutions to 350 °C and 300 bar, and revised thermodynamic properties of As(OH)30(aq), AsO(OH)30(aq) and iron sulfarsenide minerals. Geochimica et Cosmochimica Acta 72, 713–731.
) in metamorphic gold deposits (circles; Table S-11 for compilation). Also plotted are equilibrium Au(HS)2− concentrations in fluid (blue curve), Au Apy/Fluid partition coefficient (D(Au)Apy/Fluid, mass units ratio, same y scale, red curve), and Apy-Lo, Apy-pyrrhotite (Po), and Apy-pyrite (Py) equilibrium lines.