- Open access, no page charges
- Short articles (3000 words)
- Non-profit community journal
- Highest-quality research in geochemical sciences
 Top 10 most viewed articles (cumulative count of HTML views) for the last 60 days.
Top 10 most viewed articles (cumulative count of HTML views) for the last 60 days.
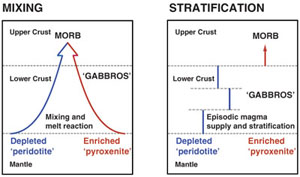
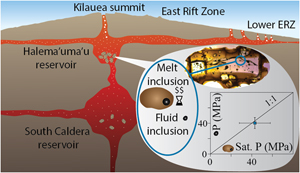
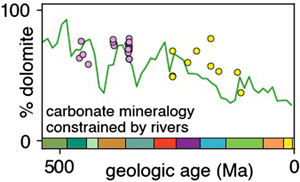 | River chemistry reveals a large decrease in dolomite abundance across the Phanerozoic Abstract: Article views: 1692 |
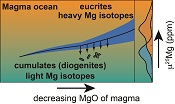 | Tracking the formation of magma oceans in the Solar System using stable magnesium isotopes Abstract: Article views: 510 |
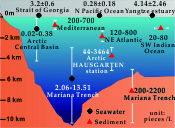 | Microplastics contaminate the deepest part of the world’s ocean Abstract: Article views: 499 |
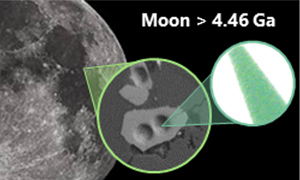 | 4.46 Ga zircons anchor chronology of lunar magma ocean Abstract: Article views: 402 |
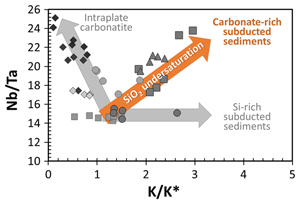 | Fractionation of Nb/Ta during subduction of carbonate-rich sediments Abstract: Article views: 359 |
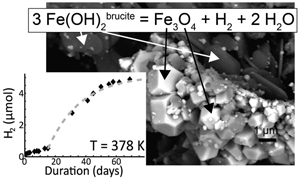 | Kinetics of low-temperature H2 production in ultramafic rocks by ferroan brucite oxidation Abstract: Article views: 313 |
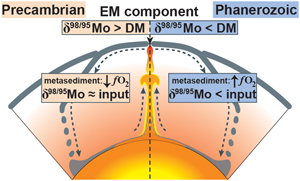 | Molybdenum isotopes in plume-influenced MORBs reveal recycling of ancient anoxic sediments Abstract: Article views: 302 |
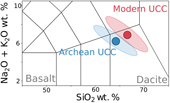 | The composition and weathering of the continents over geologic time Abstract: Article views: 296 |
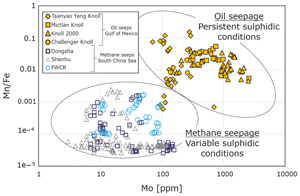 | Pyrite-based trace element fingerprints for methane and oil seepage Abstract: Article views: 295 |
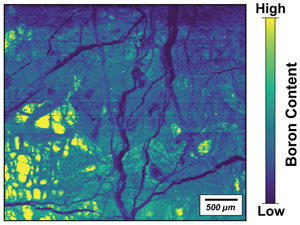 | Imaging of boron in altered mantle rocks illuminates progressive serpentinisation episodes Abstract: Article views: 280 |
 Top 10 most viewed articles (cumulative count of HTML views) for the last 12 months.
Top 10 most viewed articles (cumulative count of HTML views) for the last 12 months.
 | 4.46 Ga zircons anchor chronology of lunar magma ocean Abstract: Article views: 40450 |
 | Earth’s first glaciation at 2.9 Ga revealed by triple oxygen isotopes Abstract: Article views: 12310 |
 | Microplastics contaminate the deepest part of the world’s ocean Abstract: Article views: 11913 |
 | River chemistry reveals a large decrease in dolomite abundance across the Phanerozoic Abstract: Article views: 10641 |
 | Emergence of peraluminous crustal magmas and implications for the early Earth Abstract: Article views: 8566 |
 | A whole-lithosphere view of continental growth Abstract: Article views: 6765 |
 | Dust transport enhanced land surface weatherability in a cooling world Abstract: Article views: 5720 |
 | Environmental pressure from the 2014–15 eruption of Bárðarbunga volcano, Iceland Abstract: Article views: 5317 |
 | The composition and weathering of the continents over geologic time Abstract: Article views: 5190 |
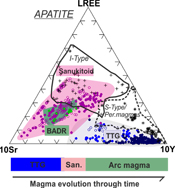 | Accessory mineral constraints on crustal evolution: elemental fingerprints for magma discrimination Abstract: Article views: 5064 |
 Top 10 most viewed articles (cumulative count of HTML views) for all time.
Top 10 most viewed articles (cumulative count of HTML views) for all time.
 | Microplastics contaminate the deepest part of the world’s ocean Abstract: Article views: 59502 |
 | 4.46 Ga zircons anchor chronology of lunar magma ocean Abstract: Article views: 40450 |
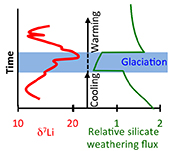 | Global climate stabilisation by chemical weathering during the Hirnantian glaciation Abstract: Article views: 37799 |
 | Copper isotope evidence for large-scale sulphide fractionation during Earth’s differentiation Abstract: Article views: 31090 |
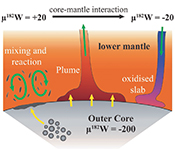 | 182W evidence for core-mantle interaction in the source of mantle plumes Abstract: Article views: 28639 |
 | Environmental pressure from the 2014–15 eruption of Bárðarbunga volcano, Iceland Abstract: Article views: 27781 |
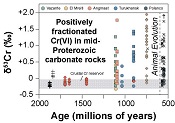 | Oxygenation of the mid-Proterozoic atmosphere: clues from chromium isotopes in carbonates Abstract: Article views: 27116 |
 | Release of subducted sedimentary nitrogen throughout Earth’s mantle Abstract: Article views: 27102 |
 | Molecular hydrogen in mantle minerals Abstract: Article views: 25445 |
 | Rapid response of silicate weathering rates to climate change in the Himalaya Abstract: Article views: 25055 |